Abstract
Opportunities for surgical treatment in metastatic melanoma patients have re-emerged due to the development of novel systemic therapeutics over the past decade. The aim of this study is to present data on outcomes of surgery in patients with unresectable stage IIIC and IV melanoma, who have previously been treated with immunotherapy or targeted therapy. Data was extracted from the Dutch Melanoma Treatment Registry (DMTR) on 154 patients obtaining disease control to systemic therapy and undergoing subsequent surgery. Disease control was defined as a complete response (CR), which was seen in 3.2% of patients; a partial response (PR), seen in 46.1% of patients; or stable disease (SD), seen in 44.2% of patients. At a median follow-up of 10.0 months (interquartile range 4–22) after surgery, the median overall survival (OS) had not been reached in our cohort and median progression-free survival (PFS) was 9.0 months (95% CI 6.3–11.7). A CR or PR at first follow-up after surgery was associated with both a better OS and PFS compared to stable or progressive disease (p < 0.001). We conclude that selected patients can benefit from surgery after achieving disease control with systemic therapy.
1. Introduction
Historically the prognosis of patients with unresectable stage III and IV melanoma has been poor, with a median overall survival (OS) of only 6.2 months [1,2]. Some patients with oligometastatic melanoma (up to three lesions) can be treated by surgery and achieve long-term survival of around 40% at 5 years, especially those with only one lesion and a long interval between their melanoma and the development of stage IV disease [3,4]. However, it is difficult to select the patients that would benefit from such surgery.
Over the past decade the development of new systemic options has drastically changed the treatment of metastatic melanoma and therefore the prognosis of these patients. Immune checkpoint inhibitors (ICI) targeting PD-1 and CTLA-4, either as monotherapy or combined treatment, have shown response rates of approximately 40% to 60% respectively, improving progression-free and overall survival [5,6,7,8,9,10,11,12,13,14,15,16,17,18]. BRAF and MEK inhibitors in patients with BRAF-mutated melanoma have even higher overall response rates (70%), but fewer durable responses due to the development of resistance [19,20,21,22,23,24,25]. This evolution in therapeutic options has also presented new opportunities for surgery in this group of patients.
In some patients experiencing a durable partial response to systemic therapy, resection of the remaining lesion(s) can contribute to obtaining a complete response. Additionally, in patients with a partial or complete response developing oligoprogression, resection of the progressive lesion(s) may be performed. However, although these surgeries are already being performed in clinical practice, little evidence has been presented to support this treatment approach.
The aim of this population-based study is to present data on the incidence and outcomes of surgery in patients with unresectable stage III and IV melanoma, who have been treated with immunotherapy or targeted therapy (TT) prior to surgery (no first line surgery included), to provide an insight into which patients may benefit from surgery after obtaining disease control with systemic therapy.
2. Patients and Methods
Data were retrieved from the Dutch Melanoma Treatment Registry (DMTR). In this nationwide prospective database, all Dutch patients undergoing treatment for unresectable stage IIIC and IV melanoma are included. This registry was set up to monitor the safety and the outcomes of the novel treatments [26]. Registration in the DMTR is a prerequisite for reimbursement, assuring nationwide coverage. In compliance with Dutch regulations, the DMTR was approved by the medical ethical committee and was not subject to the Medical Research Involving Human Subjects Act. Patients were offered an opt-out option. In the current study we included patients from the database who had commenced treatment between the start of the registry (July 2012) and July 2017 to assure sufficient follow-up at data extraction in April 2018.
2.1. Patients
Patients who had surgery after obtaining disease control with systemic therapy were selected from the registry. Disease control was defined as stable disease (SD), partial response (PR) or complete response (CR) as the best response to systemic therapy. These responses were non-confirmed investigator-assessed responses, retracted from follow-up data registered in the database, therefore this cannot be considered the same as RECIST-measured responses. Progressive disease (PD) was allowed as a most recent status of disease prior to surgery if these patients initially had a SD, PR or CR as their best response. Patients who had primarily progressive disease and underwent surgery were excluded, as we considered that this would include a substantial number of palliative surgeries for symptomatic patients, which was not the focus of this study.
