Simple Summary
Tumors in soft tissue and bones are rare, and there is limited knowledge about how they occur. Better knowledge about inherited predisposition to tumor syndromes increases the chance for the medical community to detect cancer early through targeted screening programs and to choose the most appropriate cancer treatment. In our study, we show that some inherited mutations can increase the risk for these tumors. We applied a novel approach, in which we analyzed a blood sample in tandem with a tumor sample from participants, and we found especially interesting inherited mutations. Some of the mutations we found were already known to increase the risk of cancer, although not proven to be connected to soft tissue and bone tumors before. Other mutations we present have not been shown to be connected to tumors at all before. Our findings can guide further genetic investigations of soft tissue and bone tumors.
Abstract
Background: The etiology of most mesenchymal tumors is unknown, and knowledge about syndromes with an increased risk of tumors in bone or soft tissue is sparse. Methods: We present a prospective germline analysis of 312 patients with tumors suspected of being sarcomas at a tertiary sarcoma center. Germline and tumor whole genome sequencing, tumor transcriptome, and methylome analyses were performed. Results: Germline pathogenic or likely pathogenic variants associated with an increased risk of tumors were detected in 24 patients (8%), of which 11 (4%) harbored a detectable second hit in the tumor. Second hits were confirmed in genes with (NF1, RB1, TP53, EXT2, and SDHC) and without (ATM, CDC73, MLH1, MSH6, POLG, and KCNQ1) known association with mesenchymal tumor predisposition. Sarcomas from two Lynch syndrome patients showed mismatch repair deficiency, predicting a treatment response to immune checkpoint inhibitors (Level 1 biomarker according to the FDA (Federal Drug Administration) and ESMO (European Society for Medical Oncology)). None of the three CHEK2 carriers had a second hit in the tumor, suggesting a weak link to sarcoma. Conclusions: We conclude that second-hit analyses can be used in standard of care to identify syndrome-related tumors. This approach can help distinguish true manifestations of tumor syndromes from unrelated germline findings and enhance the understanding of germline predisposition in soft tissue tumors. Prospective screening using germline whole genome sequencing should be considered when comprehensive somatic sequencing is introduced into clinical practice.
1. Introduction
Mesenchymal tumors such as lipomas, uterine leiomyomas, and fibrous histiocytomas are common in the general population. Over a hundred subtypes have been described, many having distinct genomic alterations such as fusion genes, small nucleotide variants, or copy number alterations [1]. Malignant mesenchymal tumors (sarcomas), on the other hand, are extremely rare and constitute less than 1% of all malignancies. Compared to carcinomas, sarcomas more commonly affect children and adolescents [2]. Their etiology is generally unknown, and most sarcomas are considered sporadic, without association to germline variants [3]. However, tumor syndromes such as neurofibromatosis type 1 may increase the risk of certain types of mesenchymal tumors [4], and some cancer syndromes have an established increased risk of sarcomas. For example, heritable TP53-related cancer syndrome is associated with an increased risk of osteosarcomas and soft tissue sarcomas [5], and heritable retinoblastoma syndrome (with germline pathogenic variants in the RB1 gene) increases the risk of osteosarcomas, rhabdomyosarcomas, and leiomyosarcomas [6].
We have previously described the impact of whole genome sequencing (WGS) and whole transcriptome sequencing (WTS) on sarcoma diagnostic classification and treatment prognostic biomarkers in clinical practice [7]. Such analyses can potentially reveal pathogenic germline variants, which may be associated with tumor development. Here, we describe an expanded patient cohort from a germline perspective, in which both germline WGS, tumor tissue WGS, WTS, and methylation analyses are interpreted in the light of patient phenotype (see Supplementary Figure S1 for the study pipeline).
2. Materials and Methods
2.1. Study Population
This prospective clinical research study offered inclusion for any patient treated at the Karolinska University Hospital in Stockholm, Sweden, with a primary or metastatic tumor suspicious for sarcoma during the years 2022 and 2023. The predominance of adult patients was expected in advance, since childhood sarcomas were mainly analyzed in another pipeline as part of an ongoing parallel study [8]. Family history or history of other tumors did not influence study inclusion. The research was approved by the Swedish Ethical Review Authority (ID 2022-05409-01 and 2013 1979-31 with amendment 2018/2124-32) and was designed in accordance with Swedish law and the Declaration of Helsinki. All participants, or their legal guardians for minors, had given their informed consent prior to their enrollment in the study.
2.2. Whole Genome and Transcriptome Analysis
DNA and RNA isolation from blood and tumor tissue, library generation, and sequencing were performed as previously described [7]. In short, DNA and RNA libraries were generated with the Illumina TruSeq DNA PCR-Free library preparation kit, and the Illumina Stranded mRNA Prep (Illumina, San Diego, CA, USA), respectively. Sequencing was performed on the Illumina NovaSeq 6000 or NovaSeq X Plus platforms using paired-end 150-bp sequencing, with at least 30x coverage in normal DNA and 90x in tumor DNA.
Germline WGS data were analyzed with the pipeline Mutation Identification Pipeline (versions ranging from 11.0.2 to 12.0.3) [9,10], and somatic WGTS data were analyzed with the BALSAMIC (version 12.0.2) [11] and AutoSeq pipelines [12], as previously reported [7].
2.3. Identification and Reporting of Germline Variants
Germline variants were limited by in silico filtering to 787 genes with known or suspected association to hereditary cancer (Supplementary Table S1). The gene panel was the result of a curated list of genes with potential links to cancer syndromes previously published by our group [13] and the human phenotype ontology term “neoplasia” (HP:0002664) accessed January 2022 [14]. Manual filtering of all coding (exonic or splicing) germline variants with a gnomAD [15] allele population frequency of <0.01 was performed. Variants were excluded if A) they were reported as likely benign or benign in ClinVar [16] by more than two submitters, or B) they occurred in the local variant database (9244 cases) > 100 times and were synonymous, or C) they were reported as likely benign or benign in ClinVar by only one submitter but occurred in the local variant database > 80 times.
After initial variant filtering, clinical assessment was manually performed based on participant phenotype and the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) criteria [17]. Missense variants with no reported association to a cancer syndrome in ClinVar [16] and potentially truncating variants (nonsense, frameshift, or splice site) with either no association to the phenotype of the patient, or in genes without truncation being the known mechanism of disease, were considered variants of unknown significance. All patients with a pathogenic or likely pathogenic variant were further checked for medical history, known cancer or other traits in the family, and previous medical treatment or specific carcinogenic exposure. Pathogenic or likely pathogenic variants (heterozygous for dominant disorders and homozygous or compound heterozygous for autosomal recessive disorders) determined to be clinically actionable were reported to the referring physician, who informed the patient and offered further referral for genetic counselling. Germline variants associated with a phenotype known or previously suggested to increase the risk of mesenchymal tumors were labeled “second hit expected”, and the remaining genes without a known association were labeled “second hit not expected”.
