Simple Summary
In clinical settings, patients receiving immune checkpoint inhibitors (ICIs) have different treatment criteria than those enrolled in clinical trials. There are concerns regarding the efficacy of ICIs in older adults due to age-associated decline in the immune system, and no study has directly compared the efficacy of different ICIs for the elderly in a real-world setting. We aimed to analyze ICIs’ use and treatment outcomes in Korean veterans with stage IV non-small-cell lung cancer (NSCLC). Three cohort groups were derived based on the ICI type (pembrolizumab, nivolumab, and atezolizumab treatment groups), and their clinical characteristics and survival outcomes were compared. There was no difference in the overall survival (OS) rate among the groups, no treatment-specific OS benefit was observed relative to the tumor PD-L1 expression, and bone metastasis was a poor prognostic factor for OS. Our study demonstrates that all three agents may be appropriate treatment options for elderly patients.
Abstract
Purpose: To provide a comprehensive analysis of ICI usage and treatment outcomes in elderly Korean veterans with stage IV NSCLC. Methods: Patients diagnosed with stage IV NSCLC between 2016 and 2021 were included, and three cohorts were derived according to the type of ICI received. Thereafter, the clinical characteristics and survival outcomes were compared. Results: Of the 180 patients with NSCLC (median age, 76 years) included in this study, 49 (27.7%), 61 (33.9%), and 70 (38.9%) received pembrolizumab, nivolumab, and atezolizumab, respectively, and 19.4%, 36.1%, and 34.4% had PD-L1 expressions < 1%, 1–49%, and ≥50%, respectively. The pembrolizumab, nivolumab, and atezolizumab groups, the objective response rates (ORR), and the disease control rates (DCR) were 22.4%, 8.2%, and 4.3% (p = 0.004), and 59.2, 55.7%, and 30.0% (p = 0.001), respectively. However, no difference in the overall survival (OS) rate was noted among the groups (12.6 months vs. 8.4 months vs. 7.7 months, p = 0.334). Similarly, there was no treatment specific OS benefit with respect to the tumor PD-L1 expression status. Interestingly, multivariate analysis identified bone metastasis as a significant poor prognostic factor for OS (HR = 2.75 [95% CI, 1.31–5.76], p = 0.007). Conclusion: Pembrolizumab and nivolumab showed stronger associations with increases in ORR and DCR than atezolizumab, but no statistically significant differences were observed with respect to OS.
1. Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, and non-small cell lung cancer (NSCLC) accounts for 82% of all lung cancer cases [1]. Treatment with immune checkpoint inhibitors (ICIs) targeting programmed death 1 receptor (PD-1)/programmed death ligand 1 (PD-L1) is a revolutionary development in oncology and has been approved for advanced or metastatic NSCLC with no epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) genomic alterations [2,3,4]. ICIs block the immune evasion pathway used by cancer cells for survival and proliferation, among several other mechanisms, which induce immune tolerance in cancer cells (Figure 1). Pembrolizumab (KEYNOTE-010 [5]), nivolumab (CheckMate 017 and CheckMate 057 [6]), and atezolizumab (OAK [7]) are three mono-immunotherapies recommended for patients who experience cancer progression after platinum-doublet chemotherapy. Notably, according to KEYNOTE-024 results, pembrolizumab monotherapy is the standard first-line treatment for metastatic NSCLC with a PD-L1 Tumor Proportion Score (TPS) ≥ 50% [8]. Thus, ICIs are becoming increasingly recognized as conventional treatments for NSCLC [9].
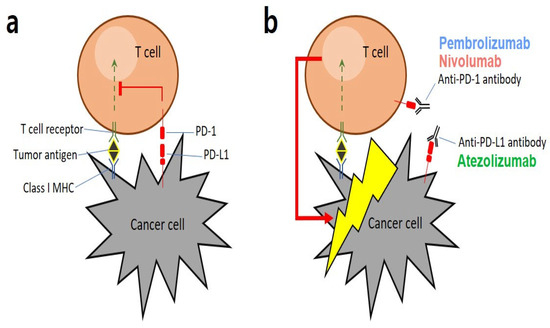
Figure 1.
Graphical representation of key mechanisms involved in PD-1/PD-L1: (a) Tumor antigens are presented by major histocompatibility complexes (MHCs) that interact with antigen-specific T-cell receptors. As a strategy for escaping the immune system, cancer cells express a specific protein called PD-L1 on their surface, which binds to PD-1 present on T cells to suppress cytotoxic T cell functions. (b) Immune checkpoint inhibitors (ICIs), such as anti-PD-1 or anti-PD-L1 antibodies, bind to the binding sites of cancer and T cells to block immune evasion signals, allowing T cells that are not hindered by immune evasion to destroy cancer cells [3].
