Engraftment of Allotransplanted Tumor Cells in Adult rag2 Mutant Xenopus tropicalis
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
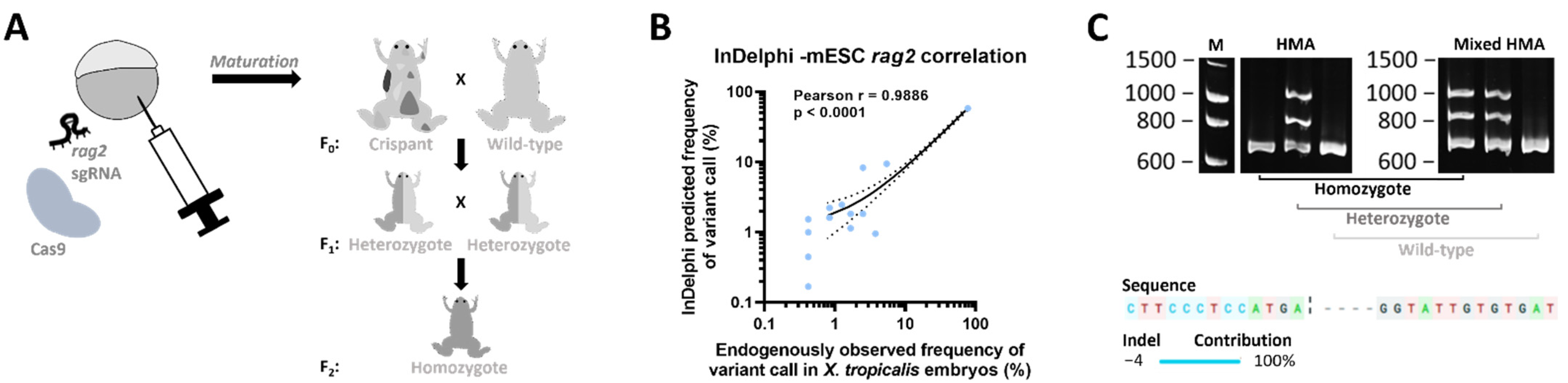
2.1. Generation of a rag2−/− Line
2.2. Transplantation of X. tropicalis Tumors in the X. tropicalis rag2−/− Line
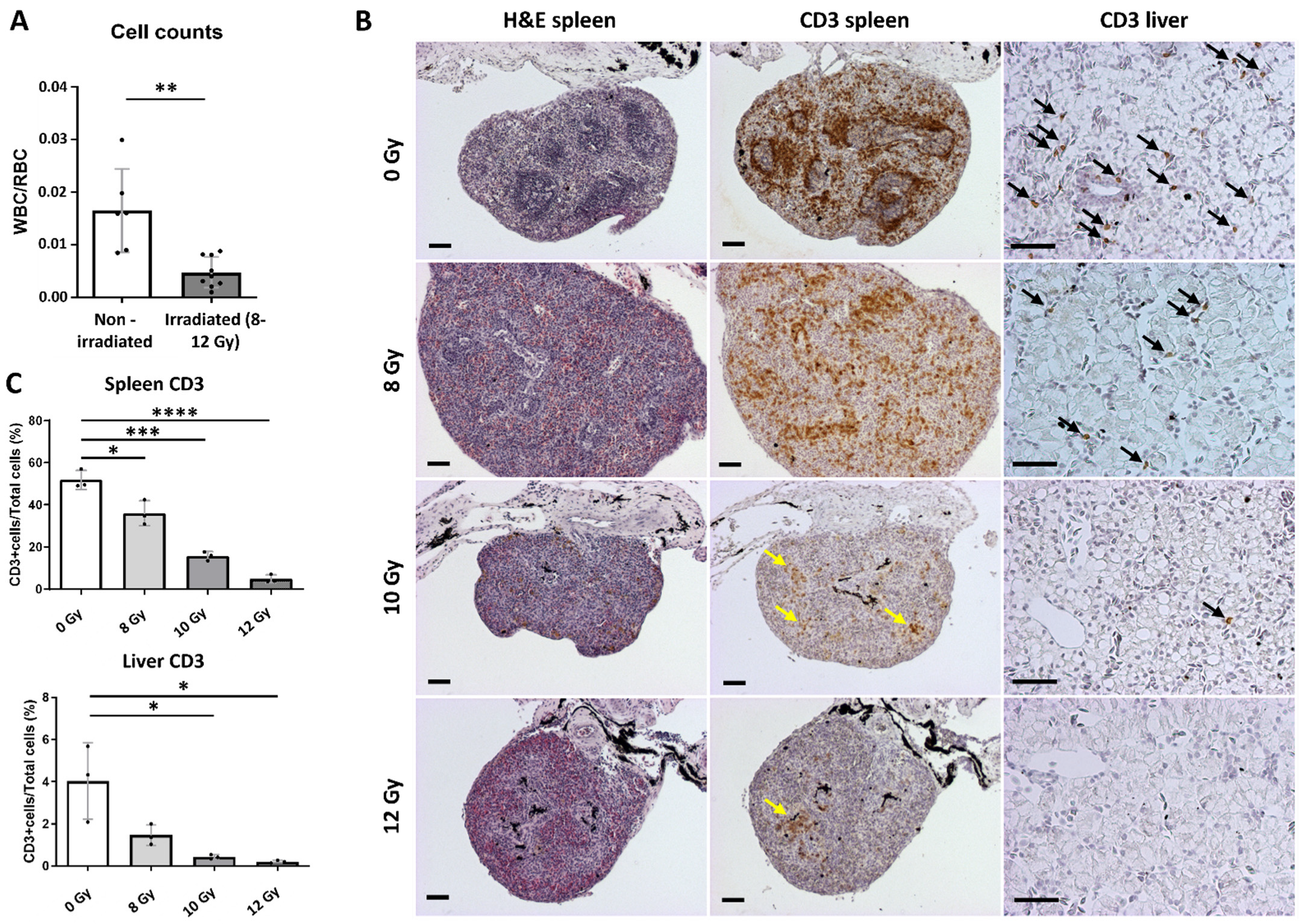
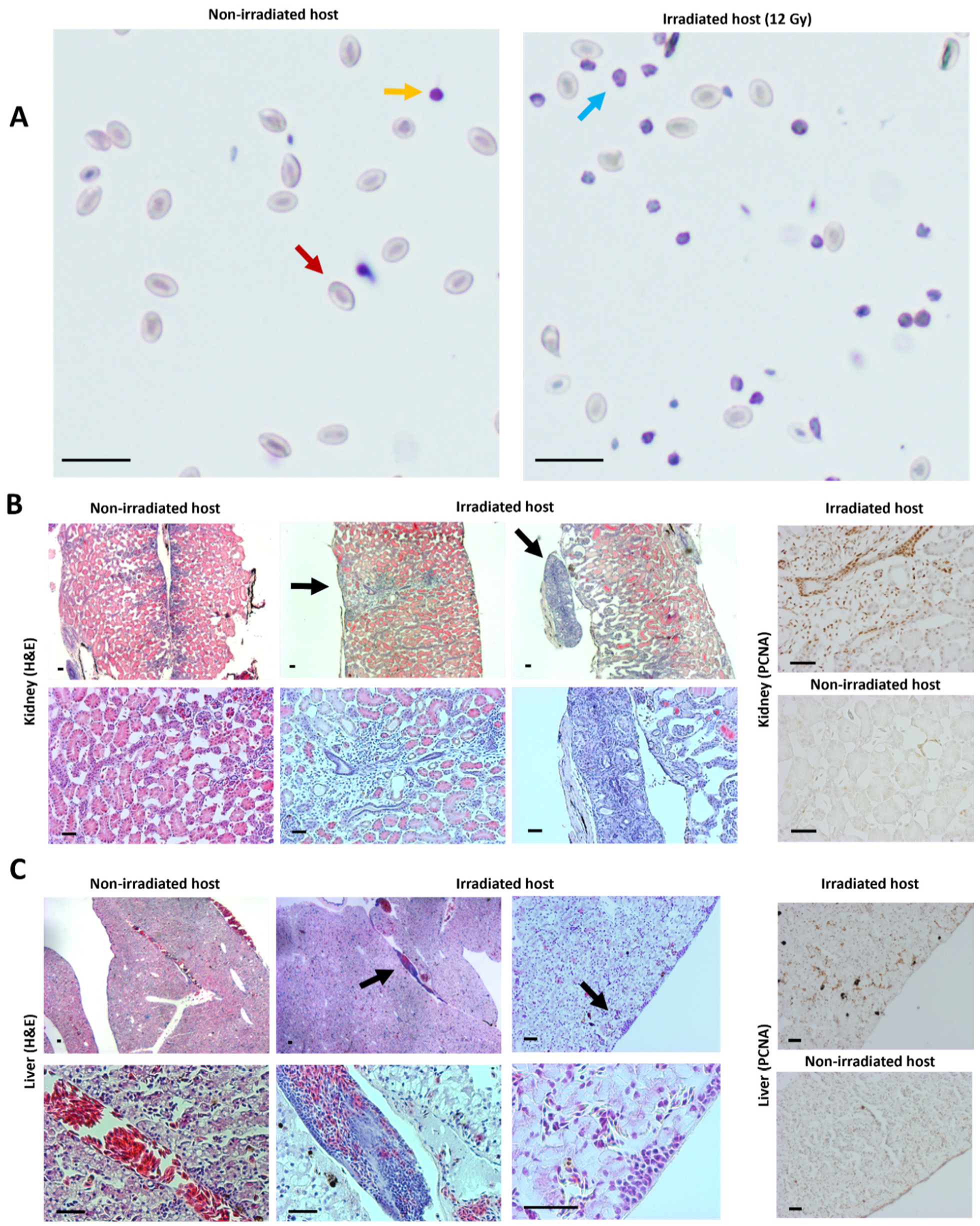
2.3. Transplantation Validation in Irradiated X. tropicalis Animals
3. Discussion
4. Material and Methods
4.1. CRISPR/Cas9 Mediated Generation of Mosaic Mutant X. tropicalis Animals
4.2. DNA Extraction and Sequencing
4.3. (Mixed) HMA Genotyping Method
4.4. Irradiation Procedure
4.5. Tumor Cell Transplantation
4.6. Blood Counts
4.7. Imaging, Histology and Immunohistochemistry
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharkey, F.; Fogh, J. Considerations in the use of nude mice for cancer research. Cancer Metastasis Rev. 1984, 3, 341–360. [Google Scholar] [CrossRef]
- Gansner, J.M.; Dang, M.; Ammerman, M.; Zon, L.I. Transplantation in zebrafish. Methods Cell Biol. 2017, 138, 629–647. [Google Scholar] [CrossRef]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, G.J. Applications of patient-derived tumor xenograft models and tumor organoids. J. Hematol. Oncol. 2020, 13, 1–16. [Google Scholar] [CrossRef]
- Traver, D.; Winzeler, A.; Stern, H.M.; Mayhall, E.A.; Langenau, D.M.; Kutok, J.L.; Look, A.T.; Zon, L.I. Effects of lethal irradiation in zebrafish and rescue by hematopoietic cell transplantation. Blood 2004, 104, 1298–1305. [Google Scholar] [CrossRef]
