Simple Summary
A large body of multidisciplinary evidence involves the topic of thyroid cancer (the most common endocrine malignancy). Nevertheless, exceptional findings such as thyroid cancer in ectopic thyroid tissue, representing 0.3–0.5% of the malignant neoplasia with any location, suggest even greater challenges. Awareness remains the key operative element since the index of suspicion is low, especially in non-cervical areas. Hence, currently, the ectopic thyroid remains a matter of individualized management. The ectopic mediastinal thyroid (EMT) is part of the less frequent sublingual ectopic sites. Here, we introduce the most complex analysis in published EMT data (N = 117 patients) that identified an unexpectedly high rate of malignancy (18.8%), papillary cancer being the most frequent histological type. A rate of 5.98% amid all EMTs represented individuals confirmed with unrelated (non-thyroid) malignancies. Thyroid anomalies (other than EMT presence) were reported in 38.33% of the benign EMT, while the overall malignancy rate in EMTs was higher than expected according to prior data when compared to other ectopic sites.
Abstract
We aimed to analyze the management of the ectopic mediastinal thyroid (EMT) with respect to EMT-related cancer and non-malignant findings related to the pathological report, clinical presentation, imaging traits, endocrine profile, connective tissue to the cervical (eutopic) thyroid gland, biopsy or fine needle aspiration (FNA) results, surgical techniques and post-operatory outcome. This was a comprehensive review based on revising any type of freely PubMed-accessible English, full-length original papers including the keywords “ectopic thyroid” and “mediastinum” from inception until March 2024. We included 89 original articles that specified EMTs data. We classified them into four main groups: (I) studies/case series (n = 10; N = 36 EMT patients); (II) malignant EMTs (N = 22 subjects; except for one newborn with immature teratoma in the EMT, only adults were reported; mean age of 62.94 years; ranges: 34 to 90 years; female to male ratio of 0.9). Histological analysis in adults showed the following: papillary (N = 11/21); follicular variant of the papillary type (N = 2/21); Hürthle cell thyroid follicular malignancy (N = 1/21); poorly differentiated (N = 1/21); anaplastic (N = 2/21); medullary (N = 1/21); lymphoma (N = 2/21); and MALT (mucosa-associated lymphoid tissue) (N = 1/21); (III) benign EMTs with no thyroid anomalies (N = 37 subjects; mean age of 56.32 years; ranges: 30 to 80 years; female to male ratio of 1.8); (IV) benign EMTs with thyroid anomalies (N = 23; female to male ratio of 5.6; average age of 52.1 years). This panel involved clinical/subclinical hypothyroidism (iatrogenic, congenital, thyroiditis-induced, and transitory type upon EMT removal); thyrotoxicosis (including autonomous activity in EMTs that suppressed eutopic gland); autoimmune thyroiditis/Graves’s disease; nodules/multinodular goiter and cancer in eutopic thyroid or prior thyroidectomy (before EMT detection). We propose a 10-item algorithm that might help navigate through the EMT domain. To conclude, across this focused-sample analysis (to our knowledge, the largest of its kind) of EMTs, the EMT clinical index of suspicion remains low; a higher rate of cancer is reported than prior data (18.8%), incident imagery-based detection was found in 10–14% of the EMTs; surgery offered an overall good outcome. A wide range of imagery, biopsy/FNA and surgical procedures is part of an otherwise complex personalized management.
Keywords:
thyroid cancer; dyspnea; thyroidectomy; mediastinum; malignancy; ectopic; VATS; thoracic surgery; fine needle aspiration; biopsy 1. Introduction
Thyroid cancer represents the most common endocrine malignancy. For the majority, there are papillary and follicular (differentiated) types followed by medullary carcinoma (in subjects harboring RET pathogenic variants, either germline in 20% of cases or sporadic in 80% of cases) and very rarely anaplastic/poorly differentiated forms. Papillary cancer represents 70–75% of all thyroid malignancies in non-endemic areas, while the follicular type accounts for 10–15% in non-endemic regions (respectively 30–40% in endemic areas); medullary thyroid cancer involves less than 5% of the thyroid malignancies. Other uncommon thyroid neoplasias include primary thyroid lymphoma, teratoma or squamous cell carcinoma [1,2,3]. While a great body of multidisciplinary evidence involves the topic of thyroid cancer, exceptional findings such as thyroid cancer in ectopic thyroid tissue (representing 0.3–0.5% of all thyroid cancers) require a complex panel of investigations in order to differentiate it from benign tissue and to decide the best surgical approach for an overall better outcome. Awareness remains the key operative element since the clinical index of suspicion is rather low, especially in non-cervical areas. Hence, currently, ectopic thyroid tissue remains a matter of individualized management [4,5,6].
The Issue of Ectopic Thyroid Tissue
Generally, ectopic thyroid tissue represents an unusual finding that associates multiple challenges and pitfalls starting with its initial recognition. The prevalence is one to three cases per 100,000 (or one 1 to 300,000) people in the general population [7,8,9]. However, the true prevalence might be underestimated. Ectopic neck thyroid is part of the neck congenital masses (also including thyroglossal duct remnants, epidermoid/dermoid cysts, laryngocele, etc.) that are identified in 21–45% of the children presenting any type of neck mass, respectively, in 5% to 14% of the adults [10]. Among the population subgroup diagnosed with any type of thyroid disease, the prevalence of ectopic thyroid tissue stands for one case in 4000 to 8000 subjects [9]. Moreover, the prevalence of ectopic thyroid tissue regardless of the site in autopsy-based studies is 7% to 10% [11].
Ectopic thyroid tissue represents an exceptional developmental anomaly regarding the impairment of the gland movement from the primitive foregut to the pre-tracheal position. The gland originates from the endodermal diverticulum at the level of the first and second pharyngeal pouch; it descends to its normal cervical site (below the larynx and hyoid bone). The eutopic thyroid is situated anterior to the second, third and fourth tracheal rings. Hence, the ectopic thyroid tissue is situated anywhere between the foramen cecum (which is situated between anterior two thirds and posterior one third of an adult tongue) and mediastinal space (across thyroglossal tract). From the embryogenesis perspective, thyroid gland represents the first endocrine tissue that is developed during intra-fetal life. The thyroid primordium starts its development since weeks three to four of gestation, while its migration takes place between the weeks five and seven; the secretion of fetal thyroid hormones begins between weeks ten and twelve [12,13]. The transcription factors that play a role in thyroid development are TTF1 (thyroid transcription factor-1 or NKX2.1) and TTF2 (or Foxe1), PAX8 (paired-box gene), HHEX (hematopoietically expressed homeobox protein) and TSH (thyroid-stimulating hormone) receptor, and they may be involved in the ectopic presentation [7,11,14,15,16].
The most common ectopic thyroid site is represented by the tongue (the lingual thyroid is involved in 90% of all ectopic cases; of note, the first ectopic thyroid tissue at this level was described in 1869 by Dr. Hickman in a newborn with rapidly fatal outcome due to respiratory obstruction) followed by various locations (that are called sublingual type [17]) such as submandibular [18], peri-tracheal, larynx, sub-diaphragmatic area [12], etc. Across these 10% of ectopic cases, the most uncommon sites that are only partially understood based on the embryogenetic perspective are at the gastrointestinal level, including the gallbladder [19], adrenal glands, ovaries (struma ovarii), lumbar/renal [19,20,21,22], axillary [23], mammary [24], supra-sellar and suprachiasmatic [25]. The mediastinal location may be easily explained by the local attachment of the primordial thyroid at this site before starting its caudal migration [8,26]. Alternatively, ectopic thyroid tissue at the level of the anterior mediastinum, lung [27], heart, and pericardium may have been dragged into the chest together with the heart and its great vessels amid physiological embryogenesis [11,28,29]. Of note, carcinoma showing thymus-like differentiation (CASTLE) of the thyroid might also be found in the mediastinum in addition to EMTs and retrosternal goiter [30].
In ectopic thyroid tissues, a female to male ratio was found of three to four; the detection may be at any age; some authors appreciated that an early identification may be registered during the teenage years (even more common than in adults) [13]. A potential explanation for the more frequent detection in women is related to the sex differences with respect to the necessary thyroid hormones across puberty, menstruation, and pregnancy [7].
Almost half of the patients with ectopic thyroid are asymptomatic [11]. Some hypotheses suggested that ectopic mass might grow and become symptomatic in subjects experiencing a TSH increase of an unrelated cause (for instance, in trauma, infections or amid traditional primary causes of hypothyroidism, including congenital forms). Since TSH controls both the gland volume increase and the thyroid hormones secretion/production, the ectopic tissue might become visible under these circumstances. Yet, the current level of understanding of TSH control over the ectopic tissue and the balance between thyroid hormone production within the follicular cells from eutopic and ectopic tissue is still low [31].
Apart from specific endocrine considerations, the anatomical perspective includes two types of ectopic thyroid: aberrant (no eutopic thyroid) and accessory (associated with normal cervical thyroid gland), which is found in 75% of cases [7]. Rarely, multiple ectopic thyroid sites (dual or triple ectopic thyroids) are described within the same patient [7,32]. One third of children with ectopic thyroid tissue might experience hypothyroidism, especially the cases with aberrant patterns [31,33]. Ectopic tissue might have a lower rate of iodine uptake and thyroid hormones production than orthotopic glands; thus, cases with aberrant thyroid are associated with hypothyroidism more often (but ectopic tissue with hyper-function has been reported, too), while decreased radioiodine uptake might prove to be a source of bias in the adequate recognition of ectopic tissue amid imaging exploration [7,34]. All types of thyroid cancer have been reported in ectopic thyroid tissue as well as different areas of thyroiditis involvement [7,34,35,36]. Notably, some authors consider that the rate of malignant transformation in ectopic sites is similar with eutopic thyroid (particularly, for the papillary thyroid cancer [37]), but the level of statistical evidence in this particular matter remains low [8,38]. For instance, the 2023 study of Gao et al. [7] (which is described by the authors as being “the world’s largest single-center sample size of comprehensive ectopic thyroid gland diagnosis and treatment” that included patients treated between 2013 and 2022) investigated 47 patients (five subjects had a double ectopic tissue, thus a total number of 52 ectopic thyroids) with different ectopic presentations showed that 61.7% of them had an accessory thyroid tissue; 78.7% were females; average age at diagnosis was of 36 years (range between 4 months and 65 years); the average maximum diameter of the ectopic tissue was of 3.2 cm; the most common sites were lingual (N = 20/52 thyroid tissues), followed by submandibular (N = 10/52), latero-cervical (N = 10/52), mediastinal (N = 4/52), ovarian (N = 7/52), and esophageal (N = 1/52). A median follow-up of 59.4 months (range between 12 and 117 months) was described. The rate of malignancy was of 1/52 (this was a single case of papillary thyroid cancer at lingual tissue requiring its surgical removal followed by lifelong TSH suppressive therapy) [7].
We aimed to analyze management in cases with an ectopic mediastinal thyroid (EMT). Multiple insights are provided with respect to EMT-related thyroid cancer and non-malignant findings regarding the pathological (histological and cytological) report, the clinical presentation, the imaging traits on first identification and the secondary assessment (meaning the need for an additional imaging tool for diagnosis), the endocrine (hormonal) profile, the presence of connective tissue to the cervical (eutopic thyroid gland), the use of biopsy or fine needle aspiration (FNA), the choice of surgical techniques and post-operatory outcome. This was a comprehensive review based on revising any type of freely PubMed-accessible English, full-length papers with the keywords “ectopic thyroid” and “mediastinum” from inception until March 2024 (a total of 318 articles have been manually screened and relevant data were analyzed). We included original articles (case reports, series or studies) that specified data with respect to EMTs (Figure 1).
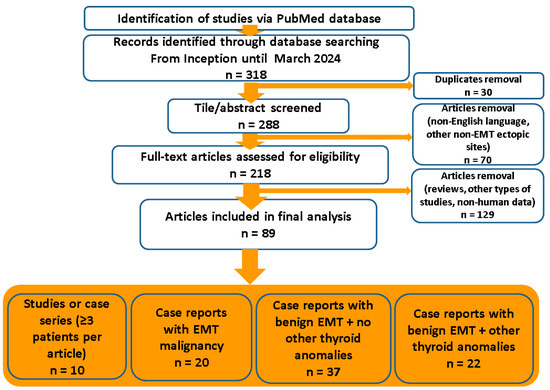
Figure 1.
Diagram flow of search according to our methods. Abbreviations: EMT = ectopic mediastinal thyroid; n = number of papers.
2. Ectopic Mediastinal Thyroid (EMT)
According to our methods, the earliest papers featuring EMTs were published in 1958 (aberrant thyroid as differential diagnosis for different mediastinal neoplasia) [39], in 1964 (the first specific mention of EMT analysis in terms of diagnosis and therapy) [40], and in 1983 (regarding the use of FNA in EMTs in the upper mediastinum) [41]. Generally, the EMT represents less than 1% of all mediastinal tumors (of any type) regardless of their origin and approximately 1% of all ectopic thyroid sites [42]. For instance, reports of thymomas, lymphomas, and germ cell tumors are more frequent in the mediastinum; alternatively, other findings are fibromas, lipomas, hemangiomas, cysts, and teratomas [29,42,43,44,45] as well as Castleman’s disease and metastases from different originating cancers [46]. Other uncommon conditions also associated with an distinct endocrine profile are represented by the intra-thoracic ectopic parathyroid glands [47] and paragangliomas [9,13].
2.1. Sample-Focused Analysis
We identified ten studies or case series (of at least three patients per series) that included the evaluation of EMTs among other outcomes (this is distinct for single case reports specifically describing patients with EMTs) [7,9,45,48,49,50,51,52,53,54] (Table 1).

Table 1.
Studies or case series that analyzed patients with EMT among other outcomes; the display starts with the most recent publication date [7,9,45,48,49,50,51,52,53,54].
To summarize, these are all retrospective studies (n = 10; N = 36 subjects with EMTs, and one them had a papillary thyroid carcinoma in the EMT [52]) with various endpoints [7,9,45,48,49,50,51,52,53,54]:

- Stanford database (N = 7 patients with benign EMTs) was analyzed across two distinct papers, but this was the same cohort [9,48];

- A single-center study on surgical outcome (between 1991 and 2006) amid approaching cervico-mediastinal goiters (N = 97 individuals) identified 11 of them as having a “forgotten” goiter in the mediastinum (an alternative name for the EMT) [51];

- A study of 3092 patients who underwent thyroidectomy (between 2000 and 2013) identified 28/3092 of them with ectopic thyroid tissue of any type; among this subgroup, 5 out of 28 had EMTs (female to male ratio of 3 to 2; mean age of 41 years) [52];

- A single-center study of ectopic thyroid tissue (N = 47) of any type (between 2013 and 2022) identified 4 out of these 47 individuals with benign EMTs (female to male ratio of 4 to 1, mean age of 55.5 years; of note, one subject had a synchronous thymus lipoma) [7];

- A case series on quality assurance protocol in endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) included three EMT cases (female to male ratio of 2 to 1; average age of 84.33 years) [49];

- A case series of benign EMT (N = 3 subjects; female to male ratio of 2 to 1, mean age of 52.33 years) focused on using endobronchial ultrasound-guided fine needle aspiration (EBUS-FNA) [50];

- An eight-patient series with huge mediastinal masses identified one benign EMT in a 44-year-old male [45];

- A retrospective study in 665 patients who underwent thyroidectomy (between 2005 and 2012) identified one subject with EMT [53];

- A study of 16 patients with benign ectopic aberrant thyroid identified one patient with EMT [54].
2.2. Clinical Presentation and Scenario of Detection in EMTs
The presentation may be asymptomatic; also, there is the typical scenario of detection according to an incidentaloma for unrelated medical and surgical issues. Alternatively, local compressive symptoms such as dysphagia, dysphonia, dyspnea, cough, chest pain, stridor, sensation of retrosternal mass, Horner’s syndrome, mediastinal syndrome [13], and superior cave vein syndrome [55] have been reported. For example, a retrospective database study of a high-volume academic surgical center, using “ectopic thyroid” as the search word, identified 202 cases of ectopic thyroid tissue (any site); EMTs were found in 7/202 subjects which firstly pointed out the rarity of the condition (hence, a rate of 3.46% amid other ectopic locations was confirmed) [48]. Initial presentation (N = 6/7 patients) included compressive symptoms or hyperthyroidism, respectively, but one out of the seven subjects with EMTs was identified with the mediastinal mass as an incidental finding amid computer tomography (CT) scan (thus, 14.28% of the EMTs may be detected as an incidentaloma) [48]. Moreover, the retrospective single-center study of Gao et al. [7] showed a rate of mediastinal involvement amid other ectopic thyroid sites of 4/47 adults (8.5% of all ectopic thyroids). The identification of an EMT was as follows: one case had cough and sputum and the other three patients were incidentally detected (asymptomatic presentation); thus, a much higher rate than Aziz’s study [48] was reported [7]. Remarkably, after EMT suspicion, a re-assessment amid anamnesis might highlight mild complains that at first seemed irrelevant such as intermittent dyspnea, chest pressure, night sweats or facial erythema [9,48]. Another interesting detection was made via assays for newly detected Graves’s disease in an adult male patient of 41 years. The case published by Agrawal et al. [34] in 2019 showed an accidental imaging detection of a suspected EMT (that was never biopsied or removed) at 99m-Tc (Technetium) sodium pertechnetate scintigraphy followed by the second imaging scan in terms of SPECT-CT (single-photon emission computed tomography); due to an increased level of thyroid hormones, only a non-contrast, low-dose CT scan was performed [34].
One of the key messages remains the fact that accidental EMT detection does not necessarily mean a completely asymptomatic patient in the matter of EMTs. In addition, we mention that thyroid incidentalomas (at the eutopic gland) represents the most common type of endocrine incidentaloma (despite the fact that the term “thyroid nodule” is more frequently used in daily practice) [56,57].
In additional to these clinical elements that are directly connected to the presence of the mediastinal lesion, highly aggressive malignancy in EMTs might be detected due to local or distant malignant spreading as the first step of further undergoing EMT identification. Here, we introduce the cases with thyroid cancer in EMTs (regardless of the scenario of detection) according to our methods [36,37,41,42,55,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72] (Table 2).

Table 2.
Case reports of EMT underlying different types of malignancy according to our methods; the display starts with the most recent publication date [36,37,41,42,55,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72].
One short note: 19 papers introduced a single case report per article [36,37,41,42,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72] and another publication presented two patients [55], leading to a total of 21 patients with any type of malignancy in EMTs. Specifically, primary thyroid cancer was identified in 20 patients (plus, a case of a one newborn displayed an immature teratoma hosted by the EMT [68]). Demographic features (N = 19, mean age of 62.94 years) showed a female to male ratio of 9 to 10 (for one subject, these data were not available [41]); the female group (N = 9) had a mean age of 63.4 years (range: 36 to 95 years); the male group (N = 10) had an average age of 66 years (range: 34 to 90) [36,37,42,55,58,59,60,61,62,63,64,65,66,67,69,70,71,72].
To summarize, the histological types of the primary thyroid malignancies in 22 subjects (Table 1 and Table 2) confirmed with EMT-related cancer were as follows:

- Papillary (the most common type; the patients had any form from microcarcinoma to severe metastatic disease); of note, one more case was introduced in prior studies-based analysis; hence, there were a total of eleven subjects [52];

- Follicular variant of the papillary type (N = 2);

- Hürthle-cell thyroid follicular malignancy (N = 1);

- Poorly differentiated (N = 1);

- Anaplastic (N = 2);

- Medullary (N = 1);

- Lymphoma (N = 2);

- MALT (mucosa-associated lymphoid tissue) (N = 1) [36,37,41,42,55,58,59,60,61,62,63,64,65,66,67,69,70,71,72];

- An additional case of immature teratoma does not belong to the specific category of primary thyroid cancer, but it was identified in ectopic tissue (EMT) [68].
2.3. Exploring the Thyroid Panel in Patients Confirmed with EMT
The endocrine profile in EMTs is essential before and after EMT removal (if any); the thyroid anomalies might share the same pathogenic traits as the EMT or they are incidental, but this is still a matter of debate [73]. Nevertheless, one subject with an EMT might have a normal endocrine status or not, and this is an important part of the overall management of EMTs. However, across our research, specific data on thyroid profile were not always available. For instance, in the study of Aziz et al. [48] (N = 7), only 4/7 had endocrine assessments, and all of them (4/4) had normal TSH [48]. The mentioned study of Gao et al. [7] (N = 4) showed that all patients with EMTs had a normal thyroid function [7].
The sample-focused analysis according to our methods identified another 37 EMT adults (22 females and 12 males; specific demographic data were not available for three cases; mean age was of 56.32 years, females subgroup: mean age of 56.04, range: 30–80 years; males subgroup: average age of 56.83, range: 31–74 years; across 35 articles featuring a single case study and two articles introducing each two subjects [74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93]), whereas benign EMTs were associated with a normal thyroid panel in terms of function, negative autoimmunity, and lack of nodules/cancer/goiter in eutopic (cervical) gland before and after EMT removal or identification if the EMT was not resected (of note, we also, included the cases whereas no specific thyroid data were provided, thus, it was presumably normal) [13,29,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107] (Table 3).

