Simple Summary
Sentinel lymph node (SLN) mapping is safe, feasible, and cost-effective to determine the lymph node status in endometrial cancer (EC). The aim of our prospective SLIM study was to investigate the incidence of SLN metastases and the contribution of SLN mapping in the management of presumed low- and intermediate-risk EC, i.e., clinical early-stage EC, endometrioid histology, grade 1 or 2. SLN metastases were present in 11.2% of 152 patients. Adjuvant management was adjusted based on the SLN status in 7.9% of patients: in 5.9% adjuvant treatment was added due to a positive sentinel node, and in 2.0% adjuvant treatment was limited due to unexpected grade 3 disease with a negative SLN. SLN mapping seems important in patients with presumed low- and intermediate-risk EC and may avoid undertreatment as well as overtreatment.
Abstract
The aim was to investigate the incidence of sentinel lymph node (SLN) metastases and the contribution of SLN mapping in presumed low- and intermediate-risk endometrial cancer (EC). A multicenter, prospective cohort study in presumed low- and intermediate-risk EC patients was performed. Patients underwent SLN mapping using cervical injections of indocyanine green and a minimally invasive hysterectomy with bilateral salpingo-oophorectomy. The primary outcome was the incidence of SLN metastases, leading to adjusted adjuvant treatment. Secondary outcomes were the SLN detection rate and the occurrence of complications. Descriptive statistics and univariate general linear model analyses were used. A total of 152 patients were enrolled, with overall and bilateral SLN detection rates of 91% and 61%, respectively. At final histology, 78.9% of patients (n = 120) had truly low- and intermediate-risk EC. Macro- and micro-metastases were present in 11.2% (n = 17/152), and three patients had isolated tumor cells (2.0%). Nine patients (5.9%) had addition of adjuvant radiotherapy based on SLN metastases only. In 2.0% of patients with high-risk disease, adjuvant therapy was more limited due to negative SLNs. This study emphasizes the importance of SLN mapping in presumed early-stage, grade 1 and 2 EC, leading to individualized adjuvant management, resulting in less undertreatment and overtreatment.
1. Introduction
The incidence of endometrial cancer (EC) in the western world is increasing, due to the increasing prevalence of obesity, a prolonged life expectancy, and changes in reproductive behavior [1]. The majority of patients (80%) are defined as presumed early-stage, with the tumor clinically and/or radiologically confined to the uterus, and grade 1 or 2 endometrioid histology on endometrial sampling. Defining the optimal treatment strategy for this patient group is of utmost importance. In contrary to most other countries, the standard surgical procedure in presumed low- and intermediate-risk EC in the Netherlands consists only of a total hysterectomy and bilateral salpingo-oophorectomy, without taking the lymph node status into account [1]. Preoperative myometrial invasion, nor biomarkers, are implemented in the preoperative risk classification. These patients are not referred to an oncology unit, unlike in all other gynaecological malignancies. Adjuvant treatment is based on postoperative patient and tumor characteristics, i.e., age, tumor stage, tumor histology and grade, depth of myometrial invasion, and the presence of lymphovascular space invasion (LVSI). The prognosis of early-stage low- and intermediate-risk EC is generally reassuring with overall survival rates of 80% to 90%, but in approximately 10% of patients with presumed early-stage EC lymph node metastases occur. Consequently, those patients are undertreated, resulting in a significant decreased disease-free and overall survival of 50–60% [2,3,4,5,6].
Worldwide, there is still no clear consensus on the surgical management of presumed early-stage, low- and intermediate-risk EC [7,8,9]. Internationally, there is ongoing debate about whether or not to perform a lymphadenectomy [10,11]. The ASTEC trial and the trial by Benedetti Panici et al. found no evidence for a therapeutic effect of a routine lymphadenectomy itself, and a lymphadenectomy does increase the risk of surgery-related morbidity and lymphedema [12,13,14,15]. These studies did not examine if adapting adjuvant therapy in case of a lymph node metastasis leads to improved survival. If the lymph node status is not examined, there is a risk of understaging, and subsequently the determination of adjuvant therapy is only based on a risk assessment. This may result in overtreatment of patients with a high-risk profile without lymph node metastases, and undertreatment of patients with a low-risk profile with lymph node metastases [16,17,18]. Sentinel lymph node (SLN) mapping has increasingly emerged as a method to determine the lymph node status with a relatively limited extent of surgery with very little morbidity and a high accuracy [19,20,21,22]. It is performed with a cervical injection of a tracer at the start of the surgical procedure. The use of indocyanine green (ICG) as tracer results in higher detection rates and higher negative predictive values compared to the traditional dyes. Overall SLN detection rates of 92% to 97% and negative predictive values of 96% to 99% have been reported [7,20,23,24]. As the involvement of lymph nodes is one of the most important prognostic factors, adding SLN mapping in presumed early-stage grade 1 or 2 EC patients may further improve outcomes for these patients.
The primary objective of this multicenter, prospective, cohort study was to examine the incidence of SLN metastases and the contribution of SLN mapping in the management of patients with presumed low- and intermediate-risk EC. Secondary objectives were to investigate the detection rate of SLN mapping and the risks and complications of SLN mapping.
2. Materials and Methods
2.1. Study Design
The SLIM study was a multicenter, prospective, cohort study in women with presumed low- and intermediate-risk EC. Patients were enrolled from eight different hospitals: four tertiary referral hospitals and four general teaching hospitals. In each of the participating hospitals, a minimum number of thirty EC patients had to be treated surgically each year. Another requirement was that each participating gynaecologist had performed at least fifty laparoscopic hysterectomies. Before the start of participation of each of the participating hospitals, the principal investigator (PZ) performed a physical or digital site visit to instruct the surgeons and to confirm standardisation of the procedures. A proctoring gynaecologist-oncologist was available in the first number of cases (varying between three and ten cases), as for most general gynaecologists SLN mapping was a relatively new procedure.
Patients were eligible to participate in the study if they (A) were 18 years or older, (B) had preoperative histologically confirmed endometrioid EC grade 1 or 2, (C) had a presumed low- or intermediate-risk profile [25], and (D) had a normal serum CA-125, or an increased serum CA-125 but no evidence of metastases on CT-scan. Classification of low- and intermediate-risk EC was performed according to the ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma, without the aid of molecular characterizations [25]. The degree of myometrial invasion was no inclusion criterion, as this cannot be assessed completely reliable in the preoperative phase. Patients were excluded from participation if they had: an intermediate-risk profile based on a grade 3 tumor, a high-risk profile, a contraindication to minimal invasive surgery, or anaesthesia, previous pelvic or abdominal radiation, a previous allergic reaction to indocyanine green or iodine, a previous pelvic or para-aortic lymph node dissection or sampling for a previous malignancy, evidence of pelvic and/or distant metastasis, pregnancy, severe renal impairment, or hyperthyroidism. Patient eligibility was reviewed per hospital. Ethical approval was obtained by the Radboudumc Committee for Ethics in Research (‘CMO’) in the region Arnhem and Nijmegen (file number 2015-1783) and Institutional Review Board approval at each site. Written informed consent was obtained from all eligible patients.