- Tang, Q.; Abdelfattah, N.S.; Blackburn, J.S.; Moore, J.C.; Martinez, S.A.; Moore, F.E.; Lobbardi, R.; Tenente, I.M.; Ignatius, M.S.; Berman, J.N.; et al. Optimized cell transplantation using adult rag2 mutant zebrafsh. Nat. Methods 2014, 11, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.C.H.; Raimondi, A.R.; Salthouse, C.D.; Ignatius, M.S.; Blackburn, J.S.; Mizgirev, I.V.; Storer, N.Y.; de Jong, J.L.O.; Chen, A.T.; Zhou, Y.; et al. high-throughput cell transplantation establishes that tumor-initiating cells are abundant in zebrafish T-cell Acute Lymphoblastic Leukemia. Blood 2010, 115, 3296–3303. [Google Scholar] [CrossRef] [PubMed]
- White, R.M.; Sessa, A.; Burke, C.; Bowman, T.; LeBlanc, J.; Ceol, C.; Bourque, C.; Dovey, M.; Goessling, W.; Burns, C.E.; et al. Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2008, 2, 183. [Google Scholar] [CrossRef]
- Yan, C.; Do, D.; Yang, Q.; Brunson, D.C.; JF, R.; Langenau, D.M. Single cell imaging of human cancer xenografts using adult immune-deficient zebrafish. Nat. Protoc. 2020, 15, 3105. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Robert, J. Tumor immunology viewed from alternative animal models—the xenopus story. Curr. Pathobiol. Rep. 2017, 5, 49–56. [Google Scholar] [CrossRef]
- Tulkens, D.; Vleminckx, K. Studying Tumor Formation and Regulation in Xenopus; CRC Press: Boca Raton, FL, USA, 2022; pp. 301–311. [Google Scholar] [CrossRef]
- Robert, J.; Guiet, C.; du Pasquier, L. Ontogeny of the alloimmune response against a transplanted tumor in xenopus laevis. Differentiation 1995, 59, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Robert, J.; Guiet, C.; Cohen, N.; Pasquier, L.D. Effects of thymectomy and tolerance induction on tumor immunity in adult xenopus laevis. Int. J. Cancer 1997, 70, 330–334. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A.; Robert, J. Lymphocyte deficiency induced by sublethal irradiation in xenopus. Cold Spring Harb. Protoc. 2019, 2019, pdb-prot097626. [Google Scholar] [CrossRef]