Recruitment for these clinical trials was limited to younger patients with good performance status (PS; Eastern Cooperative Oncology Group [ECOG], PS score 0 or 1) and minimal comorbidities other than autoimmune diseases. Lung cancer mainly affects older adults, with approximately 70% and 30% of new cases diagnosed in patients aged ≥65 and ≥75 years, respectively [1]. The Food and Drug Administration (FDA) conducted an analysis involving older patients with lung cancer enrolled in clinical trials for drug approval; approximately 40% of these patients were aged ≥65 years, which is inconsistent with the finding that 70% of all new stage IV lung cancer cases are diagnosed in patients aged ≥65 years [10]. Therefore, the patients enrolled based on the restrictive selection criteria do not represent real-world patient populations. Consequently, the data on the relative safety and efficacy of new drugs in older patients with lung cancer and multiple comorbidities are limited.
Preliminary evidence suggests that there is little difference in the efficacy of ICIs between older and younger patients; however, the impact of ICIs on older adults remains largely unknown [11]. Furthermore, some clinical factors, such as PS and metastatic sites, have emerged as potential predictors of immunotherapy efficacy [12,13,14]. Notably, some meta-analyses and systemic reviews have been conducted to assess the efficacy and safety of different ICIs; however, only one meta-analysis has compared these three drugs. Passiglia et al. [15] performed a meta-analysis of all phases II/III randomized clinical trials (RCTs) comparing PD1/PDL1 inhibitors with docetaxel in pretreated patients with NSCLC. They indirectly compared differences in the efficacy and safety profiles of atezolizumab, pembrolizumab, and nivolumab. Based on their findings, they concluded that nivolumab and pembrolizumab were associated with a significant increase in the objective response rate (ORR) compared with atezolizumab (nivolumab vs. atezolizumab: RR 1.66, 95% confidence interval [CI] 1.07–2.58 and pembrolizumab vs. atezolizumab: RR 1.94, 95% CI, 1.30–2.90); however, no statistically significant differences were observed with respect to progression-free survival (PFS) or overall survival (OS). Regarding safety, nivolumab was found to be associated with a significantly lower risk of G3/G5 adverse events.
The Veterans Health Service (VHS) Medical Center is one of Korea’s largest integrated healthcare systems. The VHS Medical Center serves a patient population comprising individuals who tend to be older, have poorer PSs and several comorbidities, and are often underrepresented in clinical trials. Herein we report the results of a real-world observational study focused on evaluating treatment outcomes in Korean veterans with advanced NSCLC treated with three different ICI monotherapies, in first-, second-, or subsequent-line settings, at the VHS Medical Center. In addition, we investigated the predictive factors for survival outcomes in patients with NSCLC.
2. Methods
2.1. Patients and Ethics Statement
In this study, we retrospectively analyzed the data of patients with histologically confirmed clinical stage IV NSCLC at the VHS Medical Center in Korea. Patients treated with ICI monotherapy, including pembrolizumab, nivolumab, and atezolizumab, between 1 January 2016 and 31 December 2021, were included. All patients were confirmed negative for EGFR mutations and ALK gene rearrangements. Patients who received ICI combination therapy or ICI plus cytotoxic chemotherapy were excluded from the study. Metastatic or recurrent NSCLC (Stage IV) was defined radiologically using contrast-enhanced computed tomography (CT), magnetic resonance imaging (MRI), or positron emission tomography-CT (PET-CT). A total of 180 patients were enrolled and classified into three groups according to the treatment regimen. We collected data on patient demographics, including age, sex, ECOG-PS, histological subtype, line of therapy, PD-L1 expression status, metastatic sites, and survival status. This study was approved and monitored by the Institutional Review Board of the Veterans Health Service Medical Center (IRB No. 2022-07-002). The IRB waived the requirement for informed consent from the patients for this study.
2.2. Study Endpoint and Measures
In this study, the patients who met the inclusion criteria were divided into three groups according to the type of ICI monotherapy (pembrolizumab, nivolumab, and atezolizumab) for comparison. Response evaluation for complete and partial responses or disease progression was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [16] based on CT imaging. The ORR was defined as the proportion of patients with complete or partial responses, whereas the disease control rate (DCR) was defined as the proportion of patients with complete, partial, or stable responses. Further, the OS was defined as the date from the start of treatment with the first ICI to the date of death from any cause. Survival was determined using the date of the last follow-up visit for patients who were alive at the time of the analysis. Furthermore, to investigate the association between PD-L1 expression and survival outcome, the entire cohort of patients was divided into three groups according to the PD-L1 expression status (PD-L1 < 1%, 1–49%, and ≥50%), and OS was further analyzed according to the PD-L1 expression level. Measurements, including survival outcomes and prognostic factors for survival, were performed until treatment discontinuation, loss of follow-up, death, or the last follow-up.
