Adrenalectomy for Metastasis: The Impact of Primary Histology on Survival Outcome
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Surgical Technique
2.2.1. Robotic Approach
2.2.2. Laparoscopic Approach
2.3. Follow Up Schedule
2.4. Statistical Analysis
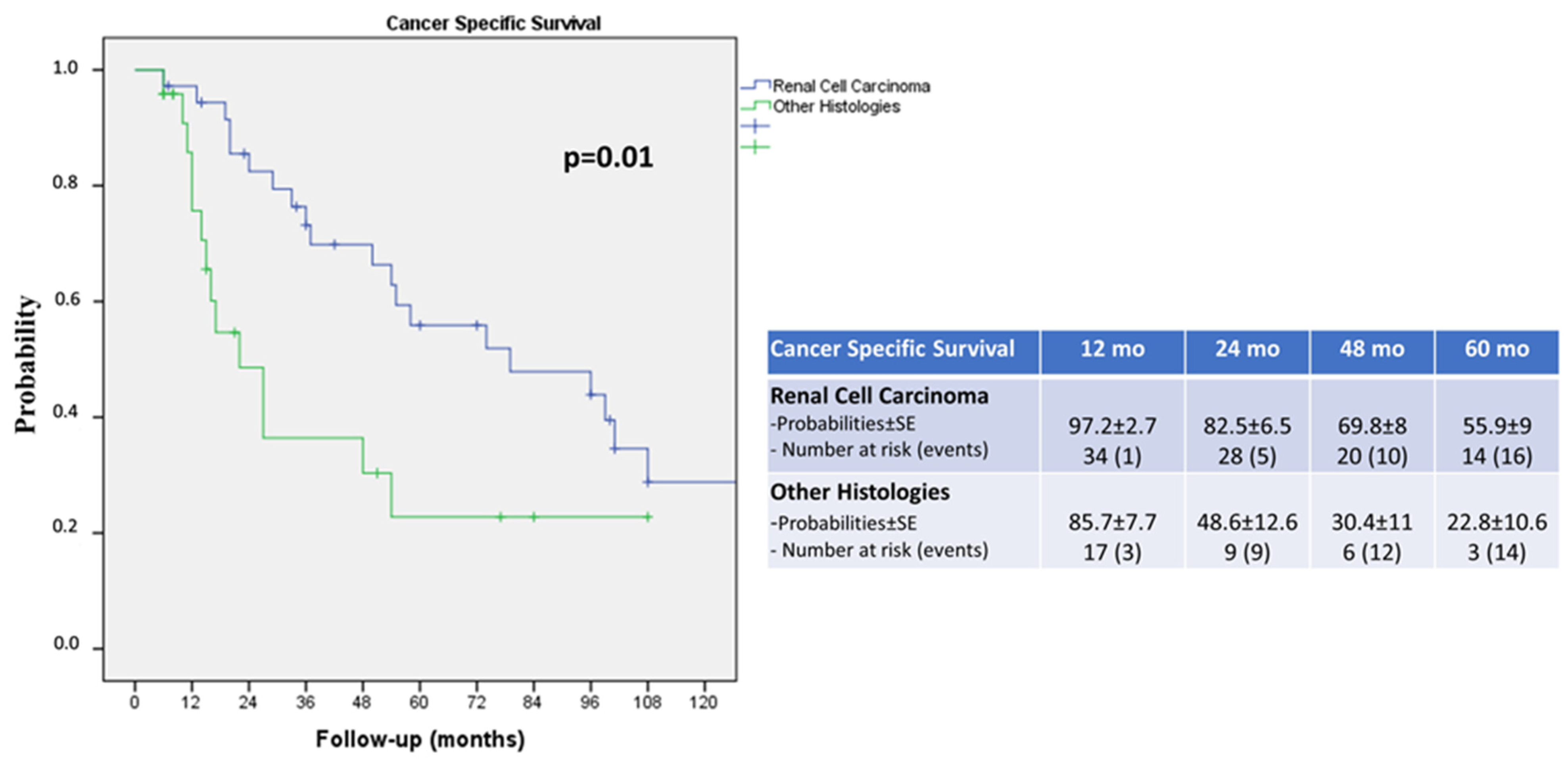
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mclean, K.; Lilienfeld, H.; Caracciolo, J.T.; Hoffe, S.; Tourtelot, J.B.; Carter, W.B. Management of Isolated Adrenal Lesions in Cancer Patients. Cancer Control 2011, 18, 113–126. [Google Scholar] [CrossRef]
- Spartalis, E.; Drikos, I.; Ioannidis, A.; Chrysikos, D.; Athanasiadis, D.I.; Spartalis, M.; Avgerinos, D. Metastatic Carcinomas of the Adrenal Glands: From Diagnosis to Treatment. Anticancer Res. 2019, 39, 2699–2710. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, M.A.; Thompson, G.B.; Duh, Q.-Y.; Hamrahian, A.H.; Angelos, P.; Elaraj, D.; Fishman, E.; Kharlip, J.; Zeiger, M.A.; Thompson, G.B.; et al. American Association of Clinical Endocrinologists and American Association of Endocrine Surgeons Medical Guidelines for The Management of Adrenal Incidentalomas. Endocr. Pract. 2009, 15, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.V.; Venkatesan, A.M.; Swerdlow, D.; DaSilva, D.; Beck, A.; Jain, N.; Wood, B.J. Image-Guided Adrenal and Renal Biopsy. Tech. Vasc. Interv. Radiol. 2010, 13, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, J.; Munver, R. Surgical Management of Metastases to the Adrenal Gland: Open, Laparoscopic, and Ablative Approaches. Curr. Urol. Rep. 2009, 10, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Vlk, E.; Ebbehoj, A.; Donskov, F.; Poulsen, P.L.; Rashu, B.S.; Bro, L.; Aagaard, M.; Rolighed, L. Outcome and Prognosis after Adrenal Metastasectomy: Nationwide Study. BJS Open 2022, 6, zrac047. [Google Scholar] [CrossRef] [PubMed]
