Simple Summary
Women with BRCA1 or BRCA2 gene mutations are at increased risk of breast and ovarian cancer and often undergo operations to remove both their ovaries in order to prevent ovarian cancer. The impact of this operation on breast cancer risk is uncertain; thus, we performed a systematic review and meta-analysis to determine it. We found that this operation was not linked with a reduced risk of developing breast cancer when considering both BRCA1 and BRCA2 carriers together but was linked with a reduced risk of breast cancer when considering BRCA2 carriers alone. If a woman had this operation after developing breast cancer, it was not related to a reduced chance of developing cancer in the other breast. However, it was associated with increased survival following breast cancer when considering BRCA1 and BRCA2 carriers together and BRCA1 carriers alone. These findings may have important implications for counselling for women in the clinic.
Abstract
Background: Risk-reducing salpingo-oophorectomy (RRSO) is the gold standard method of ovarian cancer risk reduction, but the data are conflicting regarding the impact on breast cancer (BC) outcomes. This study aimed to quantify BC risk/mortality in BRCA1/BRCA2 carriers after RRSO. Methods: We conducted a systematic review (CRD42018077613) of BRCA1/BRCA2 carriers undergoing RRSO, with the outcomes including primary BC (PBC), contralateral BC (CBC) and BC-specific mortality (BCSM) using a fixed-effects meta-analysis, with subgroup analyses stratified by mutation and menopause status. Results: RRSO was not associated with a significant reduction in the PBC risk (RR = 0.84, 95%CI: 0.59–1.21) or CBC risk (RR = 0.95, 95%CI: 0.65–1.39) in BRCA1 and BRCA2 carriers combined but was associated with reduced BC-specific mortality in BC-affected BRCA1 and BRCA2 carriers combined (RR = 0.26, 95%CI: 0.18–0.39). Subgroup analyses showed that RRSO was not associated with a reduction in the PBC risk (RR = 0.89, 95%CI: 0.68–1.17) or CBC risk (RR = 0.85, 95%CI: 0.59–1.24) in BRCA1 carriers nor a reduction in the CBC risk in BRCA2 carriers (RR = 0.35, 95%CI: 0.07–1.74) but was associated with a reduction in the PBC risk in BRCA2 carriers (RR = 0.63, 95%CI: 0.41–0.97) and BCSM in BC-affected BRCA1 carriers (RR = 0.46, 95%CI: 0.30–0.70). The mean NNT = 20.6 RRSOs to prevent one PBC death in BRCA2 carriers, while 5.6 and 14.2 RRSOs may prevent one BC death in BC-affected BRCA1 and BRCA2 carriers combined and BRCA1 carriers, respectively. Conclusions: RRSO was not associated with PBC or CBC risk reduction in BRCA1 and BRCA2 carriers combined but was associated with improved BC survival in BC-affected BRCA1 and BRCA2 carriers combined and BRCA1 carriers and a reduced PBC risk in BRCA2 carriers.
1. Introduction
BRCA1 and BRCA2 mutation carriers have a ~17–44% risk of ovarian cancer (OC) and ~69–72% risk of breast cancer (BC) [1,2,3,4]. BRCA carriers can benefit from lifestyle and reproductive advice incorporating breast feeding, contraception and informed reproductive decision making, including preimplantation genetic diagnosis (PGD) [5]. Risk-reducing mastectomy (RRM) [6], screening (breast MRI/mammography) and medical prevention (selective oestrogen receptor modulators) are available options used to reduce BC risk [7,8]. Primary surgical prevention in the form of risk-reducing salpingo-oophorectomy (RRSO) is the most effective option and gold standard for OC risk reduction, especially given the absence of an effective national OC screening program. It is associated with reductions in epithelial OC risk (80–95%) [9,10,11,12] and all-cause (60–76%) and OC-specific (75–95%) mortality. It is associated with minimal surgical morbidity and is usually undertaken through minimal access surgery, including laparoscopic, robotic and other novel approaches [13,14].
Various RRSO uptake rates reaching as high as 78% have been reported amongst BRCA1/BRCA2 mutation carriers [9,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. Decision making is a complex and dynamic process which changes with time and is influenced by multiple factors. Premenopausal RRSO leads to premature surgical menopause, which has detrimental long-term health sequelae (increased risk of heart disease, osteoporosis, vasomotor symptoms, sexual dysfunction, neurocognitive decline), particularly if women are unable to use hormone replacement-therapy (HRT) for reason such as a personal history of BC [46,47,48,49,50,51,52,53,54,55,56]. The impact of RRSO on BC risk is a critically important factor for women considering surgical prevention [57,58,59]. Earlier publications have suggested that RRSO is associated with a 46–62% reduction in primary BC risk, 41–59% reduction in contralateral BC risk and a 54–90% reduction in BC-specific mortality [10,11,22,28,58,60,61,62,63,64,65]. However, more recent data have led researchers to question this benefit of a reduction in BC risk [66,67,68,69]. As randomised controlled trials (RCTs) investigating the health effects of risk-reducing surgeries are unethical and unacceptable in the case of BRCA carriers, evaluations of efficacy are restricted to observational studies. Consequently, the risk estimates are subject to additional potential biases. Several methodological issues have been identified, which may have affected risk estimates, leading to conflicting results. These include the study design (retrospective/prospective samples; case-control/cohort studies), differing inclusion criteria, differing sample sizes and different types of selection bias (indication, cancer-induced testing, immortal person-time, familial event biases) [67,70,71].
Accurate information on the pros/cons and efficacy of risk reduction, along with the side effects and surgical risks, must form the basis of informed counselling for surgical prevention offered to BRCA carriers. Despite the methodological limitations of studies investigating the health effects of RRSO, the data consistently show a reduction in OC risk. However, given the existence of contradictory data, the same is not true for the impact on BC risk following RRSO. In order to aid clinicians in counselling BRCA carriers faced with the decision as to whether or not and when to undergo RRSO and to help patients make informed decisions, we undertook a systematic review of the available evidence regarding the association between RRSO and the risk of BC development. This is particularly important given the recent conflicting data. The aim of this review is to summarise the published evidence of BC outcomes following RRSO in BRCA carriers.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
In this systematic review, we used a comprehensive three-step search strategy to identify relevant studies. Using the NICE Evidence Service’s healthcare databases advanced search (HDAS) tool, first, we simultaneously searched the following seven databases from the time of inception to 14 June 2022: Pubmed, Medline, Embase, CINAHL, PsycINFO, PROSEPRO and Cochrane. A common search strategy (Supplementary Materials, Table S1) was developed for database searching via HDAS using a combination of free text and controlled vocabulary (MeSH terms). Second, reference lists of publications retrieved in the first step were screened for relevant studies. Third, we searched additional web-based platforms, including specialised journals, Google searches for grey literature, conference proceedings and clinical trial registries (ISRCTN and ClinicalTrials.gov [accessed on 14 June 2022]).
To increase the sensitivity of our search, no restriction was placed on language, geographical location, year of publication or the type of study. The search was limited to human studies and re-run prior to the final analyses in order to ensure that recently published studies were retrieved for inclusion.
The articles were independently screened by two authors (FG, AT) in two stages after all the identified references were transferred into a reference management software package (EndNote X8.2, Clarivate Analytics). The titles and abstracts were screened, followed by the retrieval and screening of the full-text articles, fulfilling the eligibility criteria described below. Inter-rater reliability was analysed using quantity (Q) and allocation (A) disagreements [72]. Disagreements were resolved by consensus or arbitration by a third reviewer (RM).
The predefined inclusion criteria were female BRCA1 and BRCA2 mutation carriers undergoing RRSO. The outcomes investigated were: (1) primary breast cancer (PBC), defined as the risk of invasive BC occurring in a previously unaffected individual; (2) contralateral breast cancer (CBC), defined as the risk of a second case of primary invasive BC occurring in a previously unaffected contralateral breast; and (3) BC-specific mortality (BCSM), defined as cause of death due to BC. We excluded studies that included participants who had a personal history of OC or had undergone prophylactic RRM prior to RRSO, as well as abstracts.
2.2. Data Extraction and Quality Assessment
Data were extracted by two reviewers (FG, AT) using a standardised, predesigned data extraction sheet in Microsoft Excel 2013. FG extracted data from the publications, and AT crosschecked the data for accuracy. Four main categories of data were extracted: methodological characteristics, study population, surgical interventions (RRSO/RRM) and reported outcome measures pertaining to PBC/CBC risk and BCSM. The data extraction sheet was piloted and refined before extraction. In cases where studies reported both adjusted and unadjusted data, both were collected. The risk of bias was assessed by the reviewers (FG, OB) using the Newcastle–Ottawa Scale (NOS) [73]. A low risk of bias was attributed to studies that scored four stars for selection, two stars for comparability and three stars for ascertainment of the outcome/exposure. A medium risk of bias was allocated to studies with two to three stars for selection, one for comparability and two for outcome/exposure ascertainment. All studies with scores of one for selection or outcome/exposure ascertainment or zero for any of the three domains were regarded as having a high risk of bias [74]. GRADE (Grading of Recommendations, Assessment, Development and Evaluations) was used to assess the overall quality of the evidence for each outcome. Each outcome was assigned a level of certainty in terms of the evidence. “Very low” was defined as the true effect, probably being markedly different from the estimated effect; “low” was defined as the true effect, which might be markedly different from the estimated effect; “moderate” was defined as the true effect, probably being close to the estimated effect; and “high” was defined as the true effect, being similar to the estimated effect.
