Simple Summary
The classification of myelodysplastic neoplasms (MDSs) in patients with a previous primary tumor is controversial, as an efficient consensus risk factor-based classification of secondary MDSs has not yet been established. The current classifications consider separate etiologies based on exposure to cytotoxic therapy and genetic predisposition, despite recent studies on the germline landscape and clonal hematopoiesis of indeterminate potential (CHIP) supporting the contention that these different risk factors are overlapping. This review wants to summarize the current status of knowledge of secondary MDS etiologies and foresees future classifications that assess all risk factors and their interactions for achieving a differential diagnosis of patients at risk to aid in routine clinical decision-making related to most adequate clinical management.
Abstract
There is a great deal of controversy in the hematologic community regarding the classification of secondary myelodysplastic neoplasms (MDSs). Current classifications are based on the presence of genetic predisposition and MDS post-cytotoxic therapy (MDS-pCT) etiologies. However, since these risk factors are not exclusive for secondary MDSs and there are multiple overlapping scenarios, a comprehensive and definitive classification is yet to come. In addition, a sporadic MDS might arise after a primary tumor fulfills the diagnostic criteria of MDS-pCT without a causative cytotoxicity. In this review, we describe the triggering pieces of a secondary MDS jigsaw: previous cytotoxic therapy, germline predisposition and clonal hematopoiesis. Epidemiological and translational efforts are needed to put these pieces together and ascertain the real weight of each of these pieces in each MDS patient. Future classifications must contribute to understanding the role of secondary MDS jigsaw pieces in different concomitant or independent clinical scenarios associated with the primary tumor.
1. Introduction
Myelodysplastic syndromes or, as renamed in the latest World Health Organization (WHO) classification [1], myelodysplastic neoplasms (MDSs) are an age-associated malignant condition characterized by ineffective hematopoiesis, which entails a variety of prognostic biomarkers and heterogeneous outcomes due to their diverse etiology and the risk factors involved [2]. However, the landscape of the risk factors driving different MDS etiologies is not fully understood. Previous studies reported both obesity and smoking as modifiable risk factors of MDSs [3]. Furthermore, exposure to cytotoxic therapies [4], genetic predisposition [5] or the presence of clonal hematopoiesis of indeterminate potential (CHIP) [6] have been described as risk factors for this pathology.
The development of a secondary myelodysplasia is benign and often reversible if the offending trigger is withdrawn [7]. In Vienna, in 2006, the Myelodysplastic Neoplasm Working Group conference consolidated the two prerequisite criteria for the diagnosis of MDSs: the presence of cytopenia and the absence of other hematopoietic or nonhematopoietic disorders as the etiology of cytopenia [8]. Further contributing to the confusion, the term secondary myelodysplasia was also being used to refer to MDS evolving secondarily from a previous myeloid neoplasm or to define diseases that progressed from another myeloid disease. However, in the last WHO classification, the transformation of a myeloproliferative neoplasm (MPN) to acute myeloid leukemia (AML) was retained under the MPN category, while the transformation of MDS to AML and MDS/MPN remains under AML-myelodysplasia-related (AML-MR), in the secondary myeloid neoplasm category [1]. In very early reports, Jens Pedersen-Bjergaard [9] first described secondary myeloid neoplasms in the study on “Acute Nonlymphocytic Leukemia, Preleukemia, and Acute Myeloproliferative Syndrome Secondary to Treatment of Other Malignant Diseases”, in which among 31 patients, 21 “pre-leukemia” cases predominated. The term “secondary MDS” was first used in “Proposals for the classification of the myelodysplastic syndromes”, in which the FAB group addressed the “Special features of Secondary MDS”, describing a more frequent presence of fibrosis, hypocellularity, ringed sideroblasts, a higher proportion of blasts in the peripheral blood (PB) than would be expected from the percentage in the bone marrow (BM) and abnormal and immature megakaryocyte precursors often seen in PB and BM [10]. Using sequential cytogenetics, Rowley and colleagues demonstrated in 1980 that the development of MDS after exposure to mutagens and carcinogens was related to chromosomal evolution towards complex karyotypes (–5/del(5q), –7, +8, +21) and cytological transition into acute leukemia [11].
2. The Pieces of the Jigsaw
2.1. MDS Post-Cytotoxic Therapy
MDS post-cytotoxic therapy (MDS-pCT) cases are aggressive hematologic malignant neoplasms. The incidence of these neoplasms is rare (<0.5 per 100,000), but the mortality rates are higher compared to primary MDS, with a 5-year survival rate of 10% vs. 31% in primary MDS, and a median survival of around 8–10 months [12]. The diagnosis of MDS-pCT requires fulfilment of the criteria for MDS in addition to a previous history of chemotherapy treatment or large-field radiation therapy for an unrelated neoplasm [4]. Both incidental and therapeutic radiation have been associated with MDS-pCT. Indeed, a significant linear radiation dose-response for MDS has been described in atomic bomb survivors 40 to 60 years after radiation exposure [13]. In the cancer setting, a study of patients receiving radiation therapy showed a higher risk of developing MDS than in those who did not [14]. Nevertheless, the conclusions of studies in specific cancer series are not very uniform, with conflicting data in relation to breast cancer, Hodgkin lymphoma and radiation as part of myeloablative regimens before hematopoietic stem cell transplantation [15].
It has been estimated that 10% of patients with non-Hodgkin lymphoma [16], 8.2% of patients with chronic lymphocytic leukemia [17] and 3.4% of patients with multiple myeloma (MM) develop MDS-pCT [18]. Cytogenetic abnormalities are detectable in almost 90% of MDS-pCT patients [19], while altered karyotypes are reported in 40–50% of patients with de novo MDS [20]. Likewise, while high-risk forms with a poor prognosis are prevalent in the 46–70% of MDS-pCT patients [20,21], they are only reported in 30% of patients with de novo MDS [22]. However, the same cytogenetic abnormalities are found in both groups of patients, who were undistinguishable at the karyotype level [19,23,24] (Table 1).