Patients with uveal and mucosal melanoma were excluded, since these subtypes differ in biologic behavior and in their responses to immunotherapy and targeted therapy. Moreover, patients presenting with brain metastases were excluded from this study, since these patients generally have a different prognosis.
2.2. Statistical Analysis
Data were analyzed using IBM SPSS Statistics, version 25 (IBM Corp., Armonk, NY, USA). Descriptive statistics were used to assess patient, tumor, systemic therapy, surgery and follow-up characteristics. The baseline for patient and tumor characteristics was set at start of systemic therapy. Characteristics of patients treated with ICI were compared to patients treated with targeted therapy using the chi-square test (categorical variables) and t-Test (continuous variables). Kaplan-Meier methods and log-rank tests were applied to calculate and compare progression-free survival (PFS) and OS and Cox regression models were used to analyze the influence of different variables. Variables that were (borderline) significant in the univariate analyses (and consisted of sufficient patient numbers) were used in the multivariate Cox regression models. PFS and OS were defined as the time between surgery and first disease progression or death, respectively. Patients not experiencing an event were censored at the time of last follow-up.
3. Results
3.1. Patient and Tumor Characteristics
At the time of data extraction, the DMTR database consisted of 3959 patients, of whom 876 had undergone surgery during their treatment and 463 patients received systemic treatment prior to surgery. After selecting patients obtaining disease control (SD/PR/CR) with systemic therapy, 154 patients remained. The baseline characteristics of these 154 patients and the treatment they received are listed in Table 1. The median age of our study population was 58, ranging from 24 to 87. The vast majority of patients had a good performance score, WHO zero or one (91.6%), and a normal lactate dehydrogenase (LDH) level (74.7%), which is a known prognostic factor for survival in metastatic melanoma patients [27]. The percentage of patients with a BRAF mutation was slightly higher than usual in the general patient population (68.8%), most likely due to the selection for response to systemic treatment. Most patients presented with distant metastases: 44.2% with distant metastases only and 34.4% with both distant and locoregional metastases compared to 21.4% of patients presenting with only locoregional metastases. A substantial proportion of patients presenting with distant metastases had nodal or subcutaneous metastases: 58.2% and 37.2%, respectively. The number of metastases before the start of systemic treatment was poorly documented, with missing data in 37% of patients, but most patients presented with multiple (>10) lesions.

Table 1.
Patient, tumor and treatment characteristics.
No differences were seen in baseline characteristics between patients treated with immunotherapy and targeted therapy, except for the location of the metastases (Table 1). More patients treated with targeted therapy had locoregional metastases only, compared to patients treated with ICI (31.1% vs. 11.4%, p = 0.007), of whom a larger percentage were treated for distant metastases (53.2% vs. 32.8%).
3.2. Treatment
Surgery was performed after the first line of systemic treatment in 69.5% of patients. Little over half of patients (51.3%) were treated with ICI, 39.6% with targeted therapy, and 9.1% of patients with other treatment (in trials) or the given treatment was unknown. Of patients with a BRAF mutation, the majority received targeted therapy (57.5%), the remainder received either immunotherapy (34.0%) or other treatments (8.5%). Patients receiving immunotherapy were roughly evenly divided between anti-PD1 directed therapy (48.1%) and anti-CTLA4 therapy (44.3%) and only a small percentage (7.6%) were treated with combination ICI. Of patients receiving targeted therapy, about half (50.8%) were treated with a BRAF inhibitor alone and in the remaining patients (49.2%) it was combined with a MEK inhibitor.
3.3. Response
3.3.1. Best Response
Only a very small proportion of patients (3.2%) achieved a complete response as the best response to systemic treatment prior to surgery and the fractions of patients obtaining a partial response and stable disease as a best response were similar (46.1% and 44.2%).
