Simple Summary
Identifying breast cancer patients with pathogenic mutations that run in their families may improve the follow-up care they receive and breast cancer screening of their close relatives. In this study we identified breast cancer patients with high chances of having a pathogenic mutation and their close female relatives. We developed and tested two different kinds of letters and booklets that presented either personalized or generic information about screening and breast cancer that runs in families, and we encouraged participants to seek genetic evaluation. We found that both types of letters worked equally well for breast cancer patients and for relatives, regardless of their racial background. The personalized letters had slightly better outcomes. Some breast cancer patients and their relatives used genetic services and improved their screening practices. Black patients and their relatives were more satisfied with the booklets than other participants.
Abstract
We compared a tailored and a targeted intervention designed to increase genetic testing, clinical breast exam (CBE), and mammography in young breast cancer survivors (YBCS) (diagnosed <45 years old) and their blood relatives. A two-arm cluster randomized trial recruited a random sample of YBCS from the Michigan cancer registry and up to two of their blood relatives. Participants were stratified according to race and randomly assigned as family units to the tailored (n = 637) or the targeted (n = 595) intervention. Approximately 40% of participants were Black. Based on intention-to-treat analyses, YBCS in the tailored arm reported higher self-efficacy for genetic services (p = 0.0205) at 8-months follow-up. Genetic testing increased approximately 5% for YBCS in the tailored and the targeted arm (p ≤ 0.001; p < 0.001) and for Black and White/Other YBCS (p < 0.001; p < 0.001). CBEs and mammograms increased significantly in both arms, 5% for YBCS and 10% for relatives and were similar for Blacks and White/Others. YBCS and relatives needing less support from providers reported significantly higher self-efficacy and intention for genetic testing and surveillance. Black participants reported significantly higher satisfaction and acceptability. Effects of these two low-resource interventions were comparable to previous studies. Materials are suitable for Black women at risk for hereditary breast/ovarian cancer (HBOC).
1. Introduction
Women diagnosed with breast cancer younger than 45 years old (young breast cancer survivors-YBCS) constitute approximately 25% of new breast cancer cases in the US, and are more likely to carry germline pathogenic variants associated with hereditary breast and ovarian cancer (HBOC) syndrome [1,2]. National guidelines recommend periodic screening for changes in family history and genetic evaluation (counseling and testing) of YBCS to determine HBOC status, and physical exams including clinical breast exams (CBE) and mammograms to screen for local recurrence or a new primary tumor [3,4]. However, there is underutilization of cancer genetic services and mammography surveillance among YBCS [5,6,7,8], especially among Black women [9,10,11,12], primarily due to lack of physician referral of minority women to genetic services, and due to complex barriers related to low income and low educational attainment that influence accessibility and acceptability of genetic services [13,14]. First- and second-degree relatives of YBCS have a 2.3 and 1.5 increased relative breast cancer risk respectively [15]. Relatives of HBOC cases should initiate MRI screening at age 25 (MRI) or earlier if indicated based on family history [3,4]. However, they may not always manage this risk effectively due to lack of information and inaccurate understanding of cancer risk inheritance patterns [16,17,18].
Paired with physician recommendations, theory-based and tailored interventions promote repeat screening, especially among racially diverse women [19,20,21,22,23,24,25]. However, the challenges concerning YBCS and at-risk relatives are firstly, the ability to identify them in large numbers, including racially diverse samples, and secondly, identifying low-resource ways to deliver information about the need for genetic evaluation and cancer surveillance guidelines. Two previous randomized controlled trials (RCTs) have shown that genetic counseling delivered over the telephone was not inferior to in-person counseling for cancer patients and at-risk relatives, yielded cost savings, and was equally acceptable to in-person counseling for disseminating information about screening guidelines [26,27]. A third RCT compared the efficacy of telephone genetic counseling versus a brochure and reported that about 40% of the telephone and 5% of the brochure groups obtained genetic counseling during the study period [28]. However, these studies included less than 10% of Black women and required significant resources for delivering the intervention and recruiting high-risk participants.
The present RCT builds on this prior work by oversampling for Black participants and by comparing the efficacy of two low-resource interventions delivered via postal mail, which included a targeted (more generic) versus a tailored (person-specific) intervention. The outcomes presented in this paper are initiation of genetic testing for YBCS, cascade genetic testing for relatives, and surveillance (CBE and mammography screening) consistent with national guidelines for YBCS and relatives. Satisfaction, acceptance, and perceived usefulness of the interventions were also assessed.
Interventions
The Theory of Planned Behavior (TPB) [29] guided the development of the targeted and the tailored intervention. Table 1 presents how the components of each intervention correspond to the constructs of the TPB. The main message in both interventions was that early age of breast cancer onset is a “red flag” for hereditary disease and that participants should seek genetic evaluation. The targeted intervention included a letter and a booklet written at a seventh-grade reading level, which provided information about genetic counseling, cost, a list of certified cancer genetic services in Michigan, and online genetic resources. The booklet presented mammography screening as more sensitive than CBE and more accessible compared to MRI [30] and options for low cost screening. The targeted letter also included National Comprehensive Cancer Network (NCCN) guidelines for follow-up care (YBCS) and screening (relatives), and recommended that they seek genetic evaluation and breast surveillance/screening due to their own or their relative’s early age of cancer onset.

Table 1.
Elements of the tailored and the targeted interventions.
The tailored intervention included the same booklet as above and a second booklet presenting basic principles of open communication and family support. The purpose of this second booklet was to enhance the tailored messages by encouraging participants to maintain open communication for family challenges associated with early cancer onset, and mobilize family support for obtaining genetic services and surveillance. Based on participants’ responses to the baseline survey, a computer algorithm generated a letter, which provided tailored feedback about the need to have genetic evaluation and surveillance/screening. Two messages were generated for dichotomous tailoring variables, i.e., had genetic testing (yes/no) and frequency of surveillance consistent with guidelines (yes/no). Two messages were also generated for continuous variables. Self-efficacy and intention for genetic testing and surveillance were scored from 1 to 7 and a cut-off score of ≤3.5 was used to identify participants with low versus high self-efficacy and intention. A score of “3.5” is between “Somewhat not confident/Somewhat unlikely” and “Neutral” indicating preference for a negative consciousness (e.g., low self-efficacy). Barriers for cancer surveillance, i.e., lack of physician referral, cost-related lack of access, fear of finding cancer, and perception that mammograms are unnecessary were also scored from 1 to 7 and a cut-off score of ≤5.5 was used to identify participants who reported low versus high barriers. A score of “5.5” is between “Neutral” and “Agree” and indicates the presence of a barrier. Personalized probabilities of developing cancer i.e., Gail and Claus scores were presented to relatives based on information provided in their baseline survey. Messages and tailored letters were reviewed for appropriateness and accuracy.
