Simple Summary
This study aimed to verify the prognostic value of neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) in GC patients undergoing neoadjuvant chemotherapy (NAC) and gastrectomy. Elevated NLR and PLR prior to NAC were associated with significantly higher risk of death (mOS: 36 vs. 87 months; HR = 2.21; p = 0.0255 and mOS: 30 vs. 87 months; HR = 2.89; p = 0.0034, respectively). Additionally, a significantly higher risk of death was observed in patients with elevated NLR after NAC (mOS: 35 vs. 87 months; HR = 1.94; p = 0.0368). Selected systemic inflammatory response markers (NLR, PLR) are significant prognostic factors in patients with advanced GC treated with NAC and gastrectomy, as shown in the Eastern European population.
Abstract
The prognostic value of the systemic inflammatory response markers, namely neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and platelet-to-lymphocyte ratio (PLR) has not yet been clarified in patients undergoing neoadjuvant chemotherapy (NAC) and gastrectomy for advanced gastric cancer (GC) in the Eastern European population. This study aimed to verify the prognostic value of NLR, PLR, and LMR in GC patients undergoing multimodal treatment. One hundred six GC patients undergoing NAC and gastrectomy between 2012 and 2020 were included. Analysed blood samples were obtained prior to NAC (pre-NAC group) and before surgical treatment (post-NAC group). To evaluate the prognostic value of the NLR, LMR, and PLR, univariable and multivariable overall survival (OS) analyses were performed. In the pre-NAC group, elevated NLR and PLR were associated with significantly higher risk of death (mOS: 36 vs. 87 months; HR = 2.21; p = 0.0255 and mOS: 30 vs. 87 months; HR = 2.89; p = 0.0034, respectively). Additionally, a significantly higher risk of death was observed in patients with elevated NLR in the post-NAC group (mOS: 35 vs. 87 months; HR = 1.94; p = 0.0368). Selected systemic inflammatory response markers (NLR, PLR) are significant prognostic factors in patients with advanced GC treated with NAC and gastrectomy, as shown in the Eastern European population.
1. Introduction
Gastric cancer (GC) remains a considerable burden of cancer-related mortality worldwide. However, the development of new biomarkers may improve its non-favourable outcome [1].
GC patients should undergo multidisciplinary treatment, including endoscopy, surgery, chemotherapy, and radiation therapy. In Europe, neoadjuvant chemotherapy (NAC) with a platinum and fluoropyrimidine combination is recommended for at least stage IB resectable GC patients [2]. The FLOT4 regimen (5-Fluorouracil, Leucovorin, Oxaliplatin, Docetaksel) is currently the preferred regimen for NAC, demonstrating improved survival in patients with resectable GC when compared to ECX/ECF (Epirubicin, Cisplatin, Capecitabine or 5-Fluorouracil) [3,4,5].
Personalised therapy in GC depends on the appropriate staging. Despite visible progress in computed tomography (CT) performed in specialised centres, the inadequacy of evaluating the tumour stage is a critical issue in clinical practice [6,7,8]. Staging laparoscopy enables the diagnosis of peritoneal dissemination with improved sensitivity and specificity compared to CT [7]; however, assessment of the nodal stage remains difficult [9]. There is a need for additional, cost-effective and readily available prognostic and predictive factors to select patients who may require prolonged NAC. Systemic inflammatory markers are of special interest since they are strongly correlated with the progression and response to cancer treatment [10]. These include blood count parameters, such as neutrophil, lymphocyte, monocyte and platelet number, as well as their combinations: neutrophil-to-lymphocyte ratio (NLR), lymphocyte-to-monocyte ratio (LMR), and the platelet-to-lymphocyte ratio [PLR] [11]. Among complete blood count derived markers, anaemia is recognised as an independent prognostic factor in numerous malignancies [12]. As the results of currently available studies are inconclusive, interpretation of NLR, PLR and LMR in routine practice is equivocal. Importantly, available literature provides data solely from the Asian population [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. Exploratory analysis of the REAL-2 trial revealed that a high NLR value had a significant, independent negative prognostic effect. Although the study was conducted in the Western population, it should be noted that most patients had inoperable or metastatic oesophagogastric cancer [27].
