Simple Summary
Head and neck cancers exhibit considerable heterogeneity, complicating the prediction of disease progression and treatment response. Researchers are actively investigating reliable biomarkers to forecast disease trajectories and inform therapeutic decisions. This study examines the role of BAG3, a protein involved in cell survival and stress response, as a potential prognostic marker in head and neck cancers. By analyzing BAG3 expression across various head and neck cancer types and correlating it with disease-free survival, the study aims to elucidate BAG3 positivity’s influence on cancer progression. Our findings indicate that immunohistochemical testing for BAG3 positivity could aid in diagnosing tumor progression and prognosis in head and neck squamous cell carcinoma (HNSCC), with potential implications for therapeutic strategies.
Abstract
Background: Head and neck squamous cell carcinoma (HNSCC) exhibit considerable heterogeneity, complicating the prediction of disease progression and treatment response. Consequently, researchers are actively investigating reliable biomarkers to forecast disease trajectories and inform therapeutic decisions. This study examines the role of BAG3, a protein involved in cell survival and stress response, as a potential predictive marker in HNSCC. The objective is to analyze BAG3 expression across various HNSCC types and correlate it with disease-free survival (DFS), aiming to elucidate the influence of BAG3 positivity on cancer progression. Methods: A multi-institutional retrospective study was conducted by analyzing BAG3 expression by immunohistochemistry in 104 tissue samples from patients with head and neck squamous cell carcinoma (HNSCC). The data were then correlated with DFS to assess the impact of BAG3 positivity on prognosis. Results: Immunohistochemical analysis of primary tumor samples collected from therapy-naive patients showed that BAG3 positivity was widespread across different head and neck cancer sites, with no significant correlation to sex, smoking status, HPV infection, tumor location, grade, or TNM parameters. However, BAG3 high positive patients had shorter DFS (median 23.2 months) compared to BAG3-negative patients (median 31.3 months). Cox analysis revealed that BAG3 high expression by IHC was associated with a more than 3-fold increased risk of disease recurrence. Conclusions: This study is the first to explore BAG3 as a biomarker for HNSCC recurrence. While preliminary findings suggest a link between BAG3 positivity and increased recurrence risk, further research is needed to validate these results. Prospective studies could help establish BAG3’s prognostic value and potentially lead to more personalized treatment approaches for HNSCC.
1. Introduction
Head and neck cancers (HNCs) encompass a wide array of malignancies that develop in various anatomical sites, including the oral cavity, pharynx, larynx, nasal cavity, and salivary glands [1,2]. The predominant type of these cancers is squamous cell carcinoma (HNSCC), which is frequently linked to risk factors such as tobacco and alcohol consumption, as well as human papillomavirus (HPV) infection [3,4]. Although there have been significant advancements in multimodal treatment strategies—such as surgery, radiation therapy, chemotherapy, and targeted therapies—patient outcomes continue to vary considerably due to the diverse nature of these tumors [5,6]. Histopathological biomarkers are crucial in head and neck cancers for improving prediction and prognosis. They offer detailed insights into tumor behavior, aiding personalized treatment decisions, risk stratification, and monitoring, thereby enhancing patient outcomes. These biomarkers, discovered through methods like immunohistochemistry and molecular profiling, offer valuable insights into tumor biology, aggressiveness, and responses to treatment [7,8]. The BAG3 (Bcl-2-associated athanogene 3) protein is a multifunctional co-chaperone of Hsp70 protein with an important involvement in tumor biology, particularly in cancer cell survival, resistance to therapy, and tumor development [9]. Its expression is normally raised in normal cells during stress, but it is constitutively overexpressed in a variety of human malignancies, including glioblastomas [10], pancreatic adenocarcinomas [11], melanomas [12], breast cancers [13], and thyroid carcinomas [14]. BAG3 contributes to tumor progression through several mechanisms, including anti-apoptotic activity, chemoresistance, promotion of tumor invasiveness, and maintenance of cancer stem cells [15]. In glioblastoma cells, BAG3 interacts with heat shock protein 70 (HSP70) to limit apoptosis by preventing the pro-apoptotic protein BAX from translocating to mitochondria [10]. Similarly, in thyroid carcinomas [14], leukemia [16], and ovarian cancer cells [17], BAG3 downregulation makes cells more susceptible to apoptosis-inducing therapy. Indeed, scientific evidence increasingly supports the link between high BAG3 expression and chemotherapy resistance across various malignancies. In ovarian cancer, for example, elevated BAG3 levels are associated with poor prognosis and resistance to treatments like paclitaxel and cisplatin [18], while in triple-negative breast cancer (TNBC), BAG3 overexpression contributes to apoptosis resistance and enhances cytoprotective autophagy, making these cells more resilient to chemotherapeutic agents [19]. Melanoma studies have also revealed that BAG3 positivity correlates with tumor aggressiveness and sustains NF-κB activation, further contributing to chemotherapy resistance [20]. Furthermore, BAG3 expression levels frequently correspond with tumor grade and aggressiveness. As an example, BAG3 expression in glioblastomas increases with tumor grade, and BAG3 silencing by siRNA inhibits cell growth both in vitro and in vivo [10], while elevated BAG3 levels in pancreatic ductal adenocarcinoma (PDAC) [11] and medulloblastoma [21] are associated with shorter patient survival. In PDAC, pancreatic cancer cell-secreted BAG3 activates