Low-Dose Radiation Therapy (LDRT) against Cancer and Inflammatory or Degenerative Diseases: Three Parallel Stories with a Common Molecular Mechanism Involving the Nucleoshuttling of the ATM Protein?
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Historical Features of Low-Dose Radiotherapy
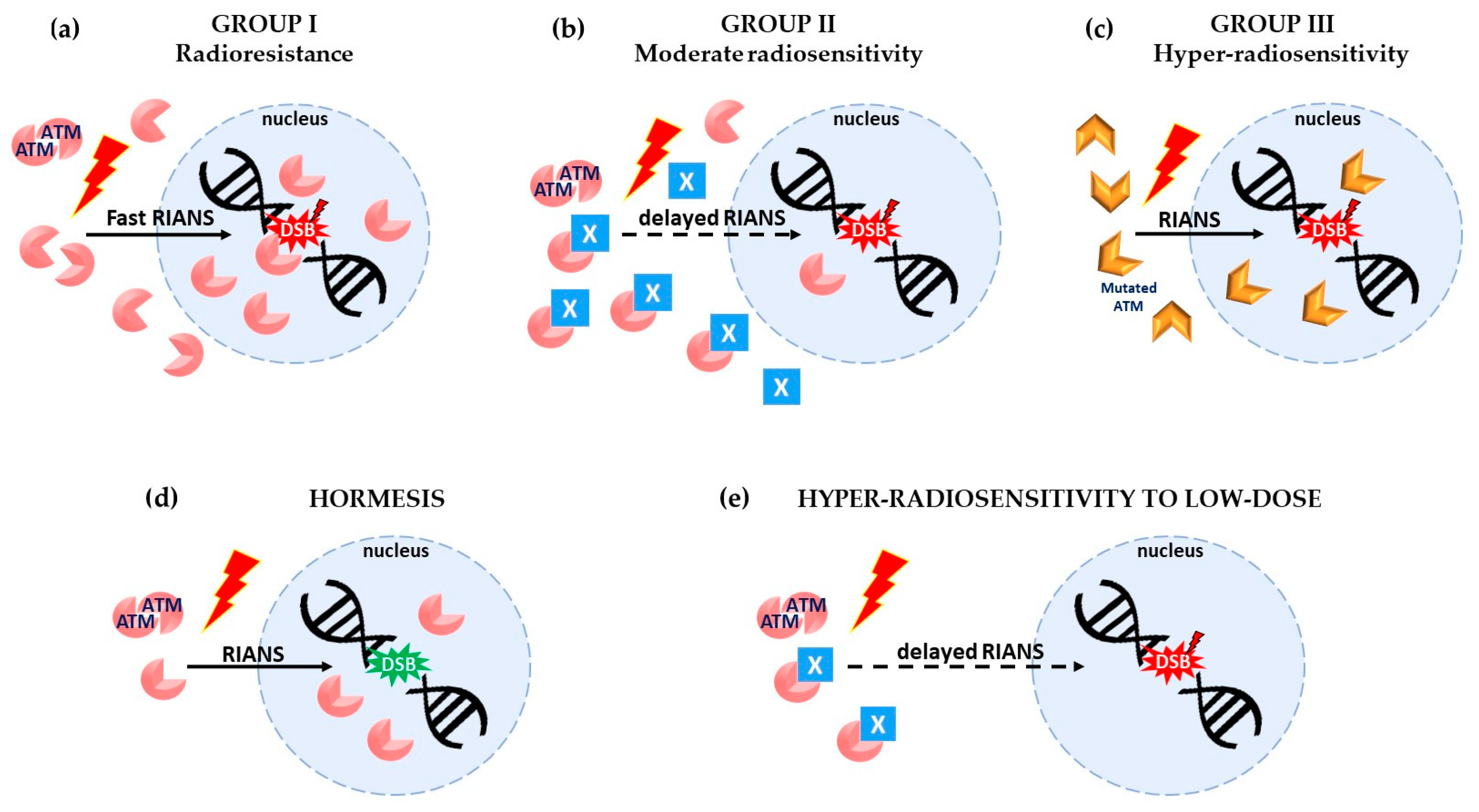
1.2. The Molecular Features of Hormesis, AR, and HRS Phenomena Explained by A Unified Model
- Hormesis: One Gy X-rays generally induce about 40 DSB and 105 to 106 ATM monomers per human non-transformed cell. Consequently, very few DSB are induced per cell at doses lower than 1/40 Gy (i.e., 25 mGy). If some spontaneous DSB are present in cells, exposure to a dose lower than 25 mGy provides some ATM monomers that may contribute to recognizing and repairing these spontaneous DSB. Hence, if these spontaneous DSB represent a risk of cancer or aging, a dose lower than 25 mGy may decrease such a risk. This phenomenon is generally observed in group I cells since the amount of ATM monomers after irradiation is significant at such low doses by comparison with group II cells, in which the flux of ATM monomers is reduced, and in group III cells, in which DSB recognition or repair are impaired [19] (Figure 1d).
- Adaptive response: In the AR scenario, a “priming” (low) dose may produce few DSB but overall a significant amount of ATM monomers that may contribute to recognizing and repairing the DSB induced by a “challenging” (high) dose, as far as the time interval between the two doses preserves the activity of the ATM monomers in the nucleus. The AR phenomenon is generally observed in group II cells since the contribution of ATM monomers provided by the priming dose is too low (due to their sequestration in the cytoplasm by the X-proteins) to recognize all the DSB induced by the priming dose. Conversely, in group I cells, all the DSB are recognized by the high flux of ATM monomers that diffuse in the nucleus [19].
- Hypersensitivity to low dose (HRS): As said above, for the priming dose, a low dose induces few DSB and few ATM monomers. In group II cells, the sequestration of ATM monomers by overexpressed X-proteins may drastically reduce the number of ATM monomers that finally diffuse in the nucleus. Consequently, in group II cells, after a single low dose (whether after the priming dose in the frame of AR scenario or after an HRS dose), the few DSB induced by the low dose may not all be recognized and repaired by the few ATM monomers available in the nucleus. The rate of unrepaired DSB after a low dose (e.g., 0.2 Gy) could produce an effect equivalent to that produced by a dose 5 to 10 times higher (e.g., 2 Gy) [20]. Again, in group I cells, such conditions are never reached since there are no ATM–X-protein complexes, and the amount of ATM monomers is always sufficient [20,23] (Figure 1e).
