Dynamic Prediction of Resectability for Patients with Advanced Ovarian Cancer Undergoing Neo-Adjuvant Chemotherapy: Application of Joint Model for Longitudinal CA-125 Levels
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Ethical Considerations
2.3. Data Collection
2.4. Management of Patients
2.5. Statistical Analysis
2.5.1. Fit a Linear Mixed-Effects (LME) Sub-Model
2.5.2. Fit a Cox Sub-Model
2.5.3. Fit the Joint Model
3. Results
3.1. Population Characteristics
3.2. Factors Related to Resectability
3.3. CA-125 Kinetics during NAC
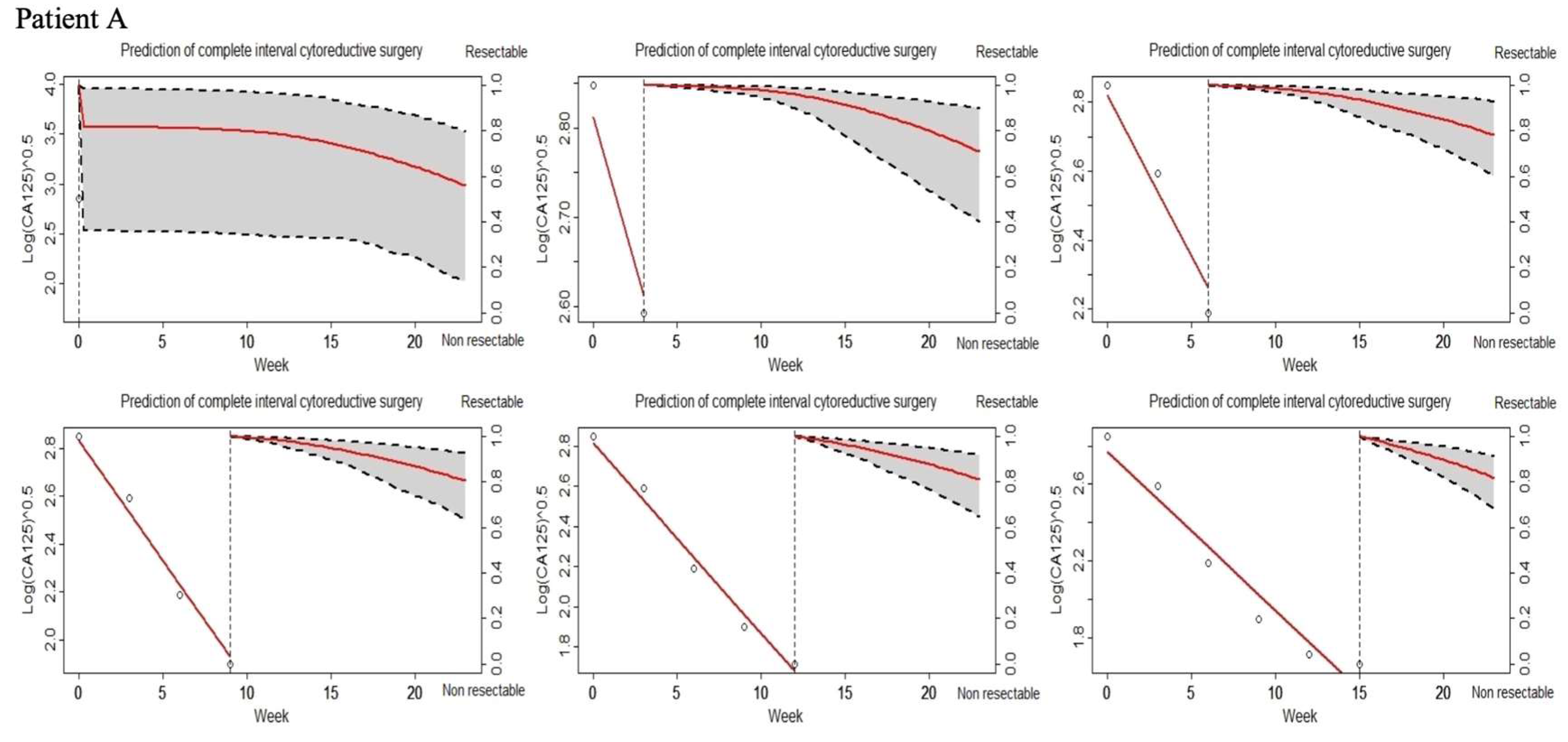
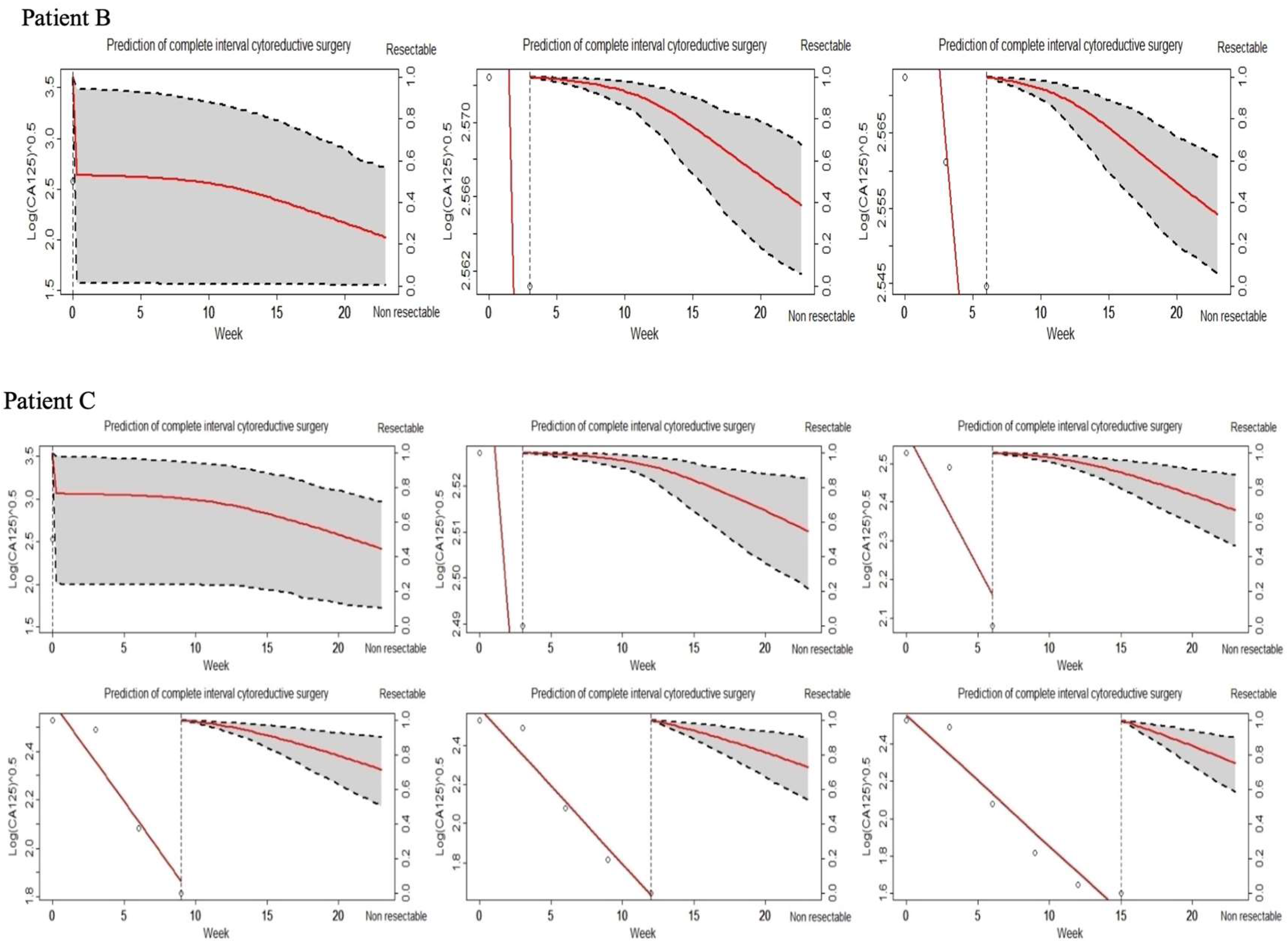
3.4. Joint Model (JM) of Longitudinal CA-125 and Tumor Resectability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kang, S.; Kim, T.-J.; Nam, B.-H.; Seo, S.-S.; Kim, B.-G.; Bae, D.-S.; Park, S.-Y. Preoperative Serum CA-125 Levels and Risk of Suboptimal Cytoreduction in Ovarian Cancer: A Meta-Analysis. J. Surg. Oncol. 2010, 101, 13–17. [Google Scholar] [CrossRef]
- Bachmann, R.; Rothmund, R.; Krämer, B.; Brucker, S.Y.; Königsrainer, A.; Königsrainer, I.; Beckert, S.; Staebler, A.; NguyenHuu, P.; Grischke, E.; et al. The Prognostic Role of Optimal Cytoreduction in Advanced, Bowel Infiltrating Ovarian Cancer. J. Investig. Surg. Off. J. Acad. Surg. Res. 2015, 28, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E. Predicting Surgical Outcome for Advanced Ovarian Cancer, Surgical Standards of Care, and the Concept of Kaizen. Gynecol. Oncol. 2009, 112, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Everett, E.N.; Heuser, C.C.; Pastore, L.M.; Anderson, W.A.; Rice, L.W.; Irvin, W.P.; Taylor, P.T. Predictors of Suboptimal Surgical Cytoreduction in Women Treated with Initial Cytoreductive Surgery for Advanced Stage Epithelial Ovarian Cancer. Am. J. Obstet. Gynecol. 2005, 193, 568–574; discussion 574–576. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Vizzielli, G.; Fanfani, F.; Costantini, B.; Ferrandina, G.; Gallotta, V.; Gueli Alletti, S.; Tortorella, L.; Scambia, G. Introduction of Staging Laparoscopy in the Management of Advanced Epithelial Ovarian, Tubal and Peritoneal Cancer: Impact on Prognosis in a Single Institution Experience. Gynecol. Oncol. 2013, 131, 341–346. [Google Scholar] [CrossRef]
- Rutten, M.J.; van de Vrie, R.; Bruining, A.; Spijkerboer, A.M.; Mol, B.W.; Kenter, G.G.; Buist, M.R. Predicting Surgical Outcome in Patients with International Federation of Gynecology and Obstetrics Stage III or IV Ovarian Cancer Using Computed Tomography: A Systematic Review of Prediction Models. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2015, 25, 407–415. [Google Scholar] [CrossRef]
