The Efficacy and Safety of (Neo)Adjuvant Therapy for Gastric Cancer: A Network Meta-analysis
Abstract
1. Introduction
2. Results
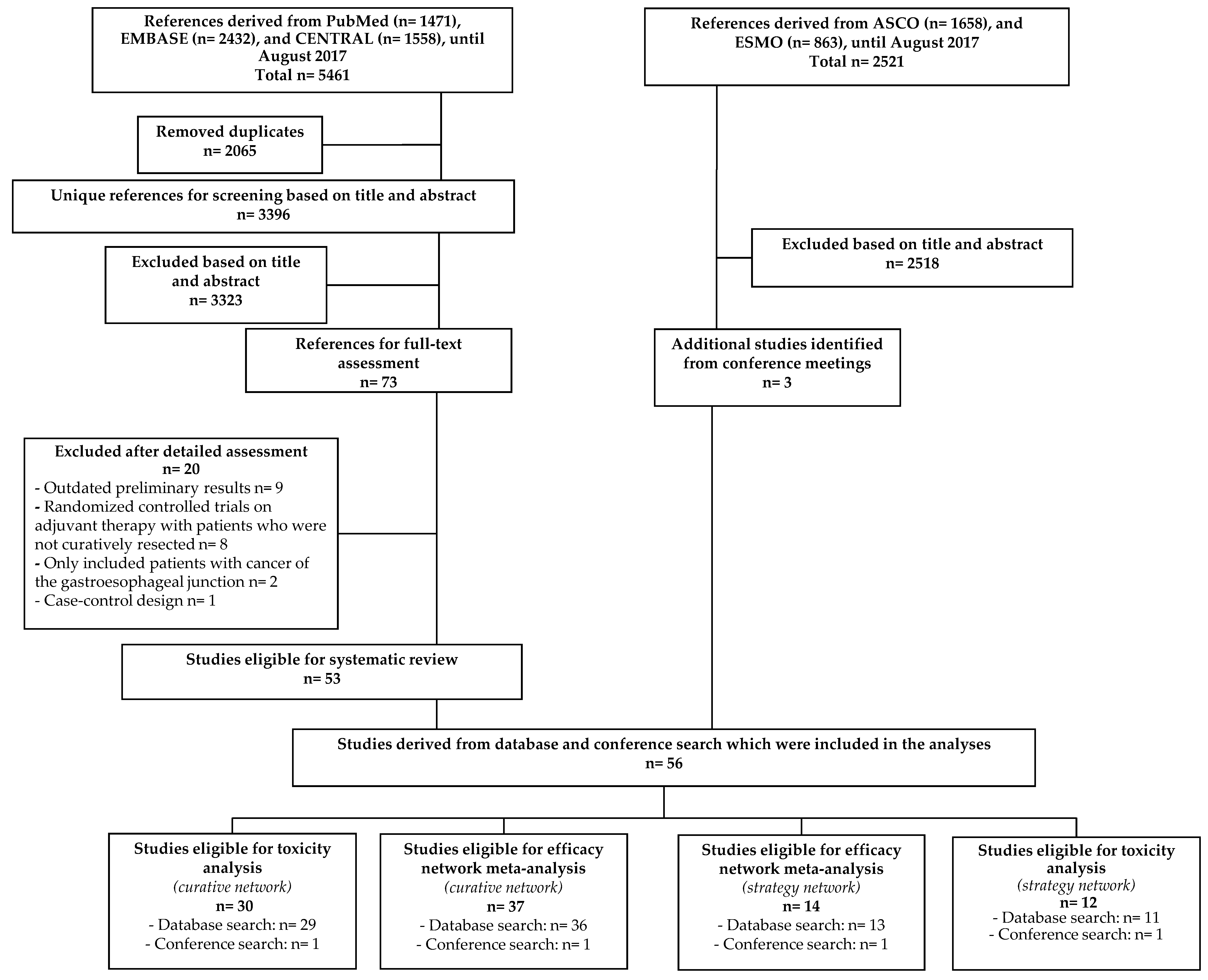
2.1. Description of the Included Studies
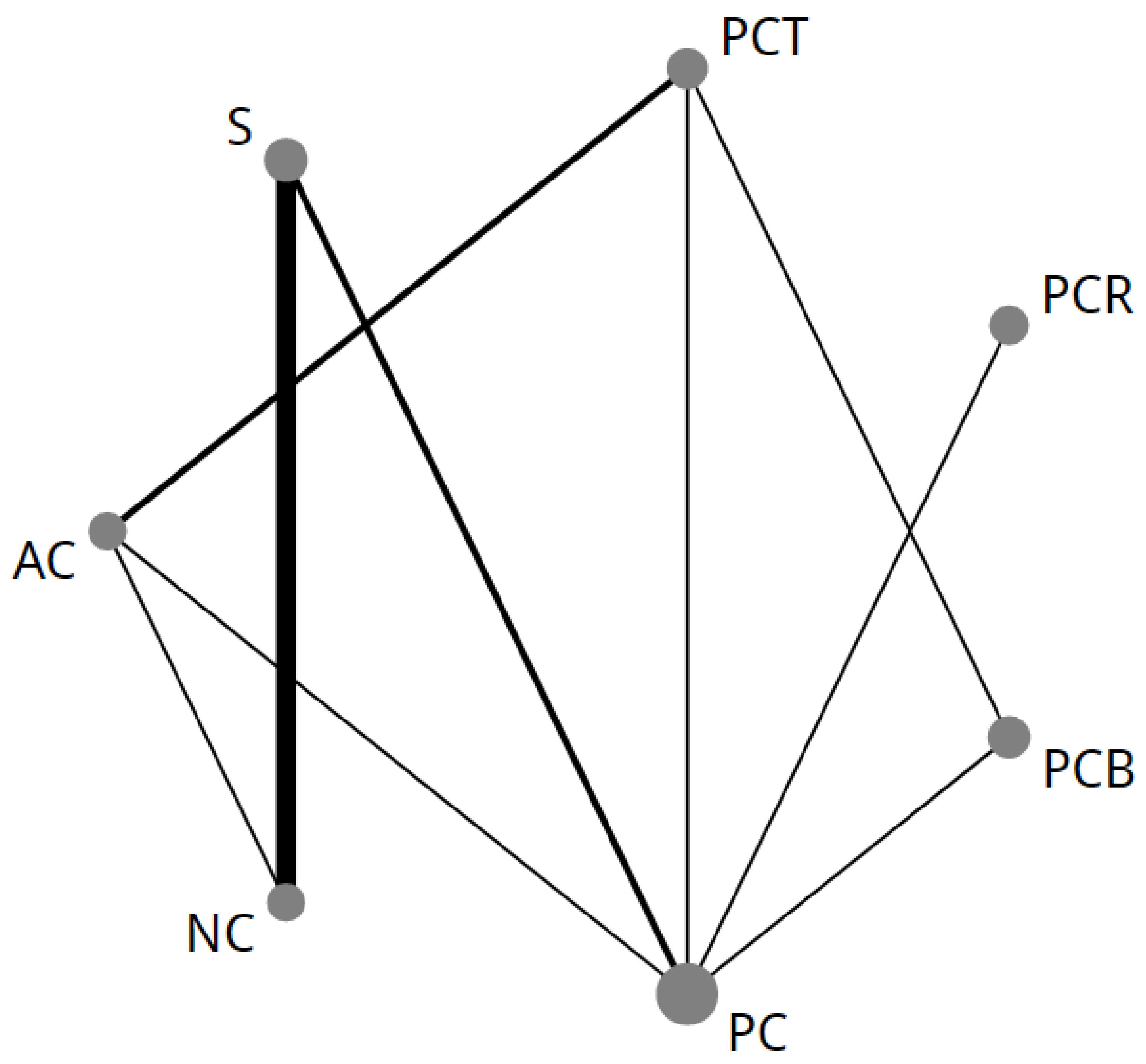
