Simple Summary
Multiple myeloma has remained largely incurable despite improvements in patient outcomes in the era of targeted anti-myeloma agents. Targeted therapies used in oncology in recent years have significantly changed the way myeloma is treated and thus improved the prognosis for patients. We sought to identify new biomarkers for patient stratification and the prediction of treatment outcomes by applying targeted capture hybridization DNA sequencing (tchDNA-Seq) technology. We have evaluated plasma and bone marrow samples from a homogenous population of 23 patients, which IG rearrangements have the potential to provide important diagnostic, prognostic, and predictive information. We will likely be able to offer a more targeted and risk-adapted therapeutic approach to MM patients at different stages of their disease guided by these potential biomarkers.
Abstract
Multiple myeloma (MM) is a hematological malignancy characterized by the clonal proliferation of pathogenic CD138+ plasma cells (PPCs) in bone marrow (BM). Recent years have seen a significant increase in the treatment options for MM; however, most patients who achieve complete the response ultimately relapse. The earlier detection of tumor-related clonal DNA would thus be very beneficial for patients with MM and would enable timely therapeutic interventions to improve outcomes. Liquid biopsy of “cell-free DNA” (cfDNA) as a minimally invasive approach might be more effective than BM aspiration not only for the diagnosis but also for the detection of early recurrence. Most studies thus far have addressed the comparative quantification of patient-specific biomarkers in cfDNA with PPCs and BM samples, which have shown good correlations. However, there are limitations to this approach, such as the difficulty in obtaining enough circulating free tumor DNA to achieve sufficient sensitivity for the assessment of minimal residual disease. Herein, we summarize current data on methodologies to characterize MM, and we present evidence that targeted capture hybridization DNA sequencing (tchDNA-Seq) can provide robust biomarkers in cfDNA, including immunoglobulin (IG) rearrangements. We also show that detection can be improved by prior purification of the cfDNA. Overall, liquid biopsies of cfDNA to monitor IG rearrangements have the potential to provide important diagnostic, prognostic, and predictive information in patients with MM.
1. Introduction
Multiple myeloma (MM) is a highly heterogeneous hematological malignancy characterized by the proliferation of neoplastic plasma cells in bone marrow (BM), which can often progress to extramedullary disease [1]. At the molecular level, MM is characterized by complex cytogenetic and molecular aberrations including chromosomal translocations involving the immunoglobulin (IG) heavy chain locus, copy number variations (CNVs), and somatic mutations in several oncogenic signaling pathways [2,3,4,5,6]. These changes are often associated with increased genomic complexity and have a relevant prognostic impact, which poses a therapeutic challenge [7].
Recent years have witnessed the emergence of novel therapeutic treatments for MM, which have substantially improved disease prognosis [7,8]. Despite the substantially prolonged survival, however, MM remains incurable in most patients and relapses are common. Hence, strategies that allow more assiduous clinical assessment over time are needed to identify early signs of therapeutic resistance and to aid clinicians in selecting the best therapeutic option before relapse occurs [9,10].
The current gold standard for the genetic diagnosis of MM remains cytogenetic profiling of plasma cells from BM samples [11], which provides data on chromosomal abnormalities but not other anomalies. More recently, the monitoring of minimal residual disease (MRD) by flow cytometry of BM samples has become a leading method to evaluate the depth of response to treatment in patients with MM by detecting persisting tumor cells [12]. We believe that a key challenge is to assess whether additional information obtained by molecular karyotyping is helpful in the management of MM. In this regard, we recently developed a new targeted capture hybridization DNA sequencing (tchDNA-Seq) gene panel [13], which combines the identification of different biomarkers in one single method, which can lead to superior genetic characterization of patients.
A liquid biopsy is defined as the detection of circulating tumor cells (CTCs) and/or small fragments of cell-free DNA released from primary and secondary neoplastic lesions [14,15,16,17] into peripheral blood (PB). Therefore, it is considered to offer a minimally invasive method to detect tumor markers and capture tumor heterogeneity [18]. Hybrid capture and targeted sequencing of cfDNA has been reported in hematological cancer genomes [19]; however, whether liquid biopsy can be used to assess genomic alterations in MM in a similar way to baseline tests is still under study. Many studies have shown that cfDNA analysis in MM also appears to accurately detect mutations identified by genetic profiling of bone marrow-derived tumor DNA, with good concordance between paired plasma samples [20,21,22]. We observed that the purification of cfDNA samples from genomic DNA using a specific magnetic beads ratio [23] clearly improves the signal detection performance of monitored biomarkers. We believe this method can potentially replace medically unnecessary BM sampling and provide an alternative non-invasive test for longitudinal genetic monitoring of patients with MM receiving targeted therapy [19].
