Abstract
Here, we critically evaluated the knowledge on cutaneous melanoma (CM) and uveal melanoma (UM). Both cancer types derive from melanocytes that share the same embryonic origin and display the same cellular function. Despite their common origin, both CM and UM display extreme differences in their genetic alterations and biological behavior. We discuss the differences in genetic alterations, metastatic routes, tumor biology, and tumor-host interactions in the context of their clinical responses to targeted- and immunotherapy.
1. Melanocytes and Their Cellular Function
Melanocytes originate from neural crest cells and are present in various parts of the human body, including the skin, eyes, cochlea, mesencephalon, and the heart. There they are responsible for the synthesis of melanin pigments within organelles called melanosomes. In the epidermis, melanocytes transfer these melanin-containing melanosomes to neighboring keratinocytes. This ensures homogeneous pigmentation, determines skin color and protects against the harmful effects of ultraviolet radiation (UVR) [1]. In the eye, melanocytes are found in the conjunctiva and all areas of the uvea (the iris, ciliary body, and choroid). Conjunctival melanoma is distinct from uveal melanoma (UM) and shares more commonalities with cutaneous melanoma (CM) [2].
The quantity and quality of melanin pigment in the iris determines its color. In contrast to the skin, the iris color is not influenced by sun exposure. The variance in melanin expressing uveal melanocytes is associated with the occurrence of various ocular diseases, including age-related macular degeneration and uveal melanoma [3,4]. Both CM and UM arise from melanocyte transformation and represent deadly forms of cancer.
2. Genetic Alterations and Treatment Implications
CM and conjunctival melanoma are genetically distinct from UM. The majority of CM cases harbor mutations in proteins associated with the mitogen-activated protein kinase (MAPK) pathway. This is an important intracellular signaling pathway involved in cell growth, differentiation, and survival. Oncogenic activation of the MAPK pathway may occur via multiple mechanisms but most commonly is driven by a constitutively activated mutated BRAF kinase. BRAF kinase mutations are present in 40–60% of the CM patients, 97% of which is located in codon 600.
BRAF-mutated melanoma tends to exhibit distinctive clinical features and is characterized by a more aggressive biological behavior than BRAF wild-type (WT) melanoma. BRAF-mutated melanoma may be associated with shorter overall survival and adverse prognostic factors, but this is still under investigation [5,6,7,8]. The second most common MAPK pathway aberration in CM is mutated NRAS, occurring in 15–30% of patients (Figure 1) [9,10,11,12]. Melanoma with mutations in the stem cell factor receptor tyrosine kinase gene (KIT) represents a relatively rare subset, seen in roughly 20% of mucosal, acral, and chronically sun-damaged skin [13].
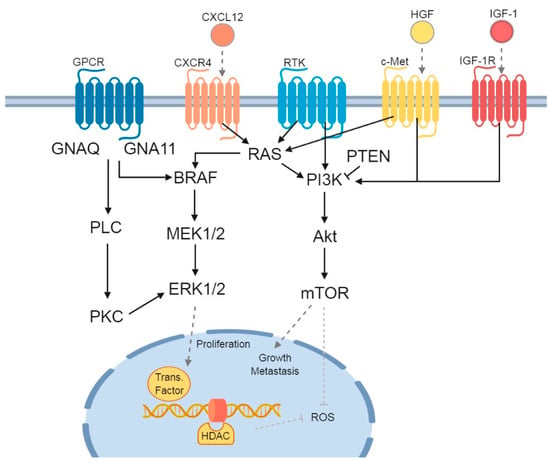
Figure 1.
Signaling pathways and receptors involved in uveal melanoma (UM) and cutaneous melanoma (CM). Three main signaling pathways affected in UM and/or CM patients are depicted. G protein-coupled receptor (GPCR) with its Guanine nucleotide-binding proteins: the first is the Guanine nucleotide binding protein (GNAQ) and subunit alpha-11 (GNA11), which downstream activate Phospholipase C (PLC) and Protein Kinase C (PKC). The second is the mitogen-activated protein kinase (MAPK) signaling pathway, consisting of BRAF-MEK1/2-ERK1/2. Finally, there is the PI3K/Akt/mTOR pathway, which can be influenced by both RAS (from the MAPK signaling pathway) and phosphatase and tensin homolog (PTEN). The previously described chemokine receptors and their influence on the signaling pathways are added: C-X-C chemokine receptor 4 (CXCR4), with its C-X-C Motif Chemokine Ligand 12 (CXCL12), tyrosine-protein kinase Met (c-Met) and its ligand Hepatocyte Growth Factor (HGF), and Insulin-like Growth Factor-1 Receptor (IGF-1R), with Insulin-like Growth Factor-1 (IGF-1). In the nucleus, the ERK1/2 stimulates transcription factors, while both histone deacetylase (HDAC) and mechanistic target of rapamycin (mTOR) inhibit the formation of Reactive Oxygen Species (ROS). Figure was created with BioRender.com.
The discovery that many CM are caused by a mutation in BRAF kinase has led to the development of selective inhibitors of the BRAF V600-mutated kinase (vemurafenib, dabrafenib, and encorafenib) and inhibitors of the downstream MEK kinase (trametinib, cobimetinib, and binimetinib). BRAF inhibition results in high response rates in patients with a BRAF V600E or V600K mutation; however, most patients ultimately develop acquired resistance. The combination of BRAF and MEK inhibitors is more effective in forestalling the development of acquired resistance when compared to BRAF monotherapy [14]. Five large phase III randomized controlled trials reported a median progression free survival for the combination treatment with BRAF and MEK inhibition of 9.3–11.4 months whereas this was 5.8–8.8 months for treatment with a BRAF inhibitor and placebo [15,16,17,18,19]. The treatment with KIT inhibitors improved the overall survival of patients with KIT-mutated gastro-intestinal stromal tumors. Following this success, multiple trials have shown that patients with metastatic melanoma harboring a KIT mutation were responsive to therapy with KIT inhibitors imatinib, sunitinib, dasatinib, and nilotinib [13]. The response rates in patients with metastatic melanoma are around 20–25%, when all KIT genetic lesions are considered, and reach 35–50% in melanomas with a KIT mutation in exon 11 or 13 [20,21,22,23,24].
Mutations in BRAF V600E occur in 29–50% and mutations in NRAS occur in up to 18% of the patients with a conjunctival melanoma. KIT mutations have only been reported in one conjunctival tumor [25,26]. As it is a rare form of ocular melanoma, clinical data after BRAF inhibition is scarce. Two case reports show mixed results [27,28]. However, the genetic similarities suggest that treatment regimens used for metastatic CM should be further investigated in metastatic conjunctival melanoma.
In UM, the most commonly mutated genes are GNA11, GNAQ, BAP1, EIF1AX, and SF3B1. More than 90% of the UM exhibit a mutation in GNA11 or GNAQ, which activate signaling between G-protein-coupled receptors and downstream effectors as well as upregulate signaling of the MAPK pathway (Figure 1) [29,30]. These mutations occur mutually exclusive in the majority of uveal melanomas, and are considered an early event in the development of UM. Mutations in GNAQ and GNA11 are not associated with a worse prognosis or with the development of metastatic disease [31,32,33,34].
However, primary UM can be stratified into four distinct, clinically relevant molecular subtypes with a significant difference in metastatic rate and prognosis [30]. Class 1A and 1B tumors retain a differentiated melanocyte phenotype, with a disomy of chromosome 3. They are further distinguished by alterations in either EIF1AX or SF3B1, respectively, with 1A having a lower metastatic rate when compared to 1B. Class 2 UM is associated with a high metastatic risk and is characterized by a monosomy of chromosome 3, followed by aberrancies in BAP1 expression and global DNA methylation. A further subdivision can be made into class 2A and 2B based on chromosome 8q copy number alterations, RNA expression, and cellular pathway activity profiles [35]. With Class 2B having a higher metastatic rate when compared to Class 2A [35,36,37].