3.3.2. Most Recent Disease Status Prior to Surgery
The most recently reported status of disease prior to surgery was PD in 46.1% of patients, versus 29.2% of patients with SD and 18.8% with a PR before surgery. As shown above, the best response to systemic therapy was not necessarily the same as the most recent status of disease prior to surgery. For example, if a patient had a CR upon systemic therapy, but developed a recurrence and was operated for this lesion in due course, then the best response was CR, but the most recent status of disease prior to surgery was classified as PD.
In the vast majority of patients, subcutaneous (39.6%) or lymph node (42.9%) metastases were resected and few serious complications occurred.
3.3.3. First Evaluation after Surgery
3.4. Survival Outcomes
At a median follow-up of 10.0 months (interquartile range 4–22) after surgery, the median OS had not been reached in our cohort (1-year OS was 70% and 2-year OS 59%) and median PFS was 9.0 months (95% CI 6.3–11.7). Figure 1a,b show Kaplan–Meier curves of the PFS and OS of the patients treated with ICI and targeted therapy separately. Figure S2 shows the PFS and OS of the entire cohort. The time to next treatment has not been shown, since this was similar to the PFS.
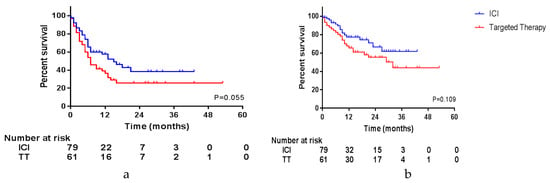
Figure 1.
Survival per type of systemic therapy. (a) Progression-free survival; (b) overall survival.
Since survival could be influenced by the response to systemic treatment, we compared Kaplan–Meier curves of these different variables. The influence of these variables was tested in the entire cohort and in patients treated with either ICI or targeted therapy separately. OS and PFS of the entire cohort were not influenced by the best response to systemic treatment. However, in patients treated with ICI, a trend was seen in PFS, favoring patients with a PR compared to patients with SD (Figure S3; CR was not shown due to the very limited number of patients). The most recent status of disease prior to surgery had an impact on PFS and OS. Patients with PD before surgery had a median PFS after surgery of 5.0 months and a median OS of 17 months, compared to a not-reached median PFS and OS in patients with a PR (p = 0.009 and p = 0.004). As shown in Figure S4, this impact is seen in patients treated with targeted therapy and is even more pronounced in patients treated with ICI. Moreover, the status of disease determined at first evaluation after surgery had a significant impact on OS and PFS in the entire cohort and both treatment groups. This is shown in Figure 2 and Figure S5, with a median OS of 7 months in patients with PD compared to 29 months in patients with SD and not reached in patients with a CR or PR after surgery (p < 0.001 in both groups). Unfortunately, further follow-up data were missing in a substantial portion of the patients achieving a CR after surgery, and since the outcomes did not significantly differ from patients achieving a PR (p = 0.966), these groups were combined.
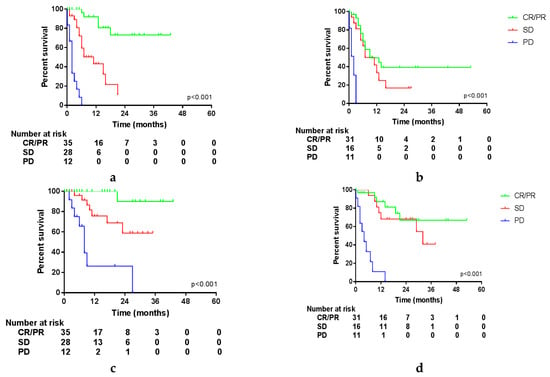
Figure 2.
Survival per status of disease at first follow-up after surgery. (a) Progression-free survival (PFS) in patients treated with ICI; (b) PFS in patients treated with targeted therapy; (c) overall survival (OS) in patients treated with ICI; (d) OS in patients treated with targeted therapy.
Interestingly, the location of the resected lesions had an impact on OS, but not on PFS, as is displayed in Figure 3. Unfortunately, it was not possible to differentiate between distant or locoregional subcutaneous and lymph node metastases in this database. However, this shows that after resection of a lymph node or subcutaneous metastasis (either locoregional or distant), patients do experience progression as quickly as after resection of a visceral metastasis, but this does not seem to influence the OS of these patients.
