Simple Summary
Improving outcomes in pancreatic cancer is achievable through the accumulation of marginal gains. There exists evidence of variation and undertreatment in many areas of the care pathway. By fully realising the existing opportunities, there is the potential for immediate improvements in outcomes and quality of life.
Abstract
Improving outcomes among patients with resectable pancreatic cancer is one of the greatest challenges of modern medicine. Major improvements in survival will result from the development of novel therapies. However, optimising existing pathways, so that patients realise benefits of already proven treatments, presents a clear opportunity to improve outcomes in the short term. This narrative review will focus on treatments and interventions where there is a clear evidence base to improve outcomes in pancreatic cancer, and where there is also evidence of variation and under-treatment. Avoidance of preoperative biliary drainage, treatment of pancreatic exocrine insufficiency, prehabiliation and enhanced recovery after surgery, reducing perioperative complications, optimising opportunities for elderly patients to receive therapy, optimising adjuvant chemotherapy and regular surveillance after surgery are some of the strategies discussed. Each treatment or pathway change represents an opportunity for marginal gain. Accumulation of marginal gains can result in considerable benefit to patients. Given that these interventions already have evidence base, they can be realised quickly and economically.
1. Introduction
Pancreatic cancer is projected to become the second leading cause of all cancer-related deaths by 2030 [1,2]. Although surgical resection is the foundation of ‘resectable’ pancreatic cancer management, alone it is associated with a less than 10% chance of cure [3]. High rates of perioperative morbidity and mortality, slow or poorly organised pathways to surgery, suboptimal preoperative management of jaundice, suboptimal use of (neo)adjuvant therapy and failure to address malnutrition all contribute to poor outcomes. It is therefore unsurprising that there can be a negative attitude and feelings of nihilism when considering the outlook for these patients.
Wide variations in care and a lack of standardised practice, however, offer an easy way to improve outcomes in the near future. Pathways to expedite surgery, prescribing of enzyme therapy, access to adjuvant chemotherapy and tackling frailty are just a few examples of how care has the potential to be optimised. Optimising an individual aspect of care represents an opportunity for a marginal gain. Individually, each gain realised may be relatively minor when observed across an entire patient cohort, but when multiple, the effect of aggregated marginal gains can be considerable. The aggregation of marginal gains was popularised in elite level cycling where its success was clear to see at Olympic level and major events such as the Tour de France. The principals have been adopted in healthcare among various patient populations from cancer surgery, stroke recovery, prehabilitation, cardiac surgery and anaesthesia [4,5,6]. This narrative review highlights common failings in the care of pancreatic cancer patients and describes where gains can be made. The aggregation of these marginal gains could improve outcomes and experience for a great many patients with resectable pancreatic cancer.
2. Pre-Operative Pathways
At presentation, resectable pancreatic cancer is in an exponential phase of growth and most primary tumours harbour cells that can metastasise [7]. Enhancing pre-operative pathways to ensure that patients are treated as quickly as possible and their functional status is optimised is essential to enabling the delivery of the highest standard of care. This section will focus on avoidance of pre-operative biliary drainage, prehabilitation, and optimisation of nutritional status.
2.1. Preoperative Biliary Drainage in Resectable Pancreatic Cancer
The majority of patients with resectable pancreatic cancer present with jaundice. Historically reluctance to operate in the presence of jaundice was related to concerns over renal, cardiac and liver dysfunction, coagulopathy and, at the time, high rates of perioperative morbidity and mortality associated with pancreatic surgery in the absence of jaundice [8].
Hence, in theory, relief of jaundice by preoperative biliary drainage (PBD) is perceived to improve these disturbances and prevent postoperative complications, though in practice, the role and propriety of PBD must be challenged.
The concept of correcting jaundice prior to resection was introduced by A.O. Whipple through staged PD, initially a cholecystogastrostomy to relieve jaundice followed by resection once the jaundice was within ‘safe’ limits. This further developed into nonoperative approaches through percutaneous transhepatic cholangiopathy (PTC) and biliary drainage in the 1960s and later followed by endoscopic retrograde cholangiopancreatography (ERCP) in the 1970s [9,10].
For many decades ERCP served both diagnostic and therapeutic purposes. However, the sensitivity and specificity of CT to diagnose pancreatic cancer has made ERCP, as a diagnostic tool, obsolete.
The main drawback of PBD is the associated rate of complications. These complications impair quality of life, can delay or prevent future surgery and are occasionally fatal. PBD itself requires resource and invariably delays treatment pathways [11,12].
The DROP trial randomised patients to upfront early surgery within 7 days or PBD-and plastic stent and delayed surgery between 4–6 weeks [13]. This landmark RCT showed the rate of serious complications at 120 days postoperatively to be far higher in the PBD group (74 vs. 39%, p < 0.001). This was largely due to drainage-related complications, cholangitis (26 vs. 2%), pancreatitis (7 vs. 0%) along with the need for change of stent in 30% of patients. There are also higher rates of perioperative infections, associated with changes in the biliary microbiome related to PBD [14,15] It is therefore advised that intraoperative bile cultures are taken and appropriate antibiotic administered to address this higher rate of complications [16].
Self-expanding metal stents (SEMS) have gained in popularity over plastic stents based on significantly greater patency and reduction in infectious complications [13,17,18]. The experience of patients undergoing PBD with fully covered SEMS, with a prospective study which mirrored the protocol of the DROP trial, superimposed the complication rates of PBD-SEMS over the cohorts within the DROP trial [19]. Though SEMS were associated with lower rates of complications than plastic stents, the rate remained significantly greater than upfront surgery (51 vs. 39%). Subsequent meta-analysis confirms advantages of metal over plastic stents with reduced rates of need for endoscopic reintervention (OR0.3), preoperative complications (OR 0.42) and cholangitis (OR 0.09) [20]. However, pancreatitis is more common (OR 3.6) and, though not reported, post hoc analysis of resection rates demonstrates a lower resection rate among patients with metal stents (plastic, 147/190 vs. metal 93/139; Chi square p = 0.04) [19,20]. A more recent network meta-analysis demonstrated a remarkably high rate of complications with plastic stents (38–93%), a much lower rate with SEMS (0–15%) whilst data regarding outcomes from percutaneous drainage is not at extensively reported (31%) [21]. This study also concluded that avoidance of PBD was associated with the best outcome.
The association between PBD and reduced resection rates was not observed in individual randomised trials. However, it is logical to associate complications such as pancreatitis or pathway delays with a reduction in resection rate. Many high-volume centres have reported that increasing time to surgery reduces resection rates, data confirmed within a recent systematic review and meta-analysis [22,23,24,25]. It may be that this higher resection rate and avoidance of complications translates into increased survival benefit, when analysed on an intention to treat basis [26]. This is controversial as some authors have associated hyperbilirbuinaemia at the time of resection with worse cancer outcomes [27].
Given the many benefits of avoiding PBD, the National Institute for Health and Care Excellence (NICE) in the United Kingdom, now recommend upfront surgery without PBD where possible [28].
What remains to be defined is a safe upper limit of bilirubin in terms of safety and oncologic outcomes and why PBD remains so widely used despite the evidence of harm. Associated venous resection appears safe in the presence of jaundice, even when levels of bilirubin exceed 300 µmol/L (>17.5 mg/dL) [29]. It may be assumed that elderly patients are safer to undergo PBD and surgery rather than upfront surgery but elderly patients are less likely to tolerate complications of PBD and remain on a surgical pathway than younger patients.
Numerous studies have established seemingly arbitrary upper limits of bilirubin at which PBD is indicated [27,30,31,32,33,34,35]. A common threshold of >250 umol/L is used and cited by The Guidelines for Perioperative Care for Pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society Recommendations [36]; Both Sauvanet and Li et al. reported higher complication rates when surgery was performed with a bilirubin of >300 umol/L [27,37]. However, contrary to this, van der Gaag, quoted a bilirubin level of ≤300 μmol/L at surgery and demonstrated significantly lower infective complications, whilst Pamecha et al. showed that a bilirubin level of ≥15 mg/dL (≥265 μmol/L) was not an independent risk factor for complications [13,38,39].
As stated above, it is the authors experience that surgery, even with venous resection, can be performed with no difference in complications with bilirubin in excess of 300 μmol/L. To summarise, there is no clear data that defines an upper limit of bilirubin at which PBD is indicated. The authors recommend an approach with prospective evaluation of outcomes and a step wise increase in threshold of bilirubin at which to undertake PBD. Variation in the rate of increase of bilirubin, the difficulty/time needed to complete staging tests and other factors such as renal function or evidence of biliary sepsis mean that there is unlikely to be a single value which can be applied to all patients.
It is useful to consider when PBD is indicated. Jaundiced patients undergoing neoadjuvant therapy clearly require PBD; if there are major diagnostic delays, though an assessment of risk of cancer progression must be weighed up against potential risks of surgery in such cases or if the patient is too frail to undergo surgery. Such patients, however, in our experience rarely improve significantly after PBD. Cholangitis has been considered a contraindication for PBD, though in our practice we frequently employ external biliary drainage, antibiotics, fluid replacement therapy and surgery within the same week if markers of infection are improving. This has controlled sepsis, avoided complications of PBD and kept patients within an early surgery pathway. See Table 1. For a summary of potential indications for PBD and Table 2 for optimizing care should PBD be undertaken.

Table 1.
Absolute and relative indications for Percutaneous biliary drainage (PBD).

Table 2.
Tips for optimising care in the event of PBD requirement.
Malnutrition associated with obstructive jaundice and its effects on outcomes following surgery is a further area of controversy. In studies by Padillo et al., malnutrition was more commonly associated with patients older than 68 years and those with high levels of bilirubin with the suggestion that PBD should be considered to allow alleviation of these modifiable factors preoperatively [40,41]. However, evidence for the optimal duration of PBD to improve nutritional status is experimental and its role in malnutrition must be considered in the broader context of pancreatic exocrine insufficiency [42,43,44,45]. Exocrine insufficiency is prevalent at diagnosis, and although not the sole cause of malnutrition, it is a major driver of malnutrition and is poorly treated; thus, delays to surgery can exacerbate malnutrition if PEI is not addressed. A balance must be struck between early surgery and better treatment of PEI versus correcting jaundice and improving nutrition. A proposed optimal pathway would be to provide PERT for all patients and provide early surgery where possible; where a patient has a poor performance status with malnutrition, a pathway of PBD, PERT, and dietician input would be advocated.
2.2. Pancreatic Exocrine Insufficiency and Overcoming Malnutrition to Improve Outcomes
Pancreatic exocrine insufficiency (PEI) and pancreatic enzyme replacement therapy (PERT) should be considered at all stages of management of pancreatic cancer, it is included in this section on pre-operative pathways as correct treatment is essential as early in the care pathway as possible. PEI is far from trivial and should be considered as organ failure. Untreated failure of other organ systems can be rapidly fatal (for example, renal, cardiac or respiratory failure or even diabetes) and withholding treatment in these settings is reserved only for those patients who are on end-of-life pathways. Consequently, it is remarkable that PEI is underdiagnosed and undertreated. In the authors view, this single issue represents the simplest pathway improvement in this review and also the intervention with the chance to achieve most gain.
Addressing pancreatic exocrine insufficiency (PEI) can have an effect upon survival as strong as that as surgery or chemotherapy and yet many patients remains untreated [46]. A 2016 systematic review of PEI demonstrated a pre-operative prevalence of 44% and a post-operative prevalence of 74% (for those undergoing pancreatico-duodenectomy for malignancy). This is likely a conservative estimate owing to the frequent use of faecal elastase (FE-1) to diagnose PEI (FE-1 has been shown to underestimate PEI following resection) [47,48]. Furthermore, there is a gradual reduction of exocrine function at a median value of 10% per month [49]. With the gold standard of testing and longer term follow up, Lemaire et al. found the post-operative incidence of PEI to be 94% [50]. In resectable disease the mechanisms underlying PEI are complex and multifactorial. Almost all physiologic control is lost after pancreatoduodenectomy, and together with other factors result in insufficient enzymes arriving at the wrong time, to the wrong place and at the wrong pH for effective function [51,52] (see Figure 1).
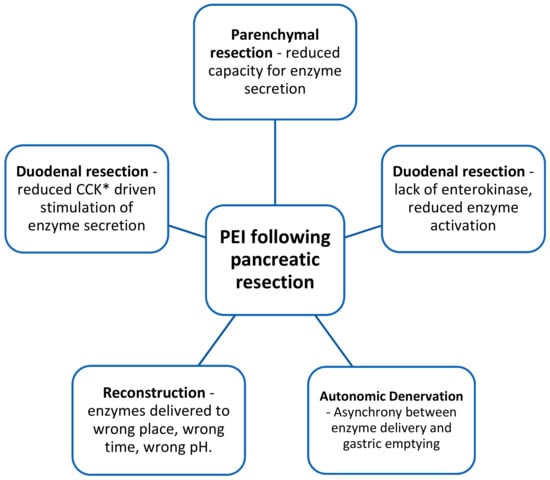
Figure 1.
Factors contributing to PEI following pancreatico-duodenectomy. *CCK = cholecystokinin.
Symptoms of PEI include pain, bloating, frequency, urgency, diarrhoea, fatty stool, flatulence, loss of appetite, nausea and vomiting and are physically and mentally distressing. The ‘classical’ sign of steathorrhoea is often absent, either because PEI is not severe enough or because the patient may have unconsciously adopted behaviours to avoid fat intake [53,54,55]. PEI is frequently untreated or undertreated worldwide. Studies from the UK, Europe and Australia demonstrate that only a minority of patients receive PERT [56,57,58,59]. Reasons for under prescribing are multifactorial and may relate to the lack of an acceptable or accurate routine diagnostic test that can yield results in near real time [47,60,61,62]. Confusing symptoms of cancer, weight loss and abdominal discomfort, further complicate diagnosis.
