Simple Summary
When evaluating new cancer therapies in clinical trials, it may take a long time to estimate their effectiveness on overall survival, an outcome typically of main interest to regulatory decision-makers. To expedite access to new therapies for patients, regulatory agencies often make their decisions based on treatment effectiveness measured on surrogate outcomes; for example looking at the impact of treatment on delaying cancer recurrence, which can be measured earlier. For such decisions to be robust, a surrogate endpoint needs to be a valid predictor of overall survival. The validation can be complex and previous research in advanced colorectal cancer has suggested that the validity of a surrogate endpoint may depend on treatment class. We have investigated this and our results indicated that the validity of surrogate endpoints is stronger within some treatment classes compared to when ignoring the treatment class. Surrogate’s validity needs careful consideration to ensure appropriate regulatory decisions.
Abstract
Background and Aim: Findings from the literature suggest that the validity of surrogate endpoints in metastatic colorectal cancer (mCRC) may depend on a treatments’ mechanism of action. We explore this and the impact of Kirsten rat sarcoma (KRAS) status on surrogacy patterns in mCRC. Methods: A systematic review was undertaken to identify randomized controlled trials (RCTs) for pharmacological therapies in mCRC. Bayesian meta-analytic methods for surrogate endpoint evaluation were used to evaluate surrogate relationships across all RCTs, by KRAS status and treatment class. Surrogate endpoints explored were progression free survival (PFS) as a surrogate endpoint for overall survival (OS), and tumour response (TR) as a surrogate for PFS and OS. Results: 66 RCTs were identified from the systematic review. PFS showed a strong surrogate relationship with OS across all data and in subgroups by KRAS status. The relationship appeared stronger within individual treatment classes compared to the overall analysis. The TR-PFS and TR-OS relationships were found to be weak overall but stronger within the Epidermal Growth Factor Receptor + Chemotherapy (EGFR + Chemo) treatment class; both overall and in the wild type (WT) patients for TR-PFS, but not in patients with the mutant (MT) KRAS status where data were limited. Conclusions: PFS appeared to be a good surrogate endpoint for OS. TR showed a moderate surrogate relationship with PFS and OS for the EGFR + Chemo treatment class. There was some evidence of impact of the mechanism of action on the strength of the surrogacy patterns in mCRC, but little evidence of the impact of KRAS status on the validity of surrogate endpoints.
1. Introduction
Metastatic colorectal cancer (mCRC) is an area in which targeted treatments have proven successful, with cetuximab and panitumumab being offered as first line treatment [1]. When evaluating novel cancer therapies in randomised controlled trials (RCTs), data on overall survival (OS) is of primary interest to regulatory and reimbursement decision-makers. However, the more successful the treatment, the longer the wait for sufficiently mature effectiveness data for OS. In such circumstances, to expedite access to new therapies to patients, a surrogate endpoint, such as tumour response (TR) or progression-free survival (PFS), may be used to determine the efficacy of the drug, for example at the regulatory decision stage, and re-evaluated when more mature OS data become available [2,3,4]. It is therefore important that surrogate endpoints are appropriately validated to ensure that they are good predictors of clinical benefit [5].
Historically, surrogate endpoint validation has been conducted based on data from RCTs of all therapies in a given disease area. With an improved understanding of cancer biology, targeted treatments are available to subgroups of patients often with specific biomarkers. This raises the question whether validity of a putative surrogate endpoint depends on the treatments’ mechanism of action. Buyse et al. [6] concluded that PFS can be used as a surrogate for OS for mCRC using data from trials comparing fluorouracil plus leucovorin with fluorouracil alone, raltitrexed, irinotecan and oxaliplatin. Subsequently, Giessen et al. [7] evaluated PFS as a surrogate endpoint for OS in mCRC, exploring surrogacy patterns in subgroups of RCTs defined by treatment classes including chemotherapy (Chemo) regimens and targeted therapies with anti-Vascular Endothelial Growth Factor (anti-VEGF) or anti-Endothelial Growth Factor Receptor (anti-EGFR) directed monoclonal antibodies. They concluded that for chemotherapy, PFS was an appropriate surrogate endpoint for OS, but for the targeted treatments explored, there was not enough RCT data available to make a conclusion with certainty. Most recently, Ciani et al. [8] explored PFS, TR and time to progression (TTP) as surrogate endpoints for OS in mCRC patients using data from RCTs of a broad range of pharmacological therapies. They concluded none of the putative surrogate endpoints had a particularly strong relationship with OS and suggested that the stronger surrogacy patterns seen previously may only apply to certain treatments or treatment classes as they may depend on treatments’ mechanism of action. In this paper, we investigate whether the validity of surrogate endpoints in mCRC depends on the mechanism of action of a treatment. We also explore whether surrogacy patterns depend on the patients’ status for the Kirsten rat sarcoma (KRAS); KRAS wild-type (WT) or KRAS mutant (MT) [9,10]. This biomarker has proven crucial in determining the response to anti-epidermal growth factor receptor (EGFR) targeted therapies [10], with the therapies performing well in the WT population. Recent meta-analysis have shown mutation status and tumour sidedness may impact survival and disease progression; KRAS mutations present in 35% of left sided and 46% of right sided tumours. Notably there are few studies reporting OS or PFS based on both of these variables [11]. Therefore it is plausible that KRAS status may also determine whether the surrogate endpoint is in the causal pathway of the disease process, and the extent to which the intervention effect is mediated through the surrogate.This assessment is important clinically, given the large proportion of patients who harbour WT tumours and are therefore eligible for EGFR based therapies.
We consider the validity of putative surrogate endpoints; PFS for OS, and TR for PFS or OS. We conducted a systematic review to identify all RCTs of pharmacological therapies in mCRC from January 2003 to April 2020 reporting treatment effects on the endpoints of interest. Subsequently, we investigated surrogacy patterns overall, in patient populations defined by KRAS status and whether the surrogacy patterns differed depending on treatment class.
2. Methods
2.1. Trial Identification
A systematic review was undertaken to identify all RCTs for pharmacological therapies in mCRC (PROSPERO ID: CRD42020167075 [12]). Three databases were reviewed, Embase [13], Medline [14] and Cochrane CENTRAL [15]. Papers published January 2003 onwards were searched. No restrictions were placed on language. Searches were carried out on 3 April 2020. Full search strategies are included in Appendix A.1.
RCTs reporting the effectiveness of therapies based on KRAS status were selected. Trials were included if they were RCTs comparing pharmacological treatments in mCRC patients and reported treatment effects on at least two of the three outcomes of interest (OS, PFS, TR). Trials had to report treatment effects for WT or MT KRAS status patient groups, or both. Trials were excluded if either arm included radiotherapy or surgery alone or in combination with a pharmacological treatment. Trials for biosimilar drugs were excluded. Titles and abstracts were screened independently by three reviewers (HP, SB, MS) until 95% agreement was reached for 10% of papers. One reviewer (HP) completed the remainder of title and abstract screening. Papers were then grouped by trial and reviewed as trials at the full text stage in a similar fashion to title and abstract review process.
2.2. Data Extraction
The following general study information was extracted from all eligible RCTs: author, title, year and journal of publication, definition of disease progression used, country(s) the RCT took place in, key inclusion and exclusion criteria, length of follow up, line of treatment and pharmacological treatments given in each arm. Treatments were classified by each drug’s mechanism of action (e.g., EGFR, VEGF, or EGFR + VEGF). Trials were grouped into treatment classes based on the mechanism of action of their experimental arm.
From the selected RCTs, data were collected on the treatment effects on TR, PFS and OS. Definitions used for each treatment effect are outlined in Appendix A.2. Treatment effects on PFS and OS were recorded as hazard ratios (HRs) with 95% Confidence Intervals (CIs) or p-values if no CIs were reported. One reviewer extracted data (HP) and 10% of the data extraction was reviewed independently by one other reviewer (LW). A risk of bias assessment was performed using a modified version of the Cochrane Risk of Bias tool [16].
Trial identification and data extraction was carried out using the systematic review software tools Covidence and EndNote.
2.3. Statistical Methods
The meta-analytic method by Daniels and Hughes [17] was used to evaluate the surrogate relationships across trials for each pair of outcomes of interest; (1) PFS as a surrogate for OS, (2) TR for PFS, and (3) TR for OS. The Daniels and Hughes approach uses Bayesian meta-regression to model the relationship between the treatment effects on the two outcomes (for example log HRs on PFS and OS), whilst taking into account the uncertainty around the effects on both outcomes and the correlation between them. The model was further applied separately to subgroups of patients defined by KRAS status.
To evaluate surrogate endpoints according to the mechanism of action jointly across treatment classes (either for the whole patient population or for KRAS sub-populations), the hierarchical method proposed by Papanikos et al. [18] was used, allowing for partial exchangeability. The method, extending the approach by Daniels and Hughes, allows for borrowing of information about the surrogacy patterns across treatment classes, which is particularly useful when the number of studies for some of the classes is small.
The surrogacy criteria outlined by Daniels and Hughes [17] were used to assess the strength of the surrogate relationships. The criteria state that a perfect surrogate relationship is defined by a regression line with intercept equal to zero (to ensure no effect on the surrogate endpoint implies no effect on the final outcome), a non-zero slope (ensuring the association between the treatment effects on the surrogate endpoint and final outcome), and zero conditional variance (ensuring a perfect prediction of the treatment effect on the final outcome is made based on the treatment effect on the surrogate endpoint). In practice, we consider a surrogate relationship strong if all the following is true: the 95% interval for the intercept includes zero, the 95% interval for the slope does not include zero and the conditional variance along with its upper interval is small. When exploring surrogacy patterns in subgroups defined by treatment class or KRAS status, we use these criteria to identify any groups where surrogacy may be stronger. In this Bayesian framework, we focus on uncertainty around these parameters rather than performing any hypothesis testing. A “take-one-out” cross-validation procedure was performed to investigate the predictive value of a putative surrogate endpoint [17]. A summary of the cross-validation procedure, and further statistical methods are included in Appendix A.3.
A Bayesian approach was used for the analyses performed in WinBUGS version 1.4.3. Analyses used 125,000 Markov chain Monte Carlo (MCMC) iterations including a 25,000 burn-in. Results are presented as a mean and 95% credible interval (CrI) for each of the parameters for surrogacy criteria. Data management and additional analyses were carried out using R version 4.1.0.