- Samsel, R.; Cichocki, A.; Roszkowska-Purska, K.; Papierska, L.; Koalasińska-Ćwikła, A.; Karpeta, E.; Ostrowski, T.; Nowak, K. Adrenal Metastases—Long-Term Results of Surgical Treatment, Single-Centre Experience. Współczesna Onkol. 2020, 24, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Wachtel, H.; Roses, R.E.; Kuo, L.E.; Lindeman, B.M.; Nehs, M.A.; Tavakkoli, A.; Parangi, S.; Hodin, R.A.; Fraker, D.L.; James, B.C.; et al. Adrenalectomy for Secondary Malignancy: Patients, Outcomes, and Indications. Ann. Surg. 2021, 274, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Simone, G.; Misuraca, L.; Tuderti, G.; Minisola, F.; Ferriero, M.; Romeo, G.; Costantini, M.; Al-Rawashdah, S.F.; Guaglianone, S.; Gallucci, M. Purely Off-Clamp Robotic Partial Nephrectomy: Preliminary 3-Year Oncological and Functional Outcomes. Int. J. Urol. 2018, 25, 606–614. [Google Scholar] [CrossRef]
- Simone, G.; Anceschi, U.; Tuderti, G.; Misuraca, L.; Celia, A.; De Concilio, B.; Costantini, M.; Stigliano, A.; Minisola, F.; Ferriero, M.; et al. Robot-Assisted Partial Adrenalectomy for the Treatment of Conn’s Syndrome: Surgical Technique, and Perioperative and Functional Outcomes. Eur. Urol. 2019, 75, 811–816. [Google Scholar] [CrossRef]
- Papalia, R.; Simone, G.; Leonardo, C.; Loreto, A.; Coppola, R.; Guaglianone, S.; Sacco, R.; Gallucci, M. Laparoscopic Transperitoneal Right Adrenalectomy for ‘Large’ Tumors. Urol. Int. 2008, 81, 437–440. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, S.-W.; Pak, K.; Kim, I.-J.; Kim, K. Diagnostic Accuracy of 18 F-FDG PET or PET/CT for the Characterization of Adrenal Masses: A Systematic Review and Meta-Analysis. Br. J. Radiol. 2018, 91, 20170520. [Google Scholar] [CrossRef]
- Wu, Q.; Luo, W.; Zhao, Y.; Xu, F.; Zhou, Q. The Utility of 18F-FDG PET/CT for the Diagnosis of Adrenal Metastasis in Lung Cancer. Nucl. Med. Commun. 2017, 38, 1117–1124. [Google Scholar] [CrossRef]
