Simple Summary
This study tested the repurposing of two rationally selected, non-anticancer drugs as a way to address the need for less toxic therapeutic options in children with gliomas. The determined recommended phase II dose of fluvastatin in combination with celecoxib in children with gliomas is 6 mg/kg/day (in 14 days on, 14 days off schedule) with a fixed daily dose of celecoxib (from 200 mg to 800 mg depending on weight). The combination is not active in HGG but could be explored as a maintenance treatment in LGG patients to avoid or delay a possible tumor recurrence, which would require a more toxic treatment. This oral strategy with very limited toxicity may be used to gain time and therefore limit treatment-related toxicities in growing children. Given its good safety profile, its low cost and all-oral administration, we think that it could be considered as an option for children with LGG living in low- and middle-income countries.
Abstract
Preclinical data support the activity of celecoxib and fluvastatin in high-grade (HGG) and low-grade gliomas (LGG). A phase I trial (NCT02115074) was designed to evaluate the safety of this combination in children with refractory/relapsed HGG and LGG using four dose levels of fluvastatin with a fixed daily dose of celecoxib. A Continual Reassessment Method was used for fluvastatin dose escalation. Dose-limiting toxicities (DLT) were determined on the first treatment cycle. Twenty patients were included. Ten LGG and ten HGG patients received a median of 3.5 treatment cycles. Two DLTs were reported: one grade 3 maculopapular rash (4 mg/kg dose level) and one grade 4 increase of Creatine Phospho-Kinase (6 mg/kg dose level). We identified the dose of 6 mg/kg/day as the recommended phase II dose (RP2D) of fluvastatin with celecoxib. Four patients with LGG continued treatment beyond 12 cycles because of stable disease, including one patient who received 23 treatment cycles. In children with refractory/relapsed glioma, the RP2D of fluvastatin with celecoxib is 6 mg/kg/day. The long-term stable diseases observed in LGG suggest a possible role of the combination in a maintenance setting, given its good tolerance and low cost for children living in low- and middle-income countries.
1. Introduction
Gliomas are the most common pediatric central nervous system (CNS) tumors [1]. Overall survival (OS) at 10 years ranges between 85% and 96% for low-grade gliomas (LGG), whereas nearly all children with high-grade gliomas (HGG) die within 1–2 years of diagnosis [1,2,3,4,5]. The therapeutic challenges dramatically differ, with the management of HGG critically in need of new thinking [6]. For LGG, the main objective is to obtain long-term tumor control while limiting treatment-induced long-term effects. While surgery is very effective, resectability is particularly challenging for optic pathway gliomas (OPG) [7]. Radiotherapy effectively controls tumor growth in most cases but carries a high risk of inducing long-term sequelae [8,9,10,11,12]. Chemotherapy has increasingly become the mainstay of pediatric LGG management. Commonly used regimens include carboplatin and vincristine, vinblastine, thioguanine, procarbazine, lomustine and vincristine (TPCV) and, more recently, bevacizumab [13,14,15,16]. Chemotherapy is however frequently only transiently effective, and multiple lines of chemotherapy are often required [13]. Therefore, there is a need for new combinations using drugs with favorable short- and long-term safety profiles.
In a preclinical study comparing the transcriptional profiles of five hypothalamo–chiasmatic and six cerebellar pilocytic astrocytomas using a microarray technique, quantitative real-time PCR and immunochemistry, Tchoghandjian et al. demonstrated that these entities are genetically distinct by showing many differentially upregulated genes [17]. These results were confirmed by Mercurio et al., who proposed that celecoxib and fluvastatin could target a set of these genes (ICAM1, CRK, CD36 and IQGAP1) differentially expressed in OPG [18]. Fluvastatin is an approved HMG-CoA reductase inhibitor that has been tested in two pediatric cancer trials with no safety concerns and encouraging efficacy [19,20]. Of relevance to glioma, statins target CD36, a scavenger receptor that is highly expressed in pilocytic astrocytoma [21]. Fluvastatin also showed an inhibitory and cytotoxic effect on several high-grade glioma cell lines [18,22,23]. Celecoxib is a cyclooxygenase 2 (COX-2) inhibitor and has a long repurposing history in oncology [24]. Sato et al. showed that the expression level of COX-2 was greater in LGG than in normal brain cells and that inhibiting COX-2 induced apoptosis and inhibited cell proliferation via the Akt/survivin and Akt/ID3 pathways in LGG [25]. Moreover, celecoxib interferes with cellular adhesion machinery by decreasing ICAM-1 expression and promotes anoikis by deregulating the focal adhesion assembly protein CRK-associated substrate, P130CAS [18]. Celecoxib has been used in several cancer trials [24] including in pediatric LGG protocols [26]. Mercurio et al. demonstrated a synergistic effect of fluvastatin and celecoxib in two glioma cell lines and reported a significant radiological response to this combination in a patient with refractory metastatic LGG [18].
Taking advantage of the safety and rationale to use these drugs against LGG, we conducted a phase I trial to determine the recommended phase II dose (RP2D) of fluvastatin in combination with a fixed dose of celecoxib in children with recurrent/refractory glioma. Considering the low toxicity of each of these two drugs and their distinct mechanism of action, no significant toxicity was expected from their combination. The trial included an expansion cohort of LGG patients to better characterize the drugs’ safety and assess the preliminary activity of the combination at the recommended phase II dose (RP2D).
2. Materials and Methods
2.1. Study Population
Patients aged 6–21 years were eligible if they met all the following criteria: refractory/recurrent LGG or HGG after at least one first-line therapy, including radiotherapy for HGG patients; Lansky play scale or Karnofsky performance status at least 70%; ability to swallow oral medications; life expectancy of at least 3 months; adequate bone marrow (absolute neutrophil count ≥1000/μL, platelet count ≥75,000/μL), renal (1.5 × age-adjusted normal serum creatinine or glomerular filtration rate ≥70 mL/min/1.73 m2 with the Schwartz formula), liver (total bilirubin ≤ 3 N and alanine aminotransferase ≤ 4 N) and muscle enzyme (with Creatine Phospho-Kinase CPK < 2 N) functions; no cytotoxic chemotherapy within 21 days (2 weeks if vincristine and 6 weeks if nitrosourea) and no radiotherapy within 6 months prior to study entry.
