The Dutch CAR-T Tumorboard Experience: Population-Based Real-World Data on Patients with Relapsed or Refractory Large B-Cell Lymphoma Referred for CD19-Directed CAR T-Cell Therapy in The Netherlands
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Study Population
2.2. Treatment and Clinical Assessments
2.3. Statistical Analyses
3. Results
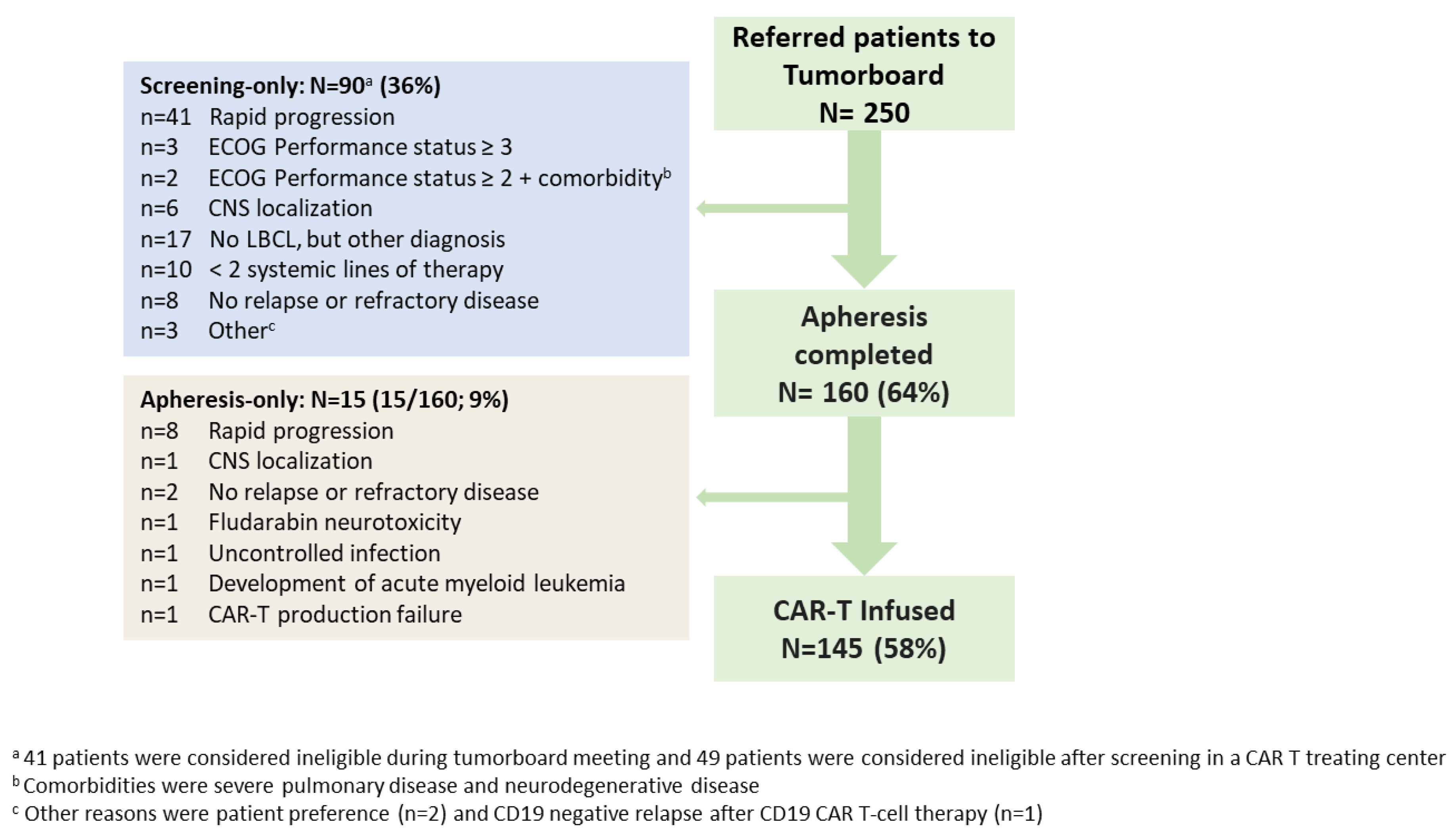
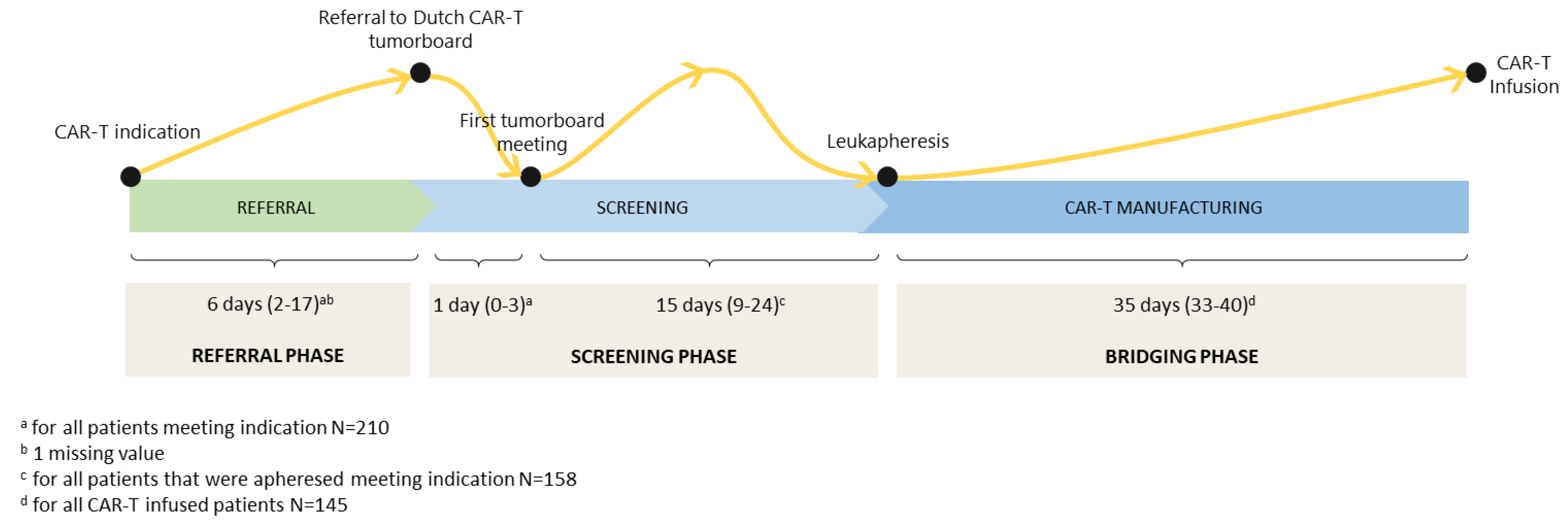
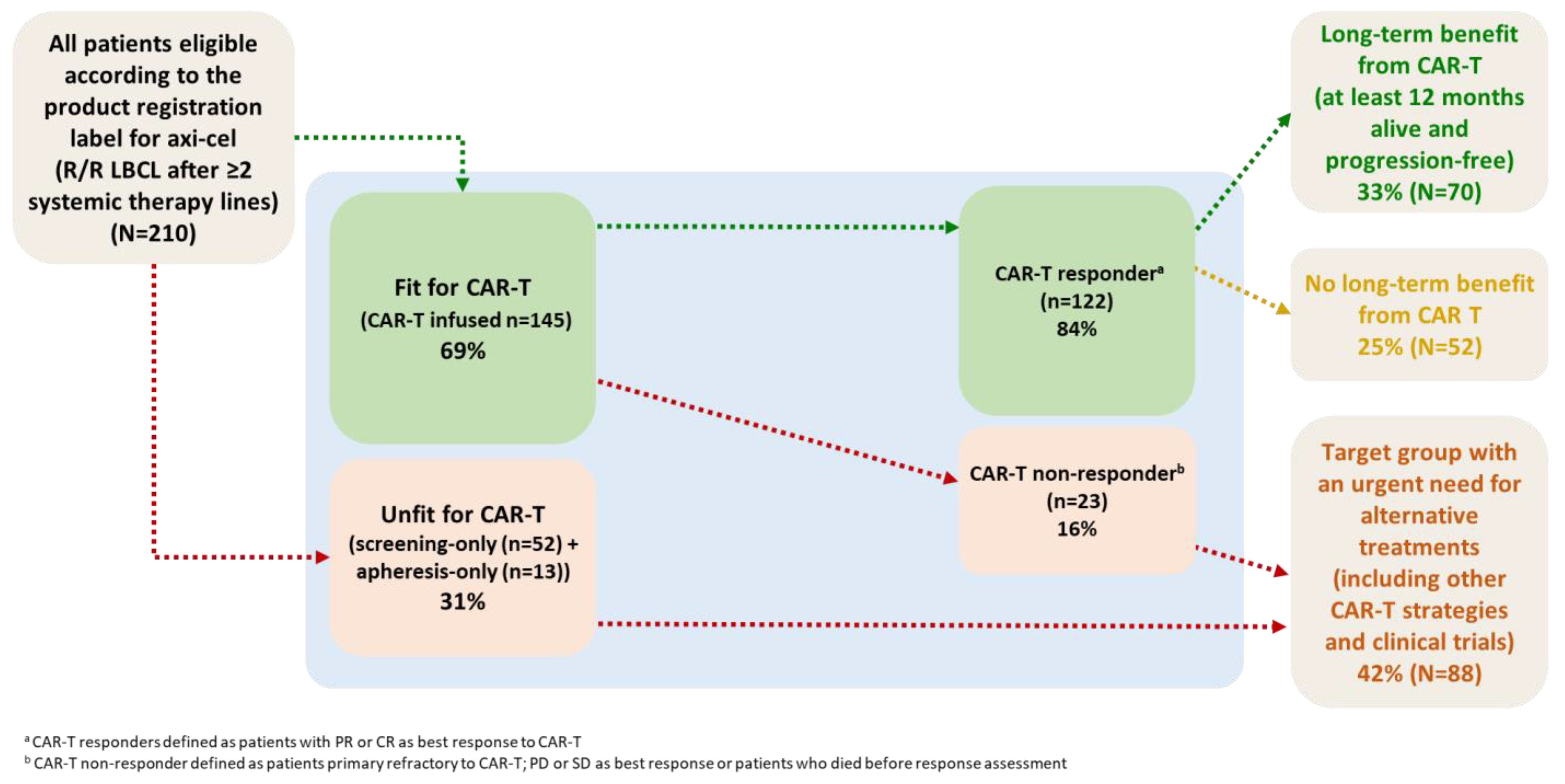
3.1. Patient Selection and Characteristics
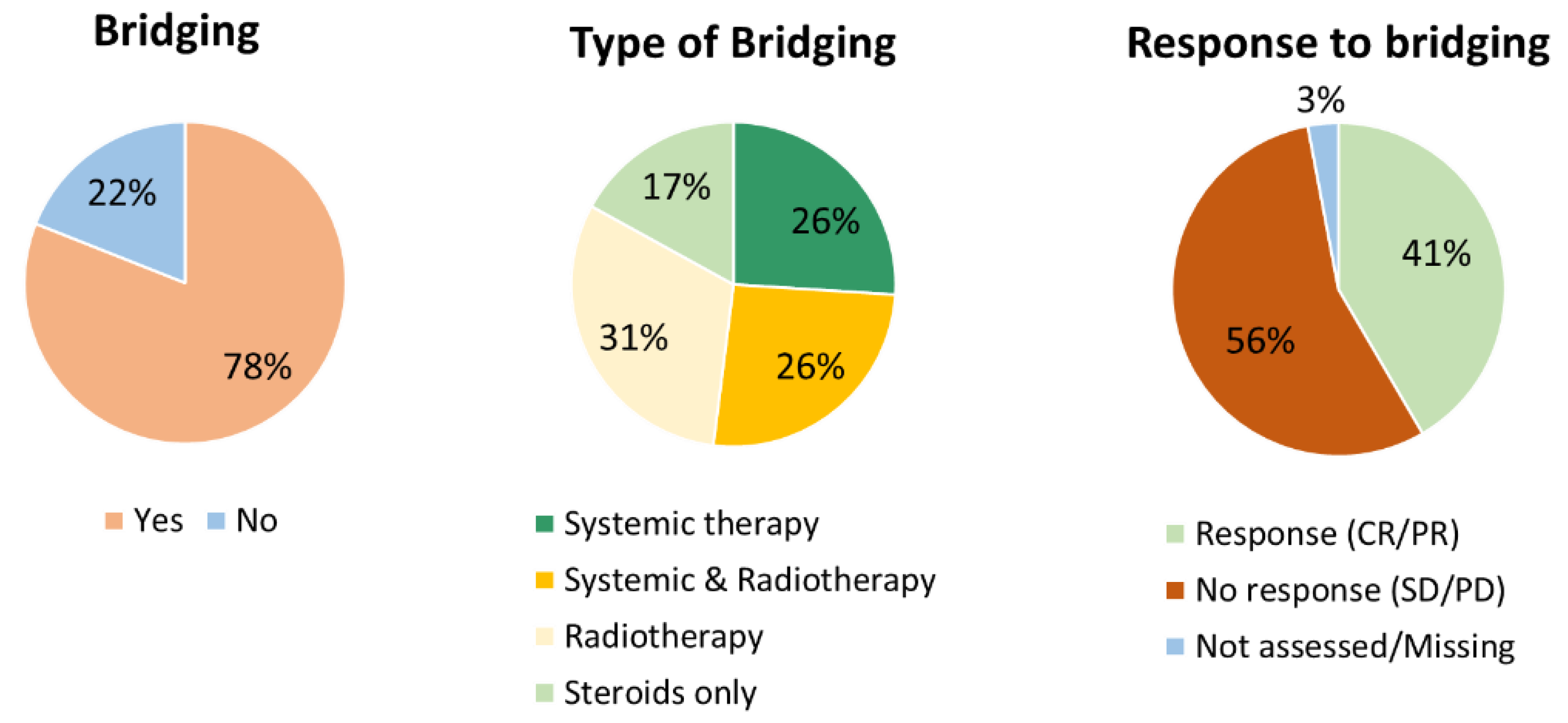
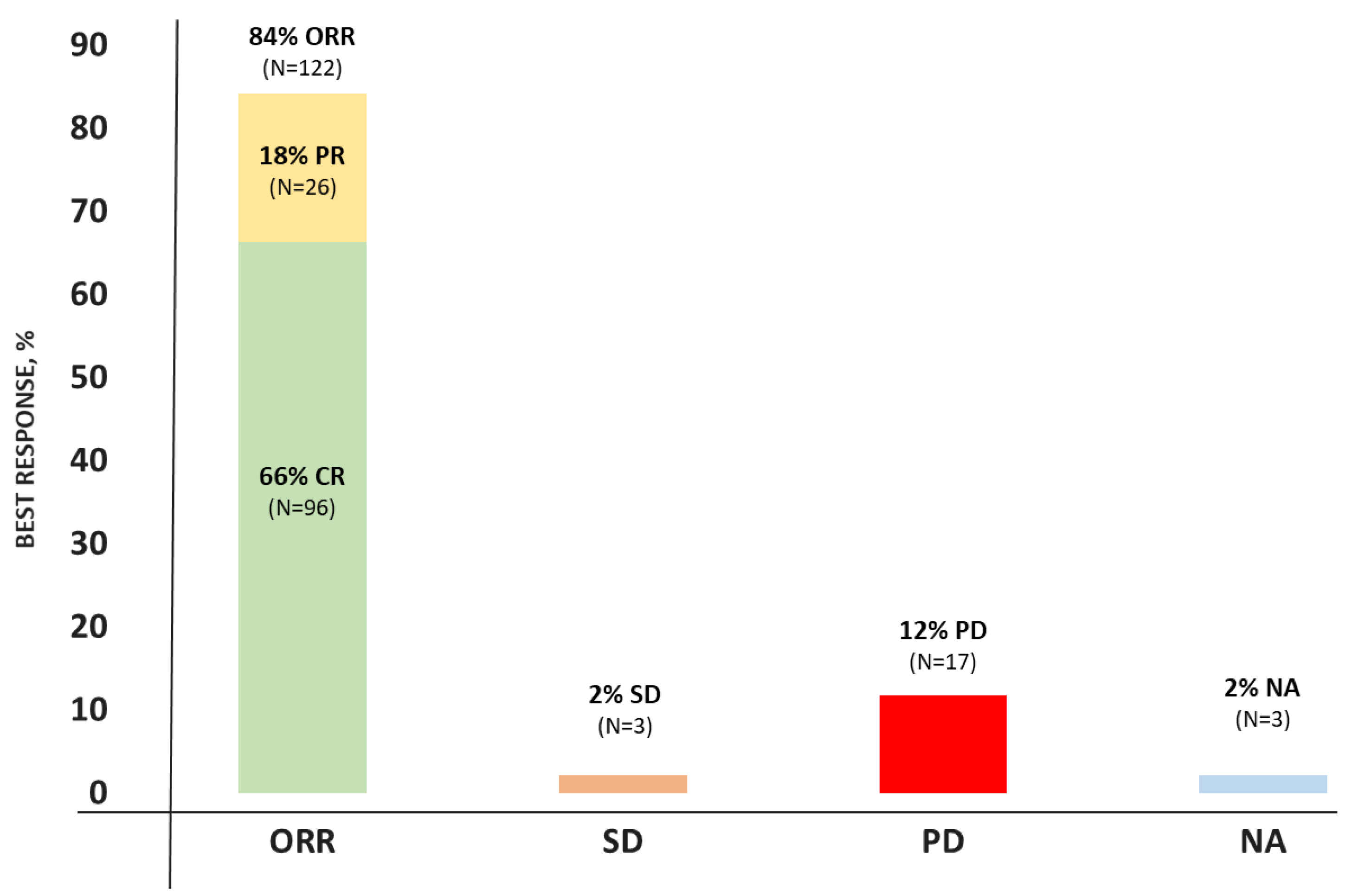
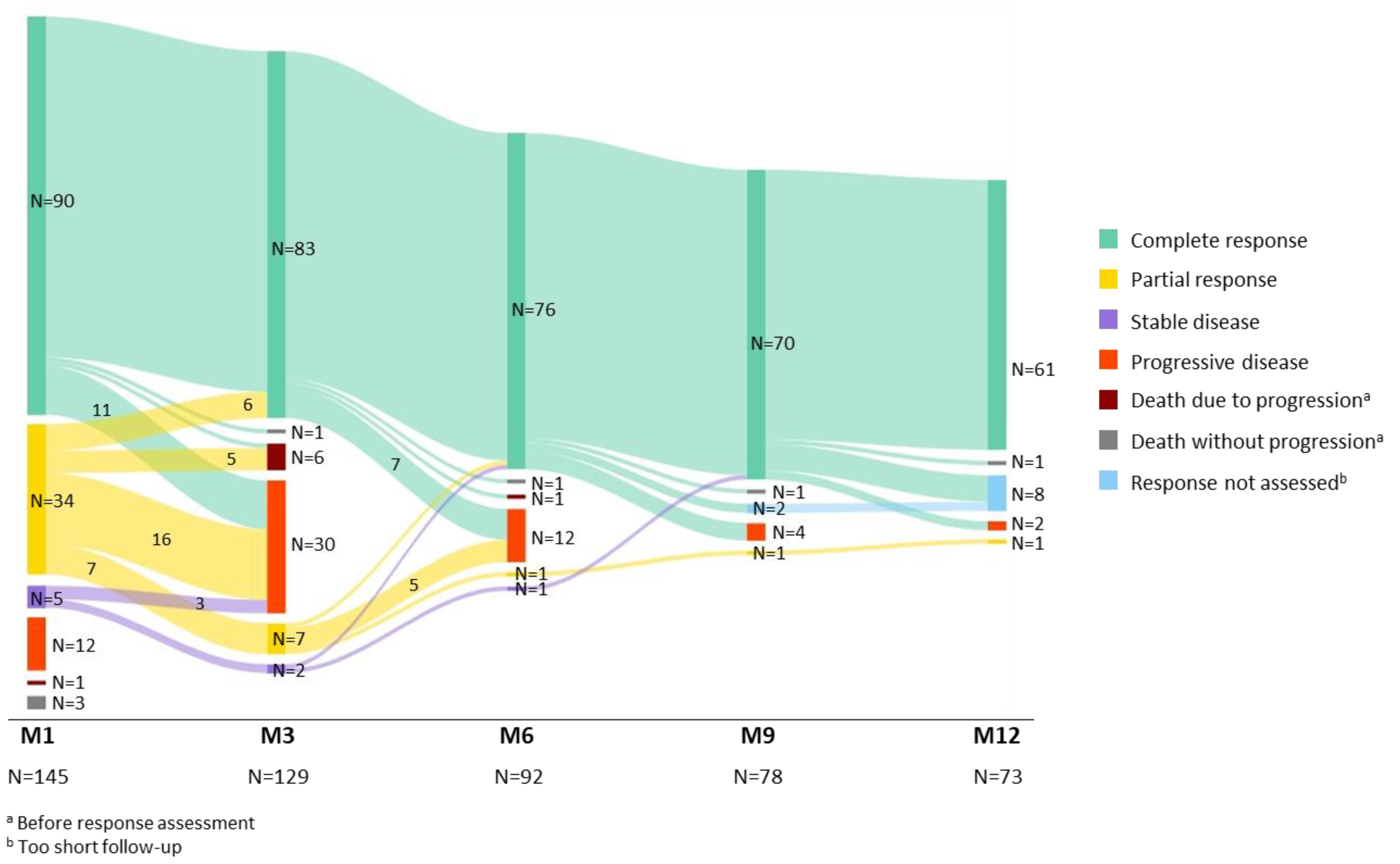
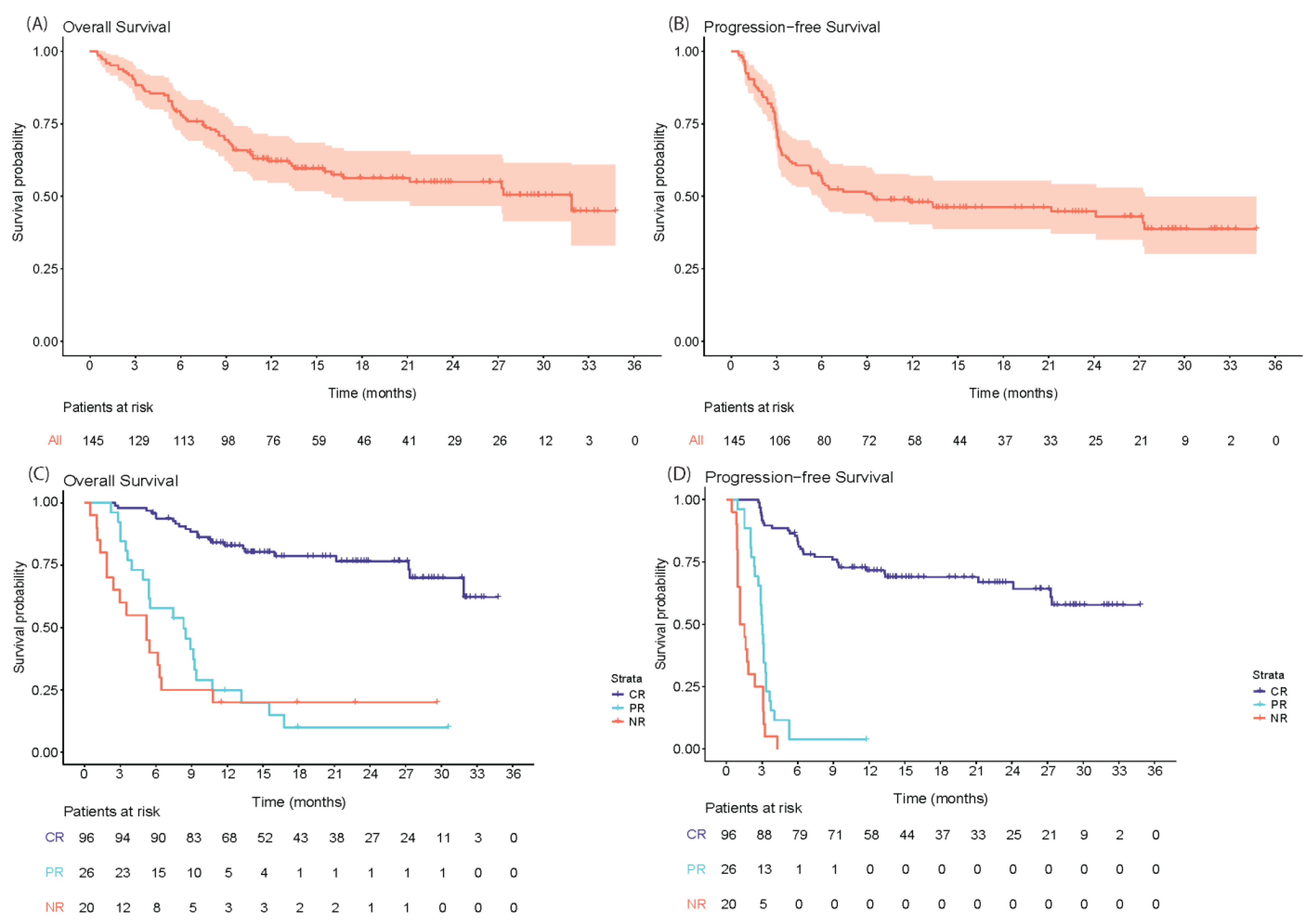
3.2. Efficacy
3.3. Toxicity, Mortality and Hospital Admission
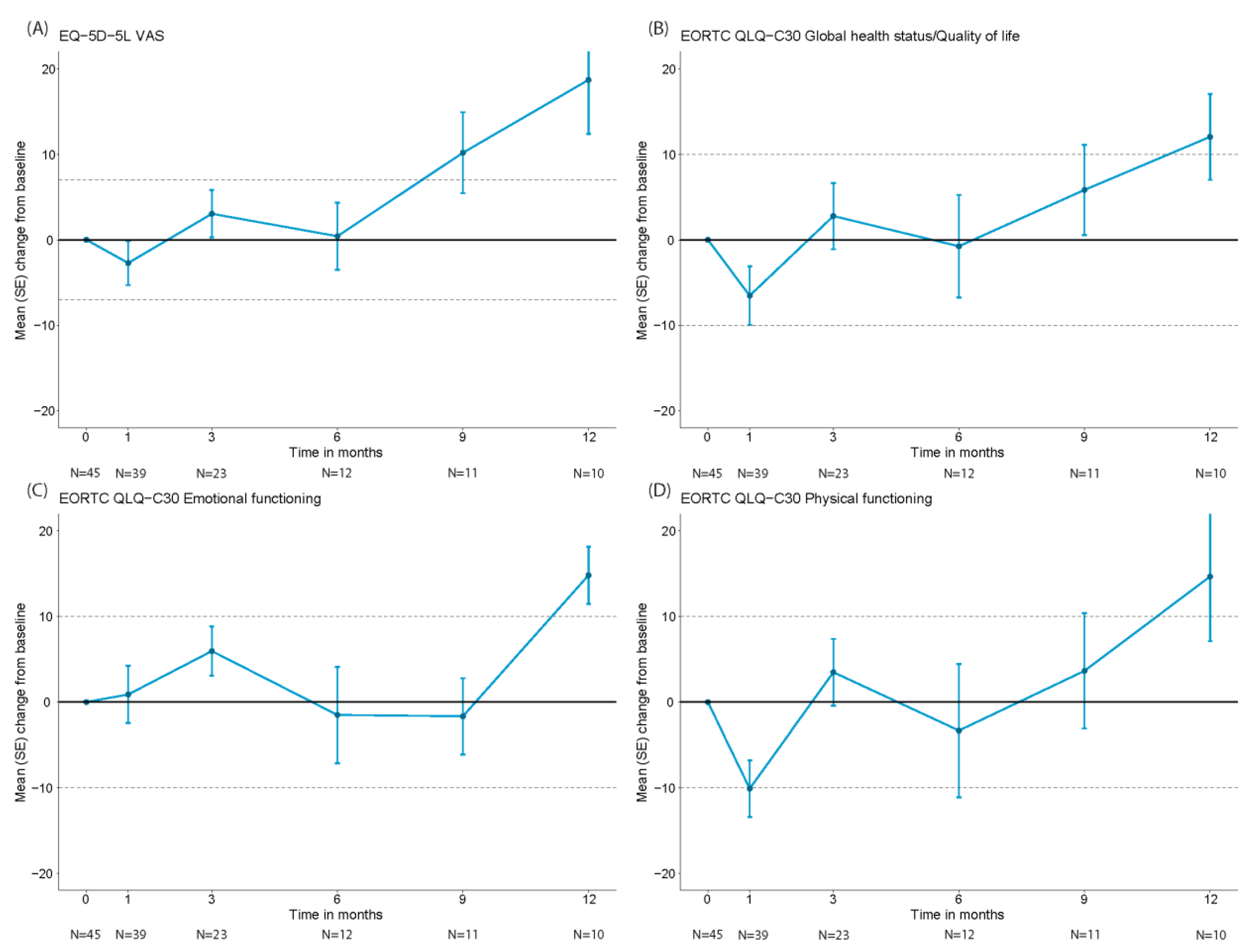
3.4. Health-Related Quality of Life
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crump, M.; Neelapu, S.S.; Farooq, U.; Van Den Neste, E.; Kuruvilla, J.; Westin, J.; Link, B.K.; Hay, A.; Cerhan, J.R.; Zhu, L.; et al. Outcomes in refractory diffuse large B-cell lymphoma: Results from the international SCHOLAR-1 study. Blood 2017, 130, 1800–1808. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Jacobson, C.A.; Ghobadi, A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 2023, 141, 2307–2315. [Google Scholar]
- Schuster, S.J.; Tam, C.S.; Borchmann, P.; Worel, N.; McGuirk, J.P.; Holte, H.; Waller, E.K.; Jaglowski, S.; Bishop, M.R.; Damon, L.E.; et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): A multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021, 22, 1403–1415. [Google Scholar] [CrossRef]
- Nastoupil, L.J.; Jain, M.D.; Feng, L.; Spiegel, J.Y.; Ghobadi, A.; Lin, Y.; Dahiya, S.; Lunning, M.; Lekakis, L.; Reagan, P.; et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results from the US Lymphoma CAR T Consortium. J. Clin. Oncol. 2020, 38, 3119–3128. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Hunter, B.D.; Redd, R.; Rodig, S.J.; Chen, P.-H.; Wright, K.; Lipschitz, M.; Ritz, J.; Kamihara, Y.; Armand, P.; et al. Axicabtagene Ciloleucel in the Non-Trial Setting: Outcomes and Correlates of Response, Resistance, and Toxicity. J. Clin. Oncol. 