Simple Summary
HER2-positive breast cancer (BC) is associated with an aggressive clinicopathological nature and is known to have a poor prognosis. Their tumor proliferation features can lead to visceral metastasis disseminating to the brain, liver, lung, and bone. Delivering standard chemotherapy HER2 blockers is strongly associated with better outcomes and can help increase the suitability for breast-conserving surgery. It is crucial to identify patients who should be selected for systemic cancer treatment before or after surgery and to decide the most appropriate option in each case. A thorough search and data collection were carried out using electronic scientific libraries and compiled with the most relevant and updated information about the stratification of BC recurrence risk and standard treatment options for each case.
Abstract
Background: The overexpression of the human epidermal growth factor receptor 2 (HER2+) accounts for 15–20% of all breast cancer phenotypes. Even after the completion of the standard combination of chemotherapy and trastuzumab, relapse events occur in approximately 15% of cases. The neoadjuvant approach has multiple benefits that include the potential to downgrade staging and convert previously unresectable tumors to operable tumors. In addition, achieving a pathologic complete response (pCR) following preoperative systemic treatment is prognostic of enhanced survival outcomes. Thus, optimal evaluation among the suitable strategies is crucial in deciding which patients should be selected for the neoadjuvant approach. Methods: A literature search was conducted in the Embase, Medline, and Cochrane electronic libraries. Conclusion: The evaluation of tumor and LN staging and, hence, stratifying BC recurrence risk are decisive factors in guiding clinicians to optimize treatment decisions between the neoadjuvant versus adjuvant approaches. For each individual case, it is important to consider the most likely postsurgical outcome, since, if the patient does not obtain pCR following neoadjuvant treatment, they are eligible for adjuvant T-DM1 in the case of residual disease. This review of HER2-positive female BC outlines suitable neoadjuvant and adjuvant systemic treatment strategies for guiding clinical decision making around the selection of an appropriate therapy.
1. Introduction
Breast cancer (BC) is a heterogenous disease with a wide range of tumor morphology phenotypes [1]. Nearly 75% of BC cases demonstrate luminal differentiation expressing endocrine receptor (ER) positivity [2]. The overexpression of the human epidermal growth factor receptor 2 (HER2+), determined by immunohistochemistry (IHC) and in situ hybridization (ISH) methods, accounts for 15–20% of all BC cases [3].
ER+ HER2– BC has the most indolent course and the best prognosis among the BC subtypes, despite the tropism for disseminating to bones [4,5]. Conversely, HER2+ BC is associated with an aggressive clinicopathological nature and a poor prognosis. Their tumor proliferation features can lead to visceral metastasis disseminating to the brain, liver, and lung, in addition to the bone [6]. Eventually, most patients who have HER2+ BC recurrence will die of their disease [7]. While most patients with luminal A, ER+ HER− BC, are treated in the adjuvant setting with oral endocrine therapy and do not require chemotherapy, the mainstay of treatment for the luminal B, ER+ HER2+ BC subtype is chemotherapy with the monoclonal antibody (mAB) trastuzumab, administered before or after surgery [5,8].
The neoadjuvant approach has multiple benefits that include the potential to improve staging, converting previously unresectable tumors to operable tumors, and, from a cosmetic perspective, an increased suitability for breast-conserving surgery. Exposing patients to neoadjuvant chemotherapy (NAC) can predict the chemosensitivity of tumors to conventional cytotoxic agents and, most importantly, is prognostic of the outcome, helping to optimize treatment decisions in patients with residual cancer after chemotherapy [9].
The advent of HER2-targeted therapies has led to a novel systematic approach, revolutionizing the treatment of HER2+-enriched tumors and improving the prognosis of this particular BC population [6]. Between 47% and 65.2% of patients receiving NAC plus trastuzumab will successfully achieve a complete eradication of malignant cells at the microscopic level, also known as pathological complete response (pCR) [10,11,12]. Many studies have demonstrated that achieving a pCR, following preoperative chemotherapy, is strongly associated with better outcomes [13,14]. The CTNeoBC pooled analysis of 12 trials including approximately 12,000 BC patients treated with preoperative chemotherapy followed by surgery showed that pCR, which included the breast and lymph node (LN), was associated with improved event-free survival (EFS) and OS when compared to pCR in the breast alone [9]. The tumor response to NAC varies depending on the BC subtype. There is a high association between pCR and improved survival in the HER2+ BC, as well as in the triple-negative breast cancer (TNBC) subtype, but it is more significant in latter group, despite HER2+ BC being a candidate for targeted treatment with trastuzumab [9]. Among the HER2+ subgroups, ER− is more susceptible to achieving pCR events as compared to the luminal B, ER+ phenotype [9,15].
Although two decades have passed and trastuzumab remains the cornerstone therapy for the HER2 populations, relapse events occur in approximately 15% of BC cases, even beyond the completion of standard chemotherapy with trastuzumab [4,16]. Out of these, 8% will reoccur within 4 years, despite the improvement in pCR rates [17,18]. Much work is needed to better understand HER2+ BC, especially as research indicates that acquired resistance may be due to the immunogenic nature of the pathophysiology. Further research will also help develop more effective therapies to counteract this acquired resistance [19].
This article highlights the latest evidence of neoadjuvant and adjuvant treatments for patients diagnosed with HER2+ BC and includes the following: chemotherapy with or without anthracycline, single or dual blockage agents against HER2, and an optimal therapy duration. In addition, this paper will briefly review critical oncodriver mutations and molecular pathways crossing with HER2+, as well as inherent caveats, pitfalls, and future directions.
2. Materials and Methods
To warrant the inclusion of updated medicine-based evidence relevant to clinical practice, a literature search was conducted with scientific journals of published systematic reviews and meta-analyses, with clinical trials and abstracts from oncology conferences, and by searching the Embase, Medline, and the Cochrane electronic libraries. The search was conducted between January 2002 and May 2023 in the English language.
3. HER2 as an Oncogenic Molecular Driver
HER2 is an oncogenic membrane-spanning receptor of a signaling pathway located in the intracellular and extracellular domains (ECD). HER2 belongs to the family of epidermal growth factor receptor tyrosine kinases known as EGFR or ErbB, encompassing four structurally analogous receptors including HER1, HER2, HER3, and HER4 [20,21]. When HER2 is overexpressed, it generates a rearrangement of the HER family of dimers, leading to a significant augmentation of the HER2–containing heterodimers and homodimers and enabling the HER2 receptor activation by dimerization with another HER family member [22,23]. Hence, by eliciting a cascade of transduction signals partaking in the PI3K/Akt/mTOR and MAPK molecular alleyways, it leads to the regulation of cellular proliferation and differentiation, thus triggering tumor growth [23].
4. Classification of HER2+ Tumors
Based on the American Society of Clinical Oncology and the College of American Pathologists (ASCO/CAP) guidelines, HER2+ is defined as IHC 3+ (circumferential membrane staining including >10% of cells) or IHC 2+ combined with HER2 amplification by an ISH assay of the invasive component of a BC specimen using single/dual-probe ISH. There are intricacies for determining HER2 positivity based on the ISH pattern. Nonetheless, to summarize, a single signal probe ISH is based on the average HER2 copy number and is considered negative when the average HER2 copy number is <4.0 signals/cell and positive when the average HER2 copy number is ≥6.0 signals/cell. However, when the estimated HER2 is ≥4.0 and <6.0 signals/cell, the HER2/chromosome enumeration probe 17 (CEP17) ratio should be ≥2.0 in order for HER2 to be considered positive [24].
5. Treatment Strategies for HER2+ Breast Cancer
5.1. Monoclonal Antibody against HER2
Trastuzumab was the first targeted humanized mAB against HER2 to be approved by the US Food and Drug Administration (FDA) [25,26]. By binding to domain-4 on the juxtamembrane region of the HER2 ECD, trastuzumab disrupts ligand-independent downstream signaling pathways to the intracellular environment, triggering antibody-dependent cell-mediated cytotoxicity. Through immune mechanisms, where antibody-coated cells target the surface of tumor-derived antigens, cascade processes are prompted to inhibit cell cycle arrest and tumor angiogenesis via antibody-dependent cellular toxicity (ADCC) [27,28].
The advent of trastuzumab has been a remarkable breakthrough in the history of BC, due to its ability to prolong survival and improve quality of life (Figure 1). Trastuzumab has dramatically changed the trajectory of HER2+ populations, not only in BC but in other malignancies such as those involving the gastro-esophageal junction, colorectal, and endometrial carcinomas [29,30,31,32].
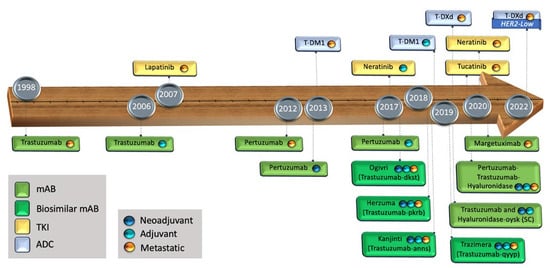
Figure 1.
Timeline of the FDA-approved targeted treatments against the HER domains. mAB: monoclonal antibody, TKI: tyrosine kinase inhibitor, ADC: antibody drug conjugate, SC: sub-cutaneous.
The first usage of trastuzumab was in combination with conventional chemotherapy in metastatic HER+ BC [33,34]. These outcomes were further replicated in non-metastatic HER+ disease [35,36]. In this manner, trastuzumab, in combination with taxane-based chemotherapy, has paved the way for understanding multimodal treatments in the adjuvant and neoadjuvant settings in HER2+ BC [35,37].
A study conducted by The Early Breast Cancer Trialists’ Collaborative group (EBCTCG), with 13,864 patients revealed noteworthy results when adding trastuzumab to standard adjuvant chemotherapy. At 10 years, there was an absolute risk reduction in breast cancer-specific mortality (BCSM) by 6.4% (95% CI, 4.9–7.8) and in all-cause mortality by 6.5% (95% CI, 5.0–8.0). The 10-year relapse event rate decreased by 9% (95% CI, 7.4–10.7) when compared to no trastuzumab. The risk of recurrence was more pronounced in the early years, as compared to 10 years and beyond [36].
5.2. Dual HER2 Blockage with mABs
Pertuzumab is a recombinant humanized mAB binding to subdomain II of the ECD of HER2, inhibiting dimerization and subsequent receptor ligand-dependent signaling [3]. In contrast to trastuzumab, pertuzumab interrupts the HER heterodimerization on the HER1 (or EGFR), HER2, HER3, and HER4 domains, stopping the molecular transduction of downstream tumor signaling [38]. Trastuzumab and pertuzumab have complementary mechanisms of action and potential synergist effects on antitumor activity when administered together [38,39].
5.3. Antibody–Drug Conjugate
Ado-trastuzumab emtansine (T-DM1) was the foremost antibody–drug conjugate (ADC) targeting HER2 to be approved by the FDA [40,41] (Figure 1). T-DM1 is composed of the monoclonal antibody trastuzumab linked to a microtubule polymerization inhibitor, mertansine (DM1), via a non-cleavable thioether linker [42,43].
5.4. TKIs
The tyrosine kinase inhibitors (TKIs) lapatinib and neratinib, through competitive molecular mechanisms, target the catalytic kinase in the HER2 intracellular domain, promoting a disruption of the ATP phosphorylation-dependent processes, which leads to cancer cell angiogenesis disruption and tumor shrinkage. While lapatinib is a reversible TKI on the EGFR and HER2 domains, neratinib is an irreversible TKI targeting the HER1, HER2, and HER4 pathways [8,44].
6. Defining Risk Categories
While achieving pCR is the most valuable predictive factor associated with better recurrence-related results, pathologic residual disease (pRD) indicates a higher risk for the poorest survival and for disease relapse [45]. At baseline, HER2+ BC patients with tumors > 20 mm and with the detection of LN+ prior to surgery, or postoperatively (following NAC with trastuzumab), and with residual tumors > 10 mm or LN+ are highly associated with early relapse events [45,46,47]. When there is pRD in the breast only, tumors > 10 mm have an estimated increased recurrence risk of 16% when compared to sub-centimeter tumor sizes or pCR. When pRD is seen in LNs, there is an increased relapse risk by at least 50% in those with ≥2 LNs+ when compared to those with 1 LN+ [46].
Other contributory factors related to increased risk and linked to worse recurrence rates are an age less than 50 years at the time of diagnosis, low levels of tumor-infiltrating lymphocytes (TILs), as well as a body mass index (BMI) ≥ 25 [45,48].
The BC recurrence risk attributed to the ER status appears to be linked to the time at which chemotherapy is delivered, whether it is administered before or after surgery, but more supportive data around this are needed. HER2+ BC with ER− is more likely to achieve pCR as compared to the ER+ phenotype, reflecting higher chances of relapse in the latter group [9,15]. However, in BC patients with pRD, many studies did not find a correlation between the ER status and OS or DFS [49,50,51]. Nevertheless, though less prone to attaining a tumor response due to decreased chemotherapy sensitivity, ER+ HER2+ patients who achieve pCR are likely to have a favorable prognosis [45].
For physician decision making, it is important to identify which patients will benefit from systemic treatment upfront versus after surgery. These decisions are typically determined by the BC risk of recurrence. The evaluation of factors such as breast cancer staging based on radiological and clinical–pathological features characterizing the breast tumor size, nodal involvement, and HER2 levels of expression is required [52]. For example, BC with uninvolved LN and tumors measuring up to 20 mm, which correspond to anatomic stages (by The American Joint Committee on Cancer—AJCC [53]) I to IIA, are preferred for upfront surgery, followed by adjuvant systemic treatment [54]. Conversely, high-risk patients with larger tumors (≥T2 stage or >20 mm) and/or with axillary LN+ involvement, encompassing BC stages IIB and III, are mostly suitable for a multimodal disciplinary approach which consists of the following: neoadjuvant systemic therapy, followed by radical or conservative breast surgical resections, usually along with LN assessment with sentinel LN biopsy or axillary LN dissection [54,55]. These approaches should be followed by adjuvant systemic treatment and, if appropriate, the subsequent administration of endocrine and local radiation therapies [52]. The selection between HER2 blockers to be given after surgery would depend on microscopic pathology appraisal, where patients with pRD on the surgical specimen should be considered for adjuvant treatment modalities [9] (Figure 2).
