Immunohistochemical Evaluation of the Expression of Specific Membrane Antigens in Patients with Pancreatic Ductal Adenocarcinoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Histopathology
2.3. Immunohistochemistry
2.4. Histopathology Analysis and Histo-Score (H-Score)
2.5. Statistics Analysis
3. Results
3.1. Clinical and Demographic Characteristics of the Study Population
3.2. Antigen Expression in the Neoplastic Tissue
3.3. Comparison of the Antigen Expression between Neoplastic and Non-Neoplastic Tissues
3.4. Clinical Outcomes and Correlation with Antigen Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryan, D.P.; Hong, T.S.; Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014, 371, 1039–1049. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.X.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Lin, Y.Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef]
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.J.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Tan, D. Diagnostic and prognostic biomarkers in pancreatic carcinoma. Int. J. Clin. Exp. Pathol. 2009, 2, 1–10. [Google Scholar]
- Goonetilleke, K.S.; Siriwardena, A.K. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur. J. Surg. Oncol. 2007, 33, 266–270. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Nolen, B.M.; Brand, R.E.; Prosser, D.; Velikokhatnaya, L.; Allen, P.J.; Zeh, H.J.; Grizzle, W.E.; Huang, Y.; Lomakin, A.; Lokshin, A.E. Prediagnostic serum biomarkers as early detection tools for pancreatic cancer in a large prospective cohort study. PLoS ONE 2014, 9, e94928. [Google Scholar] [CrossRef]
- Gulla, A.; Hashimoto, D.; Wagner, D.; Damaseviciute, R.; Strupas, K.; Satoi, S. Interdisciplinary Approach of Establishing PDAC Resectability: Biochemical, Radiological and NAT Regimen Prognostic Factors-Literature Review. Medicina 2022, 58, 756. [Google Scholar] [CrossRef]