2020, 38, 3095–3106. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Locke, F.L.; Ma, L.; Asubonteng, J.; Hu, Z.-H.; Siddiqi, T.; Ahmed, S.; Ghobadi, A.; Miklos, D.B.; Lin, Y.; et al. Real-World Evidence of Axicabtagene Ciloleucel for the Treatment of Large B Cell Lymphoma in the United States. Transplant. Cell. Ther. 2022, 28, 581.e1–581.e8. [Google Scholar] [CrossRef]
- Bethge, W.A.; Martus, P.; Schmitt, M.; Holtick, U.; Subklewe, M.; von Tresckow, B.; Ayuk, F.; Wagner-Drouet, E.M.; Wulf, G.G.; Marks, R.; et al. GLA/DRST real-world outcome analysis of CAR-T cell therapies for large B-cell lymphoma in Germany. Blood 2022, 140, 349–358. [Google Scholar] [CrossRef]
- Kuhnl, A.; Roddie, C.; Kirkwood, A.A.; Tholouli, E.; Menne, T.; Patel, A.; Besley, C.; Chaganti, S.; Sanderson, R.; O’Reilly, M.; et al. A national service for delivering CD19 CAR-T in large B-cell lymphoma–The UK real-world experience. Br. J. Haematol. 2022, 198, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Iacoboni, G.; Reguera, J.L.; Corral, L.L.; Morales, R.H.; Ortiz-Maldonado, V.; Guerreiro, M.; Caballero, A.C.; Domínguez, M.L.G.; Pina, J.M.S.; et al. Axicabtagene ciloleucel compared to tisagenlecleucel for the treatment of aggressive B-cell lymphoma. Haematologica 2023, 108, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Bachy, E.; Le Gouill, S.; Di Blasi, R.; Sesques, P.; Manson, G.; Cartron, G.; Beauvais, D.; Roulin, L.; Gros, F.X.; Rubio, M.T.; et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat. Med. 2022, 28, 2145–2154. [Google Scholar] [CrossRef]
- Buecklein, V.L.; Perez-Perez, A.; Rejeski, K.; Iacoboni, G.; Jurinovic, V.; Holtick, U.; Penack, O.; Kharboutli, S.; Blumenberg, V.; Ackermann, J.; et al. Inferior Outcomes of EU Vs. US Patients with Relapsed/Refractory Large B-Cell Lymphoma after CD19 CAR T-Cell Therapy Are Associated with Differences in Tumor Burden, Systemic Inflammation, Bridging Therapy Utilization and CAR-T Product Selection. Blood 2022, 140 (Suppl. 1), 2411–2413. [Google Scholar] [CrossRef]