3. Results
3.1. Summary of Included Trials
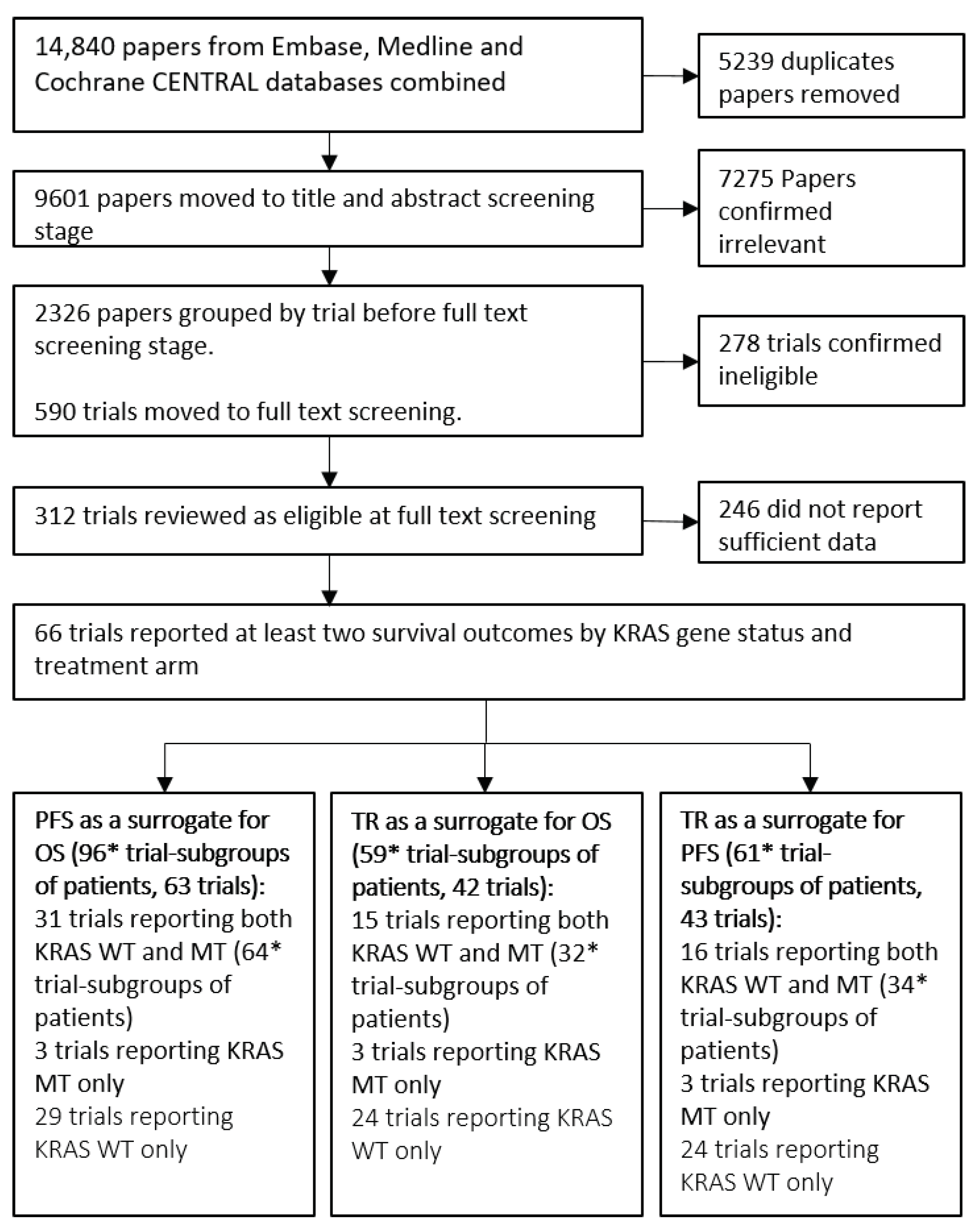
Throughout the rest of this paper we refer to “trial-subgroups” rather than trials. This is to reflect that data included in the meta-analysis is at the subgroup level; for example, two subgroups from a single trial reporting treatment effects for KRAS WT and KRAS MT are entered separately. The systematic review process, outlined in Figure 1, identified 66 trials consisting of 100 trial-subgroups that reported sufficient information to be included. The trials investigated a broad range of treatments including Chemo, EGFR and VEGF therapies. The list of treatments and classifications for the analysis are included in Table 1. Seven treatment classes were defined for the analyses investigating the impact of the mechanism of action on surrogacy patterns. Fifteen of the trials had treatment arm comparisons that were unique and therefore were not grouped into a treatment class.
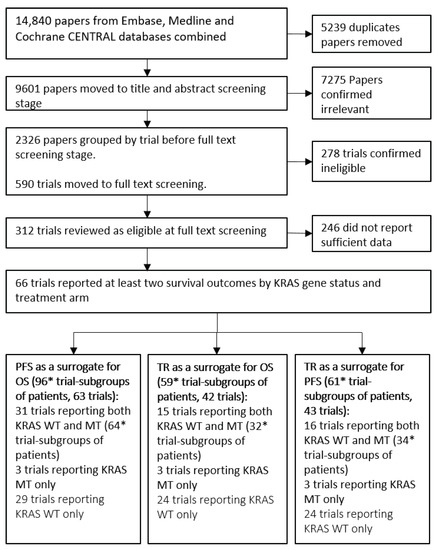
Figure 1.
Flow diagram of the systematic review process. Groups of patients are either Kirstan rat sarcoma (KRAS) wild-type (WT) or KRAS mutant (MT) patients from a trial. PFS = Progression Free Survival, OS = Overall Survival, TR = Tumour Response. * 2 trials were multi-arm trials and reported two randomised treatment contrasts for each KRAS gene status.

Table 1.
Summary of treatment arm combinations of included trials within each treatment class. Each trial was assigned to a treatment class based on the mechanism of action of the experimental arm of the trial. Chemo = Chemotherapy, EGFR = Epidermal Growth Factor Receptor, VEGF = Vascular Endothelial Growth Factor, BSC = Best Supportive Care.
The flow chart in Figure 1 shows that 96 trial-subgroups (63 trials) were available for the evaluation of PFS as a surrogate for OS, 59 trial-subgroups (42 trials) for the analysis of TR as a surrogate for OS and 61 trial-subgroups (43 trials) for the evaluation of TR as a surrogate for PFS. A full list of trials included for the evaluation of each surrogate relationship is included in Table 2.

Table 2.
Full list of trials included for each analysis.
3.2. Exploration of Surrogate Relationships
We focus here on the results for PFS as a putative surrogate endpoint for OS and TR as surrogate for PFS; both overall and according to KRAS status or treatment class. The results and conclusions for TR as a surrogate endpoint for OS can be found in Appendix B.1.
3.2.1. Surrogate Relationships Overall and by KRAS Status
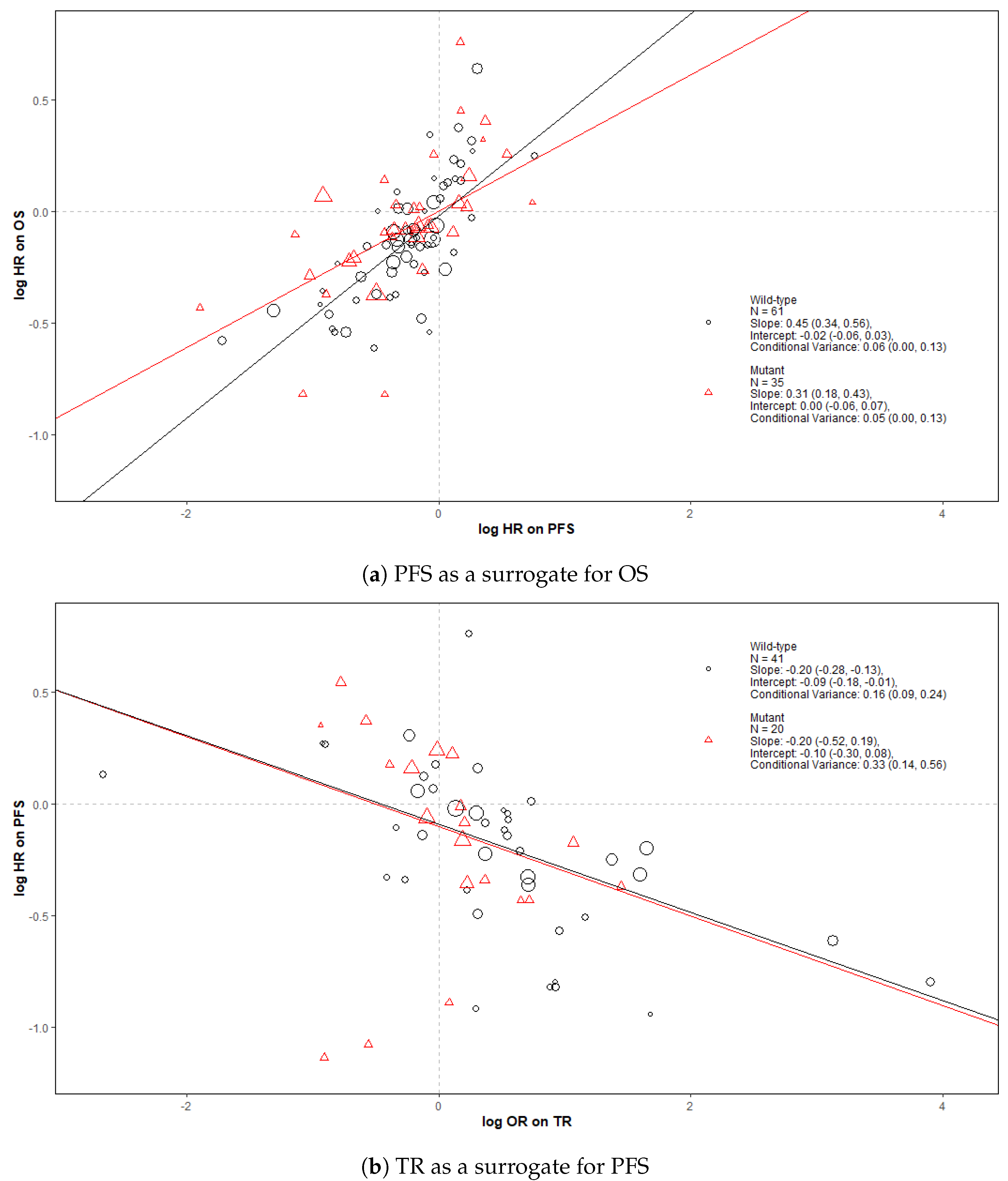
Figure 2 shows bubble plots representing data from all trial-subgroups included in the analysis, colour-coded by the KRAS status. The regression lines represent surrogate relationships by KRAS status, irrespective of treatment class, for each surrogate relationship. Surrogacy criteria for each pair of outcomes (both for all patients and KRAS subgroups) are represented in the top panels of Figure 3 and Figure 4 (marked “All data”), which correspond to the overall analysis marked by ‘All’ and the KRAS status subgroups marked by ‘MT’ and ‘WT’. Overall, the surrogacy was found to be strong for the PFS-OS surrogate relationship (Figure 2a and Figure 3). TR proved to be a sub-optimal surrogate endpoint for PFS, as indicated by a relatively large conditional variance as shown in Figure 2b and Figure 4.
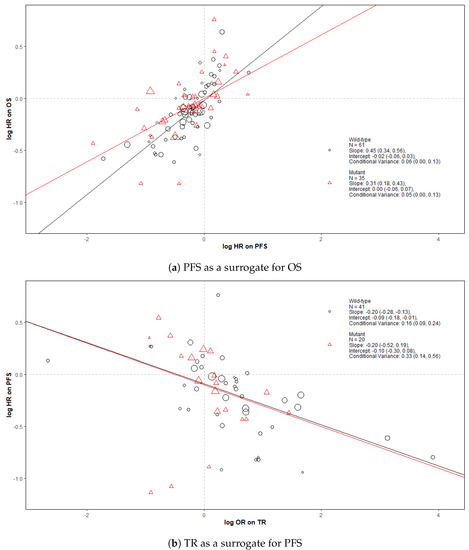
Figure 2.
Bubble plots of the surrogate relationships in trial-subgroups of patients with KRAS WT and MT metastatic colorectal cancer (mCRC). The Slope, Intercept and Conditional variance are mean estimates with 95% Credible Intervals obtained from Daniels and Hughes model. N represents the number of trial-subgroups. HR = Hazard Ratio, OR = Odds Ratio.