- A state of the art of the application of LDRT in clinical practice;
- A review of the basic mechanisms supporting the application of LDRT;
- A unified RIANS model integrating the radiobiological bases of LDRT.
2. LDRT in Oncology
2.1. Clinical Data about LDRT against Cancer
2.2. Biological Hypotheses about the Anti-Cancer Effect of LDRT
2.3. The HRS Phenomenon and the RIANS Model
3. LDRT in Inflammation-Related Pathologies
3.1. Clinical Data about LDRT against Inflammation in Rheumatology
3.2. Clinical Data about LDRT against Inflammation after COVID-19 Infection
3.3. Biological Hypotheses about the Anti-Inflammatory Effect of LDRT
3.4. Tissue Inflammation and the RIANS Model
4. LDRT in Alzheimer’s Disease (AD)
4.1. LDRT Clinical Trials in AD Patients
4.2. Biological Hypotheses about the Beneficial Effect of LDRT for AD
4.3. Degenerative Diseases and the RIANS Model
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Newmark, F.M. Radiation therapy of tuberculosis of hilar lymph nodes. JAMA 1955, 157, 532–533. [Google Scholar] [CrossRef]
- Despeignes, V. Observation concernant un cas de cancer de l’estomac traité par les rayons Roentgen. Lyon Méd. 1896, 82, 428–430. [Google Scholar]
- Despeignes, V. Observation concernant un cas de cancer de l’estomac traité par les rayons Roentgen. Lyon Méd. 1896, 82, 503–506. [Google Scholar]
- Bernier, J.; Hall, E.J.; Giaccia, A. Radiation oncology: A century of achievements. Nat. Rev. Cancer 2004, 4, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Monnard, C. Über Heilung des Gelenkrheumatismus durch Röntgenstrahlen bei. Kindern. Fortschr. Dem Geb. Der Röntgenstrahlen 1898, 1, 209. [Google Scholar]
- Stenbek, T. Om behandling of kronisk ledgangs-rheumatism med Rontgestralar. Sv Läk Förh 1898, 117. [Google Scholar]
- Rodel, F.; Keilholz, L.; Herrmann, M.; Sauer, R.; Hildebrandt, G. Radiobiological mechanisms in inflammatory diseases of low-dose radiation therapy. Int. J. Radiat. Biol. 2007, 83, 357–366. [Google Scholar] [CrossRef]
- Foray, N. Victor Despeignes (1866–1937): How a hygienist became the first radiation oncologist. Cancer Radiother. 2013, 17, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Foray, N. Victor Despeignes, the forgotten pioneer of radiation oncology. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 717–721. [Google Scholar] [CrossRef]
- Regaud, C.; Blanc, J. Action des rayons X sur les diverses générations de la lignée spermatique. Extrême sensibilité des spermatogonies à ces rayons. Comptes-Rendus Société Biol. 1906, 61, 163–165. [Google Scholar]
- Regaud, C. The influence of the duration of irradiation on the changes produced in the testicle by radium. Int. J. Radiat. Oncol. Biol. Phys. 1977, 2, 565–567. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Schull, W.J.; Awa, A.; Akiyama, M.; Otake, M. Dose-response analyses among atomic bomb survivors exposed to low-level radiation. Health Phys. 1987, 52, 645–652. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis: A fundamental concept in biology. Microb. Cell 2014, 1, 145–149. [Google Scholar] [CrossRef]
- Luckey, T.D. Hormesis with Ionizing Radiation; CRC Press: New York, NY, USA, 1980. [Google Scholar]
- Olivieri, G.; Bodycote, J.; Wolff, S. Adaptive response of human lymphocytes to low concentrations of radioactive thymidine. Science 1984, 223, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Devic, C.; Ferlazzo, M.L.; Foray, N. Influence of Individual Radiosensitivity on the Adaptive Response Phenomenon: Toward a Mechanistic Explanation Based on the Nucleo-Shuttling of ATM Protein. Dose-Response Publ. Int. Hormesis Soc. 2018, 16, 1559325818789836. [Google Scholar] [CrossRef]
- Lambin, P.; Marples, B.; Fertil, B.; Malaise, E.P.; Joiner, M.C. Hypersensitivity of a human tumour cell line to very low radiation doses. Int. J. Radiat. Biol. 1993, 63, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Joiner, M.C.; Lambin, P.; Malaise, E.P.; Robson, T.; Arrand, J.E.; Skov, K.A.; Marples, B. Hypersensitivity to very-low single radiation doses: Its relationship to the adaptive response and induced radioresistance. Mutat. Res. 1996, 358, 171–183. [Google Scholar] [CrossRef]
- Devic, C.; Ferlazzo, M.L.; Berthel, E.; Foray, N. Influence of Individual Radiosensitivity on the Hormesis Phenomenon: Toward a Mechanistic Explanation Based on the Nucleoshuttling of ATM Protein. Dose-Response Publ. Int. Hormesis Soc. 2020, 18, 1559325820913784. [Google Scholar] [CrossRef]
- Bodgi, L.; Foray, N. The nucleo-shuttling of the ATM protein as a basis for a novel theory of radiation response: Resolution of the linear-quadratic model. Int. J. Radiat. Biol. 2016, 92, 117–131. [Google Scholar] [CrossRef]
- Iliakis, G. The role of DNA double strand breaks in ionizing radiation-induced killing of eukaryotic cells. BioEssays News Rev. Mol. Cell. Dev. Biol. 1991, 13, 641–648. [Google Scholar]
- Pastwa, E.; Blasiak, J. Non-homologous DNA end joining. Acta Biochim. Pol. 2003, 50, 891–908. [Google Scholar] [CrossRef]