To the best of our knowledge, this is the first study that aimed to verify the prognostic value of NLR, PLR and LMR in GC patients undergoing multimodal treatment in the Eastern European population.
2. Materials and Methods
After receiving institutional review board approval (KE—0254/331/2018), we collected data from the database of patients diagnosed with GC treated with gastrectomy from 1 July 2012 to 31 July 2020 at the Department of Oncological Surgery of the Medical University of Lublin, Poland. The initial date of patients’ recruitment was set due to the standardisation of the NAC with 5-Fluorouracil and platinum derivatives, reflecting the current evidence-based clinical guidelines for GC [2,28,29]. The preoperative staging, evaluation of the patient’s general condition and treatment plan were carried out by the multidisciplinary team. A ypTNM stage of the disease was established according to the 8th edition of the American Joint Committee on Cancer (AJCC) [30]. In the present study, we included patients with histologically confirmed GC who underwent multimodal treatment based on NAC and surgical resection. We excluded patients in clinical stages I and IV, without NAC or gastrectomy and with adjuvant radiotherapy. One hundred six patients were eligible for the final analysis. The flow chart of the study is shown in Figure 1.
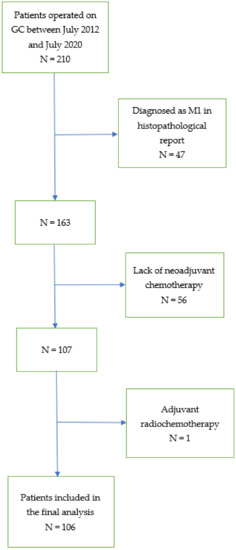
Figure 1.
Flow chart of the study. (GC—gastric cancer; M1—distant metastases; N—number of patients).
2.1. Neoadjuvant Chemotherapy (NAC)
The majority of the study group (95%) received treatment based on a combination of platinum and fluoropyrimidine derivatives. The EOX regimen included epirubicin 50 mg/m2 on the 1st day of the cycle, 130 mg/m2 oxaliplatin on the 1st day of the cycle and 625 mg/m2 of capecitabine twice a day from days 1–21; cycles were repeated every 21 days (30). Three cycles EOX before surgery and three cycles after surgery. The FLOT-4 regimen consisted of docetaxel at 50 mg/m2 on day 1, oxaliplatin at 85 mg/m2 on day 1, leucovorin at 200 mg/m2 on day 1 and 5-fluorouracil at 2600 mg/m2 on day 1 of the cycle, cycles were repeated every 14 days [4]. After 4–5 week time intervals, patients were scheduled for surgical treatment.
2.2. Complete Blood Count Based Inflammatory Response Markers
Blood samples used for analysis were obtained one day before administration of the first NAC cycle (pre-NAC group) and one day before surgical treatment (post-NAC group). The NLR was calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes in the peripheral blood. The PLR was calculated by dividing the absolute number of neutrophils by the absolute number of lymphocytes in the peripheral blood. The LMR was calculated by dividing the absolute number of lymphocytes by the absolute number of peripheral blood monocytes.
2.3. Statistical Analysis
The main outcome measured was overall survival (OS). Survival data were obtained from medical records of both hospitalisation and follow-up visits. Additionally, data from the National Health Fund were analysed, as well as personal telephone information obtained from patients and/or their families during the COVID pandemic. For this research, the observation was finalised on 1 August 2021. Statistical analysis of the data was performed using MedCalc v.15.8 (MedCalc Software, Oostende, Belgium). To assess the normality of the data distribution, the D’Agostino-Pearson test was used. Since all continuous variables had non-normal data distribution, the median was used to measure data concentration and the data spread was presented by the interquartile range and minimum-maximum range. Categorised and dichotomised variables were expressed as numbers and percentages. As an objective method of cut-off establishment, the Receiver operating characteristic (ROC) analysis was used. Determined cut-offs for pre-treatment NLR, PLR and LMR were ≤1.76, ≤132.34 and >2.75, respectively, whereas these values for the preoperative phase of POC, a day before gastrectomy were ≤1.94, ≤151.06 and >2.81, respectively. OS was defined as the time from the date of surgery to the date of the patient’s death or the date of the last follow-up. The log-rank test was used to calculate the proportional hazard ratio in univariable OS analysis (the Kaplan–Meier estimation method was used for the generation of survival curves), whereas Cox logistic regression models were used in multivariable OS analysis. Comparisons of studied ratios depending on demographic and clinical variables were performed with the use of the Mann–Whitney U test (if 2 independent groups were compared) or ANOVA Kruskal–Wallis test (if more than 2 independent groups were compared). In all analyses, we used two-tailed p-tests and results with a p-value below 0.05 were statistically significant.