The consequences of PEI must not be underestimated, these include: weight loss, malnutrition, micronutrient deficiency, cardiovascular events, osteoporosis, fractures and sarcopenia [63]. The effect of PEI on operative outcomes is considerable, being associated with higher rates of post-operative complications, longer hospital stays and increased costs [64,65,66,67].
Pancreatic enzyme replacement therapy (PERT) is cheap, mitigates against weight loss and improves quality of life. The most compelling argument for PERT is emerging evidence that it improves survival [46,58,68,69,70,71]. Given the failings of diagnostic tests for PEI and the evident benefits of PERT, it is recommended for all patients with pancreatic cancer by NICE in the United Kingdom [28].
A healthy pancreas is estimated to produce 900,000 United States Pharmacopeia (USP) of lipase in response to a meal. Sufficient absorption of fat can be maintained at around 10% of normal capacity, there is thus a need for around 90,000 USP per meal. Given that in the majority of pancreatic disease some function remains an appropriate starting dose in resected pancreatic cancer is 75,000 USP with a main meal and 25,000 with a snack [69], most effective when given across the course of, or just after, a meal rather than before [72]. Co-prescription of a proton pump inhibitor is often required after pancreatoduodenectomy as a failure to neutralise gastric acid leads to enzymes remaining inactive [73].
Pre-operative malnutrition is associated with significantly poorer post-operative outcomes for pancreatic resection, addressing this extends beyond just the prescribing of PERT [74,75]. The international Study Group on Pancreatic Surgery (ISGPS) released a position paper on nutritional support and therapy in pancreatic surgery, this emphasizes the importance of pre-operative assessment of nutritional status and recommends nutritional supplements in those who have, or are at risk of developing, moderate malnutrition [76]. Those with, or at risk of severe malnutrition may benefit from formal nutritional support with enteral or parenteral feeding [76]. Regular dietician input to asses response, compliance, diet, diabetic optimization, and the potential need for nutritional supplements is required [77].
Although outside the scope of this article to describe in detail, immunonutrition is worthy of note. Immunonutrition aims to influence the systemic immune system using nutritional supplements with immune modulating contents such as arginine, omega-3 fatty acid and RNA. This is not yet part of routine clinical practice, however, there is evidence that initiation in the pre-operative period could improve post-operative outcomes [78,79,80,81]. The most recent systematic review and meta-analysis of immunonutrition in pancreatic resection, concluded that immunonutrition reduces infectious complications (especially wound infection) and length of stay [82].
2.3. Benefits of and Access to Surgical Resection of Pancreatic Cancer in the Elderly
Benefits of resection do not diminish with increasing age [83,84]. Yet, many elderly patients are considered too frail for surgery and there is significant age-related disparity in access to surgery. Ageism is a major problem faced by elderly cancer patients; elderly patients without comorbidity are less likely to receive cancer therapy than younger patients with comorbidity. Advanced age, in the absence of comorbidity, is mistakenly considered a more significant barrier to surgery than comorbidity [85]. Elderly patients are less likely to undergo standard resection, less likely to undergo resection with concomitant venous resection and less likely to achieve negative margins [86,87,88]. An American population based study of over 45,000 patients with pancreatic adenocarcinoma observed that the rate of surgery decreased with increasing age: 21% of those under 50, 19% between the age of 50 and 70 and only 13% of those over 70 received surgery [87].
Concerns over risk and perceived lack of benefit of surgery are prohibitive and account for the discrepancies in care. However, a systematic review of surgery among elderly patients with pancreatic cancer demonstrated that over time, perioperative mortality has improved for elderly patients following pancreatic resection when compared to non-elderly patients, with mortality preceding the year 2000 being significantly higher in elderly patients, but similar from 2000 onwards [86]. Major surgical complications (post-operative pancreatic fistula, delayed gastric emptying, post pancreatectomy haemorrhage and surgical site infections) were similar between the elderly and the non-elderly; however, respiratory complications did occur more frequently in the elderly population. Prehabilitation can improve physical functioning and prevent deterioration among patients whilst waiting for cancer surgery [89,90,91,92,93,94]. The benefits of surgery are not diminished by age and therefore older patients with appropriate performance status should not be denied access based on chronological age alone. Treatment decisions for the elderly should be made in a multidisciplinary setting, should ideally include the use of a tool such as the comprehensive geriatric assessment and the input of a geriatrician to avoid discrepancies in treatment based on chronological age [95,96].
2.4. Prehabilitation
Resection is the only curative option for pancreatic cancer, however, the majority of patients with a new diagnosis are not suitable for operative management [97]. Baseline functional status influences receipt of curative resection, receipt of adjuvant chemotherapy, the rate and severity of post-operative complications and long term quality of life [89,98,99,100]. Functional status is often poor in patients with pancreatic cancer, this is contributed to by older age at diagnosis, pre-operative sarcopenia, malnutrition and obstructive jaundice [101]. Prehabilitation refers to any interventions, prior to definitive treatment that are aimed at improving patient health and lifestyle [102]. The concept centres around pre-operative conditioning to improve nutritional status and aerobic capacity. Studies of prehabilitation in other types of major surgery have suggested that these programmes can improve both access to definitive treatment, and post-operative outcomes [103] (See Table 3). A recent trial randomised patients to standard of care versus standard of care plus prehabilitation in patients having major abdominal surgery found that the prehabilitation cohort had improved aerobic capacity and a reduction in 30-day readmissions [104]. Unfortunately, systematic reviews and meta-analysis have determined that although prehabilitation programmes can potentially improve surgical outcomes, the evidence is weak, this is most likely do to the variation in prehabilitation regimens and study heterogeneity [102,103,105,106,107,108].

Table 3.
Summary of prehabilitation studies for pancreatic resection.
The majority of evidence for prehabilitation is limited to those undergoing colorectal resection, hepatic resection or major cardio-thoracic surgery, few studies look specifically at the impact of prehabilitation in pancreatic resection [109,110,111,112,113,114] (Table 1). Two studies have reported on the association between prehabilitation and post-operative outcomes in pancreatic cancer; Ausania et al. randomised patients to prehabilitation (n = 18) or standard of care (n = 22). Nakajima et al. compared a cohort of patients undergoing prehabilitation to historical patients. Neither study determined a significant difference in outcomes except for reduced rates of delayed gastric emptying in one and a shorter length of stay in the other. Several studies report an improvement in lean muscle mass prior to surgery and one reported improvement in quality of life in those undergoing prehabilitation [109,110,111,112,113,114]. Although showing promising results, the collective interpretation of these studies is difficult owing to poor standardisation of exercise and nutritional interventions and paucity of participants. Table 4: Pre-operative areas for potential gain.

Table 4.
Pre-operative areas for potential gain.
3. Peri-Operative Pathways
3.1. Enhanced Recovery after Surgery
Enhanced recovery after surgery (ERAS) is a multimodal approach to a patient’s perioperative journey which aims to facilitate early return to the preoperative state [115]. Broadly, it facilitates sustained recovery and reduces complications. A secondary benefit is frequently a reduced length of stay.
The value of ERAS pathways in pancreatic surgery has been recommended on the basis of high level evidence in domains such as avoidance of hypothermia, use of wound catheters compared to epidural analgesia (EDA), use of somatostatin analogues to reduce CR-POPF [116]. protocols for thromboprophylaxis and antimicrobials and interventions for preoperative nutrition for patients with severe weight loss [36]. As patient reported outcomes (PROs) have become of particular importance in pancreatic cancer due to the elderly cohort of patients, a recent addition has been patient-centred PROs into the ERAS pathway [117,118].
The degree of compliance with ERAS pathways is strongly associated with clinical outcome [119]. With numerous components, intensive monitoring of the pathway with regular audit and a dedicated specialist nurse input improves compliance. An evaluation into the feasibility of an ERAS pathway after PD has demonstrated over 70% compliance achieved within a multicentre cohort study and was associated with a significant reduction of overall complications and length of stay [120].
ERAS after pancreatic surgery have been associated with a reduction in mild complications (Clavien Dindo grade I-II) significant improvements in overall morbidity and length of stay without any increase in readmission [121,122,123,124,125]. Effect on pancreatic specific complications is difficult to interpret as studies have variably included PD and distal pancreatectomy and ISGPS definitions have not been consistently reported for delayed gastric emptying (DGE) and postoperative pancreatic fistula (POPF) However, a lower incidence of DGE has been observed with no effect on POPF rate [122,123,125].
Implementation of ERAS pathways has been hindered due to the economic impact associated with the necessary resources required, namely a specialist nurse, audit and data collection and patient information booklets [126]. However, these costs, have been offset by the reduction in postoperative complications and subsequent hospital length of stay [127].
Though the short-term benefits are evident, long term benefits have also been suggested by one study where a relationship between increased compliance to the ERAS pathway and survival benefit has been found [128].
3.2. Reducing Complications from Surgery
Surgery alone, is a poor treatment for pancreatic cancer. Only with associated receipt of chemotherapy do patients gain a good chance for cure. One major barrier to patients receiving adjuvant therapy is the occurrence of post-operative complications. Thus, strategies to reduce these are attractive not only to improve perioperative outcomes but improve the delivery of adjuvant therapy. Post-operative pancreatic fistula (POPF) is the most frequent and severe complication after surgery [129]. Presently there is no widely accepted approach to reducing rates of clinically relevant POPF but a national study of early detection and minimally invasive treatment of POPF aims to determine whether the severity of POPF can be reduced [130].
An individual patient’s risk of POPF varies hugely. It is somewhat remarkable that POPF rates are not routinely adjusted to take this into account. Individual surgeons risk adjustment and CUSUM analysis is a way for surgeons to objectively assess their outcomes [131]. Such strategies could help inform surgeons of optimal techniques.
Strategies to improve outcomes after complex procedures such as pancreatoduodenectomy, evolve from a critical understanding of events and outcomes. Without critical analysis background noise, variations in practice and organisational differences can make this task insensitive. Determining the root causes of mortality after pancreatectomy demonstrates this well [129]. Only by conducting in depth analyses of processes and outcomes can surgeons begin to understand fundamental reasons for failure. Solutions can then be developed which are designed to overcome problems which are prevalent at the local level. Solutions are likely to vary from centre to centre dependent upon variation in practice and outcome.
A further key improvement is to determine benchmarks of optimal outcomes. This strategy seeks to reduce the effect of variation in practice between centres by focussing on common factors between centres and avoiding outlier cases. In pancreatic cancer, this has allowed teams to compare outcomes to those of their peers after surgery for resectable cancer or with associated venous resection [132,133].
Taking the concept of assimilated gains, the Dutch are leading the way with a nationwide implementation of best practices based upon critical analysis of pathways and suboptimal outcomes with the PACAP-1 trial which seeks to improve outcomes and overall survival [134]. Table 5: Summary of Peri-operative areas for gain

Table 5.
Summary of Peri-operative areas for gain.
4. Post-Operative Pathways
Post-operative strategies should be designed to enhance functional recovery, maximise uptake of adjuvant chemotherapy and delivering appropriate surveillance. Care should be continuous and multidisciplinary, with continued nutritional consideration (as outlined in the pre-operative section). Functional decline and lack of chemotherapy uptake is more pronounced in elderly.
4.1. Adjuvant Therapy in Resectable Pancreatic Cancer
The benefit for adjuvant therapy is without question. Alone surgery achieves cure in less than 10% of patients [3,135]. Currently, a multimodal approach is the standard of care where 6 months of mFOLFIRINOX based on the results of PRODIGE-24 RCT for those with a sufficient performance status, or a combination of gemcitabine and capecitabine based on ESPAC-4 RCT [136,137]. Patients not sufficiently fit for combination therapy may still benefit from gemcitabine. The role of adjuvant therapy following neoadjuvant therapy and resection, however, is less clear. A recent multi-centre international study demonstrated that adjuvant chemotherapy following NAT, was only of benefit among patients with node positive disease [138].
Widespread variation in the use of adjuvant therapy is clear between and within countries. For example, studies demonstrate that 51% of patients receive adjuvant therapy in the USA, 54% in the Netherlands, 66% in Japan and 74% in Canada. Widespread variation within countries is also evident [139,140,141,142]. Within the Netherlands, rates of adjuvant therapy varied from 26 to 74% between health care providers. Sociodemographic variation explains some variation with deprived patients less likely to receive therapy [143]. Advanced age is a common factor associated with underuse of adjuvant therapy and will be considered separately below. However, such wide differences cannot be explained by demographic differences alone. Such data clearly points at systematic variation. Such variation is clearly undesirable and yet, despite the clear advantage of adjuvant therapy, there is little emphasis upon ensuring that patients chances of receiving therapy are optimized [144]. This low completion rate of the full therapeutic sequence may in part be explained by the reticence to initiate adjuvant chemotherapy following postoperative complications or poor performance status. However, ESPAC-3 has addressed this issue of time to initiate and optimal duration of chemotherapy [145]. Within this study, 68% of patients completed all six cycles of chemotherapy and for these patients, overall survival was significantly favoured. In those who did complete therapy, there was no difference in survival when comparing those who started earlier than eight weeks compared to those who started between 8 and 12 weeks, therefore time to initiation of chemotherapy was an important prognostic factor in favour of later treatment. Thus, this study concluded that completion of chemotherapy rather than early initiation was more important for survival.