- Khoury, J.D.; Wang, W.L.; Prieto, V.G.; Medeiros, L.J.; Kalhor, N.; Hameed, M.; Broaddus, R.; Hamilton, S.R. Validation of Immunohistochemical Assays for Integral Biomarkers in the NCI-MATCH EAY131 Clinical Trial. Clin. Cancer Res. 2018, 24, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.L.; Trufelli, D.C.; de Matos, M.G.; da Silva Pinhal, M.A. Immunohistochemistry as an important tool in biomarkers detection and clinical practice. Biomark. Insights 2010, 5, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Nicolson, G.L. Cell membrane fluid-mosaic structure and cancer metastasis. Cancer Res. 2015, 75, 1169–1176. [Google Scholar] [CrossRef] [PubMed]
- Lin, X. Functions of heparan sulfate proteoglycans in cell signaling during development. Development 2004, 131, 6009–6021. [Google Scholar] [CrossRef]
- Kleeff, J.; Ishiwata, T.; Kumbasar, A.; Friess, H.; Büchler, M.W.; Lander, A.D.; Korc, M. The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. J. Clin. Investig. 1998, 102, 1662–1673. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Montemagno, C.; Cassim, S.; Pouyssegur, J.; Broisat, A.; Pagès, G. From Malignant Progression to Therapeutic Targeting: Current Insights of Mesothelin in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2020, 21, 4067. [Google Scholar] [CrossRef]
- Lu, S.H.; Yuan, R.H.; Chen, Y.L.; Hsu, H.C.; Jeng, Y.M. Annexin A10 is an immunohistochemical marker for adenocarcinoma of the upper gastrointestinal tract and pancreatobiliary system. Histopathology 2013, 63, 640–648. [Google Scholar] [CrossRef]
- Ishikawa, A.; Kuraoka, K.; Zaitsu, J.; Saito, A.; Yamaguchi, A.; Kuwai, T.; Sudo, T.; Hadano, N.; Tashiro, H.; Taniyama, K.; et al. High Annexin A10 expression is correlated with poor prognosis in pancreatic ductal adenocarcinoma. Histol. Histopathol. 2022, 37, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Allen, P.J.; Kuk, D.; Castillo, C.F.; Basturk, O.; Wolfgang, C.L.; Cameron, J.L.; Lillemoe, K.D.; Ferrone, C.R.; Morales-Oyarvide, V.; He, J.; et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann. Surg. 2017, 265, 185–191. [Google Scholar] [CrossRef]
