Simple Summary
A significant number of prostate cancer patients will progress to metastatic castrate resistant prostate cancer despite optimal therapies. There is a growing need for alternative therapeutic strategies for this category of patients. Theragnostic refers to the ability to use an organ specific ligand and label it to both a diagnostic/imaging and therapeutic agent. Several prostate specific membrane antigen radioligands have been developed for imaging and treating PCa. Beta and alpha emitting radionuclides have been used with great success. Xerostomia is the greatest adverse event associated with radioligand therapy. More trials are necessary to determine the timing of introducing these novel therapies and to assess the efficacy as monotherapy as well as in combination with other novel agents to improve efficacy and reduce side effects to other organs.
Abstract
Prostate cancer (PCa) causes significant morbidity and mortality in men globally. While localized PCa may be managed with curative intent by surgery and/or radiation therapy, the management of advanced hormone resistant metastatic disease (mCRPC) is more challenging. Theranostics is a principle based on the ability to use an organ specific ligand and label it to both a diagnostic and a therapeutic agent. The overexpression of prostate specific membrane antigen (PSMA) on prostate cancer cells creates a unique opportunity for development of targeted radionuclide therapy. The use of both beta and alpha emitting particles has shown great success. Several clinical trials have been initiated assessing the efficacy and safety profile of these radionuclide agents. The results are encouraging with PSMA directed radioligand therapy performing well in patients who have exhausted all other standard treatment options. Future studies need to assess the timing of introduction of these radionuclide therapies in the management schema of mCRPC. Drugs or therapies are not without side effects and targeted radionuclide therapies presents a new set of toxicities including xerostomia and myelosuppression. New therapeutic strategies are being explored to improve outcomes while keeping toxicities to a minimum. This review aims to look at the various PSMA labelled tracers that form part of the theragnostic approach and subsequently delve into the progress made in the area of radionuclide therapy.
1. Introduction
Prostate cancer (PCa) is the second most frequent cause of cancer-related mortality in men globally. Therapy of PCa is a collaborative effort based on a multidisciplinary tumour board approach together with the patient and it is guided by the available resources. The widespread availability of screening tests has improved the early diagnosis of organ-confined PCa, making definitive therapy feasible [1]. Low risk organ confined disease may be managed in one of three ways, namely watchful waiting and active surveillance, surgery and/or radiation therapy (external beam therapy or brachytherapy). In localized high-risk or locally advanced disease, combination therapy with radical radiation therapy and long-term androgen deprivation therapy (ADT) with or without docetaxel is advised. Hormone sensitive metastatic disease is best managed with the combination of ADT and second generation anti-androgens (abiraterone acetate, enzalutamide, apalutamide and darolutamide) or ADT and docetaxel [2,3]. At some point during the course of the disease, PCa becomes resistant to ADT and is considered Metastatic Castrate-Resistant Prostate Cancer (mCRPC). Five years from the time of diagnosis, 10–20% patients develop mCRPC [4]. First line therapy for castrate resistant disease is second generation anti-androgens, docetaxel and 223Radium chloride (for patients with bone metastases only). Failure to respond to docetaxel may be treated with the second line agent, Cabazitaxel [3]. Metastatic cancer is one of the leading causes of prostate cancer-associated deaths. Despite great initial responses, patients soon progress and have limited treatment options.
Theranostics or theragnostics resulted from the amalgamation of the words therapy or therapeutic and diagnostics. This term was coined by Funkhouser in 2002 and has been the catchword since 2011 [5]. In the true sense this term refers to a close relationship between diagnostics and therapy whereby an appropriate diagnostic methodology is utilized to individualize therapeutic interventions [6]. Other definitions have expanded it to include monitoring of treatment response. In the discussion of the role of Nuclear Medicine in the management of prostate cancer, theranostics refers to the use of molecules that target disease-specific structures in the tumour and metastases. These molecules can be combined with an imaging agent for diagnosis/staging and subsequently to a therapeutic agent for therapy of the identified disease. Figure 1 below is an illustration of the practical aspects of theragnostics.
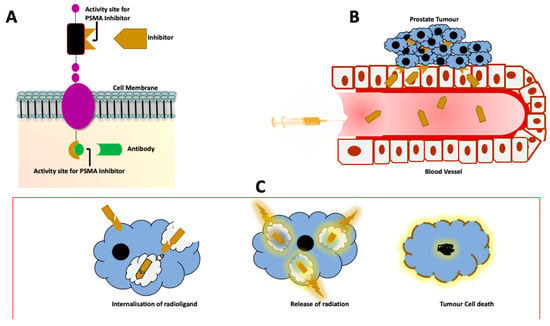
Figure 1.
Illustration of theragnostic approach to the management of PCa. (A) The PSMA protein with the different components, namely the intracellular, transmembrane and the extracellular portion. The activity sites on both the intracellular and extracellular domains have been used for binding of monoclonal antibodies, minibodies and small molecules. This enables targeting of the PSMA protein for both imaging and therapy. (B) Once the tracer is injected into the bloodstream, the PSMA ligand binds to the activity site on the tumour cells. (C) After binding to the activity site on the cell membrane, the radioligand is internalized and releases radiation from within the cell. The end product is DNA damage with resultant tumour cell death.
2. Prostate Specific Membrane Antigen (PSMA)
The emergence of Prostate Specific Membrane Antigen (PSMA) as a theragnostic agent has completely revolutionized the management of PCa. The PSMA protein is also known as glutamate carboxy peptidase II and folate hydrolase 1. It is a transmembrane glycoprotein enzyme found on the cell surface. The PSMA protein is divided into three parts, namely the internal portion, transmembrane portion, and the external portion [7,8]. The extracellular portion makes up 95% of the PSMA protein and is the accessible target for both small molecules and antibody-based agents for imaging and therapy. The internalization process that allows endocytosis of bound proteins on the cell surface of the PSMA receptor allows PSMA labelled radioisotopes to be concentrated within the cell [9]. The term “prostate specific” is a misnomer as there is minimal expression of PSMA in normal prostate tissue, kidneys, duodenum, salivary and lacrimal glands, brain, and intestines; however, it has been found to be highly expressed (up to 1000-fold) in PCa [10,11,12,13,14]. There is a strong correlation between PSMA expression and both the Gleason score and serum PSA value [15]. PSMA expression is decreased significantly during ADT confirming that PSMA expression is regulated by Androgen receptors [16]. There has been an evolution of the PSMA based approaches from antibodies and nanobodies to small molecules. The studies that provided the proof of concept for the utilization of PSMA targeting agents employed a mouse monoclonal antibody against the intracellular domain of PSMA protein. This was labelled to Indium-111 (111In-capromab pendetide) for Single Photon Emission Tomography (SPECT) imaging and marketed as Prostascint®. While it had great correlation to tissue specimen, it suffered from a lack of sensitivity [17,18,19,20]. This tracer was approved by the FDA, however it never had great take-off because of the slow kinetics which resulted in prolonged imaging duration and poor-quality images. Another antibody targeting the extracellular component (J591) was discovered by Dr Bander and colleagues. Other anti-PSMA monoclonal antibodies that bind various epitopes of the PSMA protein have also been investigated and include J415, Hybritech PEQ226.5 and PMJ2J004.5. They have been labelled with 111In, 124I, 64Cu and 89Zr and have demonstrated great promise with improved targeting of nodal, soft tissue and skeletal metastases [21,22,23]. Despite these modifications, there has been limited practical use of these tracers because they are still antibodies and suffer the same weaknesses as the 111In labelled antibody. Over the years PSMA-targeted small molecule ligands have been developed and have made significant progress. They have been labelled to 99mTc, 68Ga and 18F for imaging as well as 177Lu, 222Bi and 225Ac for therapy.
3. PSMA Based Imaging
Since the first human experience with PSMA small molecules in 2008 with the use of Iodine-123, there has been great progress made in this space with multiple variants of small molecule PSMA inhibitors being utilized for PCa imaging. The emergence of these tracers has resulted in better sensitivity and specificity for detecting locoregional and distant metastasis [24]. Although the bulk of data published is on Positron Emission Tomography (PET) imaging with PSMA ligands, there are a few SPECT tracers that have been investigated.
4. SPECT Tracers
The first SPECT tracers utilizing small molecules to image PCa were 123I-(MIP)-1072 and 123I-(MIP)-1095. Both these agents could detect the local tumour and metastases in soft tissue and bone with excellent tumour to target contrast [25,26]. The first human therapy utilizing PSMA ligands resulted from the use of these ligands substituting the 123I with 131I [26]. Technetium (99mTc) is a very convenient isotope because it is widely available and inexpensive. It has a 6-h half-life and can be labelled to a variety of tracers. There are a host of 99mTc labelled PSMA ligands (99mTc-MIP-1404, 99mTc I & S PSMA, 99mTc-HYNIC-PSMA) that have been evaluated for imaging of PCa [27,28,29,30,31]. All published work demonstrates that these tracers are useful in imaging of PCa. Caution should be applied especially in settings of low serum PSA and low volume disease; however, the value of these tracers should not be underestimated. While the tracers used for PET/CT have been shown to have greater imaging properties and results, these facilities may not always be affordable or available to remote areas. The advantage of SPECT tracers, more so 99mTc labelled PSMA ligands is that they may be of value in radioguided surgery with intraoperative probe localization [32,33,34].
5. PET Tracers
PET imaging of PCa with either Gallium-68 or Flourine-18 tracers is the most widely studied and validated method. A few trials have been undertaken to assess the diagnostic ability of PET labelled PSMA radiopharmaceuticals for PCa. Gallium-68 labelled tracers are the most extensively investigated.
5.1. Gallium-68 Labelled PSMA Ligands
Gallium-68 (68Ga) is a PET tracer with favourable pharmacokinetic characteristics that enable labelling with many peptides and other small molecules. The commercial accessibility of germanium-68/gallium-68 (68Ge/68Ga) generators and more recently cyclotron produced 68Ga, has resulted in vast data and publications focused on PSMA PET ligands. The 68Ga labelled tracers have been the most validated and as such the European Association of Urology and National Cancer Comprehensive Network have included 68Ga-PSMA PET/CT in their guidelines [1,35].
A prospective multicenter study addressed the use of 68Ga-PSMA-11 PET/CT in patients with newly diagnosed PCa and negative bone scan findings. The use of PET/CT resulted in a change in management in 12.6% of patients with a sensitivity and specificity of 41.5% and 90.9% respectively [36].
The multicenter prospective 2 phase proPSMA study set to compare conventional imaging by CT and bone scan against PSMA PET/CT in men with high risk PCa. A greater diagnostic accuracy was noted with PSMA PET/CT than conventional imaging (92% (range of 88–95%) vs. 65% (range of 60–69%); p < 0001) [37,38]. The sensitivity and specificity of PSMA PET/CT were also higher [37,38]. The imaging findings led to a change in management with a higher frequency in the PSMA PET/CT group than the conventional imaging cohort. This study provides convincing evidence and further confirms the previous findings of other authors [39]. These studies are helping cement the value of PSMA PET/CT prior to definitive therapy seeing as it impacts on the management of PCa.
A recent systematic review and meta-analysis to assess the diagnostic utility, sensitivity, and specificity of 68Ga-PSMA PET in advanced prostate cancer found an improved detection in patients with biochemical recurrence. In this analysis with close to five thousand patients from 37 articles/studies, in the setting of biochemical recurrence, there was a significant in difference in scan positivity in the prostate bed of patients who had radical prostatectomy (22%) or radiotherapy (52%). The detection at low PSA values < 0.2 ng/mL was 33% while for PSA values between 0.2 and 0.5 ng/mL were 45% [24].
There is mounting evidence to support the use of PSMA based imaging in the staging of high-risk patients as well as in patients with biochemical recurrence. These 68Ga labelled tracers have been shown to outperform conventional imaging and should form an integral part of diagnostic algorithms or guidelines.
5.2. Fluorine Labelled PSMA Ligands
Flourine-18 (18F) is a cyclotron produced radionuclide with favourable nuclear and physical properties. It has a high positron decay ratio (97%), relatively short half-life (109.7 min), and a low positron energy (0.635 MeV) which results in increased resolution because of the short diffusion range [40]. The half-life makes 18F more amenable for commercial development because it can be produced centrally and distributed to satellite sites. 18F based agents developed for PCa imaging include 18F-DCFBC, 18F-DCFPyl, 18F-rhPSMA and 18F-PSMA-1007. The Food and Drug Association (FDA) recently approved the use of 18F-DCFPyl in the work-up of PCa patients.
In their prospective study of 130 men with biochemical recurrence, Rousseau et al. localized recurrent disease in 60% of cases with PSA between ≥0.4 and <0.5, 78% with PSA between ≥0.5 and <1, 72% with PSA between ≥1 and <2 and 92% with PSA ≥ 2. They reported that there was a change in treatment intent, change in the stage of the disease and change in management plans in 65.5%, 65.5% and 87.3% of patients, respectively [41].
In a study by the National Cancer Institute (NCI), the sensitivity of 18F-DCFPyl in 90 patients with biochemical recurrence was 47.6% in patients with PSA level between 0.2 and 0.5 ng/mL, 50% in patients with PSA 0.5–1 ng/mL, 88.9% for PSA between 1–2 ng/mL and 94% in patients with PSA > 2 ng/mL. They validated their findings against histology or a composite reference of histology, imaging (pelvic magnetic resonance imaging (MRI) performed within 3 months of the PSMA PET), follow-up CT, 99mTc bone scan or 18F-sodium fluoride PET/CT at 3 to 6 months) or clinical follow-up (PSA trend after treatment). When using histopathology for validation, the positive predictive value (PPV) on a per patient basis was 93.3% while it was 96.2% when using the combination of histology, imaging and/or clinical follow-up [42]. Although 18F-DCFPyl has a high positivity rate even at low PSA values, it performs better as the PSA value rises. This is comparable to published results using 68Ga-PSMA ligands.
The fact the 18F-PSMA-1007 is predominantly excreted by the hepatobiliary system theoretically implies amended biodistribution which will lead to improved detection in the pelvis or prostate bed region as compared to the other PSMA PET agents. A systematic review by Awenat and colleagues found a high diagnostic accuracy of 18F-PSMA-1007 of 80–100% on a per patient-based analysis and from 93–95% on a per lesion-based analysis in initial staging of PCa patients [43]. When compared with other radiological and scintigraphic imaging methods 18F-PSMA-1007 had a higher sensitivity, whereas when compared to other PSMA targeted PET/CT tracers, it had a similar diagnostic accuracy for initial staging. In the setting of biochemical recurrence, where it is purported that 18F-PSMA-1007 will have an added advantage, a multi-centre study found high detection rates which were comparable to or better than 68Ga-PSMA ligands. The detection rates had a positive correlation with the serum PSA level. Local recurrence was revealed in 24.7% of the patients, while 40.6% of the patients had pelvic lymph node metastasis and 43.8% of patients had bone and visceral metastasis [44].