2.3. Statistical Analysis
All statistical analyses were performed using the statistical software SPSS version 25 (IBM, Chicago, IL, USA) and R software version 4.1.2 (R Development Core Team, Vienna, Austria). Patient demographics were analyzed using Pearson’s chi-square or Fisher’s exact test for categorical variables, and the analysis of variance or the Kruskal–Wallis test for continuous variables. The median OS was estimated using the Kaplan–Meier method with log-rank tests. Patient survival was monitored until 23 August 2022. Univariate and multivariate analyses were also performed using Cox proportional-hazards regression models to identify factors associated with survival outcomes in patients with NSCLC. Hazard ratios (HR) and 95% CIs were estimated by adjusting for age, sex, ECOG-PS, histological subtype, PD-L1 expression level, treatment, and metastasis sites. For the multivariate analysis, the above-mentioned variables were analyzed using backward selection (stopping condition: p < 0.15). Statistical significance was set at p < 0.05.
3. Results
3.1. Patient Characteristics
A total of 180 patients with recurrent or metastatic NSCLC, who received ICI monotherapy were recruited. The median age of all patients was 76 years (interquartile range, 74–78 years), and 177 patients (98.3%) were male (Table 1). Notably, most patients with NSCLC had an ECOG-PS score of 0 or 1 (96.1%) at the start of the treatment, whereas the remaining 3.9% had an ECOG-PS score of 2 or 3. Furthermore, all the patients had a current or past smoking history, and among them, 49 (27.7%), 61 (33.9%), and 70 (38.9%) were categorized into the pembrolizumab, nivolumab, and atezolizumab groups, respectively. The squamous cell histological subtype was the most common NSCLC subtype in the atezolizumab group, accounting for 60.0% of cases (squamous: pembrolizumab, n = 18 [36.7%]; nivolumab, n = 30 [49.2%]; and atezolizumab, n = 42 [60.0%]), whereas the non-squamous cell histological subtype was the least common in this group (non-squamous: pembrolizumab, n = 31 [63.3%]; nivolumab, n = 31 [50.8%]; and atezolizumab, n = 28 [40.0%]). Only 8 of the 180 patients (4.4%) in the pembrolizumab group received ICI as first-line therapy, whereas the remaining 172 (95.6%) received ICI as second-line therapy and beyond. Of all patients with known tumor PD-L1 expression statuses, 19.4%, 36.1%, and 34.4% had PD-L1 < 1%, 1–49%, and ≥50%, respectively. The most common metastatic sites were the brain (n = 21, 11.7%), bones (n = 13, 7.2%), and liver (n = 8, 4.4%).

Table 1.
Patients’ baseline clinical characteristics.
3.2. Treatment Outcomes
The median follow-up period was 10 months (range, 1–77 months), and the median number of cycles was four (range, 1–123). The ORR was 22.4% [95% CI, 11.77–36.62], 8.2% [95% CI, 2.72–18.10], and 4.3% [95% CI, 0.89–12.02] in the pembrolizumab, nivolumab, and atezolizumab groups, respectively (Table 2). Further, the DCR was 59.2% [95% CI, 44.21–73.00], 55.7% [95% CI, 42.45–68.45], and 30.0% [95% CI, 19.62–42.13] in the pembrolizumab, nivolumab, and atezolizumab groups, respectively. None of the groups showed a complete response. Partial response was achieved in 11 (22.4%), 5 (8.2%), and 3 (4.3%) patients who received pembrolizumab, nivolumab, and atezolizumab, respectively. Further, stable disease was achieved in 18 (36.7%), 29 (47.5%), and 18 (25.7%) patients who received pembrolizumab, nivolumab, and atezolizumab, respectively. The median OS was 10 months [95% CI, 7.9–12.0] (Figure 2a). There was no significant difference in the OS rate among the treatment groups (pembrolizumab [12.6 months] vs. nivolumab [8.4 months] vs. atezolizumab [7.7 months], p = 0.334; Figure 2b). Figure 3 shows the OS stratified according to the histological subtype. In the squamous cell histological subtype, the median OS was 8.5, 8.0, and 9.4 months in the pembrolizumab, nivolumab, and atezolizumab groups, respectively (Figure 3a). For the non-squamous cell histological subtype, the median OS was 14.8, 8.4, and 6.2 months in the pembrolizumab, nivolumab, and atezolizumab groups, respectively (Figure 3b). The statistical significance of OS according to the histological subtype was not confirmed (squamous, p = 0.808 and non-squamous, p = 0.257), and the OS for the pembrolizumab and nivolumab groups was shorter for patients with the squamous histological subtype than for those with the non-squamous histological subtype. Among 162 patients (90.0%) with a confirmed tumor PD-L1 expression, the OS rate according to the PD-L1 expression showed no significant difference (p = 0.210) (Figure 4). The median OS was 7.2 months, 8.0 months, and 12.5 months in the PD-L1 < 1%, 1–49%, and ≥50% groups, respectively (<1% vs. 1–49%, p = 0.805; 1–49% vs. ≥50%, p = 0.101; and <1% vs. ≥50%, p = 0.203). Regarding tumor PD-L1 expression, there was no treatment-specific OS benefit in the PD-L1 < 1%, 1–49%, and ≥50% expression groups (Figure S1a–c).