As most UM are characterized by mutations in GNAQ or GNA11, therapies that target downstream effectors of these pathways such as MEK, Akt, and protein kinase C (PKC) are being investigated. Unfortunately, the results have been disappointing with response rates generally less than 10% [38,39]. A promising new target in UM could be epigenetic dysregulation. As previously mentioned, somatic mutations in the tumor suppressor gene BAP1 are correlated with metastatic behavior [40]. The loss of BAP1 seems to sensitize UM cell lines to treatment with histone deacetylase (HDAC) inhibitors. HDAC induces a G1 cell cycle arrest with an increased cyclin D1, impaired cell proliferation, growth reduction, and induction of apoptosis in UM both in vivo and in vitro [41,42,43].
Treatment with HDAC inhibitors might prove to be beneficial for both UM and CM, as the balance between histone acetylation and deacetylation is altered in multiple cancer types. This balance defines the level of acetylation of histone and therefore plays a critical role in the regulation of gene expression [44]. While histone acetyltransferases (HAT) mediated acetylation is associated with gene transcription, HDAC-mediated histone deacetylation is associated with gene silencing. Inhibition of HDAC was shown to block tumor cell proliferation and differentiation. Currently, there are four HDAC inhibitors approved by the FDA for treatment of cancer; vorinostat, romidepsin, belinostat for T-cell lymphoma, and panobinostat for multiple myeloma [45]. Currently, several trials are studying the effect of HDAC inhibition in patients with UM or CM. Furthermore, there is pre-clinical evidence that combining HDAC inhibitors with conventional immunotherapies, targeted therapies, or cyclin-dependent kinase (CDK) inhibitors might work synergistically [46,47,48].
3. Biological Parameters Underlying Metastasis
Cutaneous and ocular melanomas have distinctly different clinical courses. For both CM and UM, the development of metastatic disease is an important determinant of the clinical course and survival. CM tends to spread via the lymphatic system, mostly to the lungs, brain, lymph nodes, and soft tissue, with 14–20% of patients developing liver metastases [49]. Because there are no lymphatics in the uveal tract, ocular melanoma spreads hematogenously, resulting in the liver as the predominant metastatic site (89% of cases) [50].
The striking liver tropism of UM metastasis is currently not fully understood. In 1889, Paget introduced the concept of ‘’seed and soil’’, which proposed that the spread of tumor cells is governed by interaction and cooperation between the tumor and the host organ [51]. More recent studies have provided a better understanding of the process of metastatic spread of multiple cancer types, including melanoma [52]. One of these studies showed that some tumors succeed in creating a premetastatic niche in the liver. They manipulate the microenvironment of different organs to render them more permissive to metastatic outgrowth before the cancer cells actually enter the organ. It was shown that integrin expression profiles of circulating plasma exosomes isolated from amongst other CM and UM could be used as a prognostic factor to predict sites of future metastasis [53].
Furthermore, a wide variety of tumors express chemokine receptors corresponding with the expression of their respective ligands in the organs bearing the highest frequency of metastases. Chemokine receptors might also influence the overall survival in patients, and may present as potential targets for treatment.
(1) CCR4-CCL17/CCL22 axis: in CM, it was shown that CCR4 overexpression might enhance the tumor’s potential to metastasize to the brain [54].
In UM, no correlation between this axis and metastatic pattern has thus far been described [55].
(2) CCR7-CCL19 axis: in CM, the CCR7-CCL19/CCL21 axis is associated with regional lymph node metastases [56,57].
In UM, the expression of CCR7 seemed to be correlated with the development of liver metastases. Both in CM and UM, this axis has been correlated with a worse patient outcome [58,59].
(3) CCR10-CCL27 axis: in a CM preclinical model, it was shown that CCR10 might play an important role in sustaining tumor viability, in protecting cells from the immune response, and in the dissemination to the draining lymph node. High expression of CCR10 was associated with a worse overall survival [57,60,61].
In UM, no correlation was found between the presence of CCR10 and/or CCL27 and the formation of liver metastases [62].
(4) CXCR3-CXCL9/CXCL10 axis: stimulation of this axis has been described to be have both pro-tumor and anti-tumor effects. This may be due to the different effects of the ligands on CXCR3. CXCL9 predominantly mediates lymphocytic infiltration and suppresses tumor growth. The induction of both CXCL9 and CCL10 expression was also seen in CM patients that responded well to interleukin 12 immunotherapy [63]. Furthermore, stage III CM patients with CXCL10 expressing CD8 T cells had a better overall survival. Conversely, CXCR3, the receptor for both CXCL9 and CXCL10, is associated with thicker primary tumors, the absence of lymphocytic infiltration, and the presence of distant metastases. It has been shown that the anti-tumor effect of this axis is induced by paracrine activation by immune cells, while the pro-tumor effect is caused by autocrine signaling mainly through the CXCR3A ligand in cancer cells [64]. The selective targeting of CXCR3A was therefore suggested to be an effective treatment option in metastatic disease.
In UM, it has been shown that CXCL10 is upregulated in a T-cell-rich environment. Recently, it was shown that in UM, mainly activated macrophages express this lymphocyte-homing chemokine CXCL10. Furthermore, CXCL10 expression may serve as an independent risk factor, inversely correlated with survival [36].
(5) CXCR4/CXC7-CXCL12 axis: in CM, high CXCR4 expression is associated with the presence of tumor ulceration, thicker lesions, as well as shorter disease-free survival, time to metastasis, and overall survival. Furthermore, its expression is associated with the development of liver and lung metastases [65,66].
The expression of CXCR4 on UM cells and the presence of CXCL12 in the liver offers an explanation for the selective colonization of the liver by UM. Interactions between CXCR4 and CXCL12 stimulate tumor cell migration and invasion of basement membrane preparation by increasing the formation of cell adhesion molecules like matrix metalloproteinases [59,67]. CXCL12 also stimulates proliferation and survival of CXCR4 positive tumor cells [68,69,70]. Furthermore, chemotaxis of uveal melanoma cells could be inhibited by anti-CXCR4 [59].
(6) c-Met, a receptor for hepatocyte growth factor (HGF): In CM overexpression of c-Met is associated with tumor growth and metastasis. Inhibition of HGF induced c-Met proliferation reduced melanoma cell line migration and invasion in vitro [71].
In UM c-Met also promotes tumor invasion and stimulates tumor growth [72]. The expression of c-Met in primary UM increases the risk of subsequent liver metastasis [73]. Cabozantinib is a tyrosine kinase inhibitor that targets the MET, AXL, and vascular endothelial growth factor (VEGF) receptors. In CM cells it inhibits HGF-induced migration and invasion [74], while in an UM xenograft model, it was shown to reduce hepatic metastasis [75]. A recent phase II randomized discontinuation trial in which the MET/VEGF receptor inhibitor cabozantinib was tested, revealed clinical activity in both metastatic CM and UM patients [76].
(7) Insulin-like growth factor-1 (IGF-1) plays an important role in tissue growth, and increases the risk for the development of many tumor types, including CM [77]. Both in CM and UM the serum IGF-1 level functioned as a potential predictive biomarker for metastatic disease. Strikingly, whereas metastatic UM patients displayed lower IGF-1 serum levels when compared to healthy controls, the IGF-1 serum levels were higher in metastatic CM patients [78,79]. In UM, a high expression of the IGF-1 receptor (IGF-1R) was found in hepatic metastasis and related to death due to metastatic disease [80,81,82]. The IGF/IGF-1R axis has been a target for new treatment combinations in both CM and UM. In CM, IGF-targeting agents have been used in combination with other treatment modalities, as it plays a role in both primary and acquired treatment resistance [83]. Preclinical research shows promising results whenIGF-1R inhibition is combined either with PI3K inhibition, Stat3 blocking, or chemotherapy (temozolomide) [84,85,86]. In metastatic UM, a trial treating patients with an anti-IGF-1R antibody (IMC-A12, cixutumumab), was conducted. However, the final results have not yet been published (NCT01413191).
Hypoxia-inducible factor (HIF) plays a key role in tumorigenesis and metastasis in multiple types of cancer [87]. It plays an important role in the development of CM from melanocytes. Even at normal oxygen levels, HIF activity is increased in melanoma, thereby accelerating the invasion of tumor cells into adjacent tissues and providing sufficient blood supply [88,89]. Recently, FBXO22 was introduced as a possible new treatment option for CM as it is supposed to regulate the expression of HIF [88].