A systematic review and meta-analysis of six 18F-PSMA PET/CT articles/studies with 645 patients with biochemical recurrence, found a pooled detection rate of 81% (95% CI: 71–88%). Using different PSA cutoff values, the pooled detection rate was 49% for PSA < 0.5 ng/mL (95% CI: 23–74%) and 86% for PSA ≥ 0.5 ng/mL (95% CI: 78–93%). The detection rate is highly dependent on PSA values with an overall better detection rate with higher PSA values ≥ 0.5 ng/mL [45].
The choice of tracer should be guided by local circumstances including health funding models/coverage, availability of skilled personnel for onsite radiolabelling for 68Ga or availability of local producers of 18F-PSMA tracers and, of course evidence and personal experience with the different tracers.
5.3. Indications for PSMA Ligand Imaging
On 1 December 2020, the FDA approved 68Ga-PSMA-11 for imaging in two clinical settings, namely in men with suspected metastases who are candidates for initial definitive therapy and secondly in men with suspected recurrence based on elevated serum PSA levels [46].
On the other hand, the European Society of Medical Oncology (ESMO) has recommended the use of PSMA PET/CT imaging in biochemical recurrence because of the superior sensitivity and specificity compared to conventional imaging [3]. While PSMA PET/CT has better sensitivity and specificity than CT or bone scan, the ESMO does not make a specific recommendation for its use in patients with intermediate or high-risk disease as it has not been shown to improve clinical outcomes [3].
The joint European Association of Nuclear Medicine (EANM) and Society of Nuclear Medicine and Molecular Imaging (SNMMI) guideline for PSMA ligand imaging is based on data from studies using 68Ga-PSMA. Currently the indications for imaging with 68Ga-PSMA or other PSMA labelled radioligands is as follows [47]:
- To localize tumour in biochemical recurrence, especially with PSA values between 0.2 and 1 ng/mL,
- High risk (Gleason score > 7, PSA > 20 ng/mL and clinical stage T2c-3a) patients to guide clinical decision making and for therapy planning,
- Staging before and during PSMA-directed therapy in patients with mCRPC,
- Targeted biopsy in patients with high suspicion of PCa with previous negative biopsy,
- Monitoring systemic therapy in metastatic Pca.
Of these indications, the first two have been validated while the last three are emerging applications. With the widespread introduction of radionuclide radiotherapy, the use of imaging for monitoring therapy will become routine.
The growth and expansion of the role of PSMA based ligands in imaging and therapy has also called for imaging specialists to adapt and formulate methods or algorithms for reporting follow-up imaging when monitoring patients that have had local or systemic therapies. This is for standardization and harmonization. In patients with mCRPC, it has been proposed that molecular response assessment may be better than morphological response criteria, especially in bone-dominant disease. In a consensus statement by international experts in PCa, the panelists agreed that patients should be broadly categorized as responders or non-responders. Patients with complete response (CR), partial response (PR), or stable disease (SD) on PSMA PET/CT should be classified as responders, while those with progressive disease (PD) on PSMA PET/CT should be considered as non-responders. The criteria to classify PSMA response into one of the four categories (CR, PR, SD, PD) mentioned above include [48,49]:
- Complete response: Disappearance of any lesion with tracer uptake
- Partial response: Reduction of uptake tumour PET volume by >30%
- Stable disease: Change of uptake and tumour PET volume by ±≤30% and no new lesions documented
- Progressive disease: Appearance of ≥2 new lesions or increase of uptake or tumour volume ≥30%
In the category of progressive disease, the panelists made special exceptions in the scenario of oligo- or polymetastatic disease. When performing follow-up imaging in patients that have had PSMA radioligand therapy, caution should be applied. This is because during the course of disease, there may be downgrading of tumour cell PSMA expression. Response assessment should be based on a composite of clinical, biochemical, and other imaging findings.
5.4. 18F-FDG for Imaging Discordance
Well differentiated early-stage prostate cancer cells demonstrate low tracer (18F-FDG) avidity. Prostate cancer is heterogenous in nature and in advanced mCRPC when there is de-differentiation, this heterogeneity is even more pronounced. There is reduced expression of PSMA molecule in advanced disease with reduced to no uptake/avidity on 68Ga-PSMA ligand imaging. These patients have been found to have discordance between 68Ga-PSMA and 18F-FDG PET/CT imaging and more aggressive phenotype with poor response to PSMA directed therapies [50,51,52,53]. Chen et al. found 13/56 (23.2%) of patients with at least one PSMA negative and FDG positive lesion. The PSA and Gleason score were higher in these patients [54,55]. Therefore, it may be of value to add 18F-FDG in the imaging algorithm of patients with mCRPC who have negative PSMA imaging despite rising serum PSA level. In the Australian TheraP trial, dual imaging with 68Ga-PSMA and 18F-FDG was performed. This was to guide the selection of patients for radiolabelled radionuclide therapy as patients with PSMA negative and FDG positive studies were excluded [56,57].
6. PSMA Radioligand Therapy
Since the discovery of PSMA theranostics with Iodinated PSMA molecules, there has been a plethora of publications on the subject of PSMA based therapy for PCa. Over the years modifications to the molecules have resulted in better tumour uptake and responses and several trials have now released the results of such therapies. These include anti-PSMA antibodies such as J591 and PSMA targeted small molecules ligands such as MIP-1095, PSMA I & T, and PSMA-617 labelled with alpha or beta particle emitting isotopes. The optimal setting to use/apply these therapies is still being sought, hence the multiple clinical trials. Currently, these therapies are usually indicated in patients with metastatic, castrate resistant PCa (mCRPC) who have exhausted all other treatment options or are ineligible for those treatment options. The condition for therapy with PSMA ligands is that there should be adequate uptake on the PSMA ligand based diagnostic scan. Adequacy of uptake is not standardized however the study by Hofman and colleagues suggested uptake in lesions that is 1.5 times higher than liver uptake [58,59].
Practical Aspects of Radionuclide Therapy with 177Lu-PSMA
The decision to proceed with radionuclide therapy incorporates imaging findings (PSMA based ligand imaging) as well as clinical and biochemical findings. Ideally, this decision is made by a multidisciplinary tumour board.
The European Association of Nuclear Medicine have published a guideline for PSMA radioligand therapy in patients with mCRPC. These guidelines provide a base for the harmonization of molecular based therapy with 177Lu-PSMA. Figure 2. Below is an algorithm for the therapeutic procedure as well as the follow-up of patients treated with 177Lu-PSMA.
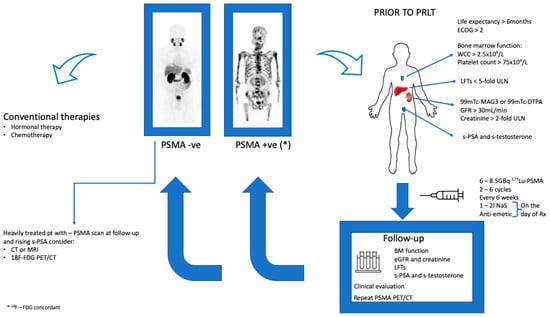
Figure 2.
Algorithm of the practical aspects of therapy from the selection of patients for targeted radionuclide therapy to the therapeutic procedure and follow-up. * 18F—FDG Concordant disease.
7. Lutetium Therapy
The most popular radionuclide for therapeutic applications across a wide spectrum of malignancies is 177Lu. The successful radiolabelling of 177Lu to a somatostatin analogue for the treatment of neuroendocrine tumours has led to the worldwide growth in interest and use of this radionuclide in therapy [60]. It is a radiometal that is reactor produced and a beta emitter (β-emitter) with a maximum energy of 498 keV (±0.5 MeV). It also has gamma rays (y-rays) with energies of 208 and 113 keV (11% and 6%, respectively) which allow for imaging and dosimetric calculations [61]. The half-life of 177Lu is 6.73 days making it long enough to impart the radiation to the tumour over an extended period of time. It has a maximal and mean tissue penetration of <2 mm (1.7 mm) and 0.23 mm, respectively which makes it ideal for small tumours. The tissue penetration of 177Lu is equivalent to 20 and 60 cell diameters, which results in a “crossfire effect”, that is DNA damage in the tumour cells as well as the surrounding cells. Of the various PSMA small molecule peptides and antibodies that have been labelled with 177Lu, PSMA-DKFZ-617 is the most widely published with PSMA I & T also fast gaining publicity and to a lesser degree, the antibody J591 also being investigated [61,62,63,64,65,66,67,68,69,70].
7.1. Efficacy of 177Lu-PSMA in mCRPC
One of the first studies to provide initial evidence of the safety and efficacy of PSMA labelled radioligand therapy is from wo-center study in Germany. In ten patients with mCRPC, seven (70%) experience a PSA decline 2 months after therapy with five (50%) having a PSA decline >50% after a single dose of 177Lu-DKFZ-617 [71]. The side effect profile included mild haematological toxicity, insignificant acute renal toxicity and transient xerostomia. The results of this early work are encouraging and offered some insight to the potential use of 177Lu-PSMA in the treatment of mCRPC.
The efficacy of 177Lu-PSMA-617 was further highlighted in the early work by Rahbar and colleagues who assessed the response and toxicity after a single dose of ±6 GBq 177Lu-PSMA-617. In this retrospective multicenter analysis, the authors found that there was a PSA decline in 35/74 patients (64%) with 31% of patients having a PSA decline of greater than 50%. These results were not confirmed by a second measurement; however, the results still demonstrated the potential efficacy of 177Lu-PSMA-617 as a therapeutic option for patients with mCRPC. Unfortunately, seventeen patients (23%) had progressive disease as evidenced by a PSA level rise of more than 25%. The patients in the study had extensive metastatic disease and had been heavily pre-treated [72].
Much of the initial work on 177Lu-PSMA radionuclide therapy is based on retrospective analysis of data acquired in centers that were offering 177Lu on a compassionate basis. A German multicenter study investigating 177Lu PSMA-617 therapy in pooled data of 145 men with mCRPCa demonstrated that this therapy is effective and safe. All patients had been pre-treated with standard of care regimen including 1st and 2nd line chemotherapy. With administered doses ranging between 2–8 GBq, there was a biochemical response (>50% decline in PSA) in 40% of the patients after the first cycle with response in 45% of patients over the entire follow-up period [73]. The authors concluded that this therapy may supersede the performance of other third-line systemic therapies. Interestingly, a metanalysis of 16 studies with a total of 671 patients also found a biochemical response in 45% of the patients and any decline in PSA in 75% of patients [74]. They went on to report on the progression free survival (PFS) and overall survival (OS) and found that the median PFS was 11 months (IQR: 7.6–13.7) from the five studies that reported PFS, while the median OS from six studies was 13.7 months (IQR: 8–14) [74]. Another meta-analysis also found a similar pooled PSA response (>50% decline) in 34.4% (95% CI: 30.14–38.97%) of patients [75].
In their single arm, single center prospective study, Hofman et al. proved that 177Lu-PSMA-617 is effective with high response rates and minimal side effects in patients with mCRPC who had progressed after conventional therapies. Thirty men fulfilled the criteria for inclusion into this study which included patients with progressive disease with high PSMA expression as evidenced by positive PSMA PET and negative FDG PET. Study participants received up to 4 cycles of 177Lu-PSMA-617 at six weekly intervals. The primary end point was PSA response and toxicity with imaging response and quality of life response being additional primary end points. All patients had been heavily treated with atleast one line of either chemotherapy or androgen axis targeting drugs. With a mean administered activity of 7.5 GBq per cycle, just over half (17/30) of the patients achieved a PSA decline (57%; 95% CI: 37–75). The median PSA PFS was 7.6 months (95% CI: 6.3–9.0) and OS was 13.5 months (95% CI: 10.4–22.7). While there was treatment related toxic effects to the salivary gland and bone marrow, none of the patients dies because of the therapy. The commonest side effect was grade 1 xerostomia (87%) with 4/30 (13%) patients presenting with grade 3 or 4 thrombocytopaenia as a result of the therapy [58].
Another trial to emerge from the same Australian group compared 177Lu-PSMA-617 to cabazitaxel in terms of efficacy and safety. It was a multicenter, phase II randomized, stratified, two-arm clinical trial in patients with mCRPC suitable for chemotherapy. Patients in the treatment arm receive standard of care treatment with cabazitaxel (20 mg/m2) intravenously every 3 weeks with Prednisolone 10 mg orally daily for a maximum of 10 cycles. The experimental group received 8.5 GBq 177Lu-PSMA-617 decreasing by 0.5 GBq with every subsequent cycle and this was repeated every 6 weeks up to a maximum of 6 cycles. The primary end point was to determine the proportion of patients with PSA response in both groups with OS and objective tumour response rate, PFS, PSA PFS, rPFS and pain response as some secondary end points. Sixty-five of 99 men (66%) randomized to 177Lu-PSMA-617 achieved PSA response compared with 31 of 85 men (37%) randomized to cabazitaxel. Treatment with 177Lu-PSMA-617 delayed progression as compared with cabazitaxel (Hazard ratio, HR = 0.63, 95% CI = 0.46–0.86, p = 0.0028). Among 78 men with evaluable lesions as per RECIST criteria, objective response occurred in 49% (95% CI = 33–65) of patients treated with 177Lu-PSMA-617 compared with 24% (95% CI = 11–38) of patients treated with cabazitaxel (Hofamn). This study also showed that 177Lu-PSMA therapy is safe as evidenced by 33% grade 3/4 adverse events as opposed to 53% in the cabazitaxel group [59].