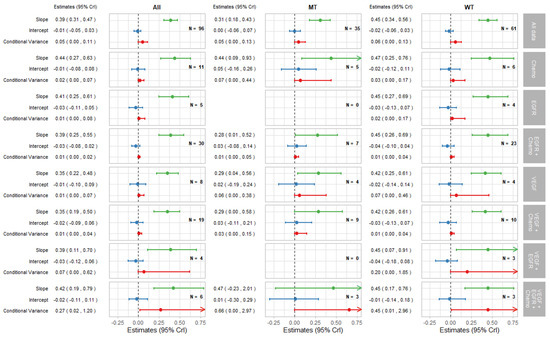
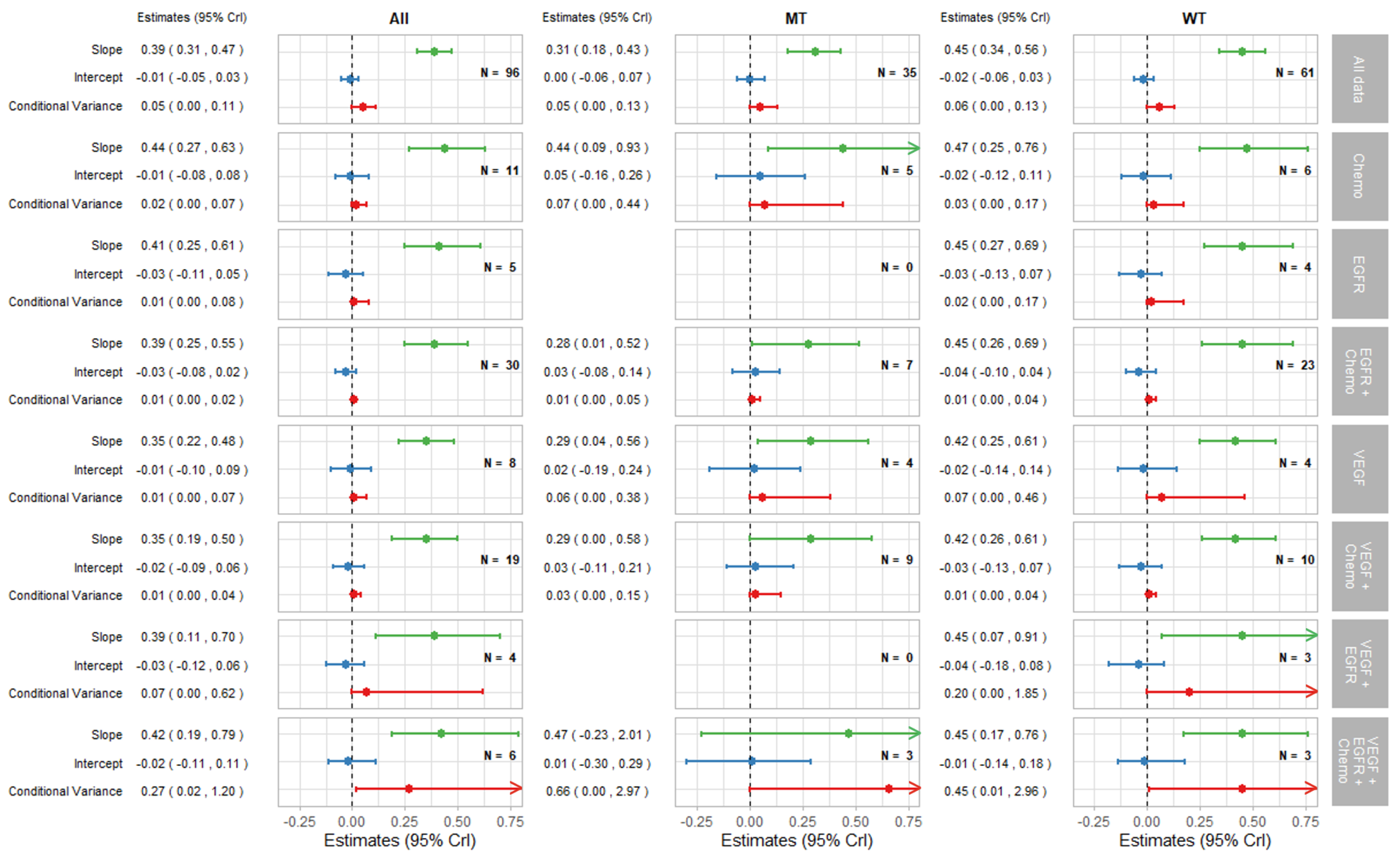
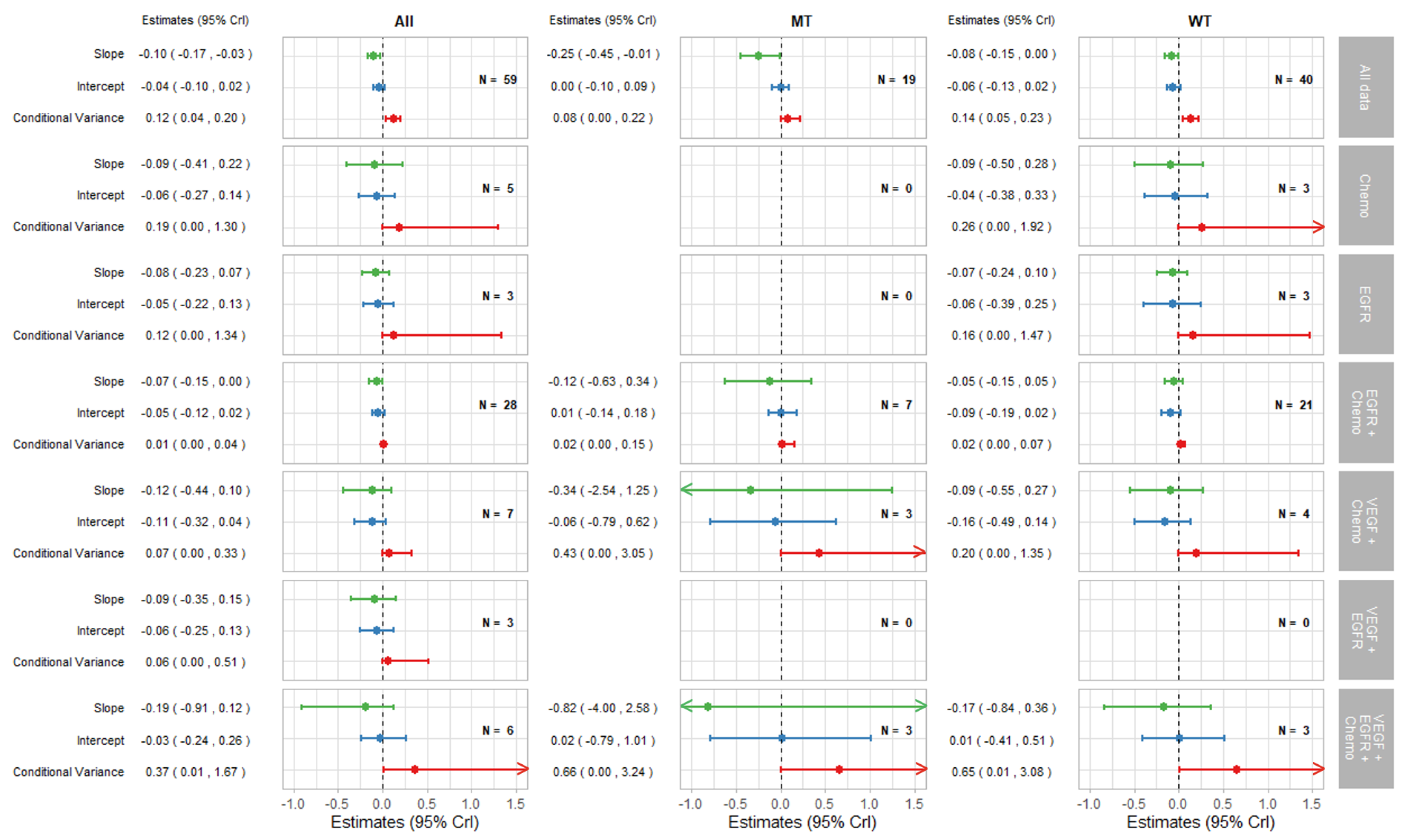
Figure 3.
Forest plot of estimates of slope (green), intercept (blue), and conditional variance (red) for PFS as a surrogate for OS. N represents the number of trial-subgroups. CrI = Credible Interval.
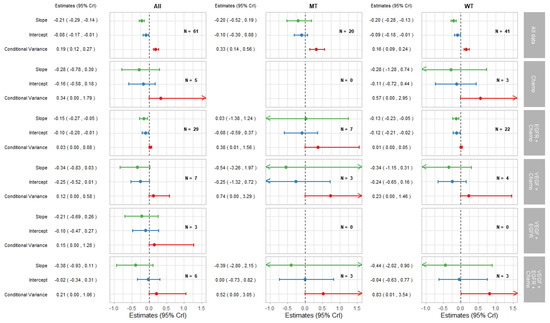
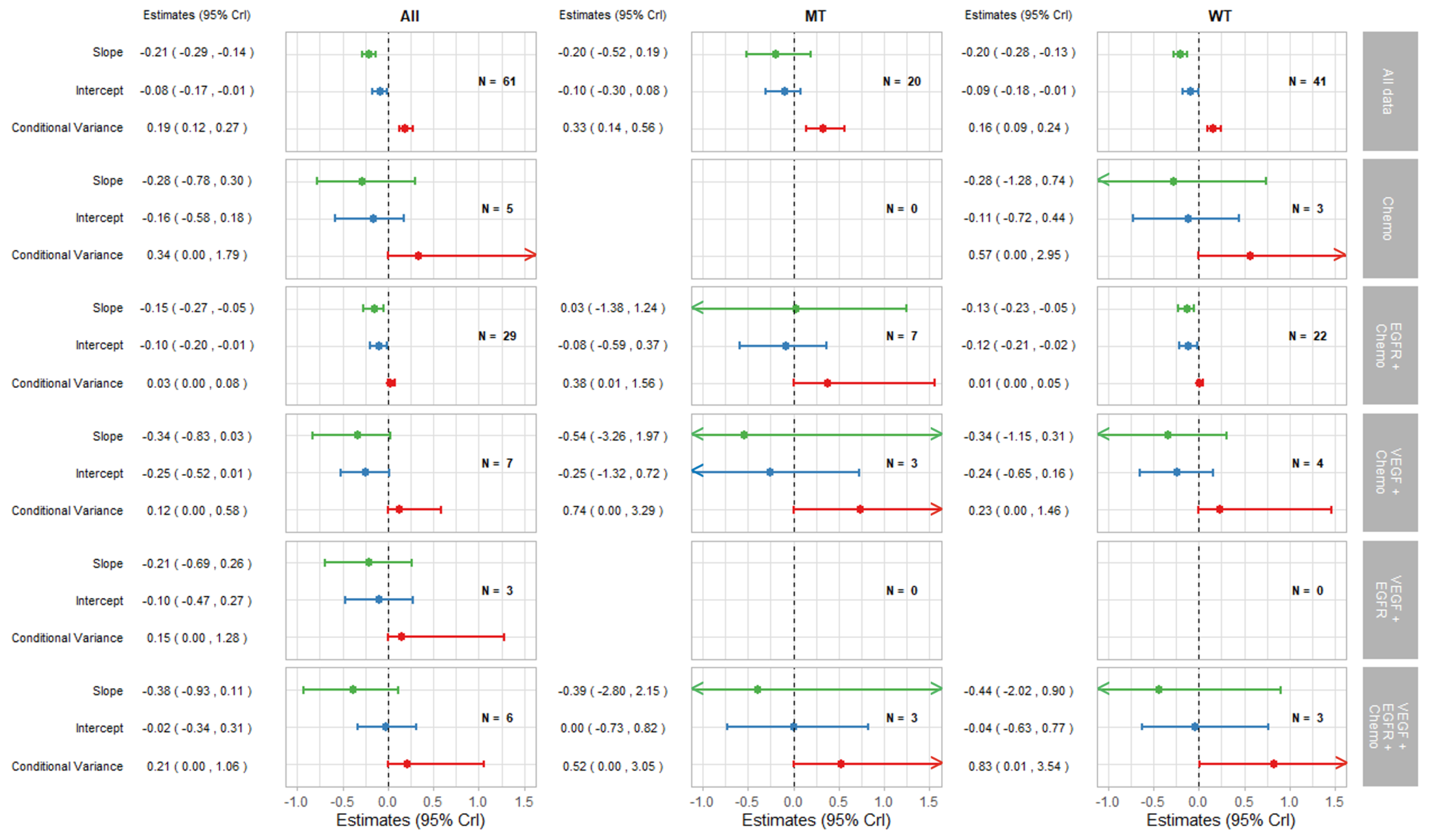
Figure 4.
Forest plot of estimates of slope (green), intercept (blue), and conditional variance (red) for TR as a surrogate for PFS. N represents the number of trial-subgroups.