- Dottermusch, M.; Krüger, S.; Behrens, H.M.; Halske, C.; Röcken, C. Expression of the potential therapeutic target claudin-18.2 is frequently decreased in gastric cancer: Results from a large Caucasian cohort study. Virchows Arch. 2019, 475, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Tsuta, K.; Wakai, S.; Arai, Y.; Asamura, H.; Shibata, T.; Furuta, K.; Kohno, T.; Kushima, R. Immunohistochemical detection of ROS1 is useful for identifying ROS1 rearrangements in lung cancers. Mod. Pathol. 2014, 27, 711–720. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Lei, H.J.; Chen, M.H.; Ho, H.L.; Chiu, L.Y.; Li, C.P.; Wang, Y.C. C-Reactive Protein (CRP) is a Promising Diagnostic Immunohistochemical Marker for Intrahepatic Cholangiocarcinoma and is Associated With Better Prognosis. Am. J. Surg. Pathol. 2017, 41, 1630–1641. [Google Scholar] [CrossRef]
- Karamitopoulou, E. Tumour microenvironment of pancreatic cancer: Immune landscape is dictated by molecular and histopathological features. Br. J. Cancer 2019, 121, 5–14. [Google Scholar] [CrossRef]
- Chang, C.H.; Pauklin, S. Extracellular vesicles in pancreatic cancer progression and therapies. Cell Death Dis. 2021, 12, 973. [Google Scholar] [CrossRef]
- Nicoletti, A.; Negri, M.; Paratore, M.; Vitale, F.; Ainora, M.E.; Nista, E.C.; Gasbarrini, A.; Zocco, M.A.; Zileri Dal Verme, L. Diagnostic and Prognostic Role of Extracellular Vesicles in Pancreatic Cancer: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 885. [Google Scholar] [CrossRef]
- O’Hurley, G.; Sjöstedt, E.; Rahman, A.; Li, B.; Kampf, C.; Pontén, F.; Gallagher, W.M.; Lindskog, C. Garbage in, garbage out: A critical evaluation of strategies used for validation of immunohistochemical biomarkers. Mol. Oncol. 2014, 8, 783–798. [Google Scholar] [CrossRef]
- Ahola, R.; Siiki, A.; Vasama, K.; Vornanen, M.; Sand, J.; Laukkarinen, J. Patients with resected, histologically re-confirmed pancreatic ductal adenocarcinoma (PDAC) can achieve long-term survival despite T3 tumour or nodal involvement. The Finnish Register Study 2000–2013. Pancreatology 2017, 17, 822–826. [Google Scholar] [CrossRef]
