Minimally Invasive Radical Nephroureterectomy: 5-Year Update of Techniques and Outcomes
Abstract
Simple Summary
Abstract
1. Introduction
2. Literature Search Methodology
3. Surgical Techniques
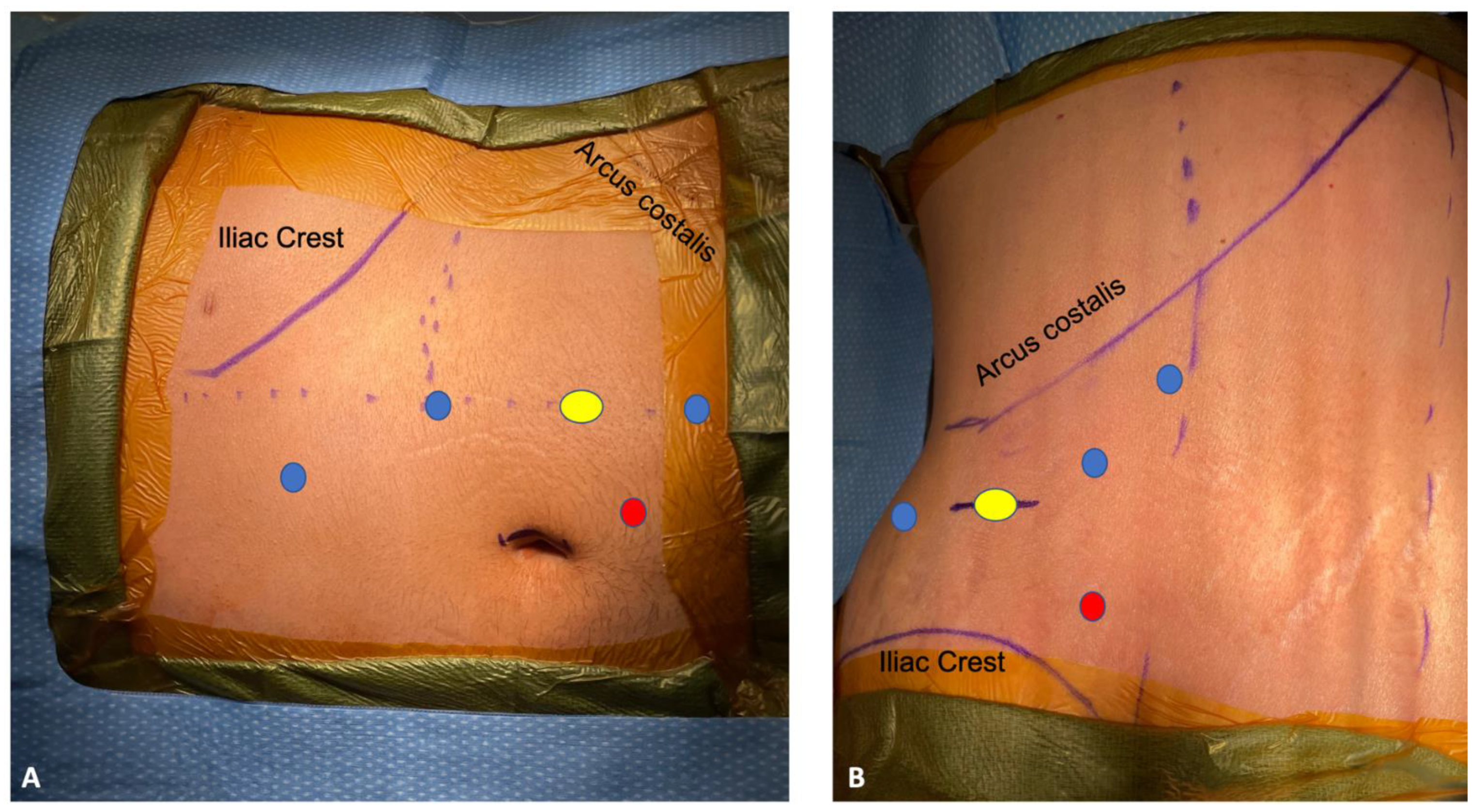
3.1. Single Stage Robotic Radical Nephroureterectomy
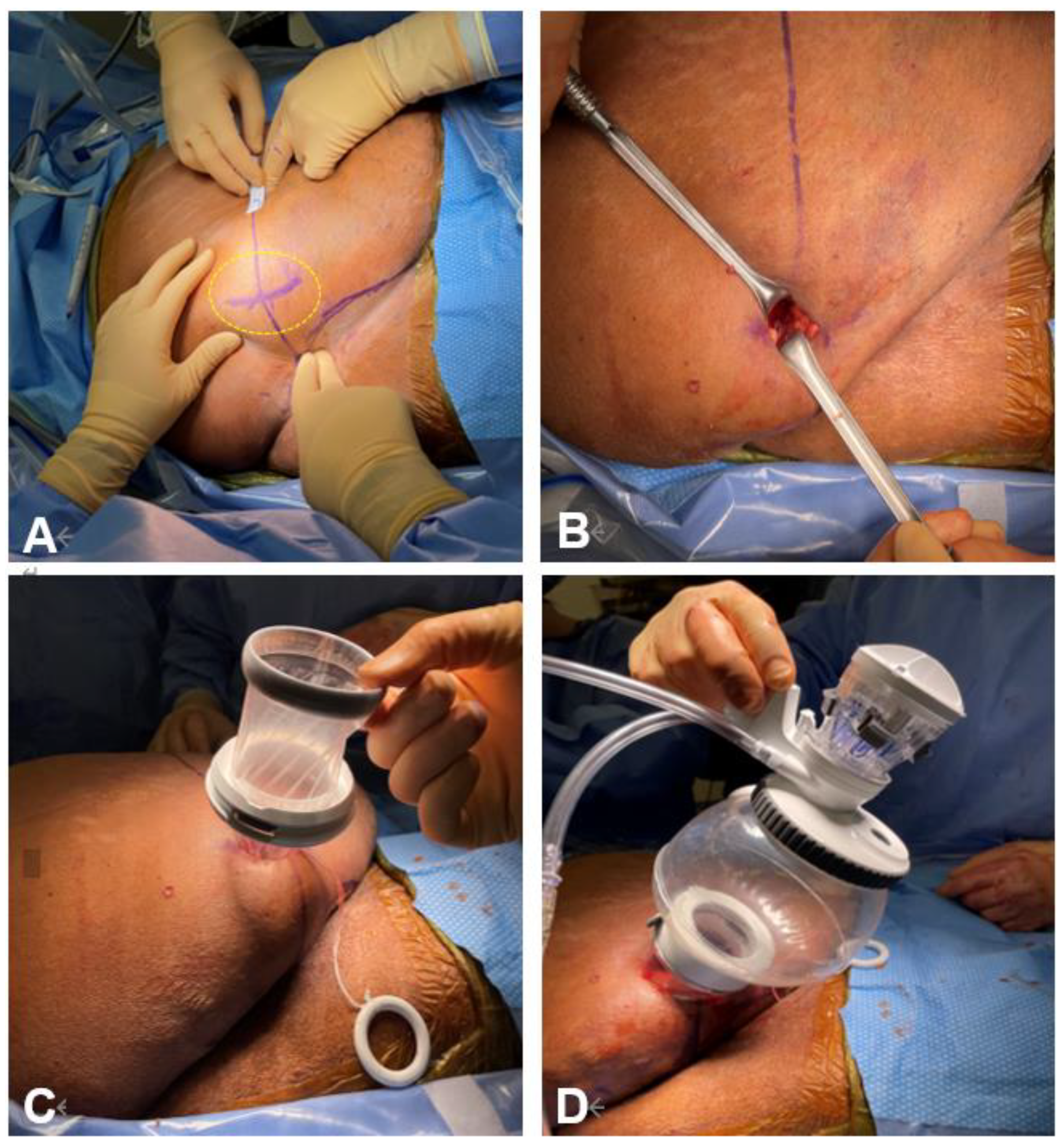
3.2. Retroperitoneal Robotic Radical Nephroureterectomy
3.3. Distal Ureterectomy and Bladder Cuff Excision
3.4. SP Robotic Radical Nephroureterectomy
4. Oncological Outcomes
4.1. Bladder Cuff vs. Non-Bladder Cuff Excision
4.2. Minimally Invasive vs. Open RNU
4.3. Lymphadenectomy
4.4. Impact of Histologic Variants
5. Renal Functional Outcomes
6. Future Perspectives
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alothman, K.I.; Mehmood, S.; Alzahrani, H.M.; Alotaibi, M.F.; Alkhudair, W.K.; Eldali, A.M. Surgical and oncological outcome after laparoscopic versus open nephroureterectomy for non-metastatic, upper-tract urothelial carcinoma: A single-centre experience. Saudi Med. J. 2020, 41, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Foerster, B.; Abufaraj, M.; Matin, S.F.; Azizi, M.; Gupta, M.; Li, W.-M.; Seisen, T.; Clinton, T.; Xylinas, E.; Mir, M.C.; et al. Pretreatment Risk Stratification for Endoscopic Kidney-sparing Surgery in Upper Tract Urothelial Carcinoma: An International Collaborative Study. Eur. Urol. 2021, 80, 507–515. [Google Scholar] [CrossRef]
- Coleman, J.A.; Clark, P.E.; Bixler, B.R.; Buckley, D.I.; Chang, S.S.; Chou, R.; Hoffman-Censits, J.; Kulkarni, G.S.; Matin, S.F.; Pierorazio, P.M.; et al. Diagnosis and Management of Non-Metastatic Upper Tract Urothelial Carcinoma: AUA/SUO Guideline. J. Urol. 2023, 209, 1071–1081. [Google Scholar] [CrossRef]
- Saini, S.; Pathak, R.A.; Hemal, A.K. Robotic nephroureterectomy in the management of upper tract urothelial cancer: Inching toward standard of care? Int. Urol. Nephrol. 2022, 54, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Clayman, R.V.; Kavoussi, L.R.; Figenshau, R.S.; Chandhoke, P.S.; Albala, D.M. Laparoscopic Nephroureterectomy: Initial Clinical Case Report. J. Laparoendosc. Surg. 1991, 1, 343–349. [Google Scholar] [CrossRef]
- Nanigian, D.K.; Smith, W.; Ellison, L.M. Robot-Assisted Laparoscopic Nephroureterectomy. J. Endourol. 2006, 20, 463–466. [Google Scholar] [CrossRef]
- Mullen, E.; Ahmed, K.; Challacombe, B. Systematic review of open versus laparoscopic versus robot-assisted nephroureterectomy. Rev. Urol. 2017, 19, 32–43. [Google Scholar] [PubMed]
- Veccia, A.; Carbonara, U.; Derweesh, I.; Mehrazin, R.; Porter, J.; Abdollah, F.; Mazzone, E.; Sundaram, C.P.; Gonzalgo, M.; Mastroianni, R.; et al. Single-stage Xi® robotic radical nephroureterectomy for upper tract urothelial carcinoma: Surgical technique and outcomes. Minerva Urol. Nephrol. 2022, 74, 233–241. [Google Scholar] [CrossRef]
- Pellegrino, A.A.; Chen, G.; Morgantini, L.; Calvo, R.S.; Crivellaro, S. Simplifying Retroperitoneal Robotic Single-port Surgery: Novel Supine Anterior Retroperitoneal Access. Eur. Urol. 2023, 84, 223–228. [Google Scholar] [CrossRef]