Table 2.
Summary of response rates.
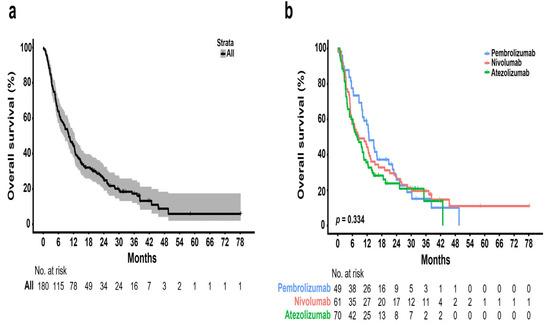
Figure 2.
Kaplan–Meier plots of overall survival (OS): (a) entire cohort of patients; (b) patients stratified according to different ICIs.
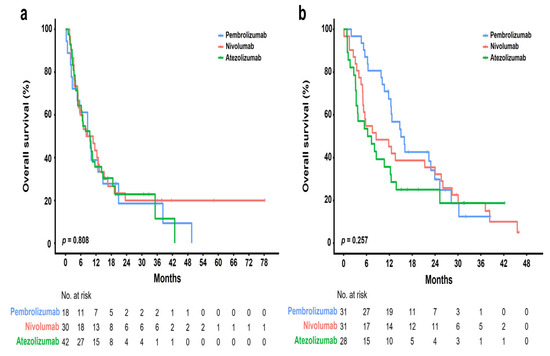
Figure 3.
Kaplan–Meier plots of OS according to different ICIs in patients with squamous and non-squamous histological subtypes: (a) squamous; (b) non-squamous.
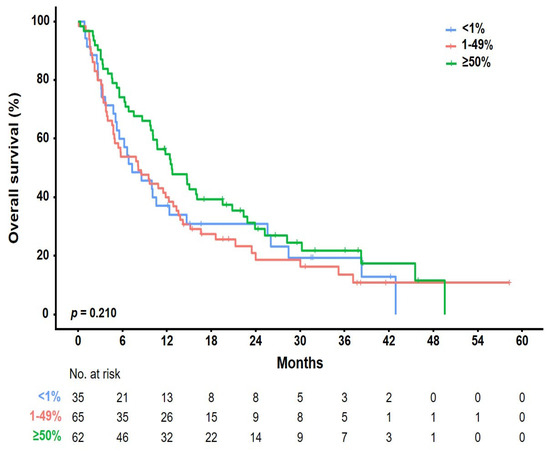
Figure 4.
Kaplan–Meier plots of OS according to PD-L1 expression status. Data of 162 patients with confirmed PD-L1 expressions were analyzed. Three different PD-L1 diagnostic assays were included: the PD-L1 IHC 22C3 pharmDx, Ventana PD-L1 SP263, and Ventana PD-L1 (SP142) assays. The most important results were based on several PD-L1 assays performed on patients individually.
3.3. Prognostic Factors Affecting Overall Survival
The univariate and multivariate Cox proportional hazards models for identifying the prognostic factors affecting the OS of patients with NSCLC are described in Table 3. No factors were independently associated with poor OS in the univariate analysis. In the multivariate analysis, bone metastasis was identified as a significantly poor prognostic factor for OS (bone metastasis: HR = 2.75 [95% CI, 1.31–5.76], p = 0.007).

Table 3.
Prognostic factors for overall survival based on uni- and multi-variate Cox proportional hazards models.
3.4. Safety Outcomes
Treatment-related adverse events were reported in 48 patients, 50% of whom experienced immune-related adverse events (irAEs) (Table 4). The most common irAEs were skin rash (5.5%), pneumonitis (3.3%), and thyroid dysfunction (3.3%). Grade ≥ 3 irAEs were reported in one patient in the pembrolizumab group and two patients in the atezolizumab group, respectively. There was no death related to irAEs.

Table 4.
Adverse events in the safety population.
4. Discussion
This study is the first in which real-world data analysis of the efficacy of nivolumab, pembrolizumab, and atezolizumab, approved by the FDA for the treatment of advanced NSCLC, was performed. These drugs are widely used in clinical practice. However, to the best of our knowledge, their efficacy as monotherapies in older patients in a real-world setting has never been directly compared.