- Rutten, M.J.; Leeflang, M.M.G.; Kenter, G.G.; Mol, B.W.J.; Buist, M. Laparoscopy for Diagnosing Resectability of Disease in Patients with Advanced Ovarian Cancer. Cochrane Database Syst. Rev. 2014, CD009786. [Google Scholar] [CrossRef]
- Petrillo, M.; Vizzielli, G.; Fanfani, F.; Gallotta, V.; Cosentino, F.; Chiantera, V.; Legge, F.; Carbone, V.; Scambia, G.; Fagotti, A. Definition of a Dynamic Laparoscopic Model for the Prediction of Incomplete Cytoreduction in Advanced Epithelial Ovarian Cancer: Proof of a Concept. Gynecol. Oncol. 2015, 139, 5–9. [Google Scholar] [CrossRef]
- Brun, J.-L.; Rouzier, R.; Selle, F.; Houry, S.; Uzan, S.; Daraï, E. Neoadjuvant Chemotherapy or Primary Surgery for Stage III/IV Ovarian Cancer: Contribution of Diagnostic Laparoscopy. BMC Cancer 2009, 9, 171. [Google Scholar] [CrossRef]
- Angioli, R.; Palaia, I.; Zullo, M.A.; Muzii, L.; Manci, N.; Calcagno, M.; Panici, P.B. Diagnostic Open Laparoscopy in the Management of Advanced Ovarian Cancer. Gynecol. Oncol. 2006, 100, 455–461. [Google Scholar] [CrossRef]
- Rutten, M.J.; van Meurs, H.S.; van de Vrie, R.; Gaarenstroom, K.N.; Naaktgeboren, C.A.; van Gorp, T.; Ter Brugge, H.G.; Hofhuis, W.; Schreuder, H.W.R.; Arts, H.J.G.; et al. Laparoscopy to Predict the Result of Primary Cytoreductive Surgery in Patients With Advanced Ovarian Cancer: A Randomized Controlled Trial. J. Clin. Oncol. 2017, 35, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Zorn, K.K.; Tian, C.; McGuire, W.P.; Hoskins, W.J.; Markman, M.; Muggia, F.M.; Rose, P.G.; Ozols, R.F.; Spriggs, D.; Armstrong, D.K. The Prognostic Value of Pretreatment CA 125 in Patients with Advanced Ovarian Carcinoma: A Gynecologic Oncology Group Study. Cancer 2009, 115, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Riedinger, J.M.; Wafflart, J.; Ricolleau, G.; Eche, N.; Larbre, H.; Basuyau, J.P.; Dalifard, I.; Hacene, K.; Pichon, M.F. CA 125 Half-Life and CA 125 Nadir during Induction Chemotherapy Are Independent Predictors of Epithelial Ovarian Cancer Outcome: Results of a French Multicentric Study. Ann. Oncol. 