Simple Summary
Pancreatic cancer (PC) is one of the deadliest cancers. Its high mortality rate is correlated with several explanations; the main one is the late disease stage at which the majority of patients are diagnosed. Since surgical resection has been recognised as the only curative treatment, a PC diagnosis at the initial stage is believed the main tool to improve survival. Therefore, patient stratification according to familial and genetic risk and the creation of screening protocol by using minimally invasive diagnostic tools would be appropriate.
Abstract
Pancreatic cancer (PC) is one of the deadliest cancers, and it is responsible for a number of deaths almost equal to its incidence. The high mortality rate is correlated with several explanations; the main one is the late disease stage at which the majority of patients are diagnosed. Since surgical resection has been recognised as the only curative treatment, a PC diagnosis at the initial stage is believed the main tool to improve survival. Therefore, patient stratification according to familial and genetic risk and the creation of screening protocol by using minimally invasive diagnostic tools would be appropriate. Pancreatic cystic neoplasms (PCNs) are subsets of lesions which deserve special management to avoid overtreatment. The current PC screening programs are based on the annual employment of magnetic resonance imaging with cholangiopancreatography sequences (MR/MRCP) and/or endoscopic ultrasonography (EUS). For patients unfit for MRI, computed tomography (CT) could be proposed, although CT results in lower detection rates, compared to MRI, for small lesions. The actual major limit is the incapacity to detect and characterize the pancreatic intraepithelial neoplasia (PanIN) by EUS and MR/MRCP. The possibility of utilizing artificial intelligence models to evaluate higher-risk patients could favour the diagnosis of these entities, although more data are needed to support the real utility of these applications in the field of screening. For these motives, it would be appropriate to realize screening programs in research settings.
1. Background
Cancer represents a leading cause of death and a critical obstacle to increasing life prospects worldwide [1]. According to World Health Organization (WHO), in 2021 [2] cancer was the first or second leading cause of death before 70 years of age in 112 of 183 states, and ranked third or fourth in a further 23 states. Pancreatic cancer (PC) is responsible for a number of deaths (466,000) almost equal to its incidence (496,000) [1], due to its poor prognosis, and represents the seventh leading cause of cancer death in both sexes, with the highest incidence rates in Europe, Northern America, and Australia/New Zealand [1,3]. Considering that incidence and mortality rates have been stable or have slightly increased in many countries, while some cancers such as breast cancer have declined, it is expected that pancreatic cancer will surpass breast cancer as the third leading cause of cancer death by 2025 in a study of 28 European countries [3,4,5,6,7,8,9,10,11,12,13].
The high mortality rate is correlated with several factors, of which the main is the late disease stage at which the majority of patients are diagnosed [14,15,16,17,18,19,20,21,22,23,24,25,26]. In fact, pancreatic cancer is asymptomatic until the disease develops to an advanced stage, and at diagnosis only about 20% of cases are eligible for surgical resection [27,28,29,30,31,32]. In addition, even after the surgical approach, the majority of patients will experience a recurrence, with five-year survival only up to 25% [3,33,34,35,36]. Another critical point is pancreatic cancer biology, which contributes not only to early recurrence and metastasis, but also a resistance to conventional treatment [4,37]. Therefore, in this context, although several alternative treatments have been introduced, as ablative therapies [34,35,36,37,38,39,40,41,42,43,44,45], an effective treatment for this type of tumor remains to be determined [14].
Most primary pancreatic cancers are pancreatic ductal adenocarcinomas (PDACs); only a small proportion are pancreatic neuroendocrine tumors [46,47,48,49,50,51,52,53] and, even rarer, malignancies [54,55,56,57,58,59]. PDACs normally rise through a process that involves several stages, from pancreatic intraepithelial neoplasia (PanIN) [60]. According to pathological sub-type, PanINs can be classified in low (PanIN-1), intermediate (PanIN-2), or high (PanIN-3) grade [60]. Several pieces of research on DNA sequencing have shown a multi-phase process in genetic mutation, with early mutations in KRAS followed by alterations in P1 and P53, among others, with a consequent progression in grade and with PDAC as the final step. [61]. Since PanINs are microscopic, diagnostic tools do not detect these entities, and the diagnosis is correlated with surgical specimen [62,63,64,65,66,67,68]. PanINs surgical resected patients have a five-year survival rate higher than 85% compared to PDAC resected patients [68]. Additionally, a precursor of PDAC could be intraductal papillary mucinous neoplasms (IPMNs) [69,70,71,72,73,74,75,76,77,78,79,80,81]. IPMNs can be classified based on histology and duct involvement (main-duct type or side branch: branch-duct type) [82]. It has been reported, on surgical specimens, that about 20–30% of IPMNs had an invasive tumor, with the highest risk for main-duct sub-types [83]. However, according to several guidelines, not all these lesions need to be resected [80,81,82,83,84,85]. Another entity correlated to PDAC are mucinous cystic neoplasms (MCN), which are slow growing cystic tumors that are normally detected in women [86,87,88]. The risk of progression to malignancy varies between IPMN and MCN, and, considering that the malignant potentials are not fully understood yet, it is critical to identify patients who are at risk in order to supply proper management [89].
2. Risk Factors
Although the specific factors correlated to PC onset are not clearly known, several modifiable and non-modifiable features are recognized [90,91,92,93,94,95,96]. Among the non-modifiable risk factors, age, sex, ethnicity, blood group, diabetes mellitus (DM), microbiota, and familial and genetics history and genetic predisposition are recognized. Modifiable risk factors include alcohol, smoking, pancreatitis history, dietary features, and obesity [90,91,92,93].
With regard to family history and genetic susceptibility, approximately 10% of PC patients have a family PC history, a feature that considerably enhances an individual’s tumor risk [94,95,96,97,98,99,100,101,102,103,104]. Nevertheless, that the genetic profile can influence PC is not well-known; although PC is recognized as a possible lesion in some genetic syndromes, it counts for few familial pancreatic cancer cases [94,95,96,97,98,99,100,101,102,103,104]. With regard to gene mutations, it is known that PC patients can have mutations in BRCA2 while the role of BRCA1 is debatable. In addition, it is known that the mutation in CDKN2A (also known as P16) is correlated with familial atypical mole melanoma syndrome (FAMM) (Figure 1); in STK11 (also known as LKB1), is correlated with Peutz–Jeghers syndrome (Figure 2 and Figure 3); and in PRSS1 and SPINK1, correlated with hereditary pancreatitis. In addition, Lynch syndrome patients, in whom there are mutations in genes encoding DNA mismatch–repair proteins, have a higher risk of PC, as do patient with mutations in PALB2. In addition, another subgroup of familial pancreatic tumor is due to germline mutations in ATM [94,95,96,97,98,99,100,101,102,103,104].
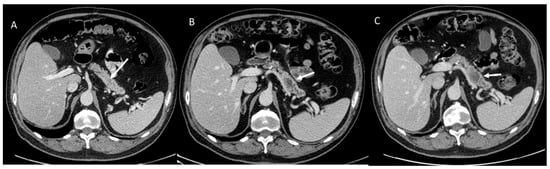
Figure 1.
CT evaluation in melanoma patient with FAMM. In (A), arrow shows non-detectable lesion. In (B) arrow shows PDAC after 1 years (respect to CT assessment of (A)) and in (C) after 3 months (respect to CT assessment of (B)).
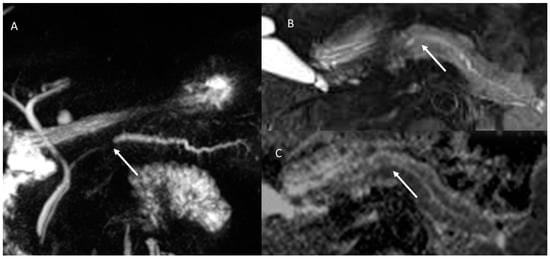
Figure 2.
MR/MRCP assessment of Peutz–Jeghers syndrome patient. In MRCP (A), the arrow shows black sign and the dilatation of the main and secondary branch ducts. In T2-W sequences the (B) arrow shows hyperintense tissue and in ADC map (C) hypointense tissue.
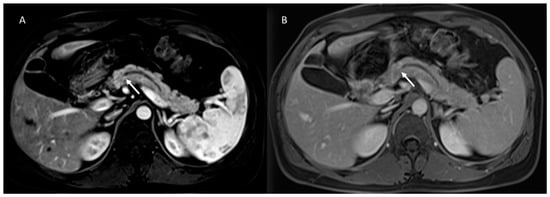
Figure 3.
The same patient as in Figure 2; post-contrast MRI evaluation. In arterial (A) and portal (B) phase of contrast study, arrows show no tissue but dilatation of the main and secondary branch ducts.
Beyond familial pancreatic cancer, the most well-recognized risk factor for pancreatic tumor is smoking, followed by chronic pancreatitis, diabetes, and obesity, specifically high body-mass index (BMI) and centralized fat distribution [105,106,107,108,109,110,111,112,113,114].
A clear knowledge of these risk factors allows a correct stratification of patients, in the perspective of a surveillance protocol centered on the patient himself [115,116,117,118,119,120,121,122,123,124,125,126,127,128]. However, to avoid the risk of over-diagnosis and to center energy on an early diagnosis, we should define who those subsets of patients are and quantify the degree of risk [115]. Once we have stratified the target population according to risk level, we should define the surveillance timing and the tools (biomarkers and imaging) [115,116,117,118,119,120,121,122,123,124,125,126,127,128]. In the PDAC scenario, we are still interpreting this multi-step process, although definite improvements have been made [115].
3. Screening Guidelines
Several working groups and scientific societies have proposed surveillance protocol for patients at risk of pancreatic cancer [129,130,131,132,133,134,135,136,137,138,139]. All working groups agree that surveillance programs should involve experienced multidisciplinary teams in dedicated oncological settings and that patient starting age should vary according to the underlying genetic condition.
According to the consensus of an international panel of experts, (in 2018, revised in 2019) the International Cancer of the Pancreas Screening (CAPS) Consortium suggested a screening PC program [129]. According to this program, patients with mutations of BRCA2, PALB2, CDKN2A, ATM, or MMR genes* and 1 first-degree relative (FDR) with PC and familial individuals with FPC or two or more BRs and 1 FDR with PC, patients with Peutz–Jeghers or FAMM syndrome, or patients with hereditary pancreatitis after their first attack should be subjected to surveillance [129]. The starting age was 50–55 years for patients with familial risk, 40 for Peutz–Jeghers syndrome or FAMM syndrome, and 45–50 for patients with other mutations. Additionally, for all high-risk patients, surveillance should begin 10 years before the youngest age at which a blood relative developed PC, if this age was lower than the general age guidelines above [129].
In 2018, the American Society of Clinical Oncology (ASCO) suggested that familial PC patients should be subjected to risk evaluation [130], proposing that for all patients who developed tumors a germline genetic analysis was useful to evaluate familial predisposition and the need for surveillance. They also suggested that patients with confirmed mutations of APC, ATM, BRCA2, BRCA1, CDKN2A, MMR genes*, PALB2, STK11, or TP53 should be subjected to surveillance [130].
In addition the American College of Gastroenterology (ACG) [140] proposed that patients with germline mutations should be subjected to surveillance. For Peutz–Jeghers syndrome patients, surveillance should start at 35 years (or 10 years younger than earliest PDAC in family); for FAMM syndrome, at 50 years (or 10 years younger than earliest PDAC in family); for Lynch syndrome, at 50 years (or 10 years younger than earliest PDAC in family); for hereditary pancreatitis, at 50 years (or 10 years younger than earliest PDAC in family); for FPC ≥2 relatives with PDAC of whom ≥1 is FDR or ≥3 relatives with PDAC, start at 50 years (or 10 years younger than earliest PDAC in family) [140].
4. Screening Modalities
The current PC screening programs are based on the annual employment of magnetic resonance imaging with cholangiopancreatography sequences (MR/MRCP) and/or endoscopic ultrasonography (EUS) [138,141,142,143,144,145,146,147,148,149,150,151,152]. For patients unfit for MRI, computed tomography (CT) could be proposed, although CT shows lower detection rates, compared to MRI, for small lesions [153,154,155,156,157,158,159,160,161,162,163]. According to some of the research, EUS and MR/MRCP could be employed alternatively, or the clinicians should consider patient preference and available expertise [127,128,129,130,131,132,133,134,135,136,137,138,139,140].
Though EUS is now considered the most sensitive imaging modality for the detection of pancreatic lesions, Wiest et al. [164], reported a sensitivity of 88–100%, a specificity of 63.4–94%, a positive predictive value (PPV) of 71.4–96.2%, and a negative (N) PV of 68.5–100% for MRI, that with MRCP has a sensitivity of 97.1–100%, a specificity of 81.8–88%, a PPV of 94.4–97%, and a NPV of 90–100%, similar to EUS, while a lower accuracy was reported for CT. CT sensitivity and specificity are correlated to detector numbers and study protocol [164]. The possibility to add EUS conventional protocol, elastography and/or contrast-enhanced ultrasound (CEUS) could improve the sensitivity of EUS for PC screening [165].
With EUS study, most solid pancreatic lesions are depicted as a heterogeneous hypoechoic mass, irrespective of the pathological type (Figure 4), and this appearance is the same found at transabdominal US [166]. With MRI study, during cholangiopancreatography study, pancreatic lesions appear as a signal blank of the pancreatic duct and in relation to lesion size, they can also be the only sign of disease [167].
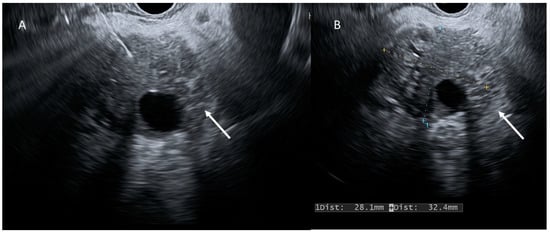
Figure 4.
EUS evaluation (A,B); PDAC (arrows) appears as a heterogeneous hypoechoic mass.
However, MRI can allow lesion detection at an earlier stage thanks to the morphological assessment of pancreatic parenchyma, as well as that of the duct. In a meta-analysis, it was reported that MRI and CT had similarly sensitive specifics in PC detection and staging [167]. In addition, the authors compared CT and PET/CT in PC diagnoses, showing inconclusive data, as in the evaluation of CT and EUS-FNA [167]. However, this meta-analysis did not evaluate studies in a screening setting, therefore they were subject to probable bias.
Conversely, in a study in which the authors evaluated high risk individuals, the overall yield for detecting pre-malignant and tumor lesions using EUS was 20% and using MRI/MRCP was 14% [168]. Notably, EUS had higher performance for solid lesions while MRI performed better for cystic lesions. Therefore, due to higher sensitivities and specificities in small lesions diagnosis, MRI and EUS should be chosen as diagnostic tools in screening programs [167,168,169,170,171,172,173,174].
The major limit of EUS and MRI technologies is the inability to reliably detect and distinguish PanINs [94].
5. Pancreatic Cystic Neoplasm Diagnostic Management
It is estimated that 2–45% of the general population have pancreatic cystic neoplasms (PCN) [175,176,177,178]. PCNs comprise numerous clinically challenging entities, since their biological behavior varies from benign (Figure 5 and Figure 6) to malignant (Figure 7). In this context, proper management should prevent progression to invasive cancer and should minimize the need for lifelong screening and related costs [179].
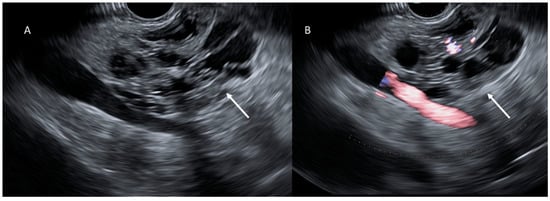
Figure 5.
EUS evaluation (A,B); cystic lesion (arrows) appears as a heterogeneous hypo-isoechoic mass.
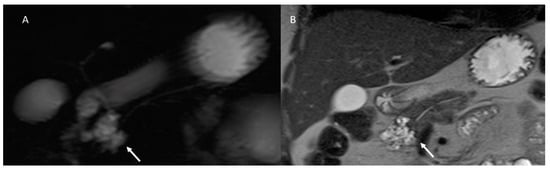
Figure 6.
Abbreviated MRI assessment (MRCP: (A) and T2-W: (B) sequences) of pancreatic cistoadenoma, which is characterized by multicyclic lesions (arrow).
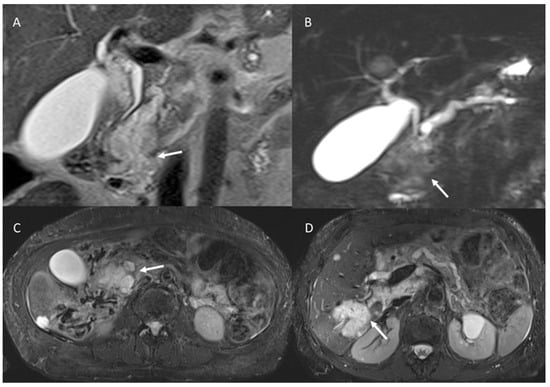
Figure 7.
MRI assessment of degenerated MD-IPMN. In T2-W (A), MRCP (B) and T2-W FS (C) sequences the lesions appear (arrows) as a dilated MD with mucinous intraluminal component (arrow). In (D) (T2-W FS) arrow shows mucinous liver metastasis.