The surrogate relationships between PFS and OS did not differ across KRAS subgroups where it was similar to the relationship in the overall cohort of patients, as can be seen in Figure 2a and Figure 3. For TR-PFS, the surrogacy pattern for KRAS WT was similar to the relationship for All data, as shown in the top panels of Figure 2b and Figure 4). However, the conditional variance was higher for the KRAS MT trial-subgroups where also the interval for the slope included zero, thus suggesting a weaker surrogate relationship compared to the KRAS WT and overall cohorts of patients.
3.2.2. Surrogate Relationships by Treatment Class: Overall and in KRAS Subgroups
The remaining parts of the forest plots in Figure 3 and Figure 4 correspond to the surrogate relationships across treatment classes. To investigate any impact of the KRAS status on surrogacy patterns within the treatment classes, all results are presented for subgroups of patients according to the KRAS status as well as for all patients (WT and MT combined). The left, middle and right columns correspond to ‘All’ (for WT and MT combined), MT trial-subgroups and WT trial-subgroups, respectively. Each column shows the overall results at the top, discussed in the previous section, followed by the results for each treatment class including the intercepts, slopes and conditional variances.
Figure 3 shows results for surrogacy patterns between the treatment effect on PFS and OS. For the All trial-subgroups analysis, there were no distinct differences in surrogacy patterns between the treatment classes. The strong surrogate relationship seen for all of the data (represented in Figure 2a and the top row of Figure 3) holds for the individual treatment classes (with the exception for those classes with small numbers of trial-subgroups). However, the surrogate relationships appeared stronger, in terms of the smaller conditional variance, within most of the individual treatment classes (apart from VEGF + EGFR and VEGF + EGFR + Chemo) compared to the analysis including All data. This was also the case for the EGFR + Chemo treatment class for both MT and WT trial-subgroups and for VEGF + Chemo for the WT trial-subgroup only.
Figure 4 shows the results for TR-PFS surrogacy patterns. The surrogacy pattern was stronger within the EGFR + Chemo treatment class with the conditional variance of 0.03 (0.00, 0.08), which was much smaller compared to the conditional variance obtained from the analysis of all data; 0.19 (0.12, 0.27). The surrogacy criteria were not fully satisfied for the EGFR + Chemo treatment class, with the CrIs for the intercept not including zero for the analysis of All data and for the WT trial-subgroups alone. For the MT trial-subgroups, the TR-PFS surrogate relationship was weak. The trial-subgroups for the EGFR + Chemo treatment class contributed the majority of the data for each analysis, with little or no studies in other treatment classes; therefore, our inferences about the other treatment classes are limited. However, the change of the results from overall analysis of all data to those using data from the EGFR + Chemo treatment class suggests the importance of the mechanism of action in this surrogate relationship, which was true for both the overall result and KRAS WT subgroup of the population.
Cross-validation results for the surrogate relationship between PFS and OS are presented in Appendix B.2. In summary, treatment effect on PFS was a good predictor of the treatment effect on OS overall and within subgroups of treatment classes and KRAS status.
4. Discussion
Overall, our analyses showed that there was a strong surrogate relationship between the treatment effect on PFS and OS for mCRC, which supports existing knowledge in this area [6,7]. However the findings are stronger than the conclusions of Ciani et al. [8], who found that overall the surrogate relationship was sub-optimal; however, the criteria used for assessing surrogacy patterns differed. When considering solely bevacizumab and chemotherapy in the first and second line setting PFS was determined to be a good candidate as a surrogate endpoint for OS in patients with mCRC [85], however others have reported OS to be the preferred primary endpoint in the second line treatment of mCRC [86]. Furthermore, exploring the relationship by treatment class suggested some evidence that the mechanism of action may contribute to the strength of surrogacy patterns in mCRC for PFS-OS, as evidenced by smaller conditional variances within the treatment classes (with zero variance indicating a perfect association).
We found that overall the surrogate relationship between the treatment effects on TR and the effects on PFS or OS was weak for mCRC. However, there was some evidence that the surrogacy patterns may vary according to the mechanism of action.For EGFR+Chemo treatment class, the surrogacy pattern between TR and PFS was relatively strong except for the intercept not being zero; however, the upper interval for the intercept was close to zero. For TR-PFS pair of outcomes the results indicated some limited evidence that there is a difference in surrogacy between KRAS subgroups of patients, with MT trial-subgroup analyses showing weaker surrogacy than the WT trial-subgroup or the All data analyses.
Additional areas to consider when evaluating surrogate endpoints in mCRC include BRAF status. This is particularly important as individuals who harbour a BRAF V600 mutation often have greater risk of recurrence and poorer prognosis than patients who do not. There is improved overall survival with combination treatment of anti-EGFR and BRAF inhibitor treatment in these patients [87], which may impact the strength of a surrogate relationship. Further analysis such as side of tumour (left or right) or evidence of a PIK3CA mutation may be helpful, however this is often not reported.
5. Conclusions
This is the first review and meta-analysis investigating surrogacy patterns based on the KRAS status of patients and differentiating surrogacy patterns according to treatment class for mCRC patients. In summary, our results showed that PFS is a good surrogate for OS when evaluating pharmacological therapies for mCRC patients. The surrogate relationships between TR and PFS or OS, however, were found to be weak overall. There was evidence that the mechanism of action may contribute to the strength of surrogacy patterns in mCRC for PFS as a surrogate for OS as well as TR for PFS. These conclusions remained the same for the subgroups of patients according to their KRAS status.
Author Contributions
Conceptualization: H.P. and S.B.; Data curation: H.P., L.W., M.S. and S.B.; Formal analysis: H.P.; Funding acquisition: S.B.; Investigation: H.P.; Methodology: H.P. and S.B.; Project administration: H.P.; Software: H.P. and S.B.; Supervision: S.K., M.S. and S.B.; Validation: L.W., M.S. and S.B.; Visualization: H.P., M.S. and S.B.; Writing—original draft: H.P.; Writing—review & editing: S.K., L.W., A.T., M.S. and S.B. All authors have read and agreed to the published version of the manuscript.
Funding
Heather Poad was funded by MRC IMPACT. (Medical Research Council, Integrated Midlands Partnership for Biomedical Training in Complex Disease, grant number: MR/N013913/1). Sylwia Bujkiewicz was funded by MRC Methodology Research Panel grant (MR/T025166/1). Lorna Wheaton was funded by a National Institute for Health Research (NIHR) Pre-doctoral Fellowship [NIHR301013]. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript. Sam Khan is funded by a NIHR ACL in Medical Oncology post. The APC was funded by the UKRI open access block grant provided to the University of Leicester.
Institutional Review Board Statement
Not applicable, this research did not require ethical approval and did not involve recruiting human subjects.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are obtained from publicly available sources, all of which are listed in Table 2 and the references section.
Acknowledgments
The authors thank Nicolas Städler from Roche for accessing RCT data and provision of values for the within-study correlations.
Conflicts of Interest
Michael Sweeting is a full-time employee of AstraZeneca. Sylwia Bujkiewicz has served as a paid consultant, providing methodological advice, to NICE, Roche, RTI Health Solutions and IQVIA, received payments for educational events from Roche and has received research funding from European Federation of Pharmaceutical Industries & Associations (EEPIA) and Johnson & Johnson. Anne Thomas has served as a paid consultant, has received payment for lectures, presentations, speakers bureaus, manuscript writing or educational events, and received payment for expert testimony from Bristol Myers Squibb.
Appendix A. Further Methods
Appendix A.1. Trial Identification: Search Strategies
All searches carried out on 3 April 2020.
Appendix A.1.1. Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library
| #1 | MeSH descriptor: [Immunotherapy] explode all trees | 7760 |
| #2 | MeSH descriptor: [Angiogenesis Modulating Agents] explode all trees | 1132 |
| #3 | MeSH descriptor: [Antibodies, Monoclonal] explode all trees | 12,528 |
| #4 | MeSH descriptor: [Antineoplastic Agents] explode all trees | 11,805 |
| #5 | MeSH descriptor: [Drug Therapy] explode all trees | 137,825 |
| #6 | MeSH descriptor: [Antigens, Neoplasm] explode all trees | 2149 |
| #7 | chemotherap* | 78,829 |
| #8 | immunotherap* | 10,758 |
| #9 | (antitum?r or "anti tum?r" or anti-tum?r) | 5131 |
| #10 | inhibitor | 52,242 |
| #11 | cytotoxic | 4112 |
| #12 | cytostatic | 501 |
| #13 | (target* next (treatment or agent or therapy or administration or drug)) | 3166 |
| #14 | (hormone* next (treatment or agent or therapy or administration or drug)) | 5148 |
| #15 | (drug next (treatment or agent or therapy or administration)) | 405,830 |