Prior histological documentation was not required in patients with Neurofibromatosis-1 (NF1) and typical radiologic low-grade OPG. Disease refractory was defined as a radiographic or clinically progressive disease while on treatment. Patients with LGG had to be considered non-eligible for complete tumor resection. Patients with HGG had to have histologically confirmed recurrent or progressive disease. Patients with completely resected HGG at relapse were eligible. Patients with LGG must have had measurable lesions according to the RANO criteria [27]. Patients with diffuse intrinsic pontine gliomas were not eligible.
Exclusion criteria were active uncontrolled infection, peptic ulcer disease, gastrointestinal bleeding, inflammatory bowel disease, history of asthma, acute rhinitis or nasal polyps, medical history of allergy or hypersensitivity induced by acetylsalicylic acid or non-steroidal anti-inflammatory drugs or known hypersensitivity to sulfonamides, non-congestive heart failure, ischemic heart disease, cardiovascular disease, preexisting muscle pathology, pregnancy, breastfeeding and organ toxicity superior to grade 2 according to NCI-CTCAE v4.0.
Patients and/or their legal guardians gave written informed consent, and assent was obtained as appropriate at the time of enrollment. The protocol and amendments received regulatory approvals from independent ethics committees and complied with the French regulations and the Declaration of Helsinki.
2.2. Study Design and Treatment
FLUVABREX (NCT02115074) was a national, multicentric, interventional, open-label, non-comparative and non-randomized dose-escalation study. The main objective was to determine a recommended phase II dose (RP2D) of fluvastatin when combined with celecoxib based on a dose-limiting toxicity (DLT) evaluation (primary endpoint). The secondary objectives were to assess the safety of the drug combination and pharmacokinetics (PK) of both drugs to assess the progression-free survival (PFS) and the overall survival (OS) and to describe the best tumor response according to the RANO criteria (secondary endpoints). Ten early-phase pediatric oncology centers from the French National Pediatric Oncology Society (SFCE) participated in this study.
Fluvastatin was orally given once daily from day 1 to day 14 of the 28-day cycle. Four dose levels, defined according to a previous study, were planned: 2 mg/kg/day (level 1), 4 mg/kg/day (level 2), 6 mg/kg/day (level 3) and 8 mg/kg/day (level 4) [19]. Celecoxib was orally given daily from day 1 to day 28 of each 28-day cycle at a fixed dose according to weight: 100 mg twice daily (BID) if <20 kg, 200 mg BID if 20–50 kg and 400 mg BID if >50 kg.
2.3. Dose-Escalation Phase
The dose-escalation scheme only concerned fluvastatin and was based on a Continual Reassessment Method Likelihood approach (CRML) model [28]. The dose associated with a probability of DLT closest to 25% was considered the recommended dose. The first patients were included at level 1 (2 mg/kg/day of Fluvastatin). Patients were included in cohorts of a minimum of 2 patients. For each dose level, the second patient could only be included after the first patient was evaluated. Escalation by a cohort of 2 patients was continued until the first DLT was observed. As soon as the first DLT was observed, the CRML model was activated to estimate a posteriori probabilities of toxicity associated with each dose level. The next patient was treated at the dose level corresponding to the estimated probability of DLT closest to the target, i.e., 25%, without skipping a level. The a priori probabilities of toxicities were as follows: 0.04, 0.07, 0.20, 0.33 and 0.50 for 1, 2, 4, 6 and 8 mg/kg/day of fluvastatin. If a patient stopped the treatment during the first cycle or received treatment at a reduced dose (<75% of the expected dose according to the protocol) for a reason other than toxicity, they were considered non-evaluable and was replaced.
The total number of patients depended on the dose level identified as the recommended dose as well as the number of DLTs observed at each dose level. The recommended dose for a possible phase II trial (RP2D) was defined as the dose level with a probability of DLT below 33% and the closest to 25%. The escalation phase ended when 6 evaluable patients had been included at the recommended dose.
2.4. Expansion Phase
Following completion of the dose escalation part, the protocol was amended to include patients with LGG only, and the number of subjects was increased to reach a total of 14 evaluable patients treated at the current recommended dose or upper dose in order to better characterize the safety and assess preliminary efficacy in a sufficient number of LGG patients. Patients of the expansion phase were included in the CRML model to confirm or not the recommended dose, allowing for subsequent dose re-escalation. If the dose was increased during the expansion phase, a minimum of 6 patients had to be treated and evaluated at the revised RP2D before stopping the trial.
During the dose-escalation and the expansion phases, intra-patient dose escalation was not allowed. Treatment was administered until progression or unacceptable toxicity for one year. Treatment continuation beyond one year was possible in case of clinical and/or radiological benefit (response or stabilization), depending on the patient and investigator’s decision.
2.5. Safety Evaluation
The safety of the study treatment was evaluated based on clinical and biological evaluations, including a complete blood count, biochemistry tests, liver and kidney functions and CPK levels at day 1 and day 14 during the first cycle, then at day 1 of each cycle. Toxicities were evaluated according to the NCI-CTC v4.0 criteria.
DLTs were evaluated during the first cycle (28 days) and were defined as follows: grade 3 or 4 neutropenia leading to temporary treatment discontinuation for more than 7 days, grade 3 or 4 thrombocytopenia requiring transfusions for more than 7 days or grade 3 or 4 non-hematologic toxicities. Exceptions were the following events: nausea and vomiting, grade 3 fever and grade 3 liver rapidly recovering toxicity, and grade 3 increase of CPK levels but rapidly reversible (back to <3 × normal within 2 weeks after treatment interruption). Toxicity leading to significant dose reduction (<75% dose as per protocol) was also considered as a DLT, even if the grade of toxicity did not in itself justify this classification. All adverse events were reported over the whole treatment duration except those related to the underlying disease or its progression.