- Efficace, F.; Cannella, L.; Sparano, F.; Giesinger, J.M.; Vignetti, M.; Baron, F.; Bruera, E.; Luppi, M.; Platzbecker, U. Chimeric Antigen Receptor T-cell Therapy in Hematologic Malignancies and Patient-reported Outcomes: A Scoping Review. Hemasphere 2022, 6, e802. [Google Scholar] [CrossRef] [PubMed]
- Sehn, L.H.; Salles, G. Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2021, 384, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Stichting Werkgroep Antibiotica Beleid. Richtlijnen SWAB. Available online: https://swab.nl/nl/richtlijnen-swab (accessed on 31 July 2023).
- Hayden, P.; Roddie, C.; Bader, P.; Basak, G.; Bonig, H.; Bonini, C.; Chabannon, C.; Ciceri, F.; Corbacioglu, S.; Ellard, R.; et al. Management of adults and children receiving CAR T-cell therapy: 2021 best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) and the European Haematology Association (EHA). Ann. Oncol. 2021, 33, 259–275. [Google Scholar]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evaluation, stag-ing, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Pickard, A.S.; Neary, M.P.; Cella, D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual. Life Outcomes 2007, 5, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Osoba, D.; Rodrigues, G.; Myles, J.; Zee, B.; Pater, J. Interpreting the significance of changes in health-related quality-of-life scores. J. Clin. Oncol. 1998, 16, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Miklos, D.B.; Jacobson, C.A.; Perales, M.-A.; Kersten, M.-J.; Oluwole, O.O.; Ghobadi, A.; Rapoport, A.P.; McGuirk, J.; Pagel, J.M.; et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 640–654. [Google Scholar] [CrossRef]
- Bishop, M.R.; Dickinson, M.; Purtill, D.; Barba, P.; Santoro, A.; Hamad, N.; Kato, K.; Sureda, A.; Greil, R.; Thieblemont, C.; et al. Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N. Engl. J. Med. 2022, 386, 629–639. [Google Scholar] [CrossRef]
- Kamdar, M.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): Results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet 2022, 399, 2294–2308. [Google Scholar]