Centralised cancer surgery is widely practiced. However, centralised chemotherapy is not standard practice. There is evidence that concentrating adjuvant therapy to a dedicated regional service increases the proportion of patients that receive therapy [146]. Overcoming nihilistic views and patients fear of therapy are important strategies as many patients choose not to pursue chemotherapy and clinicians not referring patients for chemotherapy is a mindset in great need of change [147].
A further variable that must be considered is not simply whether a patient receives adjuvant therapy but that efforts must focus upon an individualised approach where patients receive a regime that is as strong as can be tolerated for that individual. Incremental gains of multiagent therapy are seen over single agent gemcitabine [3,135,137]. Yet, there is very little data upon strategies to optimise not just the delivery of adjuvant therapy but also the regimen that is delivered. Some evidence supports centralised care to deliver more multiagent therapy [147].
Liquid biopsies are a novel way to diagnose cancer at an early stage, aid in prognostic evaluation [148], determine targets for therapy [149] and to evaluate cancer recurrence after treatment. The technique involves determining cancer DNA, vesicles and tumour cells in circulating blood [150,151]. Nomograms can be used to stratify patient risk for selection to treatment and may influence the choice of NAT, surgery or nature of adjuvant chemotherapy [152,153,154,155,156].
4.2. Benefits of and Access to Chemotherapy in the Elderly Population
After resection of pancreatic cancer, disease free survival (DFS) and disease specific survival (DSS) appear similar between elderly and younger patients, but overall survival (OS) is shorter, a possible reflection that elderly patients are less likely to receive adjuvant chemotherapy [86,157,158,159]. Elderly patients have just as much benefit from adjuvant therapy as younger patients and when chemotherapy use is stratified between young and older patients overall survival is the same [160]. The CONKO-001 trial had no upper age limit and specifically demonstrated that those over 65 show a similar improvement in OS and DFS as have numerous other studies evaluating adjuvant chemotherapy for pancreatic cancer [137,159,161,162,163]. This benefit is maintained when considering more aggressive regimens in the elderly such as FOLFIRINOX or neo-adjuvant chemotherapy [164,165,166]. Consequently, strategies to increase the use of chemotherapy in the elderly, such as centralisation of oncology services and measures to address frailty, yield major benefits [146,167]. The comprehensive geriatric assessment (CGA) is recommended by the Society for International Oncology in Geriatrics as a useful decision making tool for older people with malignancy [168]. There are several studies suggesting that the CGA can be useful in predicting functional decline, toxicity and overall survival [169,170] The CGA is lengthy and time consuming, a more practical approach, as recommended by the National Comprehensive Cancer Network, the European Organisation for Research and Treatment of Cancer and the International Society of Geriatric Oncology is to use a short frailty screening tool (such as the Fried score, the Clinical Frailty Scale or the Geriatric 8) to identify those who require a full screening [171,172,173,174,175,176,177].
Like surgery, the benefits of chemotherapy are not lessened by age and therefore older patients with appropriate performance status should not be denied access. Treatment decisions should be made in a multidisciplinary setting and include the use of a frailty screening tool and the input of a geriatrician to avoid discrepancies in treatment based on chronological age [95,96].
Since there are no published accepted standards for the proportion of patients that receive adjuvant therapy after pancreatic cancer surgery there is lack of clarity about what is acceptable practice. Thus, local audit and benchmarking one’s practice against peers is an essential step in the standardisation of practices. Quality improvement programs could further enhance delivery of adjuvant chemotherapy if they were to target those patients most at risk of not receiving therapy, i.e., to overcome barriers presented by age, frailty and good functional recovery after surgery.
4.3. Surveillance after Resection of Pancreatic Cancer
Surveillance after resection of other cancer types is routine and evidence based. The notion is simple, that early detection of recurrence is more likely to identify disease at an earlier asymptomatic phase when patients are more likely to have preserved performance status and ultimately more likely to receive treatment. Most guidelines, including those from the European Society for Medical Oncology (ESMO) and International Association of Pancreatology/European Pancreatic Club (IAP/EPC), do not recommend routine surveillance after pancreatic cancer resection due to the poor prognosis and limited treatment options available for recurrence [178,179]. The nature of surveillance protocols typically involve CT scans at 3 or 6 month intervals supplemented by CA19-9 analysis. The optimal interval and impact of surveillance programs remain to be seen. It may be that artificial intelligence may help refine and improve surveillance programs; there is currently much interest in AI as a tool to facilitate the diagnosis of breast cancer within screening programs.
In the setting of recurrent pancreatic cancer there are an increasing number of treatment strategies. Despite this, many patients fail to receive palliative therapy. However, an individualised approach to treating recurrent pancreatic cancer is logical. Around half of all patients recur with local only disease [180]. Whilst systemic therapy seeks to control occult metastatic disease local therapies in the form of high intensity radiotherapy or ablation can be delivered [181]. Given that many patients are elderly, frail or may have had poor experience with systemic therapy local therapy can be delivered with more less disruption and more satisfaction for the patient. Targeted therapy of oligometastatic disease also offers alternative options for a limited number of patients. Furthermore, surveillance is desired by patients and clinicians alike [182,183]. Table 6: Areas for Post-operative gain.

Table 6.
Areas for Post-operative gain.
5. Novel Areas for Review
Though this body of work focusses upon areas where benefit can be clearly derived using existing evidence base there are areas with an emerging evidence base where benefit may be derived if practices are adopted.
Pancreatic surgery is associated with a high rate of cancer recurrence. Improving the staging pathways and novel approaches to treatment of the surgical margin are ways to improve outcomes.
Early cancer recurrence, even within ninety days of surgery, has to be considered a failure of staging [129]. The addition of routine Positron Emission Topography (PET) or Magnetic resonance imaging (MRI) scanning can help detect occult cancer. PET scanning, within a randomised trial, upstaged 20% of patients, preventing futile surgery [184]. However, it is important to note that the routine use of PET-CT is still debatable as it cause delays to resection and the potentially false positive results that may erroneously prevent surgery [185,186]. MRI can upstage 10–24% of patients with occult liver metastases where diffusion weight images can identify lesions under 5 mm, being more sensitive than computerised tomography (CT) or PET-CT [187,188,189]. These tests need to be considered carefully, however, as false positive results can be caused by common scenarios such as abscesses secondary to cholangitis and can delay treatment.
Failure of surgery to clear the margin is an Achilles heel of pancreatoduodenectomy. Local recurrence is very high after surgery, positive margins are associated with reduced survival and therefore it is necessary to consider how outcomes can be improved [190,191]. Neoadjuvant therapy (NAT) is associated with a higher rate of R0 resections, though it is important to consider results on an intention to treat basis [192,193]. There is much interest in NAT for resectable pancreatic cancer. Ongoing clinical trials will help determine the evidence base for this treatment. There are many retrospective cohort studies which are at risk of various bias including selection and survivor bias and until well conducted trial data is available it is not possible to make a clear statement [194]. Intraoperative frozen section has been used to identify positive pancreatic transection margins and the role of extending resections has been explored in numerous studies, but systematic review fails to observe a benefit to this practice [193]. Similarly, extending lymphadenectomy is not associated with greater survival but with morbidity [195,196]. The standard approach to pancreatoduodenectomy leaves perineural tissue around the superior mesenteric artery. It is this margin which is most frequently positive on histologic analysis. An artery first approach has a theoretical advantage that it can clear periadventitial tissues but clinical studies fail to demonstrate benefit in terms of improving R0 margin status [197,198]. Intraoperative radiotherapy has been long considered as an adjunct to improving margin status but no high-level evidence yet exists [199]. The addition of stereotactic ablative radiotherapy within a NAT program does not appear to improve R0 rates [200]. Irreversible electroporation has gained interest, predominantly surrounding control of locally advanced pancreatic cancer though the technology could be applied to improve margin control at resectable cancer. However, concerns over safety and a lack of high level evidence remain [201]. There is thus much work to be done in this area and focussing efforts on the problem of the surgical margin could reduce local recurrence.
Peritoneal metastases are less common than local recurrent or liver metastases but when they occur are associated with very poor outcomes. Positive intraoperative peritoneal cytology correlates with poor oncologic outcomes, in the absence of visible metastatic disease [202]. Among patients with cytology positive, or even macroscopic peritoneal disease, intraperitoneal chemotherapy can control ascites and some patients can go on to achieve surgical resection [203,204]. Such experience is limited to Japan. The remarkable outcomes indicate a potential role for intraperitoneal therapy, challenge current beliefs about pancreatic cancer biology and prognosis and merit a wider review.
Personalised medicine in pancreatic cancer care is most strongly associated with defining an individual’s cancer genetics. Large multinational, multicentre organisations/trials are ongoing such as precisionpanc (https://precisionpanc.org, accessed on 10 January 2021). There have, however, been many negative trials of genetic targets among patients with metastatic pancreatic cancer which include MEK inhibitors, IGFR inhibitors, mTOR inhibitors, TRK inhibitors, NOTCH inhibitors, TGF-β inhibitors, immunotherapy or vaccine therapy [205]. Established genetic targets in resectable pancreatic cancer are thus largely unclear; the most actionable mutation is BRCA1-2 which is identified in approximately 5% of patients [206]. Patients with these tumours are sensitive to platinum agents and thus will be treated by inclusion of oxaliplatin in therapy regimens [207]. This target has also been exploited by the PARP inhibitor Olaparib [208].
6. Conclusions
This review sought to elucidate key areas of variation, undertreatment and pathway changes where improvements can be realised with little effort. Novel therapeutic options will present themselves in the future, but it would be remiss of any team caring for this cohort of patients to inadequately utilise current evidence and implement optimal treatment pathways. There is a disconnect between funding for research to establish novel treatments far outstripping funding to implement best care at the level of the health care provider/organisation. It is essential that surgeons understand that surgery is just one part of a complex pathway and that they are ideally placed to act as change agents to optimise broader pathway improvements. Some changes can be clearly applied to the majority, if not all patients, such as PERT among those undergoing pancreatoduodenectomy. Other interventions can never be applied to all patients. There will always be some jaundiced patients with resectable cancer that undergo PBD or some patients who never receive adjuvant therapy. Locally, nationally or internationally accepted benchmarks are required to understand what is achievable and to help teams identify areas of poor performance. Collaborative multicentre, multinational studies are an essential part of assessing and improving patient care in the 21st century and teams are encouraged to develop and take part in these ventures. Through the aggregation of marginal gains, our patients can realise better outcomes and experience in the near future.