2. Results
Recruitment, enrollment, randomization and retention are shown in a consort diagram (Figure 1). Response rates were 38.6% for White/Other and 27.5% for Black, and 801 YBCS were allocated to study arms. YBCS identified 1875 eligible relatives and they were willing to contact 1360 (72.5%). The study invited n = 853 relatives (up to two relatives per YBCS); n = 442 (51.5%) accepted participation and n = 431 relatives were allocated to study arms. Overall, 11.9% YBCS and 27.4% relatives resided in 23 and 27 different U.S. states, respectively.
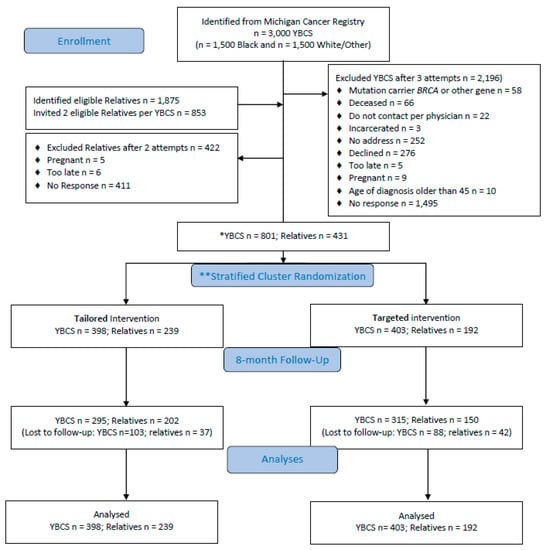
Figure 1.
Consort diagram. * YBCS = young breast cancer survivor; ** Stratified randomization of YBCS according to race (Black vs. White/Other); relatives follow randomized arm of YBCS.
After randomization, YBCS and relatives in both study arms did not differ at baseline (Table 2). YBCS were on average 51.11 (±5.74) years old and were diagnosed on average 40.13 (± 4.66) years old; approximately one in five had more than one cancer diagnoses [31]. Relatives were on average 43.35 (±11.93) years old. About one in five YBCS and one in five relatives reported cost-related lack of access to healthcare. Follow-up surveys were received from 610 YBCS (76.2% retention) and 352 relatives (81.7% retention).

Table 2.
Demographic characteristics and barriers by intervention arm (%) or mean ± SD.
2.1. (Cascade) Genetic Testing
Genetic testing was reported by 23% of YBCS and 3% of relatives at baseline, while 8 months later approximately 28% YBCS and 5% relatives reported having genetic testing at follow-up (Table 3). There were 40 new YBCS reporting having genetic testing and 9 new relative cases reporting cascade genetic testing between the baseline and the follow-up survey. From logistic regression, relatives in the tailored arm were more likely to report cascade genetic testing, although the difference was not statistically significant. From multiple linear regression analyses, YBCS in the tailored arm were more likely to report higher self-efficacy for genetic services (Beta = 0.480; CI: (0.026–0.933); p = 0.0205). Participants needing less support from providers were consistently more likely to report significant changes in outcomes related to use of genetic services, i.e., higher self-efficacy and higher intention for YBCS (Beta = 0.355; CI: (0.141–0.569); p = 0.002 and Beta = 0.490; CI: (0.334–0.645); p < 0.001), and higher self-efficacy for relatives (Beta = 0.375; CI: [0.063–0.686]; p = 0.002). YBCS who were older (Beta = 0.074; CI: (0.037–0.111); p < 0.001), Black (Beta = 0.984; CI: (0.747–1.221) p < 0.001), with cost-related barriers (Beta = 0.048; CI: (0.630–1.466); p < 0.001), and living further from genetic services (Beta = 0.014; CI: (0.007–0.022); p < 0.001) reported higher intention for genetic testing. (Appendix A Table A1).

Table 3.
Participants’ genetic testing, CBE, and mammography by intervention arms.
2.2. Breast Cancer Surveillance/Screening
At baseline, 84.27% (Tailored 85.92%; Targeted 82.63%) of YBCS and 75.41% of relatives (Tailored 74.89%; Targeted 76.04%) reported CBE consistent with NCCN guidelines (Table 3). In the 8-month follow-up survey, 89.51% of YBCS (Tailored 90.70%; Targeted 88.33%) and 84.69% of relatives (Tailored 85.36%; Targeted 83.85%) reported having CBE consistent with NCCN guidelines. At baseline, 87.41% (Tailored 87.64%; Targeted 87.16%) of YBCS and 70.50% of relatives (Tailored 69.87%; Targeted 71.31%) reported having mammograms consistent with NCCN guidelines. In the 8-month follow-up survey, 91.41% of YBCS (Tailored 92.65%; Targeted 90.15%) and 79.86% of relatives (Tailored 80.77%; Targeted 78.69%) reported having mammograms consistent with NCCN guidelines. There was about 5% and 4% increase from baseline to follow up in CBE and mammography for YBCS, while the increase was close to 10% for both outcomes for relatives. Although there were not significant differences between the two arms, there were significant changes in CBE and mammography for YBCS and relatives within each intervention arm compared to baseline. From logistic regression analyses, YBCS needing more support from providers were less likely to report CBE compared with YBCS who needed less support (OR = 0.974; CI: (0.959–0.988); p < 0.002). Older relatives were more likely to report a mammogram compared with younger relatives (OR = 1.004; CI: (1.002–1.007); p < 0.002). From multiple linear regression analyses, YBCS without health insurance reported significantly higher self-efficacy for CBE and self-efficacy for mammography (Beta = 0.696; CI: (0.278–1.113); p < 0.001; Beta = 0.830; CI: (0.406–1.254); p < 0.001). Intention to have a mammogram increased for YBCS with a routine source of care (Beta = 1.052; CI: (0.784–1.320); p < 0.001) (Appendix A Table A1).
2.3. Effects for Black and White/Other Participants
As shown in Table 4, there were not significant differences in genetic testing and surveillance/screening from baseline to follow-up between Black and White/Other participants (no differences between groups). Changes from baseline to follow-up were significantly different for both groups (significant within group differences).

Table 4.
Participants’ genetic testing, CBE, and mammography by race.
2.4. Satisfaction with the Interventions
As shown in Table 5, approximately 66% of participants reported reading the intervention materials at least once. Separate intervention effects were examined for participants reporting not reading the intervention materials (n = 131; 74 YBCS and 57 Relatives) and the main findings remained consistent. Two out of three participants reported discussing intervention materials primarily with first-degree and with non-biological relatives, most often females and/or from the maternal side of the family. We compared acceptability and perceived usefulness for YBCS versus relatives; tailored versus targeted arm; and Black versus White/Other participants. Black participants reported significantly higher satisfaction, acceptability, and usefulness of the interventions, and getting information that helped them discuss ways to lower their breast cancer risk with their provider. Relatives requested significantly more information for breast cancer risk factors and screening.

Table 5.
Evaluation of the acceptability and perceived usefulness of the interventions for YBCS vs. Relative; for Tailored vs. Targeted; and for Black vs. White/Other.