- Westin, J.R.; Oluwole, O.O.; Kersten, M.J.; Miklos, D.B.; Perales, M.-A.; Ghobadi, A.; Rapoport, A.P.; Sureda, A.; Jacobson, C.A.; Farooq, U.; et al. Survival with Axicabtagene Ciloleucel in Large B-Cell Lymphoma. N. Engl. J. Med. 2023, 389, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Solomon, S.R.; Arnason, J.; Johnston, P.B.; Glass, B.; Bachanova, V.; Ibrahimi, S.; Mielke, S.; Mutsaers, P.; Hernandez-Ilizaliturri, F.; et al. Lisocabtagene maraleucel as second-line therapy for large B-cell lymphoma: Primary analysis of the phase 3 TRANSFORM study. Blood 2023, 141, 1675–1684. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Dickinson, M.; Munoz, J.; Ulrickson, M.L.; Thieblemont, C.; Oluwole, O.O.; Herrera, A.F.; Ujjani, C.S.; Lin, Y.; Riedell, P.A.; et al. Axicabtagene ciloleucel as first-line therapy in high-risk large B-cell lymphoma: The phase 2 ZUMA-12 trial. Nat. Med. 2022, 28, 735–742. [Google Scholar] [CrossRef]
- Roddie, C.; Neill, L.; Osborne, W.; Iyengar, S.; Tholouli, E.; Irvine, D.; Chaganti, S.; Besley, C.; Bloor, A.; Jones, C.; et al. Effective bridging therapy can improve CD19 CAR-T outcomes while maintaining safety in patients with large B-cell lymphoma. Blood Adv. 2023, 7, 2872–2883. [Google Scholar] [CrossRef]
- Gal, R.; Monninkhof, E.M.; van Gils, C.H.; Groenwold, R.H.; Bongard, D.H.v.D.; Peeters, P.H.; Verkooijen, H.M.; May, A.M. The Trials within Cohorts design faced methodological advantages and disadvantages in the exercise oncology setting. J. Clin. Epidemiol. 2019, 113, 137–146. [Google Scholar] [CrossRef]
- Alkhateeb, H.B.; Mohty, R.; Greipp, P.; Bansal, R.; Hathcock, M.; Rosenthal, A.; Murthy, H.; Kharfan-Dabaja, M.; Villasboas, J.C.B.; Bennani, N.; et al. Therapy-related myeloid neoplasms following chimeric antigen receptor T-cell therapy for Non-Hodgkin Lymphoma. Blood Cancer J. 2022, 12, 113. [Google Scholar] [CrossRef]
- Vainstein, V.; Avni, B.; Grisariu, S.; Kfir-Erenfeld, S.; Asherie, N.; Nachmias, B.; Auman, S.; Saban, R.; Zimran, E.; Assayag, M.; et al. Clonal Myeloid Dysplasia Following CAR T-Cell Therapy: Chicken or the Egg? Cancers 2023, 15, 3471. [Google Scholar] [CrossRef]