Table 1.
Chromosome alterations associated with MDS with different etiologies.
On the other hand, the latency period for the appearance of MDS-pCT after treatment substantially varies depending on the type of primary cancer and the treatment regimen [21]. This is well known for drugs used for long periods in anticancer schedules. Treatment with alkylating agents has been associated with longer latency times, adverse cytogenetics with a high frequency of complex karyotypes and a poor prognosis [28]. Regarding cytogenetics, the most common clonal abnormalities include the partial or total loss of chromosomes 5 and 7 and complex karyotypes after treatment with alkylating agents [22]. The development of MDS-pCT after receiving anthracyclines and/or topoisomerase II inhibitors is associated with a median latency of 1 to 3 years and an MLL translocation at 11q23 or RUNX1/AML1 at 21q22 and a low frequency of complex karyotypes [22,26,27] (Table 1).
Exposure to PARP1 inhibitors is another criterion for the development of MN-pCT [1]. Poly (ADP-ribose) polymerase (PARP) inhibitors (PARPi) are active in cells with impaired ability to repair DNA double-strand breaks, such as cancer cells with mutations in the tumor suppressors BRCA1 or BRCA2, and have been used in subsets of patients with ovarian, breast, prostate and pancreatic cancer [28,29]. During the last 2 years, several reports have identified patients with MDS and AML following PARPi therapy [1,24,30]. Common findings include a median latency of 2 years after the initiation of PARPi treatment, complex karyotypes, the presence of germline damage response gene variants and the acquisition of TP53 mutations [31,32]. Interestingly, one of these studies reported how clonal hematopoiesis was more common in patients with ovarian cancer receiving PARPi maintenance therapy than in those not receiving this treatment, showing expansion in paired specimens pre- and post-therapy [33].
On the other hand, immunomodulatory drugs were introduced for treating MM in the late 90s, leading to significantly improved overall survival. During the following decade, lenalidomide replaced thalidomide as the most used immunomodulatory drug for MM due to its higher efficacy and lower toxicity. Several clinical trials have found significantly higher rates of secondary myeloid neoplasms in lenalidomide-treated arms than in those without lenalidomide in relapsed/refractory, transplant-eligible and transplant ineligible MM patients [34]. A definitive role for lenalidomide in the development of a secondary myeloid neoplasm after therapy for MM cannot be established in many cases since patients often receive high-dose chemotherapy during their initial treatment. However, a recent systematic analysis of 416 patients with MN-pCT and a detailed prior history of exposure found that TP53 mutations were significantly associated with previous treatment with thalidomide analogs, specifically lenalidomide. They also showed that lenalidomide treatment provides a selective advantage over TP53 (Trp53 in mice)-mutant murine hematopoietic stem and progenitor cells (HSPCs) in vitro and in vivo, and that the effect was specific to Trp53-mutant HSPCs and was not observed in HSPCs with other clonal hematopoiesis mutations [35]. A recent systematic review and meta-analysis found that lenalidomide-induced later sporadic malignancies seem to occur exclusively in patients with MM, and no significant increase was described in chronic lymphocytic leukemia and MDS trials [36].
Scarce information has been published regarding prognostic models or associations between MDS-pCT and concrete clinical outcomes and progression [37]. In addition, there are no clinical management guidelines for the appropriate monitoring of these patients, since there are no accurate diagnostic criteria that consider all etiologic factors involved in the development of MDS-pCT, thereby hampering the exploration of targeted therapies. An important clue when identifying the different etiologies in secondary MDS relies on the fact that the somatic signatures in MDS-pCT are indistinguishable from those occurring in de novo MDS [12], which would hide the incidence of the treatment effect in MDS development [38]. Only TP53 mutations were found to be enriched in MDS-pCT patients, while spliceosome mutations are more frequent in de novo MDS, which might partially explain the complex karyotype and unfavourable clinical outcomes of MDS-pCT patients [39]. Likewise, mutations in the PPM1D gene, a negative regulator of the DNA damage response pathway, are also frequent in MDS-pCT patients, as they confer a competitive advantage under the selective pressure of chemotherapy [40].
2.2. Germline Predisposition
Over the last decade, large-scale genomic studies have described the landscape of genomic variants in many of the most relevant types of cancer with the initial and fundamental objective of providing prognostic, diagnostic and pathogenic information based on the acquired alterations detected. However, the co-assessment of germ tissue in these series has transformed the understanding of how inherited variants influence cancer development [41]. Within myeloid neoplasms, it was estimated that 5% to 10% of patients with AML carried germline variants predisposing them to myeloid neoplasia [42]. In MDS, it has been estimated that germline mutations could explain at least 15% of adult and pediatric MDS cases [43]. In specific contexts, such as adolescents with MDS and monosomy of chromosome 7, this percentage could reach up to 70% [44].
The identification of clinical features and molecular biomarkers linked to this entity is important since its clinical management differs from sporadic MDS [21]. To this end, several guidelines for myeloid neoplasms with a germline predisposition have been described to identify these patients [45,46]. A growing number of inherited genetic loci that contribute to MDS has been identified [47,48]. The SAMD9, SAMD9L, SRP72, TERC and TERT genes, together with other genes typically mutated in sporadic MDS, such as TP53, GATA2, DDX41, ANKRD26, ETV6, CEBPA, ASXL1 and RUNX1, have been associated with the germline development of the disease [49]. Different correlations have been established among germline mutations, the age of onset and the severity of the myeloid neoplasm [50].