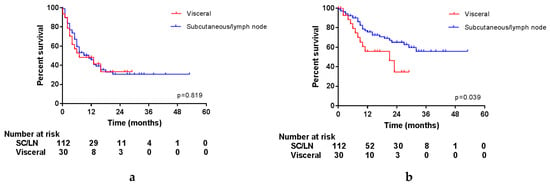
Figure 3.
Survival per location of surgery. (a) Progression-free survival; (b) overall survival.
Other factors that could influence PFS and OS were compared in a univariate Cox regression analysis. Table 2a shows factors that had a significant impact on PFS, OS or both and Table S2 shows all factors. In the univariate analysis type of systemic therapy had no statistically significant impact on PFS or OS, however, a trend favoring ICI might be visible, so this factor was included in further analyses.

Table 2.
(a) Univariate analyses (significant results). (b) Multivariate analyses.
All factors that showed (borderline) significance in both PFS and OS univariate analyses were used to perform a multivariate Cox regression analysis. These results are shown in Table 2b. Disease status after surgery is the most convincing factor, which has a significant impact on both PFS and OS. Moreover, immunotherapy prior to surgery was associated with a PFS and OS benefit compared to targeted therapy, when corrected for other factors in the multivariate analysis.
4. Discussion
We found that in metastatic melanoma patients obtaining disease control with systemic therapy and undergoing subsequent surgery, the most convincing factor associated with a more favorable outcome was the disease status (CR or PR) at first follow-up after surgery. Moreover, immunotherapy compared to targeted therapy and a duration of systemic therapy of over 3 months seemed to have a positive effect on prognosis.
OS and PFS in our cohort seem to be better than historically, with a median PFS after surgery of 9 months and OS not reached. Howard et al. retrospectively studied patients who had surgery with or without systemic therapy versus systemic therapy alone for stage IV melanoma, all of whom were initially treated in the MSLT-1 trial [4]. They described a survival benefit for surgery with or without systemic therapy versus systemic therapy alone (median OS of 15.8 vs. 6.9 months and 4-year survival of 20.8% vs. 7.0%). However, in this study by Howard, most patients had limited disease and in our cohort the majority of patients commenced systemic treatment with >10 lesions. Sosman et al. prospectively analyzed patients undergoing complete resection of stage IV melanoma and found a median recurrence-free survival of 5 months and median OS of 21 months [3]. The fact that both studies were conducted in an era without the current effective systemic therapy options explains the difference in outcomes with our cohort. Moreover, it must be noted that median follow-up in the Sosman study was substantially longer than in our cohort (5 years vs. 10 months). Follow-up in our cohort is limited because patients were treated with (novel) systemic therapy first and follow-up was measured from surgery and not from the start of systemic therapy.
The selection of patients who could benefit from surgery is crucial and, in this study, we found that expected residual tumor after surgery could be an important selection criterion. Bello et al. described a similar finding, as they had studied 237 patients who had surgery after immunotherapy. They found that a resection to no evidence of disease (NED) was associated with a better survival than residual disease after resection (OS not reached versus 10.8 months, 95% CI 7.3–14.8, p < 0.0001) [28]. Additionally, they described that OS was associated with the response to systemic therapy: patients with a response or oligoprogression did significantly better than patients with multiple progressive lesions. Unfortunately, in our database, it was not registered how many sites of progression were present in PD cases before surgery. Therefore, we could not distinguish oligoprogression from multiple progressive lesions. Klemen et al. studied patients resected to NED or non-progressive residual disease (NPRD) after progression following immunotherapy and showed a substantial 5-year disease-specific survival of 60% and no significant differences between survival in NED and NPRD patients [29]. They stratified patients for patterns of failure and patients with progression in established tumors had a significant better PFS than patients with new metastases (3-year PFS of 70% versus 6%, p = 0.001). Thus, other studies seem to confirm that the expected presence of a residual tumor after resection may be an important factor in selecting the correct patients for surgery.