2.4. Identification of Somatic Variants
Genes with pathogenic or likely pathogenic germline variants were analyzed for somatic variants in the tumor DNA, including small nucleotide variants (SNVs), copy number alterations (CNAs), loss of heterozygosity (LOH), and inactivation by structural variants. Filtering included a variant allele frequency of >5% for SNVs/deletions and >10% for structural variants. All variants were manually inspected in the integrated genome viewer (IGV) [18]. Somatic CNAs and LOH were visually addressed in AutoSeq [12,19]. Chromothripsis-like CNA profiles and large deletions were considered uninformative. Tumor mutational burden (TMB) was calculated from all somatic variants that had passed quality control (i.e., minimal read depth 10, minimal allele frequency 0.05, maximal gnomAD frequency 0.1%) and divided by 3000 Mb. TMB > 10 variants/Mb was considered “TMB-high”. Germline variants with no previously known association to the phenotype but with detectable same-gene second hits were considered novel, and these genes were further checked in the Catalogue Of Somatic Mutations In Cancer (COSMIC) database and The Cancer Genome Atlas (TCGA) datasets for further assessment of potential impact in soft tissue tumors [20,21]. For all patients with germline variants without a confirmed second hit, we expanded the search of second hits to include genes whose proteins were associated with the germline gene (including the affected signaling pathway and proteins with known interactions with the gene products).
WTS alignments (.cram files) from the RNA fusion pipeline were assembled into potential transcripts with StringTie v2.2.0. Gene expression values (FPKM) for the genes of interest were extracted. Aberrant RNA expression was determined by plotting the gene expression of the whole cohort (312 cases). Extremely low or high expression (top or bottom 5th percentile) for a gene with a germline pathogenic hit was considered aberrant and indicative of a second hit.
2.5. Methylation
Genomic DNA from fresh frozen tumors was treated with sodium bisulfite using the EZ-96 DNA methylation kit (Zymo Research, catalogue number D5004), following the manufacturer’s standard protocol. An assessment of the levels of DNA methylation of known CpG regions and promoters across the genome was performed with the Infinium MethylationEPIC v2.0 Kit (Illumina, San Diego, CA, USA) and Illumina iScan. In brief, following bisulfite conversion, approximately 500 ng of the bisulfite-converted DNA per sample was used for methylation analysis. The initial quality control and identification of signal intensities for each probe were performed with Illumina GenomeStudio Software 2011.1.
2.6. Statistical Analyses
Statistical analyses were performed in Python with the package SciPy. Student’s t-test was used for the comparison of age distribution. Odds ratios were calculated for malignancy versus non-malignancy and woman versus man. A p-value < 0.05 was considered significant.
3. Results
3.1. A Minority of Sarcoma Patients Have Tumor Predisposition Syndromes
In total, 316 patients with a preoperative suspicion of sarcoma were prospectively included in the study. After final histopathological analysis, four cases were excluded from the study (two patients with malignant melanoma, one with endometrial carcinoma, and one with colorectal cancer). This generated a study cohort of 209 (67%) sarcomas, 40 (13%) gastrointestinal stromal tumors (GISTs), and 63 (20%) benign mesenchymal tumors. A summary of all final diagnoses is available in Supplementary Table S2.
We performed whole genome sequencing from a peripheral blood sample and multiomics including DNA, RNA, and methylation analyses of the corresponding tumor tissue sample. In 8% of the whole cohort (24/312), a pathogenic or likely pathogenic germline variant associated with a dominant hereditary tumor syndrome was detected (Table 1). When excluding benign cases, the tumor syndrome detection rate was similar (8%, 21/249). Most of the carriers had no medical history of previous cancer, and no known cancer in the family. A minority of carriers would have fulfilled the testing criteria for their specific cancer syndrome (42%,10/24), and two of them had regardless not been offered a germline genetic screening. No homozygous or compound heterozygous variants in genes associated with a recessive syndrome were detected.

Table 1.
Genetic findings and clinical data. Diagnoses, germline variant details, clinical data, and second hit detection for the 24 patients with a germline, potentially disease-causing variant.
Patients with pathogenic or likely pathogenic germline variants had somewhat different tumor diagnoses than the rest of the cohort (Table 2), with a greater ratio of leiomyosarcomas. There were no significant differences regarding age distribution, malignant tumors, or sex ratio between the group with germline pathogenic findings and the group without (Supplementary Table S3).

Table 2.
Histopathological diagnoses in the potential cancer syndrome group compared to the total cohort.
3.2. Many Germline Variants Currently Have Limited Clinical Utility
Of the 24 patients with a potential hereditary tumor syndrome, 16 had findings that were considered clinically actionable, leading to genetic counselling and carrier testing in the family. Half of this group received surveillance for mesenchymal tumors and the other half for carcinomas only.
The remaining eight pathogenic and potentially actionable germline variants were not reported to the treating clinicians, in accordance with ACMG/AMP criteria and current Swedish National Guidelines. These eight variants were either associated with potential hereditary tumor syndromes without any surveillance recommendations based on patient phenotype and pedigree (variants in the genes CHEK2, EXT1, ATM), or they were weakly associated with a condition potentially increasing the risk of cancer without being likely causative for a cancer syndrome in this specific case (variants in the genes MRE11, BRIP1, ATR, DDX41, MITF, SDHAF2).
3.3. Somatic Analysis Confirms Biallelic Inactivation and Establishes Sarcoma Syndromes
For patients with a pathogenic germline variant, we used the multiomics results from the corresponding tumor tissue sample, including whole genome and transcriptome sequencing and methylation analysis, to search for a second hit in the tumor. The initial search focused on the gene affected by a first, potentially causative germline hit. A pathogenic second hit was detected in the tumor from 11 of these patients (11/24, 46%), as presented in Table 1. In addition to this, two patients with germline MSH6 pathogenic variants without detectable second hits had indirect signs of deficient MSH6: one had a mismatch repair (MMR) deficient tumor, and the other (who had received neoadjuvant treatment with complete response before the tumor sampling) had a preoperative biopsy showing complete loss of MSH6 immunoreactivity, consistent with deficient MMR.
All second hits were either missense or nonsense SNVs or focal deletions. No methylation aberration was detected as a second hit. Two illustrative examples of germline tumor suppressor inactivation with a somatic second hit and corresponding gene expression, as detected by multiomics in diagnostic patients, are depicted in Figure 1 and Figure 2.
