Simple Summary
Prostate cancer is still the most common cancer among men in the US. Current standard-of-care therapies for metastatic castration-resistant prostate cancer can offer survival benefits measured only in months. The patients eventually develop drug resistance and tumor relapse. There is strong evidence that during treatment, prostate cancer stem cells may become the predominant population within tumor bulk, and function as the “root cause” for drug resistance. microRNA-34a, a bona fide tumor-suppressive miRNA, represents a potent cancer stem cell suppressor by targeting many molecules essential for cancer stem cell survival. This article will review the tumor suppressive role of miRNA-34a in prostate cancer, and its therapeutic development strategies for advanced prostate cancer patients.
Abstract
Prostate cancer (PCa) is a highly heterogeneous disease and typically presents with multiple distinct cancer foci. Heterogeneity in androgen receptor (AR) expression levels in PCa has been observed for decades, from untreated tumors to castration-resistant prostate cancer (CRPC) to disseminated metastases. Current standard-of-care therapies for metastatic CRPC can only extend life by a few months. Cancer stem cells (CSCs) are defined as a subpopulation of cancer cells that exists in almost all treatment-naive tumors. Additionally, non-CSCs may undergo cellular plasticity to be reprogrammed to prostate cancer stem cells (PCSCs) during spontaneous tumor progression or upon therapeutic treatments. Consequently, PCSCs may become the predominant population in treatment-resistant tumors, and the “root cause” for drug resistance. microRNA-34a (miR-34a) is a bona fide tumor-suppressive miRNA, and its expression is dysregulated in PCa. Importantly, miR-34a functions as a potent CSC suppressor by targeting many molecules essential for CSC survival and functions, which makes it a promising anti-PCSC therapeutic. Here, we conducted a comprehensive literature survey of miR-34a in the context of PCa and especially PCSCs. We provided an updated overview on the mechanisms of miR-34a regulation followed by discussing its tumor suppressive functions in PCa. Finally, based on current advances in miR-34a preclinical studies in PCa, we offered potential delivery strategies for miR-34a-based therapeutics for treating advanced PCa.
1. Introduction: PCa Cell Heterogeneity, PCSCs and CRPC
Prostate cancer (PCa) is the second most common cancer in men worldwide and the most commonly diagnosed solid-organ malignancy in men in the US [1]. PCa treatment varies based on pathological parameters, i.e., the Gleason score (GS) grading and staging. Currently, the majority of low-grade (GS6-7) PCa patients are undergoing active surveillance (without any specific treatment), as recommended in clinical guidelines of the American Urological Association (AUA). The majority of these patients show good prognosis without any tumor progression. High-grade tumors (GS9-10) with lymph node (LN) metastasis are treated with drugs named AR signaling inhibitors (ARSIs). In general, ARSIs comprise two subclasses [2]. The first class aims to block AR productions in the testis (e.g., orchiectomy or using LHRH agonists or antagonists) or adrenal gland (e.g., abiraterone acetate), and the former is often called androgen deprivation therapy (ADT). The second class is to prevent the activation of AR signaling through direct binding to AR (e.g., enzalutamide, Enza). Initially, patients with advanced PCa are well responsive to ADT. However, most treated tumors inevitably recur and become resistant to ADT. These patients are subsequently treated with Enza together with chemotherapy (e.g., docetaxel). Castration-resistant PCa, or CRPC, is the general term used to refer to tumors that have failed ADT or ADT/Enza.
For CRPC treatment, ARSIs are still among the standard-of-care therapies. However, therapeutic efficacy is generally short-lived and reported survival benefit is measured only in months. What is attributed to ARSI resistance? One of the under-appreciated mechanisms is related to cell heterogeneity. Cancer cells are inherently heterogeneous both in vitro and in vivo, exhibiting unique traits phenotypically, epigenetically, and functionally [3]. Therapy resistance is significantly influenced by intra-tumor heterogeneity [4]. Over the course of PCa development and progression, from treatment-naïve primary tumors to CRPC to metastatic CRPC (mCRPC), significant heterogeneity in AR and PSA expression have been demonstrated [2,5,6].
Within tumor heterogeneity are cancer stem cells (CSCs), which generally represent a small subpopulation of the bulk tumor cells in early-stage treatment-naïve tumors. CSCs are operationally defined as the stem-like cancer cells that possess some or most of the normal stem cell properties such as relative quiescence but with great proliferative potential, the ability to self-renew and differentiate, and, importantly, the capability to regenerate and long-term propagate tumors [3]. Our group provided evidence that in untreated prostate tumors the PSA−/lo PCa cell population harbors authentic prostate cancer stem cells (PCSCs) that possess the capability of long-term self-renewal, tumor propagation in vivo, and inherent therapy-resistance [7]. Additionally, castration can reprogram PCa cells such that CRPC, which can manifest as either AR+/hi or AR−/lo, can be highly enriched in PCSCs. For example, our group reported AR−/loPSA−/lo CRPC models that exhibit prominent upregulation of CSC molecules and are refractory to Enza treatment de novo [5]. PCa progression may also be accompanied by increased ‘stemness’ based on transcriptome-based stemness score analysis, which might reflect increased CSC abundance in advanced and treatment-failed tumors [8]. These observations reinforce the notion that spontaneous tumor progression and therapeutic treatments may induce plasticity in non-CSCs and reprogram them into PCSCs. Consequently, PCSCs may become the predominant cell population in ARSI resistant tumors, and the “root cause” for drug resistance. However, current ARSI-based treatment regimens primarily target AR+/hi CRPC cells or clones but largely ignore AR−/lo cells that represent more aggressive PCSC subsets.
microRNA-34a (miR-34a) is a potent CSC suppressor by targeting many molecules essential for CSC survival and functions, which makes it a promising anti-PCSC therapeutic. However, there has not been a comprehensive review focusing on miR-34a’s role and potential therapeutic application in the context of PCa, especially with respect to targeting PCSCs. In this review, we provide an updated discussion of the mechanisms of miR-34a regulation followed by elaborating its tumor suppressive functions in the context of PCa and PCSCs. Finally, we discuss current miR-34a preclinical studies in PCa and offer potential delivery strategies for miR-34a based therapeutics for treating advanced PCa.
2. miR-34a Expression Decreases with Increasing PCa Grade but Correlates with Better Patient Survival
MicroRNAs (miRNAs) are ~22-nucleotide (nt) non-protein coding RNAs and critical posttranscriptional regulators of gene expression. The biogenesis of miRNA is a multi-step process that initially consists of generation of pre-miRNA in the nucleus by Drosha complex from the primary miRNA transcript (pri-miRNA) and subsequent Exportin 5-mediated nuclear export of pre-miRNA (Figure 1, left). The mature 20–25 bp miRNA duplex is then produced in the cytoplasm after the final cleavage by the RNase Dicer (Figure 1, left). Mature miRNAs elicit gene silencing function through either translation inhibition or messenger RNAs (mRNAs) degradation. A seed region (nt 2–8) of miRNA can recognize partially complimentary sequences in the 3’-untranslated regions (3’-UTR) of their target mRNAs. miRNAs are dysregulated in almost all malignancies, suggesting their roles in tumorigenesis [9,10,11]. miR-34a, a bona fide tumor-suppressive miRNA, is downregulated in a variety of cancers including hematologic malignancies [12]. We investigated the miR-34a expression pattern and its effect on PCa survival in TCGA (The Cancer Genome Atlas) dataset via bioinformatic approach (Figure 2A–C and Figure 3C). Interestingly and unexpectedly, we found that the expression levels of mature (22 nt) miR-34a (i.e., hsa-miR-34a-5p) are elevated in primary PCa samples compared to matching benign/normal prostatic tissues in TCGA (Figure 2A). This miR-34a expression pattern in treatment-naïve PCa is reminiscent of the expression pattern of LRIG1, an AR-regulated feedback prostate tumor suppressor [13]. PCa development involves activation of multiple oncogenic signaling pathways such as AR and c-Myc, and miR-34a upregulation, like elevated LRIG1 expression, might well represent a feedback inhibitory mechanism to antagonize oncogenic signals driven by MYC and AR (see below). In this sense, the elevated miR-34a in primary prostate tumors would still behave as a tumor suppressor. In support, miR-34a expression is found to negatively correlate with the tumor (T) stage in PCa samples (Figure 2B). To lend further support, both mature miR-34a and the stem-loop pre-miR-34a levels positively correlate with PCa patients’ overall survival (Figure 2C), suggesting that miR-34a is a prostate tumor suppressor. Extensive research has demonstrated that miR-34a suppresses tumor growth and cancer progression by directly targeting many factors involved in cancer-relevant cellular processes [14]. Notably, miR-34a functions as a potent CSC suppressor via targeting many molecules essential for CSC survival and functions in prostate, colon, breast, lung, and other cancers [14]. The role of miR-34a in inhibiting PCSCs and PCa metastasis was first reported by our group [15], indicating that miR-34a replacement therapy could be a novel therapeutic strategy to target PCSCs and aggressive PCa (Figure 1, right).
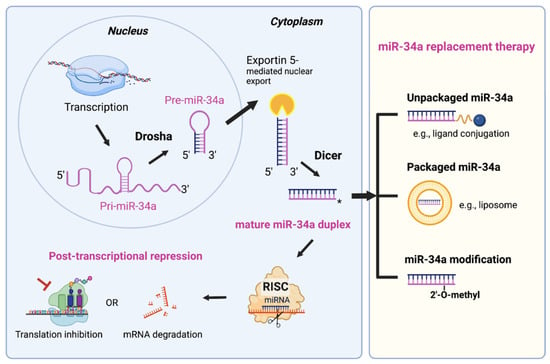
Figure 1.
miRNA biogenesis using miR-34a as an example and miR-34a replacement therapy. miR-34a encoding gene is transcribed as the primary transcript (pri-miR-34a) in the nucleus. Next, pri-miR-34a (~110 nucleotide) transcripts undergo the first cleavage by the RNase III enzyme Drosha in complex with the dsRNA-binding protein DGCR8 (also known as Pasha). The resulting product is ~70 nucleotide stem-loop-structured pre-miR-34a precursor. Pre-miR-34a is then transported to the cytoplasm by Exportin 5, where they undergo the second and final cleavage, catalyzed by the RNase Dicer. The resulting 22 bp RNA duplex consists of the mature miR-34a (guide strand) and its passenger strand (miR-34a*), which is released and degraded. Mature miR-34a is incorporated into the RNA-induced silencing complex (RISC), which mediates silencing activity of target mRNAs by either inhibiting translation or inducing mRNA degradation. For the miR-34a replacement therapy (right), the delivery platforms include unpackaged (i.e., vehicle-free) delivery and packaged vehicle delivery. In addition, chemical modification of miR-34a oligos is necessary for enhancing the stability.