| #16 | (antineoplas* or anti neoplas* or anti-neoplas*) | 29,472 |
| #17 | (anticancer* or anti cancer* or anti-cancer*) | 14,743 |
| #18 | (antiangiogen* or anti-angiogen* or anti angiogen*) | 2049 |
| #19 | infusion | 58,181 |
| #20 | immune response | 15,989 |
| #21 | (pharmacologic* next (treatment or agent or therapy or administration)) | 5686 |
| #22 | antigen | 19,362 |
| #23 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 | 556,231 |
| #24 | MeSH descriptor: [Colorectal Neoplasms] explode all trees | 7912 |
| #25 | MeSH descriptor: [Neoplasm Metastasis] explode all trees | 4995 |
| #26 | (((colorect* or colon* or rect* or anal* or intestin* or bowel* or sigmoid*) near/3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or adenoma*)) near/4 (metastas* or metastatic* or micrometastas* or micrometastatic* or advance* or "stage IV" or "stage 4" or "stage four" or irresectable or unresectable or palliati*)) | 7801 |
| #27 | (CRC near/4 (metastas* or metastatic* or micrometastas* ir micrometastatic* or advance* or "stage IV" or "stage 4" or "stage four" or irresectable or unresectable or palliati*)) | 1783 |
| #28 | aCRC or mCRC | 1662 |
| #29 | #24 and #25 | 706 |
| #30 | #26 or #27 or #28 or #29 | 8148 |
| #31 | #30 and #23 with Publication Year from 2003 to 2020, in Trials | 5047 |
Appendix A.1.2. Ovid MEDLINE
| 1 | exp Immunotherapy/ | 271,523 |
| 2 | exp Angiogenesis Modulating Agents/ | 62,615 |
| 3 | exp Antibodies, Monoclonal/ | 232,456 |
| 4 | exp Antineoplastic Agents/ | 1,085,139 |
| 5 | exp Drug Therapy/ | 1,341,564 |
| 6 | exp Antigens, Neoplasm/ | 116,062 |
| 7 | chemotherap*.mp. | 477,700 |
| 8 | immunotherap*.mp. | 111,265 |
| 9 | (antitum?r or “anti tum?r” or anti-tum?r).mp. | 166,008 |
| 10 | inhibitor.mp. | 652,707 |
| 11 | cytotoxic.mp. | 190,318 |
| 12 | cytostatic.mp. | 13,996 |
| 13 | (target* adj (treatment or agent or therapy or administration or drug)).mp. | 65,873 |
| 14 | (hormone* adj (treatment or agent or therapy or administration or drug)).mp. | 22,054 |
| 15 | (drug adj (treatment or agent or therapy or administration)).mp. | 2,373,269 |
| 16 | (antineoplas* or anti neoplas* or anti-neoplas*).mp. | 499,852 |
| 17 | (anticancer* or anti cancer* or anti-cancer*).mp. | 120,875 |
| 18 | (antiangiogen* or anti-angiogen* or anti angiogen*).mp. | 25,384 |
| 19 | infusion.mp. | 226,908 |
| 20 | immune response.mp. | 155,377 |
| 21 | (pharmacologic* adj (treatment or agent or therapy or administration)).mp. | 28,789 |
| 22 | antigen.mp. | 628,662 |
| 23 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 | 5,271,893 |
| 24 | randomized controlled trial.pt. | 503,142 |
| 25 | controlled clinical trial.pt. | 93,595 |
| 26 | randomized.ti,ab. | 513,184 |
| 27 | placebo.ti,ab. | 212,175 |
| 28 | clinical trials as topic.sh. | 190,619 |
| 29 | randomly.ti,ab. | 331,224 |
| 30 | trial.ti. | 215,880 |
| 31 | 24 or 25 or 26 or 27 or 28 or 29 or 30 | 1,287,975 |
| 32 | exp animals not humans.sh. | 4,685,426 |
| 33 | 31 not 32 | 1,185,372 |
| 34 | (((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel* or sigmoid*) adj3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or adenoma*) adj4 (metastas* or metastatic* or micrometastas* or micrometastatic* or advance* or “stage IV” or “stage 4” or “stage four” or irresectable or unresectable or palliati*)) or "aCRC" or “mCRC”).mp. | 40,955 |
| 35 | (CRC adj4 (metastas* or metastatic* or micrometastas* or micrometastatic* or advance* or “stage IV” or “stage 4” or “stage four” or irresectable or unresectable or palliati*)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] | 5279 |
| 36 | exp colorectal neoplasms/ | 197,894 |
| 37 | exp neoplasm metastasis/ | 200,932 |
| 38 | 36 and 37 | 17,461 |
| 39 | 34 or 35 or 38 | 52,675 |
| 40 | 33 and 39 | 5701 |
| 41 | 23 and 40 | 4736 |
| 42 | limit 41 to yr="2003 -Current" | 3413 |
Appendix A.1.3. Ovid EMBASE
| 1 | exp drug therapy/ | 2,759,981 |
| 2 | exp immunotherapy/ | 212,046 |
| 3 | exp drug activity/ | 2,336,899 |
| 4 | exp monoclonal antibody/ | 563,094 |
| 5 | exp cancer therapy/ | 802,327 |
| 6 | exp antigen/ | 1,561,070 |
| 7 | chemotherap*.mp. | 843,036 |
| 8 | immunotherap*.mp. | 197,910 |
| 9 | (antitum?r or “anti tum?r” or anti-tum?r).mp. | 169,575 |
| 10 | inhibitor.mp. | 1,419,535 |
| 11 | cytotoxic.mp. | 273,532 |
| 12 | cytostatic.mp. | 21,483 |
| 13 | (target* adj (treatment or agent or therapy or administration or drug)).mp. | 93,739 |
| 14 | (hormone* adj (treatment or agent or therapy or administration or drug)).mp. | 47,449 |
| 15 | (drug adj (treatment or agent or therapy or administration)).mp. | 5,633,269 |
| 16 | (antineoplas* or anti neoplas* or anti-neoplas*).mp. | 460,857 |
| 17 | (anticancer* or anti cancer* or anti-cancer*).mp. | 163,990 |
| 18 | (antiangiogen* or anti-angiogen* or anti angiogen*).mp. | 45,610 |
| 19 | infusion.mp. | 368,696 |
| 20 | immune response.mp. | 356,585 |
| 21 | (pharmacologic* adj (treatment or agent or therapy or administration)).mp. | 44,297 |
| 22 | antigen.mp. | 1,339,922 |
| 23 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 | 9,964,936 |
| 24 | random*.ab,ti. or placebo*.de,ab,ti. or (double adj1 blind*).ab,ti. | 1,772,713 |
| 25 | (exp animal/ or exp nonhuman/ or exp animal experiment/ or exp experimental animal/) not exp human/ | 6,440,460 |
| 26 | 24 not 25 | 1,566,673 |
| 27 | exp “metastatic colorectal cancer”/ | 10,749 |
| 28 | (((colorect* or colon* or rect* or anal* or anus* or intestin* or bowel* or sigmoid*) adj3 (carcinom* or neoplas* or adenocarcinom* or cancer* or tumor* or tumour* or sarcom* or adenoma*) adj4 (metastas* or metastatic* or micrometastas* or micrometastatic* or advance* or “stage IV” or “stage 4” or “stage four” or irresectable or unresectable or palliati*)) or “aCRC” or “mCRC”).mp. | 67,297 |
| 29 | (CRC adj4 (metastas* or metastatic* or micrometastas* or micrometastatic* or advance* or “stage IV” or “stage 4” or “stage four” or irresectable or unresectable or palliati*)).mp. | 9236 |
| 30 | 27 or 28 or 29 | 70,548 |
| 31 | 23 and 26 and 30 | 7155 |
| 32 | limit 31 to yr = “2003 -Current” | 6380 |
Appendix A.2. Data Extraction: Definitions of Treatment Effects
PFS was defined as time taken from randomisation or from start of treatment, until tumour progression or death (of any cause). OS was defined as time from randomisation or start of treatment until death from any cause. TR was defined as patients achieving complete or partial response at the time point specified in each RCT. For solid tumours, a partial or complete response was defined as a decrease in the tumour size usually with reference to Response Evaluation Criteria in Solid Tumours (RECIST) [88] or World Health Organisation (WHO) criteria [89] or in some cases individual trial criteria. The numbers of responders and total numbers of participants were recorded to estimate treatment effects on TR using odds ratios (ORs).
Appendix A.3. Statistical Methods
Appendix A.3.1. Cross Validation
Take-one-out cross-validation procedure was carried out to investigate the predictive value of each surrogate endpoint. The proportion of the observed effect estimates that fall within the predicted interval, the absolute difference of means of the observed and predicted effects, and the ratio of the widths between the observed and predicted intervals were calculated for each model. By chance, it is expected that around 5% of observed estimates may fall outside of the 95% predictive interval.
Appendix A.3.2. Further Statistical Methods
Within-study correlation is needed for each trial to populate the model, however this is rarely reported for RCTs. Within-study correlations between the treatment effects on PFS and OS, between the effects on TR and PFS, and between TR and OS were provided by collaborators at Roche, obtained from four RCTs for which individual patient data were available. Average correlations across the trials reported for each of the surrogate relationships were used in the analysis assuming the same correlation across trials.
Appendix B. Further Results
Appendix B.1. Results and Conclusions for TR as a Surrogate for OS
Appendix B.1.1. Results
TR as a surrogate for OS proved to be a weak surrogate endpoint (Figure A1 and Figure A2). This is due to the conditional variances being relatively large.
Exploring the results by KRAS status for TR-OS, there was no particular difference in surrogate relationship between KRAS subgroups and overall cohort of patients, as seen in Figure A1, and top row of Figure A2.
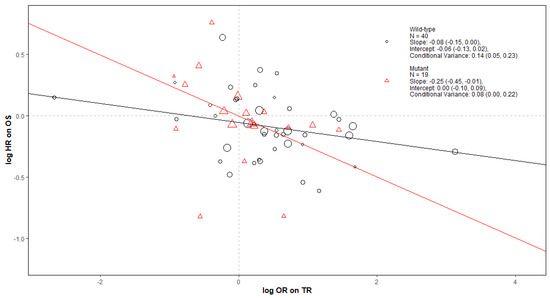
Figure A1.
Bubble plot of TR as a surrogate for OS in RCTs of KRAS WT and MT mCRC. The Slope, Intercept and Conditional variance are mean estimates with 95% Credible Intervals obtained from Daniels and Hughes model. N represents the number of trial-subgroups.
Figure A1.
Bubble plot of TR as a surrogate for OS in RCTs of KRAS WT and MT mCRC. The Slope, Intercept and Conditional variance are mean estimates with 95% Credible Intervals obtained from Daniels and Hughes model. N represents the number of trial-subgroups.
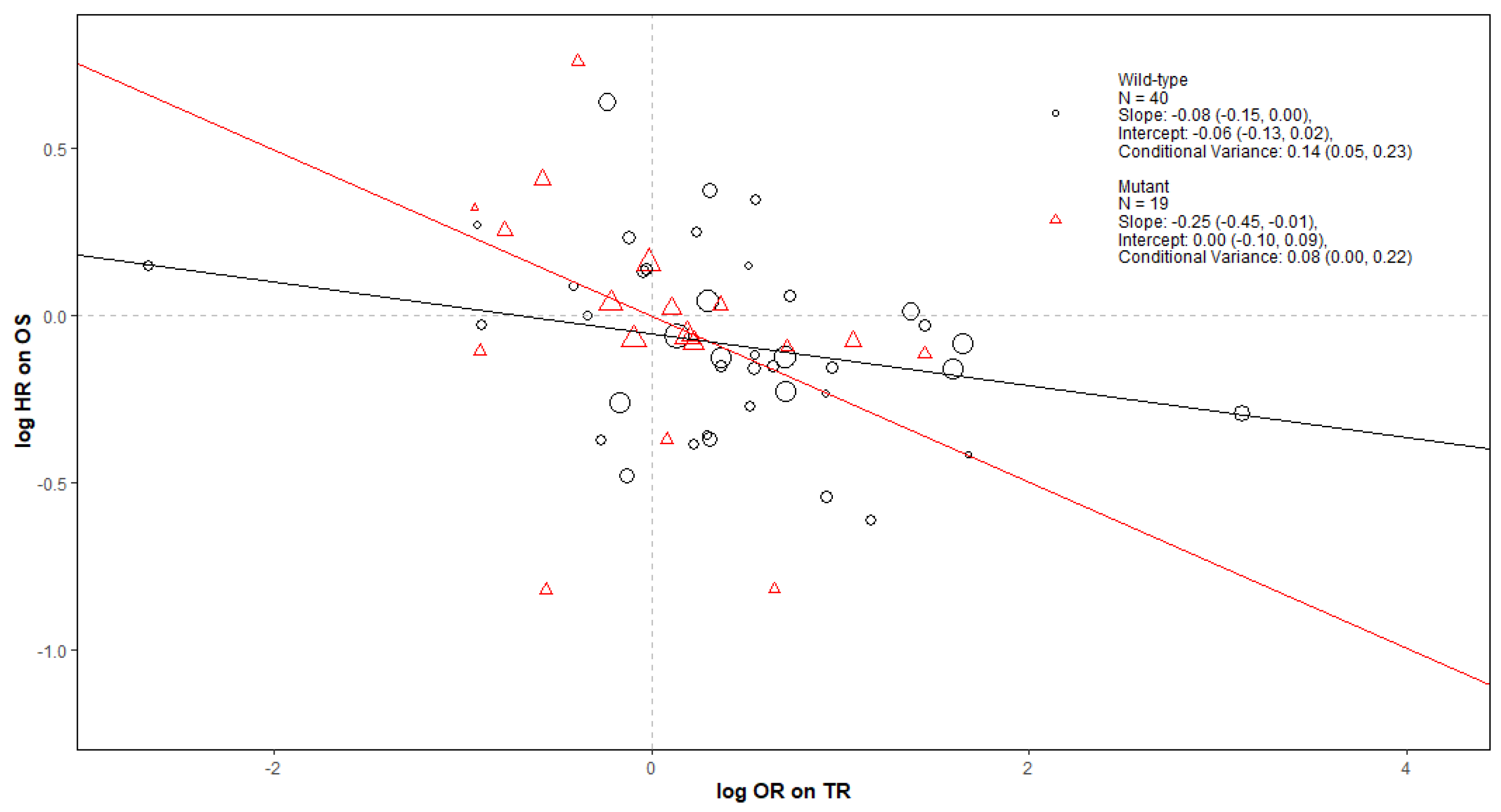
Figure A2.
Forest plot of estimates of slope (green), intercept (blue), and conditional variance (red) for TR as a surrogate for OS. N represents the number of trial-subgroups.
Figure A2.
Forest plot of estimates of slope (green), intercept (blue), and conditional variance (red) for TR as a surrogate for OS. N represents the number of trial-subgroups.
For the TR-OS pair of outcomes by KRAS status and treatment class in Figure A2, the surrogate relationship was moderate only for the EGFR + Chemo treatment class when both MT and WT trial-subgroups are included. When looking at either KRAS MT or WT trial-subgroups only, the surrogate relationships were weak within each treatment class; however, the data for these analyses were limited.