3. Discussion
Uptake of genetic testing in both arms of our RCT increased approximately 5%, which is similar to a previous RCT reporting the efficacy of a booklet on rates of genetic testing [28]. This change of 5% is commendable, given that participants received intervention materials only once and had no contact with the healthcare system, in contrast to more resource intensive studies. Given that YBCS were on average 11 years post-diagnosis, it is unlikely that this change was due to the passage of time, but most likely can be attributed to exposure to the intervention materials. At the same time, YBCS in the tailored arm were more likely to report higher self-efficacy for genetic testing, more so than the targeted intervention. Thus, the tailored intervention generated added value, since self-efficacy is an important predictor of subsequent behavior [29]. Tailored feedback improves the impact of the message on health behaviors [32,33] because it addresses personal characteristics and needs, and increases attention and information processing [34]. The lower uptake of genetic testing among YBCS may be related to other factors, including the short-term follow-up, the recruitment strategy precluding a referral from a healthcare provider, and the fact that YBCS were on average 11 years post diagnosis and genetic testing may not have been perceived as relevant or urgent [35]. Furthermore, relatives’ eligibility for cascade genetic testing depends first, on the YBCS having genetic testing as the affected relative, and second, on the YBCS’ test identifying a pathogenic variant associated with HBOC. Rates of cascade genetic testing among relatives might have been higher, if the study included the 58 YBCS reporting a known pathogenic variant in themselves or in another relative. Moreover, it is harder to improve rates of genetic testing for the 163 YBCS who had testing prior to the study, thus, influencing intervention outcomes for YBCS and relatives.
There was no difference in participant satisfaction between the two interventions. Since rates of genetic testing at baseline were low and there was little variation among study arms for this key outcome, it would be interesting to study if our targeted booklet yields better rates of genetic testing when integrated in the healthcare system and the message were reinforced by provider referrals. Future studies should also perform a cost-effectiveness analysis of tailored versus targeted interventions for genetic testing [35], since targeted messages may be as effective but less resource intensive than tailored interventions. Tailored efforts may need to focus on increasing participation of YBCS in similar initiatives and cascade genetic testing among relatives. A stepped approach with personal and timed follow-up contacts for those who do not respond to the initial invitation and those needing greater support from providers may prove efficacious and cost-effective.
An important finding of the RCT was that there was 5% to 10% increase from baseline to follow-up in CBE and mammography rates among YBCS and relatives in both study arms. Since they have already been diagnosed with the disease, YBCS are more likely to have an established relationship with the healthcare system and have cancer surveillance according to guidelines. In contrast, cancer-free relatives may not have a routine source of care and an established relationship with a provider who would guide their screening practices. Self-efficacy and intention for surveillance, which are important predictors of subsequent behavior [29], increased consistently for subgroups of participants, especially those who did not need support from providers. The booklet and the letters were an efficient and low-resource strategy for increasing screening. Given the minimal contact with participants, and that at baseline 80% of YBCS and 70% of relatives reported previous CBE and mammography leaving less room for improvement, the outcomes of the RCT indicate that both interventions addressed appropriate theory-based factors that help increase screening behaviors. Alternatively, the Healthy Michigan Plan (Medicaid expansion), which was enacted in April 2014, might have helped mitigate cost-related barriers and granted access to genetic testing and surveillance to uninsured individuals, most of whom belong to minority groups [23,36,37].
This study included a large sample of Black YBCS. Black YBCS were more likely to report higher self-efficacy and higher intention for genetic testing, higher satisfaction with their participation in the study and intervention materials, and needing additional information about genetic services and breast cancer screening compared to White/Other participants. Taken together these findings suggest that intervention booklets and letters achieved higher acceptability and perceived usefulness among Black participants, which can increase effectiveness in special populations [38,39,40]. Black participants in our study reported that underutilization of genetic services was due to lack of physician referrals and cost-related barriers [41,42,43]. Intervention materials partially addressed these barriers by encouraging participants to initiate a genetic evaluation, and by providing information about costs of genetic testing and access to low cost mammograms. Our booklets can empower minority communities and engage them in health policies for genetic screening [44].
Strengths of the study are the study design and the partnership between a state health department and a leading academic institution. Advantages of recruiting from a state cancer registry are the ability to identify retrospectively a large number of potentially eligible subjects, from diverse geographical areas and racial/ethnic backgrounds, enroll them in prospective trials, and produce results that are more representative. A disadvantage was the lower participation rate compared to recruitment from clinical sites [45,46]. However, a response rate of approximately 30% is common for RCTs recruiting participants from central cancer registries [45,46,47]. Comparisons between responders and non-responders was not possible due to lack of data for non-responders. Our RCT was underpowered to detect outcomes among Black relatives, despite their larger sample compared to previous studies. Since there was not a “no treatment” group, we can only conclude that there was no difference between the two interventions. However, the increase in genetic testing and surveillance was similar to other interventions that were compared to a “no treatment” group. It is possible that eight months was not adequate time to observe changes in outcomes. Limitations were a possible recall bias and that participants could not be blinded to study allocation, although they were not aware of materials delivered in the other arm. Additional limitations include possible recall bias, not assessing if participants received counseling but declined testing, and that information about genetic services might not be relevant for the 11.9% YBCS and 27.4% relatives not living in Michigan. Finally, findings cannot be generalized to men, to women older than 64 years old, and women who are pregnant, imprisoned, institutionalized, or non-English speaking.
4. Materials and Methods
4.1. Design and Sample
This two-arm cluster RCT was conducted in the state of Michigan (NCT01612338); the protocol and study methodology have been previously published [48]. Institutional Review Boards of the University of Michigan (HUM00055949) and the Michigan Department of Health and Human Services (201202-09-EA) approved the study protocol. The Michigan Cancer Surveillance Program identified approximately 9000 women diagnosed with breast cancer between 20 and 45 years old from the cancer registry, who were eligible for genetic evaluation due to young age of cancer onset. Cases of men with breast cancer identified in the cancer registry were not included due to their small number. Black YBCS were separated to form a separate stratum. Approximately 7% of YBCS of other racial/ethnic backgrounds (e.g., Arab Americans etc.) were grouped with White YBCS, because they could not form a separate stratum. A computer algorithm randomly selected a stratified sample of 3000 YBCS based on their cancer registry index number (1500 Black and 1500 White/Other) with oversampling of Black YBCS.
YBCS were eligible to participate if they were 20 to 45 years old when diagnosed with invasive breast cancer or ductal carcinoma in situ; 25 to 64 years old at the time of the study; Michigan residents at the time of diagnosis; and able to read English and provide informed consent. Female relatives had to be cancer-free and 25 to 64 years old; able to read English and provide informed consent; and YBCS would be willing to contact them. Up to two relatives per YBCS were included. Priority was given to younger and first-degree relatives [31]. YBCS and relatives had to be older than age 25 to assess their surveillance behavior according to NCCN guidelines. The upper age limit was set at 64 due to more limited insurance coverage for older individuals that may hinder surveillance. Excluded were pregnant, incarcerated, or institutionalized participants since they may not get mammograms.