- Berthel, E.; Foray, N.; Ferlazzo, M.L. The Nucleoshuttling of the ATM Protein: A Unified Model to Describe the Individual Response to High- and Low-Dose of Radiation? Cancers 2019, 11, 905. [Google Scholar] [CrossRef] [PubMed]
- El-Nachef, L.; Al-Choboq, J.; Restier-Verlet, J.; Granzotto, A.; Berthel, E.; Sonzogni, L.; Ferlazzo, M.L.; Bouchet, A.; Leblond, P.; Combemale, P.; et al. Human Radiosensitivity and Radiosusceptibility: What Are the Differences? Int. J. Mol. Sci. 2021, 22, 7158. [Google Scholar] [CrossRef]
- Le Reun, E.; Bodgi, L.; Granzotto, A.; Sonzogni, L.; Ferlazzo, M.L.; Al-Choboq, J.; El-Nachef, L.; Restier-Verlet, J.; Berthel, E.; Devic, C.; et al. Quantitative correlations between radiosensitivity biomarkers show that the ATM protein kinase is strongly involved in the radiotoxicities observed after radiotherapy. Int. J. Mol. Sci. 2022, 23, 10434. [Google Scholar] [CrossRef]
- Kim, S.T.; Lim, D.S.; Canman, C.E.; Kastan, M.B. Substrate specificities and identification of putative substrates of ATM kinase family members. J. Biol. Chem. 1999, 274, 37538–37543. [Google Scholar] [CrossRef]
- El Nachef, L.; Berthel, E.; Ferlazzo, M.L.; Le Reun, E.; Al-Choboq, J.; Restier-Verlet, J.; Granzotto, A.; Sonzogni, L.; Bourguignon, M.; Foray, N. Cancer and Radiosensitivity Syndromes: Is Impaired Nuclear ATM Kinase Activity the Primum Movens? Cancers 2022, 14, 6141. [Google Scholar] [CrossRef]
- Wong, J.Y.C.; Filippi, A.R.; Dabaja, B.S.; Yahalom, J.; Specht, L. Total Body Irradiation: Guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 521–529. [Google Scholar] [CrossRef]
- Holmes, G.W.; Schulz, M.D. Radiation treatment of localized malignant lymphoma. N. Engl. J. Med. 1946, 235, 789–791. [Google Scholar] [CrossRef] [PubMed]
- Lybeert, M.L.; Meerwaldt, J.H.; Deneve, W. Long-term results of low dose total body irradiation for advanced non-Hodgkin lymphoma. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 1167–1172. [Google Scholar] [CrossRef] [PubMed]
- Richaud, P.M.; Soubeyran, P.; Eghbali, H.; Chacon, B.; Marit, G.; Broustet, A.; Hoerni, B. Place of low-dose total body irradiation in the treatment of localized follicular non-Hodgkin’s lymphoma: Results of a pilot study. Int. J. Radiat. Oncol. Biol. Phys. 1998, 40, 387–390. [Google Scholar] [CrossRef]
- Harney, J.; Short, S.C.; Shah, N.; Joiner, M.; Saunders, M.I. Low dose hyper-radiosensitivity in metastatic tumors. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 1190–1195. [Google Scholar] [CrossRef] [PubMed]
- Morganti, A.G.; Cellini, F.; Mignogna, S.; Padula, G.D.; Caravatta, L.; Deodato, F.; Picardi, V.; Macchia, G.; Cilla, S.; Buwenge, M.; et al. Low-dose radiotherapy and concurrent FOLFIRI-bevacizumab: A Phase II study. Future Oncol. 2016, 12, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Sezen, D.; Patel, R.R.; Tang, C.; Onstad, M.; Nagarajan, P.; Patel, S.P.; Welsh, J.W.; Lin, L.L. Immunotherapy combined with high- and low-dose radiation to all sites leads to complete clearance of disease in a patient with metastatic vaginal melanoma. Gynecol. Oncol. 2021, 161, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.R.; He, K.; Barsoumian, H.B.; Chang, J.Y.; Tang, C.; Verma, V.; Comeaux, N.; Chun, S.G.; Gandhi, S.; Truong, M.T.; et al. High-dose irradiation in combination with non-ablative low-dose radiation to treat metastatic disease after progression on immunotherapy: Results of a phase II trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 162, 60–67. [Google Scholar] [CrossRef]
- Bourhis, J.; Overgaard, J.; Audry, H.; Ang, K.K.; Saunders, M.; Bernier, J.; Horiot, J.C.; Le Maitre, A.; Pajak, T.F.; Poulsen, M.G.; et al. Hyperfractionated or accelerated radiotherapy in head and neck cancer: A meta-analysis. Lancet 2006, 368, 843–854. [Google Scholar] [CrossRef]
- Turrisi, A.T., 3rd; Kim, K.; Blum, R.; Sause, W.T.; Livingston, R.B.; Komaki, R.; Wagner, H.; Aisner, S.; Johnson, D.H. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N. Engl. J. Med. 1999, 340, 265–271. [Google Scholar] [CrossRef]
- Joiner, M.C.; Denekamp, J. The effect of small radiation doses on mouse skin. Br. J. Cancer Suppl. 1986, 7, 63–66. [Google Scholar]
- Marples, B.; Joiner, M.C. The response of Chinese hamster V79 cells to low radiation doses: Evidence of enhanced sensitivity of the whole cell population. Radiat. Res. 1993, 133, 41–51. [Google Scholar] [CrossRef]
- Bodgi, L.; Canet, A.; Pujo-Menjouet, L.; Lesne, A.; Victor, J.M.; Foray, N. Mathematical models of radiation action on living cells: From the target theory to the modern approaches. A historical and critical review. J. Theor. Biol. 2016, 394, 93–101. [Google Scholar] [CrossRef]
- Lambin, P.; Malaise, E.P.; Joiner, M.C. The effect of very low radiation doses on the human bladder carcinoma cell line RT112. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1994, 32, 63–72. [Google Scholar] [CrossRef]
- Short, S.C.; Kelly, J.; Mayes, C.R.; Woodcock, M.; Joiner, M.C. Low-dose hypersensitivity after fractionated low-dose irradiation in vitro. Int. J. Radiat. Biol. 2001, 77, 655–664. [Google Scholar] [CrossRef]