The results obtained showed a lower ORR, as well as a similar median OS relative to previously published RCTs (ORR: 4.3–22%; overall, 10.6%; DCR: 30.0–59.2%; overall, 46.7%) (Table 5). Age-related decline in the immune system, known as “immunosenescence”, may affect immune checkpoint suppression activity in older patients [17,18]. In addition, many comorbidities may contribute to death from causes other than lung cancer in older adults [19,20]. Notably, several real-world studies on older patients have shown similar results. However, it is unreasonable to compare the efficacy of immunotherapy between the present study and the existing RCTs based on age. For an accurate comparison, many other parameters such as tumor histology, PD-L1 score, treatment regimen, and line of therapy must be corrected. Therefore, this study aimed to compare and analyze treatment efficacy and OS within the study cohort rather than directly comparing it with existing RCTs.

Table 5.
Characteristics and outcomes of the pivotal randomized clinical trials and the present study.
We classified a consecutive cohort of patients with advanced stage IV NSCLC into three groups according to the ICI monotherapy regimen. The three groups comprised older adults with an average age ≥ 75. Some vulnerable patients with an ECOG-PS score ≥ 2 were also included. Regarding efficacy, the ORRs for the pembrolizumab, nivolumab, and atezolizumab groups were 22.4%, 8.2%, and 4.3%, respectively, indicating that pembrolizumab and nivolumab were more beneficial than atezolizumab. Further, Nivolumab significantly improved ORR over atezolizumab based on correspondence analysis (nivolumab vs. atezolizumab, p = 0.006; pembrolizumab vs. atezolizumab, p = 0.471; and pembrolizumab vs. nivolumab, p = 0.066). Similarly, the DCR was better improved in the pembrolizumab (59.2%) and nivolumab (55.7%) groups than in the atezolizumab (30.0%) group. Similar results were also observed following concordance analysis (nivolumab vs. atezolizumab, p = 0.003; pembrolizumab vs. atezolizumab, p = 0.005; and pembrolizumab vs. nivolumab, p = 0.866). However, we could not conclude that pembrolizumab and nivolumab were more effective than atezolizumab in terms of efficacy. Several reasons could account for this observation. First, a squamous histology was more common for patients who received atezolizumab than those who received other drugs. In this study, the PD-L1 expression rate was high in the non-squamous histology. Therefore, patients with a non-squamous histology with these features were treated with either pembrolizumab or nivolumab. As reported in several pivotal RCTs [6] and the real-world outcome literature [23,24,25], the squamous histological subtype tends to be associated with shorter PFS and OS than the non-squamous histology. Second, in this study, we did not treat each drug evenly according to the PD-L1 expression status. Based on the results of the existing pivotal RCTs, in the present study, most patients with high PD-L1 expression levels were treated with pembrolizumab and nivolumab, whereas atezolizumab was mainly used in patients with a low or unconfirmed PD-L1 expression status. Therefore, patients in the atezolizumab group may show a relatively poor efficacy. Despite these conditions, there were no differences in OS among the three groups (12.6, 8.4, and 7.7 months for pembrolizumab, nivolumab, and atezolizumab groups, respectively), with pembrolizumab showing the longest OS duration. However, this difference was not statistically significant (p = 0.334). Additionally, we confirmed the OS of patients receiving ICIs according to PD-L1 expression status via correspondence analysis. In the PD-L1 1–49% subgroup, when the OS according to ICIs was analyzed in correspondence, no statistical significance was observed (pembrolizumab vs. atezolizumab, p = 0.092; nivolumab vs. atezolizumab, p = 0.911; and pembrolizumab vs. nivolumab, p = 0.053) (Figure S1b). In contrast, in the PD-L1 ≥ 50% subgroup, pembrolizumab showed a statistically significant OS benefit compared with the atezolizumab (pembrolizumab vs. atezolizumab, p = 0.023; nivolumab vs. atezolizumab, p = 0.153; and pembrolizumab vs. nivolumab, p = 0.406) (Figure S1a). Further, in the pembrolizumab group, eight patients treated with pembrolizumab as the first-line treatment were included, and there were only five patients with a PD-L1 expression ≥ 50% in the atezolizumab group. Therefore, more samples are needed to determine statistical significance.
Older patients are likely to be vulnerable to the side effects of cytotoxic chemotherapy; therefore, they receive many ICIs that are known to have relatively tolerable side effects [26]. However, the eligibility criteria for clinical trials are not representative of the patient population in real-world practice; most clinical trials include patients receiving conventional cytotoxic chemotherapy as controls. Therefore, the data on direct efficacy comparisons between ICIs are lacking.