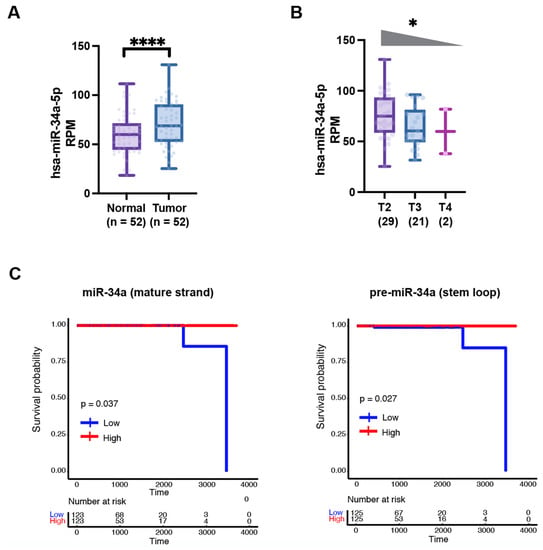
Figure 2.
miR-34a expression decreases with increasing tumor grade and correlates with better PCa patient survival. (A) Mature miR-34a expression levels are increased in treatment-naive primary tumors compared to matching normal tissues in TCGA (****, p < 0.0001, Student’s t-test). (B) Mature miR-34a expression levels decrease with increasing tumor stage in TCGA (*, p < 0.05, Jonckheere–Terpstra trend test). (C) Reduced levels of mature miR-34a (left) and pre-miR-34a (right) correlate with worse patient overall survival (TCGA dataset). p-Value was determined using the Log-Rank test.

Figure 3.
miR-34a may represent an effective ‘replacement’ therapeutic in TP53 mutated cancers. (A) In response to cellular stress signals, p53 is activated and directly binds to the promoter of miR-34a gene and induces miR-34a expression. Consequently, miR-34a expression is reduced in TP53 mutated tumors. (B) Schematic illustrating that p53 transcriptionally induces whereas Myc suppresses miR-34a expression by directly binding to the promoter region of the miR-34a gene. (C) Both mature miR-34a (left) and pre-miR-34a (right) expression levels are decreased in TP53 mutated prostate tumors compared to TP53 WT prostate tumors in TCGA (***, p < 0.001, Student’s t-test). (D) PCa progression and castration resistance are accompanied by increasing TP53 mutations and decreasing miR-34a levels but gradually increased AR and MYC expression and activity. In principle, miR-34a should be an effective therapeutic for TP53-mutated PCa.
3. Mechanisms of miR-34a Regulation
miR-34a expression is regulated transcriptionally and epigenetically. At the transcriptional level, miR-34a expression is dictated by transcription factors that bind to its promoter region [14]. In the context of cancer, miR-34a expression is largely regulated through epigenetic mechanisms, involving various long non-coding RNAs (lncRNAs) and histone-modifying enzymes.
3.1. Transcriptional Regulation of miR-34a Expression
Expression of miR-34a is predominantly regulated by the tumor suppressor p53, which is activated by a multitude of cellular stresses (Figure 3A). miR-34a is a direct transcriptional target of p53, which binds to several canonical p53 binding sites in regions proximal to the miR-34a promoter [16,17,18] (Figure 3B). miR-34a induction by DNA damage and oncogenic stresses depends on p53 both in vitro and in vivo [16]. In addition, there is a positive feedback loop between p53 and miR-34a that is partially contributed by miR-34a-mediated suppression of Mdm4, a negative p53 regulator [19]. mir-34 deficiency alone did not display significant tumor-promoting effect in a Kras-induced mouse lung cancer model. mir-34 deficiency, on the other hand, enhanced tumorigenesis only when Trp53 was haplo-insufficient, suggesting that the defective feedback loop between p53 and miR-34a can promote oncogenesis in context dependent manner [19]. TP53 mutations are enriched in PCa with progression [20,21,22,23,24]. For example, 8% TP53 mutations were observed in localized PCa, which was increased to 40% in a cohort of 429 metastatic PCa patients [21]. Of significance, we found that the levels of both mature miR-34a and pre-miR-34a correlated well with the TP53 status in PCa such that their expression levels were significantly reduced in TP53 mutated tumors (Figure 3C). In comparison to LNCaP cells that express wild-type TP53, miR-34a expression is also decreased in TP53-null PC-3 cells and TP53-mutated DU145 cells [15,25,26]. These results suggest a clear reciprocal relationship between TP53 status and the miR-34a levels during PCa progression (Figure 3D), and support the use of miR-34a replacement therapy in TP53-mutated advanced PCa.
Of interest, miR-34a expression remained high in the brain, testis, and lung tissues in Trp53 deficient mice, suggesting the existence of p53-independent mechanisms that determine basal miR-34 transcription [27]. C-Myc, the protein encoded by the MYC oncogene, is a transcription factor that regulates cell growth, cell cycle, and metabolism. In contrast to p53, Myc represses miR-34a transcription by binding to conserved promoter region of miR-34a [28] (Figure 3B). Myc-mediated repression of miR-34a was observed in many cancers such as lymphoma [28,29]. MYC amplification was found in 46% of advanced PCa samples but only 25% of clinically localized prostate tumors, [21], suggesting that MYC amplification accompanies as well as, likely, drives PCa progression. Further studies have confirmed that MYC is one of the genes significantly amplified in CRPC versus primary PCa [30], suggesting that repression of miR-34a expression may represent a fundamental component of the MYC tumorigenic program (Figure 3D).
AR, a steroid hormone receptor normally activated by androgens, is critical for PCa development, progression, and therapy response [5]. In normal prostate, androgens promote survival and differentiation, but during PCa development, AR drives uncontrolled cell growth. miR-34a was reported to mediate AR-dependent, p53-induced apoptosis in PCa cells [5]. In the study, DNA double-strand break inducing agent doxorubicin (DOX) leads to p53-induced apoptosis by upregulating miR-34a. However, no increase of miR-34a was found in PCa cells with AR knocked down after DOX treatment. Importantly, DOX did not induce miR-34a expression in LNCaP cells grown in androgen-free medium or in AR-negative PC-3 and DU145 cells [31], which implies that AR might positively regulate miR-34a expression. In contrast, another study reported an AR-miR-204-XRN1-miR-34a feedback loop in neuroendocrine-like PCa, showing negative correlation between AR and miR-34a [32]. In this loop, AR represses miR-34a by upregulating XRN1, while XRN1 raises AR expression by reducing expression of miR-34a [32]. It appears that AR might regulate miR-34a in a context dependent fashion. On the other hand, AR is proven to be a direct target of miR-34a, which binds to the 3’-UTR of AR exon 8 region [33] (Figure 4). The mutual regulation between AR and miR-34a implies that reduced miR-34a may further contribute to increased AR expression and activity during PCa progression (Figure 3D).
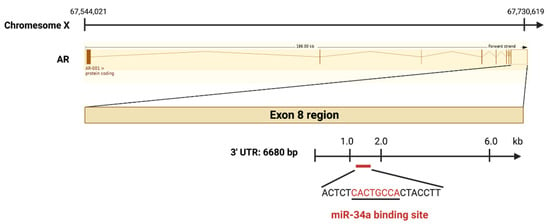
Figure 4.
AR is a direct target of miR-34a. The AR gene is located on the X chromosome at Xq11–12, and the pre-spliced AR-001 transcript is 186 kb long. The 3’-UTR region (6680 bp), located downstream of Exon 8, harbors a miR-34a binding site (marked in red).
miR-34a expression has also been reported to be transcriptionally repressed by STAT3, which is involved in IL-6-triggered IL-6R/STAT3/miR-34a feedback loop that promotes EMT-mediated colorectal cancer invasion and metastasis [34]. This study also showed that deletion of mir-34a facilitated tumor invasion in a mouse model of colitis-associated cancer, providing in vivo evidence for the tumor-suppressive functions of mir-34a. The IL-6R/STAT3/miR-34a feedback loop was also reported in PCa cell lines and to be associated with a mesenchymal phenotype [34].
3.2. Epigenetic Regulation of miR-34a Expression
Hermeking and his team were the first to report miR-34a downregulation in some cancers as a result of aberrant CpG methylation of its promoter [26]. CpG methylation in the miR-34a promoter and concurrent loss of miR-34a expression was displayed in 19 out of 24 (79.1%) primary PCa [26]. Further study confirmed that miR-34a is epigenetically downregulated by DNA methylation in PCa compared to paired normal tissues [35]. The lncRNA HOTAIR was reported to promote tumor metastasis by regulating EMT-related genes as well as miR-34a. HOTAIR recruits and binds to polycomb repressive complex 2 (PRC2), which represses miR-34a by enhancing DNA methylation of the miR-34a promoter [14]. Another lncRNA, Lnc34a, can epigenetically silence miR-34a expression via recruiting DNMT3A through PHB2 and HDAC1 to methylate and deacetylate the miR-34a promoter simultaneously [36].
5-aza-2’deoxycytidine (5Aza-2’dC) is a DNA methyltransferase inhibitor that reactivates the expression of genes silenced by CpG methylation. 5Aza-2’dC treatment reversed miR-34a silencing and upregulated miR-34a expression in PC-3 and LAPC4 cells [26]. Likewise, BR-DIM, a novel demethylating agent, increased the expression of miR-34a through demethylation of the miR-34a promoter in C4-2B and LNCaP cells, and its efficacy was shown to be more effective than 5Aza-2’dC [37]. Methylation-induced silencing of miR-34a promotes chemoresistance by directly upregulating ATG4B-induced autophagy through AMPK/mTOR pathway in PCa cells [35]. Upregulation of miR-34a by 5Aza-2′dC or ectopic miR-34a treatment sensitized PC-3 and DU145 cells to chemotherapy (e.g., DOX). Abnormal estrogen signaling, due to the decrease of androgen in favor of estrogen during aging, results in almost complete silencing of miR-34a in aggressive PCa [38]. Estrogen-dependent repression of miR-34a is prevented by treatment with HDAC inhibitors [38]. These findings suggest that epigenetic drugs as well as miR-34a replacement therapy can be beneficial to PCa patients with epigenetically downregulated miR-34a, regardless of the TP53 status.
