An Analysis of Waiting Times for the Diagnosis and Treatment of Patients with Prostate Cancer Established by the Requirements of the Fast-Track Cancer Treatment Pathway, Taking into Account Treatment Steps
Simple Summary
Abstract
1. Introduction
- Determining the characteristics of the current clinical path of patients with prostate cancer undergoing treatment, with the establishment of time standards for the initiation of individual steps.
- Developing a graphical visualization of diagnosis and treatment steps for patients within the framework of the “fast-track cancer treatment pathway”.
- Establishing the most important indicators/analytical measures for patients with a diagnosis of C61.
- Identifying the cost-intensiveness of the various steps of treatment.
- Identifying the number of patients who, treated by different methods, do not meet the required waiting time criteria set by the requirements of the fast-track cancer treatment pathway, taking into account the steps of treatment.
2. Materials and Methods
3. Results
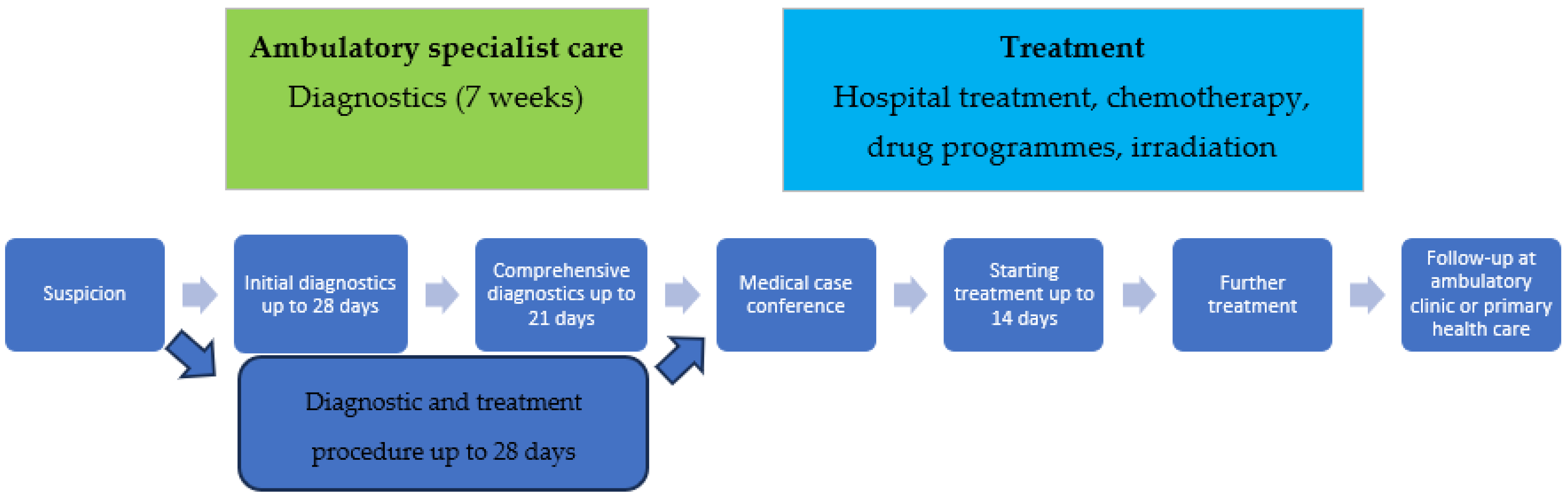
3.1. Characteristics of the Analyzed Process
- An oncological diagnostics and treatment card (DILO card): A document which entitles the patient to services provided under the terms set out in the package; the card may be issued by primary care physicians, ambulatory specialist care, and hospitals (at any step).
- Strictly defined maximum time for cancer diagnosis: Up to 7 weeks (including initial diagnostics within 28 days and comprehensive diagnostics within 21 days).
- Comprehensiveness and coordination of medical services: Following the completion of cancer diagnosis at a given facility, the patient remains in treatment with the same service provider or is referred to another facility for treatment, according to a plan established by a medical case conference.
- A multi-specialist therapeutic team (medical case conference), which develops an individual treatment plan optimized for the patient in accordance with current medical knowledge and treatment recommendations.
- Strictly defined maximum time for initiating treatment: Within 14 days from the date of the establishment of the treatment plan,
- Coordinator: The facility conducting the treatment assigns the patient a coordinator whose tasks include, among others, practical assistance in the implementation of the established treatment path.
- The unlimited nature of fast-track cancer treatment pathway services.
- Supervision (follow-up) of the patient after completing oncological treatment for a period of 5 years.
