Simple Summary
Focal radiation necrosis of the brain (fRNB) is a late side effect that can occur after treatment of brain lesions with focal radiation therapy (stereotactic radiosurgery [SRS] or stereotactic radiation therapy [SRT]). This is becoming more common as more cancer patients are receiving effective systemic therapy for brain metastases, extending survival, and putting them at risk for fRNB. Currently, treatment options are limited to long-term corticosteroid therapy, which has significant side effects, or surgery with its inherent risks. Bevacizumab, a monoclonal antibody that targets the vascular endothelial growth (VEGF), is effective in treating fRNB but its use has remained limited due to its cost. In this single-center case series, a fixed low dose of bevacizumab (400 mg loading dose followed by 100 mg every 4 weeks) was shown to be a safe and cost-effective alternative treatment option for fRNB.
Abstract
Focal radiation necrosis of the brain (fRNB) is a late adverse event that can occur following the treatment of benign or malignant brain lesions with stereotactic radiation therapy (SRT) or stereotactic radiosurgery (SRS). Recent studies have shown that the incidence of fRNB is higher in cancer patients who received immune checkpoint inhibitors. The use of bevacizumab (BEV), a monoclonal antibody that targets the vascular endothelial growth factor (VEGF), is an effective treatment for fRNB when given at a dose of 5–7.5 mg/kg every two weeks. In this single-center retrospective case series, we investigated the effectiveness of a low-dose regimen of BEV (400 mg loading dose followed by 100 mg every 4 weeks) in patients diagnosed with fRNB. A total of 13 patients were included in the study; twelve of them experienced improvement in their existing clinical symptoms, and all patients had a decrease in the volume of edema on MRI scans. No clinically significant treatment-related adverse effects were observed. Our preliminary findings suggest that this fixed low-dose regimen of BEV can be a well-tolerated and cost-effective alternative treatment option for patients diagnosed with fRNB, and it is deserving of further investigation.
1. Introduction
Stereotactic radiosurgery (SRS) and stereotactic radiotherapy (SRT) are increasingly used in the treatment of both benign and malignant (primary or metastatic) brain lesions. SRS and SRT irradiate brain lesions more accurately while sparing healthy brain structures compared to whole-brain radiation therapy (WBRT). Nevertheless, because radiation dosing delivered to the tumor and surrounding normal brain can be higher using these methods, both SRS and SRT have a potential important side effect under the form of focal radiation necrosis of the brain (fRNB) [1,2]. The incidence of fRNB is estimated to be between 7–24% [3,4,5,6]. Signs and symptoms include neurological deficits, cognitive decline, increased intracranial pressure and/or seizures [7]. The primary pathogenesis of fRNB is not yet fully understood, but damage to small blood vessels is suspected to be the principal cause [8,9]. Excessive production of vascular endothelial growth factor A (VEGF-A) and the associated neo-angiogenesis cause the formation of edema and worsening of brain tissue hypoxia and necrosis [10,11,12].
The main risk factor associated with the development of fRNB is the cumulative radiation dose, also taking into account re-irradiation [13,14]. Furthermore, fRNB is more often seen after SRS as compared to SRT, indicating a risk-lowering effect of fractionation [15]. It has recently been noted that the incidence of fRNB following SRT/SRS of brain metastases from solid tumors has been increasing in patients who received immune checkpoint inhibitors (ICI, such as anti PD-(L)1 and anti CTLA-4 monoclonal antibodies (mAb)) and possibly also targeted therapies [16,17]. In these cases, fRNB tends to occur after a median interval of 1 year (range: 8 to 46 months) following SRS/SRT [16,17,18,19,20]. Du Four et al. reported an increased incidence (12.8%) of fRNB in a series of 142 advanced melanoma patients treated with the PD-1 inhibiting mAb pembrolizumab after having previously undergone SRS/SRT [16]. In another cohort of 135 melanoma patients, almost 20% of melanoma patients that received SRS/SRT and anti-PD-1 who survived beyond 1 year developed fRNB [21]. Based on another series from our group, fRNB may also be more frequent after treatment with BRAF-/MEK inhibitors in patients treated for BRAF V600-mutant melanoma brain metastases with SRS/SRT.
The differential diagnosis between fRNB and recurring diseases can be challenging. Pathology remains the gold standard for making a diagnosis. Advanced magnetic resonance imaging (MRI), including MR-spectroscopy and perfusion MRI, can be useful for establishing the diagnosis without the need for a surgical intervention [22]. 18F-fluoro-deoxy-D-glucose positron emission tomography/computed tomography (18F-FDG PET-CT) imaging of the brain can be of additional value, as fRNB will be characterized by decreased uptake of 18F-FDG compared to malignant lesions and surrounding healthy brain tissue [22,23,24].
Currently, high doses of corticosteroids are most often used as first-line therapy for symptomatic patients that are not good candidates for neurosurgical debridement. By decreasing the secretion of pro-inflammatory cytokines and reducing the blood–brain barrier permeability, corticosteroids reduce cerebral edema and related symptoms. However, the side effects of prolonged administration of high-dose corticosteroids, which is often necessary to treat fRNB, are common. These include myopathy, hyperglycemia, atrophy of the skin, weight gain, gastritis, and immunosuppression [25,26]. The surgical debridement of fRNB can offer a permanent solution, but its indication is limited to patients that are eligible for a safe neurosurgical intervention in non-eloquent regions of the brain and it inherently carries a risk for the deterioration of neurological symptoms [27,28].