4. Tumor Suppressive Role of miR-34a in PCa
Due to aberrant transcriptional regulation, genomic deletions, and/or promoter hypermethylation, as discussed above, miR-34a expression is downregulated in a wide range of human cancers. This implies that miR-34a deficiency may have a function role in tumorigenesis. He and her group were the first to report phenotypic characterization of mir-34-deficient mice. Interestingly, mouse embryonic fibroblasts lacking mir-34 did not show any abnormalities in p53-dependent cell cycle arrest, apoptosis, or replicative senescence [39]. Moreover, the mir-34a−/−; mir-34b/34c−/− mice did not display increased susceptibility to spontaneous, irradiation-induced, or c-Myc-initiated tumorigenesis [27]. In these contexts, it is conceivable that mir-34 loss alone is insufficient for tumorigenesis and that the redundant pathways downstream from p53 could compensate for mir-34 deficiency in vivo. Indeed, in a KrasG12D lung cancer model, KrasLSL-G12D/+; Trp53+/−; mir-34a−/− mice exhibited increased tumor area and the number of high-grade tumors than KrasLSL-G12D/+; Trp53+/− mice, suggesting that mir-34a deficiency accelerated tumor progression when p53 response is compromised [19]. In addition, loss of mir-34a promoted tumor invasion in a mouse model of colitis-associated cancer [34]. Similarly, mir-34a deficiency promoted colon tumorigenesis after Citrobacter rodentium infection [40]. These observations suggest that miR-34a is a tumor suppressor.
Cheng et al. were the first to elucidate the role of miR-34a in PCa by using mir-34a deficient genetic mouse models [41]. In the study, it was demonstrated that miR-34 suppressed prostate tumorigenesis in cooperation with p53 by controlling the prostate stem cell compartment via MET [41]. Mice with mir-34 and Trp53 specifically inactivated in prostate epithelium led to expansion of the prostate stem cell compartment, development of early invasive adenocarcinomas, and high-grade prostatic intraepithelial neoplasia. Interestingly, any atypical lesions were not displayed in mice losing all mir-34 genes in the prostate epithelium (mir-34PE−/− mice) by 15 months of age [41], suggesting that mir-34 deficiency alone is insufficient to drive prostate tumorigenesis. Mice with lacking both Trp53 and mir-34 promoted self-renewal, MET-dependent growth, and motility of prostate stem cells in the proximal region of prostatic ducts [41]. These findings provide direct genetic evidence that mir-34a is a bona fide tumor suppressor, and demonstrate that miR-34 can repress PCa development by cooperating with p53.
miRNA expression profiling in PCSCs was first conducted by our group [15]. Through an unbiased profiling, we revealed that miR-34a is commonly underexpressed in all five PCSC populations purified from PCa xenografts, including three CD44+ populations, CD133+ cells, and α2β1+ population [15,42]. The underexpression of miR-34a was further confirmed in CD44+ PCa cells purified from prostate tumors [15]. These findings indicate that miR-34a is devoid in PCSCs within the prostate tumor bulk and that miR-34a reintroduction would elicit antitumor effect by targeting PCSCs. In fact, extensive studies have shown that miR-34a is a potent PCSC suppressor by targeting critical cellular processes essential for CSC survival and functions (Figure 5).

Figure 5.
miR-34a potentially offers multidimensional anti-PCSC effects. Current CSC-targeting therapy approaches focus on a single characteristic of CSCs. miR-34a replacement therapy, on the other hand, could provide multifaceted antitumor effects by targeting several cellular processes that are vital for PCSC functions and activities (Adapted from [14]).
4.1. Targeting Invasiveness and Metastasis
Our group was the first to report the role of miR-34a in inhibiting PCSCs and metastasis. Ectopic expression of miR-34a in bulk PCa cells or purified CD44+ cells exerted significant antitumor effects on tumor growth and metastasis in vivo [15]. In contrast, employing antagomirs in bulk or CD44− PCa cells to neutralize endogenous miR-34a boosted tumor regeneration and metastasis. Strikingly, systemic delivery of miR-34a through tail vein repressed lung metastasis, thereby prolonging the survival of mice bearing orthotopic human prostate tumors. Mechanistically, miR-34a suppressed PCSC properties by inhibiting prostasphere formation, migration, and invasiveness of CD44+ PCa cells, as well as serial tumor transplantation. Notably, CD44 was found to be a direct target of miR-34a [15]. Hence, our findings demonstrate that miR-34a is a PCSC suppressor that inhibits tumor development and metastasis by directly targeting CD44. The work provides compelling evidence for the development of miR-34a as a novel therapeutic against aggressive and metastatic PCa.
MET plays a vital role in promoting cell motility, invasion and metastasis of CSCs [43]. It was reported that PCa-propagating cells express MET, and depletion of MET results in a decrease in prostasphere formation [44]. Previous studies identified MET as a target of p53 and miR-34 [16,45,46]. Cheng et al. revealed that miR-34a cooperates with p53 in suppression of prostate tumorigenesis by targeting MET [41]. Loss of mir-34a and Trp53 promoted MET-dependent growth, self-renewal, and motility of prostate stem/progenitor cells, leading to tumor development [41]. In addition, ectopic overexpression of miR-34a decreased MET mRNA level in PC-3 and PC-3MM2 cells [47], whereas MET overexpression reversed miR-34a-induced suppression of invasion in PC-3 cells [48]. Importantly, systemic introduction of miR-34a decreased PCa bone metastasis by suppressing MET [47].
Epithelial-to-mesenchymal transition (EMT) is a unique process of cellular reprogramming and phenotypic changes accompanied by the loss of epithelial markers, leading to an enhancement in tumor invasive and metastatic ability. ZEB1, one factor of the ZEB family, plays a role in accelerating cell migration and invasion by promoting EMT. Zhang et al. reported that miR-34a enhanced docetaxel sensitivity in docetaxel-resistant PCa cells both in vitro and in vivo through inhibiting EMT by targeting ZEB1 [49].
Oncoprotein STMN1 (also known as stathmin 1 and oncoprotein 18) plays a role in cell proliferation, motility, and cancer metastasis [50]. STMN1 expression is elevated in metastatic PCa, and knockdown of STMN1 resulted in reduced proliferation and invasion of PCa cells in vitro as well as tumor growth and metastasis in vivo [50]. miR-34a reduced cell proliferation and colony formation in DU145 and PC-3 cells by directly targeting STMN1 [50], suggesting its negative regulation of invasion and metastasis through STMN1.
4.2. Targeting Stemness
Cancer ‘stemness’ is defined as CSC traits and properties in cancer regulated by stem cell factors, and many studies have reported that miR-34a represses cancer stemness by targeting the stem cell factors. Myc is highly expressed in PCa cells with stem-like and tumor-initiating properties [51]. Myc silencing using a promoter-targeting siRNA reduced the fraction of PCSCs, leading to reduced self-renewal, tumor-initiating and metastatic capabilities, indicating a causal role of Myc in PCSC maintenance [51]. Yamamura et al. reported that miR-34a inhibited invasion by suppressing RhoA expression through directly targeting c-Myc [48]. miR-34a suppressed the recruitment of the c-Myc–Skp2–Miz1 complex to the RhoA promoter, resulting in downregulation of RhoA [48]. In addition, delivery of miR-34a reduced subcutaneous PC-3MM2 prostate tumor burden by inducing apoptosis and downregulating c-Myc [47].
In cancers, CSCs often exhibit dysregulation in critical developmental and signaling pathways including WNT and NOTCH [14]. WNT signaling is important for stemness maintenance, and aberrant WNT signaling is driven by stabilized β-catenin which in turn mediates inappropriate gene activation. Accumulated cytoplasmic β-catenin translocates into the nucleus where it associates with members of the T-cell factor (TCF) and lymphoid enhancer factor (LEF). Subsequently, the β-catenin-TCF/LEF complex activates the transcription of target genes including c-Myc and cyclin D1. miR-34a overexpression induced G2 cell cycle arrest and promoted apoptosis in PC-3 cells by inhibiting Wnt/β-catenin activity [52]. Mechanistically, miR-34a directly targeted WNT1 and inhibited translocation of β-catenin into the nucleus, leading to suppression of PC-3 cell proliferation and migration [52]. Furthermore, TCF7, a canonical WNT response gene and a mediator of bone metastasis, has been reported to be a direct target of miR-34a [53]. Downregulated miR-34a is associated with activated WNT signaling in metastatic PCa samples compared to primary tumors and normal prostatic tissues [53]. Ectopic miR-34a expression inhibited bone metastasis and reduced cancer cell proliferation in a Ras-dependent xenograft model by repressing WNT/TCF7 signaling [53]. LEF1, another key transcription factor in the WNT signaling pathway, has been reported to regulate cancer invasion and metastasis [54]. miR-34a suppressed EMT, migration and invasion in PC-3 cells by targeting LEF1 [55].
NOTCH signaling is activated during PCa development and progression. Knockdown of NOTCH1 in C4-2R cells improved Enza sensitivity by decreasing cell proliferation and promoting apoptosis, suggesting that NOTCH1 signaling may contribute to drug resistance in PCa [56]. Ectopic miR-34a expression reduced stemness (colony formation) and enhanced sensitivity to paclitaxel by directly targeting NOTCH1 and JAG1 both in vitro and in vivo [57], indicating that miR-34a may overcome drug resistance and improve therapeutic efficacy for CRPC.
4.3. Targeting Epigenome
Sirtuin-1 (SIRT1), a class-III histone deacetylase, has a key role in the epigenetic regulation of tissue homeostasis and many diseases by deacetylating both histone and non-histone substrates [58]. SIRT1 overexpression sustains CSC activities in different cancers [59]. SIRT1 is reported to be upregulated in PCa [58]. miR-34a overexpression induced cell-cycle arrest and growth inhibition and attenuated chemoresistance to anticancer drug camptothecin by targeting SIRT1 [25]. Intriguingly, miR-34a-induced SIRT1 inhibition occurred at the transcriptional rather than post-transcriptional level despite the presence of a potential miR-34a binding site within its 3′-UTR [25]. Another study also revealed that miR-34a attenuated paclitaxel resistance in PC-3 cells by targeting SIRT1 [60]. Systemic administration of micelles carrying paclitaxel and miR-34a inducer rubone inhibited orthotopic prostate tumor growth by inhibiting CSC population and downregulating SIRT1, cyclin D1 and E-cadherin [61].