To calculate the required number of patients, a sample size calculation was performed. The null-hypothesis (H0) was that the addition of SLN mapping to the standard Dutch care (i.e., hysterectomy and bilateral salpingo-oophorectomy) will result in 10% treatment change in presumed early-stage grade 1 and 2 EC [26]. This design yielded a type I error of 0.05 and power of 0.8. The sample size was calculated by using the following data: a prevalence of lymph node metastases in presumed low- and intermediate-risk EC of 8–12% [27,28,29,30,31,32], a distribution in preoperative histological grade 1 and 2 EC (by biopsy or (micro-) curettage) of, respectively, 75% and 25%, an agreement in pre- and postoperative histological grading of 95% [33], a unilateral detection rate of SLN mapping of 90%, and a bilateral detection rate of 70% [24]. With 10% presumed lost to follow-up, this resulted in a sample size of 152 patients.
SLN mapping was performed using the cervical injection technique with ICG: after the introduction of anesthesia, ICG (1.25 mg/mL) was injected into the cervical submucosa and stroma through a spinal needle at 3 and 9 o’clock positions, superficial (1–2 mm) and deep (20–30 mm), 1 mL per injection with a total of 4 mL. The SLNs were localized by the use of a near-infrared fluorescence camera (Firefly™ Fluorescence Imaging for da Vinci® Xi ™; D-Light P system for Storz; near-infrared system for Olympus). SLNs were identified as currently described in the ‘Operation Guide’ by Moloney et al.: preserving and dividing of the Round and Infundibulopelvic ligament, identification of the external iliac vessels, internal iliac artery, ureter, umbilical ligament, and uterine artery, the opening of the paravesical space, pursuing the lymphatic channels up to the lymph node, and dissection of the lymph node in the pelvic or, rarely, para-aortic region [34]. If multiple lymphatic pathways were seen then both draining lymph nodes were dissected. Frozen section of SLN specimens was not performed. Subsequently, a laparoscopic or robot-assisted hysterectomy with bilateral salpingo-oophorectomy was performed. In case SLN mapping failed one- or both-sided, only a hysterectomy with bilateral salpingo-oophorectomy was performed, in correspondence with the current Dutch guidelines for presumed early-stage EC [1].
Consecutive patients were enrolled in the SLIM study from the start of SLN mapping in the participating hospitals. The principle was followed that if ICG was injected and the retroperitoneal space was opened, patients were included in the analysis, regardless of SLN mapping result. Patients who withdrew before the study intervention or who did not receive the study intervention (injection of ICG and opening of the retroperitoneal space) were not included in the analyses.
All removed SLNs, as well as the uterus, Fallopian tubes, ovaries, and—if present—other removed lymph nodes were analysed by specialized gynaecological pathologists. The participating pathology laboratories used a protocol in which the SLN was serially sectioned. Each SLN was sliced perpendicular to the longest axis in 2–3 mm slices. The slices were routinely processed after formalin fixation. The paraffin block was cut until the contour of the lymph node appears. Then, 4 µm sections were cut into 6 ribbons with step series spaced 200 µm apart. A central section of ribbon 4, 5 and 6 was stained with hematoxylin and eosin and an adjacent section for keratin immunohistochemical staining using the monoclonal antibody Cytokeratin 8.18 (Cam5.2; BD, OMNIS retrieval 1:20). SLN metastases were defined according to the common definitions of macro-metastasis (>2 mm), micro-metastasis (0.2–2 mm), and isolated tumor cells (ITC; <0.2 mm). Non-sentinel lymph nodes were handled according to institutional standard-of-care practices, and therefore negative H&E slides from non-sentinel lymph nodes were not subjected to immunohistochemistry.
Participating surgeons provided encrypted data of all included patients on patient characteristics, surgical procedure, pathological assessment, and peri- and postoperative complications and adverse events. Determination of the oestrogen receptor (ER) and progesterone receptor (PR) status was not routinely performed, but added to the pathological assessment in case of a positive SLN.
Adjuvant management was based on the current Dutch guidelines, local protocols, and the presence or absence of lymph node metastases [1]. The presence of a macro- or micro-metastasis resulted in FIGO stage IIIC disease; those patients received adjuvant external beam radiotherapy (EBRT) and/or chemotherapy. Patients with only ITC were managed with a watchful waiting policy, without specific adjuvant therapy. The observation was performed through CT scans of the pelvis and abdomen six and twelve months after surgery.
Patients with postoperative FIGO stage IA, low- and intermediate-risk EC, were not treated with adjuvant therapy. Patients with postoperative FIGO stage IB, low- and intermediate-risk EC, did not receive adjuvant therapy or were treated with vaginal brachytherapy when older than 60 years [35]. Patients with FIGO stage II, low- and intermediate-risk EC were treated with external beam radiotherapy, depending on histological findings (irradicality, myometrial invasion, and LVSI). The absence of SLN metastases in patients with FIGO stage II EC led in some cases to the advice for a smaller field of external beam radiation, following local protocol. Patients with FIGO stage I-II EC could be included in the PORTEC 4A study if they met the inclusion criteria of that study. Those patients were randomized in the experimental group (molecular-integrated risk profile-based adjuvant treatment) or the standard group (adjuvant vaginal brachytherapy) [36].
Patients with of presumed low- and intermediate-risk EC, but who postoperatively turned out to have high-risk features, had an indication for determining the lymph node status. In case of successful SLN mapping, an extra operative procedure could be omitted.
2.2. Statistical Analyses
Descriptive statistics were used to describe the study population, overall and bilateral SLN detection rate, prevalence of SLN metastases, risk factors for SLN metastasis, the contribution of SLN mapping to the adjustment of adjuvant treatment, and complication rate. Univariate general linear model analyses were used to study relations between determinants (e.g., age, histological grade, tumor size, etc.) and the main outcome (SLN metastasis). A p-value less than 0.05 was considered statistically significant. All analyses were performed with SPSS for Windows Version 25, release 25.0.0.1.