- Liu, Y. The Place of FDG PET/CT in Renal Cell Carcinoma: Value and Limitations. Front. Oncol. 2016, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Maisel, F.; Smolle, M.A.; Mollnar, S.; Riedl, J.M.; Barth, D.A.; Seles, M.; Terbuch, A.; Rossmann, C.H.; Eisner, F.; Mannweiler, S.; et al. Benefit of Metastasectomy in Renal Cell Carcinoma: A Propensity Score Analysis. Clin. Genitourin. Cancer 2022, 20, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Takagi, T.; Kondo, T.; Fukuda, H.; Tachibana, H.; Yoshida, K.; Iizuka, J.; Kobayashi, H.; Ishida, H.; Tanabe, K. Prognostic Impact of Metastasectomy in Renal Cell Carcinoma in the Postcytokine Therapy Era. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 77.e17–77.e25. [Google Scholar] [CrossRef] [PubMed]
- Ferriero, M.; Cacciatore, L.; Ochoa, M.; Mastroianni, R.; Tuderti, G.; Costantini, M.; Anceschi, U.; Misuraca, L.; Brassetti, A.; Guaglianone, S.; et al. The Impact of Metastasectomy on Survival Outcomes of Renal Cell Carcinoma: A 10-Year Single Center Experience. Cancers 2023, 15, 3332. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bedke, J.; Capitanio, U.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2022 Update. Eur. Urol. 2022, 82, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Goujon, A.; Schoentgen, N.; Betari, R.; Thoulouzan, M.; Vanalderwerelt, V.; Oumakhlouf, S.; Brichart, N.; Pradere, B.; Roumiguie, M.; Rammal, A.; et al. Prognostic Factors after Adrenalectomy for Adrenal Metastasis. Int. Urol. Nephrol. 2020, 52, 1869–1876. [Google Scholar] [CrossRef]
- Gryn, A.; Peyronnet, B.; Manunta, A.; Beauval, J.-B.; Bounasr, E.; Nouhaud, F.-X.; Rioux-Leclercq, N.; Caron, P.; Thoulouzan, M.; Verhoest, G.; et al. Patient Selection for Laparoscopic Excision of Adrenal Metastases: A Multicenter Cohort Study. Int. J. Surg. 2015, 24, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Krumeich, L.N.; Roses, R.E.; Kuo, L.E.; Lindeman, B.M.; Nehs, M.A.; Tavakkoli, A.; Parangi, S.; Hodin, R.A.; Fraker, D.L.; James, B.C.; et al. Survival After Adrenalectomy for Metastatic Lung Cancer. Ann. Surg. Oncol. 2022, 29, 2571–2579. [Google Scholar] [CrossRef]
- Romero Arenas, M.A.; Sui, D.; Grubbs, E.G.; Lee, J.E.; Perrier, N.D. Adrenal Metastectomy Is Safe in Selected Patients. World J. Surg. 2014, 38, 1336–1342. [Google Scholar] [CrossRef] [PubMed]