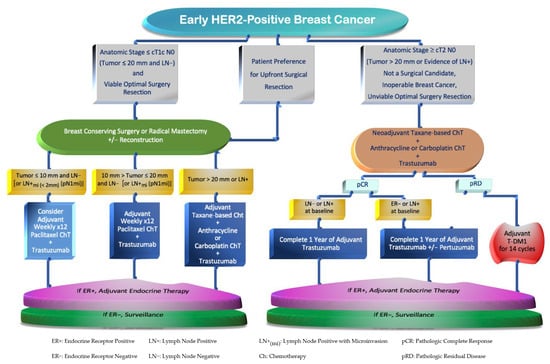
Figure 2.
Flowchart of Systemic Therapy Strategies in Early HER2-Positive Breast Cancer.
7. Adjuvant Treatment
7.1. Very Low-Risk HER2+
Most randomized controlled trials (RCTs) for early HER2+ BC excluded patients with tumors ≤ 10 mm with no LN involvement (T1abN0) [56]. The data available for this population are mostly derived from non-randomized studies with a reduced sample size in most cases [36,57]. Hence, it is not clear whether small HER2+ tumors should be treated with standard-of-care single-agent chemotherapy and trastuzumab. A recent systematic review and meta-analysis, including 12 studies and almost 7000 patients, investigated whether patients with T1abN0 HER2+ BC would benefit from adjuvant trastuzumab. A significant DFS improvement was seen in patients treated with versus without chemotherapy and trastuzumab. The absolute increased benefit of trastuzumab over no trastuzumab was 7% at a 5-year DFS and less than 1% at the 3-year OS. However, even in those patients who did not receive trastuzumab or chemotherapy, their prognosis was reasonably good, with a 5-year OS of 95.9% and a DFS of 88.3% [58]. Only a few studies evaluated the effect of chemotherapy plus trastuzumab in small HER2+ tumors based on the ER status, suggesting that a larger population sample is needed to ascertain robust conclusions regarding these subgroups [58,59]
7.2. Low-Risk HER2+
A meta-analysis that assembled data from five trials and included 4220 patients with breast tumors ≤ 20 mm (pT1c) evaluated participants who were randomized to receive adjuvant trastuzumab versus no trastuzumab. With a median follow up of 8 years, there was a substantial OS and DFS advantage for those treated with trastuzumab. However, the compiled population in this meta-analysis was highly heterogeneous, as it included patients with LN– and LN+ as well as different lengths of trastuzumab administration varying from 9 weeks to 24 months [60].
The phase II APT trial was a single-arm study that recruited HER2+ BC patients with LN– and tumors < 3 cm in size, although 91.1% of that study population had tumors ≤ 2 cm. The patients received 12 administrations of weekly paclitaxel with trastuzumab, followed by one year of maintenance trastuzumab [61,62]. The updated 7-year analysis showed an OS of 95% (95% CI, 92.4–97.7) and a DFS of 93.3% (95% CI, 90.4–96.2%) [62]. The 10-year breast cancer-specific survival (BCSS) was 98.8% (95% CI, 97.6–100%) [63]. Interestingly, despite the omission of anthracycline chemotherapy in the APT trial, the long-term benefit continues to be seen in low-risk HER2+ BC patients [62].
The ATEMP trial is a phase II study that, with a 3:1 allocation ratio, compared the efficacy between 1 year (or 17 cycles) of T-DM1 versus paclitaxel with trastuzumab, the TH regimen for HER2+ BC with stage I disease, in the adjuvant setting. The study result showed a significant 3-year iDFS advantage of 4.4% on T-DM1 [64]. Although there was no difference in the quality of life (QOL) assessment between the two arms, higher rates of treatment discontinuation and dose reductions were seen in patients treated with T-DM1 versus TH, and these were more frequent in patients with an age above 50 years. Hence, T-DM1 could potentially be an alternative option for patients who prefer to avoid alopecia and neuropathy, both of which are typical adverse events (AEs) induced by taxane chemotherapy [64,65].
In summary, very low-risk and low-risk HER2+ BC, defined as anatomic stage T1ab and T1cN0, respectively, with a clinical or radiological breast tumor size estimation of 20 mm or less and with no suspicious LN or biopsy-proven LN at baseline, are candidates for receiving 12 cycles of adjuvant paclitaxel chemotherapy and trastuzumab for 1 year, as long as the surgical findings are consistent with the pre-surgical evaluation.
7.3. High-Risk HER2+
In the higher-risk groups comprising BC stages II or III, the combined analysis of the phase III trials NSABP B-31 and NCCTG N9831 showed that, regardless of the breast tumor size, ER status, LN involvement, or age groups, trastuzumab had a substantial OS and DFS benefit in all subgroups [15,29]. In these trials, women with HER2+ BC, with LN+ or LN–, and with high-risk features (defined as ER+ with a tumor size > 20 mm and ER− with tumors > 10 mm) were randomly allocated to receive adjuvant chemotherapy with doxorubicin and cyclophosphamide, followed by weekly paclitaxel (AC→T), plus/minus 1 year of trastuzumab. At 8.4 years, those who received trastuzumab had a significant risk reduction in mortality of 37% (95% CI, 0.54–0.73) and a reduction in relapse events of 40% (95% CI, 0.53–0.68) [29].
It is a viable option to deliver systemic therapy in the adjuvant setting for HER2+ BC patients with high-risk features, with clinical or radiological staging ≥ T2 or pathological evidence of LN+. However, this high-risk population would potentially benefit more by receiving treatment in a neoadjuvant scenario. This is because up to 65% of them could achieve pCR post-NAC and hence derive additional long-term survival benefits [11,12].
7.4. Adjuvant Dual HER2 Blockage
The first phase III RCT to use the dual HER2 blockage trastuzumab and pertuzumab (H+P), in combination with taxane-based chemotherapy, was the CLEOPATRA trial, which included patients with metastatic or unresectable HER2+ BC. This study showed a survival extension of 16.3 months with the dual combination when compared to trastuzumab alone [66]. In an attempt to replicate these results in the adjuvant scenario, the phase III, double-blinded randomized APHINITY trial tested trastuzumab plus/minus pertuzumab in patients with LN+ or high-risk LN– HER2+ BC [67]. There was no significant OS advantage between trastuzumab alone and that in combination with pertuzumab, with HR 0.83 (95% CI, 0.68–1.02). The updated 8-year OS was 92.7% for dual combination therapy H+P and 92.0%, for single therapy. There was a relative IDFS improvement in reducing distant and locoregional recurrence of 23% (95% CI, 0.66–0.91). In the analysis of subgroups, there was an absolute IDFS benefit of 4.5% (95% CI, 0.59–0.87) in patients with LN+ and one of 3% (95% CI, 0.59–0.92) in those with ER− [68].
The phase III KAITLIN trial recruited HER2+ BC patients with a high risk, with 89.8% of the population having LN+. The participants were randomly assigned to receive an adjuvant anthracycline-containing regimen followed by taxane chemotherapy in combination with H+P versus anthracycline followed by pertuzumab with 18 cycles of T-DM1. The combination H+P was administered for 1 year. There was no invasive DFS difference between the two study arms [69].
None of the adjuvant trials showed a significant OS advantage by using single versus dual HER2 blockage. However, the usage of adjuvant H+P demonstrated a significant but small advantage in select HER2+ BC patients, indicating that those with LN+ and ER− benefit the most from dual combination therapy.
8. Neoadjuvant Treatment
Candidates who derive the greatest advantage from receiving upfront systemic therapy and trastuzumab are those with high-risk features in HER2+ BC, patients with inoperable diseases, or those with an unfeasible optimal surgical resection at baseline.
In the randomized phase III NOAH trial, women with HER2+ BC with locally advanced or inflammatory disease were assigned to receive NAC alone versus NAC with trastuzumab followed by 1 year of maintenance trastuzumab. At 5 years, the absolute difference in EFS was 15% (HR 0.64 (95% CI, 0.44–0.93)), and in BCSS, it was 13% (HR 0.59 (95% CI, 0.37–0.92)). These outcomes favored those treated with NAC containing trastuzumab. In these trials, there was a high correlation between pCR and enhanced EFS in patients who received trastuzumab [70].
Knowing that pCR is a potential surrogate marker associated with improved survival outcomes, a systematic review and meta-analysis of seventy-eight RCTs, with 25,150 HER2+ BC patients treated with NAC, showed a significant association between pCR and enhanced OS. The OS at 3 and 5 years revealed a significant mortality rate reduction in those who had pCR with a respective HR of 0.25 (95% CI, 0.13–0.47) and 0.26 (95% CI, 0.20–0.33) [13]. When compared to patients who had pRD, the 5-year absolute OS advantage in those who reached pCR was 13.5%, and the event-free survival was 19.6%. These results highlighted the importance of pCR as a prognostic and surrogate factor for longer survival. Although, over time, as the survival differences amplify in those who had pCR versus pRD, a precise estimate of the survival advantage remains difficult to quantify [13,14]. Notably, many trials explored whether adding a second HER2 blocker to trastuzumab in the neoadjuvant setting would maximize the treatment response, further highlighting the importance of optimizing pCR rates in HER2+ BC.
The NeoSphere is a phase II, open-label randomized trial comparing the addition of pertuzumab to standard trastuzumab with the neoadjuvant FEC→D regimen, which comprises 5-fluorouracil, epirubicin, and cyclophosphamide followed by docetaxel (FEC→DH+P) versus standard-of-care NAC with trastuzumab (FEC→DH). The NeoSphere’s result showed a pCR improvement rate of 16.8% in the arm containing pertuzumab. Notably, almost one-third of the NeoSphere study population reached pCR, and this outcome was identified in 45.8% (95% CI, 36.1–55.7) of patients treated with H+P and in 29% (95% CI, 20.6–38.5) of those who received the single-mAB trastuzumab [71]. The intervention group with dual-HER2 blockage (H+P) was associated with higher 5-year PFS rates (86% versus 81%, respectively (HR 0.69 (95% CI, 0.34–1.40))) [71,72]. In the long term, there were no issues from a safety profile standpoint with the addition of pertuzumab. The left ventricular ejection fraction (LVEF) dysfunction was found to be 3% in the H+P combination group, compared to 0% in the trastuzumab group [71,72].
Another meta-analysis of twenty-six studies which included 9,872 patients evaluated the safety and efficacy of the dual HER+ blockers. AEs such as rash, diarrhea, epistaxis, mucosal inflammation, and anemia were significantly more frequent with H+P as compared to with trastuzumab alone, whereas myalgia was less frequent (OR 0.91 (95% CI, 0.82–1.01); p = 0.072) in the combination treatment. Nevertheless, these AEs were deemed acceptable and manageable. Moreover, no significant difference in cardiac toxicity was observed between these therapies (OR 1.26 (95% CI, 0.81–1.95)) [73]. Regarding the efficacy endpoint, the study revealed that NAC with H+P significantly increased the pCR rate (OR 1.33 (95% CI, 1.08–1.63)). The ER− subgroup had the most substantial pCR outcome from the combined H+P treatment versus trastuzumab alone, with an absolute pCR rate almost three times higher, when compared to patients with ER+ status in HER2+ BC [73].
In the phase II KRISTINE trial, 444 patients were randomized to receive six cycles of neoadjuvant T-DM1 plus pertuzumab (T-DM1+P) versus docetaxel, carboplatin, and trastuzumab plus pertuzumab (TCH+P). In the adjuvant setting, patients previously allocated to each of the study arms received 12 cycles of T-DM1+P or H+P, based on randomization [74]. The pCR rates were reached in 44.4% of the study participants in the T-DM1+P and in 55.7% in the TCH+P groups. Thus, there was an absolute advantage in achieving pCR of 11.3% ((95% CI, 20.5–2.0); p = 0.016) in the TCH+P group versus the control group. Relapse events (HR 2.61 (95% CI, 1.36–4.98)) were more frequent in patients treated with T-DM1+P, including locoregional progression before surgery (6.7% versus 0%). Treatment discontinuation due to AEs was four times higher in the TDM1-containing arm as compared to the group receiving TCH+P [74,75].
A meta-analysis involving four phase II–III RTCs (CALGB-40601, ChER−LOB, NSABP-B41, NeoALTTO) included 1410 HER2+ BC patients treated with NAC and trastuzumab, lapatinib, or both combined. The pooled analysis showed that lapatinib plus trastuzumab, compared to trastuzumab alone, significantly improves OS and recurrence-free survival (RFS) by reducing the relative risk of mortality and recurrence by 35% and 37%, respectively. In the subgroup of ER− HER+ patients, pCR was correlated with a 73% decreased risk of mortality [76]. With more frequent grade ≥ 3 AEs and higher rates of early treatment discontinuation in the lapatinib-containing arms (predominantly diarrhea and rash), the role of lapatinib in early HER2+ BC has been dubious [77,78,79].
9. Adjuvant HER2 Therapy in Postoperative Pathologic Residual Disease
Knowing that patients who did not achieve pCR have worse BC outcomes, the phase III KATHERINE trial attempted to improve relapse rates in HER2+ BC patients who had pRD on the surgical specimen following taxane-based NAC (with or without anthracycline) plus trastuzumab. Notably, almost 20% of the accrued study population received dual HER2+ monoclonal antibodies in the neoadjuvant setting. The participants were assigned to receive 14 cycles of T-DM1 or trastuzumab, postoperatively. At 3 years, 88.3% of patients treated with T-DM1 and 77.0% of those treated with trastuzumab were free of recurrence, translating to a 50% risk reduction of combined invasive BC recurrence and death events in the T-DM1 arm versus the control arm with trastuzumab (HR 0.50 (95% CI, 0.39–0.64)). The OS analysis was premature (HR 0.70 (95% CI, 0.47–1.05)), as a sufficient number of death events were not statistically seen in both study arms [80]. AEs of grade three and of any grade were 5.5% and 10.3% higher, respectively, in the T-DM1 group [51,80].