3. Results
Between March 2016 and December 2021, 178 patients were identified as potential participants for the SLIM study. Twelve patients were not willing to participate, resulting in 166 inclusions. Of them, fourteen patients were excluded due to an incorrect inclusion (n = 8, reasons included poor general condition of a patient, patients’ withdrawal, or patients who met one of the exclusion criteria but signed the informed consent form), or because the retroperitoneum had not been opened during surgery (n = 6, reasons included anaesthetic difficulties during surgery, conversion to laparotomy, lack of time due to COVID-19 restrictions in surgery time). In the end, 152 patients with presumed early-stage, grade 1 or 2 EC (considered as presumed low- and intermediate-risk EC) were enrolled and underwent SLN mapping with ICG, followed by hysterectomy and bilateral salpingo-oophorectomy (Figure 1). Most of these patients were postmenopausal (88%), with a median age of 66 years (range 39–84 years). The median body-mass index of patients was 29.7 kg/m2 (range 17.6–52.2 kg/m2); 49% was obese (BMI > 30 kg/m2). Clinical, surgical, and pathological characteristics are shown in Table 1. The preoperative pathological assessment showed a grade 1 endometrioid tumor in 74% of patients and a grade 2 endometrioid tumor in 26% of patients; final histology, however, showed grade 3 or non-endometrioid EC in 8% of patients.
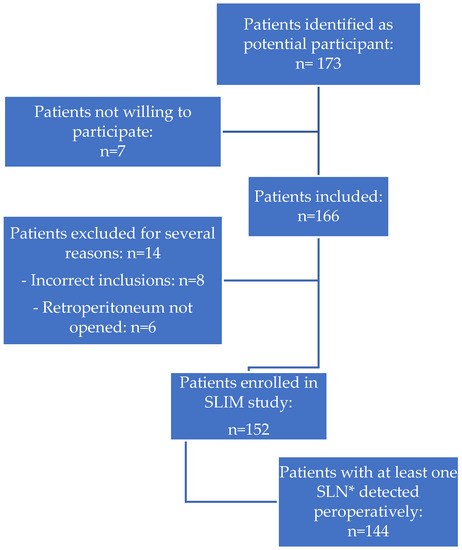
Figure 1.
Identification and inclusion of patients in the SLIM study. * SLN = sentinel lymph node.

Table 1.
Clinical, surgical and pathological characteristics.
3.1. Detection Rate of SLN Mapping
A total of 264 SLNs were dissected in 152 patients. The majority of SLNs were found in the external iliac region (38%), the obturator loge (34%), and the internal iliac region (17%). In 9% of patients, the SLN was found outside the standard region for pelvic lymph node dissection (i.e., pre-sacral, para-aortal, para-caval). In 95% of the procedures, at least one SLN was detected. Bilateral surgical SLN detection was successful in 76%. In patients with successful SLN mapping, the mean number of removed SLNs per hemipelvis was 1.2 lymph nodes. Postoperative pathological assessment of the assumed lymph node tissue showed an empty packet dissection (a dissection that does not yield a lymph node on pathological assessment) in nineteen patients (13%): in fourteen patients with assumed bilateral SLN mapping only a unilateral SLN was dissected, in two patients with assumed bilateral mapping no SLN was dissected at all, and in three patients with assumed unilateral mapping no SLN was dissected. This led to pathological SLN detection rates of 91% and 61% for overall and bilateral mapping, respectively.
3.2. Incidence of SLN Metastases
Macro- and micrometastases were found in seventeen of 152 patients (11.2%). Three patients (2.0%) had ITC. Lymphovascular space invasion (LVSI) was present in eleven of these 20 patients with a positive SLN (55%).
Eleven of the seventeen patients with macro- and micrometastases (64.7%), had grade 1–2 endometrioid EC. In final histology, nine of the eleven patients with grade 1 or 2 EC with a metastasized sentinel node, were LVSI positive. Pre-operatively, LVSI status was available in the minority (33.8%) of the included patients. Table 2 shows the characteristics of patients with and without SLN metastases (excluding the nineteen patients without successful (i.e., at least unilateral) SLN mapping, or with an empty packet dissection).

Table 2.
Characteristics of the patients with andomized SLN mapping (n = 139), with and without Sentinel Lymph Node (SLN) metastasis.
Univariate analyses showed age >60 years (p < 0.05), postoperative histological grade (p < 0.001), myometrial invasion (p < 0.005), and LVSI (p < 0.001) to be statistically significant predictors for SLN metastasis. The preoperative histological grade in itself, without other biomarkers, was no significant predictor. In patients with SLN metastases, ER and PR status were positive in 70% and in 20% only ER was positive and PR negative. In just one patient with SLN metastases, ER and PR were both negative.
Of all 152 included apparent early-stage grade 1 or 2 EC patients (apparent low- and intermediate-risk EC) only 78.9% (n = 120) were truly low- and intermediate-risk. The other 32 patients were high-risk post-operatively due to multiple reasons: seven patients had grade 3 EC (of whom four had SLN metastases), five patients had non-endometrioid EC (of whom two had SLN metastases), twelve patients with grade 1–2 EC had SLN metastases, two patients with grade 1–2 EC were postoperatively upstaged due to extension beyond the uterus with SLN metastases, and six patients with grade 1–2 EC were postoperatively upstaged due to extension beyond the uterus without SLN metastases. In thirteen of these 120 patients with true low- or intermediate-risk EC (10.8%) LVSI was present. If only the presence of LVSI was considered as an independent risk-factor that changes the risk-classification into high-risk EC, only 70.4% of the patients with apparent low- or intermediate-risk EC were correctly categorized correctly pre-operatively.
3.3. Adjusting Adjuvant Management Based on Lymph Node Status
Four of the seventeen patients with macro- and micrometastases also had another indication for adjusting the adjuvant management independent of their lymph node metastases, due to ovarian metastases (n = 1), non-endometrioid postoperative histology (n = 2), or FIGO stage II disease (n = 1).
In the remaining thirteen patients with macro- or micrometastases, adjuvant management was adjusted purely based on SLN mapping. Nine of them (5.9% of all patients) had a grade 1 or 2 endometrioid tumor after final pathological assessment without other extension beyond the uterus. SLN mapping in these patients was the only indication for upstaging to FIGO stage III disease and for adjuvant therapy: three patients were treated with external beam radiotherapy followed by chemotherapy and six patients received adjuvant external beam radiotherapy alone. The other four patients had SLN metastases but postoperative grade 3 EC. Those patients received adjuvant chemotherapy, with or without external beam radiotherapy.