A noteworthy finding of the present study was that bone metastases negatively affected the OS. Based on cancer biology, bone is a hematopoietic organ that is involved in the production of regulatory T, memory T and B, and cytotoxic T cells that control the immune system [27,28]. Therefore, pathological bone loss may impair the production of immune-related cells. The association between bone metastasis and survival has not been extensively studied; however, a prospective cohort study in Italy showed poor outcomes in terms of bone metastasis and survival rates [29]. In addition, several published retrospective studies have reported poor prognosis associated with bone metastasis [24,30]. Therefore, the results of this study are consistent with those of previous studies, indicating that bone metastases adversely affect OS in patients receiving ICIs. Additional treatment methods or adjuvant agents that can enhance the efficacy of ICI in patients with bone metastasis need to be investigated.
This study had several limitations. First, this is a retrospective, single-center observational study. Thus, differences in clinical baseline characteristics may have affected the efficacy evaluation. Pembrolizumab was used on the frontline and was administered more frequently to patients with a high PD-L1 expression. Therefore, these characteristics may have improved survival in the pembrolizumab group. Second, because various detection techniques are used to identify PD-L1 expression, the consistency between methods may be inaccurate. In the present study, three assays were used: PD-L1 22C3 pharmDx, VENTANA PD-L1 SP263, and VENTANA PD-L1 SP142 immunohistochemistry assays. PD-L1 expression was classified based on the highest value among the three test results. Notably, not all three techniques were performed in all patients; therefore, unifying them into one technique is impossible. However, several studies have indicated that the PD-L1 22C3 and SP263 techniques are highly consistent [31,32]. In the present study, none of the patients had their SP142 results reflected in the final result of PD-L1 expression. In addition, when only patients who underwent PD-L1 22C3 were analyzed for the accuracy of the results, there was no difference in OS according to ICI, as observed in previous studies. Third, comorbidities were not considered in this survival study. Meserve et al. [33] reported that in patients with pre-existing inflammatory bowel diseases (IBD) that were treated with ICIs, approximately 40% experienced IBD relapse and ICI discontinuation. Immune-checkpoint inhibitors should be used with caution as they may increase the risk of reactivation of an existing autoimmune disease. Furthermore, in the case of older adults, comorbidities may affect survival; however, since the study was based on the data from electronic medical records, information on comorbidities or the association between comorbidities and mortality could be overlooked. Despite these limitations, the survival outcomes in the present study were similar to those reported in existing pivotal RCTs. Similarly, this study had inherent limitations in identifying all adverse events due to the retrospective nature of the analysis, particularly due to the incomplete or missing data. However, several reports have mentioned that the frequency of immune-related adverse events is similar or lower in elderly patients than in younger patients [10,18]. Therefore, for safety reasons, there is no need to limit the use of ICIs.
5. Conclusions
This study is clinically significant because no previous study has directly compared the efficacy of the three different ICIs in older patients receiving ICI monotherapy. There were no statistically significant differences in survival outcomes among the three ICIs, demonstrating that they could possibly constitute an appropriate treatment option for older patients. Our results can alleviate clinicians’ concerns about deciding the type of ICI single agent and may assist in making decisions based on the patient’s outpatient visit frequency or the clinician’s preference. In addition, bone metastasis was found to be associated with poor survival outcomes after immunotherapy. Future studies are needed to determine immunotherapeutic agents with different mechanisms that are more effective according to the metastatic sites in elderly patients with lung cancer receiving monotherapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15164198/s1. Figure S1: Comparison of survival outcomes according to different ICIs: (a) PD-L1 < 1%; (b) PD-L1 1–49%; and (c) PD-L1 ≥ 50%. Data of 162 patients with confirmed PD-L1 expressions were analyzed. No patient was treated with pembrolizumab in the PD-L1 < 1% subgroup.
Author Contributions
Conceptualization, T.L.; methodology, T.L. and A.H.; investigation, A.H. and H.S.K.; software, A.H. and Y.L.; formal analysis, T.L., A.H. and Y.L.; writing—original draft, T.L. and A.H.; writing—review and editing, T.L., A.H., H.S.K. and Y.L.; A.H. and T.L. are responsible for the overall content as guarantors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the VHS Medical Center, Republic of Korea, grant number VHSMC 22050.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of the Veterans Health Service Medical Center (IRB No. 2022-07-002), Ethical approval date was 18 July 2022.
Informed Consent Statement
The IRB waived the requirement for informed consent from patients for the publication of this study.
Data Availability Statement
Data are available on reasonable request. Anonymized individual participant data and study documents can be requested from the corresponding author of this study (T.L., imegene@naver.com) upon reasonable request.
Acknowledgments
The content of this manuscript was presented as a poster at the 49th Annual Meeting of the Korean Cancer Association and the 9th International Cancer Conference, Seoul, Korea (15–16 June 2023).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cancer Stat Facts Lung and Bronchus Cancer. Available online: https://seer.cancer.gov/statfacts/html/lungb.html (accessed on 1 January 2023).