The ExteNET is a phase III RCT that recruited 2,840 participants with stages I–IIIc HER2+ BC. This study investigated whether extending the adjuvant dual blockage therapy provides a survival advantage. Patients who had received standard-of-care surgery and chemotherapy and had completed adjuvant trastuzumab were enrolled to receive 1 year of neratinib versus placebo [81,82]. The most common AE of neratinib was diarrhea, with 40% being classified as a grade 3 event. The iDFS at 5 years was 90.2% and 87.7% in the intervention and control groups, respectively. A subgroup analysis of 1,631 ER+ patients revealed a 40% iDFS risk reduction in those treated with neratinib (95% CI, 0.43–0.83) [82]. Among the ER+ group, which had an early initiation of neratinib/placebo within 1 year after the completion of adjuvant trastuzumab, the absolute iDFS improvement was 5.1% (HR 0.58 (95% CI, 0.41–0.82)) [83]. Finally, the 8-year OS analysis showed no benefit with the usage of neratinib as an extended duration of adjuvant HER2 therapy (HR 0.95 (95% CI, 0.75–1.21)). At the 8-year mark, the subgroup analysis of OS revealed no substantial differences between the study arms in the ER+ and ER− groups [84].
10. Chemotherapy Backbone with versus without Anthracycline
In terms of chemotherapy, the optimal strategies comprise taxane-based regimens plus/minus an anthracycline component. The Dutch study TRAIN-2, a controlled phase III trial, randomized patients with stage II–III HER2+ BC to receive NAC with or without anthracycline. The anthracycline arm consisted of three cycles of FEC, followed by six cycles of carboplatin with paclitaxel. In the non-anthracycline groups, the patients received six or nine cycles of carboplatin with paclitaxel. Both parties received H+P [85]. At 3 years, the estimated EFS was 92.7% versus 93.6% (HR 0.90 (95% CI, 0.50–1.63)), and the OS was 97.7% versus 98.2% (HR 0.91 (95% CI, 0.35–2.36)) in those who received NAC with versus without anthracycline, respectively. These results revealed that HER2+ BC patients can safely have anthracycline omitted without compromising BC prognosis [85,86]. No statistical difference was seen in the pCR rates between the two groups. In those treated with anthracycline, there was a higher incidence of febrile neutropenia and LVEF impairment (7.7% versus 3.2%; p = 0.04), when compared to treatment without anthracycline. In this trial, cardiac dysfunction was defined as a drop of ≥10% in LVEF from baseline or a decrease of <50% of the total LVEF. However, symptoms secondary to cardiac dysfunction were rare in both groups, with 1% versus 0, respectively. Regardless of the ER or LN status, these results support that the omission of anthracycline within NAC can be a safe and effective option and does not compromise a patient’s BC outcomes [85,86].
In the randomized phase III BCIRG 006 trial, HER2+ BC patients were allocated to receive one of the following three adjuvant treatment regimens: AC→T, AC→TH, or TCH. Approximately 72% of the accrued trial participants had LN+, from which a third of the total population had LN+ ≥ 4 axillary nodes. No significant differences in OS or DFS were identified between the two study arms containing trastuzumab that compared anthracycline versus non-anthracycline chemotherapy [35,87].
A recently published systematic review and meta-analysis included 11 RCTs with a total of 1,155 patients comparing NAC with versus without anthracycline in the HER2+ BC population. There was no difference in pCR rates between those comparators (OR 0.95 (95% CI, 0.61–1.48)). Amid these two arms, there was no difference in the rates of breast-conserving surgery (OR 1.18 (95% CI, 0.93–1.49)). However, a critical point to note in this meta-analysis was the heterogeneity of the population, comprising patients with a broad range of risk factors that could have potentially affected the study results [88].
The phase II TRYPHAENA trial, which was primarily focused on assessing cardiac safety, included 225 women with HER2+ BC who were to receive NAC with dual HER2 mAB blockage. Patients who were allocated to the anthracycline group received the FEC→DH+P regimen. The anthracycline sparring group comprised six cycles of TCH+P [89]. NAC promoted similar pCR event rates and recurrence outcomes of DFS and PFS between the study groups. And those who were exposed to anthracyclines were more likely to develop LVEF dysfunction when compared to patients exposed to the anthracycline-free regimen [89,90].
Hence, in high-risk HER2+ BC populations, doublet platinum taxane-based chemotherapy regimens, such as carboplatin with docetaxel, have been demonstrated to be a comparable alternative in select patients who should be spared from receiving anthracycline.
11. Optimal HER2 Treatment Duration
With regard to the escalation and de-escalation of treatment, the HERA trial did not show a benefit when adjuvant trastuzumab was extended to 2 years, compared to the 1-year standard duration [91]. Three of the most relevant non-inferiority trials with similar study designs, comparators, and endpoints investigated the optimal duration of mABs against HER2, comparing a 6- versus 12-month administration of adjuvant trastuzumab [92]. While the PERSEPHONE study achieved its prespecified non-inferior boundaries for OS and DFS at 4 years (HR 1.07 (95% CI, 0.93–1.24), p = 0.011), the PHARE and HORG trials failed to support that the treatment with a shorter schedule is non-inferior to the standard duration at the 7.5-year and 3-year analyses, respectively [93,94,95]. To tiebreak these contrasting results, a high-level evidence meta-analysis compiled RCTs comparing 6 versus 12 months of adjuvant trastuzumab and emphasized that the shorter treatment length conferred a significantly reduced DFS benefit, with an HR of 1.22 ((95% CI, 1.09–1.38); p = 0.0008), despite the less frequent cardiotoxicity events [96,97]. A 6-month duration of trastuzumab reflected an increased absolute DFS risk by almost 4% at 5 years [98].
Despite the many attempts to achieve comparable outcomes by abbreviating or stretching the course of trastuzumab, the 1-year duration remains the optimal time for which trastuzumab should be delivered in the adjuvant setting.
12. Multigene Profiling Assays
The genomic expression assays (GEAs) help in tailoring optimal treatment whilst also helping to avoid unnecessary chemotherapy in women with BC ER+ HER2− [99]. By means of generating predictive risk scores, GEAs identify patients in whom adjuvant chemotherapy can be advantageous [99,100].
Given similar scores among BC tissue samples from biopsy and surgical specimens, the St. Gallen International Consensus Guidelines endorsed the use of GEAs to optimize neoadjuvant treatment strategies from biopsied samples, which has helped contribute to a practice change [101,102].
For patients with HER2+ BC disease, the novel HER2DX is a prognostic and predictive GEA encompassing 27 gene expression levels including ErbB2 mRNA, which, in combination with clinical and tumor features, identifies four gene signature profiles (immune, proliferation, luminal differentiation, and HER2 amplicon) [103]. The HER2DX is a validated gene profiling assay that can estimate the BC recurrence risk and NAC tumor response, quantifying the likelihood in achieving pCR [104]. The HER2DX can be helpful by determining in whom the escalation or de-escalation of chemotherapy is the most favorable strategy [18,103].
13. Tumor-Infiltrating Lymphocytes
Antitumor immune response can lead to the recruitment and formation of well-organized clusters of T-lymphocytes (mainly, CD4+T and CD8+T), B-lymphocytes, as well as natural-killer (NK) cells. In other words, TILs are such lymphocytic cells that move towards, and surround, the microenvironment of tumor cells [105,106]. Quantifying the volume of TILs embodying the breast tumor is essential in determining TIL levels [107,108]. The greater the TIL index, the higher the ratio between CD8+ and CD4+ T cells, implying an enhanced cytotoxic T-cell reaction [109]. In these cases, a high number of TILs has been associated with a better NAC response and improved outcomes in HER2+ and TNBC, in addition to being a reliable histologic predictor in determining HER2 positivity when IHC results are equivocal (score 2+) [107,110,111].
A recent meta-analysis included 29 published trials and 9145 participants with BC treated with NAC. In this pooled-analysis, a high number of TILs were demonstrated to be predictive of pCR (OR 2.54 (95% CI, 1.50–4.29)) and prognostic for OS (HR 0.93 (95% CI, 0.87–0.99)) and DFS (HR 0.96 (95% CI, 0.94–0.98)) in HER2+ patients. However, no association was found in ER+ BC patients [112]. In another study, the secondary analysis of the NeoALTTO trial, which included patients treated with neoadjuvant lapatinib and trastuzumab, demonstrated that for each 1% increase in the level of TILs, there was a 3% decrease in the BC relapse event rate [113].
TIF can also be predictive of a better efficacy of immune checkpoint inhibitors (ICIs) in BC patients [114]. Together with TILs, the programmed death-1 (PD-1) ligand-1 (PD-L1), as immune biomarkers, can identify tumors that are sensitive to immunotherapy, helping clinicians to select potentially successful candidates to be treated with ICIs [110].
14. Immune Checkpoint Inhibitors
By blocking signaling transduction on HER2-enriched cells with targeted HER2 blockers, an immune-mediated response is triggered. Thus, HER2 blockers can be interpreted through the lens of an immunotherapeutic strategy [115]. Emerging data have demonstrated potential synergism in promoting antitumor immunity between HER2 therapies and cytotoxic T lymphocyte-associated antigen 4 (CTLA4) or PD-1/PD-L1 inhibitors in BC patients [116,117]. In the metastatic setting, the PANACEA trial showed a durable clinical benefit of pembrolizumab with trastuzumab in heavily treated HER2+ BC patients with PD-L1+ tumors [118]. On the other hand, the KATE2 study did not show an advantage of adding atezolizumab to T-DM1 in previously treated advanced HER2+ BC [119].
The phase III IMpassion050 trial recruited high-risk (T2–4; N1–3) early HER2+ BC patients. Participants were randomized to receive atezolizumab/placebo with standard anthracycline-taxane based NAC with H+P. After surgery, trial candidates continued H+P and atezolizumab/placebo until a 1-year completion of the mABs against the HER2. This study showed that those with PD-L1-positive tumors had higher pCR rates versus those with PD-L1-negative tumors. No substantial difference in pCR rates was noted when atezolizumab was added to standard chemotherapy [120].
To date, TNBC has been the only subtype among all BC phenotypes to show a substantial benefit from being exposed to the combination NAC and ICI, leading to a practice change [121,122]. Ongoing trials continue to explore a potential role for checkpoint inhibitors in the management of early HER2+ BC. The ASTEFANIA trial is evaluating adjuvant atezolizumab/placebo in combination with T-DM1 in patients with pRD after surgery [123]. The APTneo is investigating NAC with atezolizumab with H+P in BC patients with high-risk features [124]. The NeoHIP is a neoadjuvant trial accruing candidates with a high risk for BC relapse, comprising weekly paclitaxel, with H+P with versus without pembrolizumab. After surgery, the selection of systemic treatment is in accordance with their treating physician’s discretion [125].
15. Future directions
15.1. Novel Targeted Therapies and Innovative Strategies
The antibody–drug conjugate trastuzumab deruxtecan (T-DXd) is a humanized mAB against the HER2 pathways, entwined with a topoisomerase-I inhibitor payload along with a tetrapeptide-centered cleavable linker. T-DXd has shown efficacy not only in the overenhanced HER marker but also within a range of low-HER expressions in HER2+ BC [126]. The positive results of the DESTINY-Breast03 trial demonstrated durable antitumor activity and an outstanding 67% risk reduction PFS with T-DXd versus T-DM1 in metastatic HER2+ disease. These results led to questions about whether T-DXd would be a promising adjuvant treatment in patients with operable HER2+ BC disease [127]. To answer this and other queries, the DESTINY-Breast05 is continuing investigation as an ongoing phase III, randomized, active-controlled trial of T-DXd versus T-DM1 in HER2+ BC patients, with high-risk features for disease relapse, who have residual invasive disease in the breast and/or axilla after NAC completion [128]. The DESTINY-Breast11 is also a phase III trial recruiting patients with high-risk HER2+ disease, comparing T-DXd followed by paclitaxel with H+P versus standard-of-care AC→TH+P [129].
Tucatinib is a reversible TKI that selectively targets EGFR-HER2 domains in HER2 enhanced cell lines [130]. Through ATP-competitive mechanisms, tucatinib inhibits phosphorylation and blocks the proliferation of downstream HER2 signaling [131]. The HER2CLIMB trial tested the addition of oral tucatinib with capecitabine and trastuzumab in metastatic HER2+ BC and showed an increased OS and PFS in patients previously treated with H+P and T-DM1 [7,132]. Due to its small molecule, tucatinib has the ability to cross the blood–brain barrier, providing sustainable control in patients with active brain metastasis [133]. The CompassHER2 Trial is a phase III, randomized study investigating the superiority of T-DM1 with versus without tucatinib in high-risk HER2+ BC patients who had pRD after neoadjuvant treatment with HER2–directed therapy [134].
Margetuximab is an engineered modified chimeric immunoglobulin G1 (IgG1) mAB that had undergone structural modification on the IgG1 crystallizable fragment (Fc) region [135,136]. Aiming to enhance innate and adaptive immune responses against HER2, margetuximab was designed to overenhance the affinity between IgG1-Fc and CD16A and their variants relative to the IgG1 wild type [137,138]. With the aim of identifying a better drug than trastuzumab, the phase III SOPHIA trial tested margetuximab with chemotherapy versus trastuzumab with chemotherapy in metastatic HER2+ BC patients who were previously heavily treated [136]. Although margetuximab achieved the desired safety endpoints, the final analysis failed to show an OS advantage of margetuximab versus trastuzumab [136,139]. However, the SOPHIA trial showed that there is a potential survival improvement of margetuximab in selected patients harboring CD16A allelic variants [139].
