Risk of Non-Hodgkin Lymphoma among Patients with Hepatitis B Virus and Hepatitis C Virus in Taiwan: A Nationwide Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
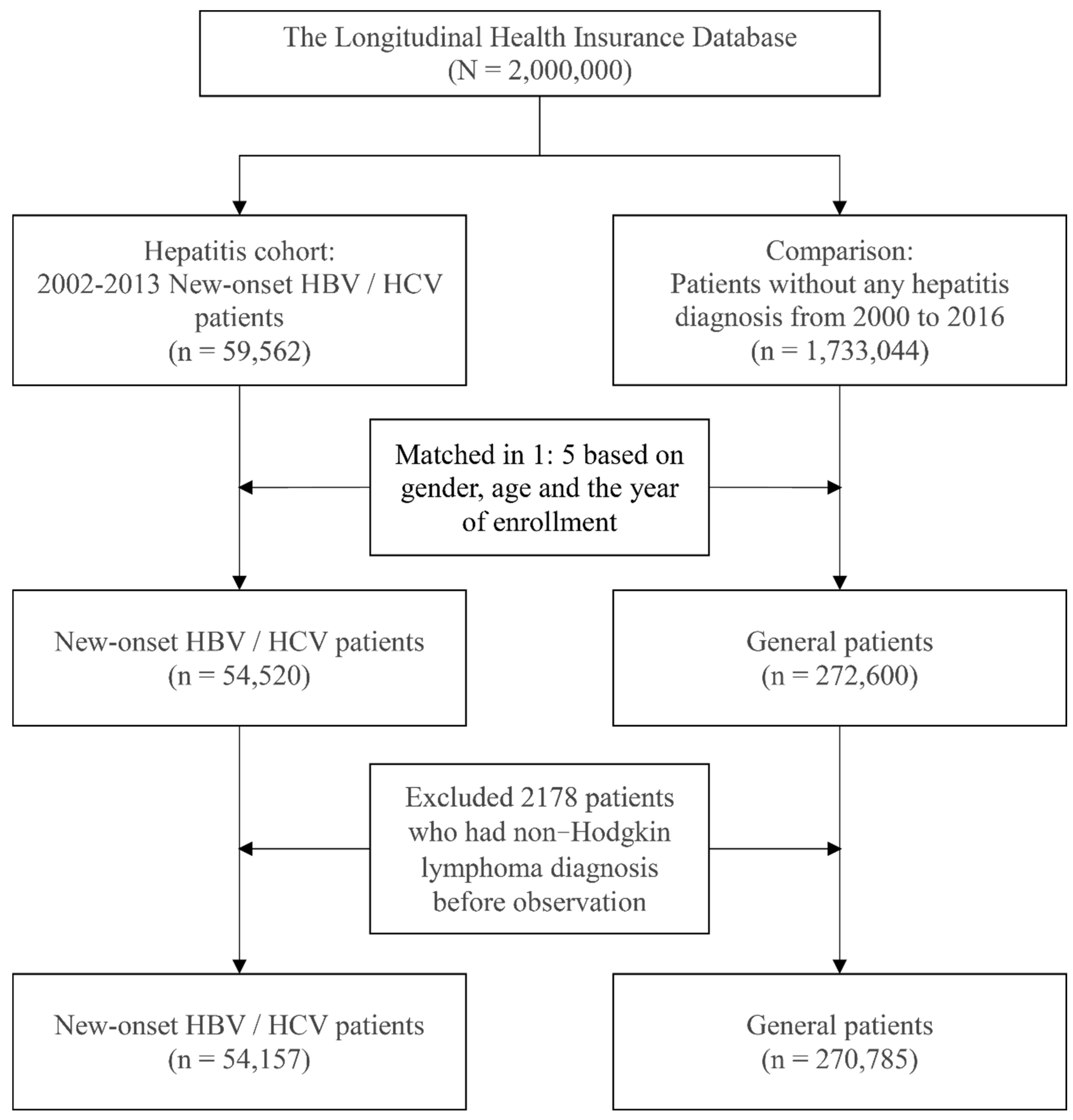
2.1. Database
2.2. Study Participants
2.3. Study Design
2.4. Statistical Analysis
3. Results
3.1. The Baseline Characteristic Distribution of Study Subjects after Matching
3.2. The Incidence Rate of Non-Hodgkin Lymphoma
3.3. Risk of Non-Hodgkin Lymphoma in Hepatitis Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.H.; Yang, P.M.; Huang, G.T.; Lee, H.S.; Sung, J.L.; Sheu, J.C. Estimation of seroprevalence of hepatitis B virus and hepatitis C virus in Taiwan from a large-scale survey of free hepatitis screening participants. J. Formos. Med. Assoc. 2007, 106, 148–155. [Google Scholar] [CrossRef]
- Yu, M.L.; Yeh, M.L.; Tsai, P.C.; Huang, C.I.; Huang, J.F.; Huang, C.F.; Hsieh, M.H.; Liang, P.C.; Lin, Y.H.; Hsieh, M.Y.; et al. Huge gap between clinical efficacy and community effectiveness in the treatment of chronic hepatitis C: A nationwide survey in Taiwan. Medicine 2015, 94, e690. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Chen, P.J. Elimination of Hepatitis B in Highly Endemic Settings: Lessons Learned in Taiwan and Challenges Ahead. Viruses 2020, 12, 815. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Filho, A.; Pineros, M.; Znaor, A.; Marcos-Gragera, R.; Steliarova-Foucher, E.; Bray, F. Global patterns and trends in the incidence of non-Hodgkin lymphoma. Cancer Causes Control 2019, 30, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Shankland, K.R.; Armitage, J.O.; Hancock, B.W. Non-Hodgkin lymphoma. Lancet 2012, 380, 848–857. [Google Scholar] [CrossRef]
- Zhang, Y.; Dai, Y.; Zheng, T.; Ma, S. Risk Factors of Non-Hodgkin Lymphoma. Expert Opin. Med. Diagn. 2011, 5, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Bang, Y.J.; Park, B.J.; Yoo, T.; Kim, C.W.; Kim, T.Y.; Heo, D.S.; Lee, H.S.; Kim, N.K. Hepatitis B virus infection and B-cell non-Hodgkin’s lymphoma in a hepatitis B endemic area: A case-control study. Jpn. J. Cancer Res. 2002, 93, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Ulcickas Yood, M.; Quesenberry, C.P., Jr.; Guo, D.; Caldwell, C.; Wells, K.; Shan, J.; Sanders, L.; Skovron, M.L.; Iloeje, U.; Manos, M.M. Incidence of non-Hodgkin’s lymphoma among individuals with chronic hepatitis B virus infection. Hepatology 2007, 46, 107–112. [Google Scholar] [CrossRef]
