Liver Transplantation for Pediatric Liver Cancer
Abstract
1. Introduction
1.1. Historical Background
1.2. Overview, Challenges, and the Rationale for Informed Decision-Making
1.3. The Liver Transplant Option and Related Considerations
2. Assessing LT Candidacy for Pediatric Liver Tumors
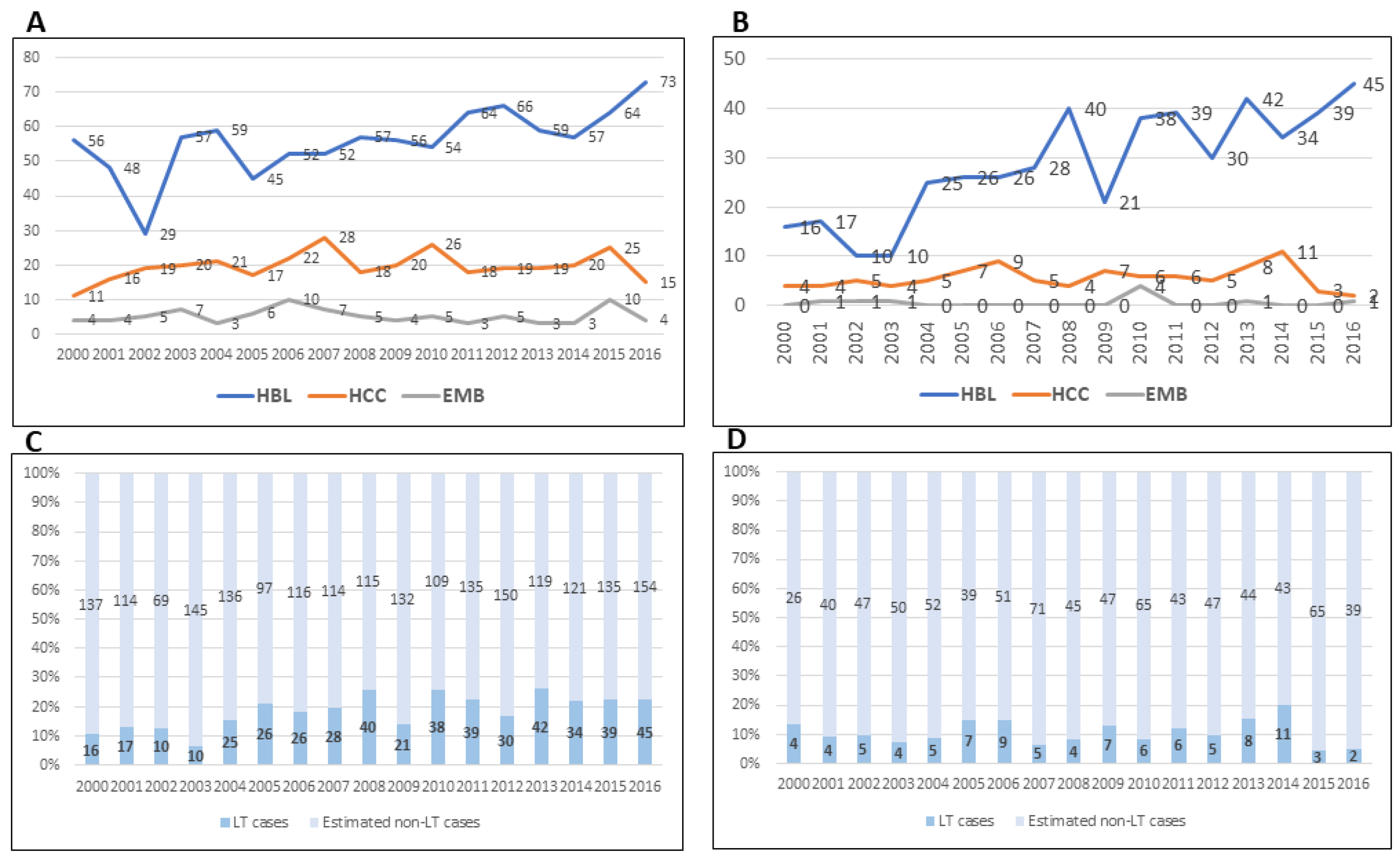
3. Changing Tumor Incidence and Use of Liver Transplantation for Unresectable Liver Cancer
4. Presentation
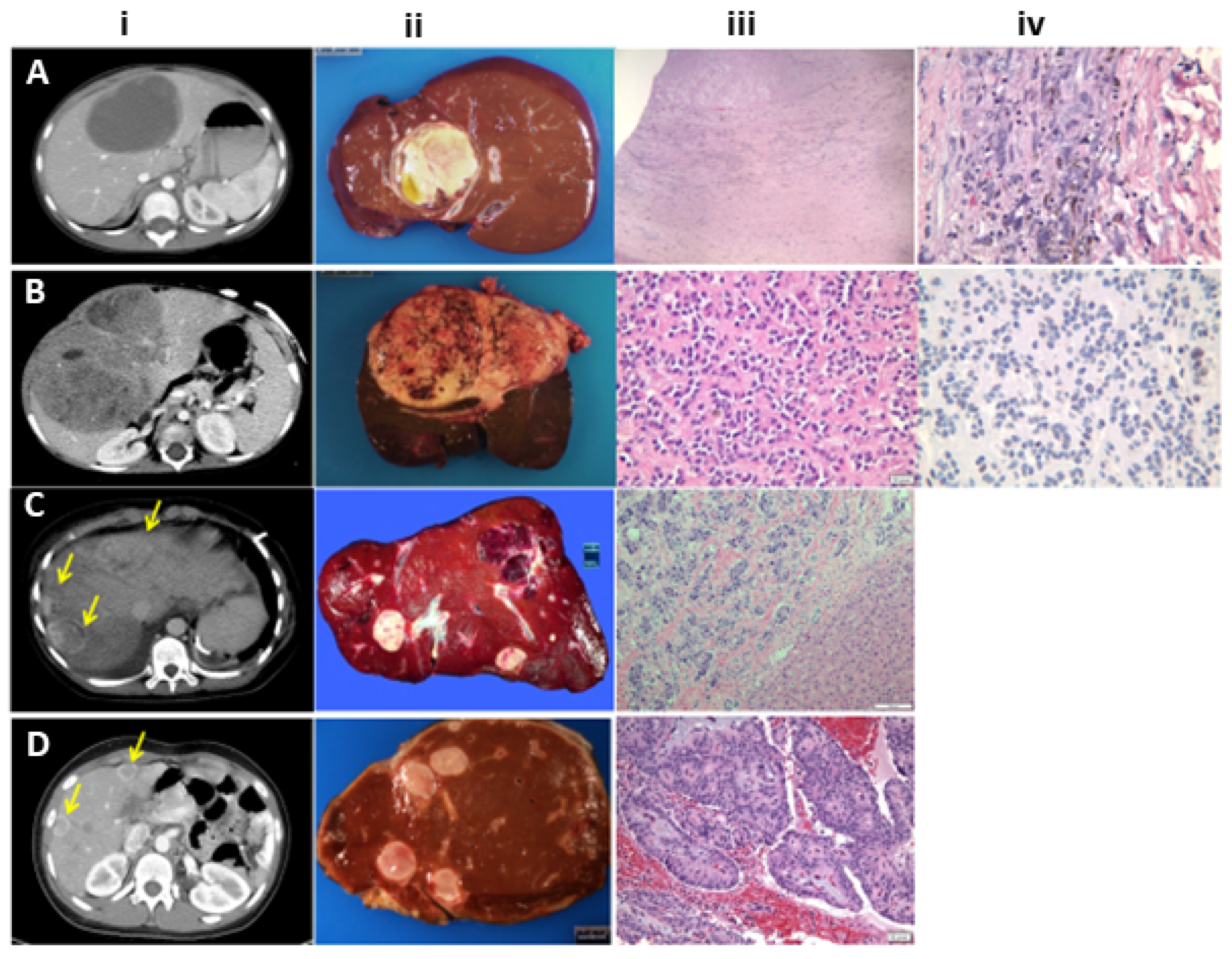
5. Pathology
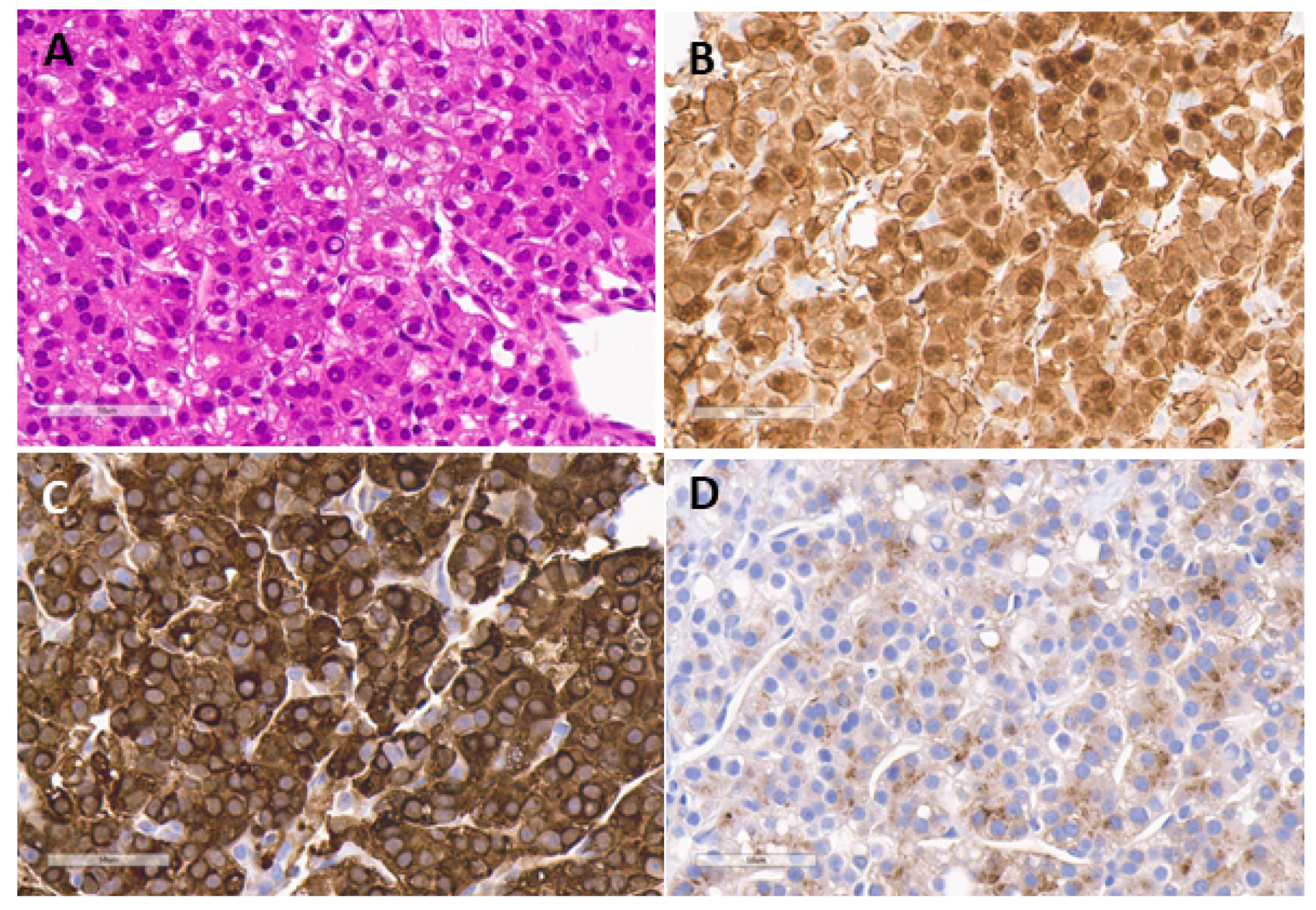
5.1. Hepatoblastoma
5.2. Hepatocellular Carcinoma
5.3. Malignant Rhabdoid Tumor (MRT)
5.4. Sarcoma
5.4.1. Undifferentiated Embryonal Sarcoma of Liver
5.4.2. Rhabdomyosarcoma of Liver
5.5. Vascular Tumors
6. Radiologic Staging
7. Chemotherapy
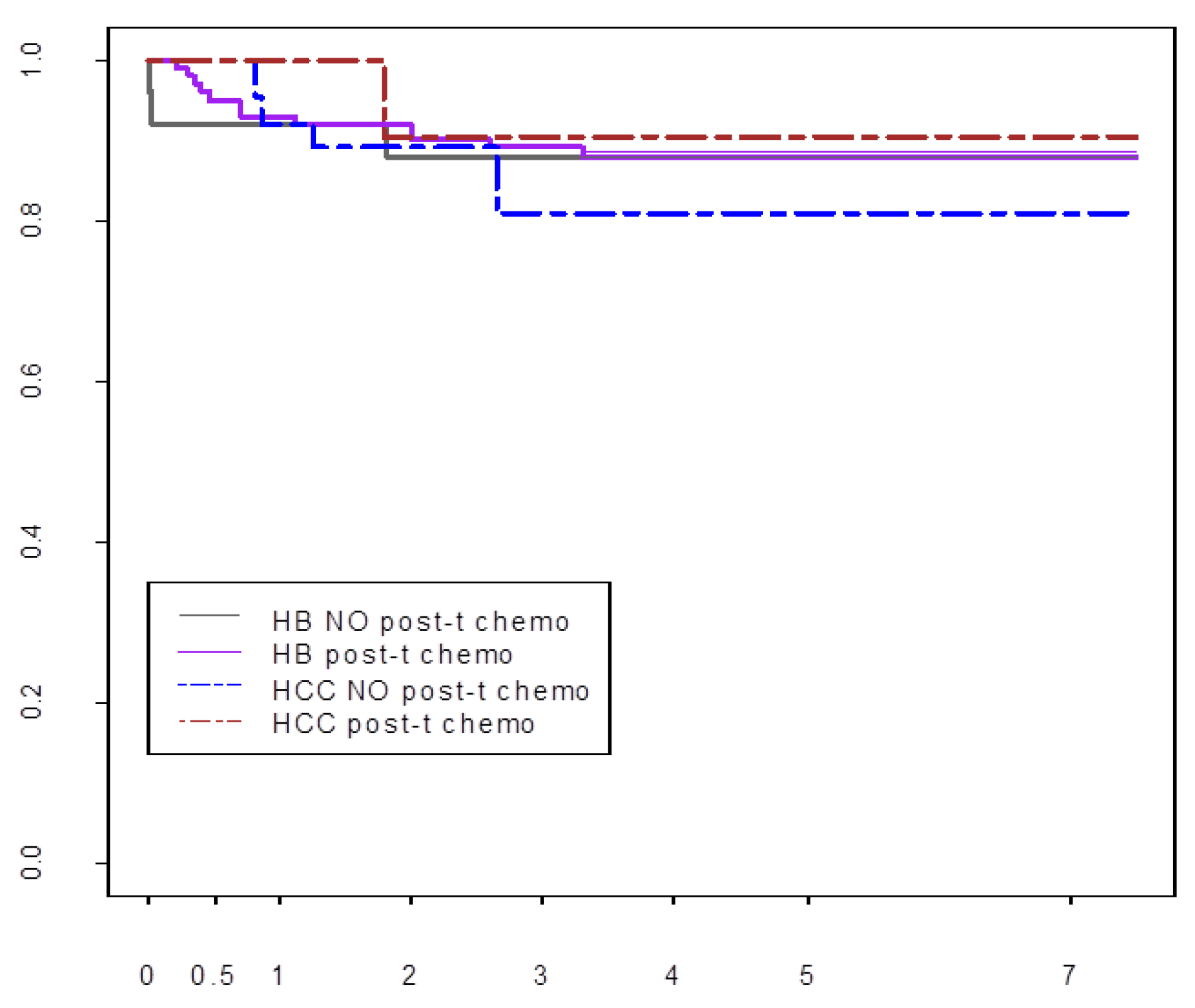
Post-Transplant Chemotherapy
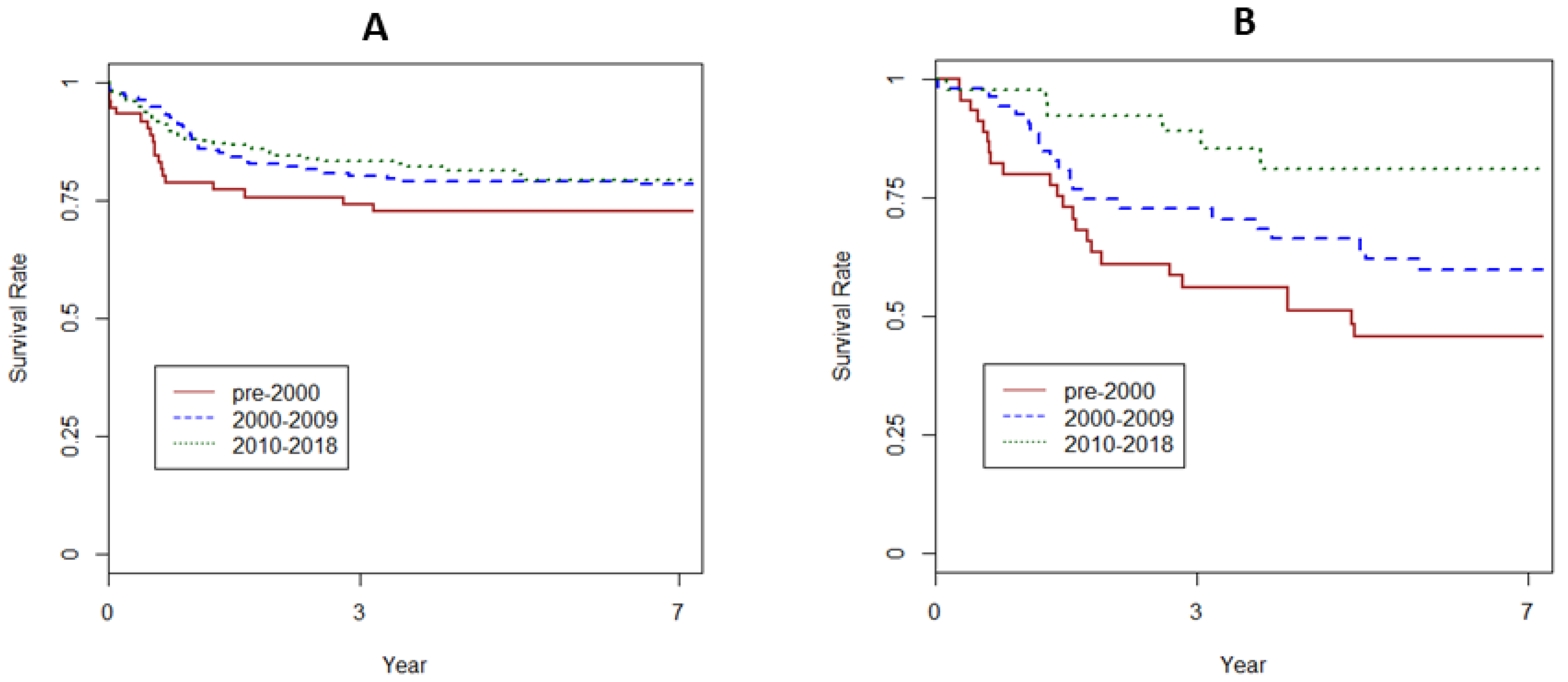
8. Outcomes after Liver Transplantation
8.1. Hepatoblastoma
8.2. Hepatocellular Carcinoma
8.3. Primary Liver Sarcoma (PLS)
8.4. Metastatic Liver Disease
9. Transplant-Related Care
10. Extreme Liver Resection and LT for Recurrent Disease
11. Genomic Landscape and Tumor Targeting
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HB | hepatoblastoma |
| HCC | Hepatocellular carcinoma |
| SEER | Surveillance, Epidemiology and End-Results |
| SRTR | Scientific Registry for Transplant Recipients |
| AFP | alphafetoprotein |
| HCN-NOS | Hepatocellular neoplasm NOS |
| SCU | small cell undifferentiated hepatoblastoma |
| N:: C | nuclear to cytoplasmic ratio |
| PHITT | Pediatric Hepatic International Tumor Trial |
| SIOPEL | Société Internationale d’Oncologie Pédiatrique—Epithelial Liver Tumor Study Group |
| PRETEXT | Pretreatment Extent of Tumor |
| POSTTEXT | Post-treatment extent of Tumor |
| COG | Cooperative Oncology group |
| JCCG | Japan Children’s Cancer Group |
| MRT | malignant rhabdoid tumor |
| PLADO | Cisplatin and doxorubicin |
| C5VD | combination of cisplatin/5-flurouricil/vincristine/doxorubicin |
| EFS | event-free survival |
| PLUTO | Pediatric Liver Unresectable Tumours Observatory |
| TMB | tumor mutation burden |
| Mut | mutations |
| ctDNA | circulating tumor DN |
| MHC | major histocompatibility complex |
References
- Starzl, T.E.; Marchioro, T.L.; Vonkaulla, K.N.; Hermann, G.; Brittain, R.S.; Waddell, W.R. Homotransplantation of the liver in humans. Surg. Gynecol. Obstet. 1963, 117, 659–676. [Google Scholar] [PubMed]
- Starzl, T.E.; Marchioro, T.L.; Rowlands, D.T., Jr.; Kirkpatrick, C.H.; Wilson, W.E.; Rifkind, D.; Waddell, W.R. Immunosuppression after experimental and clinical homotransplantation of the liver. Ann. Surg. 1964, 160, 411–439. [Google Scholar] [CrossRef] [PubMed]
- Demirleau, N.; Noureddine, B.; Vignes, P.; Prawerman, A.; Reziciner, L.; Larraud, P.; Louvier, N. Attempted hepatic homograft. Mem. Acad. Chir. 1964, 90, 177–179. [Google Scholar] [PubMed]
- Moore, F.D.; Birtch, A.G.; Dagher, F.; Veith, F.; Krisher, J.A.; Order, S.E.; Shucart, W.A.; Dammin, G.J.; Couch, N.P. Immunosuppression and vascular insufficiency in liver transplantation. Ann. N. Y. Acad. Sci. 1964, 120, 729–738. [Google Scholar] [CrossRef]
- Starzl, T.E.; Groth, C.G.; Brettschneider, L.; Penn, I.; Fulginiti, V.A.; Moon, J.B.; Blanchard, H.; Martin, A.J., Jr.; Porter, K.A. Orthotopic homotransplantation of the human liver. Ann. Surg. 1968, 168, 392–415. [Google Scholar] [CrossRef]
- Starzl, T.E.; Porter, K.A.; Putnam, C.W.; Schroter, G.P.; Halgrimson, C.G.; Weil, R., 3rd; Hoelscher, M.; Reid, H.A. Orthotopic liver transplantation in ninety-three patients. Surg. Gynecol. Obstet. 1976, 142, 487–505. [Google Scholar]