Table 3.
Case reports of benign EMT and normal thyroid profile in terms of function, autoimmunity and nodules/cancer in eutopic (cervical) gland (if available); the display starts with the most recent publication date [13,29,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107]).
Moreover, across our research, we identified a heterogeneous spectrum of thyroid anomalies in subjects confirmed with EMTs coming as a second (non-EMT) thyroid disease (affecting the cervical eutopic gland) or connected to the EMT profile (for instance, reciprocally influencing the overall thyroid function or the iodine uptake between the cervical and mediastinal thyroid tissue). Generally, one patient with an ectopic thyroid might have been admitted for prior thyroid conditions in terms of regular check-up or an unexpected change in previous thyroid status. The insights of the concurrent (orthotopic) thyroid diseases have been reported in relationship with any location of the ectopic follicular/colloidal tissue [108,109,110,111].
This large frame of interplay includes thyroid dysfunction (hyperthyroidism and hypothyroidism, either clinically manifested or mild/subclinical); a history of thyroid surgery at the orthotopic gland (for benign or malignant conditions), and the co-presence of the thyroid nodules or multinodular goiter in the cervical thyroid or different forms of thyroiditis (Hashimoto’s thyroiditis or Graves’ disease) that were confirmed by serum antibodies against thyroid or by histological report [112,113,114]. The crossroads between thyroid dysmorphogenesis and thyroid dysfunction makes no exception for EMTs. The case-focused analysis pinpointing the patients confirmed with benign EMTs that also have been affected by any type of prior, concurrent, and even early post-operatory (after EMT removal) thyroid disease are displayed in Table 4 [11,22,34,35,73,87,102,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130].

Table 4.
Case reports of benign EMT and prior or concurrent thyroid diseases; the display starts with the most recent publication date [11,22,34,35,73,87,102,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130].
The cohort of patients with benign EMTs and thyroid diseases included a total of 23 patients across 24 papers [11,22,34,35,73,87,102,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130] that have been reported as follows: 20 single case reports per article; two cases were reported in addition to another case that has already been introduced in Table 3 (two case reports per paper [87,102]); two papers addressed the same EMT patient from different perspectives [127,128]. The female to male ratio was 17 to 3 (for three patients, the demographic data were not available). The average age at EMT diagnosis was of 52.1 (range: 19 to 67) years; female group: mean age of 52.76 (range: 19 to 72) years; male group: mean age of 48.33 (range: 41 to 62) years [11,22,34,35,73,87,102,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130]. Overall, 60 patients had benign EMTs (Table 3 and Table 4, meaning the analysis based on case reports), and 38.33% of them were also affected by a second condition of the thyroid status, women being more prone.
To summarize, a total of 117 patients with any type of EMT were described across the papers we identified according to our methods:

- 36 subjects (and one of them with a malignant EMT [52]) were confirmed in studies with various endpoints (other than specifically evaluating the EMT population) or case series of at least three EMT patients per series [7,9,45,48,49,50,51,52,53,54];

- 21 subjects diagnosed with any type of malignancy in the EMT [36,37,41,42,55,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72] plus the mentioned case above [52] (N = 22 persons with malignant EMTs);

- 37 subjects with benign EMTs and otherwise normal thyroid profile [13,29,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107];

- 23 subjects with benign EMTs and a secondary thyroid condition of any type [11,22,34,35,73,87,102,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130].
2.3.1. Thyroid Dysfunction in Patients Confirmed with EMTs
All kinds of thyroid function anomalies have been reported in subjects confirmed with EMTs; sometimes, the dysfunction and the ectopic tissue share common pathogenic traits [52]. Previous data in aberrant thyroid showed a higher rate of congenital hypothyroidism and an increased prevalence in the pediatric population, particularly for ectopic cervical/lingual thyroid [131,132,133,134]. Yet, amidst our sample-based database, only one case was confirmed with congenital hypothyroidism out of the 117 subjects, suggesting that perhaps other non-EMT sites are involved in this connection [52]. Generally, various genetic and molecular studies addressed the issue of congenital hypothyroidism (for example, anomalies of TSHR, SLC26A7, JAG1, DUOX2, and FOXE1 gene) linking the thyroid dyshormonogenesis to thyroid dysgenesis [135,136,137,138,139].
Our sample-based analysis (Table 1, Table 2, Table 3 and Table 4) showed hypothyroidism as the following:

- Congenital type [52];

- Iatrogenic hypothyroidism (treated or untreated with levothyroxine replacement) following previous total or partial thyroidectomy for multinodular goiter [50,102,122], benign single nodule [60], toxic Plummer’s nodule [124], thyroid cancer in orthotopic gland [35,42,123], thyroiditis plus benign nodules [118,120];

- Primary hypothyroidism other than congenital or iatrogenic type [37,73].
Of note:

- Mild (subclinical) hypothyroidism was identified in two reports [73,126];

- Transitory hypothyroidism following EMT removal was found in a single case of 53-year-old female [73], suggesting the usefulness of thyroid assays following EMT resection.
Hyperthyroidism in patients who were confirmed with EMTs was identified on admission based on blood assays; however, a normal thyroid function might not exclude an increase iodine uptake in EMTs or in eutopic thyroids (with suppression of the other tissue); thus, the functional imagery might prove an important point in the matter of thyroid tissue exploration [140,141,142]. For instance, Kumaresan et al. [99] showed in a young lady an autonomous activity of the EMT according to 99m-technetium pertechnetate scintigraphy that suppressed the uptake of the eutopic tissue (yet, associating an overall normal thyroid function according to the blood assays on first admission); a recovery of normal thyroid gland function was registered when the scintigraphy was repeated six weeks following EMT removal [99]. The case reported in 2021 by Kola et al. [73] introduced a 42-year-old smoker male who was admitted for a 3-month history of dyspnea, chest pain, and fatigue. He was found with mildly low TSH (of 0.33, normal: 0.35–4.94 mU/L). CT showed a tumor at anterior mediastinum of 9 by 6 cm (with heterogeneous structure at CT scan). He also incidentally had a thyroid nodule on the left lobe of 0.9 cm and a right adrenal tumor of 5 by 3 cm, which was confirmed by MRI (magnetic resonance imagery). The 99m-Tc pertechnetate scintigraphy showed an orthotopic thyroid with a mildly reduced uptake rate (a potential prior asymptomatic thyroiditis that also induced TSH lowering was retrospectively suggested), but the chest scan was not included, since an EMT was not suspected at that point. Finally, the mediastinal mass was surgically removed. A post-operatory benign EMT was confirmed while TSH, T3 and T4 were found normal one month after surgery [73]. Serim et al. [121] introduced in 2016 a lady with a 2-year history of hyperthyroidism having a hyper-functional EMT but a normal thyroid (eutopic) gland according to 99m-Tc scintigraphy that actually highlighted the delicate aspect of navigating thyrotoxicosis blood serum confirmation via scintigraphy profile [121].
Hyperthyroidism/thyrotoxicosis status involved the following (according to our sample-focused analysis):

- Clinically manifested [34,52,99,116,121,122] or subclinical [73,125] forms;

- One case switched from levothyroxine replacement upon prior thyroidectomy to thyrotoxicosis that was persistent after hormone administration stopped and lead to the identification of TRAb (anti-TSH receptor antibodies) positive Graves’s disease in the EMT [122];

- One case was under tapazol for hyperthyroidism at the moment of EMT identification [129].
2.3.2. Elements of Thyroiditis in Orthotopic and Ectopic Tissue
Thyroiditis stands for the presence of autoimmune chronic Hashimoto’s thyroiditis caused by antibodies against thyroid, namely anti-thyroperoxidase antibodies (antiTPO) or anti-thyroglobulin antibodies (antiTg) usually causing hypothyroidism or, at the other end of the same autoimmune spectrum, Graves–Basedow’s disease (with or without thyroid eye disease) due to serum thyroid-activating antibodies TRAb [143,144,145]. The diagnosis itself may be established not only via high serum levels of the antibodies (which does not point out any difference between orthotopic or ectopic thyroid involvement), but, also, based on cytological and/or histological exploration, respectively, according to highly suggestive ultrasound features in the eutopic (cervical) gland. All these methods of thyroiditis confirmation have been reported in patients confirmed with different types of thyroid dysgenesis, including ectopic tissue [146,147,148,149].
To summarize, we found the following in the thyroiditis profiles of patients confirmed with EMTs:

- Positive serum antibodies [63];

- Positive serum antibodies and pathological confirmation of thyroiditis in eutopic gland [36,118,120];

- Positive serum antiTPO and antiTg in addition to thyroiditis confirmation in both cervical thyroid and an EMT [59];

- Histological report of EMT (but not in cervical eutopic thyroid) confirming focal lymphocytic thyroiditis in a 56-year-old male [105];

- In the matter of TRAb positive status, the full-blown picture of Graves’s disease on first admission was registered in one male of 41 years that was finally identified to also have EMT [34]; another case, a 67-year old female who underwent total thyroidectomy 7 years before for non-toxic goiter, had a TRAb positive EMT [122] or previous history of Graves’s disease in one case (9 year before EMT diagnosis) [130];

- Inflammatory thyroiditis pattern in benign EMTs (post-operatory confirmation) [102];

- Retrospective diagnosis of thyroiditis in eutopic thyroid due to otherwise unexplained reduced uptake amid 99m-Tc pertechnetate scintigraphy [73].
2.3.3. Thyroid Nodules/Multinodular Goiter and Cancer in Eutopic Thyroid or Prior Thyroidectomy before EMT Detection
Generally, thyroid nodules in cervical gland represent the most common endocrine finding affecting between 5% and 20% of the global population depending on the study, geographic region (iodine deficient areas) and methods of detection [150,151,152].
Nevertheless, EMT subjects might display certain elements:

- As mentioned, some EMTs were detected amidst investigations following a history of thyroidectomy for nodules or the co-presence of a multinodular goiter, autoimmune thyroiditis or Graves’s disease [50,102,122].

- The suspicion and consecutive confirmation of EMTs required the exploration of neck thyroid as a mandatory evaluation and, in cases with thyroid nodules in cervical gland, thyroid FNA was performed [11,22,58,59,69,80,87,98,117].

- Moreover, upon differentiated thyroid cancer confirmation in EMTs, total thyroidectomy was mandatory in order to start radioiodine ablative therapy (if thyroidectomy was not synchronous with the sternotomy or previously completed for unrelated issues) [55,58,59,60,61,62,64,69,71].

- Also, some cases of EMT removal, particularly, the masses located within the upper mediastinum, required thyroid resection for a better access in order to avoid a sternotomy [88,94,96].

- Concurrent multinodular goiter was described in 4/5 patients in Santangelo’s study [52] and across other single case reports [117,125,127] or single simultaneous thyroid nodules [22,54,73,87,115] or thyroid cancer in eutopic thyroid [11,22,42].
2.3.4. Serum Tumor Markers in Individuals Confirmed with EMTs
Serum tumor marker thyroglobulin is useful as a prognostic marker for differentiated thyroid cancer after its removal [153,154,155]. Its utility in benign conditions is limited unless the presence of ectopic tissue and some authors considered its very high value of being a predictor for an ectopic thyroid [55]. Of note, exceptionally elevated levels are found in malignant EMTs with metastatic disease [58].
Looking at the thyroglobulin assays in EMT patients, we conclude the following:

- High values might suggest a synchronous ectopic tissue and intact eutopic thyroid gland, but the index of suspicion remains low;

- High values after thyroidectomy of eutopic gland might suggest an ectopic tissue with an increased index of EMT suspicion;

- High values after thyroidectomy with post-operatory differentiated thyroid cancer confirmation should differentiate ectopic tissue (including EMTs) from local remnants, recurrent or metastatic thyroid malignancy;

- High values after surgery for differentiated thyroid cancer in eutopic and ectopic thyroids suggest a disease relapse or spreading, thus indicating the need for a whole body iodine scintigraphy (if confirmed, further additional surgery or a new dose of radioiodine ablative therapy should be included in the overall patient management) [36,37,41,42,55,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,122].
2.3.5. Other Blood Assays in EMTs: Endocrine and Non-Endocrine Elements
Further blood assessments amidst EMT confirmation may include the following:

- Investigations serving for differential diagnosis of the mediastinal mass such as an ectopic parathyroid tumor causing a primary hyperparathyroidism (blood calcemic and PTH assays) [54,81,115] or a paraganglioma (requiring measurements of blood and 24 h urinary metanephrines and normetanephrines at least twice) [9,76,81,102,105]. Of note, an ectopic (mediastinal) parathyroid tumor is more frequent than an EMT [156,157].

- Calcitonin and carcinoembryonic antigen are mandatory for the diagnosis and surveillance of the medullary thyroid carcinoma [65].

- Baseline ACTH (adrenocorticotropic hormone) and cortisol assays (baseline and dexamethasone suppression test) are necessary for the confirmation of an ectopic ACTH syndrome [65]. Notably, there is only an EMT case (N = 1/117) affected by this complex condition, but this paraneoplastic syndrome has been reported in relationship with the eutopic gland-related medullary malignancy or in non-endocrine cancers such as primary pulmonary carcinomas [158,159,160,161].

- The biochemistry panel might show elevated serum total calcium in primary hyperparathyroidism or hypopotassemia in Cushing’s paraneoplastic syndromes [65].

- Hemogram revealed an increased number of eosinophils and white blood cells (leukemoid reaction) in a fulminant cancer (e.g., anaplastic) in the absence of concurrent infections [66].

- Synchronous (suspected or confirmed) infections as part of the scenario concerning EMT detection such as lung actinomycosis [78] or, recently, COVID-19 [76] have been reported.