Much research has reported an accuracy in recognizing the sub-type of PCN, between 40% and 95% for MRI/MRCP and between 40% and 81% for CT [180,181,182,183,184,185,186,187,188,189], and a CT detection rate of 2.1–2.6% [189,190] and MRI/MRCP detection rate of 13.5–45% [191,192]. However, the diagnostic accuracy is low, using either single or combined imaging tools for differentiating small PCN from non-neoplastic or non-epithelial cysts, or for connection to the ductal system [180,181,182,183,184,185,186,187,188,189,190,191,192].
With regard to diagnostic management, a dedicated pancreatic protocol, either in CT or MRI/MRCP, reported similar accuracy in PCN characterization [193,194,195]. However, MRI/MRCP had a higher accuracy in identifying the communication between a lesion and the duct system, and similarly for septations or the mural nodule [196]. In addition, MRI allows the detection of multiple lesions, which favors a diagnosis of multifocal side-branch IPMN [196,197]. Although MRI is the preferred diagnostic tool, a multimodality assessment should be preferred for tumor staging, or for recurrence diagnosis [198].
No specific diagnostic study protocols are recommended for the diagnosis or surveillance of PCN patients, since there is widespread published data and a lack of dedicated comparative studies [198].
Several abbreviated protocols (AP) for PC have been proposed [198,199,200,201,202,203,204,205,206,207,208]. For PCN surveillance, AP-MRI could be a good alternative. In fact, several authors suggested MRI protocol without administration of a contrast agent [205]. Macari et al. found that contrast-enhanced images did not lead to different treatment recommendations compared to unenhanced images [205]. Similar results were found by Nougaret et al. [206]. Pedrosa et al. [207] suggested reserving the standard contrast-enhanced MRI protocol with MRCP for the first diagnosis of PCN, while for the follow-up, suggested a 10-min protocol consisting of axial and coronal SSFSE T2-weighted, 2D and 3D single-shot MRCP, and 3D T1-weighted spoiled-gradient echo. With regard to the utility of DWI in the surveillance of pancreatic cystic lesions, there is a debate in the literature [208,209,210,211,212,213]. DWI should be included to avoid the risk of missing a concomitant pancreatic cancer. A combination of T2-W sequences and DWI has been shown to have similar accuracy to a conventional contrast-enhanced MRI protocol [213]. Pozzi-Mucelli et al. showed that that an abbreviated protocol MRI is more economical, and provides equivalent clinical data for patient surveillance [214].
EUS should be considered as an adjunct to other imaging tools, since it allows identifying PCN with features that should be considered for surgical resection, although this tool does not allow us to characterize the PCN sub-type [198]. Contrast-enhanced EUS (CE-EUS) should be considered for the assessment of mural nodules and vascularity within the cyst and septations [198]. In addition, EUS fine-needle aspiration (FNA) of the lesion should be considered for differentiating mucinous versus non-mucinous lesions [198].
With regard to IPMN, jaundice, positive cytology, the presence of a solid component and/or enhancing mural nodule (≥5 mm), or a major pancreatic duct (MPD) measuring ≥10 mm are predictive of malignancy. MPD dilatation between 5 and 9.9 mm, cystic growth-rate ≥5 mm/year, increased level of serum CA 19.9 (>37 U/mL), symptoms, enhancing mural nodules (<5 mm), and/or, for MCN, a cyst diameter ≥40 mm, are also associated with an increased risk for high-grade dysplasia or cancer [138,198].
According to European-based guidelines, IPMN patients which do not meet criteria for surgical resection should be subjected a 6-month follow-up in the first year, and a yearly follow-up thereafter [198].
Regarding mucinous cystic neoplasm (MCN) patients, lesions ≥40 mm should undergo surgical resection [198]. Resection is also suggested for symptomatic patients and for lesions which have risk factors, such as mural nodules [198]. Considering that there has been reported a faster growth during pregnancy, patients with MCN should be observed closely during pregnancy [198]. According to European-based guidelines, MCN <40 mm without mural nodules or symptoms should be subjected to surveillance with MRI, EUS, or a combination of both, every 6 months for the first year, and then annually if no changes are observed [198].
Regarding to the different guidelines, the European guidelines are the most widespread and valid for all PCN patients. The AGA guidelines are designed for asymptomatic patients, but these exclude several very high-risk entities (as MD-IPMN). The ACR guidelines are for all incidental PNCs. The ACG guidelines are designed for any type of PNC, but are the only ones that exclude a possible genetic predisposition. The Fukuoka guidelines are the most specific, since the only target are the IPMN patients [87]. Today, there have not been studies designed to evaluate these guidelines in at-risk patients, consequently, it is not possible to suggest one guideline over another [87].
6. Artificial Intelligence, Radiomics, and Pancreatic Cancer
Current technological progresses have allowed the use of artificial intelligence (AI) in medical settings. Since, compared to the human brain, a computer can analyze larger amounts of data, AI could resolve several problems in oncological settings [215,216,217,218,219,220,221,222,223,224]. AI elaborates algorithms, which are qualified to execute meanings that were normally executed by the human brain [225,226,227,228,229,230,231,232,233,234]. Machine learning (ML), a sub-area of AI, uses mathematical models, through the repetition of calculations derived from large amounts of data, and can learn detailed tasks [235,236,237,238,239,240,241,242,243]. These models can be supervised or unsupervised, in relation to the desired outcome of interest in model knowledge [244,245,246,247,248,249]. In their supervised form, a training dataset is introduced to obtain the desired outcome [250,251,252,253,254,255,256,257,258,259,260,261,262,263]. This type of learning requires large amounts of training data which has been pre-labeled (“curated”) by a human operator. Once the training of the model is completed, a different dataset is used to test its performance (testing data) [264,265,266,267,268,269,270,271,272,273,274,275,276,277,278]. In unsupervised learning, the model classifies noncurated data by using the algorithm to identify features within the dataset that can be grouped and analyzed further to reach a specific outcome [279,280,281,282,283,284,285,286,287,288].
A new field of interest is radiomics, which analyzes, in a mathematical manner, data obtained by medical images [289,290,291,292,293,294,295,296,297,298,299,300,301,302]. The idea that imaging studies contain a great quantity of data, in the form of grey level patterns, which are imperceptible to the human eye, has become more and more interesting [303,304,305,306,307,308]. These texture features, when correlated with clinical–pathological data and outcomes, theoretically allow diagnostic and prognostic assessment, and could produce evidence-based clinical-decision support systems [309,310,311,312,313,314,315,316,317,318,319]. The main objective is to combine multimodal quantitative data with mathematical methods to provide clear and robust parameters allowing an outcome prediction. Radiomics offers outstanding benefits over qualitative imaging assessment, since this is clearly limited by the subjective evaluation of radiologists. A radiomic information extension can be obtained by adding genomics data (radiogenomics); in fact, genomic markers such as microRNA expression have been shown to be associated with treatment response, metastatic spread, and prognosis that could offer personalized and precision medicine [309,310,311,312,313,314,315,316,317,318,319]. The assessment of textural characteristics, obtained by conventional radiological images, such as CT or MRI, allow the extraction of biological data without an invasive approach while reducing costs and time, avoiding any risk for the patients. For several tumors, radiomic analyses have already provided an accurate evaluation of biology, allowing the identification of features correlated with clinical outcomes [309,310,311,312,313,314,315,316,317,318,319].
In the context of PC, the possibility to identify the lesion at an early stage or in a pre-malignant setting may allow proper management. Therefore, several researches have evaluated AI in PC settings.
Muhammad et al. employed AI to predict the risk of developing PDAC, by assessing demographic data, family history, and comorbidities. Their model was able to predict PC development with good accuracy [320]. Similar data were obtained by Hsieh et al. [321] in patients with type 2 diabetes (T2DM).
Several authors evaluated the accuracy of artificial neural networks (ANNs) in differentiating chronic pancreatitis (CP) from PC on EUS images. Norton et al. [14] evaluated 21 PDAC patients and 14 CP patients, showing that four features had an overall accuracy of 89% [322]. Zhu et al. [323] evaluated 262 PDAC patients and 126 CP patients, reporting that their algorithm reached an overall accuracy of 94%.
With regard to MRI AI and early detection of PC, few studies have been reported. Corral et al. [324] employed a deep learning model to categorize IPMNs. Their algorithm’s sensitivity and specificity for detecting dysplasia were 92% and 52%, respectively [324]; while for detecting high-grade dysplasia or malignancy it had a sensitivity and specificity of 75% and 78%, respectively.
In an unsupervised algorithm to categorize benign or malignant IPMNs, Hussein et al. [325] obtained an accuracy, sensitivity, and specificity of 58.04%, 58.61%, and 41.67%, respectively.
Two interesting projects are ongoing. The Felix Project, supported by the Lustgarten Foundation and by a team at Johns Hopkins University, is based on deep learning models on 156 PC cases and 300 controls. Preliminary data reported a sensitivity and specificity of 94% and 99%, respectively [326]. The second project, by the Alliance of Pancreatic Cancer Consortium Imaging Working Group, with the scope to create a repository of images, including pre- and post- PC diagnosis of CT, MRI, and US. The end point is to develop AI models that can predict PDAC appearance at an early stage [327].
7. Discussion
The main end point of a screening program is reducing PC-related mortality by early-stage tumor diagnosis and/or identifying and treating pre-malignant lesions [328]. Actually, at the time of diagnosis, PC is often metastatic or in a locally advanced stage. Published data from screening programs showed the down-staging of detected PCs, with better survival [329]. Canto et al. assessed 354 high-risk patients with a median follow-up of 5.6 years. Among them, they detected 14 PDACs: 10 (71%) in asymptomatic patients, and 9 early and resectable lesions [329]. Since surgical resection has been recognized as the only curative treatment [149,329,330], a tumor diagnosis in the initial stage is believed to be the main tool to improve survival. Therefore, patient stratification, according to familial and genetic risk, and the creation of screening protocol by using minimally invasive diagnostic tools would be appropriate [198].
The potential hazard of screening program comprise adverse events correlated to diagnostic procedures and patient anxiety [132]. Potential over-diagnosis or misdiagnosis could occur, causing an over-treatment of completely benign or low-risk neoplastic lesions [132]. For these motives, it would be appropriate to realize screening programs in the research protocol setting. In fact, the success of these programs requires patient compliance and multidisciplinary team cooperation [132].
The actual major limit is the incapacity to detect and characterize PanIN lesions by EUS and MR/MRCP. The possibility of utilizing AI models to evaluate higher-risk patients could favor the diagnosis of these entities, although more data is needed to support the real utility of these applications in the screening field.
AI is the primary choice to perform image-based extensive analysis of such minute alterations and identify potential risk predictors for disease. AI systems, as opposed to manual approaches, execute complex tasks without interruption and ensure highly accurate and precise outcomes. In the domain of automated processing and analysis of medical images, AI offers numerous techniques and tools to extract accurate measurements from different structures, and can identify nonlinear features and evaluate tissue properties. For prediction modeling, radiomic analysis and machine and deep learning are regarded as the most reliable and common AI approaches. Recent studies have found that radiomics models contribute greatly to the individualized evaluation of pancreatic lesions, such as tumor detection, classification, differentiation, and antitumor drug-effect prediction [96,331]. In addition, pancreatic cystic lesions have been categorized using radiomics methods in multiple studies [332,333]. Although these findings confirmed the feasibility of radiomics for the assessment of pancreatic cystic lesions [334,335,336,337,338,339,340,341,342,343,344], the robustness of these radiomics diagnostic models may be limited due to the relatively small datasets included in most studies. Therefore, the accumulation of additional research data is required for the study of pancreatic cystic lesions.
With regard to solid lesions, Qureshi et al. [345] proposed a PDAC risk prediction model using AI analysis of the global features of the pancreas. However, since the morphology of the pancreas was assessed “as a whole”, whether the identified precancerous changes (predictors) were merely the manifestation of local changes that occurred in a specific subregion (presumably where the tumor developed) or all subregions simultaneously adopted such changes remained unknown. To overcome this limit, Javed et al. [346], in a retrospective study, performed an extensive radiomic analysis of the precancerous pancreatic subregions using CT images. The analysis was performed using 324 pancreatic subregions identified in 108 contrast-enhanced abdominal CT scans with equal proportions of healthy control, pre-diagnostic, and diagnostic groups. In a pairwise feature analysis, several textural features were found to be potentially predictive of PDAC. A machine learning classifier was then trained to perform risk prediction of PDAC by automatically classifying the CT scans into healthy control (low-risk) and pre-diagnostic (high-risk) classes, and specifying the subregion(s) likely to develop a tumor. The proposed model was trained on CT scans from multiple phases. Using 42 CT scans from the venous phase, model validation was performed, which resulted in ~89.3% classification accuracy on average, with sensitivity and specificity reaching 86% and 93%, respectively, for predicting the development of PDAC (i.e., high-risk).
8. Conclusions
The main end point of a screening program is reducing PC-related mortality by early-stage tumor diagnosis and/or identifying and treating pre-malignant lesions. Patient stratification, according to familial and genetic risk, and the creation of screening protocol by using minimally invasive diagnostic tools would be appropriate. The actual major diagnostic limit is the incapacity to detect and characterize the PanIN. Artificial intelligence models could evaluate higher risk patients and could favor the diagnosis of these entities.
Author Contributions
Data curation and conceptualization, V.G.; investigation, V.G., R.F., S.V.S., R.G., M.D.B., E.D.G., R.P. (Renato Patrone), A.B., A.A., L.S., N.M., G.G., G.C., R.P. (Raffaele Palaia), M.C.B., F.I. and A.P.; methodology, V.G., R.F., S.V.S., F.I. and A.P.; writing—original draft, V.G.; writing—review and editing, V.G. and F.I. All authors have read and agreed to the published version of the manuscript.
Funding
Founding by the Ministry of Health—Current Research 2022.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are reported in the manuscript and at link https://zenodo.org/record/7503163#.Y7VHaHbMK3A.
Acknowledgments
The authors are grateful to Alessandra Trocino, librarian at the National Cancer Institute of Naples, Italy.
Conflicts of Interest
The authors have no conflict of interest to be disclosed. The authors confirm that the article is not under consideration for publication elsewhere. Each author has participated sufficiently to take public responsibility for the content of the manuscript.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: https://www.who.int/ (accessed on 15 November 2022).