- Park, S.C.; Jeong, S.H.; Kim, J.; Han, C.J.; Kim, Y.C.; Choi, K.S.; Cho, J.H.; Lee, M.; Jung, H.H.; Ki, S.S.; et al. High prevalence of hepatitis B virus infection in patients with B-cell non-Hodgkin’s lymphoma in Korea. J. Med. Virol. 2008, 80, 960–966. [Google Scholar] [CrossRef]
- Wang, C.; Xia, B.; Ning, Q.; Zhao, H.; Yang, H.; Zhao, Z.; Wang, X.; Wang, Y.; Yu, Y.; Zhang, Y. High prevalence of hepatitis B virus infection in patients with aggressive B cell non-Hodgkin’s lymphoma in China. Ann. Hematol. 2018, 97, 453–457. [Google Scholar] [CrossRef]
- Kim, M.; Lee, Y.K.; Park, B.; Oh, D.J.; Choi, H.G. Hepatitis virus B and C infections are associated with an increased risk of non-Hodgkin lymphoma: A nested case-control study using a national sample cohort. J. Med. Virol. 2020, 92, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Hsiao, L.T.; Chiou, T.J.; Liu, J.H.; Gau, J.P.; Teng, H.W.; Wang, W.S.; Chao, T.C.; Yen, C.C.; Chen, P.M. High prevalence of occult hepatitis B virus infection in patients with B cell non-Hodgkin’s lymphoma. Ann. Hematol. 2008, 87, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.A.; Pfeiffer, R.; Warren, J.L.; Landgren, O.; Gadalla, S.; Berndt, S.I.; Ricker, W.; Parsons, R.; Wheeler, W.; Engels, E.A. Hematopoietic malignancies associated with viral and alcoholic hepatitis. Cancer Epidemiol. Biomark. Prev. 2008, 17, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Su, T.H.; Liu, C.J.; Tseng, T.C.; Chou, S.W.; Liu, C.H.; Yang, H.C.; Wu, S.J.; Chen, P.J.; Chen, D.S.; Chen, C.L.; et al. Hepatitis C viral infection increases the risk of lymphoid-neoplasms: A population-based cohort study. Hepatology 2016, 63, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Ury, H.K. Efficiency of case-control studies with multiple controls per case: continuous or dichotomous data. Biometrics 1975, 31, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Austin P., C. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am. J. Epidemiol. 2010, 172, 1092–1097. [Google Scholar] [CrossRef]
- Marcucci, F.; Spada, E.; Mele, A.; Caserta, C.A.; Pulsoni, A. The association of hepatitis B virus infection with B-cell non-Hodgkin lymphoma—A review. Am. J. Blood Res. 2012, 2, 18–28. [Google Scholar]
