Fusobacterium Nucleatum-Induced Tumor Mutation Burden Predicts Poor Survival of Gastric Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort and Specimens
2.2. Mutation Analysis
2.3. Statistical Analysis
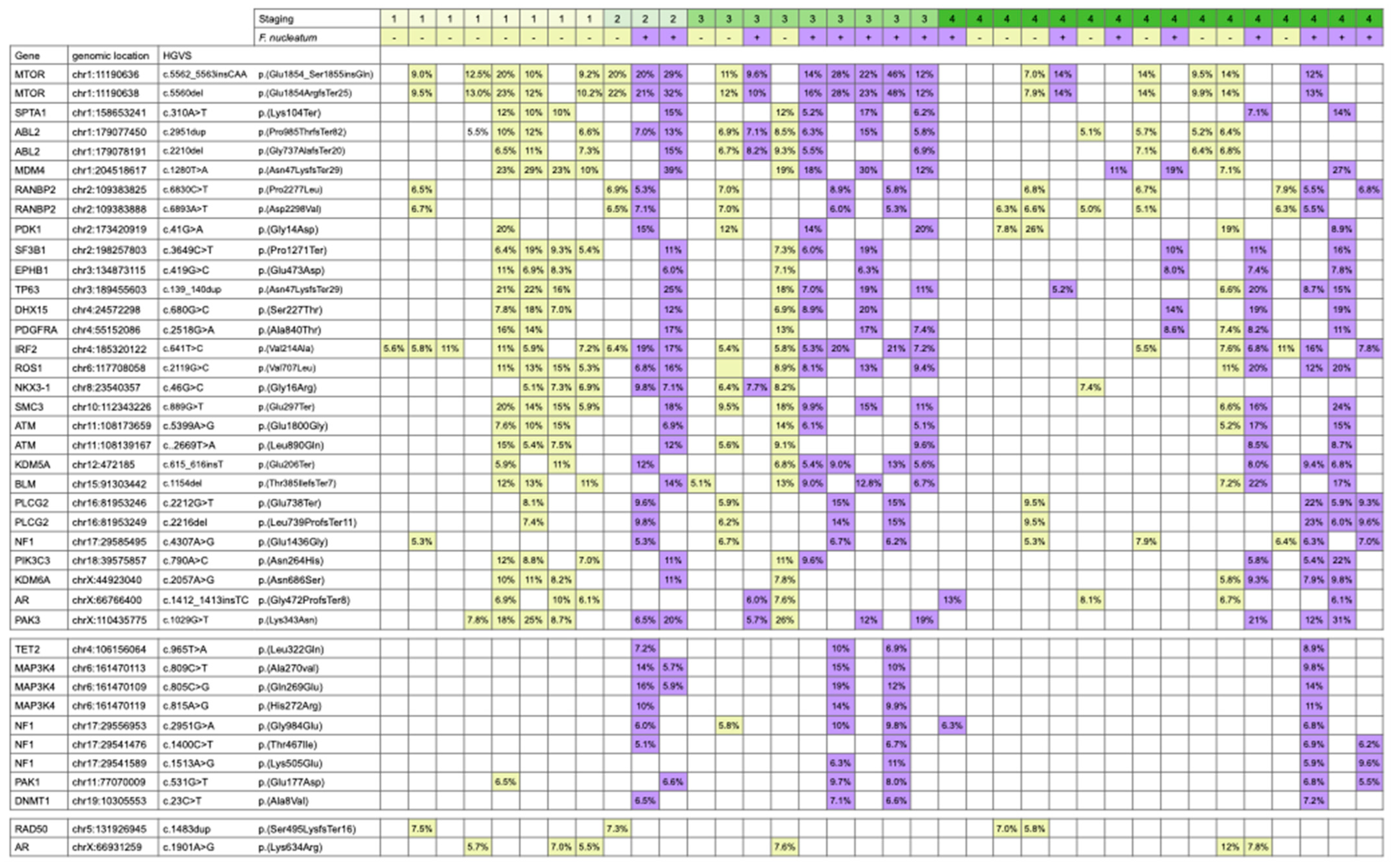
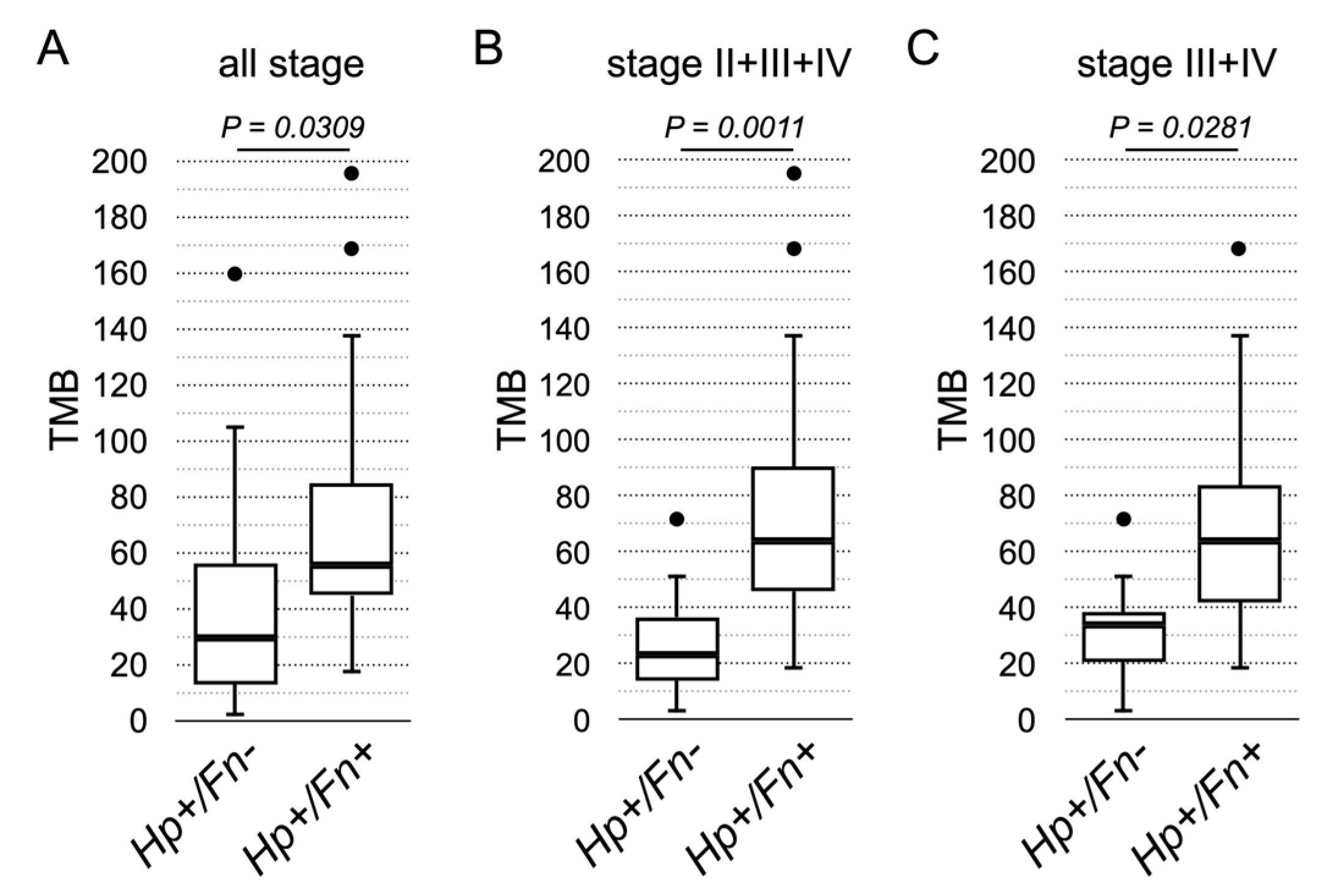
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bessede, E.; Megraud, F. Microbiota and gastric cancer. Semin. Cancer Biol. 2022, 86, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Lau, H.C.; Peppelenbosch, M.; Yu, J. Gastric Microbiota beyond H. pylori: An Emerging Critical Character in Gastric Carcinogenesis. Biomedicines 2021, 9, 1680. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.F.; Zou, K.; Xiang, C.J.; Jin, Z.J.; Ding, H.H.; Xu, S.; Wu, G.N.; Wang, Y.H.; Wu, X.Y.; Chen, C. Helicobacter pylori infection is associated with the co-occurrence of bacteria in the oral cavity and the gastric mucosa. Helicobacter 2021, 26, e12786. [Google Scholar] [CrossRef]
- Chang, J.S.; Kuo, S.-H.; Chu, P.-Y.; Shan, Y.-S.; Tsai, C.-R.; Tsai, H.-J.; Chen, L.-T. The Epidemiology of Gastric Cancers in the Era of Helicobacter pylori Eradication: A Nationwide Cancer Registry-Based Study in TaiwanEpidemiology of Gastric Cancer in Taiwan. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Cover, T.L.; Lacy, D.B.; Ohi, M.D. The Helicobacter pylori Cag type IV secretion system. Trends Microbiol. 2020, 28, 682–695. [Google Scholar] [CrossRef]
- Takahashi-Kanemitsu, A.; Knight, C.T.; Hatakeyama, M. Molecular anatomy and pathogenic actions of Helicobacter pylori CagA that underpin gastric carcinogenesis. Cell. Mol. Immunol. 2020, 17, 50–63. [Google Scholar] [CrossRef]
- Fischer, W.; Haas, R. Different roles of integrin-beta1 and integrin-alphav for type IV secretion of CagA versus cell elongation phenotype and cell lifting by Helicobacter pylori. PLoS Pathog. 2020, 16, e1008564. [Google Scholar] [CrossRef]
- Hagen, S.J.; Ang, L.-H.; Zheng, Y.; Karahan, S.N.; Wu, J.; Wang, Y.E.; Caron, T.J.; Gad, A.P.; Muthupalani, S.; Fox, J.G. Loss of tight junction protein claudin 18 promotes progressive neoplasia development in mouse stomach. Gastroenterology 2018, 155, 1852–1867. [Google Scholar] [CrossRef]