- Wykes, S.M.; Piasentin, E.; Joiner, M.C.; Wilson, G.D.; Marples, B. Low-dose hyper-radiosensitivity is not caused by a failure to recognize DNA double-strand breaks. Radiat. Res. 2006, 165, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Marples, B.; Wouters, B.G.; Collis, S.J.; Chalmers, A.J.; Joiner, M.C. Low-dose hyper-radiosensitivity: A consequence of ineffective cell cycle arrest of radiation-damaged G2-phase cells. Radiat. Res. 2004, 161, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Savage, T.; Pandey, S.; Guha, C. Postablation Modulation after Single High-Dose Radiation Therapy Improves Tumor Control via Enhanced Immunomodulation. Clin. Cancer Res. 2020, 26, 910–921. [Google Scholar] [CrossRef]
- Barsoumian, H.B.; Ramapriyan, R.; Younes, A.I.; Caetano, M.S.; Menon, H.; Comeaux, N.I.; Cushman, T.R.; Schoenhals, J.E.; Cadena, A.P.; Reilly, T.P.; et al. Low-dose radiation treatment enhances systemic antitumor immune responses by overcoming the inhibitory stroma. J. Immunother. Cancer 2020, 8, e000537. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.G.; Ronet, C.; Ochoa de Olza, M.; Barras, D.; Crespo, I.; Andreatta, M.; Corria-Osorio, J.; Spill, A.; Benedetti, F.; Genolet, R.; et al. Low-Dose Radiotherapy Reverses Tumor Immune Desertification and Resistance to Immunotherapy. Cancer Discov. 2022, 12, 108–133. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Martin, J.; Devic, C.; Diserbo, M.; Thariat, J.; Foray, N. Impact of dose-rate on the low-dose hyper-radiosensitivity and induced radioresistance (HRS/IRR) response. Int. J. Radiat. Biol. 2013, 89, 813–822. [Google Scholar] [CrossRef]
- May, E.A. Roentgen therapy in acute inflamatory conditions. Radiology 1930, 14, 411–415. [Google Scholar] [CrossRef]
- Kuhns, J.G.; Morrison, S.L. Twelve years experience in roentgenotherapy fro chronic arthritis. N. Engl. J. Med. 1946, 235, 399–405. [Google Scholar] [CrossRef]
- Gunderman, R.B.; Gonda, A.S. Radium girls. Radiology 2015, 274, 314–318. [Google Scholar] [CrossRef]
- McKeown, S.R.; Hatfield, P.; Prestwich, R.J.; Shaffer, R.E.; Taylor, R.E. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br. J. Radiol. 2015, 88, 20150405. [Google Scholar] [CrossRef] [PubMed]
- Muscoplat, C.C.; Caperton, E.M.; Dusenbery, K.E. Radiation therapy for inflammatory arthritis. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 688–689. [Google Scholar] [CrossRef] [PubMed]
- Seegenschmiedt, M.H.; Micke, O.; Muecke, R.; the German Cooperative Group on Radiotherapy for Non-malignant Diseases (GCG-BD). Radiotherapy for non-malignant disorders: State of the art and update of the evidence-based practice guidelines. Br. J. Radiol. 2015, 88, 20150080. [Google Scholar] [CrossRef]
- Micke, O.; Seegenschmiedt, M.H.; Adamietz, I.A.; Kundt, G.; Fakhrian, K.; Schaefer, U.; Muecke, R.; the German Cooperative Group on Radiotherapy for Non-malignant Diseases (GCG-BD). Low-Dose Radiation Therapy for Benign Painful Skeletal Disorders: The Typical Treatment for the Elderly Patient? Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 958–963. [Google Scholar] [CrossRef] [PubMed]
- Weissmann, T.; Ruckert, M.; Zhou, J.G.; Seeling, M.; Lettmaier, S.; Donaubauer, A.J.; Nimmerjahn, F.; Ott, O.J.; Hecht, M.; Putz, F.; et al. Low-Dose Radiotherapy Leads to a Systemic Anti-Inflammatory Shift in the Pre-Clinical K/BxN Serum Transfer Model and Reduces Osteoarthritic Pain in Patients. Front. Immunol. 2021, 12, 777792. [Google Scholar] [CrossRef]
- Van den Ende, C.H.M.; Minten, M.J.M.; Leseman-Hoogenboom, M.M.; Van den Hoogen, F.H.J.; Bden Broeder, A.A.; Mahler, E.A.M.; Poortmans, P.M.P. Long-term efficacy of low-dose radiation therapy on symptoms in patients with knee and hand osteoarthritis: Follow-up results of two parallel randomised, sham-controlled trials. Lancet Rheumatol. 2020, 2, E42–E49. [Google Scholar] [CrossRef]
- Mahler, E.A.M.; Minten, M.J.; Leseman-Hoogenboom, M.M.; Poortmans, P.M.P.; Leer, J.W.H.; Boks, S.S.; van den Hoogen, F.H.J.; den Broeder, A.A.; van den Ende, C.H.M. Effectiveness of low-dose radiation therapy on symptoms in patients with knee osteoarthritis: A randomised, double-blinded, sham-controlled trial. Ann. Rheum. Dis. 2019, 78, 83–90. [Google Scholar] [CrossRef]
- Minten, M.J.M.; Leseman-Hoogenboom, M.M.; Kloppenburg, M.; Kortekaas, M.C.; Leer, J.W.; Poortmans, P.M.P.; van den Hoogen, F.H.J.; den Broeder, A.A.; van den Ende, C.H.M. Lack of beneficial effects of low-dose radiation therapy on hand osteoarthritis symptoms and inflammation: A randomised, blinded, sham-controlled trial. Osteoarthr. Cartil. 2018, 26, 1283–1290. [Google Scholar] [CrossRef]
- Donaubauer, A.J.; Deloch, L.; Becker, I.; Fietkau, R.; Frey, B.; Gaipl, U.S. The Influence of Radiation on Bone and Bone Cells-Differential Effects on Osteoclasts and Osteoblasts. Int. J. Mol. Sci. 2020, 21, 6377. [Google Scholar] [CrossRef]
- Que, Y.; Hu, C.; Wan, K.; Hu, P.; Wang, R.; Luo, J.; Li, T.; Ping, R.; Hu, Q.; Sun, Y.; et al. Cytokine release syndrome in COVID-19: A major mechanism of morbidity and mortality. Int. Rev. Immunol. 2022, 41, 217–230. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Dhawan, D.K. How radiotherapy was historically used to treat pneunomia: Could it be useful today? Yale J. Biol. Med. 2013, 86, 555–570. [Google Scholar] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Oppenheimer, A. Roentgen therapy of interstitial pneumonia. J. Pediatr. 1943, 23, 534–538. [Google Scholar] [CrossRef]