- Kang, M.; Jeong, C.W.; Kwak, C.; Kim, H.H.; Ku, J.H. The Characteristics of Recurrent Upper Tract Urothelial Carcinoma after Radical Nephroureterectomy without Bladder Cuff Excision. Yonsei Med. J. 2015, 56, 375–381. [Google Scholar] [CrossRef]
- Krabbe, L.-M.; Westerman, M.E.; Bagrodia, A.; Gayed, B.A.; Khalil, D.; Kapur, P.; Shariat, S.F.; Raj, G.V.; Sagalowsky, A.I.; Cadeddu, J.A.; et al. Surgical management of the distal ureter during radical nephroureterectomy is an independent predictor of oncological outcomes: Results of a current series and a review of the literature. Urol. Oncol. Semin. Orig. Investig. 2014, 32, 54.e19–54.e26. [Google Scholar] [CrossRef] [PubMed]
- Kenigsberg, A.P.; Meng, X.; Ghandour, R.; Margulis, V. Oncologic outcomes of radical nephroureterectomy (RNU). Transl. Androl. Urol. 2020, 9, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Hemal, A.K.; Stansel, I.; Babbar, P.; Patel, M. Robotic-assisted Nephroureterectomy and Bladder Cuff Excision without Intraoperative Repositioning. Urology 2011, 78, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Zargar, H.; Krishnan, J.; Autorino, R.; Akca, O.; Brandao, L.F.; Laydner, H.; Samarasekera, D.; Ko, O.; Haber, G.-P.; Kaouk, J.H.; et al. Robotic Nephroureterectomy: A Simplified Approach Requiring No Patient Repositioning or Robot Redocking. Eur. Urol. 2014, 66, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Mittakanti, H.R.; Heulitt, G.; Li, H.-F.; Porter, J.R. Transperitoneal vs. retroperitoneal robotic partial nephrectomy: A matched-paired analysis. World J. Urol. 2020, 38, 1093–1099. [Google Scholar] [CrossRef]
- Lim, S.; Teo, X.L. Robot-assisted nephroureterectomy: Current perspectives. Robot. Surg. Res. Rev. 2016, 3, 37–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sparwasser, P.; Epple, S.; Thomas, A.; Dotzauer, R.; Boehm, K.; Brandt, M.P.; Mager, R.; Borgmann, H.; Kamal, M.M.; Kurosch, M.; et al. First completely robot-assisted retroperitoneal nephroureterectomy with bladder cuff: A step-by-step technique. World J. Urol. 2022, 40, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.A.; Patel, M.; Hemal, A.K. Comprehensive Approach to Port Placement Templates for Robot-Assisted Laparoscopic Urologic Surgeries. J. Endourol. 2017, 31, 1269–1276. [Google Scholar] [CrossRef]
- Sparwasser, P.; Frey, L.; Fischer, N.D.; Thomas, A.; Dotzauer, R.; Surcel, C.; Brandt, M.P.; Mager, R.; Höfner, T.; Haferkamp, A.; et al. First Comparison of Retroperitoneal Versus Transperitoneal Robot-Assisted Nephroureterectomy with Bladder Cuff: A Single Center Study. Ann. Surg. Oncol. 2023, 30, 4531–4539. [Google Scholar] [CrossRef]
- Patel, M.N.; Aboumohamed, A.; Hemal, A. Does transition from the da Vinci Si® to Xi robotic platform impact single-docking technique for robot-assisted laparoscopic nephroureterectomy? BJU Int. 2015, 116, 990–994. [Google Scholar] [CrossRef]