- Rau, L.; Cohen, N.; Robert, J. MHC-restricted and-unrestricted CD8 T Cells: An evolutionary perspective. Transplantation 2001, 72, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.W.; Arbab, M.; Hsu, J.Y.; Worstell, D.; Culbertson, S.J.; Krabbe, O.; Cassa, C.A.; Liu, D.R.; Gifford, D.K.; Sherwood, R.I. Predictable and precise template-free CRISPR editing of pathogenic variants. Nature 2018, 563, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Naert, T.; Tulkens, D.; Edwards, N.A.; Carron, M.; Shaidani, N.-I.; Wlizla, M.; Boel, A.; Demuynck, S.; Horb, M.E.; Coucke, P.; et al. Maximizing CRISPR/Cas9 phenotype penetrance applying predictive modeling of editing outcomes in xenopus and zebrafish embryos. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Foster, S.D.; Glover, S.R.; Turner, A.N.; Chatti, K.; Challa, A.K. A mixing heteroduplex mobility assay (MHMA) to genotype homozygous mutants with small indels generated by CRISPR-Cas9 nucleases. MethodsX 2019, 6, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Naert, T.; Dimitrakopoulou, D.; Tulkens, D.; Demuynck, S.; Carron, M.; Noelanders, R.; Eeckhout, L.; Van Isterdael, G.; Deforce, D.; Vanhove, C.; et al. RBL1 (P107) functions as tumor suppressor in glioblastoma and small-cell pancreatic neuroendocrine carcinoma in xenopus tropicalis. Oncogene 2020, 39, 2692–2706. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Asfari, M.; Thiébaud, C.H. Transplantation studies of a putative lymphosarcoma of xenopus. Cancer Res. 1988, 48, 954–957. [Google Scholar]