- Couronne, L.; Bachy, E.; Roulland, S.; Nadel, B.; Davi, F.; Armand, M.; Canioni, D.; Michot, J.M.; Visco, C.; Arcaini, L.; et al. From hepatitis C virus infection to B-cell lymphoma. Ann. Oncol. 2018, 29, 92–100. [Google Scholar] [CrossRef]
- Sene, D.; Limal, N.; Ghillani-Dalbin, P.; Saadoun, D.; Piette, J.C.; Cacoub, P. Hepatitis C virus-associated B-cell proliferation--the role of serum B lymphocyte stimulator (BLyS/BAFF). Rheumatology (Oxford) 2007, 46, 65–69. [Google Scholar] [CrossRef][Green Version]
- Datta, S.; Chatterjee, S.; Policegoudra, R.S.; Gogoi, H.K.; Singh, L. Hepatitis viruses and non-Hodgkin’s lymphoma: A review. World J. Virol. 2012, 1, 162–173. [Google Scholar] [CrossRef]
- Chen, C.L.; Huang, J.Y.; Wang, C.H.; Tahara, S.M.; Zhou, L.; Kondo, Y.; Schechter, J.; Su, L.; Lai, M.M.; Wakita, T.; et al. Hepatitis C virus has a genetically determined lymphotropism through co-receptor B7.2. Nat. Commun. 2017, 8, 13882. [Google Scholar] [CrossRef]
- Kuniyoshi, M.; Nakamuta, M.; Sakai, H.; Enjoji, M.; Kinukawa, N.; Kotoh, K.; Fukutomi, M.; Yokota, M.; Nishi, H.; Iwamoto, H.; et al. Prevalence of hepatitis B or C virus infections in patients with non-Hodgkin’s lymphoma. J. Gastroenterol. Hepatol. 2001, 16, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.T.; Fei, G.; Quek, R.; Lim, L.C.; Lee, L.H.; Yap, S.P.; Loong, S.; Tao, M. The relationship of hepatitis B virus infection and non-Hodgkin’s lymphoma and its impact on clinical characteristics and prognosis. Eur. J. Haematol. 2007, 79, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Taborelli, M.; Polesel, J.; Montella, M.; Libra, M.; Tedeschi, R.; Battiston, M.; Spina, M.; Di Raimondo, F.; Pinto, A.; Crispo, A.; et al. Hepatitis B and C viruses and risk of non-Hodgkin lymphoma: A case-control study in Italy. Infect. Agent. Cancer 2016, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, R.H.; Han, B.; Shi, Y.X.; Luo, H.Y.; Jiang, W.Q.; Lin, T.Y.; Huang, H.Q.; Xia, Z.J.; Guan, Z.Z. High incidence of hepatitis B virus infection in B-cell subtype non-Hodgkin lymphoma compared with other cancers. Cancer 2007, 109, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gan, Y.; Fan, C.; Yuan, H.; Zhang, X.; Shen, Y.; Wang, Q.; Meng, Z.; Xu, D.; Tu, H. Hepatitis B virus and risk of non-Hodgkin lymphoma: An updated meta-analysis of 58 studies. J. Viral Hepat. 2018, 25, 894–903. [Google Scholar] [CrossRef]