- Haidar, G.; Garner, W.; Hill, J.A. Infections after anti-CD19 chimeric antigen receptor T-cell therapy for hematologic malignancies: Timeline, prevention, and uncertainties. Curr. Opin. Infect. Dis. 2020, 33, 449–457. [Google Scholar] [CrossRef]
- Al-Rahimi, J.S.; Nass, N.M.; Hassoubah, S.A.; Wazqar, D.Y.; Alamoudi, S.A. Levels and predictors of fear and health anxiety during the current outbreak of COVID-19 in immunocompromised and chronic disease patients in Saudi Arabia: A cross-sectional correlational study. PLoS ONE 2021, 16, e0250554. [Google Scholar] [CrossRef]
- Hill, J.A.; Seo, S.K. How I prevent infections in patients receiving CD19-targeted chimeric antigen receptor T cells for B-cell malignancies. Blood 2020, 136, 925–935. [Google Scholar] [CrossRef]
- Banerjee, R.; Shah, N.; Dicker, A.P. Next-Generation Implementation of Chimeric Antigen Receptor T-Cell Therapy Using Digital Health. JCO Clin. Cancer Inform. 2021, 5, 668–678. [Google Scholar] [CrossRef]
- Spanjaart, A.M.; Pennings, E.R.; Kos, M.; Mutsaers, P.G.; Lugtenburg, P.J.; van Meerten, T.; van Doesum, J.A.; Minnema, M.C.; Jak, M.; van Dorp, S.; et al. Development of a Core Set of Patient- and Caregiver-Reported Signs and Symptoms to Facilitate Early Recognition of Acute Chimeric Antigen Receptor T-Cell Therapy Toxicities. JCO Oncol. Pract. 2023, 19, e407–e416. [Google Scholar] [CrossRef]
| CAR-T Infused Patients Cohort (N = 145) | |
|---|---|
| Median age (min–max) | 60 years (21–84) |
| Female, n (%) | 50 (34%) |
| Histological subtype, n (%) | |
| DLBCL | 73 (50%) |
| HGBCL (NOS/DH/TH) | 20 (14%) |
| PMBCL | 4 (3%) |
| tFL | 48 (33%) |
| IPI score, n (%) | |
| Low | 28 (19%) |
| Low-intermediate | 49 (34%) |
| High-intermediate | 41 (28%) |
| High | 13 (9%) |
| Incompletely assessed, n (%) | 14 (10%) |
| Stage III–IV, n (%) | 124 (86%) |
| Bulky disease (nodal ≥ 10 cm and/or extranodal ≥ 5 cm), n (%) | 50 (34%) |
| Missing | 2 (1%) |
| Extranodal sites present, n (%) | 98 (68%) |
| 2 Sites | 26 (18%) |
| ≥3 Sites | 16 (11%) |
| Unknown number of sites | 2 (1%) |
| ECOG performance status, n (%) | |
| 0 | 85 (59%) |
| 1 | 49 (34%) |
| ≥2 | 11 (8%) |
| Primary refractory a, n (%) | 88 (61%) |
| Disease status, n (%) | |
