Simple Summary
Adoptive cellular immunotherapy is one of the most effective strategies promising to eliminate tumors. Natural killer (NK) cells gain increasing attention for their stable availability and safety properties. To improve the anti-tumor efficacy of NK cells, multiple genetically engineered NK cellular products have been tested in recent years. In this review, we evaluate the latest research progress on adoptive NK cell therapy with a special focus on their alternative sources and genetic modifications.
Abstract
As an important component of the innate immune system, natural killer (NK) cells have gained increasing attention in adoptive cell therapy for their safety and efficacious tumor-killing effect. Unlike T cells which rely on the interaction between TCRs and specific peptide-MHC complexes, NK cells are more prone to be served as “off-the-shelf” cell therapy products due to their rapid recognition and killing of tumor cells without MHC restriction. In recent years, constantly emerging sources of therapeutic NK cells have provided flexible options for cancer immunotherapy. Advanced genetic engineering techniques, especially chimeric antigen receptor (CAR) modification, have yielded exciting effectiveness in enhancing NK cell specificity and cytotoxicity, improving in vivo persistence, and overcoming immunosuppressive factors derived from tumors. In this review, we highlight current advances in NK-based adoptive cell therapy, including alternative sources of NK cells for adoptive infusion, various CAR modifications that confer different targeting specificity to NK cells, multiple genetic engineering strategies to enhance NK cell function, as well as the latest clinical research on adoptive NK cell therapy.
1. Introduction
Recently, immunotherapy has attracted broad attention worldwide as an innovative approach of cancer treatment. The core of the immunotherapy strategy is to eliminate tumor cells by enhancing the patient’s immune system. As an important part of the immune system, the number and activity of immune effector cells, especially natural killer (NK) and T-cells, are closely related to the body’s anti-tumor ability. Hence, T or NK-based adoptive transfer strategy has provided new hope for patients with advanced malignant tumors including lymphoma [1,2], leukemia [3], melanoma [2], lung cancer [4], and colorectal cancer [5].
In the 1980s, Rosenberg and his group were the first to confirm that treating metastatic melanoma patients with autologous tumor-infiltrating lymphocytes (TILs) and IL-2 could achieve objective regression [6]. Since then, immunotherapeutic approaches based on adoptive cell transfer have advanced significantly with increasing sources of effector cells [7], as well as tools to enhance specificity, such as engineered specific T-cell receptors (TCR) [8], and chimeric antigen receptors (CAR) [9]. As the “first line of defense” of the human immune system, NK cells can directly recognize and rapidly kill target cells without antigen pre-sensitization and major histocompatibility complex (MHC) restriction. Compared with T cells, one of the important characteristics of NK cells is the lack of TCRs (one of the main mechanisms of allogenic T-cell activation) [10,11]. Inhibitory receptors of NK cells, such as KIR, also prevent NK cells from attacking normal host cells. Hence, NK cells are considered safer for the ACT due to much fewer possibilities of inducing graft versus host disease (GvHD). Moreover, these features of NK cells make it possible that they can be provided by any healthy donor, which breaks the limitation of T-cell-based ACT and reduces costs for large-scale production [12]. These features make NK cells an ideal candidate to become an “off-the-shelf” therapy product for adoptive immunotherapy. Specifically, autologous or allogeneic NK cells are expanded and activated under the stimulus of feeder cells, such as gene-modified K562 cells and cytokines (IL-2/IL-15) in vitro, and then infused back into patients to eliminate tumor cells in vivo through direct cytotoxicity and cytokine secretion [13,14]. Such conventional NK-based immunotherapy has shown preliminary benefits in various malignancies, especially hematological malignancies, together with limited toxicity against normal tissues [15,16,17]. Despite this, several obstacles still restrict the widespread application of NK cells in vivo, including low cytotoxicity, insufficient persistence, and inertness in the tumor microenvironment (TME) [18,19]. Encouragingly, the rapidly evolving field of adoptive NK cell therapy now encompasses a series of candidate cell sources [20], and advances in genetic engineering have further improved the specificity, persistence, and anti-tumor efficacy of NK cells [21].
In this review, we first described the basic characteristics and major functions of NK cells. We then reviewed possible sources of NK cells and ways of genetic modifications on NK cells for adoptive immunotherapy. We focused on the current application of gene-editing technologies in adoptive NK cell therapy, especially the progress made in the field of CAR NK-based therapy. Furthermore, we summarized the latest clinical results in the adoptive NK cell therapy for patients with malignancies. Finally, we propose the future directions of NK-based adoptive cell therapy (ACT).
2. An Overview of NK Cells
2.1. Biological Properties of NK Cells
NK cells are important immune effector cells and belong to the innate lymphoid cells (ILCs) family. NK cells were originally named for their natural killer activity against cancer cells [22]. During the development process, NK cells are derived from the same common lymphoid progenitor cells (CLPs) as T-cells and B-cells. CD34+ hematopoietic progenitor cells (HPCs) migrate from the bone marrow (BM) to numerous anatomical sites, including the thymus, lymph nodes, spleen, liver, etc. Through different developmental stages, HPCs drive the differentiation and maturation of NK cells by gradually down-regulating CD34 and up-regulating CD56 [23]. According to the difference in the expression density of CD56 and CD16, human NK cells can be further divided into two well-characterized subsets: CD56bright CD16− and CD56dimCD16+. The former predominantly reside in secondary lymphoid tissues, and their function is mainly to produce cytokines, including interferon-γ (IFN-γ), tumor necrosis factor (TNF), IL-10, and granulocyte-macrophage colony-stimulating factor (GM-CSF) [24,25]. In contrast, the CD56dimCD16+ subset accounts for about 90% of all NK cells in peripheral blood (PB) [26], mediating higher baseline perforin expression and stronger natural cytotoxicity [20].
Although NK cells lack somatic rearrangement receptors that specifically distinguish antigens, they inherently express a diverse repertoire of activating, co-stimulatory and inhibitory receptors. The dynamic balance between these receptors ensures a fine regulation of NK cell cytotoxic activity (Figure 1). NK cells are activated when the activation signal is stronger than the inhibitory signal; otherwise, they are in a tolerant state. Aggregation of activating receptors induces the activation of associated Src family kinases, which further phosphorylate the conserved tyrosine in the immunoreceptor tyrosine-based activation motifs (ITAM) and initiate activating signaling cascades [27]. Activating receptors include Fcγ receptor CD16, C-type lectin family (NKG2D, NKG2C), natural cytotoxicity receptors (NKp30, NKp44, and NKp46), cytokine-binding receptors, and many others [28]. These receptors, after binding with cognate ligands, induce polyfunctional NK cells degranulation and cytokine secretion (IFN-γ and MIP-1β) [29]. Killer cell immunoglobulin-like receptors (KIRs) and NKG2A/CD94 heterodimers are widely studied NK cell inhibitory receptors that recognize HLA class I molecules [20]. Immunoreceptor tyrosine-based inhibition motifs (ITIMs) in the intracellular domains act as an interface between these receptors and inhibitory signaling pathways, mediating the attenuation of NK cell activation signals [30].
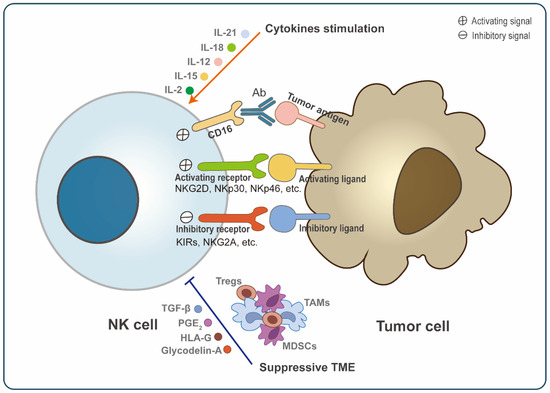
Figure 1.
General regulation of NK cell activation. Natural killer (NK) cell activation is a dynamic process mediated by multiple factors. NK cells express a range of receptors, including activating receptors (NKG2D, NKp30, NKp46, etc.) and inhibitory receptors (KIRs, NKG2A, etc.), which bind to corresponding tumor cell ligands and then transmit signals. Moreover, the Fc receptor (CD16) can also transmit activating signals into NK cells via binding with antibody-coated target cells. The stimulation of cytokines, such as IL-2, IL-15, IL-12, IL-18, and IL-21 also promotes the activation and proliferation of NK cells. However, many components in TME can suppress NK cell activation, including soluble suppressive molecules (TGF-β, prostaglandin E2, HLA-G, and glycodelin-A) and immunosuppressive cells, such as regulatory T-cells (Tregs), tumor-associated macrophages (TAMs), and myeloid-derived suppressor cells (MDSCs). Hence, the activation of NK cells ultimately depends on the sum of these signaling. When the activation signal is stronger than the inhibitory signal, NK cells will be activated and kill tumors.
2.2. Effector Function of NK Cells
2.2.1. Anti-Tumor Function
NK cells can initiate the cancer-immune defense function for the first time and respond rapidly to abnormal (pathological or malignant) cells. As a “warrior” with potent anti-cancer activity, NK cells function mainly by direct killing, antibody-dependent cell-mediated cytotoxicity (ADCC), and secreting cytokines.
In the early stages, tumor cells selectively lose MHC class I molecules to evade the T-cell attack [31]. However, these cells become sensitive to NK cell-mediated elimination because they have lost the NK cell inhibitory signal simultaneously. Additionally, cells with up-regulated expression of activating ligands are also targets of NK cells. NK cells recognize the corresponding ligands on the surface of these sensitive cells and directly kill tumor cells through non-specifically releasing killing mediators (perforin and granzyme) or by binding to death receptors (Fas-FasL and TRAIL-TRAILR) [32]. NK cells can also secrete extracellular vesicles (EVs), which contain nucleic acids, EV proteins, and cytolytic molecules that ultimately result in the apoptosis of tumor cells without cell-to-cell contact [33,34].
Another powerful anti-tumor function of NK cells is ADCC, predominantly mediated by CD16 binding to the Fc segment of antibodies [35]. Various clinically developed monoclonal antibodies (mAbs), such as rituximab [36], cetuximab [37], and infliximab [38], have been suggested to exploit this mechanism of NK cells to exert their anti-tumor effects. Moreover, activated NK cells also interact with other immune cells by synthesizing and secreting multiple cytokines, including IFN-γ, TNF, lymphotactin (XCL1), CC-chemokine ligand (CCL3, CCL4, and CCL5), IL-10, IL-13 and GM-CSF [39,40,41,42], thereby regulating the body’s immune function [41,43]. For example, secretion of IFN-γ induces T helper 1 cell polarization and stimulates tumor-specific CD8+ T-cells to enhance adaptive immunity [39,44]. Secretion of CCL5, XCL1 and FLT3L recruits dendritic cells to the tumour microenvironment and invokes proliferation and activation of these cells [41,42].
2.2.2. Pro-Tumor Function
However, NK cells also possess plasticity, which can be polarized to a pro-tumor type in response to the TME signals, as well as promote tumor angiogenesis and metastasis [45,46].
Increasing studies have found the infiltration of decidual NK-like cells in a variety of tumor tissues, including lung cancer [47], renal cell carcinoma [48], colorectal cancer [49], breast cancer [50], and melanoma [51]. Decidual NK cell is a unique NK cell subset to drive vascularization during embryo development [45]. Decidual NK-like cells possess the same CD56brightCD16− phenotype and pro-angiogenic activities as decidual NK [52]. For example, a study has shown that supernatants derived from non-small cell lung cancer (NSCLC) decidual NK-like cells can induce the formation of capillary-like structures in vitro [47]. During immunoediting, malignant cells evolve various strategies to evade NK cell attack and form macrometastases [53], such as up-regulating inhibitory ligands and down-regulating activating ligands [54,55], secreting metalloproteinases that mediate shedding of activating ligands [56,57], or reprogramming NK cells to a metastasis-promoting cell state [58]. NK cells also exist immune exhausted status under tumors or chronic infections. These cells often express markers of cell exhaustion, such as PD-1 [59,60], CD96 [61], TIGIT [62], NKG2A [63], and Tim-3 [64]. Moreover, their ability to produce cytotoxic factors (IFN-γ, granzyme, perforin) decreased significantly [63,65].
Soluble factors and cell-to-cell interactions within TME are the main reasons for inducing NK cell polarization and exhaustion [45,52,65]. Many immunosuppressive soluble factors, including TGF-β [66,67], prostaglandin E2 [68], human leukocyte antigen G (HLA-G) [69], hypoxia [67], and glycodelin-A [70], have been confirmed to induce NK cells towards a pro-tumorigenic phenotype. Moreover, several TME-associated cells, such as tumor-associated macrophages (TAMs), myeloid-derived suppressor cells (MDSCs), and regulatory T-cells (Tregs) could dampen NK cell cytotoxic activities by direct contact and secretion of soluble factors TGF-β and prostaglandin E2 [45,60,71,72] (Figure 1).
Given that these interactions could compromise the activity of NK cells, immunotherapy based on NK cells needs to consider the inherent plasticity of NK cells and design optimal strategies to promote effective phenotypes or limit the polarization to pro-tumor phenotypes.
3. NK-Based ACT
As the most commonly used immune effector cells in cellular immunotherapy, T-cells have achieved gratifying clinical efficacy in treating B-Cell Lymphoma [73,74]. CARs are artificial cell surface activating receptors that recognize specific antigens expressed on the surface of tumor cells. The clinical success of CAR-T therapy is booming in the adoption of cellular infusion [75,76,77]. To date, four CAR-T-cell products targeting CD19 and one targeting BCMA have been approved by the FDA for application [78,79,80,81]. Despite this, the field of CAR-T therapy still faces many challenges: (1) high levels of pro-inflammatory cytokines predispose to immune cell-associated neurotoxicity (ICANS) and cytokine release syndrome (CRS) with severe life-threatening toxicity [82,83,84]; (2) HLA barriers limit the cell source for large-scale production; (3) antigen escape induces treatment resistance and relapse; (4) limited migration and invasion in solid tumors [85,86]. NK cells have gained increasing attention for their advantages such as stable availability and safety properties [87]. The main preparations are similar between NK-based and T-based adoptive immunotherapy. NK cells are collected from patients or healthy volunteers typically and undergo a series of processes in vitro, including purification, activation and expansion, quality control, and eventually infusion into patients. These NK cell products not only kill target cells directly in vivo but also control cancer recurrence and metastasis by activating and/or enhancing the body’s immunity. In recent years, researchers have focused on optimizing the source and function of NK cells, especially with the advances in genetic engineering, making NK cells a unique and promising therapeutic tool in the ACT field (Figure 2).
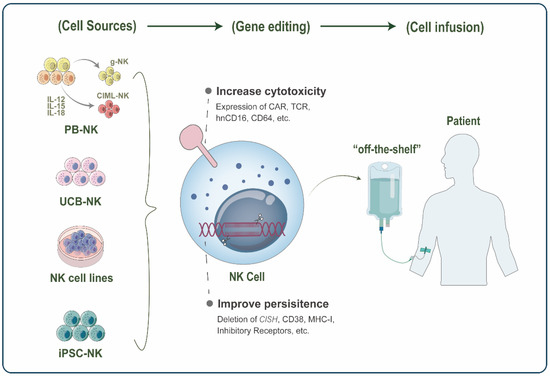
Figure 2.
Schematic representation of Natural killer (NK)-based adoptive cell therapy (ACT). NK cells for adoptive infusion come from a wide range of sources, including peripheral blood NK (PB-NK) cells, umbilical cord blood NK (UCB-NK) cells, NK cell lines, and induced pluripotent stem cell (iPSC)-derived NK cells. In PB, the novel FcRg-deficient NK(g-NK) subset and cytokine-induced memory-like NK (CIML-NK) have become promising cell sources in ACT with their enhanced antitumor activity and memory properties. Various gene editing strategies further enhance the function of therapeutic NK cells, with two main focuses: increasing NK cell cytotoxicity and improving persistence in vivo. Finally, these engineered NK cells can be infused into patients as “off-the-shelf” products for cancer therapy.
3.1. Cell Sources
To generate sufficient and high-quality NK cells, cell sources for application in immunotherapy have been largely enriched, including PB-NK [14,88,89], umbilical cord blood NK (UCB-NK) [90,91,92], NK cell lines [93,94,95,96], and stem cell-derived NK [97,98] (Figure 2).
3.1.1. PB-NK
Autologous or allogeneic PB is the most commonly used cell source in NK ACT. A phase I clinical trial indicated that autologous NK cells (expanded and activated) could exhibit preliminary anti-tumor activity on HER2-positive malignancies via trastuzumab-mediated ADCC [99]. However, for cancer immunotherapy, autologous NK cells are often exhausted or impaired by self-HLA class I levels [100]. In contrast, allogeneic PB-NK showed a stronger tumor-killing ability due to KIR-HLA incompatibility [101].