2. Technological Methods in Liquid Biopsy
2.1. Present MRD Approaches
Currently, the assessment of MRD in MM is routinely performed by multiparametric flow cytometry (MFC) of BM samples [24,25,26,27]. MFC is a robust technique for the detection of BM plasma cells and can clearly discriminate between normal and clonal plasma cells, even when they are present at very low frequencies, as occurs in MRD monitoring [28,29]. For example, the sensitivity of 8-color MFC is 1 clonal plasma cell in 105 normal cells, although highly standardized protocols from the EuroFlow consortium can reach a sensitivity of 1 in 106 [12]. It has recently been reported that a sensitivity of 10−7 can be achieved with a good correlation with BM MRD assessments using a CD138+ cell purification strategy in PB [30].
Sampling of the BM by aspiration is, nevertheless, an invasive and painful procedure for patients, and the use of liquid biopsy, which is much less invasive, has shown great potential in hematological malignancies in recent years [31]. Similarly, next-generation sequencing of IG loci has been developed in the last 5 years to overcome these limitations of conventional MFC techniques for MRD monitoring [12,32,33]. Indeed, NGS increasingly appears to offer an important advance in genetic mapping to detect new aberrations involved in disease progression. The clonoSEQ Assay manufactured by Adaptive Biotechnologies (Seattle, WA, USA) is the first and only next-generation sequencing-based MRD test authorized by the U.S. Food and Drug Administration (FDA) for MRD assessment in patients with acute lymphoblastic leukemia (ALL) or multiple myeloma. This identifies and quantifies gene sequences in DNA extracted from the bone marrow using multiplex polymerase chain reaction and NGS. The assay detects as few as 1 tumor cell within more than 1 million healthy cells, and several studies using this assay for the assessment of patients with MM demonstrated that the MRD level correlates with the outcome and that the lower the MRD level, the better the prognosis [34,35].
2.2. Disease Advancement and Relapse
The levels of V(D)J rearrangements in circulating free tumor DNA (ctDNA) correlate with clinical disease activity. Recently, Oberle et al. used NGS to detect the V(D)J clonotypic rearrangement and for subsequent follow-up by liquid biopsy after treatment initiation in MM patients. They observed that the majority of non-responders had traces of persistent myeloma cells. Positivity for CTCs and cfDNA were associated with each other in most cases, but were discordant in 30% of cases, indicating that cfDNA may not be generated entirely by CTCs and may reflect the bulk tumor burden [36]. Similarly, Biancon et al. identified clonal rearrangements of the IGH gene in PPCs and cfDNA samples from MM patients receiving second-line treatment. The same clonal IGH rearrangement identified in PPCs was detected in paired plasma samples, and levels of IGH cfDNA correlated with the outcome [37].
The detection of CNVs is also a clinically relevant approach to characterizing genomic abnormalities in MM. Guo et al. questioned whether CNVs and clonal somatic mutations could be robustly detected across the entire genome or exome using cfDNA in patients with active disease and whether the mutations found in the BM could be reliably reproduced in the cfDNA. Their study demonstrated that the comprehensive genomic interrogation by whole exome and genome sequencing of MM-derived cfDNA is feasible and allows detailed genomic insight into MM evolution and temporal progression [21].
Mutational characterization of MM is currently based on BM biopsy, which does not capture the spatial and genetic heterogeneity of this multifocal disease. Mithraprabhu et al. investigated the clinical utility of using plasma-derived ctDNA for mutational characterization and for tracking disease progression. Paired BM cell DNA and ctDNA from 33 relapsed/refractory and 15 newly diagnosed patients with MM were analyzed for KRAS, NRAS, BRAF, and TP53 mutations by NGS, which revealed a higher frequency of ctDNA-only mutations in relapsed/refractory disease, authenticating the spatial heterogeneity of MM. Accordingly, ctDNA analysis can be used as an adjunct to BM aspirate, representing a noninvasive strategy for improved mutational characterization and therapeutic monitoring of MM [38]. Along the same line, Rustad et al. explored the relationship between ctDNA and disease activity during long-term follow-up by monitoring recurrent mutations (NRAS, KRAS, and BRAF) with digital droplet PCR and comparing the results with those from the analysis of BM. They observed a good correlation between the concentration of mutated alleles in BM cells and in ctDNA, which reflects mutated cells, the total tumor mass, and the transformation to a more aggressive disease [39].
2.3. Methodological Approaches
In addition to the type of sample analyzed, it is also important to consider the technique adopted to obtain the greatest possible sensitivity and the most reliable data for patient monitoring. In this respect, a targeted NGS approach might be the most appropriate option. Gerber et al. analyzed the MM mutational landscape in the cfDNA of patients by tracking the clonotypic V(D)J rearrangement as a patient-specific marker, and by genotyping a specific set of genes. For the latter, they designed a targeted panel, including coding exons and splice sites of 14 genes (BRAF, CCND1, CYLD, DIS3, EGR1, FAM46C, IRF4, KRAS, NRAS, PRDM1, SP140, TP53, TRAF3, and ZNF462), and developed a cancer personalized profiling by deep sequencing (CAPP-seq) approach to compare the mutational profile of cfDNA and tumor genomic DNA of purified plasma cells from BM aspirates of different disease stages, including early disease. They found that the amount of cfDNA correlated with some parameters that may indicate the tumor burden, such as the percentage of plasma cell infiltration of BM [40]. Likewise, Kis et al. applied a hybrid capture-based liquid biopsy sequencing (LB-Seq) method to sequence KRAS, NRAS, BRAF, EGFR, and PIK3CA in cfDNA specimens from patients with myeloma, concluding that cfDNA analysis is comparable to myeloma molecular profiling using BM tumor cells. Their results revealed a high concordance between both fractions with high specificity, proposing LB-Seq as an implementation for genetic profiling of BM in MM. These findings have been confirmed by other studies [37,38].