- Arenas, M.; Algara, M.; De Febrer, G.; Rubio, C.; Sanz, X.; de la Casa, M.A.; Vasco, C.; Marin, J.; Fernandez-Leton, P.; Villar, J.; et al. Could pulmonary low-dose radiation therapy be an alternative treatment for patients with COVID-19 pneumonia? Preliminary results of a multicenter SEOR-GICOR nonrandomized prospective trial (IPACOVID trial). Strahlenther. Onkol. Organ Dtsch. Rontgengesellschaft 2021, 197, 1010–1020. [Google Scholar] [CrossRef]
- Sanmamed, N.; Alcantara, P.; Gomez, S.; Bustos, A.; Cerezo, E.; Gaztanaga, M.; Doval, A.; Corona, J.; Rodriguez, G.; Cabello, N.; et al. Low-dose Radiation Therapy in the Management of COVID-19 Pneumonia (LOWRAD-Cov19). Final results of a prospective phase I–II trial. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2022, 171, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, G.; Ponniah, S.; Sundaram, V.; Marimuthu, P.K.; Pitchaikannu, V.; Chandrasekaran, M.; Thangarasu, J.; Kannupaiyan, G.; Ramamoorthy, P.; Thangaraj, B.; et al. Whole lung irradiation as a novel treatment for COVID-19: Interim results of an ongoing phase 2 trial in India. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 163, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Tomas, E.; Acosta, J.C.; Torres-Royo, L.; De Febrer, G.; Baiges-Gaya, G.; Castane, H.; Jimenez, A.; Vasco, C.; Araguas, P.; Gomez, J.; et al. Effect of Low-Dose Radiotherapy on the Circulating Levels of Paraoxonase-1-Related Variables and Markers of Inflammation in Patients with COVID-19 Pneumonia. Antioxidants 2022, 11, 1184. [Google Scholar] [CrossRef]
- Papachristofilou, A.; Finazzi, T.; Blum, A.; Zehnder, T.; Zellweger, N.; Lustenberger, J.; Bauer, T.; Dott, C.; Avcu, Y.; Kohler, G.; et al. Low-Dose Radiation Therapy for Severe COVID-19 Pneumonia: A Randomized Double-Blind Study. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1274–1282. [Google Scholar] [CrossRef]
- Hess, C.B.; Buchwald, Z.S.; Stokes, W.; Nasti, T.H.; Switchenko, J.M.; Weinberg, B.D.; Steinberg, J.P.; Godette, K.D.; Murphy, D.; Ahmed, R.; et al. Low-dose whole-lung radiation for COVID-19 pneumonia: Planned day 7 interim analysis of a registered clinical trial. Cancer 2020, 126, 5109–5113. [Google Scholar] [CrossRef]
- Rodel, F.; Arenas, M.; Ott, O.J.; Fournier, C.; Georgakilas, A.G.; Tapio, S.; Trott, K.R.; Gaipl, U.S. Low-dose radiation therapy for COVID-19 pneumopathy: What is the evidence? Strahlenther. Onkol. Organ Dtsch. Rontgengesellschaft 2020, 196, 679–682. [Google Scholar] [CrossRef]
- Walsh, N.C.; Crotti, T.N.; Goldring, S.R.; Gravallese, E.M. Rheumatic diseases: The effects of inflammation on bone. Immunol. Rev. 2005, 208, 228–251. [Google Scholar] [CrossRef] [PubMed]
- Luyten, F.P.; Lories, R.J.; Verschueren, P.; de Vlam, K.; Westhovens, R. Contemporary concepts of inflammation, damage and repair in rheumatic diseases. Best Pr. Res. Clin. Rheumatol. 2006, 20, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Zoulikha, M.; Huang, F.; Wu, Z.; He, W. COVID-19 inflammation and implications in drug delivery. J. Control. Release 2022, 346, 260–274. [Google Scholar] [CrossRef]
- Rodel, F.; Schaller, U.; Schultze-Mosgau, S.; Beuscher, H.U.; Keilholz, L.; Herrmann, M.; Voll, R.; Sauer, R.; Hildebrandt, G. The induction of TGF-beta(1) and NF-kappaB parallels a biphasic time course of leukocyte/endothelial cell adhesion following low-dose X-irradiation. Strahlenther. Onkol. Organ Dtsch. Rontgengesellschaft 2004, 180, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Arenas, M.; Gil, F.; Gironella, M.; Hernandez, V.; Jorcano, S.; Biete, A.; Pique, J.M.; Panes, J. Anti-inflammatory effects of low-dose radiotherapy in an experimental model of systemic inflammation in mice. Int. J. Radiat. Oncol. Biol. Phys. 2006, 66, 560–567. [Google Scholar] [CrossRef]
- Arenas, M.; Gil, F.; Gironella, M.; Hernandez, V.; Biete, A.; Pique, J.M.; Panes, J. Time course of anti-inflammatory effect of low-dose radiotherapy: Correlation with TGF-beta(1) expression. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2008, 86, 399–406. [Google Scholar] [CrossRef]
- Rodel, F.; Hofmann, D.; Auer, J.; Keilholz, L.; Rollinghoff, M.; Sauer, R.; Beuscher, H.U. The anti-inflammatory effect of low-dose radiation therapy involves a diminished CCL20 chemokine expression and granulocyte/endothelial cell adhesion. Strahlenther. Onkol. Organ Dtsch. Rontgengesellschaft 2008, 184, 41–47. [Google Scholar] [CrossRef]
- Meziani, L.; Robert, C.; Classe, M.; Da Costa, B.; Mondini, M.; Clemenson, C.; Alfaro, A.; Mordant, P.; Ammari, S.; Le Goffic, R.; et al. Low Doses of Radiation Increase the Immunosuppressive Profile of Lung Macrophages During Viral Infection and Pneumonia. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 1283–1294. [Google Scholar] [CrossRef]
