Simple Summary
Despite BMI’s wide use as a measure of body size and its clinical and epidemiological utility, it measures weight and height without differentiating between adipose tissue and skeletal muscle. To overcome these limitations, we analyzed the third lumbar (L3) computed tomography (CT) images of 350 breast cancer patients to measure the areas of adipose tissue and five-level skeletal muscle components and assessed their relationships with triple-negative breast cancer (TNBC), an aggressive subtype leading to higher mortality. We found that higher areas of adipose tissues were associated with an increased likelihood of TNBC subtype, especially in premenopausal women. Since the risk factors associated with adiposity and skeletal muscles are modifiable, such as healthy diets, resistance training, and adequate protein intake, it is expected that a better understanding of this body composition component will lead to novel prevention and management strategies for TNBC.
Abstract
Obesity measured by anthropometrics is associated with increased risk of triple-negative breast cancer (TNBC). It is unclear to what extent specific adipose tissue components, aside from muscle, are associated with TNBC. This retrospective study included 350 breast cancer patients who received treatment between October 2011 and April 2020 with archived abdominal or pelvic computed tomography (CT) images. We measured the areas of adipose tissue and five-density levels of skeletal muscle on patients’ third lumbar vertebra (L3) image. Logistic regression was performed to examine the associations of specific adiposity and skeletal muscles components and a four-category body composition phenotype with the TNBC subtype. Results showed that higher vs. lower areas (3rd vs. 1st tertiles) of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) were associated with increased odds of TNBC vs. non-TNBC after adjusting for age, race, stage, tumor grade, tumor size, and skeletal muscle areas (adjusted odds ratio [AOR], 11.25 [95% CI = 3.46–36.52]) and (AOR, 10.34 [95% CI = 2.90–36.90]) respectively. Higher areas of low density muscle was also associated with increased odds of TNBC (AOR, 3.15 [95% CI = 1.05–10.98]). Compared to normal body composition (low adipose tissue/high muscle), high adiposity/high muscle was associated with higher odds of TNBC (AOR, 5.54 [95% CI = 2.12–14.7]). These associations were mainly in premenopausal women and among patients with the CT performed after breast cancer surgery. Specific adipose tissue and low-density muscle can be associated with the TNBC subtype in breast cancer patients. The direction of association warrants confirmation by prospective studies.
1. Introduction
Breast cancer is the most common cancer diagnosis worldwide in women [1]. In the United States, breast cancer accounts for 30% of all female cancer cases [2]. Among molecular subtypes based on protein expression status, triple-negative breast cancer (TNBC), i.e., lack of expression in estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), accounts for up to 15% of breast cancer cases [1]. TNBCs are generally more aggressive, result in poorer prognosis, and are more likely to metastasize into the liver and lungs compared to other subtypes [3].
Epidemiological studies indicate that obesity, as measured by body mass index (BMI), is positively associated with increased risk of TNBC [4,5,6]. However, the association between body size, which is in part measured by BMI, and risk of TNBC appears to differ between premenopausal and postmenopausal women. In postmenopausal women, most studies have found no association [7,8,9,10] or a reduced risk with increased BMI and body fatness [11,12]. However, in a systematic review and meta-analysis, premenopausal women with BMI ≥ 30 kg/m2 had a higher risk of developing TNBC compared to non-obese women [4]. Also, in premenopausal women, an increase in abdominal adiposity assessed with waist circumference, hip circumference, and waist-to-hip ratio (WHR) has been shown to increase the risk of TNBC subtype [12,13].
While BMI is commonly used as a measure of body size and has excellent clinical and epidemiological utility, limitations include the measurement of weight and height without differentiating between adipose and muscle tissue as well as other components in the body. Low BMI may mask excess adipose tissue, and high BMI may mask high skeletal muscle mass [14]. To overcome these limitations, recent studies have recommended the use of computed tomography (CT) image-assessed adiposity and skeletal muscle density as a measure of body composition [15,16,17], a technique that is now recognized as the gold standard in measuring body composition [18]. With semi-automated programming, one can accurately measure the areas of subcutaneous adipose tissue (SAT), visceral adipose tissue (VAT), intramuscular adipose tissue (IMAT), and various densities of skeletal muscles in a single CT slice in the abdominal area [19].
To our knowledge, no study has yet examined the association between CT-assessed body composition and TNBC subtype (versus non-TNBC subtype) in breast cancer patients. To address this knowledge gap, we used clinically acquired CT scans to investigate the relationship between adiposity, skeletal muscle densities, and TNBC subtype in a sample of breast cancer patients. We also hypothesized that the link between CT-assessed body composition and TNBC subtype (versus non-TNBC subtype) differed between premenopausal women and postmenopausal women.