4.4. Targeting Cell Survival
Our group previously demonstrated that BCL-2, an anti-apoptotic protein as well as a CSC marker, is exclusively upregulated in CRPC patient, that BCL-2 is directly induced by Enza treatment, and that BCL-2 is upregulated in both Enza-resistant AR+/hi and Enza-insensitive AR−/lo CRPC models [5]. These findings suggest that BCL-2 plays a vital role in drug resistance and cancer progression. Corcoran et al. reported that docetaxel-resistant variants of PCa cell lines harbored lower miR-34a levels as well as elevated expression of BCL-2 compared to the respective nonresistant cell lines, indicating a reciprocal relationship of miR-34a and BCL-2 in PCa chemoresistance [62]. Further study confirmed that miR-34a sensitized PCa cells to apoptosis-inducing drugs such as camptothecin by directly targeting BCL-2 [60,62]. Ectopic expression of miR-34a in paclitaxel-resistant PC-3 cells decreased BCL-2 as well as the RNA binding protein, human antigen R protein (HuR) [60]. Notably, knockdown of HuR via siRNA also reduced BCL-2 mRNA levels [60], indicating that miR-34a may regulate BCL-2 in both a direct and indirect manner.
Another anti-apoptotic protein, BIRC5, is upregulated in metastatic PCa and in prostate tumors with higher clinical grade [53]. The expression levels of BIRC5 were inversely correlated to miR-34a expression in metastatic PCa patients [53]. BIRC5 was previously reported to be a target gene of miR-34a in breast and colorectal cancers, and this relationship was also confirmed in PCa [53]. miR-34a overexpression induced dramatic increase in apoptosis in Ras signaling- activated PCa cells, and the apoptotic effect of miR-34a was reversible by overexpression of BIRC5 [53].
Autophagy, a survival mechanism conserved from yeast to mammals, is known to be widely exploited by cancer cells [63]. The association between cancer cell survival and autophagy can be partly explained by the role of autophagy in protecting cells from undergoing programmed cell death [63]. Liao et al. found that ectopic expression of miR-34a inhibited autophagy by downregulating ATG4B, Beclin-1 and LC3B II/I in DU145 and PC-3 cells [35]. Furthermore, ATG4B is directly targeted by miR-34a [35]. Mechanistical studies showed that overexpression of miR-34a resulted in a significant downregulation of p-AMPK and upregulation of p-mTOR [35], indicating that miR-34a may regulate ATG4B-induced autophagy through the AMPK/mTOR pathway.
5. miR-34a Therapeutic Development for Aggressive PCa
5.1. miR-34a Replacement Therapy
miR-34a replacement therapy is a new therapeutic concept, which aims to restore a loss of function to inhibit cancer growth by reintroduction of tumor suppressor miR-34a in tumor cells. A miR-34a therapeutic is a mature double-stranded duplex that does not require further processing by Dicer and can directly elicit gene silencing effect by entering the RISC (Figure 1, left). Dicer, an important endoribonuclease required for miRNA biogenesis, is often dysregulated in cancers. Thus, miR-34a replacement therapy could be beneficial to the cancer patients with Dicer dysfunction through circumventing endogenous miRNA processing steps.
The delivery system for miR-34a therapeutic can be categorized into two major platforms (Figure 1, right). One platform is packaged vehicles, including liposomes and other types of nanoparticles. The ability of liposomes to deliver a variety of payloads, including chemotherapy drugs, oligonucleotides, DNA, siRNA, and proteins, has made them the most widely used method for delivery of therapeutic agents [64]. Current delivery systems of miR-34a mainly utilize the packaged vehicle strategy [14]. The other platform is unpackaged or ligand-conjugates, such as folate [65,66]. Despite extensive research in the field, the translation of miR-34a therapeutics into the clinic has been hindered by several issues including delivery vehicle-associated toxicity, inefficient cellular uptake and serum stability, and limited specificity to targeted tumors. The stability problem is due to the sensitivity of unmodified miR-34a oligos to nuclease-mediated degradation, thereby requiring repetitive cycles of high-dose to achieve the desired therapeutic response [66]. Thus, chemical modifications have been pursued to overcome the issue (Figure 1, right). For example, introducing 2’-O-methyl and 2’-fluoro modifications in place of the unstable 2’-OH of the ribose sugar, may reduce immunogenic effects and enhance both stability and activity of miR-34a [66]. However, chemical modifications should be carefully optimized to prevent interference with the silencing effect of miR-34a.
The first-in-human phase I clinical trial of MRX34, a liposomal miR-34a mimic, was initiated in 2013 (NCT01829971). Unfortunately, despite that MRX34 treatment was associated with acceptable safety and showed evidence of antitumor activity in a subset of patients [67,68], the trial was terminated by FDA in September of 2016 due to severe immune-related adverse events (AE). As reviewed [14], we believe that the MRX34’s AEs were caused by packaging vehicle-associated toxicity, too high doses of miR-34a, and unselected population of cancer patients recruited in the trial. The delivery vehicle of MRX34 was SMARTICLE, an amphoteric liposome composed of combinations of anionic and cationic lipids, which may induce systemic toxicity. Additionally, the MTD of MRX34 at 93–110 mg/m2 used in the clinical trial [67,68] corresponded to ~30 mg/kg body weight, which was 30X higher than preclinically effective doses (<1 mg/kg body weight) [65,69,70,71,72,73,74,75,76,77,78,79] (also see Table 1; below). Notably, the trial used MRX34 in an unselected mixed cohort of cancer patients instead of a specific subpopulation. Therefore, these three issues should be addressed in future miR-34a clinical translation.
5.2. Preclinical Studies of miR-34a in PCa
During the past decade, several translational studies of miR-34a, mainly using packaging vehicles for delivery, have focused on PCa (Table 1). Most of these preclinical studies used ≤2 mg/kg of miR-34a mimics (Table 1). The first preclinical study of miR-34a in PCa was back in 2011, when our group demonstrated that systemic delivery of miR-34a reduced tumor burden and lung metastasis by targeting CD44 in orthotopic PC-3 and LAPC9 xenografts [15]. In this study, miR-34a was complexed with a lipid-based delivery agent, MaxSuppressor In Vivo RNA-LANCER II. It is a formulation composed of neutral lipid, non-ionic detergent, and small molecules which enable highly efficient delivery of RNAi agents into animals. Additionally, the formulation has been shown to be well tolerable and does not induce an immune response [69]. This study [15] provided the proof-of-principle that miR-34a could be a promising therapeutic targeting PCSCs and PCa. A few years later, Gaur et al. utilized chitosan (CH) nanoparticles to deliver miR-34a via tail vein [47]. CH nanoparticles comprise biodegradable natural polysaccharides with low toxicity and immunogenicity [80,81]. In the study, systemic delivery of miR-34a robustly reduced the growth of prostate tumor in the bone. Importantly, comparing an intra-femoral PCa model to a sub-cutaneous model revealed that miR-34a delivery had more potent anti-tumor effects in the former, indicating that miR-34a may mediate tumor suppressive effects by targeting the bone microenvironment in addition to the tumor. [47]. The study highlights the clinical potential of miR-34a therapeutic for treating bone metastatic PCa.
The internalization mechanism of synthetic vehicle packaged with miR-34a involves the fusion of vehicle lipids with the cell membrane followed by endocytosis and subsequent release of miR-34a from the endosome into the cytosol. Additionally, cellular uptake of miR-34a is one of the important variables that impact anti-tumor efficacy. Wang et al. reported that ultrasound-induced microbubble cavitation (UIMC) improved anti-tumor efficacy by promoting the cellular uptake of miR-34a-loaded nanoparticles in PC-3 xenograft [82]. UIMC is a safe and effective technology widely used for drug and gene delivery [83]. Microbubbles exposed to ultrasound produce cavitation microfluidic field that enlarges the capillary gaps and cell membrane permeability, thus promoting the penetration of nanomedicine into tumor tissues [82]. In the study, biodegradable methoxy polyethylene glycol-polylacticco-glycolic acid-polylysine (mPEG-PLGA-PLL) nanoparticles was used to encapsulate miR-34a mimic. mPEG-PLGA-PLL nanoparticles is biocompatible, and the PLL in the structure can be used to adsorb miR-34a oligos. In addition, the mPEG layer on the surface of nanoparticles can increase the water solubility and prevents phagocytosis by the reticuloendothelial system in vivo [82]. Notably, biodistribution study showed that UIMC increased the accumulation of miR-34a-loaded nanoparticles in tumor tissues. Additionally, the delivery vehicle did not display obvious systemic toxicity with no significant weight loss and elevated biochemical parameters [82].
In addition, the concept of combinatorial therapy has been applied to miR-34a preclinical studies. For example, co-delivery of DOX and miR-34a via an amphiphilic micellar system achieved synergistic anti-tumor effect in DU145 xenograft model compared with DOX or miR-34a alone [84]. The delivery platform is a reducible self-assembling disulfide cross-linked stearyl-peptide-based micellar system (SHRss) using poly(l-arginine)-poly(l-histidine)-stearoyl as the copolymer building unit. The nanoscale SHRss micelles facilitated the escape of miR-34a from the endosome and release of DOX into the cell nucleus, and led to downregulation of SIRT1 and inhibition of DU145 and PC-3 cell proliferation [84]. DOX and miR-34a delivered by SHRss micelles mainly accumulated in the tumor tissue with some accumulation in the liver and spleen [84].