2.3. Data Analysis
We tabulated the characteristics and reported outcome measures of all the studies for the qualitative synthesis of the data. No studies were excluded from the qualitative data synthesis based on the risk of bias scores. The decision to perform a meta-analysis (quantitative data synthesis) was made a posteriori to ensure that a sufficient number of studies with similar characteristics were available. Case-control studies were excluded from the quantitative synthesis due to their less robust study design, smaller number of outcome events and higher risk of bias. For quantitative synthesis, we compared the BC outcomes in BRCA1 and BRCA2 carriers undergoing RRSO with those of BRCA1 and BRCA2 carriers not undergoing RRSO. As the studies varied in their outcome measures, to ensure comparability between studies, the relative risk (RR) was calculated using raw data independently extracted by the authors, FG, OB or RM, using 2 × 2 tables. FG and OB extracted the data, and RM crosschecked the data for accuracy. The investigators were contacted for those studies in which raw data were missing from the published manuscript. In instances where two or more studies had overlapping datasets, the study with the least risk of bias or highest quality was used for pooling. In instances where the risk of bias/quality of the study was deemed to be equivalent between overlapping studies, the study with the largest number of events was included. All the analyses were performed using the package “meta” of the R Studio software (version 3.5.1). Since the studies differed in terms of the year of study, geographical location, confounders and reported measurements of the effect size (hazard ratio (HR)/odds ratio (OR)/relative risk (RR)), the relative risks and 95% confidence intervals calculated from the raw data were pooled based on a random effects model. The DerSimonian–Laird estimate was used to assess the between-study variance. To determine the extent of inter-study variation, we performed heterogeneity tests with Higgins’ I2 statistic to measure the proportion of the observed variance that reflected the true effect sizes [75]. An I2 ≥50% was considered to represent significant inter-study variation [76].
A baseline analysis was performed to examine the PBC risk, CBC risk and BCSM amongst both BRCA1 and BRCA2 carriers. A subgroup analysis was performed according to BRCA and menopause status (women aged <50 years were assumed to be premenopausal and those aged ≥50 years were assumed to be postmenopausal). It was not possible to investigate sources of model heterogeneity because of the small number of studies in each analysis.
We calculated numbers-needed-to-treat (NNT) values for all the statistically significant outcomes using the ‘treat as one trial’ approach: , for , and , for , where —pooled relative risk and —risk for the control (unexposed) group. The NNT values, together with 95% confidence intervals, were calculated for the minimum, maximum and mean across the correspondent studies.
Our work conformed to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Details of the protocol were registered prospectively in the international PROSPERO database (registration number CRD42018077613). Our work was exempt from Institutional Review Board approval, as our review summarizes already published data.
3. Results
3.1. Study Selection and Characteristics
Searches of electronic databases and reference lists of 789 generated references (Figure 1).
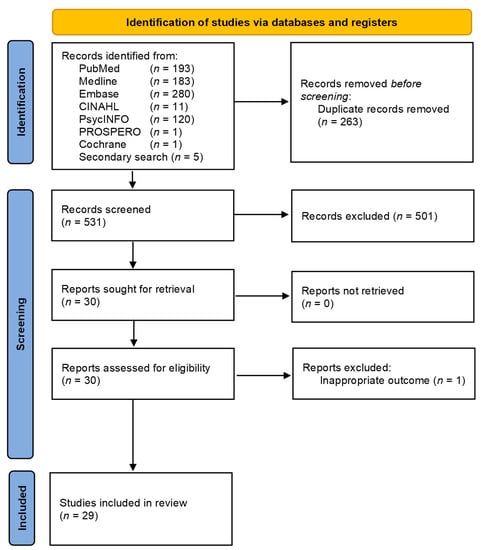
Figure 1.
PRISMA flow diagram of study selection [77].
On evaluation of all the titles and abstracts, 30/789 articles (3.8%) were potentially eligible for detailed assessment [9,10,11,22,24,57,58,59,60,61,62,63,64,65,66,67,68,69,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95]. A total of 29/30 met the predefined inclusion criteria for qualitative synthesis (Table 1). One study was excluded due to the fact it reported non-BC-related outcomes.

Table 1.
Qualitative data synthesis of studies reporting breast cancer outcomes following oophorectomy in BRCA carriers.
There were high levels of agreement between the reviews (Q = 1/29, A = 2/29). Sixteen studies reported the PBC risk [10,11,22,58,60,61,62,65,67,68,79,82,93,94,95,97]. Of these studies, there were three groups of overlapping datasets: (1) the PROSE consortium datasets: Finkelman et al. 2012 [22], Kauff et al. 2008 [11], Rebbeck et al. 1999 [65], Rebbeck et al. 2002 [10] and Domchek et al. 2010 [58]; (2) the datasets of Eisen et al. 2005 [60], Kotsopoulos et al. 2012 [61] and Kotsopoulos et al. 2017 [68]; and (3) the datasets of Chang-Claude et al. 2007 [79], Mavaddat et al. 2013 [62], Mavaddat et al. 2020 [97], Heemskerk-Gerritsen et al. 2015 [67], Choi et al. 2021 [93] and Terry et al. 2018 [95]. Six studies reported the CBC risk [58,62,64,66,69,84], with Metcalfe et al. 2004 [64], Metcalfe et al. 2011 [63] and Kotsopoulos et al. 2019 [69] having overlapping datasets. Seven studies reported on BCSM [21,57,58,59,89,90,92], with Domchek et al. 2006 [57] and Domchek et al. 2010 [58] sharing the same dataset. All 29 studies were observational, with no RCT. Five studies were case-control studies, one was cross-sectional, and twenty-three were cohort in design. The size of the studies varied from 98 [82] to 8977 [61] participants, and the follow-up period ranged from 14.1 years [82] to 1.6 years [57]. The follow-up duration was not reported in seven studies [60,61,79,91,92,93]. The outcomes were routinely assessed using hospital records and self-reported questionnaires. RRM was a censoring event in twelve studies [9,11,22,58,62,67,79,82,93,94,95,97], excluded in twelve studies [10,58,60,61,62,63,64,65,66,68,69,91], included in one study [59] and not reported in four studies [83,89,90,92]. Studies investigating the PBC risk adjusted for the following confounders: age [11,65,67,68], parity [11,60,67,68,79], HRT use [11,79,97], OCP use [60,68], mutation status [67], centre [67], country of residence [68], age at menarche [68], BC family history [68] and breast feeding [68]. Studies investigating CBC adjusted for age [64], menopause [66], ascertainment [66], mutation status [64] and BC treatment (chemotherapy/radiotherapy/surgery/tamoxifen) [63,64]. Studies investigating mortality adjusted for age [57,58,59], mutation status [57,59], tumour size [59,89], nodal status [59,89], oestrogen receptor status [59,89], progesterone receptor status [89], HER2 receptor status [89], BC treatment (chemotherapy [59,89]/surgery [59]/tamoxifen [59,89]) and centre [57,58].
3.2. Risk of Bias
Table 2 summarises the risk of bias assessment, and Table 3 summarises the GRADE assessment for certainty of evidence per outcome.

Table 2.
Risk of bias assessment using the Newcastle–Ottawa Score.

Table 3.
Grading of Recommendations, Assessment, Development and Evaluations (GRADE) assessment of certainty of evidence per outcome.
The GRADE certainty of evidence for PBC and CBC was low, and that for BCSM was moderate. According to GRADE, all the observational studies have an initially low level of evidence. For PBC and CBC, the certainty of evidence was downgraded due to the serious risks of bias and conflicting effect sizes of the individual studies. For BCSM, the certainty of evidence was also downgraded due to serious risks of bias; however, it was upgraded when taking into account the large and consistent effect sizes of the studies.
3.3. Quantitative Synthesis of Results
In the baseline meta-analysis of the PBC risk in both BRCA1 and BRCA2 carriers combined, four studies were included [22,68,94,97]. In the baseline meta-analysis of CBC in both BRCA1 and BRCA2 carriers combined, four studies were included [58,62,66,69]. It was not possible to include pooling for the PBC or CBC risk in the baseline data in the case of the studies of Kauff et al. 2002 and 2008 [9,11] and Menkiszak et al. 2016 [83], on account of the fact that both studies reported on PBC and CBC cases together, and it was not possible to differentiate between the reported cases based on the raw data. We were unable to extract raw data from two recent publications for inclusion in our meta-analysis, despite requests sent to the authors [88,90]. For BCSM in BC-affected BRCA1 and BRCA2 carriers combined, three studies were included [58,59,91]. Figure 2 depicts forest plots of the baseline and subgroup analyses.
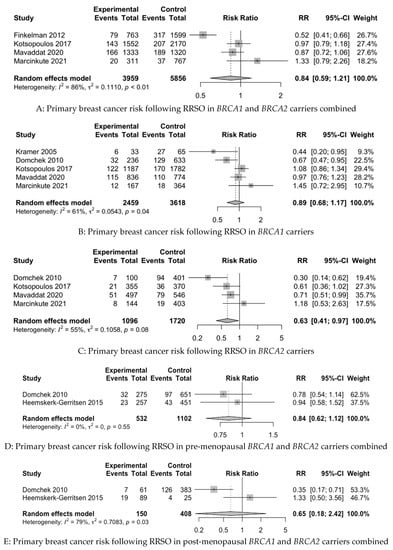
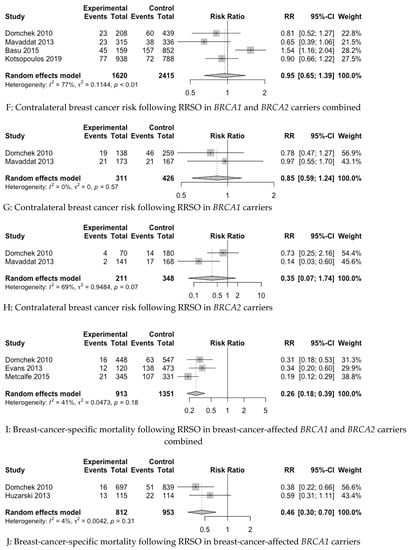
Figure 2.
Forest plots of pooled relative risk estimates evaluating breast cancer endpoints following RRSO in BRCA carriers [22,58,59,62,66,67,68,69,82,89,94,97]. RRSO—risk-reducing salpingo-oophorectomy.
Table 4 summarises the relative risks for all the baseline and subgroup analyses.

Table 4.
Summary of pooled relative risks, confidence intervals and numbers needed to treat.