7.2. Vision Trial Results
The results of the much-anticipated Phase III VISION trial are finally in and have been officially presented during the 2021 American Society of Clinical Oncology Annual Meeting Plenary session. The trial recruited 831 men with metastatic PCa and randomized them to two arms to receive either best standard of care (bSOC) therapy or best standard of care plus 177Lu-PSMA-617 targeted radionuclide therapy [76,77]. The conclusion is that bSOC plus 177Lu-PSMA-617 significantly improves overall survival than bSOC alone. The median OS for the 177Lu-PSMA-617 + bSOC arm was 15.3 months compared to 11.3 months in the bSOC only arm, with a 38% (hazard ratio: 0.62; 95% CI: 0.52–0.73) reduction in risk of death (median OS benefit of 4 months). Similarly, the median radiographic progression free survival (rPFS) was prolonged in the 177Lu-PSMA-617 arm than the bSOC with a median rPFS of 8.7 months (99.2% CI: 7.9–10.8) and 3.4 months (99.2% CI: 2.4–4), respectively. Consistently, the disease control rate and overall response rate were higher in the 177Lu-PSMA-617 and bSOC cohort than in the bSOC cohort. Unfortunately, the 177Lu-PSMA-617 cohort had higher rate of treatment related adverse events compared to the bSOC only cohort, 85.3% vs. 28.8%, respectively [76]. These results are likely to influence management decisions and future guidelines. The combination of the bSOC and 177Lu-PSMA-617 has demonstrated superior results but at the risk of increased treatment related side effects, therefore it may be worthwhile to consider a head-to-head comparison of 177Lu-PSMA-617 monotherapy against the conventional therapies or bSOC therapy [77]. The TheraP trial has already demonstrated superiority of 177Lu-PSMA against carbazitaxel, therefore the evidence is accumulating and should influence decision-makers to consider the introduction of 177Lu-PSMA in guidelines and protocols for the management of mCRPC.
7.3. Indicators of Prognosis
Several factors have been shown to be predictive of treatment response and overall survival. Based on imaging parameters on pre-therapeutic PSMA PET imaging, Seifert et al. found that the PSMA tumour volume (PSMA-TV) and PSMA tumour lesion quotient (PSMA-TLQ) were independent prognostic factors of survival [78]. Another study aimed to retrospectively identify pre-therapeutic imaging parameters that would predict which patients will achieve an early biochemical response to 177Lu-PSMA-617 therapy. Interestingly, this study found pre-therapeutic SUVmax (HR = 7.94; p = 0.001) to be correlated with a PSA change after two cycles while the PSMA-TV and whole-body total lesion (TL) PSMA were not [79]. The change in serum PSA has been shown to be the single most significant parameter correlated with overall survival. Patients with a PSA decline 2 months after the first cycle had a significantly longer median overall duration of survival (median: 63–71 weeks) than those that did not demonstrate any change in PSA (median: 33–47 weeks) [72,80,81]. The reports of the association between baseline PSA (prior to therapy) and overall survival have yielded conflicting results with other authors sighting a significant correlation between baseline PSA and overall survival following 177Lu-PSMA-PRLT [82,83]. Too much heterogeneity and controversy exist over the correlation between cumulative dose or administered activity with overall survival. Rahbar and colleagues indicated that a cumulative injected activity of ≥18.8 GBq is prognosticator of overall survival. Other independent predictors of biochemical response include an alkaline phosphatase (ALP) level < 220 U/L, the absence of visceral metastases and a higher number of therapy cycles [72]. The effect of prior therapies on overall survival was also shown by the results from the WARMTH multicenter study [84]. The presence of liver metastasis has been consistently reported to be associated with a poorer prognosis [85,86,87]. While the data is robust for the association between OS and the presence of liver metastasis, there is insufficient data on lung and brain metastasis, however a systematic review by von Eyben et al. found that patients with bone and lung metastases lived longer than patients with liver metastases [88].
7.4. Dosimetry
Dosimetric calculations require sequential imaging over several time-points, preferably using SPECT/CT techniques. Scan should be performed after 4–7 days post therapy. In patients with multiple sites of disease, estimation of clinically relevant tumour-absorbed dose is challenging [89]. Tolerance limits for the target organs, namely salivary glands, kidneys, and red marrow are 35 Gy, 28–40 Gy, and 2 Gy, respectively [89]. A dosimetric study of 26 participants imaged over a period of 7 days demonstrated that the 24-h post-injection scan had the highest target to background ratio for all normal organs whereas the 48-h image had the highest tumour to background ratio. The mean absorbed dose to the salivary gland, kidneys and red marrow were 1.24 mGy/MBq, 0.99 ± 0.31 mGy/MBq, and 0.04 mGy/MBq, respectively [90]. A higher absorbed dose was seen in patients with extensive skeletal metastasis, which is expected considering the path length of the beta particles of 177Lu. The infusion of amino acids for renoprotection does not seem to be beneficial as some groups omitted them and still found similar estimates for absorbed radiation dose to the kidney (0.88 ± 0.40 mGy/MBq and 0.6 Gy/GBq) [74,91,92,93]. Patients receiving less than 10 Gy were unlikely to achieve a fall in PSA of at least 50% [94]. In a prospective phase II trial designed to determine the dose that will be both effective and low on toxicity, Calais et al. randomized patients with mCRPC to receive either 6 GBq or 7.4 GBq of 177Lu-PSMA-617. The efficacy profile 177Lu-PSMA appeared to be favourable and comparable at both activity regimen. The PSA PFS was 2.9 months (95% CI: 0–9) in the 6 GBq group and 3.7 months (95% CI: 1.9–5.6) in the 7.4 GBq group. At the end of follow-up there were almost equal numbers of patients that had died in both treatment dose arms (86% vs. 87% for 6 GBq and 7.4 GBq, respectively) [95].
7.5. Toxicity
Systemic therapies are more likely to result in toxicities and 177Lu-PSMA is no exception. Early reports of radioligand therapy with 177Lu-PSMA have demonstrated varying grades/degrees of a wide spectrum of toxicities. These include nausea, fatigue, xerostomia, nephrotoxicity and haematological toxicity, to name a few. In a systematic review and meta-analysis of 17 original studies, low grade toxicities were the most documented [74]. Anaemia related toxicities were the commonest haematological toxicities occurring in 23% of patients (IQR: 7–35%). Leucopaenia and thrombocytopaenia were recorded in a median of 14.2% (8–25%) and 15% (6–24%) of patients, respectively [74]. Up to 10% of patients experience grade 3 or 4 haematological toxicities [96,97]. This limited bone marrow reserve may be because of prior therapies especially considering the current scheduling and application of this radionuclide therapy. Nephrotoxicity was evaluated in 10 studies with 9.5% of patients (IQR: 0–20%) experiencing renal impairment as a result of RLT. Salivary gland toxicities in the form of pain, swelling and dry mouth post therapy were described in as many as 14.5% of patients [74]. Despite all these toxicities, this therapy is effective and safe even in older patients (>75 years) [98]. In the TheraP randomized, open label, phase 2 trial comparing 177Lu-PSMA-617 to cabazitaxel, the safety profile of 177Lu-PSMA-617 was superior to that of cabazitaxel. Patients randomized to 177Lu-PSMA-617 had fewer reported grade 3–4 adverse events except thrombocytopaenia. This further supports the use of 177Lu-PSMA-617 in patients who are older or who have co-morbidities [59].
7.6. Rechallenge after Initial Therapy with 177Lu-PSMA
Almost a third of patient treated with 177Lu-PSMA do not respond to this form of therapy, whilst some patients who demonstrated an initial excellent response will relapse. Rechallenge of an oncologic treatment in patients with mCRPC who initially responded to the primary treatment has been described as a potential treatment option after a treatment “holiday”. This concept has been described for the chemotherapeutic drug with reasonable responses and moderate toxicity. Gafita et al. described their initial experience with 177Lu-PSMA rechallenge in eight patients who underwent a median of 2 cycles (range 1–4). There was a treatment (177Lu-PSMA) free period of 5.4 months (range: 3.8–14.7 months). Almost 40% (3/8) of the patients has a biochemical response with a PSA decrease of 50% [99]. There was an encouraging radiographic response in three patients while 4 patients had progressive disease. The median OS and PSA PFS were 14 (95% CI: 6.2–21.8) and 3.2 (95% CI: 2.6–3.7) months, respectively. While there is evidence of that retreatment with 177Lu-PSMA is feasible and efficacious, it does come at a risk of higher toxicities. In their cohort, Gafita et al. reported grade 3 toxicities in 3 patients. A study with a larger number of participants confirmed these findings [100]. The patients were initially treated with a median of three cycles (range: 1–5 cycles) and went on to receive another 3 cycles (range: 1–5 cycles) of retreatment. The median cumulative administered activities at baseline therapy and, after rechallenge were 17.9 GBq and 37.4 GBq, respectively. There were no grade 4 toxicities noted with grade 3 toxicities in 23% of the patients which included: anaemia (3 patients), leucopaenia (2 patients), thrombocytopaenia (1 patient), neutropaenia (1 patient) and one patient developed renal impairment. The results demonstrated that majority of the patients benefitted from additional cycles. There was a stable PSA level (<25% increase and <50% decrease) in 50–60% of the patients. A ≥50% decline in PSA was seen in 26% of patients after the first cycle with 40% and 20% declines in PSA seen after the second and third cycles of rechallenge, respectively. After a median of 3 months (range: 1–15 months), the median OS was 12 months after the retreatment which was lower than the 25 months OS after the primary therapy. The median time PSA progression was 2.8 months (range: 1–11 months). Violet et al. also found that 11/15 (73%) of patients had a PSA decline of atleast 50% after retreatment with 177Lu-PSMA at first or second relapse [94]. The median OS from the time of study enrollment was 26.6 months for patients that had retreatment [94]. This is comparable to the results of Yordonova et al. Contrary to the other two studies, there were grade 4 toxicities documented namely thrombocytoapenia and neutropaenia.
The results from these studies prove that rechallenge in patients that had an initial response to 177Lu-PSMA is feasible and should be considered in select cases. In view of the toxicities after rechallenge, ideally patients should have recovered from any toxicity resulting from the initial treatment with 177Lu-PSMA or conventional systemic therapies. This decision should be made in a multidisciplinary tumour board setting with input from all managing physicians.
8. Actinium-225 Therapy
The potential of Actinium-225 (225Ac) for the treatment of advanced cancers has been proven in several clinical trials. It is produced from a Thorium-229 (229Th) generator, however, there have been attempts of large-scale linear accelerator or cyclotron production by irradiation of Radium-226 (226Ra) [101]. It is an alpha emitting particle with a relatively long half-life of 9.9 days which make 225Ac a cytotoxic radionuclide. 225Ac is classified as a “nanogenerator” or “in-vivo generator” as the decay yields four alpha particles, three β− disintegrations and two gamma emissions [101,102,103]. There is a low abundance of gamma emissions which may be used for dosimetric calculations although their low abundance renders 225Ac unsuitable for planar SPECT imaging. While most authors do not do posttherapy scans following administration of 225Ac, some authors have reported acquisition of posttherapy scans using the 440 kEV y-coemission of 213Bi (26% probability), the 218 keV y-coemission of 221Fr (12% probability) and the Brehmstrahlung of 209Pb [104]. The alpha particles that are produced have energies ranging from 5.8–8.4 MeV with soft tissue ranges of 47–85 µm. This soft tissue penetration is equivalent to 2–3 cell diameters, which results in selective killing of targeted cells while sparing the surrounding tissues [105]. The high linear energy transfer of alpha particles deposited within a short radius cause irreparable double strand DNA breaks [106]. Beta particles are heavily dependent on the phase in the cell cycle as well as the tissue oxygenation status (production of free radicals) for the induction of DNA damage. Alpha particles on the other hand are larger in size as compared to beta particles which allows for direct DNA damage independent of the oxygenation status.
8.1. Efficacy of 225Ac-PSMA Therapy for mCRPC
In contrast to 177Lu, there are fewer clinical studies and trials on the efficacy of 225Ac in the treatment of patients with mCRPC. This may be related to the issues of production costs, logistical and supply problem of alpha emitters. Preclinical and preliminary data on the efficacy and safety of PSMA targeted alpha therapy (TAT) are encouraging [14,107].
The Heidelberg group published their initial experience of 225Ac as salvage therapy in two patients who had been heavily pretreated and with one of the patients having progressed following 177Lu-PSMA therapy. The patient who had progressed following 177Lu-PSMA therapy had liver metastases which has been considered a poor prognosticator in studies using 177Lu-PSMA therapy and has since been considered a criterion for exclusion in 177Lu-PSMA therapy trials. This patient responded well to 225Ac therapy with his serum PSA level dropping from 294 ng/mL to undetectable following three cycles of therapy [108]. In both patients there was an excellent biochemical and imaging response with none or limited bone marrow toxicities. The most significant side effect from this therapy in both patients was xerostomia. This work provided encouraging results and demonstrated the potential of 225Ac in patients with mCRPC who have exhausted all other options.
The evidence presented thus far on targeted alpha therapy (TAT) with 225Ac-PSMA in mCRPC patients is in heavily pre-treated patients who have exhausted all treatment options. In seventeen chemotherapy naive men with mCRPC, Sathekge et al. demonstrated an impressive response to 225Ac-PSMA-617 with 70% of these patients having a ≥80% decline in serum PSA after a single cycle of therapy and 82% of patients having a ≥90% decline in serum PSA level at the end of treatment. Only three patients had a rise in the serum PSA, however one of these patients went on to eventually have a serum PSA decline of 74% by the end of treatment, while the other patient progressed despite having received three cycles of therapy and subsequently received no further treatment. After a median follow-up of 13 months, just over 40% of the patients had remained in remission. While this follow-up period is limited especially for monitoring long-term responses and toxicities, the outcome in these patients is still quite impressive considering that progression free survival with some of the chemotherapeutic drugs is only up to 4.8 months [109]. Figure 3. An image of a chemo-naïve patient with a remarkable response to targeted alpha therapy with 225Ac-PSMA. This study presents evidence that supports the early introduction of TAT in the therapeutic schema of mCRPC. There is a need for phase 3 clinical trials to better define the timing of the application of 225Ac-PSMA in the treatment sequence of mCRPC. In a larger series of >70 patients, this group went on to confirm these findings of an excellent biochemical and imaging response in heavily patients with mCRPC who had failed other conventional therapies. In this study, 70% of patients had a PSA decline of ≥50% and this was the single most significant predictor of overall survival with an OS of 20.1 months for patients with serum PSA decline of ≥50% after the first treatment cycle and 10.5 months for those with a <50% decline in serum PSA level [110]. They also found that previous treatment with 177Lu-PSMA was associated with a shorter progression-free survival suggesting that prior therapy with this beta emitter may induce resistance to radiation [110].