Regarding the study of MRD using cfDNA, several reports have compared it with conventional techniques of MRD evaluation in BM, with conflicting results. For instance, Mazzotti et al. reported no correlation between ctDNA and BM for MRD analysis by NGS using only IGH gene rearrangements in patients with myeloma [41]. In a similar context, Long et al. analyzed plasma samples from 8 patients with extramedullary MM and other plasma samples from patients with MM but without extramedullary plasmacytomas. They found that patients with extramedullary disease had higher cfDNA concentrations. They designed sequencing panels targeting the coding sequence regions of 22 recurrently mutated genes, detecting 17 different somatic mutations. They concluded that cfDNA can be used as a surrogate material to track extramedullary disease progression, particularly when plasmacytomas are inaccessible [42]. However, in another study, although a relevant concordance in clonal somatic mutations (~99%) and copy number alterations (~81%) was high, a clinical correlation was not found, and the results in the case of MRD monitoring were not significant [19]. Currently, the use of cfDNA alone has no utility in the assessment of MRD in patients with MM, although, as we state, there is increasing evidence that it could be a useful complementary tool. Indeed, with further refinement cfDNA monitoring could be a relevant modality for the assessment of MRD [42,43]. Deshpande et al. also assessed whether cfDNA levels vary according to risk status using a targeted NGS approach. They found that the cfDNA levels in 77 patients were significantly higher in the high-risk MM group and correlated weakly with clinical markers. Patients with high cfDNA levels were associated with a worse progression-free and overall survival [44]. At this time, however, targeted sequencing of cfDNA cannot achieve the sensitivity of MRD detection by flow cytometry or molecular analysis in BM. Nevertheless, in the future, MRD analysis using cfDNA might have sufficient sensitivity through the use of reliable biomarkers. In this context, we have developed a new targeted method (tchDNA-Seq) combining the following molecular parameters: patient-specific mutation panels, translocations patterns, copy number alterations, and IGH rearrangements. We believe that this approach might increase the accuracy of progression-free survival prediction and the detection of false-negative MRD.
2.4. A New Liquid Biopsy Approach: Targeted Capture Hybridization Panel (tchDNA-Seq)
2.4.1. Samples
We analyzed 51 samples obtained at diagnosis from 23 patients: 23 samples of genomic DNA from PPCs, 16 samples of genomic DNA from total BM, and 12 cfDNA samples obtained from PB (the clinicopathological features are described in Table 1 from Rosa-Rosa et al., 2022) [13]. Plasma samples should be stored in tubes that do not affect cfDNA stability or promote cell lysis, which is a major concern. In this respect, we have had good results with the Cell-free DNA blood collection tubes (cfDNA BCT tubes) commercialized by Streck (La Vista, NE, USA). The plasma samples were processed using the QIAamp Circulating Nucleic Acid Kit (Qiagen, Hilden, Germany, cat. no. 1017647). Further, a liquid biopsy for cfDNA was performed across a total of 11 follow-up samples from three patients. One cfDNA sample was purified using AMPure XP (Beckman Coulter Life Sciences, Indianapolis, IN, USA) magnetic bead-based size selection to increase the signal obtained. We removed genomic DNA using a 0.6× concentration of beads, followed by a 1.8× concentration of beads to capture the remaining cfDNA. Isolated cfDNA was analyzed with an electropherogram Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) to check the optimum profile.
2.4.2. Panel Description and Sequencing
We designed a specific panel to investigate the genomic alterations present in patients with myeloma [13]. The panel contains genes involved in the development and progression of MM (ATM, ATR, CCND1, KRAS, NRAS, PRDM1, etc.), genes related to treatment or drug resistance (PSMD1, XBP1, PSMB5, PSMC2, etc.) and candidates for new immunotherapy treatments (CD38, BCMA, GPRC5D). A list of all genes is provided in Supplementary Table S1. In addition, we also considered genomic regions frequently involved in MM, including the translocations t (4;11), t (4;14), t (4;16), and t (11;14), and regions distributed in the genome for the identification of CNVs and chromosomal regions of the IGH locus. Libraries were generated with SureSelect reagent kits (Agilent Technologies, Palo Alto, CA, USA), using 50 ng of genomic DNA for PPC and BM and 10–200 ng of cfDNA. Sequencing was performed on Illumina NextSeq 500 and/or Ion Torrent platforms. A specific bioinformatics process was applied, which included alignment with the hg38 genome, identification of SNVs and Indels by combining variant calling, and IGH/K rearrangements with MiXCR 3.0.13, a software platform for analysis of NGS data for immune profiling.