3. Results
3.1. Characteristics of the Research Group
One hundred six patients were included. The majority of the study group was male (61.3%). The median age of patients was 61 years (range 38–76 years). Patients with middle (39.6%) or upper (34%) GC were predominant. According to Lauren’s classification, the intestinal type was predominant (53.8%). Patients in the ypT4 stage accounted for 13.2% of patients. Nearly half of the patients (49.5%) were node-positive (ypN1-N3b). None of the patients had distant metastases. Fifty-five patients (52%) were treated with chemotherapy according to the EOX regimen (33), whereas 32 patients (30%) according to the FLOT-4 regimen [4]. The median number of preoperative chemotherapy cycles was 4 and the postoperative cycles were 3.5. Detailed data on the characteristics of patients in terms of demographic and clinical variables are presented in Table 1.

Table 1.
Demographic and clinical characteristics of the study group.
3.2. Survival Analysis
3.2.1. Univariate Analysis
The relationship between clinical, demographic variables and systemic inflammatory response markers and the OS are presented in Table 2 and Table 3.

Table 2.
Relationship between the systemic inflammatory response markers on overall survival.

Table 3.
Influence of selected demographic and clinical variables on overall survival.
Following demographic and clinical variables were associated with a significantly higher risk of death: anatomical location in esophagogastric junction (EGJ) (mOS: 11 vs. 87 months; HR = 4.70; p = 0.0040), lymph node metastases (N1-N3b) (mOS: 29 months vs. NR; HR = 4.43; p < 0.0001), grade G3 (mOS: 33 months vs. NR; HR = 2.77; p = 0.0038) and no pathological tumour response to NAC (TRG3, TRG4) (mOS: 35 months vs. NR; HR = 6.42; p = 0.0003). On the other hand, the intestinal type was associated with a significantly lower risk of death (mOS: 87 vs. 35 months; HR = 0.51; p = 0.0394). Elevated NLR in the pre-NAC group was associated with a significantly higher risk of death (mOS: 36 vs. 87 months; HR = 2.21; p = 0.0255; Figure 2).
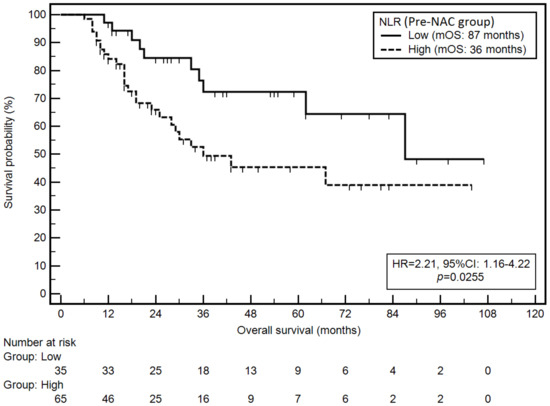
Figure 2.
Kaplan–Meier graph representing survival probability in relation to the NLR (pre-NAC group).
Similarly, elevated PLR in the pre-NAC group was associated with a significantly higher risk of death (mOS: 30 vs. 87 months; HR = 2.89; p = 0.0034; Figure 3).