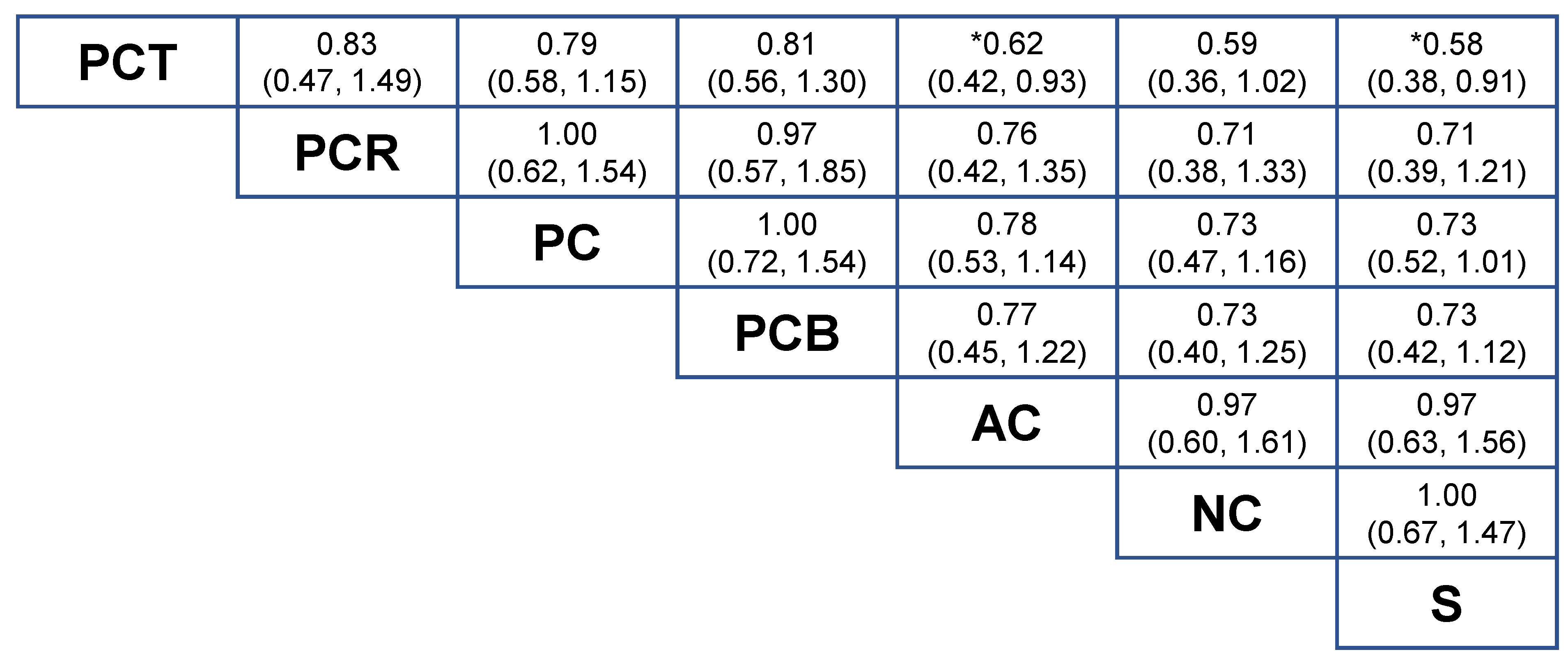
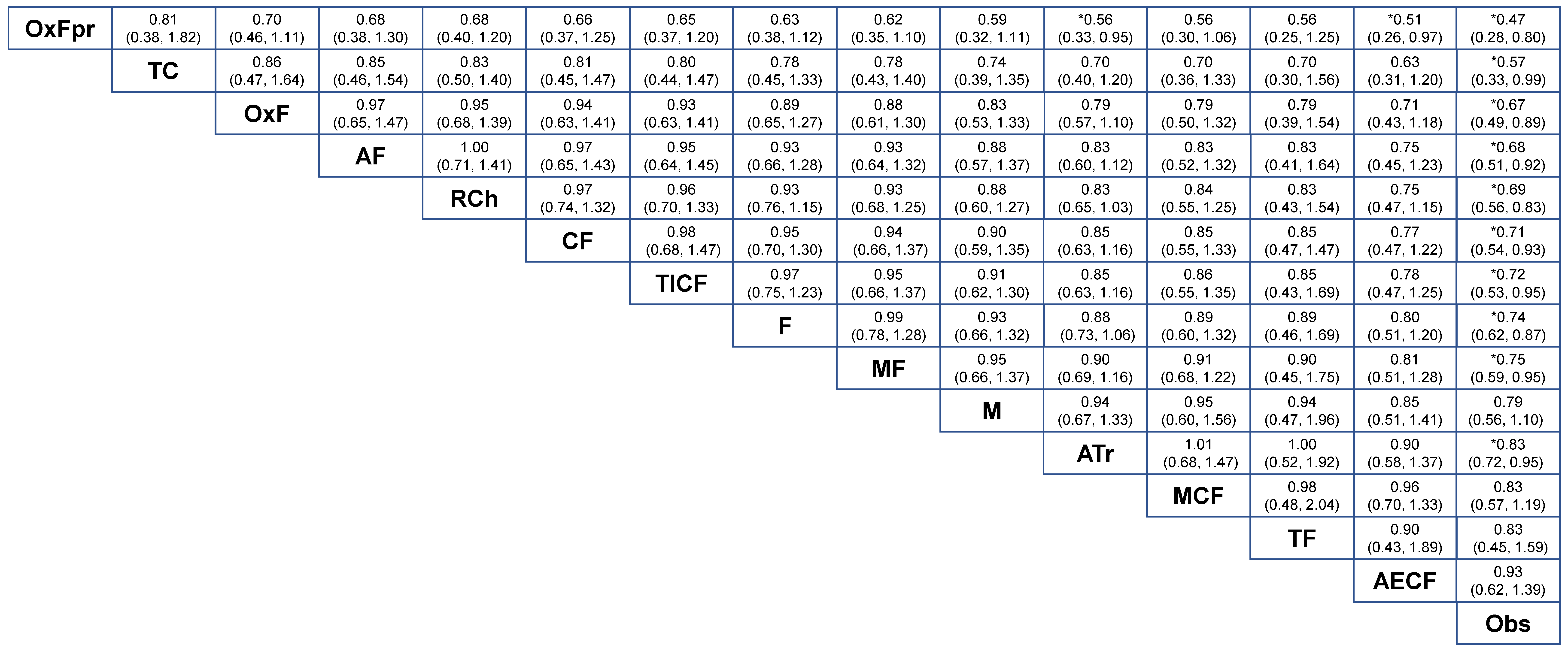
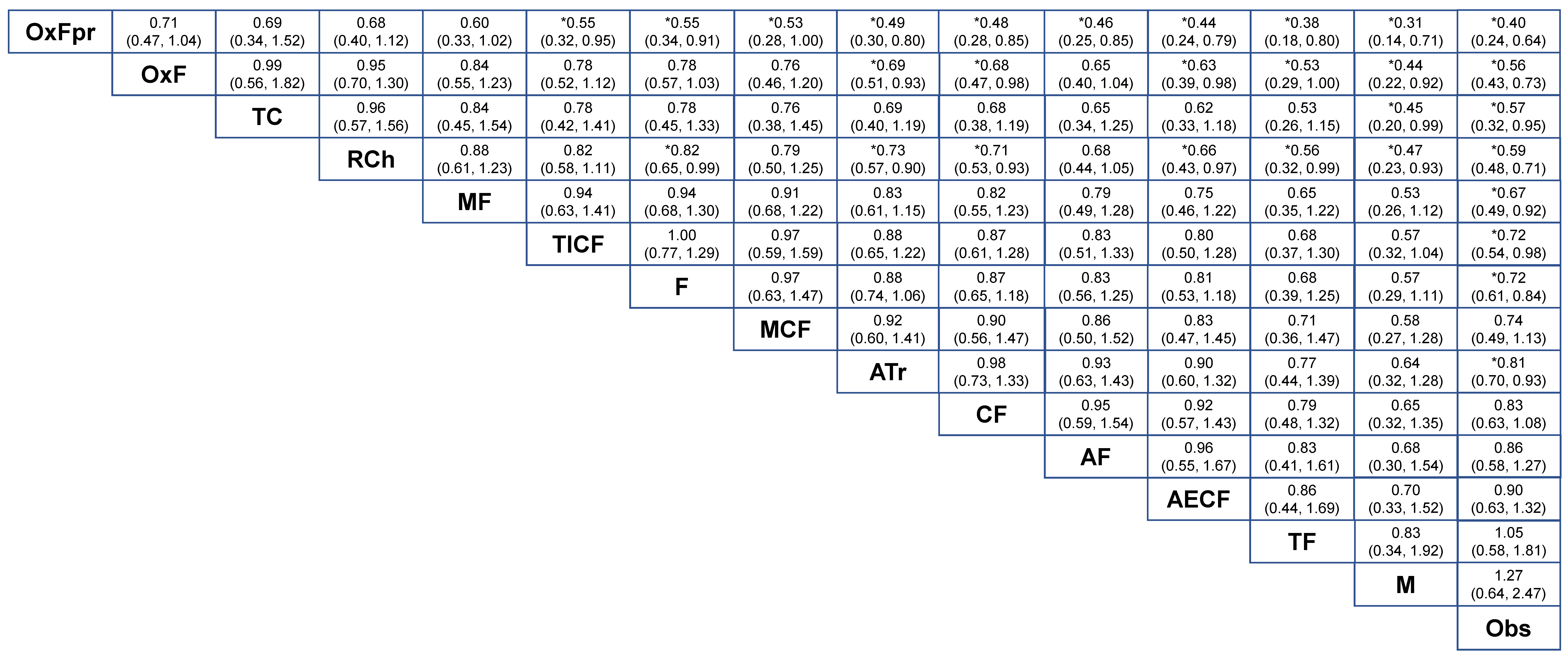
2.2. NMA-1 Comparing Different Treatment Strategies
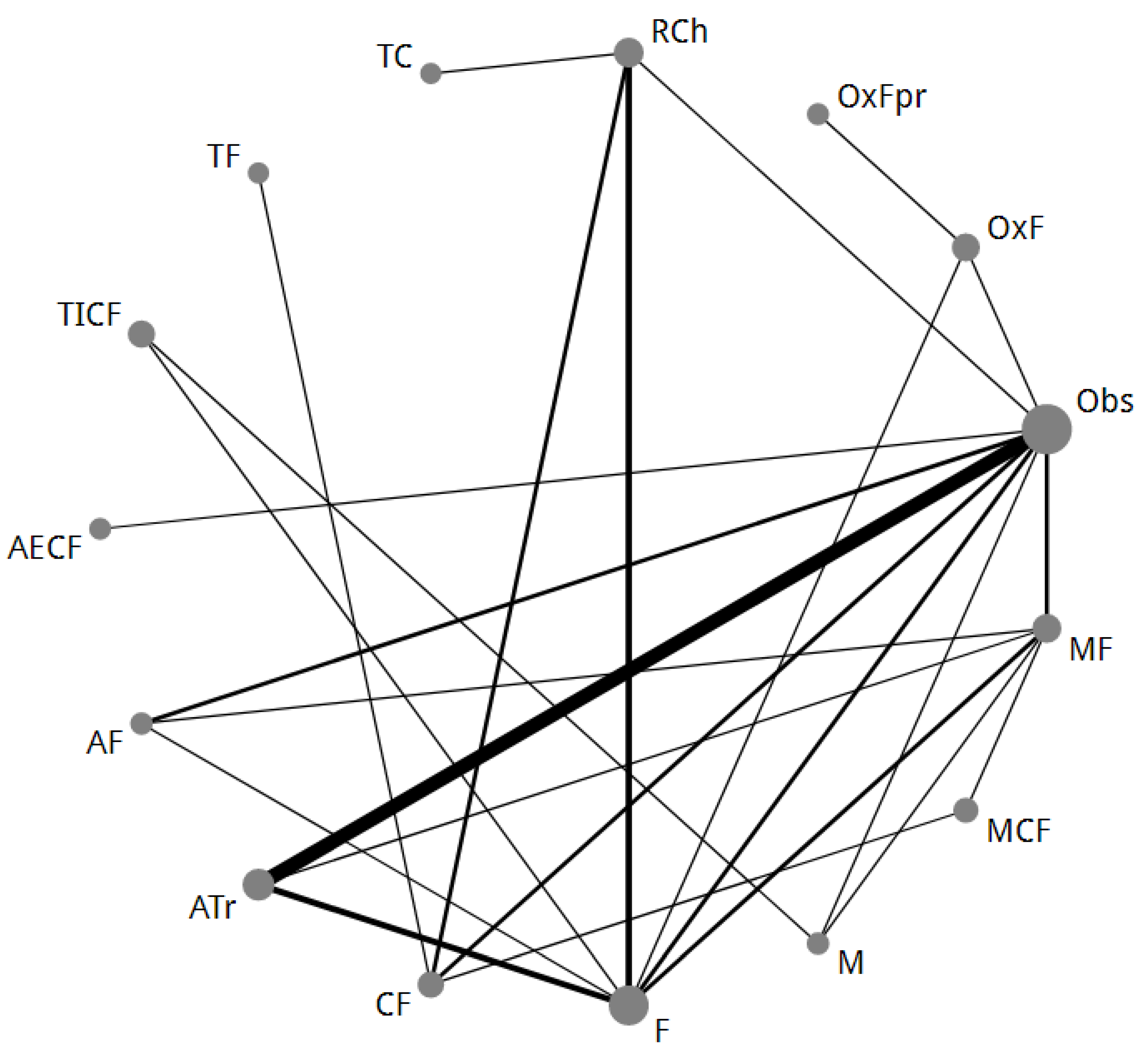
2.3. NMA-2 Comparing Adjuvant Regimens after Curative Resection
2.4. Network Consistency and Sensitivity Analyses
2.5. Toxicity and Surgical Complications
3. Discussion
4. Methods
4.1. Protocol
4.2. Literature Search
4.3. Study Selection
- (1)
- Prospective phase II or III randomized controlled trials.