Author Contributions
K.J.R.: Conception and design, Supervision, Writing—Review and editing; R.P. and S.P.-B.; Research and writing—original draft preparation, in equal parts. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rahib, L.; Smith, B.D.; Aizenberg, R.; Rosenzweig, A.B.; Fleshman, J.M.; Matrisian, L.M. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res. 2014, 74, 2913–2921. [Google Scholar] [CrossRef]
- Rawla, P.; Sunkara, T.; Gaduputi, V. Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J. Oncol. 2019, 10, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.; Dunn, J.; Stocken, D.; Almond, J.; Link, K.; Beger, H.; Bassi, C.; Falconi, M.; Pederzoli, P.; Dervenis, C.; et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: A randomised controlled trial. Lancet 2001, 358, 1576–1585. [Google Scholar] [CrossRef]
- Panagiotopoulou, I.G.; Bennett, J.; Tweedle, E.M.; Di Saverio, S.; Gourgiotis, S.; Hardwick, R.H.; Wheeler, J.; Davies, R.J. Enhancing the emergency general surgical service: An example of the aggregation of marginal gains. Ann. R. Coll. Surg. Engl. 2019, 101, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Thomson, K.J.; Peggs, K.S. Allogeneic transplantation in the UK: An aggregation of marginal gains? Br. J. Haematol. 2013, 163, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Durrand, J.W.; Batterham, A.M.; Danjoux, G.R. Pre-habilitation. I: Aggregation of marginal gains. Anaesthesia 2014, 69, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Haeno, H.; Gonen, M.; Davis, M.B.; Herman, J.M.; Iacobuzio-Donahue, C.A.; Michor, F. Computational Modeling of Pancreatic Cancer Reveals Kinetics of Metastasis Suggesting Optimum Treatment Strategies. Cell 2012, 148, 362–375. [Google Scholar] [CrossRef]
- Lygidakis, N.J.; Van Der Heyde, M.N.; Lubbers, M.J. Evaluation of preoperative biliary drainage in the surgical management of pancreatic head carcinoma. Acta Chir. Scand. 1987, 153, 665–668. [Google Scholar]
- Whipple, A.O.; Parsons, W.B.; Mullins, C.R. Treatment of Carcinoma of the Ampulla Of Vater. Ann. Surg. 1935, 102, 763–779. [Google Scholar] [CrossRef]
- Glenn, F.; Evans, J.A.; Mujahed, Z.; Thorbjarnarson, B. Percutaneous transhepatic cholangiography. Ann. Surg. 1962, 156, 451–460. [Google Scholar] [CrossRef]
- Phoa, S.S.K.S.; Reeders, J.W.A.J.; Rauws, E.A.J.; De Wit, L.; Gouma, D.J.; Laméris, J.S. Spiral computed tomography for preoperative staging of potentially resectable carcinoma of the pancreatic head. Br. J. Surg. 1999, 86, 789–794. [Google Scholar] [CrossRef]
- Katz, M.H.G.; Marsh, R.; Herman, J.M.; Shi, Q.; Collison, E.; Venook, A.P.; Kindler, H.L.; Alberts, S.R.; Philip, P.; Lowy, A.M.; et al. Borderline Resectable Pancreatic Cancer: Need for Standardization and Methods for Optimal Clinical Trial Design. Ann. Surg. Oncol. 2013, 20, 2787–2795. [Google Scholar] [CrossRef]
- Van der Gaag, N.A.; de Castro, S.M.; Rauws, E.A.; Bruno, M.J.; van Eijck, C.H.; Kuipers, E.J.; Gerritsen, J.G.M.; Rutten, J.-P.; Greve, J.W.; Hesselink, E.J.; et al. Preoperative biliary drainage for periampullary tumors causing obstructive jaundice; DR ainage vs. (direct) OP eration (DROP-trial). BMC Surg. 2007, 7, 3. [Google Scholar]
- Sahora, K.; Morales-Oyarvide, V.; Ferrone, C.; Fong, Z.V.; Warshaw, A.L.; And, K.D.L.; Castillo, C.F.-D. Preoperative biliary drainage does not increase major complications in pancreaticoduodenectomy: A large single center experience from the Massachusetts General Hospital. J. Hepatobiliary Pancreat. Sci. 2016, 23, 181–187. [Google Scholar] [CrossRef]
- Herzog, T.; Belyaev, O.; Akkuzu, R.; Hölling, J.; Uhl, W.; Chromik, A.M. The Impact of Bile Duct Cultures on Surgical Site Infections in Pancreatic Surgery. Surg. Infect. 2015, 16, 443–449. [Google Scholar] [CrossRef]
- Mohammed, S.; Evans, C.; VanBuren, G.; Hodges, S.E.; Silberfein, E.; Artinyan, A.; Mo, Q.; Issazadeh, M.; McElhany, A.L.; Fisher, W.E. Treatment of bacteriobilia decreases wound infection rates after pancreaticoduodenectomy. HPB 2014, 16, 592–598. [Google Scholar] [CrossRef]
- Cavell, L.K.; Allen, P.J.; Vinoya, C.; Eaton, A.A.; Gonen, M.; Gerdes, H.; Mendelsohn, R.B.; D’Angelica, M.I.; Kingham, P.T.; Fong, Y.; et al. Biliary Self-Expandable Metal Stents Do Not Adversely Affect Pancreaticoduodenectomy. Am. J. Gastroenterol. 2013, 108, 1168–1173. [Google Scholar] [CrossRef]
- Coates, J.M.; Beal, S.H.; Russo, J.E.; Vanderveen, K.A.; Chen, S.L.; Bold, R.J.; Canter, R.J. Negligible Effect of Selective Preoperative Biliary Drainage on Perioperative Resuscitation, Morbidity, and Mortality in Patients Undergoing Pancreaticoduodenectomy. Arch. Surg. 2009, 144, 841–847. [Google Scholar] [CrossRef]
- Tol, J.A.M.G.; Van Hooft, J.E.; Timmer, R.; Kubben, F.J.G.M.; Van Der Harst, E.; De Hingh, I.H.J.T.; Vleggaar, F.P.; Molenaar, I.Q.; Keulemans, Y.C.A.; Boerma, D.; et al. Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut 2016, 65, 1981–1987. [Google Scholar] [CrossRef]
- Liu, P.; Lin, H.; Chen, Y.; Wu, Y.-S.; Tang, M.; Liu, C. Comparison of Metal and Plastic Stents for Preoperative Biliary Drainage in Resectable and Borderline Resectable Periampullary Cancer: A Meta-Analysis and System Review. J. Laparoendosc. Adv. Surg. Tech. 2018, 28, 1074–1082. [Google Scholar] [CrossRef]
- Lee, P.J.; Podugu, A.; Wu, D.; Lee, A.C.; Stevens, T.; Windsor, J.A. Preoperative biliary drainage in resectable pancreatic cancer: A systematic review and network meta-analysis. HPB 2018, 20, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Sanjeevi, S.; Ivanics, T.; Lundell, L.; Kartalis, N.; Andrén-Sandberg, Å.; Blomberg, J.; Del Chiaro, M.; Ansorge, C. Impact of delay between imaging and treatment in patients with potentially curable pancreatic cancer. Br. J. Surg. 2016, 103, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Glant, J.A.; Waters, J.A.; House, M.G.; Zyromski, N.J.; Nakeeb, A.; Pitt, H.A.; Lillemoe, K.D.; Schmidt, C.M. Does the interval from imaging to operation affect the rate of unanticipated metastasis encountered during operation for pancreatic adenocarcinoma? Surgery 2011, 150, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.P.; Reddy, S.; Weiss, M.J.; Manos, L.L.; Cameron, J.L.; Zheng, L.; Herman, J.M.; Hruban, R.H.; Fishman, E.K.; Wolfgang, C.L. Impact of the time interval between MDCT imaging and surgery on the accuracy of identifying metastatic disease in patients with pancreatic cancer. Am. J. Roentgenol. 2015, 204, W37–W42. [Google Scholar] [CrossRef]
- Müller, P.C.; Hodson, J.; Kuemmerli, C.; Kalisvaart, M.; Pande, R.; Roberts, K.J. Effect of time to surgery in resectable pancreatic cancer: A systematic review and meta-analysis. Langenbeck’s Arch. Surg. 2020, 405, 293–302. [Google Scholar] [CrossRef]
- Pande, R.; Hodson, J.; Marudanayagam, R.; Chatzizacharis, N.A.; Dasari, B.; Muiesan, P.; Sutcliffe, R.P.; Mirza, D.F.; Isaac, J.; Roberts, K.J. Survival Advantage of Upfront Surgery for Pancreatic Head Cancer Without Preoperative Biliary Drainage. Front. Oncol. 2020, 10, 526514. [Google Scholar] [CrossRef]
- Sauvanet, A.; Boher, J.-M.; Paye, F.; Bachellier, P.; Cuhna, A.S.; Le Treut, Y.-P.; Adham, M.; Mabrut, J.-Y.; Chiche, L.; Delpero, J.-R. Severe Jaundice Increases Early Severe Morbidity and Decreases Long-Term Survival after Pancreaticoduodenectomy for Pancreatic Adenocarcinoma. J. Am. Coll. Surg. 2015, 221, 380–389. [Google Scholar] [CrossRef]
- National Guideline A. National Institute for Health and Care Excellence: Clinical Guidelines. Pancreatic Cancer in Adults: Diagnosis and Management; National Institute for Health and Care Excellence: London, UK, 2018. [Google Scholar]
- Pande, R.; Hodson, J.; Marudanayagam, R.; Mirza, D.; Isaac, J.; Roberts, K.J. Venous resection at pancreaticoduodenectomy can be safely performed in the presence of jaundice. Hepatobiliary Pancreat. Dis. Int. 2020, 19, 488–491. [Google Scholar] [CrossRef]
- Dolejs, S.; Zarzaur, B.L.; Zyromski, N.J.; Pitt, H.A.; Riall, T.S.; Hall, B.L.; Behrman, S.W. Does Hyperbilirubinemia Contribute to Adverse Patient Outcomes Following Pancreatoduodenectomy? J. Gastrointest. Surg. 2017, 21, 647–656. [Google Scholar] [CrossRef]
- El Nakeeb, A.; Salem, A.; Mahdy, Y.; El Dosoky, M.; Said, R.; Ellatif, M.A.; Ezzat, H.; Elsabbagh, A.M.; Hamed, H.; Abd Alah, T.; et al. Value of preoperative biliary drainage on postoperative outcome after pancreaticoduodenectomy: A case-control study. Asian J. Surg. 2018, 41, 155–162. [Google Scholar] [CrossRef]
- Yoon, K.W.; Heo, J.S.; Choi, D.W.; Choi, S.H. Factors affecting long-term survival after surgical resection of pancreatic ductal adenocarcinoma. J. Korean Surg. Soc. 2011, 81, 394–401. [Google Scholar] [CrossRef]
- Berberat, P.; Künzli, B.; Gulbinas, A.; Ramanauskas, T.; Kleeff, J.; Müller, M.; Wagner, M.; Friess, H.; Büchler, M. An audit of outcomes of a series of periampullary carcinomas. Eur. J. Surg. Oncol. 2009, 35, 187–191. [Google Scholar] [CrossRef]
- Topal, B.; Aerts, R.; Hendrickx, T.; Fieuws, S.; Penninckx, F. Determinants of complications in pancreaticoduodenectomy. Eur. J. Surg. Oncol. 2007, 33, 488–492. [Google Scholar] [CrossRef]
- Gilsdorf, R.B.; Spanos, P. Factors influencing morbidity and mortality in pancreaticoduodenectomy. Ann Surg. 1973, 177, 332–337. [Google Scholar]
- MMelloul, E.; Lassen, K.; Roulin, D.; Grass, F.; Perinel, J.; Adham, M.; Wellge, E.B.; Kunzler, F.; Besselink, M.G.; Asbun, H.; et al. Guidelines for Perioperative Care for Pancreatoduodenectomy: Enhanced Recovery After Surgery (ERAS) Recommendations 2019. World J. Surg. 2020, 44, 2056–2084. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z.; Hu, W.; Zeng, Y.; Liu, X.; Mai, G.; Zhang, Y.; Lu, H.; Tian, B. Pancreaticoduodenectomy with preoperative obstructive jaundice: Drainage or not. Pancreas 2009, 38, 379–386. [Google Scholar] [CrossRef]
- Pamecha, V.; Patil, N.S.; Kumar, S.; Rajendran, V.; Gupta, S.; Sasturkar, S.V.; Sinha, P.K.; Arora, A.; Agarwal, N.; Baghmar, S. Upfront pancreaticoduodenectomy in severely jaundiced patients: Is it safe? J. Hepatobiliary Pancreat. Sci. 2019, 26, 524–533. [Google Scholar] [CrossRef]
- Van der Gaag, N.A.; Rauws, E.A.; van Eijck, C.H.; Bruno, M.J.; van der Harst, E.; Kubben, F.J.; Gerritsen, J.J.G.M.; Greve, J.W.; Gerhards, M.F.; de Hingh, I.H.J.T.; et al. Preoperative biliary drainage for cancer of the head of the pancreas. N. Engl. J. Med. 2010, 362, 129–137. [Google Scholar] [CrossRef]
- Padillo, F.J.; Andicoberry, B.; Naranjo, A.; Miño, G.; Pera, C.; Sitges-Serra, A. Anorexia and the effect of internal biliary drainage on food intake in patients with obstructive jaundice. J. Am. Coll. Surg. 2001, 192, 584–590. [Google Scholar] [CrossRef]
- Padillo, F.J.; Andicoberry, B.; Muntane, J.; Lozano, J.M.; Miño, G.; Sitges-Serra, A.; Pera-Madrazo, C. Factors Predicting Nutritional Derangements in Patients with Obstructive Jaundice: Multivariate Analysis. World J. Surg. 2001, 25, 413–418. [Google Scholar] [CrossRef]
- Tomasulo, P.A.; Levin, J.; Murphy, P.A.; Winkelstein, J.A. Biological activities of tritiated endotoxins: Correlation of the Limulus lysate assay with rabbit pyrogen and complement-activation assays for endotoxin. J. Lab. Clin. Med. 1977, 89, 308–315. [Google Scholar] [PubMed]
- Mullen, J.L.; Buzby, G.P.; Matthews, D.C.; Smale, B.F.; Rosato, E.F. Reduction of operative morbidity and mortality by combined preoperative and postoperative nutritional support. Ann. Surg. 1980, 192, 604–613. [Google Scholar] [CrossRef] [PubMed]