- One single case associated an adrenal incidentaloma [73]; thus, the entire panel of adrenal hormones that serve as screening tests should be considered if incidental adrenal masses are detected at CT, MRI or PET-CT scans [162,163].
2.4. Imaging Features in EMTs
2.4.1. CT Scan
CT scan represented the most important and the mostly used imagery evaluation, and it was a mandatory step of approaching (suspected or confirmed) EMTs [7,11,13,29,35,37,42,48,55,58,59,60,61,62,63,70,71,73,74,75,76,79,80,82,89,92,93,95,96,100,102,103,105,106,117,118,119,120,121,122,124,125,129]. The largest diameter varied from 1 to 15 cm; no correlation between the size and a malignancy trait in EMTs could be established. The size groups (according to the largest diameter via our practical perspective) may be regarded as follows: <2 cm [22,59,81,89,118]; between ≥2 cm and <3 cm [11,35,55,83]; between >8 cm and <10 cm [13,73,102,117]; more than 12 cm [37,45,60,115]. The most common groups were as follows: between ≥3 cm and <5 cm [9,58,62,80,85,86,93,95,103,106,119]; between ≥5 cm and <6 cm [29,74,78,79,98,100,121,122]; between ≥6 cm and <8 cm [50,55,64,70,75,82,84,88,92,100,120,123,124].
Calcifications [13,76,115,117,121,123], heterogeneous features [80,85,86,87,95,115,121], and even necrosis [13] in EMTs were described at CT scan. They do not seem to be associated with a malignant pattern in EMTs. This is contrast to the clinical significance of identifying micro-calcifications in papillary thyroid carcinomas at the cervical gland (underlying psammoma bodies) [164,165,166,167]. In EMTs, these traits highlight a long-standing condition or the potential of a further enlargement caused by a local necrosis/hemorrhage rather than a thyroid malignancy in EMTs, but further studies are necessary.
The upper mediastinal site was the most frequent; usually, EMTs were in the anterior mediastinum, and only a few were posterior [7,22,48,77,103,124,126]. For instance, in the study published in 2023 by Aziz et al. [48], all patients (N = 7) had an initial CT scan (a description of the location is useful by using the model of the four compartments: upper (superior) compartment (N = 3/7); middle compartment (N = 1/7); anterior compartment (N = 1/7); posterior compartment (N = 2/7), thus suggesting that the upper area is the most frequent. Also, apart from CT scan, further imaging work-up seemed useful (N = 3/7): one subject had a radioiodine-based scan; another one underwent a PET scan and another was explored via cervical MRI [48]. Gao’s [7] study showed these 4/4 EMTs were within the upper mediastinum (including one posterior to trachea and one within the thymus area). The maximum diameter was larger than 2 cm in all four cases; all were accessory type, not aberrant. Radioiodine scintigraphy was not used in any case, while CT was performed in all cases [7]. The use of SPECT-CT with 99m-Tc sestamibi may confirm ectopic parathyroid tumors at the mediastinum level [168].
Intra-cardiac/intra-pericardial EMTs represent a challenging situation; however, an adequate intervention might provide a good outcome [29,79,82,83,86,89,90,119]. Kocaman et al. [82] reported in 2020 an intra-pericardial EMT, while in 2018, an adult case of EMT near the left atrium in a renal cancer survivor was introduced [86]. Another presentation of a female adult showed a successful removal of a heart-located EMT (right ventricle) [89]. Similarly, a 63-year-old woman was accidentally detected with a nodular intra-pericardial lesion while having a normal thyroid function. CT showed a heterogeneous structure; coronary CT angiography revealed no connection to the coronary artery; (18F-fluorodeoxyglucose) 18F-FDG-PET was added to further exploration that concluded with a trans-sternal approach. Post-operatory EMT confirmation (2.5 by 2 by 1.5 cm) was followed for one year and showed a good outcome [83]. This paper of Sato et al. [83] was published in 2019, and the authors showcased that since its first description in 1986, six intra-pericardial cases (included this one) were published with a median age of 49 years. In this intra-pericardial instance, the main focus points were the blood supply and the relationship with the coronary artery as being essential amid the entire case management and the fact that, unless surgically removed, thyroid tissue confirmation is most likely not feasible in this particular location despite advanced imagery scans [83].
As mentioned by Gao et al. [7], a double ectopic thyroid has been rarely reported in EMT cases (less often than lingual sites) [7,22,75,93]. Nagireddy et al. [75] introduced a 57-year-old lady with double EMTs. The presentation included a one-month history of cough and chest discomfort. CT was provided and showed two masses of 7 by 7 cm and 4.9 by 5 cm (they were on the both sides of the upper mediastinum, on the right and the left, respectively). A CT-guided biopsy-based pathological report revealed a colloidal goiter (at right tumor mass). Due to the vascular proximity, sternotomy was completed with both tumors excision [75]. Wang et al. [93] presented a 45-year-old female with a double ectopic thyroid; one was an EMT and the other one was a lateral cervical tissue (according to the authors of this paper published in 2014, this was the first case with a double ectopic thyroid in this combination: neck and mediastinum). An EMT was removed via VATS (video-assisted thoracic surgery) in addition to using a neck incision. Both tissues were completely separated from the eutopic gland and resected [93].
2.4.2. The Use of 123 or 131Iodine or 99m-Tc Thyroid Scintigraphy
The second most important imagery tool was represented by the functional imaging of the thyroid tissue. If an EMT was suspected, this tissue was targeted using whole-body (not just cervical) scintigraphy; tracers such as 99m-Tc, 131 or 123iodine play a pivotal role despite variations of radioiodine uptake in ectopic tissues [6,42]. Sometimes, the metabolic rate in ectopic thyroid tissue is different from the eutopic gland, and thus the diagnosis might be missed [13,169,170]. Also, autonomous activity (hyper-function) in one thyroid tissue might suppress the uptake on the other tissue and, essentially, an EMT might not uptake 99m-Tc or the iodine tracer at all, thus being a source of potential bias. Iodine scintigraphy was used in the benign EMT/eutopic thyroid gland, and it is mandatory across the overall management of differentiated thyroid cancer [36,52,53,65,71,76,101,123]. Alternatively, a normal uptake in one thyroid lobe in addition to the lack of the other lobe (that was prior removed) and a reduced iodine uptake in the EMT was described in one case [124]. 99m-Tc scintigraphy was equally used for EMT diagnosis [34,69,71,73,87,91,93,102,106,115]. Various aspects should be kept in mind: for example, suppression of the eutopic gland due to EMT hyperactivity, and six weeks after EMT removal, a recovery of the normal uptake in cervical gland was registered [99] or an increased Tc uptake in EMTs and normal thyroid capture was described, while the overall hormonal assays were consistent with thyrotoxicosis diagnosis [121].
2.4.3. Other Imagery Assessments
Second-line imagery tools following CT scan (and distinct from iodine/99m-Tc scintigraphy) included a large scale of investigations across our search. MRI was used for evaluating the cervical gland or distant metastases [22,53,58,59,99,100,102]. Alternatively, for bone metastases, whole body bone scintigraphy was applied [60,92]. PET-CT was performed for a prior diagnosed malignancy, for (suspected or confirmed) EMT cancer or related metastasis [37,55,58,59,61,84] showing hypermetabolic lesions. Benign EMTs might not uptake the tracer (and this stands for a potential pitfall in daily practice) [85,89,91,101], or it might actually reveal the tissue [11,48,83,87]. Rajaraman et al. [116] addressed the role of SPECT in highlighting eutopic and heterotopic thyroid glands, including patients with active thyrotoxicosis (hyperthyroidism otherwise is a contra-indication of iodine administration amid using intravenous contrasts for CT scans) [116]. SPECT-CT might be used to differentiate EMTs from ectopic parathyroid tumors [34,81,86,116].
As mentioned, the misinterpretation of various imaging procedures (that were actually performed for unrelated conditions) might lead to EMT detection. Chen Cardenas et al. [81] reported two patients who were misdiagnosed as paragangliomas during iodine-123/iodine-131 (123I/131I)-metaiodobenzylguanidine (MIBG) scan without an adequate iodine blockade at the eutopic gland. After mediastinoscopy-based resection, an EMT was confirmed by a post-operatory histological report [81]. An MIBG scan should be administered if suspicious of mediastinal paragangliomas [59,86]. Of course, ectopic thyroids at the neck level require the standard (and routinely performed) ultrasound evaluation that is not feasible for the mediastinal site unless at a very high (upper) EMT position [8].
To conclude, three main features of the ectopic thyroid, particularly the EMT, should be kept in mind: distinct blood vessels supply are found in ectopic versus eutopic tissue; another aspect is the fact that despite the presence of an EMT, there is a distinct orthotopic thyroid gland in terms of anatomy, hormonal profile, and histological report with an additional interplay (with concern to functional imagery), while a different pathologic profile/metabolic rate/tracer uptake in ectopic versus eutopic thyroid was reported in some cases.
2.5. Analyzing the Co-presence of the Eutopic (Cervical) Thyroid Gland
Ectopic tissue is identified in the presence of normal thyroid or in the absence of a eutopic (orthotopic) gland, which might represent a milestone with regard to the surgical procedure (the need to remove the cervical thyroid in order to access the EMT), or the identification of a differentiated thyroid cancer in EMTs requires further radioiodine therapy; thus, the cervical thyroid should be removed before applying it. Moreover, the virtual risk of iatrogenic hypothyroidism in subjects who lack the cervical thyroid or those to whom thyroidectomy was already administered has been already mentioned. Yet, according to our EMT-based analysis, the presence of a synchronous normal eutopic thyroid means that the EMT removal will most likely not cause hypothyroidism. On a larger scale, ectopic intra-thoracic thyroid tissue includes EMTs, but mostly substernal (or retrosternal) goiter (representing the actual extension of the cervical thyroid enlargement to the mediastinum, also involving the extension of an aggressive thyroid cancer beyond the thyroid bed [171,172] in addition to the metastases from a malignancy originating from the eutopic thyroid (including pre-sternal metastasis have been reported, as well [173]).
Most retrosternal goiters are developed within the upper and middle mediastinum and only rarely at the level of posterior compartment. Retrosternal goiter may be asymptomatic or might cause compressive symptoms according to local anatomical elements (such as dyspnea, dysphagia, cough, stridor, hemoptysis, etc.). Retrosternal goiter should be removed because of local effects (including acute respiratory insufficiency, upper cave vein syndrome, aspiration pneumonia, etc.), risk of expansion due to further enlargement (grow) and hemorrhage, respectively, risk of gaining toxic activity (hyper-function causing hyperthyroidism), and risk of malignant transformation (which is rather uncommon). The removal, depending on the location, size and surgeon’s skills, is either performed trans-cervical or via sternotomy or through thoracotomy [74,174]). In contrast to the retrosternal goiter (that has no separation line from the orthotopic thyroid and shares the same vessels), an EMT has its own blood supply (intra-thoracic vessels) [42]). The distinction between retrosternal goiter as an extension to an enlarged cervical gland and an EMT as a standalone tissue with encapsulated appearance can elegantly be made intra-operatory and/or after a post-surgery histological report in exceptional instances as reported in two cases by Sohail et al. [117] in 2019, but otherwise, the pre-operatory imagery exploration amid CT use and iodine-based scintigraphy should clarify the issue of retrosternal goiter versus an EMT [117].
2.6. Connective Tissue between EMT and Cervical (Eutopic) Thyroid
Most EMTs do not showcase a connective tissue to the cervical (physiological) thyroid (and this aspect is confirmed by our sample-based analysis), an EMT being distinct from a substernal goiter [13,48]. A thin strip of tissue between one thyroid lobe and an EMT was shown in a single case at contrast-enhanced CT [88]. Some authors agreed than only 2% (between 1% and 5%) of the intra-thoracic thyroid tissue is represented by an EMT, and the rest mostly involves a substernal goiter, which otherwise is very rare when compared to the traditional cervical goiter that comes as an easy-to-perform diagnosis due to routine neck ultrasound [48,83]. Hence, data on connective tissue are mandatory for this differential diagnosis. They may be provided based on imaging scans, intra-operatory identification or after surgery according to the histological report. Some authors suggested that potential common pathogenic factors are involved in these entities, substernal goiters and EMTs [175].
2.7. Pathological Report in EMTs
Firstly, there is the recognition of the ectopic thyroid remnants since the clinical index of suspicion is decreased followed by the biopsy/FNA and/or surgical removal depending on the EMT site, size, vascularization, and co-morbidities; overall, a decision of a multidisciplinary team is mandatory. Upon pathological confirmation of a thyroid cancer in most ectopic tissues, total thyroidectomy of the eutopic gland is necessary followed by radioiodine ablation and long-term TSH suppression therapy. Whether neck lymph nodes dissection is part of the second surgical step is a matter of personalized approach; for instance, it depends whether the ectopic gland was found in the neck area, if imaging evaluation suspected a lymph node metastasis, etc. [8].
While some studies in EMT subjects found no EMT malignancy [7,48], cancer (of any histological type) was found in 22 subjects out of the 117 (representing 18.8% of the entire cohort), according to our methods [36,37,41,42,52,55,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72], which is an unexpectedly high rate (Table 1 and Table 2, Figure 2).
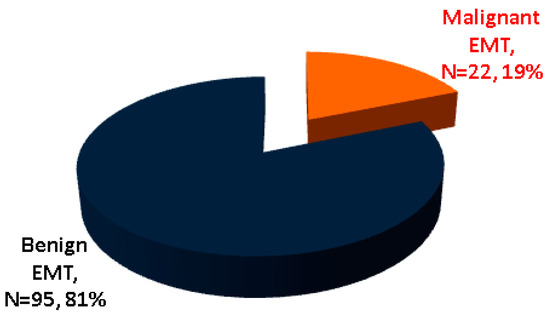
Figure 2.
The malignant profile in EMT according to our analysis (N = number of patients) [36,37,41,42,55,58,59,60,61,62,63,64,65,66,67,69,70,71,72].
2.7.1. Differentiated Thyroid Carcinoma
The earliest reports of malignant EMT were provided in 1983 (follicular type) [41] and 2003 (papillary type, specifically, columnar cell carcinoma) [71] according to our PubMed search. Differentiated type was the most frequent (N = 14/22 subjects with malignant EMT), papillary being far more frequent than follicular (N = 13/14 patients with differentiated malignancies in EMT) [36,37,41,42,55,58,59,60,61,62,63,64,65,66,67,69,70,71,72] (Figure 3).
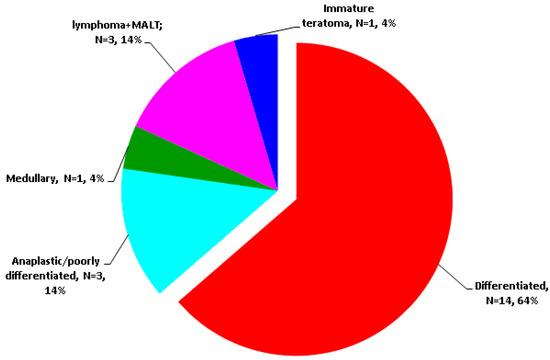
Figure 3.
Cancer-focused analysis amid our EMT research [36,37,41,42,55,58,59,60,61,62,63,64,65,66,67,69,70,71,72] (N = number of patients).
2.7.2. Anaplastic/Poorly Differentiated Thyroid Carcinoma
Yet, according to our research, a fulminant evolution was reported by Camagos et al. [66]. They published in 2010 a first case of a 95-year-old woman who died of severe respiratory insufficiency due to an EMT and associated anaplastic carcinoma but normal eutopic gland (as shown by the necropsy) [66]. In addition, Nguyen et al. [37] reported in 2023 the case of a 90-year-old male who suffered from prior non-surgical hypothyroidism and survived a prostate cancer two decades prior. He was admitted for a rash at the pre-sternal level in addition to a recent massive weight loss. A CT showed a tumor from manubrium to the mid-sternal body of 12 by 11 cm. A skin biopsy was performed and showed a papillary part (that was positive for AE1/AE3, TTF1, and PAX8) and a poorly differentiated component (that was CD58 positive in giant cells). This was followed by excisional biopsy of the mediastinal tumor. A confirmation of a rare anaplastic type was re-done, namely, the giant-cell-rich type associated with skin and sternal metastases. PET-CT identified lymph nodes metastases at the cervical and axillary level. The patient tested positive for BRAF V600E and survived 6 months since the first admission. Notably, the cervical thyroid was atrophic [37]. Median survival in anaplastic thyroid carcinoma at the eutopic gland is of 3 to 6 months. Generally, pathogenic variants of TERT, TP53, BRAF, and RAS genes have been reported in this malignancy. The same genetic pressor seems to act in ectopic malignancy as well. As seen in the eutopic site, the level of statistical evidence remains very low [37,176,177,178].
To summarize, the thyroid malignancies originating from the follicular cells at the cervical thyroid or EMT (meaning papillary, follicular, poorly differentiated and anaplastic types) embraced various patterns in EMTs as following: subject affected by a primary thyroid cancer in the eutopic gland but benign EMT [22,35,123]; multifocal carcinoma affecting both EMT and a eutopic thyroid [36,42,55]; one case was considered to have metastasis in the thyroid from an EMT cancer [55]; malignant EMT with benign features in the eutopic gland [37,41,52,55,58,60,61,62,64,66,69,70,71,72]. Notably, the distant metastatic spreading from an EMT was identified in bone [37,55,58,60,61] and pre-sternal skin [60]. Intra-mediastinal ectopic tissue was accidentally detected during surveillance or check-up for non-thyroid (unrelated) malignancies, and we identified seven such cases (a rate of 5.98% amid all EMTs); the primary carcinomas were located in the pulmonary [11,49,101], mammary [29,92,125] and ovarian [89] areas (Figure S1).
2.7.3. Thyroid Lymphoma in EMT
A single case of mucosa-associated lymphoid tissue (MALT) lymphoma in an EMT was identified (in 2020). This was a 67-year-old lady who was accidentally detected at CT scan with an upper mediastinal mass of 2 by 1.3 cm situated anterior to the trachea (poorly enhanced structure) without connection to the cervical thyroid. Further MRI was completed as well as 131iodine MIBG scintigraphy that excluded a paraganglioma. A resection via transverse neck incision was provided and confirmed an MALT lymphoma and chronic thyroiditis. The mass was 1.5 by 1.2 by 0.9 cm with lymphoma cell infiltration to follicular epithelial cells (which were positive for thyroglobulin); B lymphocytes were positive for CD79a. An FNA of the ortothopic thyroid showed thyroiditis (no MALT). Post-operatory thyroid function remained normal. Further 18F-FDG PET-CT showed a thyroid accumulation. No other therapy was added; she continued surveillance [59]. Wu et al. [63] introduced a mostly challenging case of an adult who survived renal transplant 11 years prior; he developed a neck swelling and an upper mediastinal tumor that turned out to be a primary mediastinal large B-cell lymphoma. The immunosuppressive therapy for more than a decade amid being a renal recipient in a patient who three years before was confirmed with an autoimmune Hashimoto’s thyroiditis might imply a pathogenic contribution to this type of lymphoma yet with an exceptional location in EMTs [63]. Primary ectopic thyroid B cell lymphoma arising from an ectopic thyroid in the mediastinum was first reported in 2009 [67]. Of note, chronic thyroiditis represents the ground of the primary thyroid lymphoma; its incidence in patients with Hashimoto thyroiditis has been reported as 16 cases at 10,000 persons per year [59,179,180,181].
2.7.4. Other Primary Malignancies in EMTs
As mentioned, a single case of its kind was published in 2011, namely, a mediastinal medullary thyroid carcinoma admitted for severe hypokalemia due to ectopic ACTH production [65]. Also, a dramatic pediatric case (the singular report in children we identified across our methods) involved an immature teratoma in EMTs [68].
2.8. EMT Biopsy and Fine Needle Aspiration Cytology (before or instead of Surgery)
Direct EMT access is provided by the biopsy such as transthoracic needle biopsy (TTNB) or CT-guided (or ultrasound-guided for EMTs located within superior mediastinum) FNA or EBUS-TBNA [13,182]. Generally, since most ectopic thyroid tissues are located in the neck areas, ultrasound-guided FNA-based cytology represents the most important pre-operatory tool for directly accessing the thyroid mass [7]. Gao et al. [7] had a confirmation of the thyroid tissue by FNA cytology (N = 3/4), and one case was resected via a thoracoscopic approach (surgery was performed only in this case; the others were conservatively followed upon providing the cytological analysis) [7]. FNA offers the cytological report, not the histological report, which for the orthotopic thyroid stands for the most useful tool to directly access the gland. One major pitfall concerns the follicular pattern in cytological testing, since similar features are found in thyroid adenomas and carcinomas [183,184,185]. As a general note, FNA remains a most useful tool for an ectopic thyroid within the neck area while for EMTs, a trans-esophageal or trans-bronchial approach is frequently required [49,76,186]. Metastatic thyroid carcinoma should be differentiated from EMTs (this is one of the reasons for meticulously checking the eutopic gland including thyroid nodules exploration via FNA once an EMT is suspected) [31,73].
Notably, Vuorisalo et al. [49] showed a three-case series according to a quality assurance program, whereas EBUS-TBFNA was administered in mediastinal lymph nodes in order to differentiate EMTs from metastases originating from follicular (cervical) thyroid cancer. An open issue might be represented by the high inter- and intra-laboratory variability in this particular matter [49]. Similarly, Tsai et al. [11] also raised the issue of mediastinal lymph nodes involvement in a relationship with non-thyroid cancers (such as those originating from lung) that should be differentiated from EMTs, especially if the subject is already known to have a history of a specific malignancy. This was a 50-year-old lady with a previous diagnosis of pulmonary adenocarcinoma (at the left upper lobe) who underwent surgical resection (segmentectomy) one year prior. At some point, she experienced headache, nausea and dizziness; thus, she came in for a complex imaging check-up, and two brain metastases were detected at MRI. She was offered gamma knife therapy. Moreover, CT and PET-CT scans for follow-up showed an enlargement of lymph nodes within the mediastinum and thyroid nodules. FNA at the thyroid level showed a papillary thyroid cancer. EBUS-TBFNA of the lymph nodes revealed they were cancer-free, as they were confirmed as being an EMT (2.9 by 1.6 cm). Of note, these mediastinal lesions at PET-CT were not hypermetabolic; thus, an alternative diagnosis to a cancer spreading was already taken into consideration before FNA results [11]. Notably, an immunohistochemistry panel in lung adenocarcinoma also reveals positive Ck7, TTF1, as seen in EMTs or metastatic papillary thyroid carcinoma, while PAX8 can only be found positive in thyroid tissue, as well as thyroglobulin stain; on the other hand, CD56 is positive in thyroid tissue and negative in pulmonary adenocarcinoma, but it may be positive in pulmonary typical and atypical carcinoids (whereas the well-known panel of blood neuroendocrine markers should be checked in terms of chromogranin, neuron-specific enolase, and synaptophysin; in this specific instance, TTF1 is inconstantly positive) [11].
Alternatively to FNA or EBUS-TBFNA, TTNB was used for histological rather than cytological analysis; for example, Sadidi et al. [74] performed TTNB to access an EMT located at the upper-middle mediastinum in a 54-year-old female who was admitted for cough and dyspnea for 3 years. The pathological report upon biopsy showed an adenomatous goiter; the procedure was followed by EMT removal via thoracotomy with a good post-surgical outcome. The largest diameter was 7.5 cm [74].
Overall, direct access to EMTs and/or the cervical (eutopic) thyroid was provided across a highly customized approach: mediastinoscopy for EMT diagnosis [9,87]; EBUS-guided biopsy [9,118]; trunk biopsy [13,60]; CT-guided core biopsy [69,75,92,115]; TTNB [74]; endoscopic ultrasound-guided biopsy [76]; CT-guided FNA in EMTs [96,99,101]; FNA/FNAC in EMTs with malignancy confirmation followed by its removal [7,41,58]; EBUS-FNA [50]; FNA in EMTs and cervical thyroid [80]; ultrasound-guided FNA in cervical thyroid [11,59,69,87,98,117]; EBUS-TBNA in EMTs [11,91] (of note, this investigation might be non-diagnostic [55,103] or might offer an adequate diagnosis, thus allowing the decision of surveillance, not EMT resection [62,89,95]). Some patients diagnosed with EMTs were directly referred for EMT removal after imaging evaluation, and no biopsy or cytology was completed pre-operatory. On the other hand, in small-sized EMTs, the confirmation of a benign EMT was not followed by a resection but rather by a conservative management under periodical check-up. Additionally, skin biopsy was completed in one case with pre-sternal spreading [37] or FNA in clavicle metastases [61].
Overall, the histological (or at least cytological) analysis is mandatory amid EMT exploration for a positive diagnosis, for identifying an EMT or for differential diagnosis with other malignant/benign mediastinal masses. The co-presence of the thyroid nodules in patients with EMTs requires at least FNA at this level. Non-diagnosis across different types of EMT biopsies or FNAs is not so rare; thus, an adequate EMT recognition may be affected. Also, in subjects with prior cancers, histological (or at least cytological) EMT testing is essential to differentiate it from metastasis. In patients with synchronous or prior thyroid nodular conditions (single nodules, multinodular goiter or cancer) in a eutopic gland, the second opinion (histological/cytological) should be made in relationship with the EMT profile (to check if they are similar). Metastatic lesions showing a follicular thyroid pattern may be originating from the EMT or cervical thyroid malignancies. An immunohistochemistry report (thyroglobulin, PAX8, TTF1, Ck7, and even lymphoma immune phenotyping) [55] adds value in confirming the thyroid profile in EMTs, especially in cases when pre-operatory or pre-biopsy investigations (particularly, iodine or 99m-Tc scintigraphy) were not consistent with a confirmation of ectopic thyroid tissue or in subjects suspected or confirmed with other malignancies [186,187,188,189,190].
2.9. Surgical Procedures to Remove EMTs
Median sternotomy or posterolateral thoracotomy represents the traditional approach of EMT, preferably via a minimally invasive approach as seen in recent years. Moreover, cervical incision (with immediate availability of sternotomy in case of intra-operatory complications) is preferred depending on the upper EMT location, surgeon’s experience, as well as eutopic thyroid proximity or prior/current thyroid removal (since performing a total thyroidectomy allows better access to EMT or retrosternal goiter resection to avoid a sternotomy upon skillful vessels ligature before removal) [9,13,94,96,187,191]. The indication of sternotomy depends on the EMT location and volume, vessels anatomy, the risk of hemorrhage and the pre-operatory identification of mediastinal lymph nodes enlargement without specified significance (potentially malign spreading) and the suspicion of a malignant tumor [75,192,193]. Also, as noted in the case of double upper mediastinal EMTs, synchronous removal of both tumors also required sternotomy [75].
In 2020, Imai et al. [80] reported a trans-cervical EMT resection without orthotopic thyroid removal that was feasible due to the very high position of EMTs within the upper mediastinum (at the cranial side, situated from the thoracic inlet to the superior mediastinum) with no connection to the cervical gland that seemed fine and was not enlarged. This was a 50-year-old female who was accidentally detected with an EMT after she had a 4-month history of cough. A CT scan showed a heterogeneous mass of 4 cm. The lady had normal thyroid function and negative antibodies against the thyroid. Before surgery, FNA for both thyroid sites showed no malignant traits [80]. Similarly, Uchida et al. [59] reported an EMT resection via a trans-cervical approach that was feasible due to a high position and small size (of 1.5 cm) in an MALT-positive patient [59]. Regal et al. [85] introduced the case of a young lady with an EMT of 10 cm diameter upon resection by performing midline partial sternotomy; the mass was located in the upper part of the anterior mediastinum with tracheal compression from the left side [85]. Coskun et al. [53] published a study in 2014 comprising 665 subjects who underwent thyroidectomy, and 6.3% of them (N = 42 adults) had a substernal goiter; among this subgroup, only 9.5% (N1 = 4 individuals) had a median sternotomy plus cervical incision (and one of these four patients had an EMT, thus representing 2.8% of the subgroup identified as “substernal goiter”); otherwise, only a cervical approach was used in 90.5% of the studied group) [53]. The analysis of Aziz et al. [48] showed that all individuals with EMTs (7/7) underwent surgery. The decision was individualized depending on location within the mediastinum. Two out of the three subjects with EMTs at the upper mediastinal had trans-cervical surgery, and one of these three individuals had a sternotomy (in association with concomitant valve replacement). Four patients with EMTs located at non-upper compartments underwent EMT resection through the chest with robotic assistance or posterolateral thoracotomy [48].
Some reports revealed patients who underwent total thyroidectomy in their medical history (one to 39 years prior to the moment of EMT confirmation) [35,118,120,122]. This previous neck surgical history allowed a cervical incision-based EMT removal in some cases [118,120,122] or required an additional sternotomy due to the specific EMT location (and vessels configuration) or suspected EMT malignancy [35,51]. Santangelo et al. [52] also introduced a case series of individuals with total thyroidectomy and sternotomy [52]. As mentioned, synchronous total thyroidectomy, lymph nodes resection and sternotomy for papillary thyroid cancer in EMTs was also performed by Toda et al. [55]. Metere et al. [88] used the combination of thyroidectomy via a cervical approach and longitudinal sternal splitting to remove the EMT [88].
In addition to sternotomy, thoracotomy was required, too; we mention a mostly singular case of an EMT located within the posterior mediastinum in a subject with situs inversus totalis to whom benign EMT removal was performed via a left posterolateral incision. She had a history of left hemi-thyroidectomy 30 years prior for a toxic thyroid nodule (with hyperthyroidism on first admission) with consecutive normal thyroid function including at the moment of EMT diagnosis [124].
VATS has gained success in mediastinal and non-mediastinal conditions as part of the modern thoracic surgery due to its benefits such as better recovery, shorter hospital stay, and reduced blood loss. VATS is limited by the tumor size of less than 10 cm; it seems safe even in cases of EMT malignancy as proved by the case published by Caroço et al. [42]. VATS, since its introduction in the early 2000s for non-thyroid conditions such as lung tumors, extended its indications, and an EMT is a successful candidate to VATS, as similarly seen for other endocrine (such as mediastinal parathyroid tumors) and non-endocrine conditions [194,195,196,197]. For instance, uniportal VATS was used by El Haj et al. [78] in a case of a 59-year-old lady who was synchronously identified with a mediastinal and a lung mass. She had a 4-month history of respiratory complaints. CT showed a tumor in the upper right mediastinum displacing the upper cave vein and compressing trachea on the contra-lateral site. She also had a left basal lung mass. Bronchoscopy showed pulmonary actinomycosis; thus, she underwent a 6-week course of injectable penicillin followed by a 3-month oral regime with the pulmonary mass remission. After a mediastinoscopy, an EMT (weighting 32 g) was removed via the uniportal VATS [78]. Carannante et al. [84] also reported the use of uniportal VATS in 2019 for an EMT of 98 g (histological report showed a cystic component and hemorrhage) [84].
The recent introduction of robotic-assisted thoracoscopic surgery (RATS) for ectopic parathyroid tumors [198] was extended to EMTs. Generally, the upper mediastinum is a difficult site for thoracoscopy. The location of vessels and nerves adds a challenge to this type of approach. In the case reported in 2023, RATS was used in a 40-year-old male who was accidentally detected with a mediastinal mass following COVID-19 infection that required a CT scan. A tumor of 6.1 by 7 by 6.1 cm was detected in the upper mediastinum. The tumor displaced trachea to the left. Despite being apparently incidental, retrospectively, the patient showed prior unexplained dysphagia and thoracic (and cervical expansion) pain. Once suspected, the second imaging procedure was conducted in terms of having a 131iodine scintigraphy that confirmed an EMT and a normal cervical thyroid gland. Endoscopic ultrasound-guided biopsy was performed followed by RATS with cervical tumor extraction via cervicotomy. The 2-day hospitalization was followed by an asymptomatic recovery in this non-malignant EMT case [76]. Of note, the first robotic resection of a large cervical goiter with a synchronous EMT underlying an adenoma was reported in 2004–2005 by Bodner et al. [127,128]. This was a 72-year-old lady who underwent a thoracoscopic resection with a da Vinci robot [127,128].
To summarize, the highly customized surgical approach in EMTs showed a wide area of interventions: VATS [78,84,87,93,125]; RATS [76]; resection via mediastinoscopy [81]; thoracoscopy [7,82]; thoracotomy [22,45,62,119]; right lateral thoracotomy [74,100,104] plus total thyroidectomy [69]; left posterolateral thoracotomy for EMTs [124] plus pulmonary cancer removal [101]; lateral incision for thoracotomy associated with a modified trans-manubrium approach [70]; sternotomy with total thyroidectomy [52,55,117]; sternotomy with cervical incision [53,117] and prior thyroidectomy [120]; sternotomy plus valve replacement [48]; VATS and total thyroidectomy for thyroid cancer [42]; cervical incision [38,59,106] plus right thyroid lobe removal [98]; mid-sternal body excision with sternum reconstruction and total thyroidectomy [60]; excision of clavicular metastasis and total thyroidectomy with modified neck dissection [61]; EMT and thymus removal plus total thyroidectomy [64,94]; midline sternotomy [13,35,85]; partial upper sternotomy [87]; median sternotomy for intra-pericardial EMTs [29]; sternotomy and thymectomy in a patient with a prior history of thyroidectomy for cervical thyroid cancer [123]; left hemi-thyroidectomy and isthmectomy plus median sternotomy [129]; partial sternotomy and total thyroidectomy associated with a selective parathyroidectomy via mediastinal and cervical exploration [115].
2.10. Outcome after Identification of EMTs
Regardless of the pathological traits, awareness of EMTs is essential, while removal was decided in most of the cases (rather than conservative approach) depending on the location and EMT anatomical features; the risk of malignancy (the rate of conversion from benign to a malignant EMT is not clearly understood, especially in long standing goiter-like EMT); the ectopic tissue enlargement with compressive symptoms/signs such as respiratory obstruction or compression on mediastinal organs; the risk of hemorrhage [84]; the patient’s co-morbidities and medical/surgical history as well as the general health status [7,13].
The main elements when it comes to the end results and consecutive management upon EMT identification follow:

- EMT removal: based on the published data, this is the preferred approach and it is expected to have a good outcome and no local recurrence after surgery.

- EMT surveillance: conservative approach, for instance, after EMT confirmation amid FNA results [89] is less likely preferred.

- Firstly, thyroid dysfunction impairs the surgical outcome; thus, medication with anti-thyroid drugs for newly detected hyperthyroidism [34] is mandatory, and a further decision to be taken during follow-up (for instance, propylthiouracil until thyroid function normalization followed by total thyroidectomy and EMT resection [121] or methimazol followed by EMT removal [122]).

- A second surgical step after EMT resection involves a total thyroidectomy followed by radioiodine ablative therapy, TSH-suppressive thyroxine treatment, and lifelong thyroglobulin monitoring in cases with a differentiated thyroid cancer in EMT [35,123].

- External beam radiation therapy was applied for bone metastases [61].

- Thyrosine kinase inhibitors were proposed for metastatic, aggressive thyroid cancer [61].

- Follow-up of the thyroid function after EMT removal is required in order to check for (transitory) early post-operatory hypothyroidism [73].

- The associated (iatrogenic) hypothyroidism via thyroxine replacement is corrected in benign thyroid disease, too.

- Of note, levothyroxine-based TSH suppression therapy instead of surgery for benign EMTs in euthyroid patients [102] was not successful, and they are no longer encouraged according to the modern endocrine perspective (as similarly seen in thyroid nodules of the eutopic gland in patients with normal baseline thyroid function [199]).
The longest period of follow-up since EMT diagnosis and resection according to the reported data is up to one to two years [29,53,69,70,87,103]. Alternatively, Gao et al. [7] showed that EMTs remained stable for a median follow-up of 39.5 months in three cases that were not removed upon cytological confirmation and following the resection of one benign EMT [7]. Also, a stationary EMT for one year was registered in another case; the decision of a conservative approach was based on EBUS-guided biopsy results [118].
2.11. Proposed 10-Item Algorithm of EMT Approach
There are current gaps in EMT management, making it a matter of personalized approach: a low index of suspicion; no specific criteria for providing biopsy and/or FNA, neither for opting in favor of EMT resection in each case; lack of standardized surgical approach (also, concerning additional eutopic thyroid gland removal), and the imperious need of a multidisciplinary team decision. According to the prior mentioned data [7,11,29,52,59,70,76,83,85,89,92,95,98,120], we propose a working algorithm in EMTs which stands on ten major points of a multidisciplinary approach in helping a personalized decision (of note, this is our interpretation and opinion based on the current literature evidence rather than a consensus guideline):
- Clinical presentation
This is non-specific; it is a matter of a multidisciplinary panel, and one subject may be initially admitted at various medical and surgical departments. There are no pathognomonic elements. Dyspnea, cough, dysphagia, and chest pressure are the most common features. One patient may be completely asymptomatic.
- 2.
- CT scan
This is the most important and the standard imagery assessment, and it mostly represents the starting point in EMT suspicion. Approximately one out of ten patients is detected with EMTs as an incidental finding for unrelated conditions. Intravenous contrast is recommended, but this comes with the pitfall of contra-indication to use iodine contrast in patients with uncontrolled hyperthyroidism.
- 3.
- Re-do anamnesis
This is essential step since incidental detection does not mean completely asymptomatic. Retrospective data collection showed intermittent dyspnea in some circumstances, night sweats, facial edema, etc. These are expected to remit upon EMT removal.
- 4.
- Iodine or 99m-Tc scintigraphy
This investigation is pivotal for the thyroid tissue identification. Once an EMT is suspected, this becomes a mandatory step in diagnosis management. The potential pitfalls are the following: EMTs might have a different metabolic rate and a tracer uptake than a eutopic thyroid; some EMTs do not capture the tracer at all; the functional interplay might imply an over-activity of (autonomous) EMTs and consecutive cervical thyroid suppression or quite the other way; delayed captures in EMTs have been reported; prior use of iodine contrast or anti-thyroid drugs might block the iodine uptake; 99m-Tc might be up-taken in ectopic and eutopic parathyroid tissue as well.
- 5.
- Endocrine assessments
These are mandatory in terms of thyroid function (serum TSH, free thyroxine, and triiodothyronine), serum thyroid antibodies (antiTPO and antiTg), respectively TRAb—if hyperthyroidism is identified. For differential diagnosis, calcitonin (for medullary thyroid cancer), calcium and PTH values (for primary hyperparathyroidism), and paragangliomas-related assays are helpful. Hyperthyroidism requires the immediate starting of anti-thyroid drugs since this might complicate a biopsy but mostly a cervical thyroid or EMT manipulation during surgery. Thyroglobulin should be measured in any circumstance (at baseline and during follow-up) regardless of whether differentiated thyroid cancer is present.
- 6.
- Exploration of eutopic (cervical) gland and its connective tissue to EMTs
Thyroid ultrasound at the neck area is an easy-to-use and screening investigation. If thyroid nodules are identified, FNA should be completed according to the general guidelines. The connective tissue to an EMT makes it distinct from a retrosternal goiter, and it may be analyzed via imagery exploration (in addition to the intra-operatory findings and post-operatory histological report).
- 7.
- Second-line imagery
If prior investigations are not clear, further exploration becomes optional, not mandatory; for instance, SPECT-CT (for parathyroid tumours), MIBG scintigraphy (for paragangliomas), PET-CT (for a malignancy of any origin), and MRI of non-mediastinal areas might help (this depends of the particular circumstances in one case). Potential pitfalls in using PET-CT: some EMTs are hypermetabolic but not malignant; other EMTs do not uptake the tracer at all.
- 8.
- Biopsy or FNA in EMTs
This decision is individualized, and it is a matter of a multidisciplinary team based on the patient’s co-morbidities, EMT anatomical profile (including blood vessels configuration), clinical implications, access to safely perform a biopsy of FNA, and the patient’s preference. Potential pitfalls are represented by the fact that both types of investigations might be non-diagnostic (or provide results of uncertain significance). If the decision of EMT resection is already established, biopsy or FNA might not be necessary. Direct access to EMTs (a part from its resection) is mandatory in cases with prior or concurrent malignancies, whereas metastasis should be differentiated and the patient might not become a surgery candidate under these specific circumstances.
- 9.
- Surgical resection of EMTs
Surgery candidates mostly belong to one of these six categories: (1) suspected malignancy in EMTs (a rate of 18.8% was found); (2) compression symptoms (depending on anatomical effects and clinical features); (3) at-risk traits at imagery scans (e.g., necrosis, hemorrhage, increasing dimensions during follow-up); (4) lack of biopsy or FNA data (either they were not available, or they were non-diagnostic or the patient declined it, or they are too risky to be performed due to vessels’ proximity or due to the general health status of one patient and co-morbidities); (5) prior or synchronous non-thyroid cancers whereas the presence of a mediastinal metastasis might change the entire case management; (6) prior or synchronous thyroid cancer in the eutopic gland whereas surveillance based on serum thyroglobulin and whole body iodine scintigraphy cannot be adequately used due to the presence of the ectopic tissue. The type of surgical procedure is based on the decision of a highly skilled team of cardiothoracic surgery. Thyroidectomy, if malignancy is not suspected in EMTs or the cervical gland, is not necessary unless the EMT practical approach requires it. Minimally invasive procedures are the modern approach.
- 10.
- Long-term surveillance
Post-operatory assessment of the thyroid function is necessary since transitory hypothyroidism was reported. Iatrogenic permanent hypothyroidism requires long-term levothyroxine replacement. In cases with differentiated thyroid cancer, further total thyroidectomy (if was not already provided) is mandatory followed by the application of radioiodine ablative therapy, TSH-suppressive therapy with daily oral thyroxine, and the use of thyroglobulin lifelong periodical check-up. If the conservatory approach was chosen instead of EMT surgical resection, CT serial imagery scans are needed as well as the periodical thyroid function testing. In cases with prior thyroidectomy, a second histological analysis to compare the thyroid (cervical) tissue and EMT might help. Immunohistochemistry in EMTs may be necessary for differential diagnosis in selected cases (e.g., thyroglobulin, PAX8, TTF1, AE1/3, CD56, CD58, CD79a, Ck7, etc.). Further applications of molecular and genetic testing in EMTs are yet to be confirmed (Figure 4).
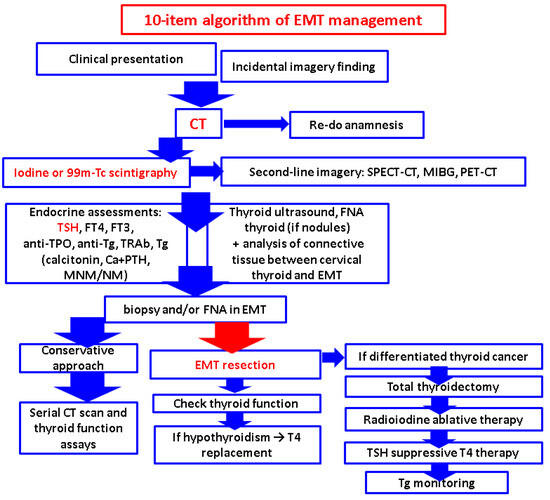
Figure 4.
Proposed 10-item algorithm of EMT management: a multidisciplinary perspective of an otherwise customized decision. Abbreviations: antiTPO = anti-thyroperoxidase antibodies; antiTg = anti-thyroglobulin antibodies; CT = computed tomography; Ca = calcium; EMT = ectopic mediastinal thyroid; FNA = fine needle aspiration; FT4 = free thyroxine; FT3 = free triiodothyronine; MIBG = metaiodobenzylguanidine; MNM = metanephrines; NM = normetanephrines; PTH = parathormone; PET-CT = positronic emission tomography-computed tomography; SPECT-CT = single-photon emission computed tomography; Tc = technetium; TSH = thyroid-stimulating hormone; TRAb = TSH receptor antibody); Tg = thyroglobulin.
3. Discussion
3.1. Considerations on the Sample-Focused Analysis of EMTs
Our study on published data included 117 cases diagnosed with EMTs and identified a rather unexpected high rate of malignancy of 18.8% (underlying various histological profiles, but mostly papillary thyroid cancer) [7,9,11,13,22,29,34,35,36,37,41,42,45,48,49,50,51,52,53,54,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130]. To our knowledge, this is the most complex analysis in EMTs that followed the clinical presentation, imagery, pathological traits, intervention, outcome, and potential pitfalls in addressing EMTs amid daily practice.
One of the most challenging aspects of EMTs includes the very first step of suspicion or recognition, since the clinical index is very low. We performed a secondary analysis upon primary data extraction regarding the findings at first admission for EMTs; they are not specific and belong to a very heterogeneous picture that might mimic multiple other conditions from cardiology, internal medicine, endocrinology, oncology or surgery domains [200,201,202,203,204,205,206]. The most frequent complaints involve sternal/chest pressure or pain, cough, dysphagia and dyspnea (Table 5).