- Uotani, T.; Murakami, K.; Uchida, T.; Tanaka, S.; Nagashima, H.; Zeng, X.L.; Akada, J.; Estes, M.K.; Graham, D.Y.; Yamaoka, Y. Changes of tight junction and interleukin-8 expression using a human gastroid monolayer model of Helicobacter pylori infection. Helicobacter 2019, 24, e12583. [Google Scholar] [CrossRef]
- Lin, A.S.; Dooyema, S.D.; Frick-Cheng, A.E.; Harvey, M.L.; Suarez, G.; Loh, J.T.; McDonald, W.H.; McClain, M.S.; Peek, R.M., Jr.; Cover, T.L. Bacterial energetic requirements for Helicobacter pylori Cag type IV secretion system-dependent alterations in gastric epithelial cells. Infect. Immun. 2020, 88, e00790-19. [Google Scholar] [CrossRef]
- Stein, S.C.; Faber, E.; Bats, S.H.; Murillo, T.; Speidel, Y.; Coombs, N.; Josenhans, C. Helicobacter pylori modulates host cell responses by CagT4SS-dependent translocation of an intermediate metabolite of LPS inner core heptose biosynthesis. PLoS Pathog. 2017, 13, e1006514. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, X.; Zhang, X.; Chen, C.; Chen, H.; She, F. CagA orchestrates eEF1A1 and PKCδ to induce interleukin-6 expression in Helicobacter pylori-infected gastric epithelial cells. Gut Pathog. 2020, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, T.; Lu, Y.; Wang, C.; Wu, Y.; Li, J.; Tao, Y.; Deng, L.; Zhang, X.; Ma, J. Helicobacter pylori infection affects the human gastric microbiome, as revealed by metagenomic sequencing. FEBS Open Bio 2022, 12, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Hale, V.L.; Jeraldo, P.; Chen, J.; Mundy, M.; Yao, J.; Priya, S.; Keeney, G.; Lyke, K.; Ridlon, J.; White, B.A. Distinct microbes, metabolites, and ecologies define the microbiome in deficient and proficient mismatch repair colorectal cancers. Genome Med. 2018, 10, 78. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Nosho, K.; Sukawa, Y.; Adachi, Y.; Ito, M.; Mitsuhashi, K.; Kurihara, H.; Kanno, S.; Yamamoto, I.; Ishigami, K.; Igarashi, H. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J. Gastroenterol. 2016, 22, 557. [Google Scholar] [CrossRef] [PubMed]
- Abed, J.; Emgård, J.E.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A. Fap2 mediates Fusobacterium nucleatum colorectal adenocarcinoma enrichment by binding to tumor-expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225. [Google Scholar] [CrossRef]