Imaging may be helpful to select patients prior to surgery. Tan et al. described that complete metabolic response on FDG-PET could be useful in predicting long-term benefits and could guide the discontinuation of therapy in metastatic melanoma patients treated with immunotherapy [30]. Perhaps this could also be used in selecting patients for surgery. However, we are unable to test this hypothesis in our database, since these data were not collected.
This is one of the limitations of our study: the DMTR contains valuable information on metastatic melanoma patients, but data in this study is limited to the data that were provided by the registry. There is no possibility to find additional unregistered clinical data, nor to perform additional translational analyses. For example, no RECIST response measurements are registered and therefore information on treatment response has to be extracted from the status of disease at follow-up. Moreover, since this is a retrospective study using prospective collected data, selection bias may still occur. The strengths of using data extracted from the DMTR are its nationwide coverage and prospective data collection by trained data managers of real-world data.
The rapid developments in treatment options in recent years have caused some heterogeneity in the registered data. For example, the type of systemic treatment patients in our cohort received does not completely reflect current practice. Anti-CTLA-4 agent ipilimumab was the first immune checkpoint inhibitor available, but anti-PD1 has proven to be superior to ipilimumab and is currently the first choice for most patients [9,13,15]. However, in our cohort, 43.6% of patients were treated with ipilimumab. Moreover, the addition of a MEK inhibitor was shown to improve response rates over a BRAF inhibitor alone, so this has become standard of care [9,19,20,23,24]. During the initial years of the registry, MEK inhibitors were not yet reimbursed and therefore half of the patients in our cohort were treated with a BRAF inhibitor alone.
Targeted therapy is known for its high and quick response rates, where ICI is known to have more durable responses. This may cause a selection bias because, in patients with a worse baseline situation, targeted therapy may be preferentially chosen over ICI. However, when comparing baseline characteristics (LDH, performance status, etc.) between patients treated with targeted therapy versus ICI in our cohort, there are no significant differences. Thus, even after correcting for a potential selection bias, surgery after ICI seems to be superior to surgery after TT treatment.
Although several retrospective studies, including ours, do suggest a benefit for surgery after a response to immunotherapy and/or targeted therapy, further studies are warranted. If patients have a deep response with only limited residual lesion(s), stopping therapy, continuing therapy and/or resection can be possible approaches. A randomized trial would be needed to appropriately address this issue.
5. Conclusions
Disease status after surgery is the most important prognostic factor for OS and PFS for unresectable stage III/stage IV melanoma patients. Therefore, we recommend that in patients with multiple metastases, surgery is only considered after systemic therapy, when a partial or complete response can be achieved after the resection.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/5/1176/s1. Figure S1: Responses, Figure S2: PFS and OS, Figure S3: PFS and OS per best response to either ICI or targeted therapy (TT), Figure S4: PFS and OS per most recent response prior to surgery in patients treated with either ICI or TT, Figure S5: PFS and OS per status of disease at first follow-up, Figure S6: PFS and OS per location of surgical resection in patients treated with either ICI or TT, Table S1: Univariate analyses.
Author Contributions
Conceptualization, S.A.B. and A.C.J.v.A.; methodology, S.A.B. and A.C.J.v.A.; formal analysis, S.A.B.; resources, S.A.B.; writing—original draft preparation, S.A.B. and A.C.J.v.A.; writing—review and editing, S.A.B., M.J.B.A., F.W.P.J.v.d.B., M.J.B.-S., A.J.M.v.d.E., M.G.F., J.W.B.d.G., J.B.A.G.H., G.A.P.H., E.K., D.P., R.S.v.R., K.P.M.S., A.J.t.T., A.A.M.V., G.V., M.W.J.M.W., A.C.J.v.A.; visualization, S.A.B.; supervision, A.C.J.v.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Netherlands Organization for Health Research and Development (ZonMW), grant number 836002002. This subsidy is part of the program of effectiveness research of high-cost medicine. The first four years (2012–2016) of the Dutch Melanoma Treatment Registry (DMTR) were sponsored by Roche Nederland B.V, Bristol-Myers Squibb (BMS), GlaxoSmithKline (GSK)/Novartis and, since 2015, also by Merck Sharp & Dohme (MSD).