- Buzby, G.P.; Williford, W.O.; Peterson, O.L.; Crosby, L.O.; Page, C.P.; Reinhardt, G.F.; Mullen, J.L. A randomized clinical trial of total parenteral nutrition in malnourished surgical patients: The rationale and impact of previous clinical trials and pilot study on protocol design. Am. J. Clin. Nutr. 1988, 47 (Suppl. S2), 357–365. [Google Scholar] [CrossRef]
- Von Meyenfeldt, M.F.; Meijerink, W.J.; Rouflart, M.M.; Builmaassen, M.T.; Soeters, P.B. Perioperative nutritional support: A randomised clinical trial. Clin. Nutr. 1992, 11, 180–186. [Google Scholar] [CrossRef]
- Roberts, K.; Bannister, C.; Schrem, H. Enzyme replacement improves survival among patients with pancreatic cancer: Results of a population based study. Pancreatology 2019, 19, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Benini, L.; Amodio, A.; Campagnola, P.; Agugiaro, F.; Cristofori, C.; Micciolo, R.; Magro, A.; Gabbrielli, A.; Cabrini, G.; Moser, L.; et al. Fecal elastase-1 is useful in the detection of steatorrhea in patients with pancreatic diseases but not after pancreatic resection. Pancreatology 2013, 13, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Tseng, D.S.; Molenaar, I.Q.; Besselink, M.G.; van Eijck, C.H.; Borel Rinkes, I.H.; van Santvoort, H.C. Pancreatic Exocrine Insufficiency in Patients With Pancreatic or Periampullary Cancer: A Systematic Review. Pancreas 2016, 45, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Sikkens, E.C.M.; Cahen, D.L.; de Wit, J.; Looman, C.W.; van Eijck, C.; Bruno, M.J. A prospective assessment of the natural course of the exocrine pancreatic function in patients with a pancreatic head tumor. J. Clin. Gastroenterol. 2014, 48, e43–e46. [Google Scholar] [CrossRef]
- Lemaire, E.; O’Toole, D.; Sauvanet, A.; Hammel, P.; Belghiti, J.; Ruszniewski, P. Functional and morphological changes in the pancreatic remnant following pancreaticoduodenectomy with pancreaticogastric anastomosis. Br. J. Surg. 2000, 87, 434–438. [Google Scholar] [CrossRef]
- Singh, V.K.; Haupt, M.E.; Geller, D.E.; Hall, J.A.; Diez, P.M.Q. Less common etiologies of exocrine pancreatic insufficiency. World J. Gastroenterol. 2017, 23, 7059–7076. [Google Scholar] [CrossRef]
- Sikkens, E.C.M.; Cahen, D.L.; de Wit, J.; Looman, C.W.; van Eijck, C.; Bruno, M.J. Prospective assessment of the influence of pancreatic cancer resection on exocrine pancreatic function. Br. J. Surg. 2014, 101, 109–113. [Google Scholar] [CrossRef]
- Dominguez-Muñoz, J.E. Diagnosis and treatment of pancreatic exocrine insufficiency. Curr. Opin. Gastroenterol. 2018, 34, 349–354. [Google Scholar] [CrossRef]
- Johnson, C.D.; Williamson, N.; Solingen, G.J.-V.; Arbuckle, R.; Johnson, C.; Simpson, S.; Staab, D.; Dominguez-Munoz, E.; Levy, P.; Connett, G.; et al. Psychometric evaluation of a patient-reported outcome measure in pancreatic exocrine insufficiency (PEI). Pancreatology 2019, 19, 182–190. [Google Scholar] [CrossRef]
- Johnson, C.D.; Arbuckle, R.; Bonner, N.; Connett, G.; Dominguez-Munoz, E.; Levy, P.; Staab, D.; Williamson, N.; Lerch, M.M. Qualitative Assessment of the Symptoms and Impact of Pancreatic Exocrine Insufficiency (PEI) to Inform the Development of a Patient-Reported Outcome (PRO) Instrument. Patient Patient Centered Outcomes Res. 2017, 10, 615–628. [Google Scholar] [CrossRef]
- Sikkens, E.C.M.; Cahen, D.L.; de Wit, J.; Looman, C.W.; van Eijck, C.; Bruno, M.J. The daily practice of pancreatic enzyme replacement therapy after pancreatic surgery: A northern European survey: Enzyme replacement after surgery. J Gastrointest Surg. 2012, 16, 1487–1492. [Google Scholar] [CrossRef]
- RICOCHET Study Group; West Midlands Research Collaborative. Receipt of Curative Resection or Palliative Care for Hepatopancreaticobiliary Tumours (RICOCHET): Protocol for a Nationwide Collaborative Observational Study. JMIR Res. Protoc. 2019, 8, e13566. [Google Scholar] [CrossRef]
- Landers, A.; Muircroft, W.; Brown, H. Pancreatic enzyme replacement therapy (PERT) for malabsorption in patients with metastatic pancreatic cancer. BMJ Support. Palliat. Care 2014, 6, 75–79. [Google Scholar] [CrossRef]
- Barkin, J.A.; Westermann, A.; Hoos, W.; Moravek, C.; Matrisian, L.; Wang, H.; Shemanski, L.; Barkin, J.S.; Rahib, L. Frequency of Appropriate Use of Pancreatic Enzyme Replacement Therapy and Symptomatic Response in Pancreatic Cancer Patients. Pancreas 2019, 48, 780–786. [Google Scholar] [CrossRef]
- Lindkvist, B. Diagnosis and treatment of pancreatic exocrine insufficiency. World J. Gastroenterol. 2013, 19, 7258–7566. [Google Scholar] [CrossRef]
- Laterza, L.; Scaldaferri, F.; Bruno, G.; Agnes, A.; Boškoski, I.; Ianiro, G.; Gerardi, V.; Ojetti, V.; Alfieri, S.; Gasbarrini, A. Pancreatic function assessment. Eur. Rev. Med. Pharmacol. Sci. 2013, 17 (Suppl. S2), 65–71. [Google Scholar]
- Dominguez-Munoz, J.E.; Nieto, L.; Vilarino, M.; Lourido, M.V.; Iglesias-Garcia, J. Development and Diagnostic Accuracy of a Breath Test for Pancreatic Exocrine Insufficiency in Chronic Pancreatitis. Pancreas 2016, 45, 241–247. [Google Scholar] [CrossRef]
- Lindkvist, B.; Phillips, M.E.; Domínguez-Muñoz, J.E. Clinical, anthropometric and laboratory nutritional markers of pancreatic exocrine insufficiency: Prevalence and diagnostic use. Pancreatology 2015, 15, 589–597. [Google Scholar] [CrossRef]
- Partelli, S.; Frulloni, L.; Minniti, C.; Bassi, C.; Barugola, G.; D’Onofrio, M.; Crippa, S.; Falconi, M. Faecal elastase-1 is an independent predictor of survival in advanced pancreatic cancer. Dig. Liver Dis. 2012, 44, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Gooden, H.M.; White, K.J. Pancreatic cancer and supportive care—Pancreatic exocrine insufficiency negatively impacts on quality of life. Support. Care Cancer 2013, 21, 1835–1841. [Google Scholar] [CrossRef]
- Van Dijk, S.M.; Heerkens, H.D.; Tseng, D.S.J.; Intven, M.; Molenaar, I.Q.; van Santvoort, H.C. Systematic review on the impact of pancreatoduodenectomy on quality of life in patients with pancreatic cancer. HPB 2018, 20, 204–215. [Google Scholar] [CrossRef]
- Heerkens, H.D.; Van Berkel, L.; Tseng, D.S.; Monninkhof, E.M.; Van Santvoort, H.C.; Hagendoorn, J.; Rinkes, I.H.B.; Lips, I.M.; Intven, M.; Molenaar, I.Q. Long-term health-related quality of life after pancreatic resection for malignancy in patients with and without severe postoperative complications. HPB 2018, 20, 188–195. [Google Scholar] [CrossRef]
- Roberts, K.J.; Schrem, H.; Hodson, J.; Angelico, R.; Dasari, B.V.M.; Coldham, C.A.; Marudanayagam, R.; Sutcliffe, R.P.; Muiesan, P.; Isaac, J.; et al. Pancreas exocrine replacement therapy is associated with increased survival following pancreatoduodenectomy for periampullary malignancy. HPB 2017, 19, 859–867. [Google Scholar] [CrossRef]
- Seiler, C.M.; Izbicki, J.; Varga-Szabó, L.; Czako, L.; Fiók, J.; Sperti, C.; Lerch, M.M.; Pezzilli, R.; Vasileva, G.; Pap, A.; et al. Randomised clinical trial: A 1-week, double-blind, placebo-controlled study of pancreatin 25 000 Ph. Eur. minimicrospheres (Creon 25000 MMS) for pancreatic exocrine insufficiency after pancreatic surgery, with a 1-year open-label extension. Aliment. Pharmacol. Ther. 2013, 37, 691–702. [Google Scholar] [CrossRef]
- Braga, M.; Cristallo, M.; De Franchis, R.; Mangiagalli, A.; Zerbi, A.; Agape, D.; Primignani, M.; Di Carlo, V. Pancreatic enzyme replacement therapy in post-pancreatectomy patients. Int. J. Pancreatol. 1989, 5, 37–44. [Google Scholar]
- Landers, A.; Brown, H.; Strother, M. The effectiveness of pancreatic enzyme replacement therapy for malabsorption in advanced pancreatic cancer, a pilot study. Palliat. Care Res. Treat. 2019, 12, 1178224218825270. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Muñoz, J.E.; Iglesias-García, J.; Iglesias-Rey, M.; Figueiras, A.; Vilariño-Insua, M. Effect of the administration schedule on the therapeutic efficacy of oral pancreatic enzyme supplements in patients with exocrine pancreatic insufficiency: A randomized, three-way crossover study. Aliment. Pharmacol. Ther. 2005, 21, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Munoz, J.E.; Iglesias-Garcia, J.; Iglesias-Rey, M.; Vilarino-Insua, M. Optimising the therapy of exocrine pancreatic insufficiency by the association of a proton pump inhibitor to enteric coated pancreatic extracts. Gut 2006, 55, 1056–1057. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kang, J.S.; Han, Y.; Kim, H.; Kwon, W.; Kim, J.R.; Kim, S.-W.; Jang, J.-Y. Influence of preoperative nutritional status on clinical outcomes after pancreatoduodenectomy. HPB 2018, 20, 1051–1061. [Google Scholar] [CrossRef]
- Kim, E.; Lee, D.-H.; Jang, J.-Y. Effects of Preoperative Malnutrition on Postoperative Surgical Outcomes and Quality of Life of Elderly Patients with Periampullary Neoplasms: A Single-Center Prospective Cohort Study. Gut Liver 2019, 13, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Gianotti, L.; Besselink, M.G.; Sandini, M.; Hackert, T.; Conlon, K.; Gerritsen, A.; Griffin, O.; Fingerhut, A.; Probst, P.; Abu Hilal, M.; et al. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS). Surgery 2018, 164, 1035–1048. [Google Scholar] [CrossRef]
- Powell-Brett, S.; Carino, N.D.L.; Roberts, K. Understanding pancreatic exocrine insufficiency and replacement therapy in pancreatic cancer. Eur. J. Surg. Oncol. 2021, 47, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Aida, T.; Furukawa, K.; Suzuki, D.; Shimizu, H.; Yoshidome, H.; Ohtsuka, M.; Kato, A.; Yoshitomi, H.; Miyazaki, M. Preoperative immunonutrition decreases postoperative complications by modulating prostaglandin E2 production and T-cell differentiation in patients undergoing pancreatoduodenectomy. Surgery 2014, 155, 124–133. [Google Scholar] [CrossRef]
- Gade, J.; Levring, T.; Hillingsø, J.; Hansen, C.P.; Andersen, J.R. The Effect of Preoperative Oral Immunonutrition on Complications and Length of Hospital Stay After Elective Surgery for Pancreatic Cancer—A Randomized Controlled Trial. Nutr. Cancer 2016, 68, 225–233. [Google Scholar] [CrossRef]
- Hamza, N.; Darwish, A.; O’Reilly, D.A.; Denton, J.; Sheen, A.J.; Chang, D.; Sherlock, D.J.; Ammori, B.J. Perioperative Enteral Immunonutrition Modulates Systemic and Mucosal Immunity and the Inflammatory Response in Patients With Periampullary Cancer Scheduled for Pancreaticoduodenectomy: A Randomized Clinical Trial. Pancreas 2015, 44, 41–52. [Google Scholar] [CrossRef]
- Silvestri, S.; Franchello, A.; Deiro, G.; Galletti, R.; Cassine, D.; Campra, D.; Bonfanti, D.; De Carli, L.; Fop, F.; Fronda, G. Preoperative oral immunonutrition versus standard preoperative oral diet in well nourished patients undergoing pancreaticoduodenectomy. Int. J. Surg. 2016, 31, 93–99. [Google Scholar] [CrossRef]
- Yang, F.-A.; Chen, Y.-C.; Tiong, C. Immunonutrition in Patients with Pancreatic Cancer Undergoing Surgical Intervention: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrition 2020, 12, 2798. [Google Scholar] [CrossRef] [PubMed]
- Riall, T.S.; Sheffield, K.M.; Kuo, Y.-F.; Townsend, C.M., Jr.; Goodwin, J.S. Resection Benefits Older Adults with Locoregional Pancreatic Cancer Despite Greater Short-Term Morbidity and Mortality. J. Am. Geriatr. Soc. 2011, 59, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Kanda, M.; Fujii, T.; Suenaga, M.; Takami, H.; Inokawa, Y.; Yamada, S.; Kobayashi, D.; Tanaka, C.; Sugimoto, H.; Nomoto, S.; et al. Pancreatoduodenectomy With Portal Vein Resection Is Feasible and Potentially Beneficial for Elderly Patients With Pancreatic Cancer. Pancreas 2014, 43, 951–958. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Equality Inititative; Pharmaceutical Oncology Initiative. The Impact of Patient Age on Clinical Decision-Making in Oncology; Department of Health Cancer Policy Team: London, UK, 2012. [Google Scholar]