- Wang, Q.; De Luca, A.; Smith, C.; Zangerle, R.; Sambatakou, H.; Bonnet, F.; Smit, C.; Schommers, P.; Thornton, A.; Berenguer, J.; et al. Chronic Hepatitis B and C Virus Infection and Risk for Non-Hodgkin Lymphoma in HIV-Infected Patients: A Cohort Study. Ann. Intern. Med. 2017, 166, 9–17. [Google Scholar] [CrossRef]
- Abe, S.K.; Inoue, M.; Sawada, N.; Iwasaki, M.; Shimazu, T.; Yamaji, T.; Sasazuki, S.; Tanaka, Y.; Mizokami, M.; Tsugane, S.; et al. Hepatitis B and C virus infection and risk of lymphoid malignancies: A population-based cohort study (JPHC Study). Cancer Epidemiol. 2015, 39, 562–566. [Google Scholar] [CrossRef]
- Franceschi, S.; Lise, M.; Trepo, C.; Berthillon, P.; Chuang, S.C.; Nieters, A.; Travis, R.C.; Vermeulen, R.; Overvad, K.; Tjonneland, A.; et al. Infection with hepatitis B and C viruses and risk of lymphoid malignancies in the European Prospective Investigation into Cancer and Nutrition (EPIC). Cancer Epidemiol. Biomark. Prev. 2011, 20, 208–214. [Google Scholar] [CrossRef]
- Andersen, E.S.; Omland, L.H.; Jepsen, P.; Krarup, H.; Christensen, P.B.; Obel, N.; Weis, N.; Study, D.C. Risk of all-type cancer, hepatocellular carcinoma, non-Hodgkin lymphoma and pancreatic cancer in patients infected with hepatitis B virus. J. Viral. Hepat. 2015, 22, 828–834. [Google Scholar] [CrossRef]
- Su, T.H.; Liu, C.J.; Tseng, T.C.; Chou, S.W.; Liu, C.H.; Yang, H.C.; Wu, S.J.; Chen, P.J.; Chen, D.S.; Chen, C.L.; et al. Chronic hepatitis B is associated with an increased risk of B-cell non-Hodgkin’s lymphoma and multiple myeloma. Aliment. Pharmacol. Ther. 2019, 49, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Kamiza, A.B.; Su, F.H.; Wang, W.C.; Sung, F.C.; Chang, S.N.; Yeh, C.C. Chronic hepatitis infection is associated with extrahepatic cancer development: A nationwide population-based study in Taiwan. BMC Cancer 2016, 16, 861. [Google Scholar] [CrossRef] [PubMed]
- Kleinstern, G.; Seir, R.A.; Perlman, R.; Abdeen, Z.; Khatib, A.; Elyan, H.; Dann, E.J.; Kedmi, M.; Ellis, M.; Nagler, A.; et al. Associations between B-cell non-Hodgkin lymphoma and exposure, persistence and immune response to hepatitis B. Haematologica 2016, 101, e303-305. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Pan, H.; Yang, P.; Ye, P.; Cao, H.; Zhou, H. Both chronic HBV infection and naturally acquired HBV immunity confer increased risks of B-cell non-Hodgkin lymphoma. BMC Cancer 2019, 19, 477. [Google Scholar] [CrossRef] [PubMed]