- Kutlu, O.C.; Vega, E.A.; Salehi, O.; Lathan, C.; Kim, S.; Krishnan, S.; Stallwood, C.; Kozyreva, O.; Conrad, C. Laparoscopic pancreatectomy for cancer in high volume centers is associated with an increased use and fewer delays of adjuvant chemotherapy. HPB 2021, 23, 625–632. [Google Scholar] [CrossRef]
- Le, K.; Wang, J.; Zhang, T.; Guo, Y.; Chang, H.; Wang, S.; Zhu, B. Overexpression of Mesothelin in Pancreatic Ductal Adenocarcinoma (PDAC). Int. J. Med. Sci. 2020, 17, 422–427. [Google Scholar] [CrossRef]
- Golfier, S.; Kopitz, C.; Kahnert, A.; Heisler, I.; Schatz, C.A.; Stelte-Ludwig, B.; Mayer-Bartschmid, A.; Unterschemmann, K.; Bruder, S.; Linden, L.; et al. Anetumab ravtansine: A novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol. Cancer Ther. 2014, 13, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Thomas, A.; Alewine, C.; Le, D.T.; Jaffee, E.M.; Pastan, I. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J. Clin. Oncol. 2016, 34, 4171–4179. [Google Scholar] [CrossRef]
- Ansari, D.; Urey, C.; Gundewar, C.; Bauden, M.P.; Andersson, R. Comparison of MUC4 expression in primary pancreatic cancer and paired lymph node metastases. Scand. J. Gastroenterol. 2013, 48, 1183–1187. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.J.; Batra, S.K.; Varshney, G.C.; Hollingsworth, M.A.; Yeo, C.J.; Cameron, J.L.; Wilentz, R.E.; Hruban, R.H.; Argani, P. MUC4 expression increases progressively in pancreatic intraepithelial neoplasia. Am. J. Clin. Pathol. 2002, 117, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Sopha, S.C.; Gopal, P.; Merchant, N.B.; Revetta, F.L.; Gold, D.V.; Washington, K.; Shi, C. Diagnostic and therapeutic implications of a novel immunohistochemical panel detecting duodenal mucosal invasion by pancreatic ductal adenocarcinoma. Int. J. Clin. Exp. Pathol. 2013, 6, 2476–2486. [Google Scholar] [PubMed]
