Simple Summary
This multicenter study delved into the outcomes of treating stage IV gastric cancer patients with positive peritoneal cytology but no other non-curative factors using chemotherapy followed by gastrectomy. The findings revealed that preoperative chemotherapy successfully eliminated peritoneal cancer cells in over 50% of patients. The median Overall and Progression-free survival stood at 20 (95% CI: 16–25) and 19 (95% CI: 11–20) months, respectively. Notably, conversion to negative cytology significantly lowered the relative risk of peritoneal progression (RR: 0.11; 95% CI: 0.03–0.47, p = 0.002). This study proposes that preoperative chemotherapy followed by gastrectomy shows promise as a viable treatment for stage IV gastric cancer patients with positive peritoneal cytology and no additional non-curative factors. The conversion of cytology status is associated with enhanced long-term outcomes and diminished risk of peritoneal relapse.
Abstract
The optimal approach for treating cytology-positive (Cy1) gastric cancer (GC) patients without additional non-curative factors remains uncertain. While neoadjuvant chemotherapy followed by gastrectomy shows promise, its suitability for Western patients is not well supported by existing data. To address this knowledge gap, a cohort study was conducted across four major GC treatment centers in Lithuania, Estonia, and Ukraine. Forty-three consecutive Cy1 GC patients who underwent neoadjuvant chemotherapy between 2016 and 2020 were enrolled. The study evaluated overall survival (OS), progression-free survival (PFS), cytology status conversion, and major pathological response rates, along with the factors influencing these outcomes. All patients underwent surgery post-neoadjuvant chemotherapy, with 53.5% experiencing cytological status conversion and 23.3% achieving a major pathological response. The median OS and PFS were 20 (95% CI: 16–25) and 19 (95% CI: 11–20) months, respectively. Conversion to negative cytology significantly reduced the relative risk of peritoneal progression (RR: 0.11; 95% CI: 0.03–0.47, p = 0.002). The study suggests that neoadjuvant chemotherapy followed by gastrectomy holds promise as a treatment option for Cy1 GC without additional non-curative factors, associating cytology status conversion with improved long-term outcomes and reduced peritoneal relapse risk.
1. Introduction
Gastric cancer (GC) ranks among the most prevalent malignancies globally, with over 1 million new cases and 750 thousand annual deaths [1]. Surgery remains the primary and only curative treatment option [2,3]. Unfortunately, up to 40% of patients present with distant metastases at the time of diagnosis, rendering them ineligible for radical surgery [4,5]. The peritoneum is the most frequent site of distant metastases [6,7]. Staging laparoscopy coupled with peritoneal lavage cytology stands as the diagnostic standard for detecting early peritoneal dissemination when only free cancer cells are present and peritoneal carcinomatosis (P1) has not yet formed [8,9,10,11]. Positive peritoneal cytology (Cy1), irrespective of other non-curative factors, emerges as a robust negative prognostic indicator for recurrence and survival [12]. Consequently, it is categorized as distant metastases (M1) and Cy1 patients are classified as stage IV, regardless of other non-curative factors [6].
Presently, there exists no international consensus on the optimal treatment for Cy1 GC patients. Western guidelines advocate for palliative care with potential re-staging post treatment [6,13]. In contrast, Eastern guidelines identify Cy1 patients as a distinct subset within the stage IV cohort, proposing the consideration of neoadjuvant chemotherapy followed by D2 gastrectomy if no other non-curative factors are present [14]. These disparities in recommendations and the absence of a widely accepted treatment for Cy1 patients stem from a knowledge gap. Hence, this study aims to explore the outcomes of a neoadjuvant approach for Cy1 GC within a Western cohort.
2. Material and Methods
2.1. Ethics
Local ethics committees or institutional review boards approved the study in each center before this study was conducted. All study-related procedures were performed following the Declaration of Helsinki of 1975, as revised in 1983.