In 2014, Tye et al. reported that the vascular endothelial growth factor A (VEGF-A), blocking monoclonal antibody bevacizumab (BEV) was an effective new therapeutic option for fRNB [29]. BEV was the first approved angiogenesis inhibitor. It is approved at a dose of 5 to 10 mg/kg every 2 weeks as a component of several chemotherapy regimens for advanced solid tumors [30]. When used to treat fRNB, it allows to reduce or replace the need for corticosteroids and avoid the complications of their long-term use [26,29,31]. Despite its well-known potential side effects, such as hypertension and decreased wound healing, BEV is a well-tolerated medical therapy. Several small studies demonstrated that doses between 5 to 7.5 mg/kg, reflecting the approved dosing regimens for oncological indications, reduce neurological symptoms and cerebral edema [32,33].
Bevacizumab has (as of 2022) not yet been registered for the treatment of fRNB, and its high drug cost has been prohibitive in its use in this indication. Gordon et al. made the observation that significantly lower doses of bevacizumab (down to 0.3 mg/kg) than those approved for oncological indications, reduced free serum VEGF concentrations below the detection limit of the assay [34]. Based on these findings, we started using a low-dose bevacizumab regimen (400 mg I.V. loading dose followed by 100 mg I.V. Q4w) for the treatment of patients diagnosed with symptomatic fRNB not eligible for neurosurgical debridement [34]. The initial reports of this regimen were presented at the 2021 ESMO-congress (European Society for Medical Oncology) [35]. Recently, other research groups demonstrated the efficacy of a low dose of bevacizumab, including regimens of 1 mg/kg and 3 mg/kg [36,37]. Here, we report a retrospective analysis of patients receiving treatment with this low-dose bevacizumab regimen at our center between 2016 and 2022.
2. Materials and Methods
2.1. Patient Selection, Clinical Outcomes, and Adverse Events
We conducted a single-center, retrospective identification of patients with benign or malignant cerebral lesions who received SRT or SRS, subsequently developed symptomatic fRNB and were treated with a low-dose bevacizumab regimen at the UZ Brussel hospital (Belgium) between 2016 and 2022. All patients that received at least one administration of 100 mg bevacizumab I.V were included. Patients were diagnosed with fRNB based on multiple arguments and consensus between physicians from complementary subspecialities (including radiologist, neurosurgeon, and radiation and medical oncologist), location within the irradiated volume, timing relative to radiotherapy, evolution of primary disease, and (in selected cases) biopsy and/or 18F-FDG PET/CT.
Data concerning patient characteristics, patient history and clinical status, diagnosis of fRNB, bevacizumab treatment disposition, adverse events (graded according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0), and response assessments was extracted from their medical records.
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of UZ Brussel (EC-2022-164). All patients signed informed consent allowing the use of their data for the purpose of this analysis.
2.2. Statistical Analysis
In this study, descriptive statistics were used to summarize and present the results. Demographic information, including age, sex, and primary tumor, were collected and summarized using frequency tables and proportions. For continuous variables, the median and standard deviation were calculated. Categorical variables, such as adverse events, were analyzed using frequency and percent distributions. As this is a retrospective case series, no statistical significance can be reported.
2.3. Imaging
T1-weighted gadolinium and T2-weighted FLAIR (fluid-attenuated inversion recovery) MRI images from one month prior to the first dose and after the last dose were reviewed. To determine the effect of the treatment, volumetry was performed on these images using a semi-automated technique (Brainlab AG, Munich, Germany).
3. Results
3.1. Baseline Characteristics
Between March 2016 and July 2022, 13 patients (six females and seven males) with a median age of 52 years (range: 33–68 years) were treated with low-dose bevacizumab for fRNB. Baseline patient characteristics are summarized in Table 1. Among the patients, six patients (46%) had melanoma as a primary disease, three patients (23%) had non-small cell lung cancer, and one patient (8%) had an arteriovenous malformation. Seven patients (54%) received SRS, three patients (23%) received SRT, and two patients (23%) received both. Two out of the seven and one out of three patients who, respectively, received SRS and SRT had multiple courses for different lesions. One patient received two SRT courses for the same lesions. Four patients (31%) were initially treated with corticosteroids prior to receiving bevacizumab but had unsatisfactory outcomes. Three out of these four patients received concomitant corticosteroids at the initiation of BEV. The steroid dose was gradually reduced in these patients. Eight (62%) and four (31%) patients received, respectively, immunotherapy and chemotherapy between SRS/SRT and the first dose of bevacizumab. The median volume of FLAIR-hyperintensity at baseline was 20.1 cm3 (range: 2.74–98.3 cm3) and 4.49 cm3 for the T1 enhancing lesion (range: 1.79–21.3 cm3) on T1-weighted gadolinium MRI. The majority (69%) of the patients had a single fRNB lesion.

Table 1.
Baseline patient characteristics. fRNB = focal radiation necrosis of the brain, WHO-PS = World Health Organization Performance Status.