- Baxter, N.T.; Ruffin, M.T.; Rogers, M.A.; Schloss, P.D. Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions. Genome Med. 2016, 8, 37. [Google Scholar] [CrossRef]
- Wong, S.H.; Kwong, T.N.; Chow, T.-C.; Luk, A.K.; Dai, R.Z.; Nakatsu, G.; Lam, T.Y.; Zhang, L.; Wu, J.C.; Chan, F.K. Quantitation of faecal Fusobacterium improves faecal immunochemical test in detecting advanced colorectal neoplasia. Gut 2017, 66, 1441–1448. [Google Scholar] [CrossRef]
- Basic, A.; Blomqvist, M.; Dahlen, G.; Svensater, G. The proteins of Fusobacterium spp. involved in hydrogen sulfide production from L-cysteine. BMC Microbiol. 2017, 17, 61. [Google Scholar] [CrossRef]
- Yoshida, Y.; Ito, S.; Kamo, M.; Kezuka, Y.; Tamura, H.; Kunimatsu, K.; Kato, H. Production of hydrogen sulfide by two enzymes associated with biosynthesis of homocysteine and lanthionine in Fusobacterium nucleatum subsp. nucleatum ATCC 25586. Microbiology 2010, 156, 2260–2269. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Wagner, E.D.; Gaskins, H.R.; Plewa, M.J. Hydrogen sulfide induces direct radical-associated DNA damage. Mol. Cancer Res. 2007, 5, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.; Rajapakse, A.; Shen, X.; Gates, K.S. Generation of DNA-damaging reactive oxygen species via the autoxidation of hydrogen sulfide under physiologically relevant conditions: Chemistry relevant to both the genotoxic and cell signaling properties of H(2)S. Chem. Res. Toxicol. 2012, 25, 1609–1615. [Google Scholar] [CrossRef] [PubMed]
- Okita, Y.; Takeda, K.; Ross, R.; Mukherjee, B.; Koeppe, E.; Stoffel, E.M.; Galanko, J.A.; McCoy, A.N.; Keku, T.O.; Okugawa, Y. Fusobacterium nucleatum infection correlates with two types of microsatellite alterations in colorectal cancer and triggers DNA damage. Gut Pathog. 2020, 12, 46. [Google Scholar] [CrossRef]
- Hsieh, Y.-Y.; Tung, S.-Y.; Pan, H.-Y.; Yen, C.-W.; Xu, H.-W.; Lin, Y.-J.; Deng, Y.-F.; Hsu, W.-T.; Wu, C.-S.; Li, C. Increased abundance of Clostridium and Fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci. Rep. 2018, 8, 158. [Google Scholar] [CrossRef]