- Asfari, M. Mycobacterium-induced infectious granuloma in xenopus: Histopathology and transmissibility. Cancer Res. 1988, 48, 958–963. [Google Scholar]
- Milas, L.; Hunter, N.; Peters, L. The tumor bed effect: Dependence of tumor take, growth rate, and metastasis on the time interval between irradiation and tumor cell transplantation. Int. J. Radiat. Oncol. Biol. Phys. 1987, 13, 379–383. [Google Scholar] [CrossRef]
- Goyos, A.; Sowa, J.; Ohta, Y.; Robert, J. Remarkable conservation of distinct nonclassical MHC class I lineages in divergent amphibian species. J. Immunol. 2011, 186, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mateos, M.A.; Vejnar, C.E.; Beaudoin, J.-D.; Fernandez, J.P.; Mis, E.K.; Khokha, M.K.; Giraldez, A.J. CRISPRscan: Designing highly efficient SgRNAs for CRISPR-Cas9 targeting in vivo. Nat. Methods 2015, 12, 982–988. [Google Scholar] [CrossRef]
- Nakayama, T.; Blitz, I.L.; Fish, M.B.; Odeleye, A.O.; Manohar, S.; Cho, K.W.Y.; Grainger, R.M. Cas9-based genome editing in xenopus tropicalis. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2014; Volume 546, pp. 355–375. [Google Scholar] [CrossRef]
- Naert, T.; Colpaert, R.; Van Nieuwenhuysen, T.; Dimitrakopoulou, D.; Leoen, J.; Haustraete, J.; Boel, A.; Steyaert, W.; Lepez, T.; Deforce, D.; et al. CRISPR/Cas9 Mediated knockout of Rb1 and Rbl1 leads to rapid and penetrant retinoblastoma development in xenopus tropicalis. Sci. Rep. 2016, 6, 35264. [Google Scholar] [CrossRef] [PubMed]
- Boel, A.; Steyaert, W.; De Rocker, N.; Menten, B.; Callewaert, B.; De Paepe, A.; Coucke, P.; Willaert, A. BATCH-GE: Batch analysis of next-generation sequencing data for genome editing assessment. Sci. Rep. 2016, 6, 30330. [Google Scholar] [CrossRef] [PubMed]