- Mittendorf, E.A.; Lim, S.J.; Schacherer, C.W.; Lucci, A.; Cormier, J.N.; Mansfield, P.F.; Gershenwald, J.E.; Ross, M.I.; Lee, J.E. Melanoma Adrenal Metastasis: Natural History and Surgical Management. Am. J. Surg. 2008, 195, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Asare, E.A.; Fisher, S.B.; Chiang, Y.; Haydu, L.E.; Patel, S.H.; Keung, E.Z.; Lucci, A.; Wargo, J.; Gershenwald, J.E.; Ross, M.I.; et al. Melanoma Metastatic to the Adrenal Gland: An Update on the Role of Adrenalectomy in Multidisciplinary Management. J. Surg. Oncol. 2023, 128, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.D.; Bhandare, M.; Deodhar, K.; Yuvaraja, T.B.; Saklani, A.P. Robotic Adrenalectomy for Sigmoid Colon Cancer Oligometastasis. Ann. Transl. Med. 2015, 3, 362. [Google Scholar] [CrossRef]
- Weiner, A.B.; Pham, M.N.; Isaacson, D.S.; Ko, O.S.; Breen, K.J.; Nadler, R.B. Predictors of Use and Overall Survival for Patients Undergoing Metastasectomy for Bladder Cancer in a National Cohort. Int. J. Urol. 2020, 27, 736–741. [Google Scholar] [CrossRef]
- Vatansever, S.; Nordenström, E.; Raffaelli, M.; Brunaud, L.; Makay, Ö.; Almquist, M.; Barczynski, M.; Bergenfelz, A.; Clerici, T.; Hansen, M.H.; et al. Robot-Assisted versus Conventional Laparoscopic Adrenalectomy: Results from the EUROCRINE Surgical Registry. Surgery 2022, 171, 1224–1230. [Google Scholar] [CrossRef]
- Chen, W.C.; Baal, J.D.; Baal, U.; Pai, J.; Gottschalk, A.; Boreta, L.; Braunstein, S.E.; Raleigh, D.R. Stereotactic Body Radiation Therapy of Adrenal Metastases: A Pooled Meta-Analysis and Systematic Review of 39 Studies with 1006 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Pan, J.; Yang, N.; Shi, H.-F.; Cao, J.; Li, Y.-M.; Zhang, H.-Z.; Wang, K.-F.; Chen, S.-H. Effectiveness and Safety of CT-Guided Percutaneous Radiofrequency Ablation of Adrenal Metastases. Br. J. Radiol. 2018, 91, 20170607. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamakado, K.; Nakatsuka, A.; Uraki, J.; Yamanaka, T.; Fujimori, M.; Miki, M.; Sasaki, T.; Sakuma, H.; Sugimura, Y. Unresectable Adrenal Metastases: Clinical Outcomes of Radiofrequency Ablation. Radiology 2015, 277, 584–593. [Google Scholar] [CrossRef]