macrophages via IFITM2-mediated signaling pathways such as PI3K and p38 MAPK phosphorilation, increasing tumor development and metastasis in a way that can be inhibited by a specific anti- BAG3, neutralizing monoclonal antibody [22]. Moreover, secreted BAG3 neutralization can enhance immunotherapy effects [23,24] while reducing fibrotic stroma [25] in PDAC animal models. Currently, there is limited specific information available on how BAG3 overexpression affects distinct subtypes of head and neck cancers. One risk factor for HNSCC is HPV positivity and patients with HPV-positive head and neck cancer (HNC) generally experience better survival outcomes; however, if recurrence occurs, it is more likely to happen in distant parts of the body and may arise later compared to HPV-negative patients [26]. A previous study showed that in HPV18-positive HeLa cells, BAG3 interacts with the viral E6 oncoprotein, stabilizing E6 and promoting degradation of the tumor suppressor p53. Downregulating BAG3 reduced E6 levels and restored p53, implicating BAG3 in HPV-driven cell transformation [27]. Furthermore, evidence suggests that BAG3 plays a key role in the tumor microenvironment, especially in fibrotic tumor phenotypes frequently seen in HNSCC [28,29,30]. Specifically, analysis using the GEPIA database revealed that BAG3 mRNA expression is higher in tumors compared to normal tissues across multiple cancers, including HNSCC, and that elevated BAG3 levels are associated with poorer clinical outcomes [28].
In this study, the expression of BAG3 has been investigated by immunohistochemistry in a series of head and neck cancers from different anatomical regions. The results were correlated with clinicopathological parameters of the tumors, including localization, tumor grade, tumor stage and survival, with the intention of providing additional tools to clinicians for predicting disease outcomes and guiding treatment decisions.
2. Materials and Methods
2.1. Patients
Patients with confirmed biopsy of SCC of oral cavity, oropharynx and larynx between January 2022 and January 2023 were retrospectively included in this study. The cohort included patients from multicenter sites, recruited in the departments of Otolaryngology—Head and Neck Surgery of San Giovanni Addolorata Hospital (Rome, Italy), IRCCS Regina Elena National Cancer Institute (Rome, Italy), and AORN Cardarelli (Naples, Italy). We conducted a retrospective review of patients’ medical records, considering the type of surgery performed, the histopathological results and the follow-up reports. For each patient, the following data were collected: gender; age at diagnosis; type of surgery performed (including any neck dissection performed at the time of the first surgery); presence of clinically evident lymph node(s) (cN) or distant metastasis at diagnosis; positive or negative tumor resection margins post-surgery; use of adjuvant radio chemotherapy (RTChT); presence of locoregional or distant recurrence; and health status at follow-up (including follow-up time in months). The TNM classification system (version 8) [31] was used to stage the tumor. Data on survival outcomes were extracted from mortality registries, outpatient visit notes and radiological follow-ups. The data collected from each center were analyzed. The exclusion criteria were as follows: (i) absence of follow-up, (ii) changes in the histological diagnosis during review of the slides, and (iii) slides unavailable for review. The data were inserted into a shared Excel file and statistical analyses were performed once the file was complete. Two authors (P.D.L. and A.R.) analyzed the data from all centers and then shared the results with the other researchers for review and conclusion-drawing. Table 1 shows the epidemiological and clinical features of the patients included in the study.

Table 1.
Patients characteristics at the time of diagnosis.
2.2. Immunohistochemistry
Four-micrometer-thick tissue sections were mounted on poly-L-lysine-coated slides and analyzed using immunohistochemistry (IHC) with a monoclonal anti-BAG3 antibody developed in our laboratories. The anti-BAG3 monoclonal antibody (mAb) was generated by immunizing four mice with a recombinant polypeptide encompassing amino acids 89 to 213 of the human BAG3 protein. Anti-BAG3 antibody titers in the mice sera were monitored using the ELISA method. Subsequently, myeloma cells were fused with splenocytes harvested from the sacrificed mice, followed by screening, selection, and expansion of positive clones producing antibodies specific to the target protein. This standard process successfully yielded the clone utilized in the present study. The IHC protocol included deparaffinization in xylene, rehydration through a graded series of ethanol to water, incubation with 3% hydrogen peroxide for 5 min to inactivate endogenous peroxidases, and enzymatic antigen retrieval in CC1 buffer (Ventana Medical System), pH 8.0, for 36 min at 95 °C. After washing with saline solution (1X PBS), the samples were blocked with 5% fetal bovine serum in 0.1% PBS/BSA and then incubated for 1 h at room temperature with the anti-BAG3 antibody (3 μg/mL) or with murine IgG as isotype control. The reaction was developed using the standard streptavidin-biotin technique, with 3,3′-diaminobenzidine (DAB) as the substrate/chromogen for peroxidase activity. Finally, cell nuclei were counterstained with hematoxylin, and coverslips were mounted using a synthetic mounting medium. BAG3 staining was negative in the non-neoplastic components of tissue samples and was also absent in 24 tested samples from human parotid tissue. In cancer samples tested, immunoreactivity was assessed using a semi-quantitative scoring system: score 0 (complete negativity), score 1 (weak reaction), score 2 (moderate reaction), and score 3 (strong reaction). In cases where there was uncertainty between scores 1 and 2, the case was reviewed collectively by the research team to reach a consensus. These scoring thresholds were applied consistently across all tissue types analyzed.