3.2. Chosen Indicators/Analytical Measures for Patients with a Diagnosis of C61
3.3. Cost-Intensity of Steps
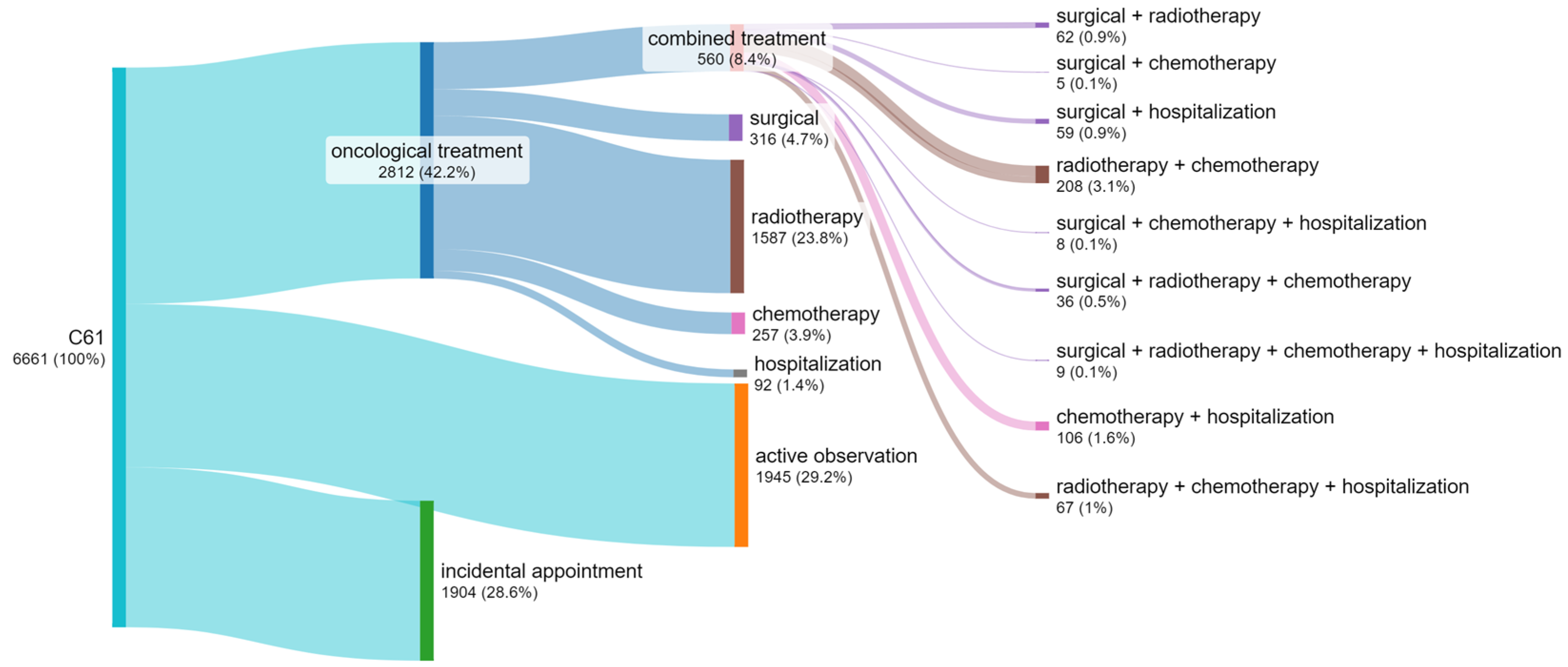
3.4. Number of Patients by Treatment Method
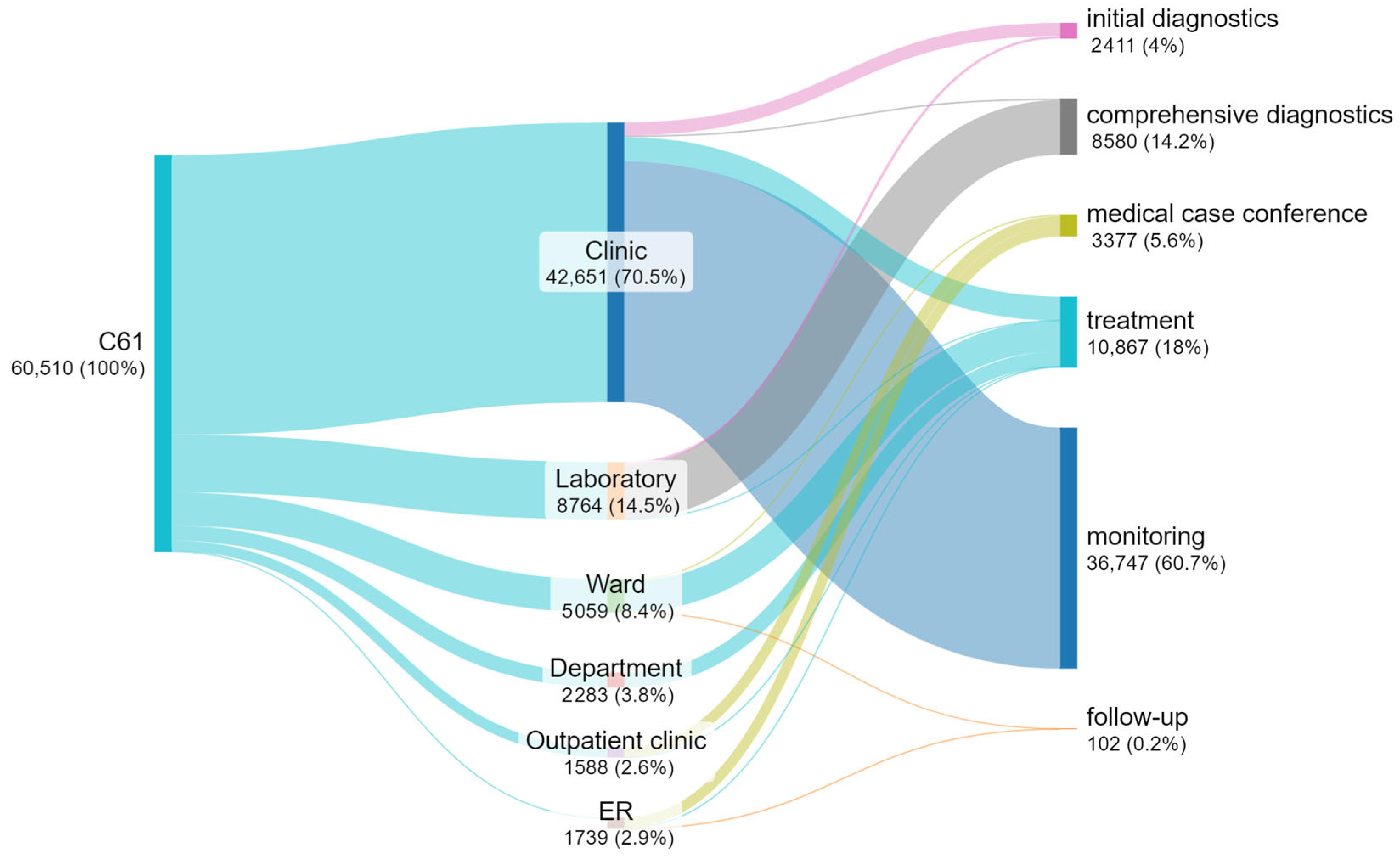
3.5. Number of Appointments at Individual Steps
3.6. Waiting Time for an Appointment
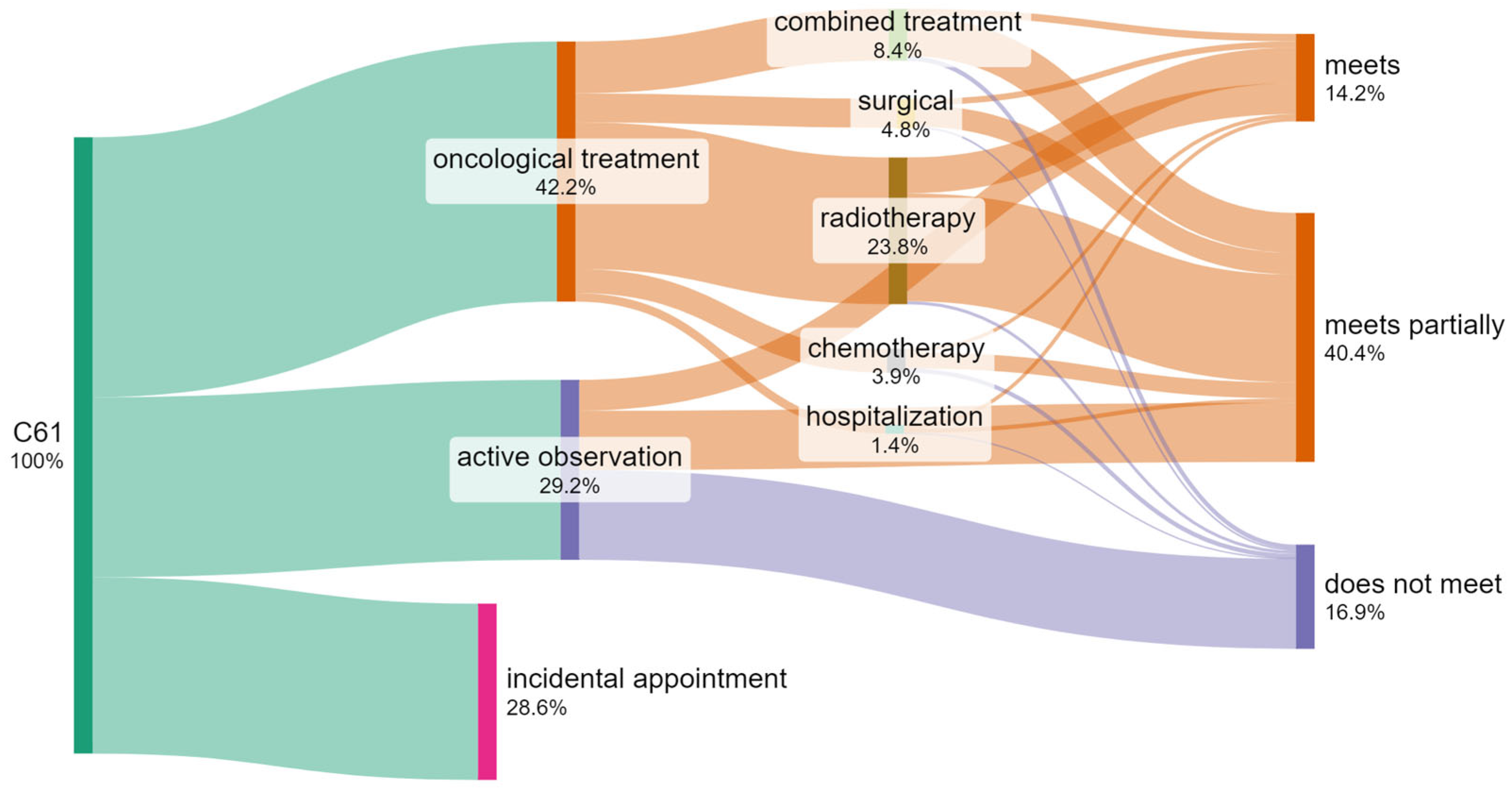
3.7. Identification of the Number of Patients Who Do Not Meet the Required Waiting Time Criteria When Treated by Different Methods
4. Discussion
5. Conclusions
- The time limits for diagnosing and commencing the treatment of patients with diagnosed prostate cancer specified by legal regulations and by guidelines of scientific associations are not observed in 42% of cases.
- The greatest delays concern the initiation of the treatment (53%) and comprehensive diagnostics (37%).
- Diagnostic pathways should be modified to facilitate early and rapid detection of prostate cancer and to allow further therapy within the time limit strictly defined by regulations and guidelines of scientific associations. The results presented herein clearly indicate that there is much to be done in terms of the timeliness of the actions taken. Many patients exceed the times established for initial diagnostics, comprehensive diagnostics, or the initiation of treatment (according to DILO).
- Analysis of the collected data according to the cost criterion indicated that the most cost-intensive methods of treatment are, in order of priority, radiotherapy (irradiation), chemotherapy, and then active observation with laboratory and imaging diagnostics. These areas should be the main areas of interest and subject to control in terms of the regularity of proceedings and implemented activities.