- Tan, E.; Song, J.; Lam, S.; D’Souza, M.; Crawford, M.; Sandroussi, C. Postoperative outcomes in elderly patients undergoing pancreatic resection for pancreatic adenocarcinoma: A systematic review and meta-analysis. Int. J. Surg. 2019, 72, 59–68. [Google Scholar] [CrossRef]
- Amin, S.; Lucas, A.L.; Frucht, H. Evidence for treatment and survival disparities by age in pancreatic adenocarcinoma: A population-based analysis. Pancreas 2013, 42, 249–253. [Google Scholar] [CrossRef]
- Nipp, R.; Tramontano, A.C.; Kong, C.Y.; Pandharipande, P.; Dowling, E.C.; Schrag, D.; Hur, C. Disparities in cancer outcomes across age, sex, and race/ethnicity among patients with pancreatic cancer. Cancer Med. 2018, 7, 525–535. [Google Scholar] [CrossRef]
- Chandrabalan, V.V.; McMillan, D.C.; Carter, R.; Kinsella, J.; McKay, C.J.; Carter, C.R.; Dickson, E.J. Pre-operative cardiopulmonary exercise testing predicts adverse post-operative events and non-progression to adjuvant therapy after major pancreatic surgery. HPB 2013, 15, 899–907. [Google Scholar] [CrossRef]
- Thomas, G.; Tahir, M.R.; Bongers, B.C.; Kallen, V.L.; Slooter, G.D.; van Meeteren, N.L. Prehabilitation before major intra-abdominal cancer surgery: A systematic review of randomised controlled trials. Eur. J. Anaesthesiol. 2019, 36, 933–945. [Google Scholar] [CrossRef]
- West, M.A.; Parry, M.G.; Lythgoe, D.; Barben, C.P.; Kemp, G.J.; Grocott, M.P.W.; Jack, S. Cardiopulmonary exercise testing for the prediction of morbidity risk after rectal cancer surgery. Br. J. Surg. 2014, 101, 1166–1172. [Google Scholar] [CrossRef]
- Minnella, E.M.; Awasthi, R.; Loiselle, S.E.; Agnihotram, R.V.; Ferri, L.E.; Carli, F. Effect of Exercise and Nutrition Prehabilitation on Functional Capacity in Esophagogastric Cancer Surgery: A Randomized Clinical Trial. JAMA Surg. 2018, 153, 1081–1089. [Google Scholar] [CrossRef]
- Dunne, D.F.J.; Jack, S.; Jones, R.P.; Jones, L.; Lythgoe, D.T.; Malik, H.Z.; Poston, G.J.; Palmer, D.H.; Fenwick, S.W. Randomized clinical trial of prehabilitation before planned liver resection. Br. J. Surg. 2016, 103, 504–512. [Google Scholar] [CrossRef]
- Bhatia, C.; Kayser, B. Preoperative high-intensity interval training is effective and safe in deconditioned patients with lung cancer: A randomized clinical trial. J. Rehabil. Med. 2019, 51, 712–718. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- Wilson, R.J.T.; Davies, S.; Yates, D.; Redman, J.; Stone, M. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br. J. Anaesth. 2010, 105, 297–303. [Google Scholar] [CrossRef]
- McGuigan, A.; Kelly, P.; Turkington, R.C.; Jones, C.; Coleman, H.G.; McCain, R.S. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J. Gastroenterol. 2018, 24, 4846–4861. [Google Scholar] [CrossRef]
- Akahori, T.; Sho, M.; Kinoshita, S.; Nagai, M.; Nishiwada, S.; Tanaka, T.; Tamamoto, T.; Ohbayashi, C.; Hasegawa, M.; Kichikawa, K.; et al. Prognostic Significance of Muscle Attenuation in Pancreatic Cancer Patients Treated with Neoadjuvant Chemoradiotherapy. World J. Surg. 2015, 39, 2975–2982. [Google Scholar] [CrossRef]
- Sandini, M.; Patino, M.; Ferrone, C.R.; Alvarez-Pérez, C.A.; Honselmann, K.C.; Paiella, S.; Catania, M.; Riva, L.; Tedesco, G.; Casolino, R.; et al. Association Between Changes in Body Composition and Neoadjuvant Treatment for Pancreatic Cancer. JAMA Surg. 2018, 153, 809–815. [Google Scholar] [CrossRef]
- Mackay, T.M.; Smits, F.J.; Roos, D.; Bonsing, B.A.; Bosscha, K.; Busch, O.R.; Creemers, G.J.; van Dam, R.M.; van Eijck, C.H.J.; Gerhardet, M.F.; et al. The risk of not receiving adjuvant chemotherapy after resection of pancreatic ductal adenocarcinoma: A nationwide analysis. HPB 2020, 22, 233–240. [Google Scholar] [CrossRef]
- Bundred, J.; Kamarajah, S.K.; Roberts, K.J. Body composition assessment and sarcopenia in patients with pancreatic cancer: A systematic review and meta-analysis. HPB 2019, 21, 1603–1612. [Google Scholar] [CrossRef]
- Giles, C.; Cummins, S. Prehabilitation before cancer treatment. BMJ 2019, 366, l5120. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Bundred, J.; Weblin, J.; Tan, B.H. Critical appraisal on the impact of preoperative rehabilitation and outcomes after major abdominal and cardiothoracic surgery: A systematic review and meta-analysis. Surgery 2020, 167, 540–549. [Google Scholar] [CrossRef] [PubMed]
- Barberan-Garcia, A.; Ubre, M.; Pascual-Argente, N.; Risco, R.; Faner, J.; Balust, J.; Lacy, A.; Puig-Junoy, J.; Roca, J.; Martinez-Palli, G. Post-discharge impact and cost-consequence analysis of prehabilitation in high-risk patients undergoing major abdominal surgery: Secondary results from a randomised controlled trial. Br. J. Anaesth. 2019, 123, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Levett, D.Z.; Edwards, M.; Grocott, M.; Mythen, M. Preparing the patient for surgery to improve outcomes. Best Pr. Res. Clin. Anaesthesiol. 2016, 30, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Carli, F.; Scheede-Bergdahl, C. Prehabilitation to Enhance Perioperative Care. Anesthesiol. Clin. 2015, 33, 17–33. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.; Buhler, K.; Bresee, L.; Carli, F.; Gramlich, L.; Culos-Reed, N.; Sajobi, T.T.; Fenton, T.R. Effects of Nutritional Prehabilitation, With and Without Exercise, on Outcomes of Patients Who Undergo Colorectal Surgery: A Systematic Review and Meta-analysis. Gastroenterology 2018, 155, 391–410.e4. [Google Scholar] [CrossRef] [PubMed]
- Heger, P.; Probst, P.; Wiskemann, J.; Steindorf, K.; Diener, M.K.; Mihaljevic, A.L. A Systematic Review and Meta-analysis of Physical Exercise Prehabilitation in Major Abdominal Surgery (PROSPERO 2017 CRD42017080366). J. Gastrointest. Surg. 2020, 24, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Ausania, F.; Senra, P.; Meléndez, R.; Caballeiro, R.; Ouviña, R.; Casal-Núñez, E. Prehabilitation in patients undergoing pancreaticoduodenectomy: A randomized controlled trial. Rev. Esp. Enferm. Dig. 2019, 111, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Yokoyama, Y.; Inoue, T.; Nagaya, M.; Mizuno, Y.; Kadono, I.; Nishiwaki, K.; Nishida, Y.; Nagino, M. Clinical Benefit of Preoperative Exercise and Nutritional Therapy for Patients Undergoing Hepato-Pancreato-Biliary Surgeries for Malignancy. Ann. Surg. Oncol. 2018, 26, 264–272. [Google Scholar] [CrossRef]
- Florez Bedoya, C.A.; Cardoso, A.C.F.; Parker, N.; Ngo-Huang, A.; Petzel, M.Q.; Kim, M.P.; Fogelman, D.; Romero, S.G.; Wang, H.; Park, M.; et al. Exercise during preoperative therapy increases tumor vascularity in pancreatic tumor patients. Sci. Rep. 2019, 9, 13966. [Google Scholar] [CrossRef]
- Marker, R.J.; Peters, J.C.; Purcell, W.T.; Jankowski, C.A. Effects of Preoperative Exercise on Physical Fitness and Body Composition in Pancreatic Cancer Survivors Receiving Neoadjuvant Therapy: A Case Series. Rehabil. Oncol. 2018, 36, E1–E9. [Google Scholar] [CrossRef]
- Parker, N.H.; Ngo-Huang, A.; Lee, R.E.; O’Connor, D.P.; Basen-Engquist, K.M.; Petzel, M.Q.; Wang, X.; Xiao, L.; Fogelman, D.R.; Schadler, K.L.; et al. Physical activity and exercise during preoperative pancreatic cancer treatment. Support. Care Cancer 2018, 27, 2275–2284. [Google Scholar] [CrossRef]
- Ngo-Huang, A.; Parker, N.H.; Bruera, E.; Lee, R.E.; Simpson, R.; O’Connor, D.P.; Petzel, M.Q.B.; Fontillas, R.C.; Schadler, K.; Xiao, L.; et al. Home-Based Exercise Prehabilitation During Preoperative Treatment for Pancreatic Cancer Is Associated With Improvement in Physical Function and Quality of Life. Integr. Cancer Ther. 2019, 18, 1534735419894061. [Google Scholar] [CrossRef]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced Recovery After Surgery: A Review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef]
- Li, T.; D’Cruz, R.T.; Lim, S.Y.; Shelat, V.G.; Tianpei, L.; Yang, L.S. Somatostatin analogues and the risk of post-operative pancreatic fistulas after pancreatic resection—A systematic review & meta-analysis. Pancreatology 2020, 20, 158–168. [Google Scholar]
- De Rosa, P.; Jewell, A. The potential use for patient reported outcome measures in people with pancreatic cancer, with a specific focus on older patients. Eur. J. Surg. Oncol. 2021, 47, 495–502. [Google Scholar] [CrossRef]
- Van Rijssen, L.B.; Gerritsen, A.; Henselmans, I.; Sprangers, M.A.; Jacobs, M.; Bassi, C.; Busch, O.R.; Fernandez-Del Castillo, C.; Fong, Z.V.; He, F.; et al. Core Set of Patient-reported Outcomes in Pancreatic Cancer (COPRAC): An International Delphi Study Among Patients and Health Care Providers. Ann. Surg. 2019, 270, 158–164. [Google Scholar] [CrossRef]
- ERAS Compliance Group. The Impact of Enhanced Recovery Protocol Compliance on Elective Colorectal Cancer Resection: Results From an International Registry. Ann. Surg. 2015, 261, 1153–1159. [Google Scholar] [CrossRef]
- Roulin, D.; Melloul, E.; Wellg, B.E.; Izbicki, J.; Vrochides, D.; Adham, M.; Hübner, M.; Demartines, N. Feasibility of an Enhanced Recovery Protocol for Elective Pancreatoduodenectomy: A Multicenter International Cohort Study. World J. Surg. 2020, 44, 2761–2769. [Google Scholar] [CrossRef]
- Coolsen, M.M.E.; Van Dam, R.M.; Van Der Wilt, A.A.; Slim, K.; Lassen, K.; DeJong, C.H.C. Systematic Review and Meta-analysis of Enhanced Recovery After Pancreatic Surgery with Particular Emphasis on Pancreaticoduodenectomies. World J. Surg. 2013, 37, 1909–1918. [Google Scholar] [CrossRef]
- Xiong, J.; Szatmary, P.; Huang, W.; de la Iglesia-Garcia, D.; Nunes, Q.M.; Xia, Q.; Hu, W.; Sutton, R.; Liu, X.; Raraty, M.G. Enhanced Recovery After Surgery Program in Patients Undergoing Pancreaticoduodenectomy: A PRISMA-Compliant Systematic Review and Meta-Analysis. Medicine 2016, 95, e3497. [Google Scholar] [CrossRef]
- Ji, H.-B.; Zhu, W.-T.; Wei, Q.; Wang, X.-X.; Wang, H.-B.; Chen, Q.-P. Impact of enhanced recovery after surgery programs on pancreatic surgery: A meta-analysis. World J. Gastroenterol. 2018, 24, 1666–1678. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gu, H.-Y.; Huang, Z.-D.; Wu, Y.-P.; Zhang, Q.; Luo, J.; Zhang, C.; Fu, Y. Impact of Enhanced Recovery After Surgery on Postoperative Recovery for Pancreaticoduodenectomy: Pooled Analysis of Observational Study. Front. Oncol. 2019, 9, 687. [Google Scholar] [CrossRef]
- Sun, Y.-M.; Wang, Y.; Mao, Y.-X.; Wang, W. The Safety and Feasibility of Enhanced Recovery after Surgery in Patients Undergoing Pancreaticoduodenectomy: An Updated Meta-Analysis. BioMed Res. Int. 2020, 2020, 7401276. [Google Scholar] [CrossRef] [PubMed]
- Roulin, D.; Najjar, P.; Demartines, N. Enhanced Recovery After Surgery Implementation: From Planning to Success. J. Laparoendosc. Adv. Surg. Tech. 2017, 27, 876–879. [Google Scholar] [CrossRef] [PubMed]
- Kagedan, D.J.; Devitt, K.S.; St-Germain, A.T.; Ramjaun, A.; Cleary, S.P.; Wei, A.C. The economics of recovery after pancreatic surgery: Detailed cost minimization analysis of an enhanced recovery program. HPB 2017, 19, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Passeri, M.; Lyman, W.B.; Murphy, K.; Iannitti, D.; Martinie, J.; Baker, E.; Vrochides, D. Implementing an ERAS Protocol for Pancreaticoduodenectomy Does Not Affect Oncologic Outcomes when Compared with Traditional Recovery. Am. Surg. 2020, 86, e81–e83. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, C.M.; The Pancreatic Surgery Mortality Study Group; Sanchez, N.; Gondek, S.; McAuliffe, J.C.; Kent, T.S.; Christein, J.D.; Callery, M.P. A Root-Cause Analysis of Mortality Following Major Pancreatectomy. J. Gastrointest. Surg. 2011, 16, 89–103. [Google Scholar] [CrossRef]