Notably, a unique cell subset in the PB of healthy individuals with prior exposure to human cytomegalovirus, termed FcRγ-deficient NK (g-NK) cells, is characterized by deficient for the signaling adaptor FcRγ and normal CD3ζ expression [102]. Compared with conventional NK cells, g-NK cells display a weaker direct killing effect on target cells but show stronger secretion of cytokines (IFN-γ and TNF-α) to CD16 stimulation [103]. Interestingly, Zhang and colleagues further found that g-NK cells presented distinct adaptive immunity features and also have a dominant advantage over memory-like T-cells in number [102]. In addition, g-NK cells exhibit robust amplification on virus-infected cells in an antibody-dependent manner and maintain a constant level in vivo for a long time [104]. In multiple myeloma (MM), the latest preclinical trial has confirmed that when combined with daratumumab, expanded g-NK cells with low CD38 expression and potent ADCC activity presented dramatically superior curative and persistence than conventional NK cells [105]. These characteristics of long-lived g-NK cells suggest that they have the potential to become a novel cell source in adoptive immunotherapy to provide more powerful therapeutic effects.
Under a series of cytokine stimulation [106], NK cells demonstrate memory-like properties similar to T-cells and B-cells. PB-NK cells preactivated briefly by the combination of IL-12, IL-15, and IL-18, show a stronger IFN-γ response upon restimulation of cytokine or K562 leukemia cells [107]. As a promising cell source in ACT, cytokine-induced memory-like NK (CIML-NK) has achieved great success in the anti-tumor response of NK-resistant lymphoma and melanoma and effectively induced complete remission in some leukemia patients [108,109,110].
3.1.2. UCB-NK
Unlike PB-NK, NK cells in UCB are more abundant (30% of lymphocytes), proliferative, and easy to collect. The current platform has been able to expand highly pure (>99.9%) clinical-grade UCB NK cells within 2 weeks for cancer immunotherapy [111]. After long-term cryopreservation, the expansion efficiency and cytotoxicity of UCB-NK cells are intact [92,112]. Compared with PB CD56dim cells, resting UCB CD56dim cells overexpressing pro-apoptotic genes are at an earlier developmental stage [113]. They typically express higher levels of NKG2A and NKG2D and functionally manifest reduced cytotoxicity in vitro against multiple non-Hodgkin’s lymphoma [113]. Of note, after stimulation with cytokines (IL-2 and IL-15), UCB-NK significantly upregulates the expression of NKp46, achieving lysis activity comparable to that of equally treated PB NK cells [114]. When cultured with γ-irradiated PLH feeder cells, UCB-NK cells were observed to have greater cytotoxicity than PB-NK cells against primary MM cells [90]. Moreover, the combination with antibody therapy also facilitates the infiltration of UCB-NK cells in the tumor bed in immunodeficient mice [91].
3.1.3. NK Cell Lines
NK cell lines, such as NK-92, KHYG-1, YTS, HANK-1, and NKL, are attractive sources for NK-based immunotherapy. The major advantage of NK cell lines is that they can be easily cultured, expanded, and genetically edited. NK-92, isolated from a 50-year-old male patient with non-Hodgkin’s lymphoma, has been tested in phase I clinical trials [95,115,116,117]. Since NK-92 is tumor-derived, it must be irradiated before administration to ensure its safety. NK-92 has also been genetically engineered with CAR to treat a variety of cancers in preclinical studies, including melanoma, high-risk rhabdomyosarcoma, and lymphoma [118,119,120]. Recently, NK-92 cells have been developed to express chimerically modified CD16 receptors to enhance the lysis efficacy against tumor cells [121,122]. Compared with CAR NK derived from a primary human donor, CAR NK-92 cells secrete more granzyme A and IL-17A. Their stronger cytotoxicity against leukemia cells also leads to the destruction of healthy cells [123]. A modified version of NK-92, NK-92MI (IL-2-independent) has been explored in various cancers treatment for its excellent expansion ability and cytotoxicity [96,124,125,126]. Notably, NK101 (from an NK/T-cell lymphoma patient) has the potential to produce immunostimulatory cytokines to mobilize host anti-tumor immunity, which is the first cell line to show superior cytotoxicity to NK-92 in the 4T1 mammary carcinoma model [93]. Terminally irradiated NK cell lines often require multiple repeat infusions with non-viable cells to achieve persistence in vivo, but there also has some concerns, such as the potential for alloimmunity and rejection.
3.1.4. Stem Cell-Derived NK
Another ideal candidate for “off-the-shelf” product development is stem cell-derived NK cells. Induced pluripotent stem cell (iPSC)-derived NK cells have several advantages, such as yielding a homogenous population of NK cells, an unlimited source of NK cells, and strong plasticity [127]. Although NK cells from different iPSC lines represent the differential expression of KIRs, their cytotoxicity is generally similar [128]. iPSC-NK cells are typically used for gene editing at the clonal level to improve the anti-tumor activity of NK cells [97,98].
By and large, the expansion of PB-derived NK cells to adequate cell doses is labor- and time-consuming in trials to date. NK cell lines may induce cell toxicity and rejection. In contrast, UCB or iPSC-derived NK cells are capable of adequate ex vivo expansion and appear to be safe, demonstrating the novel therapeutic potential for future clinical application.
3.2. Genetic Engineering
Genetic engineering technology generally refers to a series of artificial modifications of genes to create new genetic characteristics. Recently, multiple genetic engineering approaches have been tested to optimize the anti-tumor activity of NK cells and provide better adoptive NK cell therapy for cancer patients. Currently, genetic engineering on NK cells mainly includes arming NK cells with CAR or other constructions to improve the efficacy and specificity of NK cells (Figure 3 and Figure 4).
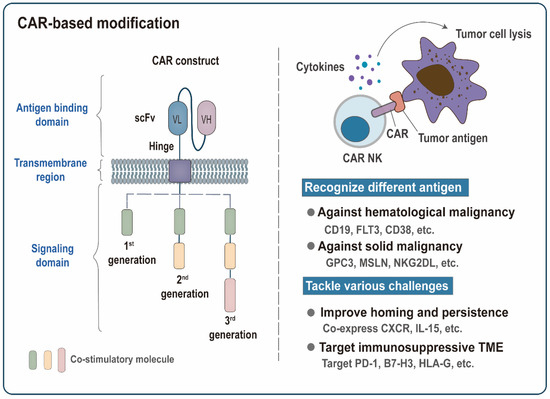
Figure 3.
Chimeric antigen receptors (CAR)-based modification. Functional CAR molecule often consists of an antigen-binding domain, a transmembrane region, and an intracellular signaling domain. The extracellular domain contains a single-chain variable fragment (scFv) that binds to tumor antigens. Conventional CAR receptors are divided into three generations. Based on the structure of the first-generation CAR, the second and third-generation CARs with enhanced proliferation and killing capability are formed by adding the intracellular part of one or two costimulatory molecules. CAR molecules confer NK cells the ability to recognize tumor antigens specifically and transmit activation signals into NK cells, thereby triggering the lysis of tumor cells. The development of CAR modification has two main focal points: recognizing different tumor antigens and tackling various challenges. The scFv structures targeting different tumor antigens endow CAK-NK cells with different specificities against multiple malignancies. Co-expression of the chemokine receptors (CXCR) or IL-15 can further promote homing of CAR NK cells to tumor sites or improve their in vivo persistence. Targeting inhibitory receptors PD-1 or inhibitory ligands (B7-H3 and HLA-G) provides an effective way to overcome the immunosuppressive tumor microenvironment (TME).
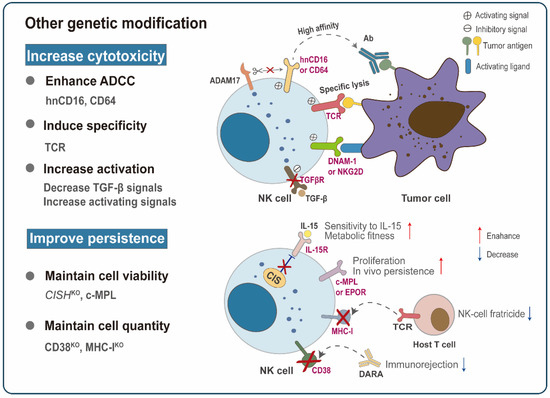
Figure 4.
Other advanced genetic modification strategies. Other non-CAR structure-based genetic modifications have also been explored to improve NK cytotoxicity and in vivo persistence. For example, the introduction of high-affinity non-ADAM17 cleavable CD16 (hnCD16) or CD64 significantly enhances the affinity of NK cells to the tumor-targeting antibodies (Abs), thereby improving antibody-dependent cell-mediated cytotoxicity (ADCC). TCR NK cells achieve similar tumor control as redirected T-cells while retaining their NK cell function. Targeting TGF-β signaling or transducing activating receptors (DNAM-1, NKG2D) can tilt NK cells toward activation. Strategies to improve in vivo persistence mainly focus on maintaining the viability and quantity of NK cells. Under low cytokine concentration, possible methods to maintain cell vitality include the deletion of CISH or the expression of c-MPL or EPOR. Prolonged persistence can also be achieved by deleting the endogenous CD38 or MHC-I of NK cells so that the infused NK cells are undetectable to the daratumumab (DARA) or the recipient’s T-lymphocytes.
3.2.1. CAR-Modified NK Cells
NK cells are a promising alternative kit in the ACT toolbox. First, NK cell products are clinically proven to be safe, and their toxicity is mild [13,129]. Second, NK cells have abundant sources with fewer graft restrictions. Third, NK cells exert CAR-dependent targeting specificity while retaining their original natural cytotoxicity, thereby helping to overcome antigen escape [130]. In addition, a variety of genetic modification strategies have further enhanced the specific and non-specific cytotoxicity, homing and infiltration capacity, in vivo persistence, and resistance to immunosuppression of CAR NK. Depending on the number of intracellular activation signals, conventional CAR receptors can be divided into three generations (Figure 3). Other more advanced CAR structures are being designed and tested to optimize the therapeutic efficacy against cancer, such as increasing the expression of cytokines [131], simultaneously targeting multiple specific antigens [132,133,134], using a construct based on nanobodies with high stability and solubility [96,135], or introducing a novel chimeric costimulatory converting receptor [136], an NK cell costimulatory domain [137,138], and a suicide gene that control the number of infused NK cells to avoid toxic side effects [139]. In the following, we presented some recent preclinical studies of CAR-NK cells (Table 1).

Table 1.
Current CAR NK cell products.
CAR NK Cells against Hematological Malignancy
As a molecule widely and specifically expressed in B-cell malignancies, CD19 serves as an ideal target for the ACT. One of the earliest studies showed that primary NK cells bearing a second-generation anti-CD19 CAR could overcome inhibitory signaling and specifically kill leukemic cells [161]. In this study, the investigators also found that co-culture with K562-mb15-41BBL favors the expansion and retroviral transduction of NK cells in vitro. CD3ζ fragment in the anti-CD19 CAR has induced superior NK-cell activity compared with DAP10, while the NK-cell activation and cytotoxicity can be further enhanced by combining both 4-1BB and CD3ζ [161]. However, after anti-CD19 CAR T/NK cell therapy, some leukemia cells develop genetic mutations and loss of CD19 antigen heterozygosity, resulting in relapsed or refractory acute leukemia [162]. This has spurred the progression of CAR NK cells that recognize alternative B-cell acute lymphoblastic leukemia (B-ALL) targets, such as FMS-like tyrosine kinase 3 (FLT3). Oelsner et al. confirmed that FLT3-positive leukemia cells specifically activated NK-92 cells expressing an anti-FLT3 CAR [139]. Interestingly, researchers also use inducible caspase 9 (iCasp9), a suicide gene that induces the rapid apoptosis of transduction cells, as a safety switch rather than traditional γ-irradiation for the CAR NK-92 cells to prevent possible adverse events [139].
CD38, a promising target for cytotoxic Abs therapy, is commonly overexpressed in MM and other hematological malignancies [163]. Nanobodies are single immunoglobulin variable domains derived from camelids’ heavy-chain antibodies, which exhibit low immunogenicity, high specificity, and stability [164]. A third-generation CAR construct based on nanobodies has been developed to target three different epitopes of CD38 [135]. These CAR NK-92 cells possess potent and specific lytic activity against CD38-expressing Burkitt lymphoma cell lines and primary MM cells in vitro, providing a basis for future clinical development [163]. In addition, to prevent CD38 expression on normal NK cells from impairing the efficacy of CAR NK treatment, researchers have utilized CRISPR/Cas9 genome editing to disrupt the expression of CD38 on primary NK cells and then combined mRNA electroporation technology to bring an affinity-optimized CD38 CAR [140]. They finally construct endogenous CD38 knockdown CAR NK cells with promising success in minimizing NK cell fratricide and specifically killing CD38+ acute myeloid leukemia (AML) cell lines and primary leukemia cells [140].
The application of CAR T cells in T-cell acute lymphoblastic leukemia(T-ALL) therapy remains restricted due to some shared antigens between effector cells and malignancies. Such as CD5 and CD7, important characteristic markers of malignant T cells, which are also commonly expressed on normal T cells. Preclinical research has revealed that anti-CD5 CAR NK-92 cells could be an alternative therapeutic strategy, and in this study, the intracellular part of 2B4, an NK-related costimulatory receptor, was integrated into the anti-CD5 CAR construct. In contrast to the T-cell-associated costimulatory domain 4-1BB, the 2B4 costimulatory domain promoted NK cells’ proliferation and their cytotoxic activity against CD5+ hematological malignant cells. The ability of anti-CD7 CAR NK-92MI to specifically eradicate CD7-positive tumor cells was observed both in vitro and in T-ALL PDX mouse models [96]. In addition, bivalent anti-CD7 nanobody sequence CAR NK-92MI displayed robust IFN-γ and Granzyme B secretion against primary T-ALL samples [96].
Recently, immunotherapies targeting CD33 and CD123 have achieved promising results in treating AML. The latest study showed that CD33-targeting CAR NK cells combined the broad expression advantage of CD33 targets and the safety of NK cells, effectively eliminating the engraftment of CD33+ AML cells in the BM and spleen [141]. In a different study, NK-92 cells stably expressed anti-CD123-CAR and IL-15 [142]. CAR-modified NK-92 cells were significantly more cytotoxic to CD123+ AML cells than unmodified NK-92 cells both in vitro and in an AML-PDX model [142]. Another study tested 8 different CAR structures that contain scFv targeting CD123 and 4 different components (DAP10, FcεRIγ, 2B4, and the ζ chain of the T-cell receptor) on PB-NK cells [143]. Among various combinations, the construct CD123-2B4-CD3ζCAR NK cells not only possessed superior target cell-specific lysis ability in vitro but also had the most pronounced tumor growth inhibitory effect in the AML xenograft model [143]. Furthermore, transgenic co-expression of IL-15 endowed CAR NK cells with enhanced activation and long-term cytolytic activity. However, it also showed lethal toxicity in mouse models [143]. These results suggest that 2B4 is a more suitable component of CAR NK, although the application of IL-15 can improve the persistence of NK, it is also essential to ensure its safety in clinical application.
CAR NK Cells against Solid Malignancy
Solid tumor-related antigens have also been tested as targets for NK-based immunotherapies. For example, Glypican-3 (GPC3), a tumor-specific antigen, is highly expressed in hepatocellular carcinoma (HCC), hepatoblastoma, and clear-cell carcinoma of the ovary (CCCO). A GPC3-specific CAR was designed and delivered into NK-92 cells to generate a novel CAR-NK cell line termed NK-92/9.28.z [148]. In vitro, NK-92/9.28.z cells exhibited specific cytotoxicity against GPC3+ HCC cells with high levels of secretion of IFN-γ, and the function of NK-92/9.28.z cells was not affected by inhibitory factors, such as hypoxic, soluble TGF-β and GPC3 [148]. In vivo, irradiated NK-92/9.28.z cells were also demonstrated to be enriched at the tumor site and efficiently inhibited the growth of GPC3+ HCC xenografts without severe adverse effects [148]. Moreover, the introduction of the NK-cell costimulatory domain (DNAM1 and 2B4) further optimized the GPC3-specific CAR construction, allowing CAR NK-92 cells to exhibit stronger proliferation and anti-apoptotic ability and achieve predominate cytotoxicity against HCC cells in vitro [138]. To make therapeutic NK cells more homogeneous, Ueda et al. established a master cell bank by selecting HLA homozygous iPSC clones with anti-GPC3 CAR modification [149]. These CAR NK cells showed effective cytotoxicity against GPC3-positive tumor cells both in vitro and in vivo and prolonged the survival of CCCO mice without any systemic toxicity and tumorigenicity [149].