2.2. Patients and Diagnostic Pathway
This cohort study screened all consecutive patients who were diagnosed with Cy1 stage IV GC without any other distant metastases between January 2016 and December 2020. The study was conducted at four major upper gastrointestinal cancer treatment centers in Lithuania, Estonia, and Ukraine: (1) National Cancer Institute, Vilnius, Lithuania; (2) Vilnius University hospital Santara Clinics, Vilnius, Lithuania; (3) National Cancer Institute, Kyiv, Ukraine; (4) North Estonia Medical Centre, Tallinn, Estonia. The standard diagnostic pathway for gastric cancer patients was consistent with current European Society for Medical Oncology (ESMO) guidelines [13] and included endoscopy with biopsy followed by chest and abdominal computed tomography (CT). If ≥cT2 or N+ disease and no distant metastases were detected at preoperative imaging, patients underwent diagnostic laparoscopy with peritoneal lavage for cytology. In the case of any suspicious peritoneal lesions, biopsies were taken to confirm or rule out peritoneal carcinomatosis (P1). After Cy1 GC without other non-curative factors was confirmed, all patients were discussed in multidisciplinary team meetings. Patients who were allocated to receive treatment with neoadjuvant chemotherapy followed by gastrectomy were included in the study; those who were allocated to receive best supportive care, upfront gastrectomy, or palliative chemotherapy were excluded (Figure 1).
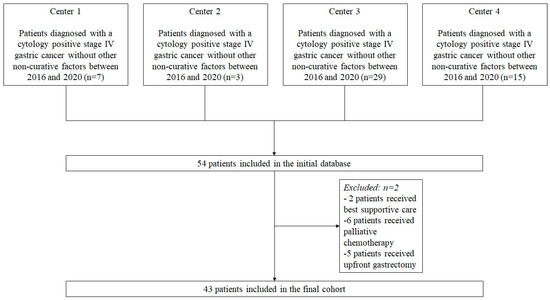
Figure 1.
Flowchart of the study patients.
2.3. Treatment and Follow-Up of Study Patients
The standard neoadjuvant treatment consisted of 3–6 cycles of chemotherapy; the exact number of cycles and regimens was selected by a medical oncologist. After neoadjuvant chemotherapy was completed, patients were scheduled for chest and abdominal CT and, if no distant metastases were detected, patients were scheduled for gastrectomy. An open or laparoscopic approach was selected by a surgeon. Subtotal gastrectomy was performed if a sufficient proximal resection margin could be ensured; otherwise, total gastrectomy was performed. In the case of an unresectable primary tumor, palliative procedures were performed if necessary. The extent of lymphadenectomy depended on the individual surgeon’s decision, but the standard lymphadenectomy was a D2 lymph node dissection performed as described in the 6th version of Japanese gastric cancer treatment guidelines [14]. All patients were considered for adjuvant chemotherapy after recovery from surgery. The standard follow-up protocol consisted of CT every 3 months for the first 2 years and, later, biannually. Also, esophagogastroduodenoscopy was performed 1 year after surgery.
2.4. Study Outcomes
The primary outcome of the study was overall survival (OS). Secondary end-points were progression-free (PFS) survival; conversion to negative cytology after neoadjuvant chemotherapy rates; major pathological response (mPR) after neoadjuvant chemotherapy rates; and postoperative complication rates. All postoperative complications were graded by Clavien–Dindo classification, and severe complications were defined as grade ≥3. OS was defined as the time between diagnosis of Cy1 stage IV GC and death. PFS was defined as the time between diagnosis of Cy1 GC and progression of the disease or death.
2.5. Statistical Analysis
All statistical analyses were conducted using the statistical program SPSS 25.0 (SPSS, Chicago, IL, USA). Continuous variables were presented as medians within the first (Q1) and third (Q3) quartiles. These variables were compared across groups using the Mann–Whitney U test or the Kruskal–Wallis test. Categorical variables were shown as proportions and were compared using the Chi-square test or Fisher’s exact test. OS and PFS rates were analyzed using the Kaplan–Meier method and were compared between the study groups using the log-rank test. To identify the factors impacting long-term outcomes in the neoadjuvant approach group, multivariable Cox proportional hazards regression analysis was used. Hazard ratios (HRs) were presented with 95% confidence intervals (CI). In all statistical analyses, two-tailed tests were used and a p-value of <0.05 was considered to be significant. The listwise deletion method was used for missing data.
3. Results
3.1. Baseline Characteristics and Neoadjuvant Chemotherapy
In total, 43 participants, with a median age of 57 (45; 65) years, were enrolled in the study. Each participant underwent a median of four (three; six) cycles of neoadjuvant chemotherapy. The most common chemotherapy regimen was fluorouracil, folinic acid, oxaliplatin, and docetaxel (FLOT), administered to 26 (60.5%) patients (Table 1).

Table 1.
Baseline characteristics of study patients.