In the baseline analyses, RRSO was not statistically significantly associated with a reduction in the risk of PBC in BRCA1 and BRCA2 carriers combined (RR 0.84, 95%CI 0.59–1.21) or the risk of CBC in BRCA1 and BRCA2 carriers combined (RR 0.95, 95%CI 0.65–1.39). However, RRSO was statistically significantly associated with a reduction in BCSM in BC-affected BRCA1 and BRCA2 carriers combined (RR 0.26, 95%CI 0.18–0.39).
For the subgroup analysis of the PBC risk, five studies were included for BRCA1 carriers alone [58,68,82,94,97], with four studies for BRCA2 carriers alone [58,68,94,97], two studies for pre-menopausal BRCA1 and BRCA2 carriers combined [58,67] and two studies for post-menopausal BRCA1 and BRCA2 carriers combined [58,67]. For the subgroup analysis of the CBC risk, two studies were included for BRCA1 carriers alone [58,62], with two studies for BRCA2 carriers alone [58,62]. Subgroup analyses stratified by menopause status for CBC were attempted; however, raw data were not available. It was possible to perform a subgroup analysis of BCSM for BC-affected BRCA1 carriers alone [58,89], but due to lack of raw data, we were unable to do this for BRCA2 carriers alone.
The subgroup analysis revealed that RRSO was not statistically significantly associated with a reduced PBC risk in BRCA1 carriers alone (RR 0.89, 95%CI 0.68–1.17) or with the PBC risk in pre-menopausal (RR 0.84, 95%CI 0.62–1.12) or post-menopausal BRCA1 and BRCA2 carriers combined (RR 0.65, 95%CI 0.18–2.42). However, RRSO was associated with a reduced risk of PBC in BRCA2 carriers alone (RR 0.63, 95%CI 0.41–0.97). The subgroup analysis revealed that RRSO was not associated with CBC risk reduction in BRCA1 carriers alone (RR 0.85, 95%CI 0.59–1.24) or BRCA2 carriers alone (RR 0.35, 95%CI 0.07–1.74). However, RRSO was associated with a reduction in the risk of BCSM in BC-affected BRCA1 carriers in the subgroup analysis (RR 0.46, 95%CI 0.30–0.70).
The heterogeneity of the baseline and subgroup models, as measured by I2, ranged from 0 to 86%. The models with low heterogeneity (I2 < 50%) were as follows: PBC risk in premenopausal BRCA1 and BRCA2 carriers combined, CBC risk in BRCA1 carriers alone, BCSM in BRCA1 and BRCA2 carriers combined and BCSM in BRCA1 carriers alone. The remainder of the models had high heterogeneity (I2 ≥ 50%).
The mean NNT for statistically significant outcomes showed that 5.6 (4.2 to 11.8) and 14.5 (9.5 to 30.3) RRSOs are needed to prevent one death from BC in BC-affected BRCA1 and BRCA2 carriers combined and one death from BC in BC-affected BRCA1 carriers alone, respectively (Table 4). Moreover, 20.6 (11.5 to 57) RRSOs are needed to prevent one PBC case in BRCA2 carriers (Table 4).
4. Discussion
4.1. Main Findings
In this systematic review, we provide relative risk estimates of PBC risk, CBC risk and BCSM in BRCA1 and BRCA2 carriers following RRSO data from fourteen publications published between 2005 and 2021. The results of this meta-analysis demonstrate that RRSO was not associated with a significant reduction in the overall PBC risk or CBC risk in the analyses incorporating both BRCA1 and BRCA2 carriers combined, nor is CBC risk significantly reduced when analysed separately by the type of BRCA mutation. RRSO was associated with a significant reduction in PBC risk in BRCA2 carriers alone, although there did not appear to be a reduction in PBC risk in BRCA1 carriers alone.
4.2. Comparison with Existing Literature
It appears that, previously, we have considerably overestimated the benefit of RRSO in regard to BC risk reduction. The impact, if any, is probably restricted to BRCA2 carriers, 70% of whom have ER-positive BC. This is in contrast with the 70% of breast cancers in BRCA1 carriers which are triple-negative (1) and is therefore in keeping with the hypothesis that a reduction in circulating oestrogens/progesterones following RRSO would most likely reduce the risk of hormone-sensitive tumours. To some extent, this is not inconsistent with the effect seen in the case of other anti-oestrogen interventions, such as Tamoxifen, which only reduces the risk of ER-positive BC in high-risk women, or the benefit of GnRH analogues in regard to the overall survival observed in women with ER-positive BC alone [98]. Additionally, the PBC risk post-RRSO was not significantly influenced by menopausal status. There is an associated reduction in BCSM in BC-affected BRCA1 and BRCA2 carriers combined and BC-affected BRCA1 carriers alone following RRSO. Although one study suggested a potential, small, non-significant reduction in BCSM in BRCA2 carriers alone following RRSO (HR 0.87, 95%CI 0.32–2.37) [58], the effect, if true, is small, and the overall paucity of published data on BCSM in BC-affected BRCA2 carriers precludes the ability to perform a subgroup analysis.
There are three previously published systematic reviews and meta-analyses investigating BC outcomes after RRSO in BRCA1 and BRCA2 carriers. Rebbeck et al., 2009 [87] investigated PBC risk over a decade ago, while more recently, Eleje et al., 2018 [99] investigated PBC risk and BCSM, and Xiao et al., 2019 [100] investigated the PBC risk, CBC risk and overall survival. The latter two analysis were published after data contradicting earlier findings on BC risk began to emerge. Nevertheless, all three of these meta-analyses concluded that, for BRCA1 and BRCA2 carriers combined, RRSO was associated with a statistically significant reduction in PBC risk (pooled HR 0.21, 95%CI 0.12–0.39; HR 0.64, 95%CI 0.43–0.96; HR 0.58, 95%CI 0.37–0.78 respectively). Xiao et al. also showed a statistically significant reduction in CBC risk following RRSO in BRCA1 and BRCA2 carriers combined (pooled HR 0.50, 95%CI 0.31–0.69). Additionally, Xiao et al. reported a statistically significant increase in overall survival in BC-affected BRCA1 and BRCA2 carriers combined following RRSO (pooled HR 0.33, 95%CI 0.28–0.38), and Eleje et al. showed increased BCSM in BRCA1 and BRCA2 carriers combined following RRSO (pooled HR 0.58, 95%CI 0.39–0.88). Whilst the improved BCSM reported by others is in keeping with the results of our meta-analysis, the significant reduction in PBC/CBC risk following RRSO in BRCA1 and BRCA2 carriers combined, as reported by these researchers, is contrary to our findings. However, our meta-analysis found a significant reduction in BRCA2 PBC risk resulting from RRSO. It is also important to point out that the overall GRADE quality assessment of studies used for the PBC meta-analysis is low, as these are observational studies. There are no RCTs which address the BC risk and mortality post-RRSO in BRCA carriers, as randomising individuals at high risk of OC/BC to a non-surgical arm would be both unethical and unacceptable for the women. It will thus not be possible to undertake an RCT on this issue, and inferences for patient care and practice will need to be drawn from well-designed observational cohort data. The previously published meta-analyses have a number of limitations. These studies did not include all/recently published data on PBC/CBC and, importantly, included overlapping datasets within the same meta-analysis, thus overstating the effect size of the earlier published literature showing a reduction in PBC/CBC risk following RRSO. They extracted reported HRs/RRs/ORs directly from the published literature and included different measurements of effect size which are not comparable within the same meta-analysis.
It appears that the conflicting risk estimates of individual studies investigating PBC/CBC risk following RRSO may be due to the various selection biases described in Table 1. Indication bias occurs because individuals with a stronger family history of OC are more likely to undergo RRSO/RRM than individuals from families with less OC/BC family history. In order to take into account possible differences in penetrance between families of BRCA carriers with and without RRSO, matching women who have undergone RRSO with relatives who have not, or a subgroup analysis restricted to individuals from families with an OC family history, would be more valid [67,70,71]. Cancer-induced testing bias occurs when individuals undergo BRCA testing because of a diagnosis of BC; thus, BC is overrepresented amongst the tested mutation carriers in the non-RRSO group, which may result in an overestimation of the risk reduction associated with RRSO. This may be overcome by commencing follow-up from the date of BRCA testing among cancer-unaffected individuals [67,70,71]. Immortal person-time bias is also an important limitation. If follow-up for the non-RRSO group starts at the date of BRCA ascertainment, for the RRSO group, the person-years of observation (PYO) between the dates of ascertainment and RRSO (cancer free by definition) should not be excluded, and these cancer-free person-years might be added to the person-years of the non-RRSO group. This allocation will reduce the cancer risk in the non-surgery group and, subsequently, prevent an overestimation of the reduction in cancer risk after RRSO [67,70,71]. Familial event bias occurs when members of the same family are selected for inclusion in the study population and the date of prophylactic surgery of the RRSO subject is not considered in the analysis. For instance, if follow-up were to start at the date of BRCA testing, the diagnosed BC would be counted as an event in the analysis. This would result in an overestimation of BC risk amongst women in the non-RRSO group and, consequently, an overestimation of the BC risk reduction after RRSO. To overcome this bias, the age of the control at the time of the relative’s RRSO should be used as the starting point of follow-up if this age is greater than the age at testing [67,70,71]. In a landmark paper by Heemskerk et al., the authors accounted for these biases by ensuring that their study cohort had no history of cancer at the date of BRCA testing, allocating all PYO before surgery, as well as a latency period of three months after RRSO, to the non-RRSO group [67]. Thereafter, PYO were allocated to the RRSO group. The follow-up of their analysis ended with the participant’s age at first BC diagnosis, age at RRM, age at diagnosis of another cancer (including OC), age at last contact, age at death or age at the study closing date, whichever came first. BC cases diagnosed during the latency period were counted as events in the non-RRSO group [67]. To estimate the association between RRSO and BC risk, the team used a Cox model with RRSO as a time-dependent variable to obtain hazard ratios and their accompanying 95% confidence intervals, using the non-RRSO group as the reference group. The variance–covariance estimation method was used to correct for non-independence of observations in the case of women from the same family [67]. The following variables were considered as potential confounders: year of birth, mutation type, centre and parity. The team concluded that the BC risk reduction after RRSO in BRCA carriers may have previously been overestimated because of bias. Using a design that maximally eliminated bias, they found no evidence for a protective effect of RRSO on PBC risk (HR 1.09, 95%CI = 0.67–1.77), whereas, when the team replicated the analysis of four previous publications [11,57,58,60] using their data, they found a ~50% PBC risk reduction, as estimated previously [67]. Data gathered by Kauff et al. and the PROSE consortium [11,58] were reanalysed by the authors to take into account RRSO as a time-dependent variable, accounting for immortal person-time bias (as per Heemskerk et al.) [67,96]. Upon reanalysis of Kauff et al.’s data, the revised HR reported a non-significant decrease in PBC risk (reanalysis: HR 0.50, 95%CI 0.20–1.25; original analysis: HR 0.53, 95%CI 0.29–0.96), having accounted for immortal person-time bias (the original analysis already accounted for the other aforementioned selection biases) [67,96]. Although the reanalysed PROSE data accounted for immortal person-time bias, the cancer-induced testing bias remained, and the revised HR continued to show a significant decrease in PBC risk (reanalysis: HR 0.59, 95%CI 0.42–0.82; original analysis: HR 0.51, 95%CI 0.36–0.70) [67,96].