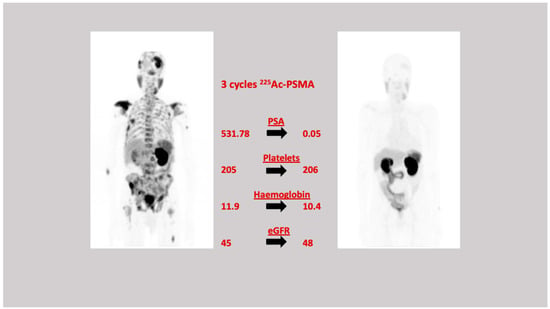
Figure 3.
A 79 yr old male with prostate adenocarcinoma. Gleason score = 4 + 3. Previous therapy—prostatectomy. 68Ga-PSMA-617 demonstrated widespread axial and appendicular skeletal metastases. He received three cycles of 225Ac-PSMA therapy (19 July 2017, 28 September 2017 and 23 November 2017). The patient had an excellent biochemical, clinical, and imaging response and was in remission.
In a detailed assessment of duration of tumour control from any 1st, 2nd, 3rd, and 4th line therapy. In this analysis of 40 men, they found that irrespective of the treatment modality, the median tumour control duration was 8, 7, 6 and 4 months. The median duration to arbiraterone, docetaxel, enzalutamide, carbazitaxel and 223Ra irrespective of treatment line was 10, 6.5, 6 and 4 months, respectively [111]. Radioligand therapy with 225Ac-PSMA which was applied as last line therapy had a mean tumour control duration of 9 months which is quite remarkable as the general observation was that the duration of tumour control decreased the further down the line the treatment was applied [111]. The findings in this study together with those of Sathekge et al. in their chemotherapy naïve patients, leave an unanswered question as to whether there would be greater benefit if 225Ac-PSMA was given earlier in the treatment line. This can only be interrogated in clinical trials. The case report by Rathke et al. of a patient with a 5-year duration of remission, supports this finding of prolonged duration of tumour control with 225Ac [112].
A group from the Netherlands looked at the clinical outcomes and molecular profiling of advanced mCRPC in patients treated with 225Ac-PSMA-617. They performed immunohistochemical staining and next generation sequencing of the pre- and post-therapy (225Ac-PSMA) biopsy specimen. When a metastatic biopsy was unavailable, they utilized archived biopsy specimen. The staining performed was for neuroendocrine markers (CD56 antigen, chromogranin and synaptophysin), PSMA, androgen receptor and Ki-67 expression. In terms of efficacy, their findings confirm those of other published work. PSMA RLT-naïve patients had a longer median OS 12.6 months as opposed to 1.3 months for the patients who previously underwent 177Lu-PSMA RLT. Patients with low baseline PSMA expression H-scores (<200; n = 2) had worse prognosis than patients with higher H-score (≥200; n = 11) with a median OS of 1.8 and 12.6 months, respectively. The H-scores were inversely correlated to the Ki-67 index [113]. The two patients with BRAC1 mutation displayed longer survival compared to those without DNA damage repair alterations. Indeed, while it is not possible and ethically justifiable to collect specimen from every metastatic site, the results from this small study do demonstrate that individual biomarkers need to be considered when planning or selecting therapy. Larger studies are necessary to confirm these findings.
As more centers gain access to 225Ac-PSMA, more data is becoming available. The published studies to date further highlight and demonstrate the promising PSA response rates as well as the survival benefit of 225Ac-PSMA as salvage therapy for patients with mCRPC who may have exhausted all other treatment options, are ineligible or refuse the standard of care treatment regimen [114,115,116,117]. The presence of visceral metastasis has been associated with a poor prognosis [115], however exceptional responses with long lasting tumour control have been noted in patients with visceral metastasis treated with 225Ac-PSMA [112,118,119].
8.2. Dosimetry
Internal dosimetry refers to the calculation of the amount and the distribution of radiation absorbed by organs in the body from a particular radionuclide [120]. Patients-specific dosimetry takes into account the individual patients physical characteristics (e.g., body habitus, etc.) and the measured radiopharmaceutical kinetics in that individual, which makes for personalized or precision medicine [121]. The idea is to deliver the maximum dose to the target tumour tissue while sparing or ensuring that the dose to the surrounding tissues is as low as reasonably achievable. The acquisition of post therapy scans from dosimetric calculation is hardly implemented following therapy with 225Ac-PSMA. There is resultant low abundance of gamma photons emitted from the daughter products in the decay scheme of 225Ac. This is because the radiopharmaceutical activities administered are quite low, in fact a thousand times lower than that administered for 177Lu-PSMA therapy. Kratochwil and colleagues estimated organ-absorbed doses for 225Ac-PSMA-617 by extrapolating pre-existing 177Lu-PSMA-617 data to the half-life of 225Ac. The salivary glands, kidneys and red marrow were found to be potential dose-limiting organs. Assuming a biologic effectiveness of 5, dose estimates from 1 MBq 225Ac-PSMA-617 were 2.3 Sv, 0.7 Sv and 0.05 Sv for salivary glands, kidneys, and red marrow, respectively [108]. The corresponding radiation absorbed doses for the salivary glands, kidneys, and red marrow for 177Lu-PSMA-617 were 1.38 Gy/GBq, 0.75 Gy/GBq, and 0.03 Gy/GBq, respectively [122]. Upon close analysis, it is evident that the salivary glands receive almost twice as much radiation dose with 225Ac-PSMA than with 177Lu-PSMA. It has been purported that the dose to normal bone marrow when treating skeletal metastases is lower with 225Ac-PSMA than with 177Lu-PSMA because of the shorter path length that alpha particles traverse.
8.3. Toxicity
The literature on therapy of mCRPC with 225Ac-PSMA does not report on a pre-defined protocol for dose estimation as most of the studies were not in a clinical trial setting so the physician determined the dose based on individual patient characteristics such as disease extent as determined by pre-therapy imaging and baseline laboratory results. Following their initial report with two patients, Kratochwil and colleagues set out to determine the ideal dose that bridges the compromise between efficacy and toxicity. Administered doses ranged from 50 kBq/kg body weight with escalations of 50 kBq/kg body weight up to a total of 200 kBq/kg body weight. They found that with increasing doses per kg body weight, the toxicity profile also amplified. While higher doses demonstrated great anti-tumour effects, one patient receiving 150 kBq/kg and all patients receiving 200 kBq/kg discontinued therapy or requested a dose reduction as the side effects became unbearable. A dose greater than 100 kBq/kg resulted in xerostomia which became intolerable at doses above 150 kBq/kg per cycle, therefore, 100 kBq/kg body weight was the dose that resulted in effective tumour control with minimal off-target toxicity [108]. This study is not powered by sufficient patient numbers but a few publications on 225Ac-PSMA seem to use these results for the justification of the dose determination [114].
There are several 225Ac-PSMA related side-effects ranging from dry eyes, anorexia, dyspepsia, nausea and vomiting, constipation, and fatigue, to name a few [108,110,115]. The vast majority of publications report on the major treatment related toxicities (xerostomia, haematological toxicity and renal toxicity) which will be discussed briefly below.
Xerostomia: Xerostomia is the most frequently encountered side effect and is by far the commonest reason for discontinuation of 225Ac-PSMA [108,110,115]. This is the area of extensive research as the pursuit for ways to reduce salivary gland toxicity continues. A few interventions have been attempted with limited success. These include external cooling with ice packs applied over the salivary glands (parotids and submandibular), injection of Botulin toxin, the co-administration of monosodium glutamate as well as saline irrigation and steroid injections [123,124,125,126]. Considering the tumour sink effect, the de-escalation method first described and implemented by Sathekge and colleagues is one intervention that seems to have success. The patients are treated with an initial dose of 8 MBq of 225Ac-PSMA with subsequent doses being titrated against biochemical and imaging (68Ga-PSMA PET/CT) findings. A follow-up dose of between 4–6 MBq is administered if there has been an acceptable response to the first treatment cycle. The South African group of patients had grade 1 and 2 toxicities while none of the patients discontinued therapy because of xerostomia [110]. Another method explored for reduction of salivary gland toxicity from TAT is a tandem approach in which low activity 225Ac-PSMA (median: 5.3 MBq; range: 1.5–7.9 MBq) is administered in the same week, typically consecutive days as a higher dose of 177Lu-PSMA (median: 6.9 GBq; range: 5–11 GBq). No grade 3 or 4 xerostomia toxicities were reported while 40% of the patients developed grade 1 and 25%, grade 2 xerostomia [127]. Of the patients with grade 2 xerostomia, two patients had already developed grade 1 xerostomia from previous 177Lu-PSMA therapy. The results from this tandem approach are similar to those from the de-escalation method. Another group found an even lower (13%) rate of grade 1/2 xerostomia using the tandem approach [128]. This tandem approach results in satisfactory tumour control with reduced toxicities and should be explored in larger studies or trials. While adjustments of the technical/practical aspects of therapy administration have shown reduced toxicities, other researchers have looked at improving the molecular aspects of the PSMA ligands in order to reduce off-target binding. One such amendment has been described by Benesova et al., in which an albumin-binding moiety altered the biodistribution of PSMA radioligands [129,130]. In pre-clinical work, this modification resulted in elevated accumulation in the tumour with fast clearance from the background organs, therefore reducing residency time, which would result in reduced toxicities [129,130].
Haematological toxicity: The skeleton is the commonest site of distant metastases in PCa, especially in the setting of mCRPC. The physical properties of 225Ac-PSMA such as high liner energy over a short path make it a better agent for therapy of diffuse bone marrow metastases. All patients had skeletal metastases in the study by Feuerecker et al. They reported a lower frequency of grade 4 haematological toxicties. The grade 3 haematological toxicities were as follows: 8 patients had anaemia (31%, 95% CI: 16–50%), three had thrombocytopaenia (12%, 95% CI: 3–29%) and leucopaenia in 7 patients (27%, 95% CI: 13–46%), while Grade 4 toxicities were anaemia in one patient (4%, 95% CI: 0–20%), thrombocytopaenia in two patients (8%, 95% CI: 1–25%) and none of the patients had grade 4 leucopaenia [115]. In the largest cohort to date, Sathekge et al. reported no grade 4 haematological toxicities and few grades 3 anaemia, thrombocytopaenia and leucopaenia toxicities in 7%, 1% and 3% of their patients [110]. The differences in the two study groups may be because the patients in the work by Feuerecker et al. had been heavily pre-treated and all patients had received prior 177Lu-PSMA which may have already compromised the bone marrow function. This is something to be borne in mind in the cases of patients that are at the end of the treatment line and have exhausted all options as is the case in most patients referred for 225Ac-PSMA.
Renal toxicity: The PSMA protein is found in the tubular cells of the kidney. A case report of two patients described the long-term renal toxicity following treatment with 225Ac-PSMA [131]. While the two patients in this report had prior or underlying renal injury, the authors attempted to provide convincing evidence implicating 225Ac-PSMA therapy by highlighting the temporal relationship between therapy and onset of renal impairment as well as histopathological evidence [131]. This provides supporting data for the careful examination of baseline renal function as patients with chronic kidney disease may develop renal toxicity. Therapy induced nephrotoxicity has also been reported in some studies on 225Ac-PSMA therapy with Grade 1/2 renal toxicities observed in 14–25% of patients receiving 225Ac-PSMA therapy [110,114]. The overall impression is that patients with underlying renal impairment had higher grade nephrotoxicity. Long term follow-up of renal function is advised to monitor for delayed renal toxicity as kidneys are late reacting organs.
Liver toxicity: Compared to the other major end organ damage/toxicity, there has been no study thus far demonstrating significant Grade II/IV hepatotoxicity following targeted alpha therapy with 225Ac-PSMA in patients with mCRPC [114].
Other PSMA Targeted Radionuclude Therapy (RNT) in Development
There is continuous research proceeding in the pursuit of improved targets for mCRPC. These include adjustments made to the antibodies or the use of other alpha radionuclides. Most of these agents are still in the pre-clinical phase of investigation, however, 213Bi-PSMA is one of the few to have been tested clinically. In the only documented clinical case thus far, Sathekge et al. treated a patient with mCRPC with 213Bi-PSMA and reported on their experience. The patient had progressed on other conventional therapies. Patient received 2 cycles of 213Bi-PSMA-617 with a cumulative dose of 592 MBq [132]. There was an overall biochemical, clinical, and imaging response with serum PSA decreasing from 237 μg/L to 43 μg/L. This case highlights the potential of this tracer for targeted alpha therapy in mCRPC. The short half-life means that the residency time is reduced, therefore potentially reducing the likelihood of severe toxicities. In the treatment of neuroendocrine tumours, this radioligand resulted in high numbers of long-lasting anti-tumour responses. In view of the short half-life, a combinatorial approach may be considered with 213Bi-PSMA being paired with another radioligand to enhance the activity of this radioligand. Table 1. below gives a list of alpha emitting radionuclides being investigated or in use for targeted alpha therapy.

Table 1.
An overview of alpha emitting radionuclides under both investigation clinical use for targeted alpha therapy for mCRPC [132,133,134,135,136].
9. Combination Therapies
Drug resistance is one of the main obstacles in the treatment of metastatic PCa. Early metastatic PCa is usually hormone sensitive and responds to ADT, however over the years the addition of docetaxel, abiraterone, apalutamide and enzalutamide has shown improved survival advantage. This was highlighted in the preliminary results of the PEACE 1 phase 3 trial in which patients were randomized to receive either standard of care (SOC—ADT/bilateral orchidectomy and docetaxel), SOC and abiraterone, SOC and radiation therapy or SOC, abiraterone and radiation therapy. In this study it was proven that the addition of abiraterone to the SOC conferred a benefit of delayed radiological progression and progression to castration resistance [140]. This is an example of improved efficacy of combination therapies. Despite great initial responses, resistance to these agents develops quickly. Resistance is a major drawback with most drugs in the treatment scheme of mCRPC and it usually sets in even with second generation androgen axis targeting drugs. Androgen receptor mutations, amplification, splice variants and bypass pathways as well as androgen independent alternative pathways have been identified as mechanisms involved in resistance in PCa [141]. Any one of these mechanisms exclusively or in concert are involved in this drug resistance. This is the reason and justification for combination therapies which may achieve superior tumour control by targeting multiple pathways involved in drug resistance. There have been many clinical studies assessing the combinations of the more conventional life prolonging PCa drugs (CHAARTED and STAMPEDE trials) which demonstrated that therapy combinations prolong survival of patients with metastatic PCa.