In the three patients with ITC (all with grade 1 or 2 endometrioid EC), no adjustment in adjuvant therapy was made, but a watchful waiting policy was performed. Two patients underwent a CT scan after six and twelve months, showing no abnormalities. The third patient underwent only one CT scan eight months after surgery, also showing no abnormalities.
132 patients had no metastasized SLN. However, no SLN was detected in thirteen of them, and in 36 patients the SLN was found only unilaterally, leaving 83 patients with a negative SLN bilaterally. LVSI was present in 13% of patients with a negative SLN (n = 15). In patients with grade 3 EC and successful bilateral SLN mapping, no staging surgery was needed after final histology as lymph node status was already known. Twenty patients without SLN metastases had an indication for vaginal brachytherapy. Six patients had FIGO stage II EC, and were treated with adjuvant external beam radiotherapy. In three of them, a smaller field of external beam radiation was given following local protocols (only on the uterus region), due to the negative lymph nodes.
In total, adjuvant treatment was adjusted based on performing SLN mapping in twelve of 152 patients (7.9%): in nine patients (5.9%) undertreatment was prevented as they received adjuvant treatment due to SLN mapping, whereas in three patients (2.0%) with grade 3 EC in final histology overtreatment was prevented by treating them with a smaller irradiation field that did not include the pelvic node region (Figure 2).
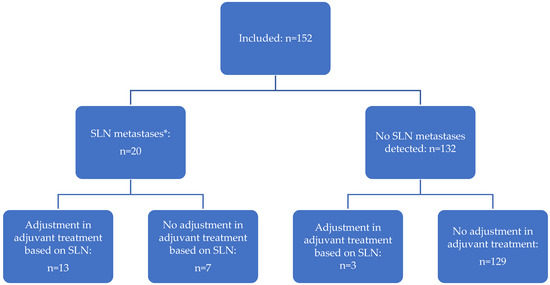
Figure 2.
The contribution of SLN mapping in the management of patients with presumed early-stage, low- and intermediate-risk EC. * Includes macro-metastases (n = 10), micro-metastasis (n = 7), and isolated tumor cells (n = 3).
3.4. Complications of SLN Mapping
A total of nine complications during surgery (5.9%) and nineteen complications in the postoperative phase (13%) were registered (Table 1). Only one surgical complication could directly be linked to SLN mapping, i.e., obturator nerve injury. One postoperative complication was linked to SLN mapping, i.e., lymphedema, and one complication may be linked to SLN mapping (i.e., a numbness feeling in the groins). All other complications were due to the hysterectomy.
4. Discussion
The SLIM study examined the contribution of SLN mapping by using ICG in the management of patients with presumed low- and intermediate-risk EC. The results of the SLIM study show that SLN mapping leads to less undertreatment of patients with presumed low- and intermediate-risk EC (presumed stage I grade 1 or 2 EC), due to upstaging if SLN metastases are present. Overtreatment can be prevented as well, due to limited therapy when final histology shows grade 3 EC without SLN metastases.
SLN metastases were found in up to 11.2% of patients with presumed pre-operative low- and intermediate-risk EC, and another 2.0% had ITC. Previous studies show comparable percentages of SLN metastases [2,3]. In the SLIM study, we calculated the percentage of lymph node metastases based on the included 152 patients. As SLN mapping had a bilateral surgical detection rate of only 76% (61% pathological), this percentage may therefore be an underestimate.
Only 78.9% of the included presumed low- and intermediate-risk EC patients were truly low- and intermediate-risk in the postoperative assessment, as the others had grade 1 and 2 EC with SLN metastases, or high-risk EC with or without SLN metastases. LVSI was present in 10.8% of the patients with true low- and intermediate-risk EC. Although these patients remained included in the low- and intermediate-risk group, as in the Netherlands LVSI is not yet implemented in the risk classification and the presence of LVSI in itself does also not lead to an adjustment in adjuvant therapy in the Netherlands, we showed that LVSI was an important risk-factor for lymph node metastases.
The preoperative identification of true low- and intermediate-risk patients versus high-risk patients remains difficult [33]. We showed that pre-operative grade 1 or 2 histology is not sufficient in predicting whom is at risk for lymph node metastases. Pre-operative characteristics, specific ultrasounds, other imaging techniques, and biomarkers, whether or not implemented in a risk model, may improve pre-operative screening to identify whom is at risk for lymph node metastases. Further studies are needed to assess if risk models may adequately identify a subgroup of patients in whom SLN detection is futile, especially since SLN mapping is cost-effective and associated with a low complication rate [37]. Furthermore, patients are willing to choose for SLN mapping, if the risk of lymph node metastases is small [38].
This study showed that SLN mapping does reduce undertreatment in patients with SLN metastases, and also reduces overtreatment. Three patients with FIGO stage II EC were treated with a smaller irradiation field than usual due to a negative SLN. In the Netherlands, this is not common practice, but per case decided in multidisciplinary meetings and conform local protocols. An advantage of this policy is that more limited radiotherapy leads to fewer side effects. Furthermore, it is supposed to be safe to limit the irradiation field, as literature exists stating that even vaginal brachytherapy alone might be sufficient to avoid recurrence for these patients, without a statistically significant difference in overall survival [39]. Another group of patients in which overtreatment might be reduced due to SLN mapping, is the group of presumed low- and intermediate-risk EC patients, who postoperatively turned out to have high-risk features (non-endometrioid EC, or grade 3 endometrioid EC, or extension beyond the uterus). A second surgery to perform a lymphadenectomy can be omitted, as the lymph node status is already known due to SLN mapping. The high diagnostic accuracy of SLN mapping is confirmed by other studies in both low- and intermediate-risk and high-risk EC [20,40,41,42,43,44,45], and SLN mapping is considered to be at least evenly safe as a (pelvic) lymphadenectomy, with a higher detection rate of SLN metastases compared to a lymphadenectomy [7]. This is probably due to the identification of lymph nodes located outside the routine lymphadenectomy area (17% of SLN metastases in the FIRES trial were found in non-traditional sites [42]), and the use of ultrastaging. Therefore, patients with postoperative high-risk features and successful SLN mapping do not have to be scheduled for a subsequent staging procedure [46,47].
We showed that SLN mapping is a feasible technique, with an overall (bilateral and/or unilateral) surgical detection rate of 95% and a bilateral surgical detection rate of 76% when using ICG as the tracer. Although multiple tracers and injection localisations can be used, current literature favours the use of a cervical injection with ICG because of the highest SLN detection rates [19,20]. Nevertheless, empty packet dissection is common during the learning curve of SLN mapping, especially in patients with a higher BMI or age [44]. Previous studies showed that the learning curve of SLN mapping in EC is achieved after thirty to forty cases, and that the risk of empty packet dissection decreases with the number of performed mappings, in which each additional procedure leads to a 3.6% reduction in the odds of an empty packet [48,49,50]. In the SLIM study, the majority of empty packet dissections were found within the first twenty consecutive cases.