Our meta-analysis corroborates more recent data [58,67,68,69,97] which show that RRSO does not reduce the PBC/CBC risk in either BRCA1 or BRCA2 carriers, contrary to the findings of earlier publications, which showed a reduction in the PBC/CBC risk post-RRSO in BRCA1 carriers [58,60,62,65,82] and BRCA2 carriers [11,58,62]. The subgroup analysis of CBC by mutation status is consistent with this finding, as the confidence intervals of the relative risk estimates includes ‘1’. Our finding of the associated reduction in PBC risk in BRCA2 carriers is in keeping with a recent publication by Mavaddat et al. 2020 [97], which reported a reduction in PBC risk after >5 years (but not ≤5 years) following RRSO in BRCA2 carriers (HR 0.51, 95%CI 0.26–0.99). However, it is important to remain cautious in the interpretation of this pooled estimate and findings regarding BRCA2 from Mavaddat et al. 2020 [97] due to the smaller number of events and wide confidence intervals observed in the BRCA2 cases compared to BRCA1. This is likely due to the smaller sample size of the BRCA2 group. Nevertheless, more prospective data are needed to improve the precision of PBC estimates. Subgroup analysis also revealed no significant reduction in PBC after RRSO in either premenopausal or postmenopausal women. This finding is in agreement with Mavaddat et al., who did not find a statistically significant reduction in PBC risk in pre- or postmenopausal BRCA1 and BRCA2 carriers (BRCA1: premenopausal HR 1.11 (95%CI 0.82–1.50), postmenopausal HR 1.69 (95%CI 0.73–3.91); BRCA2: premenopausal HR 0.69 (95%CI 0.44–1.08), postmenopausal HR 1.46 (95%CI 0.66–3.19)); Chang-Claude et al. [79], who did not find a statistically significant reduction in PBC risk post-RRSO in postmenopausal BRCA1 and BRCA2 carriers (HR 0.5, 95%CI 0.24–1.04; HR 0.39, 95%CI 0.06–2.38 respectively); and Rebbeck et al. [10], who found no risk reduction in postmenopausal BRCA1 and BRCA2 carriers combined (HR 0.52, 95%CI 0.10–2.70). However, these findings are contrary to Chang-Claude et al. [79], who found a significant reduction in PBC risk in premenopausal BRCA1 (but not BRCA2) carriers who underwent RRSO at < 35 years of age (HR 0.05, 95%CI 0.01–0.49), and Rebbeck et al. [10], who demonstrated a statistically significant risk reduction among premenopausal BRCA1 and BRCA2 carriers combined, aged 35–50 years (HR 0.49, 95%CI 0.26–0.90). In addition, Kotsopoulos et al. [61] showed a statistically significant risk reduction among postmenopausal BRCA1 and BRCA2 carriers combined (OR 0.13, 95%CI 0.05–0.54). It is possible that the aforementioned selection biases may have contributed to the conflicting results of the individual studies. However, it must be noted that it was not possible to extract data on the precise ages of patients grouped as pre-menopausal (<50 years) and post-menopausal (>50 years) from the published data included in our meta-analysis. Additionally, while the age of 50 is indicative, it may not be a true representation of the time of menopause, as some women may experience menopause earlier or later. It is therefore possible that there may be difference in breast cancer risk reduction depending on whether oophorectomy was performed in the peri-menopausal period, near the time of menopause or much earlier, in the case of pre-menopausal women, where oophorectomy would result in a significantly greater reduction in the number of lifetime ovulatory cycles. Therefore, our subgroup analysis of PBC risk and menopause status must be interpreted within this context.
The clinical management of cancer risk in BRCA carriers is complex and must consider patient preferences. RRSO remains the gold standard for preventing OC in BRCA carriers. Whether or not and when to undergo RRSO can be a complicated decision for many patients and may evolve over time [34]. Patient preferences need to be informed (and can be influenced) by accurate knowledge of the risks and benefits of the interventions considered. The previously perceived beneficial impact on BC risk is an important issue that has been routinely discussed during counselling and one of the key considerations in patient decision making. Our data suggest that BRCA carriers considering RRSO should now be counselled about the lack of consistent evidence for a reduction in BC risk. This is particularly the case for BRCA1 carriers. It is possible that this may influence more women to consider early risk-reducing salpingectomy and delayed oophorectomy, instead, as an emerging alternative preventive strategy (although this is still only recommended in clinical trials) [101,102]. However, one case of BC can be prevented for every 20.6 unaffected BRCA2 women undergoing RRSO. Despite the lack of benefit in reducing BC incidence, it is interesting that the results show a benefit for BCSM, with one BC-related death prevented for every 5.6 and 14.5 RRSOs performed on BC-affected BRCA1 and BRCA2 carriers combined and BC-affected BRCA1 carriers alone, respectively. Among the studies included in this meta-analysis, the individual analyses reported hazard ratios showing that BCSM is reduced in BC-affected BRCA1 carriers (HR 0.27, 95%CI 0.12–0.58 [58]; HR 0.38, 95%CI 0.19–0.77 [59]; HR 0.30 95%CI 0.12–0.75 [89]) but not in BC-affected BRCA2 carriers (HR 0.87, 95%CI 0.32–2.2.37 [58]; HR 0.57 95%CI 0.23–1.43 [59]). In addition, in a paper reporting on BCSM in BC-unaffected BRCA1 carriers, RRSO appeared to improve the rate of survival (HR 0.30 95%CI 0.06–1.53 [58]). The authors were unable to evaluate the impact on BC-unaffected BRCA2 carriers, as there were no events in this group. It is difficult to explain why BCSM mortality is improved by RRSO in BRCA1 while BC incidence is not. Clinicians must remain cautious in their interpretation of these findings. The observational studies evaluating BCSM outcomes are also affected by the aforementioned methodological biases (Table 1). Nevertheless, for a BC-affected woman, the current evidence suggests a potential greater benefit of RRSO compared to an early-salpingectomy-based approach. This, of course, needs to be balanced against the detrimental health impact of premature surgical menopause, which may result from oophorectomy in women who remain premenopausal following BC treatment, particularly given the inability of most of these women to take HRT.
4.3. Strengths and Limitations
Our work conformed to the PRISMA guidelines, and the protocols were prospectively registered in the PROSPERO database. The strengths of our systematic review and meta-analysis include a comprehensive search strategy, identifying all the relevant literature for inclusion, and methodologically rigorous pooled relative risk estimates of BC outcomes from published/requested raw data, resulting in standardised measures of the effect sizes of the included studies. Overlapping datasets with a greater risk of bias were excluded, ensuring that no particular dataset was overrepresented in our analyses. To limit the influence of the risk of reporting bias in our findings, all the published studies on RRSO in BRCA carriers were included.
Due to the lack of published raw data and inconsistency of the outcomes reported, the subgroup analysis was restricted, and it was not possible to fully investigate the effects of BRCA1 and BRCA2 pathogenic variants independently and the effect of menopause status on all the reported BC outcomes. This limitation, in part, could be addressed in future research through a meta-analysis based on individual patient data. Unwarranted variation in the reporting of outcomes and outcome measures between studies has been highlighted as a major limitation within women’s health research. This is being addressed by the CoRe Outcomes in Women’s and Newborn health (CROWN) initiative, which advocates for the development of a core outcome set (COS) for every woman’s health, disease and procedure. There was a large degree of statistical heterogeneity (I2 ≥ 50%); thus, a random effects meta-analysis was performed, which produced more conservative confidence intervals. This only partly removes the effects of heterogeneity. Another limitation is that the geographical location of the included studies was limited to Europe/North America/Israel. It is therefore possible that these results may not be generalizable to non-Caucasian populations.
