Association of Pulmonary Sepsis and Immune Checkpoint Inhibitors: A Pharmacovigilance Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design and Data Sources
2.2. Procedures
2.3. Statistical Analysis
3. Results
3.1. Sepsis Signal Detected Using FAERS Database
3.2. Clinical Features of Pulmonary Sepsis
3.3. Drug-Drug Interaction Signal Detection
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef] [PubMed]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef] [PubMed]
- Aroldi, F.; Saleh, R.; Jafferji, I.; Barreto, C.; Saberian, C.; Middleton, M.R. Lag3: From Bench to Bedside. Cancer Treat. Res. 2022, 183, 185–199. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol 2018, 4, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Cecconi, M.; Evans, L.; Levy, M.; Rhodes, A. Sepsis and septic shock. Lancet 2018, 392, 75–87. [Google Scholar] [CrossRef]
- Hensley, M.K.; Donnelly, J.P.; Carlton, E.F.; Prescott, H.C. Epidemiology and Outcomes of Cancer-Related Versus Non-Cancer-Related Sepsis Hospitalizations. Crit. Care Med. 2019, 47, 1310–1316. [Google Scholar] [CrossRef]
- Gotts, J.E.; Matthay, M.A. Sepsis: Pathophysiology and clinical management. BMJ 2016, 353, i1585. [Google Scholar] [CrossRef]
- Mohsin, M.; Tabassum, G.; Ahmad, S.; Ali, S.; Ali Syed, M. The role of mitophagy in pulmonary sepsis. Mitochondrion 2021, 59, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, S.; Chen, Y.; Shao, E.; Zhuang, T.; Lu, L.; Chen, X. Fatal Adverse Events Associated With Programmed Cell Death Ligand 1 Inhibitors: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2020, 11, 5. [Google Scholar] [CrossRef]
- Hsu, C.; Lee, S.H.; Ejadi, S.; Even, C.; Cohen, R.B.; Le Tourneau, C.; Mehnert, J.M.; Algazi, A.; van Brummelen, E.M.J.; Saraf, S.; et al. Safety and Antitumor Activity of Pembrolizumab in Patients With Programmed Death-Ligand 1-Positive Nasopharyngeal Carcinoma: Results of the KEYNOTE-028 Study. J. Clin. Oncol. 2017, 35, 4050–4056. [Google Scholar] [CrossRef]
- Sarangdhar, M.; Tabar, S.; Schmidt, C.; Kushwaha, A.; Shah, K.; Dahlquist, J.E.; Jegga, A.G.; Aronow, B.J. Data mining differential clinical outcomes associated with drug regimens using adverse event reporting data. Nat. Biotechnol. 2016, 34, 697–700. [Google Scholar] [CrossRef]
- Fadini, G.P.; Bonora, B.M.; Mayur, S.; Rigato, M.; Avogaro, A. Dipeptidyl peptidase-4 inhibitors moderate the risk of genitourinary tract infections associated with sodium-glucose co-transporter-2 inhibitors. Diabetes Obes. Metab. 2018, 20, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhao, Y.C.; Guo, L.; Gong, H.; Wang, Y.K.; Ma, R.; Zhang, B.K.; Sheng, Y.; Sarangdhar, M.; Noguchi, Y.; et al. Do antibody-drug conjugates increase the risk of sepsis in cancer patients? A pharmacovigilance study. Front. Pharmacol. 2022, 13, 967017. [Google Scholar] [CrossRef] [PubMed]