- Barton, G.J.; Tan, W.P.; Inman, B.A. The nephroureterectomy: A review of technique and current controversies. Transl. Androl. Urol. 2020, 9, 3168–3190. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-M.; Shen, J.-T.; Li, C.-C.; Ke, H.-L.; Wei, Y.-C.; Wu, W.-J.; Chou, Y.-H.; Huang, C.-H. Oncologic Outcomes Following Three Different Approaches to the Distal Ureter and Bladder Cuff in Nephroureterectomy for Primary Upper Urinary Tract Urothelial Carcinoma. Eur. Urol. 2010, 57, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Xylinas, E.; Rink, M.; Cha, E.K.; Clozel, T.; Lee, R.K.; Fajkovic, H.; Comploj, E.; Novara, G.; Margulis, V.; Raman, J.D.; et al. Impact of Distal Ureter Management on Oncologic Outcomes Following Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma. Eur. Urol. 2014, 65, 210–217. [Google Scholar] [CrossRef]
- Luo, H.L.; Kang, C.H.; Chen, Y.T.; Chuang, Y.C.; Cheng, Y.T.; Lee, W.C.; Chiang, P.H. Oncological impact of endoscopic bladder cuff management during nephroureterectomy varies according to upper urinary tract tumor location. Int. J. Urol. 2014, 21, 366–369. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, B.; Seisen, T.; Dominguez-Escrig, J.-L.; Bruins, H.M.; Yuan, C.Y.; Lam, T.; Maclennan, S.; N’dow, J.; Babjuk, M.; Comperat, E.; et al. Oncological Outcomes of Laparoscopic Nephroureterectomy Versus Open Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: An European Association of Urology Guidelines Systematic Review. Eur. Urol. Focus 2019, 5, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Katims, A.B.; Say, R.; Derweesh, I.; Uzzo, R.; Minervini, A.; Wu, Z.; Abdollah, F.; Sundaram, C.; Ferro, M.; Rha, K.; et al. Risk Factors for Intravesical Recurrence after Minimally Invasive Nephroureterectomy for Upper Tract Urothelial Cancer (ROBUUST Collaboration). J. Urol. 2021, 206, 568–576. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Wang, J.; Veccia, A.; Xu, Y.; Zhang, C.; Ren, J.; Yin, L.; Chen, M.; Wang, J.; et al. Pure retroperitoneoscopic extravesical standardized seeable (PRESS) excision of distal ureter and bladder cuff in radical nephroureterectomy: Step-by-step technique. Minerva Urol. Nephrol. 2021, 73, 392–400. [Google Scholar] [CrossRef]
- Medina, L.G.; Alsyouf, M.; Ghoreifi, A.; Sayegh, A.S.; Koh, K.; Yu, W.; Sobhani, S.; Douglawi, A.; Djaladat, H. Distal ureter and bladder cuff excision using the “Keyhole Technique” during Robotic Radical Nephroureterectomy. Int. Braz. J. Urol. 2022, 48, 876–877. [Google Scholar] [CrossRef]
- Kim, K.H.; Ahn, H.K.; Kim, M.; Yoon, H. Technique and perioperative outcomes of single-port robotic surgery using the da Vinci SP platform in urology. Asian J. Surg. 2023, 46, 472–477. [Google Scholar] [CrossRef]
- Martini, A.; Daza, J.; Poltiyelova, E.; Gul, Z.; Heard, J.R.; Ferket, B.S.; Waingankar, N.; Galsky, M.D.; Sfakianos, J.P. Pathological downstaging as a novel endpoint for the development of neoadjuvant chemotherapy for upper tract urothelial carcinoma. BJU Int. 2019, 124, 665–671. [Google Scholar] [CrossRef]
- Xylinas, E.; Kluth, L.; Scherr, D.; Novara, G.; Comploj, E.; Pycha, A.; Fritsche, H.-M.; Trinh, Q.-D.; Karakiewicz, P.; Weizer, A.; et al. 573 Prediction of intravesical recurrence after radical nephroureterectomy: Development of a clinical decision-making tool. Eur. Urol. Suppl. 2013, 12, e573–e574. [Google Scholar] [CrossRef]