- Koneru, B.; Flye, M.W.; Busuttil, R.W.; Shaw, B.W.; Lorber, M.I.; Emond, J.C.; Kalayoglu, M.; Freese, D.K.; Starzl, T.E. Liver transplantation for hepatoblastoma. The American experience. Ann. Surg. 1991, 213, 118–121. [Google Scholar] [CrossRef]
- Starzl, T.E.; Todo, S.; Tzakis, A.; Podesta, L.; Mieles, L.; Demetris, A.; Teperman, L.; Selby, R.; Stevenson, W.; Stieber, A. Abdominal organ cluster transplantation for the treatment of upper abdominal malignancies. Ann. Surg. 1989, 210, 374–385. [Google Scholar] [CrossRef]
- Starzl, T.E.; Todo, S.; Tzakis, A.; Alessiani, M.; Casavilla, A.; Abu-Elmagd, K.; Fung, J.J. The many faces of multivisceral transplantation. Surg. Gynecol. Obstet. 1991, 335–344. [Google Scholar]
- Le Treut, Y.P.; Gregoire, E.; Klempnauer, J.; Belghiti, J.; Jouve, E.; Lerut, J.; Castaing, D.; Soubrane, O.; Boillot, O.; Mantion, G.; et al. Liver transplantation for neuroendocrine tumors in Europe-results and trends in patient selection: A 213-case European liver transplant registry study. Ann. Surg. 2013, 257, 807–815. [Google Scholar] [CrossRef]
- King, D.R.; Ortega, J.; Campbell, J.; Haas, J.; Ablin, A.; Lloyd, D.; Newman, K.; Quinn, J.; Krailo, M.; Feusner, J.; et al. The surgical management of children with incompletely resected hepatic cancer is facilitated by intensive chemotherapy. J. Ped. Surg. 1991, 26, 1074–1780. [Google Scholar] [CrossRef]
- Douglass, E.C.; Green, A.A.; Wrenn, E.; Champion, J.; Shipp, M.; Pratt, C.B. Effective cisplatin (DDP) based chemotherapy in the treatment of hepatoblastoma. Med. Pediatr. Oncol. 1985, 13, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Polychronidis, G.; Heger, U.; Frongia, G.; Mehrabi, A.; Hoffmann, K. Incidence trends and survival prediction of hepatoblastoma in children: A population-based study. Cancer Commun. 2019, 39, 62. [Google Scholar] [CrossRef] [PubMed]
- McAteer, J.P.; Goldin, A.B.; Healey, P.J.; Gow, K.W. Surgical treatment of primary liver tumors in children: Outcomes analysis of resection and transplantation in the SEER database. Pediatr. Transplant. 2013, 17, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; He, Y.; Wei, L.; Chen, D.; Yang, H.; Tan, R.; Chen, Z. Assessment of survival of pediatric patients with hepatoblastoma who received chemotherapy following liver transplant or liver resection. JAMA Netw. Open 2019, 2, e1912676. [Google Scholar] [CrossRef]
- Cruz, R., Jr.; Ranganathan, S.; Mazariegos, G.; Soltys, K.; Nayyar, N.; Sun, Q.; Bond, G.; Shaw, P.H.; Haberman, K.; Krishnamurti, L.; et al. Analysis of national and single-center incidence and survival after liver transplantation for hepatoblastoma: New trends and future opportunities. Surgery 2013, 153, 150–159. [Google Scholar] [CrossRef]
- Vinayak, R.; Cruz, R.J., Jr.; Ranganathan, S.; Mohanka, R.; Mazariegos, G.; Soltys, K.; Bond, G.; Tadros, S.; Humar, A.; Marsh, J.W.; et al. Pediatric liver transplantation for hepatocellular cancer and rare liver malignancies: US multicenter and single-center experience (1981–2015). Liver Transpl. 2017, 23, 1577–1588. [Google Scholar] [CrossRef]
- Lupo, P.J.; Schraw, J.M.; Desrosiers, T.A.; Nembhard, W.N.; Langlois, P.H.; Canfield, M.A.; Copeland, G.; Meyer, R.E.; Brown, A.L.; Chambers, T.M.; et al. Association between birth defects and cancer risk among children and adolescents in a population-based assessment of 10 million live births. JAMA Oncol. 2019. [Google Scholar] [CrossRef]