It has been reported that anti-folate receptor alpha (αFR) CAR-engineered NK-92 cells specifically target αFR+ ovarian cancer (OC) cells [150]. Interestingly, when stimulated with αFR antigen in vitro, the third-generation αFR-specific CAR-modified NK-92 cells displayed not only potent cytokine secretion and degranulation but also high levels of proliferative and anti-apoptotic abilities [150]. Additionally, anti-αFR CAR-engineered NK-92 cells dramatically eliminate OC cells in vivo and effectively delay disease progression in tumor-bearing mice [150]. Mesothelin (MSLN) is another molecule that can be targeted by CAR-modified NK-92 cells in treating OC. MSLN-CAR-engineered NK-92 specifically lysed MSLN+ OC cell lines accompanied by high levels of IFN-γ, granzyme B, and GM-CSF. MSLN-CAR NK-92 cells also achieved even more effective tumor control and increased overall survival of NSG mice carrying OC cells, when compared with therapies using CD19-CAR NK-92 or mock control [151]. The researchers further evaluated the feasibility of using MSLN-CAR NK-92 to treat MSLN-positive gastric cancer. They demonstrated that CAR NK-92 cells efficiently infiltrated into the tumor sites and exhibited potent MSLN-dependent cytotoxic activity both in vitro and in the PDX model [151].
A previous study by Hu, Z et al. confirmed that tissue factor (TF) serves as a novel target in 50% to 85% of patients with triple-negative breast cancer (TNBC) [165]. Hu, Z further developed the TF-targeting CAR NK-92MI cell (co-expressing CD16), and a series of preclinical tests were carried out to evaluate its therapeutic effect [153]. He observed that TF-targeting CAR NK cells have direct cytotoxicity against TNBC cells and can mediate stronger ADCC when combined with therapeutic antibodies [153]. Additionally, TF-targeting CAR NK cell therapy also showed potent tumor-directed killing in mouse models of TNBC CDX and PDX [153]. Notably, the epidermal growth factor receptor (EGFR) is another therapeutic target for TNBC. In vitro, EGFR-CAR NK cells exhibited potent cytokine secretion against TNBC cells and mediated specific target cell lysis [154].
Tumor tissues often overexpress NKG2D ligands (MICA/B) that interact with the endogenous activating receptor NKG2D on NK cells. Hence, the adoptive transfer of NKG2D-modified NK cells is an attractive treatment strategy for multiple cancers. A pilot clinical trial study tested the therapeutic effect of NKG2D-modified NK cells in three patients with metastatic colorectal cancer [155]. Researchers confirmed that the extracellular domain of the NKG2D receptor specifically binds to NKG2D ligands expressed on tumor cells and further transmits NK cell activation signals through the intracellular domain of DAP12 [155]. In this study, the number of epithelial cell adhesion molecule (EpCAM)-positive cancer cells in malignant ascites of two patients was dramatically reduced after intraperitoneal infusion of CAR NK cells. Another patient with liver metastases received an ultrasound-guided percutaneous injection of CAR NK cells [155]. A complete metabolic response was detected at the injection site, and the tumor regressed rapidly in the liver region [155]. Notably, CAR modifications were achieved by non-integrating mRNA electroporation technology, and transient expression and multiple infusions ensured the efficacy and safety of clinical application [155]. Patient-derived NKG2D-CAR NK displayed superior cytotoxic activity against MM cells in vitro compared with memory NKG2D-CAR T-cells and mediated more efficient tumor control in a mouse model without any sign of adverse side effects [156]. Notably, advanced tumor cells can utilize the proteolytic shedding of MICA/B to achieve immune escape [166,167,168]. Blocking the shedding of MICA/B [169] or preparing recombinant MICA fusion proteins[170] could enhance NK cell-mediated killing. NKG2D-CAR NK as a combination partner with these immunotherapies may further improve the antitumor efficacy.
A recent study preliminarily evaluated the efficacy and safety of a CAR NK cellular product targeting human epidermal growth factor receptor 2 (HER2) [158]. In terms of product performance, the expression of HER2 CAR further enhanced the specific cytotoxicity of NK cells against various HER2-expressing tumor cell lines [158]. Unlike HER2-specific CAR T, CAR NK elicited more controllable cytokine secretion and had no enhanced cytolysis on non-malignant bronchial epithelial cells expressing basal levels of HER2 [158]. Furthermore, they observed an interesting phenomenon that lentiviral transduction up-regulated the expression of nutrient receptors CD71 and CD98 on the surface of NK cells, leading to a non-specific enhancement of their tumor-killing capacity [158].
Challenges to CAR NK Cells Application
Despite the advantages of CAR-NK cells, their clinical application is still limited by several factors, such as insufficient infiltration and homing of immune cells, low persistence in vivo, and immunosuppressive TME, etc.
In anti-tumor treatment, the infiltration of immune cells into tumor sites significantly affects the therapeutic efficacy of CAR NK cells, especially in non-hematological malignancies. To address the issue, modification of chemokine receptors provided a novel strategy to overcome the trafficking barrier and improve the immunotherapeutic efficacy of CAR NK cells against malignancies. Given the high affinity of CXCR1 to IL-8, CXCR1 transduction has been tested to augment the migration and infiltration of NK cells to IL-8-secreting tumor cells [157]. Importantly, compared with NK cells expressing only the NKG2D-specific CAR or mock control, NK cells co-expressing CXCR1 and the NKG2D CAR significantly relieved tumor burden and prolonged survival of mice carrying SKOV3 xenografts [157]. Genetic engineering of CAR NK cells with genes encoding the CXCR4 to increase tumor homing and penetration has also been explored. A recent study demonstrated that CD19-specific CAR NK cells that co-expressed CXCR4 exhibit augmented chemotaxis toward BM while retaining their CD19-specific cytotoxic activity in vitro [144]. Interestingly, it become apparent that the third-generation VSV-G pseudo-typed lentivirus using polybrene as an enhancer was important for high and stable transduction efficiency, enabling CAR expression in more than 80% of primary NK cells [144].
Lacking cytokines support with the limited persistence of CAR NK cells is also a major obstacle to clinical success. Since IL-15 is a critical cytokine required to maintain NK cells’ survival, proliferation, and activation, there has been an ongoing quest to elevate the persistence and anti-tumor activity of NK cell products. In 2018, Katayoun Rezvani’s group generated the fourth-generation anti-CD19 CAR structure [145]. Compared with conventional CAR modification, this novel construct has several improvements. First, IL-15 is expressed to support NK cell proliferation, persistence, and homing in a xenograft NSG mouse model, while retaining the specific cytotoxicity of traditional anti-CD19 CAR constructs against CD19+ tumor targets. Second, the addition of incorporating inducible caspase-9 (iC9), which can be stimulated by a small-molecule dimerizer to rapidly eliminate transduced cells, augmented the controllability and safety of adoptive therapy. Third, the source of adoptive cells was optimized by using UCB-derived allogeneic NK cells to guarantee sufficient numbers for ex vivo therapy [145]. Recently, to further boost the anti-tumor effect of IL-15-secreting CAR NK cells, investigators knocked out their CISH gene, which encodes the immune checkpoint protein, cytokine-inducible Src homology 2-containing protein (CIS) [131]. They revealed that the removal of CIS in CAR19/IL-15 NK cells completely abolished the inhibition of IL-15 signaling, which in turn activated the Akt/mTORC1-c-MYC signaling axis, thus elevating aerobic glycolysis [131]. In relapsed/refractory B-cell malignancy xenografts model, this upgraded therapy showed potent metabolic fitness and tumor control without any measurable toxicity or malignant transformation [131]. Another study revealed that anti-NKG2D-CAR NK cells also specifically killed AML cells and that ectopic expression of IL-15 could further improve the cytolytic activity and persistence of CAR NK cells [146]. Encouragingly, in a mouse model of KG-1 AML, NKG2D CAR/IL15-NK treatment significantly prolonged the survival of mice and even achieved complete tumor regression after multiple injections [146]. During this study, electroporation of a piggyBac transposon platform was critical for high transduction efficiency, enabling more than 60% of PB-NK cells to stably co-express anti-NKG2D CAR and IL-15 [146]. Moreover, a preclinical study proved that CAR-NK cells targeting prostate stem cell antigen (PSCA) also kept a potent killing effect against pancreatic cancer (PC) cells after 1 cycle of freeze-thaw, which might largely benefit from the co-expression of IL-15 [92]. And this cryopreservation cellular product could survive over 90 days in a metastatic PC model, further supporting the application of NK as an “off-the-shelf” product [92].
Another major challenge in solid cancer immunotherapy is the immunosuppression elicited by intrinsic inhibitory ligands, including PD-L1, B7-H3, and HLA-G. Encouragingly, a novel chimeric PD-1-NKG2D-41BB receptor has been investigated to reverse the immune-suppressive effects of PD-1 [136]. Compared with normal NK-92 cells, CCCR modification markedly augmented the cytotoxicity of NK-92, thereby triggering extensive pyroptosis of PD-L1-positive lung carcinoma H1299 cells in vitro [136]. B7-H3-targeting CAR-engineered NK-92MI cells were also designed to accelerate their degranulation activity and specific cytotoxicity against NSCLC. In the NSCLC xenografts model, the anti-B7-H3 CAR NK-92MI cell further unfolded their excellent immunotherapeutic efficacy. Additionally, anti-B7-H3-CAR NK-92 cells are also served as a promising cellular product for the therapy of melanoma. In either 2- or 3-dimensional assays in vitro, CAR-modified NK-92 cells can overcome the major challenges of TME, including hypoxic conditions, immunosuppressive molecules such as TGF-β, and an acidic pH environment, thus successfully migrating and infiltrating into the tumor site and specifically dissolved MM cell lines [118]. To counteract immune escape in solid tumors, Jan et al. developed a CAR construct targeting HLA-G, consisting of an anti-HLA-G antibody scFv fragment, a KIR2DS4 linkage region, the signaling adaptive molecule DAP12, and the suicide iCasp9 protein [159]. In this study, they further elucidated the anticancer mechanism of this CAR NK strategy. HLA-G CAR competes with inhibitory receptors of NK cells (LILRB1 and KIR2D4) to bind HLA-G on malignant tumors, contributing to the upregulation of phosphor-Syk/Zap70 and the downregulation of phosphor-SHP-1 [159]. Hence, anti-HLA-G CAR NK cells successfully converted immunosuppressive signals into activation signals, triggering HLA-G-depend-cytotoxicity of NK cells in vitro and effectively ablating tumors in orthotopic mice models [159]. Notably, they revealed that low-dose chemotherapy-induced accumulation of HLA-G (involving suppression of DMNT1 and TAP-1 promoter demethylation) further improves the anti-tumor efficacy of HLA-G CAR NK cells [159].
Recently, researchers found that a high concentration of hydrogen peroxide in TME significantly impaired the vitality and cytotoxicity of NK cells, whereas IL-15 could protect NK cells from redox stress induced by H2O2 via transiently up-regulating the expression of peroxidase-1 (PRDX1) [160]. To compensate for the deficiency of the antioxidant defense system of NK cells, they used a genetic modification strategy to stably over-express PRDX1 in PD-L1-CAR NK-92MI cells [160]. As expected, PRDX1 modification supported the proliferation and survival of NK-92MI under oxidative stress and further improved their anti-tumor cytotoxicity both in vitro and in the breast cancer CDX model [160]. In addition, to resist the immunosuppressive effects mediated by Treg cells in TME, an anti-CD25 CAR-expressing NK-92 cell was designed, which specifically lysed CD25-bearing Jurkat cells with appropriate cytotoxicity and IFN-γ cytokine production [147].
In summary, CAR NK is a novel and promising strategy with great potential to become an “off-the-shelf” allogeneic cell therapy against multiple cancers. To expand the application of CAR NK cells, researchers have designed different antigen receptors for different therapeutic targets to trigger the specific killing of NK cells against corresponding tumor cells. Numerous efforts have been devoted to optimizing the structure of CAR NK to alleviate immunosuppression and enhance their anti-tumor activity, including using advanced genetic engineering techniques to enable NK cells to simultaneously express IL-15 or knock out checkpoint molecules. Additionally, the introduction of the suicide gene further ensures the stability and safety of CAR NK products.
3.2.2. Enhancement of NK Cell Function
In addition to redirected modifications based on CAR structure, other genetic engineering strategies have also been explored to enhance the anti-cancer efficacy of NK cells (Table 2). These strategies mainly focus on two aspects: augmenting NK cell cytotoxicity and improving persistence in vivo (Figure 4).

Table 2.
Current genetic engineering strategies aimed at improving the functions of NK cells.
Enhancing Cytotoxicity
Rapid advances in gene-editing technologies have provided multiple strategies for optimizing the cytotoxicity of NK cells, including enhancing ADCC function, inducing target specificity, and releasing immunosuppression.
Monoclonal antibody-based immunotherapies, such as trastuzumab and rituximab, have brought hope to many cancer patients, and ADCC has been reported as one of the major mechanisms affecting its clinical efficacy [26]. As a critical receptor mediating the ADCC process, CD16a crosslinks with the Fc region of IgG Abs on immunoglobulin-opsonized cells to initiate the phosphorylation of intracellular signal adaptor CD3ζ and FcRγ, thereby inducing the activation of NK cells [186,187]. Notably, CD16 expression and function are negatively regulated by recombinant a disintegrin and metalloprotease metalloprotease-17 (ADAM17). It has been shown that ADAM17 inhibits ADCC by rapidly cleaving CD16a when NK cells are stimulated by cytokines or target cells [188,189]. Targeting ADAM17 effectively prevented CD16a shedding of NK cells and significantly enhanced INF-γ secretion [188]. However, given the broad expression of ADAM17 and the numerous hydrolysis substrates, the application of ADAM17 inhibitors lacks specificity and may impair normal biological processes. By contrast, genetic modification of CD16a is a better option to prevent the loss of CD16a on tumor-infiltrating NK cells. The antibody binding affinity of CD16a is susceptible to single nucleotide polymorphism at its amino acid position 158. Specifically, CD16a with valine at the same position (158V) has a higher affinity for mAbs compared with CD16a with phenylalanine at the position 158 [190,191,192]. A recent study demonstrated that the expression of high-affinity CD16 (allotype-V158) receptor confers high-affinity for NK-92 binding to the Fc fragment of therapeutic mAbs, thereby promoting ADCC-mediated lysis of target cells in vitro [122]. Bruce Walcheck’s team used site-directed mutagenesis to replace the proline at position 197 in the middle of the cleavage region with serine and manufactured a non-cleavable version of CD16a (CD16a/S197P) [171]. They did observe that engineered S197P mutation significantly inhibits CD16a receptors shedding from NK-92 cells or iPSC-derived NK cells without disrupting their function [171]. They further introduced a 158V high-affinity variant in the CD16a/S197P structure and successfully engineered iPSC–derived NK cells with high-affinity non-ADAM17 cleavable CD16a (hnCD16-iNK) via a human pluripotent stem cell-like platform [98]. hnCD16-iNK cells could effectively counteract activation-induced cleavage of CD16a to maintain their stable expression [98]. In an ex vivo cytological experiment, hnCD16-iNK cells outperformed PB-NK and unmodified iNK cells for numerous tumor cell-mediated ADCC activities, exhibiting higher levels of cytokine secretion and target cell lysis [98]. In Raji-Luc or SKOV-3-Luc xenograft mouse models, Ab therapy combined with multiple infusions of hnCD16-iNK cells maximized ADCC activity in vivo, contributing to tumor regression and prolonged mouse survival [98]. In a human lymphoma systemic tumor mode, three tumor-bearing mice even achieved complete remission after receiving hnCD16-iNK cells combined with anti-CD20 mAbs and survived for more than 100 days [98]. In addition to direct modification of CD16a, seeking alternative structures of CD16a is also a new direction for investigators. CD64, which also belongs to the Fcγ receptor family, has become an ideal substitute for CD16a for its high IgG affinity and non-cleavable properties. CD64 is primarily expressed on the surface of monocytes, macrophages, or activated neutrophils and initiates the transduction of downstream activation signals through the phosphorylation of ITAM on intracellular FcRγ [193,194]. Studies have shown that CD64-engineered NK-92MI cell lines can also transmit activation signals via CD3ζ to mediate ADCC [173,195]. A novel recombinant receptor, called CD64/16a, has been developed that significantly enhanced the ability of NK-92 and iPSC-derived NK cells to capture soluble tumor-targeting mAbs and to mediate stronger ADCC [172]. The latest study further revealed that intact CD64 could also be stably expressed on NK-92MI cells and induced efficient ADCC. In a prostate cancer xenograft mouse model, adoptive NK-92MICD64 cell transferred with anti-TROP2 and B12 mAb led to excellent tumor control and maintained long-term survival of tumor-bearing mice [173].