- van Hasselt, J.G.C.; Rahman, R.; Hansen, J.; Stern, A.; Shim, J.V.; Xiong, Y.; Pickard, A.; Jayaraman, G.; Hu, B.; Mahajan, M.; et al. Transcriptomic profiling of human cardiac cells predicts protein kinase inhibitor-associated cardiotoxicity. Nat. Commun. 2020, 11, 4809. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; Rastelli, F.; Bisonni, R.; Santini, D.; et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J. Immunother. Cancer 2020, 8, e001361. [Google Scholar] [CrossRef]
- Kostine, M.; Mauric, E.; Tison, A.; Barnetche, T.; Barre, A.; Nikolski, M.; Rouxel, L.; Dutriaux, C.; Dousset, L.; Prey, S.; et al. Baseline co-medications may alter the anti-tumoural effect of checkpoint inhibitors as well as the risk of immune-related adverse events. Eur. J. Cancer 2021, 157, 474–484. [Google Scholar] [CrossRef]
- Rothman, K.J.; Lanes, S.; Sacks, S.T. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol. Drug Saf. 2004, 13, 519–523. [Google Scholar] [CrossRef]
- Bate, A.; Lindquist, M.; Edwards, I.R.; Olsson, S.; Orre, R.; Lansner, A.; De Freitas, R.M. A Bayesian neural network method for adverse drug reaction signal generation. Eur. J. Clin. Pharmacol. 1998, 54, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Noren, G.N.; Hopstadius, J.; Bate, A. Shrinkage observed-to-expected ratios for robust and transparent large-scale pattern discovery. Stat. Methods Med. Res. 2013, 22, 57–69. [Google Scholar] [CrossRef]
- Johnson, D.B.; Manouchehri, A.; Haugh, A.M.; Quach, H.T.; Balko, J.M.; Lebrun-Vignes, B.; Mammen, A.; Moslehi, J.J.; Salem, J.E. Neurologic toxicity associated with immune checkpoint inhibitors: A pharmacovigilance study. J. Immunother. Cancer 2019, 7, 134. [Google Scholar] [CrossRef]
- Noguchi, Y.; Tachi, T.; Teramachi, H. Review of Statistical Methodologies for Detecting Drug-Drug Interactions Using Spontaneous Reporting Systems. Front. Pharmacol. 2019, 10, 1319. [Google Scholar] [CrossRef]
- Noren, G.N.; Sundberg, R.; Bate, A.; Edwards, I.R. A statistical methodology for drug-drug interaction surveillance. Stat. Med. 2008, 27, 3057–3070. [Google Scholar] [CrossRef]
- UMC. The UMC Measures of Disproportionate Reporting. 2016. Available online: https://www.who-umc.org/media/164041/measures-of-disproportionate-reporting_2016.pdf (accessed on 10 April 2022).
- Noguchi, Y.; Tachi, T.; Teramachi, H. Comparison of Signal Detection Algorithms Based on Frequency Statistical Model for Drug-Drug Interaction Using Spontaneous Reporting Systems. Pharm. Res. 2020, 37, 86. [Google Scholar] [CrossRef]
- Thompson, J.A.; Schneider, B.J.; Brahmer, J.; Achufusi, A.; Armand, P.; Berkenstock, M.K.; Bhatia, S.; Budde, L.E.; Chokshi, S.; Davies, M.; et al. Management of Immunotherapy-Related Toxicities, Version 1.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 387–405. [Google Scholar] [CrossRef]