2.3. Statistical Analysis
Statistical correlation between BAG3 expression and patient data were performed using MedCalc® Statistical Software version 20.115 (MedCalc Software Ltd., Ostend, Belgium). Fisher’s exact test was used to test correlations between discrete variables. For survival analysis, Kaplan–Meier estimation with a Log-rank test and Cox proportional hazards models were applied. All tests were two-sided, and p-values < 0.05 were considered statistically significant.
2.4. Ethical Considerations
Ethical approval was not required by the local ethics committee due to the retrospective and non-interventional design of the study, in accordance with the Italian legislation. The study adhered to the principles outlined by the Helsinki Declaration. All data gathered were entered into an electronic database. Patients who were alive at the time of the enrollment were contacted by phone and informed about the study; none declined participation.
3. Results
Following immunohistochemical staining, 104 paraffin-embedded primary tumor samples from therapy-naive patients were evaluated for BAG3 protein expression. The analysis encompassed 30 samples from oral cavity tumors, 8 from oropharynx tumors, and 66 from larynx tumors. Among these specimens, 14 were negative for BAG3 expression, while the remaining samples tested positive. Figure 1 illustrates the immunohistochemical scoring system for BAG3 expression, ranging from negative (no detectable staining) to high positivity (strong and widespread staining). Specifically, it shows representative tumor tissue samples stained with a monoclonal anti-BAG3 antibody, including examples of BAG3-negative samples and positive samples categorized by increasing staining intensity and extent as low positivity (BAG3 score 1), medium positivity (BAG3 score 2), and high positivity (BAG3 score 3).
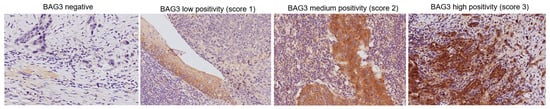
Figure 1.
Representative images from different tumor localizations are displayed based on BAG3 immunoreactivity score (images were captured at 20× magnification).
BAG3 scores did not show significant correlations with sex (p = 0.903), smoking status (p = 0.808), or HPV infection (p = 0.321). When comparing BAG3 immunostaining scores across tumors of different localizations, a relatively even distribution of negative and positive samples at different intensity signal was observed. Specifically, the analysis revealed no significant correlation between BAG3 positivity and any anatomical site of HNSCC (p = 0.729) (Figure 2A). The distribution of BAG3 score immunoreactivity was also evaluated across patients with tumor grades ranging from low (G1) to high (G3). No statistically significant differences were observed in the distribution of these scores across the different tumor grades (p = 0.540). The same observation was made when analyzing the data distribution across T (p = 0.356) and N (p = 0.235) parameters (Figure 2C,D). The observed results indicate that BAG3 negative and positive tissues are broadly and diffusely distributed across classifications of HNSCC tumors (Table 2).
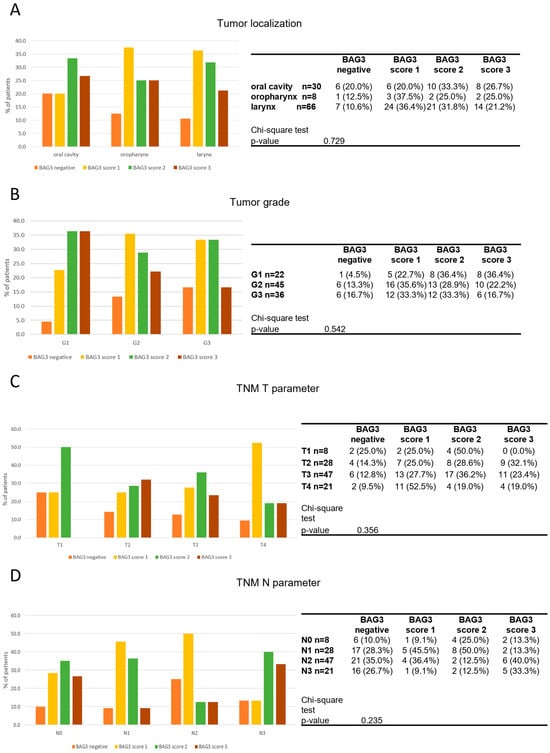
Figure 2.