Another RCT evaluating margetuximab is the MARGOT study. This is an ongoing phase II trial recruiting HER2+ BC patients with anatomic stage II–III to compare the combination paclitaxel and pertuzumab with either margetuximab or trastuzumab in the neoadjuvant setting [140].
Pyrotinib is an oral TKI that, with irreversible mechanisms, targets the HER1, HER2, and HER4 domains [141]. Pyrotinib plus capecitabine has met PFS and intracranial objective response rate (ORR) endpoints, with acceptable AEs in patients with advanced HER2+ BC [142,143]. Based on the PHOEBE and PERMEATE trials, pyrotinib has been approved in China to be administered with capecitabine following progression on pertuzumab and trastuzumab in the metastatic setting [142,143,144]. With the intent to replicate the benefit of pyrotinib in the early BC setting, the PHEDRA trial is a randomized controlled study recruiting patients with high-risk HER2+ BC, a tumor size ≥ 2 cm, and LN+. The participants are assigned to receive neoadjuvant FEC→DH, in addition to daily pyrotinib versus placebo. After the completion of NAC, the subsequent cancer therapy is selected at the physicians’ discretion following current and local guidelines. The endpoints of pCR and ORR were higher in the pyrotinib group by 19.0% (95% CI, 9.5–28.4) and 9.7% (95% CI, 2.7–16.6), respectively, compared to the control group [144]. The OS data had not yet been reached in this study. The most common AEs of pyrotinib are grade three or four diarrhea, arising in more than 44% of cases [144,145].
Ongoing RCTs include NAC with pyrotinib, pertuzumab, trastuzumab, and nab-paclitaxel for HER2+ BC [146]. Therapies compounded between ADC and TKIs are being explored, such as the ARX788, which is an anti-HER2 agent with a tubulin payload. The ARX788 with pyrotinib is being compared to a doublet platinum chemotherapy such as the TCH+P in the neoadjuvant scenario [147].
15.2. De-Escalation of Adjuvant Therapy
Aiming to identify the undefined boundaries between low- and high-risk HER2+ BC, pCR has been utilized as a prognostic tool and a surrogate marker to determine the de-escalation of adjuvant cytotoxic treatments [148]. The DECRESCENDO trial was designed to assess the efficacy of the de-escalation of adjuvant anti-HER2 therapy following pCR [149,150]. This is a dual-phase single-arm phase II trial evaluating the neoadjuvant single-agent taxane chemotherapy combined with subcutaneous (SC) applications of trastuzumab and pertuzumab. However, prior to undergoing neoadjuvant treatment, the trial candidates were required to have breast tumor sizes between 1.5 and 5.0 cm and LN uninvolved [151]. After surgery, participants who achieved pCR on breast and axillary specimens received adjuvant pertuzumab and trastuzumab via SC application for an additional 14 cycles. In those with pRD, in addition to low to intermediate levels of residual cancer burden (RCB), 14 cycles of adjuvant T-DM1 were given, and those with more substantial RCB received four cycles of AC chemotherapy. The primary analysis of the DECRESCENDO trial showed an overall pCR rate of 56.7%. Efficacy endpoints regarding the de-escalation of adjuvant therapy are still ongoing [149,150].
With an identical design and similar eligibility criteria, the DAPHNe and CompassHER2 pCR trials appraised postoperative trastuzumab and pertuzumab with the omission of adjuvant chemotherapy in HER2+ BC patients. While the phase II, CompassHER2 pCR accrued patients with stage II–IIIA, the phase I pilot study DAPHNe included participants with a tumor size ≥ 1.5 cm and stage II–III, but with the exception of inflammatory or T4d disease, as these were exclusionary conditions. In both studies, prior to trial enrolment, all candidates received taxane single chemotherapy with H+P. In the adjuvant setting, candidates who had pCR were assigned to H+P, without any further chemotherapy [149,152,153].
Following a similar intent of sparing patients from receiving chemotherapy, the phase II PHERGain trial was presented at the American Society of Clinical Oncology (ASCO) 2023 international conference. This study explored de-escalating adjuvant chemotherapy based on a pathological response-adapted approach using 18F-FDG-PET. The PHERGain recruited women with stage I–IIIA HER2+ BC with PET-evaluable and surgically operable breast tumors ≥ 1.5 cm. Participants were evaluated for an early response by PET, which corresponded to a tumor size reduction of ≥40% from baseline [154]. Participants in the chemotherapy-free arm received dual HER2 blockers with H+P for two cycles, after which nearly 80% had PET evidence of an early response. These patients, who continued on doublet mABs for an additional six cycles until surgery, had a pCR rate of 37.7% on surgical specimens. Postoperatively, patients who had pCR continued H+P for 10 additional cycles, and those who had pRD were allocated to receive 6 cycles of adjuvant chemotherapy consisting of the TCH+P regimen, followed by H+P and ET, if applicable. Conversely, patients who did not reach the desired PET response after the first two cycles of H+P following randomization were allocated to receive six cycles of TCH+P until surgery and subsequently received adjuvant H+P and ET, if ER+ [155]. The analysis of the intention-to-treat (ITT) population revealed a 3-year iDFS of 95.4% ((95% CI, 92.8–98); p < 0.001) seen in the group where chemotherapy was omitted. Among these, patients who were considered PET responders, and hence did not receive chemotherapy, had a 3-year iDFS rate of 98.8% (95% CI, 96.3–100.0) [155].
All of these studies, exploring the de-escalation of adjuvant chemotherapy, will potentially help identify which patients are the best candidates in whom omitting adjuvant chemotherapy is appropriate.
15.3. Other Oncodriver Mutations and Molecular Pathways Crossing with HER2+
PI3K/AKT/mTOR/MAPK Pathways
The phosphatidylinositol 3-kinase (PI3K) is a group of heterodimeric enzymes encoded by catalytic subunits of the PIK3CA gene [156]. The PIK3 protein kinase B (AKT), aligned with the mammalian target of rapamycin (mTOR), plays an essential role in conducting information to the intracellular domain that can lead to cell differentiation, proliferation, and apoptosis, as well as DNA repair [156,157]. Targeted therapies against HER2 act by interrupting signal conduction through the PI3K/AKT/mTOR and MAP kinase (MAPK) pathways, suppressing cellular regulation mechanisms [158,159].
Everolimus is an oral selective mTOR inhibitor approved in metastatic ER+ HER2– BC [160]. The combination of everolimus and trastuzumab was shown to have synergism by reducing tumor growth in HER2 overexpression [161]. Nevertheless, PIK3CA mutations have been associated with tumorigenesis and acquired resistance to anti-HER2 therapy [162,163]. In HER2+ BC, PIK3CA mutation has been considered predictive of a poor treatment response due to lower pCR rates and decreased DFS, as compared to wild-type PIK3CA [164,165]. Similarly, a higher risk of disease recurrence was seen in patients with metastatic HER2+ BC harboring PIK3CA gene alteration in the EMILIA and CLEOPATRA trials, despite the dual HER2 blockage [166,167].
Alpelisib is a selective PI3K inhibitor capable of inhibiting cell proliferation by suppressing signaling through both wild-type and mutated PI3K-alpha pathways [168,169]. Although alpelisib did not show survival benefits when given in ER+ HER2– BC, its usage with trastuzumab is being tested in the ALPHABET trial in advanced HER2+ BC and could provide insights about their adjunct effect in patients with PI3KCA mutations [170].
To date, none of the PI3K inhibitors have been tested in early HER2+ BC. However, harboring PIK3CA mutations is predictive of worse survival outcomes when compared to those without this mutation [171]. Thus, a better understanding of the mechanisms for evading resistance to the HER2-targeted therapies through the PI3K/AKT/mTOR/MAPK pathways is needed to improve outcomes in BC patients with PIK3CA mutations [159].
15.4. Cancer-Targeted Vaccines
Breast cancer is a heterogeneous disease with immunogenic pathogenesis. The HER2 molecular pathway plays multiple roles, including one as an oncogenic driver [19,172]. Research highlights the importance of understanding the conceivable association between ErbB2-specific immunity mechanisms and acquired treatment resistance from targeted HER2 treatments [173]. The ErbB2-antigen is targetable with vaccines that potentially trigger high levels of antibodies, which, through the mediation of lymphocyte T cells, elicits the suppression of the Th-1 response and T-cell cytotoxic proliferation in overexpressed tumor-antigens [174]. Following this approach, numerous clinical trials aim to induce anti-tumor effects with a specific HER2–directed T-cell response by using peptide-based vaccines or viral vectors and self-replicating RNA-based cancer vaccines administered alone or in conjunction with immune checkpoint inhibitor anti-PD1 mABs [19,175,176]. Although the induction of immunity by anti-cancer vaccines appears to have a crucial role in suppressing tumor activity, the magnitude and duration of their treatment effect have not yielded breakthrough results to change the treatment course of HER+ BC [175,177].
Another immunomodulatory strategy targeting tumor cells with a tumor antigen-specific antibody involves tailored-fusion proteins. This approach, designed to bind to the cell surface of the HER2 antigen, has demonstrated some biological activity in conjunction with HER2 treatments towards inhibiting the tumor growth of HER2–enriched cells [178,179]. Targeting fusion proteins may have a supplemental role, especially when administered with vaccines against the HER2 antigen to elicit a boost of immune responses in cancers where HER2 is overexpressed [179,180].
16. Conclusions
This literature review highlights the latest evidence around early HER2+ BC (Table 1). In summary, categorizing patients into appropriate BC risk groups, by way of evaluating the tumor and LN staging, is decisive in guiding clinicians towards the neoadjuvant versus adjuvant route. In patients with low-risk disease with tumors measuring up to 20 mm and LN–, there are sufficient data to support the omission of anthracycline, and in this case, upfront surgery, followed by adjuvant paclitaxel with trastuzumab, is the preferred choice. In the high-risk category comprising larger tumors or in cases of axillary lymph node involvement, the neoadjuvant approach to downsizing tumors would be the first option. Notably, systemic treatment with versus without anthracycline chemotherapy seems to have comparable results, as there is no efficacy-related difference among them. Hence, doublet platinum-based chemotherapy using carboplatin with taxane is a reasonable alternative for those in whom anthracycline is contraindicated. It is important to emphasize that, post-NAC, evidence of pCR is predictive and a surrogate marker of better survival outcomes, but in those with residual disease, adjuvant T-DM1 is the standard indication for reducing BC recurrence rates.

Table 1.
Summary of key clinical trials, systematic reviews, and meta-analyses that investigated the role of HER2 therapy in HER2-positive breast cancer.
Evidence supports that neoadjuvant treatment with the dual HER2 blockage trastuzumab and pertuzumab promotes superior pCR and PFS outcomes, when compared to single-agent trastuzumab. However, OS data are limited or non-existent around the dual combination, and the association between enhancing pCR rates and the long-term clinical advantage with the addition of pertuzumab remains unknown [181]. Nevertheless, adjuvant trastuzumab and pertuzumab uphold a significant but small recurrence reduction advantage in select HER2+ BC patients. In this case, those with LN+ and ER− benefit the most from dual combination therapy.
If there are funding restrictions or limitations in accessing the combination trastuzumab and pertuzumab in the neoadjuvant or adjuvant settings, the single HER2 mAB trastuzumab continues to be a very reasonable option.
There was a role for adjuvant neratinib in high-risk groups of ER+ HER2+ BC patients who completed adjuvant trastuzumab, but the overall recommendation in recent trials suggests the benefit is small, especially when taking into consideration treatment with the combination of pertuzumab and trastuzumab, or with T-DM1 for residual disease, all of which are less toxic and more tolerable than neratinib.
Regarding the optimal treatment duration, one year of adjuvant trastuzumab conferred an extensive survival advantage and remains the standard therapy length for early HER2+ BC. Nevertheless, shorter schedules of trastuzumab may be considered as an alternative for patients with borderline cardiac conditions or in those with a lower risk of BC relapse.
Topical areas of the current discussion, with respect to evaluating the de-escalation of adjuvant chemotherapy, include ongoing trials with forthcoming results that could help point towards patients in whom eliminating further chemotherapy is advantageous.
In this manner, tailoring the breadth of HER2+ treatment strategies hinges on a handful of factors for consideration in each individual case. Bearing in mind the patients’ personal preferences among the suitable options and medical history and identifying the BC risk category are critical in supporting optimal treatment decisions.