3.2. Outcomes of Surgical Treatment, Cytological Status Conversion, and Major Pathological Response Rates
After completing neoadjuvant treatment, all patients underwent surgery. Palliative procedures were conducted in 3 (7.0%) patients, while another 40 (93.0%) patients underwent total or subtotal gastrectomy accompanied by D2 lymphonodectomy in 35 patients (87.5%) (Table 2). Postoperative complications occurred in 19 (45.2%) patients, including severe complications (Clavien–Dindo ≥ 3) in 9 (21.4%) patients.

Table 2.
Surgical treatment outcomes in study patients.
Post-surgery, cytological and histological examinations indicated that 23 patients (53.5%) experienced a conversion to negative cytology, and 10 patients (23.3%) achieved a major pathological response (mPR), classified as TRG1a/1b by Becker, following neoadjuvant treatment (Figure 2). Notably, there was no observed correlation between conversion to negative cytology and the achievement of a major pathological response (R = −0.302; p = 0.119). Further, there were no differences between patients who converted to negative cytology and those who maintained a positive cytology in terms of sex, age, ECOG score, tumor localization, cT, cN, presence of signet ring cells, lymphovascular invasion, and HER2 status, p > 0.05.
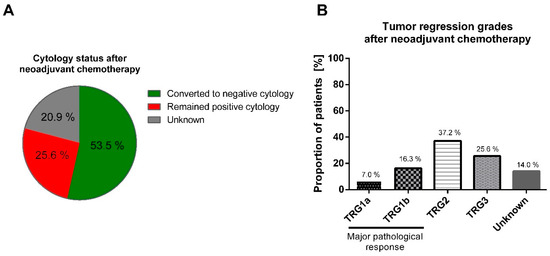
Figure 2.
Neoadjuvant chemotherapy impact on the cytological status and pathological response in the primary tumor. After neoadjuvant chemotherapy, 53.5% of patients converted from positive to negative cytology (A); major pathological response by TRG1a/b was achieved by 23.3% of patients (B). TRG: tumor regression grade by Becker classification.
The type of neoadjuvant chemotherapy, along with patient and tumor characteristics, did not show associations with the rates of conversion to negative cytology or mPR (Table 3). However, clinically negative lymph nodes were associated with higher odds (OR: 29; 95% CI: 4–210) of achieving mPR. After surgical treatment, 26 (61.9%) patients underwent adjuvant chemotherapy.

Table 3.
Factors associated with conversion to negative cytology and major histologic tumor regression after neoadjuvant chemotherapy.
3.3. Long-Term Outcomes
The median follow-up time was 16 (9; 21) months. Univariate Kaplan–Meier analysis revealed a median OS and PFS of 20 (95% CI: 16–25) and 19 (95% CI: 11–20) months, respectively. Notably, the conversion to negative cytology after neoadjuvant chemotherapy was linked to improved OS and PFS, whereas an mPR did not significantly impact long-term outcomes (Figure 3).
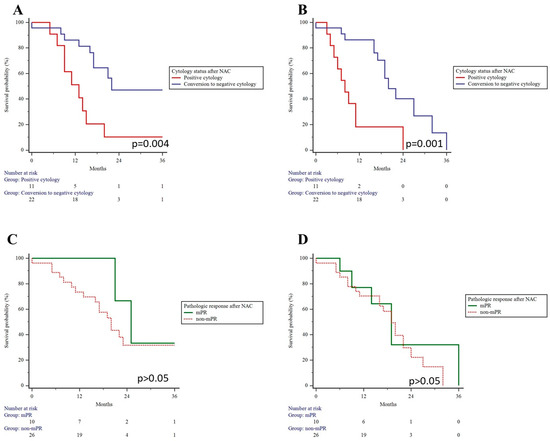
Figure 3.
Overall and progression-free survival in patients who converted to negative cytology and achieved major pathological response after neoadjuvant chemotherapy. Conversion to negative cytology resulted in better overall (A) and progression-free survival (B). Major pathological response had no impact on overall (C) and progression-free survival (D) rates.