Distribution of BAG3 score obtained in tumor samples across tumor localization (A), tumor grade (B), TNM T parameter (C) and TNM N parameter (D). Groups were compared with appropriate contingency tables and by fisher’s exact test. The graphs and tables show the case numbers.

Table 2.
Multivariate Cox regression analysis.
Given the potential role of BAG3 in driving molecular phenotypes towards more aggressive and resistant tumors, its prognostic value was investigated. Disease-free survival was correlated with available clinic-pathological patient data and BAG3 immunoreactivity obtained scores. Data collected from 104 patients during the observational period showed a mean recurrence rate of 37.5%. A significant increase in hazard ratio (HR) was observed in our cohort in relation to tumor grade (G2 vs. G1: HR = 3.4; G3 vs. G1: HR = 3.31). Although this parameter is considered prognostically and therapeutically significant in numerous cancer types, its role in HNSCC remains controversial and is not used as a staging criterion [32]. A significant association between the TNM T parameter was found only when correlating T1 with T4 survival data, as well as N0 with N3. Notably, 54.2% of patients with high BAG3 expression experienced recurrence, compared to 36.6% of patients with medium or low BAG3 scores. In contrast, only 14.3% of BAG3-negative patients showed disease recurrence. Patients with the highest BAG3 score had a shorter disease-free survival (median: 23.2 months) compared to BAG3-negative patients (median: 31.3 months) (p = 0.014) (Figure 3). Cox proportional hazards analysis further demonstrated that high BAG3 expression (score 3) was associated with more than a threefold increased risk of disease recurrence (univariate analysis: hazard ratio 3.74; 95% confidence interval, 1.30–10.77). Patients with intermediate BAG3 scores (score 1 and score 2) had mean disease-free survival times of 23.8 and 23.2 months, respectively—both shorter than BAG3-negative patients—implying a possible trend which needs further investigation.
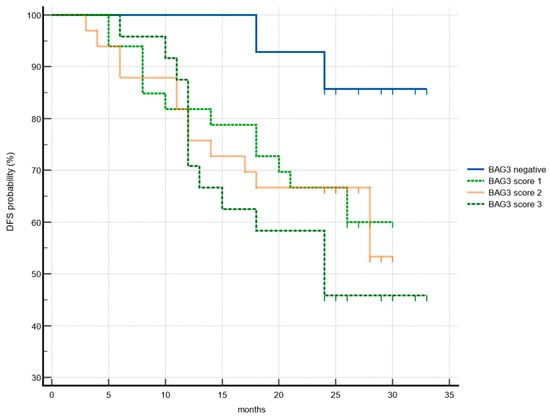
Figure 3.
Association of disease-free survival (DFS) with BAG3 immunoreactivity. Kaplan–Meier plots DFS according to the groups of BAG3 negative (blue), BAG3 score 1 (light green), BAG3 score 2 (orange), BAG3 score 3 (dark green).
4. Discussion
HNSCC is notably challenging to treat due to its aggressive nature, complex anatomical location, and high recurrence rates [33,34]. Despite advancements in surgical techniques and adjuvant therapies (RT/ChT), recurrence remains the leading cause of mortality in HNSCC patients [35,36]. Recurrence is a significant challenge, with approximately 50–60% of patients experiencing locoregional recurrence within two years post-treatment, and 20–30% developing distant metastases [37]. Therapeutic options for recurrent or metastatic HNSCC are limited and often result in poor outcomes. Platinum-based chemotherapies and immunotherapies, such as checkpoint inhibitors, offer modest survival benefits [38]. As research progresses towards more effective treatments, tissue biomarkers play a crucial role in patient risk stratification. Incorporating biomarkers into clinical practice can significantly enhance the precision of risk assessment and personalize therapeutic approaches. This strategy aims to reduce recurrence rates and improve patient outcomes [39]. Indeed, recent advancements in understanding prognostic markers and personalized treatment strategies for HNSCC emphasize the integration of molecular, clinical, and histological data. As a first example, the Accurate Prediction Model of HNSCC Overall Survival Score (APMHO), developed using transcriptional data of six key cancer driver genes (CRLF2, HSP90AA1, MAP2K1, PAFAH1B2, MYCL, and SET) alongside clinical variables like age and tumor stage, demonstrated robust predictive performance and highlighted distinct molecular and immune profiles between high- and low-APMHO groups [40]. Another study underscored the prognostic value of tumor budding (TB) as an independent biomarker in both HPV-positive and HPV-negative oropharyngeal cancer. By optimizing TB cutpoints, a superior prognostic accuracy was achieved, enabling refined risk stratification [41]. Another tumor tissue characteristic, as tumor-infiltrating lymphocyte (TIL) counts, assessed via hematoxylin and eosin (H&E)-stained samples, was studied by Torri et al. [42], who found that it was strongly associated with improved survival outcomes (OS, DFS, and DSS), demonstrating their value as a cost-effective biomarker for routine pathology workflows. Finally, histological tumor grade also emerged as a critical predictor of immunotherapy response, with high-grade tumors exhibiting increased sensitivity to immune checkpoint inhibitors, likely due to higher tumor mutational burden [43]. The evidence presented highlights the growing potential of integrating diverse prognostic markers to enhance risk stratification, refine survival predictions, and inform personalized therapeutic strategies for HNSCC. In this study, BAG3 expression was evaluated in 104 HNSCC samples from various tumor sites, and samples with high BAG3 expression were found to have a significantly higher risk of recurrence compared to BAG3-negative samples. BAG3 protein has also been described as a serum biomarker in pancreatic cancer [44], as well as in other illnesses characterized by inflammation, such as psoriasis [45], and fibrosis, such as systemic sclerosis [46,47]. Extracellular BAG3 significantly influences the tumor microenvironment by promoting fibrosis and activating stromal cells, as demonstrated in pancreatic cancer [25,26,27,28]. This fibrotic remodeling supports tumor progression and creates an immunosuppressive environment that hinders effective therapy. Neutralizing BAG3 with monoclonal antibodies has been shown to reduce fibrosis, inhibit tumor growth, and enhance the efficacy of immunotherapy by improving immune cell infiltration and activation. Given the similarities in tumor–stroma interactions, BAG3 may play a comparable role in head and neck squamous cell carcinoma (HNSCC). Therefore, targeting extracellular BAG3 holds potential as a novel therapeutic strategy to improve treatment outcomes in HNSCC.