To further elevate the specific killing ability of NK cells against malignancies, researchers have carried out a bold innovation of genetically engineering the TCR gene for an NK-based therapeutic strategy. A preclinical study endowed NK-92 cells with human CD3 polycistronic complex (CD3γ, δ, ε, ζ dimers) and TCR α/β constructs in two steps by a retrovirus, finally turning NK-92 into a T-cell-like effector cell [174]. TCR-NK-92 exhibited T cell-related phenotype, metabolic and functional characteristics while retaining its NK cell function, and achieved similar tumor control as redirected T cells in tumor-bearing mice [174]. Another study created TCR-NK92 and TCR-YTS with a similar approach, perfectly avoiding mismatches between endogenous and modified TCR subunits [175]. After successful expression of antigen-specific TCR, NK-92 and YTS triggered strong TCR-mediated antigen-specific degranulation and cytokine secretion and showed rapid targeting of tyrosinase-positive melanoma cells, effectively inhibiting tumor growth [175]. Glycoengineering is a simple and efficient approach to the genetic modification of NK cells. Arming NK-92 cells with high-affinity and specific CD22 ligands via glycan metabolic engineering or glycopolymer insertion, rather than CAR engineering, substantially improved their ability to bind and lyse CD22+ lymphoma cells in vitro and xenograft model [176]. Recently, Hong and colleagues further induced selectin ligands into CD22-targeting NK-92MI cells via cell-surface chemoenzymatic glycan editing to promote their trafficking to B lymphoma [126].
Many malignancies evade immune control by generating a pro-tumorigenic environment dominated largely by TGF-β [177,196]. In the treatment of glioblastoma multiforme (GBM), disruption of the TGFBR2 gene in NK cells could restore the antitumor activity of NK cells [177]. Smad3, a key mediator of TGF-β signaling, serves as a precise target for NK cells to attenuate TGF-β-mediated immunosuppression. Indeed, silencing Smad3 restored IFN-γ production by upregulating E4BP4 transcription, thus significantly enhancing NK-92 cell-mediated cytotoxicity against cancer [178]. Interestingly, Burga et al. genetically engineered UCB-NK cells with variant TGF-β receptors, which achieved successful conversion of TGF-β-mediated negative signaling into activating signaling [179]. They revealed that novel TGF-β receptor-modified NK cells blocked the phosphorylation of Smad2 and Smad3, and further promoted the activation of NK to kill neuroblastoma cells in a TGF-β-rich environment [179]. It is widely accepted that the E3 ligase CBLB is a critical negative regulator of lymphocytes. CBLB deletion in placental CD34+ cell-derived NK (PNK) cells improved proliferation and augmented cytotoxic effect against multiple tumor cells in vitro [180]. Moreover, CBLBKO PNK cells successfully proliferated and matured in NSG mice and showed improved leukemia control [180]. Notably, the CRISPR/Cas9 technology provides high CBLB editing efficiency in more than 90% of PNK cells, without any impact on cell proliferation, differentiation, or phenotype [180].
Activation and migration of NK cells also play a critical role in exerting their anti-tumor efficacy. Despite the overexpression of DNAM-1 ligand (CD112 or CD155) on human primary sarcoma cells, a preclinical study observed that NK cells minimally infiltrated into sarcomas and tended to downregulate the expression of activating receptors DNAM-1 and NKG2D [181]. This study further confirmed that overexpression of DNAM-1 or NKG2D enhanced the degranulation and cytokine secretion of NK cells, making them more effective in targeting sarcoma explants and multiple tumor cells [181]. Activation with IL-2, cryopreservation, and ex vivo expansion always down-regulate CXCR4 expression on NK cells, further impairing their migration to SDF-1α [182]. Inspiringly, the expression of gain-of-function (GOF) variant CXCR4R334X in PB-NK cells could reverse the reduction of CXCR4 and facilitate chemotaxis to SDF-1α [182]. Furthermore, CXCR4-expressing cells performed superior BM homing ability than control NK cells after adoptive transplantation into NSG mice [182].
Improving Persistence
It is essential to maintain the viability and number of NK cells to improve persistence in vivo. Adoptive NK cell immunotherapy often relies on IL-15 or IL-2 to support NK cell viability, but these cytokines may carry serious dose-dependent toxicities. To minimize or avoid the necessity for exogenous cytokines, novel targets of NK cell genetic engineering have been explored recently. For example, the deletion of CISH activated IL-15-mediated JAK-STAT signaling and significantly improved iPSC-derived NK in vitro proliferation and in vivo persistence [97]. Activation of IL-15 signaling further promoted mTOR-mediated metabolic fitness, including more efficient glycolysis and oxidative phosphorylation activity, which directly augmented the anti-tumor activity of CISH-knockout NK cells under low cytokines concentration [97]. Another study generated hematopoietic growth factor receptors and modified NK cells to replace the administration of exogenous cytokines. Under the stimulation of cognate ligand combined with low-dose IL-2, exogenous expression of EPOR or c-MPL boosted the proliferation and survival of NK-92 in vitro by upregulating Bcl-2 and BclxL [183]. Even in primary human NK cells, modification of c-MPL also significantly improved their proliferation, tumor cytotoxicity, and persistence in vivo [183].
A negative regulating mechanism that hinders NK cell survival is that they lyse their neighboring NK cells expressing the same target via the “missing self” mechanism, which is called fratricide. For NK cell therapy, fratricide always causes a rapid elimination of NK cells, thereby impairing the overall efficacy. As a monoclonal antibody targeting CD38, daratumumab (DARA) has dramatically improved the therapeutic outcome of patients with myeloma [197,198]. However, DARA also induces rapid depletion of CD38+ autologous NK cells during treatment, thereby crippling DARA-mediated ADCC [199]. Investigators knocked out CD38 in PB-NK cells using Cas9 ribonucleoprotein complexes, efficiently and precisely [184]. CD38KO cells successfully resisted DARA-induced fratricide with potent ADCC activity and cytotoxicity, indicating a novel strategy to reinforce the therapeutic effect of DARA [184]. A recent study simultaneously endowed CD38KO NK cells with a high-affinity CD16a-158V receptor which further elevated DARA-induced ADCC against myeloma cells [185]. In addition, triple-gene-edited CD38KO iPSC-derived NK cells, co-expressing non-cleavable CD16a and IL-15/IL-15R fusion protein, have achieved desirable results in the tests of their in vivo persistence and tumor-directed cytotoxicity [200]. In clinical practice, another potential risk of adoptive NK cell transfer is the immune rejection of alloreactive host T cells, which limits the persistence of NK cells in vivo and reduces the possibility of multiple infusions. To inhibit the surface expression of HLA class I molecules and prevent autolysis or fratricide of non-HLA-matched NK cells, Hoerster et al. manufactured a double-modified NK cell with B2M gene knockout and co-expressed single-chain HLA-E, a ligand for the inhibitory receptor NKG2A [201]. These cells successfully escaped the immune rejection of alloreactive host T-cells while still retaining the original phenotype and cytotoxicity of PB-NK cells [201].
Collectively, genetic engineering confers superior cytotoxicity and persistence to NK cells, laying a solid foundation for the broad clinical application of NK cell-directed therapies.
4. Clinical Applications
As an emerging therapeutic strategy, NK ACT has been actively tested in the clinic (Table 3 and Table 4). Current NK-based clinical trials mainly focus on leukemia, especially as consolidation therapy for AML, a common hematopoietic stem cell malignancy with high heterogeneity. In the past few years, several studies have revealed the safety and therapeutic potential of NK cell infusion in the clinical treatment of AML. When co-cultured with radiated K562-mbIL-21-41BBL, NK cells achieved 1024-fold clinical-grade expansion efficiency from PBMCs after 2 weeks of culture [88]. These expanded NK cell products exhibited stronger degranulation and achieved approximately 50% sustained remission after infusion in AML patients with persistent or relapsed minimal residual disease (MRD) [88]. The latest clinical trial indicated that six doses of cryopreserved NK cells in 13 patients with relapsed or refractory AML resulted in an overall response rate of 78.6% and a complete response rate of 50%, with no observed serious adverse events [129]. Adoptive NK therapy elicited beneficial local inflammatory responses or lesion improvement in 4 AML patients with concurrent CNS disease [129]. Recently, Katayoun Rezvani’s group conducted a pioneering clinical trial to evaluate the safety and efficacy of anti-CD19 CAR NK cell products against CD19-positive lymphoid tumors [13]. This phase I/II trial showed that infusion of CAR NK cells induced complete remission in 7 of 11 patients with CD19-positive lymphoid tumors, and all the patients are well tolerable without any dose-limiting toxicity [13]. Although patients received only a single NK cell infusion, the expansion and persistence of CAR NK cells in vivo for at least 12 months can be observed, which may largely benefit from the genetic modification of IL-15 [13]. Researchers always combine adoptive NK therapy with rhIL-2 or rhIL-15 administration to facilitate NK cell activation and expansion in vivo. For the reason of the activation and expansion of Treg cells by low-dose rhIL-2, current clinical trials mainly focus on the rhIL-15 and CAR-NK combination. For example, in a phase I trial of 26 refractory AML patients, combing haploidentical NK cells with rhIL-15 intravenously, 36% achieved potent NK-cell expansion in vivo at day 14 and 35% obtained remission [202]. A retrospective study enrolling 109 chemotherapy-refractory AML patients revealed that infused NK cells maintained well survival and expansion in the BM, and patients with higher NK cell density achieved better leukemia control [203]. Furthermore, pretreatment with CD25-targeting immunotoxin (denileukin diftitox) or low-dose radiation is beneficial for NK cell homing and persistence in the BM [203]. Although several studies have demonstrated the safety and feasibility of infusing KIR-HLA mismatched NK cells as adjuvant therapy in pediatric AML patients, the efficacy of these cell products is not obvious. Possible reasons include the suboptimal number of NK cells infused, limited persistence, and difficulty in patient recruitment. For example, a phase II clinical trial showed that the administration of unmanipulated NK cells did not substantially prolong the event-free survival in children with AML as expected [204]. In another phase II clinical trial, although NK cells were activated and expanded by K562-MB15-41BBL feeder cells before infusion, the efficacy assessment was limited by the number of participants (only 7 patients) [14]. Encouragingly, a prospective phase II clinical trial involving up to 6 institutions is currently being prepared to assess the clinical efficacy of administrating activated and expanded NK cells for AML [205]. Taken together, with proper processing, adoptive NK cell-based immunotherapy is a safe and promising strategy to complement current polychemotherapy-based leukemia treatment options.

Table 3.
Recent clinical results of NK cell infusions (2019–2021).

Table 4.
Clinical trials of NK cells for cancer immunotherapy listed on https://www.clinicaltrials.gov/ (1 November 2019–1 November 2022, accessed on 11 November 2022).
Results of clinical trials associated with solid neoplasms have also been reported, including NSCLC [4], primary liver cancer (PLC) [89], GBM[207], medulloblastoma [206], and breast cancer [99]. Interestingly, Multhoff et al. found that NK cells incubated with Hsp70 peptide (TKD) and low-dose IL2 ex vivo could specifically target membrane heat shock protein 70 (mHsp70), an indicator of high-risk tumors (including NSCLC) [208]. This group further confirmed that mHsp70-positive patients with advanced NSCLC receiving 4 cycles of TKD/IL2-activated autologous NK after radio-chemotherapy had improved progression-free survival [4]. Moreover, when combined with PD-1 inhibition, mHsp70-targeting NK cells infusion after radio-chemotherapy effectively induced anti-tumor immunity and achieved long-term tumor control in an unresectable NSCLC patient [209]. In a small-scale preliminary phase II trial, allogenic NK infusion combined with irreversible electroporation (IRE) therapy presented a synergistic effect in PLC, significantly reducing circulating tumor cells and extending patients’ median progression-free survival (15.1 months) and 1-year overall survival (23.2 months) [89]. Compared with intravenous infusion, locoregional infusion of NK cells appears to be more beneficial in achieving immune infiltration of solid neoplasms. For example, early results of a phase I study supported that intraventricular infusion of allogeneic NK cells in children with recurrent medulloblastoma and ependymoma was safe and feasible and the cryopreservation did not impair the activity and function of NK cells [206]. In addition, the safety and toxicity of intraperitoneal infusion of NK cells for the treatment of recurrent OC are also under evaluation [210]. Recently, Lee et al. showed a promising result of the combination of ex vivo expanded NK cells with trastuzumab in treating HER2-positive solid malignancies [99]. In this study, all patients are well tolerated without any severe toxicity, and 6 of 19 patients had stable disease lasting longer than 6 months [99]. Notably, one patient with a high-affinity CD16 variant (allotype v158) achieved a partial response, indicating that CD16 genotyping might be a potential biomarker for response to a therapeutic antibody [99].
Collectively, clinical anti-tumor regimens commonly choose NK cell immunotherapy as a post-remission consolidation strategy or in combination with other treatments. Most clinical trials based on NK infusion are in the early exploratory phase with a short follow-up time and a small number of enrolled patients. Although current data indicate that the safety of treatment with NK cells is better than that of T-cells, its clinical efficacy still warrants investigation in larger-scale clinical trials.
5. Conclusions and Future Direction
NK cells have extensive tumor-killing effects independent of MHC restriction. The variety of cell sources and recent preclinical advances in genetic engineering have further optimized the potential of NK cells in the ACT. Here, we summarized recent development in the field of NK cell-based adoptive transfer with an emphasis on the source and genetic modification of NK cells. We represented various sources of NK cells currently available for adoptive infusion, ranging from traditional PB-NK cells to emerging ML-NK cells, which have their characteristics to meet different clinical needs. Moreover, we described in detail diverse genetic modification strategies to improve the anti-tumor activity of NK cells, including CAR engineering for different targets, gene mutation or recombination to enhance cytotoxicity, knockout of immunosuppression-related genes, cytokine co-expression to improve migration and persistence in vivo.
However, most NK cell products are still in the early preclinical exploration stage and lack large cohorts of patients to support their clinical efficacy. Moreover, ex vivo expansion and gene transduction of primary NK cells often require high technical requirements because of their demanding culture conditions and intracellular antiviral defense mechanisms [211]. NK-92 cell line has been widely used in preclinical studies based on genetic engineering platforms for its indefinite expansion. However, due to safety concerns, the current clinical trials are mainly based on the infusion of autologous or allogeneic primary NK cells, which raised the expense.
The future direction of adoptive NK cell therapy is to continuously optimize the property of cellular products, making it a safe and effective adjuvant therapeutic strategy. Researchers are exploring multiple advanced genetic modification strategies to maximize the potential of NK cells for the treatment of multiple cancers, especially solid tumors. Meanwhile, it is also important to reduce costs and standardize the procedure of NK preparation and evaluate key preclinical discoveries in larger-scale clinical trials.
Author Contributions
J.X. and T.Z. contributed equally to this work. J.X. and T.Z. took the lead in structuring and writing the manuscript. F.G., Z.Z. and G.S. helped modify the manuscript. Y.Z. and G.Y. participated in revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Student Innovation Project of Central South University, grant number 1053320212355.