Throughout the follow-up period, a total of 12 patients (27.3%) were diagnosed with peritoneal metastasis, representing the most common site of progression. Peritoneal recurrence was almost exclusively observed in patients who retained positive cytology after neoadjuvant chemotherapy (72.7% vs. 8.7%, p = 0.001). Conversion to negative cytology significantly reduced the relative risk for peritoneal progression (RR: 0.11; 95% CI: 0.03–0.47, p = 0.002). Additionally, multivariable Cox regression analysis demonstrated that conversion to negative cytology after neoadjuvant chemotherapy correlated with a decreased risk of death (HR: 0.05; 95% CI: 0.01–0.58; p = 0.017) and recurrence (HR: 0.10; 95% CI: 0.01–0.68; p = 0.019) after adjusting for age, mPR, type of chemotherapy, pathologic tumor, and nodal status (Table 4).

Table 4.
Multivariable Cox regression analysis for overall and disease-free survival.
4. Discussion
This study elucidates the short- and long-term outcomes in Cy1 GC patients without additional non-curative factors following treatment with neoadjuvant chemotherapy. After neoadjuvant chemotherapy, 53.5% of Cy1 patients experienced a conversion to negative cytology, and 23.3% achieved a major pathological response. Importantly, the conversion in cytologic status was linked to a significant reduction in the risk of death and recurrence, and particularly a lower risk for peritoneal relapse.
Treatment for Cy1 GC patients lacks standardization due to the absence of high-quality evidence. Free cancer cells detectable on cytology from peritoneal lavage signify peritoneal dissemination and metastatic disease. Consequently, akin to other GC metastases, palliative chemotherapy emerges as a standard treatment option. Unfortunately, systemic chemotherapy exhibits limited efficacy for GC peritoneal lesions [15], yielding a median survival of only 7 months [16]. Given the unsatisfactory long-term outcomes and distinct differences between Cy1 patients and GC patients with macroscopic carcinomatosis, more aggressive treatment strategies, including surgery, may be considered. Among treatments involving gastrectomy, two different options exist: upfront gastrectomy followed by adjuvant chemotherapy and gastrectomy after neoadjuvant chemotherapy. The CCOG0301 phase II single-arm study demonstrated that upfront gastrectomy followed by adjuvant S-1 monotherapy achieved 5-year OS and relapse-free survival rates of 26% and 21%, respectively. However, the peritoneal recurrence rate after such treatment is notably high at 62% [17]. Similar outcomes for upfront gastrectomy were confirmed in a retrospective study by Kano et al., revealing a 5-year OS of 17.8% and a peritoneal recurrence rate of 52.9% [18]. Further, a recent retrospective study by Bailong et al. demonstrated comparable survival outcomes for patients who underwent upfront gastrectomy and those who had gastrectomy after neoadjuvant treatment [19]. Nevertheless, the long-term outcomes achieved by preceding gastrectomy may be significantly compromised if patients do not receive adjuvant chemotherapy. Adjuvant chemotherapy after gastrectomy for Cy1 CG patients enhances OS to 22–25 months compared to 11–12 months in patients undergoing only surgical treatment [18,20]. However, the inability to tolerate cytotoxic treatment after major surgery, such as gastrectomy, is a serious issue, as 36% of patients are unable to receive adjuvant treatment due to the deterioration of their general condition after gastrectomy. This percentage can further rise to about 63% in the case of severe postoperative complications [21]. In contrast, chemotherapy applied in a neoadjuvant setting is better tolerated, with a compliance rate of more than 90% [22]. This difference may favor the neoadjuvant approach. The present study demonstrates that treatment with neoadjuvant chemotherapy followed by gastrectomy achieves acceptable long-term outcomes, with a median OS of 20 months (95% CI: 16–25). Neoadjuvant chemotherapy downsized the disease by converting to negative cytology in 53.5% of patients, and this conversion was associated with a significantly decreased risk of death (HR: 0.05; 95% CI: 0.01–0.58; p = 0.017) and recurrence (HR: 0.10; 95% CI: 0.01–0.68; p = 0.019). Our present findings align with results from previous small-scale studies, demonstrating improved long-term outcomes in 48.9–72.2% of Cy1 patients who achieve cytology status conversion [23,24,25,26]. Poor long-term outcomes in those who remain positive on cytology underscore the necessity for re-evaluation with diagnostic laparoscopy after neoadjuvant chemotherapy, because it may help to avoid almost half of the surgeries resulting in R1 resection. Furthermore, our study reveals that the vast majority of patients (72.7%) who remain positive on cytology after neoadjuvant chemotherapy will eventually develop peritoneal carcinomatosis. Considering