2.6. Pharmacokinetic Assessments
Blood pharmacokinetic (PK) samples were collected on days 1 (D1) and 14 (D14) of cycle 1 at pre-dose, 0.5, 1, 2, 3, 4, 5, 8, 12 and 24 h post-dose of fluvastatin and 12 h after the second dose of celecoxib. Celecoxib plasma trough concentration (Ctrough) was collected on day 28 (D28) of cycle 1. Fluvastatin and celecoxib plasma concentrations were determined using a validated sensitive ultra-performance liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) method with a lower limit of quantification of 0.1 ng/mL and 10 ng/mL, respectively. Precision and accuracy were within the ±15% over the calibration range (fluvastatin from 0.1 ng/mL to 100 ng/mL and celecoxib from 10 ng/mL to 2000 ng/mL).
PK parameters of both fluvastatin and celecoxib were estimated by standard non-compartmental analysis using Monolix 2019 software (Lixoft, Orsay, France). The maximum observed plasma concentration (Cmax) and the time to maximum observed plasma concentration (Tmax) were directly determined from the plasma concentration–time profile for each patient. The area under the concentration–time curve from time zero to the last measurable concentration (AUC0-tlast), the area under the concentration–time curve extrapolated to infinity (AUC0–∞), the terminal elimination phase half-life (t1/2), the apparent oral clearance (CL/F) and the apparent volume of distribution during the terminal phase (Vz/F) were assessed. CL/F and Vz/F were normalized for body weight.
2.7. Efficacy Evaluation
Best tumor response was defined according to the RANO criteria in patients who received at least 2 cycles of treatment [27]. It was separately determined for all evaluable patients and for all patients treated at the RP2D. Brain MRI and spinal MRI, if needed, with at least 2 plans of gadolinium-enhanced T1 sequences (sagittal, axial and/or coronal), with T2 and FLAIR sequences, were performed every 3 cycles. Imaging was centrally reviewed. PFS was defined as the time from study entry to the date of progression or death, whichever occurred first. OS was defined as the time from study entry to the date of death of any cause. In the absence of any event, patients were censored at the date of the last follow-up. The distribution of follow-up was estimated using the reverse Kaplan–Meier method.
3. Results
3.1. Patient and Tumor Characteristics
From June 2014 to October 2018, 20 patients were enrolled (cf. Figure 1 and Figure 2), including 13 patients in the dose-escalation phase and 7 in the expansion phase. Characteristics of the patients are summarized in Table 1.
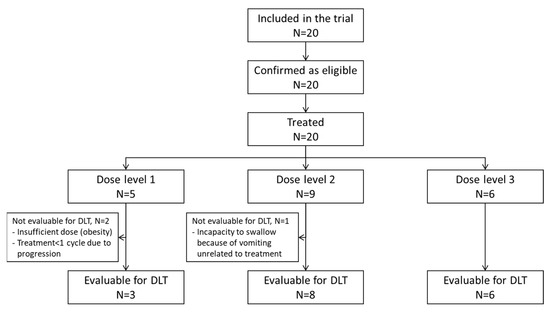
Figure 1.
CONSORT diagram.
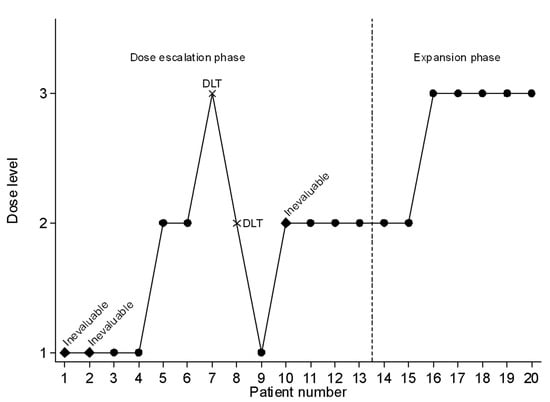
Figure 2.
Sequential inclusion of patients with the allocated dose level.

Table 1.
Characteristics of the 20 patients.
The median age of patients at inclusion is 12.5 years, with a median time from the initial diagnosis to registration in the study of 29 months (range: 3–173). Ten patients (50%) had an HGG, and ten patients (50%) had an LGG, including three NF1 patients. At inclusion, all patients had already received at least one line of chemotherapy or targeted therapy (median: 3, range 1–7). Surgery had been performed in 16 (80%) patients, and 12 (60%) patients had received prior radiation therapy.
Tumor and treatment characteristics of LGG and HGG patients are summarized in Table 2.

Table 2.
Tumor and treatment characteristics per tumor grade (LGG and HGG).
3.2. Treatment Exposure
During the dose-escalation phase, five patients were treated at dose level 1 of fluvastatin, seven patients at level 2 and one patient at level 3 (Figure 1 and Figure 2). During the expansion phase, two patients were treated at level 2 and five patients at level 3. The median number of cycles is 3.5 (range, 1–23) in the overall population, 3 (1–4) for HGG patients and 9 (1–23) for LGG patients. Among patients with LGG, three patients received 3 cycles or less, three patients received 6 to 9 cycles and four patients received 12 or more cycles (12, 18, 18 and 23 cycles).
Sixteen patients (80%) received a complete first course of treatment according to the protocol. Three (15%) patients were not evaluable for DLTs for the following reasons: lower dose received per investigator’s decision because of obesity, tumor progression during the first cycle (one patient), vomiting starting on day 4 (unrelated to study treatment) resulting in an inability to swallow the drug and leading to permanent study treatment discontinuation (one patient). One (5%) patient presented with a treatment-related, grade 3 maculopapular rash (DLT), leading to permanent treatment discontinuation after 22 days of treatment.
Most patients stopped treatment for progressive disease (n = 14, 70%), including two LGG patients who, according to the local radiological assessment, had progressive disease. Of note, both of them were later not considered as a progression by the central review, according to the RANO criteria. One patient (5%) stopped for DLT, followed by an early disease progression, and one other (5%)—as previously mentioned—stopped after 4 days of treatment because of vomiting unrelated to the study treatment. Four (20%) patients completed treatment according to the protocol with 12 cycles or more.