4.4. Implications
Our findings are important and can be useful for clinical care and decision making. They can be helpful for clinicians in regard to counselling and for women in regard to decision making while factoring in the impact on breast cancer risk and survival and considering decisions in relation to risk-reducing surgery for ovarian cancer prevention. Women need to consider a number of additional factors when making this decision, including age, menopause, impact on sexual function, cardiovascular, neurological and bone health, the potential need for and ability to take HRT and the level of cancer risk reduction [56,103]. Although oophorectomy does appear to increase BC-specific survival, there are effective alternative treatments available for improving BC outcomes. RRM reduces BCSM (HR = 0.06, 95%CI = 0.01–0.46) and overall mortality (HR = 0.40, 95%CI = 0.20–0.90) in BRCA1 carriers [104]. However, there is no significant effect on overall mortality in BRCA2 carriers (HR 0.45, 95%CI 0.15–1.36), while the effect on BCSM is unclear due to a lack of events [104]. Tamoxifen (RR = 0.69, 95%CI 0.59–0.84), Raloxifene (RR = 0.44, 95%CI 0.24–0.80) and aromatase inhibitors (RR = 0.45, 95%CI 0.26–0.70) are statistically significantly associated with a lower PBC risk after 3–5 years of use [8,105]. Adjuvant GnRH use for ER-positive BC increases progression-free survival, recurrence-free survival and overall survival [106]. It is clear that larger, prospective, well-designed studies which minimise the earlier methodological biases are needed to further improve the power and precision of risk estimates of the impacts on BC risk and BCSM following RRSO in BRCA carriers. This is necessary for women’s ability to make better-informed decisions in the future. Clinicians should emphasise that the decision to undergo salpingo-oophorectomy, in the case of both BC-unaffected and -0affected BRCA women, must primarily be for OC prevention, and that there are well-established alternative strategies available to reduce BC risk and prolong survival.
5. Conclusions
This systematic review and meta-analysis of fourteen publications found that RRSO is not associated with a significant reduction in the overall PBC risk or CBC risk in BRCA1 and BRCA2 carriers combined or in BRCA1 carriers alone but is associated with a significant reduction in PBC risk in BRCA2 carriers alone. RRSO is not associated with a reduction in CBC risk for either BRCA mutation type alone. Furthermore, RRSO is associated with improved BC survival in BC-affected BRCA1 and BRCA2 carriers combined and in BRCA1 carriers alone.
This is the most comprehensive review of this topic to date, and it indicates that, previously, the benefits of RRSO for breast cancer outcomes may have been considerably overestimated. BRCA carriers considering RRSO should be counselled about the lack of consistent evidence for a reduction in BC risk.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15051625/s1, Table S1: Search strategy for literature search.
Author Contributions
Conceptualization, F.G.; methodology, F.G., O.B., K.K. and R.M.; software, F.G. and O.B.; validation, F.G., O.B., D.M. and R.M.; formal analysis, F.G., O.B., D.M. and R.M.; investigation, F.G. and R.M.; resources, R.M.; data curation, F.G., O.B. and R.M.; writing—original draft preparation, F.G. and R.M.; writing—review and editing, all authors; visualization, F.G., O.B., A.T. and S.O.; supervision, K.K., R.L. and R.M.; project administration, F.G.; funding acquisition, R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Rosetrees Trust, grant number CF1\100001, and Barts Charity, grant number ECMG1C3R. The funders had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Institutional Review Board Statement
Ethical review and approval were waived for this study, as this is a review of previously published data.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
We thank Gareth Evans (the University of Manchester) and Timothy Rebbeck (Harvard University) for providing us with unpublished raw data for our review, the Global Gynaecological Oncology Surgical Outcomes Collaborative (GO SOAR), and the authors of the papers that we considered for inclusion. We thank Dhivya Chandrasekaran for her assistance in the development of the search strategy.
Conflicts of Interest
F.G. declares research funding from the NHS Grampian Endowment Fund, Medtronic and Karl Storz, outside the scope of this work, and an honorarium from AstraZeneca. R.M. declares research funding from the British Gynaecological Cancer Society, Eve Appeal, GSK, NHS Innovation Accelerator and Yorkshire Cancer Research, outside the scope of this work, an honorarium for grant review from the Israel National Institute for Health Policy Research and honorarium for advisory board membership from AstraZeneca/MSD/EGL/GSK. The other authors declare no conflicts of interest.
References
- Antoniou, A.; Pharoah, P.D.; Narod, S.; Risch, H.A.; Eyfjord, J.E.; Hopper, J.L.; Loman, N.; Olsson, H.; Johannsson, O.; Borg, A.; et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: A combined analysis of 22 studies. Am. J. Hum. Genet. 2003, 72, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Parmigiani, G. Meta-analysis of BRCA1 and BRCA2 penetrance. J. Clin. Oncol. 2007, 25, 1329–1333. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N.; et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Shenton, A.; Woodward, E.; Lalloo, F.; Howell, A.; Maher, E.R. Penetrance estimates for BRCA1 and BRCA2 based on genetic testing in a Clinical Cancer Genetics service setting: Risks of breast/ovarian cancer quoted should reflect the cancer burden in the family. BMC Cancer 2008, 8, 155. [Google Scholar] [CrossRef]
- Menon, U.; Harper, J.; Sharma, A.; Fraser, L.; Burnell, M.; ElMasry, K.; Rodeck, C.; Jacobs, I. Views of BRCA gene mutation carriers on preimplantation genetic diagnosis as a reproductive option for hereditary breast and ovarian cancer. Hum. Reprod. 2007, 22, 1573–1577. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Friebel, T.; Lynch, H.T.; Neuhausen, S.L.; Van’t Veer, L.; Garber, J.E.; Evans, G.R.; Narod, S.A.; Isaacs, C.; Matloff, E.; et al. Bilateral prophylactic mastectomy reduces breast cancer risk in BRCA1 and BRCA2 mutation carriers: The PROSE Study Group. J. Clin. Oncol. 2004, 22, 1055–1062. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Bonanni, B.; Costantino, J.P.; Cummings, S.; DeCensi, A.; Dowsett, M.; Forbes, J.F.; Ford, L.; LaCroix, A.Z.; et al. Selective oestrogen receptor modulators in prevention of breast cancer: An updated meta-analysis of individual participant data. Lancet 2013, 381, 1827–1834. [Google Scholar] [CrossRef]
- Cuzick, J.; Sestak, I.; Forbes, J.F.; Dowsett, M.; Cawthorn, S.; Mansel, R.E.; Loibl, S.; Bonanni, B.; Evans, D.G.; Howell, A.; et al. Use of anastrozole for breast cancer prevention (IBIS-II): Long-term results of a randomised controlled trial. Lancet 2019, 395, 117–122. [Google Scholar] [CrossRef]
- Kauff, N.D.; Satagopan, J.M.; Robson, M.E.; Scheuer, L.; Hensley, M.; Hudis, C.A.; Ellis, N.A.; Boyd, J.; Borgen, P.I.; Barakat, R.R.; et al. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N. Engl. J. Med. 2002, 346, 1609–1615. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Lynch, H.T.; Neuhausen, S.L.; Narod, S.A.; Van’t Veer, L.; Garber, J.E.; Evans, G.; Isaacs, C.; Daly, M.B.; Matloff, E.; et al. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N. Engl. J. Med. 2002, 346, 1616–1622. [Google Scholar] [CrossRef]
- Kauff, N.D.; Domchek, S.M.; Friebel, T.M.; Robson, M.E.; Lee, J.; Garber, J.E.; Isaacs, C.; Evans, D.G.; Lynch, H.; Eeles, R.A.; et al. Risk-reducing salpingo-oophorectomy for the prevention of BRCA1- and BRCA2-associated breast and gynecologic cancer: A multicenter, prospective study. J. Clin. Oncol. 2008, 26, 1331–1337. [Google Scholar] [CrossRef]
- Finch, A.; Beiner, M.; Lubinski, J.; Lynch, H.T.; Moller, P.; Rosen, B.; Murphy, J.; Ghadirian, P.; Friedman, E.; Foulkes, W.D.; et al. Salpingo-oophorectomy and the risk of ovarian, fallopian tube, and peritoneal cancers in women with a BRCA1 or BRCA2 Mutation. JAMA 2006, 296, 185–192. [Google Scholar] [CrossRef]
- Manchanda, R.; Abdelraheim, A.; Johnson, M.; Rosenthal, A.N.; Benjamin, E.; Brunell, C.; Burnell, M.; Side, L.; Gessler, S.; Saridogan, E.; et al. Outcome of risk-reducing salpingo-oophorectomy in BRCA carriers and women of unknown mutation status. Int. J. Obstet. Gynaecol. 2011, 118, 814–824. [Google Scholar] [CrossRef]