- Anand, K.; Sahu, G.; Burns, E.; Ensor, A.; Ensor, J.; Pingali, S.R.; Subbiah, V.; Iyer, S.P. Mycobacterial infections due to PD-1 and PD-L1 checkpoint inhibitors. ESMO Open 2020, 5, e000866. [Google Scholar] [CrossRef]
- Sorup, S.; Darvalics, B.; Russo, L.; Oksen, D.; Lamy, F.X.; Verpillat, P.; Aa, K.; Ht, S.; Cronin-Fenton, D. High-dose corticosteroid use and risk of hospitalization for infection in patients treated with immune checkpoint inhibitors--A nationwide register-based cohort study. Cancer Med. 2021, 10, 4957–4963. [Google Scholar] [CrossRef]
- Karam, J.D.; Noel, N.; Voisin, A.L.; Lanoy, E.; Michot, J.M.; Lambotte, O. Infectious complications in patients treated with immune checkpoint inhibitors. Eur. J. Cancer 2020, 141, 137–142. [Google Scholar] [CrossRef]
- Qin, B.D.; Jiao, X.D.; Zhou, X.C.; Shi, B.; Wang, J.; Liu, K.; Wu, Y.; Ling, Y.; Zang, Y.S. Effects of concomitant proton pump inhibitor use on immune checkpoint inhibitor efficacy among patients with advanced cancer. Oncoimmunology 2021, 10, 1929727. [Google Scholar] [CrossRef]
- Chalabi, M.; Cardona, A.; Nagarkar, D.R.; Dhawahir Scala, A.; Gandara, D.R.; Rittmeyer, A.; Albert, M.L.; Powles, T.; Kok, M.; Herrera, F.G. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: Pooled post hoc analyses of the OAK and POPLAR trials. Ann. Oncol. 2020, 31, 525–531. [Google Scholar] [CrossRef]
- Adelman, M.W.; Woodworth, M.H.; Langelier, C.; Busch, L.M.; Kempker, J.A.; Kraft, C.S.; Martin, G.S. The gut microbiome’s role in the development, maintenance, and outcomes of sepsis. Crit. Care. 2020, 24, 278. [Google Scholar] [CrossRef]
- Meijvis, S.C.; Cornips, M.C.; Voorn, G.P.; Souverein, P.C.; Endeman, H.; Biesma, D.H.; Leufkens, H.G.; van de Garde, E.M. Microbial evaluation of proton-pump inhibitors and the risk of pneumonia. Eur. Respir. J. 2011, 38, 1165–1172. [Google Scholar] [CrossRef]
- Morelli, T.; Fujita, K.; Redelman-Sidi, G.; Elkington, P.T. Infections due to dysregulated immunity: An emerging complication of cancer immunotherapy. Thorax 2022, 77, 304–311. [Google Scholar] [CrossRef]
- Fujita, K.; Yamamoto, Y.; Kanai, O.; Okamura, M.; Nakatani, K.; Mio, T. Development of Mycobacterium avium Complex Lung Disease in Patients With Lung Cancer on Immune Checkpoint Inhibitors. Open Forum Infect. Dis. 2020, 7, ofaa067. [Google Scholar] [CrossRef]
- Noguchi, Y.; Tachi, T.; Teramachi, H. Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief. Bioinform. 2021, 22, bbab347. [Google Scholar] [CrossRef]

| Categories | Overall ICIs | Full Database (Starting Q1,2011) | ROR (95%CI) | IC (95%CI) |
|---|---|---|---|---|
| Total number of ICSRs available | 215,363 | 13,943,677 | ||