The diagnosis of fRNB was based on the medical history and MRI of the brain in all patients and was further complemented with 18F-FDG PET-CT in six patients and with a surgical biopsy in six patients. Median time between the first course of SRS/SRT and the start of the low-dose BEV regimen was 39 months (range: 18–90 months).
All patients had at least one fRNB-associated neurological symptom at the start of the treatment, including focal neurological deficits (e.g., paresis) (69%, n = 9), epileptic seizures (46%, n = 6), and headache (8%, n = 1,) (Table 1 and Table 2).

Table 2.
Individual patient characteristics. BEV = bevacizumab, AVM = arteriovenous malformation, BC = breast cancer, MB = medulloblastoma, MEL = melanoma, NSCLC = non-small cell lung carcinoma, RCC = renal cell carcinoma, IPI = ipilimumab, NIVO = nivolumab, PEMBRO = pembrolizumab, Y = yes, N = no, Y/N = mixed, ICI = immune checkpoint inhibitor. 1 indicates concomitant continuation of corticosteroids. Time expressed in months.
3.2. Treatment Disposition
All patients, except three, received a loading dose of 400 mg bevacizumab I.V at the start of treatment. A median of seven maintenance doses (100 mg) was given (range: 2–38 doses). Two out of the three patients who did not receive a loading dose had previously received a conventional dose regimen of bevacizumab. Prolonging the interval between administrations from Q4w to Q6w in three patients after the initial response did not result in an increase in symptoms. In four patients, bevacizumab treatment was resumed following elective discontinuation. In one of these patients because of recurring symptoms, in two other patients following an asymptomatic increase in T1-weighted gadolinium and T2-weighted FLAIR MRI volume, and in one patient (25%, n = 4) because of both MRI changes, as well as an increase in symptoms (Figure 1). On average, patients were given 2–4 doses of BEV before their first evaluation. At this evaluation, treatment was either stopped or continued based on clinical symptoms and MRI response.
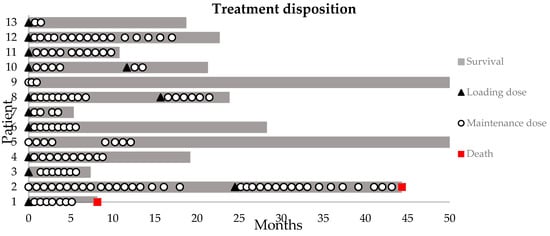
Figure 1.
Swimmer plot of the bevacizumab disposition. Loading dose = 400 mg I.V.; maintenance dose = 100 mg I.V. Survival indicates the time between first loading dose and data base lock or death.
3.3. Clinical Outcome
Eleven patients (84.6%) experienced a complete symptomatic improvement during treatment. Six patients suffered from epileptic seizures within three months preceding the initiation of BEV; three of these patients suffered from therapy refractory epilepsy (defined by refractory to two or more anti-epileptic drugs). These patients experienced a marked improvement in refractory epileptic seizures, including two patients who became seizure-free. The other three patients experiencing epileptic seizures all became seizure-free while under the low-dose regimen. All patients continued their anti-epileptic medication. The WHO-PS (World Health Organization Performance Status) remained the same in nine patients (69%) while on treatment, improved in three patients (23%), and decreased in one patient (8%) (Table 1 and Table 2)
3.4. Objective Response on MRI of the Brain
All patients underwent at least one MRI scan at baseline and on treatment. Slice thickness ranged between 0.5 and 2 mm, depending on the MRI apparatus. T2-weighted FLAIR MRI-images demonstrated a decrease in the volume of edema in all patients. The median relative decrease in the FLAIR-hyperintensity volume was 81.1% (range: 7.1–95.4%). Three patients (23%) relapsed after electively discontinuing treatment following symptomatic and radiographic improvement while on treatment. After reinitiating treatment, these patients had a renewed radiographic decrease in the edema volume.
All patients who were evaluated by T1-weighted gadolinium MRI of the brain (n = 12) had a decrease in the contrast enhancing volume. One patient had a slight increase in the contrast enhancing volume after re-challenge, but a decrease in FLAIR-hyperintensity over the same period (Figure 2).
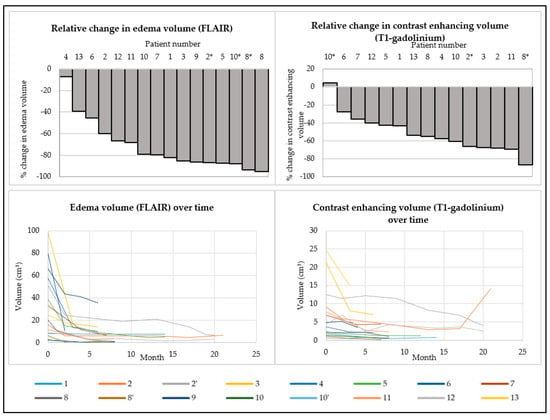
Figure 2.
Change in T2-weighted FLAIR-hyperintensity and T1-weighted gadolinium enhancing volumes between baseline MRI and the most recent MRI at the end of BEV treatment (first row). Evolution in time of T2-weighted FLAIR-hyperintensity and T1-weighted gadolinium enhancing volumes during treatment (second row). * Indicates a re-challenge with a low-dose bevacizumab regimen after an elective discontinuation of bevacizumab treatment.