2. Methods
2.1. Patient Population
Women diagnosed with breast cancer and received breast cancer treatment at the University of Florida Health Shands Hospital from October 2011 to April 2020, were identified through the local tumor registry linked to an electronic medical record (EMR) system. Eligible participants were women aged 20–75 years at diagnosis and with an abdominal or pelvic CT, including positron emission tomography-computed tomography (PET-CT) scan. In our sample, the majority (78.5%) of scans were taken after the breast cancer diagnosis, consistent with the fact that CT scans are prescribed for staging breast cancer. For patients with multiple CT images, the priority was to select a scan prior to and closest to breast cancer surgery. We excluded patients with a history of diabetes because diabetes and its treatment may influence obesity-related signaling pathways [20,21]. A total of 576 breast cancer patients had at least one recorded CT image. Out of these, we excluded breast cancer patients without an identifiable image at the 3rd lumbar vertebra (L3) (n = 73), without an eligible L3 CT image, i.e., unclear, incomplete, or distorted images (n = 50), and those with no information on hormone receptor status (n = 103), resulting in a final sample of 350 (Figure 1). Among the sample, 121 (35.2%) of women had body composition assessed pre-surgery, the average time from their CT scan to surgery was 15.20 ± 16.8 months. 223 (64.8%) women had body composition assessed post-surgery, and the average time from surgery to CT was 20.70 ± 20.7 months. The protocol was approved by the Institutional Review Board of the University of Florida.
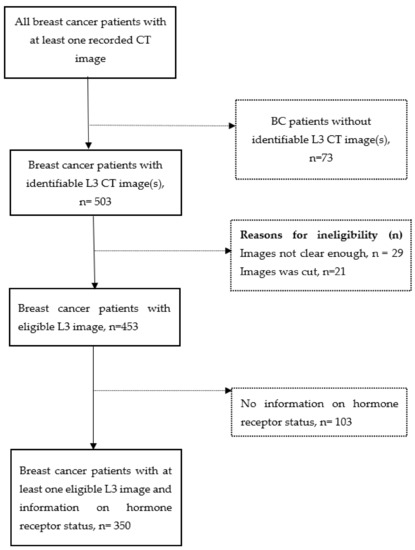
Figure 1.
Participants selection diagram. Dash line arrow and dash line square indicate exclusion. Participants were excluded if they had missing data on either estrogen receptor status, progesterone receptor status, or human epidermal growth factor receptor 2 status.
2.2. Body Composition Measurements
A single trained investigator with the guidance of two radiologists analyzed the CT images using Sliceomatic version 5.0 revision 7 (Tomovision, Montreal, QC, Canada). Adipose tissues were identified within and around the subcutaneous region, skin, inter-muscular fat, marrow, and fat around organs [22]. We used Hounsfield unit (HU) thresholds, representing the physical properties of tissues expressed in numerical form, to further segment the different types of adipose and muscle tissues. A protocol for CT image annotation was developed using their HU ranges which were keyed into a script to aid in the automaticity of segmentation and annotation of the L3 images. Areas from single-slice abdominal images at the L3 vertebra are strongly correlated with whole-body volumes of skeletal muscles and adipose tissues and have been reported in previous studies [23]. Adipose tissues comprise SAT, IMAT, and VAT. SAT was selected by limiting the measurements to the lower attenuation of −190 HU and the upper limit of −30 HU. IMAT and VAT were limited to the HU, ranging from −190 HU to −30 HU and 150 HU to −50 HU, respectively. Total Adipose tissue (TAT) area was calculated as the summation of SAT, IMAT, and VAT areas.
The skeletal muscles component of body composition was divided based on their densities. They were classified into very-low-density muscles (VLDM), low-density muscle (LDM), normal density muscle (NDM), high-density muscle (HDM), and very high-density muscle (VHDM). VLDM, LDM, and NDM were limited with the range of −29 to 0 HU, 0 to 35 HU, and 35 to 101 HU, respectively. The range for HDM was 101–151 HU, and 151 to 200 HU was calibrated as the range for VHDM. Total skeletal muscle (TSM) area was calculated as the summation of all muscle areas of the five levels of density.
2.3. Triple-Negative Breast Cancer Subtype
Information on tumor characteristics such as ER status, PR status, and HER2 status was retrieved from the local tumor registry or patients’ EMR. TNBC subtype was computed from ER, PR, and HER2 statuses.
2.4. Covariates Assessment
Demographic information on age at diagnosis, race, weight, and height was collected from EMR. If multiple data points on weight and height were available, the measurements that were closest to breast cancer diagnosis were selected. Tumor characteristics included in the dataset were tumor stage, grade, tumor size, tumor extension, lymph node status, and recurrence. Time of breast cancer diagnosis, surgery, and CT scans were also extracted from EMR.
2.5. Statistical Analysis
We first examined the relationship between patient, tumor, and body composition characteristics and TNBC subtype through chi-square analysis. Patients were grouped based on the tertile of each adiposity and skeletal muscle component. In addition, we categorized patients into four mutually exclusive phenotypes based on the levels of TAT and TSM. These groups were (i) normal (high muscle/low adiposity), i.e., highest two tertiles of TSM and lowest two tertiles of TAT; (ii) high muscle/high adiposity, i.e., highest tertile of TAT and highest two tertiles of TSM (iii) low adiposity/low muscle, i.e., lowest two tertiles of TAT and lowest tertile of TSM, and (iv) high adiposity/low muscle, i.e., 3rd tertile TAT and 1st tertile TSM [24]. To examine the associations of adiposity and skeletal muscle areas (the exposure variables) with TNBC subtype (the outcome variable), we first performed a univariate logistic regression, i.e., Model 1, analysis to calculate the unadjusted odds ratio (OR) and 95% confidence intervals (CIs). We then performed a multivariable logistic regression adjusting for age (<50 y, ≥50 y), race (White, Black American), tumor stage (0, I, II, III, IV), and tumor size (<2 cm, ≥2 cm), i.e., Model 2. In a separate model, in addition to the previously adjusted covariates, we adjusted for TAT (in tertiles) when modeling skeletal muscles and for TSM (in tertiles) when modeling adiposity, i.e., Model 3. Also, we stratified the study participants into premenopausal (age < 55 years) and postmenopausal (age ≥ 55 years) women to examine the association between body composition and TNBC subtype. A stratified analysis was also performed on CT scans taken before and after breast cancer surgery because body composition based on a CT scan taken before surgery was more consistent with the temporality compared to that taken after surgery. All statistical analyses were performed using SAS software, Version 9.4 (SAS Institute Inc., Cary, NC, USA, 2017) [25].