- The KSO system should also evolve to incorporate the assessment of clinically significant cancer risk, as well as aspects such as precise qualification for biopsy and active surveillance strategies. It is necessary to continue the work initiated by this study by including key issues, in the opinion of the authors, concerning state-of-the-art methods of management of the patient’s treatment processes (and in particular Lean Healthcare 4.0), with the use of the value stream model (VSM) from the perspective of the oncological patient as an example of visual management which can be applied in hospital practice and an indication of the potential of improvement in treatment processes using tools and methods of Lean Healthcare 4.0.
- Quality and clinical results (outcomes);
- The efficiency of the organizational process;
- The improvement in the use of medical resources;
- The reduction in waste (non-value-added activity), in the sense of the lean management methodology;
- The application of the latest standards of treatment and medical technology (best current practice);
- The optimization of process costs.
6. Limitations
- -
- The described model of care refers to data from a Polish hospital and may not be fully representative of healthcare systems in other countries. It is based on data available in Poland and the realities of the Polish healthcare system. However, similar issues to those observed in Poland also occur in many other countries with limited financial resources.
- -
- This study is based on the analysis of data from a single large oncology center in Poland, which constitutes a limitation in terms of generalizing the results to other healthcare institutions. Differences in the organizational structure, availability of services, staffing, and technological resources, as well as clinical practices between centers, may influence the implementation of the diagnostic and therapeutic pathway and the waiting times for services. It should also be noted that patient profiles may be specific to the studied center, which could impact the obtained results. Therefore, the findings presented in this study should be interpreted within the local context, and further research, involving multiple centers, is necessary to more thoroughly validate the observed associations and draw broader conclusions about the organization of oncological care for patients with prostate cancer.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KSO | National Oncological Network |
| C61 | Code for the diagnosis of prostate cancer in the International Classification of Diseases ICD-10 |
| CSV | Comma-Separated Values (text file format) |
| NFZ | Polish National Health Fund |
| DILO | Oncological Diagnostics and Treatment Card |
| PSA | Prostate Specific Antigen |
| ICD-10 | International Classification of Diseases, Tenth Revision |
| SPS | Services Provision Site |
| ODBC | Open Database Connectivity |
| PHC | Primary health care |
| ASC | Ambulatory Specialist Care |
| HT | Hospital Treatment |
| HIS | Hospital Information System |
| Industry 4.0 | Fourth Industrial Revolution |
| TPM | Total Productive Maintenance |
| MTTR | Mean Time To Recovery |
| MTBF | Mean Time Between Failures |
| DRE | Digital Rectal Exam |
| TRUS | Transrectal Ultrasound Scan |
| PET | Positron Emission Tomography |
References
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Al-Ghazawi, M.; Salameh, H.; Amo-Afful, S.; Khasawneh, S.; Ghanem, R.; Al-Ghazawi, M.; Salameh, H.; Amo-Afful, S.; Khasawneh, S.; Ghanem, R. An In-Depth Look Into the Epidemiological and Etiological Aspects of Prostate Cancer: A Literature Review. Cureus 2023, 15, e48252. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Burke, H.B.; Djakiew, D.; Euling, S.; Ho, S.M.; Landolph, J.; Morrison, H.; Sonawane, B.; Shifflett, T.; Waters, D.J.; et al. Human Prostate Cancer Risk Factors. Cancer 2004, 101, 2371–2490. [Google Scholar] [CrossRef]
- Taitt, H.E. Global Trends and Prostate Cancer: A Review of Incidence, Detection, and Mortality as in-Fluenced by Race, Ethnicity, and Geographic Location. Am. J. Men’s Health 2018, 12, 1807–1823. [Google Scholar] [CrossRef]
- Jemal, A.; Fedewa, S.A.; Ma, J.; Siegel, R.; Lin, C.C.; Brawley, O.; Ward, E.M. Prostate Cancer Incidence and PSA Testing Patterns in Relation to USPSTF Screening Recommendations. JAMA 2015, 314, 2054–2061. [Google Scholar] [CrossRef]
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect Med. 2018, 8, a030361. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Pineros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow 2019. Available online: https://gco.iarc.fr/tomorrow/en (accessed on 3 March 2025).
- Wysocki, P.; Chłosta, P.; Antoniewicz, A.; Chrzan, R.; Czech, A.K.; Dobruch, J.; Gronostaj, K.; Krzakowski, M.; Kucharz, J.; Małecki, K.; et al. Zalecenia postępowania diagnostyczno-terapeutycznego w raku gruczołu krokowego—Stanowisko Polskiego Towarzystwa Onkologii Klinicznej i Polskiego Towarzystwa Urologicznego. Onkol. W Prakt. Klin. Edukacja 2024, 10, 1–72. [Google Scholar]
- Sejm Ustawa z Dnia 27 Sierpnia 2004 r. O Świadczeniach Opieki Zdrowotnej Finansowanych ze Środków Publicznych 2004. Available online: https://lexlege.pl/ustawa-o-swiadczeniach-opieki-zdrowotnej-finansowanych-ze-srodkow-publicznych/.
- AOTMiT Kompleksowa Opieka Onkologiczna—Model Organizacji Diagnostyki i Leczenia Raka Gruczołu Krokowego 2019. Available online: https://bip.aotm.gov.pl/assets/files/zlecenia_mz/2018/089/RPT/WS.4320.1.2019_RAPORT_RGK_BIP.pdf (accessed on 5 January 2025).
- Sejm RP Ustawa z Dnia 9 Marca 2023 r. O Krajowej Sieci Onkologicznej 2023. Available online: https://isap.sejm.gov.pl/isap.nsf/download.xsp/WDU20230000650/U/D20230650Lj.pdf (accessed on 15 February 2025).