3. Preliminary Results Using the New tchDNA-Seq
3.1. Assay of Genomic DNA and cfDNA Samples at Diagnosis
Analysis of the alterations present in PPCs, genomic DNA, and cfDNA revealed a total of 97 alterations in the 23 PPC samples and at least 1 alteration was present in all samples. Sixty-seven of the alterations found in PPCs were also found in BM DNA and thirty-two in cfDNA. Positively, 1 of the 2 translocations seen in PPCs was traced in cfDNA. The results reveal a concordance between the BM and cfDNA data, detecting samples with at least one alteration in 100% of the cases (Supplementary Table S2). Although we can assume lower VAFs correlated with subclonal mutations, no studies of subclonality have been performed globally in this dataset. After an analysis of the results, we concluded that the detection of SNVs in liquid biopsy is limited by signal dilution; specifically, a reduction of approximately 1 log in cfDNA and BM compared with PPCs, whereas the median reduction in frequency observed in cfDNA and BM for IG rearrangements was not significant compared with that observed in PPCs. Moreover, based on the comparison of frequencies of SNVs observed in either cfDNA or BM with that observed in PPCs, we estimated a median tumor burden of 30% and 4% in BM and cfDNA, respectively (Table 1). It is important to mention that in the five cases where PPCs, BM, and cfDNA were available, 55% of the mutations were present in all fractions and similarly, two of the five cases with all three fractions showed the same rearrangement in PPCs, BM, and cfDNA. (Figure 1). No translocations were seen in these patients. In summary, these results confirmed the detection of IG in cfDNA as a robust biomarker of MM.

Table 1.
Comparative results among PPC, BM, and cfDNA. The VAF ratio is obtained by dividing the VAF observed in BM/cfDNA and the VAF observed in PPC for common SNVs and IG rearrangements.
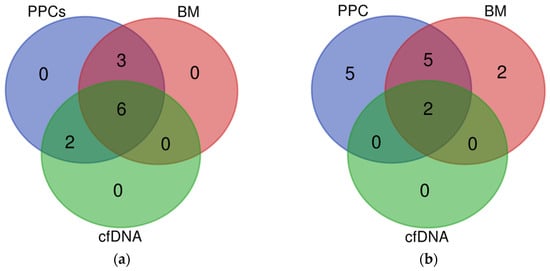
Figure 1.
Venn diagrams from capture hybridization sequencing panel of 5 matched PPCs, cfDNA, and BM tumor samples: (a) clonal mutations present in PPCs (blue) and identified in either cfDNA (green) or BM biopsies samples (red); (b) rearrangements present in PPCs samples (blue) and observed in cfDNA (green) and BM biopsies (red).
3.2. Liquid Biopsy in Follow-Up Samples
We analyzed tumor cfDNA in patients by tracking the V(D)J clonotypic rearrangement as a fingerprint of the disease and genotyping a set of cancer genes or patient-specific translocations identified at diagnosis. As described above, we performed liquid biopsy at several follow-up time points from the three patients and the results are described below as individual cases (Figure 2).
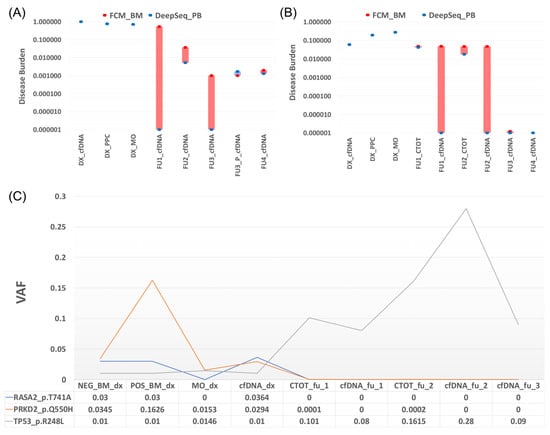
Figure 2.
Description of the alterations related to the three analyzed patients by targeted capture hybridization DNA panel. (A) Patient 1: comparison of disease burden obtained by FCM (red point) and deep sequencing (blue point). Four positive MRD follow-ups are observed by FCM, and the only alteration is found in the total bone marrow cells (CTOT) fraction of FU2 by tchDNA-Seq. (B) Patient 2: comparison of disease burden obtained by FCM (red point) and deep sequencing (blue point). Four positive MRD follow-ups are observed by FCM, and three alterations (FU2, FU3 purified, and FU4) were found in cfDNA. Bars (red and blue) show the differences between the two methods. (C) Patient 3: Mutational evolution of different types of samples at diagnosis and follow-up points.