Furthermore, several hereditary syndromes have also been associated with MDS development [51]. These MDSs arise within the genetic landscape that predisposes patients to multiple tumors [52]. Several diseases, such as Fanconi Anemia, Severe Congenital Neutropenia, Dyskeratosis Congenita or Blackfan–Diamond Anemia present with bone marrow failure triggering MDS [53]. Although a particular karyotype has not been described in most germline-predisposed MDSs, in the context of inherited bone marrow failure (BMF) syndromes, recurrent chromosomal findings have been described, including duplications of chromosome regions 1q or 3q in Fanconi Anemia, isochromosome 7q in Shwachman–Diamond Syndrome, or isolated monosomy 7 common in GATA2 haploinsufficiency, among others [54] (Table 1).
2.3. Clonal Hematopoiesis
Finally, the presence of clonal hematopoiesis has been observed to increase with age and actively participates in the development of myeloid neoplasms [55]. Clonal hematopoiesis of indeterminate potential (CHIP) is defined as the presence of clonal mutations in genes recurrently mutated in myeloid neoplasms in peripheral blood of healthy individuals at a low frequency [6,56]. The incidence of CHIP has been associated with a higher risk of developing hematologic malignant neoplasms with adverse outcomes [57]. Altered clones not only harbor genetic alterations but also numerical and structural chromosomal changes, including those found in hematopoietic malignancies, such as del(20q), del(13q), del(11q), trisomy 8 or less commonly, del(5q) or del(7q) [6,21] (Table 1). CHIP has recently been described as a risk factor for developing secondary cardiovascular diseases [58].
2.4. Current Classifications and Secondary MDS
In light of the different risk factors entailing different outcomes, the identification of risk factors must be concise for differential diagnosis and adequate clinical management [59]. However, identification of different overlapping factors in relation with the development of MDS in a patient with a previous primary tumor is challenging. This hematologic condition is considered a secondary MDS, but an efficient consensus risk factor-based classification has yet to be established.
The WHO first classified MDS occurring following cytotoxic therapy for a primary tumor as MDS-pCT independently of MDS associated with a germline predisposition [60]. The latest update of the WHO classification (2022) proposes considering the entity of secondary myeloid neoplasms, which encompass the MDSs that arise from previous exposition to cytotoxic therapy or immune intervention (MDS-pCT), as well as MDS that occurs within the context of a syndromic germline [1]. These separate subentities do not consider other risk factors or the overlapping of both conditions (MDS-pCT and germline predisposition).
On the other hand, the recent International Consensus Classification (2022) maintains the therapy-related myeloid neoplasm (TRMN) category as an entity [61]. Nevertheless, this update clarifies that TRMN should be subclassified according to its morphology and genetics, as risk factors, such as CHIP or clonal cytopenia, can occur after exposure to cytotoxic treatment. In addition, this proposal also suggests that the presence of an underlying germline condition must be explored, considering a possible relocation to germline mutation-associated disorders either as syndromes when the genetic origin is common between the two tumors or as having different molecular drivers.
Both classifications are based on fitting, including two delimited etiologies for secondary MDS, based on therapy toxicity and genetic predisposition (Figure 1A). However, this two independent etiology-based classification does not consider the genetic predisposition aside from syndromic MDSs, excluding multiple overlapping etiologies and scenarios that share the same clinical appearance. The relative contribution of risk factors, such as germline predisposition or the presence of CHIP, in the development of MDS-pCT have not yet been fully explored.

Figure 1.
Secondary myelodysplastic neoplasm (MDS) jigsaw pieces. (A) Current classifications based on delimited etiologies for secondary MDS arising from cytotoxic treatment or risk factors (genetic predisposition and clonal hematopoiesis of indeterminate potential, CHIP). MDS post-cytotoxic therapy (MDS-pCT) or therapy-related myeloid neoplasm (TRMN) were secondary MDSs arising from treatment effects with no overlap with other risk factors. (B) New classification approach. Both cytotoxic treatment and the presence of an underlying germline or CHIP conditions are overlapping etiologies and compose different susceptibility scenarios. The different contribution of several risk factors might participate in the development of MDS-pCT. Thus, treatment might promote or increase CHIP, while genetic predisposition might modulate the effect of cytotoxic therapy.
Thus, genetic predisposition might increase the susceptibility to cytotoxic agents. The treatment of a primary tumor may affect bone marrow cells differently depending on the presence of mutations affecting the DNA damage repair system. In this sense, it has been described that between 16 and 21% of cancer survivors who developed MDS-pCT had a germline mutation associated with inherited cancer susceptibility genes [12]. Several studies have reported germline mutations in BRCA1, BRCA2, PALB2, CHEK2 and TP53 [62,63] and Fanconi Anemia genes in MDS-pCT patients [64].
Similarly, recent studies showed that cancer therapy shapes the fitness landscape of clonal hematopoiesis by promoting the onset or the increment of cytopenia and clonal dysplasia, such as idiopathic cytopenia of undetermined significance (ICUS), clonal cytopenia of undetermined significance (CCUS), idiopathic dysplasia of unknown significance (IDUS) or CHIP [65]. CHIP was higher than expected according to age in patients with MDS-pCT at the time of diagnosis of the primary tumor and before treatment [66]. According to recent studies, it is estimated that 30% of patients with MDS-pCT have CHIP [65]. CHIP was detected in 66% of MDS-pCT patients previously treated for gynecologic and breast cancers, including mutations in TP53 (31%), DNMT3A (19%), IDH1/2 (13%), NRAS (13%), TET2 (12%), NPM1 (10%), PPM1D (9%) and PTPN11 (9%) [39]. Since CHIP is frequently observed in patients with MDS-pCT at the time of diagnosis of the primary tumor, it has been suggested as a predictive marker to identify patients at risk to preclude the administration of treatments that might trigger the development of MDS-pCT [67].
In addition to these overlapping scenarios, both primary and secondary tumors might not be associated with any risk factor and do not occur concomitantly. Current therapeutic strategies have reduced the mortality among cancer patients, with an increase in survival rates entailing an increased frequency of age-dependent secondary pathologies with no relationship with the primary tumor. Thus, both tumors might arise independently or from different risk factors. The MDSs of these patients would have no relationship with the cytotoxic therapy of the primary tumor or a common genetic origin (syndrome), but they mimic the clinical definition of a secondary MDS.