Acknowledgments
We thank all of the authors listed in this manuscript.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Kochenderfer, J.N.; Wilson, W.H.; Janik, J.E.; Dudley, M.E.; Stetler-Stevenson, M.; Feldman, S.A.; Maric, I.; Raffeld, M.; Nathan, D.A.; Lanier, B.J.; et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 2010, 116, 4099–4102. [Google Scholar] [CrossRef] [PubMed]
- Curti, B.D.; Ochoa, A.C.; Powers, G.C.; Kopp, W.C.; Alvord, W.G.; Janik, J.E.; Gause, B.L.; Dunn, B.; Kopreski, M.S.; Fenton, R.; et al. Phase I trial of anti-CD3-stimulated CD4+ T cells, infusional interleukin-2, and cyclophosphamide in patients with advanced cancer. J. Clin. Oncol. 1998, 16, 2752–2760. [Google Scholar] [CrossRef] [PubMed]
- Frey, N.V.; Gill, S.; Hexner, E.O.; Schuster, S.; Nasta, S.; Loren, A.; Svoboda, J.; Stadtmauer, E.; Landsburg, D.J.; Mato, A.; et al. Long-Term Outcomes from a Randomized Dose Optimization Study of Chimeric Antigen Receptor Modified T Cells in Relapsed Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2020, 38, 2862–2871. [Google Scholar] [CrossRef] [PubMed]
- Multhoff, G.; Seier, S.; Stangl, S.; Sievert, W.; Shevtsov, M.; Werner, C.; Pockley, A.G.; Blankenstein, C.; Hildebrandt, M.; Offner, R.; et al. Targeted Natural Killer Cell-Based Adoptive Immunotherapy for the Treatment of Patients with NSCLC after Radiochemotherapy: A Randomized Phase II Clinical Trial. Clin. Cancer Res. 2020, 26, 5368–5379. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Okayama, T.; Sakamoto, N.; Ideno, M.; Oka, K.; Enoki, T.; Mineno, J.; Yoshida, N.; Katada, K.; Kamada, K.; et al. Phase I clinical trial of adoptive transfer of expanded natural killer cells in combination with IgG1 antibody in patients with gastric or colorectal cancer. Int. J. Cancer 2018, 142, 2599–2609. [Google Scholar] [CrossRef]
- Rosenberg, S.; Packard, B.; Aebersold, P.; Solomon, D.; Topalian, S.; Toy, S.; Simon, P.; Lotze, M.; Yang, J.; Seipp, C. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N. Engl. J. Med. 1988, 319, 1676–1680. [Google Scholar] [CrossRef]
- Yoshida, S.; Tanaka, R.; Takai, N.; Ono, K. Local administration of autologous lymphokine-activated killer cells and recombinant interleukin 2 to patients with malignant brain tumors. Cancer Res. 1988, 48, 5011–5016. [Google Scholar]
- Morgan, R.; Dudley, M.; Wunderlich, J.; Hughes, M.; Yang, J.; Sherry, R.; Royal, R.; Topalian, S.; Kammula, U.; Restifo, N.; et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006, 314, 126–129. [Google Scholar] [CrossRef]
- Kochenderfer, J.N.; Dudley, M.E.; Stetler-Stevenson, M.; Wilson, W.H.; Rosenberg, S.A. A Phase I Clinical Trial of Treatment of B-Cell Malignancies with Autologous Anti-CD19-CAR-Transduced T Cells. Blood 2010, 116, 2865. [Google Scholar] [CrossRef]
- Goldenson, B.H.; Hor, P.; Kaufman, D.S. iPSC-Derived Natural Killer Cell Therapies—Expansion and Targeting. Front. Immunol. 2022, 13, 841107. [Google Scholar] [CrossRef]
- Minculescu, L.; Marquart, H.V.; Ryder, L.P.; Andersen, N.S.; Schjoedt, I.; Friis, L.S.; Kornblit, B.T.; Petersen, S.L.; Haastrup, E.; Fischer-Nielsen, A.; et al. Improved Overall Survival, Relapse-Free-Survival, and Less Graft-vs.-Host-Disease in Patients with High Immune Reconstitution of TCR Gamma Delta Cells 2 Months After Allogeneic Stem Cell Transplantation. Front. Immunol. 2019, 10, 1997. [Google Scholar] [CrossRef]
- Bolli, R.; Solankhi, M.; Tang, X.L.; Kahlon, A. Cell therapy in patients with heart failure: A comprehensive review and emerging concepts. Cardiovasc. Res. 2022, 118, 951–976. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.; Thompson, P.; Basar, R.; Nassif Kerbauy, L.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef]
- Gómez García, L.; Escudero, A.; Mestre, C.; Fuster Soler, J.; Martínez, A.; Vagace Valero, J.; Vela, M.; Ruz, B.; Navarro, A.; Fernández, L.; et al. Phase 2 Clinical Trial of Infusing Haploidentical K562-mb15-41BBL-Activated and Expanded Natural Killer Cells as Consolidation Therapy for Pediatric Acute Myeloblastic Leukemia. Clin. Lymphoma Myeloma Leuk. 2021, 21, 328–337. [Google Scholar] [CrossRef]
- Miller, J.; Soignier, Y.; Panoskaltsis-Mortari, A.; McNearney, S.; Yun, G.; Fautsch, S.; McKenna, D.; Le, C.; Defor, T.; Burns, L.; et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood 2005, 105, 3051–3057. [Google Scholar] [CrossRef]
- Pérez-Martínez, A.; Fernández, L.; Valentín, J.; Martínez-Romera, I.; Corral, M.; Ramírez, M.; Abad, L.; Santamaría, S.; González-Vicent, M.; Sirvent, S.; et al. A phase I/II trial of interleukin-15--stimulated natural killer cell infusion after haplo-identical stem cell transplantation for pediatric refractory solid tumors. Cytotherapy 2015, 17, 1594–1603. [Google Scholar] [CrossRef]
- Rubnitz, J.; Inaba, H.; Ribeiro, R.; Pounds, S.; Rooney, B.; Bell, T.; Pui, C.; Leung, W. NKAML: A pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 955–959. [Google Scholar] [CrossRef]
- Klingemann, H. Challenges of cancer therapy with natural killer cells. Cytotherapy 2015, 17, 245–249. [Google Scholar] [CrossRef]
- Huntington, N.; Cursons, J.; Rautela, J. The cancer-natural killer cell immunity cycle. Nat. Rev. Cancer 2020, 20, 437–454. [Google Scholar] [CrossRef]
- Myers, J.; Miller, J. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Naeimi Kararoudi, M.; Tullius, B.; Chakravarti, N.; Pomeroy, E.; Moriarity, B.; Beland, K.; Colamartino, A.; Haddad, E.; Chu, Y.; Cairo, M.; et al. Genetic and epigenetic modification of human primary NK cells for enhanced antitumor activity. Semin. Hematol. 2020, 57, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Scoville, S.; Freud, A.; Caligiuri, M. Modeling Human Natural Killer Cell Development in the Era of Innate Lymphoid Cells. Front. Immunol. 2017, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Heipertz, E.L.; Zynda, E.R.; Stav-Noraas, T.E.; Hungler, A.D.; Boucher, S.E.; Kaur, N.; Vemuri, M.C. Current Perspectives on "Off-The-Shelf" Allogeneic NK and CAR-NK Cell Therapies. Front. Immunol. 2021, 12, 732135. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Turner, S.C.; Chen, K.S.; Ghaheri, B.A.; Ghayur, T.; Carson, W.E.; Caligiuri, M.A. Human natural killer cells: A unique innate immunoregulatory role for the CD56(bright) subset. Blood 2001, 97, 3146–3151. [Google Scholar] [CrossRef]
- Crinier, A.; Narni-Mancinelli, E.; Ugolini, S.; Vivier, E. SnapShot: Natural Killer Cells. Cell 2020, 180, 1280. [Google Scholar] [CrossRef]
- Getahun, A.; Cambier, J. Of ITIMs, ITAMs, and ITAMis: Revisiting immunoglobulin Fc receptor signaling. Immunol. Rev. 2015, 268, 66–73. [Google Scholar] [CrossRef]
- Quatrini, L.; Della Chiesa, M.; Sivori, S.; Mingari, M.; Pende, D.; Moretta, L. Human NK cells, their receptors and function. Eur. J. Immunol. 2021, 51, 1566–1579. [Google Scholar] [CrossRef]
- Dorsch, M.; Urlaub, D.; Bönnemann, V.; Bröde, P.; Sandusky, M.; Watzl, C. Quantitative analysis of human NK cell reactivity using latex beads coated with defined amounts of antibodies. Eur. J. Immunol. 2020, 50, 656–665. [Google Scholar] [CrossRef]
- Rumpret, M.; Drylewicz, J.; Ackermans, L.; Borghans, J.; Medzhitov, R.; Meyaard, L. Functional categories of immune inhibitory receptors. Nat. Rev. Immunol. 2020, 20, 771–780. [Google Scholar] [CrossRef]
- Shklovskaya, E.; Rizos, H. MHC Class I Deficiency in Solid Tumors and Therapeutic Strategies to Overcome It. Int. J. Mol. Sci. 2021, 22, 6741. [Google Scholar] [CrossRef]
- Russick, J.; Torset, C.; Hemery, E.; Cremer, I. NK cells in the tumor microenvironment: Prognostic and theranostic impact. Recent advances and trends. Semin. Immunol. 2020, 48, 101407. [Google Scholar] [CrossRef]
- Federici, C.; Shahaj, E.; Cecchetti, S.; Camerini, S.; Casella, M.; Iessi, E.; Camisaschi, C.; Paolino, G.; Calvieri, S.; Ferro, S.; et al. Natural-Killer-Derived Extracellular Vesicles: Immune Sensors and Interactors. Front. Immunol. 2020, 11, 262. [Google Scholar] [CrossRef]
- Cochran, A.; Kornbluth, J. Extracellular Vesicles from the Human Natural Killer Cell Line NK3.3 Have Broad and Potent Anti-Tumor Activity. Front. Cell Dev. Biol. 2021, 9, 698639. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, Y.; Jiang, X. Antibody and antibody fragments for cancer immunotherapy. J. Control. Release Off. J. Control. Release Soc. 2020, 328, 395–406. [Google Scholar] [CrossRef]
- López-Díaz de Cerio, A.; García-Muñoz, R.; Pena, E.; Panizo, Á.; Feliu, J.; Giraldo, P.; Rodríguez-Calvillo, M.; Martínez-Calle, N.; Grande, C.; Olave, M.; et al. Maintenance therapy with ex vivo expanded lymphokine-activated killer cells and rituximab in patients with follicular lymphoma is safe and may delay disease progression. Br. J. Haematol. 2020, 189, 1064–1073. [Google Scholar] [CrossRef]
- Ottaiano, A.; Scala, S.; Normanno, N.; Napolitano, M.; Capozzi, M.; Rachiglio, A.; Roma, C.; Trotta, A.; D’Alterio, C.; Portella, L.; et al. Cetuximab, irinotecan and fluorouracile in fiRst-line treatment of immunologically-selected advanced colorectal cancer patients: The CIFRA study protocol. BMC Cancer 2019, 19, 899. [Google Scholar] [CrossRef]
- Louis, E.; El Ghoul, Z.; Vermeire, S.; Dall’Ozzo, S.; Rutgeerts, P.; Paintaud, G.; Belaiche, J.; De Vos, M.; Van Gossum, A.; Colombel, J.; et al. Association between polymorphism in IgG Fc receptor IIIa coding gene and biological response to infliximab in Crohn’s disease. Aliment. Pharmacol. Ther. 2004, 19, 511–519. [Google Scholar] [CrossRef]
- Fan, Z.; Yu, P.; Wang, Y.; Wang, Y.; Fu, M.L.; Liu, W.; Sun, Y.; Fu, Y.X. NK-cell activation by LIGHT triggers tumor-specific CD8+ T-cell immunity to reject established tumors. Blood 2006, 107, 1342–1351. [Google Scholar] [CrossRef]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef]
- Böttcher, J.P.; Bonavita, E.; Chakravarty, P.; Blees, H.; Cabeza-Cabrerizo, M.; Sammicheli, S.; Rogers, N.C.; Sahai, E.; Zelenay, S.; Reis e Sousa, C. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell 2018, 172, 1022–1037.e1014. [Google Scholar] [CrossRef] [PubMed]
- Barry, K.C.; Hsu, J.; Broz, M.L.; Cueto, F.J.; Binnewies, M.; Combes, A.J.; Nelson, A.E.; Loo, K.; Kumar, R.; Rosenblum, M.D.; et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 2018, 24, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Castro, F.; Cardoso, A.; Gonçalves, R.; Serre, K.; Oliveira, M. Interferon-Gamma at the Crossroads of Tumor Immune Surveillance or Evasion. Front. Immunol. 2018, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Bassani, B.; Baci, D.; Gallazzi, M.; Poggi, A.; Bruno, A.; Mortara, L. Natural Killer Cells as Key Players of Tumor Progression and Angiogenesis: Old and Novel Tools to Divert Their Pro-Tumor Activities into Potent Anti-Tumor Effects. Cancers 2019, 11, 461. [Google Scholar] [CrossRef]
- Corvino, D.; Kumar, A.; Bald, T. Plasticity of NK cells in Cancer. Front. Immunol. 2022, 13, 888313. [Google Scholar] [CrossRef]
- Bruno, A.; Focaccetti, C.; Pagani, A.; Imperatori, A.S.; Spagnoletti, M.; Rotolo, N.; Cantelmo, A.R.; Franzi, F.; Capella, C.; Ferlazzo, G.; et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplasia 2013, 15, 133–142. [Google Scholar] [CrossRef]
- Guan, Y.; Chambers, C.B.; Tabatabai, T.; Hatley, H.; Delfino, K.R.; Robinson, K.; Alanee, S.R.; Ran, S.; Torry, D.S.; Wilber, A. Renal cell tumors convert natural killer cells to a proangiogenic phenotype. Oncotarget 2020, 11, 2571–2585. [Google Scholar] [CrossRef]
- Bruno, A.; Bassani, B.; D’Urso, D.G.; Pitaku, I.; Cassinotti, E.; Pelosi, G.; Boni, L.; Dominioni, L.; Noonan, D.M.; Mortara, L.; et al. Angiogenin and the MMP9-TIMP2 axis are up-regulated in proangiogenic, decidual NK-like cells from patients with colorectal cancer. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2018, 32, 5365–5377. [Google Scholar] [CrossRef]
- Mamessier, E.; Sylvain, A.; Thibult, M.L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Gonçalves, A.; André, P.; Romagné, F.; Thibault, G.; et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef]
- Levi, I.; Amsalem, H.; Nissan, A.; Darash-Yahana, M.; Peretz, T.; Mandelboim, O.; Rachmilewitz, J. Characterization of tumor infiltrating natural killer cell subset. Oncotarget 2015, 6, 13835–13843. [Google Scholar] [CrossRef] [PubMed]
- Radomska-Leśniewska, D.M.; Białoszewska, A.; Kamiński, P. Angiogenic Properties of NK Cells in Cancer and Other Angiogenesis-Dependent Diseases. Cells 2021, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- López-Soto, A.; Gonzalez, S.; Smyth, M.J.; Galluzzi, L. Control of Metastasis by NK Cells. Cancer Cell 2017, 32, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Lo, H.C.; Xu, Z.; Kim, I.S.; Pingel, B.; Aguirre, S.; Kodali, S.; Liu, J.; Zhang, W.; Muscarella, A.M.; Hein, S.M.; et al. Resistance to natural killer cell immunosurveillance confers a selective advantage to polyclonal metastasis. Nat. Cancer 2020, 1, 709–722. [Google Scholar] [CrossRef] [PubMed]
- López-Soto, A.; Folgueras, A.R.; Seto, E.; Gonzalez, S. HDAC3 represses the expression of NKG2D ligands ULBPs in epithelial tumour cells: Potential implications for the immunosurveillance of cancer. Oncogene 2009, 28, 2370–2382. [Google Scholar] [CrossRef]
- Zhang, J.; Basher, F.; Wu, J.D. NKG2D Ligands in Tumor Immunity: Two Sides of a Coin. Front. Immunol. 2015, 6, 97. [Google Scholar] [CrossRef]
- Schlecker, E.; Fiegler, N.; Arnold, A.; Altevogt, P.; Rose-John, S.; Moldenhauer, G.; Sucker, A.; Paschen, A.; von Strandmann, E.P.; Textor, S.; et al. Metalloprotease-mediated tumor cell shedding of B7-H6, the ligand of the natural killer cell-activating receptor NKp30. Cancer Res. 2014, 74, 3429–3440. [Google Scholar] [CrossRef]