3.2.1. Patient 1
Presented with rearrangements in the IGH locus and one-point mutation at diagnosis (IGF1R p.T677A). No mutations were detected in the two follow-up studies with positive MRD by MFC, but the same IG rearrangement was found in the BM fraction but not in cfDNA (Figure 2A).
3.2.2. Patient 2
At diagnosis, two point mutations (KRAS G12D and DIS3 D479E), t (11,14), and different IG rearrangements were identified. From the four follow-up studies with positive MRD by flow cytometry, IG rearrangements in cfDNA could only be observed at follow-up 2 and follow-up 4. No translocations or point mutations were detected. As a cfDNA signal was detected in this assay, in contrast to patient 1, we wondered whether negative results in cfDNA could be due to purity conditions. A purification test was run on the negative cfDNA sample from follow-up 3 to determine if the signal was restored. The remaining genomic DNA was removed from the cfDNA samples by a specific ratio of magnetic beads according to Chauhan et al. [23]. Subsequently, the IG rearrangement was detected in follow-up 3. Nonetheless, after purification mutations and translocations did not manifest (Figure 2B).
3.2.3. Patient 3
Liquid biopsy was also performed on follow-up samples and the results were compared with those obtained at diagnosis. Interestingly, we observed that in the cfDNA and BM follow-up samples, the signal of the initial mutations disappeared and a TP53 mutation emerged that had not been considered before. Monitoring of the patient showed that, in the last follow-up and also coinciding with the cessation of treatment, a decrease in the TP53 allele burden was found. Although the patient is currently in remission, the TP53 mutation may condition the development of another undetected tumor different from the primary one, so follow-up remains important (Figure 2C).
4. Conclusions
Recent advances in genomics and bioinformatics have facilitated the development of precision medicine programs and allowed increasing numbers of solid tumors to undergo rapid whole-genome sequencing (WGS) according to Subhash et al. Whilst the primary focus of precision medicine has been the rapid identification of targetable genetic alterations, the WGS data can also be used to determine unique tumor-specific gene sequences and to develop patient-specific MRD assays, independent of tumor type [45], that could be extrapolated to myeloma patients. These findings highlight the potential to serve as a biomarker to track treatment response and to improve the detection of MRD following disease control.
Most of the molecular alterations manifesting in PPCs appear to be present in the cfDNA of patients, including translocations. The correlation observed between BM samples and PPCs might indicate that no separation of the positive fraction is necessary for sample processing, especially in patients with an infiltration higher than 10%. In addition, regardless of the infiltration of MM in the BM, all molecular alterations have been found at diagnosis. However, the detection of SNVs by liquid biopsy is limited by signal dilution, with an approximately 1 log reduction in cfDNA compared with PPCs, which must be considered in bioinformatics analyses.
Regarding IGH/K rearrangements, a minor signal reduction was observed, confirming that IG rearrangements are strong biomarkers of MM in cfDNA. Importantly, the purification of cfDNA and removal of the remaining genomic DNA may play an important role in improving results in terms of the ability to detect biomarker signals. We believe that the signal in cfDNA may be diluted and, therefore, purification of cfDNA in liquid biopsy could be important. In addition, we show that new molecular lesions can be identified through a capture panel.
5. Future Directions
With regard to future directions, it would be desirable to increase the cohort size to validate these promising results. Since neither point mutations nor translocations could be observed by the panel, our efforts are now directed toward improving the development of tests that will enhance detection. In this line, we have designed new primers to verify if we specifically observe the signal for translocation. Our preliminary results demonstrate that the signal from the translocation is not affected by the bias that primers can produce in a PCR although we are still working on the problems arising from customized primers. Finally, it is a priority to improve the collection of samples in appropriate tubes and conditions, as well as to implement purification methods that have proven to be effective. Although liquid biopsy is promising in MM, it is necessary to improve the sensitivity and accuracy and increase the number of studies.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers15112911/s1, Table S1: list of the 43 genes included in the targeted capture hybridization DNA panel; Table S2: summary of the frequency and alteration data collected for all analyzed sample fractions.
Author Contributions
N.B. conceptualized the review paper, wrote the drafts, edited it, and approved the final version. J.M.R.-R., I.C. and J.M.-L. performed the supervision. A.S.-d. analyzed Ig genes. A.G. and L.M. were responsible for sample management, library preparation and sequencing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Accelerator Grant: Next Steps, Early Detection, and Intervention: Understanding the Mechanisms of Transformation and Hidden Resistance of Incurable Hematological Malignancies, and grants from Instituto de Salud Carlos III PI15/01484 and CRIS Foundation 2020/0063 and 2021/0088.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Instituto de Investigación 12 de Octubre (i+12)-FIBH12O, project associated code 15/01484, 14 of July 2015.