- Hsieh, Y.-Y.; Tung, S.-Y.; Pan, H.-Y.; Chang, T.-S.; Wei, K.-L.; Chen, W.-M.; Deng, Y.-F.; Lu, C.-K.; Lai, Y.-H.; Wu, C.-S. Fusobacterium nucleatum colonization is associated with decreased survival of helicobacter pylori-positive gastric cancer patients. World J. Gastroenterol. 2021, 27, 7311. [Google Scholar] [CrossRef]
- Ramos-Paradas, J.; Hernández-Prieto, S.; Lora, D.; Sanchez, E.; Rosado, A.; Caniego-Casas, T.; Carrizo, N.; Enguita, A.B.; Muñoz-Jimenez, M.T.; Rodriguez, B. Tumor mutational burden assessment in non-small-cell lung cancer samples: Results from the TMB2 harmonization project comparing three NGS panels. J. Immunother. Cancer 2021, 9, e001904. [Google Scholar] [CrossRef]
- Wei, B.; Kang, J.; Kibukawa, M.; Arreaza, G.; Maguire, M.; Chen, L.; Qiu, P.; Lang, L.; Aurora-Garg, D.; Cristescu, R. Evaluation of the TruSight Oncology 500 Assay for Routine Clinical Testing of Tumor Mutational Burden and Clinical Utility for Predicting Response to Pembrolizumab. J. Mol. Diagn. 2022, 24, 600–608. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef]
- Hui, R.; de Boer, R.; Lim, E.; Yeo, B.; Lynch, J. CDK4/6 inhibitor plus endocrine therapy for hormone receptor-positive, HER2-negative metastatic breast cancer: The new standard of care. Asia-Pac. J. Clin. Oncol. 2021, 17, 3–14. [Google Scholar] [CrossRef]
- Lin, M.; Chen, Y.; Jin, Y.; Hu, X.; Zhang, J. Comparative overall survival of CDK4/6 inhibitors plus endocrine therapy vs. endocrine therapy alone for hormone receptor-positive, HER2-negative metastatic breast cancer. J. Cancer 2020, 11, 7127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, P.M.; Zhou, Y.; Cheng, A.S.; Yu, J.; Kang, W.; To, K.F. Targeting the oncogenic FGF-FGFR axis in gastric carcinogenesis. Cells 2019, 8, 637. [Google Scholar] [CrossRef] [PubMed]
- Mezynski, M.J.; Farrelly, A.M.; Cremona, M.; Carr, A.; Morgan, C.; Workman, J.; Armstrong, P.; McAuley, J.; Madden, S.; Fay, J. Targeting the PI3K and MAPK pathways to improve response to HER2-targeted therapies in HER2-positive gastric cancer. J. Transl. Med. 2021, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Shimozaki, K.; Shinozaki, E.; Yamamoto, N.; Imamura, Y.; Osumi, H.; Nakayama, I.; Wakatsuki, T.; Ooki, A.; Takahari, D.; Ogura, M. KRAS mutation as a predictor of insufficient trastuzumab efficacy and poor prognosis in HER2-positive advanced gastric cancer. J. Cancer Res. Clin. Oncol. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Xu, Y.; Li, J.; Zhang, X.; Peng, Z.; Hu, Y.; Zhao, X.; Dong, K.; Zhang, B. Mutations of PI3K-AKT-mTOR pathway as predictors for immune cell infiltration and immunotherapy efficacy in dMMR/MSI-H gastric adenocarcinoma. BMC Med. 2022, 20, 133. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.-d.; Chen, K.; Chen, B.; Xiong, M.-M. Positive prognostic value of HER2-HER3 co-expression and p-mTOR in gastric cancer patients. BMC cancer 2017, 17, 841. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.-D.; Chen, K.; Xiong, M.-M.; Chen, B. HER3, but not HER4, plays an essential role in the clinicopathology and prognosis of gastric cancer: A meta-analysis. PLoS ONE 2016, 11, e0161219. [Google Scholar] [CrossRef]
- Queen, J.; Domingue, J.C.; White, J.R.; Stevens, C.; Udayasuryan, B.; Nguyen, T.T.D.; Wu, S.; Ding, H.; Fan, H.; McMann, M.; et al. Comparative Analysis of Colon Cancer-Derived Fusobacterium nucleatum Subspecies: Inflammation and Colon Tumorigenesis in Murine Models. mBio 2022, 13, e0299121. [Google Scholar] [CrossRef]
- Wu, J.; Li, Q.; Fu, X. Fusobacterium nucleatum Contributes to the Carcinogenesis of Colorectal Cancer by Inducing Inflammation and Suppressing Host Immunity. Transl. Oncol. 2019, 12, 846–851. [Google Scholar] [CrossRef]
- Xu, C.; Fan, L.; Lin, Y.; Shen, W.; Qi, Y.; Zhang, Y.; Chen, Z.; Wang, L.; Long, Y.; Hou, T.; et al. Fusobacterium nucleatum promotes colorectal cancer metastasis through miR-1322/CCL20 axis and M2 polarization. Gut Microbes 2021, 13, 1980347. [Google Scholar] [CrossRef]
- Gao, Y.; Bi, D.; Xie, R.; Li, M.; Guo, J.; Liu, H.; Guo, X.; Fang, J.; Ding, T.; Zhu, H.; et al. Fusobacterium nucleatum enhances the efficacy of PD-L1 blockade in colorectal cancer. Signal Transduct. Target. Ther. 2021, 6, 398. [Google Scholar] [CrossRef]
- Hasegawa, H.; Shitara, K.; Takiguchi, S.; Takiguchi, N.; Ito, S.; Kochi, M.; Horinouchi, H.; Kinoshita, T.; Yoshikawa, T.; Muro, K. A multicenter, open-label, single-arm phase I trial of neoadjuvant nivolumab monotherapy for resectable gastric cancer. Gastric Cancer 2022, 25, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Ryu, M.