As shown in other studies, we confirmed in our study that SLN mapping is a safe procedure with low complication rates [40,43,51]. This is important, since we assess SLN mapping in a low- and intermediate-risk population. A dilemma that comes with SLN mapping is the presence of ITC. The presence of macro- and micro-metastases has therapeutic consequences since previous studies show a beneficial effect of adjuvant chemotherapy compared to radiotherapy alone in FIGO stage IIIC EC [16,17]. However, detailed pathological assessment with ultrastaging (i.e., serial sectioning and immunohistochemistry) might lead to the identification of isolated tumor cells <0.2 mm. In the SLIM study, patients with ITC did not receive adjuvant therapy (unless tailored to uterine factors). In other malignancies such as breast cancer, gastrointestinal cancers, and melanoma, ITC are related to an increased risk of recurrence and worsened prognosis [52,53,54]. In EC, there is still a lot of discussion on this topic worldwide. A prospective single centre study reported that patients with ITC have a good prognosis and that adjuvant therapy should be based on uterine factors instead of the presence of ITC [23]. This opinion is shared by other research groups [55], even though there are also groups who are more careful and suggest that the chance of recurrent disease is higher without adjuvant therapy [56]. In a web-based survey, sent by email to all members (gynaecologic oncologists) of the European Society of Gynaecological Oncologists, International Gynaecological Cancer Society, and Society of Gynaecological Oncologists, 14% of respondents recommended adjuvant therapy if ITC were identified [57].
In countries where a pelvic lymphadenectomy is a common strategy in low- and intermediate-risk EC, it might be straightforward to change the policy to SLN mapping. However, SLN mapping in presumed low- and intermediate-risk EC remains controversial in countries that use the risk stratification (low-, intermediate-, high-risk) for the indication for adjuvant therapy, such as the Netherlands and the United Kingdom. Arguments for performing SLN mapping are the number of SLN metastases in low- and intermediate-risk EC, the widespread acceptance of SLN mapping in other countries (more than 50% of international gynaecological oncologists adopted SLN mapping [57]), and the implementation of SLN mapping in international guidelines [7]. However, multiple dilemmas should be appointed. First, although SLN mapping likely leads to better oncological safety, high-level evidence on overall and disease-free survival benefit is lacking [14,15,22,51,58,59]. The PORTEC-3 trial showed beneficial effects of adjuvant chemotherapy on recurrence-free survival in FIGO stage III disease, but more research is necessary to examine the impact of SLN mapping on long-term oncological outcomes [16]. Other study groups are currently prospectively investigating the oncological safety of SLN mapping, however they do not specifically focus on presumed low- and intermediate-risk EC [60,61]. Their results are expected in the upcoming years. We, therefore, intend to continue to follow our SLIM study population, and to expand the population, to gain more knowledge on long-term oncological outcomes. Second, implementation of SLN mapping in a country such as the Netherlands might be more complicated since the presumed low- and intermediate-risk EC patients are mostly treated in the general hospitals by general gynecologists, and not by a gynaecological oncologist in one of the tertiary (academic) hospitals [1,62].
If no SLN was found during surgery, no pelvic and/or para-aortic lymphadenectomy was performed in the patients included in the SLIM study. This is because we adhered to the current Dutch guidelines, which do not include any kind of lymph node sampling in low- and intermediate-risk EC, in contrary to the NCCN SLN algorithm [8]. Our previous cost-effectiveness analysis showed that SLN mapping is cost-effective compared to the risk stratification strategy as used in the Netherlands (adjuvant therapy based on patient and uterine risk factors) and a lymphadenectomy [37]. With the obesity pandemic and the relationship between EC and obesity, this might become even more important. In case of unilateral SLN mapping, it is cost-effective to base adjuvant treatment on patient and uterine risk factors [37]. Unilateral SLN mapping will, however, become less common after performing multiple procedures and achieving the aforementioned learning curve [48,49]. Presumed low- and intermediate-risk EC patients, therefore, do not necessarily need to be treated by gynaecological oncologists. Furthermore, our previous study on patients’ and gynecologists’ views on SLN mapping showed that patients are willing to travel to another hospital, and that gynecologists on the other hand are willing to refer their patients to another hospital if SLN mapping is offered there [38].
This study also has some points that need special consideration. First, the design of our study was a prospective cohort study. Consultation of patient focus groups taught us that patients were not willing to be randomized between SLN mapping versus no lymph node dissection, as patients gained knowledge of the stage of the disease with only a limited addition to the standard surgical treatment; furthermore, SLN mapping is already implemented in international guidelines. Second, the inclusion of patients started slowly, since the approval by the ethical committees of the participating hospitals took longer than expected and a proctoring gynaecological oncologist had to be available for the first cases in the majority of the participating hospitals. This resulted in a rather long inclusion period (2016–2021). The majority of patients (75%) were included between 2019 and 2022.
5. Conclusions
This study showed that in 11.2% of patients with presumed low- and intermediate-risk EC lymph node metastases are present, and in 5.9%, adjuvant therapy was adjusted purely based on SLN metastases. As preoperative selection of true low- and intermediate-risk EC patients remains difficult, with only 78.9% of the included patients truly having a low- and intermediate-risk profile, we recommend considering SLN mapping in the management of presumed low- and intermediate-risk EC.
Author Contributions
Conceptualization, L.C.B. and P.L.M.Z.; Data curation, R.F.P.M.K., A.d.J., T.J.J.B., A.A.K., D.B., S.L., H.P.M.S., A.B., M.J.A.E., J.C.K., R.L.M.B. and P.L.M.Z.; Formal analysis, L.C.B.; Investigation, L.C.B., R.F.P.M.K., J.B., T.J.J.B., A.A.K., D.B., S.L., H.P.M.S., A.B., M.J.A.E., J.C.K., R.L.M.B. and P.L.M.Z.; Methodology, L.C.B., R.F.P.M.K., R.L.M.B. and P.L.M.Z.; Project administration, P.L.M.Z.; Resources: J.B.; Software, L.C.B.; Supervision, R.F.P.M.K., R.L.M.B. and P.L.M.Z.; Validation, L.C.B. and P.L.M.Z.; Visualization, L.C.B.; Writing—original draft, R.F.P.M.K., R.L.M.B. and P.L.M.Z.; editingWriting—review and L.C.B., A.d.J., J.B., T.J.J.B., A.A.K., D.B., S.L., H.P.M.S., A.B., M.J.A.E., J.C.K., R.L.M.B. and P.L.M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of the Radboudumc Committee for Ethics in Research (“CMO”) in the region Arnhem and Nijmegen (protocol code 2015-1783; date of approval 17 February 2016).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Federatie Medisch Specialisten, Integraal Kankercentrum Nederland. Richtlijnendatabase: Richtlijn Endometriumcarcinoom. 2011. Available online: https://richtlijnendatabase.nl/richtlijn/endometriumcarcinoom/endometriumcarcinoom_-_startpagina.html (accessed on 1 July 2022).