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data not presented in this article can be shared up on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bolli, N.; Avet-Loiseau, H.; Wedge, D.C.; Van Loo, P.; Alexandrov, L.B.; Martincorena, I.; Dawson, K.J.; Iorio, F.; Nik-Zainal, S.; Bignell, G.R.; et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat. Commun. 2014, 5, 2997. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.G.; Stojanov, P.; Carter, S.L.; Cruz-Gordillo, P.; Lawrence, M.S.; Auclair, D.; Sougnez, C.; Knoechel, B.; Gould, J.; Saksena, G.; et al. Widespread genetic heterogeneity in multiple myeloma: Implications for targeted therapy. Cancer Cell 2014, 25, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Bergsagel, P.L.; Kuehl, W.M. Molecular pathogenesis and a consequent classification of multiple myeloma. J. Clin. Oncol. 2005, 23, 6333–6338. [Google Scholar] [CrossRef]
- Robiou du Pont, S.; Cleynen, A.; Fontan, C.; Attal, M.; Munshi, N.; Corre, J.; Avet-Loiseau, H. Genomics of Multiple Myeloma. J. Clin. Oncol. 2017, 35, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Coffey, D.G.; Wu, Q.V.; Towlerton, A.M.H.; Ornelas, S.; Morales, A.J.; Xu, Y.; Green, D.J.; Warren, E.H. Ultradeep, Targeted Sequencing Reveals Distinct Mutations in Blood Compared to Matched Bone Marrow among Patients with Multiple Myeloma. Blood Cancer J. 2019, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Lawrence, M.S.; Keats, J.J.; Cibulskis, K.; Sougnez, C.; Schinzel, A.C.; Harview, C.L.; Brunet, J.P.; Ahmann, G.J.; Adli, M.; et al. Initial Genome Sequencing and Analysis of Multiple Myeloma. Nature 2011, 471, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Mahindra, A.; Laubach, J.; Raje, N.; Munshi, N.; Richardson, P.G.; Anderson, K. Latest Advances and Current Challenges in the Treatment of Multiple Myeloma. Nat. Rev. Clin. Oncol. 2012, 9, 135–143. [Google Scholar] [CrossRef]
- Kumar, S.K.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Zeldenrust, S.R.; Dingli, D.; Russell, S.J.; Lust, J.A.; et al. Improved Survival in Multiple Myeloma and the Impact of Novel Therapies. Blood 2008, 111, 2516–2520. [Google Scholar] [CrossRef]
- Keats, J.J.; Chesi, M.; Egan, J.B.; Garbitt, V.M.; Palmer, S.E.; Braggio, E.; Van Wier, S.; Blackburn, P.R.; Baker, A.S.; Dispenzieri, A.; et al. Clonal Competition with Alternating Dominance in Multiple Myeloma. Blood 2012, 120, 1067–1076. [Google Scholar] [CrossRef]
- Haertle, L.; Buenache, N.; Cuesta Hernández, H.N.; Simicek, M.; Snaurova, R.; Rapado, I.; Martinez, N.; López-Muñoz, N.; Sánchez-Pina, J.M.; Munawar, U.; et al. Genetic Alterations in Members of the Proteasome 26S Subunit, AAA-ATPase (PSMC) Gene Family in the Light of Proteasome Inhibitor Resistance in Multiple Myeloma. Cancers 2023, 5, 532. [Google Scholar] [CrossRef]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.J.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of Multiple Myeloma with High-Risk Cytogenetics: A Consensus of the International Myeloma Working Group. Blood 2016, 127, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; García-Sánchez, O.; Böttcher, S.; van der Velden, V.H.J.; Pérez-Morán, J.J.; Vidriales, M.B.; García-Sanz, R.; et al. Next Generation Flow for Highly Sensitive and Standardized Detection of Minimal Residual Disease in Multiple Myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef] [PubMed]
- Rosa-Rosa, J.M.; Cuenca, I.; Medina, A.; Vázquez, I.; Sánchez-delaCruz, A.; Buenache, N.; Sánchez, R.; Jiménez, C.; Rosiñol, L.; Gutiérrez, N.C.; et al. NGS-Based Molecular Karyotyping of Multiple Myeloma: Results from the GEM12 Clinical Trial. Cancers 2022, 14, 5169. [Google Scholar] [CrossRef] [PubMed]
- Stroun, M.; Maurice, P.; Vasioukhin, V.; Lyautey, J.; Lederrey, C.; Lefort, F.; Rossier, A.; Chen, X.Q.; Anker, P. The Origin and Mechanism of Circulating DNA. Ann. N. Y. Acad. Sci. 2000, 906, 161–168. [Google Scholar] [CrossRef]