3. Discussion: How to Put the Jigsaw Pieces Together
With a few exceptions, the cytogenetic abnormalities and molecular similarities between secondary MDSs of different etiologies hamper adequate classification of differential diagnoses [20,21,36] (Table 1). Furthermore, only a few studies with low number of patients have evaluated the proportion of altered karyotypes within the etiologies of CHIP and genetic risk factors within the context of secondary MDS (Table 1). Thus, the debate regarding the classification of secondary MDS is apparently going to continue during the coming years. The current classifications consider separate etiologies based on risk factors and cytotoxic therapy to describe either secondary MDS [1] or TRMN [61] (Figure 1A). Nevertheless, recent studies regarding the germline landscape and CHIP condition in MDS-pCT patients support the contention that these different etiologies must be considered as overlapping [37,60,66,67] (Figure 1B). Thus, genetic predisposition must be considered beyond bone marrow syndromes [12]. Likewise, CHIP may be initiated, expanded or triggered by cytotoxic therapy [67]; however, both CHIP and treatment for the primary tumor could also coexist with no association. In addition, different mutational burdens and their correlation with CHIP-related mutations are different for different hematological malignancies [68]. Thus, different MDS subtypes may be expected in different combinations of cytotoxic effects together with an individual genetic landscape and CHIP mutations.
We foresee future classifications of secondary MDSs assessing all risk factors and their interactions for a better assessment of the etiology of the cancer. Therefore, overlapping risk factors (CHIP and germline predisposition), together with the cytotoxic therapy effect, would result in the clinical definition of different concomitant scenarios, including a single or variety of responsible risk factors. Finally, clinical scenarios in which both tumors are independent or do not share risk factors must be considered as they mimic the clinical manifestation of secondary MDS but must be identified to prevent background noise.
Therefore, we suggest the following scenarios (Figure 2).
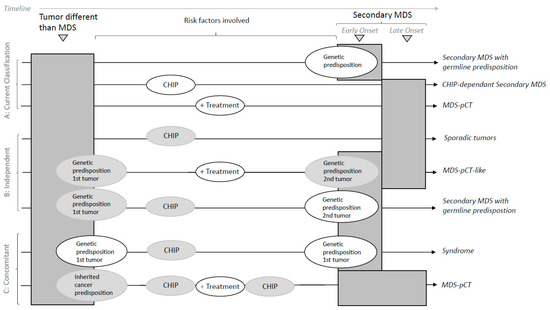
Figure 2.
Classification of secondary MDS. The risk factors involved in different etiologies are shown for the primary tumor and secondary MDS. Risk factors in white circles refer to mandatory conditions per the scenario described. Risk factors in grey circles refer to non-strictly necessary overlapping conditions co-occurring with the mandatory condition. The timeline shows the chronology of tumor events. The expected time of onset is shown for secondary MDS. Early onset is expected when MDS involves genetic predisposition. (A) Current classifications are based on well-delimited risk factors (genetic predisposition, CHIP or cytotoxic treatment). New classification approaches must consider the overlap between risk factors that compose different susceptibility scenarios of independent and concomitant tumors. (B) Independence: both tumors are independent when they do not share risk factors (sporadic) or even when the treatment of the first tumor is not related to the development of MDS (MDS-pCT-like). In addition, the primary tumor and secondary MDS might arise from a strong but not shared genetic predisposition. (C) Concomitancy is considered when secondary MDS arises in relation to the etiology of the first tumor. Typically, secondary MDS with germline predisposition within a syndromic scenario arises from genetic predisposition in common with that of the first tumor. MDS-pCT arises from exposure to a cytotoxic therapy for treating the primary tumor. The relative contribution of risk factors, such as germline predisposition or the presence of CHIP, might contribute to the development of MDS-pCT. Treatment might promote or increase CHIP, while genetic predisposition might modulate the cytotoxic effect.
(a) Concomitant tumors are related to secondary MDS when it involves the etiology of the primary tumor:
(a.1) MDS-pCT disease; the hematologic disease arises through exposure to a cytotoxic therapy when treating a primary tumor. Treatment might do the following:
- solely contribute to MDS without other known risk factors;
- trigger CHIP or drive an increase in CHIP;
- contribute depending on germline predisposition or susceptibility;
- contribute together with CHIP and the germline landscape.
(a.2) Syndrome; secondary MDS arises from a common genetic origin together with the first tumor.
(b) Independent tumors mimic the clinical appearance of the previous scenarios but do not share risk factors:
- Sporadic, correlative tumors with no shared risk factors;
- MDS-pCT-like, in which cytotoxic therapy does not participate in the development of a secondary myeloid tumor.
Secondary MDS occurs with germline predisposition, in which a primary tumor might also have a genetic predisposition but is not common with that of the MDS.
4. Conclusions and Future Directions
In summary, the role of risk factors in the different etiologies of MDS scenarios is still unclear. Furthermore, the classification of the etiologies of MDS becomes even more challenging when it occurs in a patient with a previous primary tumor. The first step to understand the molecular pathophysiology and the role of risk factors in different secondary MDSs is to have an adequate classification even with no prognostic biomarkers or molecular descriptions. Current classifications are based on etiologies related to well-delimited risk factors and cytotoxic therapy. However, the current knowledge regarding the biology of hematological malignancies supports the integration of genetic predisposition and CHIP into future MDS-pCT classifications to reflect the biological diversity and etiologies of MDS-pCT and their impact on outcomes. Thus, future classifications must consider the concomitance of single and overlapping risk factors, as well as independence regarding the relationship between primary and secondary tumors.