3.5. Safety
In general, the treatment was well-tolerated, and the adverse events were mild (Table 3). No unexpected adverse events were observed. There were no Grade 3 or higher adverse events. None of the patients needed to receive medical therapy for adverse events. One patient had to discontinue treatment because of a Grade 2 wound dehiscence (which was successfully treated with local treatment).

Table 3.
Adverse events according to the Common Terminology Criteria for Adverse Events (CTCAE) v5.0.
3.6. Survival
The median time between the first dose of bevacizumab and the database lock was 22 months (range: 5–56 months). Two patients died during this follow-up period due to progressive disease of their primary tumor (lung and melanoma). All the other patients remained alive at the time of the database lock and were still in follow up. Only one of patient remains in treatment.
4. Case Illustrations
4.1. Case Illustration 1
We present the case of a 33-year-old male patient with a cerebellar medulloblastoma, who initially presented in 2011 with orthostatic headache. Surgical resection and radiotherapy were performed in 2011 (20 × 1.8 Gy full neuraxis + 10 × 1.8 Gy boost locally) and 2013 (recurrence, 26 × 2 Gy). In 2016, perilesional edema was visualized on follow-up MRI, and the patient experienced epileptic seizures. Therefore, a treatment of standard-dosing 7.5 mg/kg Q4w bevacizumab as well as levetiracetam was initiated. After four doses of bevacizumab, T2-weighted FLAIR MRI demonstrated a decrease in the perilesional edema and the patient did not experience epileptic seizures anymore, after which the treatment was discontinued. In 2018, he experienced a new epileptic seizure and T2-weighted FLAIR MRI demonstrated an increase in perilesional edema (A). Despite increasing the dosage of the anti-epileptic drug levetiracetam, the patient still experienced seizures. Therefore, a low-dose bevacizumab regimen of 100mg Q4w was initiated (omitting the 400mg loading dose). After four doses, treatment was discontinued because the patient did not experience epileptic seizures anymore and a decrease in volume on T2-weighted FLAIR MRI was observed (C). The patient remained in follow up every three months (clinical and MRI). After six months, a T2-weighted FLAIR MRI demonstrated a new increase in edema (D) prompting a restart of low-dose BEV. Another four doses later, he again had a reduction in edema volume, after which treatment was permanently discontinued (E). Two months after discontinuing the treatment, no increase in edema was visualized (F). The patient remained asymptomatic up to the last follow up (more than 2.5 years after the last dose of bevacizumab) (Figure 3).
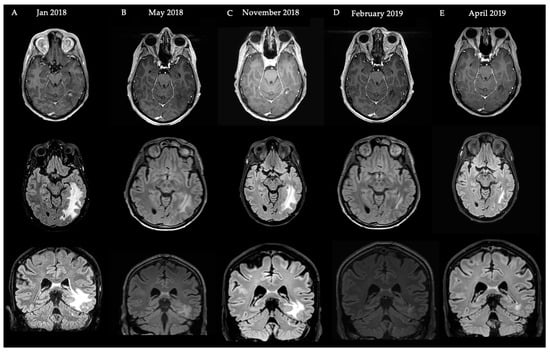
Figure 3.
Schematic overview of events and MRI images with regard to the Case Illustration 1. First row: T1-weighted gadolinium MRI. Second row: T2-weighted FLAIR MRI (axial). Third row: T2-weighted FLAIR MRI (coronal) illustrating fRNB crossing over the tentorium.
4.2. Case Illustration 2
The second case is a 52-year-old female patient who presented with two melanoma brain metastases for which she received SRT in January and March of 2019. In July 2020 (A), a diagnosis of fRNB was made based on T1-weighted gadolinium and T2-weighted FLAIR MRI, further confirmed by a hypometabolic spot on 18F-FDG PET-CT. Because of frontal and parietal left-sided headaches, a low-dose regimen bevacizumab was initiated, starting with a loading dose of 400 mg, followed by a Q4w 100 mg I.V. maintenance dose. In January 2021, a decrease in edema on T2-weighted FLAIR MRI was observed (B). Treatment was interrupted because of diarrhea and minimal improvement in symptoms. Nine months later, because of an increase in edema (C) a new low-dose regimen was started with a 400 mg I.V. loading dose. In January 2022, she received SRS for a new metastatic lesion but continued BEV maintenance. After six maintenance doses, BEV was stopped because of radiological improvement (D). Edema on this MRI is a result of the intracranial progression of her melanoma for which new systemic treatment options were started. Six weeks after stopping BEV, no subsequent increase in edema was visualized (E) (Figure 4).
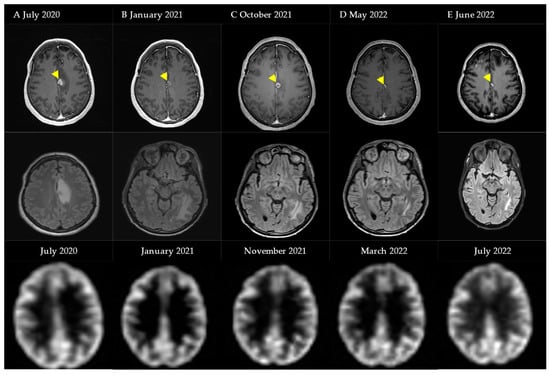
Figure 4.