In UM it was shown that relative activity of hypoxia differentiated the subgroups, irrespective of chromosome 3 status [35]. Both the previously mentioned c-Met and CXCR4 are important surface mediators of hypoxia-induced migration, invasion, and metastasis [90,91]. In addition, elevated mRNA expression of both MET and CXCR4 was found in patients with a poor prognosis and the expression levels of CXCR4, c-Met, and HIF-1 were higher in the primary tumor of patients with a subsequent metastasis. Furthermore, in cell cultures hypoxia can induce c-Met and CXCR4 expression, while these effects were inhibited by a HIF pathway inhibitor (arylsulfonamide 64B) both in vitro and in an in vivo orthotopic mouse model. In vivo treatment resulted in inhibition of primary UM growth, less liver metastasis formation, and a better survival [92].
4. The Impact of the Immune System
4.1. Primary Tumor
The distribution of immune cells varies between different tumor types. In CM, the role of the adaptive immune response in controlling tumor progression has gained a lot of attention over the past decades. In primary CM the presence of CD3+CD8+ lymphocytes, specifically activated (HLA-DR expressing) CD8+ T cells, in both the tumor and the stroma was correlated with disease-specific survival [93].
Multiple studies have investigated the role of immunosuppressive regulatory T cells (Treg) in primary CM, with conflicting results. This might be due to differences in phenotypic markers used or technical differences in staining and analyzing, as the two papers showing no difference identified Tregs as FoxP3+ cells and the paper showing a difference identified these cells as being CD25+FoxP3+ [94,95,96]. This emphasizes the need for a robust gating strategy for the analysis of Tregs [97].
Additionally, the role of macrophages has been investigated. There are two major subtypes of macrophages, being the macrophages that support an effective antitumor response (M1) and the macrophages that promote tumor growth (M2). In the early development of CM, the M1-recruited macrophages shift to the M2 phenotype, thus favoring tumor proliferation and dissemination [98].
In contrast to CM, the pronounced infiltration of UM by immune cells is associated with a poor prognosis [99]. Primary UM with monosomy 3 is associated with infiltration with a variety of immune cells, including CD8+, CD4+, and CD3+CD8-FoxP3+ T cells as well as CD68+CD163+ M2 macrophages. The Class 2B tumors that display a gain in the copy number of chromosome 8q are associated with the increased expression of macrophage-attracting chemokines and a stronger influx of myeloid cells, whereas additional aberrations in BAP1 expression seem to drive T cell infiltration, irrespective of the chromosome 3 status [100]. The presence of a CD3+ immune infiltrate in Class 2 tumors, while nearly absent in Class 1 tumors, coincides with the increased gene expression of human leukocyte antigen (HLA), suggesting the local production of type II interferon [101]. Notably, the infiltration with all these immune cells is collectively increased, the balance of the different cells was of no clinical relevance [102,103], although one study suggested that the presence of the immunosuppressive Tregs within a subgroup of COX2+ primary UM forms an independent prognostic factor for worse overall survival [104].
4.2. Metastatic Melanoma
In many metastasized tumors, including CM, the presence of effector T lymphocytes is beneficial, including CD8+ T cells and CD4+ helper T cells. The presence of CD4+CD25+ Tregs may be detrimental [105]. Our group recently identified four intratumoral parameter profile that was associated with a better survival in metastatic CM patients. Namely, the presence of tumor infiltrating CD3+CD8+FoxP3− T cells, galectin-9+ dendritic cells (DC)/DC-like macrophages, a high CD14+CD163− (M1)/CD14+CD163+ (M2) macrophage ratio, and the expression of galectin-3 by tumor cells. Patients with three or four of the described parameters present displayed the longest overall survival [106].
Currently, one of the most established treatments for metastatic CM is via immune stimulation with checkpoint blockers. This type of treatment relies on antigen-specific T cell responses by alleviating tumor-induced immunoregulatory mechanisms [107]. Immune checkpoint blockade can achieve durable responses in many CM patients and has shown to improve overall survival in this patient group. The first blocking antibody that was tested and approved for the treatment of cancer patients was against cytotoxic T-lymphocyte antigen-4 (CTLA-4). CTLA-4 increases the activation threshold of T cells, reducing immune responses to weak antigens such as self- and tumor antigens. The second blocking antibody introduced into the clinic was targeting Programmed death 1 (PD-1). While CTLA-4 mainly plays a role in the activation phase in the draining lymph node, PD-1 predominantly regulates the effector phase of T cell responses within peripheral tissues. PD-1 binding with its ligands decreases the magnitude of the immune response in T cells that are already engaged in an effector T cell response. This results in a more restricted T cell activation compared to CTLA-4 blockade, which can lead to an unspecific activation of T cells in the lymphoid organs. This could explain why PD-1 inhibition shows fever side effects and greater antitumor activity than CTLA-4 inhibition [108,109,110,111]. The updated survival data from the CheckMate 067 study showed a 3-year overall survival of 58% in the patients treated with anti-PD1 and anti-CTLA4, 52% in patients with anti-PD1 monotherapy and 34% in patients treated with anti-CTLA4 monotherapy [108].
Treatment with these checkpoint blockers has been investigated in UM. Unfortunately, the clinical response rates reported for anti-PD1 or anti-CTLA4 are unimpressive, with no significant OS benefit in UM patients [112,113,114,115,116,117,118,119,120]. A trial investigating the combination of these checkpoint inhibitors is still ongoing (NCT01585194).
Little is known about the immune microenvironment of metastatic UM (mUM). Therefore, reasons underlying the poor response to immunotherapy are unclear and have led to speculation that UM may represent an immunotherapy resistant form of melanoma. Several recent findings might help to shed some light on why UM does not respond to immunotherapy like CM.
High mutational burden is predictive of the response to immune checkpoint inhibitors across multiple cancer types [121]. The neoantigens that derive from these tumor-specific mutations are potential targets for anti-tumor immune responses, as they are foreign to the immune system. Cutaneous melanoma is one of the tumors with the highest somatic mutation prevalence [122]. In contrast UM lacks the UV-radiation mutation signature and has a low mean somatic mutation rate [123]. The lack of these targets could be a possible explanation as to why immune stimulation with checkpoint inhibitors alone is not sufficient in UM, while it can be sufficient in CM. However, low-mutational burden may also lead to the spontaneous activation of neoantigen-specific T cells [124,125].
In a recent pilot study, the immune profile of both CM and UM metastases was characterized. Overall, it seemed that the CD8 infiltration in both tumors was similar. Interestingly, the PD-1 expression levels were lower in mUM patients than those observed in metastatic CM (mCM). Furthermore, it also seemed that the expression of PD-L1 (one of the ligands of PD-1) was lower in the mUM group [126]. As activated tumor-reactive CD8+ T cells express PD-1, this may suggest that there either is a lack of tumor-antigen specific tumor infiltrating lymphocytes (TIL) in mUM or that they are locally suppressed by other means [127]. In the absence of a type 1 immune response, there is less interferon-gamma driven PD-L1 expression [128]. As the target for anti-PD1 treatment is not expressed in most mUM patients, this provides another rationale for the lack of efficacy of anti-PD1 treatment.
Preliminary data from an ongoing trial comparing the immune infiltrate of mUM and mCM show that in accordance with the previously mentioned trial, the density of CD3+CD8+, as well as the distance from CD8+ lymphocyte to tumor cell, was similar in both tumor types. However, macrophages were less numerous in mUM compared to mCM at baseline; further classification of these macrophages is still ongoing. Interestingly, the preliminary data also showed that enrichment for T cell and inflammatory gene expression was observed in a mUM patient with exceptional overall survival in contrast to an overall low CD8 and the absence of an immune gene expression profile in a patient with the shortest overall survival [129]. This suggests that some mUM are immunogenic, despite earlier reports on the immune infiltrate in primary UM. This notion is also supported by a recently published phase II clinical trial applying adoptive cell therapy to treat mUM patients. Twenty-one mUM patients were treated with autologous TIL. Of the 20 evaluable patients, seven (35%) achieved objective tumor regression (six partial response, one complete response), including mUM patients who had previously failed on anti-CTLA4 and anti-PD1 treatment. There was a strong correlation between clinical response, the autologous tumor reactivity of the infused TIL, and the number of reactive TIL infused. This clearly shows that despite the lack of an ultraviolet radiation signature, mUM do express antigens that are recognized by the adaptive immune system, suggesting that a lack of T cell activation in mUM is related to local immune suppression. Both biopsies prior and after TIL treatment were obtained from these patients, genomic and proteomic profiling is ongoing and whole exomic sequencing is being performed [130]. Despite the impressive overall response rate for patients with mUM, the durability was relatively short when compared to what has been observed in mCM. Moreover, a second phase II study is necessary, where patients with mUM are recruited with adoptive transfer of TIL to confirm the results in a larger cohort (NCT03467516).