2006, 17, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Markman, M.; Zaino, R.; Ozols, R.F.; McGuire, W.P.; Muggia, F.M.; Rose, P.G.; Spriggs, D.; Armstrong, D.K. CA-125 Change after Chemotherapy in Prediction of Treatment Outcome among Advanced Mucinous and Clear Cell Epithelial Ovarian Cancers: A Gynecologic Oncology Group Study. Cancer 2009, 115, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Almufti, R.; Wilbaux, M.; Oza, A.; Henin, E.; Freyer, G.; Tod, M.; Colomban, O.; You, B. A Critical Review of the Analytical Approaches for Circulating Tumor Biomarker Kinetics during Treatment. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Ducoulombier, S.; Golfier, F.; Colomban, O.; Benayoun, D.; Bolze, P.-A.; Tod, M.; You, B. Modeling CA-125 During Neoadjuvant Chemotherapy for Predicting Optimal Cytoreduction and Relapse Risk in Ovarian Cancer. Anticancer Res. 2017, 37, 6879–6886. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilbaux, M.; Hénin, E.; Oza, A.; Colomban, O.; Pujade-Lauraine, E.; Freyer, G.; Tod, M.; You, B. Prediction of Tumour Response Induced by Chemotherapy Using Modelling of CA-125 Kinetics in Recurrent Ovarian Cancer Patients. Br. J. Cancer 2014, 110, 1517–1524. [Google Scholar] [CrossRef]
- Musoro, J.Z.; Geskus, R.B.; Zwinderman, A.H. A Joint Model for Repeated Events of Different Types and Multiple Longitudinal Outcomes with Application to a Follow-up Study of Patients after Kidney Transplant. Biom. J. 2015, 57, 185–200. [Google Scholar] [CrossRef]
- Rizopoulos, D. Dynamic Predictions and Prospective Accuracy in Joint Models for Longitudinal and Time-to-Event Data. Biometrics 2011, 67, 819–829. [Google Scholar] [CrossRef]
- Cao, Y.; Jiang, Y.; Lin, X.; Liu, J.; Lu, T.; Cheng, W.; Yan, F. Dynamic Prediction of Outcome for Patients With Ovarian Cancer: Application of a Joint Model for Longitudinal Cancer Antigen 125 Values. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2018, 28, 85–91. [Google Scholar] [CrossRef]
- Chang, C.; Chiang, A.J.; Chen, W.-A.; Chang, H.-W.; Chen, J. A Joint Model Based on Longitudinal CA125 in Ovarian Cancer to Predict Recurrence. Biomark. Med. 2016, 10, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.J.; Chen, J.; Chung, Y.-C.; Huang, H.-J.; Liou, W.S.; Chang, C. A Longitudinal Analysis with CA-125 to Predict Overall Survival in Patients with Ovarian Cancer. J. Gynecol. Oncol. 2014, 25, 51–57. [Google Scholar] [CrossRef]
- Zeppernick, F.; Meinhold-Heerlein, I. The New FIGO Staging System for Ovarian, Fallopian Tube, and Primary Peritoneal Cancer. Arch. Gynecol. Obstet. 2014, 290, 839–842. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, P.; Sugarbaker, P.H. Clinical Research Methodologies in Diagnosis and Staging of Patients with Peritoneal Carcinomatosis. Cancer Treat. Res. 1996, 82, 359–374. [Google Scholar] [PubMed]