| Number of ICSRs by Sepsis subgroups | ||||
| Sepsis (SMQ) | 7535 | 184,080 | 2.78 (2.72–2.85) | 1.41 (1.37–1.43) |
| Sepsis | 3599 | 82,046 | 2.96 (2.86–3.06) | 1.51 (1.45–1.55) |
| Multiple organ dysfunction syndrome | 1094 | 31,510 | 2.30 (2.16–2.44) | 1.17 (1.07–1.24) |
| Bacteraemia | 312 | 7850 | 2.64 (2.36–2.96) | 1.36 (1.17–1.50) |
| Systemic inflammatory response syndrome | 252 | 3189 | 5.47 (4.81–6.23) | 2.34 (2.14–2.49) |
| Urosepsis | 277 | 7014 | 2.62 (2.33–2.96) | 1.35 (1.15–1.49) |
| Neutropenic sepsis | 242 | 5447 | 2.97 (2.61–3.37) | 1.52 (1.31–1.67) |
| Pulmonary sepsis | 120 | 1105 | 7.77 (6.43–9.39) | 2.78 (2.48–3.00) |
| Escherichia sepsis | 74 | 2123 | 2.30 (1.83–2.90) | 1.16 (0.78–1.44) |
| Escherichia bacteraemia | 59 | 1456 | 2.69 (2.08–3.49) | 1.37 (0.94–1.68) |
| Systemic candida | 55 | 1466 | 2.49 (1.90–3.25) | 1.26 (0.81–1.58) |
| Device related sepsis | 46 | 1467 | 2.06 (1.54–2.77) | 1.01 (0.52–1.36) |
| Staphylococcal sepsis | 96 | 3415 | 1.84 (1.51–2.26) | 0.86 (0.52–1.10) |
| Abdominal sepsis | 29 | 637 | 3.04 (2.09–4.41) | 1.51 (0.89–1.95) |
| Post-procedural sepsis | 13 | 464 | 1.84 (1.06–3.19) | 0.82 (−0.12–1.46) |
| Streptococcal sepsis | 26 | 791 | 2.17 (1.47–3.20) | 1.06 (0.40–1.52) |
| Procalcitonin increased | 39 | 753 | 3.48 (2.52–4.81) | 1.70 (1.17–2.08) |
| Cytomegalovirus viraemia | 26 | 2448 | 0.68 (0.46–1.01) | −0.53 (−1.19−(−0.07)) |
| Blood culture positive | 12 | 1312 | 0.59 (0.33–1.04) | −0.73 (−1.71−(−0.06)) |
| Categories | Nivolumab | Pembrolizumab | Atezolizumab | Durvalumab | Ipilimumab | Nivolumab + Ipilimumab | All ICIs |
|---|---|---|---|---|---|---|---|
| Reports of Pulmonary sepsis | 73 | 9 | 13 | 2 | 13 | 10 | 120 |
| Report Year | |||||||
| 2011–2017 | 9 (12.3%) | 2 (22.2%) | 2 (15.4%) | 2 (100.0%) | 2 (15.4%) | 0 | 17 (14.2%) |
| 2018–2021 (Q3) | 64 (87.7%) | 7 (77.8%) | 11 (84.6%) | 0 | 11 (84.6%) | 10 (100.0%) | 103 (85.8%) |
| Reporter | |||||||
| Healthcare professionals | 51 (69.9%) 22 (31.1%) | 5 (55.6%) 4 (44.4%) | 13 (100.0%) 0 | 2 (100.0%) 0 | 7 (53.8%) 6 (46.2%) | 9 (90.0%) 1 (10.0%) | 83 (69.2%) 37 (30.8%) |
| Other | |||||||
| Age Category | |||||||
| 0–14 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15–24 | 2 (2.8%) | 0 | 0 | 0 | 0 | 0 | 2 (1.7%) |
| 25–65 | 35 (49.3%) | 5 (55.6%) | 5 (38.5%) | 0 | 7 (53.8%) | 5 (50.0%) | 57 (48.3%) |
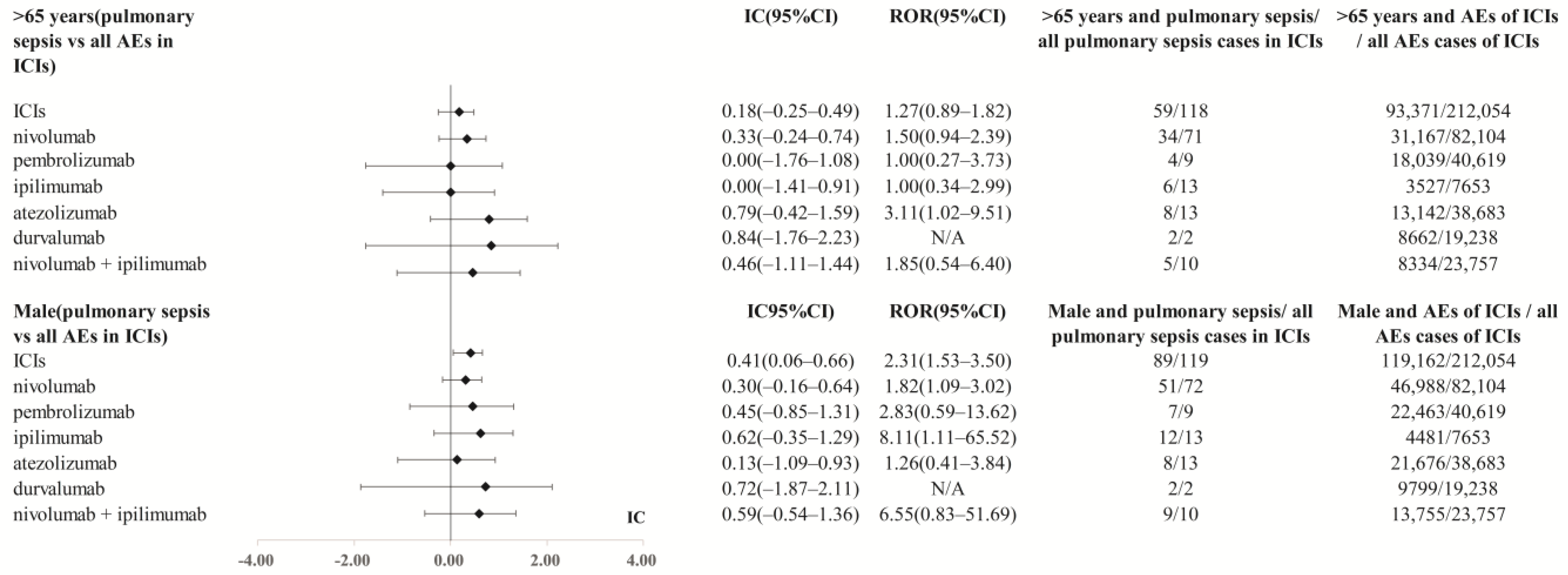
| >65 | 34 (47.9%) | 4 (44.4%) | 8 (61.5%) | 2 (100.0%) | 6 (46.2%) | 5 (50.0%) | 59 (50.0%) |
| Data available | 71 (97.3%) | 9 (100.0%) | 13 (100.0%) | 2 (100.0%) | 13 (100.0%) | 10 (100.0%) | 118 (98.3%) |
| Gender | |||||||
| Male | 51 (70.8%) | 7 (77.8%) | 8 (61.5%) | 2 (100.0%) | 12 (92.3%) | 9 (90.0%) | 89 (74.8%) |
| Female | 21 (29.2%) | 2 (22.2%) | 5 (38.5) | 0 | 1 (7.7%) | 1 (10.0%) | 30 (25.2%) |