- Jackson, M.R.; Stevenson, K.; Chahal, S.K.; Curley, E.; Finney, G.E.; Gutierrez-Quintana, R.; Onwubiko, E.; Rupp, A.; Strathdee, K.; Williams, K.; et al. Low-Dose Lung Radiation Therapy for COVID-19 Lung Disease: A Preclinical Efficacy Study in a Bleomycin Model of Pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 197–211. [Google Scholar] [CrossRef]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesth. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef]
- Lim, Y.C.; Quek, H.; Offenhauser, C.; Fazry, S.; Boyd, A.; Lavin, M.; Roberts, T.; Day, B. ATM inhibition prevents interleukin-6 from contributing to the proliferation of glioblastoma cells after ionizing radiation. J. Neurooncol. 2018, 138, 509–518. [Google Scholar] [CrossRef]
- Cipriano, R.; Kan, C.E.; Graham, J.; Danielpour, D.; Stampfer, M.; Jackson, M.W. TGF-beta signaling engages an ATM-CHK2-p53-independent RAS-induced senescence and prevents malignant transformation in human mammary epithelial cells. Proc. Natl. Acad. Sci. USA 2011, 108, 8668–8673. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Radanovic, M.; Talib, L.L.; Aprahamian, I.; Diniz, B.S.; Zetterberg, H.; Gattaz, W.F. Cerebrospinal fluid biomarkers in Alzheimer’s disease: Diagnostic accuracy and prediction of dementia. Alzheimers Dement. 2015, 1, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.; Muehlboeck, J.S.; Simmons, A. Combining MRI and CSF measures for classification of Alzheimer’s disease and prediction of mild cognitive impairment conversion. Neuroimage 2012, 62, 229–238. [Google Scholar] [CrossRef]
- Thal, D.R.; Rub, U.; Orantes, M.; Braak, H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, J.; Wang, B.; Sun, M.; Yang, H. Microglia in the Neuroinflammatory Pathogenesis of Alzheimer’s Disease and Related Therapeutic Targets. Front. Immunol. 2022, 13, 856376. [Google Scholar] [CrossRef]
- Ma, M.W.; Wang, J.; Zhang, Q.; Wang, R.; Dhandapani, K.M.; Vadlamudi, R.K.; Brann, D.W. NADPH oxidase in brain injury and neurodegenerative disorders. Mol. Neurodegener. 2017, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Cuttler, J.M.; Moore, E.R.; Hosfeld, V.D.; Nadolski, D.L. Treatment of Alzheimer Disease with CT Scans: A Case Report. Dose-Response Publ. Int. Hormesis Soc. 2016, 14, 1559325816640073. [Google Scholar] [CrossRef]
- Cuttler, J.M.; Moore, E.R.; Hosfeld, V.D.; Nadolski, D.L. Update on a Patient with Alzheimer Disease Treated with CT Scans. Dose-Response Publ. Int. Hormesis Soc. 2017, 15, 1559325817693167. [Google Scholar] [CrossRef]
- Cuttler, J.M.; Lamet, M.S.; Calabrese, E.J. Treatment of Early-Stage Alzheimer’s Disease with CT Scans of the Brain: A Case Report. Dose-Response Publ. Int. Hormesis Soc. 2022, 20, 15593258221078392. [Google Scholar] [CrossRef]
- Ruchinskas, R.A.; Curyto, K.J. Cognitive screening in geriatric rehabilitation. Rehabil. Psychol. 2003, 48, 14–22. [Google Scholar] [CrossRef]
- Ceyzeriat, K.; Zilli, T.; Fall, A.B.; Millet, P.; Koutsouvelis, N.; Dipasquale, G.; Frisoni, G.B.; Tournier, B.B.; Garibotto, V. Treatment by low-dose brain radiation therapy improves memory performances without changes of the amyloid load in the TgF344-AD rat model. Neurobiol. Aging 2021, 103, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Marples, B.; McGee, M.; Callan, S.; Bowen, S.E.; Thibodeau, B.J.; Michael, D.B.; Wilson, G.D.; Maddens, M.E.; Fontanesi, J.; Martinez, A.A. Cranial irradiation significantly reduces beta amyloid plaques in the brain and improves cognition in a murine model of Alzheimer’s Disease (AD). Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2016, 118, 579–580. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Kim, H.; Choi, Y.; Kim, H.J.; Kim, J.H.; Yoon, J.; Seo, Y.S.; Kim, H.S. Modulation of Neuroinflammation by Low-Dose Radiation Therapy in an Animal Model of Alzheimer’s Disease. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 658–670. [Google Scholar] [CrossRef]
- Diatloff-Zito, C.; Deschavanne, P.J.; Loria, E.; Malaise, E.P.; Macieira-Coelho, A. Comparison between the radiosensitivity of human, mouse and chicken fibroblast-like cells using short-term endpoints. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1981, 39, 419–430. [Google Scholar] [CrossRef]
- Parkinson, E.K.; Hume, W.J.; Potten, C.S. The radiosensitivity of cultured human and mouse keratinocytes. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1986, 50, 717–726. [Google Scholar] [CrossRef]
- Cuttler, J.M.; Abdellah, E.; Goldberg, Y.; Al-Shamaa, S.; Symons, S.P.; Black, S.E.; Freedman, M. Low Doses of Ionizing Radiation as a Treatment for Alzheimer’s Disease: A Pilot Study. J. Alzheimer’s Dis. JAD 2021, 80, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nam, Y.; Kim, C.; Lee, H.; Hong, S.; Kim, H.S.; Shin, S.J.; Park, Y.H.; Mai, H.N.; Oh, S.M.; et al. Neuroprotective and Anti-Inflammatory Effects of Low-Moderate Dose Ionizing Radiation in Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 3678. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.D.; Wilson, T.G.; Hanna, A.; Fontanesi, G.; Kulchycki, J.; Buelow, K.; Pruetz, B.L.; Michael, D.B.; Chinnaiyan, P.; Maddens, M.E.; et al. Low Dose Brain Irradiation Reduces Amyloid-beta and Tau in 3xTg-AD Mice. J. Alzheimer’s Dis. JAD 2020, 75, 15–21. [Google Scholar] [CrossRef]