- Smits, F.J.; Henry, A.C.; Van Eijck, C.H.; Besselink, M.G.; Busch, O.R.; Arntz, M.; Bollen, T.L.; Van Delden, O.M.; Heuvel, D.V.D.; Van Der Leij, C.; et al. Care after pancreatic resection according to an algorithm for early detection and minimally invasive management of pancreatic fistula versus current practice (PORSCH-trial): Design and rationale of a nationwide stepped-wedge cluster-randomized trial. Trials 2020, 21, 389. [Google Scholar] [CrossRef]
- Roberts, K.J.; Boteon, A.; Marcon, F.; Abradelo, M.; Dasari, B.; Muiesan, P.; Marudanayagam, R.; Sutcliffe, R.P.; Isaac, J.; Mirza, D.F. Risk adjusted assessment of individual surgeon’s pancreatic fistula outcomes. HPB 2020, 22, 452–460. [Google Scholar] [CrossRef]
- Sánchez-Velázquez, P.; Muller, X.; Malleo, G.; Park, J.; Hwang, H.; Napoli, N.; Javed, A.; Inoue, Y.; Beghdadi, N.; Kalisvaart, M.; et al. Benchmarks in pancreatic surgery. A novel tool for unbiased outcome comparisons. HPB 2020, 22, S383. [Google Scholar] [CrossRef]
- Raptis, D.A.; Sánchez-Velázquez, P.; Machairas, N.; Sauvanet, A.; De Leon, A.R.; Oba, A.; Koerkamp, B.G.; Lovasik, B.; Chan, C.; Yeo, C.J.; et al. Defining Benchmark Outcomes for Pancreatoduodenectomy With Portomesenteric Venous Resection. Ann. Surg. 2020, 272, 731–737. [Google Scholar] [CrossRef]
- Mackay, T.M.; Dutch Pancreatic Cancer Group; Smits, F.J.; Latenstein, A.E.J.; Bogte, A.; Bonsing, B.A.; Bos, H.; Bosscha, K.; Brosens, L.A.A.; Hol, L.; et al. Impact of nationwide enhanced implementation of best practices in pancreatic cancer care (PACAP-1): A multicenter stepped-wedge cluster randomized controlled trial. Trials 2020, 21, 334. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.-C.; Raoul, J.-L.; Chone, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. Unicancer GI PRODIGE 24/CCTG PA.6 trial: A multicenter international randomized phase III trial of adjuvant mFOLFIRINOX versus gemcitabine (gem) in patients with resected pancreatic ductal adenocarcinomas. J. Clin. Oncol. 2018, 36, LBA4001. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Palmer, D.H.; Ghaneh, P.; Psarelli, E.E.; Valle, J.W.; Halloran, C.M.; Faluyi, O.; O’Reilly, D.A.; Cunningham, D.; Wadsley, J.; et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): A multicentre, open-label, randomised, phase 3 trial. Lancet 2017, 389, 1011–1024. [Google Scholar] [CrossRef]
- Van Roessel, S.; van Veldhuisen, E.; Klompmaker, S.; Janssen, Q.P.; Abu Hilal, M.; Alseidi, A.; Balduzzi, A.; Balzano, G.; Bassi, C.; Berrevoet, F.; et al. Evaluation of Adjuvant Chemotherapy in Patients With Resected Pancreatic Cancer After Neoadjuvant FOLFIRINOX Treatment. JAMA Oncol. 2020, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sata, N.; Kurashina, K.; Nagai, H.; Nagakawa, T.; Ishikawa, O.; Ohta, T.; Oka, M.; Kinoshita, H.; Kimura, W.; Shimada, H.; et al. The effect of adjuvant and neoadjuvant chemo(radio)therapy on survival in 1679 resected pancreatic carcinoma cases in Japan: Report of the national survey in the 34th annual meeting of Japanese Society of Pancreatic Surgery. J. Hepatobiliary Pancreat. Surg. 2009, 16, 485–492. [Google Scholar] [CrossRef]
- Bakens, M.J.; van der Geest, L.G.; van Putten, M.; van Laarhoven, H.W.; Creemers, G.-J.; Besselink, M.G.; Lemmens, V.E.; de Hingh, I.H.; Dutch Pancreatic Cancer Group. The use of adjuvant chemotherapy for pancreatic cancer varies widely between hospitals: A nationwide population-based analysis. Cancer Med. 2016, 5, 2825–2831. [Google Scholar] [CrossRef]
- Kagedan, D.; Dixon, M.; Raju, R.; Li, Q.; Elmi, M.; Shin, E.; Liu, N.; El-Sedfy, A.; Paszat, L.; Kiss, A.; et al. Predictors of Adjuvant Treatment for Pancreatic Adenocarcinoma at the Population Level. Curr. Oncol. 2016, 23, 334–342. [Google Scholar] [CrossRef][Green Version]
- Mayo, S.C.; Gilson, M.M.; Herman, J.M.; Cameron, J.L.; Nathan, H.; Edil, B.H.; Choti, M.A.; Schulick, R.D.; Wolfgang, C.L.; Pawlik, T.M. Management of Patients with Pancreatic Adenocarcinoma: National Trends in Patient Selection, Operative Management, and Use of Adjuvant Therapy. J. Am. Coll. Surg. 2012, 214, 33–45. [Google Scholar] [CrossRef]
- Kagedan, D.J.; Abraham, L.; Goyert, N.; Li, Q.; Paszat, L.F.; Kiss, A.; Earle, C.C.; Mittmann, N.; Coburn, N.G. Beyond the dollar: Influence of sociodemographic marginalization on surgical resection, adjuvant therapy, and survival in patients with pancreatic cancer. Cancer 2016, 122, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Van der Geest, L.G.M.; van Eijck, C.H.J.; Groot Koerkamp, B.; Lemmens, V.; Busch, O.R.; Vissers, P.A.J.; Wilmink, J.W.; Besselink, M.G.; Dutch Pancreatic Cancer Group. Trends in treatment and survival of patients with nonresected, nonmetastatic pancreatic cancer: A population-based study. Cancer Med. 2018, 7, 4943–4951. [Google Scholar] [CrossRef] [PubMed]
- Neoptolemos, J.P.; Stocken, D.D.; Friess, H.; Bassi, C.; Dunn, J.A.; Hickey, H.; Beger, H.; Fernandez-Cruz, L.; Dervenis, C.; Lacaine, F.; et al. A Randomized Trial of Chemoradiotherapy and Chemotherapy after Resection of Pancreatic Cancer. N. Engl. J. Med. 2004, 350, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
- Faluyi, O.O.; Connor, J.L.; Chatterjee, M.; Ikin, C.; Wong, H.; Palmer, D.H. Advanced pancreatic adenocarcinoma outcomes with transition from devolved to centralised care in a regional Cancer Centre. Br. J. Cancer 2017, 116, 424–431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fisher, A.V.; Ma, Y.; Wang, X.; Campbell-Flohr, S.A.; Rathouz, P.J.; Ronnekleiv-Kelly, S.M.; Abbott, D.E.; Weber, S.M. National Trends in Centralization of Surgical Care and Multimodality Therapy for Pancreatic Adenocarcinoma. J. Gastrointest. Surg. 2019, 24, 2021–2029. [Google Scholar] [CrossRef]
- Sorenson, G.D.; Pribish, D.M.; Valone, F.H.; Memoli, V.A.; Bzik, D.J.; Yao, S.L. Soluble normal and mutated DNA sequences from single-copy genes in human blood. Cancer Epidemiol. Biomark. Prev. 1994, 3, 67–71. [Google Scholar]
- Glantz, M.J.; Cole, B.F.; Glantz, L.K.; Cobb, J.; Mills, P.; Lekos, A.; Walters, B.C.; Recht, L.D. Cerebrospinal fluid cytology in patients with cancer: Minimizing false-negative results. Cancer 1998, 82, 733–739. [Google Scholar] [CrossRef]
- Anker, P.; Stroun, M.; Maurice, P.A. Spontaneous release of DNA by human blood lymphocytes as shown in an in vitro system. Cancer Res. 1975, 35, 2375–2382. [Google Scholar]
- Stroun, M.; Lyautey, J.; Lederrey, C.; Olson-Sand, A.; Anker, P. About the possible origin and mechanism of circulating DNA apoptosis and active DNA release. Clin. Chim. Acta 2001, 313, 139–142. [Google Scholar] [CrossRef]
- Kim, N.; Han, I.W.; Ryu, Y.; Hwang, D.W.; Heo, J.S.; Choi, D.W.; Shin, S.H. Predictive Nomogram for Early Recurrence after Pancreatectomy in Resectable Pancreatic Cancer: Risk Classification Using Preoperative Clinicopathologic Factors. Cancers 2020, 12, 137. [Google Scholar] [CrossRef]
- Brennan, M.F.; Kattan, M.W.; Klimstra, D.; Conlon, K. Prognostic Nomogram for Patients Undergoing Resection for Adenocarcinoma of the Pancreas. Ann. Surg. 2004, 240, 293–298. [Google Scholar] [CrossRef]
- Ferrone, C.R.; Kattan, M.W.; Tomlinson, J.S.; Thayer, S.P.; Brennan, M.F.; Warshaw, A.L. Validation of a Postresection Pancreatic Adenocarcinoma Nomogram for Disease-Specific Survival. J. Clin. Oncol. 2005, 23, 7529–7535. [Google Scholar] [CrossRef]
- He, C.; Mao, Y.; Wang, J.; Duan, F.; Lin, X.; Li, S. Nomograms predict long-term survival for patients with periampullary adenocarcinoma after pancreatoduodenectomy. BMC Cancer 2018, 18, 327. [Google Scholar] [CrossRef]
- Li, H.-B.; Zhou, J.; Zhao, F.-Q. A Prognostic Nomogram for Disease-Specific Survival in Patients with Pancreatic Ductal Adenocarcinoma of the Head of the Pancreas Following Pancreaticoduodenectomy. Med. Sci. Monit. 2018, 24, 6313–6321. [Google Scholar] [CrossRef]
- Parmar, A.D.; Vargas, G.M.; Tamirisa, N.P.; Sheffield, K.M.; Riall, T.S. Trajectory of care and use of multimodality therapy in older patients with pancreatic adenocarcinoma. Surgery 2014, 156, 280–289. [Google Scholar] [CrossRef]
- Frakes, J.M.; Strom, T.; Springett, G.M.; Hoffe, S.E.; Balducci, L.; Hodul, P.; Malafa, M.P.; Shridhar, R. Resected pancreatic cancer outcomes in the elderly. J. Geriatr. Oncol. 2015, 6, 127–132. [Google Scholar] [CrossRef]
- Nagrial, A.M.; Chang, D.K.; Nguyen, N.Q.; Johns, A.L.; Chantrill, L.A.; Humphris, J.L.; Chin, V.T.; Samra, J.S.; Gill, A.J.; Pajic, M.; et al. Adjuvant chemotherapy in elderly patients with pancreatic cancer. Br. J. Cancer 2013, 110, 313–319. [Google Scholar] [CrossRef]
- Shin, S.H.; Park, Y.; Hwang, D.W.; Song, K.B.; Lee, J.H.; Kwon, J.; Yoo, C.; Alshammary, S.; Kim, S.C. Prognostic Value of Adjuvant Chemotherapy Following Pancreaticoduodenectomy in Elderly Patients With Pancreatic Cancer. Anticancer. Res. 2019, 39, 1005–1012. [Google Scholar] [CrossRef]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: The CONKO-001 randomized trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef]
- Neoptolemos, J.P.; Stocken, D.D.; Bassi, C.; Ghaneh, P.; Cunningham, D.; Goldstein, D.; Padbury, R.; Moore, M.J.; Gallinger, S.; Mariette, C.; et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: A randomized controlled trial. JAMA 2010, 304, 1073–1081. [Google Scholar] [CrossRef]
- Miyamoto, D.T.; Mamon, H.J.; Ryan, D.P.; Willett, C.G.; Ancukiewicz, M.; Kobayashi, W.K.; Blaszkowsky, L.; Castillo, C.F.-D.; Hong, T.S. Outcomes and Tolerability of Chemoradiation Therapy for Pancreatic Cancer Patients Aged 75 Years or Older. Int. J. Radiat. Oncol. 2010, 77, 1171–1177. [Google Scholar] [CrossRef]
- Miura, J.T.; Krepline, A.N.; George, B.; Ritch, P.S.; Erickson, B.A.; Johnston, F.M.; Oshima, K.; Christians, K.K.; Evans, D.B.; Tsai, S. Use of neoadjuvant therapy in patients 75 years of age and older with pancreatic cancer. Surgery 2015, 158, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, D.-B.; Zhang, Q.; Guo, C.-X.; Fu, Q.-H.; Zhang, X.-C.; Tang, T.-Y.; Su, W.; Chen, Y.-W.; Chen, W.; et al. The efficacy and toxicity of chemotherapy in the elderly with advanced pancreatic cancer. Pancreatology 2020, 20, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, J.D.; Rogers, J.E.; Hess, K.R.; Wolff, R.A.; Varadhachary, G.R.; Javle, M.M.; Shroff, R.T.; Ho, L.; Fogelman, D.R.; Raghav, K.P.; et al. Modified FOLFIRINOX in pancreatic cancer patients Age 75 or older. Pancreatology 2020, 20, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, T.; Babic-Illman, G.; Ross, P.; Maisey, N.; Hughes, S.; Fields, P.E.; Martin, F.C.; Wang, Y.; Harari, D. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br. J. Cancer 2015, 112, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- Overcash, J.; Ford, N.; Kress, E.; Ubbing, C.; Williams, N. Comprehensive Geriatric Assessment as a Versatile Tool to Enhance the Care of the Older Person Diagnosed with Cancer. Geriatrics 2019, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtmnan, S.M.; Gajra, A.; Bhatia, S.; Katheria, V.; et al. Predicting chemotherapy toxicity in older adults with cancer: A prospective multicenter study. J. Clin. Oncol. 2011, 29, 3457–3465. [Google Scholar] [CrossRef]
- Puts, M.T.E.; Santos, B.; Hardt, J.; Monette, J.; Girre, V.; Atenafu, E.G.; Springall, E.; Alibhai, S.M.H. An update on a systematic review of the use of geriatric assessment for older adults in oncology. Ann. Oncol. 2014, 25, 307–315. [Google Scholar] [CrossRef]