- (2)
- Patients with pathologically proven gastric adenocarcinoma stage I, II and III (T1–4, N1–3, M0).
- (3)
- The treatment of patients with gastric cancer was with curative intent.
- (4)
- Patients were treated with one or more of the following intravenous or oral cytotoxic agents; fluoropyrimidine (F; either 5-fluorouracil [5-FU], capecitabine [Cap], S-1, tegafur/uracil [UFT], tegafur, or doxifluridine). Platinum-based compounds (cisplatin [C] and oxaliplatin [Ox]). Taxanes (T; either paclitaxel, or docetaxel) or anthracyclines (A; either epirubicin, or doxorubicin). Irinotecan based regimens (I), etoposide (E) and mitomycin C (M) or methotrexate (MTX).
- (5)
- Patients treated with radiotherapy combined with one or more cytotoxic agents (RCh).
- (6)
- Patients treated with targeted agents.
4.4. Data Extraction and Quality Assessment
4.5. Statistical Analysis
- (1)
- A network comparing different treatment strategies (NMA-1).
- (2)
- A network comparing different adjuvant treatment modalities after curative resection (NMA-2).
4.6. Merging of Treatment Groups
- (1)
- In a preliminary network meta-analysis (NMA) without merging of different neoadjuvant, perioperative or adjuvant regimens, there was no significant difference between the separate original treatment regimens.
- (2)
- Taxane-based perioperative chemotherapy was kept separate from standard anthracycline-based perioperative chemotherapy due to statistically significant direct evidence for superiority of taxane-based chemotherapy provided by the recently presented results of the FLOT-4 study [4].
- (3)
- Bevacizumab combined with perioperative chemotherapy (PCB) was kept as a separate node in the network. The ST03 trial did not show any survival benefit in favor of PCB compared to perioperative anthracycline-based chemotherapy [12]. To establish if this is also the case compared to taxane-based perioperative chemotherapy, PCB was analyzed separately from perioperative chemotherapy.
4.7. Sensitivity Analysis and Assessment of Inconsistency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D.; Committee, E.G. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Al-Batran, S.-E.; Homann, N.; Schmalenberg, H.; Kopp, H.-G.; Haag, G.M.; Luley, K.B.; Schmiegel, W.H.; Folprecht, G.; Probst, S.; Prasnikar, N.; et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. J. Clin. Oncol. 2017, 35, 4004. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Smalley, S.R.; Benedetti, J.K.; Haller, D.G.; Hundahl, S.A.; Estes, N.C.; Ajani, J.A.; Gunderson, L.L.; Goldman, B.; Martenson, J.A.; Jessup, J.M.; et al. Updated analysis of SWOG-directed intergroup study 0116: A phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J. Clin. Oncol. 2012, 30, 2327–2333. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Gastric Cancer Clinical Practice Guideline Version 1.2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf (accessed on 4 December 2018).
- Cipriani, A.; Barbui, C.; Rizzo, C.; Salanti, G. What is a multiple treatments meta-analysis? Epidemiol Psychiatr. Sci. 2012, 21, 151–153. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ades, A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 2004, 23, 3105–3124. [Google Scholar] [CrossRef] [PubMed]
- Tsuburaya, A.; Yoshida, K.; Kobayashi, M.; Yoshino, S.; Takahashi, M.; Takiguchi, N.; Tanabe, K.; Takahashi, N.; Imamura, H.; Tatsumoto, N.; et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): A phase 3 factorial randomised controlled trial. Lancet Oncol. 2014, 15, 886–893. [Google Scholar] [CrossRef]
- Ychou, M.; Boige, V.; Pignon, J.P.; Conroy, T.; Bouche, O.; Lebreton, G.; Ducourtieux, M.; Bedenne, L.; Fabre, J.M.; Saint-Aubert, B.; et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J. Clin. Oncol. 2011, 29, 1715–1721. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Stenning, S.P.; Smyth, E.C.; Okines, A.F.; Allum, W.H.; Rowley, S.; Stevenson, L.; Grabsch, H.I.; Alderson, D.; Crosby, T.; et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): Primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol. 2017, 18, 357–370. [Google Scholar] [CrossRef]
- Verheij, M.; Cats, A.; Jansen Edwin, P.M.; Van Grieken Nicole, C.T.; Aaronson Neil, K.; Boot, H.; Lind Pehr, A.; Meershoek-Klein Kranenbarg, E.; Nordsmark, M.; Putter, H.; et al. A multicenter randomized phase III trial of neo-adjuvant chemotherapy followed by surgery and chemotherapy or by surgery and chemoradiotherapy in resectable gastric cancer: First results from the CRITICS study. Ann. Oncol. 2016, 27, ii140. [Google Scholar] [CrossRef]
- Nio, Y.; Koike, M.; Omori, H.; Hashimoto, K.; Itakura, M.; Yano, S.; Higami, T.; Maruyama, R. A randomized consent design trial of neoadjuvant chemotherapy with tegafur plus uracil (UFT) for gastric cancer—A single institute study. Anticancer Res. 2004, 24, 1879–1887. [Google Scholar] [PubMed]
- Ma, J.; Yao, S.; Li, X.S.; Kang, H.R.; Yao, F.F.; Du, N. Neoadjuvant Therapy of DOF Regimen Plus Bevacizumab Can Increase Surgical Resection Ratein Locally Advanced Gastric Cancer: A Randomized, Controlled Study. Medicine 2015, 94, e1489. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.B.; Ge, H.E.; Bai, X.Y.; Zhang, W.; Zhang, Y.Y.; Wang, J.; Li, X.; Xing, L.P.; Guo, S.H.; Wang, Z.Y. Effect of neoadjuvant chemotherapy combined with hyperthermic intraperitoneal perfusion chemotherapy on advanced gastric cancer. Exp. Ther. 2014, 7, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.J.; Shi, Y.R.; Liu, F.R.; Ma, S.Q.; Ma, F.Y. A clinical study of paclitaxel combined with FOLFOX4 regimen as neoadjuvant chemotherapy for advanced gastric cancer. Zhonghua Wei Chang. Wai Ke Za Zhi 2010, 13, 664–667. [Google Scholar]