Author Contributions
All authors contributed to the conceptualization, investigation, editing, and final review. Methodology, D.G.d.M.M.M.; writing—original draft preparation, D.G.d.M.M.M., R.C., M.B.H., and M.R.; visualization, D.G.d.M.M.M., J.R., and P.B.; supervision, D.G.d.M.M.M.; project administration, D.G.d.M.M.M.; creation of Figure 1 and Figure 2, D.G.d.M.M.M.; creation of Table 1, A.A.-H. and D.G.d.M.M.M. To whom it may concern: D.G.d.M.M.M. was the first author and principal investigator of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We would like to thank Mkeila Sowa and Rose Lisi for their contributions to the manuscript formatting, edits, and for the creation of the attached figure and flowchart. Neither were compensated for their work on this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mayer, E.L.; Fesl, C.; Hlauschek, D.; Garcia-Estevez, L.; Burstein, H.J.; Zdenkowski, N.; Wette, V.; Miller, K.D.; Balic, M.; Mayer, I.A.; et al. Treatment Exposure and Discontinuation in the PALbociclib CoLlaborative Adjuvant Study of Palbociclib with Adjuvant Endocrine Therapy for Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Early Breast Cancer (PALLAS/AFT-05/ABCSG-42/BIG-14-03). J. Clin. Oncol. 2022, 40, 449–458. [Google Scholar] [CrossRef]
- Alferez, D.G.; Simões, B.M.; Howell, S.J.; Clarke, R.B. The Role of Steroid Hormones in Breast and Effects on Cancer Stem Cells. Curr. Stem Cell Rep. 2018, 4, 81–94. [Google Scholar] [CrossRef]
- Loibl, S.; Gianni, L. HER2-positive breast cancer. Lancet 2017, 389, 2415–2429. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Unni, N.; Peng, Y. The Changing Paradigm for the Treatment of HER2-Positive Breast Cancer. Cancers 2020, 12, 2081. [Google Scholar] [CrossRef] [PubMed]
- Mata, D.G.D.M.M.; Carmona, A.; Eisen, C.; Trudeau, A.; Giffoni, D.; Morais Mata, M.; Carmona, C.A.; Eisen, A.; Trudeau, M. Appraising Adjuvant Endocrine Therapy in Hormone Receptor Positive HER2-Negative Breast Cancer—A Literature Review. Curr. Oncol. 2022, 29, 4956–4969. [Google Scholar] [CrossRef]
- Gradishar, W.J. HER2 Therapy—An Abundance of Riches. N. Engl. J. Med. 2012, 366, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Murthy, R.K.; Abramson, V.; Anders, C.; Bachelot, T.; Bedard, P.L.; Borges, V.; Cameron, D.; Carey, L.A.; Chien, A.J.; et al. Tucatinib vs. Placebo, Both in Combination with Trastuzumab and Capecitabine, for Previously Treated ERBB2 (HER2)-Positive Metastatic Breast Cancer in Patients with Brain Metastases: Updated Exploratory Analysis of the HER2CLIMB Randomized Clinical Trial. JAMA Oncol. 2023, 9, 197–205. [Google Scholar] [CrossRef]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting HER2-positive breast cancer: Advances and future directions. Nat. Rev. Drug Discov. 2022, 22, 101–126. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Centre, P.C.; Perrin, J.; Gligorov, J.; Coudert, B.P.; Largillier, R.; Arnould, L.; Chollet, P.; Campone, M.; Coeffic, D.; Priou, F.; et al. Multicenter Phase II Trial of Neoadjuvant Therapy with Trastuzumab, Docetaxel, and Carboplatin for Human Epidermal Growth Factor Receptor-2-Overexpressing Stage II or III Breast Ca Spontaneous Canine Model View project Projects on ABC View project Multicenter Phase II Trial of Neoadjuvant Therapy with Trastuzumab, Docetaxel, and Carboplatin for Human Epidermal Growth Factor Receptor-2-Overexpressing Stage II or III Breast Cancer: Results of the GETN(A)-1 Trial. Artic. J. Clin. Oncol. 2007, 25, 2678–2684. [Google Scholar] [CrossRef]
- Coudert, B.P.; Arnould, L.; Moreau, L.; Chollet, P.; Weber, B.; Vanlemmens, L.; Moluçon, C.; Tubiana, N.; Causeret, S.; Misset, J.L.; et al. Pre-operative systemic (neo-adjuvant) therapy with trastuzumab and docetaxel for HER2-overexpressing stage II or III breast cancer: Results of a multicenter phase II trial. Ann. Oncol. 2006, 17, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Buzdar, A.U.; Ibrahim, N.K.; Francis, D.; Booser, D.J.; Thomas, E.S.; Theriault, R.L.; Pusztai, L.; Green, M.C.; Arun, B.K.; Giordano, S.H.; et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J. Clin. Oncol. 2005, 23, 3676–3685. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.G.; Browne, F.; Miller, N.; Lowery, A.J.; Kerin, M.J. Pathological complete response as a surrogate to improved survival in human epidermal growth factor receptor-2-positive breast cancer: Systematic review and meta-analysis. BJS Open 2022, 6, zrac028. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Zhao, F.; Huo, X.; Ren, D.; Du, F.; Zheng, F.; Zhao, J. Meta-Analysis of HER2-Enriched Subtype Predicting the Pathological Complete Response within HER2-Positive Breast Cancer in Patients Who Received Neoadjuvant Treatment. Front. Oncol. 2021, 11, 632357. [Google Scholar] [CrossRef]
- Chumsri, S.; Li, Z.; Serie, D.J.; Mashadi-Hossein, A.; Colon-Otero, G.; Song, N.; Pogue-Geile, K.L.; Gavin, P.G.; Paik, S.; Moreno-Aspitia, A.; et al. Incidence of late relapses in patients with HER2-positive breast cancer receiving adjuvant trastuzumab: Combined analysis of NCCTG N9831 (Alliance) and NRG oncology/NSABP B-31. J. Clin. Oncol. 2019, 37, 3425–3435. [Google Scholar] [CrossRef]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef]
- Prat, A.; Guarneri, V.; Paré, L.; Griguolo, G.; Pascual, T.; Dieci, M.V.; Chic, N.; González-Farré, B.; Frassoldati, A.; Sanfeliu, E.; et al. A multivariable prognostic score to guide systemic therapy in early-stage HER2-positive breast cancer: A retrospective study with an external evaluation. Lancet Oncol. 2020, 21, 1455–1464. [Google Scholar] [CrossRef]
- Crosby, E.J.; Acharya, C.R.; Haddad, A.F.; Rabiola, C.A.; Lei, G.; Wei, J.P.; Yang, X.Y.; Wang, T.; Liu, C.X.; Wagner, K.U.; et al. Stimulation of oncogene-specific tumor-infiltrating T cells through combined vaccine and αPD1 enable sustained anti-tumor responses against established HER2 Breast Cancer. Clin. Cancer Res. 2020, 26, 4670–4681. [Google Scholar] [CrossRef]
- Lim, M.; Nguyen, T.H.; Niland, C.; Reid, L.E.; Jat, P.S.; Saunus, J.M.; Lakhani, S.R. Landscape of Epidermal Growth Factor Receptor Heterodimers in Brain Metastases. Cancers 2022, 14, 533. [Google Scholar] [CrossRef]
- Brockhoff, G. ‘Shedding’ light on HER4 signaling in normal and malignant breast tissues. Cell. Signal. 2022, 97, 110401. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yonesaka, K.; Tanizaki, J.; Nonagase, Y.; Takegawa, N.; Haratani, K.; Kawakami, H.; Hayashi, H.; Takeda, M.; Tsurutani, J.; et al. Targeting of the HER2/HER3 signaling axis overcomes ligand-mediated resistance to trastuzumab in HER2-positive breast cancer. Cancer Med. 2019, 8, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Moasser, M.M. The oncogene HER2: Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene 2007, 26, 6469–6487. [Google Scholar] [CrossRef]
- Wolff, A.C.; Elizabeth Hale Hammond, M.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American society of clinical oncology/ college of American pathologists clinical practice guideline focused update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Jeyakumar, A.; Younis, T. Trastuzumab for HER2-Positive Metastatic Breast Cancer: Clinical and Economic Considerations. Clin. Med. Insights Oncol. 2012, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Wilson, F.R.; Coombes, M.E.; Wylie, Q.; Yurchenko, M.; Brezden-Masley, C.; Hutton, B.; Skidmore, B.; Cameron, C. Herceptin® (trastuzumab) in HER2-positive early breast cancer: Protocol for a systematic review and cumulative network meta-analysis. Syst. Rev. 2017, 6, 196. [Google Scholar] [CrossRef]
- Baselga, J. Treatment of HER2-overexpressing breast cancer. Ann. Oncol. 2010, 21 (Suppl. S7), vii36–vii40. [Google Scholar] [CrossRef] [PubMed]
- Bleesing, J.J.H.; Oliveira, J.B. Assessment of functional immune responses. In Clinical Immunology: Principles and Practice: Fourth Edition; Saunders: Philadelphia, PA, USA, 2013; pp. 1172–1182. [Google Scholar] [CrossRef]
- Perez, E.A.; Romond, E.H.; Suman, V.J.; Jeong, J.H.; Sledge, G.; Geyer, C.E.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.M.; et al. Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Planned Joint Analysis of Overall Survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.J.; Van Cutsem, E.; Feyereislova, A.; Chung, H.C.; Shen, L.; Sawaki, A.; Lordick, F.; Ohtsu, A.; Omuro, Y.; Satoh, T.; et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010, 376, 687–697. [Google Scholar] [CrossRef]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef]
- Sukov, W.R.; Zhou, J.; Geiersbach, K.B.; Keeney, G.L.; Carter, J.M.; Schoolmeester, J.K. Frequency of HER2 protein overexpression and HER2 gene amplification in endometrial clear cell carcinoma. Hum. Pathol. 2023, 137, 94–110. [Google Scholar] [CrossRef]
- Cobleigh, M.A.; Vogel, C.L.; Tripathy, D.; Robert, N.J.; Scholl, S.; Fehrenbacher, L.; Wolter, J.M.; Paton, V.; Shak, S.; Lieberman, G.; et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J. Clin. Oncol. 1999, 17, 2639–2648. [Google Scholar] [CrossRef]
- Pegram, M.D.; Lipton, A.; Hayes, D.F.; Weber, B.L.; Baselga, J.M.; Tripathy, D.; Baly, D.; Baughman, S.A.; Twaddell, T.; Glaspy, J.A.; et al. Phase II study of receptor-enhanced chemosensitivity using recombinant humanized anti-p185HER2/neu monoclonal antibody plus cisplatin in patients with HER2/neu-overexpressing metastatic breast cancer refractory to chemotherapy treatment. J. Clin. Oncol. 2016, 16, 2659–2671. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef]
- Bradley, R.; Braybrooke, J.; Gray, R.; Hills, R.; Liu, Z.; Peto, R.; Davies, L.; Dodwell, D.; McGale, P.; Pan, H.; et al. Trastuzumab for early-stage, HER2-positive breast cancer: A meta-analysis of 13,864 women in seven randomised trials. Lancet Oncol. 2021, 22, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Leyland-Jones, B.; Shak, S.; Fuchs, H.; Paton, V.; Bajamonde, A.; Fleming, T.; Eiermann, W.; Wolter, J.; Pegram, M.; et al. Use of Chemotherapy plus a Monoclonal Antibody against HER2 for Metastatic Breast Cancer That Overexpresses HER2. N. Engl. J. Med. 2001, 344, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Lee-Hoeflich, S.T.; Crocker, L.; Yao, E.; Pham, T.; Munroe, X.; Hoeflich, K.P.; Sliwkowski, M.X.; Stern, H.M. A Central Role for HER3 in HER2-Amplified Breast Cancer: Implications for Targeted Therapy. Cancer Res. 2008, 68, 5878–5887. [Google Scholar] [CrossRef]
- Scheuer, W.; Friess, T.; Burtscher, H.; Bossenmaier, B.; Endl, J.; Hasmann, M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009, 69, 9330–9336. [Google Scholar] [CrossRef]
- Verma, S.; Miles, D.; Gianni, L.; Krop, I.E.; Welslau, M.; Baselga, J.; Pegram, M.; Oh, D.-Y.; Diéras, V.; Guardino, E.; et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N. Engl. J. Med. 2012, 367, 1783–1791. [Google Scholar] [CrossRef]
- FDA Approves Ado-Trastuzumab Emtansine for Early Breast Cancer|FDA. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ado-trastuzumab-emtansine-early-breast-cancer (accessed on 6 May 2023).