Table 5.
The clinical elements or scenario of detection in EMTs according to our methods [7,9,11,13,22,29,34,35,36,37,41,42,45,48,49,50,51,52,53,54,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130] (blue font = incidentaloma).
The symptoms/signs were described as being acute (and required an admission as emergency [118]) or relatively recent (in terms of a few days [93], weeks [50], or months [9]) or they were presented in the medical history of one subject for previous 3 [74] to 5 years [86]. Hence, an EMT plays the role, as it has been described, of a great “imitator” [11] or “mimicker” [87] or the “forgotten goiter” [120].
When it comes to cancer in EMTs, the prior mentioned study of Santangelo et al. [52] identified an overall rate of 3.6% (less than our results) concerning malignancy in ectopic thyroid tissue, particularly, papillary thyroid carcinoma (N = 3092 patients who underwent a total thyroidectomy for any type of thyroid condition at eutopic gland). The study showed across five patients with EMT that one of them had this type of malignancy only in an ectopic rather than an orthotopic site (meaning one out the five EMTs was malignant, which is close to our results) [52]. For example, Caroço et al. [42] reported in 2023 a novel case of papillary thyroid carcinoma amid EMT recognition. This was a 73-year-old male who underwent total thyroidectomy for bilateral papillary thyroid carcinoma (no lymph nodes involvement) at the level of the orthotopic gland with post-operatory high serum thyroglobulin that required additional imaging exploration. Neck ultrasound and CT scan showed a mediastinal tumor of 6 by 3 cm (upper anterior compartment). At first, this was regarded as a potential metastasis from originating thyroid malignancy, which represents the stepping stone in cases with previous diagnosis of (orthotopic) thyroid malignancy. Resection via VATS was successful. A papillary micro-carcinoma was identified at EMT (0.4 cm). Post-operatory, the thyroglobulin remained undetectable for a post-operatory 6-month period (the patient did not received further radioiodine ablation) [42]. Another case published in 2012 showed two synchronous papillary thyroid malignancies within EMTs and a micro-carcinoma in the eutopic gland (multifocal carcinoma) [36].
On the other hand, Toda et al. [55] introduced a three-patient series with so-called “occult” thyroid carcinoma, and two out of the three subjects had a thyroid malignancy in the EMT as the primary origin not in the cervical gland. The first subject was a 71-year-old male with a 9-month history of superior vein cave syndrome (eyelid edema, jugular venous distension, and persistent facial edema). CT showed a lump of 2.7 cm in the upper mediastinum on the right side causing the stenosis of the upper cave vein. PET-CT confirmed this tumor in addition to multiple bone metastasis and normal thyroid findings. Bone biopsy confirmed a thyroid papillary malignancy with positive immunostaining for thyroglobulin and PAX8. Thyroid ultrasound and function were normal. Total thyroidectomy and radioiodine ablation were completed (100 mCI) yet with no specific radioiodine uptake within the mediastinum. The patient remained under surveillance without surgery for the mediastinal mass that was considered too risky due to the vessels’ proximity. There was also an 84-year-old male admitted for cough. A mass of 7.2 cm at the upper anterior mediastinum was identified at the CT scan (with tracheal compression and no connective tissue to the thyroid). EBUS-TBNA showed indeterminate results (no EMT confirmation). Thyroid function was normal with very high serum thyroglobulin (of 2450 ng/mL). The gentleman underwent combined total thyroidectomy, central neck dissection, as well as mediastinal tumor resection via sternal incision (sternotomy). The EMT showed a papillary thyroid cancer (no lymph nodes invasion); a 0.9 cm isthmus nodule showed a papillary micro-carcinoma that was considered by the authors as being a thyroid metastasis according to the post-operatory pathological analysis. Consecutive radioiodine ablation (100 mCi) was added [55].
On the contrary, Kesici et al. [35] reported in 2015 the case of a lady who underwent a total thyroidectomy for papillary thyroid carcinoma and post-operatory iodine scintigraphy highlighted EMTs, which was completely removed amid a second operatory procedure, but no malignancy in ectopic tissue was confirmed [35]. This was similar to the case introduced by Kamaleshwaran et al. [123], also revealing the importance of iodine scintigraphy to identify non-connected mediastinal tissue that does not host similar malignancy traits with the eutopic gland [123].
Additionally, Shafiee et al. [64] also reported a papillary thyroid carcinoma in an EMT that was initially suspected as being a thymus malignancy due to its location; additionally, the eutopic thyroid showed no carcinoma [64]. Karim et al. [60] showed the finding of an EMT in a 55-year-old female underlying follicular variant of papillary thyroid carcinoma. The presentation was with a progressive tumor development amid the latest two-year period. She had a history of a right lobectomy for a benign thyroid condition with consecutive normal thyroid function, including on admission. The EMT was located within the anterior compartment (of 15 by 5 cm at CT scan) without any associated malignancy at the level of ectopic gland remnants. The lady underwent EMT removal (mid-sternal body excision with sternum reconstruction) after having a trunk biopsy that confirmed an EMT via positive TTF1 stain associated with total thyroidectomy followed by radioiodine ablative therapy (80 mCi). The initial serum thyroglobulin was very high (>6000 ng/mL). In addition, whole body scan showed several metastases [60]. Hu et al. [62] showed in 2017 primary papillary thyroid cancer features in EMTs based on a cytological report that was provided by EBUS-TBNA in a male senior later confirmed after tumor removal as having no malignancy in the eutopic thyroid gland [62]. A dramatic case was reported in an adult lady who first showed a right distal clavicular mass of 5.5 cm that turned out to be a metastasis from an EMT with primary papillary thyroid cancer and no malignancy within the eutopic gland, which was small and atrophic. FNA at clavicle mass confirmed a thyroid malignancy (positive for TTF1, thyroglobulin). 18F-FDG PET-CT showed multiple hyper-metabolic lymph nodes and EMT uptake (that displaced trachea and esophagus). The 68-year-old female underwent excision of the right clavicular mass followed by total thyroidectomy plus modified right neck dissection (levels 3, 4, and 5) followed by external beam radiation therapy to the distal clavicular region. Central neck biopsy of the superior mediastinal mass confirmed papillary thyroid cancer. She underwent radioiodine therapy; then, CT showed multiple metastatic lymph nodes the neck and upper mediastinum and additional pulmonary spreading. After additional radioiodine therapy and seemly remission, further recurrence was noted at PET-CT scan, and a neoplasia switch to a dedifferentiated type required therapy with a thyrosine kinase inhibitor. This unusual aggressive form of thyroid malignancy was considered at the moment of publication (in 2018) the most severe outcome in EMTs at that point [61].
3.2. EMT Submitting to the Scenario of an Endocrine Incidentaloma
Approximately one out of ten patients diagnosed with an EMT was accidentally detected via various imagery assessments, mostly CT scan, which were completed for different purposes (related to malignant or benign conditions) [7,11,29,52,59,70,76,83,85,89,92,95,98,120]. Awareness represents the key operating factor, since the EMT is situated at the crossroads between mediastinal masses with oncologic background and endocrine incidentalomas (in everyday endocrine practice, the traditional incidentalomas involve thyroid, adrenal and pituitary glands) [90,207,208]. Notably, one 42-year-old male was admitted for EMT-related complaints, and finally, he was identified with a right adrenal tumor of 5 cm (an adrenal incidentaloma) [73]. So far, identifying an adrenal tumor in an EMT patient seems like an accidental finding, and further research is needed to pinpoint a potential pathogenic connection. Nevertheless, the adrenal gland was exceptionally found to be involved as a host of an ectopic thyroid tissue despite the fact that the embryological rationale behind this is less understood [209,210].
3.3. The Impact of COVID-19 Pandemic in Reporting a Mediastinal Thyroid Tissue
As mentioned, some patients diagnosed with EMTs presented fever or sub-fever, and this required a differential diagnosis with an infectious disease [13,102]. Generally, many imagery investigations, particularly in the area of lung, mediastinum and cervical, were performed during or after one individual suffered a coronavirus infection during COVID-19 pandemic years, and many novel entities were described, or prior unknown conditions of one subject were detected under these circumstances while not being related to the viral infection itself [211,212,213,214,215]. For instance, a 40-year-old male was found with a 6 cm EMT amid CT scan for COVID-19 infection (the case was published in 2023) [76]. On the other hand, some authors suggested that restrictions during pandemic waves limited the access to prompt intervention in some instances, whereas an ectopic thyroid was involved [8]. Whether practice amid COVID-19 pandemic years stands as a pillar for a more frequent EMT detection or for a delay of prompt EMT recognition is yet to be proven. We performed an additional analysis on the papers with regard to the EMT diagnosis [7,11,29,52,59,70,76,83,85,89,92,95,98,120] and found that 26% of articles (regarding EMTs) were published between 2020 and the present time, representing almost half of the original articles published between 2000 and 2019. We expected an expansion of this specific topic under the umbrella of increasing awareness and elevated access to various imagery assessments (Figure 5).
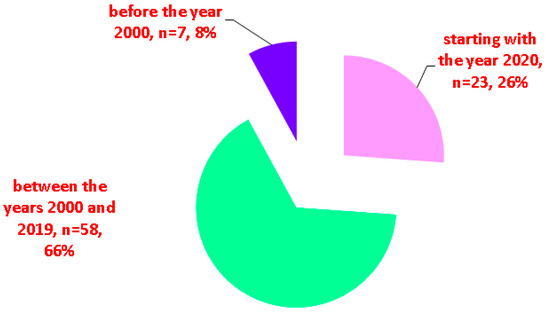
Figure 5.
The perspective of publication timeline in EMT according to our methods (n = number of original publications).
3.4. Ectopic Parathyroid versus Thyroid within Mediastinum
As mentioned, EMTs should be differentiated from ectopic parathyroid tumors. 99m-Tc sestamibi scintigraphy might identify parathyroid tumors (including at mediastinal levels). This is mostly a late capture, while the early tracer uptake may be found in eutopic and heterotopic thyroid tissue, and this aspect might be a potential source of bias in this particular instance [216]. In case of functional parathyroid tumors, additional blood assays offer the biological confirmation of primary hyperparathyroidism (high serum calcium and PTH), and further exploration of associated complications such as bone disease (including osteoporosis) or kidney anomalies is mandatory [217,218,219,220]. Of note, the interplay between ectopic thyroid and ectopic parathyroid tissue might be related to the embryological origin; as mentioned, the thyroid originates from the first and second pouches, while superior parathyroid glands come from the third pouch, and inferior parathyroid glands originate from the fourth pouch [216,219]. CT scan in addition to 99m-Tc sestamibi scintigraphy has a very high sensitivity (100%) and positive predictive value (97%) for parathyroid tumors, including for the ectopic masses. A lower rate of detection is found in small tumors with a less mitochondrial count that relates to a decreased tracer uptake [216]. Recent data agree that even better results are expected for using SPECT-CT and combined techniques such as dual-phase 99m-Tc MIBI scintigraphy and 99m-Tc MIBI SPECT-CT [216,217,218,219,220]. Approximately 3% to 16% of the population has a supra-numerary parathyroid gland (from five to eight accessory parathyroid glands), and this may be an issue of ectopic parathyroid tumor identification in cases with associated primary hyperparathyroidism that need tumor removal or in subjects with recurrent/persistent hyperparathyroidism upon the parathyroidectomy of orthotopic parathyroid tumors [216,217].
Notably, 60% of the supra-numerary parathyroid glands are in the mediastinum, and the most frequent site is the thymus [216,217,218,219,220]. For example, Muzurović et al. [115] introduced an interesting case of a 53-year-old women admitted for a 3-month history of cough and chest pain. CT scan showed an upper anterior mediastinal mass of 9.5 by 7.5 by 11.5 cm (with heterogeneous structure, central calcifications with a mild displacement of the thoracic aorta, pulmonary artery and trachea). CT-guided biopsy confirmed an EMT. Additionally, neck ultrasound showed a dominant thyroid nodule at the left lobe. FNA showed no malignancy. The initial hormonal panel was normal for the thyroid profile, but high serum calcium (of 3.11 mmol/L; normal ranges between 2.2 and 2.55 mmol/L) and PTH (of 152 pg/mL; normal ranges between 10 and 65 pg/mL) represented the biological confirmation of a primary hyperparathyroidism. The second imagery procedure, namely, 99m-Tc pertechnetate scintigraphy, identified the tracer uptake at the eutopic thyroid and the EMT. 99m-Tc sestamibi scintigraphy with SPECT-CT scan showed a right inferior parathyroid tumor and another ectopic parathyroid tumor (on the left side of the upper third of mediastinum). The complex surgical procedure included resection of an EMT with total thyroidectomy, selective parathyroidectomy and thymectomy via mediastinal and cervical exploration (partial sternotomy and Kocher cervicotomy). Post-operatory confirmation of benign features included a normal thyroid gland, colloid goiter with cystic transformation in a giant EMT plus the two parathyroid adenomas. Post-operatory PTH remained normal after the first day. Iatrogenic hypothyroidism required levothyroxine replacement. A good outcome was registered after 3 months of follow-up. Most probably, a similar embryological source might explain the co-presence of these two types of ectopic tissues, albeit this would be exceptional [115].
3.5. Limitations and Further Expansion
Our research is a non-systematic review across a single database; however, this is the largest sample-based analysis of published data when compared to prior publications. The level of statistical evidence and the heterogeneous spectrum of provided data varied, and we did not intend to limit the inclusion of interesting cases with various presentations over the years.
The topic of disorders involving thyroid morphogenesis poses some issues that are still a matter of debate in the field of EMTs such as the rate of malignant transformation in long-standing EMTs; the risk of further EMT enlargement and hemorrhage as well as the expected annual rate of growth; the importance of conducting a pre-operatory histological and/or cytological report; the best imagery option and the optimum interventional strategy as well as the selection criteria for surgery candidates. Moreover, we used the original terms regarding the pathological report, which is very important in malignant EMTs, but changes of nomenclature and classifications were registered amid this search of more than three decades [216,217,218,219,220]. Another topic that we anticipate will gain increasing interest is represented by the molecular and genetic exploration in subjects confirmed with EMTs. In our analysis, SQSTM1-NTRK3 chromosomal rearrangement was identified in one case [58], and another patient with anaplastic carcinoma harbored a BRAF pathogenic variant [37]. As mentioned, a single pediatric case was found [68], but generally, the connection between the endocrine disruptors in terms of environmental factors (such as the geographic influence, the air pollutions, etc.) or internal factors like infectious conditions (as recently shown by the COVID-19 pandemic) or autoimmune (namely, endocrine and non-endocrine autoimmunity) issues should be studied in relationship with the developmental conditions, specifically, the ectopic thyroid (either acting during pregnancy or early during childhood); yet this represents a future topic to be studied [221,222,223,224,225,226]. Further on, the exact impact on the overall thyroid cancer-associated burden with respect to ectopic thyroid-related malignancy represents an open issue according to the current level of statistical evidence; we expect a future expansion amid increased awareness and access to advanced imagery tools and performant surgical access with an overall good long-term outcome in ectopic thyroid cases [227].
4. Conclusions