- Kingston, J.E.; Herbert, A.; Draper, G.J.; Mann, J.R. Association between hepatoblastoma and polyposis coli. Arch. Dis. Child. 1983, 58, 959–962. [Google Scholar] [CrossRef]
- Hirschman, B.A.; Pollock, B.H.; Tomlinson, G.E. The spectrum of APC mutations in children with hepatoblastoma from familial adenomatous polyposis kindreds. J. Pediatr. 2005, 147, 263–266. [Google Scholar] [CrossRef]
- DeBaun, M.R.; Tucker, M.A. Risk of cancer during the first four years of life in children from the beckwith-wiedemann syndrome registry. J. Pediatr. 1998, 132, 398–400. [Google Scholar] [CrossRef]
- Venkatramani, R.; Spector, L.G.; Georgieff, M.; Tomlinson, G.; Krailo, M.; Malogolowkin, M.; Kohlmann, W.; Curtin, K.; Fonstad, R.K.; Schiffman, J.D. Congenital abnormalities and hepatoblastoma: A report from the children’s oncology group (COG) and the utah population database (UPDB). Am. J. Med. Genet. A 2014, 164a, 2250–2255. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Terrada, D.; Alaggio, R.; de Davila, M.T.; Czauderna, P.; Hiyama, E.; Katzenstein, H.; Leuschner, I.; Malogolowkin, M.; Meyers, R.; Ranganathan, S.; et al. Towards an international pediatric liver tumor consensus classification: Proceedings of the Los Angeles COG liver tumors symposium. Mod. Pathol. 2014, 27, 472–491. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.G.; Finegold, M.J. Primary hepatic tumors of childhood. Hum. Pathol. 1983, 14, 512–537. [Google Scholar] [CrossRef]
- Ranganathan, S.; Tan, X.; Monga, S.P. Beta-catenin and met deregulation in childhood Hepatoblastomas. Pediatr. Dev. Pathol. 2005, 8, 435–447. [Google Scholar] [CrossRef]
- Ranganathan, S.; Lopez-Terrada, D.; Alaggio, R. Hepatoblastoma and pediatric hepatocellular carcinoma: An update. Pediatr. Dev. Pathol. 2019. [Google Scholar] [CrossRef]
- Haines, K.; Sarabia, S.F.; Alvarez, K.R.; Tomlinson, G.; Vasudevan, S.A.; Heczey, A.A.; Roy, A.; Finegold, M.J.; Parsons, D.W.; Plon, S.E.; et al. Characterization of pediatric hepatocellular carcinoma reveals genomic heterogeneity and diverse signaling pathway activation. Pediatr. Blood Cancer 2019, 66, e27745. [Google Scholar] [CrossRef]
- Kastenhuber, E.R.; Lalazar, G.; Houlihan, S.L.; Tschaharganeh, D.F.; Baslan, T.; Chen, C.C.; Requena, D.; Tian, S.; Bosbach, B.; Wilkinson, J.E.; et al. DNAJB1-PRKACA fusion kinase interacts with beta-catenin and the liver regenerative response to drive fibrolamellar hepatocellular carcinoma. Proc. Natl. Acad. Sci. USA 2017, 114, 13076–13084. [Google Scholar] [CrossRef]
- Honeyman, J.N.; Simon, E.P.; Robine, N.; Chiaroni-Clarke, R.; Darcy, D.G.; Lim, I.I.; Gleason, C.E.; Murphy, J.M.; Rosenberg, B.R.; Teegan, L.; et al. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science 2014, 343, 1010–1014. [Google Scholar] [CrossRef]
- Hoot, A.C.; Russo, P.; Judkins, A.R.; Perlman, E.J.; Biegel, J.A. Immunohistochemical analysis of hSNF5/INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am. J. Surg. Pathol. 2004, 28, 1485–1491. [Google Scholar] [CrossRef]
- Judkins, A.R.; Mauger, J.; Ht, A.; Rorke, L.B.; Biegel, J.A. Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am. J. Surg. Pathol. 2004, 28, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Shehata, B.M.; Gupta, N.A.; Katzenstein, H.M.; Steelman, C.K.; Wulkan, M.L.; Gow, K.W.; Bridge, J.A.; Kenney, B.D.; Thompson, K.; de Chadarevian, J.P.; et al. Undifferentiated embryonal sarcoma of the liver is associated with mesenchymal hamartoma and multiple chromosomal abnormalities: A review of eleven cases. Pediatr. Dev. Pathol. 