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody–Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280–9290. [Google Scholar] [CrossRef]
- Junttila, T.T.; Li, G.; Parsons, K.; Phillips, G.L.; Sliwkowski, M.X. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res. Treat. 2011, 128, 347–356. [Google Scholar] [CrossRef]
- Collins, D.M.; Conlon, N.T.; Kannan, S.; Verma, C.S.; Eli, L.D.; Lalani, A.S.; Crown, J. Preclinical Characteristics of the Irreversible Pan-HER Kinase Inhibitor Neratinib Compared with Lapatinib: Implications for the Treatment of HER2-Positive and HER2-Mutated Breast Cancer. Cancers 2019, 11, 737. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, J.; Gradishar, W.; O’Regan, R.; Gadi, V. Risk of Recurrence in Patients with HER2+ Early-Stage Breast Cancer: Literature Analysis of Patient and Disease Characteristics. Clin. Breast Cancer 2023, 23, 350–362. [Google Scholar] [CrossRef]
- Yamashiro, H.; Iwata, H.; Masuda, N.; Yamamoto, N.; Nishimura, R.; Ohtani, S.; Sato, N.; Takahashi, M.; Kamio, T.; Yamazaki, K.; et al. Outcomes of trastuzumab therapy in HER2-positive early breast cancer patients: Extended follow-up of JBCRG-cohort study 01. Breast Cancer 2020, 27, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.H.; Chung, W.S.; Ding, R.Y.; Kuo, W.L.; Yu, C.C.; Tsai, H.P.; Shen, S.C.; Chu, C.H.; Lo, Y.F.; Chen, S.C. Factors affecting locoregional recurrence in breast cancer patients undergoing surgery following neoadjuvant treatment. BMC Surg. 2021, 21, 160. [Google Scholar] [CrossRef]
- O’Shaughnessy, J.; Robert, N.; Annavarapu, S.; Zhou, J.; Sussell, J.; Cheng, A.; Fung, A. Recurrence rates in patients with HER2+ breast cancer who achieved a pathological complete response after neoadjuvant pertuzumab plus trastuzumab followed by adjuvant trastuzumab: A real-world evidence study. Breast Cancer Res. Treat. 2021, 187, 903–913. [Google Scholar] [CrossRef]
- Moreno-Aspitia, A.; Holmes, E.M.; Jackisch, C.; de Azambuja, E.; Boyle, F.; Hillman, D.W.; Korde, L.; Fumagalli, D.; Izquierdo, M.A.; McCullough, A.E.; et al. Updated results from the international phase III ALTTO trial (BIG 2-06/Alliance N063D). Eur. J. Cancer 2021, 148, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Campbell, C.; Gelber, R.D.; Viale, G.; McCullough, A.; Hilbers, F.; Korde, L.A.; Werner, O.; Chumsri, S.; Jackisch, C.; et al. Dissecting the effect of hormone receptor status in patients with HER2-positive early breast cancer: Exploratory analysis from the ALTTO (BIG 2-06) randomized clinical trial. Breast Cancer Res. Treat. 2019, 177, 103–114. [Google Scholar] [CrossRef]
- Mamounas, E.P.; Untch, M.; Mano, M.S.; Huang, C.S.; Geyer, C.E.; von Minckwitz, G.; Wolmark, N.; Pivot, X.; Kuemmel, S.; DiGiovanna, M.P.; et al. Adjuvant T-DM1 versus trastuzumab in patients with residual invasive disease after neoadjuvant therapy for HER2-positive breast cancer: Subgroup analyses from KATHERINE. Ann. Oncol. 2021, 32, 1005–1014. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Shelley Hwang, E.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Edge, S.B.; Giuliano, A. New and Important Changes in the TNM Staging System for Breast Cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Denduluri, N.; Somerfield, M.R.; Chavez-MacGregor, M.; Comander, A.H.; Dayao, Z.; Eisen, A.; Freedman, R.A.; Gopalakrishnan, R.; Graff, S.L.; Hassett, M.J.; et al. Selection of Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2021, 39, 685–693. [Google Scholar] [CrossRef]
- Gandhi, S.; Brackstone, M.; Hong, N.J.L.; Grenier, D.; Donovan, E.; Lu, F.I.; Skarpathiotakis, M.; Lee, J.; Boileau, J.F.; Perera, F.; et al. A Canadian national guideline on the neoadjuvant treatment of invasive breast cancer, including patient assessment, systemic therapy, and local management principles. Breast Cancer Res. Treat. 2022, 193, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Gori, S.; Turazza, M.; Modena, A.; Duranti, S.; Zamboni, G.; Alongi, F.; Carbognin, G.; Massocco, A.; Salgarello, M.; Inno, A. When and how to treat women with HER2-positive, small (pT1a-b), node-negative breast cancer? Crit. Rev. Oncol./Hematol. 2018, 128, 130–138. [Google Scholar] [CrossRef]
- Vaz-Luis, I.; Ottesen, R.A.; Hughes, M.E.; Mamet, R.; Burstein, H.J.; Edge, S.B.; Gonzalez-Angulo, A.M.; Moy, B.; Rugo, H.S.; Theriault, R.L.; et al. Outcomes by tumor subtype and treatment pattern in women with small, node-negative breast cancer: A multi-institutional study. J. Clin. Oncol. 2014, 32, 2142–2150. [Google Scholar] [CrossRef]
- Hassing, C.M.S.; Nielsen, D.L.; Knoop, A.S.; Tvedskov, T.H.F.; Kroman, N.; Lænkholm, A.V.; Juhl, C.B.; Kümler, I. Adjuvant treatment with trastuzumab of patients with HER2-positive, T1a-bN0M0 breast tumors: A systematic review and meta-analysis. Crit. Rev. Oncol./Hematol. 2023, 184, 103952. [Google Scholar] [CrossRef] [PubMed]
- Fehrenbacher, L.; Capra, A.M.; Quesenberry, C.P.; Fulton, R.; Shiraz, P.; Habel, L.A. Distant invasive breast cancer recurrence risk in human epidermal growth factor receptor 2-positive T1a and T1b node-negative localized breast cancer diagnosed from 2000 to 2006: A cohort from an integrated health care delivery system. J. Clin. Oncol. 2014, 32, 2151–2158. [Google Scholar] [CrossRef]
- O’Sullivan, C.C.; Bradbury, I.; Campbell, C.; Spielmann, M.; Perez, E.A.; Joensuu, H.; Costantino, J.P.; Delaloge, S.; Rastogi, P.; Zardavas, D.; et al. Efficacy of adjuvant trastuzumab for patients with human epidermal growth factor receptor 2-positive early breast cancer and tumors ≤ 2 cm: A meta-analysis of the randomized trastuzumab trials. J. Clin. Oncol. 2015, 33, 2600–2608. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Barry, W.T.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; Albain, K.S.; Rugo, H.S.; Ellis, M.; Shapira, I.; et al. Adjuvant Paclitaxel and Trastuzumab for Node-Negative, HER2-Positive Breast Cancer. N. Engl. J. Med. 2015, 372, 134–141. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Guo, H.; Pernas, S.; Barry, W.T.; Dillon, D.A.; Ritterhouse, L.; Schneider, B.P.; Shen, F.; Fuhrman, K.; Baltay, M.; et al. Seven-year follow-up analysis of adjuvant paclitaxel and trastuzumab trial for node-negative, human epidermal growth factor receptor 2–positive breast cancer. J. Clin. Oncol. 2019, 37, 1868–1875. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Tarantino, P.; Graham, N.; Tayob, N.; Parè, L.; Villacampa, G.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer: Final 10-year analysis of the open-label, single-arm, phase 2 APT trial. Lancet Oncol. 2023, 24, 273–285. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Tayob, N.; Dang, C.; Yardley, D.A.; Isakoff, S.J.; Valero, V.; Faggen, M.; Mulvey, T.; Bose, R.; Hu, J.; et al. Adjuvant Trastuzumab Emtansine versus Paclitaxel in Combination with Trastuzumab for Stage I HER2-Positive Breast Cancer (ATEMPT): A Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 2375–2385. [Google Scholar] [CrossRef]
- Sella, T.; Zheng, Y.; Tayob, N.; Ruddy, K.J.; Freedman, R.A.; Dang, C.; Yardley, D.; Isakoff, S.J.; Valero, V.; DeMeo, M.; et al. Treatment discontinuation, patient-reported toxicities and quality-of-life by age following trastuzumab emtansine or paclitaxel/trastuzumab (ATEMPT). NPJ Breast Cancer 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Miles, D.; Kim, S.B.; Im, Y.H.; Im, S.A.; Semiglazov, V.; Ciruelos, E.; Schneeweiss, A.; Loi, S.; Monturus, E.; et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 519–530. [Google Scholar] [CrossRef] [PubMed]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Loibl, S.; Jassem, J.; Sonnenblick, A.; Parlier, D.; Winer, E.; Bergh, J.; Gelber, R.D.; Restuccia, E.; Im, Y.-H.; Huang, C.; et al. VP6-2022: Adjuvant pertuzumab and trastuzumab in patients with early HER-2 positive breast cancer in APHINITY: 8.4 years’ follow-up. Ann. Oncol. 2022, 33, 986–987. [Google Scholar] [CrossRef]
- Krop, I.E.; Im, S.A.; Barrios, C.; Bonnefoi, H.; Gralow, J.; Toi, M.; Ellis, P.A.; Gianni, L.; Swain, S.M.; Im, Y.H.; et al. Trastuzumab Emtansine Plus Pertuzumab Versus Taxane Plus Trastuzumab Plus Pertuzumab After Anthracycline for High-Risk Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer: The Phase III KAITLIN Study. J. Clin. Oncol. 2022, 40, 438–448. [Google Scholar] [CrossRef]
- Gianni, L.; Eiermann, W.; Semiglazov, V.; Lluch, A.; Tjulandin, S.; Zambetti, M.; Moliterni, A.; Vazquez, F.; Byakhov, M.J.; Lichinitser, M.; et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): Follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014, 15, 640–647. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Roman, L.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.H.; Tseng, L.M.; Liu, M.C.; Lluch, A.; Starosławska, E.; de la Haba-Rodriguez, J.; Im, S.A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Chen, S.; Liang, Y.; Feng, Z.; Wang, M. Efficacy and safety of HER2 inhibitors in combination with or without pertuzumab for HER2-positive breast cancer: A systematic review and meta-analysis. BMC Cancer 2019, 19, 973. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Martin, M.; Symmans, W.F.; Jung, K.H.; Huang, C.S.; Thompson, A.M.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; et al. Neoadjuvant trastuzumab, pertuzumab, and chemotherapy versus trastuzumab emtansine plus pertuzumab in patients with HER2-positive breast cancer (KRISTINE): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2018, 19, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Hurvitz, S.A.; Martin, M.; Jung, K.H.; Huang, C.S.; Harbeck, N.; Valero, V.; Stroyakovskiy, D.; Wildiers, H.; Campone, M.; Boileau, J.F.; et al. Neoadjuvant Trastuzumab Emtansine and Pertuzumab in Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Three-Year Outcomes from the Phase III KRISTINE Study. J. Clin. Oncol. 2019, 37, 2206–2216. [Google Scholar] [CrossRef]
- Guarneri, V.; Griguolo, G.; Miglietta, F.; Conte, P.F.; Dieci, M.V.; Girardi, F. Survival after neoadjuvant therapy with trastuzumab–lapatinib and chemotherapy in patients with HER2-positive early breast cancer: A meta-analysis of randomized trials. ESMO Open 2022, 7, 100433. [Google Scholar] [CrossRef]
- Carey, L.A.; Berry, D.A.; Cirrincione, C.T.; Barry, W.T.; Pitcher, B.N.; Harris, L.N.; Ollila, D.W.; Krop, I.E.; Henry, N.L.; Weckstein, D.J.; et al. Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab with or Without Lapatinib. J. Clin. Oncol. 2016, 34, 542. [Google Scholar] [CrossRef] [PubMed]
- Huober, J.; Holmes, E.; Baselga, J.; de Azambuja, E.; Untch, M.; Fumagalli, D.; Sarp, S.; Lang, I.; Smith, I.; Boyle, F.; et al. Survival outcomes of the NeoALTTO study (BIG 1-06): Updated results of a randomised multicenter phase III neoadjuvant clinical trial in patients with HER2-positive primary breast cancer. Eur. J. Cancer 2019, 118, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Tang, G.; Hassan, S.; Geyer, C.E.; Azar, C.A.; Magrinat, G.C.; Suga, J.M.; Bear, H.D.; Baez-Diaz, L.; Sarwar, S.; et al. Long-term outcomes of dual vs. single HER2-directed neoadjuvant therapy in NSABP B-41. Breast Cancer Res. Treat. 2023, 199, 243–252. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- Chan, A.; Delaloge, S.; Holmes, F.A.; Moy, B.; Iwata, H.; Harvey, V.J.; Robert, N.J.; Silovski, T.; Gokmen, E.; von Minckwitz, G.; et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 367–377. [Google Scholar] [CrossRef]
- Martin, M.; Holmes, F.A.; Ejlertsen, B.; Delaloge, S.; Moy, B.; Iwata, H.; von Minckwitz, G.; Chia, S.K.L.; Mansi, J.; Barrios, C.H.; et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1688–1700. [Google Scholar] [CrossRef]
- Chan, A.; Moy, B.; Mansi, J.; Ejlertsen, B.; Holmes, F.A.; Chia, S.; Iwata, H.; Gnant, M.; Loibl, S.; Barrios, C.H.; et al. Final Efficacy Results of Neratinib in HER2-positive Hormone Receptor-positive Early-stage Breast Cancer from the Phase III ExteNET Trial. Clin. Breast Cancer 2021, 21, 80–91.e7. [Google Scholar] [CrossRef]
- Holmes, F.A.; Moy, B.; Delaloge, S.; Chia, S.K.L.; Ejlertsen, B.; Mansi, J.; Iwata, H.; Gnant, M.; Buyse, M.; Barrios, C.H.; et al. Overall survival with neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): A randomised, double-blind, placebo-controlled, phase 3 trial. Eur. J. Cancer 2023, 184, 48–59. [Google Scholar] [CrossRef] [PubMed]
- van Ramshorst, M.S.; van der Voort, A.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Dezentjé, V.O.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- van der Voort, A.; van Ramshorst, M.S.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Vulink, A.J.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Three-Year Follow-up of Neoadjuvant Chemotherapy with or without Anthracyclines in the Presence of Dual ERBB2 Blockade in Patients with ERBB2-Positive Breast Cancer: A Secondary Analysis of the TRAIN-2 Randomized, Phase 3 Trial. JAMA Oncol. 2021, 7, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.; Eiermann, W.; Robert, N.; Giermek, J.; Martin, M.; Jasiowka, M.; Mackey, J.; Chan, A.; Liu, M.-C.; Pinter, T.; et al. Abstract S5-04: Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. Cancer Res. 2016, 76 (Suppl. S4), S5-04. [Google Scholar] [CrossRef]