- Moore, K. The Management of Ascites in Cirrhosis: Report on the Consensus Conference of the International Ascites Club. Hepatology 2003, 38, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Katsumata, N.; Yasuda, M.; Isonishi, S.; Takahashi, F.; Michimae, H.; Kimura, E.; Aoki, D.; Jobo, T.; Kodama, S.; Terauchi, F.; et al. Long-Term Results of Dose-Dense Paclitaxel and Carboplatin versus Conventional Paclitaxel and Carboplatin for Treatment of Advanced Epithelial Ovarian, Fallopian Tube, or Primary Peritoneal Cancer (JGOG 3016): A Randomised, Controlled, Open-Label Trial. Lancet Oncol. 2013, 14, 1020–1026. [Google Scholar] [CrossRef]
- Pomel, C.; Jeyarajah, A.; Oram, D.; Shepherd, J.; Milliken, D.; Dauplat, J.; Reynolds, K. Cytoreductive Surgery in Ovarian Cancer. Cancer Imaging 2007, 7, 210–215. [Google Scholar] [CrossRef]
- Andrinopoulou, E.-R.; Rizopoulos, D.; Jin, R.; Bogers, A.J.J.C.; Lesaffre, E.; Takkenberg, J.J.M. An Introduction to Mixed Models and Joint Modeling: Analysis of Valve Function over Time. Ann. Thorac. Surg. 2012, 93, 1765–1772. [Google Scholar] [CrossRef]
- Rizopoulos, D. The R Package JMbayes for Fitting Joint Models for Longitudinal and Time-to-Event Data Using MCMC. arXiv 2014, arXiv:1404.7625. [Google Scholar]
- Andrinopoulou, E.-R.; Eilers, P.H.C.; Takkenberg, J.J.M.; Rizopoulos, D. Improved Dynamic Predictions from Joint Models of Longitudinal and Survival Data with Time-Varying Effects Using P-Splines. Biometrics 2017, 74, 685–693. [Google Scholar] [CrossRef]
- Shen, Y.; Li, L. Serum HE4 Superior to CA125 in Predicting Poorer Surgical Outcome of Epithelial Ovarian Cancer. Tumor Biol. 2016, 37, 14765–14772. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, N.; Sasaki, Y.; Shigemitsu, A.; Akasaka, J.; Kanayama, S.; Kawaguchi, R.; Kobayashi, H. CA-125 Cut-off Value as a Predictor for Complete Interval Debulking Surgery after Neoadjuvant Chemotherapy in Patients with Advanced Ovarian Cancer. J. Gynecol. Oncol. 2013, 24, 141–145. [Google Scholar] [CrossRef][Green Version]
- Pelissier, A.; Bonneau, C.; Chéreau, E.; de La Motte Rouge, T.; Fourchotte, V.; Daraï, E.; Rouzier, R. CA125 Kinetic Parameters Predict Optimal Cytoreduction in Patients with Advanced Epithelial Ovarian Cancer Treated with Neoadjuvant Chemotherapy. Gynecol. Oncol. 2014, 135, 542–546. [Google Scholar] [CrossRef]