4.2. Randomization and Masking
The Michigan Cancer Surveillance Program inquired with the reporting facility and physician of record whether there was any reason that an YBCS should not be contacted. If a response was not received within 30 days, a recruitment package was mailed to the YBCS. Eligible YBCS received up to three mailed invitations over a period of four months. YBCS who accepted participation were asked in the baseline survey if they were willing to invite their first- and second-degree female relatives to take part in the study. In order to alleviate ethical concerns in contacting relatives without their explicit consent, recruitment materials were mailed to YBCS, who passed them on to relatives. When YBCS reported they already had genetic testing, a certified genetic counselor contacted them by phone to double-check that their response was accurate. There was n = 58 YBCS who reported that they or one of their relatives had a pathogenic variant in BRCA or other gene associated with hereditary breast cancer, or had another hereditary cancer syndrome (e.g., Li Fraumeni). These YBCS were provided appropriate information and were excluded from the RCT because intervention materials were not applicable. There was n = 163 YBCS who reported a negative genetic test result. These YBCS and their relatives (n = 103) were included. None was a “true negative” and we could not exclude the possibility that there might be updated information to justify a new genetic evaluation. We could also not exclude the possibility of a pathogenic variant in relatives.
YBCS and relatives had to return a signed informed consent before receiving the baseline survey. Recruitment of YBCS and relatives took place over six months from the date of mailing the first invitation letter to reduce bias due to sample “maturation”. Research staff at the cancer registry used a computer-generated algorithm to randomize (1:1) YBCS and relatives as stratified (Black vs. White/Other) family units (i.e., dyads and triads) and allocate them to one of the two study arms. Research staff at the cancer registry were not involved in data analyses examining efficacy of the two interventions. YBCS and relatives were randomized as stratified (Black vs. White/Other) family units (i.e., dyads and triads) to one of the two study arms (1:1) using a computer-generated allocation algorithm. All members of a family unit received intervention materials at the same time by postal mail and participants were unaware of the intervention materials delivered to the other study arm. Participants received $10 gift cards for completing the baseline survey and $20 gift cards for the follow-up survey, respectively. The study employed two research staff at 40% Full Time Equivalent (FTE) for six months for recruitment, mailing, and generating intervention materials. A certified genetic counsellor (10% FTE for three months) conducted risk assessments, and verified YBCS’ self-reports of being a mutation carrier and the content of intervention materials.
4.3. Data Collection and Measures
Eligible YBCS were mailed a baseline survey (Time 1). Following assessment of their baseline information, their relatives were recruited, and family units were randomized to the targeted or tailored intervention. The follow-up survey (Time 2) was mailed to participants approximately 8 months after the intervention to allow sufficient time for pursuing the primary outcomes within the timeframe of the study. Research staff made two attempts via phone, mail, or email to contact YBCS and relatives if they did not return the follow-up survey within six weeks.
Table 6 describes the instruments used to assess genetic testing and breast cancer surveillance/screening. The research team determined consistency of surveillance with NCCN guidelines based on items from the Centers for Disease Control and Prevention: Behavioral Risk Factor Surveillance System: 2001 Survey Questions [49]. Two single items asked participants “how often you need advice from relatives/healthcare providers to engage in behaviors aiming to find cancer at an early stage” (Likert scale 1 = never to 7 = always). The 8-month follow-up survey included additional questions assessing whether the interventions provided new and helpful information, and examined intervention acceptability, interest, usefulness, level of detail, relevance, and satisfaction (Likert scale 1 = low to 7 = high) [50,51].

Table 6.
Measures used to assess covariates and outcomes.
4.4. Sample Size and Power Evaluation
Using data from previous mammography RCTs [23,25], we calculated a sampling size that is expected to ensure 80% power to detect a small (Cohen’s d = 0.2) to medium (Cohen’s d = 0.5) effect, i.e., difference in intervention effect size between group means (d = 0.3) or between percentages (h = 0.3), using a two-tailed test with a false positive rate of α = 0.05 [60]. Power analysis with PASS software [61] determined that after attrition 176 participants were needed per group or 352 in total.
4.5. Statistical Analyses
Statistical analyses were performed using R 3.4.4. (R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, available from https://www.r-project.org/). Descriptive analyses compared means and proportions in demographic and clinical factors between and within intervention groups across time (baseline and 8-month follow-up). Differences between intervention arms were tested at baseline and follow-up with two proportions z-test for proportions and with t-test for means. We performed separate analyses for YBCS and relatives for genetic testing, CBE, and mammography.
We conducted multiple linear regressions to explored associations between outcomes (self-efficacy and intention for genetic testing, CBE, and mammography) and predictor variables for YBCS and relatives including intervention grouping, antecedents, and barriers. Changes in frequencies for outcomes were demonstrated after using Intention-To-Treat (ITT), defined as “once randomized, always analyzed” [62]. Similar to last observation carried forward (LOCF), ITT is a commonly used approach in RCTs that addresses noncompliance and missing outcomes. ITT can reduce the impact from lost to follow-up but also dilutes intervention effects, making a generally conservative estimate. Outcomes reported from drop out cases in the baseline survey were carried forward to the follow-up survey. This approach avoids overoptimistic estimates resulting from removing dropout cases. Comparisons between interventions or racial groups for genetic testing and surveillance were conducted with two-proportion z-test. Fisher’s Exact test was used for small samples. McNemar’s test was used for comparisons within interventions or racial groups, and McNemar’s Exact test for small samples. Confidence intervals were computed for parameter estimates [63]. Acceptability and perceived usefulness of the interventions for YBCS versus relatives, for each intervention arm, and for Black versus White/other participants were tested using parametric t-tests, and p-values were adjusted for multiple testing via Bonferroni corrections. Two sensitivity analyses were performed for within group comparisons: (1) excluding whoever had genetic testing at baseline but keeping dropouts (ITT); (2) excluding both whoever had genetic testing and whoever dropped out. Both analyses had shown similar results as presented in Table 3. Keeping a large baseline population (true number of subjects who received the interventions) and ITT are both conservative approaches.
We examined core features of missing data (<18% of multi-item scales) and cases who dropped out (n = 270, 21.92%) from the follow-up survey. No special patterns of missing values were identified. We used demographic variables to examine if there was a clear pattern of lost-to-follow-up across subgroups using machine-learning approaches [64,65,66]. The results indicated random drop out patterns across subgroups, thus, multiple imputations addressed missing values for subsequent analyses. Two imputation approaches were used; LOCF and multiple imputations for different analyses steps. LOCF was conducted for between group and within group comparisons for dichotomous outcomes (genetic testing, CBE and mammography), while multiple imputations were conducted for multiple linear regression modeling associations between intervention effects for continuous variables (self-efficacy and intention for genetic testing, surveillance and predictor variables for YBCS and relatives including intervention grouping, antecedents, and barriers). All variables in the final model were used to generate three imputed datasets. p-values were pooled across the three models built using the three imputed datasets and adjusted for multiple testing using Bonferroni corrections.