Schematic overview of events and MRI images with regard to Case Illustration 2. First row: T1-weighted gadolinium MRI (abnormality marked by arrow). Second row: T2-weighted FLAIR MRI. Third row: 18F-FDG PET-CT.
5. Discussion
Focal radiation necrosis of the brain (fRNB) is an important and increasingly common late adverse event of stereotactic radiotherapy and radiosurgery. Our case series confirms the observations in the literature, indicating that fRNB is more frequent after SRS, as compared to SRT (10 out of 13 patients received SRS). When symptoms occur, treatment is indicated. Depending on the localization and the prognosis of the patient, surgical debridement or systemic corticosteroids are currently considered as standard treatment options. Bevacizumab has been proven to be an effective alternative, avoiding the side effects resulting from prolonged administration of corticosteroids [29]. Gonzalez et al. reported that using a dosing regimen of 5 to 7.5 mg/kg bevacizumab administered every 2–3 weeks reduced fRNB-related neurocognitive deficits and cerebral edema [32]. Later, an observational study and a randomized controlled trial confirmed these finding [29,33]. However, until recently, bevacizumab has not been widely adapted as the preferred treatment option, as the high drug costs remain an important hurdle. More recently, bevacizumab biosimilars have been introduced, rendering the use of this drug more accessible. Our research group was among the first to report the effect of a fixed, low-dose bevacizumab regimen [35]. We have summarized all available evidence regarding the use of bevacizumab for the indication of fRNB in Table 4. Most data relate to retrospective case series, with important variations in sample size and patient heterogeneity.

Table 4.
Overview of available evidence regarding the use of bevacizumab for the treatment of focal radiation necrosis of the brain.
This study included 13 patients who received at least one administration of 100 mg I.V. bevacizumab between 2016 and 2022. Ten of these patients received an initial loading dose of 400 mg, followed by a monthly maintenance dose of 100 mg. 18F-FDG PET-CT was useful to differentiate fRNB from brain metastasis, and therefore, can aid in diagnosis. This is in line with recent reviews, although further research is still needed [48,49]. While the study population is heterogeneous in their primary disease, all patients were treated for fRNB presenting after SRS/SRT. Within this population, 92% of the patient population experienced an improvement in clinical symptoms. Almost all the patients experiencing epileptic seizures became seizure-free under anti-epileptic medication in combination with low-dose bevacizumab, including two patients with refractory epileptic seizures. All patients had a radiographic decrease in edema volume on T2-weighted FLAIR MRI after starting BEV. These findings are in line with other reports on lower-dose regimens of bevacizumab for fRNB [36,37] (see Table 4 for a full overview). We first reported the results of a fixed-dose regimen, as opposed to weight-based dosing. The fixed doses used in this regimen were generally well-tolerated with only mild adverse events, and no new safety signs were observed. Almost all patients (10 out of 13) received an initial loading dose, followed by at least two maintenance doses (100 mg) before their first evaluation (=after 3 months) to decide on whether to continue treatment. This is in line with what is described in the literature (2–4 initial courses of BEV, both for a lower dose, as well as a conventional dose). In cases where a significant beneficial effect on MRI imaging is observed, as well as symptomatic improvements without a remaining need for corticosteroids, we will discuss the possibility of treatment interruption with the patient. Further dosing is adapted to patient symptoms and imaging.
Notwithstanding the limited number of patients in this retrospective single-center data collection, our preliminary data suggest that a low-dose regimen bevacizumab comprising a fixed loading dose of a 400 mg I.V. dose and a maintenance dose of 100 mg Q4w can be a simple and effective treatment option for symptomatic fRNB. While the design comes with its inherent risk of biases, we think the real-world experience described in this manuscript demonstrates a uniform and clinically meaningful activity in all patients treated with this treatment regimen. In the future, more data on outcome according to primary pathology should be collected within the context of a prospective clinical trial. Based on our results, this regimen is a valid cost-sparing alternative for the expensive standard dose of 5 to 7.5 mg/kg of BEV every 3–4 weeks, especially since the recent availability of bevacizumab biosimilars and the subsequent reduced drug cost. We have, therefore, adapted this regimen as our institutional standard of care treatment regimen for fRNB that is not amenable for neurosurgical resection.
6. Conclusions
Our preliminary data demonstrate that treatment of fRNB with a low-dose regimen of bevacizumab can be an effective and cost-lowering alternative for standard-dose bevacizumab and likely has fewer side effects as compared to long-term high-dose corticosteroid use. 18F-FDG PET/CT can be a useful supplementary imaging modality to differentiate fRNB from malignant brain lesion recurrence or metastasis. Further research is needed to prospectively validate this low-dose treatment protocol in larger studies and homogenous patient cohorts.