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Portal, A.; Pernot, S.; Siauve, N.; Landi, B.; Lepère, C.; Colussi, O.; Rougier, P.; Zaanan, A.; Verrière, B.; Taieb, J. Sustained response with gemcitabine plus Nab-paclitaxel after folfirinox failure in metastatic pancreatic cancer: Report of an effective new strategy. Clin. Res. Hepatol. Gastroenterol. 2014, 38, e23–e26. [Google Scholar] [CrossRef] [PubMed]
- Tempero, M.A. NCCN Guidelines Updates: Pancreatic Cancer. J. Natl. Compr. Cancer Netw. 2019, 17, 603–605. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Avallone, A.; Leongito, M.; Izzo, F.; Petrillo, A. Peribiliary liver metastases MR findings. Med. Oncol. 2017, 34, 124. [Google Scholar] [CrossRef]
- Alvaro, D.; Hassan, C.; Cardinale, V.; Carpino, G.; Fabris, L.; Gringeri, E.; Granata, V.; Mutignani, M.; Morement, H.; Giuliante, F.; et al. Italian Clinical Practice Guidelines on Cholangiocarcinoma—Part I: Classification, diagnosis and staging. Dig. Liver Dis. 2020, 52, 1282–1293. [Google Scholar] [CrossRef]
- Alvaro, D.; Hassan, C.; Cardinale, V.; Carpino, G.; Fabris, L.; Gringeri, E.; Granata, V.; Mutignani, M.; Morement, H.; Giuliante, F.; et al. Italian Clinical Practice Guidelines on Cholangiocarcinoma—Part II: Treatment. Dig. Liver Dis. 2020, 52, 1430–1442. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Setola, S.V.; Castelguidone, E.D.L.D.; Piccirillo, M.; Palaia, R.; Grassi, R.; Granata, F.; Izzo, F.; et al. Multidetector computer tomography in the pancreatic adenocarcinoma assessment: An update. Infect. Agents Cancer 2016, 11, 57. [Google Scholar] [CrossRef]
- Izzo, F.; Granata, V.; Fusco, R.; D’Alessio, V.; Petrillo, A.; Lastoria, S.; Piccirillo, M.; Albino, V.; Belli, A.; Tafuto, S.; et al. Clinical Phase I/II Study: Local Disease Control and Survival in Locally Advanced Pancreatic Cancer Treated with Electrochemotherapy. J. Clin. Med. 2021, 10, 1305. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Granata, V.; Fusco, R.; D’Alessio, V.; Petrillo, A.; Lastoria, S.; Piccirillo, M.; Albino, V.; Belli, A.; Nasti, G.; et al. A Multicenter Randomized Controlled Prospective Study to Assess Efficacy of Laparoscopic Electrochemotherapy in the Treatment of Locally Advanced Pancreatic Cancer. J. Clin. Med. 2021, 10, 4011. [Google Scholar] [CrossRef] [PubMed]
- Bimonte, S.; Leongito, M.; Barbieri, A.; Del Vecchio, V.; Barbieri, M.; Albino, V.; Piccirillo, M.; Amore, A.; Di Giacomo, R.; Nasto, A.; et al. Inhibitory effect of (−)-epigallocatechin-3-gallate and bleomycin on human pancreatic cancer MiaPaca-2 cell growth. Infect. Agents Cancer 2015, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Belli, A.; Palaia, R.; Carrafiello, G.; Miele, V.; Petrillo, A.; Izzo, F. Local ablation of pancreatic tumors: State of the art and future perspectives. World J. Gastroenterol. 2021, 27, 3413–3428. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Raso, M.M.; Avallone, A.; De Stefano, A.; Nasti, G.; Palaia, R.; Delrio, P.; Petrillo, A.; et al. Liver radiologic findings of chemotherapy-induced toxicity in liver colorectal metastases patients. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9697–9706. [Google Scholar]
- Granata, V.; Fusco, R.; Avallone, A.; Cassata, A.; Palaia, R.; Delrio, P.; Grassi, R.; Tatangelo, F.; Grazzini, G.; Izzo, F.; et al. Abbreviated MRI protocol for colorectal liver metastases: How the radiologist could work in pre surgical setting. PLoS ONE 2020, 15, e0241431. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Petrillo, A. Additional Considerations on Use of Abbreviated Liver MRI in Patients With Colorectal Liver Metastases. Am. J. Roentgenol. 2021, 217, W1. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Belli, A.; Ottaiano, A.; Nasti, G.; La Porta, M.; Danti, G.; Cappabianca, S.; et al. Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: How radiologist should assess MR features. Radiol. Med. 2021, 126, 1584–1600. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Catalano, O.; Piccirillo, M.; Palaia, R.; Nasti, G.; Petrillo, A.; Izzo, F. A radiologist’s point of view in the presurgical and intraoperative setting of colorectal liver metastases. Futur. Oncol. 2018, 14, 2189–2206. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Avallone, A.; Palaia, R.; Botti, G.; Tatangelo, F.; Granata, F.; Cascella, M.; Izzo, F.; et al. Diagnostic accuracy of magnetic resonance, computed tomography and contrast enhanced ultrasound in radiological multimodality assessment of peribiliary liver metastases. PLoS ONE 2017, 12, e0179951. [Google Scholar] [CrossRef]
- Granata, V.; Palaia, R.; Izzo, F. Commentary: The Synergistic Role of Irreversible Electroporation and Chemotherapy for Locally Advanced Pancreatic Cancer. Front. Oncol. 2022, 12, 955444. [Google Scholar] [CrossRef]
- Rudno-Rudzińska, J.; Kielan, W.; Guziński, M.; Płochocki, M.; Antończyk, A.; Kulbacka, J. New therapeutic strategy: Personalization of pancreatic cancer treatment-irreversible electroporation (IRE), electrochemotherapy (ECT) and calcium electroporation (CaEP)—A pilot preclinical study. Surg. Oncol. 2021, 38, 101634. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C.; McFarland, K.; Ellis, S.; Velanovich, V. Irreversible Electroporation Therapy in the Management of Locally Advanced Pancreatic Adenocarcinoma. J. Am. Coll. Surg. 2012, 215, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.C., 2nd; McFarland, K.; Ellis, S.; Velanovich, V. Irreversible Electroporation in Locally Advanced Pancreatic Cancer: Potential Improved Overall Survival. Ann. Surg. Oncol. 2012, 20 (Suppl. 3), S443–S449. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Piccirillo, M.; Albino, V.; Palaia, R.; Belli, A.; Granata, V.; Setola, S.; Fusco, R.; Petrillo, A.; Orlando, R.; et al. Prospective screening increases the detection of potentially curable hepatocellular carcinoma: Results in 8900 high-risk patients. HPB 2013, 15, 985–990. [Google Scholar] [CrossRef]
- Argalia, G.; Tarantino, G.; Ventura, C.; Campioni, D.; Tagliati, C.; Guardati, P.; Kostandini, A.; Marzioni, M.; Giuseppetti, G.M.; Giovagnoni, A. Shear wave elastography and transient elastography in HCV patients after direct-acting antivirals. Radiol. Med. 2021, 126, 894–899. [Google Scholar] [CrossRef]
- Giovagnoni, A. A farewell from the “old” Editor-in-Chief. Radiol. Med. 2021, 126, 1–2. [Google Scholar] [CrossRef]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Salati, S.; Petrillo, A.; Di Bernardo, E.; Grassi, R.; Palaia, R.; Danti, G.; La Porta, M.; Cadossi, M.; et al. A Systematic Review about Imaging and Histopathological Findings for Detecting and Evaluating Electroporation Based Treatments Response. Int. J. Environ. Res. Public Health 2021, 18, 5592. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.V.; Palaia, R.; Belli, A.; Miele, V.; Brunese, L.; Petrillo, A.; Izzo, F. Assessment of Ablation Therapy in Pancreatic Cancer: The Radiologist’s Challenge. Front. Oncol. 2020, 10, 560952. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Avallone, A.; Palaia, R.; Grassi, R.; Izzo, F.; Petrillo, A. Radiological assessment of secondary biliary tree lesions: An update. J. Int. Med. Res. 2020, 48, 0300060519850398. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Simonetti, I.; Ianniello, S.; Villanacci, A.; Grassi, F.; Dell’Aversana, F.; Grassi, R.; Cozzi, D.; Bicci, E.; Palumbo, P.; et al. Pulmonary Lymphangitis Poses a Major Challenge for Radiologists in an Oncological Setting during the COVID-19 Pandemic. J. Pers. Med. 2022, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Tafuto, S.; von Arx, C.; De Divitiis, C.; Maura, C.T.; Palaia, R.; Albino, V.; Fusco, R.; Membrini, M.; Petrillo, A.; Granata, V.; et al. Electrochemotherapy as a new approach on pancreatic cancer and on liver metastases. Int. J. Surg. 2015, 21 (Suppl. 1), S78–S82. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Palaia, R.; Belli, A.; Petrillo, A.; Izzo, F. Comments on “Electrochemotherapy with Irreversible Electroporation and FOLFIRINOX Improves Survival in Murine Models of Pancreatic Adenocarcinoma”. Ann. Surg. Oncol. 2020, 27 (Suppl. 3), 954–955. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Palaia, R.; Albino, V.; Piccirillo, M.; Grimm, R.; Petrillo, A.; Izzo, F. Diffusion kurtosis imaging and conventional diffusion weighted imaging to assess electrochemotherapy response in locally advanced pancreatic cancer. Radiol. Oncol. 2019, 53, 15–24. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Maio, F.; Avallone, A.; Nasti, G.; Palaia, R.; Albino, V.; Grassi, R.; Izzo, F.; Petrillo, A. Qualitative assessment of EOB-GD-DTPA and Gd-BT-DO3A MR contrast studies in HCC patients and colorectal liver metastases. Infect. Agents Cancer 2019, 14, 40. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Piccirillo, M.; Leongito, M.; Palaia, R.; Granata, F.; Lastoria, S.; Izzo, F.; Petrillo, A. Early radiological assessment of locally advanced pancreatic cancer treated with electrochemotherapy. World J. Gastroenterol. 2017, 23, 4767–4778. [Google Scholar] [CrossRef]
- Bimonte, S.; Leongito, M.; Granata, V.; Barbieri, A.; DEL Vecchio, V.; Falco, M.; Nasto, A.; Albino, V.; Piccirillo, M.; Palaia, R.; et al. Electrochemotherapy in pancreatic adenocarcinoma treatment: Pre-clinical and clinical studies. Radiol. Oncol. 2016, 50, 14–20. [Google Scholar] [CrossRef]
- Stefanini, M.; Simonetti, G. Interventional Magnetic Resonance Imaging Suite (IMRIS): How to build and how to use. Radiol. Med. 2022, 127, 1063–1067. [Google Scholar] [CrossRef]
- Granata, V.; Castelguidone, E.D.L.D.; Fusco, R.; Catalano, O.; Piccirillo, M.; Palaia, R.; Izzo, F.; Gallipoli, A.D.; Petrillo, A. Irreversible electroporation of hepatocellular carcinoma: Preliminary report on the diagnostic accuracy of magnetic resonance, computer tomography, and contrast-enhanced ultrasound in evaluation of the ablated area. Radiol. Med. 2015, 121, 122–131. [Google Scholar] [CrossRef]
- Nakamura, Y.; Higaki, T.; Honda, Y.; Tatsugami, F.; Tani, C.; Fukumoto, W.; Narita, K.; Kondo, S.; Akagi, M.; Awai, K. Advanced CT techniques for assessing hepatocellular carcinoma. Radiol. Med. 2021, 126, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Barretta, M.L.; Catalano, O.; Setola, S.V.; Granata, V.; Marone, U.; Gallipoli, A.D. Gallbladder metastasis: Spectrum of imaging findings. Abdom. Imaging 2011, 36, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Ierardi, A.M.; Stellato, E.; Pellegrino, G.; Bonelli, C.; Cellina, M.; Renzulli, M.; Biondetti, P.; Carrafiello, G. Fluid-dynamic control microcatheter used with glue: Preliminary experience on its feasibility and safety. Radiol. Med. 2022, 27, 272–276. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Piccirillo, M.; Palaia, R.; Petrillo, A.; Lastoria, S.; Izzo, F. Electrochemotherapy in locally advanced pancreatic cancer: Preliminary results. Int. J. Surg. 2015, 18, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Castelguidone, E.D.L.D.; Camera, L.; Tafuto, S.; Avallone, A.; Belli, A.; Incollingo, P.; Palaia, R.; et al. The multidisciplinary team for gastroenteropancreatic neuroendocrine tumours: The radiologist’s challenge. Radiol. Oncol. 2019, 53, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Danti, G.; Flammia, F.; Matteuzzi, B.; Cozzi, D.; Berti, V.; Grazzini, G.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Gastrointestinal neuroendocrine neoplasms (GI-NENs): Hot topics in morphological, functional, and prognostic imaging. Radiol. Med. 2021, 126, 1497–1507. [Google Scholar] [CrossRef]
- Chiti, G.; Grazzini, G.; Flammia, F.; Matteuzzi, B.; Tortoli, P.; Bettarini, S.; Pasqualini, E.; Granata, V.; Busoni, S.; Messserini, L.; et al. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): A radiomic model to predict tumor grade. Radiol. Med. 2022, 127, 928–938. [Google Scholar] [CrossRef]
- Caruso, D.; Polici, M.; Rinzivillo, M.; Zerunian, M.; Nacci, I.; Marasco, M.; Magi, L.; Tarallo, M.; Gargiulo, S.; Iannicelli, E.; et al. CT-based radiomics for prediction of therapeutic response to Everolimus in metastatic neuroendocrine tumors. Radiol. Med. 2022, 127, 691–701. [Google Scholar] [CrossRef]
- Rossi, S.; Viera, F.T.; Ghittoni, G.; Cobianchi, L.; Rosa, L.L.; Siciliani, L.; Bortolotto, C.; Veronese, L.; Vercelli, A.; Gallotti, A.; et al. Radiofrequency Ablation of Pancreatic Neuroendocrine Tumors. Pancreas 2014, 43, 938–945. [Google Scholar] [CrossRef]
- Chiti, G.; Grazzini, G.; Cozzi, D.; Danti, G.; Matteuzzi, B.; Granata, V.; Pradella, S.; Recchia, L.; Brunese, L.; Miele, V. Imaging of Pancreatic Neuroendocrine Neoplasms. Int. J. Environ. Res. Public Health 2021, 18, 8895. [Google Scholar] [CrossRef]
- Granata, V.; Coppola, F.; Grassi, R.; Fusco, R.; Tafuto, S.; Izzo, F.; Reginelli, A.; Maggialetti, N.; Buccicardi, D.; Frittoli, B.; et al. Structured Reporting of Computed Tomography in the Staging of Neuroendocrine Neoplasms: A Delphi Consensus Proposal. Front. Endocrinol. 2021, 12, 748944. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, N.S.; Feldman, M.K.; Le, O.; Morris-Stiff, G. Imaging mimics of pancreatic ductal adenocarcinoma. Abdom. Imaging 2017, 43, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Dai, M.-H.; Wang, S.-T.; Jin, Z.-Y.; Wang, Q.; Denecke, T.; Hamm, B.; Xue, H.-D. Multiple solid pancreatic lesions: Prevalence and features of non-malignancies on dynamic enhanced CT. Eur. J. Radiol. 2018, 105, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Setola, S.V.; Raiano, N.; Granata, V.; Cerciello, V.; Pecori, B.; Petrillo, A. Analysis of a monocentric computed tomography dosimetric database using a radiation dose index monitoring software: Dose levels and alerts before and after the implementation of the adaptive statistical iterative reconstruction on CT images. Radiol. Med. 2022, 127, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Beatty, G.L.; Dougan, S.K. Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 2019, 156, 2056–2072. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, Y.S.; Choi, J. Dosimetric analysis of the effects of a temporary tissue expander on the radiotherapy technique. Radiol. Med. 2020, 126, 437–444. [Google Scholar] [CrossRef]
- Bozkurt, M.; Eldem, G.; Bozbulut, U.B.; Bozkurt, M.F.; Kılıçkap, S.; Peynircioğlu, B.; Çil, B.; Ergün, E.L.; Volkan-Salanci, B. Factors affecting the response to Y-90 microsphere therapy in the cholangiocarcinoma patients. Radiol. Med. 2020, 126, 323–333. [Google Scholar] [CrossRef]
- Shetty, A.S.; Menias, C.O. Rare Pancreatic Tumors. Magn. Reson. Imaging Clin. N. Am. 2018, 26, 421–437. [Google Scholar] [CrossRef]
- Haeberle, L.; Esposito, I. Pathology of pancreatic cancer. Transl. Gastroenterol. Hepatol. 2019, 4, 50. [Google Scholar] [CrossRef]
- Abramson, A.M.; Jazag, A.; Van Der Zee, J.A.; Whang, E.E. The molecular biology of pancreatic cancer. Gastrointest. Cancer Res. 2007, 1 (Suppl. 2), S7–S12. [Google Scholar]