- Koskas, M.; Rouzier, R.; Amant, F. Staging for endometrial cancer: The controversy around lymphadenectomy-Can this be resolved? Best Pract. Res. Clin. Obstet. Gynaecol. 2015, 29, 845–857. [Google Scholar] [CrossRef]
- Abdullah, N.A.; Huang, K.; Casanova, J.; Artazcoz, S.; Jarruwale, P.; Benavides, D.R.; Lee, C. Sentinel lymph node in endometrial cancer: A systematic review on laparoscopic detection. Gynecol. Minim. Invasive Ther. 2013, 2, 75–78. [Google Scholar] [CrossRef]
- Lewin, S.N.; Herzog, T.J.; Barrena Medel, N.I.; Deutsch, I.; Burke, W.M.; Sun, X.; Wright, J.D. Comparative performance of the 2009 international Federation of gynecology and obstetrics’ staging system for uterine corpus cancer. Obstet. Gynecol. 2010, 116, 1141–1149. [Google Scholar] [CrossRef] [PubMed]
- Tejerizo-García, A.; Jiménez-López, J.S.; Muñoz-González, J.L.; Bartolomé-Sotillos, S.; Marqueta-Marqués, L.; López-González, G.; Gómez, J.F. Overall survival and disease-free survival in endometrial cancer: Prognostic factors in 276 patients. OncoTargets Ther. 2013, 9, 1305–1313. [Google Scholar] [PubMed]
- Lurain, J.R.; Rice, B.L.; Rademaker, A.W.; Poggensee, L.E.; Schink, J.C.; Miller, D.S. Prognostic factors associated with recurrence in clinical stage I adenocarcinoma of the endometrium. Obstet. Gynecol. 1991, 78, 63–69. [Google Scholar]
- Holloway, R.W.; Abu-Rustum, N.R.; Backes, F.J.; Boggess, J.F.; Gotlieb, W.H.; Jeffrey Lowery, W.; Rossi, E.C.; Tanner, E.J.; Wolsky, R.J. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol. Oncol. 2017, 146, 405–415. [Google Scholar] [CrossRef]
- Koh, W.J.; Abu-Rustum, N.R.; Bean, S.; Bradley, K.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; et al. Uterine Neoplasms, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 170–199. [Google Scholar] [CrossRef]
- Ballester, M.; Bendifallah, S.; Daraï, E. European guidelines (ESMO-ESGO-ESTRO consensus conference) for the management of endometrial cancer. Bull. Cancer 2017, 104, 1032–1038. [Google Scholar] [CrossRef]
- Clark, L.H.; Soper, J.T. Endometrial Cancer and the Role of Lymphadenectomy. Obstet. Gynecol. Surv. 2016, 71, 353–360. [Google Scholar] [CrossRef]
- Frost, J.A.; Webster, K.E.; Bryant, A.; Morrison, J. Lymphadenectomy for the management of endometrial cancer. Cochrane Database Syst. Rev. 2017, 10, Cd007585. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Alektiar, K.; Iasonos, A.; Lev, G.; Sonoda, Y.; Aghajanian, C.; Chi, D.S.; Barakat, R.R. The incidence of symptomatic lower-extremity lymphedema following treatment of uterine corpus malignancies: A 12-year experience at Memorial Sloan-Kettering Cancer Center. Gynecol. Oncol. 2006, 103, 714–718. [Google Scholar] [CrossRef]
- Cardosi, R.J.; Cox, C.S.; Hoffman, M.S. Postoperative neuropathies after major pelvic surgery. Obstet. Gynecol. 2002, 100, 240–244. [Google Scholar] [CrossRef]
- Kitchener, H.; Swart, A.M.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar]
- Panici, P.B.; Basile, S.; Maneschi, F.; Lissoni, A.A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef]
- de Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. Adjuvant chemoradiotherapy versus radiotherapy alone in women with high-risk endometrial cancer (PORTEC-3): Patterns of recurrence and post-hoc survival analysis of a randomised phase 3 trial. Lancet Oncol. 2019, 20, 1273–1285. [Google Scholar] [CrossRef]
- Matei, D.; Filiaci, V.; Randall, M.E.; Mutch, D.; Steinhoff, M.M.; DiSilvestro, P.A.; Moxley, K.M.; Kim, Y.M.; Powell, M.A.; O’Malley, D.M.; et al. Adjuvant Chemotherapy plus Radiation for Locally Advanced Endometrial Cancer. N. Engl. J. Med. 2019, 380, 2317–2326. [Google Scholar] [CrossRef]
- Hogberg, T. Adjuvant chemotherapy in endometrial cancer. Int. J. Gynecol. Cancer 2010, 20 (Suppl. 2), S57–S59. [Google Scholar] [CrossRef]
- Bodurtha Smith, A.J.; Fader, A.N.; Tanner, E.J. Sentinel lymph node assessment in endometrial cancer: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 459–476.e10. [Google Scholar] [CrossRef]
- How, J.A.; O’Farrell, P.; Amajoud, Z.; Lau, S.; Salvador, S.; How, E.; Gotlieb, W.H. Sentinel lymph node mapping in endometrial cancer: A systematic review and meta-analysis. Minerva Ginecol. 2018, 70, 194–214. [Google Scholar] [CrossRef]
- Obermair, H.M.; O’Hara, M.; Obermair, A.; Janda, M. Paucity of data evaluating patient centred outcomes following sentinel lymph node dissection in endometrial cancer: A systematic review. Gynecol. Oncol. Rep. 2021, 36, 100763. [Google Scholar] [CrossRef]
- Bogani, G.; Murgia, F.; Ditto, A.; Raspagliesi, F. Sentinel node mapping vs. lymphadenectomy in endometrial cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2019, 153, 676–683. [Google Scholar] [CrossRef]