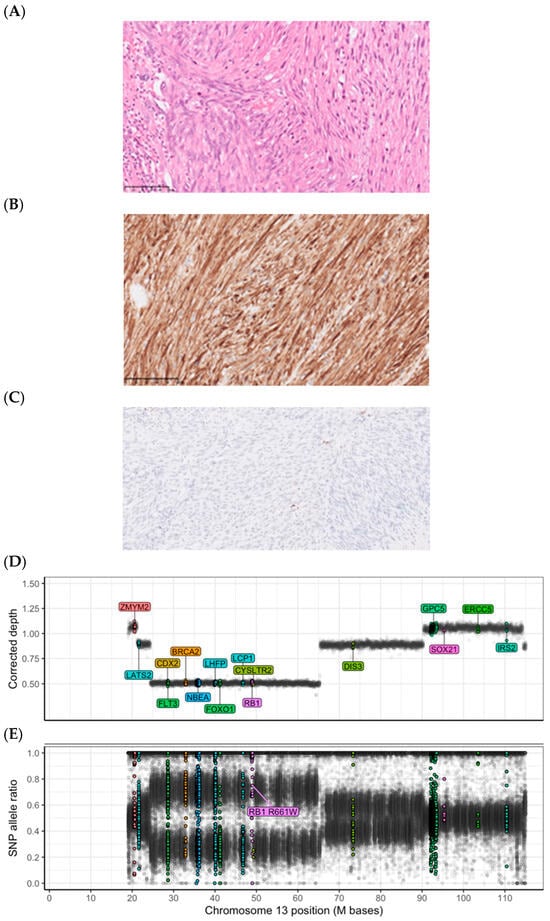
Figure 1.
Histopathology and genetics of an RB1 germline-related leiomyosarcoma. Patient 101 carried a germline RB1 pathogenic variant causing hereditary retinoblastoma syndrome. The tumor harbored a deletion of the RB1 locus (13q), resulting in the loss of heterozygosity and biallelic inactivation. (A–C): Microphotographs show (A) a routine hematoxylin–eosin stain of a leiomyomatous tumor with high grade atypia, and immunohistochemistry showing (B) positivity for desmin, and (C) a loss of Rb immunoreactivity (single cells with retained Rb expression are tumor-infiltrating immune cells), as discovered in the clinical workup of the tumor. All microphotographs were captured at 400x magnification. (D): DNA abundance, measured as the bias-corrected sequence depth ratio for 10kb bins along the reference genome, appears at distinct levels corresponding to the number of copies per cancer cell. The RB1-containing segment displays a low DNA abundance, typical of a deletion. (E): The SNP (single nucleotide polymorphism) allele frequency for the RB1-containing segment shows distinct allelic imbalance, also consistent with a deletion. The high allele ratio of the pathogenic germline RB1 variant confirms the retention of the alternative allele in the tumor genome. The estimated average copy number (ploidy) is about 3.6 and the cancer cell fraction is about 60%. Colored dots represent probes located in sarcoma-associated genes.
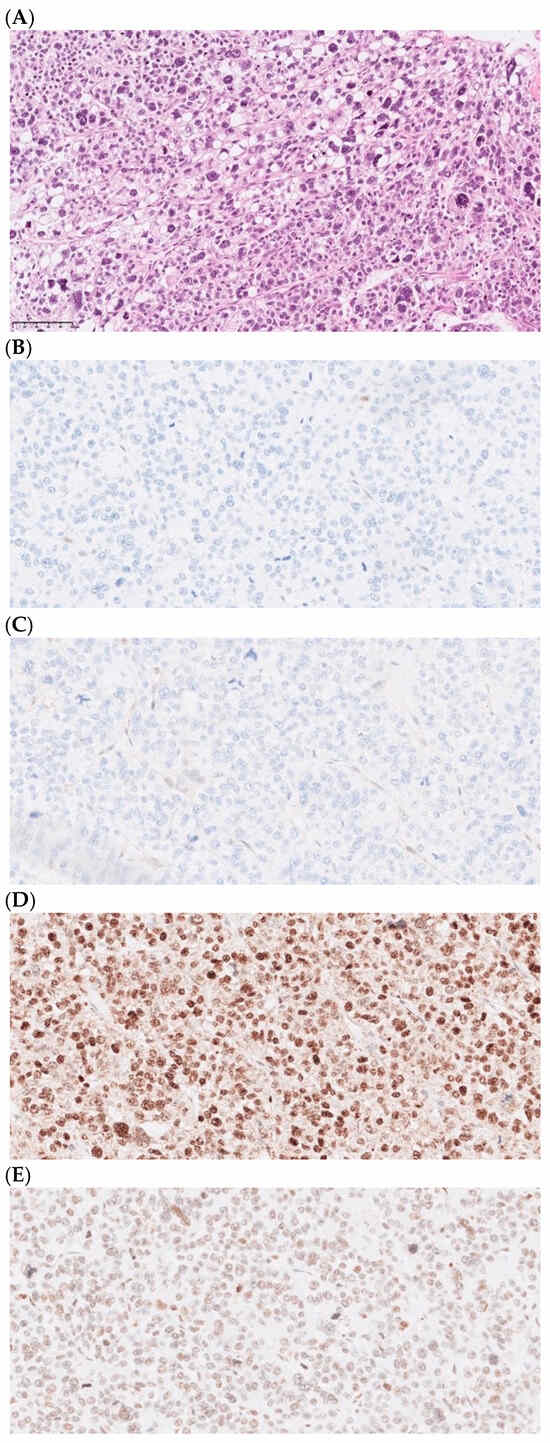
Figure 2.
Histopathology and genetics of a Lynch syndrome-associated pleomorphic liposarcoma. Patient 76 was shown to have Lynch syndrome caused by a germline MLH1 inactivation. Microphotographs of the tumor histology and immunohistochemistry, as performed in the clinical workup. All microphotographs were captured at 400x magnification. (A) Routine hematoxylin–eosin stain depicting a pleomorphic liposarcoma and immunohistochemistry with loss of (B) MLH1 and (C) PMS2 expression, with retained expression of MSH2 (D) and MSH6 (E), compatible with deficient mismatch repair (dMMR).
Genetic counselling was recommended for all patients whose tumors harbored a second hit, except for the ATM, POLG, and KCNQ1 carriers. Most of the germline variants with a second hit had previously been linked with an increased risk of mesenchymal tumors, or an association had previously been suggested, and were thereby considered as “second hit expected” (Figure 3). Vice versa, all patients with a germline hit considered “second hit not expected” (in genes without a previously known association with mesenchymal tumors or sarcoma), except for CDC73 and ATM, had no second hit (Figure 3). For Lynch syndrome, the second hit was considered semi-expected based on the current literature.