- Van Poppel, H.; Roobol, M.J.; Chapple, C.R.; Catto, J.W.F.; N’Dow, J.; Sønksen, J.; Stenzl, A.; Wirth, M. Prostate-Specific Antigen Testing as Part of a Risk-Adapted Early Detection Strategy for Prostate Cancer: European Association of Urology Position and Recommendations for 2021. Eur. Urol. 2021, 80, 703–711. [Google Scholar] [CrossRef]
- Wojciechowska, U.; Bauer, A.; Didkowska, J.; Zatoński, W. Nowotwory Złośliwe w Polsce—Wskaźniki 5-Letnich Przeżyć Według Województw =: Cancer in Poland—Five-Year Survival Rates by Regions; Centrum Onkologii—Instytut im Marii Skłodowskiej-Curie: Warsaw, Poland, 2010; ISBN 978-83-88681-78-3. [Google Scholar]
- Mithal, P.; Howard, L.E.; Aronson, W.J.; Kane, C.J.; Cooperberg, M.R.; Terris, M.K.; Amling, C.L.; Freedland, S.J. Prostate-Specific Antigen Level, Stage or Gleason Score: Which Is Best for Predicting Outcomes after Radical Prostatectomy, and Does It Vary by the Outcome Being Measured? Results from Shared Equal Access Regional Cancer Hospital Database. Int. J. Urol. 2015, 22, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Lamer, A.; Laurent, G.; Pelayo, S.; El Amrani, M.; Chazard, E.; Marcilly, R. Exploring Patient Path Through Sankey Diagram: A Proof of Concept. In Digital Personalized Health and Medicine; IOS Press: Amsterdam, The Netherlands, 2020; pp. 218–222. [Google Scholar]
- European Central Bank Euro Foreign Exchange Reference Rates. Available online: https://www.ecb.europa.eu/stats/policy_and_exchange_rates/euro_reference_exchange_rates/html/index.en.html (accessed on 3 March 2025).
- Vileikis, L. Optimizing MySQL for Big Data. In Hacking MySQL: Breaking, Optimizing, and Securing MySQL for Your Use Case; Vileikis, L., Ed.; Apress: Berkeley, CA, USA, 2024; pp. 195–208. [Google Scholar]
- Pauw, V.M.P.; Číž, D.; Mlýnský, V.; Banáš, P.; Otyepka, M.; Hachinger, S.; Martinovič, J. Management of Physics Simulations with Databases. In Proceedings of International Symposium on Grids and Clouds (ISGC) 2024—PoS(ISGC2024), Taipei, Taiwan, 24–29 March 2024; SISSA Medialab: Trieste, Italia, 2024; Volume 458, p. 31. [Google Scholar]
- Wang, C.; Xu, J.; Feng, X.; Wu, W.; Wang, J. Database Interface Application Based on ODBC Development. In Proceedings of the 2022 2nd Asia-Pacific Conference on Communications Technology and Computer Science (ACCTCS), Shenyang, China, 25–27 February 2022; pp. 362–365. [Google Scholar]
- Bakhshi, S. Expert Data Modeling with Power BI: Get the Best out of Power BI by Building Optimized Data Models for Reporting and Business Needs; Packt Publishing Ltd.: Birmingham, UK, 2021. [Google Scholar]
- Bogart, S. About SankeyMATIC. Available online: https://sankeymatic.com/about/ (accessed on 16 February 2025).
- Daniel, D.; West-Mitchell, K. The Sankey Diagram: An Exploratory Application of a Data Visualization Tool. Transfusion 2024, 64, 967–968. [Google Scholar] [CrossRef]
- Bousdekis, A.; Lepenioti, K.; Apostolou, D.; Mentzas, G. Data Analytics in Quality 4.0: Literature Review and Future Research Directions. Int. J. Comput. Integr. Manuf. 2023, 36, 678–701. [Google Scholar] [CrossRef]
- Guo, B.; Chen, J. Analysis of MTBF/MTTR for Logistics Service System. In Proceedings of the ICTE 2013, Chengdu, China, 19–20 October 2013; American Society of Civil Engineers: Chengdu, China, 2013; pp. 2868–2875. [Google Scholar]
- Tortorella, G.L.; Fogliatto, F.S.; Cauchick-Miguel, P.A.; Kurnia, S.; Jurburg, D. Integration of Industry 4.0 Technologies into Total Productive Maintenance Practices. Int. J. Prod. Econ. 2021, 240, 108224. [Google Scholar] [CrossRef]
- Sanghera, S.; Coast, J.; Martin, R.M.; Donovan, J.L.; Mohiuddin, S. Cost-Effectiveness of Prostate Cancer Screening: A Systematic Review of Decision-Analytical Models. BMC Cancer 2018, 18, 84. [Google Scholar] [CrossRef]