- Wilbaux, M.; Hénin, E.; Oza, A.; Colomban, O.; Pujade-Lauraine, E.; Freyer, G.; Tod, M.; You, B. Dynamic Modeling in Ovarian Cancer: An Original Approach Linking Early Changes in Modeled Longitudinal CA-125 Kinetics and Survival to Help Decisions in Early Drug Development. Gynecol. Oncol. 2014, 133, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Saygili, U.; Guclu, S.; Uslu, T.; Erten, O.; Dogan, E. The Effect of Ascites, Mass Volume, and Peritoneal Carcinomatosis on Serum CA125 Levels in Patients with Ovarian Carcinoma. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2002, 12, 438–442. [Google Scholar]
- Crespo Valadés, E.; Malmierca Corral, M. [Elevated CA 125 in chronic liver disease with ascites]. Gastroenterol. Hepatol. 2004, 27, 558. [Google Scholar] [CrossRef]
- Aletti, G.D.; Gostout, B.S.; Podratz, K.C.; Cliby, W.A. Ovarian Cancer Surgical Resectability: Relative Impact of Disease, Patient Status, and Surgeon. Gynecol. Oncol. 2006, 100, 33–37. [Google Scholar] [CrossRef]
- Suzuki, C.; Wallgren, H.; Abraham-Nordling, M.; Palmer, G. Preoperative CT-Based Predictive Factors for Resectability and Medium-Term Overall Survival in Patients with Peritoneal Carcinomatosis from Colorectal Cancer. Clin. Radiol. 2018, 73, 756.e11–756.e16. [Google Scholar] [CrossRef]
- Barlow, T.S.; Przybylski, M.; Schilder, J.M.; Moore, D.H.; Look, K.Y. The Utility of Presurgical CA125 to Predict Optimal Tumor Cytoreduction of Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2006, 16, 496–500. [Google Scholar] [CrossRef]
- Arits, A.H.M.M.; Stoot, J.E.G.M.; Botterweck, A.A.M.; Roumen, F.J.M.E.; Voogd, A.C. Preoperative Serum CA125 Levels Do Not Predict Suboptimal Cytoreductive Surgery in Epithelial Ovarian Cancer. Int. J. Gynecol. Cancer 2008, 18, 621–628. [Google Scholar] [CrossRef]
- Bendifallah, S.; Body, G.; Daraï, E.; Ouldamer, L. Diagnostic and prognostic value of tumor markers, scores (clinical and biological) algorithms, in front of an ovarian mass suspected of an epithelial ovarian cancer: Article drafted from the French Guidelines in oncology entitled “Initial management of patients with epithelial ovarian cancer” developed by FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY under the aegis of CNGOF and endorsed by INCa. Gynecol. Obstet. Fertil. Senol. 2019, 47, 134–154. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Robelin, P.; Tod, M.; Louvet, C.; Lotz, J.-P.; Abadie-Lacourtoisie, S.; Fabbro, M.; Desauw, C.; Bonichon-Lamichhane, N.; Kurtz, J.-E.; et al. CA-125 ELIMination Rate Constant K (KELIM) Is A Marker Of Chemosensitivity In Patients With Ovarian Cancer: Results from the Phase II CHIVA Trial. Clin. Cancer Res. 2020, 26, 4625–4632. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | Overall Population (%) | Complete Interval Cytoreductive Surgery (%) | p | |