3. Results
Our study participants consisted of 350 breast cancer patients who had data on hormone receptor status; 24.6% of these participants received a diagnosis of TNBC. Overall, the mean age of the participants was 58.5 ± 9.6. The prevalence of obesity and overweight were 34.7% and 33.3% respectively. Patients with non-TNBC subtypes had higher proportion of obesity (BMI > 30 kg/m2) than those with TNBC subtype (34.7% vs. 18.4%; p = 0.027; Table 1). A majority of the study participants were White (86.0%); the proportion of Blacks in patients with the TNBC subtype (19.8%) was higher than those without TNBC (12.1%). Patients with TNBC vs. those with non-TNBC had a higher percentage of stage III/IV diseases (30.1% vs. 15.1%), grade III tumors (85.9% vs. 42.8%), and larger tumors (84.0% vs. 32.0%) (all p < 0.05).

Table 1.
Patient and tumor characteristics by Triple-Negative Breast Cancer (TNBC) subtype (N = 350).
Regarding body composition, the distributions of visceral adipose tissue, total adipose tissue, and total skeletal muscle in tertiles were significantly different in participants with TNBC subtype compared to those without TNBC subtype (p < 0.05). Body composition categories were significantly different between participants with and without TNBC subtype (p = 0.037); the proportion of individuals with the high adiposity/low muscle phenotype was 4.9% in TNBC patients and 7.1% in non-TNBC patients (Table 1).
Table 2 shows the regression analysis results for the associations between specific body composition components and the TNBC subtype. In the unadjusted model, high (T3) vs. low (T1) SAT was not associated with TNBC subtype (OR = 1.28 [95% CI = 0.71–2.31], Model 1). In the adjusted model, high vs. low of SAT was associated with higher odds of TNBC subtype after adjusting for age, race, tumor stage, tumor grade, and tumor size (adjusted odds ratio [AOR], 6.10 [95% CI = 1.93–19.32], Model 2). The association was stronger after additionally adjusting for TSM areas (AOR, 10.34 [95% CI = 2.90–36.90], Model 3). The associations were similar for VAT (AOR, 11.25 [95% CI = 3.46–36.25], Model 3) and TAT (AOR = 3.99 [95% CI = 1.37–11.68], Model 3). Also, middle vs. low VAT was associated with higher odds of TNBC subtype after adjusting for age, race, tumor stage, tumor grade, and tumor size (adjusted odds ratio [AOR], 6.72 [95% CI = 2.12–21.36], Model 2); this association was stronger after further adjusting for TSMFor skeletal muscle with different densities, high vs. low LDM (AOR = 4.25 [95% CI = 1.05–10.38], Model 3) was associated with increased odds of TNBC subtype. Also, middle vs. low LDM was associated with higher odds of TNBC subtype (AOR = 3.35 [95% CI = 1.11–10.10], Model 3). However, high vs. low TSM was not associated with TNBC subtype (AOR = 0.75 [95% CI = 0.29–1.96], Model 3).

Table 2.
Odds ratio (95% CI) for the association between specific body composition components and TNBC subtype.
For Table 3 and Table 4, participants were stratified into premenopausal and postmenopausal women, and the association between their body composition and TNBC subtype was assessed. In premenopausal women, the unadjusted model showed that high vs. low SAT (OR = 3.30 [95% CI = 1.25–8.74], Model 1), IMAT (OR = 3.82 [95% CI = 1.17–12.44], Model 1), VAT (OR = 4.16 [95% CI = 1.46–11.88], Model 1), and TAT (OR = 4.44 [95% CI = 1.53–12.90], Model 1) were associated with higher odds of TNBC subtype (Table 3). In the adjusted model, middle vs. low VAT (OR = 213 [95% CI = 14.26–603.78], model 2) and TAT (OR = 21.11 [95% CI = 2.56–174.08], model 2) were associated with increased odds of TNBC subtype. Similarly, high vs. low SAT (OR = 32.90 [95% CI= 7.27–148.98], model 2), VAT (OR = 59.91 [95% CI = 6.85–524.19], model 2), and TAT (OR = 108.64 [95% CI = 10.40–442.75], model 2) were associated with higher odds of TNBC subtype. The association was stronger after adjusting for age, race, tumor stage, tumor grade, tumor size, and TSM. Among different densities of muscle tissue, high vs. low VLDM (OR = 3.10 [95% CI = 1.14–8.47], model 1) and middle vs. low TSM (OR = 4.02 [1.27–12.71], model 1) were associated with higher odds of TNBC subtype. Also, high vs. low VLDM (AOR = 7.62 [95% CI = 1.40–41.49], Model 2), middle vs. low NDM (OR = 6.15 [95% CI = 1.16–32.75], model 2), and middle vs. low TSM (OR = 6.15 [95% CI = 1.13–33.34], model 2) were associated with higher odds of TNBC after adjusting for covariates, but there was no association after further adjusting for TAT. In postmenopausal women, adipose tissues and skeletal muscles were not associated with the TNBC subtype (Table 4).