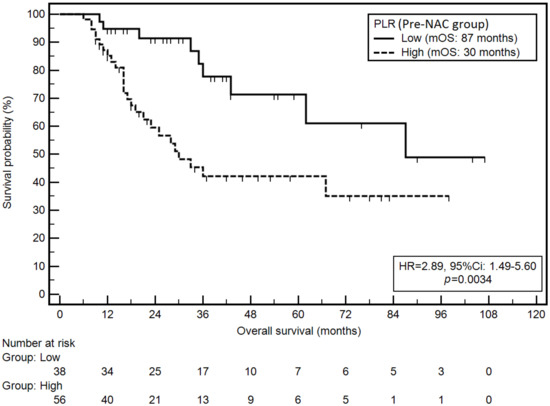
Figure 3.
Kaplan–Meier graph representing survival probability in relation to the PLR (pre-NAC group).
Moreover, high NLR in the post-NAC group was associated with a significantly higher risk of death (mOS: 35 vs. 87 months; HR = 1.94; p = 0.0368; Figure 4).
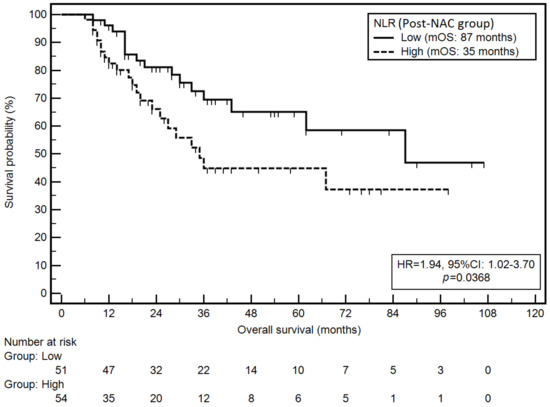
Figure 4.
Kaplan–Meier graph representing survival probability in relation to the NLR (post-NAC group).
3.2.2. Multivariate Analysis
Multivariate analysis confirmed the independent, unfavourable prognostic value of anatomical location (EGJ) (HR = 6.63; p = 0.0042), positive lymph nodes (HR = 3.94; p = 0.0009), grade G3 (HR = 2.66; p = 0.0304) and no pathological tumour response to NAC (TRG3, TRG4; HR = 5.92, p = 0.0066).
Moreover, in multivariate analysis, elevated NLR in the pre-NAC group was associated with a non-significantly higher risk of death (HR = 1.29; p = 0.5263). Whereas elevated PLR in the pre-NAC group was associated with a significantly higher risk of death (HR = 2.75; p = 0.0143). Elevated NLR in the post-NAC group was associated with a significantly higher risk of death (HR = 1.97; p = 0.0414).
3.3. Relationship of Demographic and Clinical Variables with the Systemic Inflammatory Response Markers
The age of patients was the only factor related to one of the systemic inflammatory response markers. Patients under 61 years old (median, Table 1) had significantly lower LMR compared to patients over 61 years old (Me: 3.58 vs. 2.87; p = 0.0188). There were no statistically significant relationships between other demographic and clinical variables and the systemic inflammatory response markers.
4. Discussion
This study aimed to verify the prognostic value of the systemic inflammatory response markers in patients with advanced GC treated with NAC and gastrectomy. PLR in the pre-NAC group and NLR in the post-NAC group were independent prognostic factors in patients undergoing multimodal treatment. Although tumour-related neutrophils are significant factors in promoting angiogenesis, tumour growth or metastasis, the exact correlation between higher NLR values and poor prognosis remains unclear [10,31]. Recently, Asian research confirmed PLR to be an independent prognostic factor for OS also in patients undergoing adjuvant chemoradiotherapy after radical gastrectomy [32]. Moreover, the prognostic model, including systemic inflammation, was more precise than the model without systemic inflammation.
Various original studies and meta-analyses have evaluated systemic inflammatory response markers in GC patients, as shown in Table 4.

Table 4.
Baseline characteristics of the selected studies in comparison to the results of the present study.