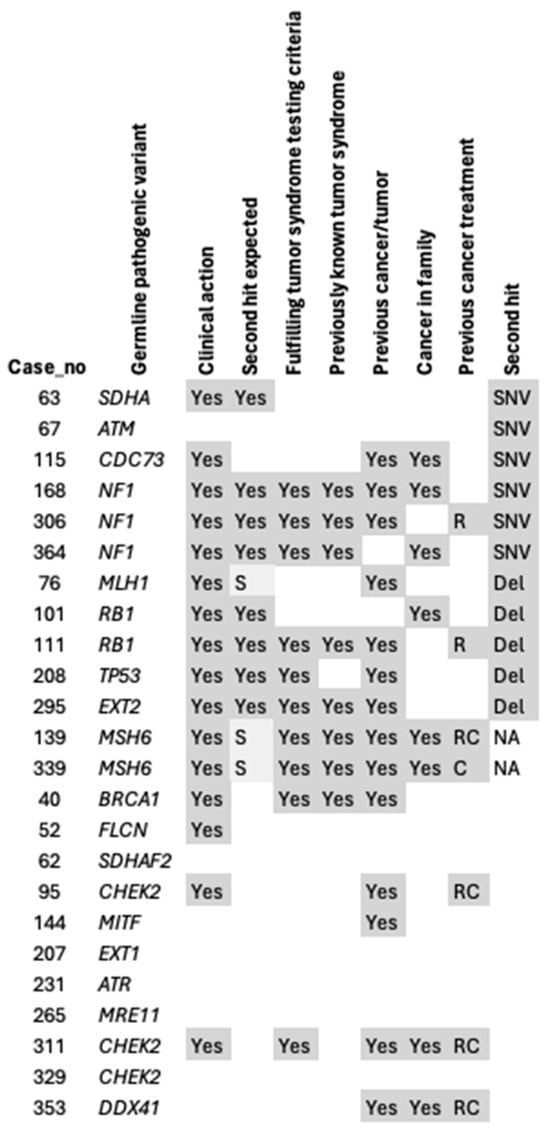
Figure 3.
Second hits in relation to phenotype. C: chemotherapy, del: deletion, NA: no tumor tissue available, or second hit detected through immunohistochemistry, R: radiotherapy, RC: radiotherapy combined with chemotherapy, SNV: single nucleotide variant, S: suggested.
For one female patient with a history of paraganglioma, pulmonary hamartomas, and SDH-deficient GIST (as determined by SDHB loss in the immunohistochemistry analysis), and no detectable germline variant, somatic methylation analysis detected biallelic hypermethylation of the SDHC promoter. This is consistent with the constitutional hypermethylation of SDHC that results in the somatic syndrome Carney triad (Figure 4).
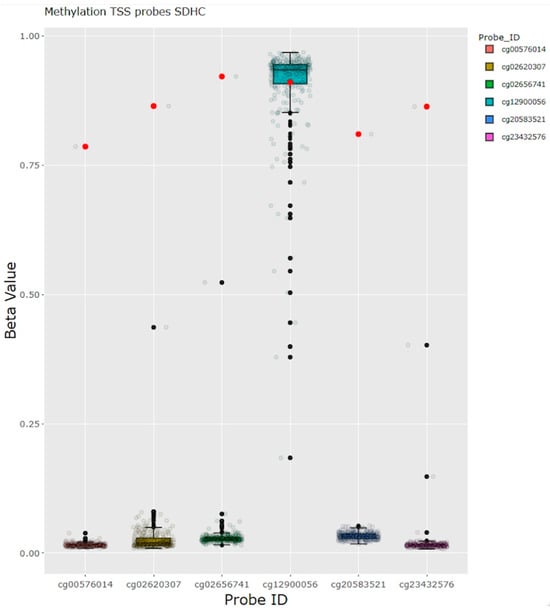
Figure 4.
Methylation analysis in a patient with paraganglioma and GIST. Hypermethylation of the SDHC promoter in a patient with the clinical diagnosis Carney triad, without any germline findings and no somatic gene dose abnormalities in the SDHC region. Red dots represent the beta values of methylation from the case of interest. GIST: gastrointestinal stromal tumor, TSS: transcription start site.
The common c.1100delC (current nomenclature c.1229del) variant in the CHEK2 gene was detected in three participants, and none of them had a second hit in CHEK2. Two of them, a woman with a history of breast cancer and a woman whose sister had breast cancer, were referred for genetic counselling, whereas the third case was a man without a first-degree relative with breast cancer, thereby not fulfilling the criteria for surveillance recommendation according to Swedish national guidelines [22].
In cases with germline pathogenic findings but without detectable second hits, genes with functions in the same pathways as the germline gene were assessed. No additional pathogenic somatic lesions were detected through this approach.
We also performed second hit analysis for 12 patients with pathogenic or likely pathogenic germline variants without established association with hereditary tumor syndromes (Supplementary Table S4). Of these, second hits in the tumors were identified in two patients, harboring germline variants in the KCNQ1 and POLG genes and diagnosed with fibromatosis and GIST, respectively. In patients with heterozygous germline variants in genes associated with a recessive disorder, no second hits were detected in the tumor.
4. Discussion
In this prospective cohort of 312 patients with mesenchymal tumors suspicious for sarcoma, we confirmed a causal relationship between the tumors and an underlying tumor syndrome in 4% of the patients. An additional 3% of patients had germline variants associated with tumor syndromes or an increased risk of tumors, but without second hits, suggesting these variants were unrelated to the current diagnosis.
The prevalence of tumor predisposition in our cohort is similar to previous publications, with differences related to the clinical assessment of actionability [23,24].
Studies of potential causative mesenchymal tumor syndromes are sparse. Recently, Ballinger et al. published the largest study so far regarding sarcoma genetic predisposition. They identified two sarcoma-specific pathways involved in mitotic and telomere functions and concluded that further studies are needed to map their connection to an increased risk of sarcomas [25]. In our study, 16 individuals were referred for genetic counselling, and carrier testing was offered to their family members. Even though the risk of sarcomas is usually low for most cancer syndromes, thereby not warranting surveillance programs, the concurrent risk of developing a carcinoma might be high. Additionally, some germline findings directly impact the sarcoma treatment, such as avoidance of radiation therapy in the case of pathogenic TP53 variants and potential immunotherapy for Lynch syndrome patients [26,27].
To differentiate causative germline variants from incidental findings in a diagnostic setting, we applied a somatic second hit omics approach. In clinical practice, this procedure is not widely used, as it requires both a tissue biopsy and a blood sample. However, with the growing use of comprehensive genetic screening for tumors, using blood samples as a normal reference to filter for somatic driver events, the potential for this approach is expanding.
Several groups have shown the usefulness of evaluating germline findings in the light of second hits, showing that the rate of detectable same-gene second hits was higher in patients carrying a germline variant with a known association to their tumor than those with a non-associated tumor [24,28,29,30]. Yap et al. published a retrospective study with the aim to evaluate whether the identification of germline variants adds benefit to paired tumor/normal sequencing. They found that 7.3% of their large cohort harbored pathogenic or likely pathogenic variants, but for tumor types lacking genetic testing guidelines, such as sarcomas, this proportion was lower [24]. Also, second hits were only detected in a minority of the cases, for instance only in 29% of the NF1 carriers and 31% of the MSH6 carriers. Fiala et al. found that 12% (28/229) of pediatric patients with sarcoma had a germline pathogenic or likely pathogenic variant, including both those associated with dominant and recessive inheritance. In 81% of the cases with an expected germline finding, a second hit was found [29]. In a recent study by Tesi et al., the prevalence of childhood cancer predisposition in a cohort of children with tumors was 11% (35/309), with a second hit and/or a relevant mutational signature detected in 19/21 (90%) of tumors with informative data [8].