Table 3.
Odds ratio (95% CI) for the association between specific body composition components and TNBC subtype among premenopausal women (<55 years).

Table 4.
Odds ratio (95% CI) for the association between specific body composition components and TNBC subtype among postmenopausal women (≥55 years).
Compared to normal body composition (low adipose tissue/high muscle), high adiposity/high muscle was associated with higher odds of TNBC subtype (OR = 2.71 [95% CI = 1.33–5.51], Model 1] (Table 5); this association was more pronounced after adjusting for age, race, tumor stage, tumor grade, tumor size (AOR = 5.54 [95% CI = 2.12–14.7], Model 2). A similar observation was made in premenopausal women. In premenopausal women, high adiposity/high muscle was associated with higher odds of TNBC subtype, compared to normal body composition (AOR = 20.92 [95% CI = 4.46–166.65], Model 2). In postmenopausal women, adipose tissues and skeletal muscles were not associated with the TNBC subtype.

Table 5.
Four-category of body composition phenotype in relation to TNBC subtype.
We stratified the analyses by the time of CT in relation to surgery, i.e., CT scans taken before or after surgery. For patients with CT scans taken before surgery, specific body composition components were not associated with the TNBC subtype (Table S1). For those with scans taken after surgery, high vs. low SAT was associated with higher odds of TNBC subtype, (OR = 3.13 [95% CI = 1.37–7.14], Model 1) (Table S2). Also, high vs. low IMAT and VAT were associated with increased odds of TNBC subtype (OR = 3.31 [95% CI = 1.42–7.72], Model 1) and (OR = 5.59 [95% CI = 2.39–13.10], respectively. In addition, middle vs. low VAT was associated with increased of TNBC subtype (OR = 3.75 [1.68–8.37], model 1). These associations were more pronounced after adjusting for age, race, tumor stage, tumor grade, tumor size, and TSM. For skeletal muscle tissues, VLDM (OR = 7.58 [95% CI = 1.54–37.25]) was positively associated with TNBC subtype, but NDM (AOR = 0.16 [95% CI = 0.04–0.74], model 3) and TSM (AOR = 0.24 [95% CI = 0.06–0.98], Model 3) were inversely associated with the TNBC subtype, after adjusting for age, race, tumor stage, tumor grade, tumor size, and TSM. In addition, middle vs. low LDM was associated with increased odds of TNBC subtype (AOR = 7.46 [1.83–30.34], model 2). However, the 4-body composition phenotypes were not associated with TNBC after stratifying by the time of CT in relation to surgery (Table S3).
4. Discussion
To our knowledge, this is the first study to examine the associations of CT-assessed adiposity and skeletal muscles with TNBC subtype in breast cancer patients. Higher vs. lower areas (3rd vs. 1st tertiles) of VAT, SAT, and TAT were associated with increased odds of TNBC after adjusting for age, race, tumor stage, tumor grade, and tumor size. Compared to normal body composition (low adiposity/high muscle), high adiposity was associated with a higher odds of TNBC subtype. The associations were mainly in premenopausal women but not in postmenopausal women.
Optimal measurement of adipose tissue would require both the amount and sites of disposition of the adipose tissue [26]. Our study quantified SAT, VAT, and IMAT separately, as VAT has been seen to be more metabolically active than SAT due to its high lipolytic activity and release of large amounts of free fatty acids [27,28]. However, most studies examining the association between body size and TNBC subtype have used BMI and waist circumference measurements, which do not differentiate between adipose tissues and skeletal muscles components. CT is a highly accurate method for measuring fat distribution and skeletal muscle mass due to its ability to provide cross-sectional images which can be compartmentalized [23]. In this study, high vs. low SAT and VAT were found to be associated with the TNBC subtype. Although no previous study has been done on CT-assessed adiposity-TNBC association, research reported that higher CT-assessed abdominal distribution of VAT was associated with elevated odds of ER-negative, PR-negative, and HER2 negative breast cancers compared to ER, PR, and HER2 positive breast cancers [29]. In a population-based case-control study, there was an increased risk of developing TNBC in women with higher WHR [30]. In a case-only analysis, women with TNBC were more likely to be obese compared to those with non-TNBC subtypes [31].
Mechanisms directly linking SAT and VAT to TNBC subtype are unclear. However, studies identifying the potential mechanistic links between obesity and TNBC subtypes have suggested some mechanisms. First, obesity-mediated inflammatory cytokines, such as leptin, and activation pathways that stimulate invasion and metastasis have been suggested [32]. Circulating levels of insulin and leptin are high in obesity [33]. Insulin stimulates the overexpression of leptin which sets up an autocrine loop to stimulate breast cancer cell growth [32]. Also, the activation of mammalian target of rapamycin (mTOR) by insulin has been seen as a predictor of poor prognosis in women with TNBC [34,35,36]. Moreover, mTOR promotes a switch from mitochondrial respiration to aerobic respiration, i.e., the Warburg effect [37,38]. The Warburg effect increases glucose uptake that supplies anabolic precursors for the rapid growth of TNBCs through mitochondrial dysfunction [33]. Also, ethyl-CpG-binding domain protein 2 (MBD2) may play a role in the tumor initiation capacity in inoculated TNBC cell lines [39].