EMT stands for one of the most fascinating and challenging medical and surgical entities; a multidisciplinary team is mandatory. The sample-based analysis identified 117 patients with EMTs, and 18.8% of them displayed a malignancy in the EMT. The most common histological type was papillary thyroid cancer. While most cases had a good outcome, emergency admission due to local compressive effects needs a life-saving intervention; on the other hand, a very aggressive fulminant evolution has been reported in a small number of patients with follicular-cell-derived carcinoma. One of the most striking aspects in analyzing EMTs involves a wide area of imaging procedures but mostly of biopsies/FNA and surgical interventions. Despite the lack of a standardized approach, the customized management may be synthetized in a 10-item algorithm as we mentioned. CT scan remains the major imagery element, and iodine or 99m-Tc scintigraphy represents the most practical one once the ectopic intra-thoracic tissue is suspected. An endocrine panel is required at any point from baseline to long-term follow-up. The selection of biopsy or FNA methods varies depending on EMT profile, co-morbidities and available procedures. A skillful surgical team for EMT removal with or without thyroidectomy offers a very good prognostic in majority of EMT patients.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16101868/s1, Figure S1: Qualitative perspective of the malignancies in EMT patients (red = cancer originating from the follicular thyroid cell at any differentiation level; white = benign thyroid tissue; yellow = non-thyroid type of cancer): 1 = subjects with primary cancer within the eutopic thyroid and benign EMT [22,35,123]; 2 = subjects with thyroid cancer in EMT and cervical thyroid (distinct foci) [36,42,55]; 3 = cancer in EMT, not in cervical thyroid [37,41,52,55,58,60,61,62,64,66,69,70,71,72]; 4 = malignancy in EMT with metastasis in cervical thyroid [55]; 5 = prior or concurrent non-thyroid malignancies at the moment of EMT identification in terms of originating from lung [11,49,101], breast [29,92,125] or ovary [89].
Author Contributions
Conceptualization, M.C., M.-L.C., O.-C.S., A.C., O.P.-V., M.S., F.L.P. and C.N.; methodology, M.C., M.-L.C., A.C., O.P.-V., M.S., F.L.P. and C.N.; software, M.C., M.-L.C., O.-C.S., O.P.-V., M.S., F.L.P. and C.N.; validation, M.C., M.-L.C., O.-C.S., A.C., O.P.-V., F.L.P. and C.N.; formal analysis, M.C., M.-L.C., O.-C.S., A.C., O.P.-V., M.S. and C.N.; investigation, M.C., O.-C.S., A.C., O.P.-V., M.S., F.L.P. and C.N.; resources, M.C., M.-L.C., O.-C.S., A.C., M.S., F.L.P. and C.N.; data curation, M.C., M.-L.C., A.C., O.P.-V., M.S., F.L.P. and C.N.; writing—original draft preparation, M.C.; writing—review and editing, M.C., M.-L.C., O.-C.S., A.C., O.P.-V., M.S., F.L.P. and C.N.; visualization, M.C., M.-L.C., O.-C.S., A.C., O.P.-V., M.S. and C.N.; supervision, M.C., M.-L.C., A.C. and C.N.; project administration, M.C., M.-L.C., O.-C.S., A.C., O.P.-V., M.S., F.L.P. and C.N.; funding acquisition, M.C., M.-L.C., O.-C.S., A.C., O.P.-V., M.S., F.L.P. and C.N. All authors have read and agreed to the published version of the manuscript.
Funding
Publication of this paper was supported by the “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania through the institutional program “Publish not Perish” 2024.
Acknowledgments
Publication of this paper was supported by the “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania through the institutional program “Publish not Perish”.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| antiTPO | anti-thyroperoxidase antibodies |
| antiTg | anti-thyroglobulin antibodies |
| CT | computer tomography |
| CASTLE | carcinoma showing thymus-like differentiation |
| EBUS-TBFNA | endobronchial ultrasound-guided trans-bronchial needle aspiration |
| EMT | ectopic mediastinal thyroid |
| FNA | fine needle aspiration |
| HHEX | hematopoietically-expressed homeobox protein |
| MIBG | metaiodobenzylguanidine |
| MALT | mucosa-associated lymphoid tissue |
| MRI | magnetic resonance imagery |
| n | number of studies |
| N | number of patients |
| PAX | paired-box gene |
| RATS | robotic-assisted thoracoscopic surgery |
| SPECT-CT | Single-photon emission computed tomography |
| TTNB | transthoracic needle biopsy |
| TTF1 | thyroid transcription factor-1 |
| TSH | thyroid-stimulating hormone |
| Tc | Technetium |
| TRAb | TSH receptor antibody |
| VATS | video-assisted thoracic surgery |
References
- Khatami, F.; Larijani, B.; Nikfar, S.; Hasanzad, M.; Fendereski, K.; Tavangar, S.M. Personalized treatment options for thyroid cancer: Current perspectives. Pharmacogenom. Pers. Med. 2019, 12, 235–245. [Google Scholar] [CrossRef]
- San Juan, M.D.; Paz-Pacheco, E. Incidence, Recurrence and Mortality among Filipinos with Differentiated Thyroid Cancer: A Systematic Review. J. ASEAN Fed. Endocr. Soc. 2023, 38, 100–107. [Google Scholar] [CrossRef]
- Agosto Salgado, S.; Kaye, E.R.; Sargi, Z.; Chung, C.H.; Papaleontiou, M. Management of Advanced Thyroid Cancer: Overview, Advances, and Opportunities. Am. Soc. Clin. Oncol. Educ. Book 2023, 43, e389708. [Google Scholar] [CrossRef]
- Fu, G.; Guo, F.; Zhang, W.; Ruan, X.; Zheng, X.; Wang, Z.; Gao, M. Diagnosis and treatment of ectopic thyroid carcinoma: A case report and literature review. Front. Oncol. 2022, 12, 1072607. [Google Scholar] [CrossRef]
- Mitchell, A.L.; Gandhi, A.; Scott-Coombes, D.; Perros, P. Management of thyroid cancer: United Kingdom National Multidisciplinary Guidelines. J. Laryngol. Otol. 2016, 130, S150–S160. [Google Scholar] [CrossRef]
- Nisa, L.; Morrison, S.; Levi, E. Airway management in patients with lingual thyroid: A case report and review of the literature. Eur. Arch. Oto-Rhino-Laryngol. 2022, 279, 3289–3295. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; He, Q.; Li, L.; Ji, F.; Ding, Y.; Sun, Q.; Qiu, X. The clinicopathological features, treatment outcomes and follow-up results of 47 ectopic thyroid gland cases: A single-center retrospective study. Front. Endocrinol. 2023, 14, 1278734. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hayati, F.; Azizan, N.; Sharif, S.Z. Ectopic Papillary Thyroid Carcinoma of the Posterior Pharynx. Iran. J. Otorhinolaryngol. 2023, 35, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Motlaghzadeh, Y.; Nesbit, S.; Guo, H.H.; Yang, E.; Desai, K.; Lui, N.S. Surgical resection of mediastinal ectopic thyroid tissue: A case series. J. Thorac. Dis. 2023, 15, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Pupić-Bakrač, J.; Pupić-Bakrač, A.; Novaković, J.; Skitarelić, N. Congenital Neck Masses. J. Craniofac. Surg. 2021, 32, 1417–1420. [Google Scholar] [CrossRef]
- Tsai, A.; Rafferty, W.; Ren, S. Mediastinal ectopic thyroid tissue, an imitator of an enlarged lymph node with metastatic pulmonary neoplasia. Diagn. Cytopathol. 2021, 49, E471–E474. [Google Scholar] [CrossRef] [PubMed]
- Mak, C.-W. Submental ectopic thyroid in a patient with an orthotopic thyroid gland. J. Med. Ultrasound 2022, 30, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Kolwalkar, J.; Samant, D.; Borkar, S.; Vidyasagar, M.S.C.; Vaggar, J.N. Ectopic colloid goiter in mediastinum with normal thyroid gland. J. Cardiothorac. Surg. 2024, 19, 103. [Google Scholar] [CrossRef] [PubMed]
- Nettore, I.C.; Cacace, V.; De Fusco, C.; Colao, A.; Macchia, P.E. The molecular causes of thyroid dysgenesis: A systematic review. J. Endocrinol. Investig. 2013, 36, 654–664. [Google Scholar] [CrossRef]
- Kostopoulou, E.; Miliordos, K.; Spiliotis, B. Genetics of primary congenital hypothyroidism—A review. Hormones 2021, 20, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Mio, C.; Grani, G.; Durante, C.; Damante, G. Molecular defects in thyroid dysgenesis. Clin. Genet. 2019, 97, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.D.; Khandeparkar, S.G.S.; Gulati, H.K.; Naik, C.S. Microfollicular adenoma of ectopic thyroid gland masquerading as salivary gland tumor—A diagnostic and therapeutic challenge: A case report. J. Med. Case Rep. 2014, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Düzer, S.; Akyiğit, A.; Sakallıoğlu, Ö.; Solmaz, Ö.A.; Polat, C. Ectopic thyroid tissue localized in submandibular region. Turk. J. Ear Nose Throat 2016, 26, 172–175. [Google Scholar] [CrossRef]
- Cassol, C.A.; Noria, D.; Asa, S.L. Ectopic Thyroid Tissue Within the Gall Bladder: Case Report and Brief Review of the Literature. Endocr. Pathol. 2010, 21, 263–265. [Google Scholar] [CrossRef]
- Kajaia, D.; Hager, B.; Kliebisch, S.; Weingärtner, K.; Seitz, G.; von Streitberg, U.; Seggewiß-Bernhardt, R.; Zugor, V. A rare case of renal ectopic thyroid tissue. Aktuelle Urol. 2020, 52, 64–66. [Google Scholar] [CrossRef]
- Mera, C.M.; Kreutzer, N.; Lorenzen, J.; Truß, M. A rare renal tumour: Ectopic thyroid tissue in the kidney. Urologe 2018, 57, 944–946. [Google Scholar] [CrossRef]
- Khan, S.; Waleed, M.S.; Verma, D.; Rahman, M. Ectopic Mediastinal and Lumbar Thyroid Tissue. Cureus 2021, 13, e18598. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.H.; Yap, Y.L.; Chin, H.H.; Sarina, S. A rare presentation of ectopic thyroid gland at right axilla. Med. J. Malays. 2021, 76, 600–602. [Google Scholar]
- Moon, A.; Kim, H.; Chang, K.; Do, S. Multifocal ectopic thyroid tissues including breast: A case report. Mol. Clin. Oncol. 2020, 12, 117–119. [Google Scholar] [CrossRef]
- Vaillant, P.-F.; Devalckeneer, A.; Csanyi-Bastien, M.; Ares, G.S.; Marks, C.; Mallea, M.; Cortet-Rudelli, C.; Maurage, C.-A.; Aboukaïs, R. An unusual ectopic thyroid tissue location & review of literature. Neurochirurgie 2023, 69, 101497. [Google Scholar] [CrossRef] [PubMed]
- De Falco, M.; Oliva, G.; Santangelo, G.; Del Giudice, S.; Parmeggiani, U. Thyroid ectopia: Problems in diagnosis and therapy. G. Chir. 2010, 31, 279–281. [Google Scholar] [PubMed]
- Sumiya, R.; Sekihara, K.; Sugimura, A.; Miyazaki, H.; Igari, T.; Ikeda, T.; Nagasaka, S. Ectopic intrapulmonary follicular adenoma diagnosed by surgical resection. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 564–567. [Google Scholar] [CrossRef]
- Liang, X.M.; Li, J.; Huang, Y.M.; Peng, S.; Wei, Z. Ectopic Pulmonary Goiter Simulating Malignant Tumor Detected on SPECT/CT. Clin. Nucl. Med. 2021, 46, e579–e581. [Google Scholar] [CrossRef] [PubMed]
- Chataigner, O.; Mussot, S.; Fadel, E.; Dartevelle, P.G. Two cases of intra-pericardial tumors arising from the ascending aorta in adults. Eur. J. Cardio-Thorac. Surg. 2007, 32, 174–175. [Google Scholar] [CrossRef][Green Version]
- Chan, L.P.; Chiang, F.Y.; Lee, K.W.; Kuo, W.R. Carcinoma Showing Thymus-Like Differentiation (CASTLE) of Thyroid: A Case Report and Literature Review. Kaohsiung J. Med. Sci. 2008, 24, 591–597. [Google Scholar] [CrossRef]
- Kola, E.; Musa, J.; Guy, A.; Kola, I.; Horjeti, E.; Filaj, V.; Alimehmeti, M. Ectopic Thyroid Papillary Carcinoma with Cervical Lymph Node Metastasis as the Initial Presentation, Accompanied by Benign Thyroid Gland. Med. Arch. 2021, 75, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Pierro, A.; Cilla, S.; Modugno, P.; Sallustio, G. Incidental Finding of Dual Ectopic Thyroid on Computed Tomography Angiography. J. Clin. Imaging Sci. 2017, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Kumar, M.; Thukral, A.; Biswas, D.; Jain, R.; Ghosh, S.; Mukhopadhyay, S.; Chowdhury, S. Medical Management of Thyroid Ectopia: Report of Three Cases. J. Clin. Res. Pediatr. Endocrinol. 2013, 5, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Sradha Patro, P.S.; Behera, K.K. Graves’ disease in eutopic thyroid with ectopic mediastinal thyroid tissue: Role of single photon emission computed tomography-computed tomography. Indian J. Nucl. Med. 2019, 34, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Kesici, U.; Koral, Ö.; Karyağar, S.; Kesici, S.; Yılbaş, A.; Karyağar, S.; Mataracı, E.; Mataracı, İ. Missed retrosternal ectopic thyroid tissue in a patient operated for multinodular goiter. Turk. J. Surg. 2016, 32, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Piciu, D.; Piciu, A.; Irimie, A. Papillary Thyroid Microcarcinoma and Ectopic Papillary Thyroid Carcinoma in Mediastinum: A case report. Clin. Nucl. Med. 2012, 37, 214–215. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Htun, N.N.; Wang, B.; Lee, B.; Johnson, C. An Anaplastic Thyroid Carcinoma of the Giant-Cell Type from a Mediastinal Ectopic Thyroid Gland. Diagnostics 2023, 13, 2941. [Google Scholar] [CrossRef]
- Lianos, G.; Bali, C.; Tatsis, V.; Anastasiadi, Z.; Lianou, E.; Papathanasiou, V.; Messinis, T. Ectopic thyroid carcinoma. Case report. G. Chir. 2013, 34, 114–116. [Google Scholar]
- Iglauer, E.; Ernst, H. Use of radioiodine for exclusion of aberrant thyroid tissue in the differential diagnosis of mediastinal tumors. Dtsch. Gesundheitsw. 1958, 13, 1024–1025. [Google Scholar]
- Mark, J.B. Ectopic Mediastinal Thyroid: Features in Diagnosis and Factors in Treatment. Dis. Chest 1964, 45, 412–415. [Google Scholar] [CrossRef]
- Mishriki, Y.Y.; Lane, B.P.; Lozowski, M.S.; Epstein, H. Hurthle-cell tumor arising in the mediastinal ectopic thyroid and diagnosed by fine needle aspiration. Light microscopic and ultrastructural features. Acta Cytol. 1983, 27, 188–192. [Google Scholar]
- Caroço, T.V.; Saraiva, R.P.; Baião, J.M.; Nogueira, T.; Garcia, A.L.; Costa Almeida, C.E. Mediastinal papillary thyroid carcinoma treated by video-assisted thoracic surgery—Case report. Int. J. Surg. Case Rep. 2023, 106, 108140. [Google Scholar] [CrossRef]
- Rashidfarokhi, M.; Gupta, J.; Leytin, A.; Epelbaum, O. Ectopic Anterior Mediastinal Pathology in the Chest: Radiologic-pathologic Correlation of Unexpected Encounters with the “Terrible Ts”. J. Clin. Imaging Sci. 2016, 6, 49. [Google Scholar] [CrossRef]
- Paradies, G.; Zullino, F.; Orofino, A.; Leggio, S. Mediastinal teratomas in children. Case reports and review of the literature. Ann. Ital. Chir. 2013, 84, 395–403. [Google Scholar]
- Sunam, G.S.; Öncel, M.; Ceran, S.; Ödev, K.; Yıldıran, H. Giant Benign Mediastinal Masses Extending into the Pleural Cavity. Surg. J. 2016, 2, e46–e50. [Google Scholar] [CrossRef][Green Version]
- Kumar, A.; Kumar, S.; Kushwaha, J.; Raj, V.; Mishra, A. Unusual Anterior Mediastinal Tumors Treated at a Tertiary Thoracic Center: A Case Series Analysis. Cureus 2021, 13, e17625. [Google Scholar] [CrossRef]
- Mitsuboshi, S.; Maeda, H.; Aoshima, H.; Isaka, T.; Matsumoto, T.; Onizuka, H.; Kanzaki, M. Thoracoscopic surgical case of an ectopic mediastinal parathyroid adenoma detected by chance: A case report. BMC Surg. 2019, 19, 171. [Google Scholar] [CrossRef]
- Aziz, J.; Murthy, R. Mediastinal ectopic thyroid tissue: A rare but important diagnosis. J. Thorac. Dis. 2023, 15, 6388–6389. [Google Scholar] [CrossRef] [PubMed]
- Vuorisalo, A.; Tommola, E.; Eloranta, P.; Vanhelo, T.; Paavonen, T.; Kholová, I. Ectopic thyroid in EBUS: Experience from a quality assurance programme. APMIS 2023, 131, 217–225. [Google Scholar] [CrossRef]
- Ozturk, A.; Cicek, T.; Aktas, Z.; Demirag, F.; Yilmaz, A. Mediastinal ectopic thyroid diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration: Report of three cases. J. Clin. Ultrasound 2018, 46, 299–301. [Google Scholar] [CrossRef]
- Di Crescenzo, V.; Vitale, M.; Valvano, L.; Napolitano, F.; Vatrella, A.; Zeppa, P.; De Rosa, G.; Amato, B.; Laperuta, P. Surgical management of cervico-mediastinal goiters: Our experience and review of the literature. Int. J. Surg. 2016, 28 (Suppl. S1), S47–S53. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, G.; Pellino, G.; De Falco, N.; Colella, G.; D’Amato, S.; Maglione, M.G.; De Luca, R.; Canonico, S.; De Falco, M. Prevalence, diagnosis and management of ectopic thyroid glands. Int. J. Surg. 2016, 28 (Suppl. S1), S1–S6. [Google Scholar] [CrossRef]
- Coskun, A.; Yildirim, M.; Erkan, N. Substernal Goiter: When is a Sternotomy Required? Int. Surg. 2014, 99, 419–425. [Google Scholar] [CrossRef]
- Kozol, R.A.; Geelhoed, G.W.; Flynn, S.D.; Kinder, B. Management of ectopic thyroid nodules. Surgery 1993, 114, 1103. [Google Scholar]
- Toda, S.; Iwasaki, H.; Suganuma, N.; Okubo, Y.; Hayashi, H.; Masudo, K.; Nakayama, H.; Masuda, M. Occult Thyroid Carcinoma without Malignant Thyroid Gland Findings during Preoperative Examination: Report of Three Cases. Case Rep. Endocrinol. 2020, 2020, 4249067. [Google Scholar] [CrossRef] [PubMed]
- Reidelberger, K.; Fingeret, A. Management of Incidentalomas. Surg. Clin. N. Am. 2021, 101, 1081–1096. [Google Scholar] [CrossRef]
- Chooi, J.E.; Ravindiran, A.; Balasubramanian, S.P. The influence of incidental detection of thyroid nodule on thyroid cancer risk and prognosis—A systematic review. Clin. Endocrinol. 2022, 96, 246–254. [Google Scholar] [CrossRef]
- Khthir, R.; Binegar, N.B. Primary Intrathoracic Ectopic Papillary Thyroid Carcinoma, Presenting With Thoracic Spine Metastasis: A Case Presentation and Literature Review. Cureus 2024, 16, e55329. [Google Scholar] [CrossRef] [PubMed]
- Uchida, N.; Yoshida, M. Mucosa-associated lymphoid tissue (MALT) lymphoma developing in ectopic mediastinal thyroid tissue: A case report. Surg. Case Rep. 2020, 6, 97. [Google Scholar] [CrossRef]
- Karim, F.; Inam, H.; Choudry, U.K.; Hasnain Fatimi, S. Ectopic follicular variant of papillary thyroid carcinoma in anterior mediastinum with a normal thyroid gland. A case report. Int. J. Surg. Case Rep. 2018, 51, 213–217. [Google Scholar] [CrossRef]
- Vázquez, O.R.; Silva, F.; Acosta-Pumarejo, E.; Marín, M.L. Ectopic Papillary Thyroid Cancer with Distant Metastasis. Case Rep. Endocrinol. 2018, 2018, 8956712. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, M.; Xu, L. Ectopic thyroid cancer diagnosed by endobronchial ultrasound-guided transbronchial needle aspiration. Thorac. Cancer 2017, 8, 703–705. [Google Scholar] [CrossRef]
- Wu, F.; Qu, L.; Li, D.Q.; Hu, C.H. Primary mediastinal large B-cell lymphoma arising from thyroid in a renal recipient with Hashimoto’s thyroiditis. Int. J. Clin. Exp. Pathol. 2015, 8, 5944–5946. [Google Scholar]
- Shafiee, S.; Sadrizade, A.; Jafarian, A.; Zakavi, S.R.; Ayati, N. Ectopic papillary thyroid carcinoma in the mediastinum without any tumoral involvement in the thyroid gland. A Case report. Asia Ocean J. Nucl Med. Biol. 2013, 1, 44–46. [Google Scholar] [CrossRef]
- Wang, X.-L.; Mu, Y.-M.; Dou, J.-T.; Zhong, W.-W.; Lü, Z.-H.; Lu, J.-M.; Pan, C.-Y. Medullar thyroid carcinoma in mediastinum initially presenting as Ectopic ACTH syndrome. A case report. Neuro Endocrinol. Lett. 2011, 32, 421–424. [Google Scholar] [PubMed]