Study Limitations
When assessing the importance and generalizability of the data given in this study, numerous limitations must be considered. As a retrospective analysis, the study has inherent methodological limitations. While the sample size appears small for a general oncology cohort, it is very representative considering the rarity of HNSCC, especially oral and oropharyngeal squamous cell carcinoma. Furthermore, like with other potential tissue biomarkers, the findings presented here need to be validated in larger clinical populations. Future research will be required to completely establish the clinical value of these techniques and confirm their role in guiding HNSCC patients’ care.
5. Conclusions
This study is inherently exploratory in nature, as it is the first investigation to our knowledge into the possible involvement of the BAG3 protein as a tissue biomarker related to disease recurrence in HNSCC patients. While the findings are promising, they are still preliminary and highlight the need for additional research. Prospective, multicenter studies are required to evaluate BAG3’s prognostic importance and investigate its potential as a molecular biomarker for disease progression monitoring and adjuvant therapy response prediction. Such developments may open the door for more precise and individualized ways to manage HNSCC.
Author Contributions
Conceptualization, P.D.L., M.D.M., L.M. and A.R.; methodology, A.L.C., P.M., J.D., L.C. and R.C.; investigation, P.D.L., A.C., L.C., R.P., G.P., L.V., F.R., G.R. and A.I.; data curation, P.D.L., A.C., R.P., G.R., A.M. and F.A.S.; writing—original draft preparation, P.D.L. and A.R.; writing—review and editing, F.A.S., P.D.L., M.D.M. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical approval was waived by the local ethics committee in view of the retrospective non-interventional nature of the study, in accordance with the Italian Law Number 3/2018. This study was conducted in compliance with the Helsinki Declaration. All the collected data were recorded in a computerized database. Patients who were still alive at the time of the enrollment were informed about the study via telephone, and none expressed opposition to inclusion.
Informed Consent Statement
Verbal informed consent to use clinical data from living patients was obtained from the participants. Verbal consent was chosen over written consent due to the retrospective, non-interventional nature of the study, in accordance with Italian law.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Acknowledgments
FIBROSYS s.r.l., an academic spin-off, provided the anti-BAG3 mAb free of charge.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, Y.; Han, J.; Zhu, Y.; Huang, N.; Qu, N. New advances in the therapeutic strategy of head and neck squamous cell carcinoma: A review of latest therapies and cutting-edge research. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2024, 1880, 189230. [Google Scholar] [CrossRef]
- Smith, C.D.L.; McMahon, A.D.; Purkayastha, M.; Creaney, G.; Clements, K.; Inman, G.J.; Bhatti, L.A.; Douglas, C.M.; Paterson, C.; Conway, D.I. Head and Neck Cancer Incidence Is Rising but the Sociodemographic Profile Is Unchanging: A Population Epidemiological Study (2001–2020). BJC Rep. 2024, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, M.E.; Chiocca, S. Human papillomavirus as a driver of head and neck cancers. Br. J. Cancer 2020, 122, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Sun, P.; Dahlstrom, K.R.; Gross, N.; Li, G. Joint effect of human papillomavirus exposure, smoking and alcohol on risk of oral squamous cell carcinoma. BMC Cancer 2023, 23, 457. [Google Scholar] [CrossRef]
- Rao, Y.J.; Goodman, J.F.; Haroun, F.; Bauman, J.E. Integrating Immunotherapy into Multimodal Treatment of Head and Neck Cancer. Cancers 2023, 15, 672. [Google Scholar] [CrossRef]