We also assessed the association between 4 mutually exclusive body composition phenotypes. We found that compared to normal body composition (low adiposity/high muscle), high adiposity was associated with higher odds of TNBC subtype. The distribution of the four body composition phenotypes was similar between our study and the previous studies of colorectal cancer patients and healthy individuals [24,40]. With the same phenotype classification, colorectal cancer patients with the high adiposity/low muscle phenotype had a 64% higher risk of overall mortality compared to those with adequate muscle and lower adiposity phenotype, signifying the importance of body composition phenotypes in assessing cancer survival [24]. However, there is no report on the association between these four phenotypes and survival in breast cancer.
In our study, the associations between adiposity and TNBC subtype were mainly seen in premenopausal women but not postmenopausal women. In premenopausal women, high vs. low SAT, IMAT, and VAT were associated with higher odds of TNBC subtype. This is in line with the findings of two large case-control studies [4,41] and a consortium of African American/Black women [12] that larger vs. smaller body size measured with WHR was associated with increased risk of TNBC among premenopausal women. We, however, found no association between adiposity and TNBC subtype in postmenopausal women. Menopausal status may be a factor mitigating the effect of body size on the incidence of TNBC [4]. Postmenopausal women often present with less aggressive phenotypes and estrogen-dependent lesions, most likely resulting from the production of steroidal hormones from adipocytes [42]. On the contrary, premenopausal women often present with more aggressive phenotypes (large size, high tumor grade, and high proliferation rate) and hormone-independent lesions [42,43].
In our sample of breast cancer patients, TSM area was not associated with TNBC subtype; however, high vs. low LDM was associated with TNBC subtype. Also in premenopausal women, high vs. low HDM was associated decreased odds of TNBC subtype. In spite of the lack of studies investigating the association between skeletal muscle areas and TNBC subtype, 6 previous studies [14,44,45,46,47,48] have assessed the association between skeletal muscle areas and survival, 3 in metastatic disease [45,46,47] and 3 in non-metastatic disease [14,44,48]. Only one study did not show an increased risk of death among breast cancer patients with low muscle mass [47]. In our study, when assessing the association between adipose tissues and TNBC subtype, further adjusting for TSM in a multivariable model increased the odds ratio of TNBC. These findings indicate that skeletal muscle areas may be an important confounder of the adiposity-TNBC relationship and should be considered in study design and analyses.
Limitations of this study include its limited generalizability beyond breast cancer patients who did not have a CT scan, the use of cross-sectional design, and relatively small sample size in the stratified analyses. CT images were obtained based on availability, so a high proportion of CT scans were taken after breast cancer surgery. When we stratified the analysis by the time of CT scan taken, i.e., pre-or post-surgery, we found that the associations were mainly in scans taken after the surgery. The reason for the discrepancy is not clear. One possibility for this observation may be due to the relatively small sample size in the group that had scans before surgery. In our post-hoc analysis of sample size, for the four-categories body composition, comparing high adiposity/low muscle group to normal group, one would need 34 TNBC cases and 104 controls to have sufficient power for the effect overall (effect size = 4.87, alpha = 0.05, power = 80%) and 31 cases and 128 controls for the group that had scans before surgery for the overall effect of (effect size = 3.13, alpha = 0.05, power = 80%). It is also unclear if the pre-surgery scans were taken in the window that can influence breast cancer subtypes. After surgery, cancer patients are usually less mobile and less physically active, which may lead to fat accumulation and skeletal muscle loss. Also, our data lacked information on chemotherapy as well as anti-lipid therapy, both of which may influence body composition. Patients under statin therapy seem to be more vulnerable to the aging-associated lowering of fat-free mass in a prospective cohort study [49]. In a randomized controlled trial, statins decreased HUs of epicardial adipose tissues over one year, although there was no change in subcutaneous adipose tissue HUs [50]. Despite these limitations, we used L3 images from CT scans which provide more details on the extent and location of adipose tissues and muscle mass, all of which provide different profiles of metabolic markers that may be associated with the TNBC subtype.
5. Conclusions
We demonstrate that adiposity measured by L3 CT images is associated with the TNBC subtype, especially in premenopausal breast cancer patients. This finding supports the need for prospective studies with larger sample sizes and non-breast cancer controls evaluating body composition and risk of TNBC. As adiposity and muscle mass are modifiable risk factors in cancer patients, e.g., diet-induced weight-loss interventions, resistance training, and adequate protein intake, a better understanding of these body composition components may lead to novel preventive strategies and contribute to the management of TNBC.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14071846/s1, Table S1: Associations of computed tomography image-assessed adiposity and skeletal muscles with triple-negative breast cancer; Table S2: Associations of computed tomography image-assessed adiposity and skeletal muscles with triple-negative breast cancer; Table S3: Associations of computed tomography image-assessed adiposity and skeletal muscles with triple-negative breast cancer.
Author Contributions
L.A.-P. conceptualized and designed the study, analyzed images and data, and drafted the manuscript. T.-Y.D.C. conceptualized and designed the study, edited the manuscript, and provided the funding. J.B. acquired data and assisted in study design. D.R.G., M.H. and C.L. provided essential materials and assisted in image analysis. S.M.F., S.M. and A.J. assisted in data acquisition and interpretation. All authors have read and agreed to the published version of the manuscript.