- Wang, S.; You, L.; Dai, M.; Zhao, Y. Mucins in pancreatic cancer: A well-established but promising family for diagnosis, prognosis and therapy. J. Cell Mol. Med. 2020, 24, 10279–10289. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, J.; Pei, X.; Tan, Z.; Shi, J.; Lubman, D.M. Annexin A10 is a candidate marker associated with the progression of pancreatic precursor lesions to adenocarcinoma. PLoS ONE 2017, 12, e0175039. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Kälsch, J.; Padden, J.; Bertram, S.; Pott, L.L.; Reis, H.; Westerwick, D.; Schaefer, C.M.; Sowa, J.P.; Möllmann, D.; Fingas, C.; et al. Annexin A10 optimally differentiates between intrahepatic cholangiocarcinoma and hepatic metastases of pancreatic ductal adenocarcinoma: A comparative study of immunohistochemical markers and panels. Virchows Arch. 2017, 470, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Ferrone, C.R.; Finkelstein, D.M.; Thayer, S.P.; Muzikansky, A.; Fernandez-delCastillo, C.; Warshaw, A.L. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J. Clin. Oncol. 2006, 24, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Kiliç, M.; Göçmen, E.; Tez, M.; Ertan, T.; Keskek, M.; Koç, M. Value of preoperative serum CA 19-9 levels in predicting resectability for pancreatic cancer. Can. J. Surg. 2006, 49, 241–244. [Google Scholar] [PubMed]
- Gonzalez, R.S.; Bagci, P.; Basturk, O.; Reid, M.D.; Balci, S.; Knight, J.H.; Kong, S.Y.; Memis, B.; Jang, K.T.; Ohike, N.; et al. Intrapancreatic distal common bile duct carcinoma: Analysis, staging considerations, and comparison with pancreatic ductal and ampullary adenocarcinomas. Mod. Pathol. 2016, 29, 1358–1369. [Google Scholar] [CrossRef]
- Van Roessel, S.; Soer, E.C.; Daamen, L.A.; van Dalen, D.; Fariña Sarasqueta, A.; Stommel, M.W.J.; Molenaar, I.Q.; van Santvoort, H.C.; van de Vlasakker, V.C.J.; de Hingh, I.H.J.T.; et al. Preoperative misdiagnosis of pancreatic and periampullary cancer in patients undergoing pancreatoduodenectomy: A multicentre retrospective cohort study. Eur. J. Surg. Oncol. 2021, 47, 2525–2532. [Google Scholar] [CrossRef]
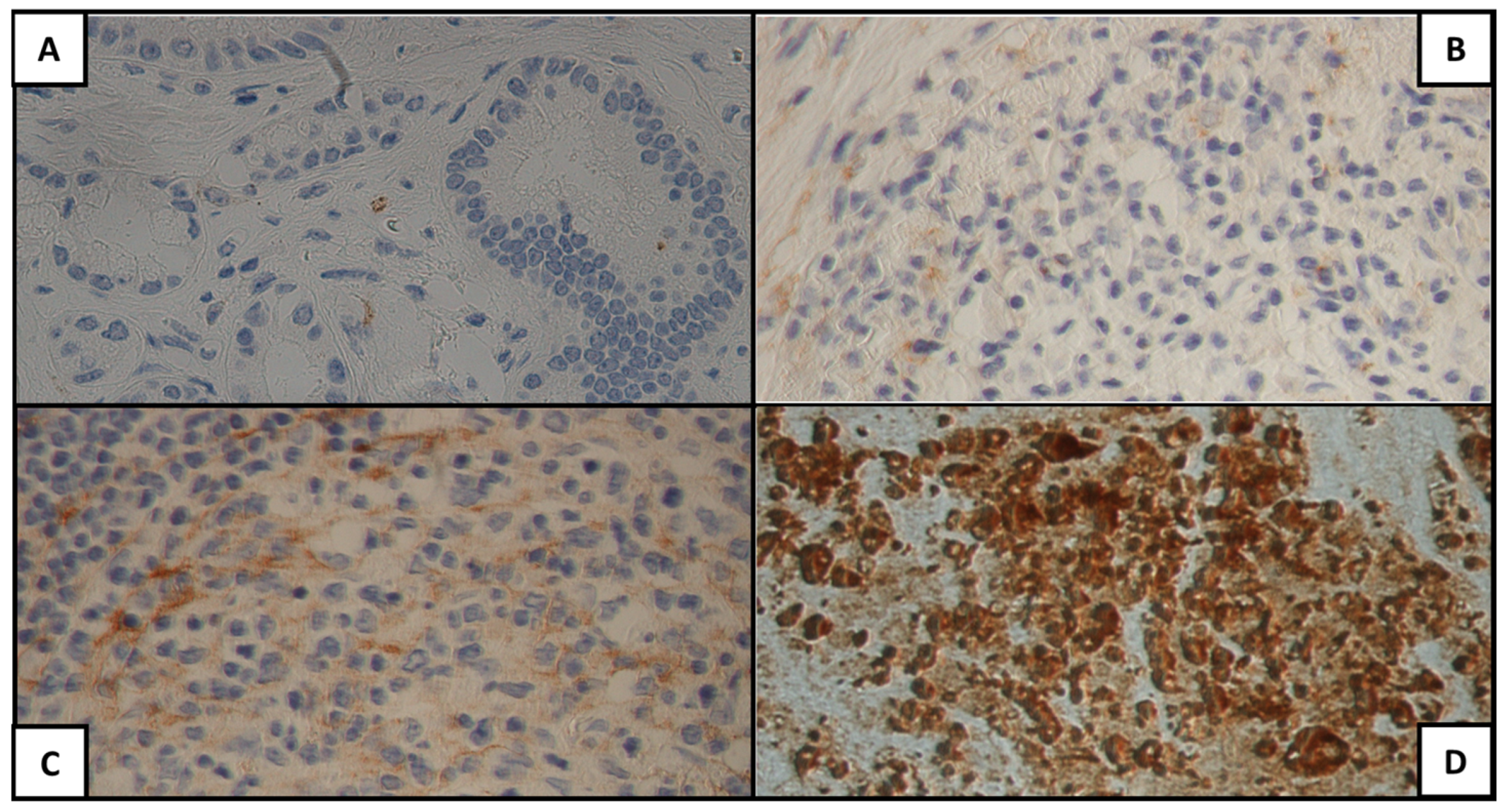

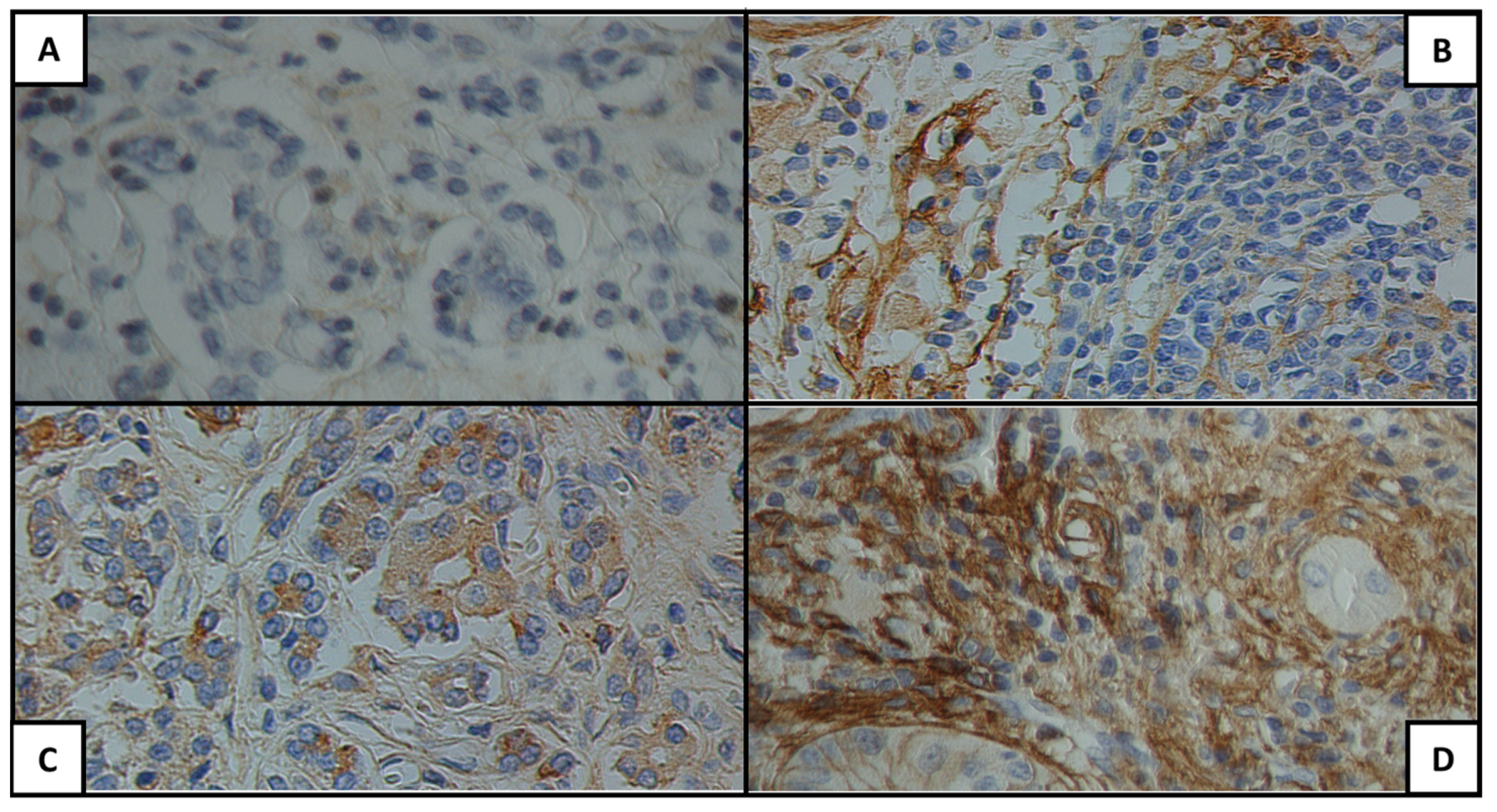
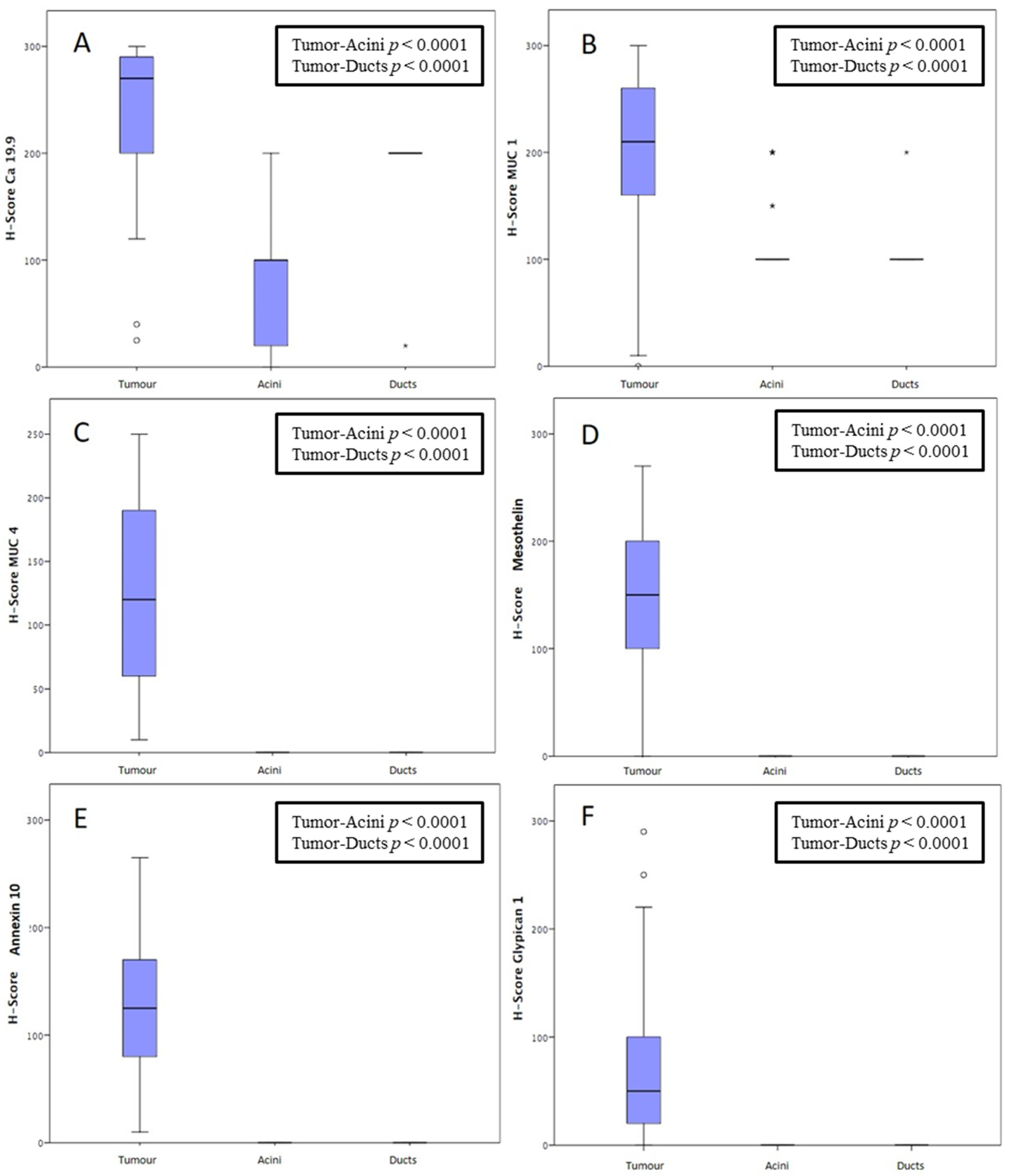