- Bhatia, A.; Burtness, B. Treating Head and Neck Cancer in the Age of Immunotherapy: A 2023 Update. Drugs 2023, 83, 217–248. [Google Scholar] [CrossRef]
- Broggi, G.; Maniaci, A.; Lentini, M.; Palicelli, A.; Zanelli, M.; Zizzo, M.; Koufopoulos, N.; Salzano, S.; Mazzucchelli, M.; Caltabiano, R. Artificial Intelligence in Head and Neck Cancer Diagnosis: A Comprehensive Review with Emphasis on Radiomics, Histopathological, and Molecular Applications. Cancers 2024, 16, 3623. [Google Scholar] [CrossRef]
- Saini, K.S.; Somara, S.; Ko, H.C.; Thatai, P.; Quintana, A.; Wallen, Z.D.; Green, M.F.; Mehrotra, R.; McGuigan, S.; Pang, L.; et al. Biomarkers in head and neck squamous cell carcinoma: Unraveling the path to precision immunotherapy. Front. Oncol. 2024, 14, 1473706. [Google Scholar] [CrossRef]
- Brenner, C.M.; Choudhary, M.; McCormick, M.G.; Cheung, D.; Landesberg, G.P.; Wang, J.F.; Song, J.; Martin, T.G.; Cheung, J.Y.; Qu, H.Q.; et al. BAG3: Nature’s Quintessential Multi-Functional Protein Functions as a Ubiquitous Intra-Cellular Glue. Cells. 2023, 12, 937. [Google Scholar] [CrossRef]
- Festa, M.; Del Valle, L.; Khalili, K.; Franco, R.; Scognamiglio, G.; Graziano, V.; De Laurenzi, V.; Turco, M.C.; Rosati, A. BAG3 Protein Is Overexpressed in Human Glioblastoma and Is a Potential Target for Therapy. Am. J. Pathol. 2011, 178, 2504–2512. [Google Scholar] [CrossRef]
- Rosati, A.; Bersani, S.; Tavano, F.; Pozza, E.D.; De Marco, M.; Palmieri, M.; De Laurenzi, V.; Franco, R.; Scognamiglio, G.; Palaia, R.; et al. Expression of the Antiapoptotic Protein BAG3 Is a Feature of Pancreatic Adenocarcinoma and Its Overexpression Is Associated with Poorer Survival. Am. J. Pathol. 2012, 181, 1524–1529. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Franco, R.; Scognamiglio, G.; Salerno, V.; Sebastiani, A.; Cennamo, G.; Ascierto, P.A.; Botti, G.; Turco, M.C.; Rosati, A. Expression of the Anti-Apoptotic Protein BAG3 in Human Melanomas. J. Investig. Dermatol. 2012, 132, 252–254. [Google Scholar] [CrossRef]
- Liu, B.Q.; Zhan, S.; Li, S.; An, M.X.; Li, C.; Yan, J.; Wang, J.M.; Wang, H.Q. BAG3 promotes stem cell-like phenotype in breast cancer by upregulation of CXCR4 via interaction with its transcript. Cell Death Dis. 2017, 8, e2933. [Google Scholar] [CrossRef]
- Chiappetta, G.; Ammirante, M.; Basile, A.; Rosati, A.; Festa, M.; Monaco, M.; Vuttariello, E.; Pasquinelli, R.; Arra, C.; Zerilli, M.; et al. The Antiapoptotic Protein BAG3 Is Expressed in Thyroid Carcinomas and Modulates Apoptosis Mediated by Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand. J. Clin. Endocrinol. Metab. 2007, 92, 1159–1163. [Google Scholar] [CrossRef]
- Kögel, D.; Linder, B.; Brunschweiger, A.; Chines, S.; Behl, C. At the Crossroads of Apoptosis and Autophagy: Multiple Roles of the Co-Chaperone BAG3 in Stress and Therapy Resistance of Cancer. Cells 2020, 9, 574. [Google Scholar] [CrossRef]
- Rosati, A.; Basile, A.; Falco, A.; D’Avenia, M.; Festa, M.; Graziano, V.; De Laurenzi, V.; Arra, C.; Pascale, M.; Turco, M.C. Role of BAG3 protein in leukemia cell survival and response to therapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2012, 1826, 365–369. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, J. Knockdown of Bag3 Synergizes with Olaparib to Kill Ovarian Cancer Cells via Repressing Autophagy. J. Investig. Med. 2021, 69, 878–883. [Google Scholar] [CrossRef]
- Qiu, S.; Sun, L.; Zhang, Y.; Han, S. Downregulation of BAG3 attenuates cisplatin resistance by inhibiting autophagy in human epithelial ovarian cancer cells. Oncol. Lett. 2019, 18, 1969–1978. [Google Scholar] [CrossRef]
- Das, C.K.; Linder, B.; Bonn, F.; Rothweiler, F.; Dikic, I.; Michaelis, M.; Cinatl, J.; Mandal, M.; Kögel, D. BAG3 Overexpression and Cytoprotective Autophagy Mediate Apoptosis Resistance in Chemoresistant Breast Cancer Cells. Neoplasia 2018, 20, 263–279. [Google Scholar] [CrossRef]