In this study, the second hit approach suggested ATM and POLG as new candidates for association with sarcoma and identified a potential association between KCNQ1 and desmoid fibromatosis.
ATM is a tumor suppressor gene with multiple protein functions, such as DNA-repair and cell cycle regulation. In current clinical practice, this gene is classified as a moderate penetrance gene associated with an increased risk of breast cancer [31]. There is growing evidence that pathogenic ATM variants increase the risk of several tumor types, including melanoma, ovarian, and pancreatic cancer, among others [31,32,33]. In Sweden, only truncating ATM variants are considered actionable [22], but the missense variant detected in our study is a Finnish founder, with an increased risk of breast cancer [34]. The somatic second hit detected in our patient, c.5188C>T, is a well-established truncating pathogenic germline variant [16]. In the COSMIC database, somatic ATM variants are reported in 8% (82/1077) of soft tissue tumors, of which 16 are angiosarcomas. The c.5188C>T variant is reported in 13 cases (carcinomas (N = 9), malignant melanomas (N = 3), and one carcinoid) [20]. Aberrations in ATM are present in 5% (12/255) of the PanCancer Atlas Sarcoma dataset, mostly in myxofibrosarcomas [21]. The detection of the biallelic loss of ATM in an angiosarcoma tumor in our study contributes to the growing knowledge about ATM in tumorigenesis.
The POLG gene is primarily known to cause the autosomal recessive Mitochondrial DNA Depletion Syndrome 4B, but autosomal dominant inheritance of progressive ophtalmoplegia has also been described [35]. There is so far no known connection to sarcomas, but there are limited reports suggesting tumor suppressor properties [36]. The POLG germline variant identified in our study is reported as pathogenic in mitochondrial disease cohorts by multiple submitters in ClinVar [16], and somatic variants in POLG are reported in 1% (10/902) of soft tissue tumors in COMIC, of which 30% (3/10) are GISTs [20]. A second hit in a GIST is intriguing, and further studies are needed to determine the functional role of biallelic POLG inactivation in tumor development.
Pathogenic (mainly truncating) variants in the KCNQ1 gene lead to autosomal dominant arrhythmia syndromes and recessive Jervell and Lange–Nielsen syndromes [37]. Both the germline and somatic variants detected in our patient are known pathogenic variants associated with long QT syndrome, and none of these specific variants are reported in tumor samples in the COSMIC database. However, other KCNQ1 variants are reported in a variety of tumor types, including 4% (33/867) of soft tissue tumors, mainly in unclassified sarcoma from fibrous tissue/uncertain origin (23/33) [20]. Missense variants in the same gene have been associated with inherited gingival fibrosis [38,39]. Mice carrying a targeted deletion in the KCNQ1 gene developed significantly more intestinal tumors than non-mutant mice, and low expression of KCNQ1 was associated with poor overall survival for colorectal cancer patients [40]. There are no reports of KCNQ1 involvement in desmoid fibromatosis, and our patient’s tumor harbored the well-characterized CTNNB1 somatic activating variant. There is evidence that KCNQ1 is involved in the regulation of the Wnt/b-catenin signaling pathway, which is normally upregulated and serves as the primary driver of desmoid fibromatosis [41]. This suggests a potential mechanistic link between the biallelic loss of KCNQ1 and desmoid fibromatosis in this case. However, further studies are needed to clarify this connection.
These new candidates for mesenchymal tumor syndromes highlight the potential for rare, previously unrecognized low-penetrance syndromes.
The somatic second hit approach also verified pathogenic variants in the genes SDHA, CDC73, NF1, RB1, TP53, and EXT2 as causative in their carriers. Germline variants in the CDC73 gene, including the truncating variant detected in our patient, are known to cause hyperparathyroid-jaw tumor syndrome (HPT-JT), which has variable penetrance for parathyroid carcinomas, ossifying fibromas of the jaw, and uterine lesions (adenofibromas and rarely adenosarcomas) [42]. While the truncating somatic variant detected in our patient has been reported solely in parathyroid carcinoma (N = 4) in the COSMIC database, other somatic variants in CDC73 are reported in 2% (30/1679) of soft tissue tumors [20]. Our findings further substantiate the association between germline CDC73 variants and the infrequent development of adenosarcoma.
For MLH1 and MSH6, our results support previous studies suggesting that sarcomas are rare manifestations of Lynch syndrome. Similar to the cases in our study, a small subset of sarcomas presenting in Lynch syndrome have been radiation-induced [43,44].
We considered the pathogenic variants in genes with no detected second hit to be unsolicited findings. For instance, the risk of mesenchymal tumors in CHEK2 c.1100delC carriers is not known. Näslund-Kock et al. found a sex-adjusted hazard ratio for heterozygous carriers compared to non-carriers of 3.45 (95% confidence interval, 1.09 to 10.9) for sarcomas [45], which was not significant after correcting for multiple comparisons. Bychkovsky et al. showed that pathogenic variants in the CHEK2 gene are not associated with an increased risk of sarcomas [46]. Abdelghani et al. found that 6/300 pediatric cancer patients had germline CHEK2 pathogenic variants, one of whom had Ewing sarcoma, while none of the others had mesenchymal tumors [47]. Of course, a germline pathogenic variant might be associated with an increased risk for a tumor regardless of a somatic second hit. The second hit approach is merely one of the available tools for interpreting the pathogenicity of genetic variants. The ACMG/AMP criteria [17], including the statistical correlation between diagnosis frequency and carrier frequency, bioinformatic information such as how conserved the affected amino acid is, etc., are widely used in the clinic. For mesenchymal tumors, however, their rarity makes it harder to achieve statistical power and to prove a phenotype–genotype correlation.
The diverse nature of our cohort reflects the typical presentation of patients at a sarcoma reference center, including benign tumors suspicious for sarcoma based on radiological or clinical findings and true sarcomas. When we analyzed different subgroups separately, leiomyosarcomas and GIST were the most prevalent within the cohort with germline pathogenic findings, which is to be expected since these are also among the more common diagnoses. Two participants with leiomyosarcoma carried pathogenic variants and same-gene second hits in RB1 and TP53, which have known connections to this condition [48,49,50], and one carried a pathogenic variant without a detectable in-gene second hit in the DDX41 gene, without any known connection to leiomyosarcoma. Among the patients with GIST, four had germline pathogenic findings. In total, there were five participants with GISTs without mutations in KIT, PDGFRA, or BRAF (sometimes referred to as “wild-type GISTs”), of whom two had germline pathogenic findings (in the genes SDHA and NF1). The number of wild-type GIST patients in our study is too small to base any statistical analyses on. Mandelker et al. reported a cohort with 35 wild-type GIST patients, in which they found germline pathogenic variants (in the genes SDHA, SDHB, SDHC, NF1, and KIT) in 70% (24/35) [51]. These results highlight the importance of germline genomic analysis for this patient group.