Funding
Research reported in this publication was supported by NIH/NCI K07CA201334 and R37CA248371 and the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1 TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Institutional Review Board Statement
All procedures performed in studies involving human participants were in accordance with ethical standards of the Institutional Review Board of the University of Florida (reference number: IRB201800102) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The requirement of consent was waived by the Institutional Review Board of the University of Florida.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will not be available to the public to protect patient privacy.
Acknowledgments
We would like to acknowledge the Integrated Data Repository team, part of the Clinical and Translational Institute (CTSI) at the University of Florida, for assisting this project.
Conflicts of Interest
The authors declare that they have no competing interest.
References
- American Cancer Society. Cancer Facts and Statistics. Available online: https://www.cancer.org/research/cancer-facts-statistics.html (accessed on 20 May 2021).
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Miller, K.D.; Sauer, A.G.; Jemal, A.; Siegel, R.L. Breast Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; López-Tarruella, S.; García-Saenz, J.A.; Khan, Q.J.; Gómez, H.L.; Prat, A.; Moreno, F.; Jerez-Gilarranz, Y.; Barnadas, A.; Picornell, A.C.; et al. Pathological Response and Survival in Triple-Negative Breast Cancer Following Neoadjuvant Carboplatin plus Docetaxel. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 5820–5829. [Google Scholar] [CrossRef] [PubMed]
- Pierobon, M.; Frankenfeld, C.L. Obesity as a Risk Factor for Triple-Negative Breast Cancers: A Systematic Review and Meta-Analysis. Breast Cancer Res. Treat. 2013, 137, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Michels, K.B.; Terry, K.L.; Willett, W.C. Longitudinal Study on the Role of Body Size in Premenopausal Breast Cancer. Arch. Intern. Med. 2006, 166, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Ogundiran, T.O.; Huo, D.; Adenipekun, A.; Campbell, O.; Oyesegun, R.; Akang, E.; Adebamowo, C.; Olopade, O.I. Case-Control Study of Body Size and Breast Cancer Risk in Nigerian Women. Am. J. Epidemiol. 2010, 172, 682–690. [Google Scholar] [CrossRef]
- Ahn, J.; Schatzkin, A.; Lacey, J.V.; Albanes, D.; Ballard-Barbash, R.; Adams, K.F.; Kipnis, V.; Mouw, T.; Hollenbeck, A.R.; Leitzmann, M.F. Adiposity, Adult Weight Change, and Postmenopausal Breast Cancer Risk. Arch. Intern. Med. 2007, 167, 2091–2102. [Google Scholar] [CrossRef]
- Feigelson, H.S.; Patel, A.V.; Teras, L.R.; Gansler, T.; Thun, M.J.; Calle, E.E. Adult Weight Gain and Histopathologic Characteristics of Breast Cancer among Postmenopausal Women. Cancer 2006, 107, 12–21. [Google Scholar] [CrossRef]
- Palmer, J.R.; Adams-Campbell, L.L.; Boggs, D.A.; Wise, L.A.; Rosenberg, L. A Prospective Study of Body Size and Breast Cancer in Black Women. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2007, 16, 1795–1802. [Google Scholar] [CrossRef]
- Suzuki, R.; Rylander-Rudqvist, T.; Ye, W.; Saji, S.; Wolk, A. Body Weight and Postmenopausal Breast Cancer Risk Defined by Estrogen and Progesterone Receptor Status among Swedish Women: A Prospective Cohort Study. Int. J. Cancer 2006, 119, 1683–1689. [Google Scholar] [CrossRef]
- Setiawan, V.W.; Monroe, K.R.; Wilkens, L.R.; Kolonel, L.N.; Pike, M.C.; Henderson, B.E. Breast Cancer Risk Factors Defined by Estrogen and Progesterone Receptor Status. Am. J. Epidemiol. 2009, 169, 1251–1259. [Google Scholar] [CrossRef]
- Bandera, E.V.; Chandran, U.; Hong, C.-C.; Troester, M.A.; Bethea, T.N.; Adams-Campbell, L.L.; Haiman, C.A.; Park, S.-Y.; Olshan, A.F.; Ambrosone, C.B.; et al. Obesity, Body Fat Distribution, and Risk of Breast Cancer Subtypes in African American Women Participating in the AMBER Consortium. Breast Cancer Res. Treat. 2015, 150, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Willett, W.C.; Terry, K.L.; Michels, K.B. Body Fat Distribution and Risk of Premenopausal Breast Cancer in the Nurses’ Health Study II. J. Natl. Cancer Inst. 2011, 103, 273–278. [Google Scholar] [CrossRef]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography With Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef]