- Tanaka, N.; Kikuchi, E.; Kanao, K.; Matsumoto, K.; Kobayashi, H.; Ide, H.; Miyazaki, Y.; Obata, J.; Hoshino, K.; Shirotake, S.; et al. Metastatic Behavior of Upper Tract Urothelial Carcinoma After Radical Nephroureterectomy: Association with Primary Tumor Location. Ann. Surg. Oncol. 2014, 21, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Jeldres, C.; Sun, M.; Isbarn, H.; Lughezzani, G.; Budäus, L.; Alasker, A.; Shariat, S.F.; Lattouf, J.-B.; Widmer, H.; Pharand, D.; et al. A Population-based Assessment of Perioperative Mortality After Nephroureterectomy for Upper-tract Urothelial Carcinoma. Urology 2010, 75, 315–320. [Google Scholar] [CrossRef]
- Morselli, S.; Vitelli, F.D.; Verrini, G.; Sebastianelli, A.; Campi, R.; Liaci, A.; Spatafora, P.; Barzaghi, P.; Ferrari, G.; Gacci, M.; et al. Comparison of Tumor Seeding and Recurrence Rate After Laparoscopic vs. Open Nephroureterectomy for Upper Urinary Tract Transitional Cell Carcinoma. Front. Surg. 2021, 8, 769527. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Shariat, S.F.; Isbarn, H.; Weizer, A.; Remzi, M.; Roscigno, M.; Kikuchi, E.; Raman, J.D.; Bolenz, C.; Bensalah, K.; et al. Comparison of Oncologic Outcomes for Open and Laparoscopic Nephroureterectomy: A Multi-Institutional Analysis of 1249 Cases. Eur. Urol. 2009, 56, 1–9. [Google Scholar] [CrossRef]
- Guo, R.; Zhu, Y.; Xiong, G.; Li, X.; Zhang, K.; Zhou, L. Role of lymph node dissection in the management of upper tract urothelial carcinomas: A meta-analysis. BMC Urol. 2018, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Lughezzani, G.; Sun, M.; Perrotte, P.; Shariat, S.F.; Jeldres, C.; Buraus, L.; Alasker, A.; Duclos, A.; Widmer, H.; Latour, M.; et al. Should Bladder Cuff Excision Remain the Standard of Care at Nephroureterectomy in Patients with Urothelial Carcinoma of the Renal Pelvis? A Population-based Study. Eur. Urol. 2010, 57, 956–962. [Google Scholar] [CrossRef]
- Veccia, A.; Antonelli, A.; Francavilla, S.; Simeone, C.; Guruli, G.; Zargar, H.; Perdoná, S.; Ferro, M.; Carrieri, G.; Hampton, L.J.; et al. Robotic versus other nephroureterectomy techniques: A systematic review and meta-analysis of over 87,000 cases. World J. Urol. 2020, 38, 845–852. [Google Scholar] [CrossRef]
- Carrion, A.; Huguet, J.; García-Cruz, E.; Izquierdo, L.; Mateu, L.; Musquera, M.; Ribal, M.J.; Alcaraz, A. Intraoperative prognostic factors and atypical patterns of recurrence in patients with upper urinary tract urothelial carcinoma treated with laparoscopic radical nephroureterectomy. Scand. J. Urol. 2016, 50, 305–312. [Google Scholar] [CrossRef]
- Rouprêt, M.; Hupertan, V.; Seisen, T.; Colin, P.; Xylinas, E.; Yates, D.R.; Fajkovic, H.; Lotan, Y.; Raman, J.D.; Zigeuner, R.; et al. Prediction of Cancer Specific Survival after Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: Development of an Optimized Postoperative Nomogram Using Decision Curve Analysis. J. Urol. 2013, 189, 1662–1669. [Google Scholar] [CrossRef]