Overviewed studies are mostly retrospective, published between 2020–2021 and conducted in the Far East (China and Japan). Moreover, all patients were treated with NAC followed by surgical resection. Notably, the rate of NAC administration in the Asian GC population is rather low when compared with the West. A meta-analysis of 28,929 patients from 51 international cohorts (10 from Europe and the USA and 41 from Asia), evaluating the role of PLR in GC patients treated with surgery and chemotherapy, concluded high PLR is associated with a shorter OS in regard to more frequent involvement of the serosa, lymph node metastases and a higher rate of stage III/IV [25]. Elevated PLR had a high prognostic impact only in the Asian population. Furthermore, considering different treatment methods, high PLR was a significant predictor of shorter OS in patients undergoing surgery alone. However, it was of no prognostic value in patients receiving chemotherapy or combined treatment [25]. Another meta-analysis of 28 studies confirmed the association between high PLR values and unfavourable OS [26]. Twenty-one studies were conducted in Asia and seven in Europe/America. Patients with elevated PLR had significantly shorter OS.
In patients undergoing gastrectomy with curative intent, increasing evidence underlines that systemic inflammatory response, triggered by higher neutrophil and platelet levels, worsens the prognosis [11]. Mohri et al. concluded that evaluation of NLR prior to the treatment remains the crucial host-related parameter affecting prognosis in patients with GC [33], possibly due to intracellular instability through atypical cell proliferation leading to further deterioration of the cell’s environment [34]. Our study confirms the prognostic value of these markers in the Caucasian population. Elevated NLR and PLR values were associated with a higher risk of death.
A Chinese study aimed to verify the prognostic relationship between NAC and conversion rates of NLR and PLR in patients with unresectable and metastatic GC. Patients with low inflammatory response markers rates after NAC had significantly longer progression-free survival when compared to patients with sustained high-NLR/PLR [35]. In our study, patients with poor or no response to NAC (Tumour Regression Grade 3 and 4) had a significantly higher risk of death in both univariable and multivariable analyses.
Limitations of our study include its retrospective and single-centre nature and relatively small sample size. A prospective multi-centre study with a large sample size would overcome these shortcomings and possibly validate our current findings.
5. Conclusions
Selected systemic inflammatory response markers (NLR, PLR) are significant prognostic factors in patients with advanced GC treated with NAC and gastrectomy, as shown in the Eastern European population.
Author Contributions
Conceptualisation, A.P., M.S., Z.P. and K.R.-P.; methodology, R.M., K.S. and K.G.; software, R.M., K.S. and K.G.; validation, W.P.P., K.R.-P., M.K. and Z.P.; formal analysis, A.P., M.S., K.R.-P. and R.M.; investigation, B.C., M.S., A.P. and Z.P.; data curation, K.S., K.G., M.K. and B.C.; writing—original draft preparation, A.P., M.S., Z.P. and K.R.-P.; writing—review and editing, K.R.-P., W.P.P. and M.S.; visualisation, R.M., K.G. and K.S.; supervision, W.P.P.; project administration, A.P., M.S., Z.P., R.M., K.G., K.S., B.C., M.K., K.R.-P. and W.P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted after obtaining institutional review board approval (Bioethical Committee of Medical University of Lublin, Ethic Code: KE—0254/331/2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data available on special request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; Committee, E.G. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef]