that current systemic chemotherapy does not benefit these patients, it is crucial to explore and embrace new biomarkers. These biomarkers would play a key role in predicting the response to systemic neoadjuvant chemotherapy and allowing for personalized treatment for every patient [27]. This becomes particularly important as alternative treatment modalities, like intraperitoneal cytotoxic therapy, emerge as potential options for patients. A pilot study by Imano et al. showed that 80 mg/m2 paclitaxel applied intraperitoneally at the end of radical D2 gastrectomy can clear peritoneal cytology. Moreover, this study showed a promising 3-year survival rate of 56% and a peritoneal recurrence rate of 30% [28]. However, conflicting data exist on the effectiveness of intraperitoneal chemotherapy. A randomized controlled study from Japan showed a poor 5-year OS of 4.6% and 0% in patients who received gastrectomy and intraperitoneal chemotherapy with 100 mg cisplatin or gastrectomy alone. Thus, this approach remains controversial. Interestingly, the same study demonstrated promising outcomes with a 5-year OS rate of 43.8% in patients who received extensive peritoneal lavage with 10 L of a saline solution together with gastrectomy and intraperitoneal chemotherapy. Furthermore, intraperitoneal lavage reduced the peritoneal progression rate to 40.0% compared to 79.3% in the IPC group and 89.7% in the group receiving gastrectomy alone [29]. However, these techniques are rare outside of East Asia and would be considered experimental treatment in West.
Another available option for peritoneal disease, including GC, is hyperthermic intraperitoneal chemotherapy (HIPEC). A recent meta-analysis of randomized and high-quality non-randomized trials showed that HIPEC had no impact on long-term outcomes in GC patients with peritoneal carcinomatosis but may have a role in a prophylactic setting. HIPEC reduces the risk of peritoneal metastases (RR = 0.63; 95% CI: 0.45–0.88; p < 0.01) in high-risk patients, including Cy1 GC patients [30]. HIPEC can also find application in a neoadjuvant setting. A phase II study conducted by Badgwell et al. revealed that administering five cycles of neoadjuvant laparoscopic HIPEC after initial systemic chemotherapy resulted in cytology status conversion in 66.6% of patients [31]. However, this conversion rate does not significantly surpass the 53.5% achieved in our study with neoadjuvant chemotherapy alone. The broader acceptance of HIPEC for Cy1 GC patients is hindered by the scarcity of data from high-quality randomized controlled trials. The ongoing GASTRICHIP study, which explores the use of HIPEC in patients at high risk of peritoneal recurrence, including Cy1 patients after neoadjuvant chemotherapy, is anticipated to contribute more data to the field [32]. Another innovative technique for delivering chemotherapy intraperitoneally for GC peritoneal metastases is pressurized intraperitoneal chemotherapy (PIPAC) [33]. However, there are a lack of data regarding its efficacy, specifically in Cy1 patients.
The current study has some limitations that have to be considered. Firstly, being a retrospective study, it inherently carries typical disadvantages, including the potential for selection bias. Participants were chosen based on their eligibility for neoadjuvant chemotherapy, possibly excluding individuals with specific characteristics or conditions. Secondly, the relatively small sample size could impact the statistical power of the study, making it challenging to discern subtle differences in outcomes. Thirdly, the study’s single-arm design, focusing on the neoadjuvant approach, lacks a robust comparison with alternative treatment modalities like upfront gastrectomy or palliative care. This limitation restricts the assessment of the relative effectiveness of different strategies. Notably, the low number of patients treated with alternative methods in our initial database (n = 6 palliative chemotherapy; n = 5 upfront gastrectomy) precluded their inclusion for meaningful comparison. Fourthly, the median follow-up time of 16 months might not suffice to capture long-term outcomes and evaluate the enduring efficacy of the neoadjuvant treatment strategy. Longer follow-up durations would offer a more comprehensive understanding of survival and recurrence patterns. Fifthly, our present study exclusively involved patients of the Caucasian race from Lithuania, Estonia, and Ukraine. Consequently, the generalization of our findings to other Western cohorts may be somewhat restricted. Despite these limitations, it is crucial to interpret the findings cautiously and underscore the necessity for further research to address these constraints. Notably, this study represents the largest cohort of Western patients, showcasing the efficacy of the neoadjuvant approach in Cy1 GC patients given the current knowledge landscape.