|---|---|---|---|---|
| No n = 37 (48%) | Yes n = 40 (52%) | 0.20 | ||
| Age > 65 (years) | 40 (52) | 22 (55) | 18 (45) | 0.46 |
| BMI (kg/m2) | 24.2 [21.5–29.7] | 25.1 [21.8–29.7] | 22.6 [21.3–28.8] | 0.36 |
| PS | ||||
| 0 | 44 (57.1) | 18 (48.6) | 26 (65) | |
| 1 | 26 (33.8) | 14 (37.8) | 12 (30) | |
| 2 | 6 (7.8) | 4 (10.8) | 2 (5) | |
| 3 | 1 (1.3) | 1 (2.7) | 0 (0) | |
| Age at menopause (years), Median [IQR] | 50 [50–53] | 50.0 [50.0–53.5] | 50.0 [46.8–53.0] | 0.53 |
| Histology sub-type | 0.42 | |||
| Serous | 71 (92.2) | 33 (89.2) | 38 (95) | |
| Mixed or Undifferentiated | 6 (7.8) | 4 (10.8) | 2 (5) | |
| Tumor grade | <0.01 | |||
| I | 3 (4.6) | 3 (9.7) | 0(0) | |
| II | 49 (75.4) | 17 (54.8) | 32 (94.1) | |
| III | 13 (20) | 11 (35.5) | 2 (5.9) | |
| FIGO stage | 0.43 | |||
| IIIc | 66 (85.7) | 30 (81.1) | 36 (90) | |
| IVa | 5 (6.5) | 4 (10.8) | 1 (2.5) | |
| IVb | 6 (7.8) | 3 (8.1) | 3 (7.5) | |
| Extra-abdominal metastases | 0.58 | |||
| Extra-abdominal Lymph nodes | 4 (5.2) | 2 (5.4) | 2 (5) | |
| Liver | 2 (2.6) | 1 (2.7) | 1 (2.5) | |
| Pleural | 5 (6.5) | 4 (10.8) | 1 (2.5) | |
| CA-125 Pre-NAC (IU/mL), median [range] | 926 [23–27,350] | 1115 [23–27,350] | 848 [39–24,465] | 0.75 |
| Ascites at diagnosis | 68 (88.3) | 34 (91.9) | 34 (85) | 0.48 |
| Number of ascites punctures | 0.06 | |||
| 1 | 3 (3.9) | 1 (2.7) | 2 (5) | |
| 2 | 7 (9.1) | 6 (16.2) | 1 (2.5) | |
| 4 | 1 (1.3) | 1 (2.7) | 0 (0) | |
| 6 | 1 (1.3) | 1 (2.7) | 0 (0) | |
| PCI at diagnosis, median [range] | 21 [18–29] | 24 [18–29] | 20 [17–23] | 0.11 |
| PC location details at diagnosis | ||||
| Douglas | 67 (87) | 34 (91.9) | 33 (82.5) | 0.31 |
| Hepatic hilum or stomach | 30 (39) | 20 (54.1) | 10 (25) | <0.01 |
| Small intestine | 52 (67.5) | 28 (75.7) | 24 (60) | 0.14 |
| Large intestine | 49 (64.5) | 23 (63.9) | 26 (65) | 0.92 |
| Right diaphragmatic dome | 58 (76.3) | 31 (83.8) | 27 (69.2) | 0.14 |
| Left diaphragmatic dome | 50 (65.8) | 28 (75.7) | 22 (56.4) | 0.08 |
| NAC regimen | 1.00 | |||
| Carboplatin-paclitaxel | 73 (94.8) | 35 (94.6) | 38 (95) | |
| Carboplatin | 4 (5.2) | 2 (5.4) | 2 (5) | |