| Characteristic | Median (IQR)/Patients n (%) |
|---|---|
| Age (years) | 67.5 (57.7–72.2) |
| Sex | M: 31 (62); F: 19 (38) |
| Diabetes mellitus | 14 (28) |
| Smokers | 32 (64) |
| BMI > 25 kg/m2 | 9 (18) |
| Familiar history | 17 (34) |
| Chronic pancreatitis | 1 (2) |
| Tumor Site | Patients n (%) |
|---|---|
| Head Body Tail | 41 (82) 6 (12) 3 (6) |
| T | Patients n (%) |
| 1 2 3 4 | 3 (6) 42 (84) 5 (8) 0 (0) |
| N | Patients n (%) |
| 0 1 2 | 13 (26) 23 (46) 14 (28) |
| Tumor Stage | Patients n (%) |
| IA IB IIA IIB III IV | 3 (6) 10 (20) 0 (0) 23 (46) 14 (28) 0 (0) |
| Grading | Patients n (%) |
| G1 G2 G3 | 1 (2) 44 (88) 5 (10) |
| CA 19-9 Tumor | Patients n (%) | CA 19-9 Acini | Patients n (%) | CA 19-9 Ducts | Patients n (%) |
|---|---|---|---|---|---|
| H-score 0 H-score 1+ H-score 2+ H-score 3+ | 0 (0) 2 (4) 5 (10) 43 (86) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 10 (20) 6 (12) 25 (50) 9 (18) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 0 (0) 1 (2) 0 (0) 49 (98) |
| MUC1 Tumor | Patients n (%) | MUC1 Acini | Patients n (%) | MUC1 Ducts | Patients n (%) |
| H-score 0 H-score 1+ H-score 2+ H-score 3+ | 1 (2) 4 (8) 7 (14) 38 (76) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 0 (0) 0 (0) 44 (88) 6 (12) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 0 (0) 0 (0) 49 (98) 1 (2) |
| MUC4 Tumor | Patients n (%) | MUC4 Acini | Patients n (%) | MUC4 Ducts | Patients n (%) |
| H-score 0 H-score 1+ H-score 2+ H-score 3+ | 0 (0) 12 (24) 18 (36) 20 (40) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 50 (100) 0 (0) 0 (0) 0 (0) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 50 (100) 0 (0) 0 (0) 0 (0) |
| MSLN Tumor | Patients n (%) | MSLN Acini | Patients n (%) | MSLN Ducts | Patients n (%) |
| H-score 0 H-score 1+ H-score 2+ H-score 3+ | 1 (2) 7 (14) 20 (40) 22(44) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 50 (100) 0 (0) 0 (0) 0 (0) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 50 (100) 0 (0) 0 (0) 0 (0) |
| ANXA10 Tumor | Patients n (%) | ANXA10 Acini | Patients n (%) | ANXA10 Ducts | Patients n (%) |