- Imano, M.; Itoh, T.; Satou, T.; Sogo, Y.; Hirai, H.; Kato, H.; Yasuda, A.; Peng, Y.F.; Shinkai, M.; Yasuda, T.; et al. Prospective randomized trial of short-term neoadjuvant chemotherapy for advanced gastric cancer. Eur. J. Surg. Oncol. 2010, 36, 963–968. [Google Scholar] [CrossRef]
- Schuhmacher, C.; Gretschel, S.; Lordick, F.; Reichardt, P.; Hohenberger, W.; Eisenberger, C.F.; Haag, C.; Mauer, M.E.; Hasan, B.; Welch, J.; et al. Neoadjuvant chemotherapy compared with surgery alone for locally advanced cancer of the stomach and cardia: European Organisation for Research and Treatment of Cancer randomized trial 40954. J. Clin. Oncol. 2010, 28, 5210–5218. [Google Scholar] [CrossRef]
- Zhao, W.H.; Wang, S.F.; Ding, W.; Sheng, J.M.; Ma, Z.M.; Teng, L.S.; Wang, M.; Wu, F.S.; Luo, B. Apoptosis induced by preoperative oral 5′-DFUR administration in gastric adenocarcinoma and its mechanism of action. World J. Gastroenterol. 2006, 12, 1356–1361. [Google Scholar] [CrossRef]
- Hartgrink, H.H.; van de Velde, C.J.; Putter, H.; Songun, I.; Tesselaar, M.E.; Kranenbarg, E.K.; de Vries, J.E.; Wils, J.A.; van der Bijl, J.; van Krieken, J.H.; et al. Neo-adjuvant chemotherapy for operable gastric cancer: Long term results of the Dutch randomised FAMTX trial. Eur. J. Surg. Oncol. 2004, 30, 643–649. [Google Scholar] [CrossRef]
- Fazio, N.; Biffi, R.; Maibach, R.; Hayoz, S.; Thierstein, S.; Brauchli, P.; Bernhard, J.; Stupp, R.; Andreoni, B.; Renne, G.; et al. Preoperative versus postoperative docetaxel-cisplatin-fluorouracil (TCF) chemotherapy in locally advanced resectable gastric carcinoma: 10-year follow-up of the SAKK 43/99 phase III trial. Ann. Oncol. 2016, 27, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Blackstone, E.H.; Rusch, V.W. 7th edition of the AJCC Cancer Staging Manual: Esophagus and esophagogastric junction. Ann. Surg. Oncol. 2010, 17, 1721–1724. [Google Scholar] [CrossRef]
- Neri, B.; Cini, G.; Andreoli, F.; Boffi, B.; Francesconi, D.; Mazzanti, R.; Medi, F.; Mercatelli, A.; Romano, S.; Siliani, L.; et al. Randomized trial of adjuvant chemotherapy versus control after curative resection for gastric cancer: 5-year follow-up. Br. J. Cancer 2001, 84, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Krook, J.E.; O’Connell, M.J.; Wieand, H.S.; Beart, R.W., Jr.; Leigh, J.E.; Kugler, J.W.; Foley, J.F.; Pfeifle, D.M.; Twito, D.I. A prospective, randomized evaluation of intensive-course 5-fluorouracil plus doxorubicin as surgical adjuvant chemotherapy for resected gastric cancer. Cancer 1991, 67, 2454–2458. [Google Scholar] [CrossRef]
- Kulig, J.; Kolodziejczyk, P.; Sierzega, M.; Bobrzynski, L.; Jedrys, J.; Popiela, T.; Dadan, J.; Drews, M.; Jeziorski, A.; Krawczyk, M.; et al. Adjuvant chemotherapy with etoposide, adriamycin and cisplatin compared with surgery alone in the treatment of gastric cancer: A phase III randomized, multicenter, clinical trial. Oncology 2010, 78, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Di Costanzo, F.; Gasperoni, S.; Manzione, L.; Bisagni, G.; Labianca, R.; Bravi, S.; Cortesi, E.; Carlini, P.; Bracci, R.; Tomao, S.; et al. Adjuvant chemotherapy in completely resected gastric cancer: A randomized phase III trial conducted by GOIRC. J. Natl. Cancer Inst. 2008, 100, 388–398. [Google Scholar] [CrossRef] [PubMed]
- De Vita, F.; Giuliani, F.; Orditura, M.; Maiello, E.; Galizia, G.; Di Martino, N.; Montemurro, F.; Carteni, G.; Manzione, L.; Romito, S.; et al. Adjuvant chemotherapy with epirubicin, leucovorin, 5-fluorouracil and etoposide regimen in resected gastric cancer patients: A randomized phase III trial by the Gruppo Oncologico Italia Meridionale (GOIM 9602 Study). Ann. Oncol. 2007, 18, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Tentes, A.A.; Markakidis, S.K.; Karanikiotis, C.; Fiska, A.; Tentes, I.K.; Manolopoulos, V.G.; Dimitriou, T. Intraarterial chemotherapy as an adjuvant treatment in locally advanced gastric cancer. Langenbecks Arch. Surg. 2006, 391, 124–129. [Google Scholar] [CrossRef]
- Tsavaris, N.; Tentas, K.; Kosmidis, P.; Mylonakis, N.; Sakelaropoulos, N.; Kosmas, C.; Lisaios, B.; Soumilas, A.; Mandrekas, D.; Tsetis, A.; et al. A randomized trial comparing adjuvant fluorouracil, epirubicin, and mitomycin with no treatment in operable gastric cancer. Chemotherapy 1996, 42, 220–226. [Google Scholar] [CrossRef]
- Lise, M.; Nitti, D.; Marchet, A.; Sahmoud, T.; Buyse, M.; Duez, N.; Fiorentino, M.; Dos Santos, J.G.; Labianca, R.; Rougier, P.; et al. Final results of a phase III clinical trial of adjuvant chemotherapy with the modified fluorouracil, doxorubicin, and mitomycin regimen in resectable gastric cancer. J. Clin. Oncol. 1995, 13, 2757–2763. [Google Scholar] [CrossRef]
- Coombes, R.C.; Schein, P.S.; Chilvers, C.E.; Wils, J.; Beretta, G.; Bliss, J.M.; Rutten, A.; Amadori, D.; Cortes-Funes, H.; Villar-Grimalt, A. A randomized trial comparing adjuvant fluorouracil, doxorubicin, and mitomycin with no treatment in operable gastric cancer. International Collaborative Cancer Group. J. Clin. Oncol. 1990, 8, 1362–1369. [Google Scholar] [CrossRef] [PubMed]