Appendix B.1.2. Conclusions
Our results indicated a sub-optimal surrogate relationship between the treatment effects on TR and the effects on OS for mCRC, reaching a similar conclusion as Ciani et al. [8]. This was the case in all three analyses; of all data and the KRAS status subgropus.There was some evidence from the results that the surrogacy patterns may vary according to the mechanism of action, with relatively strong surrogate relationship for EGFR+Chemo therapies.
Fewer trials reported TR than PFS and OS results, which led to more uncertainty around the estimates produced from the analyses for TR. In addition, TR was defined at a specific time point and using a particular criteria, e.g., RECIST [88], WHO criteria [89] or individual trial criteria, which varied between RCTs and could account for the increased between-studies heterogeneity of the treatment effect on TR and therefore potentially weaker surrogate relationship. Further analysis could be undertaken to explore how TR was defined within each trial and whether this affects the strength of the surrogate relationship.
In summary the results from this investigation suggest that TR is not a strong surrogate endpoint for OS when evaluating pharmacological therapies for mCRC patients overall, but could potentially be used as a surrogate endpoint when evaluating EGFR+Chemo therapies. The overall conclusions also hold for subgroups of population by KRAS status, but there was no evidence of the importance of the mechanism of action, potentially due to the limited data.
Appendix B.2. Cross Validation and Predictions
Table A1 shows the results of the cross validation procedure for PFS as a surrogate for OS. The Daniels and Hughes method showed a large coverage in terms of the proportion of the 95% predicted intervals containing the observed effect estimate. For the Hierarchical model, taking into account treatment class, the cross validation for the KRAS MT and WT trial-subgroups resulted in all 95% prediction intervals including the observed estimates of the effect on OS, whereas cross validation using the Daniels and Hughes method had 3.61% of the predicted intervals not including the observed estimates of the treatment effect on OS.

Table A1.
Summary of cross validation results for all analyses for PFS as a surrogate for OS.
Table A1.
Summary of cross validation results for all analyses for PFS as a surrogate for OS.
| Trial-Subgroups | All | MT | WT | |||
|---|---|---|---|---|---|---|
| Model | Daniels and Hughes | Hierarchical | Daniels and Hughes | Hierarchical | Daniels and Hughes | Hierarchical |
| Percentage of observed effect estimates within 95% predicted interval | 94.79% | 96.39% | 94.29% | 100.00% | 95.08% | 100.00% |
| Average absolute difference between the observed and predicted effect estimates | 0.16 | 0.16 | 0.17 | 0.20 | 0.15 | 0.16 |
| Average ratio of the width of intervals between the predicted and observer treatment effects | 1.14 | 1.51 | 1.13 | 2.14 | 1.21 | 2.02 |
There was 0.16 average absolute difference between the observed effect estimate and the predicted effect for OS for each trial from both the Daniels and Hughes model and the Hierarchical model including all trial-subgroups and also for KRAS WT trial-subgroups when using the hierarchical model, with a slightly smaller average of 0.15 when using Daniels and Hughes model. For KRAS MT, the average discrepancy was slightly higher, 0.20 from the hierarchical model and 0.17 from Daniels and Hughes model.
The ratios of the width of intervals indicate that the predictions obtained from the hierarchical model were obtained with larger uncertainty compared to the predictions from the Daniels and Hughes model of all data on all treatment classes combined. This is likely due to the predictions in the treatment classes with small number of studies being obtained with large uncertainty from the hierarchical model.
References
- Managing Metastatic Colorectal Cancer—NICE Pathways. Available online: https://pathways.nice.org.uk/pathways/colorectal-cancer/managing-metastatic-colorectal-cancer (accessed on 3 August 2020).
- Bujkiewicz, S.; Achana, F.; Papanikos, T.; Riley, R.D.; Abrams, K.R. NICE DSU Technical Support Document 20: Multivariate Meta-Analysis of Summary Data for Combining Treatment Effects on Correlated Outcomes and Evaluating Surrogate Endpoints. 2019. Available online: http://www.nicedsu.org.uk (accessed on 5 September 2022).
- Ciani, O.; Buyse, M.; Drummond, M.; Rasi, G.; Saad, E.D.; Taylor, R.S. Time to Review the Role of Surrogate End Points in Health Policy: State of the Art and the Way Forward. Value Health. 2017, 20, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Downing, N.S.; Aminawung, J.A.; Shah, N.D.; Krumholz, H.M.; Ross, J.S. Clinical Trial Evidence Supporting FDA Approval of Novel Therapeutic Agents, 2005–2012. JAMA 2014, 311, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Dawoud, D.; Naci, H.; Ciani, O.; Bujkiewicz, S. Raising the bar for using surrogate endpoints in drug regulation and health technology assessment. BMJ 2021, 374, n2191. [Google Scholar] [CrossRef]
- Buyse, M.; Burzykowski, T.; Carroll, K.; Michiels, S.; Sargent, D.J.; Miller, L.L.; Elfring, G.L.; Pignon, J.P.; Piedbois, P. Progression-Free Survival Is a Surrogate for Survival in Advanced Colorectal Cancer. J. Clin. Oncol. 2007, 25, 5218–5224. [Google Scholar] [CrossRef] [PubMed]
- Giessen, C.; Laubender, R.P.; Ankerst, D.P.; Stintzing, S.; Modest, D.P.; Mansmann, U.; Heinemann, V. Progression-Free Survival as a Surrogate Endpoint for Median Overall Survival in Metastatic Colorectal Cancer: Literature-Based Analysis from 50 Randomized First-Line Trials. Clin. Cancer Res. 2013, 19, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ciani, O.; Buyse, M.; Garside, R.; Peters, J.; Saad, E.D.; Stein, K.; Taylor, R.S. Meta-analyses of randomized controlled trials show suboptimal validity of surrogate outcomes for overall survival in advanced colorectal cancer. J. Clin. Epidemiol. 2015, 68, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef]
- Cox, A.D.; Der, C.J. Ras history. Small GTPases 2010, 1, 2–27. [Google Scholar] [CrossRef]
- Bylsma, L.C.; Gillezeau, C.; Garawin, T.A.; Kelsh, M.A.; Fryzek, J.P.; Sangaré, L.; Lowe, K.A. Prevalence of RAS and BRAF mutations in metastatic colorectal cancer patients by tumor sidedness: A systematic review and meta-analysis. Cancer Med. 2020, 9, 1044–1057. [Google Scholar] [CrossRef]
- PROSPERO, International Prospective Register of Systematic Reviews. Available online: https://www.crd.york.ac.uk/prospero/ (accessed on 22 July 2020).
- Embase (Excerpta Medica Database). Available online: https://www.embase.com/landing?status=grey (accessed on 3 December 2021).
- MEDLINE®: National Library of Medicine (United States). Available online: https://www.nlm.nih.gov/bsd/medline.html (accessed on 22 July 2020).
- Cochrane Controlled Register of Trials (CENTRAL) | Cochrane Library. Available online: https://www.cochranelibrary.com/central/about-central (accessed on 22 July 2020).
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Daniels, M.J.; Hughes, M.D. Meta-analysis for the evaluation of potential surrogate markers. In Statistics in Medicine; John Wiley & Sons Ltd.: New York, NY, USA, 1997; Volume 16, pp. 1965–1982. [Google Scholar]
- Papanikos, T.; Thompson, J.R.; Abrams, K.R.; Städler, N.; Ciani, O.; Taylor, R.; Bujkiewicz, S. Bayesian hierarchical meta-analytic methods for modeling surrogate relationships that vary across treatment classes using aggregate data. Stat. Med. 2020, 39, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Hagman, H.; Frödin, J.E.; Berglund, Å.; Sundberg, J.; Vestermark, L.W.; Albertsson, M.; Fernebro, E.; Johnsson, A. A randomized study of KRAS-guided maintenance therapy with bevacizumab, erlotinib or metronomic capecitabine after first-line induction treatment of metastatic colorectal cancer: The Nordic ACT2 trial. Ann. Oncol. 2016, 27, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.J.; Philip, P.; Saunders, M.; Kolevska, T.; Mukherjee, K.; Samuel, L.; Bondarde, S.; Dobbs, T.; Tagliaferri, M.; Hoch, U.; et al. Randomized study of etirinotecan pegol versus irinotecan as second-line treatment for metastatic colorectal cancer. Cancer Chemother. Pharmacol. 2017, 80, 1161–1169. [Google Scholar] [CrossRef]
- Maughan, T.S.; Adams, R.A.; Smith, C.G.; Meade, A.M.; Seymour, M.T.; Wilson, R.H.; Idziaszczyk, S.; Harris, R.; Fisher, D.; Kenny, S.L.; et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: Results of the randomised phase 3 MRC COIN trial. Lancet 2011, 377, 2103–2114. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Passardi, A.; Scarpi, E.; Gelsomino, F.; Palladino, M.A.; Casadei Gardini, A.; Turci, D.; Chiuri, V.E.; Mucciarini, C.; Tassinari, D.; Ragazzini, A.; et al. Impact of second-line cetuximab-containing therapy in patients with KRAS wild-type metastatic colorectal cancer: Results from the ITACa randomized clinical trial. Sci. Rep. 2017, 7, 10426. [Google Scholar] [CrossRef]
- Reinacher-Schick, A.; Schulmann, K.; Modest, D.P.; Bruns, N.; Graeven, U.; Jaworska, M.; Greil, R.; Porschen, R.; Arnold, D.; Schmiegel, W.; et al. Effect of KRAS codon13 mutations in patients with advanced colorectal cancer (advanced CRC) under oxaliplatin containing chemotherapy. Results from a translational study of the AIO colorectal study group. BMC Cancer 2012, 12, 349. [Google Scholar] [CrossRef]
- Richman, S.D.; Seymour, M.T.; Chambers, P.; Elliott, F.; Daly, C.L.; Meade, A.M.; Taylor, G.; Barrett, J.H.; Quirke, P. KRAS and BRAF mutations in advanced colorectal cancer are associated with poor prognosis but do not preclude benefit from oxaliplatin or irinotecan: Results from the MRC FOCUS trial. J. Clin. Oncol. 2009, 27, 5931–5937. [Google Scholar] [CrossRef]
- Yoshino, T.; Mizunuma, N.; Yamazaki, K.; Nishina, T.; Komatsu, Y.; Baba, H.; Tsuji, A.; Yamaguchi, K.; Muro, K.; Sugimoto, N.; et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: A double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol. 2012, 13, 993–1001. [Google Scholar] [CrossRef]
- Aranda, E.; Garcia Alfonso, P.; Benavides, M.; Sanchez Ruiz, A.; Guillen-Ponce, C.; Safont, M.J.; Alcaide, J.; Gomez, A.; Lopez, R.; Manzano, J.L.; et al. First-line mFOLFOX plus cetuximab followed by mFOLFOX plus cetuximab or single-agent cetuximab as maintenance therapy in patients with metastatic colorectal cancer: Phase II randomised MACRO2 TTD study. Eur. J. Cancer 2018, 101, 263–272. [Google Scholar] [CrossRef]