- Restaino, S.; Finelli, A.; Pellecchia, G.; Biasioli, A.; Mauro, J.; Ronsini, C.; Martina, M.D.; Arcieri, M.; Della Corte, L.; Sorrentino, F.; et al. Scar-Free Laparoscopy in BRCA-Mutated Women. Medicina 2022, 58, 943. [Google Scholar] [CrossRef]
- Beattie, M.S.; Crawford, B.; Lin, F.; Vittinghoff, E.; Ziegler, J. Uptake, time course, and predictors of risk-reducing surgeries in BRCA carriers. Genet. Test. Mol. Biomark. 2009, 13, 51–56. [Google Scholar] [CrossRef]
- Botkin, J.R.; Smith, K.R.; Croyle, R.T.; Baty, B.J.; Wylie, J.E.; Dutson, D.; Chan, A.; Hamann, H.A.; Lerman, C.; McDonald, J.; et al. Genetic testing for a BRCA1 mutation: Prophylactic surgery and screening behavior in women 2 years post testing. Am. J. Med. Genet. Part A 2003, 118A, 201–209. [Google Scholar] [CrossRef]
- Bradbury, A.R.; Ibe, C.N.; Dignam, J.J.; Cummings, S.A.; Verp, M.; White, M.A.; Artioli, G.; Dudlicek, L.; Olopade, O.I. Uptake and timing of bilateral prophylactic salpingo-oophorectomy among BRCA1 and BRCA2 mutation carriers. Genet. Med. 2008, 10, 161–166. [Google Scholar] [CrossRef]
- Chai, X.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Use of risk-reducing surgeries in a prospective cohort of 1,499 BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2014, 148, 397–406. [Google Scholar] [CrossRef]
- Cragun, D.; Weidner, A.; Lewis, C.; Bonner, D.; Kim, J.; Vadaparampil, S.T.; Pal, T. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer 2017, 123, 2497–2505. [Google Scholar] [CrossRef]
- D’Alonzo, M.; Pecchio, S.; Liberale, V.; Modaffari, P.; Biglia, N.; Piva, E.; Ponzone, R. Satisfaction and Impact on Quality of Life of Clinical and Instrumental Surveillance and Prophylactic Surgery in BRCA-mutation Carriers. Clin. Breast Cancer 2018, 18, e1361–e1366. [Google Scholar] [CrossRef]
- Evans, D.G.R.; Lalloo, F.; Shenton, A.; Clancy, T.; Hopwood, P.; Ashcroft, L.; Howell, A.; Baildam, A.D.; Brain, A. Uptake of risk-reducing surgery in unaffected women at high risk of breast and ovarian cancer is risk, age, and time dependent. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2318–2324. [Google Scholar] [CrossRef] [PubMed]
- Finkelman, B.S.; Rubinstein, W.S.; Friedman, S.; Friebel, T.M.; Dubitsky, S.; Schonberger, N.S.; Shoretz, R.; Singer, C.F.; Blum, J.L.; Tung, N.; et al. Breast and ovarian cancer risk and risk reduction in Jewish BRCA1/2 mutation carriers. J. Clin. Oncol. 2012, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Flippo-Morton, T.; Walsh, K.; Sarantou, T.; White, R.L.; Chambers, K.; Amacker-North, L.; White, B.; Boselli, D.M. Surgical Decision Making in the BRCA-Positive Population: Institutional Experience and Comparison with Recent Literature. Breast J. 2016, 22, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Friebel, T.M.; Domchek, S.M.; Neuhausen, S.L.; Wagner, T.; Evans, D.G.; Isaacs, C.; Garber, J.E.; Daly, M.B.; Eeles, R.; Matloff, E.; et al. Bilateral prophylactic oophorectomy and bilateral prophylactic mastectomy in a prospective cohort of unaffected BRCA1 and BRCA2 mutation carriers. Clin. Breast Cancer 2007, 7, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Lyon, L.; Conell, C.; Littell, R.D.; Powell, C.B. Osteoporosis risk and management in BRCA1 and BRCA2 carriers who undergo risk-reducing salpingo-oophorectomy. Gynecol. Oncol. 2015, 138, 723–726. [Google Scholar] [CrossRef]
- Holman, L.L.; Friedman, S.; Daniels, M.S.; Sun, C.C.; Lu, K.H. Acceptability of prophylactic salpingectomy with delayed oophorectomy as risk-reducing surgery among BRCA mutation carriers. Gynecol. Oncol. 2014, 133, 283–286. [Google Scholar] [CrossRef]
- Kim, S.I.; Lim, M.C.; Lee, D.O.; Seo, S.S.; Kang, S.; Park, S.Y.; Kong, S.Y.; Lee, E.S. Uptake of risk-reducing salpingo-oophorectomy among female BRCA mutation carriers: Experience at the National Cancer Center of Korea. J. Cancer Res. Clin. Oncol. 2016, 142, 333–340. [Google Scholar] [CrossRef]
- Kram, V.; Peretz, T.; Sagi, M. Acceptance of Preventive Surgeries by Israeli Women Who had Undergone BRCA Testing. Fam. Cancer 2006, 5, 327–335. [Google Scholar] [CrossRef]
- Kwong, A.; Wong, C.H.; Shea, C.; Suen, D.T.; Choi, C.L. Choice of management of southern Chinese BRCA mutation carriers. World J. Surg. 2010, 34, 1416–1426. [Google Scholar] [CrossRef]
- Lerman, C.; Hughes, C.; Croyle, R.T.; Main, D.; Durham, C.; Snyder, C.; Bonney, A.; Lynch, J.F.; Narod, S.A.; Lynch, H.T. Prophylactic surgery decisions and surveillance practices one year following BRCA1/2 testing. Prev. Med. 2000, 31, 75–80. [Google Scholar] [CrossRef]
- Lodder, L.N.; Frets, P.G.; Trijsburg, R.W.; Meijers-Heijboer, E.J.; Klijn, J.G.M.; Seynaeve, C.; van Geel, A.N.; Tilanus, M.M.A.; Bartels, C.C.M.; Verhoog, L.C.; et al. One Year Follow-Up of Women Opting for Presymptomatic Testing for BRCA1 and BRCA2: Emotional Impact of the Test Outcome and Decisions on Risk Management (Surveillance or Prophylactic Surgery). Breast Cancer Res. Treat. 2002, 73, 97–112. [Google Scholar] [CrossRef]
- Madalinska, J.B.; Hollenstein, J.; Bleiker, E.; van Beurden, M.; Valdimarsdottir, H.B.; Massuger, L.F.; Gaarenstroom, K.N.; Mourits, M.J.E.; Verheijen, R.H.M.; van Dorst, E.B.L.; et al. Quality-of-Life Effects of Prophylactic Salpingo-Oophorectomy Versus Gynecologic Screening Among Women at Increased Risk of Hereditary Ovarian Cancer. J. Clin. Oncol. 2005, 23, 6890–6898. [Google Scholar] [CrossRef]
- Mai, P.L.; Piedmonte, M.; Han, P.K.; Moser, R.P.; Walker, J.L.; Rodriguez, G.; Boggess, J.; Rutherford, T.J.; Zivanovic, O.; Cohn, D.E.; et al. Factors associated with deciding between risk-reducing salpingo-oophorectomy and ovarian cancer screening among high-risk women enrolled in GOG-0199: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017, 145, 122–129. [Google Scholar] [CrossRef]
- Manchanda, R.; Burnell, M.; Abdelraheim, A.; Johnson, M.; Sharma, A.; Benjamin, E.; Brunell, C.; Saridogan, E.; Gessler, S.; Oram, D.; et al. Factors influencing uptake and timing of risk reducing salpingo-oophorectomy in women at risk of familial ovarian cancer: A competing risk time to event analysis. Int. J. Obstet. Gynaecol. 2012, 119, 527–536. [Google Scholar] [CrossRef]
- Meijers-Heijboer, E.J.; Verhoog, L.C.; Brekelmans, C.T.; Seynaeve, C.; Tilanus-Linthorst, M.M.; Wagner, A.; Dukel, L.; Devilee, P.; van den Ouweland, A.M.; van Geel, A.N.; et al. Presymptomatic DNA testing and prophylactic surgery in families with a BRCA1 or BRCA2 mutation. Lancet 2000, 355, 2015–2020. [Google Scholar] [CrossRef]
- Metcalfe, K.A.; Ghadirian, P.; Rosen, B.; Foulkes, W.; Kim-Sing, C.; Eisen, A.; Ainsworth, P.; Horsman, D.; Maugard, C.; Provencher, D.; et al. Variation in rates of uptake of preventive options by Canadian women carrying the BRCA1 or BRCA2 genetic mutation. Open Med. A Peer-Rev. Indep. Open-Access J. 2007, 1, e92–e98. [Google Scholar]
- Metcalfe, K.A.; Liede, A.; Hoodfar, E.; Scott, A.; Foulkes, W.D.; Narod, S.A. An evaluation of needs of female BRCA1 and BRCA2 carriers undergoing genetic counselling. J. Med. Genet. 2000, 37, 866–874. [Google Scholar] [CrossRef]
- Nebgen, D.R.; Hurteau, J.; Holman, L.L.; Bradford, A.; Munsell, M.F.; Soletsky, B.R.; Sun, C.C.; Chisholm, G.B.; Lu, K.H. Bilateral salpingectomy with delayed oophorectomy for ovarian cancer risk reduction: A pilot study in women with BRCA1/2 mutations. Gynecol. Oncol. 2018, 150, 79–84. [Google Scholar] [CrossRef]
- Pezaro, C.; James, P.; McKinley, J.; Shanahan, M.; Young, M.A.; Mitchell, G. The consequences of risk reducing salpingo-oophorectomy: The case for a coordinated approach to long-term follow up post surgical menopause. Fam. Cancer 2012, 11, 403–410. [Google Scholar] [CrossRef]
- Ray, J.A.; Loescher, L.J.; Brewer, M. Risk-reduction surgery decisions in high-risk women seen for genetic counseling. J. Genet. Couns. 2005, 14, 473–484. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Isaacs, C.; Graves, K.D.; Poggi, E.; Peshkin, B.N.; Gell, C.; Finch, C.; Kelly, S.; Taylor, K.L.; Perley, L. Long-term outcomes of BRCA1/BRCA2 testing: Risk reduction and surveillance. Cancer 2012, 118, 510–517. [Google Scholar] [CrossRef]