- Bajetta, E.; Buzzoni, R.; Mariani, L.; Beretta, E.; Bozzetti, F.; Bordogna, G.; Aitini, E.; Fava, S.; Schieppati, G.; Pinotti, G.; et al. Adjuvant chemotherapy in gastric cancer: 5-year results of a randomised study by the Italian Trials in Medical Oncology (ITMO) Group. Ann. Oncol. 2002, 13, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Cascinu, S.; Labianca, R.; Barone, C.; Santoro, A.; Carnaghi, C.; Cassano, A.; Beretta, G.D.; Catalano, V.; Bertetto, O.; Barni, S.; et al. Adjuvant treatment of high-risk, radically resected gastric cancer patients with 5-fluorouracil, leucovorin, cisplatin, and epidoxorubicin in a randomized controlled trial. J. Natl. Cancer Inst. 2007, 99, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Kim, S.Y.; Shin, I.; Cho, K.S.; Joo, H.Z.; Yoon, C. Randomized Phase III Trial of Cisplatin, Epirubicin, Leucovorin, 5-Fluorouracil (PELF) Combination versus 5-fluorouracil Alone as Adjuvant Chemotherapy in Curative Resected Stage III Gastric Cancer. Cancer Res. Treat. 2004, 36, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Tsujinaka, T.; Shiozaki, H.; Inoue, M.; Furukawa, H.; Hiratsuka, M.; Kikkawa, N.; Takami, M.; Suzuki, T.; Monden, M. Evaluation of effectiveness of chemotherapy in patients with gastric cancer after curative resection. Int. J. Clin. Oncol. 2000, 5, 372–379. [Google Scholar] [CrossRef]
- Chang, H.M.; Jung, K.H.; Kim, T.Y.; Kim, W.S.; Yang, H.K.; Lee, K.U.; Choe, K.J.; Heo, D.S.; Bang, Y.J.; Kim, N.K. A phase III randomized trial of 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil and mitomycin C versus 5-fluorouracil alone in curatively resected gastric cancer. Ann. Oncol. 2002, 13, 1779–1785. [Google Scholar] [CrossRef] [PubMed]
- Bouche, O.; Ychou, M.; Burtin, P.; Bedenne, L.; Ducreux, M.; Lebreton, G.; Baulieux, J.; Nordlinger, B.; Martin, C.; Seitz, J.F.; et al. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the FFCD randomized phase III trial (8801). Ann. Oncol. 2005, 16, 1488–1497. [Google Scholar] [CrossRef]
- Chipponi, J.; Huguier, M.; Pezet, D.; Basso, N.; Hay, J.M.; Quandalle, P.; Jaeck, D.; Fagniez, P.L.; Gainant, A. Randomized trial of adjuvant chemotherapy after curative resection for gastric cancer. Am. J. Surg. 2004, 187, 440–445. [Google Scholar] [CrossRef]
- Sasako, M.; Sakuramoto, S.; Katai, H.; Kinoshita, T.; Furukawa, H.; Yamaguchi, T.; Nashimoto, A.; Fujii, M.; Nakajima, T.; Ohashi, Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J. Clin. Oncol. 2011, 29, 4387–4393. [Google Scholar] [CrossRef]
- Nakajima, T.; Kinoshita, T.; Nashimoto, A.; Sairenji, M.; Yamaguchi, T.; Sakamoto, J.; Fujiya, T.; Inada, T.; Sasako, M.; Ohashi, Y.; et al. Randomized controlled trial of adjuvant uracil-tegafur versus surgery alone for serosa-negative, locally advanced gastric cancer. Br. J. Surg. 2007, 94, 1468–1476. [Google Scholar] [CrossRef]
- Grau, J.J.; Estape, J.; Alcobendas, F.; Pera, C.; Daniels, M.; Teres, J. Positive results of adjuvant mitomycin-C in resected gastric cancer: A randomised trial on 134 patients. Eur. J. Cancer 1993, 29A, 340–342. [Google Scholar] [CrossRef]
- Cirera, L.; Balil, A.; Batiste-Alentorn, E.; Tusquets, I.; Cardona, T.; Arcusa, A.; Jolis, L.; Saigi, E.; Guasch, I.; Badia, A.; et al. Randomized clinical trial of adjuvant mitomycin plus tegafur in patients with resected stage III gastric cancer. J. Clin. Oncol. 1999, 17, 3810–3815. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.P.; Kwon, O.J.; Oh, S.T.; Yang, H.K. Results of surgery on 6589 gastric cancer patients and immunochemosurgery as the best treatment of advanced gastric cancer. Ann. Surg. 1992, 216, 269–278; discussion 278–269. [Google Scholar] [CrossRef] [PubMed]
- Grau, J.J.; Estapé, J.; Fuster, J.; Filella, X.; Visa, J.; Terés, J.; Soler, G.; Albiol, S.; García-Valdecasas, J.C.; Grande, L.; et al. Randomized trial of adjuvant chemotherapy with mitomycin plus ftorafur versus mitomycin alone in resected locally advanced gastric cancer. J. Clin. Oncol. 1998, 16, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.K.; Chang, H.M.; Yook, J.H.; Ryu, M.H.; Park, I.; Min, Y.J.; Zang, D.Y.; Kim, G.Y.; Yang, D.H.; Jang, S.J.; et al. Adjuvant chemotherapy for gastric cancer: A randomised phase 3 trial of mitomycin-C plus either short-term doxifluridine or long-term doxifluridine plus cisplatin after curative D2 gastrectomy (AMC0201). Br. J. Cancer 2013, 108, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, S.; Shimizu, N.; Kaminishi, M. Type-oriented intraoperative and adjuvant chemotherapy and survival after curative resection of advanced gastric cancer. World J. Surg. 1999, 23, 284–291; discussion 291–282. [Google Scholar] [CrossRef]
- Noh, S.H.; Park, S.R.; Yang, H.K.; Chung, H.C.; Chung, I.J.; Kim, S.W.; Kim, H.H.; Choi, J.H.; Kim, H.K.; Yu, W.; et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1389–1396. [Google Scholar] [CrossRef]
- Zhang, X.L.; Shi, H.J.; Cui, S.Z.; Tang, Y.Q.; Ba, M.C. Prospective, randomized trial comparing 5-FU/LV with or without oxaliplatin as adjuvant treatment following curative resection of gastric adenocarcinoma. Eur. J. Surg. Oncol. 2011, 37, 466–472, Erratum in 2013, 39, 525. [Google Scholar] [CrossRef]
- Feng, W.M.; Tang, C.W.; Guo, H.H.; Bao, Y.; Fei, M.Y. Prolonged adjuvant capecitabine chemotherapy improved survival of stage IIIA gastric cancer after D2 gastrectomy. Biomed. Pharmacother. 2015, 72, 140–143. [Google Scholar] [CrossRef]
- Kim, T.H.; Park, S.R.; Ryu, K.W.; Kim, Y.W.; Bae, J.M.; Lee, J.H.; Choi, I.J.; Kim, Y.J.; Kim, D.Y. Phase 3 trial of postoperative chemotherapy alone versus chemoradiation therapy in stage III-IV gastric cancer treated with R0 gastrectomy and D2 lymph node dissection. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e585–e592. [Google Scholar] [CrossRef]
- Yu, C.; Yu, R.; Zhu, W.; Song, Y.; Li, T. Intensity-modulated radiotherapy combined with chemotherapy for the treatment of gastric cancer patients after standard D1/D2 surgery. J. Cancer Res. Clin. Oncol. 2012, 138, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.G.; Xua, D.F.; Pu, J.; Zong, C.D.; Li, T.; Tao, G.Z.; Ji, F.Z.; Zhou, X.L.; Han, J.H.; Wang, C.S.; et al. A randomized, controlled, multicenter study comparing intensity-modulated radiotherapy plus concurrent chemotherapy with chemotherapy alone in gastric cancer patients with D2 resection. Radiother. Oncol. 2012, 104, 361–366. [Google Scholar] [CrossRef]