2011, 14, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Geller, J.; Coots, A.; Towbin, A.; Nathan, J.; Alonso, M.; Sheridan, R.; Tiao, G. Multimodal therapy including liver transplantation for hepatic undifferentiated embryonal sarcoma. Liver Transpl. 2014, 20, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, M.; Beaunoyer, M.; Samson, Y.; Dal Soglio, D.; Dubois, J.; Lallier, M.; Alvarez, F. A child with unresectable biliary rhabdomyosarcoma: 48-month disease-free survival after liver transplantation. Pediatr. Transplant. 2014, 18, E146–E151. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.H.; Dong, K.R.; Tao, Y.F.; Chen, G.; Li, R.D.; Zhang, Q.B.; Zhang, X.F.; Zheng, S.; Wang, Z.X. Liver transplantation for biliary rhabdomyosarcoma with liver metastasis: Report of one case. Transpl. Proc. 2017, 49, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Perruccio, K.; Cecinati, V.; Scagnellato, A.; Provenzi, M.; Milano, G.M.; Basso, E.; Manzitti, C.; Cecchetto, G.; Alaggio, R.; Di Martino, M.; et al. Biliary tract rhabdomyosarcoma: A report from the soft tissue sarcoma committee of the associazione Italiana ematologia oncologia pediatrica. Tumori J. 2018, 104, 232–237. [Google Scholar] [CrossRef]
- Sharif, K.; English, M.; Ramani, P.; Alberti, D.; Otte, J.B.; McKiernan, P.; Gosseye, S.; Jenney, M.; de Ville de Goyet, J. Management of hepatic epithelioid haemangio-endothelioma in children: What option? Br. J. Cancer 2004, 90, 1498–1501. [Google Scholar] [CrossRef]
- Lerut, J.P.; Orlando, G.; Adam, R.; Schiavo, M.; Klempnauer, J.; Mirza, D.; Boleslawski, E.; Burroughs, A.; Selles, C.F.; Jaeck, D.; et al. The place of liver transplantation in the treatment of hepatic epitheloid hemangioendothelioma: Report of the European liver transplant registry. Ann. Surg. 2007, 246, 949–957. [Google Scholar] [CrossRef]
- Guiteau, J.J.; Cotton, R.T.; Karpen, S.J.; O’Mahony, C.A.; Goss, J.A. Pediatric liver transplantation for primary malignant liver tumors with a focus on hepatic epithelioid hemangioendothelioma: The UNOS experience. Pediatr. Transplant. 2010, 14, 326–331. [Google Scholar] [CrossRef]
- Otte, J.B.; Zimmerman, A. The role of liver transplantation for pediatric epithelioid hemangioendothelioma. Pediatr. Transplant. 2010, 14, 295–297. [Google Scholar] [CrossRef]
- Orlando, G.; Adam, R.; Mirza, D.; Soderdahl, G.; Porte, R.J.; Paul, A.; Burroughs, A.K.; Seiler, C.A.; Colledan, M.; Graziadei, I.; et al. Hepatic hemangiosarcoma: An absolute contraindication to liver transplantation—The European liver transplant registry experience. Transplantation 2013, 95, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Masand, P.; Thompson, P.; Finegold, M.; Leung, D.H. Angiosarcoma successfully treated with liver transplantation and sirolimus. Pediatr. Transplant. 2014, 18, E114–E119. [Google Scholar] [CrossRef] [PubMed]
- Pilbeam, K.; Eidenschink, B.; Sulciner, M.; Luquette, M.; Neglia, J.; Chinnakotla, S. Success of chemotherapy and a liver transplant in a pediatric patient with hepatic angiosarcoma: A case report. Pediatr. Transplant. 2019, 23, e13410. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, O.; Fabre, M.; Franchi, S.; Pariente, D.; Debray, D.; Jacquemin, E.; Gauthier, F.; Bernard, O.J. Widening spectrum of liver angiosarcoma in children. Pediatr. Gastroenterol. Nutr. 2011, 53, 615–619. [Google Scholar]
- Towbin, A.J.; Meyers, R.L.; Woodley, H.; Miyazaki, O.; Weldon, C.B.; Morland, B.; Hiyama, E.; Czauderna, P.; Roebuck, D.J.; Tiao, G.M. 2017 PRETEXT: Radiologic staging system for primary hepatic malignancies of childhood revised for the paediatric hepatic international tumour trial (PHITT). Pediatr. Radiol. 2018, 48, 536–554. [Google Scholar] [CrossRef]