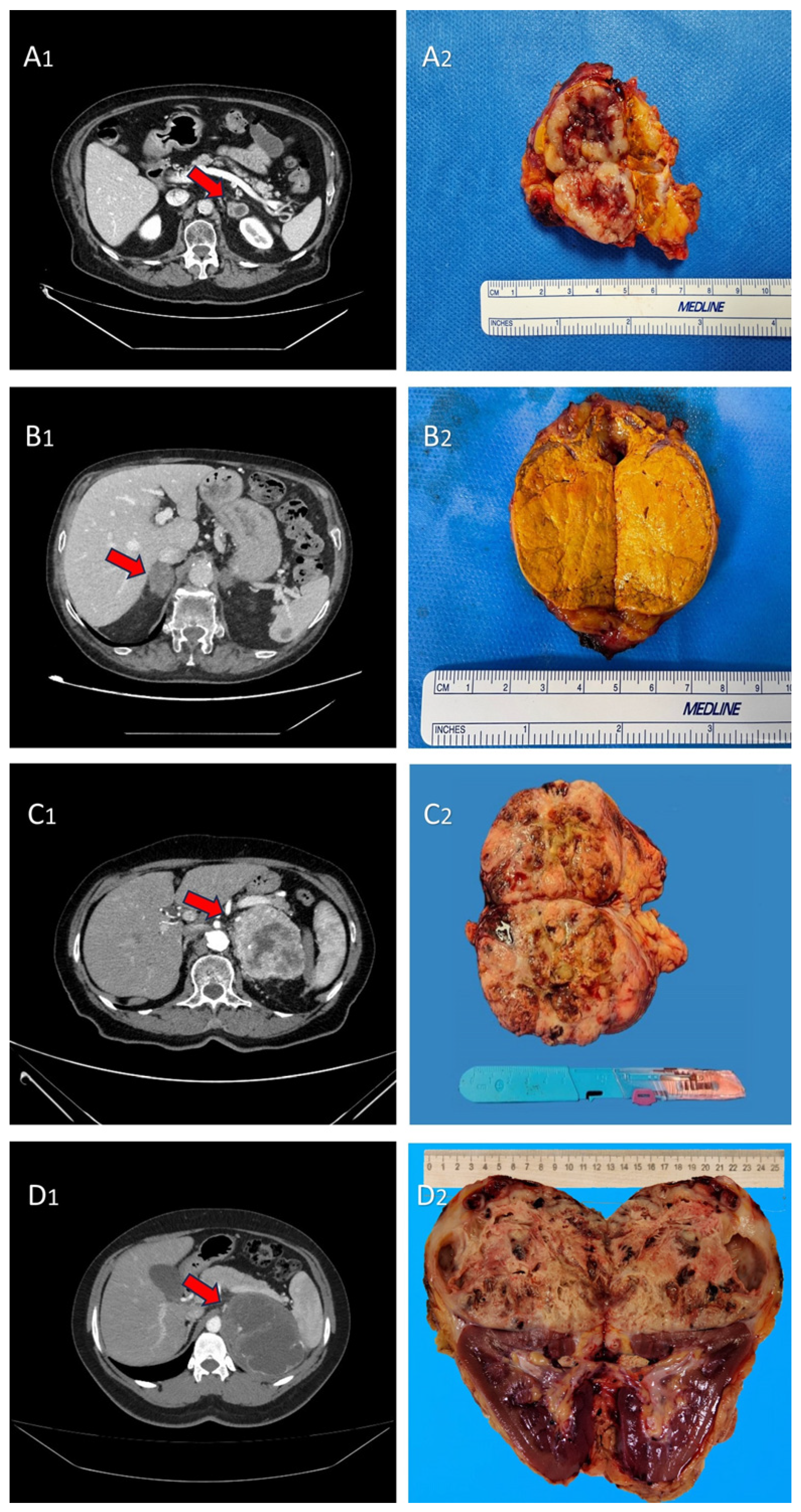
| DATA | Median (IQR) or n (%) |
|---|---|
| Age (years) | 66 (58–72) |
| Race | |
| White Caucasian | 60 (100) |
| Gender | |
| M | 41 (68.3) |
| F | 19 (31.7) |
| Side | |
| Right | 22 (36.7) |
| Left | 30 (50) |
| Bilateral | 8 (13.3) |
| ASA Score | 1–2 (43) 3 (17) |
| Hospital Stay (days) | 3 (3–5) |
| High Grade Complication Rate (Clavien–Dindo Classification 3–5) | 1 (1.7) |
| Histology | |
| Renal Cell Carcinoma | 36 (60) |
| Lung Cancer | 9 (15) |
| Bladder Cancer | 2 (3.3) |
| Colon Cancer | 6 (10) |
| Melanoma | 3 (5) |
| Sarcoma | 4 (6.7) |
| Tumor size (cm) | 5.5 (3.5–7) |
| Adrenal size (cm) | 6 (5–8) |
| Follow-Up (mo) | 34.5 (15–75.2) |
| RCC (36) | Other Histology (24) | p | |
|---|---|---|---|
| DATA | Median (IQR) or n (%) | Median (IQR) or n (%) | |
| Age (years) | 63 (55–72) | 68 (59–73) | |
| Race | |||
| White Caucasian | 36 (100) | 24 (100) | 1 |
| Gender | 0.42 | ||
| M | 25 (69.4) | 14 (58.3) | |
| F | 11 (30.6) | 10 (41.7) | |
| Side | 0.41 | ||
| Right | 11 (30.6) | 11 (45.8) | |
| Left | 19 (52.8) | 11 (45.8) | |
| Bilateral | 6 (16.7) | 2 (8.3) | |
| ASA Score | 0.57 | ||
| 2 | 26 (72.2) | 17 (70.8) | |
| 3 | 10 (27.8) | 7 (29.2) | |
| Hospital Stay (days) | 3 (3–3) | 3 (3–3) | 0.84 |
| High Grade Complication Rate (Clavien–Dindo Classification 3–5) | 1 (2.8) | 0 | 0.6 |
| Tumor size (cm) | 5 (3.2–6.5) | 5.5 (3.5–7) | 0.3 |
| Adrenal size (cm) | 6 (5–7) | 6 (3.7–9.5) | 0.71 |
| Follow-Up (mo) | 54 (23–98) | 15 (10–43) | 0.004 |
| Univariable Analysis | Multivariable Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |||
| Age | 1.01 | 0.97 | 1.04 | 0.63 | - | - | - | - |
| Gender | 1.79 | 0.90 | 3.53 | 0.09 | - | - | - | - |
| Bilateral Adrenal Metastasis | 0.94 | 0.36 | 2.45 | 0.89 | - | - | - | - |