- Zhu, J.; Min, N.; Chen, Y.; Li, X. Neoadjuvant therapy with vs. without anthracyclines for HER2-positive breast cancer: A systematic review and meta-analysis. Ann. Transl. Med. 2023, 11, 200. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Waldron-Lynch, M.; Eng-Wong, J.; Kirk, S.; Cortés, J. Long-term efficacy analysis of the randomised, phase II TRYPHAENA cardiac safety study: Evaluating pertuzumab and trastuzumab plus standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer. Eur. J. Cancer 2018, 89, 27–35. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Gelber, R.D.; Piccart-Gebhart, M.J.; De Azambuja, E.; Procter, M.; Suter, T.M.; Jackisch, C.; Cameron, D.; Weber, H.A.; Heinzmann, D.; et al. 2 years versus 1 year of adjuvant trastuzumab for HER2-positive breast cancer (HERA): An open-label, randomised controlled trial. Lancet 2013, 382, 1021–1028. [Google Scholar] [CrossRef]
- Pondé, N.; Gelber, R.D.; Piccart, M. PERSEPHONE: Are we ready to de-escalate adjuvant trastuzumab for HER2-positive breast cancer? Npj Breast Cancer 2019, 5, 1. [Google Scholar] [CrossRef]
- Earl, H.M.; Hiller, L.; Vallier, A.L.; Loi, S.; McAdam, K.; Hughes-Davies, L.; Harnett, A.N.; Ah-See, M.L.; Simcock, R.; Rea, D.; et al. 6 versus 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 2019, 393, 2599. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X.; Romieu, G.; Debled, M.; Pierga, J.Y.; Kerbrat, P.; Bachelot, T.; Lortholary, A.; Espié, M.; Fumoleau, P.; Serin, D.; et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): Final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet 2019, 393, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, D.; Saloustros, E.; Malamos, N.; Kakolyris, S.; Boukovinas, I.; Papakotoulas, P.; Kentepozidis, N.; Ziras, N.; Georgoulias, V. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: A multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann. Oncol. 2015, 26, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, W.; Hu, X.; Yi, M.; Ye, C.; Yao, G. Short-duration versus 1-year adjuvant trastuzumab in early HER2 positive breast cancer: A meta-analysis of randomized controlled trials. Cancer Treat. Rev. 2019, 75, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X.; Romieu, G.; Debled, M.; Pierga, J.Y.; Kerbrat, P.; Bachelot, T.; Lortholary, A.; Espié, M.; Fumoleau, P.; Serin, D.; et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.; Blanchette, P.; Shah, P.S.; Ye, X.Y.; Boldt, R.G.; Fernandes, R.; Vandenberg, T.; Raphael, J. Do all patients with HER2 positive breast cancer require one year of adjuvant trastuzumab? A systematic review and meta-analysis. Breast 2020, 54, 203–210. [Google Scholar] [CrossRef]
- Buus, R.; Sestak, I.; Kronenwett, R.; Ferree, S.; Schnabel, C.A.; Baehner, F.L.; Mallon, E.A.; Cuzick, J.; Dowsett, M. Molecular Drivers of Oncotype DX, Prosigna, EndoPredict, and the Breast Cancer Index: A TransATAC Study. J. Clin. Oncol. 2021, 39, 126. [Google Scholar] [CrossRef] [PubMed]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837. [Google Scholar] [CrossRef]
- Griguolo, G.; Bottosso, M.; Vernaci, G.; Miglietta, F.; Dieci, M.V.; Guarneri, V. Gene-expression signatures to inform neoadjuvant treatment decision in HR+/HER2− breast cancer: Available evidence and clinical implications. Cancer Treat. Rev. 2022, 102, 102323. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Aebi, S.; et al. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- Waks, A.G.; Ogayo, E.R.; Paré, L.; Marín-Aguilera, M.; Brasó-Maristany, F.; Galván, P.; Castillo, O.; Martínez-Sáez, O.; Vivancos, A.; Villagrasa, P.; et al. Assessment of the HER2DX Assay in Patients with ERBB2-Positive Breast Cancer Treated With Neoadjuvant Paclitaxel, Trastuzumab, and Pertuzumab. JAMA Oncol. 2023, 9, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Guarneri, V.; Pascual, T.; Brasó-Maristany, F.; Sanfeliu, E.; Paré, L.; Schettini, F.; Martínez, D.; Jares, P.; Griguolo, G.; et al. Development and validation of the new HER2DX assay for predicting pathological response and survival outcome in early-stage HER2-positive breast cancer. EBioMedicine 2022, 75, 103801. [Google Scholar] [CrossRef]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4+T Cells: Differentiation and Functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Jewett, A.; Tseng, H.C. Immunotherapy. In Pharmacology and Therapeutics for Dentistry, 7th ed.; Mosby: St. Louis, MO, USA, 2017; pp. 504–529. [Google Scholar] [CrossRef]
- Hwang, H.W.; Hong, S.A.; Nam, S.J.; Kim, S.W.; Lee, J.E.; Yu, J.H.; Lee, S.K.; Cho, S.Y.; Cho, E.Y. Histologic analysis according to HER2 gene status in HER2 2 + invasive breast cancer: A study of 280 cases comparing ASCO/CAP 2013 and 2018 guideline recommendations. Virchows Arch. 2022, 480, 749–758. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef]
- Savas, P.; Virassamy, B.; Ye, C.; Salim, A.; Mintoff, C.P.; Caramia, F.; Salgado, R.; Byrne, D.J.; Teo, Z.L.; Dushyanthen, S.; et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat. Med. 2018, 24, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Michiels, S.; Adams, S.; Loibl, S.; Budczies, J.; Denkert, C.; Salgado, R. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: Clinical utility in an era of checkpoint inhibition. Ann. Oncol. 2021, 32, 1236–1244. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Martinez, A.; Pascual, T.; Singh, B.; Nuciforo, P.; Rashid, N.U.; Ballman, K.V.; Campbell, J.D.; Hoadley, K.A.; Spears, P.A.; Pare, L.; et al. Prognostic and Predictive Value of Immune-Related Gene Expression Signatures vs. Tumor-Infiltrating Lymphocytes in Early-Stage ERBB2/HER2-Positive Breast Cancer: A Correlative Analysis of the CALGB 40601 and PAMELA Trials. JAMA Oncol. 2023, 9, 490–499. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Zhang, P.; Xue, S.; Chen, Y.; Sun, L.; Yang, R. Predictive and prognostic values of tumor infiltrating lymphocytes in breast cancers treated with neoadjuvant chemotherapy: A meta-analysis. Breast 2022, 66, 97–109. [Google Scholar] [CrossRef]
- Salgado, R.; Denkert, C.; Campbell, C.; Savas, P.; Nuciforo, P.; Aura, C.; De Azambuja, E.; Eidtmann, H.; Ellis, C.E.; Baselga, J.; et al. Tumor-Infiltrating Lymphocytes and Associations With Pathological Complete Response and Event-Free Survival in HER2-Positive Early-Stage Breast Cancer Treated with Lapatinib and Trastuzumab: A Secondary Analysis of the NeoALTTO Trial. JAMA Oncol. 2015, 1, 448–455. [Google Scholar] [CrossRef]
- Gonzalez-Ericsson, P.I.; Stovgaard, E.S.; Sua, L.F.; Reisenbichler, E.; Kos, Z.; Carter, J.M.; Michiels, S.; Le Quesne, J.; Nielsen, T.O.; Lænkholm, A.V.; et al. The path to a better biomarker: Application of a risk management framework for the implementation of PD-L1 and TILs as immuno-oncology biomarkers in breast cancer clinical trials and daily practice. J. Pathol. 2020, 250, 667–684. [Google Scholar] [CrossRef]
- Rimawi, M.F.; Schiff, R.; Osborne, C.K. Targeting HER2 for the Treatment of Breast Cancer. Annu. Rev. Med. 2015, 66, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Krasniqi, E.; Barchiesi, G.; Pizzuti, L.; Mazzotta, M.; Venuti, A.; Maugeri-Saccà, M.; Sanguineti, G.; Massimiani, G.; Sergi, D.; Carpano, S.; et al. Immunotherapy in HER2-positive breast cancer: State of the art and future perspectives. J. Hematol. Oncol. 2019, 12, 111. [Google Scholar] [CrossRef]
- Kyriazoglou, A.; Kaparelou, M.; Goumas, G.; Liontos, M.; Zakopoulou, R.; Zografos, E.; Zygogianni, A.; Dimopoulos, M.A.; Zagouri, F. Immunotherapy in HER2-Positive Breast Cancer: A Systematic Review. Breast Care 2022, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): A single-arm, multicentre, phase 1b-2 trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.B.; Im, S.A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef]
- Huober, J.; Barrios, C.H.; Niikura, N.; Jarząb, M.; Chang, Y.C.; Huggins-Puhalla, S.L.; Pedrini, J.; Zhukova, L.; Graupner, V.; Eiger, D.; et al. Atezolizumab with Neoadjuvant Anti-Human Epidermal Growth Factor Receptor 2 Therapy and Chemotherapy in Human Epidermal Growth Factor Receptor 2-Positive Early Breast Cancer: Primary Results of the Randomized Phase III IMpassion050 Trial. J. Clin. Oncol. 2022, 40, 2946–2956. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Dent, R.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; et al. Event-free Survival with Pembrolizumab in Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2022, 386, 556–567. [Google Scholar] [CrossRef]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kümmel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Bachelot, T.; Bianchini, G.; Harbeck, N.; Loi, S.; Park, Y.H.; Prat, A.; Gilham, L.; Boulet, T.; Monturus, N.G.E.; et al. ASTEFANIA: Adjuvant ado-trastuzumab emtansine and atezolizumab for high-risk, HER2-positive breast cancer. Future Oncol. 2022, 18, 3563–3572. [Google Scholar] [CrossRef]
- Neoadjuvant Treatment of HER2 Positive Early High-Risk and Locally Advanced Breast Cancer. ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03595592 (accessed on 1 June 2023).
- Neoadjuvant Her2-targeted Therapy and Immunotherapy with Pembrolizumab. ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT03747120?term=HER2-POSITIVE+ADJUVANT+PEMBROLIZUMAB&cond=BREAST+CANCER&draw=2&rank=1 (accessed on 1 June 2023).
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Hurvitz, S.A.; Hegg, R.; Chung, W.P.; Im, S.A.; Jacot, W.; Ganju, V.; Chiu, J.W.Y.; Xu, B.; Hamilton, E.; Madhusudan, S.; et al. Trastuzumab deruxtecan versus trastuzumab emtansine in patients with HER2-positive metastatic breast cancer: Updated results from DESTINY-Breast03, a randomised, open-label, phase 3 trial. Lancet 2023, 401, 105–117. [Google Scholar] [CrossRef] [PubMed]
- A Study of Trastuzumab Deruxtecan (T-DXd) versus Trastuzumab Emtansine (T-DM1) in High-Risk HER2-Positive Participants with Residual Invasive Breast Cancer Following Neoadjuvant Therapy (DESTINY-Breast05). ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04622319?cond=DESTINY-Breast05&draw=2&rank=1 (accessed on 5 May 2023).
- Trastuzumab Deruxtecan (T-DXd) Alone or in Sequence with THP, versus Standard Treatment (ddAC-THP), in HER2-Positive Early Breast Cancer. ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT05113251?term=DESTINY+11&draw=2&rank=1 (accessed on 25 May 2023).
- Borges, V.F.; Ferrario, C.; Aucoin, N.; Falkson, C.; Khan, Q.; Krop, I.; Welch, S.; Conlin, A.; Chaves, J.; Bedard, P.L.; et al. Tucatinib Combined with Ado-Trastuzumab Emtansine in Advanced ERBB2/HER2-Positive Metastatic Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol. 2018, 4, 1214–1220. [Google Scholar] [CrossRef]
- Moulder, S.L.; Borges, V.F.; Baetz, T.; Mcspadden, T.; Fernetich, G.; Murthy, R.K.; Chavira, R.; Guthrie, K.; Barrett, E.; Chia, S.K. Phase I Study of ONT-380, a HER2 Inhibitor, in Patients with HER2+-Advanced Solid Tumors, with an Expansion Cohort in HER2+ Metastatic Breast Cancer (MBC). Clin. Cancer Res. 2017, 23, 3529–3536. [Google Scholar] [CrossRef] [PubMed]
- Murthy, R.K.; Loi, S.; Okines, A.; Paplomata, E.; Hamilton, E.; Hurvitz, S.A.; Lin, N.U.; Borges, V.; Abramson, V.; Anders, C.; et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N. Engl. J. Med. 2020, 382, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Mueller, V.; Borges, V.; Hamilton, E.; Hurvitz, S.; Loi, S.; Murthy, R.; Okines, A.; Paplomata, E.; Cameron, D.; et al. Tucatinib versus placebo added to trastuzumab and capecitabine for patients with pretreated HER2+ metastatic breast cancer with and without brain metastases (HER2CLIMB): Final overall survival analysis. Ann. Oncol. 2022, 33, 321–329. [Google Scholar] [CrossRef]
- O’Sullivan, C.C.; Ballman, K.V.; McCall, L.; Kommalapati, A.; Zemla, T.; Weiss, A.; Mitchell, M.; Blinder, V.; Tung, N.M.; Irvin, W.J.; et al. Alliance A011801 (compassHER2 RD): Postneoadjuvant T-DM1 + tucatinib/placebo in patients with residual HER2-positive invasive breast cancer. Future Oncol. 2021, 17, 4665–4676. [Google Scholar] [CrossRef]
- Bang, Y.J.; Giaccone, G.; Im, S.A.; Oh, D.Y.; Bauer, T.M.; Nordstrom, J.L.; Li, H.; Chichili, G.R.; Moore, P.A.; Hong, S.; et al. First-in-human phase 1 study of margetuximab (MGAH22), an Fc-modified chimeric monoclonal antibody, in patients with HER2-positive advanced solid tumors. Ann. Oncol. 2017, 28, 855. [Google Scholar] [CrossRef]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortés, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Wright, G.S.; Saura, C.; Escrivá-De-Romaní, S.; et al. Efficacy of Margetuximab vs. Trastuzumab in Patients with Pretreated ERBB2-Positive Advanced Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2021, 7, 573–584. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Z.; Chen, W.; Feng, Y.; Dimitrov, D.S. Crystallizable Fragment Glycoengineering for Therapeutic Antibodies Development. Front. Immunol. 2017, 8, 1554. [Google Scholar] [CrossRef]
- Coënon, L.; Villalba, M. From CD16a Biology to Antibody-Dependent Cell-Mediated Cytotoxicity Improvement. Front. Immunol. 2022, 13, 913215. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Im, S.A.; Cardoso, F.; Cortes, J.; Curigliano, G.; Musolino, A.; Pegram, M.D.; Bachelot, T.; Wright, G.S.; Saura, C.; et al. Margetuximab versus Trastuzumab in Patients with Previously Treated HER2-Positive Advanced Breast Cancer (SOPHIA): Final Overall Survival Results from a Randomized Phase 3 Trial. J. Clin. Oncol. 2023, 41, 198–205. [Google Scholar] [CrossRef] [PubMed]
- MARGetuximab or Trastuzumab (MARGOT). ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04425018?cond=Margetuximab+breast+cancer&draw=2&rank=5 (accessed on 16 May 2023).