Researchers from Australia paired the tumour-specific radiation sensitizer idronoxil (NOX66) with 177Lu-PSMA-617 (LuPIN) with the hope to improve outcomes in heavily treated mCRPC. In this Phase I/II study the researchers divided the patients into two groups receiving different doses of NOX66. The first cohort of eight (8) patients received 400 mg NOX66 suppositories on days 1–10 of a 6-week cycle while the second cohort of twenty-four (24) patients received 800 mg NOX66 suppositories on the same schedule [142]. After a median of five cycles, there was a biochemical (PSA) response in almost two-thirds (63%) of the patients, with 91% of the patients having any decline in PSA. The median PSA PFS was 6.1 months (95% CI: 2.8–9.2) with a median overall survival of 17.1 months (95% CI: 6.5–27.1). The most frequent adverse effects (non-specific grading) were anaemia (88%), xerostomia (59%) and fatigue (69%). Nine (28%) patients also reported grade 1 and 2 anal inflammation as a result of the NOX66 suppositories [142]. In this dose escalation study, it is evident that the combination therapy was well tolerated with no increase in toxicity of 177Lu-PSMA-617. While this is encouraging, these results are similar to those found in single agent 177Lu-PSMA trials/studies without the additional adverse effects related to the suppositories. At this point there appears to be no added advantage from the combination therapy.
In addition to upregulating PSMA messenger RNA production and PSMA receptor density on the cell surface, but androgen receptor blockade may also be a radiosensitizer. A phase 2 multicenter trial testing enzalutamide and 177Lu-PSMA-617 vs. enzalutamide monotherapy (ENZA-P, ANZUP 1901; NCT04419402) is in the recruitment phase and aims to recruit 160 patients with a 1:1 randomization. Patients randomized to the combination treatment arm will receive 4 cycles of 7.5 GBq 177Lu-PSMA-617 every 6 weeks in together with 160 mg enzalutamide daily. The primary outcome measure will be PSA-PFS with radiographic PFS (rPFS), PSA response rate, pain response, clinical PFS (cPFS), health-related quality of life and lastly frequency and severity of adverse events.
Another ongoing trial is the 177Lu-PSMA-617 Therapy and Olaparib in patients with mCRPC (LuPARP; NCT03874884). Poly Adenosine diphosphate Ribose Polymerase (PARP) inhibitors play an important role as radiosensitizers as they help minimize and block the catalytic and DNA reparative role of the PARP enzyme. This is a single arm study where patients will receive up to four cycles of 7.4 GBq 177Lu-PSMA-617 and Olaparib (PARP inhibitor) on days 2–15 of each cycle. This study also will also have a dose escalation component where varying doses (range: 50–300 mg twice daily) of Olaparib are administered. The primary outcome is to calculate the maximum tolerable doses and dose limiting toxicities. It is envisioned that the synergistic activity of these two therapeutic regimens will result in improved and sustained patient outcomes because evidence has been emerging suggesting that there is an interaction between the immune system and the tumour DNA damage. This therapy would be more beneficial in patients with proven BRCA1/2 mutations.
Another exciting prospect is combining 177Lu-PSMA with immune checkpoint inhibitors. Immune checkpoint inhibitors such as anti-programmed death 1 (anti-PD-1) monoclonal antibodies (nivolumab and pembrolizumab) and anti-CTLA-4/CD28T monoclonal antibodies enhance T cell effector function and have found application in many malignancies including melanoma and lung cancers [143,144]. Immunotherapeutic drugs that can be combined with 177Lu-PSMA or 225Ac-PSMA include anti-PD1/antiPD-L1 and anti-CTLA-4 + anti-PD-1 to name a few [145]. Early preclinical work assessing the synergistic activity of 225Ac and PD-1 demonstrated an improved therapeutic efficacy (OS: 51.5 days) as opposed to when either was used as monotherapy (anti-PD-1: 37 days and RNT: 32 days). The time to progression was prolonged for the combination group (47.5 days) than with the anti-PD-1 (33.5 days) and RNT group (30 days) [145]. The combination of anti-PD-1 and RNT synergistically reduces tumour burden and improves the time to progression and overall survival. This pre-clinical work offers promising results and to this end, the phase 1b/II PRINCE trial (NCT03658447) has been registered. It aims to test efficacy, safety, and tolerability of the combination of pembrolizumab with 177Lu-PSMA-617 in patients with mCRPC. Patients will receive 200 mg pembrolizumab given 3-weekly for up to 35 cycles and 6-weekly 177Lu-PSMA-617 therapy up to 6 cycles starting at 8.5 GBq and reducing the dose by 0.5 GBq at every subsequent cycle. This therapy may only be feasible in a select few with a high load of neoantigens, therefore further interrogation of the tumour microenvironment may offer insight to the application of these therapies in PCa.
The trials reported thus far aim to combine a novel agent/drug with radionuclide therapy to enhance the therapeutic effect. Other trials include combination of PSMA labelled radionuclide therapies with immune checkpoint inhibitors and some have even attempted combinations of radionuclide therapies such as 177Lu-PSMA small molecule with radiolabelled antibody J591. These combinations are trying to improve efficacy while reducing off target toxicities. Other classes of novel treatments such as androgen receptor (AR) degraders are still in clinical development. Further interrogation of these novel treatment agents, either alone or in combination with prostate-cancer targeting therapies, will be essential to frame optimal management strategies for this challenging disease. The landscape of treatment options thus continues to expand for patients with mCRPC.
10. Future Perspectives
It is evident that radionuclide therapy with alpha or beta particles has an impact on survival in men with mCRPC, however There is a need to look at improved methods of increasing the tumor target binding whilst reducing the normal tissue (off-target) binding. To this end several researchers have looked at developing antibody or albumin based PSMA targeting agents both of which alter the biodistribution of these tracers with possibilities of reducing xerostomia and dry eye effects [129,130]. A phase 1 trial testing 225Ac-J591 (which is an antibody-based imaging) has been initiated. The study recruited 22 patients and varying doses were administered with a determined safe dose of 93.8 kBq/kg. Over two thirds (14/22) of the patients experienced any PSA decline with 9 (41%) of the patients having a PSA decline of >50% [146]. The multicenter expansion trial stemming from this study is already underway. Small molecules with an albumin binding moiety are thought to improve tumour retention of PSMA radioligands whilst reducing residence time in normal tissues. This may translate to dose reduction and adjusted frequency of therapy. The pre-clinical work has demonstrated improved pharmacokinetics and efficacy when labelled to 177Lu or 225Ac [129,130]. The first clinical studies with these modified PSMA ligands have demonstrated great promise but further modifications and trials are still necessary to optimize tumour retention while reducing non-tumour uptake which leads to significant morbidity [147,148]. The optimization strategies and the clinical trials on combinatorial therapies are welcome development and the outcomes of these are eagerly anticipated.
11. Conclusions
Despite developments in screening and diagnosis, PCa remains a significant cause of morbidity and mortality in men. There is increasing evidence supporting the use of molecular targets for imaging and therapy. Accumulating data is showing the improved accuracy of PSMA labelled tracers for imaging PCa. This has catapulted the acceptance of these tracers for guiding therapy. The availability of PRLT as a treatment option for the challenging pathology of mCRPC has encouraged researchers to better define the role and timing of this therapy. The results thus far have demonstrated good efficacy when used as salvage therapy. 177Lu-PSMA has shown great PSA responses and with the results of the phase three VISION trial finally out, it is anticipated that 177Lu-PSMA will get approval for treatment in selected patients with mCRPC. 225Ac-PSMA has also resulted in great responses when used as salvage therapy. The physical characteristics of 225Ac-PSMA result in more remarkable PSA responses, nevertheless at the risk of severe salivary gland toxicity. Supply challenges of 225Ac-PSMA as well as significant xerostomia are issues that need to be addressed for wider clinical acceptance. Ligand modifications and combinatorial therapies are promising areas of continued research. With all these developments happening in the field of PCa, we can only look forward to what promises to be an exciting era in PCa theranostics.
Author Contributions
Conceptualization, K.M. and M.S.; methodology, K.M., I.L., M.S.; validation, K.M. and M.S.; investigation, K.M., I.L., M.V.; writing—original draft preparation, K.M., M.S.; writing—review and editing, K.M., I.L., T.L., M.K., F.L.G., M.V., M.S.; supervision, M.S.; project administration, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest. F.L.G. is a medical advisor for ABX Advanced Biochemical Compound, Telix Pharmaceuticals, and SOFIE Biosciences. FLG has patent applications for PSMA-1007.
References
- Mottet, N.; van den Bergh, R.C.; Briers, E.; Van den Broeck, T.V.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer—2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate Cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Aprikian, A.; Finelli, A.; Fleshner, N.E.; Gleave, M.; Kapoor, A.; Niazi, T.; North, S.A.; Pouliot, F.; Rendon, R.A.; et al. 2021 Canadian Urological Association (CUA)—Canadian Uro Oncology Group (CUOG) guideline: Management of castration-resistant prostate cancer (CRPC). Can. Urol. Assoc. J. 2021, 15, E81–E90. [Google Scholar] [CrossRef] [PubMed]
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjug. Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef] [PubMed]
- Wiesing, U. Theranostics: Is it really a revolution? Evaluating a new term in medicine. Med. Health Care Philos. 2019, 22, 593–597. [Google Scholar] [CrossRef]
- Chang, S.S.; Reuter, V.E.; Heston, W.D.; Bander, N.H.; Grauer, L.S.; Gaudin, P.B. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999, 59, 3192–3198. [Google Scholar] [PubMed]
- Chang, S.S.; Heston, W.D. The clinical role of prostate-specific membrane antigen (PSMA). Urol. Oncol. Semin. Orig. Investig. 2002, 7, 7–12. [Google Scholar] [CrossRef]
- Liu, H.; Moy, P.; Kim, S.; Xia, Y.; Rajasekaran, A.; Navarro, V.; Knudsen, B.; Bander, N.H. Monoclonal antibodies to the extracellular domain of prostate-specific membrane antigen also react with tumor vascular endothelium. Cancer Res. 1997, 57, 3629–3634. [Google Scholar]
- Sokoloff, R.L.; Norton, K.C.; Gasior, C.L.; Marker, K.M.; Grauer, L.S. A dual-monoclonal sandwich assay for prostate-specific membrane antigen: Levels in tissues, seminal fluid and urine. Prostate 2000, 43, 150–157. [Google Scholar] [CrossRef]
- Hupe, M.C.; Philippi, C.; Roth, D.; Kümpers, C.; Ribbat-Idel, J.; Becker, F.; Joerg, V.; Duensing, S.; Lubczyk, V.H.; Kirfel, J.; et al. Expression of Prostate-Specific Membrane Antigen (PSMA) on Biopsies Is an Independent Risk Stratifier of Prostate Cancer Patients at Time of Initial Diagnosis. Front. Oncol. 2018, 8, 623. [Google Scholar] [CrossRef]
- Paschalis, A.; Tombal, B.; de Bono, J.S. Prostate-specific Membrane Antigen Theranostics for Prostate Cancer Care: A Need to Prove Clinical Utility. Eur. Urol. Focus 2021, 7, 231–233. [Google Scholar] [CrossRef] [PubMed]
- Kasperzyk, J.L.; Finn, S.; Flavin, R.; Fiorentino, M.; Lis, R.; Hendrickson, W.K.; Clinton, S.K.; Sesso, H.D.; Giovannucci, E.L.; Stampfer, M.J.; et al. Prostate-Specific Membrane Antigen Protein Expression in Tumor Tissue and Risk of Lethal Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2013, 22, 2354–2363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juzeniene, A.; Stenberg, V.Y.; Bruland, Ø.S.; Larsen, R.H. Preclinical and Clinical Status of PSMA-Targeted Alpha Therapy for Metastatic Castration-Resistant Prostate Cancer. Cancers 2021, 13, 779. [Google Scholar] [CrossRef]
- Cimadamore, A.; Gasparrini, S.; Scarpelli, M.; Doria, A.; Mazzucchelli, R.; Massari, F.; Cheng, L.; López-Beltrán, A.; Montironi, R. Epigenetic Modifications and Modulators in Prostate Cancer. Crit. Rev. Oncog. 2017, 22, 439–450. [Google Scholar] [CrossRef]