- Plante, M.; Stanleigh, J.; Renaud, M.C.; Sebastianelli, A.; Grondin, K.; Grégoire, J. Isolated tumor cells identified by sentinel lymph node mapping in endometrial cancer: Does adjuvant treatment matter? Gynecol. Oncol. 2017, 146, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Jewell, E.L.; Huang, J.J.; Abu-Rustum, N.R.; Gardner, G.J.; Brown, C.L.; Sonoda, Y.; Barakat, R.R.; Levine, D.A.; Leitao, M.M. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecol. Oncol. 2014, 133, 274–277. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2020, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rustum, N.R. Update on sentinel node mapping in uterine cancer: 10-year experience at Memorial Sloan-Kettering Cancer Center. J. Obstet. Gynaecol. Res. 2014, 40, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.S.; Barakat, R.R.; Palayekar, M.J.; Levine, D.A.; Sonoda, Y.; Alektiar, K.; Brown, C.L.; Abu-Rustum, N.R. The incidence of pelvic lymph node metastasis by FIGO staging for patients with adequately surgically staged endometrial adenocarcinoma of endometrioid histology. Int. J. Gynecol. Cancer 2008, 18, 269–273. [Google Scholar] [CrossRef]
- Canlorbe, G.; Bendifallah, S.; Laas, E.; Raimond, E.; Graesslin, O.; Hudry, D.; Coutant, C.; Touboul, C.; Bleu, G.; Collinet, P.; et al. Tumor Size, an Additional Prognostic Factor to Include in Low-Risk Endometrial Cancer: Results of a French Multicenter Study. Ann. Surg. Oncol. 2016, 23, 171–177. [Google Scholar] [CrossRef]
- Creasman, W.T.; Morrow, C.P.; Bundy, B.N.; Homesley, H.D.; Graham, J.E.; Heller, B. Surgical pathologic spread patterns of endometrial cancer. A Gynecologic Oncology Group Study. Cancer 1987, 60 (Suppl. 8), 2035–2041. [Google Scholar] [CrossRef]
- Masubuchi, S.; Fujimoto, I.; Masubuchi, K. Lymph node metastasis and prognosis of endometrial carcinoma. Gynecol. Oncol. 1979, 7, 36–46. [Google Scholar] [CrossRef]
- Boronow, R.C.; Morrow, C.P.; Creasman, W.T.; Disaia, J.; Silverberg, S.G.; Miller, A.; Blessing, J.A. Surgical staging in endometrial cancer: Clinical-pathologic findings of a prospective study. Obstet. Gynecol. 1984, 63, 825–832. [Google Scholar]
- Milam, M.R.; Java, J.; Walker, J.L.; Metzinger, D.S.; Parker, L.P.; Coleman, R.L. Nodal metastasis risk in endometrioid endometrial cancer. Obstet. Gynecol. 2012, 119 Pt 1, 286–292. [Google Scholar] [CrossRef]
- Visser, N.C.M.; Reijnen, C.; Massuger, L.; Nagtegaal, I.D.; Bulten, J.; Pijnenborg, J.M.A. Accuracy of Endometrial Sampling in Endometrial Carcinoma: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2017, 130, 803–813. [Google Scholar] [CrossRef]
- Moloney, K.; Janda, M.; Frumovitz, M.; Leitao, M.; Abu-Rustum, N.R.; Rossi, E.; Nicklin, J.L.; Plante, M.; Lecuru, F.R.; Buda, A.; et al. Development of a surgical competency assessment tool for sentinel lymph node dissection by minimally invasive surgery for endometrial cancer. Int. J. Gynecol. Cancer 2021, 31, 647–655. [Google Scholar] [CrossRef]
- Nout, R.A.; Smit, V.T.; Putter, H.; Jürgenliemk-Schulz, I.M.; Jobsen, J.J.; Lutgens, L.C.; van der Steen-Banasik, E.M.; Mens, J.W.; Slot, A.; Kroese, M.C.; et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): An open-label, non-inferiority, randomised trial. Lancet 2010, 375, 816–823. [Google Scholar] [CrossRef]
- van den Heerik, A.; Horeweg, N.; Nout, R.A.; van der Steen-Banasik, E.M.L.L.; Westerveld, G.H.; van den Berg, H.A.; Slot, A.; Koppe FL, A.; Kommoss, S. PORTEC-4a: International randomized trial of molecular profile-based adjuvant treatment for women with high-intermediate risk endometrial cancer. Int. J. Gynecol. Cancer 2020, 30, 2002–2007. [Google Scholar] [CrossRef]
- Burg, L.C.; Vermeulen, R.J.; Bekkers RL, M.; Wijn SR, W.; Rovers, M.M.; Govers, T.M.; Zusterzeel, L.M. A cost-effectiveness analysis of three approaches for lymph node assessment in patients with low- and intermediate-risk endometrial cancer. Gynecol. Oncol. 2021, 161, 251–260. [Google Scholar] [CrossRef]
- Aarts, J.W.M.; Burg, L.C.; Kasius, J.C.; Groenewoud, H.; A Kraayenbrink, A.; Stalmeier, P.; Zusterzeel, P.L.M. Patients’ and gynecologists’ views on sentinel lymph node mapping in low- and intermediate-risk endometrial cancer: A Dutch vignette study. Int. J. Gynecol. Cancer 2020, 30, 813–818. [Google Scholar] [CrossRef]
- Paydar, I.; DeWees, T.; Powell, M.; Mutch, D.G.; Grigsby, W.; Schwarz, J.K. Adjuvant radiotherapy in Stage II endometrial carcinoma: Is brachytherapy alone sufficient for local control? Brachytherapy 2015, 14, 427–432. [Google Scholar] [CrossRef]
- Khoury-Collado, F.; St Clair, C.; Abu-Rustum, N.R. Sentinel Lymph Node Mapping in Endometrial Cancer: An Update. Oncologist 2016, 21, 461–466. [Google Scholar] [CrossRef]
- Sullivan, S.A.; Rossi, E.C. Sentinel Lymph Node Biopsy in Endometrial Cancer: A New Standard of Care? Curr. Treat. Options Oncol. 2017, 18, 62. [Google Scholar] [CrossRef]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Geppert, B.; Lönnerfors, C.; Bollino, M.; Persson, J. Sentinel lymph node biopsy in endometrial cancer-Feasibility, safety and lymphatic complications. Gynecol. Oncol. 2018, 148, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Buda, A.; Puppo, A.; Capozzi, V.A.; Sozzi, G.; Casarin, J.; Gallitelli, V.; Murgia, F.; Vizzielli, G.; Baroni, A.; et al. Anatomical distribution of sentinel lymph nodes in patients with endometrial cancer: A multicenter study. Int. J. Gynecol. Cancer 2022, 32, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Marchocki, Z.; Cusimano, M.C.; Clarfield, L.; Kim, S.R.; Fazelzad, R.; Espin-Garcia, O.; Bouchard-Fortier, G.; Rossi, E.C.; Stewart, K.I.; Soliman, T.; et al. Sentinel lymph node biopsy in high-grade endometrial cancer: A systematic review and meta-analysis of performance characteristics. Am. J. Obstet. Gynecol. 2021, 225, e1–e367. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.W.; Gupta, S.; Stavitzski, N.M.; Zhu, X.; Takimoto, E.L.; Gubbi, A.; Bigsby, G.E.; Brudie, L.A.; Kendrick, J.E.; Ahmad, S. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol. Oncol. 2016, 141, 206–210. [Google Scholar] [CrossRef]