Interestingly, rubone, a chalcone derivative, was reported to be a miR-34a inducer to upregulate the intracellular miR-34a and elicit anti-cancer effects [61]. Co-delivery of rubone and paclitaxel via a micellar system significantly reduced the tumor burden in orthotopic paclitaxel-resistant PC-3 xenograft [61]. Mechanistically, rubone inhibited invasion and decreased the CSC population of paclitaxel-resistant PCa cells, resulting in the reversal of drug resistance. Additionally, rubone synergized with paclitaxel to promote drug effect by downregulating the levels of E-cadherin, SIRT1, as well as ALDH (aldehyde dehydrogenase) activity, which is a CSC marker [61]. Similarly, another study revealed that co-delivery of docetaxel and rubone by dual responsive micelles sensitized taxane and inhibited tumor growth in orthotopic taxane-resistant PC-3 xenograft [85]. This dual response micelle is a nano-platform responsive to pH and glutathione (GSH) levels that are altered in tumor microenvironment. Upon endocytosis by tumor cells, low pH value of endocytic vehicles promotes the dissociation of the micelles, leading to efficient drug release and sufficient exposure to GSH for the cleavage of disulfide bond [85]. The treatment with rubone micelles of taxane-resistant PCa cells robustly reduced the ALDHhi CSC subpopulation in a dose dependent manner [85], indicating that miR-34a can reverse the chemoresistance by targeting PCSCs.
In a very recent study, recombinant adeno-associated virus (rAAV) was used as the delivery platform of miR-34a to investigate its therapeutic effect in transgenic adenocarcinoma mouse prostate (TRAMP) model [86]. rAAV-based gene therapy shows advantages of long-term expression, low immunogenicity, and nonchromosomal integration. Notably, a rAAV-based drug for the treatment of Leber’s congenital amaurosis was approved by the FDA in 2017 [87], suggesting potential clinical application of rAAV-based drug delivery in cancer. miR-34a expression was significantly downregulated in the anterior prostate (AP) and dorsal lateral prostate (DLP) of TRAMP mice compared to wild type (WT) mice [86]. Moreover, miR-34a expression is significantly decreased in mouse prostatic intraepithelial neoplasia (PIN) and prostate tumors compared to normal prostatic tissues, indicating a negative role of miR-34a in PCa development. After a single dose of intraprostatic injection, rAAV9-miR-34a improved the survival of TRAMP mice compared to mice that received PBS (median survival 307.5 and 220 days, respectively) [86]. miR-34a overexpression significantly reduced the neoplastic area in both AP and DLP, and downregulated cyclin D1 expression in TRAMP tumors [86]. These findings suggest that intraprostatic delivery of miR-34a effectively inhibits PCa progression in vivo by targeting cyclin D1. However, the body weight of rAAV9-miR-34a-treated group was markedly reduced after 30 weeks of injection [86], raising the safety concern of rAAV-based delivery platform.

Table 1.
Preclinical studies of miR-34a in PCa.
Table 1.
Preclinical studies of miR-34a in PCa.
| Delivery System | Mouse Model | Route of Administration | Dose | Dose Schedule | Reference |
|---|---|---|---|---|---|
| Lipid-based transfection reagent | Orthotopic PC3 and DU145 xenografts | i.v. | 1 mg/kg | Every 2 days for 5 times | [15] |
| Chitosan nanoparticle | s.c PC3MM2 xenograft; intra-femoral model | i.v. | 250 μg/kg | Every 3 days for three weeks | [47] |
| Cationic polypeptide-based micelles | s.c DU145 xenograft | i.v. | 2 mg/kg | Every 4 days for 4 times | [84] |
| PEG-PCD micelles | Orthotopic PC3-TXR xenograft | i.v. | 10 mg/kg (Rubone) | Every 2 days for 5 times | [61] |
| pH and GSH responsive micelles | Orthotopic PC3-TXR xenograft | i.v. | 25 mg/kg (Rubone) | Every 2 days for 9 times | [85] |
| mPEG-PLGA-PLL nanoparticles | s.c. PC3 xenograft | i.v. | 1.5 mg/kg | Every 3 days for 7 times | [82] |
| rAAV9-miR-34a vector | TRAMP genetic model | i.p. | 1 × 1011 genome copy | Single dose | [86] |
s.c.: subcutaneous injection; i.v.: intravenous injection; i.p.: intraprostatic injection.
5.3. Novel miR-34a Delivery Systems for Targeting PCa
One of the apparent limitations using packaged vehicles is the lack of specific delivery of miR-34a to tumors. This could be the cause of systemic toxicity due to off-target effects. Several targeted delivery approaches have been developed to overcome the challenge. One strategy is via ligand-mediated delivery of miRNA mimics. The principle is direct conjugation of a targeting ligand to the miRNA in the absence of a delivery vehicle. When designing a ligand-conjugated miRNA therapeutic, several critical features should be considered (reviewed in [66]). To achieve specificity, the ligand of choice should bind to a high-affinity receptor that is highly expressed on tumor cell surface. Moreover, the same receptor should not be expressed, or expressed at a relatively low level, on normal cells, or should be inconsequential for targeted delivery. In order to establish the potent interaction between the ligand and the receptor, it should also be taken into consideration to include a linker between the ligand and the miRNA. The linker itself can be chemically modified to improve binding affinity and the pharmacokinetics properties of the ligand. Chemical modification of the miRNA can also be considered to improve serum stability and increase intracellular half-life. Several small molecule ligands have been developed for delivery of miR-34a specifically and robustly to tumor cells. One good example is folate, an essential vitamin and a high-affinity ligand for the folate receptor (FR) [88]. FR expression is highly upregulated in several cancer types including ovarian, lung and breast cancers [66]. Orellana et al. were the first to directly conjugate miR-34a to folate (folate-miR-34a), and they showed that folate-miR-34a is selectively targeted to FR-expressing tumors, downregulates target genes, and suppressed the growth of lung and breast cancer models in vivo [65].
Prostate-specific membrane antigen (PSMA) is a promising therapeutic target for advanced PCa. PSMA, also known as folate hydrolase 1 (FOLH1), is a glycoprotein originally discovered on the membrane of prostatic epithelial cells [89]. PSMA is highly, but heterogeneously, upregulated in PCa cells [89,90]. It is also expressed in the neovasculature of several solid tumors and its expression has been associated with PCa progression [90,91,92]. Currently two PSMA-targeted Positron Emission Tomography (PET) imaging drugs, piflufolastat F-18 and Ga-68 PSMA-11, have been approved by FDA for men with suspected PCa metastasis. As radioactive drugs that emit positrons, PET imaging using these probes can indicate the presence of PSMA-positive PCa metastases in the patient’s body. Furthermore, multiple PSMA-targeting therapies are currently in clinical development, including radioligand therapy, PSMA-targeting immunotherapies (bi- and tri-specific T-cell engagers), antibody-drug conjugates, photodynamic therapy, imaging-guided surgery, and ultrasound-mediated nanobubble destruction (reviewed in [92,93]). The study led by Liu et al. demonstrated that PSMA is constitutively internalized via endocytosis and that the rate of internalization is increased by binding to anti-PSMA antibodies [66]. Following internalization, PSMA undergoes receptor cycling which allows additional internalization cycles [66]. With the consideration of the key features of ligand-conjugated miRNA therapeutics, PSMA may represent a golden receptor for specific targeting of PCa.
Interestingly, DUPA, a synthetic urea-based ligand, can bind to PSMA with nanomolar affinity, saturating the receptor quickly [90], suggesting DUPA could be an ideal ligand for conjugated miR-34a therapeutic to target PCa. In fact, DUPA was used to specifically deliver siRNAs to PSMA-expressing PCa cells both in vitro and in vivo [94]. However, therapeutic efficacy of DUPA-siRNA conjugates needs to be further studied. Notably, PSMA expression is increased in advanced-stage PCa and CRPC [95]. Moreover, treatment with Enza increased PSMA expression of PSMA-low PCa [96]. Therefore, DUPA-conjugated miR-34a could be a novel PSMA-targeted therapeutic for aggressive PCa by targeting treatment-reprogramed and PSMA-expressing PCSCs.
6. Conclusions and Perspectives
miR-34a is a bona fide tumor suppressor that represses PCa development by cooperating with p53 in genetic mouse models. In addition, miR-34a, commonly underexpressed in PCSCs, is a potent PCSC inhibitor by targeting cellular processes essential for CSC survival and functions including invasiveness and metastasis, stemness, epigenome, and cell survival. These discussions suggest that miR-34a could be developed into a specific anti-PCSC therapeutic for treating aggressive (advanced, metastatic, and therapy-resistant) PCa that are highly enriched in PCSCs. In reality, optimal miR-34a delivery specifically to prostate tumors still represents a major challenge for its clinical translation. Great efforts have been made towards the use of packaging vehicles for miR-34a delivery for PCa treatment. However, vehicle-associated toxicity, reduced stability, and lack of specificity are the main roadblocks that limit the clinical development of packaged miR-34a therapeutics. In this regard, ligand-mediated delivery of miR-34a represents a promising strategy to achieve specific targeting to prostate tumors while minimizing systemic toxicity. PSMA is highly expressed in PCa, and continuously expressed and upregulated during progression and therapeutic treatment, which makes it an attractive target in PCa. Therefore, DUPA-conjugated miR-34a could be a potential anti-CSC therapeutic for aggressive PCa that continue to express PSMA. In-depth preclinical studies are required to validate the efficacy and safety of this approach prior to its clinical application. In addition, chemical modification of the miR-34a mimics represents another future avenue to improve its serum stability.
Author Contributions
W.L. and D.G.T. conceptualized the current review. W.L. collected data from published studies and wrote the manuscript draft. E.M.D. and X.L. provided some bioinformatics data. X.L. and D.G.T. discussed the review content and critically reviewed the manuscript draft. D.G.T. aided in manuscript writing and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Work in the authors’ lab was supported, in part, by grants from the U.S National Institutes of Health (NIH) R01CA237027, R01CA240290, R21CA237939, and R21CA218635, and by RPCCC and NCI center grant P30CA016056 (all to D.G.T.).
Acknowledgments
We acknowledge the help from Zinian Wang on providing bioinformatics assistance on survival analysis.
Conflicts of Interest
The authors declare no conflict of interest.