Acknowledgments
The authors thank all physicians and data managers who registered the patient data in the Dutch Melanoma Treatment Registry.
Conflicts of Interest
M.J.B.A. has consulting/advisory relationships with BMS, MSD, Merck, Pfizer, Pierre Fabre, Roche, Ipsen, Novartis, Astellas and Sanofi. M.J.B.-S. has consulting/advisory relationships with BMS, Pierre Fabre and Novartis. A.J.M.v.d.E. has consulting/advisory relationships with BMS, Roche, MSD Oncology, Amgen, Sanofi, Pfizer, Ipsen, Merck, Pierre Fabre and Novartis. He received a study grant from Sanofi, Roche and BMS. He received travel expenses from MSD Oncology, Roche, Pfizer and Sanofi. He received speaker honoraria from BMS and Novartis. M.G.F. received study grants form Abbvie, Daiichi Sankyo, PamGene, Gilead Sciences Netherlands B.V., Roche Nederland B.V. J.W.B.d.G. has consulting/advisory relationships with BMS, MSD, Novartis, Pierre Fabre and Servier. J.B.A.G.H. has provided consultation, attended advisory boards, and/or provided lectures for: Amgen, AZ, Bayer, BMS, Celsius Therapeutics, GSK, Ipsen, Merck Serono, MSD, Novartis, Pfizer, Roche/Genentech, Sanofi, Seattle Genetics, AIMM, Neogene Therapeutics, Gadeta, Immunocore, Vaximm, and Neon Therapeutics and received grant support from, BMS, MSD, Novartis, Neon Therapeutics. G.A.P.H. has consulting/advisory relationships with Amgen, Roche, MSD, BMS, Pfizer, Novartis and received research grants from BMS and Seerave. E.K. has consulting/advisory relationships with Amgen, Bristol-Myers Squibb, Novartis, Roche, Merck, Pierre-Fabre, EISAI, Bayer, Genzyme-Sanofi and received research grants from Novartis and Bristol-Myers Squibb. K.P.M.S. has consulting/advisory relationships with BMS, MSD, Novartis, Pierre Fabre and Roche. A.A.M.v.d.V. has consulting/advisory relationships with BMS, MSD, Merck, Pfizer, Pierre Fabre, Roche, Eisai, Ipsen and Novartis. A.C.J.v.A. has consulting/advisory relationships with Amgen, BMS, MSD-Merck, Merck-Pfizer, Novartis, 4SC and Sanofi and has received research funding from Amgen, BMS and Novartis. The other authors report no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Korn, E.L.; Liu, P.-Y.; Lee, S.J.; Chapman, J.-A.W.; Niedzwiecki, D.; Suman, V.J.; Moon, J.; Sondak, V.K.; Atkins, M.B.; Eisenhauer, E.A.; et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J. Clin. Oncol. 2008, 26, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Svedman, F.C.; Pillas, D.; Taylor, A.; Kaur, M.; Linder, R.; Hansson, J. Stage-specific survival and recurrence in patients with cutaneous malignant melanoma in Europe—A systematic review of the literature. Clin. Epidemiol. 2016, 8, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Sosman, J.A.; Moon, J.; Tuthill, R.J.; Warneke, J.A.; Vetto, J.T.; Redman, B.G.; Liu, P.Y.; Unger, J.M.; Flaherty, L.E.; Sondak, V.K. A phase 2 trial of complete resection for stage IV melanoma: Results of Southwest Oncology Group Clinical Trial S9430. Cancer 2011, 117, 4740–4806. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.H.; Thompson, J.F.; Mozzillo, N.; Nieweg, O.E.; Hoekstra, H.J.; Roses, D.F.; Sondak, V.K.; Reintgen, D.S.; Kashani-Sabet, M.; Karakousis, C.P.; et al. Metastasectomy for distant metastatic melanoma: Analysis of data from the first Multicenter Selective Lymphadenectomy Trial (MSLT-I). Ann. Surg. Oncol. 2012, 19, 2547–2555. [Google Scholar] [CrossRef] [PubMed]