- Ammirante, M.; Rosati, A.; Arra, C.; Basile, A.; Falco, A.; Festa, M.; Pascale, M.; D’Avenia, M.; Marzullo, L.; Belisario, M.A.; et al. IKKγ protein is a target of BAG3 regulatory activity in human tumor growth. Proc. Natl. Acad. Sci. USA 2010, 107, 7497–7502. [Google Scholar] [CrossRef]
- Yang, D.; Zhou, J.; Wang, H.; Wang, Y.; Yang, G.; Zhang, Y. High Expression of BAG3 Predicts a Poor Prognosis in Human Medulloblastoma. Tumor Biol. 2016, 37, 13215–13224. [Google Scholar] [CrossRef] [PubMed]
- Rosati, A.; Basile, A.; D’auria, R.; D’avenia, M.; De Marco, M.; Falco, A.; Festa, M.; Guerriero, L.; Iorio, V.; Parente, R.; et al. BAG3 promotes pancreatic ductal adenocarcinoma growth by activating stromal macrophages. Nat. Commun. 2015, 6, 8695. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M.; Gauttier, V.; Pengam, S.; Mary, C.; Ranieri, B.; Basile, A.; Festa, M.; Falco, A.; Reppucci, F.; Cammarota, A.L.; et al. Concerted BAG3 and SIRPα blockade impairs pancreatic tumor growth. Cell Death Discov. 2022, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Iorio, V.; Rosati, A.; D’Auria, R.; De Marco, M.; Marzullo, L.; Basile, A.; Festa, M.; Pascale, M.; Remondelli, P.; Capunzo, M.; et al. Combined effect of anti-BAG3 and anti-PD-1 treatment on macrophage infiltrate, CD8+ T cell number and tumour growth in pancreatic cancer. Gut 2017, 67, 780–782. [Google Scholar] [CrossRef]
- Iorio, V.; De Marco, M.; Basile, A.; Eletto, D.; Capunzo, M.; Remondelli, P.; Sala, G.; Marzullo, L.; Rosati, A.; De Laurenzi, V.; et al. CAF-Derived IL6 and GM-CSF Cooperate to Induce M2-like TAMs-Letter. Clin. Cancer Res. 2019, 25, 892–893. [Google Scholar] [CrossRef]
- Haring, C.T.; Kana, L.A.; Dermody, S.M.; Brummel, C.; McHugh, J.B.; Casper, K.A.; Chinn, S.B.; Malloy, K.M.; Mierzwa, M.; Prince, M.E.P.; et al. Patterns of Recurrence in Head and Neck Squamous Cell Carcinoma to Inform Personalized Surveillance Protocols. Cancer 2023, 129, 2817–2827. [Google Scholar] [CrossRef]
- Cotugno, R.; Basile, A.; Romano, E.; Gallotta, D.; Belisario, M.A. BAG3 Down-Modulation Sensitizes HPV18+ HeLa Cells to PEITC-Induced Apoptosis and Restores P53. Cancer Lett. 2014, 354, 263–271. [Google Scholar] [CrossRef]
- De Marco, M.; Del Papa, N.; Reppucci, F.; Iorio, V.; Basile, A.; Falco, A.; Iaccarino, R.; Brongo, S.; De Caro, F.; Capunzo, M.; et al. BAG3 induces α-SMA expression in human fibroblasts and its over-expression correlates with poorer survival in fibrotic cancer patients. J. Cell. Biochem. 2021, 123, 91–101. [Google Scholar] [CrossRef]
- Guo, Z.; Li, K.; Ren, X.; Wang, X.; Yang, D.; Ma, S.; Zeng, X.; Zhang, P. The Role of the Tumor Microenvironment in HNSCC Resistance and Targeted Therapy. Front. Immunol. 2025, 16, 1554835. [Google Scholar] [CrossRef]
- El Herch, I.; Tornaas, S.; Dongre, H.N.; Costea, D.E. Heterogeneity of Cancer-Associated Fibroblasts and Tumor-Promoting Roles in Head and Neck Squamous Cell Carcinoma. Front. Mol. Biosci. 2024, 11, 1340024. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Luu, M.; Balzer, B.L.; Scher, K.S.; Mita, A.C.; Lu, D.J.; Shiao, S.L.; Clair, J.M.; Ho, A.S.; Zumsteg, Z.S. Variations in the association of grade with survival across the head and neck cancer landscape. Head Neck 2020, 43, 1105–1115. [Google Scholar] [CrossRef]
- Tahara, M.; Kiyota, N.; Yokota, T.; Hasegawa, Y.; Muro, K.; Takahashi, S.; Onoe, T.; Homma, A.; Taguchi, J.; Suzuki, M.; et al. Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02). Ann. Oncol. 2018, 29, 1004–1009. [Google Scholar] [CrossRef]
- Williamson, A.; Lim, A.E.; Green, F.; Lee, Y.K.; Li, L.; Moen, C.; Vasanthan, R.; Wharf, O.; Wong, J.; Paleri, V. The Burden of Recurrent Head and Neck Squamous Cell Carcinoma Across the United Kingdom: Results from a National Snapshot Study. Head Neck 2024, 47, 1399–1410. [Google Scholar] [CrossRef]