- Grob, G.; Rogers, D.; Pandolfo, S.D.; Vourganti, S.; Buscarini, M.; Mehrazin, R.; Grob, B.M.; Mir, M.C.; Perdonà, S.; Derweesh, I.H.; et al. Oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma: A literature review. Transl. Androl. Urol. 2023, 12, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Nazzani, S.; Preisser, F.; Mazzone, E.; Tian, Z.; Mistretta, F.A.; Soulières, D.; Montanari, E.; Acquati, P.; Briganti, A.; Shariat, S.F.; et al. Nephroureterectomy with or without Bladder Cuff Excision for Localized Urothelial Carcinoma of the Renal Pelvis. Eur. Urol. Focus 2020, 6, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Rouprêt, M.; Seisen, T.; Birtle, A.J.; Capoun, O.; Compérat, E.M.; Dominguez-Escrig, J.L.; Andersson, I.G.; Liedberg, F.; Mariappan, P.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2023 Update. Eur. Urol. 2023, 84, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Correia, J.; Mendes, G.; Texeira, B.; Madanelo, M.; Fraga, A.; Silva-Ramos, M. Perioperative and oncological outcomes of laparoscopic and open radical nephroureterectomy for locally advanced upper tract urothelial carcinoma: A single-center cohort study. Cent. Eur. J. Urol. 2022, 75, 257–264. [Google Scholar] [CrossRef]
- Motzer, R.J.; Jonasch, E.; Bhayani, S.; Bro, W.P.; Chang, S.S.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; Ellis, R.; Fishman, M.; et al. NCCN Guidelines Index Table of Contents Discussion. Kidney Cancer; National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2018. [Google Scholar]
- Rouprêt, M.; Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Cowan, N.C.; Dominguez-Escrig, J.L.; Gontero, P.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2021, 79, 62–79. [Google Scholar] [CrossRef]
- Seisen, T.; Peyronnet, B.; Dominguez-Escrig, J.L.; Bruins, H.M.; Yuan, C.Y.; Babjuk, M.; Böhle, A.; Burger, M.; Compérat, E.M.; Cowan, N.C.; et al. Oncologic Outcomes of Kidney-sparing Surgery Versus Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the EAU Non-muscle Invasive Bladder Cancer Guidelines Panel. Eur. Urol. 2016, 70, 1052–1068. [Google Scholar] [CrossRef]
- Duquesne, I.; Ouzaid, I.; Loriot, Y.; Moschini, M.; Xylinas, E. Lymphadenectomy for Upper Tract Urothelial Carcinoma: A Systematic Review. J. Clin. Med. 2019, 8, 1190. [Google Scholar] [CrossRef] [PubMed]
- Ouzzane, A.; Colin, P.; Ghoneim, T.P.; Zerbib, M.; De La Taille, A.; Audenet, F.; Saint, F.; Hoarau, N.; Adam, E.; Azemar, M.D.; et al. The impact of lymph node status and features on oncological outcomes in urothelial carcinoma of the upper urinary tract (UTUC) treated by nephroureterectomy. World J. Urol. 2013, 31, 189–197. [Google Scholar] [CrossRef]
- Dominguez-Escrig, J.L.; Peyronnet, B.; Seisen, T.; Bruins, H.M.; Yuan, C.Y.; Babjuk, M.; Böhle, A.; Burger, M.; Compérat, E.M.; Gontero, P.; et al. Potential Benefit of Lymph Node Dissection During Radical Nephroureterectomy for Upper Tract Urothelial Carcinoma: A Systematic Review by the European Association of Urology Guidelines Panel on Non–muscle-invasive Bladder Cancer. Eur. Urol. Focus 2019, 5, 224–241. [Google Scholar] [CrossRef]