- Govers, T.M.; Hessels, D.; Vlaeminck-Guillem, V.; Schmitz-Dräger, B.J.; Stief, C.G.; Martinez-Ballesteros, C.; Ferro, M.; Borque-Fernando, A.; Rubio-Briones, J.; Sedelaar, J.P.M.; et al. Cost-Effectiveness of SelectMDx for Prostate Cancer in Four European Countries: A Comparative Modeling Study. Prostate Cancer Prostatic Dis. 2019, 22, 101–109. [Google Scholar] [CrossRef]
- Pan, H.Y.; Jiang, J.; Hoffman, K.E.; Tang, C.; Choi, S.L.; Nguyen, Q.N.; Frank, S.J.; Anscher, M.S.; Tina Shih, Y.-C.; Smith, B.D. Comparative Toxicities and Cost of Intensity-Modulated Radiotherapy, Proton Radiation, and Stereotactic Body Radiotherapy among Younger Men with Prostate Cancer. J. Clin. Oncol. 2018, 36, 1823–1830. [Google Scholar] [CrossRef]
- Fleshner, N.; Dranitsaris, G.; Finelli, A.; Tsihlias, J.; Bell, D.; Gleave, M. Canadian surgical wait times (SWAT) initiative Surgical Wait Times for Patients with Urological Cancers: A Survey of Canadian Surgeons. Can. J. Urol. 2006, 13, 3–13. [Google Scholar]
- Omri, N.; Kamil, M.; Alexander, K.; Alexander, K.; Edmond, S.; Ariel, Z.; David, K.; Gilad, A.E.; Azik, H. Association between PSA Density and Pathologically Significant Prostate Cancer: The Impact of Prostate Volume. Prostate 2020, 80, 1444–1449. [Google Scholar] [CrossRef]
- Smeenge, M.; Barentsz, J.; Cosgrove, D.; de la Rosette, J.; de Reijke, T.; Eggener, S.; Frauscher, F.; Kovacs, G.; Matin, S.F.; Mischi, M.; et al. Role of Transrectal Ultrasonography (TRUS) in Focal Therapy of Prostate Cancer: Report from a Consensus Panel. BJU Int. 2012, 110, 942–948. [Google Scholar] [CrossRef]
- Shen, G.; Deng, H.; Hu, S.; Jia, Z. Comparison of Choline-PET/CT, MRI, SPECT, and Bone Scintigraphy in the Diagnosis of Bone Metastases in Patients with Prostate Cancer: A Meta-Analysis. Skelet. Radiol. 2014, 43, 1503–1513. [Google Scholar] [CrossRef] [PubMed]
- Corfield, J.; Perera, M.; Bolton, D.; Lawrentschuk, N. Ga-Prostate Specific Membrane Antigen (PSMA) Positron Emission Tomography (PET) for Primary Staging of High-Risk Prostate Cancer: A Systematic Review. World J. Urol. 2018, 36, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Minister Zdrowia Obwieszczenie Ministra Zdrowia z Dnia 21 Lutego 2022 r. w Sprawie Wykazu Refundowanych Leków, Środków Spożywczych Specjalnego Przeznaczenia Żywieniowego Oraz Wyrobów Medycznych na 1 Marca 2022 r.—Ministerstwo Zdrowia-Portal Gov.pl 2022. Available online: https://www.gov.pl/web/zdrowie/obwieszczenie-ministra-zdrowia-z-dnia-21-lutego-2022-r-w-sprawie-wykazu-refundowanych-lekow-srodkow-spozywczych-specjalnego-przeznaczenia-zywieniowego-oraz-wyrobow-medycznych-na-1-marca-2022-r (accessed on 23 December 2022).
- Minister Zdrowia Obwieszczenie Ministra Zdrowia z Dnia 20 Lutego 2023 r. w Sprawie Wykazu Leków, środków Spożywczych Specjalnego Przeznaczenia Żywieniowego Oraz Wyrobów Medycznych na 1 Marca 2023 r.—Ministerstwo Zdrowia-Portal Gov.pl 2023. Available online: https://www.gov.pl/web/zdrowie/obwieszczenie-ministra-zdrowia-z-dnia-20-lutego-2023-r-w-sprawie-wykazu-lekow-srodkow-spozywczych-specjalnego-przeznaczenia-zywieniowego-na-1-marca-2023-r (accessed on 13 December 2023).
- Michalski, J.M.; Moughan, J.; Purdy, J.; Bosch, W.; Bruner, D.W.; Bahary, J.-P.; Lau, H.; Duclos, M.; Parliament, M.; Morton, G.; et al. Effect of Standard vs Dose-Escalated Radiation Therapy for Patients With Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e180039. [Google Scholar] [CrossRef]
- Walz, J. Re: Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients with Localized Prostate Cancer: Results from the FLAME Randomized Phase III Trial. Eur. Urol. 2022, 81, 544–545. [Google Scholar] [CrossRef]