- Chavhan, G.B.; Siddiqui, I.; Ingley, K.M.; Gupta, A.A. Rare malignant liver tumors in children. Pediatr. Radiol. 2019, 49, 1404–1421. [Google Scholar] [CrossRef]
- Shelmerdine, S.C.; Roebuck, D.J.; Towbin, A.J.; McHugh, K. MRI of paediatric liver tumours: How we review and report. Cancer Imaging 2016, 16, 21. [Google Scholar] [CrossRef]
- Warmann, S.W.; Schenk, A.; Schaefer, J.F.; Ebinger, M.; Blumenstock, G.; Tsiflikas, I.; Fuchs, J. Computer-assisted surgery planning in children with complex liver tumors identifies variability of the classical Couinaud classification. J. Pediatr. Surg. 2016, 51, 1801–1806. [Google Scholar] [CrossRef]
- Zhang, G.; Zhou, X.J.; Zhu, C.Z.; Dong, Q.; Su, L. Usefulness of three-dimensional (3D) simulation software in hepatectomy for pediatric hepatoblastoma. Surg. Oncol. 2016, 25, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, D.J.; Aronson, D.; Clapuyt, P.; Czauderna, P.; de Ville de Goyet, J.; Gauthier, F.; Mackinlay, G.; Maibach, R.; McHugh, K.; Olsen, O.E.; et al. International childhood liver tumor strategy group. 2005 PRETEXT: A revised staging system for primary malignant liver tumours of childhood developed by the SIOPEL group. Pediatr. Radiol. 2007, 37, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Maibach, R.; Roebuck, D.; Brugieres, L.; Capra, M.; Brock, P.; Dall’Igna, P.; Otte, J.B.; De Camargo, B.; Zsiros, J.; Zimmermann, A.; et al. Prognostic stratification for children with hepatoblastoma: The SIOPEL experience. Eur. J. Cancer 2012, 48, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Meyers, R.L.; Maibach, R.; Hiyama, E.; Häberle, B.; Krailo, M.; Rangaswami, A.; Aronson, D.C.; Malogolowkin, M.H.; Perilongo, G.; von Schweinitz, D.; et al. Risk-stratified staging in paediatric hepatoblastoma: A unified analysis from the children’s hepatic tumors international collaboration. Lancet Oncol. 2017, 18, 122–131. [Google Scholar] [CrossRef]
- Ortega, J.A.; Douglass, E.C.; Feusner, J.H.; Reynolds, M.; Quinn, J.J.; Finegold, M.J.; Haas, J.E.; King, D.R.; Liu-Mares, W.; Sensel, M.G.; et al. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the children’s cancer group and the pediatric oncology group. J. Clin. Oncl. 2000, 18, 2665–2675. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, H.M.; Langham, M.R.; Malogolowkin, M.H.; Krailo, M.D.; Towbin, A.J.; McCarville, M.B.; Finegold, M.J.; Ranganathan, S.; Dunn, S.; McGahren, E.D. Minimal adjuvant chemotherapy for children with hepatoblastoma resected at diagnosis (AHEP0731): A Children’s oncology group, multicentre, phase 3 trial. Lancet Oncol. 2019, 20, 719–727. [Google Scholar] [CrossRef]
- Pritchard, J.; Brown, J.; Shafford, E.; Perilongo, G.; Brock, P.; Dicks-Mireaux, C.; Keeling, J.; Phillips, A.; Vos, A.; Plaschkes, J. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: A successful approach—Results of the first prospective study of the international society of pediatric oncology. J. Clin. Oncol. 2000, 18, 3819–3828. [Google Scholar] [CrossRef]
- Perilongo, G.; Shafford, E.; Maibach, R.; Aronson, D.; Brugieres, L.; Brock, P.; Childs, M.; Czauderna, P.; MacKinlay, G.; Otte, J.B.; et al. Risk-adapted treatment for childhood hepatoblastoma. Final report of the second study of the international society of paediatric oncology—SIOPEL 2. Eur. J. Cancer 2004, 40, 411–421. [Google Scholar] [CrossRef]