List of Abbreviations
| PCa | Prostate Cancer |
| AR | Androgen Receptor |
| CRPC | Castration Resistant Prostate Cancer |
| CSCs | Cancer Stem Cells |
| PCSCs | Prostate Cancer Stem Cells |
| miR-34a | microRNA-34a |
| AUA | American Urological Association |
| GS | Gleason Score |
| LN | Lymph Node |
| ARSIs | AR Signaling Inhibitors |
| ADT | Androgen-Deprivation Therapy |
| Enza | Enzalutamide |
| metastatic CRPC | mCRPC |
| miRNAs | microRNAs |
| nt | Nucleotide |
| pri-miRNA | primary miRNA |
| mRNAs | Messenger RNAs |
| 3’-UTR | 3’-Untranslated Regions |
| TCGA | The Cancer Genome Atlas |
| T | Tumor |
| RISC | RNA-Induced Silencing Complex |
| lncRNAs | Long non-coding RNAs |
| DOX | Doxorubicin |
| PRC2 | Polycomb Repressive Complex 2 |
| 5Aza-2′dC | 5-aza-2′deoxycytidine |
| EMT | Epithelial-to-Mesenchymal Transition |
| STMN1 | Stathmin 1 |
| TCF | T-Cell Factor |
| LEF | Lymphoid Enhancer Factor |
| SIRT1 | Sirtuin-1 |
| HuR | Human antigen R protein |
| AE | Adverse Events |
| UIMC | Ultrasound Induces Microbubble Cavitation |
| mPEG-PLGA-PLL | Methoxy polyethylene glycol-polylacticco-glycolic acid-polylysine |
| SHRss | Self-assembling disulfide cross-linked stearyl-peptide-based micellar system |
| GSH | Glutathione |
| rAAV | Recombinant Adeno-Associated Virus |
| TRAMP | Transgenic Adenocarcinoma Mouse Prostate |
| AP | Anterior Prostate |
| DLP | Dorsal Lateral Prostate |
| WT | Wild Type |
| PIN | Prostatic Intraepithelial Neoplasia |
| FR | Folate Receptor |
| PSMA | Prostate-Specific Membrane Antigen |
| FOLH1 | Folate Hydrolase 1 |
| PET | Positron Emission Tomography |
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.G. Understanding and targeting prostate cancer cell heterogeneity and plasticity. Semin. Cancer Biol. 2021, 82, 68–93. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.G. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012, 22, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The Rosetta Stone of Therapy Resistance. Cancer Cell 2020, 37, 471–484. [Google Scholar] [CrossRef]
- Li, Q.; Deng, Q.; Chao, H.-P.; Liu, X.; Lu, Y.; Lin, K.; Liu, B.; Tang, G.W.; Zhang, D.; Tracz, A.; et al. Linking prostate cancer cell AR heterogeneity to distinct castration and enzalutamide responses. Nat. Commun. 2018, 9, 3600. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Rycaj, K.; Chao, H.-P.; Deng, Q.; Jeter, C.; Liu, C.; Honorio, S.; Li, H.; Davis, T.; et al. Systematic dissection of phenotypic, functional, and tumorigenic heterogeneity of human prostate cancer cells. Oncotarget 2015, 6, 23959–23986. [Google Scholar] [CrossRef]
- Qin, J.; Liu, X.; Laffin, B.; Chen, X.; Choy, G.; Jeter, C.R.; Calhoun-Davis, T.; Li, H.; Palapattu, G.S.; Pang, S.; et al. The PSA(-/lo) prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration. Cell Stem Cell 2012, 10, 556–569. [Google Scholar] [CrossRef]
- Liu, X.; Li, W.; Puzanov, I.; Goodrich, D.W.; Chatta, G.; Tang, D.G. Prostate cancer as a dedifferentiated organ: Androgen receptor, cancer stem cells, and cancer stemness. Essays Biochem. 2022, 66, 291–303. [Google Scholar] [CrossRef]
- Lionetti, M.; Musto, P.; Di Martino, M.T.; Fabris, S.; Agnelli, L.; Todoerti, K.; Tuana, G.; Mosca, L.; Cantafio, M.E.G.; Grieco, V.; et al. Biological and Clinical Relevance of miRNA Expression Signatures in Primary Plasma Cell Leukemia. Clin. Cancer Res. 2013, 19, 3130–3142. [Google Scholar] [CrossRef]
- Ling, H.; Fabbri, M.; Calin, G.A. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat. Rev. Drug Discov. 2013, 12, 847–865. [Google Scholar] [CrossRef]
- Garzon, R.; Marcucci, G.; Croce, C.M. Targeting microRNAs in cancer: Rationale, strategies and challenges. Nat. Rev. Drug Discov. 2010, 9, 775–789. [Google Scholar] [CrossRef]
- Bader, A.G. MiR-34—A microRNA replacement therapy is headed to the clinic. Front. Genet. 2012, 3, 120. [Google Scholar] [CrossRef]
- Li, Q.; Liu, B.; Chao, H.P.; Ji, Y.; Lu, Y.; Mehmood, R.; Jeter, C.; Chen, T.; Moore, J.R.; Li, W.; et al. LRIG1 is a pleiotropic androgen receptor-regulated feedback tumor suppressor in prostate cancer. Nat. Commun. 2019, 10, 5494. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Liu, R.; Kasinski, A.L.; Shen, H.; Slack, F.J.; Tang, D.G. MicroRNA-34a: Potent Tumor Suppressor, Cancer Stem Cell Inhibitor, and Potential Anticancer Therapeutic. Front. Cell Dev. Biol. 2021, 9, 640587. [Google Scholar] [CrossRef]
- Liu, C.; Kelnar, K.; Liu, B.; Chen, X.; Calhoun-Davis, T.; Li, H.; Patrawala, L.; Yan, H.; Jeter, C.; Honorio, S.; et al. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011, 17, 211–215. [Google Scholar] [CrossRef]
- He, L.; He, X.; Lim, L.P.; de Stanchina, E.; Xuan, Z.; Liang, Y.; Xue, W.; Zender, L.; Magnus, J.; Ridzon, D.; et al. A microRNA component of the p53 tumour suppressor network. Nature 2007, 447, 1130–1134. [Google Scholar] [CrossRef]
- Chang, T.C.; Wentzel, E.A.; Kent, O.A.; Ramachandran, K.; Mullendore, M.; Lee, K.H.; Feldmann, G.; Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J.; et al. Transactivation of miR-34a by p53 broadly influences gene expression and promotes apoptosis. Mol. Cell 2007, 26, 745–752. [Google Scholar] [CrossRef]
- Raver-Shapira, N.; Marciano, E.; Meiri, E.; Spector, Y.; Rosenfeld, N.; Moskovits, N.; Bentwich, Z.; Oren, M. Transcriptional activation of miR-34a contributes to p53-mediated apoptosis. Mol. Cell 2007, 26, 731–743. [Google Scholar] [CrossRef]
- Okada, N.; Lin, C.P.; Ribeiro, M.C.; Biton, A.; Lai, G.; He, X.; Bu, P.; Vogel, H.; Jablons, D.M.; Keller, A.C. A positive feedback between p53 and miR-34 miRNAs mediates tumor suppression. Genes Dev. 2014, 28, 438–450. [Google Scholar] [CrossRef]
- Shen, M.M.; Abate-Shen, C. Molecular genetics of prostate cancer: New prospects for old challenges. Genes Dev. 2010, 24, 1967–2000. [Google Scholar] [CrossRef]
- Cotter, K.; Rubin, M.A. The evolving landscape of prostate cancer somatic mutations. Prostate 2022, 82, S13–S24. [Google Scholar] [CrossRef]
- Hamid, A.A.; Gray, K.P.; Shaw, G.; MacConaill, L.E.; Evan, C.; Bernard, B.; Loda, M.; Corcoran, N.M.; Van Allen, E.M.; Choudhury, A.D.; et al. Compound Genomic Alterations of TP53, PTEN, and RB1 Tumor Suppressors in Localized and Metastatic Prostate Cancer. Eur. Urol. 2019, 76, 89–97. [Google Scholar] [CrossRef]
- Mateo, J.; Seed, G.; Bertan, C.; Rescigno, P.; Dolling, D.; Figueiredo, I.; Miranda, S.; Nava Rodrigues, D.; Gurel, B.; Clarke, M.; et al. Genomics of lethal prostate cancer at diagnosis and castration resistance. J. Clin. Investig. 2020, 130, 1743–1751. [Google Scholar] [CrossRef]
- Teroerde, M.; Nientiedt, C.; Duensing, A.; Hohenfellner, M.; Stenzinger, A.; Duensing, S. Revisiting the Role of p53 in Prostate Cancer. In Prostate Cancer; Bott, S.R.J., Ng, K.L., Eds.; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- Fujita, Y.; Kojima, K.; Hamada, N.; Ohhashi, R.; Akao, Y.; Nozawa, Y.; Deguchi, T.; Ito, M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem. Biophys. Res. Commun. 2008, 377, 114–119. [Google Scholar] [CrossRef]
- Lodygin, D.; Tarasov, V.; Epanchintsev, A.; Berking, C.; Knyazeva, T.; Körner, H.; Knyazev, P.; Diebold, J.; Hermeking, H. Inactivation of miR-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle 2008, 7, 2591–2600. [Google Scholar] [CrossRef]
- Concepcion, C.P.; Han, Y.-C.; Mu, P.; Bonetti, C.; Yao, E.; D’Andrea, A.; Vidigal, J.A.; Maughan, W.P.; Ogrodowski, P.; Ventura, A. Intact p53-dependent responses in miR-34-deficient mice. PLoS Genet. 2012, 8, e1002797. [Google Scholar] [CrossRef]
- Chang, T.C.; Yu, D.; Lee, Y.-S.; Wentzel, E.A.; Arking, D.E.; West, K.M.; Dang, C.V.; Thomas-Tikhonenko, A.; Mendell, J.T. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat. Genet. 2008, 40, 43–50. [Google Scholar] [CrossRef]
- Craig, V.J.; Cogliatti, S.B.; Imig, J.; Renner, C.; Neuenschwander, S.; Rehrauer, H.; Schlapbach, R.; Dirnhofer, S.; Tzankov, A.; Müller, A. Myc-mediated repression of microRNA-34a promotes high-grade transformation of B-cell lymphoma by dysregulation of FoxP1. Blood 2011, 117, 6227–6236. [Google Scholar] [CrossRef]