| Relapse after last therapy line | 29 (20%) |
| Refractory to last therapy line | 116 (80%) |
| Prior therapy lines, median (min–max) | 2 (2–6) |
| ≥3 | 38 (26%) |
| Prior transplant, n (%) | |
| Autologous SCT | 42 (29%) |
| Allogeneic SCT | 3 (2%) |
| LDH, n (%) | |
| >1x ULN–2x ULN | 54 (37%) |
| ≥2x ULN | 22 (15%) |
| Missing | 12 (8%) |
| Hemoglobin < 6 mmol/L, n (%) | 44 (30%) |
| Missing | 2 (1%) |
| Ferritin > 1000 µg/L, n (%) | 20 (14%) |
| Missing | 82 (57%) |
| CRP > 50 mg/L, n (%) | 28 (19%) |
| Missing | 43 (30%) |
| Platelets < 75 × 109/L, n (%) | 17 (12%) |
| Missing | 3 (2%) |
| Neutrophil count < 0.5 × 109/L, n (%) | 7 (5%) |
| Missing | 30 (21%) |
| Lymphocyte count < 0.5 × 109/L, n (%) | 27 (19%) |
| Missing | 40 (28%) |
| Factor | HR (95% CI) | p-Value |
|---|---|---|
| (A). Multivariable Analysis for OS | ||
| LDH elevated ≥ 2x ULN at time of infusion | 7.40 (1.56–35.00) | 0.0116 * |
| No response to bridging therapy | 3.73 (0.75–18.45) | 0.1072 |
| Hemoglobin < 6 mmol/L at time of infusion | 3.18 (1.16–8.75) | 0.0247 * |
| LDH elevated 1 − 2x ULN at time of infusion | 2.68 (0.98–7.36) | 0.0553 |
| Response to bridging therapy | 2.35 (0.43–12.88) | 0.3240 |
| IPI score high intermediate and high risk (≥3) | 2.35 (1.06–5.2) | 0.0354 * |
| Male sex | 2.33 (0.82–6.62) | 0.1125 |
| Platelets < 75 × 109/L at time of infusion | 1.92 (0.72–5.15) | 0.1935 |
| ECOG performance status ≥ 2 at time of infusion | 1.44 (0.24–8.56) | 0.6899 |
| ECOG performance status = 1 at time of infusion | 1.07 (0.47–2.47) | 0.8661 |
| No CR to first-line treatment (primary refractory disease) | 1.07 (0.43–2.65) | 0.8864 |
| LDH elevated 1 − 2x ULN at screening | 0.91 (0.37–2.24) | 0.8290 |
| CRP > 50 mg/L at time of infusion | 0.82 (0.27–2.49) | 0.7230 |
| Ferritin > 1000 µg/L at time of infusion | 0.53 (0.21–1.35) | 0.1856 |
| LDH elevated ≥ 2x ULN at screening | 0.44 (0.10–1.85) | 0.2627 |
| (B). Multivariable analysis for PFS | ||
| No response to bridging therapy | 2.68 (1.00–7.17) | 0.0497 * |
| LDH elevated ≥ 2x ULN at time of infusion | 2.31 (0.71–7.53) | 0.1630 |
| IPI score high intermediate and high risk (≥3) | 2.28 (1.20–4.33) | 0.0123 * |