5. Conclusions
In this prospective study, we screened patients at a sarcoma reference center for germline variants. A significant proportion of patients (4%) harbored germline variants associated with a tumor predisposition syndrome and a second hit in the tumor. Pathogenic germline variants and somatic second hits were found in NF1, RB1, TP53, EXT2, and SDHC, in patients with a malignant peripheral nerve sheath tumor and GIST, leiomyosarcoma, leiomyosarcoma, secondary peripheral chondrosarcoma, and GIST, respectively. Both germline and somatic hits were found in the ATM, CDC73, MLH1, MSH6, POLG, and KCNQ1 genes, which have no previously known connection with sarcoma. As WGS becomes routine clinical practice for sarcomas and other rare tumors, an integrated somatic–germline analysis is a feasible and efficient approach to evaluate germline findings in the clinical setting, and it could be employed in the reality of the rapidly expanding WGS analyses performed in cancer centers. Importantly, germline variants need to be interpreted in their clinical context, and knowledge about how to handle unsolicited findings and variants of unknown significance is crucial.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16223816/s1, Supplementary Table S1: Genes included in the in-silico germline list. Supplementary Table S2: Diagnoses of all study participants. GIST: Gastronintestinal stromal tumor, GYN: gynaecological tumor, N: no, Y: yes. Supplementary Table S3: Clinical characteristics of the potential cancer syndrome group compared to the total cohort. * Subcohort with no pathogenic variant in a gene associated with a hereditary cancer syndrome (N = 288). ** Subcohort with detected pathogenic variant in a gene associated with a hereditary cancer syndrome (N = 24). NS: not significant. Supplementary Table S4: Second hits for all pathogenic and likely pathogenic non-tumor syndrome variants. Supplementary Figure S1: Overview of study design. After inclusion, WGS from blood and tumor tissue was performed in parallel, with additional RNA and methylation analysis for the tissue sample. Germline pathogenic or likely pathogenic variants were selected by the bioinformatic filtering of normal DNA. Variants were evaluated individually together with the patient tumor type, digital clinical records, and available evidence for pathogenicity. All germline variants were further investigated through analysis of the somatic DNA. Variants classified as clinically relevant for the carrier were reported to the treating physician, and genetic counselling was offered. WGS: whole genome sequencing, WTS: whole transcriptome sequencing, P/LP: pathogenic or likely pathogenic according to American College of Medical Genetics and genomics classification.
Author Contributions
F.H.d.F., K.W., V.W. and J.L. conceived and supervised the study; F.H.d.F. processed and archived the biopsy tissue and performed the histopathological assessment of tissue samples; Y.L. processed the tissue samples and performed nucleic acid extraction for sequencing; K.W., J.M., J.H., F.H.d.F., I.Ö. and Y.L. performed the manual variant filtering analyses for germline and somatic events; K.W., I.Ö. and C.H. provided clinical information; K.W. and I.Ö. performed the clinical assessment of germline variants; V.C., M.M. and A.G. developed the bioinformatic pipelines for DNA and RNA sequencing; P.T. and R.B. included patients in the study and managed the clinical follow-up of molecular findings. F.H.d.F., K.W. and I.Ö. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
HdF and KW were sponsored by fellowships from Swedish cancer society (Cancerfonden, grant IDs 19 0029 JCIA; and 22 0607 FE). This study was sponsored by the Swedish Sarcoma Association (Sarkomföreningen), Swedish Cancer Society (Cancerfonden, grant 22 2124 Pj), the Cancer Society in Stockholm (Radiumhemmets forskningsfonder) and Karolinska Institutet.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Swedish Ethical Review Authority (ID 2022-05409-01 and 2013 1979-31 with amendment 2018/2124-32, year 2022, 2013, and 2018, respectively).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data generated in this study are available from the corresponding author upon request. Raw data is not publicly available, because it contains information that could compromise research participants’ anonymity.
Acknowledgments
We are grateful to all patients that participated in this project. We are also thankful to the clinicians, David Goldstein, Marie Jalsenius, and others, at Karolinska University Hospital, without whom the project would not have been possible. We thank the Translational Analysis in Molecular Medicine (TAMM) at the Karolinska University Hospital for their support with this work. We acknowledge the Clinical Genomics Stockholm facility at the Science for Life Laboratory for providing expertise and service with sequencing analysis.
Conflicts of Interest
Sample reagents for massive parallel sequencing were kindly sponsored by Illumina Inc. as part of an investigator-initiated study (PI F.H.d.F., study ID MR-000103).
References
- The WHO Classification of Tumours Editorial Board. WHO Classification of Tumours Soft Tissue and Bone Tumours, 5th ed.; IARC Press: Lyon, France, 2020; Volume 5. [Google Scholar]
- Burningham, Z.; Hashibe, M.; Spector, L.; Schiffman, J.D. The epidemiology of sarcoma. Clin. Sarcoma Res. 2012, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Ngeow, J. Sarcomas Associated with Genetic Cancer Predisposition Syndromes: A Review. Oncologist 2016, 21, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Yang, Q.; Friedman, J.M. Mortality in neurofibromatosis 1: An analysis using U.S. death certificates. Am. J. Hum. Genet. 2001, 68, 1110–1118. [Google Scholar] [CrossRef]
- Frebourg, T.; Bajalica Lagercrantz, S.; Oliveira, C.; Magenheim, R.; Evans, D.G. Guidelines for the Li-Fraumeni and heritable TP53-related cancer syndromes. Eur. J. Hum. Genet. 2020, 28, 1379–1386. [Google Scholar] [CrossRef]
- Kleinerman, R.A.; Schonfeld, S.J.; Tucker, M.A. Sarcomas in hereditary retinoblastoma. Clin. Sarcoma Res. 2012, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Öfverholm, I.; Wallander, K.; Haglund, C.; Chellappa, V.; Wejde, J.; Gellerbring, A.; Wirta, V.; Renevey, A.; Caceres, E.; Tsagkozis, P.; et al. Comprehensive Genomic Profiling Alters Clinical Diagnoses in a Significant Fraction of Tumors Suspicious of Sarcoma. Clin. Cancer Res. 2024, 30, 2647–2658. [Google Scholar] [CrossRef]
- Tesi, B.; Robinson, K.L.; Abel, F.; Díaz de Ståhl, T.; Orrsjö, S.; Poluha, A.; Hellberg, M.; Wessman, S.; Samuelsson, S.; Frisk, T.; et al. Diagnostic yield and clinical impact of germline sequencing in children with CNS and extracranial solid tumors-a nationwide, prospective Swedish study. Lancet Reg. Health Eur. 2024, 39, 100881. [Google Scholar] [CrossRef]
- MIP Piepline. Available online: https://github.com/Clinical-Genomics/MIP/blob/develop/documentation/README.md (accessed on 10 October 2023).