- Wright, W.; Chan, K.; Sundaram, K.; Bardin, C.W. New Observation on Androgen Action: Androgen Receptor Stabilization and Antisteroid Effects of LHRH Agonists. Horm. Cancer 1982, 138, 325–336. [Google Scholar]
- Bermejo, C.E.; Coursey, J.; Basler, J.; Austenfeld, M.; Thompson, I. Histologic confirmation of lesions identified by Prostascint scan following definitive treatment. Urol. Oncol. Semin. Orig. Investig. 2003, 21, 349–352. [Google Scholar] [CrossRef]
- Lau, H.Y.; Kindrachuk, G.; Carter, M.; Prestage, K.; Webber, D.; Stauffer, E.; Haseman, M. Surgical confirmation of ProstaScint abnormalities in two patients with high risk prostate cancer. Can. J. Urol. 2001, 8, 1199–1202. [Google Scholar]
- Hinkle, G.H.; Burgers, J.K.; Olsen, J.O.; Williams, B.S.; Lamatrice, R.A.; Barth, R.F.; Rogers, B.; Maguire, R.T. Prostate cancer abdominal metastases detected with indium-111 capromab pendetide. J. Nucl. Med. 1998, 39, 650–652. [Google Scholar]
- Hinkle, G.H.; Burgers, J.K.; Neal, C.E.; Texter, J.H.; Kahn, D.; Williams, R.D.; Maguire, R.; Rogers, B.; Olsen, J.O.; Badalament, R.A. Multicenter radioimmunoscintigraphic evaluation of patients with prostate carcinoma using indium-111 capromab pendetide. Cancer 1998, 83, 739–747. [Google Scholar] [CrossRef]
- Foss, C.A.; Mease, R.C.; Fan, H.; Wang, Y.; Ravert, H.T.; Dannals, R.F.; Olszewski, R.T.; Heston, W.D.; Kozikowski, A.P.; Pomper, M.G. Radiolabeled Small-Molecule Ligands for Prostate-Specific Membrane Antigen: In vivo Imaging in Experimental Models of Prostate Cancer. Clin. Cancer Res. 2005, 11, 4022–4028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandit-Taskar, N.; O’Donoghue, J.A.; Morris, M.J.; Wills, E.A.; Schwartz, L.H.; Gonen, M.; Scher, H.I.; Larson, S.M.; Divgi, C.R. Antibody Mass Escalation Study in Patients with Castration-Resistant Prostate Cancer Using 111In-J591: Lesion Detectability and Dosimetric Projections for 90Y Radioimmunotherapy. J. Nucl. Med. 2008, 49, 1066–1074. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.X.; Takach, E.J.; Solomon, M.; Zhu, Q.; Law, S.-J.; Hsieh, F.Y. Mass Spectral Analyses of Labile DOTA-NHS and Heterogeneity Determination of DOTA or DM1 Conjugated Anti-PSMA Antibody for Prostate Cancer Therapy. J. Pharm. Sci. 2005, 94, 788–797. [Google Scholar] [CrossRef]
- Perera, M.; Papa, N.; Roberts, M.; Williams, M.; Udovicich, C.; Vela, I.; Christidis, D.; Bolton, D.; Hofman, M.S.; Lawrentschuk, N.; et al. Gallium-68 Prostate-specific Membrane Antigen Positron Emission Tomography in Advanced Prostate Cancer—Updated Diagnostic Utility, Sensitivity, Specificity, and Distribution of Prostate-specific Membrane Antigen-avid Lesions: A Systematic Review and Meta-analysis. Eur. Urol. 2020, 77, 403–417. [Google Scholar]
- Barrett, J.A.; Coleman, R.E.; Goldsmith, S.J.; Vallabhajosula, S.; Petry, N.A.; Cho, S.; Armor, T.; Stubbs, J.B.; Maresca, K.P.; Stabin, M.G.; et al. First-in-Man Evaluation of 2 High-Affinity PSMA-Avid Small Molecules for Imaging Prostate Cancer. J. Nucl. Med. 2013, 54, 380–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zechmann, C.M.; Afshar-Oromieh, A.; Armor, T.; Stubbs, J.B.; Mier, W.; Hadaschik, B.; Joyal, J.; Kopka, K.; Debus, J.; Babich, J.W.; et al. Radiation dosimetry and first therapy results with a 124I/131I-labeled small molecule (MIP-1095) targeting PSMA for prostate cancer therapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1280–1292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, P.; Neumann, C.; Eiber, M.; Wester, H.J.; Schottelius, M. [99cmTc] Tc-PSMA-I&S-SPECT/CT: Experience in prostate cancer imaging in an outpatient center. EJNMMI1 Res. 2020, 10, 1–10. [Google Scholar]
- Schmidkonz, C.; Hollweg, C.; Beck, M.; Reinfelder, J.; Goetz, T.I.; Sanders, J.C.; Schmidt, D.; Prante, O.; Bäuerle, T.; Cavallaro, A.; et al. 99mTc-MIP-1404-SPECT/CT for the detection of PSMA-positive lesions in 225 patients with biochemical recurrence of prostate cancer. Prostate 2017, 78, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Schmidkonz, C.; Goetz, T.I.; Kuwert, T.; Ritt, P.; Prante, O.; Bäeuerle, T.; Goebell, P.; Cordes, M. PSMA SPECT/CT with 99mTc-MIP-1404 in biochemical recurrence of prostate cancer: Predictive factors and efficacy for the detection of PSMA-positive lesions at low and very-low PSA levels. Ann. Nucl. Med. 2019, 33, 891–898. [Google Scholar] [CrossRef]
- Schmidkonz, C.; Cordes, M.; Beck, M.; Goetz, T.I.; Schmidt, D.; Prante, O.; Bauerle, T.; Uder, M.; Wullich, B.; Goebell, P.; et al. SPECT/CT With the PSMA Ligand 99mTc-MIP-1404 for Whole-Body Primary Staging of Patients With Prostate Cancer. Clin. Nucl. Med. 2018, 43, 225–231. [Google Scholar] [CrossRef]
- Lawal, I.O.; Ankrah, A.O.; Mokgoro, N.P.; Vorster, M.; Maes, A.; Sathekge, M.M. Diagnostic sensitivity of Tc-99m HYNIC PSMA SPECT/CT in prostate carcinoma: A comparative analysis with Ga-68 PSMA PET/CT. Prostate 2017, 77, 1205–1212. [Google Scholar] [CrossRef]
- Rauscher, I.; Eiber, M.; Maurer, T. PSMA-Radioguided Surgery for Salvage Lymphadenectomy in Recurrent Prostate Cancer. Aktuelle Urol. 2017, 48, 148–152. [Google Scholar]
- Maurer, T.; Robu, S.; Schottelius, M.; Schwamborn, K.; Rauscher, I.; van den Berg, N.S.; van Leeuwen, F.W.; Haller, B.; Horn, T.; Heck, M.M.; et al. 99mTechnetium-based Prostate-specific Membrane Antigen–radioguided Surgery in Recurrent Prostate Cancer. Eur. Urol. 2019, 75, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Robu, S.; Schottelius, M.; Eiber, M.; Maurer, T.; Gschwend, J.; Schwaiger, M.; Wester, H.J. Preclinical Evaluation and First Patient Application of 99mTc-PSMA-I&S for SPECT Imaging and Radioguided Surgery in Prostate Cancer. J. Nucl. Med. 2017, 58, 235–242. [Google Scholar] [PubMed] [Green Version]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021: Featured Updates to the NCCN Guidelines. J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef] [PubMed]
- van Kalmthout, L.W.M.; van Melick, H.H.E.; Lavalaye, J.; Meijer, R.P.; Kooistra, A.; de Klerk, J.M.H.; Braat, A.; Kaldeway, H.P.; de Bruin, P.C.; de Keizer, B.; et al. Prospective Validation of Gallium-68 Prostate Specific Membrane Antigen-Positron Emission Tomography/Computerized Tomography for Primary Staging of Prostate Cancer. J. Urol. 2020, 203, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Murphy, D.G.; Williams, S.G.; Nzenza, T.; Herschtal, A.; Lourenco, R.D.A.; Bailey, D.L.; Budd, R.; Hicks, R.J.; Francis, R.; et al. A prospective randomized multicentre study of the impact of gallium-68 prostate-specific membrane antigen (PSMA) PET/CT imaging for staging high-risk prostate cancer prior to curative-intent surgery or radiotherapy (proPSMA study): Clinical trial protocol. BJU Int. 2018, 122, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M. PSMA PET/CT for staging and treatment of prostate cancer. Clin. Adv. Hematol. Oncol. 2019, 17, 370–373. [Google Scholar]
- Lengana, T.; Lawal, I.O.; Boshomane, T.G.; Popoola, G.O.; Mokoala, K.M.G.; Moshokoa, E.; Maes, A.; Mokgoro, N.P.; Van de Wiele, C.; Vorster, M.; et al. 68Ga-PSMA PET/CT Replacing Bone Scan in the Initial Staging of Skeletal Metastasis in Prostate Cancer: A Fait Accompli? Clin. Genitourin. Cancer 2018, 16, 392–401. [Google Scholar] [CrossRef]
- Jacobson, O.; Kiesewetter, D.O.; Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjug. Chem. 2015, 26, 1–18. [Google Scholar] [CrossRef]
- Rousseau, E.; Wilson, D.; Lacroix-Poisson, F.; Krauze, A.; Chi, K.; Gleave, M.; McKenzie, M.; Tyldesley, S.; Goldenberg, S.L.; Bénard, F. A Prospective Study on 18F-DCFPyL PSMA PET/CT Imaging in Biochemical Recurrence of Prostate Cancer. J. Nucl. Med. 2019, 60, 1587–1593. [Google Scholar] [CrossRef] [Green Version]
- Mena, E.; Lindenberg, M.L.; Turkbey, I.B.; Shih, J.H.; Harmon, S.A.; Lim, I.; Lin, F.; Adler, S.; Eclarinal, P.; McKinney, Y.L.; et al. 18F-DCFPyL PET/CT Imaging in Patients with Biochemically Recurrent Prostate Cancer After Primary Local Therapy. J. Nucl. Med. 2020, 61, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Awenat, S.; Piccardo, A.; Carvoeiras, P.; Signore, G.; Giovanella, L.; Prior, J.O.; Treglia, G. Diagnostic Role of 18F-PSMA-1007 PET/CT in Prostate Cancer Staging: A Systematic Review. Diagnostics 2021, 11, 552. [Google Scholar] [CrossRef]
- Giesel, F.L.; Will, L.; Kesch, C.; Freitag, M.; Kremer, C.; Merkle, J.; Neels, O.; Cardinale, J.; Hadaschik, B.; Hohenfellner, M.; et al. Biochemical Recurrence of Prostate Cancer: Initial Results with [18F] PSMA-1007 PET/CT. J. Nucl. Med. 2018, 59, 632–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treglia, G.; Annunziata, S.; Pizzuto, D.A.; Giovanella, L.; Prior, J.O.; Ceriani, L. Detection Rate of 18F-Labeled PSMA PET/CT in Biochemical Recurrent Prostate Cancer: A Systematic Review and a Meta-Analysis. Cancers 2019, 11, 710. [Google Scholar] [CrossRef] [Green Version]
- Masters, S.C.; Hofling, A.A.; Gorovets, A.; Marzella, L. FDA Approves Ga 68 PSMA-11 for Prostate Cancer Imaging. Int. J. Radiat. Oncol. 2021, in press. [Google Scholar] [CrossRef]
- Fendler, W.P.; Eiber, M.; Beheshti, M.; Bomanji, J.; Ceci, F.; Cho, S.; Giesel, F.; Haberkorn, U.; Hope, T.A.; Kopka, K.; et al. 68Ga-PSMA PET/CT: Joint EANM and SNMMI procedure guideline for prostate cancer imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1014–1024. [Google Scholar] [CrossRef]
- Fanti, S.; Goffin, K.; Hadaschik, B.A.; Herrmann, K.; Maurer, T.; MacLennan, S.; Oprea-Lager, D.E.; Oyen, W.J.; Rouvière, O.; Mottet, N.; et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 469–476. [Google Scholar] [CrossRef]
- Fanti, S.; Minozzi, S.; Morigi, J.J.; Giesel, F.; Ceci, F.; Uprimny, C.; Hofman, M.; Eiber, M.; Schwarzenbock, S.; Castellucci, P.; et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1622–1635. [Google Scholar] [CrossRef]
- Emmett, L.; Crumbaker, M.; Ho, B.; Willowson, K.; Eu, P.; Ratnayake, L.; Epstein, R.; Blanksby, A.; Horvath, L.; Guminski, A.; et al. Results of a Prospective Phase 2 Pilot Trial of 177Lu-PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer Including Imaging Predictors of Treatment Response and Patterns of Progression. Clin. Genitourin. Cancer 2019, 17, 15–22. [Google Scholar] [CrossRef]
- Lavallée, E.; Bergeron, M.; Buteau, F.-A.; Blouin, A.-C.; Duchesnay, N.; Dujardin, T.; Tiguert, R.; Lacombe, L.; Fradet, V.; Makao-Nguile, M.; et al. Increased Prostate Cancer Glucose Metabolism Detected by 18F-fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Localised Gleason 8–10 Prostate Cancers Identifies Very High–risk Patients for Early Recurrence and Resistance to Castration. Eur. Urol. Focus 2019, 5, 998–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thang, S.P.; Violet, J.; Sandhu, S.; Iravani, A.; Akhurst, T.; Kong, G.; Kumar, A.R.; Murphy, D.G.; Williams, S.G.; Hicks, R.J.; et al. Poor Outcomes for Patients with Metastatic Castration-resistant Prostate Cancer with Low Prostate-specific Membrane Antigen (PSMA) Expression Deemed Ineligible for 177Lu-labelled PSMA Radioligand Therapy. Eur. Urol. Oncol. 2019, 2, 670–676. [Google Scholar] [CrossRef]
- Jadvar, H.; Ballas, L.K.; Choyke, P.L.; Fanti, S.; Gulley, J.L.; Herrmann, K.; Hope, T.A.; Klitzke, A.K.; Oldan, J.D.; Pomper, M.G.; et al. Appropriate Use Criteria for Imaging Evaluation of Biochemical Recurrence of Prostate Cancer After Definitive Primary Treatment. J. Nucl. Med. 2020, 61, 552–562. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Wang, Y.; Zhu, Y.; Shi, Y.; Xu, L.; Huang, G.; Liu, J. The added value of 18F-FDG PET/CT compared to 68Ga-PSMA PET/CT in patients with castration-resistant prostate cancer. J. Nucl. Med. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, Y.; Shi, Y.; Zhu, Y.; Xu, L.; Huang, G.; Liu, J. Diagnostic value of 18F-FDG PET/CT in patients with biochemical recurrent prostate cancer and negative 68Ga-PSMA PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2970–2977. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L. Tumour Heterogeneity and Resistance to Therapy in Prostate Cancer: A Fundamental Limitation of Prostate-specific Membrane Antigen Theranostics or a Key Strength? Eur. Urol. 2019, 76, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Hofman, M.S.; Emmett, L.; Violet, J.; Zhang, A.Y.; Lawrence, N.J.; Stockler, M.; Francis, R.J.; Iravani, A.; Williams, S.; Azad, A.; et al. TheraP: A randomized phase 2 trial of 177 Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU Int. 2019, 124 (Suppl. 1), 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Hofman, M.S.; Emmett, L.; Sandhu, S.; Iravani, A.; Joshua, A.M.; Goh, J.C.; Pattison, D.A.; Tan, T.H.; Kirkwood, I.D.; Ng, S.; et al. [177Lu] Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomised, open-label, phase 2 trial. Lancet 2021, 397, 797–804. [Google Scholar] [CrossRef]