- Ottenhof, B.N.A.; Milne, A.N.A.; Morsink, B.F.H.M.; Drillenburg, P.; Kate, F.J.W.T.; Maitra, M.A.; Offerhaus, G.J. Pancreatic Intraepithelial Neoplasia and Pancreatic Tumorigenesis: Of Mice and Men. Arch. Pathol. Lab. Med. 2009, 133, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Arslan, A.; Aktas, E.; Sengul, B.; Tekin, B. Dosimetric evaluation of left ventricle and left anterior descending artery in left breast radiotherapy. Radiol. Med. 2020, 126, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Haugk, B. Pancreatic intraepithelial neoplasia—Can we detect early pancreatic cancer? Histopathology 2010, 57, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Cionfoli, N.; Paladini, A.; Vallone, M.; Corvino, F.; Teodoli, L.; Moramarco, L.; Quaretti, P.; Catalano, C.; Niola, R.; et al. PHIL® (precipitating hydrophobic injectable liquid): Retrospective multicenter experience on 178 patients in peripheral embolizations. Radiol. Med. 2022, 127, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xie, D.; Wei, D. Pancreatic Acinar-to-Ductal Metaplasia and Pancreatic Cancer. Pancreat. Cancer 2018, 1882, 299–308. [Google Scholar] [CrossRef]
- Longnecker, D.S.; Suriawinata, A.A. Incidence of Pancreatic Intraepithelial Neoplasia in an Autopsy Series. Pancreas 2022, 51, 305–309. [Google Scholar] [CrossRef]
- Egawa, S.; Toma, H.; Ohigashi, H.; Okusaka, T.; Nakao, A.; Hatori, T.; Maguchi, H.; Yanagisawa, A.; Tanaka, M. Japan Pancreatic Cancer Registry; 30th Year Anniversary. Pancreas 2012, 41, 985–992. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-del Castillo, C.; Adsay, V.; Chari, S.; Falconi, M.; Jang, J.-Y.; Kimura, W.; Levy, P.; Pitman, M.B.; Schmidt, C.M.; et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012, 12, 183–197. [Google Scholar] [CrossRef]
- Hussein, M.A.M.; Cafarelli, F.P.; Paparella, M.T.; Rennie, W.J.; Guglielmi, G. Phosphaturic mesenchymal tumors: Radiological aspects and suggested imaging pathway. Radiol. Med. 2021, 126, 1609–1618. [Google Scholar] [CrossRef]
- Ansari, D.; Amini, J.; Edman, M.; Andersson, R. IPMN of the pancreas—Does histological subtyping allow for improved stratification and follow-up? Scand. J. Gastroenterol. 2021, 56, 862–864. [Google Scholar] [CrossRef]
- Granata, V.; Catalano, O.; Fusco, R.; Tatangelo, F.; Rega, D.; Nasti, G.; Avallone, A.; Piccirillo, M.; Izzo, F.; Petrillo, A. The target sign in colorectal liver metastases: An atypical Gd-EOB-DTPA “uptake” on the hepatobiliary phase of MR imaging. Abdom. Imaging 2015, 40, 2364–2371. [Google Scholar] [CrossRef] [PubMed]
- Hirono, S.; Yamaue, H. Surgical strategy for intraductal papillary mucinous neoplasms of the pancreas. Surg. Today 2019, 50, 50–55. [Google Scholar] [CrossRef] [PubMed]
- De Muzio, F.; Cutolo, C.; Dell’Aversana, F.; Grassi, F.; Ravo, L.; Ferrante, M.; Danti, G.; Flammia, F.; Simonetti, I.; Palumbo, P.; et al. Complications after Thermal Ablation of Hepatocellular Carcinoma and Liver Metastases: Imaging Findings. Diagnostics 2022, 12, 1151. [Google Scholar] [CrossRef] [PubMed]
- Hecht, E.M.; Khatri, G.; Morgan, D.; Kang, S.; Bhosale, P.R.; Francis, I.R.; Gandhi, N.S.; Hough, D.M.; Huang, C.; Luk, L.; et al. Intraductal papillary mucinous neoplasm (IPMN) of the pancreas: Recommendations for Standardized Imaging and Reporting from the Society of Abdom.inal Radiology IPMN disease focused panel. Abdom. Radiol. 2020, 46, 1586–1606. [Google Scholar] [CrossRef] [PubMed]
- Pizzini, F.B.; Conti, E.; Bianchetti, A.; Splendiani, A.; Fusco, D.; Caranci, F.; Bozzao, A.; Landi, F.; Gandolfo, N.; Farina, L.; et al. Radiological assessment of dementia: The Italian inter-society consensus for a practical and clinically oriented guide to image acquisition, evaluation, and reporting. Radiol. Med. 2022, 127, 998–1022. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Catalano, O.; Filice, S.; Amato, D.M.; Nasti, G.; Avallone, A.; Izzo, F.; Petrillo, A. Early Assessment of Colorectal Cancer Patients with Liver Metastases Treated with Antiangiogenic Drugs: The Role of Intravoxel Incoherent Motion in Diffusion-Weighted Imaging. PLoS ONE 2015, 10, e0142876. [Google Scholar] [CrossRef]
- Li, N.; Wakim, J.; Koethe, Y.; Huber, T.; Schenning, R.; Gade, T.P.; Hunt, S.J.; Park, B.J. Multicenter assessment of augmented reality registration methods for image-guided interventions. Radiol. Med. 2022, 127, 857–865. [Google Scholar] [CrossRef]
- Izzo, F.; Palaia, R.; Albino, V.; Amore, A.; Di Giacomo, R.; Piccirillo, M.; Leongito, M.; Nasto, A.; Granata, V.; Petrillo, A.; et al. Hepatocellular carcinoma and liver metastases: Clinical data on a new dual-lumen catheter kit for surgical sealant infusion to prevent perihepatic bleeding and dissemination of cancer cells following biopsy and loco-regional treatments. Infect. Agents Cancer 2015, 10, 11. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Castelguidone, E.D.L.D.; Avallone, A.; Palaia, R.; Delrio, P.; Tatangelo, F.; Botti, G.; Grassi, R.; Izzo, F.; et al. Diagnostic performance of gadoxetic acid-enhanced liver MRI versus multidetector CT in the assessment of colorectal liver metastases compared to hepatic resection. BMC Gastroenterol. 2019, 19, 129. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernández-del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Mino-Kenudson, M.; Fernández-del Castillo, C.; Baba, Y.; Valsangkar, N.P.; Liss, A.S.; Hsu, M.; Correa-Gallego, J.C.; Ingkakul, T.; Perez Johnston, R.; Turner, B.G.; et al. Prognosis of invasive intraductal papillary mucinous neoplasm depends on histological and precursor epithelial subtypes. Gut 2011, 60, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.G.; Beleva Guthrie, V.; Braxton, A.M.; Zheng, L.; Wang, P.; Song, Q.; Griffin, J.F.; Chianchiano, P.E.; Hosoda, W.; Niknafs, N.; et al. Intraductal Papillary Mucinous Neoplasms Arise From Multiple Independent Clones, Each With Distinct Mutations. Gastroenterology 2019, 157, 1123–1137.e22. [Google Scholar] [CrossRef] [PubMed]
- Levink, I.; Bruno, M.; Cahen, D. Management of Intraductal Papillary Mucinous Neoplasms: Controversies in Guidelines and Future Perspectives. Curr. Treat. Options Gastroenterol. 2018, 16, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.G.; Smith, D.; Ojili, V.; Paspulati, R.M.; Ramaiya, N.H.; Tirumani, S.H. Pancreatic cystic neoplasms: A review of current recommendations for surveillance and management. Abdom. Radiol. 2021, 46, 3946–3962. [Google Scholar] [CrossRef]
- Hasan, A.; Visrodia, K.; Farrell, J.J.; Gonda, A.T. Overview and comparison of guidelines for management of pancreatic cystic neoplasms. World J. Gastroenterol. 2019, 25, 4405–4413. [Google Scholar] [CrossRef]
- van Huijgevoort, N.C.M.; del Chiaro, M.; Wolfgang, C.L.; van Hooft, J.E.; Besselink, M.G. Diagnosis and management of pancreatic cystic neoplasms: Current evidence and guidelines. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 676–689. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, G. Comparison of clinicopathologic characteristics and survival outcomes between invasive IPMN and invasive MCN: A population-based analysis. Front. Oncol. 2022, 12, 899761. [Google Scholar] [CrossRef]
- Hu, J.-X.; Zhao, C.-F.; Chen, W.-B.; Liu, Q.-C.; Li, Q.-W.; Lin, Y.-Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef]
- Cai, J.; Chen, H.; Lu, M.; Zhang, Y.; Lu, B.; You, L.; Zhang, T.; Dai, M.; Zhao, Y. Advances in the epidemiology of pancreatic cancer: Trends, risk factors, screening, and prognosis. Cancer Lett. 2021, 520, 1–11. [Google Scholar] [CrossRef]
- De Re, V.; Caggiari, L.; De Zorzi, M.; Repetto, O.; Zignego, A.L.; Izzo, F.; Tornesello, M.L.; Buonaguro, F.M.; Mangia, A.; Sansonno, D.; et al. Genetic Diversity of the KIR/HLA System and Susceptibility to Hepatitis C Virus-Related Diseases. PLoS ONE 2015, 10, e0117420. [Google Scholar] [CrossRef] [PubMed]
- Capurso, G.; Paiella, S.; Falconi, M. Screening for pancreatic cancer—A compelling challenge. Hepatobiliary Surg. Nutr. 2021, 10, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, H.R.; Lee, J.H.; Canto, M.I. AGA Clinical Practice Update on Pancreas Cancer Screening in High-Risk Individuals: Expert Review. Gastroenterology 2020, 159, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Baron, T.H.; DiMaio, C.J.; Wang, A.Y.; Morgan, K.A. American Gastroenterological Association Clinical Practice Update: Management of Pancreatic Necrosis. Gastroenterology 2020, 158, 67–75.e1. [Google Scholar] [CrossRef]
- Bartoli, M.; Barat, M.; Dohan, A.; Gaujoux, S.; Coriat, R.; Hoeffel, C.; Cassinotto, C.; Chassagnon, G.; Soyer, P. CT and MRI of pancreatic tumors: An update in the era of radiomics. JPN. J. Radiol. 2020, 38, 1111–1124. [Google Scholar] [CrossRef]
- Hruban, R.H.; Canto, M.I.; Goggins, M.; Schulick, R.; Klein, A.P. Update on Familial Pancreatic Cancer. Adv. Surg. 2010, 44, 293–311. [Google Scholar] [CrossRef]
- Zhen, D.B.; Rabe, K.G.; Gallinger, S.; Syngal, S.; Schwartz, A.G.; Goggins, M.G.; Hruban, R.H.; Cote, M.L.; McWilliams, R.R.; Roberts, N.J.; et al. BRCA1, BRCA2, PALB2, and CDKN2A mutations in familial pancreatic cancer: A PACGENE study. Genet. Med. 2015, 17, 569–577. [Google Scholar] [CrossRef]
- Salo-Mullen, E.E.; O’Reilly, E.M.; Kelsen, D.P.; Ashraf, A.M.; Lowery, M.A.; Yu, K.H.; Reidy, D.L.; Epstein, A.S.; Lincoln, A.; Saldia, A.; et al. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer 2015, 121, 4382–4388. [Google Scholar] [CrossRef]
- Puccini, A.; Ponzano, M.; Dalmasso, B.; Vanni, I.; Gandini, A.; Puglisi, S.; Borea, R.; Cremante, M.; Bruno, W.; Andreotti, V.; et al. Clinical Significance of Germline Pathogenic Variants among 51 Cancer Predisposition Genes in an Unselected Cohort of Italian Pancreatic Cancer Patients. Cancers 2022, 14, 4447. [Google Scholar] [CrossRef]
- Falcinelli, L.; Mendichi, M.; Chierchini, S.; Tenti, M.V.; Bellavita, R.; Saldi, S.; Ingrosso, G.; Reggioli, V.; Bini, V.; Aristei, C. Pulmonary function in stereotactic body radiotherapy with helical tomotherapy for primary and metastatic lung lesions. Radiol. Med. 2020, 126, 163–169. [Google Scholar] [CrossRef]
- Bono, M.; Fanale, D.; Incorvaia, L.; Cancelliere, D.; Fiorino, A.; Calò, V.; Dimino, A.; Filorizzo, C.; Corsini, L.; Brando, C.; et al. Impact of deleterious variants in other genes beyond BRCA1/2 detected in breast/ovarian and pancreatic cancer patients by NGS-based multi-gene panel testing: Looking over the hedge. ESMO Open 2021, 6, 100235. [Google Scholar] [CrossRef]
- Merlotti, A.; Bruni, A.; Borghetti, P.; Ramella, S.; Scotti, V.; Trovò, M.; Chiari, R.; Lohr, F.; Ricardi, U.; Bria, E.; et al. Sequential chemo-hypofractionated RT versus concurrent standard CRT for locally advanced NSCLC: GRADE recommendation by the Italian Association of Radiotherapy and Clinical Oncology (AIRO). Radiol. Med. 2021, 126, 1117–1128. [Google Scholar] [CrossRef]
- Catts, Z.A.-K.; Baig, M.K.; Milewski, B.; Keywan, C.; Guarino, M.; Petrelli, N. Statewide Retrospective Review of Familial Pancreatic Cancer in Delaware, and Frequency of Genetic Mutations in Pancreatic Cancer Kindreds. Ann. Surg. Oncol. 2016, 23, 1729–1735. [Google Scholar] [CrossRef]
- Yuan, C.; Babic, A.; Khalaf, N.; Nowak, J.A.; Brais, L.K.; Rubinson, D.A.; Ng, K.; Aguirre, A.J.; Pandharipande, P.V.; Fuchs, C.S.; et al. Diabetes, Weight Change, and Pancreatic Cancer Risk. JAMA Oncol. 2020, 6, e202948. [Google Scholar] [CrossRef]
- Sharma, A.; Kandlakunta, H.; Nagpal, S.J.S.; Feng, Z.; Hoos, W.; Petersen, G.M.; Chari, S.T. Model to Determine Risk of Pancreatic Cancer in Patients With New-Onset Diabetes. Gastroenterology 2018, 155, 730–739.e3. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Rega, D.; Russo, C.; Pace, U.; Pecori, B.; Tatangelo, F.; Botti, G.; Izzo, F.; Cascella, M.; et al. Morphological and functional features prognostic factor of magnetic resonance imaging in locally advanced rectal cancer. Acta Radiol. 2018, 60, 815–825. [Google Scholar] [CrossRef]
- Mueller, A.M.; Meier, C.R.; Jick, S.S.; Schneider, C. Weight change and blood glucose concentration as markers for pancreatic cancer in subjects with new-onset diabetes mellitus: A matched case-control study. Pancreatology 2019, 19, 578–586. [Google Scholar] [CrossRef]
- Fusco, R.; Petrillo, M.; Granata, V.; Filice, S.; Sansone, M.; Catalano, O.; Petrillo, A. Magnetic resonance imaging evaluation in neoadjuvant therapy of locally advanced rectal cancer: A systematic review. Radiol. Oncol. 2017, 51, 252–262. [Google Scholar] [CrossRef]
- Dunne, R.F.; Roeland, E.J. The Interplay Among Pancreatic Cancer, Cachexia, Body Composition, and Diabetes. Hematol. Clin. N. Am. 2022, 36, 897–910. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Avallone, A.; Catalano, O.; Filice, F.; Leongito, M.; Palaia, R.; Izzo, F.; Petrillo, A. Major and ancillary magnetic resonance features of LI-RADS to assess HCC: An overview and update. Infect. Agents Cancer 2017, 12, 23. [Google Scholar] [CrossRef]
- Santos, R.; Coleman, H.G.; Cairnduff, V.; Kunzmann, A.T. Clinical Prediction Models for Pancreatic Cancer in General and At-Risk Populations: A Systematic Review. Am. J. Gastroenterol. 2022, 10, 14309. [Google Scholar] [CrossRef] [PubMed]
- Chiloiro, G.; Cusumano, D.; de Franco, P.; Lenkowicz, J.; Boldrini, L.; Carano, D.; Barbaro, B.; Corvari, B.; Dinapoli, N.; Giraffa, M.; et al. Does restaging MRI radiomics analysis improve pathological complete response prediction in rectal cancer patients? A prognostic model development. Radiol. Med. 2022, 127, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pergolini, I.; Jäger, C.; Safak, O.; Göß, R.; Novotny, A.; Ceyhan, G.O.; Friess, H.; Demir, I.E. Diabetes and Weight Loss Are Associated With Malignancies in Patients With Intraductal Papillary Mucinous Neoplasms. Clin. Gastroenterol. Hepatol. 2020, 19, 171–179. [Google Scholar] [CrossRef]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Barra, S.; Guarnieri, A.; Bastia, M.B.D.M.E.; Marcenaro, M.; Tornari, E.; Belgioia, L.; Magrini, S.M.; Ricardi, U.; Corvò, R. Short fractionation radiotherapy for early prostate cancer in the time of COVID-19: Long-term excellent outcomes from a multicenter Italian trial suggest a larger adoption in clinical practice. Radiol. Med. 2020, 126, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Cellini, F.; Di Franco, R.; Manfrida, S.; Borzillo, V.; Maranzano, E.; Pergolizzi, S.; Morganti, A.G.; Fusco, V.; Deodato, F.; Santarelli, M.; et al. Palliative radiotherapy indications during the COVID-19 pandemic and in future complex logistic settings: The NORMALITY model. Radiol. Med. 2021, 126, 1619–1656. [Google Scholar] [CrossRef]
- Lancellotta, V.; Del Regno, L.; Di Stefani, A.; Fionda, B.; Marazzi, F.; Rossi, E.; Balducci, M.; Pampena, R.; Morganti, A.G.; Mangoni, M.; et al. The role of stereotactic radiotherapy in addition to immunotherapy in the management of melanoma brain metastases: Results of a systematic review. Radiol. Med. 2022, 127, 773–783. [Google Scholar] [CrossRef]
- Laurelli, G.; Falcone, F.; Gallo, M.S.; Scala, F.; Losito, S.; Granata, V.; Cascella, M.; Greggi, S. Long-Term Oncologic and Reproductive Outcomes in Young Women With Early Endometrial Cancer Conservatively Treated: A Prospective Study and Literature Update. Int. J. Gynecol. Cancer 2016, 26, 1650–1657. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Barretta, M.L.; Picone, C.; Avallone, A.; Belli, A.; Patrone, R.; Ferrante, M.; Cozzi, D.; Grassi, R.; et al. Radiomics in hepatic metastasis by colorectal cancer. Infect. Agents Cancer 2021, 16, 39. [Google Scholar] [CrossRef]