- Borga, M.; West, J.; Bell, J.D.; Harvey, N.C.; Romu, T.; Heymsfield, S.B.; Dahlqvist Leinhard, O. Advanced Body Composition Assessment: From Body Mass Index to Body Composition Profiling. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2018, 66, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zopfs, D.; Theurich, S.; Große Hokamp, N.; Knuever, J.; Gerecht, L.; Borggrefe, J.; Schlaak, M.; Pinto Dos Santos, D. Single-Slice CT Measurements Allow for Accurate Assessment of Sarcopenia and Body Composition. Eur. Radiol. 2020, 30, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Faron, A.; Sprinkart, A.M.; Kuetting, D.L.R.; Feisst, A.; Isaak, A.; Endler, C.; Chang, J.; Nowak, S.; Block, W.; Thomas, D.; et al. Body Composition Analysis Using CT and MRI: Intra-Individual Intermodal Comparison of Muscle Mass and Myosteatosis. Sci. Rep. 2020, 10, 11765. [Google Scholar] [CrossRef]
- Pickhardt, P.J.; Jee, Y.; O’Connor, S.D.; del Rio, A.M. Visceral Adiposity and Hepatic Steatosis at Abdominal CT: Association With the Metabolic Syndrome. Am. J. Roentgenol. 2012, 198, 1100–1107. [Google Scholar] [CrossRef]
- Mühlberg, A.; Museyko, O.; Laredo, J.-D.; Engelke, K. A Reproducible Semi-Automatic Method to Quantify the Muscle-Lipid Distribution in Clinical 3D CT Images of the Thigh. PLoS ONE 2017, 12, e0175174. [Google Scholar] [CrossRef][Green Version]
- Bronsveld, H.K.; De Bruin, M.L.; Wesseling, J.; Sanders, J.; Hofland, I.; Jensen, V.; Bazelier, M.T.; Ter Braak, B.; de Boer, A.; Vestergaard, P.; et al. The Association of Diabetes Mellitus and Insulin Treatment with Expression of Insulin-Related Proteins in Breast Tumors. BMC Cancer 2018, 18, 224. [Google Scholar] [CrossRef]
- Dowling, R.J.O.; Niraula, S.; Chang, M.C.; Done, S.J.; Ennis, M.; McCready, D.R.; Leong, W.L.; Escallon, J.M.; Reedijk, M.; Goodwin, P.J.; et al. Changes in Insulin Receptor Signaling Underlie Neoadjuvant Metformin Administration in Breast Cancer: A Prospective Window of Opportunity Neoadjuvant Study. Breast Cancer Res. BCR 2015, 17, 32. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Z.; Punyanita, M.; Lei, J.; Sinav, A.; Kral, J.G.; Imielinska, C.; Ross, R.; Heymsfield, S.B. Adipose Tissue Quantification by Imaging Methods: A Proposed Classification. Obes. Res. 2003, 11, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Punyanitya, M.; Wang, Z.; Gallagher, D.; St-Onge, M.-P.; Albu, J.; Heymsfield, S.B.; Heshka, S. Total Body Skeletal Muscle and Adipose Tissue Volumes: Estimation from a Single Abdominal Cross-Sectional Image. J. Appl. Physiol. Bethesda Md 1985 2004, 97, 2333–2338. [Google Scholar] [CrossRef] [PubMed]
- Caan, B.J.; Meyerhardt, J.A.; Kroenke, C.H.; Alexeeff, S.; Xiao, J.; Weltzien, E.; Feliciano, E.C.; Castillo, A.L.; Quesenberry, C.P.; Kwan, M.L.; et al. Explaining the Obesity Paradox: The Association between Body Composition and Colorectal Cancer Survival (C-SCANS Study). Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2017, 26, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- SAS Analytics. Artificial Intelligence and Data Management. Available online: https://www.sas.com/en_sa/home.html (accessed on 20 May 2021).
- Renehan, A.G.; Zwahlen, M.; Egger, M. Adiposity and Cancer Risk: New Mechanistic Insights from Epidemiology. Nat. Rev. Cancer 2015, 15, 484–498. [Google Scholar] [CrossRef]
- Heymsfield, S.B.; Shen, W. Obesity: BAI as a New Measure of Adiposity--Throw Away Your Scale? Nat. Rev. Endocrinol. 2011, 7, 321–322. [Google Scholar] [CrossRef]
- Hamilton, E.J.; Gianatti, E.; Strauss, B.J.; Wentworth, J.; Lim-Joon, D.; Bolton, D.; Zajac, J.D.; Grossmann, M. Increase in Visceral and Subcutaneous Abdominal Fat in Men with Prostate Cancer Treated with Androgen Deprivation Therapy. Clin. Endocrinol. 2011, 74, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Choi, Y.-J.; Lee, Y.H. Visceral Fat Measured by Computed Tomography and the Risk of Breast Cancer. Transl. Cancer Res. 2019, 8, 1939. [Google Scholar] [CrossRef]
- Millikan, R.C.; Newman, B.; Tse, C.-K.; Moorman, P.G.; Conway, K.; Dressler, L.G.; Smith, L.V.; Labbok, M.H.; Geradts, J.; Bensen, J.T.; et al. Epidemiology of Basal-like Breast Cancer. Breast Cancer Res. Treat. 2008, 109, 123–139. [Google Scholar] [CrossRef]
- Trivers, K.F.; Lund, M.J.; Porter, P.L.; Liff, J.M.; Flagg, E.W.; Coates, R.J.; Eley, J.W. The Epidemiology of Triple-Negative Breast Cancer, Including Race. Cancer Causes Control CCC 2009, 20, 1071–1082. [Google Scholar] [CrossRef]
- Rose, D.P.; Vona-Davis, L. The Cellular and Molecular Mechanisms by Which Insulin Influences Breast Cancer Risk and Progression. Endocr. Relat. Cancer 2012, 19, R225–R241. [Google Scholar] [CrossRef] [PubMed]