- Hurria, A.; Wildes, T.; Blair, S.L.; Browner, I.S.; Cohen, H.J.; DeShazo, M.; Dotan, E.; Edil, B.H.; Extermann, M.; Ganti, A.K.P.; et al. Senior Adult Oncology, Version 2.2014. J. Natl. Compr. Cancer Netw. 2014, 12, 82–126. [Google Scholar] [CrossRef]
- Wildiers, H.; Heeren, P.; Puts, M.; Topinkova, E.; Janssen-Heijnen, M.L.G.; Extermann, M.; Falandry, C.; Artz, A.; Brain, E.; Colloca, G.; et al. International Society of Geriatric Oncology Consensus on Geriatric Assessment in Older Patients With Cancer. J. Clin. Oncol. 2014, 32, 2595–2603. [Google Scholar] [CrossRef]
- Extermann, M.; Aapro, M.; Bernabei, R.; Cohen, H.J.; Droz, J.-P.; Lichtman, S.; Mor, V.; Monfardini, S.; Repetto, L.; Sørbye, L.; et al. Use of comprehensive geriatric assessment in older cancer patients: Recommendations from the task force on CGA of the International Society of Geriatric Oncology (SIOG). Crit. Rev. Oncol. Hematol. 2005, 55, 241–252. [Google Scholar] [CrossRef]
- Pallis, A.; Fortpied, C.; Wedding, U.; Van Nes, M.; Penninckx, B.; Ring, A.; Lacombe, D.; Monfardini, S.; Scalliet, P.; Wildiers, H. EORTC elderly task force position paper: Approach to the older cancer patient. Eur. J. Cancer 2010, 46, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.D.; Newman, A.B.; Hirsch, C.; Gottdiener, J.S.; Seeman, T.E.; Tracy, R.P.; Kop, W.J.; Burke, G.L.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Rockwood, K.; Song, X.; Macknight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. Can. Med. Assoc. J. 2005, 173, 489–495. [Google Scholar] [CrossRef]
- Bellera, C.A.; Rainfray, M.; Mathoulin-Pélissier, S.; Mertens, C.; Delva, F.; Fonck, M.; Soubeyran, P.L. Screening older cancer patients: First evaluation of the G-8 geriatric screening tool. Ann. Oncol. 2012, 23, 2166–2172. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef]
- Takaori, K.; Bassi, C.; Biankin, A.; Brunner, T.B.; Cataldo, I.; Campbell, F.; Cunningham, D.; Falconi, M.; Frampton, A.E.; Furuse, J.; et al. International Association of Pancreatology (IAP)/European Pancreatic Club (EPC) consensus review of guidelines for the treatment of pancreatic cancer. Pancreatology 2016, 16, 14–27. [Google Scholar] [CrossRef]
- Jones, R.P.; Psarelli, E.E.; Jackson, R.; Ghaneh, P.; Halloran, C.M.; Palmer, D.H.; Campbell, F.; Valle, J.W.; Faluyi, O.; O’Reilly, D.; et al. Patterns of Recurrence After Resection of Pancreatic Ductal Adenocarcinoma: A Secondary Analysis of the ESPAC-4 Randomized Adjuvant Chemotherapy Trial. JAMA Surg. 2019, 154, 1038–1048. [Google Scholar] [CrossRef]
- Goldsmith, C.; Plowman, P.N.; Green, M.M.; Dale, R.G.; Price, P.M. Stereotactic ablative radiotherapy (SABR) as primary, adjuvant, consolidation and re-treatment option in pancreatic cancer: Scope for dose escalation and lessons for toxicity. Radiat. Oncol. 2018, 13, 204. [Google Scholar] [CrossRef]
- Elberg Dengsø, K.; Tjørnhøj-Thomsen, T.; Oksbjerg Dalton, S.; Marcel Christensen, B.; Hillingsø, J.; Thomsen, T. It’s all about the CA-19-9. A longitudinal qualitative study of patients’ experiences and perspectives on follow-up after curative surgery for cancer in the pancreas, duodenum or bile-duct. Acta Oncol. 2019, 58, 642–649. [Google Scholar] [CrossRef]
- Deobald, R.G.; Cheng, E.S.W.; Ko, Y.-J.; Wright, F.C.; Karanicolas, P.J. A qualitative study of patient and clinician attitudes regarding surveillance after a resection of pancreatic and peri-ampullary cancer. HPB 2015, 17, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Ghaneh, P.; Hanson, R.; Titman, A.; Lancaster, G.; Plumpton, C.; Lloyd-Williams, H.; Yeo, S.T.; Edwards, R.T.; Johnson, C.; Abu Hilal, M.; et al. PET-PANC: Multicentre prospective diagnostic accuracy and health economic analysis study of the impact of combined modality 18fluorine-2-fluoro-2-deoxy-d-glucose positron emission tomography with computed tomography scanning in the diagnosis and management of pancreatic cancer. Health Technol. Assess. 2018, 22, 1–114. [Google Scholar] [PubMed]
- Palmieri, L.-J.; Coriat, R. 18F-FDG PET/CT in pancreatic adenocarcinoma: On the edge of a paradigm shift? Diagn. Interv. Imaging 2019, 100, 731–733. [Google Scholar] [CrossRef]
- Wang, L.; Dong, P.; Wang, W.; Tian, B. Positron emission tomography modalities prevent futile radical resection of pancreatic cancer: A meta-analysis. Int. J. Surg. 2017, 46, 119–125. [Google Scholar] [CrossRef]
- Riviere, D.M.; Van Geenen, E.J.M.; Van Der Kolk, B.M.; Nagtegaal, I.D.; Radema, S.A.; Van Laarhoven, C.J.H.M.; Hermans, J.J. Improving preoperative detection of synchronous liver metastases in pancreatic cancer with combined contrast-enhanced and diffusion-weighted MRI. Abdom. Radiol. 2019, 44, 1756–1765. [Google Scholar] [CrossRef]
- Marion-Audibert, A.-M.; Vullierme, M.-P.; Ronot, M.; Mabrut, J.-Y.; Sauvanet, A.; Zins, M.; Cuilleron, M.; Sa-Cunha, A.; Lévy, P.; Rode, A. Routine MRI With DWI Sequences to Detect Liver Metastases in Patients With Potentially Resectable Pancreatic Ductal Carcinoma and Normal Liver CT: A Prospective Multicenter Study. Am. J. Roentgenol. 2018, 211, W217–W225. [Google Scholar] [CrossRef]
- Jeon, S.K.; Lee, J.M.; Joo, I.; Lee, N.H.; Ahn, S.J.; Woo, H.; Lee, M.S.; Jang, J.-Y.; Han, J.K. Correction to: Magnetic resonance with diffusion-weighted imaging improves assessment of focal liver lesions in patients with potentially resectable pancreatic cancer on CT. Eur. Radiol. 2018, 28, 4475. [Google Scholar] [CrossRef]
- Kalisvaart, M.; Broadhurst, D.; Marcon, F.; Pande, R.; Schlegel, A.; Sutcliffe, R.; Marudanayagam, R.; Mirza, D.; Chatzizacharias, N.; Abradelo, M.; et al. Recurrence patterns of pancreatic cancer after pancreatoduodenectomy: Systematic review and a single-centre retrospective study. HPB 2020, 22, 1240–1249. [Google Scholar] [CrossRef]
- Butler, J.R.; Ahmad, S.A.; Katz, M.H.; Cioffi, J.L.; Zyromski, N.J. A systematic review of the role of periadventitial dissection of the superior mesenteric artery in affecting margin status after pancreatoduodenectomy for pancreatic adenocarcinoma. HPB 2016, 18, 305–311. [Google Scholar] [CrossRef]
- Versteijne, E.; Vogel, J.A.; Besselink, M.G.; Busch, O.R.C.; Wilmink, J.W.; Daams, J.G.; van Eijck, C.H.J.; Koerkamp, B.G.; Rasch, C.R.N.; van Tienhoven, G. Meta-analysis comparing upfront surgery with neoadjuvant treatment in patients with resectable or borderline resectable pancreatic cancer. Br. J. Surg. 2018, 105, 946–958. [Google Scholar] [CrossRef]
- Petrucciani, N.; Nigri, G.; Debs, T.; Giannini, G.; Sborlini, E.; Antolino, L.; Aurello, P.; D’Angelo, F.; Gugenheim, J.; Ramacciato, G. Frozen section analysis of the pancreatic margin during pancreaticoduodenectomy for cancer: Does extending the resection to obtain a secondary R0 provide a survival benefit? Results of a systematic review. Pancreatology 2016, 16, 1037–1043. [Google Scholar] [CrossRef]
- Schwarz, L.; Vernerey, D.; Bachet, J.B.; Tuech, J.J.; Portales, F.; Michel, P.; Cunha, A.S. Resectable pancreatic adenocarcinoma neo-adjuvant FOLF(IRIN)OX-based chemotherapy—A multicenter, non-comparative, randomized, phase II trial (PANACHE01-PRODIGE48 study). BMC Cancer 2018, 18, 762. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; Wu, L.; Ye, L.; Yao, L.; Tang, Z. Efficacy of extended versus standard lymphadenectomy in pancreatoduodenectomy for pancreatic head adenocarcinoma. An update meta-analysis. Pancreatology 2019, 19, 1074–1080. [Google Scholar] [CrossRef]
- Dasari, B.V.M.; Pasquali, S.; Vohra, R.S.; Smith, A.M.; Taylor, M.A.; Sutcliffe, R.P.; Muiesan, P.; Roberts, K.J.; Isaac, J.; Mirza, D.F. Extended Versus Standard Lymphadenectomy for Pancreatic Head Cancer: Meta-Analysis of Randomized Controlled Trials. J. Gastrointest. Surg. 2015, 19, 1725–1732. [Google Scholar] [CrossRef]
- Sabater, L.; Cugat, E.; Serrablo, A.; Suarez-Artacho, G.; Diez-Valladares., L.; Santoyo-Santoyo, J.; Martín-Pérez, E.; Ausania, F.; Lopez-Ben, S.; Jover-Navalon, J.M.; et al. Does the Artery-first Approach Improve the Rate of R0 Resection in Pancreatoduodenectomy?: A Multicenter, Randomized, Controlled Trial. Ann. Surg. 2019, 270, 738–746. [Google Scholar] [CrossRef]
- Negoi, I.; Hostiuc, S.; Runcanu, A.; Negoi, R.I.; Beuran, M. Superior mesenteric artery first approach versus standard pancreaticoduodenectomy: A systematic review and meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2017, 16, 127–138. [Google Scholar] [CrossRef]
- Hiraoka, T.; Uchino, R.; Kanemitsu, K.; Toyonaga, M.; Saitoh, N.; Nakamura, I.; Tashiro, S.; Miyauchi, Y. Combination of intraoperative radiation with resection of cancer of the pancreas. Int. J. Pancreatol. 1990, 7, 201–207. [Google Scholar]
- Katz, M.H.G.; Ou, F.S.; Herman, J.M.; Ahmad, S.A.; Wolpin, B.; Marsh, R.; Behr, S.; Shi, Q.; Chuong, M.; Schwartz, L.H.; et al. Alliance for clinical trials in oncology (ALLIANCE) trial A021501: Preoperative extended chemotherapy vs. chemotherapy plus hypofractionated radiation therapy for borderline resectable adenocarcinoma of the head of the pancreas. BMC Cancer 2017, 17, 505. [Google Scholar] [CrossRef]
- Lafranceschina, S.; Brunetti, O.; DelVecchio, A.; Conticchio, M.; Ammendola, M.; Currò, G.; Piardi, T.; De’Angelis, N.; Silvestris, N.; Memeo, R. Systematic Review of Irreversible Electroporation Role in Management of Locally Advanced Pancreatic Cancer. Cancers 2019, 11, 1718. [Google Scholar] [CrossRef]
- Tsuchida, H.; Fujii, T.; Mizuma, M.; Satoi, S.; Igarashi, H.; Eguchi, H.; Kuroki, T.; Shimizu, Y.; Tani, M.; Tanno, S.; et al. Prognostic importance of peritoneal washing cytology in patients with otherwise resectable pancreatic ductal adenocarcinoma who underwent pancreatectomy: A nationwide, cancer registry–based study from the Japan Pancreas Society. Surgery 2019, 166, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Fujii, T.; Yamamoto, T.; Takami, H.; Yoshioka, I.; Yamaki, S.; Sonohara, F.; Shibuya, K.; Motoi, F.; Hirano, S.; et al. Phase I/II study of adding intraperitoneal paclitaxel in patients with pancreatic cancer and peritoneal metastasis. BJS 2020, 107, 1811–1817. [Google Scholar] [CrossRef]
- Satoi, S.; Fujii, T.; Yanagimoto, H.; Motoi, F.; Kurata, M.; Takahara, N.; Yamada, S.; Yamamoto, T.; Mizuma, M.; Honda, G.; et al. Multicenter Phase II Study of Intravenous and Intraperitoneal Paclitaxel With S-1 for Pancreatic Ductal Adenocarcinoma Patients With Peritoneal Metastasis. Ann. Surg. 2017, 265, 397–401. [Google Scholar] [CrossRef]
- Lai, E.; Puzzoni, M.; Ziranu, P.; Pretta, A.; Impera, V.; Mariani, S.; Liscia, N.; Soro, P.; Musio, F.; Persano, M.; et al. New therapeutic targets in pancreatic cancer. Cancer Treat. Rev. 2019, 81, 101926. [Google Scholar] [CrossRef] [PubMed]
- Holter, S.; Borgida, A.; Dodd, A.; Grant, R.; Semotiuk, K.; Hedley, D.; Dhani, N.; Narod, S.; Akbari, M.; Moore, M.; et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. J. Clin. Oncol. 2015, 33, 3124–3129. [Google Scholar] [CrossRef] [PubMed]
- Waddell, N.; Initiative, A.P.C.G.; Pajic, M.; Patch, A.-M.; Chang, D.K.; Kassahn, K.S.; Bailey, P.; Johns, A.L.; Miller, D.; Nones, K.; et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nat. Cell Biol. 2015, 518, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Golan, T.; Hammel, P.; Reni, M.; Van Cutsem, E.; Macarulla, T.; Hall, M.J.; Park, J.-O.; Hochhauser, D.; Arnold, D.; Oh, D.-Y.; et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019, 381, 317–327. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).