- Cherny, N.I.; Dafni, U.; Bogaerts, J.; Latino, N.J.; Pentheroudakis, G.; Douillard, J.-Y.; Tabernero, J.; Zielinski, C.; Piccart, M.J.; de Vries, E.G.E. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann. Oncol. 2017, 28, 2340–2366. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.-G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Hsu, J.-S.; Wu, D.-C.; Kang, W.-Y.; Hsieh, J.-S.; Jaw, T.-S.; Wu, M.-T.; Liu, G.-C. Gastric Cancer: Preoperative Local Staging with 3D Multi-Detector Row CT—Correlation with Surgical and Histopathologic Results. Radiology 2007, 242, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Leake, P.-A.; Cardoso, R.; Seevaratnam, R.; Lourenco, L.; Helyer, L.; Mahar, A.; Law, C.; Coburn, N.G. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer 2012, 15 (Suppl. 1), S38–S47. [Google Scholar] [CrossRef] [Green Version]
- Fairweather, M.; Jajoo, K.; Sainani, N.; Bertagnolli, M.M.; Wang, J. Accuracy of EUS and CT imaging in preoperative gastric cancer staging. J. Surg. Oncol. 2015, 111, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Kwee, R.M.; Kwee, T.C. Imaging in assessing lymph node status in gastric cancer. Gastric Cancer 2009, 12, 6–22. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.J.; Salas, M.; Ward, A.; Goss, G. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer 2001, 91, 2214–2221. [Google Scholar] [CrossRef]
- Yin, X.; Fang, T.; Wang, Y.; Wang, Y.; Zhang, D.; Li, C.; Xue, Y. Prognostic significance of serum inflammation indexes in different Lauren classification of gastric cancer. Cancer Med. 2021, 10, 1103–1119. [Google Scholar] [CrossRef]
- Sun, X.-J.; Ai, L.; Feng, Y.-C. The Value of ABO Blood Group and Complete Blood Count for the Prognosis Analysis of Gastric Cancer Patients. OncoTargets Ther. 2020, 13, 4627–4633. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-X.; Lin, J.-P.; Xie, J.-W.; Wang, J.-B.; Lu, J.; Chen, Q.-Y.; Cao, L.-L.; Lin, M.; Tu, R.; Zheng, C.-H.; et al. Complete blood count-based inflammatory score (CBCS) is a novel prognostic marker for gastric cancer patients after curative resection. BMC Cancer 2020, 20, 11. [Google Scholar] [CrossRef]
- Lin, J.-X.; Wang, Z.-K.; Huang, Y.-Q.; Xie, J.-W.; Wang, J.-B.; Lu, J.; Chen, Q.-Y.; Lin, M.; Tu, R.-H.; Huang, Z.-N.; et al. Dynamic Changes in Pre- and Postoperative Levels of Inflammatory Markers and Their Effects on the Prognosis of Patients with Gastric Cancer. J. Gastrointest. Surg. 2021, 25, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.-B.; Xu, Y.; Lu, J.; Wu, Y.; Wang, J.-B.; Lin, J.-X.; Xie, J.-W.; Li, P.; Zheng, C.-H.; Huang, A.-M.; et al. Prognostic significance of combined Lymphocyte-monocyte Ratio and Tumor-associated Macrophages in Gastric Cancer Patients after Radical Resection. J. Cancer 2020, 11, 5078–5087. [Google Scholar] [CrossRef]
- Nakamura, N.; Kinami, S.; Tomita, Y.; Miyata, T.; Fujita, H.; Takamura, H.; Ueda, N.; Kosaka, T. The neutrophil/lymphocyte ratio as a predictor of successful conversion surgery for stage IV gastric cancer: A retrospective study. BMC Cancer 2020, 20, 363. [Google Scholar] [CrossRef]
- Gu, L.; Wang, M.; Cui, X.; Mo, J.; Yuan, L.; Mao, F.; Zhang, K.; Ng, D.M.; Chen, P.; Wang, D. Clinical significance of peripheral blood-derived inflammation markers in advanced gastric cancer after radical resection. BMC Surg. 2020, 20, 219. [Google Scholar] [CrossRef]
- Kudou, K.; Nakashima, Y.; Haruta, Y.; Nambara, S.; Tsuda, Y.; Kusumoto, E.; Ando, K.; Kimura, Y.; Hashimoto, K.; Yoshinaga, K.; et al. Comparison of Inflammation-Based Prognostic Scores Associated with the Prognostic Impact of Adenocarcinoma of Esophagogastric Junction and Upper Gastric Cancer. Ann. Surg. Oncol. 2021, 28, 2059–2067. [Google Scholar] [CrossRef]
- Hirahara, N.; Matsubara, T.; Fujii, Y.; Kaji, S.; Kawabata, Y.; Hyakudomi, R.; Yamamoto, T.; Taniura, T.; Tajima, Y. Comparison of the prognostic value of immunoinflammation-based biomarkers in patients with gastric cancer. Oncotarget 2020, 11, 2625–2635. [Google Scholar] [CrossRef] [PubMed]