| Hemoglobin < 6 mmol/L at time of infusion | 2.27 (1.04–4.95) | 0.0392 * |
| LDH elevated 1 − 2x ULN at time of infusion | 1.75 (0.87–3.55) | 0.1185 |
| Response to bridging therapy | 1.24 (0.44–3.49) | 0.6876 |
| Platelets < 75 × 109/L at time of infusion | 1.09 (0.52–2.28) | 0.8101 |
| CRP > 50 mg/L at time of infusion | 1.06 (0.51–2.19) | 0.8756 |
| Ferritin > 1000 µg/L at time of infusion | 0.79 (0.40–1.56) | 0.4957 |
| LDH elevated 1 − 2x ULN at screening | 0.73 (0.36–1.49) | 0.3861 |
| LDH elevated ≥ 2x ULN at screening | 0.64 (0.21–1.89) | 0.4160 |
| Event | |
|---|---|
| CRS, any grade, n (%) | 133 (92%) |
| Grade ≥3 | 7 (5%) |
| Median time to onset (min–max) | 2 days (0–9) |
| Median duration | 5 days (1–7) |
| ICANS, any grade, n (%) | 90 (62%) |
| Grade ≥3 | 32 (22%) |
| Median time to onset (min–max) | 6 days (1–28) |
| Median duration | 5 days (1–28) |
| Tocilizumab use, n (%) | 103 (71%) |
| Anakinra use, n (%) | 3 (2%) |
| Steroids use, n (%) | 93 (64%) |
| Any grade and any type infections, n (%) | |
| Month <1 | 38 (26%) |
| Months 1–12 | 61 (43%) |
| Months >12 | 33 (43%) |
| Thrombocytopenia at Month 3, n (%) | 59 (45%) |
| Grade ≥3 | 19 (15%) |
| Missing | 5 (4%) |
| Anemia at Month 3, n (%) | 95 (73%) |
| Grade ≥3 | 9 (7%) |
| Missing | 5 (4%) |
| Neutropenia at Month 3, n (%) | 62 (47%) |
| Grade ≥3 | 45 (34%) |
| Missing | 6 (5%) |
| CD4 < 0.2 × 109/L at 1 year, n (%) | 37 (49%) |
| Missing | 27 (36%) |
| CD8 < 0.2 × 109/L at 1 year, n (%) | 25 (33%) |
| Missing | 27 (36%) |
| B-cell aplasia (B-cells/lymphocytes < 0.5%) at 1 year, n (%) | 40 (53%) |
| Missing | 26 (34%) |
| Hypogammaglobulinemia (IgG < 6 g/L) at 1 year, n (%) | 52 (68%) |
| Missing | 17 (22%) |
| Median hospital admission duration (min–max) | 14 days (7–78) |
| Patients that died during admission, n (%) | 4 (3%) |
| ICU admission, n (%) | 20 (14%) |
| Median ICU admission duration (min–max) | 3 days (1–19) |
| ER visits and readmissions < Month 1, n (%) | 18 (12%) |
| Readmission for CAR-T related toxicity, n | 7 (neurotoxicity 6, infection 1) |
| ER visits and readmissions between Month 1–3, n (%) | 26 (18%) |