- Bodei, L.; Pepe, G.; Paganelli, G. Peptide receptor radionuclide therapy (PRRT) of neuroendocrine tumors with somatostatin analogues. Eur. Rev. Med. Pharmacol. Sci. 2010, 14, 347–351. [Google Scholar]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II Study of Lutetium-177–Labeled Anti-Prostate-Specific Membrane Antigen Monoclonal Antibody J591 for Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagawa, S.T.; Vallabhajosula, S.; Christos, P.J.; Jhanwar, Y.S.; Batra, J.S.; Lam, L.; Osborne, J.; Beltran, H.; Molina, A.M.; Goldsmith, S.J.; et al. Phase 1/2 study of fractionated dose lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 (177Lu-J591) for metastatic castration-resistant prostate cancer. Cancer 2019, 125, 2561–2569. [Google Scholar] [CrossRef]
- Niaz, M.; Sun, M.; Ramirez-Fort, M.K.; Niaz, M.J. Review of Lutetium-177-labeled Anti-prostate-specific Membrane Antigen Monoclonal Antibody J591 for the Treatment of Metastatic Castration-resistant Prostate Cancer. Cureus 2020, 12, e7107. [Google Scholar] [CrossRef] [Green Version]
- Niaz, M.J.; Batra, J.S.; Walsh, R.D.; Ramirez-Fort, M.K.; Vallabhajosula, S.; Jhanwar, Y.S.; Molina, A.M.; Nanus, D.M.; Osborne, J.R.; Bander, N.H.; et al. Pilot Study of Hyperfractionated Dosing of Lutetium-177-Labeled Antiprostate-Specific Membrane Antigen Monoclonal Antibody J591 (177Lu-J591) for Metastatic Castration-Resistant Prostate Cancer. Oncologist 2020, 25, 477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallabhajosula, S.; Nikolopoulou, A.; Jhanwar, Y.S.; Kaur, G.; Tagawa, S.T.; Nanus, D.M.; Bander, N.H.; Goldsmith, S.J. Radioimmunotherapy of Metastatic Prostate Cancer with; Lu-DOTAhuJ591 Anti Prostate Specific Membrane Antigen Specific Monoclonal Antibody. Curr. Radiopharm. 2016, 9, 44–53. [Google Scholar] [CrossRef]
- Vallabhajosula, S.; Kuji, I.; Hamacher, K.A.; Konishi, S.; Kostakoglu, L.; Kothari, P.A.; Milowski, M.I.; Nanus, D.M.; Bander, N.H.; Goldsmith, S.J. Pharmacokinetics and biodistribution of 111In- and 177Lu-labeled J591 antibody specific for prostate-specific membrane antigen: Prediction of 90Y-J591 radiation dosimetry based on 111In or 177Lu? J. Nucl. Med. 2005, 46, 634–641. [Google Scholar]
- Bander, N.H.; Trabulsi, E.J.; Kostakoglu, L.; Yao, D.; Vallabhajosula, S.; Smith-Jones, P.; Joyce, M.A.; Milowsky, M.; Nanus, D.M.; Goldsmith, S.J. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J. Urol. 2003, 170, 1717–1721. [Google Scholar] [CrossRef] [PubMed]
- Bander, N.H.; Nanus, D.M.; Milowsky, M.I.; Kostakoglu, L.; Vallabahajosula, S.; Goldsmith, S.J. Targeted systemic therapy of prostate cancer with a monoclonal antibody to prostate-specific membrane antigen. Semin. Oncol. 2003, 30, 667–676. [Google Scholar] [CrossRef]
- Bander, N.H. Technology Insight: Monoclonal antibody imaging of prostate cancer. Nat. Clin. Pract. Urol. 2006, 3, 216–225. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Rahbar, K.; Essler, M.; Biersack, H.J. PSMA-Based Theranostics: A Step-by-Step Practical Approach to Diagnosis and Therapy for mCRPC Patients. Semin. Nucl. Med. 2020, 50, 98–109. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Rahbar, K.; Kürpig, S.; Bögemann, M.; Claesener, M.; Eppard, E.; Gärtner, F.; Rogenhofer, S.; Schäfers, M.; Essler, M. Early side effects and first results of radioligand therapy with 177Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: A two-centre study. EJNMMI Res. 2015, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahbar, K.; Schmidt, M.; Heinzel, A.; Eppard, E.; Bode, A.; Yordanova, A.; Claesener, M.; Ahmadzadehfar, H. Response and Tolerability of a Single Dose of 177Lu-PSMA-617 in Patients with Metastatic Castration-Resistant Prostate Cancer: A Multicenter Retrospective Analysis. J. Nucl. Med. 2016, 57, 1334–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahbar, K.; Ahmadzadehfar, H.; Kratochwil, C.; Haberkorn, U.; Schäfers, M.; Essler, M.; Baum, R.P.; Kulkarni, H.R.; Schmidt, M.; Drzezga, A.; et al. German Multicenter Study Investigating 177 Lu-PSMA-617 Radioligand Therapy in Advanced Prostate Cancer Patients. J. Nucl. Med. 2017, 58, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Dwivedi, S.N.; Bal, C. Radioligand Therapy With 177Lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Am. J. Roentgenol. 2019, 213, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tang, T.; Liu, X.P.; Zhou, Z.H.; Li, F.J. Evaluation of the efficiency of peptide receptor radionuclide therapy in patients with metastatic prostate carcinoma: A protocol for systematic review and meta-analysis. Medicine 2021, 100, e25612. [Google Scholar] [CrossRef]
- Morris, M.J.; De Bono, J.S.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Phase III study of lutetium-177-PSMA-617 in patients with metastatic castration-resistant prostate cancer (VISION). J. Clin. Oncol. 2021, 39 (Suppl. 18), LBA4. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Seifert, R.; Kessel, K.; Schlack, K.; Weber, M.; Herrmann, K.; Spanke, M.; Fendler, W.P.; Hadaschik, B.; Kleesiek, J.; Schäfers, M.; et al. PSMA PET total tumor volume predicts outcome of patients with advanced prostate cancer receiving [177Lu] Lu-PSMA-617 radioligand therapy in a bicentric analysis. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1200–1210. [Google Scholar] [CrossRef]
- Widjaja, L.; Werner, R.; Ross, T.; Bengel, F.; Derlin, T. PSMA Expression Predicts Early Biochemical Response in Patients with Metastatic Castration-Resistant Prostate Cancer under 177Lu-PSMA-617 Radioligand Therapy. Cancers 2021, 13, 2938. [Google Scholar] [CrossRef]
- Ahmadzadehfar, H.; Essler, M. Predictive Factors of Response and Overall Survival in Patients with Castration-Resistant Metastatic Prostate Cancer Undergoing 177Lu-PSMA Therapy. J. Nucl. Med. 2018, 59, 1033–1034. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, K.; Ahmadzadehfar, H.; Seifert, R.; Boegemann, M. [177Lu]-PSMA-617 radionuclide therapy in patients with metastatic castration-resistant prostate cancer. Lancet Oncol. 2018, 19, e371. [Google Scholar] [CrossRef] [Green Version]
- Yordanova, A.; Becker, A.; Eppard, E.; Kurpig, S.; Fisang, C.; Feldmann, G.; Essler, M.; Ahmadzadehfar, H. The impact of repeated cycles of radioligand therapy using [177Lu] Lu-PSMA-617 on renal function in patients with hormone refractory metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- Gafita, A.; Fendler, W.P.; Hui, W.; Sandhu, S.; Weber, M.; Esfandiari, R.; Calais, J.; Rauscher, I.; Rathke, H.; Tauber, R.; et al. Efficacy and Safety of 177Lu-labeled Prostate-specific Membrane Antigen Radionuclide Treatment in Patients with Diffuse Bone Marrow Involvement: A Multicenter Retrospective Study. Eur. Urol. 2020, 78, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadehfar, H.; Rahbar, K.; Baum, R.P.; Seifert, R.; Kessel, K.; Bögemann, M.; Kulkarni, H.R.; Zhang, J.; Gerke, C.; Fimmers, R.; et al. Prior therapies as prognostic factors of overall survival in metastatic castration-resistant prostate cancer patients treated with [177Lu] Lu-PSMA-617. A WARMTH multicenter study (the 617 trial). Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with 177Lu-PSMA-I&T in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar]
- Satapathy, S.; Mittal, B.R.; Sood, A. Visceral Metastases as Predictors of Response and Survival Outcomes in Patients of Castration-Resistant Prostate Cancer Treated With 177Lu-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy: A Systematic Review and Meta-analysis. Clin. Nucl. Med. 2020, 45, 935–942. [Google Scholar] [CrossRef]
- Halabi, S.; Kelly, W.K.; Ma, H.; Zhou, H.; Solomon, N.C.; Fizazi, K.; Tangen, C.M.; Rosenthal, M.; Petrylak, D.P.; Hussain, M.; et al. Meta-Analysis Evaluating the Impact of Site of Metastasis on Overall Survival in Men With Castration-Resistant Prostate Cancer. J. Clin. Oncol. 2016, 34, 1652–1659. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Roviello, G.; Kiljunen, T.; Uprimny, C.; Virgolini, I.; Kairemo, K.; Joensuu, T. Third-line treatment and 177Lu-PSMA radioligand therapy of metastatic castration-resistant prostate cancer: A systematic review. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 496–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Bolton, R.C.D.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.P.; Ballal, S.; Tripathi, M.; Damle, N.A.; Sahoo, R.K.; Seth, A.; Bal, C. Post-therapeutic dosimetry of 177Lu-DKFZ-PSMA-617 in the treatment of patients with metastatic castration-resistant prostate cancer. Nucl. Med. Commun. 2017, 38, 91–98. [Google Scholar] [CrossRef]
- Kabasakal, L.; Toklu, T.; Yeyin, N.; Demirci, E.; Abuqbeitah, M.; Ocak, M.; Aygun, A.; Karayel, E.; Pehlivanoğlu, H.; Selcuk, N.A. Lu-177-PSMA-617 Prostate-Specific Membrane Antigen Inhibitor Therapy in Patients with Castration-Resistant Prostate Cancer: Stability, Bio-distribution and Dosimetry. Mol. Imaging Radionucl. Ther. 2017, 26, 62–68. [Google Scholar] [CrossRef]
- Kabasakal, L.; Abuqbeitah, M.; Aygün, A.; Yeyin, N.; Ocak, M.; Demirci, E.; Toklu, T. Pre-therapeutic dosimetry of normal organs and tissues of 177Lu-PSMA-617 prostate-specific membrane antigen (PSMA) inhibitor in patients with castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1976–1983. [Google Scholar] [CrossRef] [PubMed]
- Delker, A.; Fendler, W.P.; Kratochwil, C.; Brunegraf, A.; Gosewisch, A.; Gildehaus, F.J.; Tritschler, S.; Stief, C.G.; Kopka, K.; Haberkorn, U.; et al. Dosimetry for 177Lu-DKFZ-PSMA-617: A new radiopharmaceutical for the treatment of metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Violet, J.; Sandhu, S.; Iravani, A.; Ferdinandus, J.; Thang, S.-P.; Kong, G.; Kumar, A.R.; Akhurst, T.; Pattison, D.A.; Beaulieu, A.; et al. Long-Term Follow-up and Outcomes of Retreatment in an Expanded 50-Patient Single-Center Phase II Prospective Trial of 177Lu-PSMA-617 Theranostics in Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2019, 61, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Calais, J.; Gafita, A.; Eiber, M.R.; Armstrong, W.R.; Gartmann, J.; Thin, P.; Nguyen, K.; Lok, V.; Gosa, L.; Grogan, T.; et al. Prospective phase 2 trial of PSMA-targeted molecular RadiothErapy with 177Lu-PSMA-617 for metastatic Castration-reSISTant Prostate Cancer (RESIST-PC): Efficacy results of the UCLA cohort. J. Nucl. Med. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Violet, J.; Sandhu, S.; Hicks, R.J.; Kumar, A.S.R.; Iravani, A.; Kong, G.; Akhurst, T.; Thang, S.P.; Murphy, D.G.; et al. Prognostic biomarkers in men with metastatic castration-resistant prostate cancer receiving [177Lu]-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2322–2327. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Eppard, E.; Gaertner, F.C.; Kürpig, S.; Fimmers, R.; Yordanova, A.; Hauser, S.; Feldmann, G.; Essler, M.; Ahmadzadehfar, H. Predictors of Response to Radioligand Therapy of Metastatic Castrate-Resistant Prostate Cancer with 177 Lu-PSMA-617. J. Nucl. Med. 2016, 58, 312–319. [Google Scholar] [CrossRef]
- Leibowitz, R.; Davidson, T.; Gadot, M.; Aharon, M.; Malki, A.; Levartovsky, M.; Oedegaard, C.; Saad, A.; Sandler, I.; Ben-Haim, S.; et al. A Retrospective Analysis of the Safety and Activity of Lutetium-177-Prostate-Specific Membrane Antigen Radionuclide Treatment in Older Patients with Metastatic Castration-Resistant Prostate Cancer. Oncologist 2020, 25, 787–792. [Google Scholar] [CrossRef]
- Gafita, A.; Rauscher, I.; Retz, M.; Knorr, K.; Heck, M.; Wester, H.-J.; D’Alessandria, C.; Weber, W.A.; Eiber, M.; Tauber, R.; et al. Early Experience of Rechallenge 177Lu-PSMA Radioligand Therapy After an Initial Good Response in Patients with Advanced Prostate Cancer. J. Nucl. Med. 2019, 60, 644–648. [Google Scholar] [CrossRef] [Green Version]
- Yordanova, A.; Linden, P.; Hauser, S.; Meisenheimer, M.; Kürpig, S.; Feldmann, G.; Gaertner, F.C.; Essler, M.; Ahmadzadehfar, H. Outcome and safety of rechallenge [177Lu] Lu-PSMA-617 in patients with metastatic prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 46, 1073–1080. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Andersson, J.D.; Wuest, F. Targeted Alpha Therapy: Progress in Radionuclide Production, Radiochemistry, and Applications. Pharmaceutics 2020, 13, 49. [Google Scholar] [CrossRef] [PubMed]
- Miederer, M.; Henriksen, G.; Alke, A.; Mossbrugger, I.; Quintanilla-Martinez, L.; Senekowitsch-Schmidtke, R.; Essler, M. Preclinical Evaluation of the α-Particle Generator Nuclide 225Ac for Somatostatin Receptor Radiotherapy of Neuroendocrine Tumors. Clin. Cancer Res. 2008, 14, 3555–3561. [Google Scholar] [CrossRef] [Green Version]