Another potentially interesting cell-based therapy is treatment with chimeric antigen receptor (CAR) T cells. In hematological malignancies two CAR-T cell constructs targeting CD19 have been approved, both in the United States and in the European Union. One of the pilot trials currently recruiting melanoma patients uses c-Met as a target antigen (NCT03060356). As c-Met plays an important role in both CM and UM, this might be a promising treatment strategy for both melanoma subtypes.
5. Conclusions
Cutaneous and uveal melanoma both arise from melanocytes. However, they are biologically distinct tumor types. In recent years, many new treatment options have become available for patients with advanced cutaneous melanoma, improving the disease free and overall survival. Unfortunately, most of these new treatment options do not show the same responses in patients with metastatic uveal melanoma. Chemokine receptors, which play a role in both tumor growth and the formation of metastases, have shown to be promising new targets. Based on the pre-clinical work with anti-CXCR4 and anti-IGF-1R, as well as the first clinical results with a MET/VEGF receptor inhibitor, several treatment options are now (further) investigated in the clinic. Multiple trials with both UM and CM patients that are treated with HDAC-inhibitors are also ongoing.
Recent studies indicate that the role of the adaptive immune system in primary versus metastatic UM might be very different. Where immune infiltrate in primary uveal melanoma is correlated with a worse overall survival, this difference was so far not seen in metastatic lesions. However, even when immune cells succeed in infiltrating metastatic UM lesions, these cells do not seem to be activated. Adoptive cell therapy trials in mUM indicate that metastatic UM are immunogenic and able to trigger tumor-reactive T cells; however, potentially, they are locally suppressed, similar to what is seen in primary UM.
As there is not yet a gold standard in the systemic treatment of metastatic UM, early detection and enrolment in clinical trials seems crucial.
Author Contributions
Conceptualization, M.K.v.d.K., S.H.v.d.B., and E.K.; writing—original draft preparation, M.K.v.d.K.; writing—review and editing, M.K.v.d.K., F.M.S., S.H.v.d.B., and E.K.; visualization, M.K.v.d.K.; supervision, E.K., and S.H.v.d.B.
Funding
This research received no external funding.
Conflicts of Interest
E.K. has consulting/advisory relationships with Bristol-Myers Squibb, Novartis, Roche, Amgen, Pierre-Fabre (honoraria paid to institution). She received research grants from BMS and Novartis. In the contribution to this specific manuscript the authors declare no conflict of interest.
References
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Westekemper, H.; Murali, R.; Mach, M.; Schilling, B.; Wiesner, T.; Schimming, T.; Livingstone, E.; Sucker, A.; Grabellus, F.; et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin. Cancer Res. 2013, 19, 3143–3152. [Google Scholar] [CrossRef] [PubMed]
- Sarna, T. Properties and function of the ocular melanin—A photobiophysical view. J. Photochem. Photobiol. B 1992, 12, 215–258. [Google Scholar] [CrossRef]
- Weis, E.; Shah, C.P.; Lajous, M.; Shields, J.A.; Shields, C.L. The association between host susceptibility factors and uveal melanoma—A meta-analysis. Arch. Ophthalmol. 2006, 124, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ny, L.; Nyakas, M.; Hernberg, M.; Koivunen, J.; Oddershede, L.; Yoon, M.-R.; Wang, X.; Guyot, P.; Geisler, J. BRAF mutation as a prognostic marker for survival in malignant melanoma: A systematic review and meta-analysis. J. Clin. Oncol. 2018, 36, e21566. [Google Scholar] [CrossRef]
- Spathis, A.; Katoulis, A.C.; Damaskou, V.; Liakou, A.I.; Kottaridi, C.; Leventakou, D.; Sgouros, D.; Mamantopoulos, A.; Rigopoulos, D.; Karakitsos, P.; et al. BRAF mutation status in primary, recurrent, and metastatic malignant melanoma and its relation to histopathological parameters. Dermatol. Pract. Concept. 2019, 9, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Siepmann, T.; Engel, J.; Schubert-Fritschle, G.; Eckel, R.; Mirlach, L.; Kirchner, T.; Jung, A.; Gesierich, A.; Ruzicka, T.; et al. Prognostic significance of BRAF and NRAS mutations in melanoma: A German study from routine care. BMC Cancer 2017, 17, 536. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Menzies, A.M.; Nagrial, A.M.; Haydu, L.E.; Hamilton, A.L.; Mann, G.J.; Hughes, T.M.; Thompson, J.F.; Scolyer, R.A.; Kefford, R.F. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. 2011, 29, 1239–1246. [Google Scholar] [CrossRef]
- Colombino, M.; Capone, M.; Lissia, A.; Cossu, A.; Rubino, C.; De Giorgi, V.; Massi, D.; Fonsatti, E.; Staibano, S.; Nappi, O.; et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J. Clin. Oncol. 2012, 30, 2522–2529. [Google Scholar] [CrossRef]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Akbani, R.; Akdemir, K.C.; Aksoy, B.A.; Albert, M.; Ally, A.; Amin, S.B.; Arachchi, H.; Arora, A.; Auman, J.T.; Ayala, B.; et al. Genomic classification of cutaneous melanoma. Cell 2015, 161, 1681–1696. [Google Scholar] [CrossRef] [PubMed]
- Ihle, M.A.; Fassunke, J.; Konig, K.; Grunewald, I.; Schlaak, M.; Kreuzberg, N.; Tietze, L.; Schildhaus, H.U.; Buttner, R.; Merkelbach-Bruse, S. Comparison of high resolution melting analysis, pyrosequencing, next generation sequencing and immunohistochemistry to conventional Sanger sequencing for the detection of p.V600E and non-p.V600E BRAF mutations. BMC Cancer 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Carvajal, R.D. KIT as an oncogenic driver in melanoma: An update on clinical development. Am. J. Clin. Dermatol. 2019, 20, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Yang, X.; Liu, J.; Zhao, B.; Cai, W.; Li, Y.; Hu, D. Efficacy and safety of BRAF inhibition alone versus combined BRAF and MEK inhibition in melanoma: A meta-analysis of randomized controlled trials. Oncotarget 2017, 8, 32258–32269. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Dabrafenib and trametinib versus dabrafenib and placebo for Val600 BRAF-mutant melanoma: A multicentre, double-blind, phase 3 randomised controlled trial. Lancet 2015, 386, 444–451. [Google Scholar] [CrossRef]
- Robert, C.; Karaszewska, B.; Schachter, J.; Rutkowski, P.; Mackiewicz, A.; Stroiakovski, D.; Lichinitser, M.; Dummer, R.; Grange, F.; Mortier, L.; et al. Improved Overall Survival in Melanoma with Combined Dabrafenib and Trametinib. N. Eng. J. Med. 2015, 372, 30–39. [Google Scholar] [CrossRef]
- Larkin, J.; Ascierto, P.A.; Dreno, B.; Atkinson, V.; Liszkay, G.; Maio, M.; Mandala, M.; Demidov, L.; Stroyakovskiy, D.; Thomas, L.; et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N. Eng. J. Med. 2014, 371, 1867–1876. [Google Scholar] [CrossRef]