- Aggarwal, A.; Nossiter, J.; Cathcart, P.; van der Meulen, J.; Rashbass, J.; Clarke, N.; Payne, H. Organisation of Prostate Cancer Services in the English National Health Service. Clin. Oncol. 2016, 28, 482–489. [Google Scholar] [CrossRef]
- Aggarwal, A.; Nossiter, J.; Parry, M.; Sujenthiran, A.; Zietman, A.; Clarke, N.; Payne, H.; Meulen, J. van der Public Reporting of Outcomes in Radiation Oncology: The National Prostate Cancer Audit. Lancet Oncol. 2021, 22, e207–e215. [Google Scholar] [CrossRef]
- Dodkins, J.; Morris, M.; Nossiter, J.; van der Meulen, J.; Payne, H.; Clarke, N.; Aggarwal, A. Practicalities, Challenges and Solutions to Delivering a National Organisational Survey of Cancer Service and Processes: Lessons from the National Prostate Cancer Audit. J. Cancer Policy 2022, 33, 100344. [Google Scholar] [CrossRef]
- Nguyen, D.-D.; Haeuser, L.; Paciotti, M.; Reitblat, C.; Cellini, J.; Lipsitz, S.R.; Kibel, A.S.; Choudhury, A.D.; Cone, E.B.; Trinh, Q.-D. Systematic Review of Time to Definitive Treatment for Intermediate Risk and High Risk Prostate Cancer: Are Delays Associated with Worse Outcomes? J. Urol. 2021, 205, 1263–1274. [Google Scholar] [CrossRef]
- Saad, F.; Finelli, A.; Dranitsaris, G.; Goldenberg, L.; Bagnell, S.; Gleave, M.; Fleshner, N. Does Prolonging the Time to Prostate Cancer Surgery Impact Long-Term Cancer Control: A Systematic Review of the Literature. Can. J. Urol. 2006, 13, 16–24. [Google Scholar]


| Indicator Name | Description | Definition | Measurement Unit |
|---|---|---|---|
| Value | Value of services provided over a given period, taking into account selection criteria | =[Total Value provided] | EUR |
| Number of patients | Number of unique patients who received services during a given period | =[Number of separate patient ID values] | Integer |
| Number of appointments | Number of unique appointments carried out during a given period in the framework of the services provided | =[Number of separate appointment ID values] | Integer |
| Value/Patient | Average value of services per patient in a given period | =[Total Value provided]/[Number of separate patient ID values] | EUR/patient |
| Value/appointment | Average value of services per appointment in a given period | =[Total Value provided]/[Number of separate appointment ID values] | EUR/appointment |
| Number of appointments/patient | Average number of appointments per patient in a given period | =[Number of separate appointment ID values]/[Number of separate patient ID values] | Decimal |
| Length of stay/appointment | Average length of stay of patients per appointment in a given period. Average number of days during one appointment | =AVERAGE([Appointments_Discharge_Date]-[Appointments_Admission_Date]) | Decimal |
| Waiting time/appointment | Average patient waiting time per appointment in a given period. Average number of days between appointments | =AVERAGE([Appointment_N_Admission_Date_N]-[Appointment_N-1_Discharge_Date]) | Decimal |
| Number of appointments/day | Average number of appointments per working day during a given period, taking into account selection criteria | =[Number of separate appointment ID values]/[Number of working days] | Decimal |
| Year | Value | Number of Patients | Number of Appointments | Value/ Patient | Value/ Appointment | Number of Appointments/ Patient | Length of Stay/ Appointments | Waiting time/ Appointment | Number of Appointments/Day |
|---|---|---|---|---|---|---|---|---|---|
| 2018 | 3,461,145 | 2784 | 12,225 | 1243 | 283 | 4.4 | 2.8 | 34.1 | 48.5 |
| 2019 | 3,947,451 | 3000 | 12,748 | 1316 | 310 | 4.2 | 3.1 | 44.1 | 50.8 |
| 2020 | 3,860,859 | 2705 | 11,141 | 1427 | 347 | 4.1 | 3.0 | 46.5 | 43.7 |
| 2021 | 4,023,156 | 2798 | 11,963 | 1438 | 336 | 4.3 | 3.0 | 44.4 | 47.1 |