- Ascierto, P.A.; Long, G.V.; Robert, C.; Brady, B.; Dutriaux, C.; Di Giacomo, A.M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: Three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Topalian, S.L.; Sznol, M.; McDermott, D.F.; Kluger, H.M.; Carvajal, R.D.; Sharfman, W.H.; Brahmer, J.R.; Lawrence, D.P.; Atkins, M.B.; Powderly, J.D.; et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J. Clin. Oncol. 2014, 32, 1020–1030. [Google Scholar] [CrossRef]
- Weber, J.S.; D’Angelo, S.P.; Minor, D.; Hodi, F.S.; Gutzmer, R.; Neyns, B.; Hoeller, C.; Khushalani, N.I.; Miller, W.H., Jr.; Lao, C.D.; et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): A randomised, controlled, open-label, phase 3 trial. Lancet. Oncol. 2015, 16, 375–384. [Google Scholar] [CrossRef]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet (Lond. Engl.) 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Ascierto, P.A.; Del Vecchio, M.; Robert, C.; Mackiewicz, A.; Chiarion-Sileni, V.; Arance, A.; Lebbe, C.; Bastholt, L.; Hamid, O.; Rutkowski, P.; et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: A randomised, double-blind, multicentre, phase 3 trial. Lancet. Oncol. 2017, 18, 611–622. [Google Scholar] [CrossRef]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.; O’Day, S.; Weber, J.; Garbe, C.; Lebbe, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall survival with combined nivolumab and ipilimumab in advanced melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1315–1327. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 603–615. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dreno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandala, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Engl. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Flaherty, K.T.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 2017, 28, 1631–1639. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Jochems, A.; Schouwenburg, M.G.; Leeneman, B.; Franken, M.G.; van den Eertwegh, A.J.; Haanen, J.B.; Gelderblom, H.; Uyl-de Groot, C.A.; Aarts, M.J.; van den Berkmortel, F.W.; et al. Dutch Melanoma Treatment Registry: Quality assurance in the care of patients with metastatic melanoma in the Netherlands. Eur. J. Cancer 2017, 72, 156–165. [Google Scholar] [CrossRef]
- Balch, C.M.; Gershenwald, J.E.; Soong, S.J.; Thompson, J.F.; Atkins, M.B.; Byrd, D.R.; Buzaid, A.C.; Cochran, A.J.; Coit, D.G.; Ding, S.; et al. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009, 27, 6199–6206. [Google Scholar] [CrossRef]
- Bello, D.M.; Panageas, K.S.; Hollmann, T.; Shoushtari, A.N.; Momtaz, P.; Chapman, P.B.; Postow, M.A.; Callahan, M.K.; Wolchok, J.D.; Brady, M.S.; et al. Survival outcomes after metastasectomy in melanoma patients categorized by response to checkpoint blockade. Ann. Surg. Oncol. 2019. [Google Scholar] [CrossRef]
- Klemen, N.D.; Wang, M.; Feingold, P.L.; Cooper, K.; Pavri, S.N.; Han, D.; Detterbeck, F.C.; Boffa, D.J.; Khan, S.A.; Olino, K.; et al. Patterns of failure after immunotherapy with checkpoint inhibitors predict durable progression-free survival after local therapy for metastatic melanoma. J. Immunother. Cancer 2019, 7, 196. [Google Scholar] [CrossRef]
- Tan, A.C.; Emmett, L.; Lo, S.; Liu, V.; Guminski, A.D.; Long, G.V.; Menzies, A.M. Utility of 1-year FDG-PET (PET) to determine outcomes from anti-PD-1 (PD1) based therapy in patients (pts) with metastatic melanoma (MM). J. Clin. Oncol. 2018, 36, 9517. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).