Deciphering the contribution of risk factors in combination with cytotoxic treatments will allow for the differential diagnoses of patients at risk to aid in routine clinical decision-making at the translational level to provide adequate clinical management. In addition, understanding the pathologic mechanisms underlying different etiologies will improve the development of prognostic biomarkers and therapy-oriented guidance of primary tumors for patients at risk of developing secondary MDS and will also help in genetic counseling related to the suitability of hematopoietic progenitor cell transplants (HPTs) from a related donor.
Author Contributions
O.C., F.S., J.M. and A.J. wrote, reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Instituto de Salud Carlos III, Ministerio de Economia y Competividad, grant numbers PI/17/0575, PI19/00374, PI 20/00531 and PI22/00102; TRANSCAN, grant numbers AECC AC 18/000002 and ISCIII; and Generalitat de Catalunya, grant numbers 2017 SGR288 (GRC) and SGR00560 (GRC).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th Edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef] [PubMed]
- Bersanelli, M.; Travaglino, E.; Meggendorfer, M.; Matteuzzi, T.; Sala, C.; Mosca, E.; Chiereghin, C.; di Nanni, N.; Gnocchi, M.; Zampini, M.; et al. Classification and Personalized Prognostic Assessment on the Basis of Clinical and Genomic Features in Myelodysplastic Syndromes. J. Clin. Oncol. 2021, 39, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Lim, U.; Park, Y.; Mayne, S.T.; Wang, R.; Hartge, P.; Hollenbeck, A.R.; Schatzkin, A. Obesity, Lifestyle Factors, and Risk of Myelodysplastic Syndromes in a Large US Cohort. Am. J. Epidemiol. 2009, 169, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Kuendgen, A.; Nomdedeu, M.; Tuechler, H.; Garcia-Manero, G.; Komrokji, R.S.; Sekeres, M.A.; della Porta, M.G.; Cazzola, M.; DeZern, A.E.; Roboz, G.J.; et al. Therapy-Related Myelodysplastic Syndromes Deserve Specific Diagnostic Sub-Classification and Risk-Stratification—An Approach to Classification of Patients with t-MDS. Leukemia 2021, 35, 835–849. [Google Scholar] [CrossRef]
- Furutani, E.; Shimamura, A. Germline Genetic Predisposition to Hematologic Malignancy. J. Clin. Oncol. 2017, 35, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Steensma, D.P.; Bejar, R.; Jaiswal, S.; Lindsley, R.C.; Sekeres, M.A.; Hasserjian, R.P.; Ebert, B.L. Clonal Hematopoiesis of Indeterminate Potential and Its Distinction from Myelodysplastic Syndromes. Blood 2015, 126, 9–16. [Google Scholar] [CrossRef]
- Zeidan, A.M.; Faltas, B.; Douglas Smith, B.; Gore, S. Myelodysplastic Syndromes: What Do Hospitalists Need to Know? J. Hosp. Med. 2013, 8, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Horny, H.-P.; Bennett, J.M.; Fonatsch, C.; Germing, U.; Greenberg, P.; Haferlach, T.; Haase, D.; Kolb, H.-J.; Krieger, O.; et al. Definitions and Standards in the Diagnosis and Treatment of the Myelodysplastic Syndromes: Consensus Statements and Report from a Working Conference. Leuk. Res. 2007, 31, 727–736. [Google Scholar] [CrossRef]
- Pedersen-Bjergaard, J.; Philip, P.; Mortensen, B.T.; Ersbøll, J.; Jensen, G.; Panduro, J.; Thomsen, M. Acute Nonlymphocytic Leukemia, Preleukemia, and Acute Myeloproliferative Syndrome Secondary to Treatment of Other Malignant Diseases. Clinical and Cytogenetic Characteristics and Results of in Vitro Culture of Bone Marrow and HLA Typing. Blood 1981, 57, 712–723. [Google Scholar] [CrossRef]
- Bennett, J.M.; Catovsky, D.; Daniel, M.T.; Flandrin, G.; Galton, D.A.; Gralnick, H.R.; Sultan, C. Proposals for the Classification of the Myelodysplastic Syndromes. Br. J. Haematol. 1982, 51, 189–199. [Google Scholar] [CrossRef]
- Streuli, R.A.; Testa, J.R.; Vardiman, J.W.; Mintz, U.; Golomb, H.M.; Rowley, J.D. Dysmyelopoietic Syndrome: Sequential Clinical and Cytogenetic Studies. Blood 1980, 55, 636–644. [Google Scholar] [CrossRef]
- McNerney, M.E.; Godley, L.A.; le Beau, M.M. Therapy-Related Myeloid Neoplasms: When Genetics and Environment Collide. Nat. Rev. Cancer 2017, 17, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M.; Hsu, W.-L.; Soda, M.; Takasaki, Y.; Tawara, M.; Joh, T.; Amenomori, T.; Yamamura, M.; Yoshida, Y.; Koba, T.; et al. Risk of Myelodysplastic Syndromes in People Exposed to Ionizing Radiation: A Retrospective Cohort Study of Nagasaki Atomic Bomb Survivors. J. Clin. Oncol. 2011, 29, 428–434. [Google Scholar] [CrossRef]
- Sun, L.-M.; Lin, C.-L.; Lin, M.-C.; Liang, J.-A.; Kao, C.-H. Radiotherapy- and Chemotherapy-Induced Myelodysplasia Syndrome. Medicine 2015, 94, e737. [Google Scholar] [CrossRef] [PubMed]