- Fiorino, S.; Bacchi-Reggiani, L.; de Biase, D.; Fornelli, A.; Masetti, M.; Tura, A.; Grizzi, F.; Zanello, M.; Mastrangelo, L.; Lombardi, R.; et al. Possible association between hepatitis C virus and malignancies different from hepatocellular carcinoma: A systematic review. World J. Gastroenterol. 2015, 21, 12896–12953. [Google Scholar] [CrossRef]
- Mahale, P.; Torres, H.A.; Kramer, J.R.; Hwang, L.Y.; Li, R.; Brown, E.L.; Engels, E.A. Hepatitis C virus infection and the risk of cancer among elderly US adults: A registry-based case-control study. Cancer 2017, 123, 1202–1211. [Google Scholar] [CrossRef]
- Morton, L.M.; Slager, S.L.; Cerhan, J.R.; Wang, S.S.; Vajdic, C.M.; Skibola, C.F.; Bracci, P.M.; de Sanjose, S.; Smedby, K.E.; Chiu, B.C.; et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: The InterLymph Non-Hodgkin Lymphoma Subtypes Project. J. Natl. Cancer Inst. Monogr. 2014, 2014, 130–144. [Google Scholar] [CrossRef]
- Caccamo, G.; Saffioti, F.; Raimondo, G. Hepatitis B virus and hepatitis C virus dual infection. World J. Gastroenterol. 2014, 20, 14559–14567. [Google Scholar] [CrossRef]
- Chen, C.J.; You, S.L.; Hsu, W.L.; Yang, H.I.; Lee, M.H.; Chen, H.C.; Chen, Y.Y.; Liu, J.; Hu, H.H.; Lin, Y.J.; et al. Epidemiology of Virus Infection and Human Cancer. Recent Results Cancer Res. 2021, 217, 13–45. [Google Scholar] [CrossRef]
- Picardi, M.; Della Pepa, R.; Giordano, C.; Zacheo, I.; Pugliese, N.; Mortaruolo, C.; Trastulli, F.; Giordano, A.; Lucignano, M.; Di Perna, M.; et al. Tenofovir vs lamivudine for the prevention of hepatitis B virus reactivation in advanced-stage DLBCL. Blood 2019, 133, 498–501. [Google Scholar] [CrossRef]
- Picardi, M.; Giordano, C.; Pepa, R.D.; Pugliese, N.; Leone, A.; Gentile, G.; Pane, F. Correspondence in reference to the previously published Epub manuscript: “Murt Ahmet et al. Hepatitis B reactivation in hematopoietic stem cell transplanted patients: 20 years of experience of a single center from a middle endemic country. Ann. Hematol. 2020, 99, 2671–2677. [Google Scholar] [CrossRef]
- Picardi, M.; Giordano, C.; Della Pepa, R.; Pugliese, N.; Leone, A.; Delle Cave, G.; Iula, R., Pane; Gentile, G. Hepatitis B Surface Antigen Positivity Is an Independent Unfavorable Prognostic Factor in Diffuse Large B-Cell Lymphoma in the Rituximab Era. Oncologist 2021, 26, e1083–e1084. [Google Scholar] [CrossRef] [PubMed]