Author Contributions
Conceptualization, B.N.; Formal analysis, J.T., E.C. and B.N.; Investigation, J.T., I.D., M.V., A.M.V., H.E., L.S., D.V.D.B., J.D. and B.N.; Methodology, J.T., A.M.V. and B.N.; Project administration, B.N.; Resources, B.N.; Supervision, J.D. and B.N.; Visualization, E.C.; Writing—original draft, J.T. and E.C.; Writing—review and editing, J.T., E.C., I.D., M.V., R.K., A.M.V., H.E., L.S., D.V.D.B., J.D. and B.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of UZ Brussel (EC-2022-164).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data are available on reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vellayappan, B.; Tan, C.L.; Yong, C.; Khor, L.K.; Koh, W.Y.; Yeo, T.T.; Detsky, J.; Lo, S.; Sahgal, A. Diagnosis and Management of Radiation Necrosis in Patients with Brain Metastases. Front. Oncol. 2018, 8, 395. [Google Scholar] [CrossRef]
- Ali, F.S.; Arevalo, O.; Zorofchian, S.; Patrizz, A.; Riascos, R.; Tandon, N.; Blanco, A.; Ballester, L.Y.; Esquenazi, Y. Cerebral Radiation Necrosis: Incidence, Pathogenesis, Diagnostic Challenges, and Future Opportunities. Curr. Oncol. Rep. 2019, 21, 66. [Google Scholar] [CrossRef]
- Kohutek, Z.A.; Yamada, Y.; Chan, T.A.; Brennan, C.W.; Tabar, V.; Gutin, P.H.; Jonathan Yang, T.; Rosenblum, M.K.; Ballangrud, Å.; Young, R.J.; et al. Long-term risk of radionecrosis and imaging changes after stereotactic radiosurgery for brain metastases. J. Neuro-Oncol. 2015, 125, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Clarke, E.; Lanzetta, G.; Osti, M.F.; Trasimeni, G.; Bozzao, A.; Romano, A.; Enrici, R.M. Stereotactic radiosurgery for brain metastases: Analysis of outcome and risk of brain radionecrosis. Radiat. Oncol. 2011, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Varlotto, J.M.; Flickinger, J.C.; Niranjan, A.; Bhatnagar, A.K.; Kondziolka, D.; Lunsford, L.D. Analysis of tumor control and toxicity in patients who have survived at least one year after radiosurgery for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Blonigen, B.J.; Steinmetz, R.D.; Levin, L.; Lamba, M.A.; Warnick, R.E.; Breneman, J.C. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 996–1001. [Google Scholar] [CrossRef]
- Pan, D.; Rong, X.; Chen, D.; Jiang, J.; Ng, W.T.; Mai, H.; Li, Y.; Li, H.; Cai, J.; Cheng, J.; et al. Mortality of early treatment for radiation-induced brain necrosis in head and neck cancer survivors: A multicentre, retrospective, registry-based cohort study. EClinicalMedicine 2022, 52, 101618. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Nonoguchi, N.; Kawabata, S.; Miyatake, S.; Kuroiwa, T. Delayed brain radiation necrosis: Pathological review and new molecular targets for treatment. Med. Mol. Morphol. 2015, 48, 183–190. [Google Scholar] [CrossRef]
- Miyatake, S.; Nonoguchi, N.; Furuse, M.; Yoritsune, E.; Miyata, T.; Kawabata, S.; Kuroiwa, T. Pathophysiology, Diagnosis, and Treatment of Radiation Necrosis in the Brain. Neurol. Med. Chir. 2015, 55 (Suppl. 1), 50–59. [Google Scholar] [CrossRef]
- Calvo, W.; Hopewell, J.W.; Reinhold, H.S.; Yeung, T.K. Time- and dose-related changes in the white matter of the rat brain after single doses of X rays. Br. J. Radiol. 1988, 61, 1043–1052. [Google Scholar] [CrossRef]
- Reinhold, H.S.; Calvo, W.; Hopewell, J.W.; van der Berg, A.P. Development of blood vessel-related radiation damage in the fimbria of the central nervous system. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 37–42. [Google Scholar] [CrossRef]
- Lyubimova, N.; Hopewell, J.W. Experimental evidence to support the hypothesis that damage to vascular endothelium plays the primary role in the development of late radiation-induced CNS injury. Br. J. Radiol. 2004, 77, 488–492. [Google Scholar] [CrossRef]
- Sneed, P.K.; Mendez, J.; Vemer-van den Hoek, J.G.; Seymour, Z.A.; Ma, L.; Molinaro, A.M.; Fogh, S.E.; Nakamura, J.L.; McDermott, M.W. Adverse radiation effect after stereotactic radiosurgery for brain metastases: Incidence, time course, and risk factors. J. Neurosurg. 2015, 123, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Kerschbaumer, J.; Demetz, M.; Krigers, A.; Nevinny-Stickel, M.; Thome, C.; Freyschlag, C.F. Risk Factors for Radiation Necrosis in Patients Undergoing Cranial Stereotactic Radiosurgery. Cancers 2021, 13, 4736. [Google Scholar] [CrossRef] [PubMed]
- Milano, M.T.; Grimm, J.; Niemierko, A.; Soltys, S.G.; Moiseenko, V.; Redmond, K.J.; Yorke, E.; Sahgal, A.; Xue, J.; Mahadevan, A.; et al. Single- and Multifraction Stereotactic Radiosurgery Dose/Volume Tolerances of the Brain. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 68–86. [Google Scholar] [CrossRef]