- Ceyzeriat, K.; Tournier, B.B.; Millet, P.; Dipasquale, G.; Koutsouvelis, N.; Frisoni, G.B.; Garibotto, V.; Zilli, T. Low-Dose Radiation Therapy Reduces Amyloid Load in Young 3xTg-AD Mice. J. Alzheimer’s Dis. JAD 2022, 86, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Mims, P.N.; Roman, R.J.; Fan, F. Is beta-amyloid accumulation a cause or a consequence of Alzheimer’s disease? J Alzheimer Park. Dement. 2016, 1, 007. [Google Scholar]
- Ferlazzo, M.; Berthel, E.; Granzotto, A.; Devic, C.; Sonzogni, L.; Bachelet, J.T.; Pereira, S.; Bourguignon, M.; Sarasin, A.; Mezzina, M.; et al. Some mutations in the xeroderma pigmentosum D gene may lead to moderate but significant radiosensitivity associated with a delayed radiation-induced ATM nuclear localization. Int. J. Radiat. Biol. 2019, 96, 394–410. [Google Scholar] [CrossRef]
- Ferlazzo, M.L.; Bach-Tobdji, M.K.E.; Djerad, A.; Sonzogni, L.; Burlet, S.F.; Devic, C.; Granzotto, A.; Bodgi, L.; Djeffal-Kerrar, A.; Foray, N. Radiobiological characterization of tuberous sclerosis: A delay in the nucleo-shuttling of ATM may be responsible for radiosensitivity. Mol. Neurobiol. 2017, 55, 4973–4983. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, M.L.; Sonzogni, L.; Granzotto, A.; Bodgi, L.; Lartin, O.; Devic, C.; Vogin, G.; Pereira, S.; Foray, N. Mutations of the Huntington’s Disease Protein Impact on the ATM-Dependent Signaling and Repair Pathways of the Radiation-Induced DNA Double-Strand Breaks: Corrective Effect of Statins and Bisphosphonates. Mol. Neurobiol. 2014, 49, 1200–1211. [Google Scholar] [CrossRef]
- Combemale, P.; Sonzogni, L.; Devic, C.; Bencokova, Z.; Ferlazzo, M.L.; Granzotto, A.; Burlet, S.F.; Pinson, S.; Amini-Adle, M.; Al-Choboq, J.; et al. Individual Response to Radiation of Individuals with Neurofibromatosis Type I: Role of the ATM Protein and Influence of Statins and Bisphosphonates. Mol. Neurobiol. 2022, 59, 556–573. [Google Scholar] [CrossRef]
- Soares, H.D.; Morgan, J.I.; McKinnon, P.J. Atm expression patterns suggest a contribution from the peripheral nervous system to the phenotype of ataxia-telangiectasia. Neuroscience 1998, 86, 1045–1054. [Google Scholar] [CrossRef]
- Robbins, J.H.; Otsuka, F.; Tarone, R.E.; Polinsky, R.J.; Brumback, R.A.; Nee, L.E. Parkinson’s disease and Alzheimer’s disease: Hypersensitivity to X rays in cultured cell lines. J. Neurol. Neurosurg. Psychiatry 1985, 48, 916–923. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Itzhaki, R.F. Radiosensitivity of lymphocytes from patients with Alzheimer’s disease. Mutat. Res. 1989, 217, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Tobi, S.E.; Moquet, J.E.; Edwards, A.A.; Lloyd, D.C.; Itzhaki, R.F. Chromosomal radiosensitivity of lymphocytes from Alzheimer’s disease patients. J. Med. Genet. 1990, 27, 437–440. [Google Scholar] [CrossRef] [PubMed]
| Target | Study Design | Irradiation Scheme | Response | Reference | |
|---|---|---|---|---|---|
| Haemato-oncology | |||||
| 68 patients with NHL (90% stage III and IV) | Whole body | Retrospective | LD-TBI: midline 0.1 Gy per session (1.78 Gy total) | Recurrence-free survival: 32% at 5 years, 27% at 10 years | [30] |
| 26 patients with NHL (stage III and IV) | Whole body | Prospective, non-randomized (pilot study) | Chemotherapy and 2 courses of LD-TBI: 5 × 0.15 Gy per session (separated by 2 weeks), followed 1 month later by radical involved-field RT (20 × 2 Gy per session) | Complete remission in 92.3% (24/26) of patients after LD-TBI and before IF-RT; Complete remission in 96.2% (25/26) of patients after IF-RT | [31] |
| Distant metastases | |||||
| 8 patients with metastatic tumor nodules | Metastases | Randomized study | Standard fractionated RT: 12 × 1.5 Gy per session or ultra-fractionated RT: 0.5 Gy/4 h over 12 days | Significantly increased growth delay in nodules treated with the ultra-fractionated RT scheme | [32] |
| 18 patients with metastatic colorectal cancer | Metastases | Phase II | 0.2 Gy per session every 6 h interval (on each chemotherapy cycle) | Clinical or pathological complete response in 38.9% (7/18) of patients | [33] |
| A 73-yr woman with metastatic vaginal mucosal melanoma progressing on immunotherapy | Metastases | Case report | LDRT: 5 × 1 Gy per session (liver metastasis); 6 × 1 Gy per session (inguinal lymph node) | Clinical and radiographic complete response | [34] |
| 74 patients with metastatic cancer (NSCLC, n = 38; melanoma, n = 21) progressing on immunotherapy within 6 months | Metastases | Phase II | HDRT alone: 3–12.5 Gy per session (20–70 Gy total) or HDRT + LDRT: 0.5–2 Gy per session (1–10 Gy total) | 4-month disease control response: 47% in HDRT + LDRT vs. 37% in HDRT alone (p = 0.38); Overall response: 26% in HDRT + LDRT vs. 13% in HDRT (p = 0.27) | [35] |
| Target | Study Design | Irradiation Scheme | Response | Reference | |
|---|---|---|---|---|---|
| Rheumatology | |||||