-H.; Park, Y.S.; Ma, J.; Lee, S.Y.; Kim, D.; Kang, Y.-K. Predictive biomarkers for the efficacy of nivolumab as ≥ 3rd-line therapy in patients with advanced gastric cancer: A subset analysis of ATTRACTION-2 phase III trial. BMC Cancer 2022, 22, 378. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, N.; Li, X.; Chen, X.; Shen, B.; Zhu, D.; Zhu, L.; Xu, Y.; Yu, Y.; Shu, Y. Tumor mutation burden as a biomarker in resected gastric cancer via its association with immune infiltration and hypoxia. Gastric Cancer 2021, 24, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.C.; Duong, C.P.; Gopalakrishnan, V.; Iebba, V.; Chen, W.-S.; Derosa, L.; Khan, M.A.W.; Cogdill, A.P.; White, M.G.; Wong, M.C. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat. Med. 2021, 27, 1432–1441. [Google Scholar] [CrossRef]
- He, D.; Li, X.; An, R.; Wang, L.; Wang, Y.; Zheng, S.; Chen, X.; Wang, X. Response to PD-1-based immunotherapy for non-small cell lung cancer altered by gut microbiota. Oncol. Ther. 2021, 9, 647–657. [Google Scholar] [CrossRef]
- Newsome, R.C.; Gharaibeh, R.Z.; Pierce, C.M.; da Silva, W.V.; Paul, S.; Hogue, S.R.; Yu, Q.; Antonia, S.; Conejo-Garcia, J.R.; Robinson, L.A. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 2022, 14, 35. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-Y.; Kuo, W.-L.; Hsu, W.-T.; Tung, S.-Y.; Li, C. Fusobacterium Nucleatum-Induced Tumor Mutation Burden Predicts Poor Survival of Gastric Cancer Patients. Cancers 2023, 15, 269. https://doi.org/10.3390/cancers15010269
Hsieh Y-Y, Kuo W-L, Hsu W-T, Tung S-Y, Li C. Fusobacterium Nucleatum-Induced Tumor Mutation Burden Predicts Poor Survival of Gastric Cancer Patients. Cancers. 2023; 15(1):269. https://doi.org/10.3390/cancers15010269
Chicago/Turabian StyleHsieh, Yung-Yu, Wen-Lin Kuo, Wan-Ting Hsu, Shui-Yi Tung, and Chin Li. 2023. "Fusobacterium Nucleatum-Induced Tumor Mutation Burden Predicts Poor Survival of Gastric Cancer Patients" Cancers 15, no. 1: 269. https://doi.org/10.3390/cancers15010269
APA StyleHsieh, Y.-Y., Kuo, W.-L., Hsu, W.-T., Tung, S.-Y., & Li, C. (2023). Fusobacterium Nucleatum-Induced Tumor Mutation Burden Predicts Poor Survival of Gastric Cancer Patients. Cancers, 15(1), 269. https://doi.org/10.3390/cancers15010269