| Biotherapy | 0.22 | |||
| Bevacizumab | 4 (5.2) | 3 (8.1) | 1 (2.5) | |
| Pembrolizumab | 8 (10.4) | 3 (8.1) | 5 (12.5) | |
| TSR 042 or placebo | 3 (3.9) | 3 (8.1) | 0 (0) | |
| Cycles of NAC median [range] | 6 [3–9] | 6 [3–9] | 6 [3–7] | 0.95 |
| CA-125 < 75 after 3rd NAC | 46 (59.7) | 13 (35.1) | 33 (82.5) | <0.01 |
| Post-NAC CA-125 (IU/mL), median [range] | 35 [16–144] | 85 [35–402] | 22 [13–38] | <0.01 |
| Author (Year) | Type of Study | Endpoint | Number of Patients | CA-125 Cut-Off (IU/mL) | AUC (%)(CI) | AUC in Our Study Using Literature Cut-Off |
|---|---|---|---|---|---|---|
| Shen et al., 2016 [31] | Retrospective | ICRS | 43 | 58.58 | 66 [50–83] | 71 [62–80] |
| Furukawa et al., 2013 [32] | Retrospective | Complete and non-complete ICRS | 75 | 20 | NA | 63 [53–73] |
| Pelissier et al., 2013 [33] | Retrospective | Optimal ICRS | 148 | 75 after the 3rd NAC | 73 [62–82] | 70 [59–80] |
| Our study | Retrospective | Complete ICRS | 77 | - | 88 [82–100] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amroun, K.; Chaltiel, R.; Reyal, F.; Kianmanesh, R.; Savoye, A.-M.; Perrier, M.; Djerada, Z.; Bouché, O. Dynamic Prediction of Resectability for Patients with Advanced Ovarian Cancer Undergoing Neo-Adjuvant Chemotherapy: Application of Joint Model for Longitudinal CA-125 Levels. Cancers 2023, 15, 231. https://doi.org/10.3390/cancers15010231
Amroun K, Chaltiel R, Reyal F, Kianmanesh R, Savoye A-M, Perrier M, Djerada Z, Bouché O. Dynamic Prediction of Resectability for Patients with Advanced Ovarian Cancer Undergoing Neo-Adjuvant Chemotherapy: Application of Joint Model for Longitudinal CA-125 Levels. Cancers. 2023; 15(1):231. https://doi.org/10.3390/cancers15010231
Chicago/Turabian StyleAmroun, Koceila, Raphael Chaltiel, Fabien Reyal, Reza Kianmanesh, Aude-Marie Savoye, Marine Perrier, Zoubir Djerada, and Olivier Bouché. 2023. "Dynamic Prediction of Resectability for Patients with Advanced Ovarian Cancer Undergoing Neo-Adjuvant Chemotherapy: Application of Joint Model for Longitudinal CA-125 Levels" Cancers 15, no. 1: 231. https://doi.org/10.3390/cancers15010231
APA StyleAmroun, K., Chaltiel, R., Reyal, F., Kianmanesh, R., Savoye, A.-M., Perrier, M., Djerada, Z., & Bouché, O. (2023). Dynamic Prediction of Resectability for Patients with Advanced Ovarian Cancer Undergoing Neo-Adjuvant Chemotherapy: Application of Joint Model for Longitudinal CA-125 Levels. Cancers, 15(1), 231. https://doi.org/10.3390/cancers15010231