- Armenia, J.; Wankowicz, S.A.M.; Liu, D.; Gao, J.; Kundra, R.; Reznik, E.; Chatila, W.K.; Chakravarty, D.; Han, G.C.; Coleman, I.; et al. The long tail of oncogenic drivers in prostate cancer. Nat. Genet. 2018, 50, 645–651. [Google Scholar] [CrossRef]
- Rokhlin, O.W.; Scheinker, V.S.; Taghiyev, A.F.; Bumcrot, D.; Glover, R.A.; Cohen, M.B. MicroRNA-34 mediates AR-dependent p53-induced apoptosis in prostate cancer. Cancer Biol. Ther. 2008, 7, 1288–1296. [Google Scholar] [CrossRef]
- Ding, M.; Lin, B.; Li, T.; Liu, Y.; Li, Y.; Zhou, X.; Miao, M.; Gu, J.; Pan, H.; Yang, F.; et al. A dual yet opposite growth-regulating function of miR-204 and its target XRN1 in prostate adenocarcinoma cells and neuroendocrine-like prostate cancer cells. Oncotarget 2015, 6, 7686–7700. [Google Scholar] [CrossRef]
- Ostling, P.; Leivonen, S.-K.; Aakula, A.; Kohonen, P.; Mäkelä, R.; Hagman, Z.; Edsjö, A.; Kangaspeska, S.; Edgren, H.; Nicorici, D.; et al. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 2011, 71, 1956–1967. [Google Scholar] [CrossRef] [PubMed]
- Rokavec, M.; Öner, M.G.; Li, H.; Jackstadt, R.; Jiang, L.; Lodygin, D.; Kaller, M.; Horst, D.; Ziegler, P.K.; Schwitalla, S.; et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J. Clin. Investig. 2014, 124, 1853–1867. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Xiao, Y.; Hu, Y.; Xiao, Y.; Yin, Z.; Liu, L.; Kang, X.; Chen, Y. Methylation-induced silencing of miR-34a enhances chemoresistance by directly upregulating ATG4B-induced autophagy through AMPK/mTOR pathway in prostate cancer. Oncol. Rep. 2016, 35, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Bu, P.; Ai, Y.; Srinivasan, T.; Chen, H.J.; Xiang, K.; Lipkin, S.M.; Shen, X. A long non-coding RNA targets microRNA miR-34a to regulate colon cancer stem cell asymmetric division. eLife 2016, 5, e14620. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Heath, E.; Chen, W.; Cher, M.; Powell, I.; Heilbrun, L.; Li, Y.; Ali, S.; Sethi, S.; Hassan, O.; et al. Epigenetic silencing of miR-34a in human prostate cancer cells and tumor tissue specimens can be reversed by BR-DIM treatment. Am. J. Transl. Res. 2012, 4, 14–23. [Google Scholar] [PubMed]
- Nanni, S.; Aiello, A.; Re, A.; Guffanti, A.; Benvenuti, V.; Colussi, C.; Castro-Vega, L.J.; Felsani, A.; Londono-Vallejo, A.; Capogrossi, M.C.; et al. Estrogen-dependent dynamic profile of eNOS-DNA associations in prostate cancer. PLoS ONE 2013, 8, e62522. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lin, C.-P.; Ho, J.J.; He, X.; Okada, N.; Bu, P.; Zhong, Y.; Kim, S.Y.; Bennett, M.J.; Chen, C.; et al. MiR-34 miRNAs provide a barrier for somatic cell reprogramming. Nat. Cell Biol. 2011, 13, 1353–1360. [Google Scholar] [CrossRef]
- Wang, L.; Wang, E.; Wang, Y.; Mines, R.; Xiang, K.; Sun, Z.; Zhou, G.; Chen, K.-Y.; Rakhilin, N.; Chao, S.; et al. MiR-34a is a microRNA safeguard for Citrobacter-induced inflammatory colon oncogenesis. eLife 2018, 7, e39479. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Hwang, C.-I.; Corney, D.C.; Flesken-Nikitin, A.; Jiang, L.; Öner, G.M.; Munroe, R.J.; Schimenti, J.C.; Hermeking, H.; Nikitin, A.Y. MiR-34 cooperates with p53 in suppression of prostate cancer by joint regulation of stem cell compartment. Cell Rep. 2014, 6, 1000–1007. [Google Scholar] [CrossRef]
- Liu, C.; Kelnar, K.; Vlassov, A.V.; Brown, D.; Wang, J.; Tang, D.G. Distinct microRNA expression profiles in prostate cancer stem/progenitor cells and tumor-suppressive functions of let-7. Cancer Res. 2012, 72, 3393–3404. [Google Scholar] [CrossRef]
- Boccaccio, C.; Comoglio, P.M. Comoglio, Invasive growth: A MET-driven genetic programme for cancer and stem cells. Nat. Rev. Cancer 2006, 6, 637–645. [Google Scholar] [CrossRef]
- Rajasekhar, V.K.; Studer, L.; Gerald, W.; Socci, N.D.; Scher, H.I. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-kappaB signalling. Nat. Commun. 2011, 2, 162. [Google Scholar] [CrossRef]
- Corney, D.C.; Hwang, C.-I.; Matoso, A.; Vogt, M.; Flesken-Nikitin, A.; Godwin, A.K.; Kamat, A.A.; Sood, A.K.; Ellenson, L.H.; Hermeking, H.; et al. Frequent downregulation of miR-34 family in human ovarian cancers. Clin. Cancer Res. 2010, 16, 1119–1128. [Google Scholar] [CrossRef]
- Hwang, C.I.; Matoso, A.; Corney, D.C.; Flesken-Nikitin, A.; Körner, S.; Wang, W.; Boccaccio, C.; Thorgeirsson, S.S.; Comoglio, P.M.; Hermeking, H.; et al. Wild-type p53 controls cell motility and invasion by dual regulation of MET expression. Proc. Natl. Acad. Sci. USA 2011, 108, 14240–14245. [Google Scholar] [CrossRef]
- Gaur, S.; Wen, Y.; Song, J.H.; Parikh, N.U.; Mangala, L.S.; Blessing, A.M.; Ivan, C.; Wu, S.Y.; Varkaris, A.; Shi, Y.; et al. Chitosan nanoparticle-mediated delivery of miRNA-34a decreases prostate tumor growth in the bone and its expression induces non-canonical autophagy. Oncotarget 2015, 6, 29161–29177. [Google Scholar] [CrossRef]
- Yamamura, S.; Saini, S.; Majid, S.; Hirata, H.; Ueno, K.; Deng, G.; Dahiya, R. MicroRNA-34a modulates c-Myc transcriptional complexes to suppress malignancy in human prostate cancer cells. PLoS ONE 2012, 7, e29722. [Google Scholar] [CrossRef]
- Zhang, G.; Tian, X.; Li, Y.; Wang, Z.; Li, X.; Zhu, C. MiR-27b and miR-34a enhance docetaxel sensitivity of prostate cancer cells through inhibiting epithelial-to-mesenchymal transition by targeting ZEB1. Biomed. Pharm. 2018, 97, 736–744. [Google Scholar] [CrossRef]
- Chakravarthi, B.; Chandrashekar, D.S.; Agarwal, S.; Balasubramanya, S.A.H.; Pathi, S.S.; Goswami, M.T.; Jing, X.; Wang, R.; Mehra, R.; Asangani, I.A.; et al. miR-34a Regulates Expression of the Stathmin-1 Oncoprotein and Prostate Cancer Progression. Mol. Cancer Res. 2018, 16, 1125–1137. [Google Scholar] [CrossRef]
- Civenni, G.; Malek, A.; Albino, D.; Garcia-Escudero, R.; Napoli, S.; Di Marco, S.; Pinton, S.; Sarti, M.; Carbone, G.M.; Catapano, C.V. RNAi-mediated silencing of Myc transcription inhibits stem-like cell maintenance and tumorigenicity in prostate cancer. Cancer Res. 2013, 73, 6816–6827. [Google Scholar] [CrossRef]
- Dong, B.; Xu, G.C.; Liu, S.T.; Liu, T.; Geng, B. MiR-34a affects G2 arrest in prostate cancer PC3 cells via Wnt pathway and inhibits cell growth and migration. Eur. Rev. Med. Pharm. Sci. 2020, 24, 8349–8358. [Google Scholar]
- Chen, W.Y.; Liu, S.-Y.; Chang, Y.-S.; Yin, J.J.; Yeh, H.-L.; Mouhieddine, T.H.; Hadadeh, O.; Abou-Kheir, W.; Liu, Y.-N. MicroRNA-34a regulates WNT/TCF7 signaling and inhibits bone metastasis in Ras-activated prostate cancer. Oncotarget 2015, 6, 441–457. [Google Scholar] [CrossRef]
- Nguyen, D.X.; Chiang, A.C.; Zhang, X.H.F.; Kim, J.Y.; Kris, M.G.; Ladanyi, M.; Gerald, W.L.; Massagué, J. WNT/TCF Signaling through LEF1 and HOXB9 Mediates Lung Adenocarcinoma Metastasis. Cell 2009, 138, 51–62. [Google Scholar] [CrossRef]
- Liang, J.; Li, Y.; Daniels, G.; Sfanos, K.; De Marzo, A.; Wei, J.; Li, X.; Chen, W.; Wang, J.; Zhong, X.; et al. LEF1 Targeting EMT in Prostate Cancer Invasion Is Regulated by miR-34a. Mol. Cancer Res. 2015, 13, 681–688. [Google Scholar] [CrossRef]
- Farah, E.; Li, C.; Cheng, L.; Kong, Y.; Lanman, N.A.; Pascuzzi, P.; Lorenz, G.R.; Zhang, Y.; Ahmad, N.; Li, L.; et al. NOTCH signaling is activated in and contributes to resistance in enzalutamide-resistant prostate cancer cells. J. Biol. Chem. 2019, 294, 8543–8554. [Google Scholar] [CrossRef]
- Liu, X.; Luo, X.; Wu, Y.; Xia, D.; Chen, W.; Fang, Z.; Deng, J.; Hao, Y.; Yang, X.; Zhang, T.; et al. MicroRNA-34a Attenuates Paclitaxel Resistance in Prostate Cancer Cells via Direct Suppression of JAG1/Notch1 Axis. Cell. Physiol. Biochem. 2018, 50, 261–276. [Google Scholar] [CrossRef]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef]
- Liu, T.F.; McCall, C.E. Deacetylation by SIRT1 Reprograms Inflammation and Cancer. Genes Cancer 2013, 4, 135–147. [Google Scholar] [CrossRef]
- Kojima, K.; Fujita, Y.; Nozawa, Y.; Deguchi, T.; Ito, M. MiR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate 2010, 70, 1501–1512. [Google Scholar] [CrossRef]
- Wen, D.; Peng, Y.; Lin, F.; Singh, R.K.; Mahato, R.I. Micellar Delivery of miR-34a Modulator Rubone and Paclitaxel in Resistant Prostate Cancer. Cancer Res. 2017, 77, 3244–3254. [Google Scholar] [CrossRef]