5. Conclusions
This RCT is aligned with evidence-based recommendations for public health action relevant to cancer predisposition cascade genetic screening [67,68]. Adoption of these recommendations will achieve a population-level reduction in cancer morbidity and mortality. A combination of targeting and tailoring health messages and recruitment efforts will likely maximize resources [69] and help achieve optimal outcomes for genetic testing and cancer surveillance.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/9/2526/s1, Supplementary Material: Breast Cancer and Your Family: How to Improve Screening and Monitoring A guide for young breast cancer survivors and their female relatives.
Author Contributions
Conceptualization, M.C.K., L.L.N. and S.A.D.; Data curation, K.E.M.-V.; Formal analysis, C.M. and I.D.D.; Funding acquisition, M.C.K., L.L.N., S.A.D., D.D. and S.D.M.; Investigation, M.C.K. and D.D.; Methodology, M.C.K. and L.L.N.; Project administration, D.D. and K.E.M.-V.; Software, K.E.M.-V.; Supervision, M.C.K.; Validation, L.L.N., S.A.D., D.D., K.J.M., S.D.M. and N.K.J.; Writing—original draft, C.M.; Writing—review and editing, M.C.K., L.L.N., S.A.D., K.J.M. and N.K.J. All authors have read and agreed to the published version of the manuscript.
Funding
Funding was provided by the Centers for Disease Control and Prevention, 5U48DP001901-03, PI: M.C.K., and the Robert Wood Johnson Foundation, Nurse Faculty Scholars Award 68039, PI: M.C.K.
Acknowledgments
Beth Anderson, Epidemiologist—Michigan Department of Health and Human Services for patient and relative identification, recruitment, and assessment of eligibility. Glenn Copeland—Michigan Department of Health and Human Services, Michigan Cancer Prevention Program for patient identification and recruitment. Jenna McLosky, Cancer Genomics Program—Michigan Department of Health and Human Services for patient and relative identification, recruitment, and assessment of eligibility.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Significant associations for primary and secondary outcomes (post-minus pre-intervention) from regression analyses.
Table A1.
Significant associations for primary and secondary outcomes (post-minus pre-intervention) from regression analyses.
| Antecedents | Barriers | Subjective Norms | Family Trait | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| YBCS * Outcomes Odds Ratio or Coefficient (p) | Intervention 1 Tailored 2 Targeted | Age | Race 0 White/Other 1 Black | Education ≤High school >High school | Caregiving 0 No, 1 Yes | Anxiety 0 No, 1 Yes | Depression: 0 No, 1 Yes | Comorbidities 0 No, 1 Yes | Income:≤$40,000, >$40,000 | Insurance 0 No, 1 Yes | Routine source of care 0 No, 1 Yes | Cost-related no access to care 0 No, 1 Yes | Distance genetic services | Perc. expect family (1–7) | Perc. expect providers (1–7) | Motivation comply family (1–7) | Motivation comply provider (1–7) | Family coherence (1–20) |
| Genetic testing and surveillance a | ||||||||||||||||||
| Had Genetic Testing | 0.4631 (0.0465) | 0.9829 (0.0478) | ||||||||||||||||
| CBE - NCCN** Guidelines | 0.9741 (0.0017) | |||||||||||||||||
| Mammography - NCCN** Guidelines1 | 0.3223 (0.0060) | 1.7535 (0.0185) | 0.6149 (0.0222) | |||||||||||||||
| Self-Efficacy b | ||||||||||||||||||
| Self-efficacy for genetic testing (1–7) | −0.4797 (0.0205) | −0.7221 (0.0037) | −0.3547 (0.0020) | |||||||||||||||
| Self-efficacy for CBE (1–7) | −0.6961 (0.0007) | −0.1369 (0.0070) | ||||||||||||||||
| Self-efficacy for mammography (1–7)1 | −0.8296 (0.0001) | 0.4030 (0.0457) | −0.1086 (0.0372) | 0.0987 (0.0225) | −0.1639 (0.0039) | |||||||||||||
| Intention b | ||||||||||||||||||
| Intention for genetic testing (1–7) | 0.0739 (0.0002) | 0.9838 (0.0000) | 1.0475 (0.0001) | 0.0139 (0.0000) | −0.4903 (0.0000) | |||||||||||||
| Intention for CBE (1–7) | −0.2229 (0.0076) | −0.1780 (0.0098) | ||||||||||||||||
| Intention for Mammography (1–7)1 | 1.0522 (0.0006) | −0.1703 (0.0323) | ||||||||||||||||
| Antecedents | Barriers | Subjective Norms | Family Trait | |||||||||||||||
| Relative Outcomes Odds Ratio or Coefficient (p) | Intervention 1 Tailored 2 Targeted | Age | Race 0 White/Other 1 Black | Education ≤High school >High school | Caregiving 0 No, 1 Yes | Anxiety 0 No, 1 Yes | Depression: 0 No, 1 Yes | Comorbidities 0 No, 1 Yes | Income ≤$40,000, >$40,000 | Insurance 0 No, 1 Yes | Routine source of care 0 No, 1 Yes | Cost-related no access to care 0 No; 1 Yes | Distance genetic services | Perc. expect family (1–7) | Perc. expect providers (1–7) | Motivation comply family (1–7) | Motivation comply provider (1–7) | Family coherence (1–20) |
| Genetic testing and surveillance a | ||||||||||||||||||
| Had Genetic Testing | 0.0983 (0.0468) | |||||||||||||||||
| CBE - NCCN** Guidelines | 0.3720 (0.0414) | |||||||||||||||||
| Mammography - NCCN** Guidelines 2 | 1.0040 (0.0019) | 2.9500 (0.0273) | 3.659 (0.0147) | 0.2252 (0.0039) | 1.8172 (0.0113) | |||||||||||||
| Self-Efficacyb | ||||||||||||||||||
| Self-efficacy for genetic testing (1–7) | −0.7305 (0.0210) | −0.3751 (0.0016) | ||||||||||||||||
| Self-efficacy for CBE (1–7) | 0.4329 (0.0414) | −0.5037 (0.0475) | −0.2106 (0.0146) | |||||||||||||||
| Self-efficacy for mammography (1–7)2 | −0.0431 (0.0329) | −0.8418 (0.0407) | −0.2897 (0.0337) | |||||||||||||||
| Intentionb | ||||||||||||||||||
| Intention for genetic testing (1–7) | −0.3532 (0.0048) | 0.3122 (0.0294) | ||||||||||||||||
| Intention for CBE (1–7) | −0.0088 (0.0005) | −0.2442 (0.0118) | ||||||||||||||||
| Intention for Mammography (1–7)2 | −0.0301 (0.0089) | −1.014 (0.0129) | −1.0914 (0.0258) | −0.0089 (0.0271) | −0.3514 (0.0411) | |||||||||||||
* YBCS = young breast cancer survivor; **NCCN = National Comprehensive Cancer Network; a Logistic regression; b Linear regression; 1 Tailored n = 293; Targeted n = 382, after excluding YBCS with double mastectomy (excluded Tailored n = 31; Targeted n = 95); 2 Tailored n = 60; Targeted n = 218, after excluding relatives younger than 35 years old AND relatives between 35 to 40 with Gail lifetime risk <20% according to NCCN guidelines (excluded Tailored n = 27; Targeted n = 126). Bold = still significant after Bonferroni corrections.