- Raimond, E.; Ballester, M.; Hudry, D.; Bendifallah, S.; Daraï, E.; Graesslin, O.; Coutant, C. Impact of sentinel lymph node biopsy on the therapeutic management of early-stage endometrial cancer: Results of a retrospective multicenter study. Gynecol. Oncol. 2014, 133, 506–511. [Google Scholar] [CrossRef]
- Tucker, K.; Staley, S.A.; Gehrig, A.; Soper, J.T.; Boggess, J.F.; Ivanova, A.; Rossi, E. Defining the learning curve for successful staging with sentinel lymph node biopsy for endometrial cancer among surgeons at an academic institution. Int. J. Gynecol. Cancer 2020, 30, 346–351. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, K.J.; Min, K.J.; Lee, S.; Jung, U.S.; Hong, J.H.; Song, J.Y.; Lee, J.K.; Lee, N.W. Learning curve for sentinel lymph node mapping in gynecologic malignancies. J. Surg. Oncol. 2020, 121, 599–604. [Google Scholar] [CrossRef]
- Thomaier, L.; Jager, L.; Stone, R.; Wethington, S.; Fader, A.; Tanner, E.J. Risk of empty lymph node packets in sentinel lymph node mapping for endometrial cancer using indocyanine green. Int. J. Gynecol. Cancer 2019, 29, 513–517. [Google Scholar] [CrossRef]
- Zorzato, P.C.; Bosco, M.; Franchi, M.P.; Mariani, A.; Cianci, S.; Garzon, S.; Uccella, S. Sentinel lymph node for endometrial cancer treatment: Review of the literature. Minerva Med. 2021, 112, 70–80. [Google Scholar] [CrossRef]
- de Boer, M.; van Deurzen, C.H.; van Dijck, J.A.; Borm, G.F.; van Diest, J.; Adang, E.M.; Nortier, J.W.; Rutgers, E.J.; Seynaeve, C.; Menke-Pluymers, M.B.; et al. Micrometastases or isolated tumor cells and the outcome of breast cancer. N. Engl. J. Med. 2009, 361, 653–663. [Google Scholar] [CrossRef]
- Weixler, B.; Warschkow, R.; Güller, U.; Zettl, A.; von Holzen, U.; Schmied, B.M.; Zuber, M. Isolated tumor cells in stage I & II colon cancer patients are associated with significantly worse disease-free and overall survival. BMC Cancer 2016, 16, 106. [Google Scholar]
- Scheri, R.P.; Essner, R.; Turner, R.R.; Ye, X.; Morton, D.L. Isolated tumor cells in the sentinel node affect long-term prognosis of patients with melanoma. Ann. Surg. Oncol. 2007, 14, 2861–2866. [Google Scholar] [CrossRef]
- Bogani, G.; Mariani, A.; Paolini, B.; Ditto, A.; Raspagliesi, F. Low-volume disease in endometrial cancer: The role of micrometastasis and isolated tumor cells. Gynecol. Oncol. 2019, 153, 670–675. [Google Scholar] [CrossRef]
- Todo, Y.; Kato, H.; Okamoto, K.; Minobe, S.; Yamashiro, K.; Sakuragi, N. Isolated tumor cells and micrometastases in regional lymph nodes in stage I to II endometrial cancer. J. Gynecol. Oncol. 2016, 27, e1. [Google Scholar] [CrossRef]
- Casarin, J.; Multinu, F.; Abu-Rustum, N.; Cibula, D.; A Cliby, W.; Ghezzi, F.; Leitao, M.; Konishi, I.; Nam, J.-H.; Querleu, D.; et al. Factors influencing the adoption of the sentinel lymph node technique for endometrial cancer staging: An international survey of gynecologic oncologists. Int. J. Gynecol. Cancer 2019, 29, 60–67. [Google Scholar] [CrossRef]
- Skanjeti, A.; Dhomps, A.; Paschetta, C.; Tordo, J.; Giammarile, F. Sentinel Node Mapping in Gynecologic Cancers: A Comprehensive Review. Semin Nucl. Med. 2019, 49, 521–533. [Google Scholar] [CrossRef]
- How, J.; Gauthier, C.; Abitbol, J.; Lau, S.; Salvador, S.; Gotlieb, R.; Pelmus, M.; Ferenczy, A.; Probst, S.; Brin, S.; et al. Impact of sentinel lymph node mapping on recurrence patterns in endometrial cancer. Gynecol. Oncol. 2017, 144, 503–509. [Google Scholar] [CrossRef]
- Obermair, A.; Nicklin, J.; Gebski, V.; Hayes, S.C.; Graves, N.; Mileshkin, L.; Lin, M.Y.; Beale, P.; Baxter, E.; Robledo, K.; et al. A phase III randomized clinical trial comparing sentinel node biopsy with no retroperitoneal node dissection in apparent early-stage endometrial cancer-ENDO-3: ANZGOG trial 1911/2020. Int. J. Gynecol. Cancer 2021, 31, 1595–1601. [Google Scholar] [CrossRef]
- Grassi, T.; Mariani, A.; Cibula, D.; Soliman, T.; Suman, V.J.; Weaver, A.L.; Pedra Nobre, S.; Weigelt, B.; Glaser, G.E.; Cappuccio, S.; et al. A prospective multicenter international single-arm observational study on the oncological safety of the sentinel lymph node algorithm in stage I intermediate-risk endometrial cancer (SELECT, SEntinel Lymph node Endometrial Cancer Trial). Int. J. Gynecol. Cancer 2020, 30, 1627–1632. [Google Scholar] [CrossRef]
- Nederlandse Kanker Registratie (NKR), Integraal Kankercentrum Nederland (IKNL). 2022. Available online: https://iknl.nl/nkr-cijfers (accessed on 14 January 2022).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).