- Hakimi, K.; Carbonara, U.; Djaladat, H.; Mehrazin, R.; Eun, D.; Reese, A.; Gonzalgo, M.L.; Margulis, V.; Uzzo, R.G.; Porter, J.; et al. Outcomes of Lymph Node Dissection in Nephroureterectomy in the Treatment of Upper Tract Urothelial Carcinoma: Analysis of the ROBUUST Registry. J. Urol. 2022, 208, 268–276. [Google Scholar] [CrossRef]
- Chung, H.S.; Hwang, E.C.; Kim, M.S.; Yu, S.H.; Jung, S.I.; Kang, T.W.; Choi, C.; Choi, S.H.; Kwon, T.G.; Noh, J.H.; et al. Effects of Variant Histology on the Oncologic Outcomes of Patients with Upper Urinary Tract Carcinoma after Radical Nephroureterectomy: A Propensity Score-Matched Analysis. Clin. Genitourin. Cancer 2019, 17, e394–e407. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Janisch, F.; Parizi, M.K.; Mostafaei, H.; Lysenko, I.; Kimura, S.; Enikeev, D.V.; Egawa, S.; Shariat, S.F. Prognostic Value of Variant Histology in Upper Tract Urothelial Carcinoma Treated with Nephroureterectomy: A Systematic Review and Meta-Analysis. J. Urol. 2020, 203, 1075–1084. [Google Scholar] [CrossRef]
- Douglawi, A.; Ghoreifi, A.; Carbonara, U.; Yip, W.; Uzzo, R.G.; Margulis, V.; Ferro, M.; De Cobelli, O.; Wu, Z.; Simone, G.; et al. Impact of Variant Histology on Oncological Outcomes in Upper Tract Urothelial Carcinoma: Results from the ROBUUST Collaborative Group. Clin. Genitourin. Cancer 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Inamoto, T.; Matsuyama, H.; Ibuki, N.; Komura, K.; Takahara, K.; Fujimoto, K.; Shiina, H.; Sakano, S.; Nagao, K.; Miyake, M.; et al. Biological Behavior and Long-Term Outcomes of Carcinoma In Situ in Upper Urinary Tract Managed by Radical Nephroureterectomy. J. Urol. 2018, 199, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Minervini, A.; Sandri, M.; Bertini, R.; Bertolo, R.; Carini, M.; Furlan, M.; Larcher, A.; Mantica, G.; Mari, A.; et al. Below Safety Limits, Every Unit of Glomerular Filtration Rate Counts: Assessing the Relationship Between Renal Function and Cancer-specific Mortality in Renal Cell Carcinoma. Eur. Urol. 2018, 74, 661–667. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakagawa, T.; Miyakawa, J.; Kawai, T.; Tabata, M.; Kaneko, T.; Taguchi, S.; Naito, A.; Hikatsu, M.; Sato, Y.; et al. Smaller decline of renal function after nephroureterectomy predicts poorer prognosis of upper tract urothelial carcinoma: A multicentre retrospective study. Jpn. J. Clin. Oncol. 2021, 51, 1577–1586. [Google Scholar] [CrossRef]
- Xylinas, E.; Rink, M.; Margulis, V.; Clozel, T.; Lee, R.K.; Comploj, E.; Novara, G.; Raman, J.D.; Lotan, Y.; Weizer, A.; et al. Impact of renal function on eligibility for chemotherapy and survival in patients who have undergone radical nephro-ureterectomy. BJU Int. 2013, 112, 453–461. [Google Scholar] [CrossRef]
- Kaag, M.G.; O’Malley, R.L.; O’Malley, P.; Godoy, G.; Chen, M.; Smaldone, M.C.; Hrebinko, R.L.; Raman, J.D.; Bochner, B.; Dalbagni, G.; et al. Changes in Renal Function Following Nephroureterectomy May Affect the Use of Perioperative Chemotherapy. Eur. Urol. 2010, 58, 581–587. [Google Scholar] [CrossRef]