- Xuhong, J.; Qi, X.; Tang, P.; Fan, L.; Chen, L.; Zhang, F.; Tan, X.; Yan, W.; Zhong, L.; He, C.; et al. Neoadjuvant Pyrotinib plus Trastuzumab and Chemotherapy for Stage I-III HER2-Positive Breast Cancer: A Phase II Clinical Trial. Oncologist 2020, 25, e1909–e1920. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Yan, M.; Ma, F.; Hu, X.; Feng, J.; Ouyang, Q.; Tong, Z.; Li, H.; Zhang, Q.; Sun, T.; et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): A multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Ouyang, Q.; Sun, T.; Niu, L.; Yang, J.; Li, L.; Song, Y.; Hao, C.; Chen, Z.; Orlandi, A.; et al. Pyrotinib plus capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases (PERMEATE): A multicentre, single-arm, two-cohort, phase 2 trial. Lancet Oncol. 2022, 23, 353–361. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Z.; Liu, Z.; Yang, B.; Yang, H.; Tang, J.; Wang, K.; Liu, Y.; Wang, H.; Fu, P.; et al. Neoadjuvant pyrotinib, trastuzumab, and docetaxel for HER2-positive breast cancer (PHEDRA): A double-blind, randomized phase 3 trial. BMC Med. 2022, 20, 498. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Wu, Z.; Ye, Y.; Zhou, L.; Xu, S.; Lin, Y.; Du, Y.; Yan, T.; Yang, F.; et al. Neoadjuvant Trastuzumab and Pyrotinib for Locally Advanced HER2-Positive Breast Cancer (NeoATP): Primary Analysis of a Phase II Study. Clin. Cancer Res. 2022, 28, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Pyrotinib, Trastuzumab, Pertuzumab and Nab-Paclitaxel as Neoadjuvant Therapy in HER2-Positive Breast Cancer. ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04398914?term=NCT04398914&draw=2&rank=1 (accessed on 9 May 2023).
- A Study of ARX788 Combined with Pyrotinib Maleate versus TCBHP (Trastuzumab Plus Pertuzumab with Docetaxel and Carboplatin) as Neoadjuvant Treatment in HER2-Positive Breast Cancer Patients. ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT05426486?term=NCT05426486&draw=2&rank=1 (accessed on 9 May 2023).
- Piccart, M.J.; Hilbers, F.S.; Bliss, J.M.; Caballero, C.; Frank, E.S.; Renault, P.; Kaoudjt, R.N.; Schumacher, E.; Spears, P.A.; Regan, M.M.; et al. Road map to safe and well-designed de-escalation trials of systemic adjuvant therapy for solid tumors. J. Clin. Oncol. 2020, 38, 4120–4129. [Google Scholar] [CrossRef]
- Waks, A.G.; Desai, N.V.; Li, T.; Poorvu, P.D.; Partridge, A.H.; Sinclair, N.; Spring, L.M.; Faggen, M.; Constantine, M.; Metzger, O.; et al. A prospective trial of treatment de-escalation following neoadjuvant paclitaxel/trastuzumab/pertuzumab in HER2-positive breast cancer. Npj Breast Cancer 2022, 8, 63. [Google Scholar] [CrossRef]
- Debien, V.; Adam, V.; Caparica, R.; Fumagalli, D.; Velghe, C.; Gaye, J.; De Nobrega, V.C.; Arahmani, A.; Zoppoli, G.; Piccart-Gebhart, M.J. DECRESCENDO: De-escalation of adjuvant chemotherapy in patients with HER2+/HR-/node-negative early breast cancer who achieve pCR after neoadjuvant taxane and subcutaneous dual anti-HER2 blockade. J. Clin. Oncol. 2022, 40 (Suppl. S16), TPS621. [Google Scholar] [CrossRef]
- De-Escalation Adjuvant Chemo in HER2+/ER-/Node-neg Early BC Patients Who Achieved pCR after Neoadjuvant Chemo & Dual HER2 Blockade. ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04675827?term=DECRESCENDO+HER2&draw=2&rank=1 (accessed on 27 May 2023).
- CompassHER2-pCR: Decreasing Chemotherapy for Breast Cancer Patients after Pre-surgery Chemo and Targeted Therapy. ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/show/NCT04266249?term=CompassHER2+pCR+trial&draw=2&rank=1 (accessed on 31 May 2023).
- DAPHNe De-escalation HER2|Breast Cancer—List Results. ClinicalTrials.gov. (n.d.). Available online: https://clinicaltrials.gov/ct2/results?cond=Breast+Cancer&term=DAPHNe+De-escalation+HER2&cntry=&state=&city=&dist= (accessed on 31 May 2023).
- Pérez-García, J.M.; Gebhart, G.; Ruiz Borrego, M.; Stradella, A.; Bermejo, B.; Schmid, P.; Marmé, F.; Escrivá-de-Romani, S.; Calvo, L.; Ribelles, N.; et al. Chemotherapy de-escalation using an 18F-FDG-PET-based pathological response-adapted strategy in patients with HER2-positive early breast cancer (PHERGain): A multicentre, randomised, open-label, non-comparative, phase 2 trial. Lancet Oncol. 2021, 22, 858–871. [Google Scholar] [CrossRef] [PubMed]
- Cortes, J. ASCO 2023—Oral Abstract Session: 3-Year Invasive Disease-Free Survival (iDFS) of the Strategy-Based, Randomized Phase II PHERGain Trial Evaluating Chemotherapy (CT) De-escalation in Human Epidermal Growth Factor Receptor 2-Positive (HER2[+]) Early Breast Cancer (EBC). Available online: https://meetings.asco.org/abstracts-presentations/219848 (accessed on 5 June 2023).
- Vogt, P.K.; Hart, J.R.; Gymnopoulos, M.; Jiang, H.; Kang, S.; Bader, A.G.; Zhao, L.; Denley, A. Phosphatidylinositol 3-kinase (PI3K): The Oncoprotein. Curr. Top. Microbiol. Immunol. 2011, 347, 79. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.I.; Badoiu, S.C.; Stefani, C.; Greabu, M. PI3K/AKT/mTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Campone, M.; Bachelot, T.; Treilleux, I.; Pistilli, B.; Salleron, J.; Seegers, V.; Arnedos, M.; Loussouarn, D.; Wang, Q.; Vanlemmens, L.; et al. A phase II randomised study of preoperative trastuzumab alone or combined with everolimus in patients with early HER2-positive breast cancer and predictive biomarkers (RADHER trial). Eur. J. Cancer 2021, 158, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Occhiuzzi, M.A.; Lico, G.; Ioele, G.; De Luca, M.; Garofalo, A.; Grande, F. Recent advances in PI3K/PKB/mTOR inhibitors as new anticancer agents. Eur. J. Med. Chem. 2023, 246, 114971. [Google Scholar] [CrossRef]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A.; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, X.; Liu, Y.; Zhang, S.; Liu, J.; Ma, Y.; Zhang, J. Antitumor effect of the mTOR inhibitor everolimus in combination with trastuzumab on human breast cancer stem cells in vitro and in vivo. Tumor Biol. 2012, 33, 1349–1362. [Google Scholar] [CrossRef]
- Seo, Y.; Park, Y.H.; Ahn, J.S.; Im, Y.H.; Nam, S.J.; Cho, S.Y.; Cho, E.Y. PIK3CA Mutations and Neoadjuvant Therapy Outcome in Patients with Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: A Sequential Analysis. J. Breast Cancer 2018, 21, 382. [Google Scholar] [CrossRef]
- Martínez-Saéz, O.; Chic, N.; Pascual, T.; Adamo, B.; Vidal, M.; González-Farré, B.; Sanfeliu, E.; Schettini, F.; Conte, B.; Brasó-Maristany, F.; et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 2020, 22, 45. [Google Scholar] [CrossRef]
- Irelli, A.; Parisi, A.; D’orazio, C.; Sidoni, T.; Rotondaro, S.; Patruno, L.; Pavese, F.; Bafile, A.; Resta, V.; Pizzorno, L.; et al. Anthracycline-Free Neoadjuvant Treatment in Patients with HER2-Positive Breast Cancer: Real-Life Use of Pertuzumab, Trastuzumab and Taxanes Association with an Exploratory Analysis of PIK3CA Mutational Status. Cancers 2022, 14, 3003. [Google Scholar] [CrossRef]
- Kim, J.W.; Lim, A.R.; You, J.Y.; Lee, J.H.; Song, S.E.; Lee, N.K.; Jung, S.P.; Cho, K.R.; Kim, C.Y.; Park, K.H. PIK3CA Mutation is Associated with Poor Response to HER2-Targeted Therapy in Breast Cancer Patients. Cancer Res. Treat. 2023, 55, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Phillips, G.D.L.; Verma, S.; Ro, J.; Huober, J.; Guardino, A.E.; Samant, M.K.; Olsen, S.; De Haas, S.L.; Pegram, M.D. Relationship between Tumor Biomarkers and Efficacy in EMILIA, a Phase III Study of Trastuzumab Emtansine in HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2016, 22, 3755–3763. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Im, S.A.; Clark, E.; Ross, G.; Kiermaier, A.; Swain, S.M. Biomarker Analyses in CLEOPATRA: A phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J. Clin. Oncol. 2014, 32, 3753–3761. [Google Scholar] [CrossRef]
- Galstyan, A.; Cho, J.; Johnson, D.E.; Grandis, J.R. Modulation of the PI3K/mTOR pathways. In Improving the Therapeutic Ratio in Head and Neck Cancer; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–105. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Fidalgo, J.A.; Criscitiello, C.; Carrasco, E.; Regan, M.M.; Di Leo, A.; Ribi, K.; Adam, V.; Bedard, P.L. A phase III trial of alpelisib + trastuzumab ± fulvestrant versus trastuzumab + chemotherapy in HER2+ PIK3CA-mutated breast cancer. Future Oncol. 2022, 18, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Xuhong, J.; Tian, H.; Qu, M.; Zhang, Y.; Jiang, J.; Qi, X. Predictive and prognostic value of PIK3CA mutations in HER2-positive breast cancer treated with tyrosine kinase inhibitors: A systematic review and meta-analysis. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188847. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Mauro, F.L.; Schillaci, R. Emerging Targeted Therapies for HER2-Positive Breast Cancer. Cancers 2023, 15, 1987. [Google Scholar] [CrossRef]
- Knutson, K.L.; Clynes, R.; Shreeder, B.; Yeramian, P.; Kemp, K.P.; Ballman, K.; Tenner, K.S.; Erskine, C.L.; Norton, N.; Northfelt, D.; et al. Improved Survival of HER2+ Breast Cancer Patients Treated with Trastuzumab and Chemotherapy Is Associated with Host Antibody Immunity against the HER2 Intracellular Domain. Cancer Res. 2016, 76, 3702–3710. [Google Scholar] [CrossRef]
- Disis, M.L.; Guthrie, K.A.; Liu, Y.; Coveler, A.L.; Higgins, D.M.; Childs, J.S.; Dang, Y.; Salazar, L.G. Safety and Outcomes of a Plasmid DNA Vaccine Encoding the ERBB2 Intracellular Domain in Patients with Advanced-Stage ERBB2-Positive Breast Cancer: A Phase 1 Nonrandomized Clinical Trial. JAMA Oncol. 2023, 9, 71–78. [Google Scholar] [CrossRef]
- Morse, M.A.; Crosby, E.J.; Force, J.; Osada, T.; Hobeika, A.C.; Hartman, Z.C.; Berglund, P.; Smith, J.; Lyerly, H.K. Clinical trials of self-replicating RNA-based cancer vaccines. Cancer Gene Ther. 2023, 2023, 803–811. [Google Scholar] [CrossRef]
- Kang, J.; Lee, H.J.; Lee, J.; Hong, J.; Hong Kim, Y.; Disis, M.L.; Gim, J.A.; Park, K.H. Novel peptide-based vaccine targeting heat shock protein 90 induces effective antitumor immunity in a HER2+ breast cancer murine model. J. Immunother. Cancer 2022, 10, e004702. [Google Scholar] [CrossRef] [PubMed]
- Crosby, E.J.; Gwin, W.; Blackwell, K.; Marcom, P.K.; Chang, S.; Maecker, H.T.; Broadwater, G.; Hyslop, T.; Kim, S.; Rogatko, A.; et al. Vaccine-induced memory CD8+ T cells provide clinical benefit in HER2 expressing breast cancer: A mouse to human translational study. Clin. Cancer Res. 2019, 25, 2725. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Farshdari, F.; Nematollahi, L.; Behdani, M.; Mohit, E. Anti-HER2 scFv Expression in Escherichia coli SHuffle®T7 Express Cells: Effects on Solubility and Biological Activity. Mol. Biotechnol. 2020, 62, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Mohit, E. Therapeutic potential of a novel IP-10-(anti-HER2 scFv) fusion protein for the treatment of HER2-positive breast cancer. Biotechnol. Lett. 2023, 45, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, M.; Farshdari, F.; Behdani, M.; Nematollahi, L.; Mohit, E. Cloning, Expression and One-Step Purification of a Novel IP-10-(anti-HER2 scFv) Fusion Protein in Escherichia coli. Int. J. Pept. Res. Ther. 2021, 27, 433–446. [Google Scholar] [CrossRef]
- Trudeau, M.; Fraser, B. The CADTH pCODR Expert Review Committee Process Explained. Comment on Rayson et al. Access to Neoadjuvant Pertuzumab for HER2 Positive Breast Cancer in Canada: A Dilemma Increasingly Difficult to Explain. Curr. Oncol. 2022, 29, 9891–9895. Curr. Oncol. 2023, 30, 5047–5049. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).