- Stranneheim, H.; Lagerstedt-Robinson, K.; Magnusson, M.; Kvarnung, M.; Nilsson, D.; Lesko, N.; Engvall, M.; Anderlid, B.M.; Arnell, H.; Johansson, C.B.; et al. Integration of whole genome sequencing into a healthcare setting: High diagnostic rates across multiple clinical entities in 3219 rare disease patients. Genome Med. 2021, 13, 40. [Google Scholar] [CrossRef]
- Balsamic Pipline. Available online: https://balsamic.readthedocs.io/en/v12.0.2/ (accessed on 22 December 2023).
- Mayrhofer, M.; De Laere, B.; Whitington, T.; Van Oyen, P.; Ghysel, C.; Ampe, J.; Ost, P.; Demey, W.; Hoekx, L.; Schrijvers, D.; et al. Cell-free DNA profiling of metastatic prostate cancer reveals microsatellite instability, structural rearrangements and clonal hematopoiesis. Genome Med. 2018, 10, 85. [Google Scholar] [CrossRef]
- Wallander, K.; Thonberg, H.; Nilsson, D.; Tham, E. Massive parallel sequencing in individuals with multiple primary tumours reveals the benefit of re-analysis. Hered. Cancer Clin. Pract. 2021, 19, 46. [Google Scholar] [CrossRef]
- Gargano, M.A.; Matentzoglu, N.; Coleman, B.; Addo-Lartey, E.B.; Anagnostopoulos, A.V.; Anderton, J.; Avillach, P.; Bagley, A.M.; Bakštein, E.; Balhoff, J.P.; et al. The Human Phenotype Ontology in 2024: Phenotypes around the world. Nucleic Acids Res. 2024, 52, D1333–D1346. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.J.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.J.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.S.; Hart, J.; Hoffman, D.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018, 46, D1062–D1067. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Andeer, R.; Dalberg, M.; Laaksonen, M.; Magnusson, M.; Nilsson, D.; Rasi, C. Scout, Clinical DNA variant visualizer and browser, repository code. Available online: https://github.com/Clinical-Genomics/scout (accessed on 17 September 2020).
- Sondka, Z.; Dhir, N.B.; Carvalho-Silva, D.; Jupe, S.; Madhumita; McLaren, K.; Starkey, M.; Ward, S.; Wilding, J.; Ahmed, M.; et al. COSMIC: A curated database of somatic variants and clinical data for cancer. Nucleic Acids Res. 2024, 52, D1210–D1217. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Nationellt Vårdprogram för Bröstcancer (Regional Cancer Centra, Sweden). Available online: https://kunskapsbanken.cancercentrum.se/diagnoser/brostcancer/vardprogram/ (accessed on 17 September 2020).
- Carvalho, N.A.; Santiago, K.M.; Maia, J.M.L.; Costa, F.D.; Formiga, M.N.; Soares, D.C.Q.; Paixão, D.; Mello, C.A.L.; Costa, C.; Rocha, J.; et al. Prevalence and clinical implications of germline pathogenic variants in cancer predisposing genes in young patients across sarcoma subtypes. J. Med. Genet. 2023, 61, 61–68. [Google Scholar] [CrossRef]
- Yap, T.A.; Ashok, A.; Stoll, J.; Mauer, E.; Nepomuceno, V.M.; Blackwell, K.L.; Garber, J.E.; Meric-Bernstam, F. Prevalence of Germline Findings Among Tumors From Cancer Types Lacking Hereditary Testing Guidelines. JAMA Netw. Open 2022, 5, e2213070. [Google Scholar] [CrossRef]
- Ballinger, M.L.; Pattnaik, S.; Mundra, P.A.; Zaheed, M.; Rath, E.; Priestley, P.; Baber, J.; Ray-Coquard, I.; Isambert, N.; Causeret, S.; et al. Heritable defects in telomere and mitotic function selectively predispose to sarcomas. Science 2023, 379, 253–260. [Google Scholar] [CrossRef]
- Thariat, J.; Chevalier, F.; Orbach, D.; Ollivier, L.; Marcy, P.Y.; Corradini, N.; Beddok, A.; Foray, N.; Bougeard, G. Avoidance or adaptation of radiotherapy in patients with cancer with Li-Fraumeni and heritable TP53-related cancer syndromes. Lancet Oncol. 2021, 22, e562–e574. [Google Scholar] [CrossRef]
- Therkildsen, C.; Jensen, L.H.; Rasmussen, M.; Bernstein, I. An Update on Immune Checkpoint Therapy for the Treatment of Lynch Syndrome. Clin. Exp. Gastroenterol. 2021, 14, 181–197. [Google Scholar] [CrossRef]
- Parsons, D.W.; Roy, A.; Yang, Y.; Wang, T.; Scollon, S.; Bergstrom, K.; Kerstein, R.A.; Gutierrez, S.; Petersen, A.K.; Bavle, A.; et al. Diagnostic Yield of Clinical Tumor and Germline Whole-Exome Sequencing for Children With Solid Tumors. JAMA Oncol. 2016, 2, 616–624. [Google Scholar] [CrossRef]
- Fiala, E.M.; Jayakumaran, G.; Mauguen, A.; Kennedy, J.A.; Bouvier, N.; Kemel, Y.; Fleischut, M.H.; Maio, A.; Salo-Mullen, E.E.; Sheehan, M.; et al. Prospective pan-cancer germline testing using MSK-IMPACT informs clinical translation in 751 patients with pediatric solid tumors. Nat. Cancer 2021, 2, 357–365. [Google Scholar] [CrossRef]
- Mutetwa, T.; Goudie, C.; Foulkes, W.D.; Polak, P. Companion Tumor Sequencing to Assess the Clinical Significance of Germline Sequencing in Children With Cancer. JAMA Netw. Open 2021, 4, e2135135. [Google Scholar] [CrossRef]
- Borja, N.A.; Silva-Smith, R.; Huang, M.; Parekh, D.J.; Sussman, D.; Tekin, M. Atypical ATMs: Broadening the phenotypic spectrum of ATM-associated hereditary cancer. Front. Oncol. 2023, 13, 1068110. [Google Scholar] [CrossRef]
- Hall, M.J.; Bernhisel, R.; Hughes, E.; Larson, K.; Rosenthal, E.T.; Singh, N.A.; Lancaster, J.M.; Kurian, A.W. Germline Pathogenic Variants in the Ataxia Telangiectasia Mutated (ATM) Gene are Associated with High and Moderate Risks for Multiple Cancers. Cancer Prev. Res. 2021, 14, 433–440. [Google Scholar] [CrossRef]