- Long, G.V.; Stroyakovskiy, D.; Gogas, H.; Levchenko, E.; de Braud, F.; Larkin, J.; Garbe, C.; Jouary, T.; Hauschild, A.; Grob, J.J.; et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Eng. J. Med. 2014, 371, 1877–1888. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Infante, J.R.; Daud, A.; Gonzalez, R.; Kefford, R.F.; Sosman, J.; Hamid, O.; Schuchter, L.; Cebon, J.; Ibrahim, N.; et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Eng. J. Med. 2012, 367, 1694–1703. [Google Scholar] [CrossRef]
- Carvajal, R.D.; Antonescu, C.R.; Wolchok, J.D.; Chapman, P.B.; Roman, R.-A.; Teitcher, J.; Panageas, K.S.; Busam, K.J.; Chmielowski, B.; Lutzky, J.; et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011, 305, 2327–2334. [Google Scholar] [CrossRef]
- Guo, J.; Carvajal, R.D.; Dummer, R.; Hauschild, A.; Daud, A.; Bastian, B.C.; Markovic, S.N.; Queirolo, P.; Arance, A.; Berking, C.; et al. Efficacy and safety of nilotinib in patients with KIT-mutated metastatic or inoperable melanoma: Final results from the global, single-arm, phase II TEAM trial. Ann. Oncol. 2017, 28, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Si, L.; Kong, Y.; Flaherty, K.T.; Xu, X.; Zhu, Y.; Corless, C.L.; Li, L.; Li, H.; Sheng, X.; et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J. Clin. Oncol. 2011, 29, 2904–2909. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Corless, C.L.; Giobbie-Hurder, A.; Fletcher, J.A.; Zhu, M.; Marino-Enriquez, A.; Friedlander, P.; Gonzalez, R.; Weber, J.S.; Gajewski, T.F.; et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J. Clin. Oncol. 2013, 31, 3182–3190. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, T.M.; Kim, Y.J.; Jang, K.T.; Lee, H.J.; Lee, S.N.; Ahn, M.S.; Hwang, I.G.; Lee, S.; Lee, M.H.; et al. Phase II Trial of nilotinib in patients with metastatic malignant melanoma harboring kit gene aberration: A multicenter trial of korean cancer study group (UN10-06). Oncologist 2015, 20, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.F.; Heijkants, R.C.; Jochemsen, A.G.; Dogrusoz, M.; de Lange, M.J.; van der Velden, P.A.; van der Burg, S.H.; Jager, M.J.; Verdijk, R.M. Targeting of the MAPK and AKT pathways in conjunctival melanoma shows potential synergy. Oncotarget 2017, 8, 58021–58036. [Google Scholar] [CrossRef] [PubMed]
- Beadling, C.; Jacobson-Dunlop, E.; Hodi, F.S.; Le, C.; Warrick, A.; Patterson, J.; Town, A.; Harlow, A.; Cruz, F., 3rd; Azar, S.; et al. KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. 2008, 14, 6821–6828. [Google Scholar] [CrossRef]
- Pahlitzsch, M.; Bertelmann, E.; Mai, C. Conjunctival melanoma and BRAF inhibitor therapy. J. Clin. Exp. Ophtalmol. 2014, 5, 322. [Google Scholar] [CrossRef]
- Weber, J.L.; Smalley, K.S.M.; Sondak, V.K.; Gibney, G.T. Conjunctival Melanomas Harbor BRAF and NRAS Mutations-Letter. Clin. Cancer Res. 2013, 19, 6329–6330. [Google Scholar] [CrossRef]
- Van Raamsdonk, C.D.; Bezrookove, V.; Green, G.; Bauer, J.; Gaugler, L.; O’Brien, J.M.; Simpson, E.M.; Barsh, G.S.; Bastian, B.C. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009, 457, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, C.D.; Griewank, K.G.; Crosby, M.B.; Garrido, M.C.; Vemula, S.; Wiesner, T.; Obenauf, A.C.; Wackernagel, W.; Green, G.; Bouvier, N.; et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 2010, 363, 2191–2199. [Google Scholar] [CrossRef]
- Koopmans, A.E.; Vaarwater, J.; Paridaens, D.; Naus, N.C.; Kilic, E.; de Klein, A. Patient survival in uveal melanoma is not affected by oncogenic mutations in GNAQ and GNA11. Br. J. Cancer 2013, 109, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Staby, K.M.; Gravdal, K.; Mork, S.J.; Heegaard, S.; Vintermyr, O.K.; Krohn, J. Prognostic impact of chromosomal aberrations and GNAQ, GNA11 and BAP1 mutations in uveal melanoma. Acta Ophthalmol. 2018, 96, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Onken, M.D.; Worley, L.A.; Long, M.D.; Duan, S.; Council, M.L.; Bowcock, A.M.; Harbour, J.W. Oncogenic mutations in GNAQ occur early in uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2008, 49, 5230–5234. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Kilic, E.; Vaarwater, J.; Bastian, B.C.; Garbe, C.; de Klein, A. Oncogenic GNAQ mutations are not correlated with disease-free survival in uveal melanoma. Br. J. Cancer 2009, 101, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer Cell 2017, 32, 204–220. [Google Scholar] [CrossRef] [PubMed]
- De Lange, M.J.; Nell, R.J.; Lalai, R.N.; Versluis, M.; Jordanova, E.S.; Luyten, G.P.M.; Jager, M.J.; van der Burg, S.H.; Zoutman, W.H.; van Hall, T.; et al. Digital PCR-based T-cell quantification-assisted deconvolution of the microenvironment reveals that activated macrophages drive tumor inflammation in uveal melanoma. Mol. Cancer Res. 2018, 16, 1902–1911. [Google Scholar] [CrossRef] [PubMed]
- De Lange, M.J.; van Pelt, S.I.; Versluis, M.; Jordanova, E.S.; Kroes, W.G.; Ruivenkamp, C.; van der Burg, S.H.; Luyten, G.P.; van Hall, T.; Jager, M.J.; et al. Heterogeneity revealed by integrated genomic analysis uncovers a molecular switch in malignant uveal melanoma. Oncotarget 2015, 6, 37824–37835. [Google Scholar] [CrossRef]
- Yang, J.; Manson, D.K.; Marr, B.P.; Carvajal, R.D. Treatment of uveal melanoma: Where are we now? Ther. Adv. Med. Oncol. 2018, 10, 1758834018757175. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Kapiteijn, E.; Larkin, J.M.G.; Carvajal, R.D.; Luke, J.J.; Seifert, H.; Roozen, I.; Zoubir, M.; Yang, L.; Choudhury, S.; et al. Phase I dose-escalation study of the protein kinase C (PKC) inhibitor AEB071 in patients with metastatic uveal melanoma. J. Clin. Oncol. 2014, 32, 9030. [Google Scholar] [CrossRef]
- Harbour, J.W.; Onken, M.D.; Roberson, E.D.; Duan, S.; Cao, L.; Worley, L.A.; Council, M.L.; Matatall, K.A.; Helms, C.; Bowcock, A.M. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science 2010, 330, 1410–1413. [Google Scholar] [CrossRef]
- Landreville, S.; Agapova, O.A.; Matatall, K.A.; Kneass, Z.T.; Onken, M.D.; Lee, R.S.; Bowcock, A.M.; Harbour, J.W. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin. Cancer Res. 2012, 18, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Zhou, J.; Jin, B.; Pan, J. Class III-specific HDAC inhibitor Tenovin-6 induces apoptosis, suppresses migration and eliminates cancer stem cells in uveal melanoma. Sci. Rep. 2016, 6, 22622. [Google Scholar] [CrossRef]
- Hornig, E.; Heppt, M.V.; Graf, S.A.; Ruzicka, T.; Berking, C. Inhibition of histone deacetylases in melanoma-a perspective from bench to bedside. Exp. Dermatol. 2016, 25, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Bi, G.; Jiang, G. The molecular mechanism of HDAC inhibitors in anticancer effects. Cell Mol. Immunol. 2006, 3, 285–290. [Google Scholar] [PubMed]
- Moschos, M.M.; Dettoraki, M.; Androudi, S.; Kalogeropoulos, D.; Lavaris, A.; Garmpis, N.; Damaskos, C.; Garmpi, A.; Tsatsos, M. The role of histone deacetylase inhibitors in uveal melanoma: Current evidence. Anticancer Res. 2018, 38, 3817–3824. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Jin, L.; Gallagher, S.; Mijatov, B.; Zhang, X.D.; Hersey, P. Histone deacetylases (HDACs) as mediators of resistance to apoptosis in melanoma and as targets for combination therapy with selective BRAF inhibitors. Adv. Pharmacol. 2012, 65, 27–43. [Google Scholar] [CrossRef]