3.3. Toxicities
Seventeen patients (85%) were evaluable for the DLTs (Table 3). DLTs were observed in two patients during the dose-escalation phase. One patient treated at level 2 (4 mg/kg/d) presented with a grade 3 cutaneous rash after 17 days, resulting in definitive treatment discontinuation. The second patient presented with a DLT at level 3 (6 mg/kg/d) with a grade 4 increase in CPK after 13 days of treatment. The patient continued the treatment at level 2 with a reduced dose of fluvastatin for a total of 18 cycles. At the end of the escalation dose, level 2 and level 3 were associated with a probability of DLT of 20.6% (95% CI: 2.6–50.5%) and 33.6% (95% CI: 8.1–62.4%), respectively.

Table 3.
Number of patients per fluvastatin dose levels and DLTs.
In the expansion phase, the dose was finally re-escalated to dose level 3 after two patients were treated at dose level 2 with no DLT, in accordance with the study’s protocol. The last five patients were treated at dose level 3 with no DLT. At the end of the study, the probability of DLT was 9.5% (95% CI: 1.3–28.0%) at level 2, 19.8% (95% CI: 5.0–41.6%) at level 3 and 36.3% (95% CI: 15.4–57.8%) at level 4. The RP2D was therefore determined to be 6 mg/kg/day (level 3).
All patients were evaluable for toxicity. Table 4 summarizes all grades ≥ 1 toxicity related to the fluvastatin–celecoxib combination observed during the whole study period. Of note, one patient presented with perturbation of hepatic function with transaminase and bilirubin increase (grade 3). The perturbation was transient (it had recovered at the subsequent evaluation, performed 16 days later) and was not considered a DLT.

Table 4.
Treatment-related adverse events were observed during all cycles with grade and dose levels (DL) at which they occurred.
The RP2D of fluvastatin in combination with celecoxib is 6 mg/kg/d given from day 1 to day 14 in 28-day cycles. Celecoxib is given daily from day 1 to day 28 at a fixed, body-weight-adjusted dose (see Methods).
3.4. Pharmacokinetic Analysis
Fluvastatin PK data were available for 13 and 10 patients on days 1 and 14, respectively. A summary of the PK parameters at each dose level and sampling day is reported in Table 5. An important inter-individual variability was reported, but there was no evidence of drug accumulation between D1 and D14, with a ratio ranging from 0.9 to 1.8, except for two patients at 4 mg/kg (accumulation ratio of 3.2 and 3.6). Celecoxib PK parameters from day 1 were obtained for 12 patients (Table 5). PK parameters at day 14 could not be calculated for all patients due to missing samples. Celecoxib Ctrough at day 28 of treatment was available in seven patients with a mean value (CV%) of 347 ng/mL (56.5%).

Table 5.
Summary of the fluvastatin and celecoxib pharmacokinetic parameters (mean ± standard deviation unless otherwise specified).
3.5. Efficacy
A total of 18 patients were evaluable for best response. Considering the nine evaluable LGG patients, the best response was a stable disease for eight patients (89%) and progression for one patient (11%). A minor radiological response was actually seen in one patient (Figure 3).
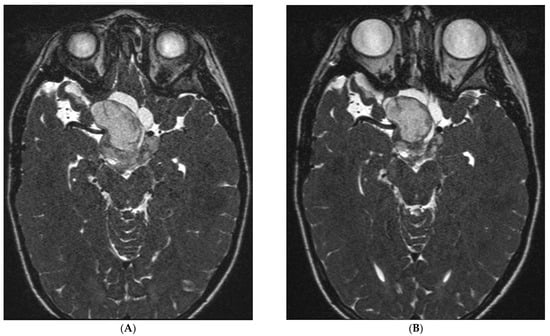
Figure 3.
MRI (T2 gadolinium) of an LGG patient at inclusion (A) and 15 weeks later after 4 cycles of fluvastatin and celecoxib (B). This 8-year-old male patient experienced stable disease and remained on treatment for 23 cycles. A minor response, not meeting the RANO criteria, was observed when comparing the MRI at inclusion (A) with the MRI at week 15 (B).
All nine evaluable patients with HGG glioma had an early disease progression. All patients were evaluable for survival analysis. The median follow-up of living patients at the latest news was 40 months (range, 16.8–83.7 months). Twelve patients (ten HGG and two LGG) died after having experienced a disease progression (four at dose level 1, seven at dose level 2 and one at dose level 3). Median OS was 13.5 months (95% CI 3.7—not achieved) in the entire study population, 7.4 months (95% CI, 1.5–13.5 months) for HGG patients and not reached for LGG patients (Figure 4A). Median PFS was 2.8 months (95% CI 1.7–7.4 months) for the entire study population, 2.1 months (95% CI 0.95–2.6) for HGG patients and not achieved for LGG patients. Among patients with LGG, four patients are alive and free of disease progression at 17, 35, 41 and 84 months after study entry. The two patients who discontinued treatment because of radiological progression not confirmed by a central review were censored at the time of their last tumor evaluation in the study at 6 and 9 months, respectively (Figure 4B).
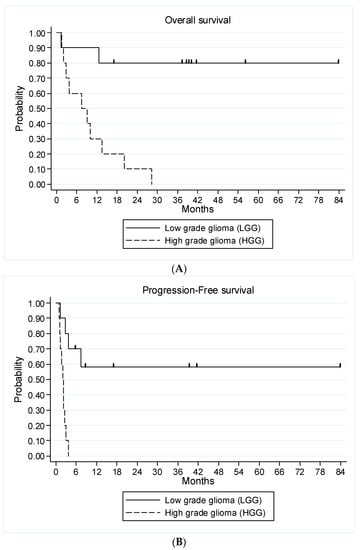
Figure 4.
Kaplan–Meier curves for overall survival (A) and progression-free survival (B) of all 20 patients according to the type of glioma.