- Kustanovich, A.; Schwartz, R.; Peretz, T.; Grinshpun, A. Life and Death of Circulating Cell-Free DNA. Cancer Biol. Ther. 2019, 20, 1057–1067. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Heitzer, E.; Ulz, P.; Geigl, J.B. Circulating Tumor DNA as a Liquid Biopsy for Cancer. Clin. Chem. 2015, 61, 112–123. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Bardelli, A. Liquid Biopsies: Genotyping Circulating Tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Kis, O.; Kaedbey, R.; Chow, S.; Danesh, A.; Dowar, M.; Li, T.; Li, Z.; Liu, J.; Mansour, M.; Masih-Khan, E.; et al. Circulating Tumour DNA Sequence Analysis as an Alternative to Multiple Myeloma Bone Marrow Aspirates. Nat. Commun. 2017, 8, 15086. [Google Scholar] [CrossRef]
- Manier, S.; Park, J.; Capelletti, M.; Bustoros, M.; Freeman, S.S.; Ha, G.; Rhoades, J.; Liu, C.J.; Huynh, D.; Reed, S.C.; et al. Whole-Exome Sequencing of Cell-Free DNA and Circulating Tumor Cells in Multiple Myeloma. Nat. Commun. 2018, 9, 1691. [Google Scholar] [CrossRef]
- Guo, G.; Raje, N.S.; Seifer, C.; Kloeber, J.; Isenhart, R.; Ha, G.; Yee, A.J.; O’Donnell, E.K.; Tai, Y.T.; Richardson, P.G.; et al. Genomic Discovery and Clonal Tracking in Multiple Myeloma by Cell-Free DNA Sequencing. Leukemia 2018, 32, 1838–1841. [Google Scholar] [CrossRef] [PubMed]
- Garcés, J.J.; Bretones, G.; Burgos, L.; Valdes-Mas, R.; Puig, N.; Cedena, M.T.; Alignani, D.; Rodriguez, I.; Puente, D.Á.; Álvarez, M.G.; et al. Circulating tumor cells for comprehensive and multiregional non-invasive genetic characterization of multiple myeloma. Leukemia 2020, 34, 3007–3018. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Chen, K.; Babbra, R.K.; Feng, W.; Pejovic, N.; Nallicheri, A.; Harris, P.K.; Dienstbach, K.; Atkocius, A.; Maguire, L.; et al. Urine Tumor DNA Detection of Minimal Residual Disease in Muscle-Invasive Bladder Cancer Treated with Curative-Intent Radical Cystectomy: A Cohort Study. PLoS Med. 2021, 18, e1003732. [Google Scholar]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.C.; Auclair, D.; Kelloff, G.J.; Sigman, C.C.; Avet-Loiseau, H.; Farrell, A.T.; Gormley, N.J.; Kumar, S.K.; Landgren, O.; Munshi, N.C.; et al. The Role of Minimal Residual Disease Testing in Myeloma Treatment Selection and Drug Development: Current Value and Future Applications. Clin. Cancer Res. 2017, 23, 3980–3993. [Google Scholar] [CrossRef] [PubMed]
- Kubaczkova, V.; Vrabel, D.; Sedlarikova, L.; Besse, L.; Sevcikova, S. Cell-Free DNA—Minimally Invasive Marker of Hematological Malignancies. Eur. J. Haematol. 2017, 99, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Lopez, J.; Lahuerta, J.J.; Pepin, F.; González, M.; Barrio, S.; Ayala, R.; Puig, N.; Montalban, M.A.; Paiva, B.; Weng, L.; et al. Prognostic value of deep sequencing method for minimal residual disease detection in multiple myeloma. Blood 2014, 123, 3073–3079. [Google Scholar] [CrossRef]
- Flores-Montero, J.; Sanoja, L.; Pérez, J.J.; Pojero, F.; Puig, N.; Vidriales, M.B.; Orfao, A. Plasma Cell Disorders. In Manual of Molecular and Clinical Laboratory Immunology; Detrick, B., Schmitz, J.L., Hamilton, R.G., Eds.; ASM Press: Washington, DC, USA, 2016; pp. 235–250. [Google Scholar]
- Paiva, B.; Almeida, J.; Pérez-Andrés, M.; Mateo, G.; López, A.; Rasillo, A.; Vídriales, M.B.; López-Berges, M.C.; Miguel, J.F.; Orfao, A. Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell-related disorders. Cytom. B Clin. Cytom. 2010, 78, 239–252. [Google Scholar] [CrossRef]
- Notarfranchi, L.; Zherniakova, A.; Lasa, M.; Puig, N.; Cedena, M.T.; Martínez-López, J.; Calasanz, M.J.; Alignani, D.; Burgos, L.; Manrique, I.; et al. Ultra-Sensitive Assessment of Measurable Residual Disease (MRD) in Peripheral Blood (PB) of Multiple Myeloma (MM) Patients Using Bloodflow. Blood 2022, 140 (Suppl. 1), 2095–2097. [Google Scholar] [CrossRef]
- Colmenares, R.; Álvarez, N.; Barrio, S.; Martínez-López, J.; Ayala, R. The Minimal Residual Disease Using Liquid Biopsies in Hematological Malignancies. Cancers 2022, 14, 1310. [Google Scholar] [CrossRef]