| Tumor Size | 0.98 | 0.85 | 1.14 | 0.85 | - | - | - | - |
| Adrenal Size | 0.88 | 0.70 | 1.12 | 0.89 | - | - | - | - |
| ASA Score (3 vs. 2) | 1.34 | 0.64 | 2.80 | 0.44 | - | - | - | - |
| Primary Histology (RCC as ref category) | <0.001 | 0.005 | ||||||
| Colon | 6.94 | 2.56 | 18.81 | <0.001 | 5.33 | 1.85 | 15.34 | 0.002 |
| Bladder | 1230.45 | 10.36 | 1470.56 | <0.001 | 75.49 | 5.90 | 965 | 0.001 |
| Sarcoma | 0.94 | 0.12 | 7.06 | 0.95 | 1.04 | 0.13 | 7.9 | 0.97 |
| Lung | 1.51 | 0.51 | 4.45 | 0.46 | 1.52 | 0.51 | 4.51 | 0.45 |
| Melanoma | 1.41 | 0.18 | 10.69 | 0.74 | 1.49 | 0.19 | 11.28 | 0.70 |
| DFI < 12 mo | 3.05 | 1.46 | 6.33 | 0.003 | 1.89 | 0.80 | 4.46 | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferriero, M.; Iannuzzi, A.; Bove, A.M.; Tuderti, G.; Anceschi, U.; Misuraca, L.; Brassetti, A.; Mastroianni, R.; Guaglianone, S.; Leonardo, C.; et al. Adrenalectomy for Metastasis: The Impact of Primary Histology on Survival Outcome. Cancers 2024, 16, 763. https://doi.org/10.3390/cancers16040763
Ferriero M, Iannuzzi A, Bove AM, Tuderti G, Anceschi U, Misuraca L, Brassetti A, Mastroianni R, Guaglianone S, Leonardo C, et al. Adrenalectomy for Metastasis: The Impact of Primary Histology on Survival Outcome. Cancers. 2024; 16(4):763. https://doi.org/10.3390/cancers16040763
Chicago/Turabian StyleFerriero, Mariaconsiglia, Andrea Iannuzzi, Alfredo Maria Bove, Gabriele Tuderti, Umberto Anceschi, Leonardo Misuraca, Aldo Brassetti, Riccardo Mastroianni, Salvatore Guaglianone, Costantino Leonardo, and et al. 2024. "Adrenalectomy for Metastasis: The Impact of Primary Histology on Survival Outcome" Cancers 16, no. 4: 763. https://doi.org/10.3390/cancers16040763
APA StyleFerriero, M., Iannuzzi, A., Bove, A. M., Tuderti, G., Anceschi, U., Misuraca, L., Brassetti, A., Mastroianni, R., Guaglianone, S., Leonardo, C., Papalia, R., Gallucci, M., & Simone, G. (2024). Adrenalectomy for Metastasis: The Impact of Primary Histology on Survival Outcome. Cancers, 16(4), 763. https://doi.org/10.3390/cancers16040763