- Abou Zahr, A.; Kavi, A.M.; Mukherjee, S.; Zeidan, A.M. Therapy-Related Myelodysplastic Syndromes, or Are They? Blood Rev. 2017, 31, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Armitage, J.O.; Carbone, P.P.; Connors, J.M.; Levine, A.; Bennett, J.M.; Kroll, S. Treatment-Related Myelodysplasia and Acute Leukemia in Non-Hodgkin’s Lymphoma Patients. J. Clin. Oncol. 2003, 21, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.R.; Neuberg, D.; Flinn, I.W.; Grever, M.R.; Lazarus, H.M.; Rowe, J.M.; Dewald, G.; Bennett, J.M.; Paietta, E.M.; Byrd, J.C.; et al. Incidence of Therapy-Related Myeloid Neoplasia after Initial Therapy for Chronic Lymphocytic Leukemia with Fludarabine-Cyclophosphamide versus Fludarabine: Long-Term Follow-up of US Intergroup Study E2997. Blood 2011, 118, 3525–3527. [Google Scholar] [CrossRef]
- Pemmaraju, N.; Shah, D.; Kantarjian, H.; Orlowski, R.Z.; Nogueras González, G.M.; Baladandayuthapani, V.; Jain, N.; Wagner, V.; Garcia-Manero, G.; Shah, J.; et al. Characteristics and Outcomes of Patients with Multiple Myeloma Who Develop Therapy-Related Myelodysplastic Syndrome, Chronic Myelomonocytic Leukemia, or Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2015, 15, 110–114. [Google Scholar] [CrossRef]
- Bendari, M.; Khoubila, N. Cytogenetic and Genetic Advances in Myelodysplasia Syndromes. In Cytogenetics—Classical and Molecular Strategies for Analysing Heredity Material; IntechOpen: London, UK, 2021. [Google Scholar]
- Schanz, J.; Braulke, F.; Haase, D. Rare Cytogenetic Abnormalities in Myelodysplastic Syndromes. Mediterr. J. Hematol. Infect. Dis. 2015, 7, e2015034. [Google Scholar] [CrossRef]
- Churpek, J.E.; Larson, R.A. The Evolving Challenge of Therapy-Related Myeloid Neoplasms. Best Pract. Res. Clin. Haematol. 2013, 26, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Shahjahani, M.; Hadad, E.H.; Azizidoost, S.; Nezhad, K.C.; Shahrabi, S. Complex Karyotype in Myelodysplastic Syndromes: Diagnostic Procedure and Prognostic Susceptibility. Oncol. Rev. 2019, 13, 389. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.F.; Malik, U.A.; Sohail, M.; Hassan, I.N.; Ali, S.; Shaukat, M.H.S. Cytogenetic Abnormalities in Myelodysplastic Syndromes: An Overview. Int. J. Hematol. Oncol. Stem Cell Res. 2017, 11, 231–239. [Google Scholar] [PubMed]
- Leone, G.; Fabiani, E.; Voso, M.T. De Novo and Therapy-Related Myelodysplastic Syndromes: Analogies and Differences. Mediterr. J. Hematol. Infect. Dis. 2022, 14, e2022030. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Wang, F.; Kantarjian, H.; Song, X.; Patel, K.; Neelapu, S.; Gumbs, C.; Little, L.; Tippen, S.; Thornton, R.; et al. Copy Number Alterations Detected as Clonal Hematopoiesis of Indeterminate Potential. Blood Adv. 2017, 1, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A. Therapy-Related Myeloid Neoplasms. Haematologica 2009, 94, 454–459. [Google Scholar] [CrossRef]
- Haase, D.; Germing, U.; Schanz, J.; Pfeilstöcker, M.; Nösslinger, T.; Hildebrandt, B.; Kundgen, A.; Lübbert, M.; Kunzmann, R.; Giagounidis, A.A.N.; et al. New Insights into the Prognostic Impact of the Karyotype in MDS and Correlation with Subtypes: Evidence from a Core Dataset of 2124 Patients. Blood 2007, 110, 4385–4395. [Google Scholar] [CrossRef]
- Heuser, M. Therapy-Related Myeloid Neoplasms: Does Knowing the Origin Help to Guide Treatment? Hematology 2016, 2016, 24–32. [Google Scholar] [CrossRef]
- Rowley, J.D.; Olney, H.J. International Workshop on the Relationship of Prior Therapy to Balanced Chromosome Aberrations in Therapy-Related Myelodysplastic Syndromes and Acute Leukemia: Overview Report. Genes Chromosomes Cancer 2002, 33, 331–345. [Google Scholar] [CrossRef]
- Armand, P.; Kim, H.T.; DeAngelo, D.J.; Ho, V.T.; Cutler, C.S.; Stone, R.M.; Ritz, J.; Alyea, E.P.; Antin, J.H.; Soiffer, R.J. Impact of Cytogenetics on Outcome of De Novo and Therapy-Related AML and MDS after Allogeneic Transplantation. Biol. Blood Marrow Transplant. 2007, 13, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Csizmar, C.M.; Saliba, A.N.; Swisher, E.M.; Kaufmann, S.H. PARP Inhibitors and Myeloid Neoplasms: A Double-Edged Sword. Cancers 2021, 13, 6385. [Google Scholar] [CrossRef]
- Todisco, E.; Gigli, F.; Ronchini, C.; Amato, V.; Sammassimo, S.; Pastano, R.; Parma, G.; Lapresa, M.T.; Bertolini, F.; Corsini, C.; et al. Hematological Disorders after Salvage PARPi Treatment for Ovarian Cancer: Cytogenetic and Molecular Defects and Clinical Outcomes. Int. J. Cancer 2022, 151, 1791–1803. [Google Scholar] [CrossRef]