- Skytte, A.B.; Gerdes, A.M.; Andersen, M.K.; Sunde, L.; Brondum-Nielsen, K.; Waldstrom, M.; Kolvraa, S.; Cruger, D. Risk-reducing mastectomy and salpingo-oophorectomy in unaffected BRCA mutation carriers: Uptake and timing. Clin. Genet. 2010, 77, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Tiller, K.; Meiser, B.; Butow, P.; Clifton, M.; Thewes, B.; Friedlander, M.; Tucker, K. Psychological Impact of Prophylactic Oophorectomy in Women at Increased Risk of Developing Ovarian Cancer: A Prospective Study. Gynecol. Oncol. 2002, 86, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Uyei, A.; Peterson, S.K.; Erlichman, J.; Broglio, K.; Yekell, S.; Schmeler, K.; Lu, K.; Meric-Bernstam, F.; Amos, C.; Strong, L.; et al. Association between clinical characteristics and risk-reduction interventions in women who underwent BRCA1 and BRCA2 testing: A single-institution study. Cancer 2006, 107, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Westin, S.N.; Sun, C.C.; Lu, K.H.; Schmeler, K.M.; Soliman, P.T.; Lacour, R.A.; Johnson, K.G.; Daniels, M.S.; Bodurka, D.C.; Arun, B.K.; et al. Satisfaction with ovarian carcinoma risk-reduction strategies among women at high risk for breast and ovarian carcinoma. Cancer 2011, 117, 2659–2667. [Google Scholar] [CrossRef]
- Parker, W.H.; Feskanich, D.; Broder, M.S.; Chang, E.; Shoupe, D.; Farquhar, C.M.; Berek, J.S.; Manson, J.E. Long-term mortality associated with oophorectomy compared with ovarian conservation in the nurses’ health study. Obs. Gynecol. 2013, 121, 709–716. [Google Scholar] [CrossRef]
- Fakkert, I.E.; Abma, E.M.; Westrik, I.G.; Lefrandt, J.D.; Wolffenbuttel, B.H.; Oosterwijk, J.C.; Slart, R.H.; van der Veer, E.; de Bock, G.H.; Mourits, M.J. Bone mineral density and fractures after risk-reducing salpingo-oophorectomy in women at increased risk for breast and ovarian cancer. Eur. J. Cancer 2015, 51, 400–408. [Google Scholar] [CrossRef]
- Fakkert, I.E.; van der Veer, E.; Abma, E.M.; Lefrandt, J.D.; Wolffenbuttel, B.H.; Oosterwijk, J.C.; Slart, R.H.; Westrik, I.G.; de Bock, G.H.; Mourits, M.J. Elevated Bone Turnover Markers after Risk-Reducing Salpingo-Oophorectomy in Women at Increased Risk for Breast and Ovarian Cancer. PLoS ONE 2017, 12, e0169673. [Google Scholar] [CrossRef]
- Shuster, L.T.; Gostout, B.S.; Grossardt, B.R.; Rocca, W.A. Prophylactic oophorectomy in premenopausal women and long-term health. Menopause Int. 2008, 14, 111–116. [Google Scholar] [CrossRef]
- Rocca, W.A.; Bower, J.H.; Maraganore, D.M.; Ahlskog, J.E.; Grossardt, B.R.; de Andrade, M.; Melton, L.J. 3rd. Increased risk of cognitive impairment or dementia in women who underwent oophorectomy before menopause. Neurology 2007, 69, 1074–1083. [Google Scholar] [CrossRef]
- Rocca, W.A.; Bower, J.H.; Maraganore, D.M.; Ahlskog, J.E.; Grossardt, B.R.; de Andrade, M.; Melton, L.J. 3rd. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology 2008, 70, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Rocca, W.A.; Grossardt, B.R.; de Andrade, M.; Malkasian, G.D.; Melton, L.J. 3rd. Survival patterns after oophorectomy in premenopausal women: A population-based cohort study. Lancet Oncol. 2006, 7, 821–828. [Google Scholar] [CrossRef]
- Atsma, F.; Bartelink, M.L.; Grobbee, D.E.; van der Schouw, Y.T. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: A meta-analysis. Menopause 2006, 13, 265–279. [Google Scholar] [CrossRef]
- Rivera, C.M.; Grossardt, B.R.; Rhodes, D.J.; Brown, R.D., Jr.; Roger, V.L.; Melton, L.J., 3rd; Rocca, W.A. Increased cardiovascular mortality after early bilateral oophorectomy. Menopause 2009, 16, 15–23. [Google Scholar] [CrossRef]
- Gaba, F.; Manchanda, R. Systematic review of acceptability, cardiovascular, neurological, bone health and HRT outcomes following risk reducing surgery in BRCA carriers. Best Pract. Res. Clin. Obs. Gynaecol. 2020, 65, 46–65. [Google Scholar] [CrossRef]
- Manchanda, R.; Gaba, F.; Talaulikar, V.; Pundir, J.; Gessler, S.; Davies, M.; Menon, U.; Royal College of Obstetricians and Gynaecologists. Risk-Reducing Salpingo-Oophorectomy and the Use of Hormone Replacement Therapy Below the Age of Natural Menopause: Scientific Impact Paper No. 66 October 2021: Scientific Impact Paper No. 66. Int. J. Obstet. Gynaecol. 2022, 129, e16–e34. [Google Scholar] [CrossRef]
- Domchek, S.M.; Friebel, T.M.; Neuhausen, S.L.; Wagner, T.; Evans, G.; Isaacs, C.; Garber, J.E.; Daly, M.B.; Eeles, R.; Matloff, E.; et al. Mortality after bilateral salpingo-oophorectomy in BRCA1 and BRCA2 mutation carriers: A prospective cohort study. Lancet Oncol. 2006, 7, 223–229. [Google Scholar] [CrossRef]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of risk-reducing surgery in BRCA1 or BRCA2 mutation carriers with cancer risk and mortality. J. Am. Med. Assoc. 2010, 304, 967–975. [Google Scholar] [CrossRef]
- Metcalfe, K.; Lynch, H.T.; Foulkes, W.D.; Tung, N.; Kim-Sing, C.; Olopade, O.I.; Eisen, A.; Rosen, B.; Snyder, C.; Gershman, S.; et al. Effect of Oophorectomy on Survival After Breast Cancer in BRCA1 and BRCA2 Mutation Carriers. JAMA Oncol. 2015, 1, 306–313. [Google Scholar] [CrossRef]
- Eisen, A.; Lubinski, J.; Klijn, J.; Moller, P.; Lynch, H.T.; Offit, K.; Weber, B.; Rebbeck, T.; Neuhausen, S.L.; Ghadirian, P.; et al. Breast cancer risk following bilateral oophorectomy in BRCA1 and BRCA2 mutation carriers: An international case-control study. J. Clin. Oncol. 2005, 23, 7491–7496. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Lubinski, J.; Lynch, H.T.; Kim-Sing, C.; Neuhausen, S.; Demsky, R.; Foulkes, W.D.; Ghadirian, P.; Tung, N.; Ainsworth, P.; et al. Oophorectomy after menopause and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Mavaddat, N.; Peock, S.; Frost, D.; Ellis, S.; Platte, R.; Fineberg, E.; Evans, D.G.; Izatt, L.; Eeles, R.A.; Adlard, J.; et al. Cancer Risks for BRCA1 and BRCA2 Mutation Carriers: Results From Prospective Analysis of EMBRACE. J. Natl. Cancer Inst. 2013, 105, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.; Gershman, S.; Lynch, H.T.; Ghadirian, P.; Tung, N.; Kim-Sing, C.; Olopade, O.I.; Domchek, S.; McLennan, J.; Eisen, A.; et al. Predictors of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. Br. J. Cancer 2011, 104, 1384–1392. [Google Scholar] [CrossRef]
- Metcalfe, K.; Lynch, H.T.; Ghadirian, P.; Tung, N.; Olivotto, I.; Warner, E.; Olopade, O.I.; Eisen, A.; Weber, B.; McLennan, J.; et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 2004, 22, 2328–2335. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Eisen, A.; Weber, B.L.; Levin, A.M.; Snyder, C.; Watson, P.; Lynch, H.T.; Cannon-Albright, L.; Neuhausen, S.L.; Isaacs, C.; et al. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J. Natl. Cancer Inst. 1999, 91, 1475–1479. [Google Scholar] [CrossRef]
- Basu, N.N.; Ingham, S.; Howell, A.; Evans, D.G.; Hodson, J.; Lalloo, F.; Bulman, M. Risk of contralateral breast cancer in BRCA1 and BRCA2 mutation carriers: A 30-year semi-prospective analysis. Fam. Cancer 2015, 14, 531–538. [Google Scholar] [CrossRef]
- Heemskerk-Gerritsen, B.A.M.; Seynaeve, C.; van Asperen, C.J.; Ausems, M.G.E.M.; Collée, J.M.; van Doorn, H.C.; Gomez Garcia, E.B.; Kets, C.M.; van Leeuwen, F.E.; Meijers-Heijboer, H.E.J.; et al. Breast Cancer Risk After Salpingo-Oophorectomy in Healthy BRCA1/2 Mutation Carriers: Revisiting the Evidence for Risk Reduction. J. Natl. Cancer Inst. 2015, 107, djv217. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Huzarski, T.; Gronwald, J.; Singer, C.F.; Moller, P.; Lynch, H.T.; Armel, S.; Karlan, B.; Foulkes, W.D.; Neuhausen, S.L.; et al. Bilateral Oophorectomy and Breast Cancer Risk in BRCA1 and BRCA2 Mutation Carriers. J. Natl. Cancer Inst. 2017, 109, djw177. [Google Scholar] [CrossRef]
- Kotsopoulos, J.; Lubinski, J.; Lynch, H.T.; Tung, N.; Armel, S.; Senter, L.; Singer, C.F.; Fruscio, R.; Couch, F.; Weitzel, J.N.; et al. Oophorectomy and risk of contralateral breast cancer among BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 175, 443–449. [Google Scholar] [CrossRef]
- Klaren, H.M.; van’t Veer, L.J.; van Leeuwen, F.E.; Rookus, M.A. Potential for bias in studies on efficacy of prophylactic surgery for BRCA1 and BRCA2 mutation. J. Natl. Cancer Inst. 2003, 95, 941–947. [Google Scholar] [CrossRef]
- Wacholder, S. Bias in Intervention Studies That Enroll Patients From High-Risk Clinics. J. Natl. Cancer Inst. 2004, 96, 1204–1207. [Google Scholar] [CrossRef] [PubMed]
- Warrens, M.J. Properties of the quantity disagreement and the allocation disagreement. Int. J. Remote Sens. 2015, 36, 1439–1446. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-randomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 9 January 2022).