4. Discussion
The overall therapeutic management of patients with LGG exposes them to possible transient toxicities but also to long-term sequelae [8,9,10,11,12]. New treatment modalities with MEK inhibitors, BRAF inhibitors and metronomic scheduling could lead to long-term treatment with control of the disease [26,29,30]. In this trial, we investigated an oral drug repurposing strategy to reach this goal [31]. Treatment of HGG requires new agents and new thinking because of poor prognosis [6,32]. In this phase I study, the RP2D of fluvastatin in combination with celecoxib is 6 mg/kg/d given from day 1 to day 14 in 28-day cycles with continuous fixed doses of celecoxib. We observed two DLTs: one grade 3 maculopapular rash and one grade 4 elevation of CPK. In patients with LGG, seven of ten patients received the treatment for 6 cycles or more with stable disease. No partial or complete responses were observed. No activity was seen in HGG.
The experimental treatment displayed very good tolerability. Only two patients experienced DLT during the first cycle of treatment. One patient had a maculopapular rash grade 3 at the dose of 4 mg/kg/day but continued the treatment without toxicity at the dose of 2 mg/kg/day for 17 months. The second patient had a grade 4 increase in CPK at the dose of 6 mg/kg/day and stopped treatment. No patients stopped treatment because of treatment toxicities after the first cycle. More importantly, seven out of the ten LGG patients safely received at least 6 treatment cycles. The tolerability of the experimental treatment compares favorably to other therapeutic options [13,14,15]. Ater et al. reported peripheral nervous system grade 3–4 toxicity for 19% of patients treated with vincristine [15]. In a European randomized study, 84% of patients experienced grade 3–4 hematologic toxicity, 10% had an allergic reaction to carboplatin and 24% had grade 3–4 infections [13]. No patients treated with fluvastatin/celecoxib experienced hematologic toxicity or neurotoxicity. Verschuur et al. used celecoxib in a multidrug metronomic regimen in LGG patients and reported grade 3–4 neutropenia in 11 of 18 patients [26]. BRAF inhibitors offer a better safety profile, with the most frequent grade 3–4 adverse events being elevated Creatine Phospho-Kinase (10%) and maculopapular rash (10%) [30]. Similarly, the most frequent toxicities of MEK inhibitors were minor to moderately severe skin rash and gastrointestinal symptoms [29]. Here, we confirm the good safety profile of fluvastatin that López-Aguilar et al. reported in their pediatric cancer trial, with only low-grade gastrointestinal toxicity and myalgia [19].
The high inter-individual variability of fluvastatin we observed is consistent with prior PK findings [33]. Of note, no PK data are available for fluvastatin in the pediatric population, but PK parameters were consistent with those reported in the adult population except for the half-life, which was two-fold longer in our study (2.3 ± 0.9 h in adults) [34].
In terms of efficacy in LGG, 6-month PFS was 70% in this heavily pre-treated population. We did not observe any objective response, though seven of the ten patients received treatment for ≥6 cycles, and four patients were alive and free of progression with long follow-ups. Several therapeutic approaches have greater response rates and PFS than in our study. Gururangan et al. reported 3/30 (10%) partial responses (PR) and a 2-year PFS of 49% in patients treated with temozolomide [35]. In a metronomic phase II trial, Verschuur et al. reported two PR and six stable diseases (SD) in 10 LGG patients, including patients who had relapsed after or progressed on vinblastine. Additionally, 2-year PFS was 70%, and seven patients continued treatment beyond one year [26]. Bouffet et al. reported one CR and ten PR in 50 patients (22%) receiving weekly vinblastine with a 2-year PFS of 62% [14]. A recent PBTC study reported 2/35 (6%) PR and a 2-year PFS of 48% (±9%) for bevacizumab with irinotecan (B + I), but most patients relapse within 5 months after treatment cessation [36,37,38]. Roux et al. treated 16 patients with B + I, followed by metronomic maintenance with weekly vinblastine. After a median follow-up of 3.9 years after B + I cessation, nine of the sixteen patients were progression-free [39,40]. Because of the SD observed in our LGG patients, we think that the association of celecoxib and fluvastatin may represent an interesting maintenance approach for a patient treated with bevacizumab as those patients rapidly progressed after stopping treatment [37,38]. The potential place of this combination in the context of targeted therapies for LGG could also be explored. Aberrations of the MAPK pathway are key to oncogenesis in low-grade gliomas [41]. Sustained responses (36–40%) have now been observed in early-phase trials with MEK inhibitors, even in patients with multiple recurrences [29,30,42]. In our study, three patients presented with a KIAA1549-BRAF gene fusion. Interestingly, an 8-year-old male LGG patient had progressed on a MEK inhibitor before joining our trial. The patient experienced stable disease for 23 months while on treatment. A minor radiological response is actually seen in this patient (Figure 1). Modulation of autophagy might be an explanation of the observed response to the combination as was observed for chloroquine, though no strong biological data have been reported to date to support this hypothesis for fluvastatin and celecoxib [43,44].
5. Conclusions
In conclusion, this combination of fluvastatin with celecoxib displayed a good safety profile with interesting preliminary activity in children with LGG. Its use as a maintenance treatment may be worthy of further investigation in children with LGG. Of note, both drug repositioning and metronomic chemotherapy have been proposed as interesting therapeutic options for patients with cancer living in low- and middle-income countries (LMIC) as these treatments are orally available, are inexpensive, and display only limited toxicities [45]. Recently an international survey performed among pediatric oncologist working in LMIC has confirmed a growing interest in these strategies, which therefore represents a unique opportunity and shall be evaluated properly in this specific setting [46,47].
Author Contributions
Conceptualization, P.L. and N.A.; methodology, M.-C.L.D. and E.T.-B.; formal analysis, M.-C.L.D., E.T.-B., T.B., P.L., C.S., N.N. and N.A.; writing—original draft preparation, P.L., G.B. and N.A.; N.N. and C.S. were in charge of all PK analyses and their statistical correlations. writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by an INCa PHRC-K grant and a grant from the Anticancer Fund (grant A29).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee CPP Nord-Ouest IV (23/APR/2020), and the French National Medicines Safety Agency ANSM (avis favorable 07/APR/ 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data supporting reported results can be obtained on request from the corresponding author.