References
- King, M.C.; Levy-Lahad, E.; Lahad, A. Population-Based Screening forBRCA1andBRCA. JAMA 2014, 312, 1091–1092. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.; Newman, L.A.; Partridge, A.H.; Rosenberg, S.S.M. Breast Cancer in Young Women. JAMA Oncol. 2015, 1, 877. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.B.; Pilarski, R.; Axilbund, J.E.; Buys, S.S.; Crawford, B.; Friedman, S.; Garber, J.E.; Horton, C.; Kaklamani, V.G.; Klein, C.; et al. Genetic/familial high-risk assessment: Breast and ovarian, version 1. J. Natl. Compr. Cancer Netw. 2014, 12, 1326–1338. [Google Scholar] [CrossRef] [PubMed]
- Runowicz, C.D.; Leach, C.R.; Henry, N.L.; Henry, K.S.; Mackey, H.T.; Cowens-Alvarado, R.L.; Cannady, R.S.; Pratt-Chapman, M.L.; Edge, S.B.; Jacobs, L.A.; et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J. Clin. Oncol. 2016, 34, 611–635. [Google Scholar] [CrossRef]
- Katz, M.L.; Donohue, K.A.; Alfano, C.M.; Day, J.M.; Herndon, J.E.; Paskett, E.D. Cancer surveillance behaviors and psychosocial factors among long-term survivors of breast cancer. Cancer Leuk. Group B Cancer 2009, 115, 480–488. [Google Scholar] [CrossRef]
- Sabatino, S.A.; Thompson, T.D.; Richardson, L.C.; Miller, J. Health Insurance and Other Factors Associated With Mammography Surveillance Among Breast Cancer Survivors. Med. Care 2012, 50, 270–276. [Google Scholar] [CrossRef]
- Shelby, R.A.; Scipio, C.D.; Somers, T.J.; Soo, M.S.; Weinfurt, K.P.; Keefe, F.J. Prospective Study of Factors Predicting Adherence to Surveillance Mammography in Women Treated for Breast Cancer. J. Clin. Oncol. 2012, 30, 813–819. [Google Scholar] [CrossRef]
- Wirtz, H.S.; Boudreau, D.M.; Gralow, J.R.; Barlow, W.E.; Gray, S.; Bowles, E.J.A.; Buist, D.S.M. Factors associated with long-term adherence to annual surveillance mammography among breast cancer survivors. Breast Cancer Res. Treat. 2014, 143, 541–550. [Google Scholar] [CrossRef]
- Adams, I.; Christopher, J.; Williams, K.P.; Sheppard, V.B. What black women know and want to know about counseling and testing for brca1/2. J. Cancer Educ. 2015, 30, 344–352. [Google Scholar] [CrossRef]
- Glenn, B.A.; Chawla, N.; Bastani, R. Barriers to genetic testing for breast cancer risk among ethnic minority women: An exploratory study. Ethn. Dis. 2012, 22, 267–273. [Google Scholar]
- Levy, D.E.; Byfield, S.D.; Comstock, C.B.; Garber, J.E.; Syngal, S.; Crown, W.H.; Shields, A.E. Underutilization of BRCA1/2 testing to guide breast cancer treatment: Black and Hispanic women particularly at risk. Genet. Med. 2011, 13, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, V.B.; Graves, K.D.; Christopher, J.; Hurtado-De-Mendoza, A.; Talley, C.; Williams, K.P. African American Women’s Limited Knowledge and Experiences with Genetic Counseling for Hereditary Breast Cancer. J. Genet. Couns. 2013, 23, 311–322. [Google Scholar] [CrossRef]
- Kolb, B.; Wallace, A.M.; Hill, D.; Royce, M. Disparities in cancer care among racial and ethnic minorities. Oncology 2006, 20, 1256–1261. [Google Scholar] [PubMed]
- Mai, P.L.; Vadaparampil, S.T.; Breen, N.; McNeel, T.S.; Wideroff, L.; Graubard, B.I. Awareness of cancer susceptibility genetic testing: The 2000, 2005, and 2010 National Health Interview Surveys. Am. J. Prev. Med. 2014, 46, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Eccles, S.A.; Aboagye, E.O.; Ali, S.; Anderson, A.S.; Armes, J.; Berditchevski, F.; Blaydes, J.; Brennan, K.; Brown, N.; Bryant, H.E.; et al. Critical research gaps and translational priorities for the successful prevention and treatment of breast cancer. Breast Cancer Res. 2013, 15, R92. [Google Scholar] [CrossRef] [PubMed]
- Glassey, R.; Investigators, K.; O’Connor, M.; Ives, A.; Saunders, C.; O’Sullivan, S.; Hardcastle, S.J. Heightened perception of breast cancer risk in young women at risk of familial breast cancer. Fam. Cancer 2017, 17, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.; Gold, R.; Levin, N.; Tainsky, M.A.; Berry-Bobovski, L. Communication and decision-making about seeking inherited cancer risk information: Findings from female survivor-relative focus groups. Psycho. Oncol. 2006, 15, 193–208. [Google Scholar] [CrossRef]
- Underhill-Blazey, M.; Habin, K.; Shannon, K.M. Perceptions of Cancer Risk, Cause, and Needs in Participants from Low Socioeconomic Background at Risk for Hereditary Cancer. Behav. Med. 2016, 43, 259–267. [Google Scholar] [CrossRef]
- Brevik, T.B.; Laake, P.; Bjørkly, S. Effect of culturally tailored education on attendance at mammography and the Papanicolaou test. Health Serv. Res. 2020, 55, 457–468. [Google Scholar] [CrossRef]
- Champion, V.L.; Skinner, C.S.; Hui, S.; Monahan, P.; Juliar, B.; Daggy, J.K.; Menon, U. The effect of telephone versus print tailoring for mammography adherence. Patient Educ. Couns. 2007, 65, 416–423. [Google Scholar] [CrossRef]
- Champion, V.L.; Springston, J.K.; Zollinger, T.W.; Saywell, R.M.; Monahan, P.O.; Zhao, Q.; Russell, K.M. Comparison of three interventions to increase mammography screening in low income African American women. Cancer Detect. Prev. 2006, 30, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Copeland, V.C.; Kim, Y.J.; Eack, S.M. Effectiveness of Interventions for Breast Cancer Screening in African American Women: A Meta-Analysis. HealTH Serv. Res. 2017, 53, 3170–3188. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-R.; Lee, J.-E.; Kim, J.; Hedlin, H.K.; Song, H.; Kim, M.T. A Meta-Analysis of Interventions to Promote Mammography Among Ethnic Minority Women. Nurs. Res. 2009, 58, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Sohl, S.J.; Moyer, A. Tailored interventions to promote mammography screening: A meta-analytic review. Prev. Med. 2007, 45, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Vernon, S.W.; McQueen, A.; Tiro, J.A.; Del Junco, D.J. Interventions to Promote Repeat Breast Cancer Screening With Mammography: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2010, 102, 1023–1039. [Google Scholar] [CrossRef]