| Variables | Control Group | Case Group | Total | p-Value 3 | |||
|---|---|---|---|---|---|---|---|
| Patients without Hepatitis | Patients with Hepatitis | ||||||
| n | % | n | % | n | % | ||
| Total | 270,785 | 100 | 54,157 | 100 | 324,942 | 100 | |
| Gender 1 | 1.000 | ||||||
| Female | 114,960 | 42.45 | 22,992 | 42.45 | 137,952 | 42.45 | |
| Male | 155,825 | 57.55 | 31,165 | 57.55 | 186,990 | 57.55 | |
| Age (years) 1 | 48.59 ± 15.39 | 48.78 ± 14.28 | 48.62 ± 15.21 | 1.000 | |||
| 20–44 | 49,060 | 18.12 | 9812 | 18.12 | 58,872 | 18.12 | |
| 45–54 | 94,555 | 34.92 | 18,911 | 34.92 | 113,466 | 34.92 | |
| 55–64 | 85,985 | 31.75 | 17,197 | 31.75 | 103,182 | 31.75 | |
| ≥65 | 41,185 | 15.21 | 8237 | 15.21 | 49,422 | 15.21 | |
| DM 2 | <0.001 | ||||||
| No | 223,161 | 82.41 | 40,342 | 74.49 | 263,503 | 81.09 | |
| Yes | 47,624 | 17.59 | 13,815 | 25.51 | 61,439 | 18.91 | |
| HTN 2 | <0.001 | ||||||
| No | 178,351 | 65.86 | 32,619 | 60.23 | 210,970 | 64.93 | |
| Yes | 92,434 | 34.14 | 21,538 | 39.77 | 113,972 | 35.07 | |
| HPL 2 | <0.001 | ||||||
| No | 199,223 | 73.57 | 35,406 | 65.38 | 234,629 | 72.21 | |
| Yes | 71,562 | 26.43 | 18,751 | 34.62 | 90,313 | 27.79 | |
| CKD 2 | <0.001 | ||||||
| No | 251,014 | 92.70 | 47,747 | 88.16 | 298,761 | 91.94 | |
| Yes | 19,771 | 7.30 | 6410 | 11.84 | 26,181 | 8.06 | |
| RA 2 | <0.001 | ||||||
| No | 266,645 | 98.47 | 52,658 | 97.23 | 319,303 | 98.26 | |
| Yes | 4140 | 1.53 | 1499 | 2.77 | 5639 | 1.74 | |
| SLE 2 | |||||||
| No | 265,912 | 98.20 | 52,396 | 96.75 | 318,308 | 97.96 | |
| Yes | 4873 | 1.8 | 1761 | 3.25 | 6634 | 2.04 | |
| Psoriasis | <0.001 | ||||||
| No | 267,992 | 98.97 | 53,389 | 98.58 | 321,381 | 98.90 | |
| Yes | 2793 | 1.03 | 768 | 1.42 | 3561 | 1.10 | |
| HIV 2 | <0.001 | ||||||
| No | 270,562 | 99.92 | 53,774 | 99.29 | 324,336 | 99.81 | |
| Yes | 223 | 0.08 | 383 | 0.71 | 606 | 0.19 | |
| Organ transplant | <0.001 | ||||||
| No | 270,594 | 99.93 | 53,903 | 99.53 | 324,497 | 99.86 | |
| Yes | 191 | 0.07 | 254 | 0.47 | 445 | 0.14 | |
| Variables | Non-Hodgkin Lymphoma | Total | p-Value 2 | ||||
|---|---|---|---|---|---|---|---|
| No | Yes | ||||||
| n | % | n | % | n | % | ||
| Total | 324,523 | 99.87 | 419 | 0.13 | 324,942 | 100 | |
| Hepatitis | <0.001 | ||||||
| No | 270,503 | 99.90 | 282 | 0.10 | 270,785 | 83.33 | |