- Toyokawa, T.; Muguruma, K.; Yoshii, M.; Tamura, T.; Sakurai, K.; Kubo, N.; Tanaka, H.; Lee, S.; Yashiro, M.; Ohira, M. Clinical significance of prognostic inflammation-based and/or nutritional markers in patients with stage III gastric cancer. BMC Cancer 2020, 20, 517. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ge, H.; Miao, Z.; Shao, S.; Shi, H.; Dong, C. Dynamic Changes in the Systemic Inflammation Response Index Predict the Outcome of Resectable Gastric Cancer Patients. Front. Oncol. 2021, 11, 577043. [Google Scholar] [CrossRef] [PubMed]
- Ohe, Y.; Fushida, S.; Yamaguchi, T.; Kinoshita, J.; Saito, H.; Okamoto, K.; Nakamura, K.; Tajima, H.; Ninomiya, I.; Ohta, T. Peripheral Blood Platelet-Lymphocyte Ratio Is Good Predictor of Chemosensitivity and Prognosis in Gastric Cancer Patients. Cancer Manag. Res. 2020, 12, 1303–1311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Zhao, W.; Yu, Y.; Qi, X.; Song, L.; Zhang, C.; Li, G.; Yang, L. Clinicopathological and prognostic significance of platelet-lymphocyte ratio (PLR) in gastric cancer: An updated meta-analysis. World J. Surg. Oncol. 2020, 18, 191. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Yao, X.; Cen, D.; Zhi, Y.; Zhu, N.; Xu, L. The prognostic role of platelet-to-lymphocyte ratio on overall survival in gastric cancer: A systematic review and meta-analysis. BMC Gastroenterol. 2020, 20, 16. [Google Scholar] [CrossRef] [Green Version]
- Grenader, T.; Waddell, T.; Peckitt, C.; Oates, J.; Starling, N.; Cunningham, D.; Bridgewater, J. Prognostic value of neutrophil-to-lymphocyte ratio in advanced oesophago-gastric cancer: Exploratory analysis of the REAL-2 trial. Ann. Oncol. 2016, 27, 687–692. [Google Scholar] [CrossRef]
- Cunningham, D.; Starling, N.; Rao, S.; Iveson, T.; Nicolson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R. Capecitabine and Oxaliplatin for Advanced Esophagogastric Cancer. N Engl. J. Med. 2008, 358, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Ychou, M.; Boige, V.; Pignon, J.-P.; Conroy, T.; Bouché, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.-M.; Saint-Aubert, B.; et al. Perioperative Chemotherapy Compared with Surgery Alone for Resectable Gastroesophageal Adenocarcinoma: An FNCLCC and FFCD Multicenter Phase III Trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef]
- In, H.; Solsky, I.; Palis, B.; Langdon-Embry, M.; Ajani, J.A.; Sano, T. Validation of the 8th Edition of the AJCC TNM Staging System for Gastric Cancer using the National Cancer Database. Ann. Surg. Oncol. 2017, 24, 3683–3691. [Google Scholar] [CrossRef]
- McMillan, D.C. Systemic inflammation, nutritional status and survival in patients with cancer. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, W.-B.; Yang, L.; Wang, Q.-Y.; Dai, J.; Xia, L.; Peng, J.; Zhou, F.-X.; Wei, Y.-C.; Shi, H.-P. The combination of body composition conditions and systemic inflammatory markers has prognostic value for patients with gastric cancer treated with adjuvant chemoradiotherapy. Nutrition 2022, 93, 111464. [Google Scholar] [CrossRef] [PubMed]
- Mohri, Y.; Tanaka, K.; Ohi, M.; Yokoe, T.; Miki, C.; Kusunoki, M. Prognostic Significance of Host- and Tumor-Related Factors in Patients with Gastric Cancer. World J. Surg. 2010, 34, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, J.; Chen, X. Lymphocyte-to-Monocyte Ratio is Associated with the Poor Prognosis of Breast Cancer Patients Receiving Neoadjuvant Chemotherapy. Cancer Manag. Res. 2021, 13, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Z.-Y.; Xia, Y.-Y.; Zhou, C.; Shen, X.-M.; Li, X.-L.; Han, S.-G.; Zheng, Y.; Mao, Z.-Q.; Gong, F.-R.; et al. Changes in neutrophil/lymphocyte and platelet/lymphocyte ratios after chemotherapy correlate with chemotherapy response and prediction of prognosis in patients with unresectable gastric cancer. Oncol. Lett. 2015, 10, 3411–3418. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).