- Corcoran, C.; Rani, S.; O’Driscoll, L. miR-34a is an intracellular and exosomal predictive biomarker for response to docetaxel with clinical relevance to prostate cancer progression. Prostate 2014, 74, 1320–1334. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Ozpolat, B.; Sood, A.K.; Lopez-Berestein, G. Liposomal siRNA nanocarriers for cancer therapy. Adv. Drug Deliv. Rev. 2014, 66, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Orellana, E.A.; Tenneti, S.; Rangasamy, L.; Lyle, L.T.; Low, P.S.; Kasinski, A.L. FolamiRs: Ligand-targeted, vehicle-free delivery of microRNAs for the treatment of cancer. Sci. Transl. Med. 2017, 9, eaam9327. [Google Scholar] [CrossRef]
- Abdelaal, A.M.; Kasinski, A.L. Ligand-mediated delivery of RNAi-based therapeutics for the treatment of oncological diseases. NAR Cancer 2021, 3, zcab030. [Google Scholar] [CrossRef]
- Beg, M.S.; Brenner, A.J.; Sachdev, J.; Borad, M.; Kang, Y.-K.; Stoudemire, J.; Smith, S.; Bader, A.G.; Kim, S.; Hong, D.S. Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investig. New Drugs 2017, 35, 180–188. [Google Scholar] [CrossRef]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 12, 1630–1637. [Google Scholar] [CrossRef]
- Wiggins, J.F.; Ruffino, L.; Kelnar, K.; Omotola, M.; Patrawala, L.; Brown, D.; Bader, A.G. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010, 70, 5923–5930. [Google Scholar] [CrossRef]
- Daige, C.L.; Wiggins, J.F.; Priddy, L.; Nelligan-Davis, T.; Zhao, J.; Brown, D. Systemic delivery of a miR34a mimic as a potential therapeutic for liver cancer. Mol. Cancer Ther. 2014, 13, 2352–2360. [Google Scholar] [CrossRef]
- Cortez, M.A.; Ivan, C.; Valdecanas, D.; Wang, X.; Peltier, H.J.; Ye, Y.; Araujo, L.; Carbone, D.P.; Shilo, K.; Giri, D.K.; et al. PDL1 Regulation by p53 via miR-34. J. Natl. Cancer Inst. 2016, 108, djv303. [Google Scholar]
- Kasinski, A.L.; Kelnar, K.; Stahlhut, C.; Orellana, E.; Zhao, J.; Shimer, E.; Dysart, S.; Chen, X.; Bader, A.G.; Slack, F.J. A combinatorial microRNA therapeutics approach to suppressing non-small cell lung cancer. Oncogene 2015, 34, 3547–3555. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, X.; Zhang, X.; Liu, B.; Huang, L. Nanoparticles modified with tumor-targeting scFv deliver siRNA and miRNA for cancer therapy. Mol. Ther. 2010, 18, 1650–1656. [Google Scholar] [CrossRef]
- Trang, P.; Wiggins, J.F.; Daige, C.L.; Cho, C.; Omotola, M.; Brown, D.; Weidhaas, J.B.; Bader, A.G.; Slack, F.J. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther. 2011, 19, 1116–1122. [Google Scholar] [CrossRef]
- Craig, V.J.; Tzankov, A.; Flori, M.; Schmid, C.; Bader, A.G.; Müller, A. Systemic microRNA-34a delivery induces apoptosis and abrogates growth of diffuse large B-cell lymphoma in vivo. Leukemia 2012, 26, 2421–2424. [Google Scholar] [CrossRef]
- Hu, Q.L.; Jiang, Q.Y.; Jin, X.; Shen, J.; Wang, K.; Li, Y.B.; Xu, F.J.; Tang, G.P.; Li, Z.H. Cationic microRNA-delivering nanovectors with bifunctional peptides for efficient treatment of PANC-1 xenograft model. Biomaterials 2013, 34, 2265–2276. [Google Scholar] [CrossRef]
- Lin, X.; Chen, W.; Wei, F.; Zhou, B.P.; Hung, M.-C.; Xie, X. Nanoparticle Delivery of miR-34a Eradicates Long-term-cultured Breast Cancer Stem Cells via Targeting C22ORF28 Directly. Theranostics 2017, 7, 4805–4824. [Google Scholar] [CrossRef]
- Gibori, H.; Eliyahu, S.; Krivitsky, A.; Ben-Shushan, D.; Epshtein, Y.; Tiram, G.; Blau, R.; Ofek, P.; Lee, J.S.; Ruppin, E.; et al. Amphiphilic nanocarrier-induced modulation of PLK1 and miR-34a leads to improved therapeutic response in pancreatic cancer. Nat. Commun. 2018, 9, 16. [Google Scholar] [CrossRef]
- Jiang, G.; Chen, H.; Huang, J.; Song, Q.; Chen, Y.; Gu, X.; Jiang, Z.; Huang, Y.; Lin, Y.; Feng, J.; et al. Tailored Lipoprotein-Like miRNA Delivery Nanostructure Suppresses Glioma Stemness and Drug Resistance through Receptor-Stimulated Macropinocytosis. Adv. Sci. 2020, 7, 1903290. [Google Scholar] [CrossRef]
- Lu, C.; Han, H.D.; Mangala, L.S.; Ali-Fehmi, R.; Newton, C.S.; Ozbun, L.; Armaiz-Pena, G.N.; Hu, W.; Stone, R.L.; Munkarah, A.; et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell 2010, 18, 185–197. [Google Scholar] [CrossRef]
- Han, H.D.; Mangala, L.S.; Lee, J.W.; Shahzad, M.M.; Kim, H.S.; Shen, D.; Nam, E.J.; Mora, E.M.; Stone, R.L.; Lu, C.; et al. Targeted gene silencing using RGD-labeled chitosan nanoparticles. Clin. Cancer Res. 2010, 16, 3910–3922. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, C.; Tao, Y.; Zou, P.; Gao, F.; Jia, C.; Liu, L.; Li, G.; Zhang, G.; Duan, Y.; et al. Ultrasound-Induced Microbubble Cavitation Combined with miR-34a-Loaded Nanoparticles for the Treatment of Castration-Resistant Prostate Cancer. J. Biomed. Nanotechnol. 2021, 17, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Paliwal, S.; Mitragotri, S. Ultrasound-induced cavitation: Applications in drug and gene delivery. Expert Opin. Drug Deliv. 2006, 3, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Liu, J.; Wu, X.; Tai, Z.; Gao, Y.; Zhu, Q.; Li, J.; Zhang, L.; Hu, C.; Gu, F.; et al. Reducible self-assembling cationic polypeptide-based micelles mediate co-delivery of doxorubicin and microRNA-34a for androgen-independent prostate cancer therapy. J. Control. Release 2016, 232, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Wen, D.; Wang, X.; Mahato, R.I. Dual responsive micelles capable of modulating miRNA-34a to combat taxane resistance in prostate cancer. Biomaterials 2019, 192, 95–108. [Google Scholar] [CrossRef]
- Ai, J.; Li, J.; Su, Q.; Ma, H.; He, R.; Wei, Q.; Li, H.; Gao, G. rAAV-based and intraprostatically delivered miR-34a therapeutics for efficient inhibition of prostate cancer progression. Gene Ther. 2021, 29, 418–424. [Google Scholar] [CrossRef]
- Bainbridge, J.W.; Mehat, M.S.; Sundaram, V.; Robbie, S.J.; Barker, S.E.; Ripamonti, C.; Georgiadis, A.; Mowat, F.M.; Beattie, S.G.; Gardner, P.J.; et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N. Engl. J. Med. 2015, 372, 1887–1897. [Google Scholar] [CrossRef]
- Leamon, C.; Low, P.S. Folate-mediated targeting: From diagnostics to drug and gene delivery. Drug Discov. Today 2001, 6, 44–51. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M.; Wang, C.Y.; Haas, G.P. Expression of prostate-specific membrane antigen in normal and malignant human tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef]
- Kularatne, S.A.; Wang, K.; Santhapuram, H.-K.R.; Low, P.S. Prostate-Specific Membrane Antigen Targeted Imaging and Therapy of Prostate Cancer Using a PSMA Inhibitor as a Homing Ligand. Mol. Pharm. 2009, 6, 780–789. [Google Scholar] [CrossRef]
- Bravaccini, S.; Puccetti, M.; Bocchini, M.; Ravaioli, S.; Celli, M.; Scarpi, E.; De Giorgi, U.; Tumedei, M.M.; Raulli, G.; Cardinale, L.; et al. PSMA expression: A potential ally for the pathologist in prostate cancer diagnosis. Sci. Rep. 2018, 8, 4254. [Google Scholar] [CrossRef]
- Sheehan, B.; Guo, C.; Neeb, A.; Paschalis, A.; Sandhu, S.; de Bono, J.S. Prostate-specific Membrane Antigen Biology in Lethal Prostate Cancer and its Therapeutic Implications. Eur. Urol. Focus 2021. [Google Scholar] [CrossRef]
- Wang, F.; Li, Z.; Feng, X.; Yang, D.; Lin, M. Advances in PSMA-targeted therapy for prostate cancer. Prostate Cancer Prostatic Dis. 2022, 25, 11–26. [Google Scholar] [CrossRef]
- Thomas, M.; Kularatne, S.A.; Qi, L.; Kleindl, P.; Leamon, C.P.; Hansen, M.J.; Low, P.S. Ligand-targeted delivery of small interfering RNAs to malignant cells and tissues. Ann. N. Y. Acad. Sci. 2009, 1175, 32–39. [Google Scholar] [CrossRef]
- Maurer, T.; Eiber, M.; Schwaiger, M.; Gschwend, J.E. Current use of PSMA–PET in prostate cancer management. Nat. Rev. Urol. 2016, 13, 226–235. [Google Scholar] [CrossRef]
- Staniszewska, M.; Costa, P.F.; Eiber, M.; Klose, J.; Wosniack, J.; Reis, H.; Szarvas, T.; Hadaschik, B.; Lückerath, K.; Herrmann, K.; et al. Enzalutamide Enhances PSMA Expression of PSMA-Low Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 7431. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).