| Yes | 54,020 | 99.75 | 137 | 0.25 | 54,157 | 16.67 | |
| Hepatitis types | <0.001 | ||||||
| Hepatitis B | 37,573 | 99.78 | 83 | 0.22 | 37,656 | 11.59 | |
| Hepatitis C | 14,315 | 99.65 | 50 | 0.35 | 14,365 | 4.42 | |
| Both | 2132 | 99.81 | 4 | 0.19 | 2136 | 0.66 | |
| Gender | 0.422 | ||||||
| Female | 137,766 | 99.87 | 186 | 0.13 | 137,952 | 42.45 | |
| Male | 186,757 | 99.88 | 233 | 0.12 | 186,990 | 57.55 | |
| Age (year) | 48.59 ± 15.39 | 48.77 ± 14.28 | 48.62 ± 15.21 | <0.001 | |||
| 20–44 | 58,833 | 99.93 | 39 | 0.07 | 58,872 | 18.12 | |
| 45–54 | 113,386 | 99.93 | 80 | 0.07 | 113,466 | 34.92 | |
| 55–64 | 103,021 | 99.84 | 161 | 0.16 | 103,182 | 31.75 | |
| ≥65 | 49,283 | 99.72 | 139 | 0.28 | 49,422 | 15.21 | |
| DM 1 | 0.003 | ||||||
| No | 263,187 | 99.88 | 316 | 0.12 | 263,503 | 81.09 | |
| Yes | 61,336 | 99.83 | 103 | 0.17 | 61,439 | 18.91 | |
| HTN 1 | <0.001 | ||||||
| No | 210,749 | 99.9 | 221 | 0.1 | 210,970 | 64.93 | |
| Yes | 113,774 | 99.83 | 198 | 0.17 | 113,972 | 35.07 | |
| HPL 1 | 0.960 | ||||||
| No | 234,326 | 99.87 | 303 | 0.13 | 234,629 | 72.21 | |
| Yes | 90,197 | 99.87 | 116 | 0.13 | 90,313 | 27.79 | |
| CKD 1 | 0.193 | ||||||
| No | 298,383 | 99.87 | 378 | 0.13 | 298,761 | 91.94 | |
| Yes | 26,140 | 99.84 | 41 | 0.16 | 26,181 | 8.06 | |
| RA 1 | <0.001 | ||||||
| No | 318,902 | 99.87 | 401 | 0.13 | 319,303 | 98.26 | |
| Yes | 5621 | 99.68 | 18 | 0.32 | 5639 | 1.74 | |
| SLE 1 | <0.001 | ||||||
| No | 317,911 | 99.88 | 397 | 0.12 | 318,308 | 97.96 | |
| Yes | 6612 | 99.67 | 22 | 0.33 | 6634 | 2.04 | |
| Psoriasis | 0.110 | ||||||
| No | 320,970 | 99.87 | 411 | 0.13 | 321,381 | 98.90 | |
| Yes | 3553 | 99.78 | 8 | 0.22 | 3561 | 1.10 | |
| HIV 1 | <0.001 | ||||||
| No | 323,921 | 99.87 | 415 | 0.13 | 324,336 | 99.81 | |
| Yes | 602 | 99.34 | 4 | 0.66 | 606 | 0.19 | |
| Organ transplant | <0.001 | ||||||
| No | 324,084 | 99.87 | 413 | 0.13 | 324,497 | 99.86 | |
| Yes | 439 | 98.65 | 6 | 1.35 | 445 | 0.14 | |
| Variables | Model 1 | Model 2 | ||||
|---|---|---|---|---|---|---|
| HR 1 | 95% CI | p-Value | HR 1 | 95% CI | p-Value | |
| Hepatitis | ||||||
| No (ref.) | 1 | 1 | ||||
| Yes | 2.37 | 1.93–2.92 | <0.001 | - | ||
| Hepatitis B | - | - | 2.49 | 1.94–3.19 | <0.001 | |
| Hepatitis C | - | - | 2.36 | 1.73–3.22 | <0.001 | |
| Both | - | - | 1.20 | 0.45–3.23 | 0.720 | |
| Gender | ||||||
| Female (ref.) | 1 | 1 | ||||