- Osmani, L.; Askin, F.; Gabrielson, E.; Li, Q.K. Current who guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (nsclc): Moving from targeted therapy to immunotherapy. Semin. Cancer Biol. 2018, 52, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Xu, A.; Xu, J. Roles of pd-1/pd-l1 pathway: Signaling, cancer, and beyond. Adv. Exp. Med. Biol. 2020, 1248, 33–59. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Sanmamed, M.F.; Hastings, K.; Politi, K.; Rimm, D.L.; Chen, L.; Melero, I.; Schalper, K.A.; Herbst, R.S. Immunotherapy in non-small cell lung cancer: Facts and hopes. Clin. Cancer Res. 2019, 25, 4592–4602. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, pd-l1-positive, advanced non-small-cell lung cancer (keynote-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-year outcomes from the randomized, phase iii trials checkmate 017 and 057: Nivolumab versus docetaxel in previously treated non-small-cell lung cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef]
- Rittmeyer, A.; Barlesi, F.; Waterkamp, D.; Park, K.; Ciardiello, F.; von Pawel, J.; Gadgeel, S.M.; Hida, T.; Kowalski, D.M.; Dols, M.C.; et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (oak): A phase 3, open-label, multicentre randomised controlled trial. Lancet 2017, 389, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for pd-l1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. Nccn Guidelines Insights: Non-small Cell Lung Cancer, version 2.2021. J. Natl. Compr. Canc. Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Marur, S.; Singh, H.; Mishra-Kalyani, P.; Larkins, E.; Keegan, P.; Sridhara, R.; Blumenthal, G.M.; Pazdur, R. Fda analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of pd-1/pd-l1 blocking antibodies. Semin. Oncol. 2018, 45, 220–225. [Google Scholar] [CrossRef]
- Zhang, S.; Pease, D.F.; Kulkarni, A.A.; Kumar, M.; Shanley, R.M.; Xu, B.; Joshi, S.P.; Patel, M.R. Real-world outcomes and clinical predictors of immune checkpoint inhibitor monotherapy in advanced lung cancer. Clin. Med. Insights Oncol. 2021, 15, 11795549211004489. [Google Scholar] [CrossRef]
- Botticelli, A.; Salati, M.; Di Pietro, F.R.; Strigari, L.; Cerbelli, B.; Zizzari, I.G.; Giusti, R.; Mazzotta, M.; Mazzuca, F.; Roberto, M.; et al. A nomogram to predict survival in non-small cell lung cancer patients treated with nivolumab. J. Transl. Med. 2019, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Tamiya, M.; Tamiya, A.; Inoue, T.; Kimura, M.; Kunimasa, K.; Nakahama, K.; Taniguchi, Y.; Shiroyama, T.; Isa, S.I.; Nishino, K.; et al. Metastatic site as a predictor of nivolumab efficacy in patients with advanced non-small cell lung cancer: A retrospective multicenter trial. PLoS ONE 2018, 13, e0192227. [Google Scholar] [CrossRef]
- Schmid, S.; Diem, S.; Li, Q.; Krapf, M.; Flatz, L.; Leschka, S.; Desbiolles, L.; Klingbiel, D.; Jochum, W.; Früh, M. Organ-specific response to nivolumab in patients with non-small cell lung cancer (nsclc). Cancer Immunol. Immunother. 2018, 67, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Passiglia, F.; Galvano, A.; Rizzo, S.; Incorvaia, L.; Listì, A.; Bazan, V.; Russo, A. Looking for the best immune-checkpoint inhibitor in pre-treated nsclc patients: An indirect comparison between nivolumab, pembrolizumab and atezolizumab. Int. J. Cancer 2018, 142, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Casaluce, F.; Sgambato, A.; Maione, P.; Spagnuolo, A.; Gridelli, C. Lung cancer, elderly and immune checkpoint inhibitors. J. Thorac. Dis. 2018, 10 (Suppl. S13), S1474–S1481. [Google Scholar] [CrossRef] [PubMed]
- Tagliamento, M.; Frelaut, M.; Baldini, C.; Naigeon, M.; Nencioni, A.; Chaput, N.; Besse, B. The use of immunotherapy in older patients with advanced non-small cell lung cancer. Cancer Treat. Rev. 2022, 106, 102394. [Google Scholar] [CrossRef]
- Zeber, J.E.; Copeland, L.A.; Hosek, B.J.; Karnad, A.B.; Lawrence, V.A.; Sanchez-Reilly, S.E. Cancer rates, medical comorbidities, and treatment modalities in the oldest patients. Crit. Rev. Oncol. Hematol. 2008, 67, 237–242. [Google Scholar] [CrossRef]