| Readmission for CAR-T related toxicity, n | 19 (neurotoxicity 2, infection 17) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanjaart, A.M.; Pennings, E.R.A.; Mutsaers, P.G.N.J.; van Dorp, S.; Jak, M.; van Doesum, J.A.; de Boer, J.W.; Niezink, A.G.H.; Kos, M.; Vermaat, J.S.P.; et al. The Dutch CAR-T Tumorboard Experience: Population-Based Real-World Data on Patients with Relapsed or Refractory Large B-Cell Lymphoma Referred for CD19-Directed CAR T-Cell Therapy in The Netherlands. Cancers 2023, 15, 4334. https://doi.org/10.3390/cancers15174334
Spanjaart AM, Pennings ERA, Mutsaers PGNJ, van Dorp S, Jak M, van Doesum JA, de Boer JW, Niezink AGH, Kos M, Vermaat JSP, et al. The Dutch CAR-T Tumorboard Experience: Population-Based Real-World Data on Patients with Relapsed or Refractory Large B-Cell Lymphoma Referred for CD19-Directed CAR T-Cell Therapy in The Netherlands. Cancers. 2023; 15(17):4334. https://doi.org/10.3390/cancers15174334
Chicago/Turabian StyleSpanjaart, Anne M., Elise R. A. Pennings, Pim G. N. J. Mutsaers, Suzanne van Dorp, Margot Jak, Jaap A. van Doesum, Janneke W. de Boer, Anne G. H. Niezink, Milan Kos, Joost S. P. Vermaat, and et al. 2023. "The Dutch CAR-T Tumorboard Experience: Population-Based Real-World Data on Patients with Relapsed or Refractory Large B-Cell Lymphoma Referred for CD19-Directed CAR T-Cell Therapy in The Netherlands" Cancers 15, no. 17: 4334. https://doi.org/10.3390/cancers15174334
APA StyleSpanjaart, A. M., Pennings, E. R. A., Mutsaers, P. G. N. J., van Dorp, S., Jak, M., van Doesum, J. A., de Boer, J. W., Niezink, A. G. H., Kos, M., Vermaat, J. S. P., Sijs-Szabo, A., van der Poel, M. W. M., Nijhof, I. S., Kuipers, M. T., Chamuleau, M. E. D., Lugtenburg, P. J., Doorduijn, J. K., Serroukh, Y. I. M., Minnema, M. C., ... Kersten, M. J., on behalf of the Dutch CAR-T Tumorboard Consortium. (2023). The Dutch CAR-T Tumorboard Experience: Population-Based Real-World Data on Patients with Relapsed or Refractory Large B-Cell Lymphoma Referred for CD19-Directed CAR T-Cell Therapy in The Netherlands. Cancers, 15(17), 4334. https://doi.org/10.3390/cancers15174334