| 2022 | 5,129,134 | 2998 | 12,433 | 1711 | 413 | 4.1 | 2.8 | 44.6 | 49.3 |
| Total | 20,421,745 | 6661 | 60,510 | 3066 | 337 | 9.1 | 2.9 | 42.7 | 47.9 |
| Step | Value | Number of Patients | Number of Appointments | Value/ Patient | Value/ Appointment | Number of Appointments/ Patient | Length of Stay/ Appointments | Waiting time/Appointment | Number of Appointments/Day |
|---|---|---|---|---|---|---|---|---|---|
| Initial diagnosis | 47,696 | 2180 | 2411 | 22 | 20 | 1.1 | 1.0 | 19.6 | 1.9 |
| Comprehensive diagnostics | 3,747,604 | 4287 | 8580 | 874 | 437 | 2.0 | 1.0 | 27.7 | 6.8 |
| Medical case conference | 119,823 | 1801 | 1803 | 67 | 66 | 1.0 | 1.6 | 14.0 | 1.4 |
| Treatment | 15,850,522 | 2866 | 10,867 | 5531 | 1459 | 3.8 | 11.4 | 18.3 | 8.6 |
| Follow-up | 656,100 | 5321 | 36,849 | 123 | 18 | 6.9 | 1.0 | 56.3 | 29.2 |
| Total | 20,421,745 | 6661 | 60,510 | 3066 | 337 | 9.1 | 2.9 | 42.7 | 47.9 |
| Step | 2018 | 2019 | 2020 | 2021 | 2022 | Total |
|---|---|---|---|---|---|---|
| Initial diagnosis | 12.8 | 17.6 | 21.3 | 21.0 | 24.4 | 19.6 |
| Comprehensive diagnostics | 19.1 | 26.9 | 33.5 | 28.5 | 31.5 | 27.7 |
| Medical case conference | 9.9 | 14.7 | 14.3 | 15.7 | 15.0 | 14.0 |
| Treatment | 18.6 | 18.4 | 17.9 | 18.0 | 18.7 | 18.3 |
| Follow-up | 44.1 | 58.4 | 60.8 | 59.3 | 59.5 | 56.3 |
| Total | 34.1 | 44.1 | 46.5 | 44.4 | 44.6 | 42.7 |
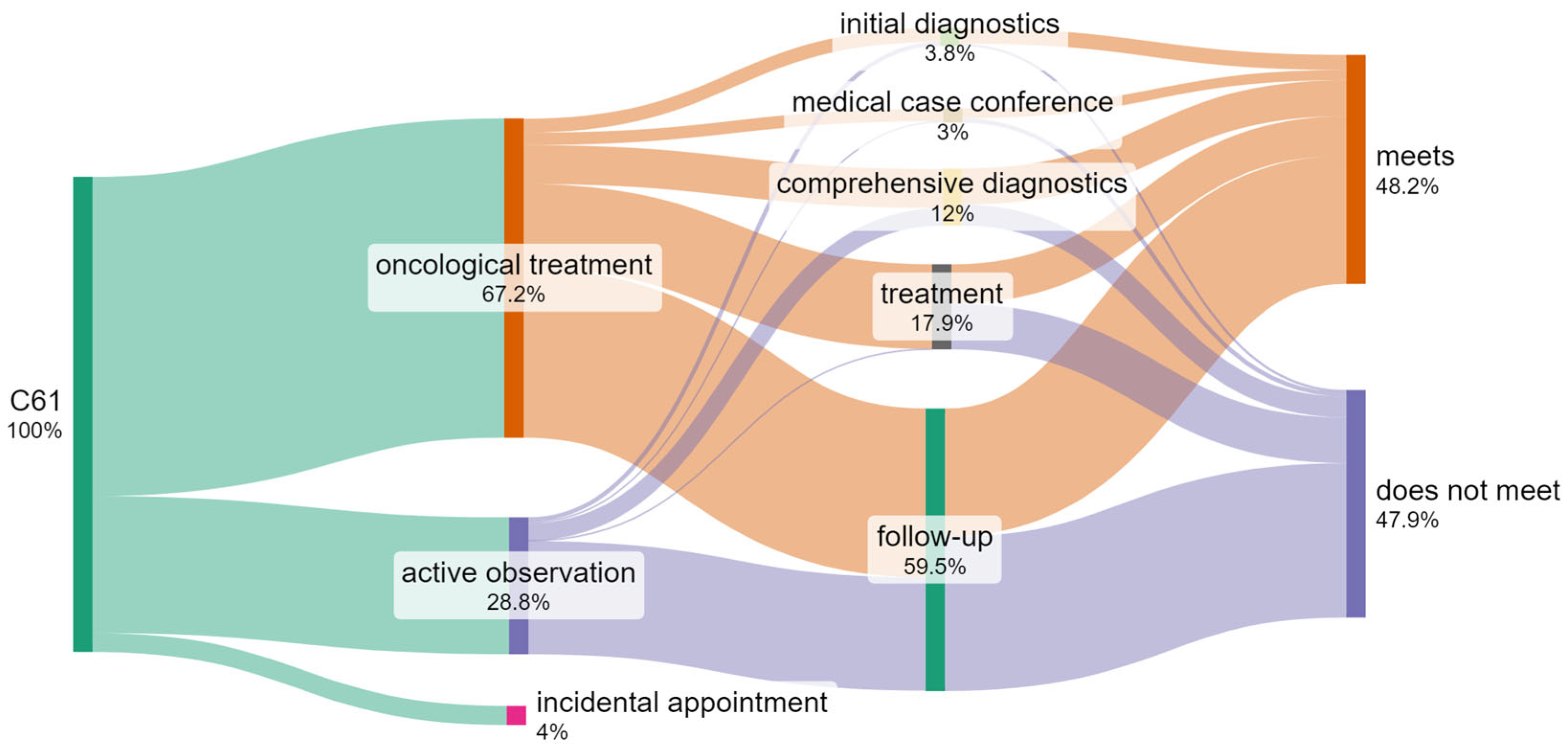
| Step | Meet | % | Do Not Meet | % | Total |
|---|---|---|---|---|---|
| Initial diagnosis | 1951 | 86% | 321 | 14% | 2272 |
| Comprehensive diagnostics | 4569 | 63% | 2678 | 37% | 7247 |
| Medical case conference | 1269 | 71% | 520 | 29% | 1789 |
| Treatment | 5050 | 47% | 5799 | 53% | 10,849 |
| Follow-up | 16,309 | 45% | 19,640 | 55% | 35,949 |
| Incidental appointment | 2144 | 89% | 260 | 11% | 2404 |
| Total | 31,292 | 52% | 29,218 | 48% | 60,510 |
| Step | Meet | % | Do Not Meet | % | Total |
|---|---|---|---|---|---|
| Initial diagnosis | 1951 | 86% | 321 | 14% | 2272 |
| Comprehensive diagnostics | 4569 | 63% | 2678 | 37% | 7247 |
| Medical case conference | 1269 | 71% | 520 | 29% | 1789 |
| Treatment | 5050 | 47% | 5799 | 53% | 10,849 |
| Total | 12,839 | 58% | 9318 | 42% | 22,157 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierocka, A.; Brzozowski, S.; Marczak, M.; Bednarek, M.; Kozłowski, R. An Analysis of Waiting Times for the Diagnosis and Treatment of Patients with Prostate Cancer Established by the Requirements of the Fast-Track Cancer Treatment Pathway, Taking into Account Treatment Steps. Cancers 2025, 17, 1842. https://doi.org/10.3390/cancers17111842
Sierocka A, Brzozowski S, Marczak M, Bednarek M, Kozłowski R. An Analysis of Waiting Times for the Diagnosis and Treatment of Patients with Prostate Cancer Established by the Requirements of the Fast-Track Cancer Treatment Pathway, Taking into Account Treatment Steps. Cancers. 2025; 17(11):1842. https://doi.org/10.3390/cancers17111842
Chicago/Turabian StyleSierocka, Aleksandra, Stanisław Brzozowski, Michał Marczak, Mariusz Bednarek, and Remigiusz Kozłowski. 2025. "An Analysis of Waiting Times for the Diagnosis and Treatment of Patients with Prostate Cancer Established by the Requirements of the Fast-Track Cancer Treatment Pathway, Taking into Account Treatment Steps" Cancers 17, no. 11: 1842. https://doi.org/10.3390/cancers17111842
APA StyleSierocka, A., Brzozowski, S., Marczak, M., Bednarek, M., & Kozłowski, R. (2025). An Analysis of Waiting Times for the Diagnosis and Treatment of Patients with Prostate Cancer Established by the Requirements of the Fast-Track Cancer Treatment Pathway, Taking into Account Treatment Steps. Cancers, 17(11), 1842. https://doi.org/10.3390/cancers17111842