- Woods, D.M.; Sodre, A.L.; Villagra, A.; Sarnaik, A.; Sotomayor, E.M.; Weber, J. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol. Res. 2015, 3, 1375–1385. [Google Scholar] [CrossRef]
- Heijkants, R.; Willekens, K.; Schoonderwoerd, M.; Teunisse, A.; Nieveen, M.; Radaelli, E.; Hawinkels, L.; Marine, J.-C.; Jochemsen, A. Combined inhibition of CDK and HDAC as a promising therapeutic strategy for both cutaneous and uveal metastatic melanoma. Oncotarget 2018, 9, 6174–6187. [Google Scholar] [CrossRef]
- Leiter, U.; Meier, F.; Schittek, B.; Garbe, C. The natural course of cutaneous melanoma. J. Surg. Oncol. 2004, 86, 172–178. [Google Scholar] [CrossRef]
- Diener-West, M.; Reynolds, S.M.; Agugliaro, D.J.; Caldwell, R.; Cumming, K.; Earle, J.D.; Hawkins, B.S.; Hayman, J.A.; Jaiyesimi, I.; Jampol, L.M.; et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative ocular melanoma study group report No. 26. Arch. Ophthalmol. 2005, 123, 1639–1643. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [PubMed]
- Akhtar, M.; Haider, A.; Rashid, S.; Al-Nabet, A.D.M.H. Paget’s “Seed and Soil” Theory of cancer metastasis: An idea whose time has come. Adv. Anat. Pathol. 2019, 26, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329. Available online: https://www.nature.com/articles/nature15756#supplementary-information (accessed on 24 May 2019). [CrossRef] [PubMed]
- Klein, A.; Sagi-Assif, O.; Meshel, T.; Telerman, A.; Izraely, S.; Ben-Menachem, S.; Bayry, J.; Marzese, D.M.; Ohe, S.; Hoon, D.S.B.; et al. CCR4 is a determinant of melanoma brain metastasis. Oncotarget 2017, 8, 31079–31091. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Mantovani, A. Macrophage plasticity and interaction with lymphocyte subsets: Cancer as a paradigm. Nat. Immunol. 2010, 11, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Wiley, H.E.; Gonzalez, E.B.; Maki, W.; Wu, M.T.; Hwang, S.T. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J. Natl. Cancer Inst. 2001, 93, 1638–1643. [Google Scholar] [CrossRef] [PubMed]
- Kuhnelt-Leddihn, L.; Muller, H.; Eisendle, K.; Zelger, B.; Weinlich, G. Overexpression of the chemokine receptors CXCR4, CCR7, CCR9, and CCR10 in human primary cutaneous melanoma: A potential prognostic value for CCR7 and CCR10? Arch. Dermatol. Res. 2012, 304, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Van den Bosch, T.; Koopmans, A.E.; Vaarwater, J.; van den Berg, M.; de Klein, A.; Verdijk, R.M. Chemokine receptor CCR7 expression predicts poor outcome in uveal melanoma and relates to liver metastasis whereas expression of CXCR4 is not of clinical relevance. Invest. Ophthalmol. Vis. Sci. 2013, 54, 7354–7361. [Google Scholar] [CrossRef]
- Li, H.; Alizadeh, H.; Niederkorn, J.Y. Differential expression of chemokine receptors on uveal melanoma cells and their metastases. Invest. Ophthalmol. Vis. Sci. 2008, 49, 636–643. [Google Scholar] [CrossRef]
- Murakami, T.; Cardones, A.R.; Finkelstein, S.E.; Restifo, N.P.; Klaunberg, B.A.; Nestle, F.O.; Castillo, S.S.; Dennis, P.A.; Hwang, S.T. Immune evasion by murine melanoma mediated through CC chemokine receptor-10. J. Exp. Med. 2003, 198, 1337–1347. [Google Scholar] [CrossRef]
- Simonetti, O.; Goteri, G.; Lucarini, G.; Filosa, A.; Pieramici, T.; Rubini, C.; Biagini, G.; Offidani, A. Potential role of CCL27 and CCR10 expression in melanoma progression and immune escape. Eur. J. Cancer 2006, 42, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Dobner, B.C.; Riechardt, A.I.; Joussen, A.M.; Englert, S.; Bechrakis, N.E. Expression of haematogenous and lymphogenous chemokine receptors and their ligands on uveal melanoma in association with liver metastasis. Acta Ophth. 2012, 90, e638–e644. [Google Scholar] [CrossRef] [PubMed]
- Alatrash, G.; Hutson, T.E.; Molto, L.; Richmond, A.; Nemec, C.; Mekhail, T.; Elson, P.; Tannenbaum, C.; Olencki, T.; Finke, J.; et al. Clinical and immunologic effects of subcutaneously administered interleukin-12 and interferon alfa-2b: Phase I trial of patients with metastatic renal cell carcinoma or malignant melanoma. J. Clin. Oncol. 2004, 22, 2891–2900. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, R.; Zhang, W.; Naseem, M.; Puccini, A.; Berger, M.D.; Soni, S.; McSkane, M.; Baba, H.; Lenz, H.J. CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation—A target for novel cancer therapy. Cancer Treat. Rev. 2018, 63, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A.; Fricker, S.P. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin. Cancer Res. 2010, 16, 2927–2931. [Google Scholar] [CrossRef]
- O’Boyle, G.; Swidenbank, I.; Marshall, H.; Barker, C.E.; Armstrong, J.; White, S.A.; Fricker, S.P.; Plummer, R.; Wright, M.; Lovat, P.E. Inhibition of CXCR4-CXCL12 chemotaxis in melanoma by AMD11070. Br. J. Cancer 2013, 108, 1634–1640. [Google Scholar] [CrossRef]
- Muller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Scotton, C.J.; Wilson, J.L.; Scott, K.; Stamp, G.; Wilbanks, G.D.; Fricker, S.; Bridger, G.; Balkwill, F.R. Multiple actions of the chemokine CXCL12 on epithelial tumor cells in human ovarian cancer. Cancer Res. 2002, 62, 5930–5938. [Google Scholar]
- Sun, Y.X.; Wang, J.; Shelburne, C.E.; Lopatin, D.E.; Chinnaiyan, A.M.; Rubin, M.A.; Pienta, K.J.; Taichman, R.S. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J. Cell Biochem. 2003, 89, 462–473. [Google Scholar] [CrossRef]
- Zhou, Y.; Larsen, P.H.; Hao, C.H.; Yong, V.W. CXCRA is a major chemokine receptor on glioma cells and mediates their survival. J. Biol. Chem. 2002, 277, 49481–49487. [Google Scholar] [CrossRef]
- Cao, H.H.; Cheng, C.Y.; Su, T.; Fu, X.Q.; Guo, H.; Li, T.; Tse, A.K.W.; Kwan, H.Y.; Yu, H.; Yu, Z.L. Quercetin inhibits HGF/c-Met signaling and HGF-stimulated melanoma cell migration and invasion. Mol. Cancer 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Van der Ent, W.; Burrello, C.; de Lange, M.J.; van der Velden, P.A.; Jochemsen, A.G.; Jager, M.J.; Snaar-Jagalska, B.E. Embryonic zebrafish: Different phenotypes after injection of human uveal melanoma cells. Ocul. Oncol. Pathol. 2015, 1, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Bakalian, S.; Marshall, J.C.; Logan, P.; Faingold, D.; Maloney, S.; Di Cesare, S.; Martins, C.; Fernandes, B.F.; Burnier, M.N., Jr. Molecular pathways mediating liver metastasis in patients with uveal melanoma. Clin. Cancer Res. 2008, 14, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Yakes, F.M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.C.; Yu, P.W.; Qian, F.; Chu, F.L.; Bentzien, F.; Cancilla, B.; et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011, 10, 2298–2308. [Google Scholar] [CrossRef] [PubMed]
- Yeh, I.; Griewank, K.; Ding, V.W.; Bastian, B.C. XL184: C-Met inhibition is effective in a mouse xenograft model of metastatic uveal melanoma. Cancer Res. 2011, 71, 3587. [Google Scholar] [CrossRef]