- Robertson, A.K.H.; Ramogida, C.; Schaffer, P.; Radchenko, V. Development of 225Ac Radiopharmaceuticals: TRIUMF Perspectives and Experiences. Curr. Radiopharm. 2018, 11, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. 225Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [Green Version]
- Morgenstern, A.; Bruchertseifer, F. Development of Targeted Alpha Therapy from Bench to Bedside. J. Med. Imaging Radiat. Sci. 2019, 50, S18–S20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgouros, G. Dosimetry, Radiobiology and Synthetic Lethality: Radiopharmaceutical Therapy (RPT) With Alpha-Particle-Emitters. Semin. Nucl. Med. 2020, 50, 124–132. [Google Scholar] [CrossRef]
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Bagni, O. The potential of PSMA-targeted alpha therapy in the management of prostate cancer. Expert Rev. Anticancer Ther. 2020, 20, 823–829. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Bronzel, M.; Apostolidis, C.; Weichert, W.; Haberkorn, U.; Giesel, F.L.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J. Nucl. Med. 2017, 58, 1624–1631. [Google Scholar] [CrossRef] [Green Version]
- Sathekge, M.; Bruchertseifer, F.; Knoesen, O.; Reyneke, F.; Lawal, I.; Lengana, T.; Davis, C.; Mahapane, J.; Corbett, C.; Vorster, M.; et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: A pilot study. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, I.; Knoesen, O.; Mahapane, J.; Davis, C.; Reyneke, F.; Maes, A.; Kratochwil, C.; et al. Predictors of Overall and Disease-Free Survival in Metastatic Castration-Resistant Prostate Cancer Patients Receiving 225Ac-PSMA-617 Radioligand Therapy. J. Nucl. Med. 2019, 61, 62–69. [Google Scholar] [CrossRef]
- Kratochwil, C.; Bruchertseifer, F.; Rathke, H.; Hohenfellner, M.; Giesel, F.L.; Haberkorn, U.; Morgenstern, A. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225Ac-PSMA-617: Swimmer-Plot Analysis Suggests Efficacy Regarding Duration of Tumor Control. J. Nucl. Med. 2018, 59, 795–802. [Google Scholar] [CrossRef] [Green Version]
- Rathke, H.; Bruchertseifer, F.; Kratochwil, C.; Keller, H.; Giesel, F.L.; Apostolidis, C.; Haberkorn, U.; Morgenstern, A. First patient exceeding 5-year complete remission after 225Ac-PSMA-TAT. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 311–312. [Google Scholar] [CrossRef]
- van der Doelen, M.J.; Mehra, N.; van Oort, I.M.; Looijen-Salamon, M.G.; Janssen, M.J.; Custers, J.A.; Slootbeek, P.H.; Kroeze, L.I.; Bruchertseifer, F.; Morgenstern, A.; et al. Clinical outcomes and molecular profiling of advanced metastatic castration-resistant prostate cancer patients treated with 225Ac-PSMA-617 targeted alpha-radiation therapy. Urol. Oncol. Semin. Orig. Investig. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Yadav, M.P.; Ballal, S.; Sahoo, R.K.; Tripathi, M.; Seth, A.; Bal, C. Efficacy and safety of 225Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics 2020, 10, 9364–9377. [Google Scholar] [CrossRef] [PubMed]
- Feuerecker, B.; Tauber, R.; Knorr, K.; Heck, M.; Beheshti, A.; Seidl, C.; Bruchertseifer, F.; Pickhard, A.; Gafita, A.; Kratochwil, C.; et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur. Urol. 2021, 79, 343–350. [Google Scholar] [CrossRef]
- Satapathy, S.; Sood, A.; Das, C.K.; Mittal, B.R. Evolving role of 225Ac-PSMA radioligand therapy in metastatic castration-resistant prostate cancer—A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Novruzov, F.; Aliyev, J.A.; Allahverdiyeva, Z.; Mehdi, E.; Giammarile, F. Positive effect of Actinium 225-PSMA treatment on ECOG performance status: A case report on a patient with castration resistant stage IV prostate carcinoma. Rev. Española Med. Nucl. Imagen Mol. 2019, 38, 260–261. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.M.; Bruchertseifer, F.; Lawal, I.; Vorster, M.; Knoesen, O.; Lengana, T.; Boshomane, T.G.; Mokoala, K.; Morgenstern, A. Treatment of brain metastases of castration-resistant prostate cancer with 225Ac-PSMA-617. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1756–1757. [Google Scholar] [CrossRef] [PubMed]
- Maserumule, L.C.; Mokoala, K.M.; Hlongwa, K.N.; Ndlovu, H.; Reed, J.D.; Ismail, A.; Boshomane, T.M.; Lawal, I.O.; Mokgoro, N.P.; Vorster, M.; et al. Exceptional initial response of prostate cancer lung metastases to 225Ac-PSMA: A case report. Curr. Probl. Cancer: Case Rep. 2021, 3, 100038. [Google Scholar]
- Zanzonico, P.B. Internal radionuclide radiation dosimetry: A review of basic concepts and recent developments. J. Nucl. Med. 2000, 41, 297–308. [Google Scholar]
- Zanzonico, P.B.; Bigler, R.E.; Sgouros, G.; Strauss, A. Quantitative SPECT in radiation dosimetry. Semin. Nucl. Med. 1989, 19, 47–61. [Google Scholar] [CrossRef]
- Kratochwil, C.; Giesel, F.L.; Stefanova, M.; Benešová, M.; Bronzel, M.; Afshar-Oromieh, A.; Mier, W.; Eder, M.; Kopka, K.; Haberkorn, U. PSMA-Targeted Radionuclide Therapy of Metastatic Castration-Resistant Prostate Cancer with 177Lu-Labeled PSMA-617. J. Nucl. Med. 2016, 57, 1170–1176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rousseau, E.; Lau, J.; Kuo, H.-T.; Zhang, Z.; Merkens, H.; Hundal-Jabal, N.; Colpo, N.; Lin, K.-S.; Bénard, F. Monosodium Glutamate Reduces 68Ga-PSMA-11 Uptake in Salivary Glands and Kidneys in a Preclinical Prostate Cancer Model. J. Nucl. Med. 2018, 59, 1865–1868. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, B.; Nisli, S.; Ergul, N.; Gursu, R.U.; Acikgoz, O.; Cermik, T.F. Effect of External Cooling on 177Lu-PSMA Uptake by the Parotid Glands. J. Nucl. Med. 2019, 60, 1388–1393. [Google Scholar] [CrossRef] [Green Version]
- Kalidindi, T.M.; Lee, S.-G.; Jou, K.; Chakraborty, G.; Skafida, M.; Tagawa, S.T.; Bander, N.H.; Schoder, H.; Bodei, L.; Pandit-Taskar, N.; et al. A simple strategy to reduce the salivary gland and kidney uptake of PSMA-targeting small molecule radiopharmaceuticals. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Rathke, H.; Kratochwil, C.; Hohenberger, R.; Giesel, F.L.; Bruchertseifer, F.; Flechsig, P.; Morgenstern, A.; Hein, M.; Plinkert, P.; Haberkorn, U.; et al. Initial clinical experience performing sialendoscopy for salivary gland protection in patients undergoing 225Ac-PSMA-617 RLT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 139–147. [Google Scholar] [CrossRef]
- Khreish, F.; Ebert, N.; Ries, M.; Maus, S.; Rosar, F.; Bohnenberger, H.; Stemler, T.; Saar, M.; Bartholoma, M.; Ezziddin, S. 225Ac-PSMA-617/177Lu-PSMA-617 tandem therapy of metastatic castration-resistant prostate cancer: Pilot experience. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Rosar, F.; Krause, J.; Bartholomä, M.; Maus, S.; Stemler, T.; Hierlmeier, I.; Linxweiler, J.; Ezziddin, S.; Khreish, F. Efficacy and Safety of [225Ac] Ac-PSMA-617 Augmented [177Lu] Lu-PSMA-617 Radioligand Therapy in Patients with Highly Advanced mCRPC with Poor Prognosis. Pharmaceutics 2021, 13, 722. [Google Scholar] [CrossRef]
- Benešová, M.; Umbricht, C.A.; Schibli, R.; Müller, C. Albumin-Binding PSMA Ligands: Optimization of the Tissue Distribution Profile. Mol. Pharm. 2018, 15, 934–946. [Google Scholar] [CrossRef]
- Deberle, L.M.; Benešová, M.; Umbricht, C.A.; Borgna, F.; Büchler, M.; Zhernosekov, K.; Schibli, R.; Müller, C. Development of a new class of PSMA radioligands comprising ibuprofen as an albumin-binding entity. Theranostics 2020, 10, 1678–1693. [Google Scholar] [CrossRef]
- Pelletier, K.; Côté, G.; Fallah-Rad, N.; John, R.; Kitchlu, A. CKD After 225Ac-PSMA617 Therapy in Patients with Metastatic Prostate Cancer. Kidney Int. Rep. 2021, 6, 853–856. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Knoesen, O.; Meckel, M.; Modiselle, M.; Vorster, M.; Marx, S. 213Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1099–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mease, R.C.; Kang, C.; Kumar, V.; Ray, S.; Minn, I.; Brummet, M.; Gabrielson, K.; Feng, Y.; Park, A.; Kiess, A.; et al. An improved 211At-labeled agent for PSMA-targeted alpha therapy. J. Nucl. Med. 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Stenberg, V.Y.; Juzeniene, A.; Chen, Q.; Yang, X.; Bruland, Ø.S.; Larsen, R.H. Preparation of the alpha-emitting prostate-specific membrane antigen targeted radioligand [212Pb] Pb-NG001 for prostate cancer. J. Label. Compd. Radiopharm. 2020, 63, 129–143. [Google Scholar] [CrossRef] [Green Version]
- Umbricht, C.A.; Köster, U.; Bernhardt, P.; Gracheva, N.; Johnston, K.; Schibli, R.; Van Der Meulen, N.P.; Müller, C. Alpha-PET for Prostate Cancer: Preclinical investigation using 149Tb-PSMA-617. Sci. Rep. 2019, 9, 1–10. [Google Scholar]
- Hammer, S.; Hagemann, U.B.; Zitzmann-Kolbe, S.; Larsen, A.; Ellingsen, C.; Geraudie, S.; Grant, D.; Indrevoll, B.; Smeets, R.; Von Ahsen, O.; et al. Preclinical Efficacy of a PSMA-Targeted Thorium-227 Conjugate (PSMA-TTC), a Targeted Alpha Therapy for Prostate Cancer. Clin. Cancer Res. 2020, 26, 1985–1996. [Google Scholar] [CrossRef] [Green Version]
- Kratochwil, C.; Giesel, F.L.; Heussel, C.-P.; Kazdal, D.; Endris, V.; Nientiedt, C.; Bruchertseifer, F.; Kippenberger, M.; Rathke, H.; Leichsenring, J.; et al. Patients Resistant Against PSMA-Targeting α-Radiation Therapy Often Harbor Mutations in DNA Damage–Repair–Associated Genes. J. Nucl. Med. 2020, 61, 683–688. [Google Scholar] [CrossRef]
- Sen, I.; Thakral, P.; Tiwari, P.; Pant, V.; Das, S.S.; Manda, D.; Raina, V. Therapeutic efficacy of 225Ac-PSMA-617 targeted alpha therapy in patients of metastatic castrate resistant prostate cancer after taxane-based chemotherapy. Ann. Nucl. Med. 2021, 35, 794–810. [Google Scholar] [CrossRef]
- Zacherl, M.J.; Gildehaus, F.J.; Mittlmeier, L.; Boning, G.; Gosewisch, A.; Wenter, V.; Unterrainer, M.; Schmidt-Hegemann, N.; Belka, C.; Kretschmer, A.; et al. First Clinical Results for PSMA-Targeted alpha-Therapy Using (225)Ac-PSMA-I&T in Advanced-mCRPC Patients. J. Nucl. Med. 2021, 62, 669–674. [Google Scholar]
- Fizazi, K.; Maldonado, X.; Foulon, S.; Roubaud, G.; McDermott, R.S.; Flechon, A.; Tombal, B.F.; Supiot, S.; Berthold, D.R.; Ronchin, P.; et al. A phase 3 trial with a 2 × 2 factorial design of abiraterone acetate plus prednisone and/or local radiotherapy in men with de novo metastatic castration-sensitive prostate cancer (mCSPC): First results of PEACE-1. J. Clin. Oncol. 2021, 39, 5000. [Google Scholar] [CrossRef]
- Watson, P.A.; Arora, V.K.; Sawyers, C.L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 2015, 15, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Crumbaker, M.; Pathmanandavel, S.; Yam, A.O.; Nguyen, A.; Ho, B.; Chan, L.; Ende, J.A.; Rofe, C.; Kongrak, K.; Kwan, E.M.; et al. Phase I/II Trial of the Combination of 177Lutetium Prostate specific Membrane Antigen 617 and Idronoxil (NOX66) in Men with End-stage Metastatic Castration-resistant Prostate Cancer (LuPIN). Eur. Urol. Oncol. 2020. online ahead of print. [Google Scholar] [CrossRef]
- Kgatle, M.M.; Boshomane, T.M.; Lawal, I.O.; Mokoala, K.M.; Mokgoro, N.P.; Lourens, N.; Kairemo, K.; Zeevaart, J.R.; Vorster, M.; Sathekge, M. Immune Checkpoints, Inhibitors and Radionuclides in Prostate Cancer: Promising Combinatorial Therapy Approach. Int. J. Mol. Sci. 2021, 22, 4109. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, A.K.; Pienta, K.J.; Babich, J.W.; Bander, N.H.; Calais, J.; Choyke, P.; Hofman, M.S.; Larson, S.M.; Lin, F.I.; Morris, M.J.; et al. Meeting report from the Prostate Cancer Foundation PSMA theranostics state of the science meeting. Prostate 2020, 80, 1273–1296. [Google Scholar] [CrossRef]
- Czernin, J.; Current, K.; Mona, C.E.; Nyiranshuti, L.; Hikmat, F.; Radu, C.G.; Lueckerath, K. Immune-Checkpoint Blockade Enhances 225Ac-PSMA617 Efficacy in a Mouse Model of Prostate Cancer. J. Nucl. Med. 2021, 62, 228–231. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Osborne, J.; Niaz, M.J.; Vallabhajosula, S.; Vlachostergios, P.J.; Thomas, C.; Molina, A.M.; Sternberg, C.N.; Singh, S.; Fernandez, E.; et al. Dose-escalation results of a phase I study of 225Ac-J591 for progressive metastatic castration resistant prostate cancer (mCRPC). J. Clin. Oncol. 2020, 38 (Suppl. 6), 114. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Jacobson, O.; Cheng, Y.; Niu, G.; Li, F.; Bai, C.; Zhu, Z.; Chen, X. Safety, Pharmacokinetics, and Dosimetry of a Long-Acting Radiolabeled Somatostatin Analog 177Lu-DOTA-EB-TATE in Patients with Advanced Metastatic Neuroendocrine Tumors. J. Nucl. Med. 2018, 59, 1699–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kramer, V.; Fernández, R.; Lehnert, W.; Jiménez-Franco, L.D.; Soza-Ried, C.; Eppard, E.; Ceballos, M.; Meckel, M.; Benešová, M.; Umbricht, C.A.; et al. Biodistribution and dosimetry of a single dose of albumin-binding ligand [177Lu] Lu-PSMA-ALB-56 in patients with mCRPC. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 893–903. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).