- Kinney, A.Y.; Steffen, L.E.; Brumbach, B.H.; Kohlmann, W.; Du, R.; Lee, J.-H.; Gammon, A.; Butler, K.; Buys, S.S.; Stroup, A.M.; et al. Randomized Noninferiority Trial of Telephone Delivery of BRCA1/2 Genetic Counseling Compared With In-Person Counseling: 1-Year Follow-Up. J. Clin. Oncol. 2016, 34, 2914–2924. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Valdimarsdottir, H.B.; Peshkin, B.N.; Mandelblatt, J.; Nusbaum, R.; Huang, A.-T.; Chang, Y.; Graves, K.; Isaacs, C.; Wood, M.; et al. Randomized Noninferiority Trial of Telephone Versus In-Person Genetic Counseling for Hereditary Breast and Ovarian Cancer. J. Clin. Oncol. 2014, 32, 618–626. [Google Scholar] [CrossRef]
- Pasick, R.J.; Joseph, G.; Stewart, S.L.; Kaplan, C.; Lee, R.; Luce, J.; Davis, S.; Marquez, T.; Nguyen, T.; Guerra, C. Effective Referral of Low-Income Women at Risk for Hereditary Breast and Ovarian Cancer to Genetic Counseling: A Randomized Delayed Intervention Control Trial. Am. J. Public Health 2016, 106, 1842–1848. [Google Scholar] [CrossRef]
- Ajzen, I. The theory of planned behaviour: Reactions and reflections. Psychol. Health 2011, 26, 1113–1127. [Google Scholar] [CrossRef]
- Tadros, A.; Arditi, B.; Weltz, C.; Port, E.R.; Margolies, L.R.; Schmidt, H. Utility of surveillance MRI in women with a personal history of breast cancer. Clin. Imaging 2017, 46, 33–36. [Google Scholar] [CrossRef]
- Katapodi, M.C.; Duquette, D.; Yang, J.J.; Mendelsohn-Victor, K.; Anderson, B.; Nikolaidis, C.; Mancewicz, E.; Northouse, L.L.; Duffy, S.; Ronis, D.; et al. Recruiting families at risk for hereditary breast and ovarian cancer from a statewide cancer registry: A methodological study. Cancer Causes Control. 2017, 28, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.P.; Kreuter, M.; Resnicow, K.; Fishbein, M.; Dijkstra, A. Understanding tailoring in communicating about health. Health Educ. Res. 2008, 23, 454–466. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.W.; Farrell, D.W.; Olevitch, L.R.; Brennan, L.K. Tailoring Health Messages; Informa UK Limited: London, UK, 2013. [Google Scholar]
- Kreuter, M.W.; Wray, R.J. Tailored and targeted health communication: Strategies for enhancing information relevance. Am. J. Health Behav. 2003, 27, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, C.; Ming, C.; Pedrazzani, C.; Van Der Horst, T.; Kaiser-Grolimund, A.; Ademi, Z.; Bührer-Landolt, R.; Bürki, N.; Caiata-Zufferey, M.; Champion, V.; et al. Challenges and Opportunities for Cancer Predisposition Cascade Screening for Hereditary Breast and Ovarian Cancer and Lynch Syndrome in Switzerland: Findings from an International Workshop. Public Heal. Genom. 2018, 21, 121–132. [Google Scholar] [CrossRef]
- Ayanian, J.Z.; Ehrlich, G.M.; Grimes, D.R.; Levy, H. Economic effects of medicaid expansion in michigan. N. Engl. J. Med. 2017, 376, 407–410. [Google Scholar] [CrossRef]
- Cragun, D.; Weidner, A.; Lewis, C.; Bonner, D.; Kim, J.; Vadaparampil, S.T.; Pal, T. Racial disparities in BRCA testing and cancer risk management across a population-based sample of young breast cancer survivors. Cancer 2017, 123, 2497–2505. [Google Scholar] [CrossRef]
- Brach, C.; Fraser, I. Can cultural competency reduce racial and ethnic health disparities? A review and conceptual model. Med. Care Res. Rev. 2000, 57 (Suppl. 1), 181–217. [Google Scholar] [CrossRef]
- Kreuter, M.W.; Lukwago, S.N.; Bucholtz, D.C.; Clark, I.M.; Sanders-Thompson, V. Achieving Cultural Appropriateness in Health Promotion Programs: Targeted and Tailored Approaches. Heal. Educ. Behav. 2003, 30, 133–146. [Google Scholar] [CrossRef]
- Resnicow, K.; Baranowski, T.; Ahluwalia, J.S.; Braithwaite, R.L. Cultural sensitivity in public health: Defined and demystified. Ethn. Dis. 1999, 9, 10–21. [Google Scholar]
- Jones, T.; Duquette, D.; Underhill, M.; Ming, C.; Mendelsohn-Victor, K.E.; Anderson, B.; Milliron, K.J.; Copeland, G.; Janz, N.K.; Northouse, L.L.; et al. Surveillance for cancer recurrence in long-term young breast cancer survivors randomly selected from a statewide cancer registry. Breast Cancer Res. Treat. 2018, 169, 141–152. [Google Scholar] [CrossRef]
- Jones, T.; Lockhart, J.S.; Mendelsohn-Victor, K.E.; Duquette, D.; Northouse, L.L.; Duffy, S.A.; Donley, R.; Merajver, S.D.; Milliron, K.J.; Roberts, J.S.; et al. Use of Cancer Genetics Services in African-American Young Breast Cancer Survivors. Am. J. Prev. Med. 2016, 51, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, C.; Duquette, D.; Mendelsohn-Victor, K.E.; Anderson, B.; Copeland, G.; Milliron, K.J.; Merajver, S.D.; Janz, N.K.; Northouse, L.L.; Duffy, S.A.; et al. Disparities in genetic services utilization in a random sample of young breast cancer survivors. Genet. Med. 2018, 21, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Bonham, V.L.; Citrin, T.; Modell, S.M.; Franklin, T.H.; Bleicher, E.W.B.; Fleck, L.M. Community-based dialogue: Engaging communities of color in the United states’ genetics policy conversation. J. Heal. Politi. Policy Law 2009, 34, 325–359. [Google Scholar] [CrossRef]
- Bonner, D.; Cragun, D.; Bs, M.R.; Vadaparampil, S.T.; Pal, T. Recruitment of a Population-Based Sample of Young Black Women with Breast Cancer through a State Cancer Registry. Breast J. 2015, 22, 166–172. [Google Scholar] [CrossRef]
- Tan, M.; Thomas, M.; Mac Eachern, M. Using registries to recruit subjects for clinical trials. Contemp. Clin. Trials 2014, 41, 31–38. [Google Scholar] [CrossRef]
- Millar, M.M.; Kinney, A.Y.; Camp, N.J.; Cannon-Albright, L.A.; Hashibe, M.; Penson, D.F.; Kirchhoff, A.C.; Neklason, D.W.; Gilsenan, A.W.; Dieck, G.S.; et al. Predictors of Response Outcomes for Research Recruitment Through a Central Cancer Registry: Evidence From 17 Recruitment Efforts for Population-Based Studies. Am. J. Epidemiol. 2019, 188, 928–939. [Google Scholar] [CrossRef] [PubMed]
- Katapodi, M.C.; Northouse, L.L.; Schafenacker, A.M.; Duquette, D.; Duffy, S.A.; Ronis, D.L.; Anderson, B.; Janz, N.K.; McLosky, J.; Milliron, K.J.; et al. Using a state cancer registry to recruit young breast cancer survivors and high-risk relatives: Protocol of a randomized trial testing the efficacy of a targeted versus a tailored intervention to increase breast cancer screening. BMC Cancer 2013, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Questionnaire. Available online: https://www.cdc.gov/brfss/questionnaires/pdf-ques/2001brfss.pdf (accessed on 18 January 2019).