- Park, S.H.; Sohn, T.S.; Lee, J.; Lim, D.H.; Hong, M.E.; Kim, K.M.; Sohn, I.; Jung, S.H.; Choi, M.G.; Lee, J.H.; et al. Phase III trial to compare adjuvant chemotherapy with capecitabine and cisplatin versus concurrent chemoradiotherapy in gastric cancer: Final report of the adjuvant chemoradiotherapy in stomach tumors trial, including survival and subset analyses. J. Clin. Oncol. 2015, 33, 3130–3136. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.C.; Kim, M.C.; Kim, K.H.; Jang, J.S.; Oh, S.Y.; Kim, S.H.; Kwon, K.A.; Lee, S.; Lee, H.S.; Kim, H.J. Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection. Asia Pac. J. Clin. Oncol. 2010, 6, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Bamias, A.; Karina, M.; Papakostas, P.; Kostopoulos, I.; Bobos, M.; Vourli, G.; Samantas, E.; Christodoulou, C.; Pentheroudakis, G.; Pectasides, D.; et al. A randomized phase III study of adjuvant platinum/docetaxel chemotherapy with or without radiation therapy in patients with gastric cancer. Cancer Chemother. Pharmacol. 2010, 65, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Jung, M.; Kim, H.S.; Jung, I.; Shin, D.B.; Kang, S.Y.; Zang, D.Y.; Kim, K.H.; Lee, M.H.; Kim, B.S.; et al. An update on the randomized phase III POST trial: S-1 based doublet as an adjuvant chemotherapy for curatively resected stage III gastric cancer. J. Clin. Oncol. 2016, 34, 4042. [Google Scholar] [CrossRef]
- Bajetta, E.; Floriani, I.; Di Bartolomeo, M.; Labianca, R.; Falcone, A.; Di Costanzo, F.; Comella, G.; Amadori, D.; Pinto, C.; Carlomagno, C.; et al. Randomized trial on adjuvant treatment with FOLFIRI followed by docetaxel and cisplatin versus 5-fluorouracil and folinic acid for radically resected gastric cancer. Ann. Oncol. 2014, 25, 1373–1378. [Google Scholar] [CrossRef]
- Di Bartolomeo, M.; Buzzoni, R.; Mariani, L.; Ferrario, E.; Katia, D.; Gevorgyan, A.; Zilembo, N.; Bordonaro, R.; Bochicchio, A.M.; Massidda, B.; et al. Feasibility of sequential therapy with FOLFIRI followed by docetaxel/cisplatin in patients with radically resected gastric adenocarcinoma. A randomized phase III trial. Oncology 2006, 71, 341–346, Erratum in 2007, 73, 406. [Google Scholar] [CrossRef]
- Zhao, J.H.; Gao, P.; Song, Y.X.; Sun, J.X.; Chen, X.W.; Ma, B.; Yang, Y.C.; Wang, Z.N. Which is better for gastric cancer patients, perioperative or adjuvant chemotherapy: A meta-analysis. BMC Cancer 2016, 16, 631. [Google Scholar] [CrossRef]
- Zhou, M.L.; Kang, M.; Li, G.C.; Guo, X.M.; Zhang, Z. Postoperative chemoradiotherapy versus chemotherapy for R0 resected gastric cancer with D2 lymph node dissection: An up-to-date meta-analysis. World J. Surg. Oncol. 2016, 14, 209. [Google Scholar] [CrossRef]
- Paoletti, X.; Oba, K.; Burzykowski, T.; Michiels, S. Benefit of Adjuvant Chemotherapy for Resectable Gastric Cancer A Meta-analysis. JAMA 2010, 303, 1729–1737. [Google Scholar]
- Ronellenfitsch, U.; Schwarzbach, M.; Hofheinz, R.; Kienle, P.; Kieser, M.; Slanger, T.E.; Jensen, K.; GE Adenocarcinoma Meta-analysis Group. Perioperative chemo(radio)therapy versus primary surgery for resectable adenocarcinoma of the stomach, gastroesophageal junction, and lower esophagus. Cochrane Database Syst. Rev. 2013, 31, CD008107. [Google Scholar] [CrossRef]
- Bang, Y.J.; Kim, Y.W.; Yang, H.K.; Chung, H.C.; Park, Y.K.; Lee, K.H.; Lee, K.W.; Kim, Y.H.; Noh, S.I.; Cho, J.Y.; et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012, 379, 315–321. [Google Scholar] [CrossRef]
- Sakuramoto, S.; Sasako, M.; Yamaguchi, T.; Kinoshita, T.; Fujii, M.; Nashimoto, A.; Furukawa, H.; Nakajima, T.; Ohashi, Y.; Imamura, H.; et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N. Engl. J. Med. 2007, 357, 1810–1820, Erratum in 2008, 358, 1977. [Google Scholar] [CrossRef] [PubMed]
- Ter Veer, E.; Haj Mohammad, N.; van Valkenhoef, G.; Ngai, L.L.; Mali, R.M.A.; Anderegg, M.C.; van Oijen, M.G.H.; van Laarhoven, H.W.M. The Efficacy and Safety of First-line Chemotherapy in Advanced Esophagogastric Cancer: A Network Meta-analysis. J. Natl. Cancer Inst. 2016, 108. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.D.; Syn, N.L.; Moehler, M.; Grothe, W.; Yong, W.P.; Tai, B.C.; Ho, J.; Unverzagt, S. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2017, 8, CD004064. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Wang, F.; Xu, R.H. Platinum-based versus non-platinum-based chemotherapy as first line treatment of inoperable, advanced gastric adenocarcinoma: A meta-analysis. PLoS ONE 2013, 8, e68974. [Google Scholar] [CrossRef]
- Petrelli, F.; Zaniboni, A.; Coinu, A.; Cabiddu, M.; Ghilardi, M.; Sgroi, G.; Barni, S. Cisplatin or not in advanced gastric cancer: A systematic review and meta-analysis. PLoS ONE 2013, 8, e83022. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Hartmann, J.T.; Probst, S.; Schmalenberg, H.; Hollerbach, S.; Hofheinz, R.; Rethwisch, V.; Seipelt, G.; Homann, N.; Wilhelm, G.; et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: A study of the Arbeitsgemeinschaft Internistische Onkologie. J. Clin. Oncol. 2008, 26, 1435–1442. [Google Scholar] [CrossRef]
- Cunningham, D.; Starling, N.; Rao, S.; Iveson, T.; Nicolson, M.; Coxon, F.; Middleton, G.; Daniel, F.; Oates, J.; Norman, A.R.; et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N. Engl. J. Med. 2008, 358, 36–46. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, Y.; Xu, Y.; Zhu, Y.; Huang, J.; Liu, Y.; Zhao, L.; Li, Z.; Liu, H.; Wang, Q.L.; et al. Comparative effectiveness and safety between oxaliplatin-based and cisplatin-based therapy in advanced gastric cancer: A meta-analysis of randomized controlled trials. Oncotarget 2016, 7, 34824–34831. [Google Scholar] [CrossRef] [PubMed]