5. Conclusions
In conclusion, this study provides novel evidence that neoadjuvant chemotherapy followed by gastrectomy is a promising treatment option for cytology-positive gastric cancer patients without other non-curative factors in a Western setting. Clearance of cytology is associated with improved outcomes and a lower risk for peritoneal relapse; thus, cytological status re-evaluation should be standard before considering radical surgery.
Author Contributions
A.B.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing; T.Ü.: Conceptualization, Data curation, Investigation, Methodology, Writing—original draft, Writing—review & editing; O.D.: Conceptualization, Data curation, Investigation, Methodology, Writing—original draft, Writing—review & editing; M.L.: Conceptualization, Data curation, Investigation, Methodology, Writing—original draft, Writing—review & editing; Y.K.: Conceptualization, Data curation, Investigation, Methodology, Project administration, Supervision, Writing—review & editing; A.R.: Data curation, Investigation, Writing—original draft, Writing—review & editing; M.V.: Data curation, Visualization, Writing—review & editing; B.B.: Data curation, Investigation, Writing—original draft, Writing—review & editing; K.B.: Data curation, Investigation, Writing—original draft, Writing—review & editing; K.R.: Data curation, Investigation, Writing—original draft, Writing—review & editing; R.L.-L.: Data curation, Formal analysis, Investigation, Writing—review & editing; R.B.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing; K.S.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study did not receive any funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Vilnius University Regional Bioethics Committee issued on 28 January 2020 and amended on 29 June 2021 (protocol code 2020/1-1185-675). Additional approvals were obtained from responsible institutions in Estonia and Ukraine.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. Some of the results presented in the manuscript have been communicated to society at scientific meetings.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Stratilatovas, E.; Baušys, A.; Baušys, R.; Sangaila, E. Mortality after gastrectomy: A 10 year single institution experience. Acta Chir. Belg. 2015, 115, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Bausys, R.; Bausys, A.; Vysniauskaite, I.; Maneikis, K.; Stratilatovas, E.; Strupas, K. Surgical treatment outcomes of patients with T1-T2 gastric cancer: Does the age matter when excellent treatment results are expected? World J. Surg. Oncol. 2018, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, Y.; Duan, J.; Xu, K.; Mao, M.; Wang, X. A Population-Based Analysis of Distant Metastasis in Stage IV Gastric Cancer. J. Pharmacol. Exp. Ther. 2020, 26, e923867-1–e923867-18. [Google Scholar] [CrossRef] [PubMed]
- Bernards, N.; Creemers, G.J.; Nieuwenhuijzen, G.A.P.; Bosscha, K.; Pruijt, J.F.M.; Lemmens, V.E.P.P. No improvement in median survival for patients with metastatic gastric cancer despite increased use of chemotherapy. Ann. Oncol. 2013, 24, 3056–3060. [Google Scholar] [CrossRef] [PubMed]
- Bausys, A.; Gricius, Z.; Aniukstyte, L.; Luksta, M.; Bickaite, K.; Bausys, R.; Strupas, K. Current treatment strategies for patients with only peritoneal cytology positive stage IV gastric cancer. World J. Clin. Cases 2021, 9, 9711–9721. [Google Scholar] [CrossRef] [PubMed]
- Shi, B.; Lin, H.; Zhang, M.; Lu, W.; Qu, Y.; Zhang, H. Gene Regulation and Targeted Therapy in Gastric Cancer Peritoneal Metastasis: Radiological Findings from Dual Energy CT and PET/CT. J. Vis. Exp. 2018, 131, 56526. [Google Scholar]
- Nakajima, T.; Harashima, S.; Hirata, M.; Kajitani, T. Prognostic and therapeutic values of peritoneal cytology in gastric cancer. Acta Cytol. 1978, 22, 225–229. [Google Scholar]