- Paiva, B.; van Dongen, J.J.; Orfao, A. New criteria for response assessment: Role of minimal residual disease in multiple myeloma. Blood 2015, 125, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Berger, N.; Kim-Schulze, S.; Parekh, S. Minimal residual disease in multiple myeloma: Impact on response assessment, prognosis and tumor heterogeneity. Adv. Exp. Med. Biol. 2018, 1100, 141–159. [Google Scholar] [PubMed]
- Perrot, A.; Lauwers-Cances, V.; Corre, J.; Robillard, N.; Hulin, C.; Chretien, M.L.; Dejoie, T.; Maheo, S.; Stoppa, A.M.; Pegourie, B.; et al. Minimal residual disease negativity using deep sequencing is a major prognostic factor in multiple myeloma. Blood 2018, 132, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Weisel, K.; Spencer, A.; Lentzsch, S.; Avet-Loiseau, H.; Mark, T.M.; Spicka, I.; Masszi, T.; Lauri, B.; Levin, M.D.; Bosi, A.; et al. Daratumumab, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma: Subgroup analysis of CASTOR based on cytogenetic risk. J. Hematol. Oncol. 2020, 13, 115. [Google Scholar] [CrossRef]
- Oberle, A.; Brandt, A.; Voigtlaender, M.; Thiele, B.; Radloff, J.; Schulenkorf, A.; Alawi, M.; Akyüz, N.; März, M.; Ford, C.T.; et al. Monitoring Multiple Myeloma by Next-Generation Sequencing of V(D)J Rearrangements from Circulating Myeloma Cells and Cell-Free Myeloma DNA. Haematologica 2017, 102, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Biancon, G.; Gimondi, S.; Vendramin, A.; Carniti, C.; Corradini, P. Noninvasive Molecular Monitoring in Multiple Myeloma Patients Using Cell-Free Tumor DNA. J. Mol. Diagn. 2018, 20, 859–870. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Khong, T.; Ramachandran, M.; Chow, A.; Klarica, D.; Mai, L.; Walsh, S.; Broemeling, D.; Marziali, A.; Wiggin, M.; et al. Circulating Tumour DNA Analysis Demonstrates Spatial Mutational Heterogeneity That Coincides with Disease Relapse in Myeloma. Leukemia 2017, 31, 1695–1705. [Google Scholar] [CrossRef]
- Rustad, E.H.; Coward, E.; Skytøen, E.R.; Misund, K.; Holien, T.; Standal, T.; Børset, M.; Beisvag, V.; Myklebost, O.; Meza-Zepeda, L.A.; et al. Monitoring Multiple Myeloma by Quantification of Recurrent Mutations in Serum. Haematologica 2017, 102, 1266–1272. [Google Scholar] [CrossRef]
- Gerber, B.; Manzoni, M.; Spina, V.; Bruscaggin, A.; Lionetti, M.; Fabris, S.; Barbieri, M.; Ciceri, G.; Pompa, A.; Forestieri, G.; et al. Circulating Tumor DNA as a Liquid Biopsy in Plasma Cell Dyscrasias. Haematologica 2018, 103, e245–e248. [Google Scholar] [CrossRef]
- Mazzotti, C.; Buisson, L.; Maheo, S.; Perrot, A.; Chretien, M.L.; Leleu, X.; Hulin, C.; Manier, S.; Hébraud, B.; Roussel, M.; et al. Myeloma MRD by Deep Sequencing from Circulating Tumor DNA Does Not Correlate with Results Obtained in the Bone Marrow. Blood Adv. 2018, 2, 2811–2813. [Google Scholar] [CrossRef]
- Long, X.; Xu, Q.; Lou, Y.; Li, C.; Gu, J.; Cai, H.; Wang, D.; Xu, J.; Li, T.; Zhou, X.; et al. The Utility of Non-invasive Liquid Biopsy for Mutational Analysis and Minimal Residual Disease Assessment in Extramedullary Multiple Myeloma. Br. J. Haematol. 2020, 189, e45–e48. [Google Scholar] [CrossRef] [PubMed]
- Ntanasis-Stathopoulos, I.; Gavriatopoulou, M.; Terpos, E.; Fotiou, D.; Kastritis, E.; Dimopoulos, M.A. Monitoring Plasma Cell Dyscrasias with Cell-Free DNA Analysis. Clin. Lymphoma Myeloma Leuk 2020, 20, e905–e909. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, S.; Tytarenko, R.G.; Wang, Y.; Boyle, E.M.; Ashby, C.; Schinke, C.D.; Thanendrarajan, S.; Zangari, M.; Zhan, F.; Davies, F.E.; et al. Monitoring treatment response and disease progression in myeloma with circulating cell-free DNA. Eur. J. Haematol. 2020, 106, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Subhash, V.V.; Huang, L.; Kamili, A.; Wong, M.; Chen, D.; Venn, N.C.; Atkinson, C.; Mayoh, C.; Venkat, P.; Tyrrell, V.; et al. Whole-genome sequencing facilitates patient-specific quantitative PCR-based minimal residual disease monitoring in acute lymphoblastic leukaemia, neuroblastoma and Ewing sarcoma. Br. J. Cancer 2021, 126, 482–491. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).