- Lee, B.H.; Zabor, E.C.; Tennenbaum, D.; Furberg, H.; Benfante, N.; Coleman, J.A.; Jaimes, E.A.; Russo, P. Renal function recovery after radical nephroureterectomy for upper tract urothelial carcinoma. World J. Urol. 2018, 36, 257–263. [Google Scholar] [CrossRef]
- Tafuri, A.; Marchioni, M.; Cerrato, C.; Mari, A.; Tellini, R.; Odorizzi, K.; Veccia, A.; Amparore, D.; Shakir, A.; Carbonara, U.; et al. Changes in renal function after nephroureterectomy for upper urinary tract carcinoma: Analysis of a large multicenter cohort (Radical Nephroureterectomy Outcomes (RaNeO) Research Consortium). World J. Urol. 2022, 40, 2771–2779. [Google Scholar] [CrossRef]
- Hashimoto, T.; Ohno, Y.; Nakashima, J.; Gondo, T.; Nakagami, Y.; Namiki, K.; Horiguchi, Y.; Yoshioka, K.; Ohori, M.; Tachibana, M. Prediction of renal function after nephroureterectomy in patients with upper tract urothelial carcinoma. Jpn. J. Clin. Oncol. 2015, 45, 1064–1068. [Google Scholar] [CrossRef]
- Rodríguez Faba, O.; Palou, J.; Breda, A.; Maroto, P.; Gómez, J.F.; Wong, A.; Villavicencio, H. Predictive Factors for Impaired Renal Function following Nephroureterectomy in Upper Urinary Tract Urothelial Cell Carcinoma. Urol. Int. 2014, 92, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Chen, Q.; Djaladat, H.; Minervini, A.; Uzzo, R.G.; Sundaram, C.P.; Rha, K.H.; Gonzalgo, M.L.; Mehrazin, R.; Mazzone, E.; et al. A Preoperative Nomogram to Predict Renal Function Insufficiency for Cisplatin-based Adjuvant Chemotherapy Following Minimally Invasive Radical Nephroureterectomy (ROBUUST Collaborative Group). Eur. Urol. Focus 2022, 8, 173–181. [Google Scholar] [CrossRef]
- Necchi, A.; Madison, R.; Pal, S.K.; Ross, J.S.; Agarwal, N.; Sonpavde, G.; Joshi, M.; Yin, M.; Miller, V.A.; Grivas, P.; et al. Comprehensive Genomic Profiling of Upper-tract and Bladder Urothelial Carcinoma. Eur. Urol. Focus 2021, 7, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Rao, P.; Matin, S.F. Lynch syndrome and urologic malignancies: A contemporary review. Curr. Opin. Urol. 2019, 29, 357–363. [Google Scholar] [CrossRef]
- Catto, J.W.; Azzouzi, A.-R.; Rehman, I.; Feeley, K.M.; Cross, S.S.; Amira, N.; Fromont, G.; Sibony, M.; Cussenot, O.; Meuth, M.; et al. Promoter Hypermethylation Is Associated with Tumor Location, Stage, and Subsequent Progression in Transitional Cell Carcinoma. J. Clin. Oncol. 2005, 23, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Amira, N.; Rivet, J.; Soliman, H.; Cancel-Tassin, G.; LE Duc, A.; Janin, A.; Cussenot, O. Microsatellite Instability in Urothelial Carcinoma of the Upper Urinary Tract. J. Urol. 2003, 170, 1151–1154. [Google Scholar] [CrossRef]
- Sfakianos, J.P.; Cha, E.K.; Iyer, G.; Scott, S.N.; Zabor, E.C.; Shah, R.H.; Ren, Q.; Bagrodia, A.; Kim, P.H.; Hakimi, A.A.; et al. Genomic Characterization of Upper Tract Urothelial Carcinoma. Eur. Urol. 2015, 68, 970–977. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How To Build and Interpret a Nomogram for Cancer Prognosis. J. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef]