- Camargos, E.F.; Pandolfi, M.B.; Toledo, M.A.V.; Quintas, J.L.; Moreira, S., Jr.; de Azevedo, A.E.B.; Tavares, A.C. A 95-year-old woman with leucocytosis and eosinophilia: Anaplastic carcinoma in an ectopic thyroid. BMJ Case Rep. 2010, 2010, 2823. [Google Scholar] [CrossRef] [PubMed]
- Demirag, F.; Cakir, E.; Aydin, E.; Kaya, S.; Akyurek, N. Ectopic Primary B Cell Lymphoma of the Thyroid Presenting as an Anterior Mediastinal Mass. A Case Report. Acta Chir. Belg. 2009, 109, 802–804. [Google Scholar] [CrossRef]
- Ranaldi, R.; Morichetti, D.; Goteri, G.; Martino, A. Immature teratoma of the mediastinum arising in ectopic thyroid tissue: A case report. Anal. Quant. Cytol. Histol. 2009, 31, 233–238. [Google Scholar]
- Shah, B.C.; Ravichand, C.S.; Juluri, S.; Agarwal, A.; Pramesh, C.S.; Mistry, R.C. Ectopic thyroid cancer. Ann. Thorac. Cardiovasc. Surg. 2007, 13, 122–124. [Google Scholar]
- Yoshino, M.; Mizobuchi, T.; Fujiwara, T.; Noro, M.; Akikusa, B.; Iwai, N. Large mediastinal cyst of an ectopic thyroid with small nodules diagnosed as papillary carcinoma. Jpn. J. Thorac. Cardiovasc. Surg. 2006, 54, 550–554. [Google Scholar] [CrossRef]
- Zagar, I.; Vidergar-Kralj, B.; Schwarzbartl-Pevec, A.A.; Pompe, F. Columnar cell thyroid carcinoma—Diagnostic dilemmas and pitfalls. Nucl. Med. Rev. Cent. East. Eur. 2003, 6, 155–158. [Google Scholar] [PubMed]
- Dominguez-Malagon, H.; Guerrero-Medrano, J.; Suster, S. Ectopic Poorly Differentiated (Insular) Carcinoma of the Thyroid: Report of a Case Presenting as an Anterior Mediastinal Mass. Am. J. Clin. Pathol. 1995, 104, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Kola, E.; Gjata, A.; Kola, I.; Guy, A.; Musa, J.; Biba, V.; Filaj, V.; Horjeti, E.; Nakuci, D.; Cobo, A.; et al. Ectopic thyroid tissue in the anterior mediastinum along with a normally located gland. Radiol. Case Rep. 2021, 16, 3191–3195. [Google Scholar] [CrossRef] [PubMed]
- Sadidi, H.; Mehri, A.; Rezaei, R. An accessory mediastinal thyroid: A case report. Clin. Case Rep. 2023, 11, e7232. [Google Scholar] [CrossRef] [PubMed]
- Nagireddy, T.V.; Vaidya, A.A.; Gupta, S.; Kshirsagar, P.; Kamble, T. A rare case of double ectopic thyroid in the superior mediastinum: A case report. J. Surg. Case Rep. 2023, 2023, rjad058. [Google Scholar] [CrossRef] [PubMed]
- Melinte, A.; Saftoiu, A.; Vlaicu-Melinte, A.; Dobritoiu, F.; Copaescu, C. Robotic Resection of Ectopic Thyroid Tissue of the Mediastinum—Case Report and Literature Review. Chirurgia 2023, 118, 96–102. [Google Scholar] [CrossRef]
- Arjun, P.; Palangadan, S.; Abraham, R.; Haque, A.; Ramachandran, R. An Unusual Posterior Mediastinal Mass—Ectopic Thyroid within the Oesophagus. J. Assoc. Physicians India 2021, 69, 11–12. [Google Scholar]
- El Haj, N.I.; Hafidi, S.; Khoaja, A.; Boubia, S.; Karkouri, M.; Ridai, M. Ectopic mediastinal thyroid removed by U-VATS approach. A case report. Int. J. Surg. Case Rep. 2021, 78, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.L.; Yang, J.; Xie, X.; Han, D. Ectopic thyroid tissue: An unusual cause of intracardiac mass. Echocardiography 2021, 38, 500–503. [Google Scholar] [CrossRef]
- Imai, T.; Morishita, Y.; Ito, S.; Saijo, S.; Asada, Y. A Case of Ectopic Thyroid Presenting as a Superior Mediastinal Mass. Cureus 2020, 12, e9541. [Google Scholar] [CrossRef]
- Chen Cardenas, S.M.; Duan, D.; Rooper, L.M.; Santhanam, P.; Cooper, D.S.; Ladenson, P.W. Misdiagnosis of Paraganglioma by 123I-mIBG Without Stable Iodine Blockade of Thyroidal Radioiodine Uptake. J. Endocr. Soc. 2020, 4, bvaa099. [Google Scholar] [CrossRef]
- Kocaman, G.; Yenigün, B.M.; Çoruh, A.G.; Koçak, E.M.; Memmedyarow, İ.; Tural, M.; Sak, S.D.; Yazıcıoğlu, L.; Akal, R.M. Intrapericardial goiter. Gen. Thorac. Cardiovasc. Surg. 2020, 68, 1051–1054. [Google Scholar] [CrossRef]
- Sato, D.; Hayashi, S.; Sakata, S.; Kawachi, R.; Shimamura, M.; Sakurai, H. Intrapericardial Ectopic Goiter: A Very Unusual Presentation. Ann. Thorac. Cardiovasc. Surg. 2022, 28, 72–74. [Google Scholar] [CrossRef]
- Carannante, F.; Frasca, L.; Depalma, M.; Longo, F.; Crucitti, P. Ectopic thoracic thyroid removed by uniportal VATS approach. A case report. Int. J. Surg. Case Rep. 2019, 61, 111–114. [Google Scholar] [CrossRef]
- Regal, M.; Kamel, M.M.; Alyami, H.; Al-Osail, E.M. Mediastinal ectopic thyroid mass with normal thyroid function and location: Case report. Int. J. Surg. Case Rep. 2018, 52, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, K.; Bhandari, T.; Banait-Deshmane, S.; Crowe, D.R.; Sonavane, S.K. Incidentally detected ectopic thyroid in juxta cardiac location—Imaging and pathology. Radiol. Case Rep. 2018, 13, 909–913. [Google Scholar] [CrossRef]
- Raji, Y.; Gupta, S.; Pucar, D.; Keshavamurthy, J.H. Ectopic thyroid: The great mimicker. Lung India 2018, 35, 248–250. [Google Scholar] [CrossRef]
- Metere, A.; De Giacomo, T.; Vergine, M.; Biffoni, M.; Giacomelli, L. Diagnosis and management of a mediastinal ectopic thyroid laying on the right bronchus: Case report and review of literature. BMC Surg. 2018, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Robitaille, C.; Ofiara, L.; Auger, M. Off the beaten path: A case of mediastinal ectopic thyroid tissue. Diagn. Cytopathol. 2018, 46, 53–55. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, W.; Wang, X.; Gao, Y.; Xu, H. Ectopic thyroid mass in the right ventricle: A case report. Echocardiography 2017, 34, 1096–1098. [Google Scholar] [CrossRef]
- Hardy, J.; Jeyabalan, A.R.; Bhatt, N.; Medford, A.R. An Unusual Mediastinal Mass. J. R. Coll. Physicians Edinb. 2016, 46, 93–95. [Google Scholar] [CrossRef]
- Abdel Aal, M.; Scheer, F.; Andresen, R. Ectopic Mediastinal Thyroid Tissue with a Normally Located Thyroid Gland. Iran. J. Radiol. 2015, 12, e7054. [Google Scholar] [CrossRef]
- Wang, J.; Fang, J. Ectopic thyroid mass in the left lateral neck and anterior mediastinum: A case report. J. Med. Case Rep. 2014, 8, 351. [Google Scholar] [CrossRef]
- Scognamillo, F.; Attene, F.; Paliogiannis, P.; Ruggiu, M.W.; Cossu, A.; Trignano, M. Is sternotomy always necessary for the treatment of mediastinal ectopic thyroid goiter? Ann. Ital. Chir. 2014, 85, 304–307. [Google Scholar]
- Roh, E.; Hong, E.S.; Ahn, H.Y.; Park, S.-Y.; Yoon, H.I.; Park, K.S.; Park, Y.J. A case of mediastinal ectopic thyroid presenting with a paratracheal mass. Korean J. Intern. Med. 2013, 28, 361–364. [Google Scholar] [CrossRef]
- Walz, P.C.; Iwenofu, O.H.; Essig, G.F. Ectopic mediastinal goiter successfully managed via cervical approach: Case report and review of the literature. Head Neck 2013, 35, E94–E97. [Google Scholar] [CrossRef]
- Thuillier, F.; Venot, J. Ectopic thyroid tissue in the anterior mediastinum with a normally located gland: A case report. Ann. Endocrinol. 2012, 73, 34–36. [Google Scholar] [CrossRef]
- Mace, A.D.; Taghi, A.; Khalil, S.; Sandison, A. Ectopic Sequestered Thyroid Tissue: An Unusual Cause of a Mediastinal Mass. ISRN Surg. 2011, 2011, 313626. [Google Scholar] [CrossRef]
- Kumaresan, K.; Rao, N.S.; Mohan, A.R.; Rao, M.S. Autonomously functioning nodule arising from accessory mediastinal thyroid tissue. Indian J. Nucl. Med. 2011, 26, 153–154. [Google Scholar] [CrossRef]
- Pilavaki, M.; Kostopoulos, G.; Asimaki, A.; Papachristodoulou, A.; Papaemanouil, S.; Palladas, P. Imaging of ectopic intrathoracic multinodular goiter with pathologic correlation: A case report. Cases J. 2009, 2, 8554. [Google Scholar] [CrossRef]
- Topcu, S.; Liman, S.T.; Elicora, A.; Zanuzi, F.; Isgoren, S.; Filinte, D. A difficult case: Ectopic thyroid, bronchial anomalies, and incidentaloma in a patient with lung carcinoma. J. Thorac. Cardiovasc. Surg. 2009, 138, 231–233. [Google Scholar] [CrossRef][Green Version]
- Guimarães, M.J.; Valente, C.M.; Santos, L.; Baganha, M.F. Ectopic thyroid in the anterior mediastinum. J. Bras. Pneumol. 2009, 35, 383–387. [Google Scholar] [CrossRef]
- Karapolat, S.; Bulut, I. Ectopic posterior mediastinal thyroid: A case report. Cases J. 2008, 1, 53. [Google Scholar] [CrossRef]
- Sakorafas, G.H.; Vlachos, A.; Tolumis, G.; Kassaras, G.A.; Anagnostopoulos, G.K.; Gorgogiannis, D. Ectopic intrathoracic thyroid: Case report. Mt. Sinai J. Med. 2004, 71, 131–133. [Google Scholar]
- Van Schil, P.; Vanmaele, R.; Ehlinger, P.; Schoofs, E.; Goovaerts, G. Primary intrathoracic goitre. Acta Chir. Belg. 1989, 89, 206–208. [Google Scholar]
- Arriaga, M.A.; Myers, E.N. Ectopic Thyroid in the Retroesophageal Superior Mediastinum. Otolaryngol. Head Neck Surg. 1988, 99, 338–340. [Google Scholar] [CrossRef]
- Asp, A.; Hasbargen, J.; Blue, P.; Kidd, G.S. Ectopic Thyroid Tissue on Thallium/ Technetium Parathyroid Scan. Arch. Intern. Med. 1987, 147, 595–596. [Google Scholar] [CrossRef]
- Parida, P.K.; Herkel, K.; Preetam, C.; Pradhan, P.; Samal, D.K.; Sarkar, S. Management of Lingual Thyroid with Second Thyroid Anomaly: An Institutional Experience. Indian J. Otolaryngol. Head Neck Surg. 2022, 74, 5329–5337. [Google Scholar] [CrossRef]
- Mikosch, P.; Weixlbaumer, V.; Irrgang, M.; Aistleitner, A.; Trifina-Mikosch, E. Hemiagenesis of the thyroid gland detected by coincidence—What is the clinical relevance?: Case report and review of the literature. Wien. Med. Wochenschr. 2020, 170, 403–409. [Google Scholar] [CrossRef]
- Barbieri, A.; Prasad, M.L.; Gilani, S.M. Thyroid tissue outside the thyroid gland: Differential diagnosis and associated diagnostic challenges. Ann. Diagn. Pathol. 2020, 48, 151584. [Google Scholar] [CrossRef]
- Szczepanek-Parulska, E.; Hernik, A.; Ruchała, M. Thyroid ectopy—Diagnostic and therapeutic challenges before and in the era of TSH neonatal screening. Endokrynol. Polska 2017, 68, 708–721. [Google Scholar] [CrossRef]
- Nilsson, M.; Fagman, H. Development of the thyroid gland. Development 2017, 144, 2123–2140. [Google Scholar] [CrossRef]
- Abu-Khudir, R.; Larrivée-Vanier, S.; Wasserman, J.D.; Deladoëy, J. Disorders of thyroid morphogenesis. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 143–159. [Google Scholar] [CrossRef]
- Park, E.S.; Yoon, J.Y. Factors associated with permanent hypothyroidism in infants with congenital hypothyroidism. BMC Pediatr. 2019, 19, 453. [Google Scholar] [CrossRef]
- Muzurović, E.; Smolović, B.; Miladinović, M.; Muhović, D.; Čampar, B. Diagnosis and treatment of mediastinal ectopic thyroid tissue with normally located thyroid gland and primary hyperparathyroidism: A case report. Gland. Surg. 2021, 10, 1532–1541. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, V.; Ponnusamy, M. Retrosternal ectopic thyroid mimicking esophagus in Tc-99m pertechnetate thyroid scan. Indian J. Nucl. Med. 2019, 34, 351–352. [Google Scholar] [CrossRef]
- Sohail, A.A.; Shahabuddin, S.; Siddiqui, M.I. Ectopic Thyroid Mass Separately Present in Mediastinum and Not a Retrosternal Extension: A Report of Two Cases. Case Rep. Surg. 2019, 2019, 3821767. [Google Scholar] [CrossRef]
- Hummel, J.; Wachsmann, J.; Carrick, K.; Oz, O.K.; Mathews, D.; Peng, F. Ectopic Thyroid Tissue in the Mediastinum Characterized by Histology and Functional Imaging with I-123 SPECT/CT. Case Rep. Radiol. 2017, 2017, 9084207. [Google Scholar] [CrossRef]
- Wang, L.-J.; Liu, C.-M.; Chen, X.; Zhang, L.; Zhou, H.-W. An intracardiac accessory thyroid gland mimicking cardiac tumor: A case report and literature review. Medicine 2017, 96, e9465. [Google Scholar] [CrossRef]
- Patel, K.M.; Parsons, C.C. Forgotten goiter: Diagnosis and management. A case report and literature review. Int. J. Surg. Case Rep. 2016, 27, 192–194. [Google Scholar] [CrossRef]
- Serim, B.D.; Korkmaz, U.; Can, U.; Altun, G.D. Intrathoracic toxic thyroid nodule causing hyperthyroidism with a multinodular normal functional cervical thyroid gland. Indian J. Nucl. Med. 2016, 31, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Cunha, F.M.; Rodrigues, E.; Oliveira, J.; Saavedra, A.; Vinhas, L.S.; Carvalho, D. Graves’ disease in a mediastinal mass presenting after total thyroidectomy for nontoxic multinodular goiter: A case report. J. Med. Case Rep. 2016, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Kamaleshwaran, K.K.; Rajan, F.; Asokumar, P.; Mohanan, V.; Shinto, A.S. Mediastinal ectopic benign colloid goitre detected using iodine-131 whole body scintigraphy and single-photon emission computed tomography-computed tomography. Indian J. Nucl. Med. 2015, 30, 180–182. [Google Scholar] [CrossRef]
- Lee, W.S.; Kim, Y.H.; Chee, H.K.; Hwang, J.J.; Lee, S.A.; Lee, J.H.; Kim, H.Y. A 7.35.33.5-cm heterotopic thyroid in the posterior mediastinum in a patient with situs inversus totalis. J. Thorac. Dis. 2014, 6, E39–E42. [Google Scholar] [CrossRef]
- Barker, T.A.; Daultrey, C.R.; Trotter, S.E.; Kalkat, M. Intrathymic Primary Intrathoracic Goiter in a Patient With Breast Malignancy. Ann. Thorac. Surg. 2012, 93, e35–e36. [Google Scholar] [CrossRef] [PubMed]
- Demirhan, R.; Onan, B.; Oz, K.; Keser, S.H.; Gul, A.E.; Onan, I.S. Posterior Mediastinal Ectopic Thyroid: An Unusual Cause for Dysphagia. Ann. Thorac. Surg. 2009, 88, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Bodner, J.; Fish, J.; Lottersberger, A.C.; Wetscher, G.; Schmid, T. Robotic Resection of an Ectopic Goiter in the Mediastinum. Surg. Laparosc. Endosc. Percutaneous Tech. 2005, 15, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Bodner, J.; Lottersberger, C.A.; Kirchmayr, W.; Schmid, T. Ectopic mediastinal thyroid adenoma. Eur. J. Cardio-Thorac. Surg. 2004, 26, 211–212. [Google Scholar] [CrossRef] [PubMed]
- Gamblin, T.C.; Jennings, G.R.; Christie, D.B., 3rd; Thompson, W.M., Jr.; Dalton, M.L. Ectopic thyroid. Ann. Thorac. Surg. 2003, 75, 1952–1953. [Google Scholar] [CrossRef]
- Basaria, S.; Cooper, D.S. Graves’ Disease and Recurrent Ectopic Thyroid Tissue. Thyroid 1999, 9, 1261–1264. [Google Scholar] [CrossRef]
- Stoupa, A.; Kariyawasam, D.; Nguyen Quoc, A.; Polak, M.; Carré, A. Approach to the Patient With Congenital Hypothyroidism. J. Clin. Endocrinol. Metab. 2022, 107, 3418–3427. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.; Cannupp, A.; Myers, J.; Jnah, A.J. Congenital Hypothyroidism. Neonatal Netw. 2021, 40, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Stępniewska, A.; Wójcik, M.; Starzyk, J.B. Congenital hypothyroidism due to thyroid ectopy not detected in neonatal screening—Case report. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 53–56. [Google Scholar] [CrossRef]
- Stoupa, A.; Kariyawasam, D.; Carré, A.; Polak, M. Update of Thyroid Developmental Genes. Endocrinol. Metab. Clin. N. Am. 2016, 45, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Stoupa, A.; Kariyawasam, D.; Polak, M.; Carré, A. Genetics of congenital hypothyroidism: Modern concepts. Pediatr. Investig. 2022, 6, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Acar, S.; Gürsoy, S.; Arslan, G.; Nalbantoğlu, Ö.; Hazan, F.; Köprülü, Ö.; Özkaya, B.; Özkan, B. Screening of 23 candidate genes by next-generation sequencing of patients with permanent congenital hypothyroidism: Novel variants in TG, TSHR, DUOX2, FOXE1, and SLC26A7. J. Endocrinol. Investig. 2022, 45, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Kizys, M.M.L.; Louzada, R.A.; Mitne-Neto, M.; Jara, J.R.; Furuzawa, G.K.; de Carvalho, D.P.; Dias-Da-Silva, M.R.; Nesi-França, S.; Dupuy, C.; Maciel, R.M.B. DUOX2 Mutations Are Associated With Congenital Hypothyroidism With Ectopic Thyroid Gland. J. Clin. Endocrinol. Metab. 2017, 102, 4060–4071. [Google Scholar] [CrossRef]
- Li, M.; Wang, X.; Wang, F.; Wang, F.; Zhao, D.; Liu, S. JAG1 Variants Confer Genetic Susceptibility to Thyroid Dysgenesis and Thyroid Dyshormonogenesis in 813 Congenital Hypothyroidism in China. Int. J. Gen. Med. 2024, 17, 885–894. [Google Scholar] [CrossRef] [PubMed]
- Chaker, L.; Cooper, D.S.; Walsh, J.P.; Peeters, R.P. Hyperthyroidism. Lancet 2024, 403, 768–780. [Google Scholar] [CrossRef]
- Petranović Ovčariček, P.; Görges, R.; Giovanella, L. Autoimmune Thyroid Diseases. Semin. Nucl. Med. 2024, 54, 219–236. [Google Scholar] [CrossRef]
- Lee, S.Y.; Pearce, E.N. Hyperthyroidism: A Review. JAMA 2023, 330, 1472–1483. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Meng, Z.; Yu, H.; Gao, Z.; Li, H.; Liu, N. A case report of 131I therapy for Graves’ disease patient with hemiagenesis. Medicine 2019, 98, e14606. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, L.; Dharia, R.; Massey, B. Ectopic Thyroid Tissue With Hashimoto’s Thyroiditis. WMJ 2016, 115, 47–48. [Google Scholar]
- Tan, H.M.; Nga, M.E.; Petersson, F. Ectopic Cervical Thyroid Tissue Affected by Fibrosing Hashimoto’s Thyroiditis Mimicking Multifocal Metastatic Papillary Thyroid Carcinoma—A Hard Lesson Learnt from an Unusual Case. Head Neck Pathol. 2021, 15, 328–333. [Google Scholar] [CrossRef]
- Pandey, P.; Dixit, A.; Bedi, S.; Kapoor, K.; Mahajan, N.C. Hashimoto’s thyroiditis in ectopic thyroid tissue: A diagnostic surprise. Cytopathology 2012, 23, 419–421. [Google Scholar] [CrossRef]
- Pederzoli, S.; Salviato, T.; Mattioli, F.; Di Massa, G.; Brigante, G. Chronic thyroiditis in lateral ectopic thyroid mimicking cervical metastasis of thyroid cancer. Endocrinol. Diabetes Metab. Case Rep. 2021, 2021, 21-0052. [Google Scholar] [CrossRef]
- Chopra, A.; Singh, Y.; Kaushal, M.; Taneja, A.; Kulshreshtha, B. Simultaneous Occurrence of Thyroiditis in Ectopic and Eutopic Thyroid Masquerading as Thyroglossal Cyst. J. Clin. Diagn. Res. 2017, 11, OD17–OD19. [Google Scholar] [CrossRef]
- Cakir, M.; Gonen, S.; Dikbas, O.; Ozturk, B. Thyroid Hemiagenesis with Graves’ Disease, Graves’ Ophthalmopathy and Multinodular Goiter. Intern. Med. 2009, 48, 1047–1049. [Google Scholar] [CrossRef]
- Meringolo, D.; Campi, I.; Costante, G. Severe Graves’ Orbitopathy occurring in a patient with thyroid hemiagenesis. Endocrine 2018, 62, 490–491. [Google Scholar] [CrossRef]