- Zsiros, J.; Brugieres, L.; Brock, P.; Roebuck, D.; Maibach, R.; Zimmermann, A.; Childs, M.; Pariente, D.; Laithier, V.; Otte, J.B.; et al. Dose-dense cisplatin-based chemotherapy and surgery for children with high-risk hepatoblastoma (SIOPEL-4): A prospective, single-arm, feasibility study. Lancet Oncol. 2013, 14, 834–842. [Google Scholar] [CrossRef]
- Zsiros, J.; Maibach, R.; Shafford, E.; Brugieres, L.; Brock, P.; Czauderna, P.; Roebuck, D.; Childs, M.; Zimmermann, A.; Laithier, V.; et al. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: Final results of the SIOPEL-3HR study. J. Clin. Oncol. 2010, 28, 2584–2590. [Google Scholar] [CrossRef]
- Lautz, T.B.; Ben-Ami, T.; Tantemsapya, N.; Gosiengfiao, Y.; Superina, R.A. Successful non-transplant resection of POST-TEXT III and IV hepatoblastoma. Cancer 2011, 117, 1976–1983. [Google Scholar] [CrossRef]
- La Quaglia, M.P. State of the art in oncology: High risk neuroblastoma, alveolar rhabdomyosarcoma, desmoplastic small round cell tumor, and POST-TEXT 3 and 4 hepatoblastoma. J. Pediatr. Surg. 2014, 49, 233–240. [Google Scholar] [CrossRef]
- De Freitas, P.G.; Tannuri, A.C.A.; Dantas Marques, A.C.; Torres, R.R.; Mendes Gibelli, N.E.; Tannuri, U. Extensive hepatectomy as an alternative to liver transplant in advanced hepatoblastoma: A new protocol used in a pediatric liver transplantation center. Transplant. Proc. 2019, 51, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials.gov [Internet]. Bethesda (MD): National Library of Medicine (USA). 23 May 2018. Identifier NCT03533582 Pediatric Hepatic International Tumor Trial (PHITT). Available online: https://clinicaltrials.gov/ct2/show/NCT03533582?term=Pediatric+Hepatic+Malignancy+International+Therapeutic+Trial+%28PHITT%29&rank=1 (accessed on 22 October 2019).
- Lim, I.I.P.; Bondoc, A.J.; Geller, J.I.; Tiao, M.G. Hepatoblastoma, the evolution of biology, surgery and transplantation. Children 2019, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Murawski, M.; Weeda, V.B.; Maibach, R.; Morland, B.; Roebuck, D.J.; Zimmerman, A.; Casanova, M.; Perilongo, G.; Laithier, V.; Kebudi, R.; et al. Hepatocellular carcinoma in children: Does modified platinum- and doxorubicin-based chemotherapy increase tumor resectability and change outcome? Lessons learned from the SIOPEL 2 and 3 studies. J. Clin. Oncol. 2016, 34, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, H.M.; Krailo, M.D.; Malogolowkin, M.H.; Ortega, J.A.; Liu-Mares, W.; Douglass, E.C.; Feusner, J.H.; Reynolds, M.; Quinn, J.J.; Newman, K.; et al. Hepatocellular carcinoma in children and adolescents: Results from the pediatric oncology group and the children’s cancer group intergroup study. J. Clin. Oncol. 2002, 20, 2789–2797. [Google Scholar] [CrossRef]
- Wu, Z.; Wei, Y.; Cai, Z.; Zhou, Y. Long-term survival outcomes of undifferentiated embryonal sarcoma of the liver: A pooled analysis of 308 patients. ANZ J. Surg. 2020. [Google Scholar] [CrossRef]
- Trobaugh-Lotrario, A.D.; Finegold, M.J.; Feusner, J.H. Rhabdoid tumors of the liver: Rare, aggressive, and poorly responsive to standard cytotoxic chemotherapy. Pediatr. Blood Cancer 2011, 57, 423–428. [Google Scholar] [CrossRef]
- Geoerger, B.; Bourdeaut, F.; DuBois, S.G.; Fischer, M.; Geller, J.I.; Gottardo, N.G.; Marabelle, A.; Pearson, A.D.J.; Modak, S.; Cash, T.; et al. A phase I study of the CDK4/6 inhibitor ribociclib (LEE011) in pediatric patients with malignant rhabdoid tumors, neuroblastoma, and other solid tumors. Clin. Cancer Res. 2017, 23, 2433–2441. [Google Scholar] [CrossRef]
- Otte, J.B.; Meyers, R. PLUTO first report. Pediatr. Transplant. 2010, 14, 830–835. [Google Scholar] [CrossRef]