Acknowledgments
We are indebted to all patients and families for their participation in this study; staff members Fanny Ben Oune, Julie Courtial, Caroline Decamps and Marie Vanseymortier, sponsorship unit at the Centre Oscar Lambret, Lille, France; methodologists and biostatisticians Andrew Kramar, André Michel Bimbai and Emilie Bogart; Diane Braguer, Xavier Paoletti and Nicolas Sirvent for participating in the Independent Data Monitoring Committee; Dory-Lautrec for his help in the radiological review; and data managers from the Centre de Traitement des Données du Cancéropôle Nord-Ouest (CTD-CNO) in charge of the trial data management. The CTD-CNO clinical research platform was funded by the French National Cancer Institute (INCa) and the “La Ligue Nationale Contre le Cancer”. We thank all funders, investigators and teams who participated in this trial.
Conflicts of Interest
G.B., E.T.-B., M.-C.L.D., A.P., N.N., C.S., A.S., T.B., I.A., C.F.-C., A.-I.B., P.C., N.E.-W. and E.D.C. have no conflict of interest to declare. N.A. reports receiving grants and drugs for trials from Bristol Myers Squibb and Pierre Fabre Oncology, receiving travel support from Bristol Myers Squibb for an International Society of Paediatric Oncology meeting and participating as a scientific advisory board member (without receiving personal fees) for Bayer and Bristol Myers Squibb and Partners Therapeutics related to metronomic chemotherapy. P.L. reports receiving drugs for a clinical trial from Pierre Fabre Oncology, receiving drugs and grants for a clinical trial from Bristol Myers Squibb and participating as a scientific advisory board member (receiving personal fees) for AstraZeneca.
References
- Northcott, P.A.; Pfister, S.M.; Jones, D.T.W. Next-generation (epi)genetic drivers of childhood brain tumours and the outlook for targeted therapies. Lancet Oncol. 2015, 16, e293–e302. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; de Blank, P.M.; Kruchko, C.; Petersen, C.M.; Liao, P.; Finlay, J.L.; Stearns, D.S.; Wolff, J.E.; Wolinsky, Y.; Letterio, J.J.; et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro Oncol. 2015, 16 (Suppl. 1), x1–x36. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhayay, P.; Bergthold, G.; London, W.B.; Goumnerova, L.C.; Morales La Madrid, A.; Marcus, K.J.; Guo, D.; Ullrich, N.J.; Robison, N.J.; Chi, S.N.; et al. Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: An analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr. Blood Cancer 2014, 61, 1173–1179. [Google Scholar] [CrossRef] [PubMed]
- Krishnatry, R.; Zhukova, N.; Guerreiro Stucklin, A.S.; Pole, J.D.; Mistry, M.; Fried, I.; Ramaswamy, V.; Bartels, U.; Huang, A.; Laperriere, N.; et al. Clinical and treatment factors determining long-term outcomes for adult survivors of childhood low-grade glioma: A population-based study. Cancer 2016, 122, 1261–1269. [Google Scholar] [CrossRef]
- Colin, C.; Padovani, L.; Chappé, C.; Mercurio, S.; Scavarda, D.; Loundou, A.; Frassineti, F.; Andre, N.; Bouvier, C.; Korshunov, A.; et al. Outcome analysis of childhood pilocytic astrocytomas: A retrospective study of 148 cases at a single institution. Neuropathol. Appl. Neurobiol. 2013, 39, 693–705. [Google Scholar] [CrossRef]
- Jones, C.; Karajannis, M.A.; Jones, D.T.W.; Kieran, M.W.; Monje, M.; Baker, S.J.; Becher, O.J.; Cho, Y.J.; Gupta, N.; Hawkins, C.; et al. Pediatric high-grade glioma: Biologically and clinically in need of new thinking. Neuro Oncol. 2017, 19, 153–161. [Google Scholar] [CrossRef]
- Fried, I.; Tabori, U.; Tihan, T.; Reginald, A.; Bouffet, E. Optic pathway gliomas: A review. CNS Oncol. 2013, 2, 143–159. [Google Scholar] [CrossRef]
- Ris, M.D.; Beebe, D.W.; Armstrong, F.D.; Fontanesi, J.; Holmes, E.; Sanford, R.A.; Wisoff, J.H.; Children’s Oncology Group. Cognitive and adaptive outcome in extracerebellar low-grade brain tumors in children: A report from the Children’s Oncology Group. J. Clin. Oncol. 2008, 26, 4765–4770. [Google Scholar] [CrossRef]
- Tacke, U.; Karger, D.; Spreer, J.; Berlis, A.; Nikkhah, G.; Korinthenberg, R. Incidence of vasculopathy in children with hypothalamic/chiasmatic gliomas treated with brachytherapy. Childs Nerv. Syst. 2011, 27, 961–966. [Google Scholar] [CrossRef]
- Grill, J.; Couanet, D.; Cappelli, C.; Habrand, J.L.; Rodriguez, D.; Sainte-Rose, C.; Kalifa, C. Radiation-induced cerebral vasculopathy in children with neurofibromatosis and optic pathway glioma. Ann. Neurol. 1999, 45, 393–396. [Google Scholar] [CrossRef]
- Perkins, S.M.; Fei, W.; Mitra, N.; Shinohara, E.T. Late causes of death in children treated for CNS malignancies. J. Neurooncol. 2013, 115, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, B.A.; Pulsifer, M.B.; Ebb, D.H.; MacDonald, S.M.; Jones, R.M.; Butler, W.E.; Huang, M.S.; Marcus, K.J.; Oberg, J.A.; Tarbell, N.J.; et al. Clinical outcomes and late endocrine, neurocognitive, and visual profiles of proton radiation for pediatric low-grade gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Gnekow, A.K.; Walker, D.A.; Kandels, D.; Picton, S.; Perilongo, G.; Grill, J.; Stokland, T.; Sandstrom, P.E.; Warmuth-Metz, M.; Pietsch, T.; et al. A European randomised controlled trial of the addition of etoposide to standard vincristine and carboplatin induction as part of an 18-month treatment programme for childhood (≤16 years) low grade glioma—A final report. Eur. J. Cancer 2017, 81, 206–225. [Google Scholar] [CrossRef] [PubMed]