- Capone, F.; Costantini, S.; Guerriero, E.; Calemma, R.; Napolitano, M.; Scala, S.; Izzo, F.; Castello, G. Serum cytokine levels in patients with hepatocellular carcinoma. Eur. Cytokine Netw. 2010, 21, 99–104. [Google Scholar] [CrossRef]
- Taieb, J.; Svrcek, M.; Cohen, R.; Basile, D.; Tougeron, D.; Phelip, J.-M. Deficient mismatch repair/microsatellite unstable colorectal cancer: Diagnosis, prognosis and treatment. Eur. J. Cancer 2022, 175, 136–157. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Costa, M.; Picone, C.; Cozzi, D.; Moroni, C.; La Casella, G.; Montanino, A.; Monti, R.; Mazzoni, F.; et al. Preliminary Report on Computed Tomography Radiomics Features as Biomarkers to Immunotherapy Selection in Lung Adenocarcinoma Patients. Cancers 2021, 13, 3992. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Simonetti, I.; Fusco, R.; Setola, S.V.; Izzo, F.; Scarpato, L.; Vanella, V.; Festino, L.; Simeone, E.; Ascierto, P.A.; et al. Management of cutaneous melanoma: Radiologists challenging and risk assessment. Radiol. Med. 2022, 127, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, L.; Rustici, A.; Toni, F.; Zoli, M.; Bartiromo, F.; Gramegna, L.L.; Cicala, D.; Tonon, C.; Caranci, F.; Lodi, R. Vessel Wall MRI: Clinical implementation in cerebrovascular disorders—Technical aspects. Radiol. Med. 2022, 127, 645–651. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Grassi, F.; Belli, A.; Silvestro, L.; Ottaiano, A.; et al. Radiomics and machine learning analysis based on magnetic resonance imaging in the assessment of liver mucinous colorectal metastases. Radiol. Med. 2022, 127, 763–772. [Google Scholar] [CrossRef]
- Tagliafico, A.S.; Campi, C.; Bianca, B.; Bortolotto, C.; Buccicardi, D.; Francesca, C.; Prost, R.; Rengo, M.; Faggioni, L. Blockchain in radiology research and clinical practice: Current trends and future directions. Radiol. Med. 2022, 127, 391–397. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Grassi, R.; Grassi, F.; Ottaiano, A.; Nasti, G.; Tatangelo, F.; et al. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol. Med. 2022, 127, 461–470. [Google Scholar] [CrossRef]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of patients with increased risk for familial pancreatic cancer: Updated recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2019, 69, 7–17. [Google Scholar] [CrossRef]
- Stoffel, E.M.; McKernin, S.E.; Brand, R.; Canto, M.; Goggins, M.; Moravek, C.; Nagarajan, A.; Petersen, G.M.; Simeone, D.M.; Yurgelun, M.; et al. Evaluating Susceptibility to Pancreatic Cancer: ASCO Provisional Clinical Opinion. J. Clin. Oncol. 2019, 37, 153–164. [Google Scholar] [CrossRef]
- Greenhalf, W.; Lévy, P.; Gress, T.; Rebours, V.; Brand, R.E.; Pandol, S.; Chari, S.; Jørgensen, M.T.; Mayerle, J.; Lerch, M.M.; et al. International consensus guidelines on surveillance for pancreatic cancer in chronic pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and European Pancreatic Club. Pancreatology 2020, 20, 910–918. [Google Scholar] [CrossRef]
- Vanek, P.; Urban, O.; Zoundjiekpon, V.; Falt, P. Current Screening Strategies for Pancreatic Cancer. Biomedicines 2022, 10, 2056. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Sansone, M.; Rega, D.; Delrio, P.; Tatangelo, F.; Romano, C.; Avallone, A.; Pupo, D.; Giordano, M.; et al. Validation of the standardized index of shape tool to analyze DCE-MRI data in the assessment of neo-adjuvant therapy in locally advanced rectal cancer. Radiol. Med. 2021, 126, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Renzulli, M.; Brandi, N.; Argalia, G.; Brocchi, S.; Farolfi, A.; Fanti, S.; Golfieri, R. Morphological, dynamic and functional characteristics of liver pseudolesions and benign lesions. Radiol. Med. 2022, 127, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Gaujoux, S.; Williet, N.; Bachet, J.-B.; Bauguion, L.; Durand, L.C.; Conroy, T.; Dahan, L.; Gilabert, M.; Huguet, F.; et al. Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC). Dig. Liver Dis. 2018, 50, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Ledda, R.E.; Silva, M.; McMichael, N.; Sartorio, C.; Branchi, C.; Milanese, G.; Nayak, S.M.; Sverzellati, N. The diagnostic value of grey-scale inversion technique in chest radiography. Radiol. Med. 2022, 127, 294–304. [Google Scholar] [CrossRef]
- Tempero, M.A.; Arnoletti, J.P.; Behrman, S.W.; Ben-Josef, E.; Benson, A.B.; Casper, E.S.; Cohen, S.J.; Czito, B.; Ellenhorn, J.D.I.; Hawkins, W.G.; et al. Pancreatic Adenocarcinoma, Version 2.2012. J. Natl. Compr. Cancer Netw. 2012, 10, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Henrikson, N.B.; Bowles, E.J.A.; Blasi, P.R.; Morrison, C.C.; Nguyen, M.; Pillarisetty, V.G.; Lin, J.S. Screening for Pancreatic Cancer. JAMA 2019, 322, 445–454. [Google Scholar] [CrossRef]
- Joergensen, M.T.; Gerdes, A.-M.; Sorensen, J.; de Muckadell, O.S.; Mortensen, M.B. Is screening for pancreatic cancer in high-risk groups cost-effective?—Experience from a Danish national screening program. Pancreatology 2016, 16, 584–592. [Google Scholar] [CrossRef]
- Syngal, S.; Brand, E.R.; Church, J.M.; Giardiello, F.M.; Hampel, H.L.; Burt, R.W. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes. Am. J. Gastroenterol. 2015, 110, 223–262. [Google Scholar] [CrossRef]
- Barnes, C.A.; Krzywda, E.; Lahiff, S.; McDowell, D.; Christians, K.K.; Knechtges, P.; Tolat, P.; Hohenwalter, M.; Dua, K.; Khan, A.H.; et al. Development of a high risk pancreatic screening clinic using 3.0 T MRI. Fam. Cancer 2017, 17, 101–111. [Google Scholar] [CrossRef]
- Owens, D.K.; Davidson, K.W.; Krist, A.H.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Curry, S.J.; Doubeni, C.A.; Epling, J.W.; Kubik, M.; et al. Screening for pancreatic cancer: US preventive services Task force reaffirmation recommendation statement. JAMA 2019, 322, 438–444. [Google Scholar] [PubMed]
- Bianchi, A.; Mazzoni, L.N.; Busoni, S.; Pinna, N.; Albanesi, M.; Cavigli, E.; Cozzi, D.; Poggesi, A.; Miele, V.; Fainardi, E.; et al. Assessment of cerebrovascular disease with computed tomography in COVID-19 patients: Correlation of a novel specific visual score with increased mortality risk. Radiol. Med. 2020, 126, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Cartocci, G.; Colaiacomo, M.C.; Lanciotti, S.; Andreoli, C.; De Cicco, M.L.; Brachetti, G.; Pugliese, S.; Capoccia, L.; Tortora, A.; Scala, A.; et al. Correction to: Chest CT for early detection and management of coronavirus disease (COVID-19): A report of 314 patients admitted to Emergency Department with suspected pneumonia. Radiol. Med. 2020, 126, 642. [Google Scholar] [CrossRef]
- Trikudanathan, G.; Lou, E.; Maitra, A.; Majumder, S. Early detection of pancreatic cancer: Current state and future opportu-nities. Curr. Opin. Gastroenterol. 2021, 37, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Polesel, J.; Talamini, R.; Montella, M.; Maso, L.D.; Crovatto, M.; Parpinel, M.; Izzo, F.; Tommasi, L.G.; Serraino, D.; La Vecchia, C.; et al. Nutrients intake and the risk of hepatocellular carcinoma in Italy. Eur. J. Cancer 2007, 43, 2381–2387. [Google Scholar] [CrossRef] [PubMed]
- Sansone, M.; Marrone, S.; Di Salvio, G.; Belfiore, M.P.; Gatta, G.; Fusco, R.; Vanore, L.; Zuiani, C.; Grassi, F.; Vietri, M.T.; et al. Comparison between two packages for pectoral muscle removal on mammographic images. Radiol. Med. 2022, 127, 848–856. [Google Scholar] [CrossRef]
- Cutolo, C.; Dell’Aversana, F.; Fusco, R.; Grazzini, G.; Chiti, G.; Simonetti, I.; Bruno, F.; Palumbo, P.; Pierpaoli, L.; Valeri, T.; et al. Combined Hepatocellular-Cholangiocarcinoma: What the Multidisciplinary Team Should Know. Diagnostics 2022, 12, 890. [Google Scholar] [CrossRef]
- Pignata, S.; Gallo, C.; Daniele, B.; Elba, S.; Giorgio, A.; Capuano, G.; Adinolfi, L.E.; De Sio, I.; Izzo, F.; Farinati, F.; et al. Characteristics at presentation and outcome of hepatocellular carcinoma (HCC) in the elderly. Crit. Rev. Oncol. 2006, 59, 243–249. [Google Scholar] [CrossRef]
- Calderwood, A.H.; Sawhney, M.S.; Thosani, N.C.; Rebbeck, T.R.; Wani, S.; Canto, M.I.; Fishman, D.S.; Golan, T.; Hidalgo, M.; Kwon, R.S.; et al. American Society for Gastrointestinal Endoscopy guideline on screening for pancreatic cancer in individuals with genetic susceptibility: Methodology and review of evidence. Gastrointest. Endosc. 2022, 95, 827–854.e3. [Google Scholar] [CrossRef]
- Burra, P.; Bretthauer, M.; Ferret, M.B.; Dugic, A.; Fracasso, P.; Leja, M.; Budnik, T.M.; Michl, P.; Ricciardiello, L.; Seufferlein, T.; et al. Digestive cancer screening across Europe. United Eur. Gastroenterol. J. 2022, 10, 435–437. [Google Scholar] [CrossRef]
- Han, D.; Yu, N.; Yu, Y.; He, T.; Duan, X. Performance of CT radiomics in predicting the overall survival of patients with stage III clear cell renal carcinoma after radical nephrectomy. Radiol. Med. 2022, 127, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Masci, G.M.; Ciccarelli, F.; Mattei, F.I.; Grasso, D.; Accarpio, F.; Catalano, C.; Laghi, A.; Sammartino, P.; Iafrate, F. Role of CT texture analysis for predicting peritoneal metastases in patients with gastric cancer. Radiol. Med. 2022, 127, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Mazzei, M.A.; Di Meglio, N.; Del Roscio, D.; Moroni, C.; Monti, R.; Cappabianca, C.; Picone, C.; Neri, E.; et al. Quantitative imaging decision support (QIDSTM) tool consistency evaluation and radiomic analysis by means of 594 metrics in lung carcinoma on chest CT scan. Cancer Control 2021, 28, 1073274820985786. [Google Scholar] [CrossRef] [PubMed]
- Zerunian, M.; Pucciarelli, F.; Caruso, D.; Polici, M.; Masci, B.; Guido, G.; De Santis, D.; Polverari, D.; Principessa, D.; Benvenga, A.; et al. Artificial intelligence based image quality enhancement in liver MRI: A quantitative and qualitative evaluation. Radiol. Med. 2022, 127, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Cho, J.-H.; Hwang, S.H. Diagnostic value of various criteria for deep lobe involvement in radiologic studies with parotid mass: A systematic review and meta-analysis. Radiol. Med. 2022, 127, 1124–1133. [Google Scholar] [CrossRef]
- Borgheresi, A.; De Muzio, F.; Agostini, A.; Ottaviani, L.; Bruno, A.; Granata, V.; Fusco, R.; Danti, G.; Flammia, F.; Grassi, R.; et al. Lymph Nodes Evaluation in Rectal Cancer: Where Do We Stand and Future Perspective. J. Clin. Med. 2022, 11, 2599. [Google Scholar] [CrossRef]
- Fusco, R.; Sansone, M.; Granata, V.; Grimm, R.; Pace, U.; Delrio, P.; Tatangelo, F.; Botti, G.; Avallone, A.; Pecori, B.; et al. Diffusion and perfusion MR parameters to assess preoperative short-course radiotherapy response in locally advanced rectal cancer: A comparative explorative study among Standardized Index of Shape by DCE-MRI, intravoxel incoherent motion- and diffusion kurtosis imaging-derived parameters. Abdom. Radiol. 2018, 44, 3683–3700. [Google Scholar] [CrossRef]
- Scola, E.; Desideri, I.; Bianchi, A.; Gadda, D.; Busto, G.; Fiorenza, A.; Amadori, T.; Mancini, S.; Miele, V.; Fainardi, E. Assessment of brain tumors by magnetic resonance dynamic susceptibility contrast perfusion-weighted imaging and computed tomography perfusion: A comparison study. Radiol. Med. 2022, 127, 664–672. [Google Scholar] [CrossRef]
- Vicini, S.; Bortolotto, C.; Rengo, M.; Ballerini, D.; Bellini, D.; Carbone, I.; Preda, L.; Laghi, A.; Coppola, F.; Faggioni, L. A narrative review on current imaging applications of artificial intelligence and radiomics in oncology: Focus on the three most common cancers. Radiol. Med. 2022, 127, 819–836. [Google Scholar] [CrossRef]
- Petrillo, A.; Fusco, R.; Petrillo, M.; Granata, V.; Delrio, P.; Bianco, F.; Pecori, B.; Botti, G.; Tatangelo, F.; Caracò, C.; et al. Standardized Index of Shape (DCE-MRI) and Standardized Uptake Value (PET/CT): Two quantitative approaches to discriminate chemo-radiotherapy locally advanced rectal cancer responders under a functional profile. Oncotarget 2016, 8, 8143–8153. [Google Scholar] [CrossRef]
- Masci, G.M.; Iafrate, F.; Ciccarelli, F.; Pambianchi, G.; Panebianco, V.; Pasculli, P.; Ciardi, M.R.; Mastroianni, C.M.; Ricci, P.; Catalano, C.; et al. Tocilizumab effects in COVID-19 pneumonia: Role of CT texture analysis in quantitative assessment of response to therapy. Radiol. Med. 2021, 126, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Francolini, G.; Desideri, I.; Stocchi, G.; Ciccone, L.P.; Salvestrini, V.; Garlatti, P.; Aquilano, M.; Greto, D.; Bonomo, P.; Meattini, I.; et al. Impact of COVID-19 on workload burden of a complex radiotherapy facility. Radiol. Med. 2021, 126, 717–721. [Google Scholar] [CrossRef]
- Wiest, N.E.; Moktan, V.P.; Oman, S.P.; Chirilă, R.M. Screening for pancreatic cancer: A review for general clinicians. Romanian J. Intern. Med. 2020, 58, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Kitano, M.; Yoshida, T.; Itonaga, M.; Tamura, T.; Hatamaru, K.; Yamashita, Y. Impact of endoscopic ultrasonography on diagnosis of pancreatic cancer. J. Gastroenterol. 2018, 54, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sanagapalli, S.; Stoita, A. Challenges in diagnosis of pancreatic cancer. World J. Gastroenterol. 2018, 24, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Treadwell, J.R.; Mitchell, M.D.; Eatmon, K.; Jue, J.; Zafar, H.; Teitelbaum, U.; Schoelles, K. Imaging Tests for the Diagnosis and Staging of Pancreatic Adenocarcinoma: A Meta-Analysis. Pancreas 2016, 45, 789–795. [Google Scholar] [CrossRef]
- Capurso, G.; Signoretti, M.; Valente, R.; Arnelo, U.; Lohr, M.; Poley, J.-W.; Fave, G.D.; Del Chiaro, M. Methods and outcomes of screening for pancreatic adenocarcinoma in high-risk individuals. World J. Gastrointest. Endosc. 2015, 7, 833–842. [Google Scholar] [CrossRef]
- Bruno, F.; Granata, V.; Bellisari, F.C.; Sgalambro, F.; Tommasino, E.; Palumbo, P.; Arrigoni, F.; Cozzi, D.; Grassi, F.; Brunese, M.C.; et al. Advanced Magnetic Resonance Imaging (MRI) Techniques: Technical Principles and Applications in Nanomedicine. Cancers 2022, 14, 1626. [Google Scholar] [CrossRef]
- De Robertis, R.; Geraci, L.; Tomaiuolo, L.; Bortoli, L.; Beleù, A.; Malleo, G.; D’Onofrio, M. Liver metastases in pancreatic ductal adenocarcinoma: A predictive model based on CT texture analysis. Radiol. Med. 2022, 127, 1079–1084. [Google Scholar] [CrossRef]
- Kamisawa, T.; Imai, M.; Chen, P.Y.; Tu, Y.; Egawa, N.; Tsuruta, K.; Okamoto, A.; Suzuki, M.; Kamata, N. Strategy for Differentiating Autoimmune Pancreatitis From Pancreatic Cancer. Pancreas 2008, 37, e62–e67. [Google Scholar] [CrossRef]
- Gurgitano, M.; Angileri, S.A.; Rodà, G.M.; Liguori, A.; Pandolfi, M.; Ierardi, A.M.; Wood, B.J.; Carrafiello, G. Interventional Radiology ex-machina: Impact of Artificial Intelligence on practice. Radiol. Med. 2021, 126, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Kawa, S.; Kamisawa, T.; Ikeura, T.; Itoi, T.; Ito, T.; Inui, K.; Irisawa, A.; Uchida, K.; Ohara, H.; et al. Amendment of the Japanese consensus guidelines for autoimmune pancreatitis, 2020. J. Gastroenterol. 2022, 57, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Campbell, D.H.; Walsh, B.J.; Packer, N.H.; Liu, D.; Wang, Y. Cancer-derived small extracellular vesicles: Emerging biomarkers and therapies for pancreatic ductal adenocarcinoma diagnosis/prognosis and treatment. J. Nanobiotechnol. 2022, 20, 446. [Google Scholar] [CrossRef] [PubMed]