- Plant, A.S.; Busuttil, R.W.; Rana, A.; Nelson, S.D.; Auerbach, M.; Federman, N.C. A single-institution retrospective cases series of childhood undifferentiated embryonal liver sarcoma (UELS): Success of combined therapy and the use of orthotopic liver transplant. J. Pediatr. Hematol. Oncol. 2013, 35, 451–455. [Google Scholar] [CrossRef]
- Ezekian, B.; Mulvihill, M.S.; Schroder, P.M.; Gilmore, B.F.; Leraas, H.J.; Gulack, B.C.; Jane Commander, S.; Mavis, A.M.; Kreissman, S.G.; Knechtle, S.J.; et al. Improved contemporary outcomes of liver transplantation for pediatric hepatoblastoma and hepatocellular carcinoma. Pediatr. Transplant. 2018, 22, e13305. [Google Scholar] [CrossRef]
- Schmid, I.; von Schweinitz, D. Pediatric hepatocellular carcinoma: Challenges and solutions. J. Hepatocell. Carcinoma 2017, 4, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Ruth, N.D.; Kelly, D.; Sharif, K.; Morland, B.; Lloyd, C.; McKiernan, P.J. Rejection is less common in children undergoing liver transplantation for hepatoblastoma. Pediatr. Transplant. 2014, 18, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Buque, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological effects of conventional chemotherapy and targeted anticancer agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [PubMed]
- De Biasi, A.R.; Villena-Vargas, J.; Adusumilli, P.S. Cisplatin-induced antitumor immunomodulation: A review of preclinical and clinical evidence. Clin. Cancer Res. 2014, 20, 5384–5391. [Google Scholar] [CrossRef]
- Zitvogel, L.; Apetoh, L.; Ghiringhelli, F.; Andre, F.; Tesniere, A.; Kroemer, G. The anticancer immune response: Indispensable for therapeutic success? J. Clin. Investig. 2008, 118, 1991–2001. [Google Scholar] [CrossRef]
- Tran, L.; Allen, C.T.; Xiao, R.; Moore, E.; Davis, R.; Park, S.J.; Spielbauer, K.; Van Waes, C.; Schmitt, N.C. Cisplatin alters antitumor immunity and synergizes with PD-1/PD-L1 inhibition in head and neck squamous cell carcinoma. Cancer Immunol. Res. 2017, 5, 1141–1151. [Google Scholar] [CrossRef]
- Walhof, C.M.; van Sonderen, L.; Voute, P.A.; Delemarre, J.F. Half-life of alpha-fetoprotein in patients with a teratoma, endodermal sinus tumor, or hepatoblastoma. Pediatr. Hematol. Oncol. 1988, 5, 217–227. [Google Scholar] [CrossRef]
- Fonseca, A.; Gupta, A.; Shaikh, F.; Ramphal, R.; Ng, V.; McGilvray, I.; Gerstle, J.T. Extreme hepatic resections for the treatment of advanced hepatoblastoma: Are planned close margins an acceptable approach? Pediatr. Blood Cancer. 2018, 65. [Google Scholar] [CrossRef]
- Li, J.; Girotti, P.; Königsrainer, I.; Ladurner, R.; Königsrainer, A.; Nadalin, S. ALPPS in right trisectionectomy: A safe procedure to avoid postoperative liver failure? J. Gastrointest. Surg. 2013, 17, 956–961. [Google Scholar] [CrossRef]
- Tanaka, K.; Matsuo, K.; Murakami, T.; Kawaguchi, D.; Hiroshima, Y.; Koda, K.; Endo, I.; Ichikawa, Y.; Taguri, M.; Tanabe, M. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): Short-term outcome, functional changes in the future liver remnant, and tumor growth activity. Eur. J. Surg. Oncol. 2015, 41, 506–512. [Google Scholar] [CrossRef]