| Data available | 72 (98.6%) | 9 (100.0%) | 13 (100.0%) | 2 (100.0%) | 13 (100.0%) | 10 (100.0%) | 119 (99.2%) |
| Indication | |||||||
| Non-small Lung cancer | 17 (23.3%) | 5 (55.6%) | 2 (15.4%) | 1 (50.0%) | 0 | 0 | 25 (19.2%) |
| Lung neoplasm malignant | 14 (19.2%) | 1 (11.1%) | 0 | 0 | 0 | 0 | 15 (12.5%) |
| Other | 42 (57.5%) | 3 (33.3%) | 11 (84.6%) | 1 (50.0%) | 13 (100.0%) | 10 (100.0%) | 80 (66.7%) |
| Co-administration drugs Glucocorticoids or corticosteroids | 28 (38.4%) | 0 | 1 (7.7%) | 2 (100.0%) | 9 (69.2%) | 8 (80.0%) | 48 (40.0%) |
| proton pump inhibitors | 26 (35.6%) | 2 (22.2%) | 4 (92.3%) | 2 (100.0%) | 5 (38.5%) | 5 (50.0%) | 44 (36.7%) |
| Outcome | |||||||
| Death | 49 (67.1%) | 6 (66.7%) | 10 (76.9%) | 2 (100.0%) | 9 (69.2%) | 6 (60.0%) | 82 (68.3%) |
| Life-threatening | 15 (20.5%) | 2 (22.2%) | 2 (15.4%) | 1 (50.0%) | 6 (46.2%) | 5 (50.0%) | 31 (25.8%) |
| Disability | 1 (1.4%) | 0 | 0 | 0 | 0 | 0 | 1 (0.8%) |
| Hospitalization | 64 (87.7%) | 9 (100.0%) | 13 (100.0%) | 2 (100.0%) | 10 (76.9%) | 8 (80.0%) | 106 (88.3%) |
| Other Serious | 71 (97.3%) | 6 (66.7%) | 1 (7.7%) | 0 | 11 (84.6%) | 10 (100.0%) | 99 (82.5%) |
| Drug 1 | Drug 2 | AEs | Ω (95%CI) |
|---|---|---|---|
| Nivolumab | Glucocorticoids or corticosteroids | Pulmonary sepsis | 1.45 (0.91–1.98) |
| Ipilimumab | Glucocorticoids or corticosteroids | Pulmonary sepsis | 1.72 (0.77–2.66) |
| Nivolumab plus ipilimumab | Glucocorticoids or corticosteroids | Pulmonary sepsis | 2.04 (1.04–3.04) |
| Nivolumab | Proton pump inhibitors | Pulmonary sepsis | 1.55 (1.00–2.11) |
| Nivolumab plus ipilimumab | Proton pump inhibitors | Pulmonary sepsis | 1.44 (0.17–2.70) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, S.; Gong, H.; Zhao, Y.; Guo, L.; Wang, Y.; Zhang, B.; Sarangdhar, M.; Noguchi, Y.; Yan, M. Association of Pulmonary Sepsis and Immune Checkpoint Inhibitors: A Pharmacovigilance Study. Cancers 2023, 15, 240. https://doi.org/10.3390/cancers15010240
Xia S, Gong H, Zhao Y, Guo L, Wang Y, Zhang B, Sarangdhar M, Noguchi Y, Yan M. Association of Pulmonary Sepsis and Immune Checkpoint Inhibitors: A Pharmacovigilance Study. Cancers. 2023; 15(1):240. https://doi.org/10.3390/cancers15010240
Chicago/Turabian StyleXia, Shuang, Hui Gong, Yichang Zhao, Lin Guo, Yikun Wang, Bikui Zhang, Mayur Sarangdhar, Yoshihiro Noguchi, and Miao Yan. 2023. "Association of Pulmonary Sepsis and Immune Checkpoint Inhibitors: A Pharmacovigilance Study" Cancers 15, no. 1: 240. https://doi.org/10.3390/cancers15010240
APA StyleXia, S., Gong, H., Zhao, Y., Guo, L., Wang, Y., Zhang, B., Sarangdhar, M., Noguchi, Y., & Yan, M. (2023). Association of Pulmonary Sepsis and Immune Checkpoint Inhibitors: A Pharmacovigilance Study. Cancers, 15(1), 240. https://doi.org/10.3390/cancers15010240