- Maxham, L.A.; Forzán, M.J.; Hogan, N.S.; Vanderstichel, R.V.; Gilroy, C.V. Hematologic reference intervals for Xenopus Tropicalis with partial use of automatic counting methods and reliability of long-term stored samples. Vet. Clin. Pathol. 2016, 45, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Natt, M.P.; Herrick, C.A. A new blood diluent for counting the erythrocytes and leucocytes of the chicken. Poult. Sci. 1952, 31, 735–738. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tulkens, D.; Dimitrakopoulou, D.; Boelens, M.; Van Nieuwenhuysen, T.; Demuynck, S.; Toussaint, W.; Creytens, D.; Van Vlierberghe, P.; Vleminckx, K. Engraftment of Allotransplanted Tumor Cells in Adult rag2 Mutant Xenopus tropicalis. Cancers 2022, 14, 4560. https://doi.org/10.3390/cancers14194560
Tulkens D, Dimitrakopoulou D, Boelens M, Van Nieuwenhuysen T, Demuynck S, Toussaint W, Creytens D, Van Vlierberghe P, Vleminckx K. Engraftment of Allotransplanted Tumor Cells in Adult rag2 Mutant Xenopus tropicalis. Cancers. 2022; 14(19):4560. https://doi.org/10.3390/cancers14194560
Chicago/Turabian StyleTulkens, Dieter, Dionysia Dimitrakopoulou, Marthe Boelens, Tom Van Nieuwenhuysen, Suzan Demuynck, Wendy Toussaint, David Creytens, Pieter Van Vlierberghe, and Kris Vleminckx. 2022. "Engraftment of Allotransplanted Tumor Cells in Adult rag2 Mutant Xenopus tropicalis" Cancers 14, no. 19: 4560. https://doi.org/10.3390/cancers14194560
APA StyleTulkens, D., Dimitrakopoulou, D., Boelens, M., Van Nieuwenhuysen, T., Demuynck, S., Toussaint, W., Creytens, D., Van Vlierberghe, P., & Vleminckx, K. (2022). Engraftment of Allotransplanted Tumor Cells in Adult rag2 Mutant Xenopus tropicalis. Cancers, 14(19), 4560. https://doi.org/10.3390/cancers14194560