| 166 patients with painful skeletal disorders (calcaneodynia, n = 51; achillodynia, n = 8; gonarthrosis, n = 80; bursitis trochanterica, n = 27) | Joints/bone | Prospective | 0.5–1 Gy per session (6 Gy total) | Good response in 37.3% (62/166) of patients immediately on completion of RT and in 49.5% (54/109) of patients after a median follow-up of 29 months (p = 0.001) | [55] |
| 196 patients with ankle/foot osteoarthritis | Joints | Prospective | 0.5–1 Gy per session (3–6 Gy total) over 3 weeks | Subjective improvement of 80–100% in 37% (71/196) of patients | [56] |
| 56 patients with knee/hand osteoarthritis | Joints | Randomised, sham-controlled | LDRT: 6 × 1 Gy per session or sham | No significant evidence of beneficial LDRT effect | [57] |
| 55 patients with knee osteoarthritis | Joints | Randomised, double-blinded, sham-controlled | LDRT: 6 × 1 Gy per session or sham | No significant evidence of beneficial LDRT effect | [58] |
| 56 patients with hand osteoarthritis | Joints | Randomised, blinded, sham-controlled | LDRT: 6 × 1 Gy per session or sham | No significant evidence of beneficial LDRT effect | [59] |
| COVID-19 | |||||
| 36 COVID-19 patients | Bilateral whole lungs | Prospective | 1 × 0.5 Gy per session | SAFI improved from 255 mmHg to 283 mmHg at 24 h and to 381 mmHg at 1 week, respectively | [65] |
| 41 COVID-19 patients | Bilateral whole lungs | Prospective phase I-II | 1 × 1 Gy per session | SAFI significantly improved on day +3 and +7 (p < 0.01) | [66] |
| 25 COVID-19 patients | Bilateral whole lungs | Phase II | 1 × 0.5 Gy per session | SAFI significantly improved between pre-RT and day +2 (p < 0.05), +3 (p < 0.001) and +7 (p < 0.001) post-RT; oxygen supply significantly decreased between pre-RT and day +2 (p < 0.05), +3 (p < 0.001), and +7 (p < 0.001) post-RT | [67] |
| 30 COVID-19 patients | Bilateral whole lungs | Multicenter, prospective, observational | 1 × 0.5 Gy per session | SAFI significantly improved; oxygen supply decreased | [68] |
| 20 COVID-19 patients | Bilateral whole lungs | Randomized, double-blinded | 1 × 1 Gy per session or sham | No significant evidence in 15-day ventilator-free days (p = 1.00) nor overall survival at 28 days (p = 0.69) in both arms; lymphocyte counts significantly decreased after LDRT (p < 0.01) | [69] |
| 100 COVID-19 patients | Bilateral whole lungs | Phase II, randomized | LDRT: 1 × 0.35 Gy per session or 1 × 1 Gy per session or sham | Recruiting since 2020 | NCT04466683 (Ohio State University Comprehensive Cancer Center, Columbus, OH, USA) |
| 52 COVID-19 patients | Bilateral whole lungs | Phase III, randomized | 1 × 1.5 Gy per session or sham | Recruiting since 2020 | NCT04433949 (Emory University Atlanta, GA, USA) [70] |
| Target | Study Design | Irradiation Scheme | Response | Reference | |
|---|---|---|---|---|---|
| An 81-yr-old woman with AD | Brain | Case report | 5 × 40 mGy/CT over 3 months | Clinical cognitive improvement allowing discharge from hospice care | [93] |
| A 73-yr-old man with AD | Brain | Case report | 6 × 45–50 mGy/CT over 18 months | Elevation of MMSE score from 22/30 up to 26/30 | [95] |
| 4 AD patients | Brain | Single-arm (pilot study) | 4 × 40 mGy/CT over 1 month | Slight cognition and behavior improvements on quantitative measures (SIB, ADL) | [102] |
| 30 AD patients | Brain | Phase I (single-arm pilot study) | 5 × 2 Gy per session 10 × 2 Gy per session | Suspended due to staffing and budget limitations | NCT02359864 (William Beaumont Hospitals, Royal Oak, MI, USA) |
| 5 AD patients | Brain | Phase IIa (single-arm pilot study) | 5 × 2 Gy per session 10 × 2 Gy per session | Interrupted due to COVID-19 | NCT02769000 (Virgina Commonwealth University, Richmond, VA, USA) |
| 20 AD patients | Brain | Randomized, monocentric, prospective (pilot study) | 5 × 2 Gy per session | Recruiting since 2017 | NCT03352258 (Geneva University Hospital, Geneva, Switzerland) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Reun, E.; Foray, N. Low-Dose Radiation Therapy (LDRT) against Cancer and Inflammatory or Degenerative Diseases: Three Parallel Stories with a Common Molecular Mechanism Involving the Nucleoshuttling of the ATM Protein? Cancers 2023, 15, 1482. https://doi.org/10.3390/cancers15051482
Le Reun E, Foray N. Low-Dose Radiation Therapy (LDRT) against Cancer and Inflammatory or Degenerative Diseases: Three Parallel Stories with a Common Molecular Mechanism Involving the Nucleoshuttling of the ATM Protein? Cancers. 2023; 15(5):1482. https://doi.org/10.3390/cancers15051482
Chicago/Turabian StyleLe Reun, Eymeric, and Nicolas Foray. 2023. "Low-Dose Radiation Therapy (LDRT) against Cancer and Inflammatory or Degenerative Diseases: Three Parallel Stories with a Common Molecular Mechanism Involving the Nucleoshuttling of the ATM Protein?" Cancers 15, no. 5: 1482. https://doi.org/10.3390/cancers15051482
APA StyleLe Reun, E., & Foray, N. (2023). Low-Dose Radiation Therapy (LDRT) against Cancer and Inflammatory or Degenerative Diseases: Three Parallel Stories with a Common Molecular Mechanism Involving the Nucleoshuttling of the ATM Protein? Cancers, 15(5), 1482. https://doi.org/10.3390/cancers15051482