- Dietze, E.C.; Chavez, T.A.; Seewaldt, V.L. Obesity and Triple-Negative Breast Cancer: Disparities, Controversies, and Biology. Am. J. Pathol. 2018, 188, 280–290. [Google Scholar] [CrossRef]
- Massihnia, D.; Galvano, A.; Fanale, D.; Perez, A.; Castiglia, M.; Incorvaia, L.; Listì, A.; Rizzo, S.; Cicero, G.; Bazan, V.; et al. Triple Negative Breast Cancer: Shedding Light onto the Role of Pi3k/Akt/Mtor Pathway. Oncotarget 2016, 7, 60712–60722. [Google Scholar] [CrossRef]
- Ueng, S.-H.; Chen, S.-C.; Chang, Y.-S.; Hsueh, S.; Lin, Y.-C.; Chien, H.-P.; Lo, Y.-F.; Shen, S.-C.; Hsueh, C. Phosphorylated MTOR Expression Correlates with Poor Outcome in Early-Stage Triple Negative Breast Carcinomas. Int. J. Clin. Exp. Pathol. 2012, 5, 806–813. [Google Scholar] [PubMed]
- Iqbal, J.; Thike, A.A.; Cheok, P.Y.; Tse, G.M.-K.; Tan, P.H. Insulin Growth Factor Receptor-1 Expression and Loss of PTEN Protein Predict Early Recurrence in Triple-Negative Breast Cancer. Histopathology 2012, 61, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Elstrom, R.L.; Bauer, D.E.; Buzzai, M.; Karnauskas, R.; Harris, M.H.; Plas, D.R.; Zhuang, H.; Cinalli, R.M.; Alavi, A.; Rudin, C.M.; et al. Akt Stimulates Aerobic Glycolysis in Cancer Cells. Cancer Res. 2004, 64, 3892–3899. [Google Scholar] [CrossRef]
- Robey, R.B.; Hay, N. Is Akt the “Warburg Kinase”?—Akt-Energy Metabolism Interactions and Oncogenesis. Semin. Cancer Biol. 2009, 19, 25. [Google Scholar] [CrossRef]
- Teslow, E.A.; Mitrea, C.; Bao, B.; Mohammad, R.M.; Polin, L.A.; Dyson, G.; Purrington, K.S.; Bollig-Fischer, A. Obesity-Induced MBD2_v2 Expression Promotes Tumor-Initiating Triple-Negative Breast Cancer Stem Cells. Mol. Oncol. 2019, 13, 894–908. [Google Scholar] [CrossRef]
- Prado, C.M.M.; Siervo, M.; Mire, E.; Heymsfield, S.B.; Stephan, B.C.M.; Broyles, S.; Smith, S.R.; Wells, J.C.K.; Katzmarzyk, P.T. A Population-Based Approach to Define Body-Composition Phenotypes. Am. J. Clin. Nutr. 2014, 99, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Phipps, A.I.; Malone, K.E.; Porter, P.L.; Daling, J.R.; Li, C.I. Body Size and Risk of Luminal, HER2-Overexpressing, and Triple-Negative Breast Cancer in Postmenopausal Women. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2008, 17, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Vona-Davis, L. Interaction between Menopausal Status and Obesity in Affecting Breast Cancer Risk. Maturitas 2010, 66, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.P.; Vona-Davis, L. Influence of Obesity on Breast Cancer Receptor Status and Prognosis. Expert Rev. Anticancer Ther. 2009, 9, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Del Fabbro, E.; Parsons, H.; Warneke, C.L.; Pulivarthi, K.; Litton, J.K.; Dev, R.; Palla, S.L.; Brewster, A.; Bruera, E. The Relationship between Body Composition and Response to Neoadjuvant Chemotherapy in Women with Operable Breast Cancer. Oncologist 2012, 17, 1240–1245. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Baracos, V.; McCargar, L.; Reiman, T.; Tonkin, K.; Mackey, J.; Koski, S.; Pituskin, E.; Sawyer, M. Sarcopenia as a Determinant of Chemotherapy Toxicity and Time to Tumor Progression in Metastatic Breast Cancer Patients Receiving Capecitabine Treatment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef]
- Rier, H.N.; Jager, A.; Sleijfer, S.; van Rosmalen, J.; Kock, M.C.J.M.; Levin, M.-D. Low Muscle Attenuation Is a Prognostic Factor for Survival in Metastatic Breast Cancer Patients Treated with First Line Palliative Chemotherapy. Breast Edinb. Scotl. 2017, 31, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Villaseñor, A.; Ballard-Barbash, R.; Baumgartner, K.; Baumgartner, R.; Bernstein, L.; McTiernan, A.; Neuhouser, M.L. Prevalence and Prognostic Effect of Sarcopenia in Breast Cancer Survivors: The HEAL Study. J. Cancer Surviv. Res. Pract. 2012, 6, 398–406. [Google Scholar] [CrossRef]
- Dzien, A.; Winner, H.; Theurl, E.; Dzien-Bischinger, C.; Lechleitner, M. Fat-Free Mass and Fasting Glucose Values in Patients with and without Statin Therapy Assigned to Age Groups between <60 and >75 Years. Obes. Facts 2013, 6, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Gadiyaram, V.; Zhang, C.; Chen, Z.; Lopaschuk, G.; Stillman, A.E. Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. J. Am. Heart Assoc. 2019, 8, e013104. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).