| H-score 0 H-score 1+ H-score 2+ H-score 3+ | 1 (2) 7 (14) 20 (40) 22(44) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 50 (100) 0 (0) 0 (0) 0 (0) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 50 (100) 0 (0) 0 (0) 0 (0) |
| GPC1 Tumor | Patients n (%) | GPC1 Acini | Patients n (%) | GPC1 Ducts | Patients n (%) |
| H-score 0 H-score 1+ H-score 2+ H-score 3+ | 2 (4) 24 (48) 20 (40) 4 (8) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 50 (100) 0 (0) 0 (0) 0 (0) | H-score 0 H-score 1+ H-score 2+ H-score 3+ | 50 (100) 0 (0) 0 (0) 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoletti, A.; Vitale, F.; Quero, G.; Paratore, M.; Fiorillo, C.; Negri, M.; Carlino, A.; Inzani, F.; Gasbarrini, A.; Alfieri, S.; et al. Immunohistochemical Evaluation of the Expression of Specific Membrane Antigens in Patients with Pancreatic Ductal Adenocarcinoma. Cancers 2023, 15, 4586. https://doi.org/10.3390/cancers15184586
Nicoletti A, Vitale F, Quero G, Paratore M, Fiorillo C, Negri M, Carlino A, Inzani F, Gasbarrini A, Alfieri S, et al. Immunohistochemical Evaluation of the Expression of Specific Membrane Antigens in Patients with Pancreatic Ductal Adenocarcinoma. Cancers. 2023; 15(18):4586. https://doi.org/10.3390/cancers15184586
Chicago/Turabian StyleNicoletti, Alberto, Federica Vitale, Giuseppe Quero, Mattia Paratore, Claudio Fiorillo, Marcantonio Negri, Angela Carlino, Frediano Inzani, Antonio Gasbarrini, Sergio Alfieri, and et al. 2023. "Immunohistochemical Evaluation of the Expression of Specific Membrane Antigens in Patients with Pancreatic Ductal Adenocarcinoma" Cancers 15, no. 18: 4586. https://doi.org/10.3390/cancers15184586
APA StyleNicoletti, A., Vitale, F., Quero, G., Paratore, M., Fiorillo, C., Negri, M., Carlino, A., Inzani, F., Gasbarrini, A., Alfieri, S., & Zileri Dal Verme, L. (2023). Immunohistochemical Evaluation of the Expression of Specific Membrane Antigens in Patients with Pancreatic Ductal Adenocarcinoma. Cancers, 15(18), 4586. https://doi.org/10.3390/cancers15184586