- Chaffee, K.G.; Oberg, A.L.; McWilliams, R.R.; Majithia, N.; Allen, B.A.; Kidd, J.; Singh, N.; Hartman, A.R.; Wenstrup, R.J.; Petersen, G.M. Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history. Genet. Med. 2018, 20, 119–127. [Google Scholar] [CrossRef]
- Nurmi, A.; Muranen, T.A.; Pelttari, L.M.; Kiiski, J.I.; Heikkinen, T.; Lehto, S.; Kallioniemi, A.; Schleutker, J.; Bützow, R.; Blomqvist, C.; et al. Recurrent moderate-risk mutations in Finnish breast and ovarian cancer patients. Int. J. Cancer 2019, 145, 2692–2700. [Google Scholar] [CrossRef]
- Van Goethem, G.; Dermaut, B.; Löfgren, A.; Martin, J.J.; Van Broeckhoven, C. Mutation of POLG is associated with progressive external ophthalmoplegia characterized by mtDNA deletions. Nat. Genet. 2001, 28, 211–212. [Google Scholar] [CrossRef]
- Lv, M.; Zhang, S.; Dong, Y.; Cao, L.; Guo, S. PolG Inhibits Gastric Cancer Glycolysis and Viability by Suppressing PKM2 Phosphorylation. Cancer Manag. Res. 2021, 13, 1559–1570. [Google Scholar] [CrossRef]
- Vyas, B.; Puri, R.D.; Namboodiri, N.; Nair, M.; Sharma, D.; Movva, S.; Saxena, R.; Bohora, S.; Aggarwal, N.; Vora, A.; et al. KCNQ1 mutations associated with Jervell and Lange-Nielsen syndrome and autosomal recessive Romano-Ward syndrome in India-expanding the spectrum of long QT syndrome type 1. Am. J. Med. Genet. A 2016, 170, 1510–1519. [Google Scholar] [CrossRef]
- Tommiska, J.; Känsäkoski, J.; Skibsbye, L.; Vaaralahti, K.; Liu, X.; Lodge, E.J.; Tang, C.; Yuan, L.; Fagerholm, R.; Kanters, J.K.; et al. Two missense mutations in KCNQ1 cause pituitary hormone deficiency and maternally inherited gingival fibromatosis. Nat. Commun. 2017, 8, 1289. [Google Scholar] [CrossRef]
- Gao, Q.; Yang, C.; Meng, L.; Wang, Z.; Chen, D.; Peng, Y.; Yang, K.; Bian, Z. Activated KCNQ1 channel promotes fibrogenic response in hereditary gingival fibromatosis via clustering and activation of Ras. J. Periodontal Res. 2021, 56, 471–481. [Google Scholar] [CrossRef]
- Than, B.L.; Goos, J.A.; Sarver, A.L.; O’Sullivan, M.G.; Rod, A.; Starr, T.K.; Fijneman, R.J.; Meijer, G.A.; Zhao, L.; Zhang, Y.; et al. The role of KCNQ1 in mouse and human gastrointestinal cancers. Oncogene 2014, 33, 3861–3868. [Google Scholar] [CrossRef]
- Rapetti-Mauss, R.; Berenguier, C.; Allegrini, B.; Soriani, O. Interplay Between Ion Channels and the Wnt/β-Catenin Signaling Pathway in Cancers. Front. Pharmacol. 2020, 11, 525020. [Google Scholar] [CrossRef]
- van der Tuin, K.; Tops, C.M.J.; Adank, M.A.; Cobben, J.M.; Hamdy, N.A.T.; Jongmans, M.C.; Menko, F.H.; van Nesselrooij, B.P.M.; Netea-Maier, R.T.; Oosterwijk, J.C.; et al. CDC73-Related Disorders: Clinical Manifestations and Case Detection in Primary Hyperparathyroidism. J. Clin. Endocrinol. Metab. 2017, 102, 4534–4540. [Google Scholar] [CrossRef]
- Poumeaud, F.; Valentin, T.; Vande Perre, P.; Jaffrelot, M.; Bonnet, D.; Chibon, F.; Chevreau, C.; Selves, J.; Guimbaud, R.; Fares, N. Special features of sarcomas developed in patients with Lynch syndrome: A systematic review. Crit. Rev. Oncol. Hematol. 2023, 188, 104055. [Google Scholar] [CrossRef]
- de Angelis de Carvalho, N.; Niitsuma, B.N.; Kozak, V.N.; Costa, F.D.; de Macedo, M.P.; Kupper, B.E.C.; Silva, M.L.G.; Formiga, M.N.; Volc, S.M.; Aguiar Junior, S.; et al. Clinical and Molecular Assessment of Patients with Lynch Syndrome and Sarcomas Underpinning the Association with MSH2 Germline Pathogenic Variants. Cancers 2020, 12, 1848. [Google Scholar] [CrossRef]
- Naslund-Koch, C.; Nordestgaard, B.G.; Bojesen, S.E. Increased Risk for Other Cancers in Addition to Breast Cancer for CHEK2(star)1100delC Heterozygotes Estimated From the Copenhagen General Population Study. J. Clin. Oncol. 2016, 34, 1208–1216. [Google Scholar] [CrossRef]
- Bychkovsky, B.L.; Agaoglu, N.B.; Horton, C.; Zhou, J.; Yussuf, A.; Hemyari, P.; Richardson, M.E.; Young, C.; LaDuca, H.; McGuinness, D.L.; et al. Differences in Cancer Phenotypes Among Frequent CHEK2 Variants and Implications for Clinical Care-Checking CHEK2. JAMA Oncol. 2022, 8, 1598–1606. [Google Scholar] [CrossRef] [PubMed]
- Abdelghani, E.; Schieffer, K.M.; Cottrell, C.E.; Audino, A.; Zajo, K.; Shah, N. CHEK2 Alterations in Pediatric Malignancy: A Single-Institution Experience. Cancers 2023, 15, 1649. [Google Scholar] [CrossRef] [PubMed]
- Ognjanovic, S.; Olivier, M.; Bergemann, T.L.; Hainaut, P. Sarcomas in TP53 germline mutation carriers: A review of the IARC TP53 database. Cancer 2012, 118, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Kleinerman, R.A.; Tucker, M.A.; Abramson, D.H.; Seddon, J.M.; Tarone, R.E.; Fraumeni, J.F., Jr. Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J. Natl. Cancer Inst. 2007, 99, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, P.; Mughal, S.S.; Sanders, M.A.; Hübschmann, D.; Chung, I.; Deeg, K.I.; Wong, S.H.; Rabe, S.; Hlevnjak, M.; Zapatka, M.; et al. Integrative genomic and transcriptomic analysis of leiomyosarcoma. Nat. Commun. 2018, 9, 144. [Google Scholar] [CrossRef]
- Mandelker, D.; Marra, A.; Mehta, N.; Selenica, P.; Yelskaya, Z.; Yang, C.; Somar, J.; Mehine, M.; Misyura, M.; Basturk, O.; et al. Expanded genetic testing of GIST patients identifies high proportion of non-syndromic patients with germline alterations. NPJ Precis. Oncol. 2023, 7, 1. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).