- Viale, L.; Allotey, J.; Cheong-See, F.; Arroyo-Manzano, D.; McCorry, D.; Bagary, M.; Mignini, L.; Khan, K.S.; Zamora, J.; Thangaratinam, S. Epilepsy in pregnancy and reproductive outcomes: A systematic review and meta-analysis. Lancet 2015, 386, 1845–1852. [Google Scholar] [CrossRef]
- Michael, B.; Larry, V.H.; Julian, H.; Hannah, R.W. Introduction to Meta-Analysis; John Wiley and Sons Ltd.: Chichester, UK, 2009. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence--inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Barlin, J.; Pike, M.; Otegbeye, E.; Arnold, A.; Stadler, Z.; Robson, M.; Aghajanian, C.; Offit, K.; Barakat, R.; Kauff, N. Does postmenopausal risk-reducing salpingo-oophorectomy reduce the risk of BRCA-associated breast cancer? Gynecol. Oncol. 2013, 130, e103–e104. [Google Scholar] [CrossRef]
- Chang-Claude, J.; Andrieu, N.; Rookus, M.; Brohet, R.; Antoniou, A.C.; Peock, S.; Davidson, R.; Izatt, L.; Cole, T.; Nogues, C.; et al. Age at menarche and menopause and breast cancer risk in the International BRCA1/2 Carrier Cohort Study. Cancer Epidemiol. Biomark. Prev. 2007, 16, 740–746. [Google Scholar] [CrossRef]
- Kauff, N.D.; Barakat, R.R. Risk-reducing salpingo-oophorectomy in patients with germline mutations in BRCA1 or BRCA2. J. Clin. Oncol. 2007, 25, 2921–2927. [Google Scholar] [CrossRef]
- Kauff, N.D.; Domchek, S.M.; Friebel, T.M.; Lee, J.B.; Roth, R.; Robson, M.E.; Barakat, R.R.; Norton, L.; Offit, K.; Rebbeck, T.R.; et al. Multi-center prospective analysis of risk-reducing salpingo-oophorectomy to prevent BRCA-associated breast and ovarian cancer. J. Clin. Oncol. 2006, 24, 1003. [Google Scholar] [CrossRef]
- Kramer, J.L.; Velazquez, I.A.; Chen, B.E.; Rosenberg, P.S.; Struewing, J.P.; Greene, M.H. Prophylactic oophorectomy reduces breast cancer penetrance during prospective, long-term follow-up of BRCA1 mutation carriers. J. Clin. Oncol. 2005, 23, 8629–8635. [Google Scholar] [CrossRef]
- Menkiszak, J.; Chudecka-Głaz, A.; Gronwald, J.; Cymbaluk-Płoska, A.; Celewicz, A.; Świniarska, M.; Wężowska, M.; Bedner, R.; Zielińska, D.; Tarnowska, P.; et al. Prophylactic salpingo-oophorectomy in BRCA1 mutation carriers and postoperative incidence of peritoneal and breast cancers. J. Ovarian Res. 2016, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.; Sun, P.; Narod, S.A.; Lynch, H.T.; Ghadirian, P.; Tung, N.; Kim-Sing, C.; Olopade, O.I.; Domchek, S.; Eisen, A.; et al. Risk of ipsilateral breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2011, 127, 287–296. [Google Scholar] [CrossRef]
- Metcalfe, K.A.; Lynch, H.T.; Snyder, C.L.; Foulkes, W.; Tung, N.M.; Kim-Sing, C.; Olopade, O.I.; Eisen, A.; Rosen, B.; Sun, P.; et al. The impact of oophorectomy on survival after breast cancer in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 2014, 32, 1507. [Google Scholar] [CrossRef]
- Rebbeck, T.R. Prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 2000, 18, 100s. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Kauff, N.D.; Domchek, S.M.; Rebbeck, T.R.; Kauff, N.D.; Domchek, S.M. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in BRCA1 or BRCA2 mutation carriers. J. Natl. Cancer Inst. 2009, 101, 80–87. [Google Scholar] [CrossRef]
- Valachis, A.; Nearchou, A.D.; Lind, P. Surgical management of breast cancer in BRCA-mutation carriers: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2014, 144, 443–455. [Google Scholar] [CrossRef]
- Huzarski, T.; Byrski, T.; Gronwald, J.; Górski, B.; Domagała, P.; Cybulski, C.; Oszurek, O.; Szwiec, M.; Gugała, K.; Stawicka, M.; et al. Ten-Year Survival in Patients With BRCA1-Negative and BRCA1-Positive Breast Cancer. J. Clin. Oncol. 2013, 31, 3191–3196. [Google Scholar] [CrossRef]
- Brekelmans, C.T.; Seynaeve, C.; Menke-Pluymers, M.; Bruggenwirth, H.T.; Tilanus-Linthorst, M.M.; Bartels, C.C.; Kriege, M.; van Geel, A.N.; Crepin, C.M.; Blom, J.C.; et al. Survival and prognostic factors in BRCA1-associated breast cancer. Ann. Oncol. 2006, 17, 391–400. [Google Scholar] [CrossRef]
- Evans, D.G.; Ingham, S.L.; Baildam, A.; Ross, G.L.; Lalloo, F.; Buchan, I.; Howell, A. Contralateral mastectomy improves survival in women with BRCA1/2-associated breast cancer. Breast Cancer Res. Treat. 2013, 140, 135–142. [Google Scholar] [CrossRef]
- Van Sprundel, T.C.; Schmidt, M.K.; Rookus, M.A.; Brohet, R.; van Asperen, C.J.; Rutgers, E.J.; Van’t Veer, L.J.; Tollenaar, R.A. Risk reduction of contralateral breast cancer and survival after contralateral prophylactic mastectomy in BRCA1 or BRCA2 mutation carriers. Br. J. Cancer 2005, 93, 287–292. [Google Scholar] [CrossRef]
- Choi, Y.H.; Terry, M.B.; Daly, M.B.; MacInnis, R.J.; Hopper, J.L.; Colonna, S.; Buys, S.S.; Andrulis, I.L.; John, E.M.; Kurian, A.W.; et al. Association of Risk-Reducing Salpingo-Oophorectomy With Breast Cancer Risk in Women With BRCA1 and BRCA2 Pathogenic Variants. JAMA Oncol. 2021, 7, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Marcinkute, R.; Woodward, E.R.; Gandhi, A.; Howell, S.; Crosbie, E.J.; Wissely, J.; Harvey, J.; Highton, L.; Murphy, J.; Holland, C.; et al. Uptake and efficacy of bilateral risk reducing surgery in unaffected female BRCA1 and BRCA2 carriers. J. Med. Genet. 2022, 59, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.B.; Daly, M.B.; Phillips, K.A.; Ma, X.; Zeinomar, N.; Leoce, N.; Dite, G.S.; MacInnis, R.J.; Chung, W.K.; Knight, J.A.; et al. Risk-Reducing Oophorectomy and Breast Cancer Risk Across the Spectrum of Familial Risk. J. Natl. Cancer Inst. 2019, 111, 331–334. [Google Scholar] [CrossRef]
- Heemskerk-Gerritsen, B.A.M.; Hooning, M.J.; Rookus, M.A. Response. J. Natl. Cancer Inst. 2015, 107, 218. [Google Scholar] [CrossRef]
- Mavaddat, N.; Antoniou, A.C.; Mooij, T.M.; Hooning, M.J.; Heemskerk-Gerritsen, B.A.; Noguès, C.; Laborde, L.; Breysse, E.; Stoppa-Lyonnet, D.; Gauthier-Villars, M.; et al. Risk-reducing salpingo-oophorectomy, natural menopause, and breast cancer risk: An international prospective cohort of BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2020, 22, 8. [Google Scholar] [CrossRef]
- Bui, K.T.; Willson, M.L.; Goel, S.; Beith, J.; Goodwin, A. Ovarian suppression for adjuvant treatment of hormone receptor-positive early breast cancer. Cochrane Database Syst. Rev. 2020, 3, CD013538. [Google Scholar] [CrossRef]
- Eleje, G.U.; Eke, A.C.; Ezebialu, I.U.; Ikechebelu, J.I.; Ugwu, E.O.; Okonkwo, O.O. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst. Rev. 2018, 8, CD012464. [Google Scholar] [CrossRef]
- Xiao, Y.L.; Wang, K.; Liu, Q.; Li, J.; Zhang, X.; Li, H.Y. Risk Reduction and Survival Benefit of Risk-Reducing Salpingo-oophorectomy in Hereditary Breast Cancer: Meta-analysis and Systematic Review. Clin. Breast Cancer 2019, 19, e48–e65. [Google Scholar] [CrossRef]
- Gaba, F.; Goyal, S.; Marks, D.; Chandrasekaran, D.; Evans, O.; Robbani, S.; Tyson, C.; Legood, R.; Saridogan, E.; McCluggage, W.G.; et al. Surgical decision making in premenopausal BRCA carriers considering risk-reducing early salpingectomy or salpingo-oophorectomy: A qualitative study. J. Med. Genet. 2021, 59, 122–132. [Google Scholar] [CrossRef]
- Gaba, F.; Robbani, S.; Singh, N.; McCluggage, W.G.; Wilkinson, N.; Ganesan, R.; Bryson, G.; Rowlands, G.; Tyson, C.; Arora, R.; et al. Preventing Ovarian Cancer through early Excision of Tubes and late Ovarian Removal (PROTECTOR): Protocol for a prospective non-randomised multi-center trial. Int. J. Gynecol. Cancer 2020, 31, 286–291. [Google Scholar] [CrossRef]
- Nitschke, A.S.; do Valle, H.A.; Dawson, L.; Kwon, J.S.; Hanley, G.E. Long-Term Non-Cancer Risks in People with BRCA Mutations following Risk-Reducing Bilateral Salpingo-Oophorectomy and the Role of Hormone Replacement Therapy: A Review. Cancers 2023, 15, 711. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk-Gerritsen, B.A.M.; Jager, A.; Koppert, L.B.; Obdeijn, A.I.; Collee, M.; Meijers-Heijboer, H.E.J.; Jenner, D.J.; Oldenburg, H.S.A.; van Engelen, K.; de Vries, J.; et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 177, 723–733. [Google Scholar] [CrossRef]
- Nelson, H.D.; Fu, R.; Zakher, B.; Pappas, M.; McDonagh, M. Medication Use for the Risk Reduction of Primary Breast Cancer in Women: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2019, 322, 868–886. [Google Scholar] [CrossRef]
- Robertson, J.F.R.; Blamey, R.W. The use of gonadotrophin-releasing hormone (GnRH) agonists in early and advanced breast cancer in pre- and perimenopausal women. Eur. J. Cancer 2003, 39, 861–869. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).