- Ip, I.K.; Mortele, K.J.; Prevedello, L.M.; Khorasani, R. Focal cystic pancreatic lesions: Assessing variation in radiologists’ management recommendations. Radiology 2011, 259, 136–141. [Google Scholar] [CrossRef]
- Girometti, R.; Intini, S.; Brondani, G.; Como, G.; Londero, F.; Bresadola, F.; Zuiani, C.; Bazzocchi, M. Incidental pancreatic cysts on 3D turbo spin echo magnetic resonance cholangiopan-creatography: Prevalence and relation with clinical and imaging features. Abdom. Imaging 2011, 36, 196–205. [Google Scholar] [CrossRef]
- Chang, Y.R.; Park, J.K.; Jang, J.-Y.; Kwon, W.; Yoon, J.H.; Kim, S.-W. Incidental pancreatic cystic neoplasms in an asymptomatic healthy population of 21,745 individuals. Medicine 2016, 95, e5535. [Google Scholar] [CrossRef]
- de Jong, K.; Nio, C.Y.; Hermans, J.J.; Dijkgraaf, M.G.; Gouma, D.J.; Van Eijck, C.H.; van Heel, E.; Klass, G.; Fockens, P.; Bruno, M.J. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin. Gastroenterol. Hepatol. 2010, 8, 806–811. [Google Scholar] [CrossRef]
- Del Chiaro, M.; Segersvärd, R.; Lohr, M.; Verbeke, C. Early detection and prevention of pancreatic cancer: Is it really possible today? World J. Gastroenterol. 2014, 20, 12118–12131. [Google Scholar] [CrossRef]
- Jang, D.K.; Song, B.J.; Ryu, J.K.; Chung, K.H.; Lee, B.S.; Park, J.K.; Lee, S.H.; Kim, Y.T.; Lee, J.Y. Preoperative diagnosis of pancreatic cystic lesions: The accuracy of endoscopic ultrasound and cross-sectional imaging. Pancreas 2015, 44, 1329–1333. [Google Scholar] [CrossRef]
- Petralia, G.; Zugni, F.; Summers, P.E.; Colombo, A.; Pricolo, P.; Grazioli, L.; Colagrande, S.; Giovagnoni, A.; Padhani, A.R. On behalf of the Italian Working Group on Magnetic Resonance Whole-body magnetic resonance imaging (WB-MRI) for cancer screening: Recommendations for use. Radiol. Med. 2021, 126, 1434–1450. [Google Scholar] [CrossRef]
- Assadsangabi, R.; Babaei, R.; Songco, C.; Ivanovic, V.; Bobinski, M.; Chen, Y.J.; Nabavizadeh, S.A. Multimodality oncologic evaluation of superficial neck and facial lymph nodes. Radiol. Med. 2021, 126, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Kim, M.-J.; Choi, J.-Y.; Hong, H.-S.; Kim, K. Relative accuracy of CT and MRI in the differentiation of benign from malignant pancreatic cystic lesions. Clin. Radiol. 2011, 66, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Giurazza, F.; Contegiacomo, A.; Calandri, M.; Mosconi, C.; Modestino, F.; Corvino, F.; Scrofani, A.R.; Marra, P.; Coniglio, G.; Failla, G.; et al. IVC filter retrieval: A multicenter proposal of two score systems to predict application of complex technique and procedural outcome. Radiol. Med. 2021, 126, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Sainani, N.I.; Saokar, A.; Deshpande, V.; Castillo, C.F.-D.; Hahn, P.; Sahani, D.V. Comparative Performance of MDCT and MRI With MR Cholangiopancreatography in Characterizing Small Pancreatic Cysts. Am. J. Roentgenol. 2009, 193, 722–731. [Google Scholar] [CrossRef]
- Visser, B.; Muthusamy, V.; Yeh, B.; Coakley, F.; Way, L. Diagnostic evaluation of cystic pancreatic lesions. HPB 2008, 10, 63–69. [Google Scholar] [CrossRef]
- Barile, A. Some thoughts and greetings from the new Editor-in-Chief. Radiol. Med. 2021, 126, 3–4, Erratum in 2021, 126, 1377. [Google Scholar] [CrossRef]
- Song, S.J.; Lee, J.M.; Kim, Y.J.; Kim, S.H.; Lee, J.Y.; Han, J.K.; Choi, B.I. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: Comparison of multirow-detector CT and MR imaging using ROC analysis. J. Magn. Reson. Imaging 2007, 26, 86–93. [Google Scholar] [CrossRef]
- Laffan, T.A.; Horton, K.M.; Klein, A.P.; Berlanstein, B.; Siegelman, S.S.; Kawamoto, S.; Johnson, P.T.; Fishman, E.K.; Hruban, R.H. Prevalence of Unsuspected Pancreatic Cysts on MDCT. Am. J. Roentgenol. 2008, 191, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, K.S.; Fromwiller, T.E.; Daniel, R.A.; Kiely, J.M.; Nakeeb, A.; Komorowski, R.A.; Wilson, S.D.; Pitt, H.A. Cystic pancreatic neoplasms: Observe or operate. Ann. Surg. 2004, 239, 651–657. [Google Scholar] [CrossRef]
- Lee, K.S.; Sekhar, A.; Rofsky, N.M.; Pedrosa, I. Prevalence of Incidental Pancreatic Cysts in the Adult Population on MR Imaging. Am. J. Gastroenterol. 2010, 105, 2079–2084. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Mitchell, D.G.; Dohke, M.; Holland, G.A.; Parker, L. Pancreatic Cysts: Depiction on Single-Shot Fast Spin-Echo MR Images. Radiology 2002, 223, 547–553. [Google Scholar] [CrossRef]
- Sahani, D.V.; Kambadakone, A.; Macari, M.; Takahashi, N.; Chari, S.; Castillo, C.F.-D. Diagnosis and Management of Cystic Pancreatic Lesions. Am. J. Roentgenol. 2013, 200, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, V.V.; Raman, S.S.; Vuong, N.L.; Zimmerman, P.; Farrell, J.; Reber, H.; Sayre JLu, D.S.K. Pancreatic cystic lesions: Discrimination accuracy based on clinical data and high resolution CT features. J. Comput. Assist. Tomogr. 2007, 31, 860–867. [Google Scholar] [CrossRef] [PubMed]
- de Jong, K.; Nio, C.Y.; Mearadji, B.; Phoa, S.S.; Engelbrecht, M.R.; Dijkgraaf, M.G.; Bruno, M.J.; Fockens, P. Disappointing interobserver agreement among radiologists for a classifying diagnosis of pancreatic cysts using magnetic resonance imaging. Pancreas 2012, 41, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Waters, J.A.; Schmidt, C.M.; Pinchot, J.W.; White, P.B.; Cummings, O.W.; Pitt, H.A.; Sandrasegaran, K.; Akisik, F.; Howard, T.J.; Nakeeb, A.; et al. CT vs. MRCP: Optimal Classification of IPMN Type and Extent. J. Gastrointest. Surg. 2007, 12, 101–109. [Google Scholar] [CrossRef]
- Pilleul, F.; Rochette, A.; Partensky, C.; Scoazec, J.-Y.; Bernard, P.; Valette, P.-J. Preoperative evaluation of intraductal papillary mucinous tumors performed by pancreatic magnetic resonance imaging and correlated with surgical and histopathologic findings. J. Magn. Reson. Imaging 2005, 21, 237–244. [Google Scholar] [CrossRef]
- Kim, T.S.; Castillo, C.F.-D. Diagnosis and Management of Pancreatic Cystic Neoplasms. Hematol. Clin. N. Am. 2015, 29, 655–674. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Bicchierai, G.; Cozzi, D.; Grazzini, G.; Danti, G.; De Muzio, F.; Maggialetti, N.; Smorchkova, O.; D’Elia, M.; et al. Diagnostic protocols in oncology: Workup and treatment planning: Part 1: The optimitation of CT protocol. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6972–6994. [Google Scholar]
- Granata, V.; Bicchierai, G.; Fusco, R.; Cozzi, D.; Grazzini, G.; Danti, G.; De Muzio, F.; Maggialetti, N.; Smorchkova, O.; D’Elia, M.; et al. Diagnostic protocols in oncology: Workup and treatment planning. Part 2: Abbreviated MR protocol. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 6499–6528. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Belli, A.; Danti, G.; Bicci, E.; Cutolo, C.; Petrillo, A.; Izzo, F. Diffusion weighted imaging and diffusion kurtosis imaging in abdominal oncological setting: Why and when. Infect. Agents Cancer 2022, 17, 25. [Google Scholar] [CrossRef]
- Granata, V.; Grassi, R.; Fusco, R.; Setola, S.; Belli, A.; Piccirillo, M.; Pradella, S.; Giordano, M.; Cappabianca, S.; Brunese, L.; et al. Abbreviated MRI Protocol for the Assessment of Ablated Area in HCC Patients. Int. J. Environ. Res. Public Health 2021, 18, 3598. [Google Scholar] [CrossRef] [PubMed]
- Izzo, F.; Granata, V.; Grassi, R.; Fusco, R.; Palaia, R.; Delrio, P.; Carrafiello, G.; Azoulay, D.; Petrillo, A.; Curley, A.S. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist 2019, 24, e990–e1005. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Grassi, R.; Fusco, R.; Galdiero, R.; Setola, S.V.; Palaia, R.; Belli, A.; Silvestro, L.; Cozzi, D.; Brunese, L.; et al. Pancreatic cancer detection and characterization: State of the art and radiomics. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 3684–3699. [Google Scholar] [CrossRef] [PubMed]
- Macari, M.; Lee, T.; Kim, S.; Jacobs, S.; Megibow, A.J.; Hajdu, C.; Babb, J. Is Gadolinium Necessary for MRI Follow-Up Evaluation of Cystic Lesions in the Pancreas? Preliminary Results. Am. J. Roentgenol. 2009, 192, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Reinhold, C.; Chong, J.; Escal, L.; Mercier, G.; Fabre, J.M.; Guiu, B.; Molinari, N. Incidental pancreatic cysts: Natural history and diagnostic accuracy of a limited serial pancreatic cyst MRI protocol. Eur. Radiol. 2014, 24, 1020–1029. [Google Scholar] [CrossRef]
- Pedrosa, I. A 10-min MRI Protocol for Follow Up Incidental Cystic Pancreatic Lesions. In Radiological Society of North America scientific Assembly and Annual Meeting Program; Radiological Society of North America: Oak Brook, IL, USA, 2017. [Google Scholar]
- Malla, S.; Kumar, P.; Madhusudhan, K.S. Radiology of the neuroendocrine neoplasms of the gastrointestinal tract: A comprehensive review. Abdom. Imaging 2020, 46, 919–935. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Risi, C.; Ottaiano, A.; Avallone, A.; De Stefano, A.; Grimm, R.; Grassi, R.; Brunese, L.; Izzo, F.; et al. Diffusion-Weighted MRI and Diffusion Kurtosis Imaging to Detect RAS Mutation in Colorectal Liver Metastasis. Cancers 2020, 12, 2420. [Google Scholar] [CrossRef]
- Perillo, T.; Paolella, C.; Perrotta, G.; Serino, A.; Caranci, F.; Manto, A. Reversible cerebral vasoconstriction syndrome: Review of neuroimaging findings. Radiol. Med. 2022, 127, 981–990. [Google Scholar] [CrossRef]
- Petrillo, A.; Fusco, R.; Granata, V.; Filice, S.; Sansone, M.; Rega, D.; Delrio, P.; Bianco, F.; Romano, G.M.; Tatangelo, F.; et al. Assessing response to neo-adjuvant therapy in locally advanced rectal cancer using Intra-voxel Incoherent Motion modelling by DWI data and Standardized Index of Shape from DCE-MRI. Ther. Adv. Med. Oncol. 2018, 10, 1758835918809875. [Google Scholar] [CrossRef]
- De Felice, F.; Boldrini, L.; Greco, C.; Nardone, V.; Salvestrini, V.; Desideri, I. ESTRO vision 2030: The young Italian Association of Radiotherapy and Clinical Oncology (yAIRO) commitment statement. Radiol. Med. 2021, 126, 1374–1376. [Google Scholar] [CrossRef]
- Pozzi-Mucelli, R.M.; Rinta-Kiikka, I.; Wünsche, K.; Laukkarinen, J.; Labori, K.J.; Ånonsen, K.; Verbeke, C.; Del Chiaro, M.; Kartalis, N. Pancreatic MRI for the surveillance of cystic neoplasms: Comparison of a short with a comprehensive imaging protocol. Eur. Radiol. 2016, 27, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Ladd, A.M.; Diehl, D.L. Artificial intelligence for early detection of pancreatic adenocarcinoma: The future is promising. World J. Gastroenterol. 2021, 27, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Bera, K.; Braman, N.; Gupta, A.; Velcheti, V.; Madabhushi, A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat. Rev. Clin. Oncol. 2021, 19, 132–146. [Google Scholar] [CrossRef]
- Taghavi, M.; Trebeschi, S.; Simões, R.; Meek, D.B.; Beckers, R.C.J.; Lambregts, D.M.J.; Verhoef, C.; Houwers, J.B.; van der Heide, U.A.; Beets-Tan, R.G.H.; et al. Machine learning-based analysis of CT radiomics model for prediction of colorectal metachronous liver metastases. Abdom. Radiol. 2020, 46, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Brunese, M.C.; Santone, A.; Avella, P.; Bianco, P.; Scacchi, A.; Scaglione, M.; Bellifemine, F.; Danzi, R.; Varriano, G.; et al. Early Diagnosis of Liver Metastases from Colorectal Cancer through CT Radiomics and Formal Methods: A Pilot Study. J. Clin. Med. 2021, 11, 31. [Google Scholar] [CrossRef]
- Wei, J.; Cheng, J.; Gu, D.; Chai, F.; Hong, N.; Wang, Y.; Tian, J. Deep learning-based radiomics predicts response to chemotherapy in colorectal liver metastases. Med. Phys. 2020, 48, 513–522. [Google Scholar] [CrossRef]
- Saini, A.; Breen, I.; Pershad, Y.; Naidu, S.; Knuttinen, M.G.; Alzubaidi, S.; Sheth, R.; Albadawi, H.; Kuo, M.; Oklu, R. Radiogenomics and Radiomics in Liver Cancers. Diagnostics 2018, 9, 4. [Google Scholar] [CrossRef]
- Petrillo, A.; Fusco, R.; Di Bernardo, E.; Petrosino, T.; Barretta, M.L.; Porto, A.; Granata, V.; Di Bonito, M.; Fanizzi, A.; Massafra, R.; et al. Prediction of Breast Cancer Histological Outcome by Radiomics and Artificial Intelligence Analysis in Contrast-Enhanced Mammography. Cancers 2022, 14, 2132. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Belli, A.; Romano, C.; Ottaiano, A.; Nasti, G.; et al. Magnetic Resonance Features of Liver Mucinous Colorectal Metastases: What the Radiologist Should Know. J. Clin. Med. 2022, 11, 2221. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.-Y.; Yin, X.-P.; Gao, B.-L. Radiomics and Radiogenomics in Evaluation of Colorectal Cancer Liver Metastasis. Front. Oncol. 2022, 11, 5451. [Google Scholar] [CrossRef]
- Costa, G.; Cavinato, L.; Masci, C.; Fiz, F.; Sollini, M.; Politi, L.; Chiti, A.; Balzarini, L.; Aghemo, A.; di Tommaso, L.; et al. Virtual Biopsy for Diagnosis of Chemotherapy-Associated Liver Injuries and Steatohepatitis: A Combined Radiomic and Clinical Model in Patients with Colorectal Liver Metastases. Cancers 2021, 13, 3077. [Google Scholar] [CrossRef] [PubMed]
- Donato, H.; França, M.; Candelária, I.; Caseiro-Alves, F. Liver MRI: From basic protocol to advanced techniques. Eur. J. Radiol. 2017, 93, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ligero, M.; Jordi-Ollero, O.; Bernatowicz, K.; Garcia-Ruiz, A.; Delgado-Muñoz, E.; Leiva, D.; Mast, R.; Suarez, C.; Sala-Llonch, R.; Calvo, N.; et al. Minimizing acquisition-related radiomics variability by image resampling and batch effect correction to allow for large-scale data analysis. Eur. Radiol. 2020, 31, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.; Galdiero, R.; Picone, C.; Izzo, F.; D’Aniello, R.; Miele, V.; Grassi, R.; Grassi, R.; et al. Lymphadenopathy after BNT162b2 Covid-19 Vaccine: Preliminary Ultrasound Findings. Biology 2021, 10, 214. [Google Scholar] [CrossRef]
- Scapicchio, C.; Gabelloni, M.; Barucci, A.; Cioni, D.; Saba, L.; Neri, E. A deep look into radiomics. Radiol. Med. 2021, 126, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Morin, O.; Vallières, M.; Jochems, A.; Woodruff, H.C.; Valdes, G.; Braunstein, S.E.; Wildberger, J.E.; Villanueva-Meyer, J.E.; Kearney, V.; Yom, S.; et al. A Deep Look Into the Future of Quantitative Imaging in Oncology: A Statement of Working Principles and Proposal for Change. Int. J. Radiat. Oncol. 2018, 102, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Cellina, M.; Pirovano, M.; Ciocca, M.; Gibelli, D.; Floridi, C.; Oliva, G. Radiomic analysis of the optic nerve at the first episode of acute optic neuritis: An indicator of optic nerve pathology and a predictor of visual recovery? Radiol. Med. 2021, 126, 698–706. [Google Scholar] [CrossRef]
- Santone, A.; Brunese, M.C.; Donnarumma, F.; Guerriero, P.; Mercaldo, F.; Reginelli, A.; Miele, V.; Giovagnoni, A.; Brunese, L. Radiomic features for prostate cancer grade detection through formal verification. Radiol. Med. 2021, 126, 688–697. [Google Scholar] [CrossRef]
- Agazzi, G.M.; Ravanelli, M.; Roca, E.; Medicina, D.; Balzarini, P.; Pessina, C.; Vermi, W.; Berruti, A.; Maroldi, R.; Farina, D. CT texture analysis for prediction of EGFR mutational status and ALK rearrangement in patients with non-small cell lung cancer. Radiol. Med. 2021, 126, 786–794. [Google Scholar] [CrossRef]