- Martin, J.E.; Khalife-Hachem, S.; Grinda, T.; Kfoury, M.; Garciaz, S.; Pasquier, F.; Vargaftig, J.; Uzunov, M.; Belhabri, A.; Bertoli, S.; et al. Therapy-Related Myeloid Neoplasms Following Treatment with PARP Inhibitors: New Molecular Insights. Ann. Oncol. 2021, 32, 1046–1048. [Google Scholar] [CrossRef] [PubMed]
- Landgren, O.; Mailankody, S. Update on Second Primary Malignancies in Multiple Myeloma: A Focused Review. Leukemia 2014, 28, 1423–1426. [Google Scholar] [CrossRef] [PubMed]
- Sperling, A.S.; Guerra, V.A.; Kennedy, J.A.; Yan, Y.; Hsu, J.I.; Wang, F.; Nguyen, A.T.; Miller, P.G.; McConkey, M.E.; Quevedo Barrios, V.A.; et al. Lenalidomide Promotes the Development of TP53 -Mutated Therapy-Related Myeloid Neoplasms. Blood 2022, 140, 1753–1763. [Google Scholar] [CrossRef] [PubMed]
- Saleem, K.; Franz, J.; Klem, M.L.; Yabes, J.G.; Boyiadzis, M.; Jones, J.R.; Shaikh, N.; Lontos, K. Second Primary Malignancies in Patients with Haematological Cancers Treated with Lenalidomide: A Systematic Review and Meta-Analysis. Lancet Haematol. 2022, 9, e906–e918. [Google Scholar] [CrossRef]
- Quintás-Cardama, A.; Daver, N.; Kim, H.; Dinardo, C.; Jabbour, E.; Kadia, T.; Borthakur, G.; Pierce, S.; Shan, J.; Cardenas-Turanzas, M.; et al. A Prognostic Model of Therapy-Related Myelodysplastic Syndrome for Predicting Survival and Transformation to Acute Myeloid Leukemia. Clin. Lymphoma Myeloma Leuk. 2014, 14, 401–410. [Google Scholar] [CrossRef]
- Singhal, D.; Wee, L.Y.A.; Kutyna, M.M.; Chhetri, R.; Geoghegan, J.; Schreiber, A.W.; Feng, J.; Wang, P.P.-S.; Babic, M.; Parker, W.T.; et al. The Mutational Burden of Therapy-Related Myeloid Neoplasms Is Similar to Primary Myelodysplastic Syndrome but Has a Distinctive Distribution. Leukemia 2019, 33, 2842–2853. [Google Scholar] [CrossRef]
- Khalife-Hachem, S.; Saleh, K.; Pasquier, F.; Willekens, C.; Tarabay, A.; Antoun, L.; Grinda, T.; Castilla-Llorente, C.; Duchmann, M.; Quivoron, C.; et al. Molecular Landscape of Therapy-Related Myeloid Neoplasms in Patients Previously Treated for Gynecologic and Breast Cancers. Hemasphere 2021, 5, e632. [Google Scholar] [CrossRef] [PubMed]
- DeZern, A.E.; Malcovati, L.; Ebert, B.L. CHIP, CCUS, and Other Acronyms: Definition, Implications, and Impact on Practice. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 400–410. [Google Scholar] [CrossRef]
- Huang, K.; Mashl, R.J.; Wu, Y.; Ritter, D.I.; Wang, J.; Oh, C.; Paczkowska, M.; Reynolds, S.; Wyczalkowski, M.A.; Oak, N.; et al. Pathogenic Germline Variants in 10,389 Adult Cancers. Cell 2018, 173, 355–370. [Google Scholar] [CrossRef]
- Churpek, J.E.; Pyrtel, K.; Kanchi, K.-L.; Shao, J.; Koboldt, D.; Miller, C.A.; Shen, D.; Fulton, R.; O’Laughlin, M.; Fronick, C.; et al. Genomic Analysis of Germ Line and Somatic Variants in Familial Myelodysplasia/Acute Myeloid Leukemia. Blood 2015, 126, 2484–2490. [Google Scholar] [CrossRef]
- Tawana, K.; Brown, A.L.; Churpek, J.E. Integrating Germline Variant Assessment into Routine Clinical Practice for Myelodysplastic Syndrome and Acute Myeloid Leukaemia: Current Strategies and Challenges. Br. J. Haematol. 2022, 196, 1293–1310. [Google Scholar] [CrossRef] [PubMed]
- Wlodarski, M.W.; Hirabayashi, S.; Pastor, V.; Starý, J.; Hasle, H.; Masetti, R.; Dworzak, M.; Schmugge, M.; van den Heuvel-Eibrink, M.; Ussowicz, M.; et al. Prevalence, Clinical Characteristics, and Prognosis of GATA2-Related Myelodysplastic Syndromes in Children and Adolescents. Blood 2016, 127, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Baliakas, P.; Tesi, B.; Wartiovaara-Kautto, U.; Stray-Pedersen, A.; Friis, L.S.; Dybedal, I.; Hovland, R.; Jahnukainen, K.; Raaschou-Jensen, K.; Ljungman, P.; et al. Nordic Guidelines for Germline Predisposition to Myeloid Neoplasms in Adults: Recommendations for Genetic Diagnosis, Clinical Management and Follow-Up. Hemasphere 2019, 3, e321. [Google Scholar] [CrossRef] [PubMed]
- Schlegelberger, B.; Mecucci, C.; Wlodarski, M. Review of Guidelines for the Identification and Clinical Care of Patients with Genetic Predisposition for Hematological Malignancies. Fam. Cancer 2021, 20, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Churpek, J.E. Familial Myelodysplastic Syndrome/Acute Myeloid Leukemia. Best Pract. Res. Clin. Haematol. 2017, 30, 287–289. [Google Scholar] [CrossRef]
- Brown, A.L.; Churpek, J.E.; Malcovati, L.; Döhner, H.; Godley, L.A. Recognition of Familial Myeloid Neoplasia in Adults. Semin. Hematol. 2017, 54, 60–68. [Google Scholar] [CrossRef]
- Babushok, D.V.; Bessler, M.; Olson, T.S. Genetic Predisposition to Myelodysplastic Syndrome and Acute Myeloid Leukemia in Children and Young Adults. Leuk. Lymphoma 2016, 57, 520–536. [Google Scholar] [CrossRef]