- Chan, I.S.; Knútsdóttir, H.; Ramakrishnan, G.; Padmanaban, V.; Warrier, M.; Ramirez, J.C.; Dunworth, M.; Zhang, H.; Jaffee, E.M.; Bader, J.S.; et al. Cancer cells educate natural killer cells to a metastasis-promoting cell state. J. Cell Biol. 2020, 219, e202001134. [Google Scholar] [CrossRef]
- Beldi-Ferchiou, A.; Lambert, M.; Dogniaux, S.; Vély, F.; Vivier, E.; Olive, D.; Dupuy, S.; Levasseur, F.; Zucman, D.; Lebbé, C.; et al. PD-1 mediates functional exhaustion of activated NK cells in patients with Kaposi sarcoma. Oncotarget 2016, 7, 72961–72977. [Google Scholar] [CrossRef]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immunol. 2017, 139, 335–346.e333. [Google Scholar] [CrossRef]
- Sun, H.; Huang, Q.; Huang, M.; Wen, H.; Lin, R.; Zheng, M.; Qu, K.; Li, K.; Wei, H.; Xiao, W.; et al. Human CD96 Correlates to Natural Killer Cell Exhaustion and Predicts the Prognosis of Human Hepatocellular Carcinoma. Hepatology 2019, 70, 168–183. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, H.; Li, J.; Hou, X.; Li, L.; Wang, W.; Shi, Y.; Li, D.; Li, L.; Zhao, Z.; et al. Involvement of TIGIT in Natural Killer Cell Exhaustion and Immune Escape in Patients and Mouse Model with Liver Echinococcus multilocularis Infection. Hepatology 2021, 74, 3376–3393. [Google Scholar] [CrossRef]
- Sun, C.; Xu, J.; Huang, Q.; Huang, M.; Wen, H.; Zhang, C.; Wang, J.; Song, J.; Zheng, M.; Sun, H.; et al. High NKG2A expression contributes to NK cell exhaustion and predicts a poor prognosis of patients with liver cancer. Oncoimmunology 2017, 6, e1264562. [Google Scholar] [CrossRef]
- Ju, Y.; Hou, N.; Meng, J.; Wang, X.; Zhang, X.; Zhao, D.; Liu, Y.; Zhu, F.; Zhang, L.; Sun, W.; et al. T cell immunoglobulin- and mucin-domain-containing molecule-3 (Tim-3) mediates natural killer cell suppression in chronic hepatitis B. J. Hepatol. 2010, 52, 322–329. [Google Scholar] [CrossRef]
- Bi, J.; Tian, Z. NK Cell Exhaustion. Front. Immunol 2017, 8, 760. [Google Scholar] [CrossRef]
- Gao, Y.; Souza-Fonseca-Guimaraes, F.; Bald, T.; Ng, S.S.; Young, A.; Ngiow, S.F.; Rautela, J.; Straube, J.; Waddell, N.; Blake, S.J.; et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 2017, 18, 1004–1015. [Google Scholar] [CrossRef]
- Cerdeira, A.S.; Rajakumar, A.; Royle, C.M.; Lo, A.; Husain, Z.; Thadhani, R.I.; Sukhatme, V.P.; Karumanchi, S.A.; Kopcow, H.D. Conversion of peripheral blood NK cells to a decidual NK-like phenotype by a cocktail of defined factors. J. Immunol. 2013, 190, 3939–3948. [Google Scholar] [CrossRef]
- Mao, Y.; Sarhan, D.; Steven, A.; Seliger, B.; Kiessling, R.; Lundqvist, A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin. Cancer Res. 2014, 20, 4096–4106. [Google Scholar] [CrossRef]
- Wan, R.; Wang, Z.W.; Li, H.; Peng, X.D.; Liu, G.Y.; Ou, J.M.; Cheng, A.Q. Human Leukocyte Antigen-G Inhibits the Anti-Tumor Effect of Natural Killer Cells via Immunoglobulin-Like Transcript 2 in Gastric Cancer. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 1828–1841. [Google Scholar] [CrossRef]
- Lee, C.L.; Vijayan, M.; Wang, X.; Lam, K.K.W.; Koistinen, H.; Seppala, M.; Li, R.H.W.; Ng, E.H.Y.; Yeung, W.S.B.; Chiu, P.C.N. Glycodelin-A stimulates the conversion of human peripheral blood CD16-CD56bright NK cell to a decidual NK cell-like phenotype. Hum. Reprod. 2019, 34, 689–701. [Google Scholar] [CrossRef]
- Law, A.M.K.; Valdes-Mora, F.; Gallego-Ortega, D. Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Langhans, B.; Alwan, A.W.; Krämer, B.; Glässner, A.; Lutz, P.; Strassburg, C.P.; Nattermann, J.; Spengler, U. Regulatory CD4+ T cells modulate the interaction between NK cells and hepatic stellate cells by acting on either cell type. J. Hepatol. 2015, 62, 398–404. [Google Scholar] [CrossRef]
- Neelapu, S.; Locke, F.; Bartlett, N.; Lekakis, L.; Miklos, D.; Jacobson, C.; Braunschweig, I.; Oluwole, O.; Siddiqi, T.; Lin, Y.; et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 2531–2544. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Bishop, M.; Tam, C.; Waller, E.; Borchmann, P.; McGuirk, J.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef]
- Maude, S.; Frey, N.; Shaw, P.; Aplenc, R.; Barrett, D.; Bunin, N.; Chew, A.; Gonzalez, V.; Zheng, Z.; Lacey, S.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Garfall, A.; Maus, M.; Hwang, W.; Lacey, S.; Mahnke, Y.; Melenhorst, J.; Zheng, Z.; Vogl, D.; Cohen, A.; Weiss, B.; et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N. Engl. J. Med. 2015, 373, 1040–1047. [Google Scholar] [CrossRef]
- June, C.; O’Connor, R.; Kawalekar, O.; Ghassemi, S.; Milone, M. CAR T cell immunotherapy for human cancer. Science 2018, 359, 1361–1365. [Google Scholar] [CrossRef]
- American Association for Cancer Research. First-Ever CAR T-cell Therapy Approved in U.S. Cancer Discov. 2017, 7, OF1. [Google Scholar] [CrossRef]
- FDA Approves Second CAR T-cell Therapy. Cancer Discov. 2018, 8, 5–6. [CrossRef]
- Mullard, A. FDA approves fourth CAR-T cell therapy. Nat. Rev. Drug Discov. 2021, 20, 166. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef]
- Hay, K.; Hanafi, L.; Li, D.; Gust, J.; Liles, W.; Wurfel, M.; López, J.; Chen, J.; Chung, D.; Harju-Baker, S.; et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood 2017, 130, 2295–2306. [Google Scholar] [CrossRef]
- Leahy, A.; Newman, H.; Li, Y.; Liu, H.; Myers, R.; DiNofia, A.; Dolan, J.; Callahan, C.; Baniewicz, D.; Devine, K.; et al. CD19-targeted chimeric antigen receptor T-cell therapy for CNS relapsed or refractory acute lymphocytic leukaemia: A post-hoc analysis of pooled data from five clinical trials. Lancet Haematol. 2021, 8, e711–e722. [Google Scholar] [CrossRef]
- Berdeja, J.; Madduri, D.; Usmani, S.; Jakubowiak, A.; Agha, M.; Cohen, A.; Stewart, A.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Zhao, Y.; Shao, Q.; Peng, G. Exhaustion and senescence: Two crucial dysfunctional states of T cells in the tumor microenvironment. Cell. Mol. Immunol. 2020, 17, 27–35. [Google Scholar] [CrossRef]
- Sterner, R.; Sterner, R. CAR-T cell therapy: Current limitations and potential strategies. Blood Cancer J. 2021, 11, 69. [Google Scholar] [CrossRef]
- Titov, A.; Zmievskaya, E.; Ganeeva, I.; Valiullina, A.; Petukhov, A.; Rakhmatullina, A.; Miftakhova, R.; Fainshtein, M.; Rizvanov, A.; Bulatov, E. Adoptive Immunotherapy beyond CAR T-Cells. Cancers 2021, 13, 743. [Google Scholar] [CrossRef]
- Zhao, X.; Jiang, Q.; Jiang, H.; Hu, L.; Zhao, T.; Yu, X.; Huang, X. Expanded clinical-grade membrane-bound IL-21/4-1BBL NK cell products exhibit activity against acute myeloid leukemia in vivo. Eur. J. Immunol. 2020, 50, 1374–1385. [Google Scholar] [CrossRef]
- Yang, Y.; Qin, Z.; Du, D.; Wu, Y.; Qiu, S.; Mu, F.; Xu, K.; Chen, J. Safety and Short-Term Efficacy of Irreversible Electroporation and Allogenic Natural Killer Cell Immunotherapy Combination in the Treatment of Patients with Unresectable Primary Liver Cancer. Cardiovasc. Interv. Radiol. 2019, 42, 48–59. [Google Scholar] [CrossRef]
- Reina-Ortiz, C.; Constantinides, M.; Fayd-Herbe-de-Maudave, A.; Présumey, J.; Hernandez, J.; Cartron, G.; Giraldos, D.; Díez, R.; Izquierdo, I.; Azaceta, G.; et al. Expanded NK cells from umbilical cord blood and adult peripheral blood combined with daratumumab are effective against tumor cells from multiple myeloma patients. Oncoimmunology 2020, 10, 1853314. [Google Scholar] [CrossRef]
- Xu, C.; Liu, D.; Chen, Z.; Zhuo, F.; Sun, H.; Hu, J.; Li, T. Umbilical Cord Blood-Derived Natural Killer Cells Combined with Bevacizumab for Colorectal Cancer Treatment. Hum. Gene Ther. 2019, 30, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Teng, K.; Mansour, A.; Zhu, Z.; Li, Z.; Tian, L.; Ma, S.; Xu, B.; Lu, T.; Chen, H.; Hou, D.; et al. Off-the-Shelf Prostate Stem Cell Antigen–Directed Chimeric Antigen Receptor Natural Killer Cell Therapy to Treat Pancreatic Cancer. Gastroenterology 2022, 162, 1319–1333. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Kang, M.; Kim, T.; Hwang, I.; Jin, H.; Sung, Y.; Eom, K.; Kim, S. Discovery of a novel natural killer cell line with distinct immunostimulatory and proliferative potential as an alternative platform for cancer immunotherapy. J. Immunother. Cancer 2019, 7, 138. [Google Scholar] [CrossRef] [PubMed]
- Suck, G.; Odendahl, M.; Nowakowska, P.; Seidl, C.; Wels, W.; Klingemann, H.; Tonn, T. NK-92: An ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. CII 2016, 65, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Law, A.; Routy, B.; denHollander, N.; Gupta, V.; Wang, X.; Chaboureau, A.; Viswanathan, S.; Keating, A. A phase I trial of NK-92 cells for refractory hematological malignancies relapsing after autologous hematopoietic cell transplantation shows safety and evidence of efficacy. Oncotarget 2017, 8, 89256–89268. [Google Scholar] [CrossRef]
- You, F.; Wang, Y.; Jiang, L.; Zhu, X.; Chen, D.; Yuan, L.; An, G.; Meng, H.; Yang, L. A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am. J. Cancer Res. 2019, 9, 64–78. [Google Scholar]
- Zhu, H.; Blum, R.; Bernareggi, D.; Ask, E.; Wu, Z.; Hoel, H.; Meng, Z.; Wu, C.; Guan, K.; Malmberg, K.; et al. Metabolic Reprograming via Deletion of CISH in Human iPSC-Derived NK Cells Promotes In Vivo Persistence and Enhances Anti-tumor Activity. Cell Stem Cell 2020, 27, 224–237.e226. [Google Scholar] [CrossRef]
- Zhu, H.; Blum, R.; Bjordahl, R.; Gaidarova, S.; Rogers, P.; Lee, T.; Abujarour, R.; Bonello, G.; Wu, J.; Tsai, P.; et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood 2020, 135, 399–410. [Google Scholar] [CrossRef]
- Lee, S.; Shimasaki, N.; Lim, J.; Wong, A.; Yadav, K.; Yong, W.; Tan, L.; Koh, L.; Poon, M.; Tan, S.; et al. Phase I Trial of Expanded, Activated Autologous NK-cell Infusions with Trastuzumab in Patients with HER2-positive Cancers. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2020, 26, 4494–4502. [Google Scholar] [CrossRef]
- Parkhurst, M.; Riley, J.; Dudley, M.; Rosenberg, S. Adoptive transfer of autologous natural killer cells leads to high levels of circulating natural killer cells but does not mediate tumor regression. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2011, 17, 6287–6297. [Google Scholar] [CrossRef]
- Igarashi, T.; Wynberg, J.; Srinivasan, R.; Becknell, B.; McCoy, J.; Takahashi, Y.; Suffredini, D.; Linehan, W.; Caligiuri, M.; Childs, R. Enhanced cytotoxicity of allogeneic NK cells with killer immunoglobulin-like receptor ligand incompatibility against melanoma and renal cell carcinoma cells. Blood 2004, 104, 170–177. [Google Scholar] [CrossRef]
- Zhang, T.; Scott, J.; Hwang, I.; Kim, S. Cutting edge: Antibody-dependent memory-like NK cells distinguished by FcRγ deficiency. J. Immunol. 2013, 190, 1402–1406. [Google Scholar] [CrossRef]
- Hwang, I.; Zhang, T.; Scott, J.; Kim, A.; Lee, T.; Kakarla, T.; Kim, A.; Sunwoo, J.; Kim, S. Identification of human NK cells that are deficient for signaling adaptor FcRγ and specialized for antibody-dependent immune functions. Int. Immunol. 2012, 24, 793–802. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, T.; Hwang, I.; Kim, A.; Nitschke, L.; Kim, M.; Scott, J.; Kamimura, Y.; Lanier, L.; Kim, S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015, 42, 431–442. [Google Scholar] [CrossRef]
- Bigley, A.; Spade, S.; Agha, N.; Biswas, S.; Tang, S.; Malik, M.; Dai, L.; Masoumi, S.; Patiño-Escobar, B.; Hale, M.; et al. FcεRIγ-negative NK cells persist in vivo and enhance efficacy of therapeutic monoclonal antibodies in multiple myeloma. Blood Adv. 2021, 5, 3021–3031. [Google Scholar] [CrossRef]
- Cooper, M.; Elliott, J.; Keyel, P.; Yang, L.; Carrero, J.; Yokoyama, W. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 1915–1919. [Google Scholar] [CrossRef]
- Romee, R.; Rosario, M.; Berrien-Elliott, M.; Wagner, J.; Jewell, B.; Schappe, T.; Leong, J.; Abdel-Latif, S.; Schneider, S.; Willey, S.; et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 2016, 8, 357ra123. [Google Scholar] [CrossRef]
- Gang, M.; Marin, N.; Wong, P.; Neal, C.; Marsala, L.; Foster, M.; Schappe, T.; Meng, W.; Tran, J.; Schaettler, M.; et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood 2020, 136, 2308–2318. [Google Scholar] [CrossRef]
- Marin, N.; Krasnick, B.; Becker-Hapak, M.; Conant, L.; Goedegebuure, S.; Berrien-Elliott, M.; Robbins, K.; Foltz, J.; Foster, M.; Wong, P.; et al. Memory-like Differentiation Enhances NK Cell Responses to Melanoma. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2021, 27, 4859–4869. [Google Scholar] [CrossRef]
- Dong, H.; Ham, J.; Hu, G.; Xie, G.; Vergara, J.; Liang, Y.; Ali, A.; Tarannum, M.; Donner, H.; Baginska, J.; et al. Memory-like NK cells armed with a neoepitope-specific CAR exhibit potent activity against NPM1 mutated acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2022, 119, e2122379119. [Google Scholar] [CrossRef]
- Liu, E.; Ang, S.; Kerbauy, L.; Basar, R.; Kaur, I.; Kaplan, M.; Li, L.; Tong, Y.; Daher, M.; Ensley, E.; et al. GMP-Compliant Universal Antigen Presenting Cells (uAPC) Promote the Metabolic Fitness and Antitumor Activity of Armored Cord Blood CAR-NK Cells. Front. Immunol. 2021, 12, 626098. [Google Scholar] [CrossRef] [PubMed]
- Nham, T.; Poznanski, S.; Fan, I.; Vahedi, F.; Shenouda, M.; Lee, A.; Chew, M.; Hogg, R.; Lee, D.; Ashkar, A. Ex Vivo-expanded Natural Killer Cells Derived from Long-term Cryopreserved Cord Blood are Cytotoxic Against Primary Breast Cancer Cells. J. Immunother. 2018, 41, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Shereck, E.; Day, N.; Awasthi, A.; Ayello, J.; Chu, Y.; McGuinn, C.; van de Ven, C.; Lim, M.; Cairo, M. Immunophenotypic, cytotoxic, proteomic and genomic characterization of human cord blood vs. peripheral blood CD56 NK cells. Innate Immun. 2019, 25, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Herrera, L.; Santos, S.; Vesga, M.; Anguita, J.; Martin-Ruiz, I.; Carrascosa, T.; Juan, M.; Eguizabal, C. Adult peripheral blood and umbilical cord blood NK cells are good sources for effective CAR therapy against CD19 positive leukemic cells. Sci. Rep. 2019, 9, 18729. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Maki, G.; Klingemann, H. Characterization of a human cell line (NK-92) with phenotypical and functional characteristics of activated natural killer cells. Leukemia 1994, 8, 652–658. [Google Scholar]