- Harbison, C.T.; Horak, C.E.; Ledeine, J.M.; Mukhopadhyay, P.; Malone, D.P.; O’Callaghan, C.; Jonker, D.J.; Karapetis, C.S.; Khambata-Ford, S.; Gustafson, N.; et al. Validation of companion diagnostic for detection of mutations in codons 12 and 13 of the KRAS gene in patients with metastatic colorectal cancer: Analysis of the NCIC CTG CO.17 trial. Arch. Pathol. Lab. Med. 2013, 137, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Elme, A.; Park, J.O.; Udrea, A.A.; Kim, S.Y.; Ahn, J.B.; Valencia, R.V.; Krishnan, S.; Manojlovic, N.; Guan, X.; et al. Final Analysis of Outcomes and RAS/BRAF Status in a Randomized Phase 3 Study of Panitumumab and Best Supportive Care in Chemorefractory Wild Type KRAS Metastatic Colorectal Cancer. Clin. Colorectal. Cancer 2018, 17, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Montagut, C.; Argilés, G.; Ciardiello, F.; Poulsen, T.T.; Dienstmann, R.; Kragh, M.; Kopetz, S.; Lindsted, T.; Ding, C.; Vidal, J.; et al. Efficacy of Sym004 in Patients With Metastatic Colorectal Cancer With Acquired Resistance to Anti-EGFR Therapy and Molecularly Selected by Circulating Tumor DNA Analyses: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e175245. [Google Scholar] [CrossRef] [PubMed]
- Price, T.; Kim, T.W.; Li, J.; Cascinu, S.; Ruff, P.; Suresh, A.S.; Thomas, A.; Tjulandin, S.; Guan, X.; Peeters, M. Final results and outcomes by prior bevacizumab exposure, skin toxicity, and hypomagnesaemia from ASPECCT: Randomized phase 3 non-inferiority study of panitumumab versus cetuximab in chemorefractory wild-type KRAS exon 2 metastatic colorectal cancer. Eur. J. Cancer 2016, 68, 51–59. [Google Scholar] [CrossRef][Green Version]
- Bokemeyer, C.; Bondarenko, I.; Hartmann, J.T.; de Braud, F.; Schuch, G.; Zubel, A.; Celik, I.; Schlichting, M.; Koralewski, P. Efficacy according to biomarker status of cetuximab plus FOLFOX-4 as first-line treatment for metastatic colorectal cancer: The OPUS study. Ann. Oncol. 2011, 22, 1535–1546. [Google Scholar] [CrossRef]
- Bridgewater, J.; Pugh, S.; Whitehead, A.; Stanton, L.; Eminton, Z.; Mellor, J.; Allen, A.; Finch-Jones, M.; Falk, S.; Iveson, T.; et al. Perioperative chemotherapy with or without cetuximab in patients (pts) with resectable colorectal liver metastasis (CRLM): Mature analysis of overall survival (OS) in the New EPOC randomised controlled trial. Ann. Oncol. 2017, 28, v162. [Google Scholar] [CrossRef]
- Brodowicz, T.; Ciuleanu, T.E.; Radosavljevic, D.; Shacham-Shmueli, E.; Vrbanec, D.; Plate, S.; Mrsic-Krmpotic, Z.; Dank, M.; Purkalne, G.; Messinger, D.; et al. Folfox4 plus cetuximab administered weekly or every second week in the first-line treatment of patients with kras wild-type metastatic colorectal cancer: A randomized phase ii cecog study. Ann. Oncol. 2013, 24, 1769–1777. [Google Scholar] [CrossRef]
- Carrato, A.; Abad, A.; Massuti, B.; Gravalos, C.; Escudero, P.; Longo-Munoz, F.; Manzano, J.L.; Gomez, A.; Safont, M.J.; Gallego, J.; et al. First-line panitumumab plus FOLFOX4 or FOLFIRI in colorectal cancer with multiple or unresectable liver metastases: A randomised, phase II trial (PLANET-TTD). Eur. J. Cancer 2017, 81, 191–202. [Google Scholar] [CrossRef]
- Cascinu, S.; Rosati, G.; Nasti, G.; Lonardi, S.; Zaniboni, A.; Marchetti, P.; Leone, F.; Bilancia, D.; Iaffaioli, R.V.; Zagonel, V.; et al. Treatment sequence with either irinotecan/cetuximab followed by FOLFOX-4 or the reverse strategy in metastatic colorectal cancer patients progressing after first-line FOLFIRI/bevacizumab: An Italian Group for the Study of Gastrointestinal Cancer phase III, randomised trial comparing two sequences of therapy in colorectal metastatic patients. Eur. J. Cancer 2017, 83, 106–115. [Google Scholar] [CrossRef]
- Ciardiello, F.; Normanno, N.; Martinelli, E.; Troiani, T.; Pisconti, S.; Cardone, C.; Nappi, A.; Bordonaro, A.R.; Rachiglio, M.; Lambiase, M.; et al. Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): A randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX. Ann. Oncol. 2016, 27, 1055–1061. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Final results from PRIME: Randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014, 25, 1346–1355. [Google Scholar] [CrossRef] [PubMed]
- Douillard, J.Y.; Zemelka, T.; Fountzilas, G.; Barone, C.; Schlichting, M.; Heighway, J.; Eggleton, S.P.; Srimuninnimit, V. FOLFOX4 with cetuximab vs. UFOX with cetuximab as first-line therapy in metastatic colorectal cancer: The randomized phase II FUTURE study. Clin. Colorectal. Cancer 2014, 13, 14–26.e1. [Google Scholar] [CrossRef] [PubMed]
- Hara, H.; Sakai, D.; Takano, T.; Shinozaki, K.; Goto, M.; Taniguchi, H.; Kishimoto, J.; Boku, N.; Hyodo, I.; Muro, K. WJOG6510G: Randomized phase II of Pmab + IRI vs. Cmab + IRI for KRAS WT mCRC previously treated after FU, IRI, and L-OHP. Ann. Oncol. 2017, 28, 74–75. [Google Scholar] [CrossRef]
- Hecht, J.R.; Cohn, A.; Dakhil, S.; Saleh, M.; Piperdi, B.; Cline-Burkhardt, M.; Tian, Y.; Go, W.Y. SPIRITT: A randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin. Colorectal. Cancer 2015, 14, 72–80. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmuller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Maughan, T.S.; Meade, A.M.; Adams, R.A.; Richman, S.D.; Butler, R.; Fisher, D.; Wilson, R.H.; Jasani, B.; Taylor, G.R.; Williams, G.T.; et al. A feasibility study testing four hypotheses with phase II outcomes in advanced colorectal cancer (MRC FOCUS3): A model for randomised controlled trials in the era of personalised medicine? Br. J. Cancer 2014, 110, 2178–2186. [Google Scholar] [CrossRef]
- Munemoto, Y.; Nakamura, M.; Takahashi, M.; Kotaka, M.; Kuroda, H.; Kato, T.; Minagawa, N.; Noura, S.; Fukunaga, M.; Kuramochi, H.; et al. SAPPHIRE: A randomised phase II study of planned discontinuation or continuous treatment of oxaliplatin after six cycles of modified FOLFOX6 plus panitumumab in patients with colorectal cancer. Eur. J. Cancer 2019, 119, 158–167. [Google Scholar] [CrossRef]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; Andre, T.; Chan, E.; Lordick, F.; Punt, C.J.A.; et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J. Clin. Oncol. 2010, 28, 4706–4713. [Google Scholar] [CrossRef]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; Andre, T.; Chan, E.; Lordick, F.; Punt, C.J.A.; et al. Final results from a randomized phase 3 study of FOLFIRI +/- panitumumab for second-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014, 25, 107–116. [Google Scholar] [CrossRef]
- Qin, S.; Li, J.; Wang, L.; Xu, J.; Cheng, Y.; Bai, Y.; Li, W.; Xu, N.; Lin, L.Z.; Wu, Q.; et al. Efficacy and Tolerability of First-Line Cetuximab Plus Leucovorin, Fluorouracil, and Oxaliplatin (FOLFOX-4) Versus FOLFOX-4 in Patients With RAS Wild-Type Metastatic Colorectal Cancer: The Open-Label, Randomized, Phase III TAILOR Trial. J. Clin. Oncol. 2018, 36, 3031–3039. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Rivera, F.; Karthaus, M.; Fasola, G.; Canon, J.L.; Hecht, J.R.; Yu, H.; Oliner, K.S.; Go, W.Y. PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J. Clin. Oncol. 2014, 32, 2240–2247. [Google Scholar] [CrossRef] [PubMed]
- Seymour, M.T.; Brown, S.R.; Middleton, G.; Maughan, T.; Richman, S.; Gwyther, S.; Lowe, C.; Seligmann, J.F.; Wadsley, J.; Maisey, N.; et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): A prospectively stratified randomised trial. Lancet Oncol. 2013, 14, 749–759. [Google Scholar] [CrossRef]
- Shapiro, J.D.; Thavaneswaran, S.; Underhill, C.R.; Robledo, K.P.; Karapetis, C.S.; Day, F.L.; Nott, L.M.; Jefford, M.; Chantrill, L.A.; Pavlakis, N.; et al. Cetuximab Alone or With Irinotecan for Resistant KRAS-, NRAS-, BRAF- and PIK3CA-wild-type Metastatic Colorectal Cancer: The AGITG Randomized Phase II ICECREAM Study. Clin. Colorectal. Cancer 2018, 17, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Shitara, K.; Yonesaka, K.; Denda, T.; Yamazaki, K.; Moriwaki, T.; Tsuda, M.; Takano, T.; Okuda, H.; Nishina, T.; Sakai, K.; et al. Randomized study of FOLFIRI plus either panitumumab or bevacizumab for wild-type KRAS colorectal cancer-WJOG 6210G. Cancer Sci. 2016, 107, 1843–1850. [Google Scholar] [CrossRef]
- Tveit, K.M.; Guren, T.; Glimelius, B.; Pfeiffer, P.; Sorbye, H.; Pyrhonen, S.; Sigurdsson, F.; Kure, E.; Ikdahl, T.; Skovlund, E.; et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in first-line treatment of metastatic colorectal cancer: The NORDIC-VII study. J. Clin. Oncol. 2012, 30, 1755–1762. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Kohne, C.H.; Lang, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef]
- Venook, A.P.; Niedzwiecki, D.; Lenz, H.J.; Innocenti, F.; Fruth, B.; Meyerhardt, J.A.; Schrag, D.; Greene, C.; O’Neil, B.H.; Atkins, J.N.; et al. Effect of First-Line Chemotherapy Combined With Cetuximab or Bevacizumab on Overall Survival in Patients With KRAS Wild-Type Advanced or Metastatic Colorectal Cancer: A Randomized Clinical Trial. JAMA 2017, 317, 2392–2401. [Google Scholar] [CrossRef]
- Ye, L.C.; Liu, T.S.; Ren, L.; Wei, Y.; Zhu, D.X.; Zai, S.Y.; Ye, Q.H.; Yu, Y.; Xu, B.; Qin, X.Y.; et al. Randomized controlled trial of cetuximab plus chemotherapy for patients with KRAS wild-type unresectable colorectal liver-limited metastases. J. Clin. Oncol. 2013, 31, 1931–1938. [Google Scholar] [CrossRef]
- Garcia, E.D.R.; Gomez, A.; Yuste, A.; Puente, J.; Lopez-Lopez, C.; Safont, M.J.; Layos, L.; Reboredo, M.; Benavides, M.; Aranda, E. Role of kras status in patients with metastatic colorectal cancer receiving first-line chemotherapy plus bevacizumab—A TTD Spanish group cooperative study. PLoS ONE 2012, 47, S391–S392. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.; Yau, T.C.C.; Ma, B.; Pan, H.; Xu, J.; Bai, Y.; Chi, Y.; Wang, L.; et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015, 16, 619–629. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.H.; Shen, L.; Xu, J.; Bai, Y.; Yang, L.; Deng, Y.; Chen, Z.D.; Zhong, H.; et al. Effect of Fruquintinib vs. Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018, 319, 2486–2496. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Lenz, H.J.; Siena, S.; Sobrero, A.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouche, O.; Mineur, L.; Barone, C.; et al. Analysis of circulating DNA and protein biomarkers to predict the clinical activity of regorafenib and assess prognosis in patients with metastatic colorectal cancer: A retrospective, exploratory analysis of the CORRECT trial. Lancet Oncol. 2015, 16, 937–948. [Google Scholar] [CrossRef]