- Favaretto, R.L.; Shariat, S.F.; Chade, D.C.; Godoy, G.; Adamy, A.; Kaag, M.; Bochner, B.H.; Coleman, J.; Dalbagni, G. The Effect of Tumor Location on Prognosis in Patients Treated with Radical Nephroureterectomy at Memorial Sloan-Kettering Cancer Center. Eur. Urol. 2010, 58, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Pallauf, M.; König, F.; D’andrea, D.; Laukhtina, E.; Mostafaei, H.; Motlagh, R.S.; Quhal, F.; Aydh, A.; Yanagisawa, T.; Kawada, T.; et al. A Systematic Review and Meta-Analysis of Prognostic Nomograms After UTUC Surgery. Front. Oncol. 2022, 12, 907975. [Google Scholar] [CrossRef] [PubMed]
| Study Name | Year | Type of Study | N of Cases | Topic | Main Results |
|---|---|---|---|---|---|
| Inamoto [34] | 2018 | Retrospective two-arm comparative study | 163 | Variant Histology p-CIS vs. c-CIS | 10 yrs CSS: p-CIS 111.8 months c-CIS 85.9 months |
| Upfill-Brown [35] | 2019 | Retrospective two-arm comparative study (NCDB Database) | 16,783 | Nephroureterectomy vs. Endoscopic Management | ET worse OS vs. RNU (HR 1.43; p = 0.006) |
| Nazzani [36] | 2020 | Retrospective two-arm comparative study (SEER Database) | 4266 | RNU + BCE vs. RNU | 5 yrs CSM: BCE 19.7% vs. No BCE 23.5% (p = 0.005) ± BCE (HR 1.14; p = 0.1) |
| Peyronnet [22] | 2019 | Meta-analysis | 7554 | Laparoscopic vs. Open RNU | CSS, RFS, MFS: p = 0.2, p = 0.86, and p = 0.12 pT3/HG Open vs. Lap (p < 0.05) |
| Veccia [37] | 2020 | Meta-analysis | 87,291 | Robotic vs. Lap vs. Open RNU | RANU vs. Lap vs. Open RFS: 0.99; CSS: 0.83 |
| Mori [38] | 2020 | Meta-analysis | 12,865 | Variant Histology | CSS: HR 2.00 OS: HR 1.76 RFS: HR 1.64 |
| Kawada [39] | 2023 | Meta-analysis | N/A | Nephroureterectomy vs. Endoscopic Management | OS: HR 1.27 CSS: HR 1.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, A.; Ditonno, F.; Feng, C.; Manfredi, C.; Sturgis, M.R.; Farooqi, M.; Del Giudice, F.; Coogan, C.; Ferro, M.; Zhang, C.; et al. Minimally Invasive Radical Nephroureterectomy: 5-Year Update of Techniques and Outcomes. Cancers 2023, 15, 4585. https://doi.org/10.3390/cancers15184585
Franco A, Ditonno F, Feng C, Manfredi C, Sturgis MR, Farooqi M, Del Giudice F, Coogan C, Ferro M, Zhang C, et al. Minimally Invasive Radical Nephroureterectomy: 5-Year Update of Techniques and Outcomes. Cancers. 2023; 15(18):4585. https://doi.org/10.3390/cancers15184585
Chicago/Turabian StyleFranco, Antonio, Francesco Ditonno, Carol Feng, Celeste Manfredi, Morgan R. Sturgis, Mustafa Farooqi, Francesco Del Giudice, Christopher Coogan, Matteo Ferro, Chao Zhang, and et al. 2023. "Minimally Invasive Radical Nephroureterectomy: 5-Year Update of Techniques and Outcomes" Cancers 15, no. 18: 4585. https://doi.org/10.3390/cancers15184585
APA StyleFranco, A., Ditonno, F., Feng, C., Manfredi, C., Sturgis, M. R., Farooqi, M., Del Giudice, F., Coogan, C., Ferro, M., Zhang, C., Wu, Z., Yang, B., Wang, L., & Autorino, R. (2023). Minimally Invasive Radical Nephroureterectomy: 5-Year Update of Techniques and Outcomes. Cancers, 15(18), 4585. https://doi.org/10.3390/cancers15184585