| Male | 1.06 | 0.87–1.29 | 0.567 | 1.06 | 0.87–1.29 | 0.574 |
| Age (year) | ||||||
| 20–44 (ref.) | 1 | 1 | ||||
| 45–54 | 1.29 | 0.87–1.89 | 0.203 | 1.29 | 0.88–1.90 | 0.194 |
| 55–64 | 3.30 | 2.29–4.75 | <0.001 | 3.33 | 2.31–4.81 | <0.001 |
| ≥65 | 6.28 | 4.26–9.26 | <0.001 | 6.37 | 4.31–9.42 | <0.001 |
| DM 1 | ||||||
| No | 1 | 1 | ||||
| Yes | 0.98 | 0.77–1.26 | 0.893 | 0.99 | 0.77–1.27 | 0.928 |
| HTN 1 | ||||||
| No | 1 | 1 | ||||
| Yes | 0.94 | 0.75–1.18 | 0.584 | 0.94 | 0.75–1.18 | 0.586 |
| HPL 1 | ||||||
| No | 1 | 1 | ||||
| Yes | 0.69 | 0.54–0.87 | 0.002 | 0.68 | 0.54–0.86 | 0.002 |
| CKD 1 | ||||||
| No | 1 | 1 | ||||
| Yes | 0.64 | 0.46–0.90 | 0.010 | 0.64 | 0.46–0.90 | 0.011 |
| RA 1 | ||||||
| No | 1 | 1 | ||||
| Yes | 1.58 | 0.97–2.57 | 0.066 | 1.59 | 0.98–2.58 | 0.063 |
| SLE 1 | ||||||
| No | 1 | 1 | ||||
| Yes | 1.82 | 1.17–2.83 | 0.008 | 1.82 | 1.17–2.83 | 0.008 |
| Psoriasis | ||||||
| No | 1 | 1 | ||||
| Yes | 1.43 | 0.71–2.88 | 0.317 | 1.43 | 0.71–2.89 | 0.316 |
| HIV 1 | ||||||
| No | 1 | 1 | ||||
| Yes | 7.09 | 2.62–19.22 | <0.001 | 7.39 | 2.72–20.10 | <0.001 |
| Organ transplant | ||||||
| No | 1 | 1 | ||||
| Yes | 6.59 | 2.92–14.88 | <0.001 | 6.67 | 2.96–15.05 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-R.; Chang, Y.-L.; Lee, C.-H.; Tsai, T.-H.; Huang, K.-H.; Lee, C.-Y. Risk of Non-Hodgkin Lymphoma among Patients with Hepatitis B Virus and Hepatitis C Virus in Taiwan: A Nationwide Cohort Study. Cancers 2022, 14, 583. https://doi.org/10.3390/cancers14030583
Lai Y-R, Chang Y-L, Lee C-H, Tsai T-H, Huang K-H, Lee C-Y. Risk of Non-Hodgkin Lymphoma among Patients with Hepatitis B Virus and Hepatitis C Virus in Taiwan: A Nationwide Cohort Study. Cancers. 2022; 14(3):583. https://doi.org/10.3390/cancers14030583
Chicago/Turabian StyleLai, Yung-Rung, Ya-Lan Chang, Chiu-Hsiang Lee, Tung-Han Tsai, Kuang-Hua Huang, and Chien-Ying Lee. 2022. "Risk of Non-Hodgkin Lymphoma among Patients with Hepatitis B Virus and Hepatitis C Virus in Taiwan: A Nationwide Cohort Study" Cancers 14, no. 3: 583. https://doi.org/10.3390/cancers14030583
APA StyleLai, Y.-R., Chang, Y.-L., Lee, C.-H., Tsai, T.-H., Huang, K.-H., & Lee, C.-Y. (2022). Risk of Non-Hodgkin Lymphoma among Patients with Hepatitis B Virus and Hepatitis C Virus in Taiwan: A Nationwide Cohort Study. Cancers, 14(3), 583. https://doi.org/10.3390/cancers14030583