- Runnels, J.; Bloom, J.R.; Hsieh, K.; Dickstein, D.R.; Shi, Y.; Jones, B.M.; Lehrer, E.J.; Bakst, R.L. Combining Radiotherapy and Immunotherapy in Head and Neck Cancer. Biomedicines 2023, 11, 2097. [Google Scholar] [CrossRef]
- Pannunzio, S.; Di Bello, A.; Occhipinti, D.; Scala, A.; Messina, G.; Valente, G.; Quirino, M.; Di Salvatore, M.; Tortora, G.; Cassano, A. Multimodality treatment in recurrent/metastatic squamous cell carcinoma of head and neck: Current therapy, challenges, and future perspectives. Front. Oncol. 2024, 13, 1288695. [Google Scholar] [CrossRef]
- Barham, W.T.; Stagg, M.P.; Mualla, R.; DiLeo, M.; Kansara, S. Recurrent and Metastatic Head and Neck Cancer: Mechanisms of Treatment Failure, Treatment Paradigms, and New Horizons. Cancers 2025, 17, 144. [Google Scholar] [CrossRef]
- Ganesan, P.; Sekaran, S.; Ramasamy, P.; Ganapathy, D. Systematic analysis of chemotherapy, immunotherapy, and combination therapy in Head and Neck Squamous Cell Carcinoma (HNSCC) clinical trials: Focusing on overall survival and progression-free survival outcomes. Oral Oncol. Rep. 2024, 12, 100673. [Google Scholar] [CrossRef]
- Jayawickrama, S.M.; Ranaweera, P.M.; Pradeep, R.G.G.R.; Jayasinghe, Y.A.; Senevirathna, K.; Hilmi, A.J.; Rajapakse, R.M.G.; Kanmodi, K.K.; Jayasinghe, R.D. Developments and future prospects of personalized medicine in head and neck squamous cell carcinoma diagnoses and treatments. Cancer Rep. 2024, 7, e2045. [Google Scholar] [CrossRef]
- Zeng, W.; Xie, F.; Pan, Y.; Chen, Z.; Chen, H.; Liu, X.; Tian, K.; Xu, D. A comprehensive prognostic score for head and neck squamous cancer driver genes and phenotype traits. Discov. Oncol. 2023, 14, 193. [Google Scholar] [CrossRef]
- Stögbauer, F.; Beck, S.; Ourailidis, I.; Hess, J.; Poremba, C.; Lauterbach, M.; Wollenberg, B.; Buchberger, A.M.S.; Jesinghaus, M.; Schirmacher, P.; et al. Tumour budding-based grading as independent prognostic biomarker in HPV-positive and HPV-negative head and neck cancer. Br. J. Cancer 2023, 128, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Torri, M.; Sandell, A.; Al-Samadi, A. The prognostic value of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Biomed. Pharmacother. 2024, 180, 117544. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, H.H.; Maroun, C.A.; Amin, N.; Zhu, G.; Guller, M.; Herberg, M.E.; Wu, E.S.; Seiwert, T.Y.; Rooper, L.M.; Eisele, D.W.; et al. Tumor Histological Grade and Immunotherapy Response in Patients With Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma. Arch. Otolaryngol. Neck Surg. 2022, 148, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Falco, A.; Rosati, A.; Festa, M.; Basile, A.; De Marco, M.; D’Avenia, M.; Pascale, M.; Piaz, F.D.; Tavano, F.; Di Mola, F.F.; et al. BAG3 Is a Novel Serum Biomarker for Pancreatic Adenocarcinomas. Am. J. Gastroenterol. 2013, 108, 1178–1180. [Google Scholar] [CrossRef]
- Falco, A.; Basile, A.; Raimondo, A.; Guglielmi, G.; Rosati, A.; De Marco, M.; Turco, M.C.; Pascale, M.; Lembo, S. Presence of BAG3 protein in serum samples from patients affected by psoriasis. J. Transl. Med. 2024, 22, 461. [Google Scholar] [CrossRef]
- De Marco, M.; Basile, A.; Cammarota, A.L.; Iannone, C.; Falco, A.; Marzullo, L.; Rosati, A.; Caporali, R.; Turco, M.C.; Del Papa, N. Response to antifibrotic therapy and decrease of circulating BAG3 protein levels in systemic sclerosis patients with reduced forced vital capacity. Biomed. Pharmacother. 2024, 174, 116578. [Google Scholar] [CrossRef]
- De Marco, M.; Armentaro, G.; Falco, A.; Minniti, A.; Cammarota, A.L.; Iannone, C.; Basile, A.; D’Ardia, A.; Zeppa, P.; Marzullo, L.; et al. Overexpression of BAG3 (Bcl2-associated athanogene 3) in serum and skin of patients with systemic sclerosis. Clin. Exp. Rheumatol. 2024, 42, 1623–1628. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).