- Bando, E.; Yonemura, Y.; Taniguchi, K.; Yasui, T.; Fushida, S.; Fujimura, T.; Nishimura, G.-I.; Miwa, K. Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am. J. Surg. 1999, 178, 256–262. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takashima, A.; Nagashima, K.; Makuuchi, R.; Aizawa, M.; Ohashi, M.; Tashiro, K.; Yamada, T.; Kinoshita, T.; Hata, H.; et al. Efficacy of Postoperative Chemotherapy After Resection that Leaves No Macroscopically Visible Disease of Gastric Cancer with Positive Peritoneal Lavage Cytology (CY1) or Localized Peritoneum Metastasis (P1a): A Multicenter Retrospective Study. Ann. Surg. Oncol. 2020, 27, 284–292. [Google Scholar] [CrossRef]
- Schena, C.A.; Laterza, V.; De Sio, D.; Quero, G.; Fiorillo, C.; Gunawardena, G.; Strippoli, A.; Tondolo, V.; De’angelis, N.; Alfieri, S.; et al. The Role of Staging Laparoscopy for Gastric Cancer Patients: Current Evidence and Future Perspectives. Cancers 2023, 15, 3425. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.J.; Newhook, T.E.; Vreeland, T.J.; Das, P.; Minsky, B.D.; Blum, M.; Song, S.; Ajani, J.; Ikoma, N.; Mansfield, P.F.; et al. Yield of peritoneal cytology in staging patients with gastric and gastroesophageal cancer. J. Surg. Oncol. 2019, 120, 1350–1357. [Google Scholar] [CrossRef]
- Lordick, F.; Carneiro, F.; Cascinu, S.; Fleitas, T.; Haustermans, K.; Piessen, G.; Vogel, A.; Smyth, E. Gastric cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 1005–1020. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese Gastric Cancer Treatment Guidelines 2021 (6th edition). Gastric Cancer 2023, 26, 1–25. [Google Scholar] [CrossRef]
- Sun, B.J.; Lee, B. Review of Regional Therapies for Gastric Cancer with Peritoneal Metastases. Cancers 2022, 14, 570. [Google Scholar] [CrossRef]
- Badgwell, B.; Cormier, J.N.; Krishnan, S.; Yao, J.; Staerkel, G.A.; Lupo, P.J.; Pisters, P.W.; Feig, B.; Mansfield, P. Does Neoadjuvant Treatment for Gastric Cancer Patients with Positive Peritoneal Cytology at Staging Laparoscopy Improve Survival? Ann. Surg. Oncol. 2008, 15, 2684–2691. [Google Scholar] [CrossRef]
- Kodera, Y.; Ito, S.; Mochizuki, Y.; Ohashi, N.; Tanaka, C.; Kobayashi, D.; Kojima, H.; Matsui, T.; Kondo, K.; Fujiwara, M. Long-term follow up of patients who were positive for peritoneal lavage cytology: Final report from the CCOG0301 study. Gastric Cancer 2012, 15, 335–337. [Google Scholar] [CrossRef]
- Kano, K.; Aoyama, T.; Maezawa, Y.; Nakajima, T.; Ikeda, K.; Yamada, T.; Sato, T.; Oshima, T.; Rino, Y.; Masuda, M.; et al. The survival and prognosticators of peritoneal cytology-positive gastric cancer patients who received upfront gastrectomy and subsequent S-1 chemotherapy. Int. J. Clin. Oncol. 2017, 22, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Miao, R.; Shan, F.; Li, S.; Jia, Y.; Xue, K.; Li, Z.; Ying, X.; Pang, F.; Zhang, Y.; et al. Efficacy of chemotherapy versus surgery as initial treatment for gastric cancer with positive peritoneal cytology. World J. Surg. Oncol. 2023, 21, 204. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.-J.; Kim, H.-J.; Lee, S.H.; Bae, W.-K.; Hwang, E.-C.; Cho, S.-H.; Chung, I.-J.; Bang, H.-J.; Hwang, J.E. Observational Study of Peritoneal Washing Cytology-Positive Gastric Cancer without Gross Peritoneal Metastasis in Patients who Underwent Radical D2 Gastrectomy. Sci. Rep. 2020, 10, 1–6. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, S.; Choi, S.; Cho, M.; Kwon, I.G.; Kim, Y.M.; Son, T.; Kim, H.-I.; Jung, M.; Hyung, W.J. Adverse Prognostic Impact of Postoperative Complications After Gastrectomy for Patients with Stage II/III Gastric Cancer: Analysis of Prospectively Collected Real-World Data. Front. Oncol. 2021, 11, 611510. [Google Scholar] [CrossRef]