- Tonn, T.; Schwabe, D.; Klingemann, H.; Becker, S.; Esser, R.; Koehl, U.; Suttorp, M.; Seifried, E.; Ottmann, O.; Bug, G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013, 15, 1563–1570. [Google Scholar] [CrossRef]
- Boyiadzis, M.; Agha, M.; Redner, R.; Sehgal, A.; Im, A.; Hou, J.; Farah, R.; Dorritie, K.; Raptis, A.; Lim, S.; et al. Phase 1 clinical trial of adoptive immunotherapy using “off-the-shelf” activated natural killer cells in patients with refractory and relapsed acute myeloid leukemia. Cytotherapy 2017, 19, 1225–1232. [Google Scholar] [CrossRef]
- Grote, S.; Ureña-Bailén, G.; Chan, K.; Baden, C.; Mezger, M.; Handgretinger, R.; Schleicher, S. In Vitro Evaluation of CD276-CAR NK-92 Functionality, Migration and Invasion Potential in the Presence of Immune Inhibitory Factors of the Tumor Microenvironment. Cells 2021, 10, 1020. [Google Scholar] [CrossRef]
- Gossel, L.; Heim, C.; Pfeffermann, L.; Moser, L.; Bönig, H.; Klingebiel, T.; Bader, P.; Wels, W.; Merker, M.; Rettinger, E. Retargeting of NK-92 Cells against High-Risk Rhabdomyosarcomas by Means of an ERBB2 (HER2/Neu)-Specific Chimeric Antigen Receptor. Cancers 2021, 13, 1443. [Google Scholar] [CrossRef]
- Grote, S.; Traub, F.; Mittelstaet, J.; Seitz, C.; Kaiser, A.; Handgretinger, R.; Schleicher, S. Adapter Chimeric Antigen Receptor (AdCAR)-Engineered NK-92 Cells for the Multiplex Targeting of Bone Metastases. Cancers 2021, 13, 1124. [Google Scholar] [CrossRef]
- Fabian, K.; Padget, M.; Donahue, R.; Solocinski, K.; Robbins, Y.; Allen, C.; Lee, J.; Rabizadeh, S.; Soon-Shiong, P.; Schlom, J.; et al. PD-L1 targeting high-affinity NK (t-haNK) cells induce direct antitumor effects and target suppressive MDSC populations. J. Immunother. Cancer 2020, 8, e000450. [Google Scholar] [CrossRef]
- Zhao, H.; Zhou, Z.; Li, G.; Liu, G.; Lin, S.; Chen, W.; Xiong, S. An NK cell line (NK92-41BB) expressing high levels of granzyme is engineered to express the high affinity chimeric genes CD16/CAR. Cytotechnology 2021, 73, 539–553. [Google Scholar] [CrossRef]
- Kloess, S.; Oberschmidt, O.; Dahlke, J.; Vu, X.; Neudoerfl, C.; Kloos, A.; Gardlowski, T.; Matthies, N.; Heuser, M.; Meyer, J.; et al. Preclinical Assessment of Suitable Natural Killer Cell Sources for Chimeric Antigen Receptor Natural Killer-Based "Off-the-Shelf" Acute Myeloid Leukemia Immunotherapies. Hum. Gene Ther. 2019, 30, 381–401. [Google Scholar] [CrossRef]
- Zhu, L.; Li, X.; Kalimuthu, S.; Gangadaran, P.; Lee, H.; Oh, J.; Baek, S.; Jeong, S.; Lee, S.; Lee, J.; et al. Natural Killer Cell (NK-92MI)-Based Therapy for Pulmonary Metastasis of Anaplastic Thyroid Cancer in a Nude Mouse Model. Front. Immunol. 2017, 8, 816. [Google Scholar] [CrossRef]
- Yang, S.; Cao, B.; Zhou, G.; Zhu, L.; Wang, L.; Zhang, L.; Kwok, H.; Zhang, Z.; Zhao, Q. Targeting B7-H3 Immune Checkpoint with Chimeric Antigen Receptor-Engineered Natural Killer Cells Exhibits Potent Cytotoxicity Against Non-Small Cell Lung Cancer. Front. Pharmacol. 2020, 11, 1089. [Google Scholar] [CrossRef]
- Hong, S.; Yu, C.; Wang, P.; Shi, Y.; Cao, W.; Cheng, B.; Chapla, D.; Ma, Y.; Li, J.; Rodrigues, E.; et al. Glycoengineering of NK Cells with Glycan Ligands of CD22 and Selectins for B-Cell Lymphoma Therapy. Angew. Chem. Int. Ed. 2021, 60, 3603–3610. [Google Scholar] [CrossRef]
- Saetersmoen, M.; Hammer, Q.; Valamehr, B.; Kaufman, D.; Malmberg, K. Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells. Semin. Immunopathol. 2019, 41, 59–68. [Google Scholar] [CrossRef]
- Goldenson, B.; Zhu, H.; Wang, Y.; Heragu, N.; Bernareggi, D.; Ruiz-Cisneros, A.; Bahena, A.; Ask, E.; Hoel, H.; Malmberg, K.; et al. Umbilical Cord Blood and iPSC-Derived Natural Killer Cells Demonstrate Key Differences in Cytotoxic Activity and KIR Profiles. Front. Immunol. 2020, 11, 561553. [Google Scholar] [CrossRef]
- Silla, L.; Valim, V.; Pezzi, A.; da Silva, M.; Wilke, I.; Nobrega, J.; Vargas, A.; Amorin, B.; Correa, B.; Zambonato, B.; et al. Adoptive immunotherapy with double-bright (CD56/CD16) expanded natural killer cells in patients with relapsed or refractory acute myeloid leukaemia: A proof-of-concept study. Br. J. Haematol. 2021, 195, 710–721. [Google Scholar] [CrossRef]
- Burger, M.; Zhang, C.; Harter, P.; Romanski, A.; Strassheimer, F.; Senft, C.; Tonn, T.; Steinbach, J.; Wels, W. CAR-Engineered NK Cells for the Treatment of Glioblastoma: Turning Innate Effectors Into Precision Tools for Cancer Immunotherapy. Front. Immunol. 2019, 10, 2683. [Google Scholar] [CrossRef]
- Daher, M.; Basar, R.; Gokdemir, E.; Baran, N.; Uprety, N.; Nunez Cortes, A.; Mendt, M.; Kerbauy, L.; Banerjee, P.; Shanley, M.; et al. Targeting a cytokine checkpoint enhances the fitness of armored cord blood CAR-NK cells. Blood 2021, 137, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Luanpitpong, S.; Poohadsuan, J.; Klaihmon, P.; Issaragrisil, S. Selective Cytotoxicity of Single and Dual Anti-CD19 and Anti-CD138 Chimeric Antigen Receptor-Natural Killer Cells against Hematologic Malignancies. J. Immunol. Res. 2021, 2021, 5562630. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Rong, L.; Jiang, N.; Wayne, A.; Xie, J. Targeting HLA-DR loss in hematologic malignancies with an inhibitory chimeric antigen receptor. Mol. Ther. J. Am. Soc. Gene Ther. 2022, 30, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Roex, G.; Campillo-Davo, D.; Flumens, D.; Shaw, P.; Krekelbergh, L.; De Reu, H.; Berneman, Z.; Lion, E.; Anguille, S. Two for one: Targeting BCMA and CD19 in B-cell malignancies with off-the-shelf dual-CAR NK-92 cells. J. Transl. Med. 2022, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Hambach, J.; Riecken, K.; Cichutek, S.; Schütze, K.; Albrecht, B.; Petry, K.; Röckendorf, J.; Baum, N.; Kröger, N.; Hansen, T.; et al. Targeting CD38-Expressing Multiple Myeloma and Burkitt Lymphoma Cells In Vitro with Nanobody-Based Chimeric Antigen Receptors (Nb-CARs). Cells 2020, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Guo, C.; Chen, H.; Zhang, H.; Zhi, L.; Lv, T.; Li, M.; Niu, Z.; Lu, P.; Zhu, W. A novel chimeric PD1-NKG2D-41BB receptor enhances antitumor activity of NK92 cells against human lung cancer H1299 cells by triggering pyroptosis. Mol. Immunol. 2020, 122, 200–206. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Q.; Zhong, M.; Wang, Z.; Chen, Z.; Zhang, Y.; Xing, H.; Tian, Z.; Tang, K.; Liao, X.; et al. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J. Hematol. Oncol. 2019, 12, 49. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, J.; Liu, T.; Xu, Q.; Song, X.; Zeng, J. DNAM1 and 2B4 Costimulatory Domains Enhance the Cytotoxicity of Anti-GPC3 Chimeric Antigen Receptor-Modified Natural Killer Cells Against Hepatocellular Cancer Cells in vitro. Cancer Manag. Res. 2020, 12, 3247–3255. [Google Scholar] [CrossRef]
- Oelsner, S.; Waldmann, A.; Billmeier, A.; Röder, J.; Lindner, A.; Ullrich, E.; Marschalek, R.; Dotti, G.; Jung, G.; Große-Hovest, L.; et al. Genetically engineered CAR NK cells display selective cytotoxicity against FLT3-positive B-ALL and inhibit in vivo leukemia growth. Int. J. Cancer 2019, 145, 1935–1945. [Google Scholar] [CrossRef]
- Gurney, M.; Stikvoort, A.; Nolan, E.; Kirkham-McCarthy, L.; Khoruzhenko, S.; Shivakumar, R.; Zweegman, S.; Van de Donk, N.; Mutis, T.; Szegezdi, E.; et al. CD38 knockout natural killer cells expressing an affinity optimized CD38 chimeric antigen receptor successfully target acute myeloid leukemia with reduced effector cell fratricide. Haematologica 2022, 107, 437–445. [Google Scholar] [CrossRef]
- Albinger, N.; Pfeifer, R.; Nitsche, M.; Mertlitz, S.; Campe, J.; Stein, K.; Kreyenberg, H.; Schubert, R.; Quadflieg, M.; Schneider, D.; et al. Primary CD33-targeting CAR-NK cells for the treatment of acute myeloid leukemia. Blood Cancer J. 2022, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.; Kloos, A.; Lenz, D.; Kattre, N.; Nowak, J.; Bentele, M.; Keisker, M.; Dahlke, J.; Zimmermann, K.; Sauer, M.; et al. Improved Activity against Acute Myeloid Leukemia with Chimeric Antigen Receptor (CAR)-NK-92 Cells Designed to Target CD123. Viruses 2021, 13, 1365. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, I.; Ho, W.; Marple, A.; Ravich, J.; Tam, A.; Rahnama, R.; Fearnow, A.; Rietberg, C.; Yanik, S.; Solomou, E.; et al. Engineering CAR-NK cells to secrete IL-15 sustains their anti-AML functionality but is associated with systemic toxicities. J. Immunother. Cancer 2021, 9, e003894. [Google Scholar] [CrossRef] [PubMed]
- Jamali, A.; Hadjati, J.; Madjd, Z.; Mirzaei, H.; Thalheimer, F.; Agarwal, S.; Bonig, H.; Ullrich, E.; Hartmann, J. Highly Efficient Generation of Transgenically Augmented CAR NK Cells Overexpressing CXCR4. Front. Immunol. 2020, 11, 2028. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Tong, Y.; Dotti, G.; Shaim, H.; Savoldo, B.; Mukherjee, M.; Orange, J.; Wan, X.; Lu, X.; Reynolds, A.; et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia 2018, 32, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Ng, Y.; Zha, S.; Wang, S. piggyBac system to co-express NKG2D CAR and IL-15 to augment the in vivo persistence and anti-AML activity of human peripheral blood NK cells. Mol. Therapy. Methods Clin. Dev. 2021, 23, 582–596. [Google Scholar] [CrossRef]
- Dehbashi, M.; Hojati, Z.; Motovali-Bashi, M.; Ganjalikhany, M.; Cho, W.; Shimosaka, A.; Navabi, P.; Ganjalikhani-Hakemi, M. A Novel CAR Expressing NK Cell Targeting CD25 With the Prospect of Overcoming Immune Escape Mechanism in Cancers. Front. Oncol. 2021, 11, 649710. [Google Scholar] [CrossRef]
- Yu, M.; Luo, H.; Fan, M.; Wu, X.; Shi, B.; Di, S.; Liu, Y.; Pan, Z.; Jiang, H.; Li, Z. Development of GPC3-Specific Chimeric Antigen Receptor-Engineered Natural Killer Cells for the Treatment of Hepatocellular Carcinoma. Mol. Ther. J. Am. Soc. Gene Ther. 2018, 26, 366–378. [Google Scholar] [CrossRef]
- Ueda, T.; Kumagai, A.; Iriguchi, S.; Yasui, Y.; Miyasaka, T.; Nakagoshi, K.; Nakane, K.; Saito, K.; Takahashi, M.; Sasaki, A.; et al. Non-clinical efficacy, safety and stable clinical cell processing of induced pluripotent stem cell-derived anti-glypican-3 chimeric antigen receptor-expressing natural killer/innate lymphoid cells. Cancer Sci. 2020, 111, 1478–1490. [Google Scholar] [CrossRef]
- Ao, X.; Yang, Y.; Li, W.; Tan, Y.; Guo, W.; Ao, L.; He, X.; Wu, X.; Xia, J.; Xu, X.; et al. Anti-αFR CAR-engineered NK-92 Cells Display Potent Cytotoxicity Against αFR-positive Ovarian Cancer. J. Immunother. 2019, 42, 284–296. [Google Scholar] [CrossRef]
- Cao, B.; Liu, M.; Wang, L.; Liang, B.; Feng, Y.; Chen, X.; Shi, Y.; Zhang, J.; Ye, X.; Tian, Y.; et al. Use of chimeric antigen receptor NK-92 cells to target mesothelin in ovarian cancer. Biochem. Biophys. Res. Commun. 2020, 524, 96–102. [Google Scholar] [CrossRef]
- Cao, B.; Liu, M.; Huang, J.; Zhou, J.; Li, J.; Lian, H.; Huang, W.; Guo, Y.; Yang, S.; Lin, L.; et al. Development of mesothelin-specific CAR NK-92 cells for the treatment of gastric cancer. Int. J. Biol. Sci. 2021, 17, 3850–3861. [Google Scholar] [CrossRef]
- Hu, Z. Tissue factor as a new target for CAR-NK cell immunotherapy of triple-negative breast cancer. Sci. Rep. 2020, 10, 2815. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Huang, K.; Fang, X.; Li, Y.; Wang, F.; An, L.; Chen, Q.; Zhang, Y.; Shi, A.; et al. Targeting epidermal growth factor-overexpressing triple-negative breast cancer by natural killer cells expressing a specific chimeric antigen receptor. Cell Prolif. 2020, 53, e12858. [Google Scholar] [CrossRef]
- Xiao, L.; Cen, D.; Gan, H.; Sun, Y.; Huang, N.; Xiong, H.; Jin, Q.; Su, L.; Liu, X.; Wang, K.; et al. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol. Ther. J. Am. Soc. Gene Ther. 2019, 27, 1114–1125. [Google Scholar] [CrossRef]
- Leivas, A.; Valeri, A.; Córdoba, L.; García-Ortiz, A.; Ortiz, A.; Sánchez-Vega, L.; Graña-Castro, O.; Fernández, L.; Carreño-Tarragona, G.; Pérez, M.; et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J. 2021, 11, 146. [Google Scholar] [CrossRef]
- Ng, Y.; Tay, J.; Wang, S. CXCR1 Expression to Improve Anti-Cancer Efficacy of Intravenously Injected CAR-NK Cells in Mice with Peritoneal Xenografts. Mol. Ther. Oncolytics 2020, 16, 75–85. [Google Scholar] [CrossRef]
- Portillo, A.; Hogg, R.; Poznanski, S.; Rojas, E.; Cashell, N.; Hammill, J.; Chew, M.; Shenouda, M.; Ritchie, T.; Cao, Q.; et al. Expanded human NK cells armed with CAR uncouple potent anti-tumor activity from off-tumor toxicity against solid tumors. iScience 2021, 24, 102619. [Google Scholar] [CrossRef]
- Jan, C.; Huang, S.; Canoll, P.; Bruce, J.; Lin, Y.; Pan, C.; Lu, H.; Chiu, S.; Cho, D. Targeting human leukocyte antigen G with chimeric antigen receptors of natural killer cells convert immunosuppression to ablate solid tumors. J. Immunother. Cancer 2021, 9, e003050. [Google Scholar] [CrossRef]
- Klopotowska, M.; Bajor, M.; Graczyk-Jarzynka, A.; Kraft, A.; Pilch, Z.; Zhylko, A.; Firczuk, M.; Baranowska, I.; Lazniewski, M.; Plewczynski, D.; et al. PRDX-1 supports the survival and antitumor activity of primary and CAR-modified NK cells under oxidative stress. Cancer Immunol. Res. 2021, 10, 228–244. [Google Scholar] [CrossRef]