- Otte, J.B.; Pritchard, J.; Aronson, D.C.; Brown, J.; Czauderna, P.; Maibach, R.; Perilongo, G.; Shafford, E.; Plaschkes, J. International society of pediatric oncology (SIOP). Liver transplantation for hepatoblastoma:results from the international society of pediatric oncology(SIOP) study SIOPEL-1 and review of the world experience. Pediatr. Blood Cancer 2004, 42, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; El Jabbour, T.; Ainechi, S.; Gay, L.M.; Elvin, J.A.; Vergilio, J.A.; Suh, J.; Ramkissoon, S.H.; Ali, S.M.; Schrock, A.; et al. General paucity of genomic alteration and low tumor mutation burden in refractory and metastatic hepatoblastoma: Comprehensive genomic profiling study. Hum. Pathol. 2017, 70, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Eichenmuller, M.; Trippel, F.; Kreuder, M.; Beck, A.; Schwarzmayr, T.; Haberle, B.; Cairo, S.; Leuschner, I.; von Schweinitz, D.; Strom, T.M.; et al. The genomic landscape of hepatoblastoma and their progenies with HCC-like features. J. Hepatol. 2014, 61, 1312–1320. [Google Scholar] [CrossRef]
- Jia, D.; Dong, R.; Jing, Y.; Xu, D.; Wang, Q.; Chen, L.; Li, Q.; Huang, Y.; Zhang, Y.; Zhang, Z.; et al. Exome sequencing of hepatoblastoma reveals novel mutations and cancer genes in the Wnt pathway and ubiquitin ligase complex. Hepatology 2014, 60, 1686–1696. [Google Scholar] [CrossRef]
- Grobner, S.N.; Worst, B.C.; Weischenfeldt, J.; Buchhalter, I.; Kleinheinz, K.; Rudneva, V.A.; Johann, P.D.; Balasubramanian, G.P.; Segura-Wang, M.; Brabetz, S.; et al. The landscape of genomic alterations across childhood cancers. Nature 2018, 555, 321–327. [Google Scholar] [CrossRef]
- Ma, X.; Liu, Y.; Liu, Y.; Alexandrov, L.B.; Edmonson, M.N.; Gawad, C.; Zhou, X.; Li, Y.; Rusch, M.C.; Easton, J.; et al. Pan-cancer genome and transcriptome analyses of 1699 paediatric leukaemias and solid tumours. Nature 2018, 555, 371–376. [Google Scholar] [CrossRef]
- Cabel, L.; Proudhon, C.; Romano, E.; Girard, N.; Lantz, O.; Stern, M.H.; Pierga, J.Y.; Bidard, F.C. Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy. Nat. Rev. Clin. Oncol. 2018, 15, 639–650. [Google Scholar] [CrossRef]
- Liao, W.; Mao, Y.; Ge, P.; Yang, H.; Xu, H.; Lu, X.; Sang, X.; Zhong, S. Value of quantitative and qualitative analyses of circulating cell-free DNA as diagnostic tools for hepatocellular carcinoma: A meta-analysis. Medicine 2015, 94, e722. [Google Scholar] [CrossRef]
- Lee, J.H.; Long, G.V.; Menzies, A.M.; Lo, S.; Guminski, A.; Whitbourne, K.; Peranec, M.; Scolyer, R.; Kefford, R.F.; Rizos, H.; et al. Association between circulating tumor DNA and pseudoprogression in patients with metastatic melanoma treated with anti-programmed cell death 1 antibodies. JAMA Oncol. 2018, 4, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Robbins, P.F.; Rosenberg, S.A. ’Final common pathway’ of human cancer immunotherapy: Targeting random somatic mutations. Nat. Immunol. 2017, 18, 255–262. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sindhi, R.; Rohan, V.; Bukowinski, A.; Tadros, S.; de Ville de Goyet, J.; Rapkin, L.; Ranganathan, S. Liver Transplantation for Pediatric Liver Cancer. Cancers 2020, 12, 720. https://doi.org/10.3390/cancers12030720
Sindhi R, Rohan V, Bukowinski A, Tadros S, de Ville de Goyet J, Rapkin L, Ranganathan S. Liver Transplantation for Pediatric Liver Cancer. Cancers. 2020; 12(3):720. https://doi.org/10.3390/cancers12030720
Chicago/Turabian StyleSindhi, Rakesh, Vinayak Rohan, Andrew Bukowinski, Sameh Tadros, Jean de Ville de Goyet, Louis Rapkin, and Sarangarajan Ranganathan. 2020. "Liver Transplantation for Pediatric Liver Cancer" Cancers 12, no. 3: 720. https://doi.org/10.3390/cancers12030720
APA StyleSindhi, R., Rohan, V., Bukowinski, A., Tadros, S., de Ville de Goyet, J., Rapkin, L., & Ranganathan, S. (2020). Liver Transplantation for Pediatric Liver Cancer. Cancers, 12(3), 720. https://doi.org/10.3390/cancers12030720