- Benedetti, G.; Mori, M.; Panzeri, M.M.; Barbera, M.; Palumbo, D.; Sini, C.; Muffatti, F.; Andreasi, V.; Steidler, S.; Doglioni, C.; et al. CT-derived radiomic features to discriminate histologic characteristics of pancreatic neuroendocrine tumors. Radiol. Med. 2021, 126, 745–760. [Google Scholar] [CrossRef]
- Calloni, S.F.; Panni, P.; Calabrese, F.; del Poggio, A.; Roveri, L.; Squarza, S.; Pero, G.C.; Paolucci, A.; Filippi, M.; Falini, A.; et al. Cerebral hyperdensity on CT imaging (CTHD) post-reperfusion treatment in patients with acute cerebral stroke: Understanding its clinical meaning. Radiol. Med. 2022, 127, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Avallone, A.; De Stefano, A.; Ottaiano, A.; Sbordone, C.; Brunese, L.; Izzo, F.; Petrillo, A. Radiomics-Derived Data by Contrast Enhanced Magnetic Resonance in RAS Mutations Detection in Colorectal Liver Metastases. Cancers 2021, 13, 453. [Google Scholar] [CrossRef] [PubMed]
- Halefoglu, A.M.; Ozagari, A.A. Tumor grade estimation of clear cell and papillary renal cell carcinomas using contrast-enhanced MDCT and FSE T2 weighted MR imaging: Radiology-pathology correlation. Radiol. Med. 2021, 126, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; Setola, S.V.; Simonetti, I.; Cozzi, D.; Grazzini, G.; Grassi, F.; Belli, A.; Miele, V.; Izzo, F.; et al. An update on radiomics techniques in primary liver cancers. Infect. Agents Cancer 2022, 17, 6. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, C.; Traverso, A.; Zhovannik, I.; Dekker, A.; Wee, L.; Bermejo, I. Generative models improve radiomics reproducibility in low dose CTs: A simulation study. Phys. Med. Biol. 2021, 66, 165002. [Google Scholar] [CrossRef]
- Arrigoni, F.; Mazzoleni, M.G.; Calvisi, V.; Masciocchi, C. In-Office Needle Arthroscopy (IONA): May a traditionally orthopedic procedure enter the portfolio of interventional radiology? Radiol. Med. 2022, 127, 784–787. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Sansone, M.; Grassi, R.; Maio, F.; Palaia, R.; Tatangelo, F.; Botti, G.; Grimm, R.; Curley, S.; et al. Magnetic resonance imaging in the assessment of pancreatic cancer with quantitative parameter extraction by means of dynamic contrast-enhanced magnetic resonance imaging, diffusion kurtosis imaging and intravoxel incoherent motion diffusion-weighted imaging. Ther. Adv. Gastroenterol. 2020, 13, 1756284819885052. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; Picone, C.; Vallone, P.; Belli, A.; Incollingo, P.; Albino, V.; Tatangelo, F.; Izzo, F.; et al. Microvascular invasion and grading in hepatocellular carcinoma: Correlation with major and ancillary features according to LIRADS. Abdom. Radiol. 2019, 44, 2788–2800. [Google Scholar] [CrossRef]
- Granata, V.; Palaia, R.; Albino, V.; Piccirillo, M.; Setola, S.V.; Petrillo, A.; Izzo, F. Electrochemotherapy of cholangiocellular carcinoma at hepatic hilum: A case report. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7051–7057. [Google Scholar]
- Grassi, R.; Cappabianca, S.; Urraro, F.; Feragalli, B.; Montanelli, A.; Patelli, G.; Granata, V.; Giacobbe, G.; Russo, G.; Grillo, A.; et al. Chest CT Computerized Aided Quantification of PNEUMONIA Lesions in COVID-19 Infection: A Comparison among Three Commercial Software. Int. J. Environ. Res. Public Health 2020, 17, 6914. [Google Scholar] [CrossRef]
- Fusco, R.; Grassi, R.; Granata, V.; Setola, S.V.; Grassi, F.; Cozzi, D.; Pecori, B.; Izzo, F.; Petrillo, A. Artificial Intelligence and COVID-19 Using Chest CT Scan and Chest X-ray Images: Machine Learning and Deep Learning Approaches for Diagnosis and Treatment. J. Pers. Med. 2021, 11, 993. [Google Scholar] [CrossRef]
- Özel, M.; Aslan, A.; Araç, S. Use of the COVID-19 Reporting and Data System (CO-RADS) classification and chest computed tomography involvement score (CT-IS) in COVID-19 pneumonia. Radiol. Med. 2021, 126, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, D.; Giandola, T.; Maino, C.; Pecorelli, A.; Capodaglio, C.; Ragusi, M.; Porta, M.; Gandola, D.; Masetto, A.; Drago, S.; et al. Acute pulmonary embolism in hospitalized patients with SARS-CoV-2-related pneumonia: Multicentric experience from Italian endemic area. Radiol. Med. 2021, 126, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Moroni, C.; Cozzi, D.; Albanesi, M.; Cavigli, E.; Bindi, A.; Luvarà, S.; Busoni, S.; Mazzoni, L.N.; Grifoni, S.; Nazerian, P.; et al. Chest X-ray in the emergency department during COVID-19 pandemic descending phase in Italy: Correlation with patients’ outcome. Radiol. Med. 2021, 126, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Cereser, L.; Girometti, R.; Da Re, J.; Marchesini, F.; Como, G.; Zuiani, C. Inter-reader agreement of high-resolution computed tomography findings in patients with COVID-19 pneumonia: A multi-reader study. Radiol. Med. 2021, 126, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Rawashdeh, M.A.; Saade, C. Radiation dose reduction considerations and imaging patterns of ground glass opacities in coronavirus: Risk of over exposure in computed tomography. Radiol. Med. 2020, 126, 380–387. [Google Scholar] [CrossRef]
- Granata, V.; Ianniello, S.; Fusco, R.; Urraro, F.; Pupo, D.; Magliocchetti, S.; Albarello, F.; Campioni, P.; Cristofaro, M.; Di Stefano, F.; et al. Quantitative Analysis of Residual COVID-19 Lung CT Features: Consistency among Two Commercial Software. J. Pers. Med. 2021, 11, 1103. [Google Scholar] [CrossRef]
- Fusco, R.; Granata, V.; Petrillo, A. Introduction to Special Issue of Radiology and Imaging of Cancer. Cancers 2020, 12, 2665. [Google Scholar] [CrossRef]
- Fusco, R.; Sansone, M.; Filice, S.; Granata, V.; Catalano, O.; Amato, D.M.; Di Bonito, M.; D’Aiuto, M.; Capasso, I.; Rinaldo, M.; et al. Integration of DCE-MRI and DW-MRI Quantitative Parameters for Breast Lesion Classification. BioMed Res. Int. 2015, 2015, 237863. [Google Scholar] [CrossRef]
- Nakamoto, T.; Haga, A.; Takahashi, W. An Introduction to Radiomics: Toward a New Era of Precision Medicine. Igaku Butsuri. 2018, 38, 129–134. (In Japanese) [Google Scholar] [CrossRef] [PubMed]
- Vuong, D.; Tanadini-Lang, S.; Wu, Z.; Marks, R.; Unkelbach, J.; Hillinger, S.; Eboulet, E.I.; Thierstein, S.; Peters, S.; Pless, M.; et al. Radiomics Feature Activation Maps as a New Tool for Signature Interpretability. Front. Oncol. 2020, 10, 578895. [Google Scholar] [CrossRef] [PubMed]
- Yip, S.S.F.; Aerts, H.J.W.L. Applications and limitations of radiomics. Phys. Med. Biol. 2016, 61, R150–R166. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Simonetti, I.; Dell’Aversana, F.; Grassi, F.; Bruno, F.; Belli, A.; et al. Complications Risk Assessment and Imaging Findings of Thermal Ablation Treatment in Liver Cancers: What the Radiologist Should Expect. J. Clin. Med. 2022, 11, 2766. [Google Scholar] [CrossRef]
- Wilson, R.; Devaraj, A. Radiomics of pulmonary nodules and lung cancer. Transl. Lung Cancer Res. 2017, 6, 86–91. [Google Scholar] [CrossRef]
- Binczyk, F.; Prazuch, W.; Bozek, P.; Polanska, J. Radiomics and artificial intelligence in lung cancer screening. Transl. Lung Cancer Res. 2021, 10, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Beig, N.; Bera, K.; Tiwari, P. Introduction to radiomics and radiogenomics in neuro-oncology: Implications and challenges. Neuro-Oncol. Adv. 2020, 2, iv3–iv14. [Google Scholar] [CrossRef] [PubMed]
- Barile, A.; Lanni, G.; Conti, L.; Mariani, S.; Calvisi, V.; Castagna, A.; Rossi, F.; Masciocchi, C. Lesions of the biceps pulley as cause of anterosuperior impingement of the shoulder in the athlete: Potentials and limits of MR arthrography compared with arthroscopy. Radiol. Med. 2012, 118, 112–122. [Google Scholar] [CrossRef]
- Masciocchi, C.; Lanni, G.; Conti, L.; Conchiglia, A.; Fascetti, E.; Flamini, S.; Coletti, G.; Barile, A. Soft-tissue inflammatory myofibroblastic tumors (IMTs) of the limbs: Potential and limits of diagnostic imaging. Skelet. Radiol. 2011, 41, 643–649. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, L.; Liu, S.; You, J.; Chen, L.; Jin, Z.; Zhang, S.; Zhang, B. Radiomics in precision medicine for gastric cancer: Opportunities and challenges. Eur. Radiol. 2022, 32, 5852–5868. [Google Scholar] [CrossRef]
- Shi, Z.; Traverso, A.; van Soest, J.; Dekker, A.; Wee, L. Technical Note: Ontology-guided radiomics analysis workflow (O-RAW). Med. Phys. 2019, 46, 5677–5684. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; Setola, S.V.; De Muzio, F.; Aversana, F.D.; Cutolo, C.; Faggioni, L.; Miele, V.; Izzo, F.; Petrillo, A. CT-Based Radiomics Analysis to Predict Histopathological Outcomes Following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1648. [Google Scholar] [CrossRef]
- Neri, E.; Granata, V.; Montemezzi, S.; Belli, P.; Bernardi, D.; Brancato, B.; Caumo, F.; Calabrese, M.; Coppola, F.; Cossu, E.; et al. Structured reporting of x-ray mammography in the first diagnosis of breast cancer: A Delphi consensus proposal. Radiol. Med. 2022, 127, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, H.; Gao, J.; Li, J.; Li, M.; Zhou, Z.; Peng, Y. Performance evaluation of a deep learning image reconstruction (DLIR) algorithm in “double low” chest CTA in children: A feasibility study. Radiol. Med. 2021, 126, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Faggioni, L.; Grassi, R.; Fusco, R.; Reginelli, A.; Rega, D.; Maggialetti, N.; Buccicardi, D.; Frittoli, B.; Rengo, M.; et al. Structured reporting of computed tomography in the staging of colon cancer: A Delphi consensus proposal. Radiol. Med. 2021, 127, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Dell’Aversana, F.; Ottaiano, A.; Nasti, G.; Grassi, R.; Pilone, V.; et al. EOB-MR Based Radiomics Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases. Cancers 2022, 14, 1239. [Google Scholar] [CrossRef]
- Fushimi, Y.; Yoshida, K.; Okawa, M.; Maki, T.; Nakajima, S.; Sakata, A.; Okuchi, S.; Hinoda, T.; Kanagaki, M.; Nakamoto, Y. Vessel wall MR imaging in neuroradiology. Radiol. Med. 2022, 127, 1032–1045. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Guo, W.; Zeng, P.; Liu, Y.; Lang, N.; Yuan, H. A preliminary study using spinal MRI-based radiomics to predict high-risk cytogenetic abnormalities in multiple myeloma. Radiol. Med. 2021, 126, 1226–1235. [Google Scholar] [CrossRef]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Setola, S.V.; Aversana, F.D.; Ottaiano, A.; Avallone, A.; Nasti, G.; Grassi, F.; et al. Contrast MR-Based Radiomics and Machine Learning Analysis to Assess Clinical Outcomes following Liver Resection in Colorectal Liver Metastases: A Preliminary Study. Cancers 2022, 14, 1110. [Google Scholar] [CrossRef] [PubMed]
- Granata, V.; Fusco, R.; De Muzio, F.; Cutolo, C.; Raso, M.M.; Gabelloni, M.; Avallone, A.; Ottaiano, A.; Tatangelo, F.; Brunese, M.C.; et al. Radiomics and Machine Learning Analysis Based on Magnetic Resonance Imaging in the Assessment of Colorectal Liver Metastases Growth Pattern. Diagnostics 2022, 12, 1115. [Google Scholar] [CrossRef]
- Chianca, V.; Albano, D.; Messina, C.; Vincenzo, G.; Rizzo, S.; Del Grande, F.; Sconfienza, L.M. An update in musculoskeletal tumors: From quantitative imaging to radiomics. Radiol. Med. 2021, 126, 1095–1105. [Google Scholar] [CrossRef]
- Qin, H.; Que, Q.; Lin, P.; Li, X.; Wang, X.-R.; He, Y.; Chen, J.-Q.; Yang, H. Magnetic resonance imaging (MRI) radiomics of papillary thyroid cancer (PTC): A comparison of predictive performance of multiple classifiers modeling to identify cervical lymph node metastases before surgery. Radiol. Med. 2021, 126, 1312–1327. [Google Scholar] [CrossRef]
- Fusco, R.; Di Bernardo, E.; Piccirillo, A.; Rubulotta, M.R.; Petrosino, T.; Barretta, M.L.; Raso, M.M.; Vallone, P.; Raiano, C.; Di Giacomo, R.; et al. Radiomic and Artificial Intelligence Analysis with Textural Metrics Extracted by Contrast-Enhanced Mammography and Dynamic Contrast Magnetic Resonance Imaging to Detect Breast Malignant Lesions. Curr. Oncol. 2022, 29, 1947–1966. [Google Scholar] [CrossRef] [PubMed]
- Brunese, L.; Brunese, M.C.; Carbone, M.; Ciccone, V.; Mercaldo, F.; Santone, A. Automatic PI-RADS assignment by means of formal methods. Radiol. Med. 2021, 127, 83–89. [Google Scholar] [CrossRef]
- Bellardita, L.; Colciago, R.R.; Frasca, S.; De Santis, M.C.; Gay, S.; Palorini, F.; La Rocca, E.; Valdagni, R.; Rancati, T.; Lozza, L. Breast cancer patient perspective on opportunities and challenges of a genetic test aimed to predict radio-induced side effects before treatment: Analysis of the Italian branch of the REQUITE project. Radiol. Med. 2021, 126, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Pucciarelli, F.; Zerunian, M.; Ganeshan, B.; De Santis, D.; Polici, M.; Rucci, C.; Polidori, T.; Guido, G.; Bracci, B.; et al. Chest CT texture-based radiomics analysis in differentiating COVID-19 from other interstitial pneumonia. Radiol. Med. 2021, 126, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, S.; Scaggiante, J.; Schuldt, B.R.; Smith, C.J.; Chennareddy, S.; Kalagara, R.; Majidi, S.; Bederson, J.B.; Fifi, J.T.; Mocco, J.; et al. Accuracy of artificial intelligence for the detection of intracranial hemorrhage and chronic cerebral microbleeds: A systematic review and pooled analysis. Radiol. Med. 2022, 127, 1106–1123. [Google Scholar] [CrossRef] [PubMed]
- Karmazanovsky, G.; Gruzdev, I.; Tikhonova, V.; Kondratyev, E.; Revishvili, A. Computed tomography-based radiomics approach in pancreatic tumors characterization. Radiol. Med. 2021, 126, 1388–1395. [Google Scholar] [CrossRef]
- Satake, H.; Ishigaki, S.; Ito, R.; Naganawa, S. Radiomics in breast MRI: Current progress toward clinical application in the era of artificial intelligence. Radiol. Med. 2021, 127, 39–56. [Google Scholar] [CrossRef]
- Gregucci, F.; Fiorentino, A.; Mazzola, R.; Ricchetti, F.; Bonaparte, I.; Surgo, A.; Figlia, V.; Carbonara, R.; Caliandro, M.; Ciliberti, M.P.; et al. Radiomic analysis to predict local response in locally advanced pancreatic cancer treated with stereotactic body radiation therapy. Radiol. Med. 2021, 127, 100–107. [Google Scholar] [CrossRef]
- Ji, G.W.; Wang, K.; Xia, Y.X.; Li, X.C.; Wang, X.H. Application and challenge of radiomics technique in the era of precision medicine for hepatobiliary disease. Zhonghua Wai Ke Za Zhi. 2020, 58, 749–753. (In Chinese) [Google Scholar] [CrossRef]
- Wu, J.; Tha, K.; Xing, L.; Li, R. Radiomics and radiogenomics for precision radiotherapy. J. Radiat. Res. 2018, 59 (Suppl. 1), i25–i31. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Orlhac, F.; Nioche, C.; Klyuzhin, I.; Rahmim, A.; Buvat, I. Radiomics in PET Imaging:: A Practical Guide for Newcomers. PET Clin. 2021, 16, 597–612. [Google Scholar] [CrossRef]
- Avanzo, M.; Stancanello, J.; El Naqa, I. Beyond imaging: The promise of radiomics. Phys. Med. 2017, 38, 122–139. [Google Scholar] [CrossRef]
- Da-Ano, R.; Visvikis, D.; Hatt, M. Harmonization strategies for multicenter radiomics investigations. Phys. Med. Biol. 2020, 65, 24TR02. [Google Scholar] [CrossRef]
- Bogowicz, M.; Vuong, D.; Huellner, M.W.; Pavic, M.; Andratschke, N.; Gabrys, H.S.; Guckenberger, M.; Tanadini-Lang, S. CT radiomics and PET radiomics: Ready for clinical implementation? Q. J. Nucl. Med. Mol. Imaging 2019, 63, 355–370. [Google Scholar] [CrossRef]