- Chai-Ho, W.; Schiller, G.J. Myelodysplastic Syndromes: An Update on Pathophysiology and Management. In Recent Developments in Myelodysplastic Syndromes; IntechOpen: London, UK, 2019. [Google Scholar]
- Kennedy, A.L.; Shimamura, A. Genetic Predisposition to MDS: Clinical Features and Clonal Evolution. Blood 2019, 133, 1071–1085. [Google Scholar] [CrossRef]
- Palomino-Echeverría, S.; Vázquez, I.; Alfonso-Piérola, A.; José Larrayoz, M.; Aguilera-Díaz, A.; Ariceta, B.; Daniela Urribarri, A.; Mañú, A.; Blasco-Iturri, Z.; Prósper, F.; et al. Predisposición a Neoplasias Mieloides: El Nuevo Desafío En La Consulta de Hematología; Genética medica y genómica: Valencia, Spain, 2020; Volume 4. [Google Scholar]
- Crysandt, M.; Brings, K.; Beier, F.; Thiede, C.; Brümmendorf, T.H.; Jost, E. Germ Line Predisposition to Myeloid Malignancies Appearing in Adulthood. Expert Rev. Hematol. 2018, 11, 625–636. [Google Scholar] [CrossRef]
- Babushok, D.V.; Bessler, M. Genetic Predisposition Syndromes: When Should They Be Considered in the Work-up of MDS? Best Pract. Res. Clin. Haematol. 2015, 28, 55–68. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef]
- Pich, O.; Reyes-Salazar, I.; Gonzalez-Perez, A.; Lopez-Bigas, N. Discovering the Drivers of Clonal Hematopoiesis. Nat. Commun. 2022, 13, 4267. [Google Scholar] [CrossRef] [PubMed]
- Valent, P. ICUS, IDUS, CHIP and CCUS: Diagnostic Criteria, Separation from MDS and Clinical Implications. Pathobiology 2019, 86, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Senguttuvan, N.B.; Subramanian, V.; Venkatesan, V.; Muralidharan, T.R.; Sankaranarayanan, K. Clonal Hematopoiesis of Indeterminate Potential (CHIP) and Cardiovascular Diseases—An Updated Systematic Review. J. Genet. Eng. Biotechnol. 2021, 19, 105. [Google Scholar] [CrossRef]
- Bernard, E.; Tuechler, H.; Greenberg, P.L.; Hasserjian, R.P.; Arango Ossa, J.E.; Nannya, Y.; Devlin, S.M.; Creignou, M.; Pinel, P.; Monnier, L.; et al. Molecular International Prognostic Scoring System for Myelodysplastic Syndromes. NEJM Evid. 2022, 1, 7. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.; Thiele, J.; Borowitz, M.J.; le Beau, M.M.; Bloomfield, C.D.; Cazzola, M.; Vardiman, J.W. The 2016 Revision to the World Health Organization Classification of Myeloid Neoplasms and Acute Leukemia. Blood 2016, 127, 2391–2405. [Google Scholar] [CrossRef]
- Arber, D.A.; Orazi, A.; Hasserjian, R.P.; Borowitz, M.J.; Calvo, K.R.; Kvasnicka, H.M.; Wang, S.A.; Bagg, A.; Barbui, T.; Branford, S.; et al. International Consensus Classification of Myeloid Neoplasms and Acute Leukemia: Integrating Morphological, Clinical, and Genomic Data. Blood 2022, 140, 1200–1228. [Google Scholar] [CrossRef]
- Schulz, E.; Valentin, A.; Ulz, P.; Beham-Schmid, C.; Lind, K.; Rupp, V.; Lackner, H.; Wölfler, A.; Zebisch, A.; Olipitz, W.; et al. Germline Mutations in the DNA Damage Response Genes BRCA1, BRCA2, BARD1 and TP53 in Patients with Therapy Related Myeloid Neoplasms. J. Med. Genet. 2012, 49, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Churpek, J.E.; Marquez, R.; Neistadt, B.; Claussen, K.; Lee, M.K.; Churpek, M.M.; Huo, D.; Weiner, H.; Bannerjee, M.; Godley, L.A.; et al. Inherited Mutations in Cancer Susceptibility Genes Are Common among Survivors of Breast Cancer Who Develop Therapy-Related Leukemia. Cancer 2016, 122, 304–311. [Google Scholar] [CrossRef]
- Voso, M.T.; Fabiani, E.; Zang, Z.; Fianchi, L.; Falconi, G.; Padella, A.; Martini, M.; Li Zhang, S.; Santangelo, R.; Larocca, L.M.; et al. Fanconi Anemia Gene Variants in Therapy-Related Myeloid Neoplasms. Blood Cancer J. 2015, 5, e323. [Google Scholar] [CrossRef]
- Bolton, K.L.; Ptashkin, R.N.; Gao, T.; Braunstein, L.; Devlin, S.M.; Kelly, D.; Patel, M.; Berthon, A.; Syed, A.; Yabe, M.; et al. Cancer Therapy Shapes the Fitness Landscape of Clonal Hematopoiesis. Nat. Genet. 2020, 52, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Gillis, N.K.; Ball, M.; Zhang, Q.; Ma, Z.; Zhao, Y.; Yoder, S.J.; Balasis, M.E.; Mesa, T.E.; Sallman, D.A.; Lancet, J.E.; et al. Clonal Haemopoiesis and Therapy-Related Myeloid Malignancies in Elderly Patients: A Proof-of-Concept, Case-Control Study. Lancet Oncol. 2017, 18, 112–121. [Google Scholar] [CrossRef]
- Desai, P.; Roboz, G.J. Clonal Hematopoiesis and Therapy Related MDS/AML. Best Pract. Res. Clin. Haematol. 2019, 32, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Stengel, A.; Baer, C.; Walter, W.; Meggendorfer, M.; Kern, W.; Haferlach, T.; Haferlach, C. Mutational Patterns and Their Correlation to CHIP-Related Mutations and Age in Hematological Malignancies. Blood Adv. 2021, 5, 4426–4434. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).