- Yao, W.; Peng, X.; Guan, Y.; Du, X.; Xia, C.; Liu, F. Thyroid Nodules: Emerging Trends in Detection and Visualization based on Citespace. Endocr. Metab. Immune Disord.—Drug Targets 2024, 24, 130–141. [Google Scholar] [CrossRef]
- Papini, E.; Crescenzi, A.; D’Amore, A.; Deandrea, M.; De Benedictis, A.; Frasoldati, A.; Garberoglio, R.; Guglielmi, R.; Lombardi, C.P.; Mauri, G.; et al. Italian Guidelines for the Management of Non-Functioning Benign and Locally Symptomatic Thyroid Nodules. Endocr. Metab. Immune Disord.—Drug Targets 2023, 23, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Borson-Chazot, F.; Borget, I.; Mathonnet, M.; Leenhardt, L. SFE-AFCE-SFMN 2022 consensus on the management of thyroid nodules: Epidemiology and challenges in the management of thyroid nodules. Ann. Endocrinol. 2022, 83, 378–379. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Shengelia-de Lange, N.; Einspieler, H.; Hacker, M.; Haug, A.; Kretschmer-Chott, E.; Karanikas, G. Value of follow-up diagnostic radioiodine scans in differentiated thyroid cancer. Endocr. Connect. 2024, 13, e240007. [Google Scholar] [CrossRef] [PubMed]
- Giovanella, L.; D’aurizio, F.; Algeciras-Schimnich, A.; Görges, R.; Petranovic Ovcaricek, P.; Tuttle, R.M.; Visser, W.E.; Verburg, F.A.; hsTg&TgAb Consensus Working Group. Thyroglobulin and thyroglobulin antibody: An updated clinical and laboratory expert consensus. Eur. J. Endocrinol. 2023, 189, R11–R27. [Google Scholar] [CrossRef] [PubMed]
- van Kinschot, C.M.; Peeters, R.P.; van den Berg, S.A.; Verburg, F.A.; van Noord, C.; van Ginhoven, T.M.; Visser, W.E. Thyroglobulin and thyroglobulin antibodies: Assay-dependent management consequences in patients with differentiated thyroid carcinoma. Clin. Chem. Lab. Med. 2022, 60, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ngiam, K.Y.; Parameswaran, R. Mediastinal parathyroid adenomas and their surgical implications. Ann. R. Coll. Surg. Engl. 2015, 97, 259–261. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Stanciu-Găvan, C.; Vasilescu, F.; Dumitru, A.V.; Ciuche, A. Attitude of the surgical approach in hyperparathyroidism: A retrospective study. Exp. Ther. Med. 2021, 22, 959. [Google Scholar] [CrossRef]
- Corsello, A.; Ramunno, V.; Locantore, P.; Pacini, G.; Rossi, E.D.; Torino, F.; Pontecorvi, A.; De Crea, C.; Paragliola, R.M.; Raffaelli, M.; et al. Medullary Thyroid Cancer with Ectopic Cushing’s Syndrome: A Case Report and Systematic Review of Detailed Cases from the Literature. Thyroid 2022, 32, 1281–1298. [Google Scholar] [CrossRef] [PubMed]
- Wagner-Bartak, N.A.; Baiomy, A.; Habra, M.A.; Mukhi, S.V.; Morani, A.C.; Korivi, B.R.; Waguespack, S.G.; Elsayes, K.M. Cushing Syndrome: Diagnostic Workup and Imaging Features, With Clinical and Pathologic Correlation. AJR Am. J. Roentgenol. 2017, 209, 19–32. [Google Scholar] [CrossRef]
- Ragnarsson, O.; Juhlin, C.C.; Torpy, D.J.; Falhammar, H. A clinical perspective on ectopic Cushing’s syndrome. Trends Endocrinol. Metab. 2023, 35, 347–360. [Google Scholar] [CrossRef]
- Nistor, C.-E.; Staden, R.-I.S.-V.; Dumitru, A.V.; Stanciu Găvan, C. A Screening Test for Early Diagnosis of Microcellular Bronchopulmonary Cancer-Pilot Study. J. Clin. Med. 2019, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Kim, J.H. Recent Updates on the Management of Adrenal Incidentalomas. Endocrinol. Metab. 2023, 38, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Glazer, D.I.; Mayo-Smith, W.W. Management of incidental adrenal masses: An update. Abdom. Radiol. 2019, 45, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Cardisciani, L.; Policardo, F.; Tralongo, P.; Fiorentino, V.; Rossi, E.D. What psammoma bodies can represent in the thyroid. What we recently learnt from a story of lack of evidence. Pathologica 2022, 114, 373–375. [Google Scholar] [CrossRef]
- Ferreira, L.B.; Gimba, E.; Vinagre, J.; Sobrinho-Simões, M.; Soares, P. Molecular Aspects of Thyroid Calcification. Int. J. Mol. Sci. 2020, 21, 7718. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Wang, Y.-X.J.; Feng, X.-L.; Zhao, F.; Zou, S.-M.; Hao, Y.-Z.; Ying, J.-M.; Zhou, C.-W. Ultrasound Findings of Papillary Thyroid Microcarcinoma: A Review of 113 Consecutive Cases with Histopathologic Correlation. Ultrasound Med. Biol. 2012, 38, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Bonfiglio, R.; Granaglia, A.; Giocondo, R.; Scimeca, M.; Bonanno, E. Molecular Aspects and Prognostic Significance of Microcalcifications in Human Pathology: A Narrative Review. Int. J. Mol. Sci. 2020, 22, 120. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.M., Jr.; Sharkey, E.; Kwatampora, L.; Ranzinger, M.; Von Holzen, U. Mediastinal Intrathymic Parathyroid Adenoma: A Case Report and Review of the Literature. Cureus 2023, 15, e42306. [Google Scholar] [CrossRef]
- Garberoglio, S.; Testori, O. Role of Nuclear Medicine in the Diagnosis of Benign Thyroid Diseases. In Imaging in Endocrine Disorders; Karger Publishers: Basel, Switzerland, 2016; Volume 45, pp. 24–36. [Google Scholar] [CrossRef]
- Triggiani, V.; Giagulli, V.A.; Licchelli, B.; Resta, F.; Fiore, G.; DE Pergola, G.; Sabbà, C.; Guastamacchia, E. Ectopic Thyroid Gland: Description of a Case and Review of the Literature. Endocr. Metab. Immune Disord.—Drug Targets 2013, 13, 275–281. [Google Scholar] [CrossRef]
- Ferreira, M.; Leal, C.T.d.S.; Ferreira, L.V.; Ezequiel, D.G.A.; Costa, M.B. Atypical Presentation of a Medullary Thyroid Carcinoma Producing Acth and Serotonin. Case Rep. Oncol. 2019, 12, 742–748. [Google Scholar] [CrossRef]
- Tabchouri, N.; Anil, Z.; Marques, F.; Michot, N.; Dumont, P.; Arnault, V.; De Calan, L. Morbidity of total thyroidectomy for substernal goiter: A series of 70 patients. J. Visc. Surg. 2018, 155, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Bains, L.; Bhatia, S.; Kaushik, R.; Jain, S.K.; Singh, C.B.; Mandal, S.; Kaur, D. Pre-sternal thyroid swellings: A case of rare aberrant site recurrence and review of literature. Thyroid. Res. 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Asif, M.; Khan, W.J.; Aslam, S.; Nadeem, I.; Hericks, A. Thyroid Cancer Presenting as Aspiration Pneumonia: A Tale of Retrosternal Goiters. Cureus 2023, 15, e35861. [Google Scholar] [CrossRef] [PubMed]
- Unlu, M.T.; Aygun, N.; Kostek, M.; Isgor, A.; Uludag, M. Substernal goiter: From definitions to treatment. Sisli Etfal Hastan. Tip Bul. 2022, 56, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tan, J.; Wu, B.; Wu, R.; Han, Y.; Wang, C.; Gao, Z.; Jiang, D.; Xia, X. Customizing cancer treatment at the nanoscale: A focus on anaplastic thyroid cancer therapy. J. Nanobiotechnol. 2023, 21, 374. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, V.; Kyrodimos, E.; Mastronikolis, N.; Asimakopoulos, A.D.; Papanastasiou, G.; Tsiambas, E.; Spyropoulou, D.; Katsinis, S.; Manoli, A.; Papouliakos, S.; et al. Anti-EGFR/BRAF-Tyrosine Kinase Inhibitors in Thyroid Carcinoma. Cancer Diagn. Progn. 2023, 3, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Scheffel, R.S.; Dora, J.M.; Maia, A.L. BRAF mutations in thyroid cancer. Curr. Opin. Oncol. 2022, 34, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Rovira, A.; Carroll, P.; Simo, R. Thyroid lymphoma. Curr. Opin. Otolaryngol. Head Neck Surg. 2023, 31, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Travaglino, A.; Pace, M.; Varricchio, S.; Insabato, L.; Giordano, C.; Picardi, M.; Pane, F.; Staibano, S.; Mascolo, M. Hashimoto Thyroiditis in Primary Thyroid Non-Hodgkin Lymphoma. Am. J. Clin. Pathol. 2020, 153, 156–164. [Google Scholar] [CrossRef]
- Mancuso, S.; Carlisi, M.; Napolitano, M.; Siragusa, S. Lymphomas and thyroid: Bridging the gap. Hematol. Oncol. 2018, 36, 519–524. [Google Scholar] [CrossRef]
- Stewart, B.D.; VandenBussche, C.J.; Leon, M.E. Benign lesions of the mediastinum: A review with emphasis on cytology and small biopsy specimens. Semin. Diagn. Pathol. 2020, 37, 199–210. [Google Scholar] [CrossRef]
- Papazian, M.R.; Dublin, J.C.; Patel, K.N.; Oweity, T.; Jacobson, A.S.; Brandler, T.C.; Givi, B. Repeat Fine-Needle Aspiration With Molecular Analysis in Management of Indeterminate Thyroid Nodules. Otolaryngol. Head Neck Surg. 2022, 168, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Bongiovanni, M.; Bellevicine, C.; Troncone, G.; Sykiotis, G.P. Approach to cytological indeterminate thyroid nodules. Gland. Surg. 2019, 8 (Suppl. S2), S98–S104. [Google Scholar] [CrossRef] [PubMed]
- Belovarac, B.; Zhou, F.; Sharma, J.; Brandler, T.C. Indeterminate Thyroid Nodules and Advances in Molecular Pathology. Semin. Diagn. Pathol. 2023, 40, 349–352. [Google Scholar] [CrossRef]
- Sanker, V.; Mohamed, A.; Pranala, M.; Tharakan, V. A Unique Presentation of Ectopic Thyroid Tissue: Case Report and Management Principles. Cureus 2022, 14, e28717. [Google Scholar] [CrossRef]
- Anikin, V.; Welman, K.; Asadi, N.; Dalal, P.; Reshetov, I.; Beddow, E. Retrosternal goiter in thoracic surgical practice. Khirurgiia 2021, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Brcic, L.; Roden, A.C. Histopathological features of giant mediastinal tumors—A literature review. Mediastinum 2023, 7, 37. [Google Scholar] [CrossRef]
- Ciuche, A.; Nistor, C.; Motaş, C.; Horvat, T. Minimally invasive surgery in the treatment of malignant pleuro-pericardial effusions. Chirurgia 2012, 107, 206–212. [Google Scholar]
- Gerber, T.S.; Porubsky, S. Benign lesions of the mediastinum. Histopathology 2024, 84, 183–195. [Google Scholar] [CrossRef]
- Nakaya, M.; Ito, A.; Mori, A.; Oka, M.; Omura, S.; Kida, W.; Inayoshi, Y.; Inoue, A.; Fuchigami, T. Surgical treatment of substernal goiter: An analysis of 44 cases. Auris Nasus Larynx 2017, 44, 111–115. [Google Scholar] [CrossRef]
- Snyder, R.W.; McIvor, D.W.; Mishel, H.S. Spontaneous Hemorrhage of an Ectopic Mediastinal Thyroid. Chest 1990, 98, 1548. [Google Scholar] [CrossRef] [PubMed]
- Haciyanli, S.G.; Karaisli, S.; Acar, N.; Eygi, B.; Haciyanli, M. Split Sternotomy in Retrosternal Thyroid and Mediastinal Parathyroid Pathologies. Sisli Etfal Hastan. Tip Bul. 2021, 55, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Abdulsalam, M.S.; Devanayagam, S.; Santosham, R.; Ganapathy, V.; Menon, M.; Simon, S. Mediastinal parathyroid adenoma removal by video-assisted thoracoscopic surgery. Ann. Afr. Med. 2021, 20, 150–153. [Google Scholar]
- Nistor, C.-E.; Ciuche, A.; Cucu, A.P.; Nitipir, C.; Slavu, C.; Serban, B.; Cursaru, A.; Cretu, B.; Cirstoiu, C. Management of Lung Cancer Presenting with Solitary Bone Metastasis. Medicina 2022, 58, 1463. [Google Scholar] [CrossRef]
- Chen-Yoshikawa, T.F.; Fukui, T.; Nakamura, S.; Ito, T.; Kadomatsu, Y.; Tsubouchi, H.; Ueno, H.; Sugiyama, T.; Goto, M.; Mori, S.; et al. Current trends in thoracic surgery. Nagoya J. Med. Sci. 2020, 82, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.; Ranetti, A.E.; Ciuche, A.; Pantile, D.; Constatin, L.M.; Brincoveanu, R. Betadine in chemical pleurodesis. Farmacia 2014, 62, 897–906. [Google Scholar]
- Anemoulis, M.; Kachtsidis, V.; Geropoulos, G.; Panagiotopoulos, N. Robot-Assisted Thoracoscopic Resection of Ectopic Parathyroid Tissue in Mediastinum: A Scoping Review. Innovations 2024, 19, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Reverter, J.L.; Lucas, A.; Salinas, I.; Audi, L.; Foz, M.; Sanmarti, A. Suppressive therapy with levothyroxinefor solitary thyroid nodules. Clin. Endocrinol. 1992, 36, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.-E.; Ciuche, A.; Cucu, A.-P.; Serban, B.; Cursaru, A.; Cretu, B.; Cirstoiu, C. Clavicular Malignancies: A Borderline Surgical Management. Medicina 2022, 58, 910. [Google Scholar] [CrossRef]
- Ionescu, O.P.; Stanciu, S.M.; Ciobica, M.L. Atherosclerosis in rheumatoid arthritis—The importance of imaging tests. Rom. J. Mil. Med. 2020, CXXIII, 26–31. [Google Scholar] [CrossRef]
- Anghel, D.; Ciobîcă, L.M.; Stanciu, S.M.; Jurcuț, C.V.; Stoicescu, G.D.; Răduță, I.A.; Coca, A. Ankylosing spondylitis and cardiovascular risk—Case report. Rom. J. Mil. Med. 2016, CXIX, 39–42. [Google Scholar]
- Ciobîcă, L.M.; Sârbu, I.; Stanciu, S.M.; Coca, A. Behçet disease—Case presentation. Rom. J. Mil. Med. 2016, CXIX, 43–46. [Google Scholar] [CrossRef]
- Schwalk, A.J.; Niroula, A.; Schimmel, M. What is new in mediastinal staging? Curr. Opin. Pulm. Med. 2024, 30, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Xiu, W.; Ma, X. Application of Artificial Intelligence in the Diagnosis, Treatment, and Prognostic Evaluation of Mediastinal Malignant Tumors. J. Clin. Med. 2023, 12, 2818. [Google Scholar] [CrossRef] [PubMed]
- Jinga, M.; Jurcuţ, C.; Vasilescu, F.; Becheanu, G.; Stancu, S.H.; Ciobaca, L.; Mircescu, G.; Jinga, V. A rare case of digestive hemorrhage in an elderly patient: Diagnosis and treatment difficulties. Rom. J. Morphol. Embryol. 2012, 53 (Suppl. S3), 831–834. [Google Scholar] [PubMed]
- Fassnacht, M.; Tsagarakis, S.; Terzolo, M.; Tabarin, A.; Sahdev, A.; Newell-Price, J.; Pelsma, I.; Marina, L.; Lorenz, K.; Bancos, I.; et al. European Society of Endocrinology clinical practice guidelines on the management of adrenal incidentalomas, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2023, 189, G1–G42. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Kang, J.; Liu, X.; Yao, Y.; Wang, H.; Wang, R. How to Classify and Define Pituitary Tumors: Recent Advances and Current Controversies. Front. Endocrinol. 2021, 12, 604644. [Google Scholar] [CrossRef] [PubMed]
- Paunovic, I.; Rovcanin, B.; Jovanovic, M.; Buzejic, M.; Dundjerovic, D.; Zivaljevic, V. Ectopic thyroid tissue in adrenal gland, case report and review of literature. Gland. Surg. 2020, 9, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Rawitzer, J.; Kapakoglou, A.; Walz, M.K.; Schmid, K.W.; Reis, H. Ectopic thyroid tissue in the adrenal gland: A case report and review of the literature. Pathologe 2019, 41, 177–180. [Google Scholar] [CrossRef]
- Nistor, C.-E.; Pantile, D.; Stanciu-Gavan, C.; Ciuche, A.; Moldovan, H. Diagnostic and Therapeutic Characteristics in Patients with Pneumotorax Associated with COVID-19 versus Non-COVID-19 Pneumotorax. Medicina 2022, 58, 1242. [Google Scholar] [CrossRef]
- Valluri, S.; Lakshmi, H.N.; Sunkavalli, C. Incidental Findings in CT Scans on Screening for COVID-19. Indian J. Surg. Oncol. 2023, 14, 318–323. [Google Scholar] [CrossRef]
- Nistor, C.E.; Pantile, D.; Gavan, C.S.; Ciuche, A. Pneumothorax on COVID-19 Patients—Retrospective Clinical Observations. Rom. J. Leg. Med. 2022, 30, 112–116. [Google Scholar] [CrossRef]
- Jantarapootirat, M.; Traiwanatham, S.; Hirunpat, P.; Boonsomsuk, W.; Sungkanuparph, S.; Sriphrapradang, C. Thyroid Incidentalomas in Hospitalized Patients With COVID-19: A Single-Center Retrospective Analysis. J. Endocr. Soc. 2023, 7, bvad060. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Gavan, C.S.; Pantile, D.; Tanase, N.V.; Ciuche, A. Cervico-Thoracic Air Collections in COVID-19 Pneumonia Patients—Our Experience and Brief Review. Chirurgia 2022, 117, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Palot Manzil, F.F.; Eichhorn, J.; Vattoth, S. Synchronous Ectopic Thyroid Gland and Ectopic Parathyroid Adenoma on99mTc-Sestamibi Scintigraphy and Correlative Imaging. J. Nucl. Med. Technol. 2023, 51, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Che Kadir, S.; Mustaffa, B.E.; Ghazali, Z.; Hasan, Z.; Imisairi, A.H.; Mustafa, S. Mediastinal parathyroid adenoma: Diagnostic and management challenges. Singap. Med. J. 2011, 52, e70–e74. [Google Scholar]
- Ciobîcă, M.L.; Ionescu, O.P.; Săndulescu, B.A. Osteoporosis and the fracture risk in systemic lupus erythematosus. Rom. J. Mil. Med. 2020, CXXIII, 341–347. [Google Scholar] [CrossRef]
- Sunny, S.A.; Singh, A.; Adhikary, A.B. Ectopic Parathyroid Adenoma: Surgical Correction and its Complication Management. Mymensingh Med. J. 2019, 28, 245–249. [Google Scholar]
- Tizu, M.; Mărunțelu, I.; Cristea, B.M.; Nistor, C.; Ishkitiev, N.; Mihaylova, Z.; Tsikandelova, R.; Miteva, M.; Caruntu, A.; Sabliov, C.; et al. PLGA Nanoparticles Uptake in Stem Cells from Human Exfoliated Deciduous Teeth and Oral Keratinocyte Stem Cells. J. Funct. Biomater. 2022, 13, 109. [Google Scholar] [CrossRef]
- Shim, S.R.; Kim, H.J.; Hong, M.; Kwon, S.K.; Kim, J.H.; Lee, S.J.; Lee, S.W.; Han, H.W. Effects of meteorological factors and air pollutants on the incidence of COVID-19 in South Korea. Environ. Res. 2022, 212, 113392. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, S.; Lee, C.S.; Byeon, S.H.; Kim, S.S.; Lee, S.W.; Kim, Y.J. Retinal vascular occlusion risks during the COVID-19 pandemic and after SARS-CoV-2 infection. Sci. Rep. 2023, 13, 16851. [Google Scholar] [CrossRef]
- Lee, E.; Kim, J.H.; Cha, H.R.; Ha, E.K.; Shin, J.; Lee, W.S.; Lee, S.W.; Han, M.Y. Association between atopic dermatitis and school readiness in preschool children. Pediatr. Allergy Immunol. 2023, 34, e13996. [Google Scholar] [CrossRef]
- Martinez-Arguelles, D.B.; Campioli, E.; Culty, M.; Zirkin, B.R.; Papadopoulos, V. Fetal origin of endocrine dysfunction in the adult: The phthalate model. J. Steroid Biochem. Mol. Biol. 2013, 137, 5–17. [Google Scholar] [CrossRef]
- Sánchez, R.M.; Bermeo Losada, J.F.; Marín Martínez, J.A. The research landscape concerning environmental factors in neurodevelopmental disorders: Endocrine disrupters and pesticides—A review. Front. Neuroendocrinol. 2024, 73, 101132. [Google Scholar] [CrossRef]
- Kirtana, A.; Seetharaman, B. Comprehending the Role of Endocrine Disruptors in Inducing Epigenetic Toxicity. Endocr. Metab. Immune Disord.—Drug Targets 2022, 22, 1059–1072. [Google Scholar] [CrossRef]
- GBD 2019 IMID Collaborators. Global, regional, and national incidence of six major immune-mediated inflammatory diseases: Findings from the global burden of disease study 2019. eClinicalMedicine 2023, 64, 102193. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).