- Arimura, H.; Soufi, M.; Kamezawa, H.; Ninomiya, K.; Yamada, M. Radiomics with artificial intelligence for precision medicine in radiation therapy. J. Radiat. Res. 2018, 60, 150–157. [Google Scholar] [CrossRef]
- Lambin, P.; Rios-Velazquez, E.; Leijenaar, R.; Carvalho, S.; van Stiphout, R.G.P.M.; Granton, P.; Zegers, C.M.L.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef]
- Gebauer, L.; Moltz, J.H.; Mühlberg, A.; Holch, J.W.; Huber, T.; Enke, J.; Jäger, N.; Haas, M.; Kruger, S.; Boeck, S.; et al. Quantitative Imaging Biomarkers of the Whole Liver Tumor Burden Improve Survival Prediction in Metastatic Pancreatic Cancer. Cancers 2021, 13, 5732. [Google Scholar] [CrossRef]
- Rompianesi, G.; Pegoraro, F.; Ceresa, C.D.; Montalti, R.; Troisi, R.I. Artificial intelligence in the diagnosis and management of colorectal cancer liver metastases. World J. Gastroenterol. 2022, 28, 108–122. [Google Scholar] [CrossRef]
- Euler, A.; Laqua, F.C.; Cester, D.; Lohaus, N.; Sartoretti, T.; dos Santos, D.P.; Alkadhi, H.; Baessler, B. Virtual Monoenergetic Images of Dual-Energy CT—Impact on Repeatability, Reproducibility, and Classification in Radiomics. Cancers 2021, 13, 4710. [Google Scholar] [CrossRef]
- Kelahan, L.C.; Kim, D.; Soliman, M.; Avery, R.J.; Savas, H.; Agrawal, R.; Magnetta, M.; Liu, B.P.; Velichko, Y.S. Role of hepatic metastatic lesion size on inter-reader reproducibility of CT-based radiomics features. Eur. Radiol. 2022, 32, 4025–4033. [Google Scholar] [CrossRef]
- Bracco, S.; Zanoni, M.; Casseri, T.; Castellano, D.; Cioni, S.; Vallone, I.M.; Gennari, P.; Mazzei, M.A.; Romano, D.G.; Piano, M.; et al. Endovascular treatment of acute ischemic stroke due to tandem lesions of the anterior cerebral circulation: A multicentric Italian observational study. Radiol. Med. 2021, 126, 804–817. [Google Scholar] [CrossRef]
- Michallek, F.; Genske, U.; Niehues, S.M.; Hamm, B.; Jahnke, P. Deep learning reconstruction improves radiomics feature stability and discriminative power in abdominal CT imaging: A phantom study. Eur. Radiol. 2022, 32, 4587–4595. [Google Scholar] [CrossRef]
- Fusco, R.; Sansone, M.; Granata, V.; Setola, S.V.; Petrillo, A.; Fusco, R.; Sansone, M.; Granata, V.; Setola, S.V.; Petrillo, A. A systematic review on multiparametric MR imaging in prostate cancer detection. Infect. Agents Cancer 2017, 12, 57. [Google Scholar] [CrossRef]
- Cappabianca, S.; Granata, V.; Di Grezia, G.; Mandato, Y.; Reginelli, A.; Di Mizio, V.; Grassi, R.; Rotondo, A. The role of nasoenteric intubation in the MR study of patients with Crohn’s disease: Our experience and literature review. Radiol. Med. 2010, 116, 389–406. [Google Scholar] [CrossRef]
- De Filippo, M.; Puglisi, S.; D’Amuri, F.; Gentili, F.; Paladini, I.; Carrafiello, G.; Maestroni, U.; Del Rio, P.; Ziglioli, F.; Pagnini, F. CT-guided percutaneous drainage of abdominopelvic collections: A pictorial essay. Radiol. Med. 2021, 126, 1561–1570. [Google Scholar] [CrossRef]
- Pecoraro, M.; Cipollari, S.; Marchitelli, L.; Messina, E.; Del Monte, M.; Galea, N.; Ciardi, M.R.; Francone, M.; Catalano, C.; Panebianco, V. Cross-sectional analysis of follow-up chest MRI and chest CT scans in patients previously affected by COVID-19. Radiol. Med. 2021, 126, 1273–1281. [Google Scholar] [CrossRef]
- Gabelloni, M.; Faggioni, L.; Cioni, D.; Mendola, V.; Falaschi, Z.; Coppola, S.; Corradi, F.; Isirdi, A.; Brandi, N.; Coppola, F.; et al. Extracorporeal membrane oxygenation (ECMO) in COVID-19 patients: A pocket guide for radiologists. Radiol. Med. 2022, 13, 369–382. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Materka, A.; Langs, G.; Häggström, I.; Szczypiński, P.; Gibbs, P.; Cook, G. Introduction to Radiomics. J. Nucl. Med. 2020, 61, 488–495. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, L.; Wang, Y.; Wang, J.; Zhang, H.; Xia, F.; Wan, J.; Zhang, Z. MRI Radiomics Signature as a Potential Biomarker for Predicting KRAS Status in Locally Advanced Rectal Cancer Patients. Front. Oncol. 2021, 11, 614052. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dong, D.; Fang, M.; Zhu, Y.; Zang, Y.; Liu, Z.; Zhang, H.; Ying, J.; Zhao, X.; Tian, J. Can CT-based radiomics signature predict KRAS/NRAS/BRAF mutations in colorectal cancer? Eur. Radiol. 2018, 28, 2058–2067. [Google Scholar] [CrossRef]
- Wen, Y.L.; Leech, M. Review of the Role of Radiomics in Tumour Risk Classification and Prognosis of Cancer. Anticancer. Res. 2020, 40, 3605–3618. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Borgheresi, A.; Carotti, M.; Ottaviani, L.; Badaloni, M.; Floridi, C.; Giovagnoni, A. Third-generation iterative reconstruction on a dual-source, high-pitch, low-dose chest CT protocol with tin filter for spectral shaping at 100 kV: A study on a small series of COVID-19 patients. Radiol. Med. 2020, 126, 388–398. [Google Scholar] [CrossRef]
- Palmisano, A.; Scotti, G.M.; Ippolito, D.; Morelli, M.J.; Vignale, D.; Gandola, D.; Sironi, S.; De Cobelli, F.; Ferrante, L.; Spessot, M.; et al. Chest CT in the emergency department for suspected COVID-19 pneumonia. Radiol. Med. 2020, 126, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.F.; Afsahi, A.M.; Gupta, A.; Gholamrezanezhad, A. Severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), influenza, and COVID-19, beyond the lungs: A review article. Radiol. Med. 2021, 126, 561–569. [Google Scholar] [CrossRef]
- Golia Pernicka, J.S.; Gagniere, J.; Chakraborty, J.; Yamashita, R.; Nardo, L.; Creasy, J.M.; Petkovska, I.; Do, R.R.K.; Bates, D.D.B.; Gollub, M.J.; et al. Radiomics-Based Prediction of Mi-crosatellite Instability in Colorectal Cancer at Initial Computed Tomography Evaluation. Abdom. Radiol 2019, 44, 3755–3763. [Google Scholar] [CrossRef]
- Wu, J.; Lv, Y.; Wang, N.; Zhao, Y.; Zhang, P.; Liu, Y.; Chen, A.; Li, J.; Li, X.; Guo, Y.; et al. The value of single-source dual-energy CT imaging for discriminating microsatellite instability from microsatellite stability human colorectal cancer. Eur. Radiol. 2019, 29, 3782–3790. [Google Scholar] [CrossRef]
- Sun, R.; Limkin, E.J.; Vakalopoulou, M.; Dercle, L.; Champiat, S.; Han, S.R.; Verlingue, L.; Brandao, D.; Lancia, A.; Ammari, S.; et al. A radiomics approach to assess tumour-infiltrating CD8 cells and response to an-ti-PD-1 or anti-PD-L1 immunotherapy: An imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018, 19, 1180–1191. [Google Scholar] [CrossRef]
- Tunali, I.; Tan, Y.; Gray, J.E.; Katsoulakis, E.; Eschrich, S.A.; Saller, J.; Aerts, H.J.W.L.; Boyle, T.; Qi, J.; Guvenis, A.; et al. Hypoxia-Related Radiomics and Immunotherapy Response: A Multicohort Study of Non-Small Cell Lung Cancer. JNCI Cancer Spectr. 2021, 5, pkab048. [Google Scholar] [CrossRef]
- Zanfardino, M.; Franzese, M.; Pane, K.; Cavaliere, C.; Monti, S.; Esposito, G.; Salvatore, M.; Aiello, M. Bringing radiomics into a multi-omics framework for a comprehensive genotype–phenotype characterization of oncological diseases. J. Transl. Med. 2019, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Lafata, K.J.; Wang, Y.; Konkel, B.; Yin, F.-F.; Bashir, M.R. Radiomics: A primer on high-throughput image phenotyping. Abdom. Imaging 2021, 47, 2986–3002. [Google Scholar] [CrossRef]
- Lenga, L.; Bernatz, S.; Martin, S.; Booz, C.; Solbach, C.; Mulert-Ernst, R.; Vogl, T.; Leithner, D. Iodine Map Radiomics in Breast Cancer: Prediction of Metastatic Status. Cancers 2021, 13, 2431. [Google Scholar] [CrossRef] [PubMed]
- Frix, A.-N.; Cousin, F.; Refaee, T.; Bottari, F.; Vaidyanathan, A.; Desir, C.; Vos, W.; Walsh, S.; Occhipinti, M.; Lovinfosse, P.; et al. Radiomics in Lung Diseases Imaging: State-of-the-Art for Clinicians. J. Pers. Med. 2021, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Fusco, R.; Granata, V.; Grazzini, G.; Pradella, S.; Borgheresi, A.; Bruno, A.; Palumbo, P.; Bruno, F.; Grassi, R.; Giovagnoni, A.; et al. Radiomics in medical imaging: Pitfalls and challenges in clinical management. JPN. J. Radiol. 2022, 40, 919–929. [Google Scholar] [CrossRef]
- Li, Y.; Eresen, A.; Lu, Y.; Yang, J.; Shangguan JVelichko, Y.; Yaghmai, V.; Zhang, Z. Radiomics signature for the preoperative assessment of stage in advanced colon cancer. Am. J. Cancer Res. 2019, 9, 1429–1438. [Google Scholar]
- Gang, G.J.; Deshpande, R.; Stayman, J.W. Standardization of histogram- and gray-level co-occurrence matrices-based radiomics in the presence of blur and noise. Phys. Med. Biol. 2021, 66, 074004. [Google Scholar] [CrossRef]
- Muhammad, W.; Hart, G.R.; Nartowt, B.; Farrell, J.J.; Johung, K.; Liang, Y.; Deng, J. Pancreatic Cancer Prediction Through an Artificial Neural Network. Front. Artif. Intell. 2019, 2, 2. [Google Scholar] [CrossRef]
- Hsieh, M.H.; Sun, L.-M.; Lin, C.-L.; Hsieh, M.J.; Hsu, C.Y.; Kao, C.H. Development of a prediction model for pancreatic cancer in patients with type 2 diabetes using logistic regression and artificial neural network models. Cancer Manag. Res. 2018, 10, 6317–6324. [Google Scholar] [CrossRef]
- Norton, I.D.; Zheng, Y.; Wiersema, M.S.; Greenleaf, J.; Clain, J.E.; DiMagno, E.P. Neural network analysis of EUS images to differentiate between pancreatic malignancy and pancreatitis. Gastrointest. Endosc. 2001, 54, 625–629. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, C.; Yu, J.; Wu, Y.; Li, C.; Zhang, M.; Jin, Z.; Li, Z. Differentiation of Pancreatic Cancer and Chronic Pancreatitis Using Computer-Aided Diagnosis of Endoscopic Ultrasound (EUS) Images: A Diagnostic Test. PLoS ONE 2013, 8, e63820. [Google Scholar] [CrossRef] [PubMed]
- Corral, J.E.; Hussein, S.; Kandel, P.; Bolan, C.W.; Bagci, U.; Wallace, M.B. Deep Learning to Classify Intraductal Papillary Mucinous Neoplasms Using Magnetic Resonance Imaging. Pancreas 2019, 48, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Hussein, S.; Kandel, P.; Bolan, C.W.; Wallace, M.B.; Bagci, U. Lung and Pancreatic Tumor Characterization in the Deep Learning Era: Novel Supervised and Unsupervised Learning Approaches. IEEE Trans. Med. Imaging 2019, 38, 1777–1787. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.C.; Park, S.; Kawamoto, S.; Wang, Y.; Zhou, Y.; Shen, W.; Zhu, Z.; Xia, Y.; Xie, L.; Liu, F.; et al. Application of Deep Learning to Pancreatic Cancer Detection: Lessons Learned From Our Initial Experience. J. Am. Coll. Radiol. 2019, 16 Pt B, 1338–1342. [Google Scholar] [CrossRef]
- Young, M.R.; Abrams, N.; Ghosh, S.; Rinaudo, J.A.S.; Marquez, G.; Srivastava, S. Prediagnostic Image Data, Artificial Intelligence, and Pancreatic Cancer. Pancreas 2020, 49, 882–886. [Google Scholar] [CrossRef]
- Canto, M.I.; Harinck, F.; Hruban, R.H.; Offerhaus, G.J.; Poley, J.-W.; Kamel, I.; Nio, Y.; Schulick, R.S.; Bassi, C.; Kluijt, I.; et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2012, 62, 339–347. [Google Scholar] [CrossRef]
- Canto, M.I.; Almario, J.A.; Schulick, R.D.; Yeo, C.J.; Klein, A.; Blackford, A.; Shin, E.J.; Sanyal, A.; Yenokyan, G.; Lennon, A.M.; et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018, 155, 740–751.e2. [Google Scholar] [CrossRef]
- Perrone, F.; Gallo, C.; Daniele, B.; Gaeta, G.B.; Izzo, F.; Capuano, G.; Adinolfi, L.E.; Mazzanti, R.; Farinati, F.; Elba, S.; et al. Tamoxifen in the treatment of Hepatocellular Carcinoma: 5-Year Results of the CLIP-1 Multicentre Randomized Controlled Trial. Curr. Pharm. Des. 2002, 8, 1013–1019. [Google Scholar] [CrossRef]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early detection of pancreatic cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef]
- Gorris, M.; Hoogenboom, S.A.; Wallace, M.B.; van Hooft, J.E. Artificial intelligence for the management of pancreatic diseases. Dig Endosc. 2021, 33, 231–241. [Google Scholar] [CrossRef]
- Abunahel, B.M.; Pontre, B.; Kumar, H.; Petrov, M.S. Pancreas image mining: A systematic review of radiomics. Eur. Radiol. 2020, 31, 3447–3467. [Google Scholar] [CrossRef] [PubMed]
- Virarkar, M.; Wong, V.K.; Morani, A.C.; Tamm, E.P.; Bhosale, P. Update on quantitative radiomics of pancreatic tumors. Abdom. Radiol. 2021, 47, 3118–3160. [Google Scholar] [CrossRef]
- Dalal, V.; Carmicheal, J.; Dhaliwal, A.; Jain, M.; Kaur, S.; Batra, S.K. Radiomics in stratification of pancreatic cystic lesions: Machine learning in action. Cancer Lett. 2019, 469, 228–237. [Google Scholar] [CrossRef]
- Machicado, J.D.; Koay, E.J.; Krishna, S.G. Radiomics for the Diagnosis and Differentiation of Pancreatic Cystic Lesions. Diagnostics 2020, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Lin, K.; Yan, W.; Guo, Y.; Wang, Y.; Li, J.; Zhu, J. Computer-Aided Diagnosis of Pancreas Serous Cystic Neoplasms: A Radiomics Method on Preoperative MDCT Images. Technol. Cancer Res. Treat. 2019, 18, 1533033818824339. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, X.; Ou, X.; Zhang, W.; Ma, X. Discrimination of Pancreatic Serous Cystadenomas From Mucinous Cystadenomas With CT Textural Features: Based on Machine Learning. Front. Oncol. 2019, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Awe, A.M.; Vanden Heuvel, M.M.; Yuan, T.; Rendell, V.R.; Shen, M.; Kampani, A.; Liang, S.; Morgan, D.D.; Winslow ERLubner, M.G. Machine learning principles applied to CT radiomics to predict mucinous pancreatic cysts. Abdom. Radiol. 2021, 47, 221–231. [Google Scholar] [CrossRef]
- Xie, H.; Ma, S.; Guo, X.; Zhang, X.; Wang, X. Preoperative differentiation of pancreatic mucinous cystic neoplasm from macrocystic serous cystic adenoma using radiomics: Preliminary findings and comparison with radiological model. Eur. J. Radiol. 2019, 122, 108747. [Google Scholar] [CrossRef]
- Polk, S.L.; Choi, J.W.; Mcgettigan, M.J.; Rose, T.; Ahmed, A.; Kim, J.; Jiang, K.; Balagurunathan, Y.; Qi, J.; Farah, P.T.; et al. Multiphase computed tomography radiomics of pancreatic intraductal papillary mucinous neoplasms to predict malignancy. World J. Gastroenterol. 2020, 26, 3458–3471. [Google Scholar] [CrossRef]
- Han, X.; Yang, J.; Luo, J.; Chen, P.; Zhang, Z.; Alu, A.; Xiao, Y.; Ma, X. Application of CT-Based Radiomics in Discriminating Pancreatic Cystadenomas From Pancreatic Neuroendocrine Tumors Using Machine Learning Methods. Front. Oncol. 2021, 11, 606677. [Google Scholar] [CrossRef]
- Xie, T.; Wang, X.; Zhang, Z.; Zhou, Z. CT-Based Radiomics Analysis for Preoperative Diagnosis of Pancreatic Mucinous Cystic Neoplasm and Atypical Serous Cystadenomas. Front. Oncol. 2021, 11, 621520. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Yang, F.; Yang, P.; Yang, M.; Xu, L.; Zhuo, J.; Xu, X. A Contrast-Enhanced Computed Tomography Based Radiomics Approach for Preoperative Differentiation of Pancreatic Cystic Neoplasm Subtypes: A Feasibility Study. Front. Oncol. 2020, 10, 248. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, T.A.; Gaddam, S.; Wachsman, A.M.; Wang, L.; Azab, L.; Asadpour, V.; Chen, W.; Xie, Y.; Wu, B.; Pandol, S.J.; et al. Predicting pancreatic ductal adenocarcinoma using artificial intelligence analysis of pre-diagnostic computed tomography images. Cancer Biomark. 2022, 33, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Qureshi, T.A.; Gaddam, S.; Wang, L.; Azab, L.; Wachsman, A.M.; Chen, W.; Asadpour, V.; Jeon, C.Y.; Wu, B.; et al. Risk prediction of pancreatic cancer using AI analysis of pancreatic subregions in computed tomography images. Front. Oncol. 2022, 12, 1007990. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).