- Burdett, N.; Vincent, A.D.; O’Callaghan, M.; Kichenadasse, G. Competing risks in older patients with cancer: A systematic review of geriatric oncology trials. J. Natl. Cancer Inst. 2018, 110, 825–830. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, pd-l1-expressing, locally advanced or metastatic non-small-cell lung cancer (keynote-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Jassem, J.; Herbst, R.S.; de Marinis, F.d.; Cadranel, J.; Csőszi, T.; Isla, D.; Chen, G.; Syrigos, K.N.; Cortinovis, D.; Hida, T.; et al. Impower110: Clinical safety in a phase III study of atezolizumab (atezo) monotherapy (mono) vs platinum-based chemotherapy (chemo) in first-line non-small cell lung cancer (NSCLC) [Mono. ]. J. Clin. Oncol. 2020, 38, e21623. [Google Scholar] [CrossRef]
- Stenehjem, D.D.; Lubinga, S.J.; Gupte-Singh, K.; Zhang, Y.; Le, T.K.; Penrod, J.R.; Smith, C.B. Real-world effectiveness of nivolumab monotherapy after prior systemic therapy in advanced non-small-cell lung cancer in the united states. Clin. Lung Cancer 2021, 22, e35–e47. [Google Scholar] [CrossRef] [PubMed]
- Morita, R.; Okishio, K.; Shimizu, J.; Saito, H.; Sakai, H.; Kim, Y.H.; Hataji, O.; Yomota, M.; Nishio, M.; Aoe, K.; et al. Real-world effectiveness and safety of nivolumab in patients with non-small cell lung cancer: A multicenter retrospective observational study in japan. Lung Cancer 2020, 140, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, D.; Lam, J.; Betts, K.A.; Yin, L.; Gao, S.; Yuan, Y.; Hartman, J.; Rao, S.; Lubinga, S.; Stenehjem, D. Real-world outcomes of immunotherapy-based regimens in first-line advanced non-small cell lung cancer. Lung Cancer 2021, 156, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Magee, D.E.; Hird, A.E.; Klaassen, Z.; Sridhar, S.S.; Nam, R.K.; Wallis, C.J.D.; Kulkarni, G.S. Adverse event profile for immunotherapy agents compared with chemotherapy in solid organ tumors: A systematic review and meta-analysis of randomized clinical trials. Ann. Oncol. 2020, 31, 50–60. [Google Scholar] [CrossRef]
- Botticelli, A.; Cirillo, A.; Scagnoli, S.; Cerbelli, B.; Strigari, L.; Cortellini, A.; Pizzuti, L.; Vici, P.; De Galitiis, F.; Di Pietro, F.R.; et al. The agnostic role of site of metastasis in predicting outcomes in cancer patients treated with immunotherapy. Vaccines 2020, 8, 203. [Google Scholar] [CrossRef]
- Zhao, E.; Xu, H.; Wang, L.; Kryczek, I.; Wu, K.; Hu, Y.; Wang, G.; Zou, W. Bone marrow and the control of immunity. Cell Mol. Immunol. 2012, 9, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Landi, L.; D’Incà, F.; Gelibter, A.; Chiari, R.; Grossi, F.; Delmonte, A.; Passaro, A.; Signorelli, D.; Gelsomino, F.; Galetta, D.; et al. Bone metastases and immunotherapy in patients with advanced non-small-cell lung cancer. J. Immunother. Cancer 2019, 7, 316. [Google Scholar] [CrossRef]
- Yoneda, T.; Sone, T.; Koba, H.; Shibata, K.; Suzuki, J.; Tani, M.; Nishitsuji, M.; Nishi, K.; Kobayashi, T.; Shirasaki, H.; et al. Long-term survival of patients with non-small cell lung cancer treated with immune checkpoint inhibitor monotherapy in real-world settings. Clin. Lung Cancer 2022, 23, 467–476. [Google Scholar] [CrossRef]
- Velcheti, V.; Patwardhan, P.D.; Liu, F.X.; Chen, X.; Cao, X.; Burke, T. Real-world pd-l1 testing and distribution of pd-l1 tumor expression by immunohistochemistry assay type among patients with metastatic non-small cell lung cancer in the united states. PLoS ONE 2018, 13, e0206370. [Google Scholar] [CrossRef]
- Yuan, P.; Guo, C.Y.; Li, Y.; Jiang, L.L.; Liu, Y.P.; Liu, X.Y.; Ying, J.M. Consistency of pd-l1 immunohistochemical detection platforms in biopsy samples with advanced lung adenocarcinoma: A multicenter study. Zhonghua Bing Li Xue Za Zhi 2018, 47, 840–844. [Google Scholar] [CrossRef]
- Meserve, J.; Facciorusso, A.; Holmer, A.K.; Annese, V.; Sandborn, W.J.; Singh, S. Systematic review with meta-analysis: Safety and tolerability of immune checkpoint inhibitors in patients with pre-existing inflammatory bowel diseases. Aliment. Pharmacol. Ther. 2021, 53, 374–382. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).