- Bennouna, J.; Sastre, J.; Arnold, D.; Osterlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Vieitez, J.M.; Bouche, O.; Borg, C.; et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef]
- Bennouna, J.; Hiret, S.; Bertaut, A.; Bouche, O.; Deplanque, G.; Borel, C.; Francois, E.; Conroy, T.; Ghiringhelli, F.; Des Guetz, G.; et al. Continuation of Bevacizumab vs. Cetuximab Plus Chemotherapy after First Progression in KRAS Wild-Type Metastatic Colorectal Cancer: The UNICANCER PRODIGE18 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 83–90. [Google Scholar] [CrossRef]
- Goey, K.K.H.; Elias, S.G.; van Tinteren, H.; Lacle, M.M.; Willems, S.M.; De Leng, W.W.J.; Strengman, E.; Vreuls, C.; Creemers, G.J.; Van Der Velden, A.; et al. Predictive value of KRAS mutation status in metastatic colorectal cancer (mCRC) patients treated with capecitabine and bevacizumab (CAP-B) maintenance treatment vs. observation in the phase III CAIRO3 study. J. Clin. Oncol. 2016, 34, 3525. [Google Scholar] [CrossRef]
- Hurwitz, H.I.; Yi, J.; Ince, W.; Novotny, W.F.; Rosen, O. The clinical benefit of bevacizumab in metastatic colorectal cancer is independent of K-ras mutation status: Analysis of a phase III study of bevacizumab with chemotherapy in previously untreated metastatic colorectal cancer. Oncologist 2009, 14, 22–28. [Google Scholar] [CrossRef]
- Nakayama, G.; Mitsuma, A.; Sunagawa, Y.; Ishigure, K.; Yokoyama, H.; Matsui, T.; Nakayama, H.; Nakata, K.; Ishiyama, A.; Asada, T.; et al. Randomized Phase II Trial of CapOX plus Bevacizumab and CapIRI plus Bevacizumab as First-Line Treatment for Japanese Patients with Metastatic Colorectal Cancer (CCOG-1201 Study). Oncologist 2018, 23, 919–927. [Google Scholar] [CrossRef]
- Price, T.J.; Hardingham, J.E.; Lee, C.K.; Weickhardt, A.; Townsend, A.R.; Wrin, J.W.; Chua, A.; Shivasami, A.; Cummins, M.M.; Murone, C.; et al. Impact of KRAS and BRAF Gene Mutation Status on Outcomes From the Phase III AGITG MAX Trial of Capecitabine Alone or in Combination With Bevacizumab and Mitomycin in Advanced Colorectal Cancer. J. Clin. Oncol. 2011, 29, 2675–2682. [Google Scholar] [CrossRef]
- Smith, J.C.; Brooks, L.; Hoff, P.M.; McWalter, G.; Dearden, S.; Morgan, S.R.; Wilson, D.; Robertson, J.D.; Jurgensmeier, J.M. KRAS mutations are associated with inferior clinical outcome in patients with metastatic colorectal cancer, but are not predictive for benefit with cediranib. Eur. J. Cancer 2013, 49, 2424–2432. [Google Scholar] [CrossRef]
- Tabernero, J.; Garcia-Carbonero, R.; Cassidy, J.; Sobrero, A.; Van Cutsem, E.; Kohne, C.H.; Tejpar, S.; Gladkov, O.; Davidenko, I.; Salazar, R.; et al. Sorafenib in combination with oxaliplatin, leucovorin, and fluorouracil (modified FOLFOX6) as first-line treatment of metastatic colorectal cancer: The RESPECT trial. Clin. Cancer Res. 2013, 19, 2541–2550. [Google Scholar] [CrossRef]
- Tabernero, J.; Yoshino, T.; Cohn, A.L.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.E.; Portnoy, D.C.; Van Cutsem, E.; Grothey, A.; et al. Ramucirumab versus placebo in combination with second-line FOLFIRI in patients with metastatic colorectal carcinoma that progressed during or after first-line therapy with bevacizumab, oxaliplatin, and a fluoropyrimidine (RAISE): A randomised, double-blind, multicentre, phase 3 study. Lancet Oncol. 2015, 16, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Maria Vieitez, J.; Bouche, O.; Osterlund, P.; Bennouna, J.; Andre, T.; Sastre, J.; Alonso-Orduna, V.; Kubicka, S.; Greil, R.; et al. Randomised phase III study of bevacizumab + chemotherapy beyond progression in bevacizumab-treated patients with metastatic colorectal cancer: TML study KRAS subgroup findings. Ann. Oncol. 2012, 23 (Suppl. 4). [Google Scholar] [CrossRef]
- Siu, L.L.; Shapiro, J.D.; Jonker, D.J.; Karapetis, C.S.; Zalcberg, J.R.; Simes, J.; Couture, F.; Moore, M.J.; Price, T.J.; Siddiqui, J.; et al. Phase III randomized, placebo-controlled study of cetuximab plus brivanib alaninate versus cetuximab plus placebo in patients with metastatic, chemotherapy-refractory, wild-type K-RAS colorectal carcinoma: The NCIC Clinical Trials Group and AGITG CO.20 trial. J. Clin. Oncol. 2013, 31, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Tournigand, C.; Chibaudel, B.; Samson, B.; Scheithauer, W.; Vernerey, D.; Mesange, P.; Lledo, G.; Viret, F.; Ramee, J.F.; Tubiana-Mathieu, N.; et al. Bevacizumab with or without erlotinib as maintenance therapy in patients with metastatic colorectal cancer (GERCOR DREAM; OPTIMOX3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 1493–1505. [Google Scholar] [CrossRef]
- Liu, Y.; Luan, L.; Wang, X. A randomized Phase II clinical study of combining panitumumab and bevacizumab, plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for patients with metastatic colorectal cancer and KRAS mutation. Onco. Targets Ther. 2015, 8, 1061–1068. [Google Scholar] [CrossRef]
- Hecht, J.R.; Mitchell, E.; Chidiac, T.; Scroggin, C.; Hagenstad, C.; Spigel, D.; Marshall, J.; Cohn, A.; McCollum, D.; Stella, P.; et al. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J. Clin. Oncol. 2009, 27, 672–680. [Google Scholar] [CrossRef]
- Bendell, J.C.; Hochster, H.; Hart, L.L.; Firdaus, I.; Mace, J.R.; McFarlane, J.J.; Kozloff, M.; Catenacci, D.; Hsu, J.J.; Hack, S.P.; et al. A Phase II Randomized Trial (GO27827) of First-Line FOLFOX Plus Bevacizumab with or Without the MET Inhibitor Onartuzumab in Patients with Metastatic Colorectal Cancer. Oncologist 2017, 22, 264–271. [Google Scholar] [CrossRef]
- Cohn, A.L.; Tabernero, J.; Maurel, J.; Nowara, E.; Sastre, J.; Chuah, B.Y.S.; Kopp, M.V.; Sakaeva, D.D.; Mitchell, E.P.; Dubey, S.; et al. A randomized, placebo-controlled phase 2 study of ganitumab or conatumumab in combination with FOLFIRI for second-line treatment of mutant KRAS metastatic colorectal cancer. Ann. Oncol. 2013, 24, 1777–1785. [Google Scholar] [CrossRef]
- Elez, E.; Kocakova, I.; Hohler, T.; Martens, U.M.; Bokemeyer, C.; Van cutsem, E.; Melichar, B.; Smakal, M.; Csoszi, T.; Topuzov, E.; et al. Abituzumab combined with cetuximab plus irinotecan versus cetuximab plus irinotecan alone for patients with KRAS wild-type metastatic colorectal cancer: The randomised phase I/II POSEIDON trial. Ann. Oncol. 2015, 26, 132–140. [Google Scholar] [CrossRef]
- Randolph Hecht, J.; Benson, A.B.; Vyushkov, D.; Yang, Y.; Bendell, J.; Verma, U. A phase II, randomized, double-blind, placebo-controlled study of simtuzumab in combination with FOLFIRI for the Second-Line treatment of metastatic KRAS mutant colorectal adenocarcinoma. Oncologist 2017, 22, 243-e23. [Google Scholar] [CrossRef]
- Hill, A.G.; Findlay, M.P.; Burge, M.E.; Jackson, C.; Alfonso, P.G.; Samuel, L.; Ganju, V.; Karthaus, M.; Amatu, A.; Jeffery, M.; et al. Phase II study of the dual EGFR/her3 inhibitor duligotuzumab (mehd7945a) versus cetuximab in combination with folfiri in second-line ras wild-type metastatic colorectal cancer. Clin. Cancer Res. 2018, 24, 2276–2284. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Strickland, A.H.; Lichinitser, M.; Suresh, A.V.S.; Manikhas, G.; Shapiro, J.; Rogowski, W.; Huang, X.; Wu, B.; Warner, D.; et al. A randomised, double-blind, placebo-controlled phase 2 study of trebananib (AMG 386) in combination with FOLFIRI in patients with previously treated metastatic colorectal carcinoma. Br. J. Cancer 2013, 108, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Peeters, M.; Oliner, K.; Siena, S.; Van Cutsem, E.; Humblet, Y.; Van Laethem, J.L.; Andre, T.; Tian, Y.; Sidhu, R.; Patterson, S. Comprehensive kras and nras mutation analysis: Predictive biomarkers in a phase 3 panitumumab monotherapy study of metastatic colorectal cancer (MCRC). Ann. Oncol. 2013, 24. [Google Scholar] [CrossRef]
- Sclafani, F.; Kim, T.Y.; Cunningham, D.; Kim, T.W.; Tabernero, J.; Schmoll, H.J.; Roh, J.K.; Kim, S.Y.; Park, Y.S.; Guren, T.K.; et al. A Randomized Phase II/III Study of Dalotuzumab in Combination With Cetuximab and Irinotecan in Chemorefractory, KRAS Wild-Type, Metastatic Colorectal Cancer. J. Natl. Cancer Inst. 2015, 107, djv258. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Eng, C.; Nowara, E.; Swieboda-Sadlej, A.; Tebbutt, N.C.; Mitchell, E.; Davidenko, I.; Stephenson, J.; Elez, E.; Prenen, H.; et al. Randomized phase Ib/II trial of rilotumumab or ganitumab with panitumumab versus panitumumab alone in patients with wild-type KRAS metastatic colorectal cancer. Clin. Cancer Res. 2014, 20, 4240–4250. [Google Scholar] [CrossRef]
- Watkins, D.J.; Ayers, M.; Cunningham, D.; Tabernero, J.; Tejpar, S.; Kim, T.Y.; Kim, T.W.; Kim, S.Y.; Roh, J.K.; Beale, P.J.; et al. Molecular analysis of the randomized phase II/III study of the anti-IGF-1R antibody dalotuzumab (MK-0646) in combination with cetuximab (Cx) and irinotecan (Ir) in the treatment of chemorefractory KRAS wild-type metastatic colorectal cancer (mCRC). J. Clin. Oncol. 2012, 30, 3531. [Google Scholar] [CrossRef]
- Xu, R.H.; Muro, K.; Morita, S.; Iwasa, S.; Han, S.W.; Wang, W.; Kotaka, M.; Nakamura, M.; Ahn, J.B.; Deng, Y.H.; et al. Modified XELIRI (capecitabine plus irinotecan) versus FOLFIRI (leucovorin, fluorouracil, and irinotecan), both either with or without bevacizumab, as second-line therapy for metastatic colorectal cancer (AXEPT): A multicentre, open-label, randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2018, 19, 660–671. [Google Scholar] [CrossRef]
- Cicero, G.; De Luca, R.; Dieli, F. Progression-free survival as a surrogate endpoint of overall survival in patients with metastatic colorectal cancer. Onco. Targets Ther. 2018, 11, 3059. [Google Scholar] [CrossRef]
- Cremolini, C.; Antoniotti, C.; Pietrantonio, F.; Berenato, R.; Tampellini, M.; Baratelli, C.; Salvatore, L.; Marmorino, F.; Borelli, B.; Nichetti, F.; et al. Surrogate endpoints in second-line trials of targeted agents in metastatic colorectal cancer: A literature-based systematic review and meta-analysis. Cancer Res. Treat. Off. J. Korean Cancer Assoc. 2017, 49, 834–845. [Google Scholar] [CrossRef]
- Kopetz, S.; Grothey, A.; Yaeger, R.; Van Cutsem, E.; Desai, J.; Yoshino, T.; Wasan, H.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E–Mutated Colorectal Cancer. N. Engl. J. Med. 2019, 381, 1632–1643. [Google Scholar] [CrossRef]
- Eisenhauer, E.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System; World Health Organization: Geneva, Switzerland, 2019.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).