- Bausys, A.; Senina, V.; Luksta, M.; Anglickiene, G.; Molnikaite, G.; Bausys, B.; Rybakovas, A.; Baltruskeviciene, E.; Laurinavicius, A.; Poskus, T.; et al. Histologic Lymph Nodes Regression after Preoperative Chemotherapy as Prognostic Factor in Non-metastatic Advanced Gastric Adenocarcinoma. J. Cancer 2021, 12, 1669–1677. [Google Scholar] [CrossRef]
- Aizawa, M.; Nashimoto, A.; Yabusaki, H.; Nakagawa, S.; Matsuki, A.; Homma, K.; Kawasaki, T. The clinical significance of potentially curative resection for gastric cancer following the clearance of free cancer cells in the peritoneal cavity by induction chemotherapy. Surg. Today 2015, 45, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Mezhir, J.J.; Shah, M.A.; Jacks, L.M.; Brennan, M.F.; Coit, D.G.; Strong, V.E. Positive Peritoneal Cytology in Patients with Gastric Cancer: Natural History and Outcome of 291 Patients. Ann. Surg. Oncol. 2010, 17, 3173–3180. [Google Scholar] [CrossRef] [PubMed]
- Yasufuku, I.; Nunobe, S.; Ida, S.; Kumagai, K.; Ohashi, M.; Hiki, N.; Sano, T. Conversion therapy for peritoneal lavage cytology-positive type 4 and large type 3 gastric cancer patients selected as candidates for R0 resection by diagnostic staging laparoscopy. Gastric Cancer 2020, 23, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Valletti, M.; Eshmuminov, D.; Gnecco, N.; Gutschow, C.A.; Schneider, P.M.; Lehmann, K. Gastric cancer with positive peritoneal cytology: Survival benefit after induction chemotherapy and conversion to negative peritoneal cytology. World J. Surg. Oncol. 2021, 19, 245. [Google Scholar] [CrossRef] [PubMed]
- Derouane, F.; van Marcke, C.; Berlière, M.; Gerday, A.; Fellah, L.; Leconte, I.; Van Bockstal, M.R.; Galant, C.; Corbet, C.; Duhoux, F.P. Predictive Biomarkers of Response to Neoadjuvant Chemotherapy in Breast Cancer: Current and Future Perspectives for Precision Medicine. Cancers 2022, 14, 3876. [Google Scholar] [CrossRef] [PubMed]
- Imano, M.; Imamoto, H.; Itoh, T.; Satou, T.; Peng, Y.; Yasuda, A.; Kato, H.; Nishiki, K.; Shiraishi, O.; Shinkai, M.; et al. Impact of Intraperitoneal Chemotherapy after Gastrectomy with Positive Cytological Findings in Peritoneal Washings. Eur. Surg. Res. 2011, 47, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, M.; Shimada, S.; Ikeshima, S.; Matsuo, A.; Yagi, Y.; Matsuda, M.; Yonemura, Y.; Baba, H. Extensive Intraoperative Peritoneal Lavage as a Standard Prophylactic Strategy for Peritoneal Recurrence in Patients with Gastric Carcinoma. Ann. Surg. 2009, 250, 242–246. [Google Scholar] [CrossRef]
- Desiderio, J.; Chao, J.; Melstrom, L.; Warner, S.; Tozzi, F.; Fong, Y.; Parisi, A.; Woo, Y. The Thirty-Year Experience—A Meta-analysis of Randomized and High Quality Non-Randomized Studies of Hyperthermic Intraperitoneal Chemotherapy (HIPEC) in the Treatment of Gastric Cancer. Eur. J. Cancer 2017, 79, 1–14. [Google Scholar] [CrossRef]
- Badgwell, B.; Blum, M.; Das, P.; Estrella, J.; Wang, X.; Ho, L.; Fournier, K.; Royal, R.; Mansfield, P.; Ajani, J.A. Phase II Trial of Laparoscopic Hyperthermic Intraperitoneal Chemoperfusion for Peritoneal Carcinomatosis or Positive Peritoneal Cytology in Patients with Gastric Adenocarcinoma. Ann. Surg. Oncol. 2017, 24, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Passot, G.; Villeneuve, L.; Vaudoyer, D.; Bin-Dorel, S.; Boschetti, G.; Piaton, E.; Garofalo, A. GASTRICHIP: D2 resection and hyperthermic intraperitoneal chemotherapy in locally advanced gastric carcinoma: A randomized and multicenter phase III study. BMC Cancer 2014, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Račkauskas, R.; Baušys, A.; Lukšta, M.; Jurgaitis, J.; Paškonis, M.; Strupas, K. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal malignancy: Initial experience of the first program in the Baltic countries. World J. Surg. Oncol. 2021, 19, 236. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).