- Durand, M.-A.; Witt, J.; Joseph-Williams, N.; Newcombe, R.G.; Politi, M.C.; Sivell, S.; Elwyn, G. Minimum standards for the certification of patient decision support interventions: Feasibility and application. Patient Educ. Couns. 2015, 98, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Northouse, L.L.; Walker, J.; Schafenacker, A.; Mood, D.; Mellon, S.; Galvin, E.; Harden, J.; Freeman-Gibb, L. A Family-Based Program of Care for Women With Recurrent Breast Cancer and Their Family Members. Oncol. Nurs. Forum 2002, 29, 1411–1419. [Google Scholar] [CrossRef]
- Bliss, R.L.; Katz, J.N.; Wright, E.A.; Losina, E. Estimating Proximity to Care. Med. Care 2012, 50, 99–106. [Google Scholar] [CrossRef]
- Anderson, B.; McLosky, J.; Wasilevich, E.; Lyon-Callo, S.; Duquette, D.; Copeland, G. Barriers and Facilitators for Utilization of Genetic Counseling and Risk Assessment Services in Young Female Breast Cancer Survivors. J. Cancer Epidemiol. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.; Kuchenbacker, K.; Engel, C.; Zachariae, S.; Rhiem, K.; Meindl, A.; Rahner, N.; Dikow, N.; Plendl, H.; Debatin, I.; et al. Evaluating the performance of the breast cancer genetic risk models boadicea, ibis, brcapro and claus for predicting brca1/2 mutation carrier probabilities: A study based on 7352 families from the german hereditary breast and ovarian cancer consortium. J. Med. Genet. 2013, 50, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Gail, M.H.; Brinton, L.A.; Byar, D.P.; Corle, D.K.; Green, S.B.; Schairer, C.; Mulvihill, J.J. Projecting Individualized Probabilities of Developing Breast Cancer for White Females Who Are Being Examined Annually. J. Natl. Cancer Inst. 1989, 81, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Rockhill, B.; Spiegelman, N.; Byrne, C.; Hunter, D.J.; Colditz, G.A. Validation of the Gail et al. model of breast cancer risk prediction and implications for chemoprevention. J. Natl. Cancer Inst. 2001, 93, 358–366. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, H.I.; Thompson, A.I.; McCubbin, M.A. Family Assessment: Resiliency, Coping and Adaptation: Inventories for Research and Practice; University of Wisconsin-Madison: Madison, WI, USA, 1996. [Google Scholar]
- Rakowski, W.; Andersen, M.R.; Stoddard, A.M.; Urban, N.; Al, E. Confirmatory analysis of opinions regarding the pros and cons of mammography. Health Psychol. 1997, 16, 433–442. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Anderson, B.O.; Balassanian, R.; Blair, S.L.; Burstein, H.J.; Cyr, A.; Elias, A.D.; Farrar, W.B.; Forero, A.; Giordano, S.H.; et al. NCCN Guidelines Insights: Breast Cancer, Version 1. J. Natl. Compr. Cancer Netw. 2017, 15, 433–451. [Google Scholar] [CrossRef]
- Chu, A.; Cui, J.; Dinov, I.D. SOCR Analyses: Implementation and Demonstration of a New Graphical Statistics Educational Toolkit. J. Stat. Softw. 2009, 30, 1–19. [Google Scholar] [CrossRef]
- Hintze, J. Pass 2008 User’s Guide; Number Cruncher Statistical Software Google Scholar: Kaysville, UT, USA, 2008. [Google Scholar]
- Wright, C.; Sim, J. Intention-to-treat approach to data from randomized controlled trials: A sensitivity analysis. J. Clin. Epidemiol. 2003, 56, 833–842. [Google Scholar] [CrossRef]
- Christou, N.; Dinov, I.D. Confidence Interval Based Parameter Estimation—A New SOCR Applet and Activity. PLoS ONE 2011, 6, e19178. [Google Scholar] [CrossRef]
- Dinov, I.D. Data Science and Predictive Analytics; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Dinov, I.D.; Heavner, B.D.; Tang, M.; Glusman, G.; Chard, K.; Darcy, M.; Madduri, R.; Pa, J.; Spino, C.; Kesselman, C.; et al. Predictive Big Data Analytics: A Study of Parkinson’s Disease Using Large, Complex, Heterogeneous, Incongruent, Multi-Source and Incomplete Observations. PLoS ONE 2016, 11, e0157077. [Google Scholar] [CrossRef]
- Toga, A.W.; Dinov, I.D. Sharing big biomedical data. J. Big Data 2015, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Rogowski, W.; Ross, L.; Cornel, M.; Dondorp, W.; Khoury, M. Population Screening for Genetic Disorders in the 21st Century: Evidence, Economics, and Ethics. Public Health Genom. 2010, 13, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Khoury, M.J.; Evans, J.P. A public health perspective on a national precision medicine cohort: Balancing long-term knowledge generation with early health benefit. JAMA 2015, 313, 2117–2118. [Google Scholar] [CrossRef] [PubMed]
- Schmid, K.L.; Rivers, S.E.; Latimer, A.E.; Salovey, P. Targeting or tailoring? Maximizing resources to create effective health communications. Mark. Health Serv. 2008, 28, 32. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).