- Du Four, S.; Janssen, Y.; Michotte, A.; Van Binst, A.M.; Van den Begin, R.; Duerinck, J.; Neyns, B. Focal radiation necrosis of the brain in patients with melanoma brain metastases treated with pembrolizumab. Cancer Med. 2018, 7, 4870–4879. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.M.; Cagney, D.N.; Catalano, P.J.; Alexander, B.M.; Redig, A.J.; Schoenfeld, J.D.; Aizer, A.A. Immunotherapy and Symptomatic Radiation Necrosis in Patients With Brain Metastases Treated With Stereotactic Radiation. JAMA Oncol. 2018, 4, 1123–1124. [Google Scholar] [CrossRef]
- Du Four, S.; Wilgenhof, S.; Duerinck, J.; Michotte, A.; Van Binst, A.; De Ridder, M.; Neyns, B. Radiation necrosis of the brain in melanoma patients successfully treated with ipilimumab, three case studies. Eur. J. Cancer 2012, 48, 3045–3051. [Google Scholar] [CrossRef]
- Du Four, S.; Maenhout, S.K.; Niclou, S.P.; Thielemans, K.; Neyns, B.; Aerts, J.L. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am. J. Cancer Res. 2016, 6, 2514–2531. [Google Scholar]
- Fang, P.; Jiang, W.; Allen, P.; Glitza, I.; Guha, N.; Hwu, P.; Ghia, A.; Phan, J.; Mahajan, A.; Tawbi, H.; et al. Radiation necrosis with stereotactic radiosurgery combined with CTLA-4 blockade and PD-1 inhibition for treatment of intracranial disease in metastatic melanoma. J. Neurooncol. 2017, 133, 595–602. [Google Scholar] [CrossRef]
- Pires da Silva, I.; Glitza, I.C.; Haydu, L.E.; Johnpulle, R.; Banks, P.D.; Grass, G.D.; Goldinger, S.M.A.; Smith, J.L.; Everett, A.S.; Koelblinger, P.; et al. Incidence, features and management of radionecrosis in melanoma patients treated with cerebral radiotherapy and anti-PD-1 antibodies. Pigment. Cell Melanoma Res. 2019, 32, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Furuse, M.; Nonoguchi, N.; Yamada, K.; Shiga, T.; Combes, J.D.; Ikeda, N.; Kawabata, S.; Kuroiwa, T.; Miyatake, S.I. Radiological diagnosis of brain radiation necrosis after cranial irradiation for brain tumor: A systematic review. Radiat. Oncol. 2019, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Galldiks, N.; Stoffels, G.; Filss, C.P.; Piroth, M.D.; Sabel, M.; Ruge, M.I.; Herzog, H.; Shah, N.J.; Fink, G.R.; Coenen, H.H.; et al. Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl. Med. 2012, 53, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Ceccon, G.; Lohmann, P.; Stoffels, G.; Judov, N.; Filss, C.P.; Rapp, M.; Bauer, E.; Hamisch, C.; Ruge, M.I.; Kocher, M.; et al. Dynamic O-(2-18F-fluoroethyl)-L-tyrosine positron emission tomography differentiates brain metastasis recurrence from radiation injury after radiotherapy. Neuro-Oncology 2017, 19, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Vellayappan, B.A.; McGranahan, T.; Graber, J.; Taylor, L.; Venur, V.; Ellenbogen, R.; Sloan, A.E.; Redmond, K.J.; Foote, M.; Chao, S.T.; et al. Radiation Necrosis from Stereotactic Radiosurgery-How Do We Mitigate? Curr. Treat. Options Oncol. 2021, 22, 57. [Google Scholar] [CrossRef]
- Winter, S.F.; Loebel, F.; Loeffler, J.; Batchelor, T.T.; Martinez-Lage, M.; Vajkoczy, P.; Dietrich, J. Treatment-induced brain tissue necrosis: A clinical challenge in neuro-oncology. Neuro-Oncology 2019, 21, 1118–1130. [Google Scholar] [CrossRef]
- McPherson, C.M.; Warnick, R.E. Results of contemporary surgical management of radiation necrosis using frameless stereotaxis and intraoperative magnetic resonance imaging. J. Neurooncol. 2004, 68, 41–47. [Google Scholar] [CrossRef]
- Nath, S.K.; Sheridan, A.D.; Rauch, P.J.; Yu, J.B.; Minja, F.J.; Vortmeyer, A.O.; Chiang, V.L. Significance of histology in determining management of lesions regrowing after radiosurgery. J. Neurooncol. 2014, 117, 303–310. [Google Scholar] [CrossRef]
- Tye, K.; Engelhard, H.H.; Slavin, K.V.; Nicholas, M.K.; Chmura, S.J.; Kwok, Y.; Ho, D.S.; Weichselbaum, R.R.; Koshy, M. An analysis of radiation necrosis of the central nervous system treated with bevacizumab. J. Neurooncol. 2014, 117, 321–327. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin(R)) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef]
- Brandes, A.A.; Bartolotti, M.; Tosoni, A.; Poggi, R.; Franceschi, E. Practical management of bevacizumab-related toxicities in glioblastoma. Oncologist 2015, 20, 166–175. [Google Scholar] [CrossRef]
- Gonzalez, J.; Kumar, A.J.; Conrad, C.A.; Levin, V.A. Effect of bevacizumab on radiation necrosis of the brain. Int. J. Radiat. Oncol. Biol Phys 2007, 67, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Levin, V.A.; Bidaut, L.; Hou, P.; Kumar, A.J.; Wefel, J.S.; Bekele, B.N.; Grewal, J.; Prabhu, S.; Loghin, M.; Gilbert, M.R.; et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Gordon, M.S.; Margolin, K.; Talpaz, M.; Sledge, G.W., Jr.; Holmgren, E.; Benjamin, R.; Stalter, S.; Shak, S.; Adelman, D. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J. Clin. Oncol. 2001, 19, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Abstract 371P: Low-dose bevacizumab for the treatment of focal post-radiation necrosis of the brain. In Proceedings of the European Society of Medical Oncology Congress, Lugano, Switzerland, 16–21 September 2021.