- Daud, A.; Kluger, H.M.; Kurzrock, R.; Schimmoller, F.; Weitzman, A.L.; Samuel, T.A.; Moussa, A.H.; Gordon, M.S.; Shapiro, G.I. Phase II randomised discontinuation trial of the MET/VEGF receptor inhibitor cabozantinib in metastatic melanoma. Br. J. Cancer 2017, 116, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Weroha, S.J.; Haluska, P. The insulin-like growth factor system in cancer. Endocrinol. Metab. Clin. N. Am. 2012, 41, 335. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, S.; Zloto, O.; Pe’er, J.; Barak, V. Insulin-like growth factor-1 as a predictive biomarker for metastatic uveal melanoma in humans. Invest. Ophth. Vis. Sci. 2013, 54, 490–493. [Google Scholar] [CrossRef] [PubMed]
- Kucera, R.; Treskova, I.; Vrzalova, J.; Svobodova, S.; Topolcan, O.; Fuchsova, R.; Rousarova, M.; Treska, V.; Kydlicek, T. Evaluation of IGF1 serum levels in malignant melanoma and healthy subjects. Anticancer Res. 2014, 34, 5217–5220. [Google Scholar]
- Yoshida, M.; Selvan, S.; McCue, P.A.; DeAngelis, T.; Baserga, R.; Fujii, A.; Rui, H.; Mastrangelo, M.J.; Sato, T. Expression of insulin-like growth factor-1 receptor in metastatic uveal melanoma and implications for potential autocrine and paracrine tumor cell growth. Pigment. Cell Melanoma Res. 2014, 27, 297–308. [Google Scholar] [CrossRef]
- All-Ericsson, C.; Girnita, L.; Seregard, S.; Bartolazzi, A.; Jager, M.J.; Larsson, O. Insulin-like growth factor-1 receptor in uveal melanoma: A predictor for metastatic disease and a potential therapeutic target. Invest. Ophth. Vis. Sci. 2002, 43, 1–8. [Google Scholar]
- Economou, M.A.; All-Ericsson, C.; Bykov, V.; Girnita, L.; Bartolazzi, A.; Larsson, O.; Seregard, S. Receptors for the liver synthesized growth factors IGF-1 and HGF/SF in uveal melanoma: Intercorrelation and prognostic implications. Invest. Ophth. Vis. Sci. 2005, 46, 4372–4375. [Google Scholar] [CrossRef] [PubMed]
- Von Manstein, V.; Yang, C.M.; Richter, D.; Delis, N.; Vafaizadeh, V.; Groner, B. Resistance of cancer cells to targeted therapies through the activation of compensating signaling loops. Curr. Signal Transd. Ther. 2013, 8, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Ramcharan, R.; Aleksic, T.; Kamdoum, W.P.; Gao, S.; Pfister, S.X.; Tanner, J.; Bridges, E.; Asher, R.; Watson, A.J.; Margison, G.P.; et al. IGF-1R inhibition induces schedule-dependent sensitization of human melanoma to temozolomide. Oncotarget 2015, 6, 39877–39890. [Google Scholar] [CrossRef] [PubMed]
- Herkert, B.; Kauffmann, A.; Molle, S.; Schnell, C.; Ferrat, T.; Voshol, H.; Juengert, J.; Erasimus, H.; Marszalek, G.; Kazic-Legueux, M.; et al. Maximizing the efficacy of MAPK-targeted treatment in PTENLOF/BRAF(MUT) melanoma through PI3K and IGF1R inhibition. Cancer Res. 2016, 76, 390–402. [Google Scholar] [CrossRef] [PubMed]
- Flashner-Abramson, E.; Klein, S.; Mullin, G.; Shoshan, E.; Song, R.; Shir, A.; Langut, Y.; Bar-Eli, M.; Reuveni, H.; Levitzki, A. Targeting melanoma with NT157 by blocking Stat3 and IGF1R signaling. Oncogene 2016, 35, 2675–2680. [Google Scholar] [CrossRef] [PubMed]
- Giaccia, A.; Siim, B.G.; Johnson, R.S. HIF-1 as a target for drug development. Nat. Rev. Drug Discov. 2003, 2, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, H.; Zhao, Y.; Zhang, X.; Liu, J.; Pan, Y.; Bai, J.; Zhang, H. Knockdown of FBXO22 inhibits melanoma cell migration, invasion and angiogenesis via the HIF-1alpha/VEGF pathway. Invest. New Drugs 2019, 1–9. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Riveiro-Falkenbach, E.; Rodriguez-Peralto, J.L.; Nagore, E.; Martorell-Calatayud, A.; Campos-Rodriguez, F.; Farre, R.; Hernandez Blasco, L.; Banuls Roca, J.; Chiner Vives, E.; et al. A prospective multicenter cohort study of cutaneous melanoma: Clinical staging and potential associations with HIF-1alpha and VEGF expressions. Melanoma Res. 2017, 27, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, E.; Birchmeier, W.; Birchmeier, C.; Vande Woude, G. Targeting MET in cancer: Rationale and progress. Nat. Rev. Cancer 2012, 12, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; You, S.; Zhang, Q.; Osuka, S.; Devi, N.S.; Kaluz, S.; Ferguson, J.H.; Yang, H.; Chen, G.; Wang, B.; et al. Arylsulfonamide 64B inhibits hypoxia/HIF-induced expression of c-met and CXCR4 and reduces primary tumor growth and metastasis of uveal melanoma. Clin. Cancer Res. 2019, 25, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Gartrell, R.D.; Marks, D.K.; Hart, T.D.; Li, G.; Davari, D.R.; Wu, A.; Blake, Z.; Lu, Y.; Askin, K.N.; Monod, A.; et al. Quantitative analysis of immune infiltrates in primary melanoma. Cancer Immunol. Res. 2018, 6, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Ladanyi, A.; Mohos, A.; Somlai, B.; Liszkay, G.; Gilde, K.; Fejos, Z.; Gandi, I.; Timar, J. FOXP3(+) Cell density in primary tumor has no prognostic impact in patients with cutaneous malignant melanoma. Pathol. Oncol. Res. 2010, 16, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Hillen, F.; Baeten, C.I.M.; van de Winkel, A.; Creytens, D.; van der Schaft, D.W.J.; Winnepenninckx, V.; Griffioen, A.W. Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol. Immun. 2008, 57, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Miracco, C.; Mourmouras, V.; Biagioli, M.; Rubegni, P.; Mannucci, S.; Monciatti, I.; Cosci, E.; Tosi, P.; Luzi, P. Utility of tumour-infiltrating CD25(+)FOXP3(+) regulatory T cell evaluation in predicting local recurrence in vertical growth phase cutaneous melanoma. Oncol. Rep. 2007, 18, 1115–1122. [Google Scholar] [PubMed]
- Santegoets, S.J.A.M.; Dijkgraaf, E.M.; Battaglia, A.; Beckhove, P.; Britten, C.M.; Gallimore, A.; Godkin, A.; Gouttefangeas, C.; de Gruijl, T.D.; Koenen, H.J.P.M.; et al. Monitoring regulatory T cells in clinical samples: Consensus on an essential marker set and gating strategy for regulatory T cell analysis by flow cytometry. Cancer Immunol. Immun. 2015, 64, 1271–1286. [Google Scholar] [CrossRef] [PubMed]
- Falleni, M.; Savi, F.; Tosi, D.; Agape, E.; Cerri, A.; Moneghini, L.; Bulfamante, G.P. M1 and M2 macrophages’ clinicopathological significance in cutaneous melanoma. Melanoma Res. 2017, 27, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Whelchel, J.C.; Farah, S.E.; Mclean, I.W.; Burnier, M.N. Immunohistochemistry of infiltrating lymphocytes in uveal malignant-melanoma. Invest. Ophth. Vis. Sci. 1993, 34, 2603–2606. [Google Scholar]
- Gezgin, G.; Dogrusoz, M.; van Essen, T.H.; Kroes, W.G.M.; Luyten, G.P.M.; van der Velden, P.A.; Walter, V.; Verdijk, R.M.; van Hall, T.; van der Burg, S.H.; et al. Genetic evolution of uveal melanoma guides the development of an inflammatory microenvironment. Cancer Immunol. Immunother. 2017, 66, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, T.H.; van Pelt, S.I.; Bronkhorst, I.H.G.; Versluis, M.; Nemati, F.; Laurent, C.; Luyten, G.P.M.; van Hall, T.; van den Elsen, P.J.; Decaudin, D.; et al. Upregulation of HLA expression in primary uveal melanoma by infiltrating leukocytes. PLoS ONE 2016, 11, e0164292. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, I.H.G.; Ly, L.V.; Jordanova, E.S.; Vrolijk, J.; Versluis, M.; Luyten, G.P.M.; Jager, M.J. Detection of M2-macrophages in uveal melanoma and relation with survival. Invest. Ophth. Vis. Sci. 2011, 52, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, I.H.G.; Vu, T.H.K.; Jordanova, E.S.; Luyten, G.P.M.; van der Burg, S.H.; Jager, M.J. Different subsets of tumor-infiltrating lymphocytes correlate with macrophage influx and monosomy 3 in uveal melanoma. Invest. Ophth. Vis. Sci. 2012, 53, 5370–5378. [Google Scholar] [CrossRef] [PubMed]