- Bouffet, E.; Jakacki, R.; Goldman, S.; Hargrave, D.; Hawkins, C.; Shroff, M.; Hukin, J.; Bartels, U.; Foreman, N.; Kellie, S.; et al. Phase II study of weekly vinblastine in recurrent or refractory pediatric low-grade glioma. J. Clin. Oncol. 2012, 30, 1358–1363. [Google Scholar] [CrossRef]
- Ater, J.L.; Zhou, T.; Holmes, E.; Mazewski, C.M.; Booth, T.N.; Freyer, D.R.; Lazarus, K.H.; Packer, R.J.; Prados, M.; Sposto, R.; et al. Randomized study of two chemotherapy regimens for treatment of low-grade glioma in young children: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 2641–2647. [Google Scholar] [CrossRef]
- Lu, V.M.; Welby, J.P.; Nesvick, C.L.; Daniels, D.J. Efficacy and safety of bevacizumab in progressive pediatric low-grade glioma: A systematic review and meta-analysis of outcome rates. Neuro-Oncol. Pract. 2020, 7, 359–368. [Google Scholar] [CrossRef]
- Tchoghandjian, A.; Fernandez, C.; Colin, C.; El Ayachi, I.; Voutsinos-Porche, B.; Fina, F.; Scavarda, D.; Piercecchi-Marti, M.-D.; Intagliata, D.; Ouafik, L.; et al. Pilocytic astrocytoma of the optic pathway: A tumour deriving from radial glia cells with a specific gene signature. Brain 2009, 132, 1523–1535. [Google Scholar] [CrossRef]
- Mercurio, S.; Padovani, L.; Colin, C.; Carre, M.; Tchoghandjian, A.; Scavarda, D.; Lambert, S.; Baeza-Kallee, N.; Fernandez, C.; Chappe, C.; et al. Evidence for new targets and synergistic effect of metronomic celecoxib/fluvastatin combination in pilocytic astrocytoma. Acta Neuropathol. Commun. 2013, 1, 17. [Google Scholar] [CrossRef]
- López-Aguilar, E.; Sepúlveda-Vildósola, A.C.; Rivera-Márquez, H.; Cerecedo-Diaz, F.; Valdez-Sánchez, M.; Villasis-Keever, M.A. Security and maximal tolerated doses of fluvastatin in pediatric cancer patients. Arch. Med. Res. 1999, 30, 128–131. [Google Scholar] [CrossRef]
- López-Aguilar, E.; Sepúlveda-Vildósola, A.C.; Betanzos-Cabrera, Y.; Rocha-Moreno, Y.G.; Gascon-Lastiri, G.; Rivera-Marquez, H.; Wanzke-del-Angel, V.; Cerecedo-Diaz, F.; de la Cruz-Yanez, H. Phase II Study of Metronomic Chemotherapy with Thalidomide, Carboplatin-Vincristine-Fluvastatin in the Treatment of Brain Stem Tumors in Children. Arch. Med. Res. 2008, 39, 655–662. [Google Scholar] [CrossRef]
- Silverstein, R.L.; Febbraio, M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009, 2, re3. [Google Scholar] [CrossRef]
- Sławińska-Brych, A.; Zdzisińska, B.; Kandefer-Szerszeń, M. Fluvastatin inhibits growth and alters the malignant phenotype of the C6 glioma cell line. Pharmacol. Rep. 2014, 66, 121–129. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Huang, L.-C.; Chen, Y.-C.; Wang, Y.-W.; Hueng, D.-Y.; Huang, S.-M. The synergistic effects of valproic acid and fluvastatin on apoptosis induction in glioblastoma multiforme cell lines. Int. J. Biochem. Cell Biol. 2017, 92, 155–163. [Google Scholar] [CrossRef]
- Tołoczko-Iwaniuk, N.; Dziemiańczyk-Pakieła, D.; Nowaszewska, B.K.; Celińska-Janowicz, K.; Miltyk, W. Celecoxib in Cancer Therapy and Prevention-Review. Curr. Drug Targets 2019, 20, 302–315. [Google Scholar] [CrossRef]
- Sato, A.; Mizobuchi, Y.; Nakajima, K.; Shono, K.; Fujihara, T.; Kageji, T.; Kitazato, K.; Matsuzaki, K.; Mure, H.; Kuwayama, K.; et al. Blocking COX-2 induces apoptosis and inhibits cell proliferation via the Akt/survivin- and Akt/ID3 pathway in low-grade-glioma. J. Neurooncol. 2017, 132, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Verschuur, A.; Heng-Maillard, M.-A.; Dory-Lautrec, P.; Truillet, R.; Jouve, E.; Chastagner, P.; Leblond, P.; Aerts, I.; Honore, S.; Entz-Werle, N.; et al. Metronomic Four-Drug Regimen Has Anti-tumor Activity in Pediatric Low-Grade Glioma; The Results of a Phase II Clinical Trial. Front. Pharmacol. 2018, 9, 00950. [Google Scholar] [CrossRef] [PubMed]
- van den Bent, M.J.; Wefel, J.S.; Schiff, D.; Taphoorn, M.J.B.; Jaeckle, K.; Armstrong, T.; Choucair, A.; Waldman, A.D.; Gorlia, T.; Chamberlain, M.; et al. Response assessment in neuro-oncology (a report of the RANO group): Assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011, 12, 583–593. [Google Scholar] [CrossRef]
- Iasonos, A.; O’Quigley, J. Adaptive dose-finding studies: A review of model-guided phase I clinical trials. J. Clin. Oncol. 2014, 32, 2505–2511. [Google Scholar] [CrossRef]
- Banerjee, A.; Jakacki, R.I.; Onar-Thomas, A.; Wu, S.; Nicolaides, T.; Poussaint, T.Y.; Fangusaro, J.; Phillips, J.; Perry, A.; Turner, D.; et al. A phase I trial of the MEK inhibitor selumetinib (AZD6244) in pediatric patients with recurrent or refractory low-grade glioma: A Pediatric Brain Tumor Consortium (PBTC) study. Neuro Oncol. 2017, 19, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Fangusaro, J.; Onar-Thomas, A.; Young Poussaint, T.; Wu, S.; Ligon, A.H.; Lindeman, N.; Banerjee, A.; Packer, R.J.; Kilburn, L.B.; Glodman, S.; et al. Selumetinib in paediatric patients with BRAF-aberrant or neurofibromatosis type 1-associated recurrent, refractory, or progressive low-grade glioma: A multicentre, phase 2 trial. Lancet Oncol. 2019, 20, 1011–1022. [Google Scholar] [CrossRef]
- Bertolini, F.; Sukhatme, V.P.; Bouche, G. Drug repurposing in oncology--patient and health systems opportunities. Nat. Rev. Clin. Oncol. 2015, 12, 732–742. [Google Scholar] [CrossRef]
- Kline, C.; Felton, E.; Allen, I.E.; Tahir, P.; Mueller, S. Survival outcomes in pediatric recurrent high-grade glioma: Results of a 20-year systematic review and meta-analysis. J. Neurooncol. 2018, 137, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Fluvastatin-Summary of Product Characteristics. Available online: https://www.medicines.org.uk/emc/product/4465/smpc (accessed on 8 February 2023).