- Imai, C.; Iwamoto, S.; Campana, D. Genetic modification of primary natural killer cells overcomes inhibitory signals and induces specific killing of leukemic cells. Blood 2005, 106, 376–383. [Google Scholar] [CrossRef] [PubMed]
- Orlando, E.; Han, X.; Tribouley, C.; Wood, P.; Leary, R.; Riester, M.; Levine, J.; Qayed, M.; Grupp, S.; Boyer, M.; et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat. Med. 2018, 24, 1504–1506. [Google Scholar] [CrossRef] [PubMed]
- Horenstein, A.; Bracci, C.; Morandi, F.; Malavasi, F. CD38 in Adenosinergic Pathways and Metabolic Re-programming in Human Multiple Myeloma Cells: In-tandem Insights from Basic Science to Therapy. Front. Immunol. 2019, 10, 760. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.R.; Schmidt, F.I.; Ploegh, H.L. Exploiting Nanobodies’ Singular Traits. Annu. Rev. Immunol. 2018, 36, 695–715. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Shen, R.; Campbell, A.; McMichael, E.; Yu, L.; Ramaswamy, B.; London, C.; Xu, T.; Carson, W. Targeting Tissue Factor for Immunotherapy of Triple-Negative Breast Cancer Using a Second-Generation ICON. Cancer Immunol. Res. 2018, 6, 671–684. [Google Scholar] [CrossRef]
- Waldhauer, I.; Goehlsdorf, D.; Gieseke, F.; Weinschenk, T.; Wittenbrink, M.; Ludwig, A.; Stevanovic, S.; Rammensee, H.; Steinle, A. Tumor-associated MICA is shed by ADAM proteases. Cancer Res. 2008, 68, 6368–6376. [Google Scholar] [CrossRef]
- Boutet, P.; Agüera-González, S.; Atkinson, S.; Pennington, C.; Edwards, D.; Murphy, G.; Reyburn, H.; Valés-Gómez, M. Cutting edge: The metalloproteinase ADAM17/TNF-alpha-converting enzyme regulates proteolytic shedding of the MHC class I-related chain B protein. J. Immunol. 2009, 182, 49–53. [Google Scholar] [CrossRef]
- Luo, Q.; Luo, W.; Zhu, Q.; Huang, H.; Peng, H.; Liu, R.; Xie, M.; Li, S.; Li, M.; Hu, X.; et al. Tumor-Derived Soluble MICA Obstructs the NKG2D Pathway to Restrain NK Cytotoxicity. Aging Dis. 2020, 11, 118–128. [Google Scholar] [CrossRef]
- Ferrari de Andrade, L.; Tay, R.; Pan, D.; Luoma, A.; Ito, Y.; Badrinath, S.; Tsoucas, D.; Franz, B.; May, K.; Harvey, C.; et al. Antibody-mediated inhibition of MICA and MICB shedding promotes NK cell-driven tumor immunity. Science 2018, 359, 1537–1542. [Google Scholar] [CrossRef]
- Zou, Y.; Luo, W.; Guo, J.; Luo, Q.; Deng, M.; Lu, Z.; Fang, Y.; Zhang, C. NK cell-mediated anti-leukemia cytotoxicity is enhanced using a NKG2D ligand MICA and anti-CD20 scfv chimeric protein. Eur. J. Immunol. 2018, 48, 1750–1763. [Google Scholar] [CrossRef]
- Jing, Y.; Ni, Z.; Wu, J.; Higgins, L.; Markowski, T.; Kaufman, D.; Walcheck, B. Identification of an ADAM17 cleavage region in human CD16 (FcγRIII) and the engineering of a non-cleavable version of the receptor in NK cells. PLoS ONE 2015, 10, e0121788. [Google Scholar] [CrossRef]
- Snyder, K.; Hullsiek, R.; Mishra, H.; Mendez, D.; Li, Y.; Rogich, A.; Kaufman, D.; Wu, J.; Walcheck, B. Expression of a Recombinant High Affinity IgG Fc Receptor by Engineered NK Cells as a Docking Platform for Therapeutic mAbs to Target Cancer Cells. Front. Immunol. 2018, 9, 2873. [Google Scholar] [CrossRef]
- Hintz, H.; Snyder, K.; Wu, J.; Hullsiek, R.; Dahlvang, J.; Hart, G.; Walcheck, B.; LeBeau, A. Simultaneous Engagement of Tumor and Stroma Targeting Antibodies by Engineered NK-92 Cells Expressing CD64 Controls Prostate Cancer Growth. Cancer Immunol. Res. 2021, 9, 1270–1282. [Google Scholar] [CrossRef]
- Mensali, N.; Dillard, P.; Hebeisen, M.; Lorenz, S.; Theodossiou, T.; Myhre, M.; Fåne, A.; Gaudernack, G.; Kvalheim, G.; Myklebust, J.; et al. NK cells specifically TCR-dressed to kill cancer cells. EBioMedicine 2019, 40, 106–117. [Google Scholar] [CrossRef]
- Parlar, A.; Sayitoglu, E.; Ozkazanc, D.; Georgoudaki, A.; Pamukcu, C.; Aras, M.; Josey, B.; Chrobok, M.; Branecki, S.; Zahedimaram, P.; et al. Engineering antigen-specific NK cell lines against the melanoma-associated antigen tyrosinase via TCR gene transfer. Eur. J. Immunol. 2019, 49, 1278–1290. [Google Scholar] [CrossRef]
- Wang, X.; Lang, S.; Tian, Y.; Zhang, J.; Yan, X.; Fang, Z.; Weng, J.; Lu, N.; Wu, X.; Li, T.; et al. Glycoengineering of Natural Killer Cells with CD22 Ligands for Enhanced Anticancer Immunotherapy. ACS Cent. Sci. 2020, 6, 382–389. [Google Scholar] [CrossRef]
- Shaim, H.; Shanley, M.; Basar, R.; Daher, M.; Gumin, J.; Zamler, D.; Uprety, N.; Wang, F.; Huang, Y.; Gabrusiewicz, K.; et al. Targeting the αv integrin/TGF-β axis improves natural killer cell function against glioblastoma stem cells. J. Clin. Investig. 2021, 131, e142116. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, P.; Lian, G.; Li, C.; Li, J.; Huang, X.; To, K.; Lan, H. Enhanced Cancer Immunotherapy with Smad3-Silenced NK-92 Cells. Cancer Immunol. Res. 2018, 6, 965–977. [Google Scholar] [CrossRef]
- Burga, R.; Yvon, E.; Chorvinsky, E.; Fernandes, R.; Cruz, C.; Bollard, C. Engineering the TGFβ Receptor to Enhance the Therapeutic Potential of Natural Killer Cells as an Immunotherapy for Neuroblastoma. Clin. Cancer Res. An. Off. J. Am. Assoc. Cancer Res. 2019, 25, 4400–4412. [Google Scholar] [CrossRef]
- Guo, X.; Mahlakõiv, T.; Ye, Q.; Somanchi, S.; He, S.; Rana, H.; DiFiglia, A.; Gleason, J.; van der Touw, W.; Hariri, R.; et al. CBLB ablation with CRISPR/Cas9 enhances cytotoxicity of human placental stem cell-derived NK cells for cancer immunotherapy. J. Immunother. Cancer 2021, 9, e001975. [Google Scholar] [CrossRef]
- Sayitoglu, E.; Georgoudaki, A.; Chrobok, M.; Ozkazanc, D.; Josey, B.; Arif, M.; Kusser, K.; Hartman, M.; Chinn, T.; Potens, R.; et al. Boosting Natural Killer Cell-Mediated Targeting of Sarcoma Through DNAM-1 and NKG2D. Front. Immunol. 2020, 11, 40. [Google Scholar] [CrossRef] [PubMed]
- Levy, E.; Reger, R.; Segerberg, F.; Lambert, M.; Leijonhufvud, C.; Baumer, Y.; Carlsten, M.; Childs, R. Enhanced Bone Marrow Homing of Natural Killer Cells Following mRNA Transfection with Gain-of-Function Variant CXCR4. Front. Immunol. 2019, 10, 1262. [Google Scholar] [CrossRef] [PubMed]
- Chanswangphuwana, C.; Allan, D.; Chakraborty, M.; Reger, R.; Childs, R. Augmentation of NK Cell Proliferation and Anti-tumor Immunity by Transgenic Expression of Receptors for EPO or TPO. Mol. Ther. J. Am. Soc. Gene Ther. 2021, 29, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Naeimi Kararoudi, M.; Nagai, Y.; Elmas, E.; de Souza Fernandes Pereira, M.; Ali, S.; Imus, P.; Wethington, D.; Borrello, I.; Lee, D.; Ghiaur, G. CD38 deletion of human primary NK cells eliminates daratumumab-induced fratricide and boosts their effector activity. Blood 2020, 136, 2416–2427. [Google Scholar] [CrossRef] [PubMed]
- Clara, J.; Levy, E.; Reger, R.; Barisic, S.; Chen, L.; Cherkasova, E.; Chakraborty, M.; Allan, D.; Childs, R. High-affinity CD16 integration into a CRISPR/Cas9-edited CD38 locus augments CD38-directed antitumor activity of primary human natural killer cells. J. Immunother. Cancer 2022, 10, e003804. [Google Scholar] [CrossRef]
- Bruhns, P.; Jönsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef]
- Lanier, L. Up on the tightrope: Natural killer cell activation and inhibition. Nat. Immunol. 2008, 9, 495–502. [Google Scholar] [CrossRef]
- Romee, R.; Foley, B.; Lenvik, T.; Wang, Y.; Zhang, B.; Ankarlo, D.; Luo, X.; Cooley, S.; Verneris, M.; Walcheck, B.; et al. NK cell CD16 surface expression and function is regulated by a disintegrin and metalloprotease-17 (ADAM17). Blood 2013, 121, 3599–3608. [Google Scholar] [CrossRef]
- Lajoie, L.; Congy-Jolivet, N.; Bolzec, A.; Gouilleux-Gruart, V.; Sicard, E.; Sung, H.; Peiretti, F.; Moreau, T.; Vié, H.; Clémenceau, B.; et al. ADAM17-mediated shedding of FcγRIIIA on human NK cells: Identification of the cleavage site and relationship with activation. J. Immunol. 2014, 192, 741–751. [Google Scholar] [CrossRef]
- Koene, H.; Kleijer, M.; Algra, J.; Roos, D.; von dem Borne, A.; de Haas, M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood 1997, 90, 1109–1114. [Google Scholar] [CrossRef]
- Cartron, G.; Dacheux, L.; Salles, G.; Solal-Celigny, P.; Bardos, P.; Colombat, P.; Watier, H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood 2002, 99, 754–758. [Google Scholar] [CrossRef]
- Bibeau, F.; Lopez-Crapez, E.; Di Fiore, F.; Thezenas, S.; Ychou, M.; Blanchard, F.; Lamy, A.; Penault-Llorca, F.; Frébourg, T.; Michel, P.; et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 1122–1129. [Google Scholar] [CrossRef]
- DiLillo, D.; Ravetch, J. Fc-Receptor Interactions Regulate Both Cytotoxic and Immunomodulatory Therapeutic Antibody Effector Functions. Cancer Immunol. Res. 2015, 3, 704–713. [Google Scholar] [CrossRef]
- Lu, J.; Sun, P. Structural mechanism of high affinity FcγRI recognition of immunoglobulin G. Immunol. Rev. 2015, 268, 192–200. [Google Scholar] [CrossRef]
- Chen, Y.; You, F.; Jiang, L.; Li, J.; Zhu, X.; Bao, Y.; Sun, X.; Tang, X.; Meng, H.; An, G.; et al. Gene-modified NK-92MI cells expressing a chimeric CD16-BB-ζ or CD64-BB-ζ receptor exhibit enhanced cancer-killing ability in combination with therapeutic antibody. Oncotarget 2017, 8, 37128–37139. [Google Scholar] [CrossRef]
- Wang, D.; Sun, Z.; Zhu, X.; Zheng, X.; Zhou, Y.; Lu, Y.; Yan, P.; Wang, H.; Liu, H.; Jin, J.; et al. GARP-mediated active TGF-β1 induces bone marrow NK cell dysfunction in AML patients with early relapse post-allo-HSCT. Blood 2022. [Google Scholar] [CrossRef]
- Usmani, S.; Weiss, B.; Plesner, T.; Bahlis, N.; Belch, A.; Lonial, S.; Lokhorst, H.; Voorhees, P.; Richardson, P.; Chari, A.; et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016, 128, 37–44. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Casneuf, T.; Xu, X.; Adams, H.; Axel, A.; Chiu, C.; Khan, I.; Ahmadi, T.; Yan, X.; Lonial, S.; Plesner, T.; et al. Effects of daratumumab on natural killer cells and impact on clinical outcomes in relapsed or refractory multiple myeloma. Blood Adv. 2017, 1, 2105–2114. [Google Scholar] [CrossRef]
- Woan, K.; Kim, H.; Bjordahl, R.; Davis, Z.; Gaidarova, S.; Goulding, J.; Hancock, B.; Mahmood, S.; Abujarour, R.; Wang, H.; et al. Harnessing features of adaptive NK cells to generate iPSC-derived NK cells for enhanced immunotherapy. Cell Stem Cell 2021, 28, 2062–2075. [Google Scholar] [CrossRef]
- Hoerster, K.; Uhrberg, M.; Wiek, C.; Horn, P.; Hanenberg, H.; Heinrichs, S. HLA Class I Knockout Converts Allogeneic Primary NK Cells Into Suitable Effectors for “Off-the-Shelf” Immunotherapy. Front. Immunol. 2020, 11, 586168. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.; He, F.; Bachanova, V.; Vercellotti, G.; DeFor, T.; Curtsinger, J.; Robertson, P.; Grzywacz, B.; Conlon, K.; Waldmann, T.; et al. First-in-human trial of rhIL-15 and haploidentical natural killer cell therapy for advanced acute myeloid leukemia. Blood Adv. 2019, 3, 1970–1980. [Google Scholar] [CrossRef] [PubMed]
- Grzywacz, B.; Moench, L.; McKenna, D.; Tessier, K.; Bachanova, V.; Cooley, S.; Miller, J.; Courville, E. Natural Killer Cell Homing and Persistence in the Bone Marrow After Adoptive Immunotherapy Correlates with Better Leukemia Control. J. Immunother. 2019, 42, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Wu, H.; Pounds, S.; Inaba, H.; Ribeiro, R.; Cullins, D.; Rooney, B.; Bell, T.; Lacayo, N.; Heym, K.; et al. A phase II clinical trial of adoptive transfer of haploidentical natural killer cells for consolidation therapy of pediatric acute myeloid leukemia. J. Immunother. Cancer 2019, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Builes, M.; Vela Cuenca, M.; Fuster Soler, J.; Astigarraga, I.; Pascual Martínez, A.; Vagace Valero, J.; Tong, H.; Valentín Quiroga, J.; Fernández Casanova, L.; Escudero López, A.; et al. Study protocol for a phase II, multicentre, prospective, non-randomised clinical trial to assess the safety and efficacy of infusing allogeneic activated and expanded natural killer cells as consolidation therapy for paediatric acute myeloblastic leukaemia. BMJ Open 2020, 10, e029642. [Google Scholar] [CrossRef]
- Khatua, S.; Cooper, L.; Sandberg, D.; Ketonen, L.; Johnson, J.; Rytting, M.; Liu, D.; Meador, H.; Trikha, P.; Nakkula, R.; et al. Phase I study of intraventricular infusions of autologous ex vivo expanded NK cells in children with recurrent medulloblastoma and ependymoma. Neuro-Oncology 2020, 22, 1214–1225. [Google Scholar] [CrossRef]
- Lim, J.; Park, Y.; Ahn, J.; Sim, J.; Kang, S.; Hwang, S.; Chun, J.; Choi, H.; Kim, S.; Chun, D.; et al. Autologous adoptive immune-cell therapy elicited a durable response with enhanced immune reaction signatures in patients with recurrent glioblastoma: An open label, phase I/IIa trial. PLoS ONE 2021, 16, e0247293. [Google Scholar] [CrossRef]
- Multhoff, G.; Pfister, K.; Gehrmann, M.; Hantschel, M.; Gross, C.; Hafner, M.; Hiddemann, W. A 14-mer Hsp70 peptide stimulates natural killer (NK) cell activity. Cell Stress Chaperones 2001, 6, 337–344. [Google Scholar] [CrossRef]
- Kokowski, K.; Stangl, S.; Seier, S.; Hildebrandt, M.; Vaupel, P.; Multhoff, G. Radiochemotherapy combined with NK cell transfer followed by second-line PD-1 inhibition in a patient with NSCLC stage IIIb inducing long-term tumor control: A case study. Strahlenther. Onkol. 2019, 195, 352–361. [Google Scholar] [CrossRef]
- Hoogstad-van Evert, J.; Bekkers, R.; Ottevanger, N.; Schaap, N.; Hobo, W.; Jansen, J.; Massuger, L.; Dolstra, H. Intraperitoneal infusion of ex vivo-cultured allogeneic NK cells in recurrent ovarian carcinoma patients (a phase I study). Medicine 2019, 98, e14290. [Google Scholar] [CrossRef]
- Sutlu, T.; Nyström, S.; Gilljam, M.; Stellan, B.; Applequist, S.; Alici, E. Inhibition of intracellular antiviral defense mechanisms augments lentiviral transduction of human natural killer cells: Implications for gene therapy. Hum. Gene Ther. 2012, 23, 1090–1100. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).