- Zhuang, H.; Zhuang, H.; Shi, S.; Wang, Y. Ultra-Low-Dose Bevacizumab for Cerebral Radiation Necrosis: A Prospective Phase II Clinical Study. OncoTargets Ther. 2019, 12, 8447–8453. [Google Scholar] [CrossRef]
- Weng, Y.; Shen, J.; Zhang, L.; Fang, Z.; Xiao, F.; Zhang, C.; Fan, Z.; Huang, K.; Wang, L.; Huang, B.; et al. Low-Dosage Bevacizumab Treatment: Effect on Radiation Necrosis After Gamma Knife Radiosurgery for Brain Metastases. Front. Surg. 2021, 8, 720506. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, L.; Sheng, X.; Mao, Y.; Yao, Y.; Wang, E.; Zhang, N.; Dai, J. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. Eur. J. Med. Res. 2012, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Boothe, D.; Young, R.; Yamada, Y.; Prager, A.; Chan, T.; Beal, K. Bevacizumab as a treatment for radiation necrosis of brain metastases post stereotactic radiosurgery. Neuro-Oncology 2013, 15, 1257–1263. [Google Scholar] [CrossRef]
- Furuse, M.; Nonoguchi, N.; Kawabata, S.; Yoritsune, E.; Takahashi, M.; Inomata, T.; Kuroiwa, T.; Miyatake, S. Bevacizumab treatment for symptomatic radiation necrosis diagnosed by amino acid PET. Jpn. J. Clin. Oncol. 2013, 43, 337–341. [Google Scholar] [CrossRef]
- Yonezawa, S.; Miwa, K.; Shinoda, J.; Nomura, Y.; Asano, Y.; Nakayama, N.; Ohe, N.; Yano, H.; Iwama, T. Bevacizumab treatment leads to observable morphological and metabolic changes in brain radiation necrosis. J. Neurooncol. 2014, 119, 101–109. [Google Scholar] [CrossRef]
- Sadraei, N.H.; Dahiya, S.; Chao, S.T.; Murphy, E.S.; Osei-Boateng, K.; Xie, H.; Suh, J.H.; Peereboom, D.M.; Stevens, G.H.; Ahluwalia, M.S. Treatment of cerebral radiation necrosis with bevacizumab: The Cleveland clinic experience. Am. J. Clin. Oncol. 2015, 38, 304–310. [Google Scholar] [CrossRef]
- Zhuang, H.; Yuan, X.; Zheng, Y.; Li, X.; Chang, J.Y.; Wang, J.; Wang, X.; Yuan, Z.; Wang, P. A study on the evaluation method and recent clinical efficacy of bevacizumab on the treatment of radiation cerebral necrosis. Sci. Rep. 2016, 6, 24364. [Google Scholar] [CrossRef]
- Li, Y.; Huang, X.; Jiang, J.; Hu, W.; Hu, J.; Cai, J.; Rong, X.; Cheng, J.; Xu, Y.; Wu, R.; et al. Clinical Variables for Prediction of the Therapeutic Effects of Bevacizumab Monotherapy in Nasopharyngeal Carcinoma Patients with Radiation-Induced Brain Necrosis. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Rong, X.; Hu, W.; Huang, X.; Li, Y.; Zheng, D.; Cai, Z.; Zuo, Z.; Tang, Y. Bevacizumab Monotherapy Reduces Radiation-induced Brain Necrosis in Nasopharyngeal Carcinoma Patients: A Randomized Controlled Trial. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Alessandretti, M.; Buzaid, A.C.; Brandao, R.; Brandao, E.P. Low-dose bevacizumab is effective in radiation-induced necrosis. Case Rep. Oncol. 2013, 6, 598–601. [Google Scholar] [CrossRef] [PubMed]
- Glitza, I.C.; Guha-Thakurta, N.; D’Souza, N.M.; Amaria, R.N.; McGovern, S.L.; Rao, G.; Li, J. Bevacizumab as an effective treatment for radiation necrosis after radiotherapy for melanoma brain metastases. Melanoma Res. 2017, 27, 580–584. [Google Scholar] [CrossRef]
- Singnurkar, A.; Poon, R.; Detsky, J. 18F-FET-PET imaging in high-grade gliomas and brain metastases: A systematic review and meta-analysis. J. Neurooncol. 2023, 161, 1–12. [Google Scholar] [CrossRef]
- Mayo, Z.S.; Halima, A.; Broughman, J.R.; Smile, T.D.; Tom, M.C.; Murphy, E.S.; Suh, J.H.; Lo, S.S.; Barnett, G.H.; Wu, G.; et al. Radiation necrosis or tumor progression? A review of the radiographic modalities used in the diagnosis of cerebral radiation necrosis. J. Neurooncol. 2023, 161, 23–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).