- Mougiakakos, D.; Johansson, C.C.; Trocme, E.; All-Ericsson, C.; Economou, M.A.; Larsson, O.; Seregard, S.; Kiessling, R. Intratumoral forkhead box P3-positive regulatory T cells predict poor survival in cyclooxygenase-2 positive uveal melanoma. Cancer 2010, 116, 2224–2233. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Fu, Y.X. Tumor-infiltrating T lymphocytes: Friends or foes? Lab. Invest. 2006, 86, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Melief, S.M.; Visconti, V.V.; Visser, M.; van Diepen, M.; Kapiteijn, E.H.W.; van den Berg, J.H.; Haanen, J.B.A.G.; Smit, V.T.H.B.M.; Oosting, J.; van der Burg, S.H.; et al. Long-term survival and clinical benefit from adoptive T-cell transfer in stage IV melanoma patients is determined by a four-parameter tumor immune signature. Cancer Immunol. Res. 2017, 5, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.P.; Salama, A.K. Updates in therapy for advanced melanoma. Cancers Basel 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Eng. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Ott, P.A.; Hodi, F.S.; Robert, C. CTLA-4 and PD-1/PD-L1 blockade: New immunotherapeutic modalities with durable clinical benefit in melanoma patients. Clin. Cancer Res. 2013, 19, 5300–5309. [Google Scholar] [CrossRef]
- Luke, J.J.; Callahan, M.K.; Postow, M.A.; Romano, E.; Ramaiya, N.; Bluth, M.; Giobbie-Hurder, A.; Lawrence, D.P.; Ibrahim, N.; Ott, P.A.; et al. Clinical activity of ipilimumab for metastatic uveal melanoma: A retrospective review of the Dana-Farber Cancer Institute, Massachusetts General Hospital, Memorial Sloan-Kettering Cancer Center, and University Hospital of Lausanne experience. Cancer 2013, 119, 3687–3695. [Google Scholar] [CrossRef] [PubMed]
- Kelderman, S.; van der Kooij, M.K.; van den Eertwegh, A.J.; Soetekouw, P.M.; Jansen, R.L.; van den Brom, R.R.; Hospers, G.A.; Haanen, J.B.; Kapiteijn, E.; Blank, C.U. Ipilimumab in pretreated metastastic uveal melanoma patients. Results of the Dutch working group on immunotherapy of oncology (WIN-O). Acta Oncol. 2013, 52, 1786–1788. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, L.; Vaubel, J.; Mohr, P.; Hauschild, A.; Utikal, J.; Simon, J.; Garbe, C.; Herbst, R.; Enk, A.; Kampgen, E.; et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS ONE 2015, 10, e0118564. [Google Scholar] [CrossRef] [PubMed]
- Maio, M.; Danielli, R.; Chiarion-Sileni, V.; Pigozzo, J.; Parmiani, G.; Ridolfi, R.; De, R.F.; Del, V.M.; Di, G.L.; Queirolo, P.; et al. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann. Oncol. 2013, 24, 2911–2915. [Google Scholar] [CrossRef] [PubMed]
- Kottschade, L.A.; McWilliams, R.R.; Markovic, S.N.; Block, M.S.; Villasboas Bisneto, J.; Pham, A.Q.; Esplin, B.L.; Dronca, R.S. The use of pembrolizumab for the treatment of metastatic uveal melanoma. Melanoma Res. 2016, 26, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.; Enk, A.; Gutzmer, R.; Hassel, J.C. Anti-PD-1 antibodies in metastatic uveal melanoma: A treatment option? Cancer Med. 2017, 6, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Algazi, A.P.; Tsai, K.K.; Shoushtari, A.N.; Munhoz, R.R.; Eroglu, Z.; Piulats, J.M.; Ott, P.A.; Johnson, D.B.; Hwang, J.; Daud, A.I.; et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer 2016, 122, 3344–3353. [Google Scholar] [CrossRef] [PubMed]
- Heppt, M.V.; Heinzerling, L.; Kahler, K.C.; Forschner, A.; Kirchberger, M.C.; Loquai, C.; Meissner, M.; Meier, F.; Terheyden, P.; Schell, B.; et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur. J. Cancer 2017, 82, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Van der Kooij, M.K.; Joosse, A.; Speetjens, F.M.; Hospers, G.A.; Bisschop, C.; de Groot, J.W.; Koornstra, R.; Blank, C.U.; Kapiteijn, E. Anti-PD1 treatment in metastatic uveal melanoma in the Netherlands. Acta Oncol. 2017, 56, 101–103. [Google Scholar] [CrossRef]
- Samstein, R.M.; Lee, C.H.; Shoushtari, A.N.; Hellmann, M.D.; Shen, R.; Janjigian, Y.Y.; Barron, D.A.; Zehir, A.; Jordan, E.J.; Omuro, A.; et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019, 51, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.P.; Kim, I.K.; Esmaeli, B.; Amin-Mansour, A.; Treacy, D.J.; Carter, S.L.; Hodis, E.; Wagle, N.; Seepo, S.; Yu, X.; et al. Systematic genomic and translational efficiency studies of uveal melanoma. PLoS ONE 2017, 12, e0178189. [Google Scholar] [CrossRef] [PubMed]
- Kalaora, S.; Wolf, Y.; Feferman, T.; Barnea, E.; Greenstein, E.; Reshef, D.; Tirosh, I.; Reuben, A.; Patkar, S.; Levy, R.; et al. Combined analysis of antigen presentation and t-cell recognition reveals restricted immune responses in melanoma. Cancer Discov. 2018, 8, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Ahmadzadeh, M.; Lu, Y.C.; Gros, A.; Turcotte, S.; Robbins, P.F.; Gartner, J.J.; Zheng, Z.; Li, Y.F.; Ray, S.; et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science 2015, 350, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Petaccia de Macedo, M.; Reuben, A.; Forget, M.A.; Haymaker, C.; Bernatchez, C.; Spencer, C.N.; Gopalakrishnan, V.; Reddy, S.; Cooper, Z.A.; et al. Parallel profiling of immune infiltrate subsets in uveal melanoma versus cutaneous melanoma unveils similarities and differences: A pilot study. Oncoimmunology 2017, 6, e1321187. [Google Scholar] [CrossRef]
- Gros, A.; Robbins, P.F.; Yao, X.; Li, Y.F.; Turcotte, S.; Tran, E.; Wunderlich, J.R.; Mixon, A.; Farid, S.; Dudley, M.E.; et al. PD-1 identifies the patient-specific CD8(+) tumor-reactive repertoire infiltrating human tumors. J. Clin. Investig. 2014, 124, 2246–2259. [Google Scholar] [CrossRef]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-regulation of PD-L1, IDO, and T-regs in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef]
- Komatsubara, K.M.; Gartrell, R.D.; Bayan, C.-A.; Pradhan, J.S.; Hasan, S.S.; Hart, T.D.; Borgardus, M.; Lu, Y.; Marks, D.K.; Yang, J.; et al. Characterization and spatial localization of the tumor immune microenvironment in metastatic uveal melanoma. J. Clin. Oncol. 2018, 36, 9570. [Google Scholar] [CrossRef]
- Chandran, S.S.; Somerville, R.P.T.; Yang, J.C.; Sherry, R.M.; Klebanoff, C.A.; Goff, S.L.; Wunderlich, J.R.; Danforth, D.N.; Zlott, D.; Paria, B.C.; et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: A single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 2017, 18, 792–802. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).