- Stempak, D.; Gammon, J.; Klein, J.; Koren, G.; Baruchel, S. Single-dose and steady-state pharmacokinetics of celecoxib in children. Clin. Pharmacol. Ther. 2002, 72, 490–497. [Google Scholar] [CrossRef] [PubMed]
- de Blank, P.; Bandopadhayay, P.; Haas-Kogan, D.; Fouladi, M.; Fangusaro, J. Management of pediatric low-grade glioma. Curr. Opin. Pediatr. 2019, 31, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Gururangan, S.; Fangusaro, J.; Poussaint, T.Y.; McLendon, R.E.; Onar-Thomas, A.; Wu, S.; Packer, R.J.; Banerjee, A.; Gilbertson, R.J.; Fahey, F.; et al. Efficacy of bevacizumab plus irinotecan in children with recurrent low-grade gliomas--a Pediatric Brain Tumor Consortium study. Neuro Oncol. 2014, 16, 310–317. [Google Scholar] [CrossRef]
- Hwang, E.I.; Jakacki, R.I.; Fisher, M.J.; Kilburn, L.B.; Horn, M.; Vezina, G.; Rood, B.R.; Packer, R.J. Long-term efficacy and toxicity of bevacizumab-based therapy in children with recurrent low-grade gliomas. Pediatr. Blood Cancer 2013, 60, 776–782. [Google Scholar] [CrossRef]
- Couec, M.-L.; André, N.; Thebaud, E.; Minckes, O.; Rialland, X.; Corradini, N.; Aerts, I.; Marec-Berard, P.; Bourdeaut, F.; Leblond, P.; et al. Bevacizumab and irinotecan in children with recurrent or refractory brain tumors: Toxicity and efficacy trends. Pediatr. Blood Cancer 2012, 59, 34–38. [Google Scholar] [CrossRef]
- Roux, C.; Revon-Rivière, G.; Gentet, J.C.; Verschuur, A.; Scavarda, D.; Saultier, P.; Appay, R.; Padovani, L.; Andre, N. Metronomic Maintenance with Weekly Vinblastine after Induction With Bevacizumab-Irinotecan in Children with Low-grade Glioma Prevents Early Relapse. J. Pediatr. Hematol. Oncol. 2021, 43, e630–e634. [Google Scholar] [CrossRef] [PubMed]
- Heng, M.A.; Padovani, L.; Dory-Lautrec, P.; Gentet, J.-C.; Verschuur, A.; Pasquier, E.; Figarella-Branger, D.; Scavarda, D.; Andre, N. Can metronomic maintenance with weekly vinblastine prevent early relapse/progression after bevacizumab-irinotecan in children with low-grade glioma? Cancer Med. 2016, 5, 1542–1545. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, G.; Miller, C.P.; Tatevossian, R.G.; Dalton, J.D.; Tang, B.; Orisme, W.; Punchihewa, C.; Parker, M.; Qaddoumi, I.; et al. Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat. Genet. 2013, 45, 602–612. [Google Scholar]
- Kondyli, M.; Larouche, V.; Saint-Martin, C.; Ellezam, B.; Pouliot, L.; Sinnett, D.; Legault, G.; Crevier, L.; Weil, A.; Farmer, J.P.; et al. Trametinib for progressive pediatric low-grade gliomas. J. Neurooncol. 2018, 140, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Thompson, J.C.; Griesinger, A.M.; Amani, V.; Donson, A.D.; Birks, D.K.; Morgan, M.J.; Mirsky, D.M.; Handler, M.H.; Foreman, N.K.; et al. Autophagy Inhibition Improves Chemosensitivity in BRAFV600E Brain Tumors. Cancer Discov. 2014, 4, 773–780. [Google Scholar] [CrossRef]
- Mulcahy Levy, J.M.; Zahedi, S.; Griesinger, A.M.; Morin, A.; Davies, K.D.; Aisner, D.L.; Kleinschmidt-DeMasters, B.K.; Fitzwalter, B.E.; Goodall, M.L.; Thorburn, J.; et al. Autophagy inhibition overcomes multiple mechanisms of resistance to BRAF inhibition in brain tumors. Elife. 2017, 6, 358–367. [Google Scholar] [CrossRef]
- André, N.; Banavali, S.; Snihur, Y.; Pasquier, E. Has the time come for metronomics in low-income and middle-income countries? Lancet Oncol. 2013, 14, e239–e248. [Google Scholar] [CrossRef] [PubMed]
- Revon-Rivière, G.; Banavali, S.; Heississen, L.; Gomez Garcia, W.; Abdolkarimi, B.; Vaithilingum, M.; Li, C.-K.; Leung, P.C.; Malik, P.; Pasquier, E.; et al. Metronomic Chemotherapy for Children in Low- and Middle-Income Countries: Survey of Current Practices and Opinions of Pediatric Oncologists. J. Glob. Oncol. 2019, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Bouche, G.; Crisp, N.; Andre, N. Challenges and opportunities for cancer clinical trials in low- and middle-income countries. Nat. Cancer 2020, 1, 142–145. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).