- Ter Veer, E.; Creemers, A.; de Waal, L.; van Oijen, M.G.H.; van Laarhoven, H.W.M. Comparison of cytotoxic backbones for first line trastuzumab-containing regimens in HER2-positive advanced esophagogastric cancer: A meta-analysis. Ann. Oncol. 2017, 28 (Suppl. 5), V209–V268. [Google Scholar] [CrossRef]
- Montagnani, F.; Turrisi, G.; Marinozzi, C.; Aliberti, C.; Fiorentini, G. Effectiveness and safety of oxaliplatin compared to cisplatin for advanced, unresectable gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2011, 14, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Alderson, D.; Cunningham, D.; Nankivell, M.; Blazeby, J.M.; Griffin, S.M.; Crellin, A.; Grabsch, H.I.; Langer, R.; Pritchard, S.; Okines, A.; et al. Neoadjuvant cisplatin and fluorouracil versus epirubicin, cisplatin, and capecitabine followed by resection in patients with oesophageal adenocarcinoma (UK MRC OE05): An open-label, randomised phase 3 trial. Lancet Oncol. 2017, 18, 1249–1260. [Google Scholar] [CrossRef]
- Ashraf, N.; Kim, R. Treatment of Gastric and Gastroesophageal Cancers-Do We Really Need Anthracyclines? JAMA Oncol. 2017, 3, 1172–1173. [Google Scholar] [CrossRef]
- Cats, A.; Jansen, E.P.M.; van Grieken, N.C.T.; Sikorska, K.; Lind, P.; Nordsmark, M.; Meershoek-Klein Kranenbarg, E.; Boot, H.; Trip, A.K.; Swellengrebel, H.A.M.; et al. Chemotherapy versus chemoradiotherapy after surgery and preoperative chemotherapy for resectable gastric cancer (CRITICS): An international, open-label, randomised phase 3 trial. Lancet Oncol. 2018, 19, 616–628. [Google Scholar] [CrossRef]
- Rhome, R.M.; Moshier, E.; Sarpel, U.; Ohri, N.; Mazumdar, M.; Buckstein, M.H. Predictors of Positive Margins After Definitive Resection for Gastric Adenocarcinoma and Impact of Adjuvant Therapies. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 1106–1115. [Google Scholar] [CrossRef]
- Hagen, P.; Hulshof, M.C.; Lanschot, J.J.; Steyerberg, E.W.; Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Andre, T.; Boni, C.; Mounedji-Boudiaf, L.; Navarro, M.; Tabernero, J.; Hickish, T.; Topham, C.; Zaninelli, M.; Clingan, P.; Bridgewater, J.; et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004, 350, 2343–2351. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Rice, T.W.; Patil, D.T.; Blackstone, E.H. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann. Cardiothorac. Surg. 2017, 6, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.K.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Van Valkenhoef, G.; Lu, G.; de Brock, B.; Hillege, H.; Ades, A.E.; Welton, N.J. Automating network meta-analysis. Res. Synth. Methods 2012, 3, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Gelman, A.; Rubin, D.B. Markov chain Monte Carlo methods in biostatistics. Stat. Methods Med. Res. 1996, 5, 339–355. [Google Scholar] [CrossRef] [PubMed]
- Ellis, L.M.; Bernstein, D.S.; Voest, E.E.; Berlin, J.D.; Sargent, D.; Cortazar, P.; Garrett-Mayer, E.; Herbst, R.S.; Lilenbaum, R.C.; Sima, C.; et al. American Society of Clinical Oncology perspective: Raising the bar for clinical trials by defining clinically meaningful outcomes. J. Clin. Oncol. 2014, 32, 1277–1280. [Google Scholar] [CrossRef]
- Donegan, S.; Williamson, P.; D’Alessandro, U.; Tudur Smith, C. Assessing key assumptions of network meta-analysis: A review of methods. Res. Synth. Methods 2013, 4, 291–323. [Google Scholar] [CrossRef]
| Studies | No. | Regimen | Node | Stage 1 | D2 or > LND No. (%) | Descent | Age, Median, (Range), y | Men No. (%) |
|---|---|---|---|---|---|---|---|---|
| Perioperative Chemotherapy vs. Surgery | ||||||||
| Ychou 2011 [11] | 113 | Peri + Cis + 5-FU | PC | I–IV | D2 | W | 63 (36-75) | 96 (85) |
| 111 | Surg | S | I–IV | W | 63 (38–75) | 91 (82) | ||
| Cunningham 2006 [3] | 250 | Peri + Epi + Cis + 5-FU | PC | II–III | 93 (37) | W | 62 (29–85) | 205 (82) |
| 253 | Surg | S | II–III | 96 (38) | W | 62 (23–81) | 191 (76) | |
| Perioperative Chemotherapy vs. Perioperative Chemotherapy + Bevacizumab | ||||||||
| Cunningham 2017 [12] | 533 | Peri + Epi + Cis + Cap | PC | II–III | D1+D2 | W | 63 (31–79) | 434 (82) |
| 530 | Peri + Epi + Cis + Cap + BEV | PCB | II–III | W | 64 (28–82) | 425 (80) | ||
| Perioperative Chemotherapy vs. Perioperative Chemotherapy + Radiotherapy | ||||||||
| Verheij 2016 [13] | 393 | Peri + Epi + Cis/Ox + Cap | PC | I–III | 40 (6) | W | 62 | 264 (67) |
| 395 | Peri + Epi + Cis/Ox + Cap + RT | PCR | I–III | W | 265 (67) | |||
| Perioperative Chemotherapy vs. Adjuvant Chemotherapy | ||||||||
| Nio 2004 [14] | 102 | Peri + UFT | PC | I–IV | 58 (57) | A | 64 (±12) | 70 (69) |
| 193 | UFT | AC | I–IV | 95 (49) | A | 65 (±12) | 141 (73) | |
| Perioperative Chemotherapy Taxane Based vs. Perioperative Chemotherapy | ||||||||
| Al-Batran 2017 [4] | 356 | Peri + Dtx + Ox + 5-FU/Lv | PCT | II–III | D2 | W | 62 | 530 (74) |
| 360 | Peri + Epi + Cis + 5-FU/Cap | PC | II–III | W | ||||
| Perioperative Chemotherapy Taxane Based vs. Perioperative Chemotherapy + Bevacuzimab | ||||||||
| Ma 2015 [15] | 40 | Peri + Dtx + Ox + 5-FU/Lv | PCT | II–III | 21 (53) | A | 53 * | 22 (55) |
| 40 | Peri + Dtx + Ox + 5-FU/Lv + BEV | PCB | II–III | 31 (78) | A | 55 * | 24 (60) | |
| Perioperative Chemotherapy Taxane Based vs. Adjuvant Chemotherapy | ||||||||
| Cui 2014 [16] | 48 | Peri + Ptx + Cis + Tgf | PCT | II–III | NR | A | 55 (41–69) * | 19 (40) |
| 48 | Ptx + Cis + Tgf | AC | II–III | NR | A | 56 (39–72) * | 21 (44) | |
| Qu 2010 [17] | 39 | Peri + Ptx + Ox + 5-FU/Lv | PCT | II–III | NA | A | NA | NA |
| 39 | Ptx + Ox + 5-FU/Lv | AC | II–III | NA | A | NA | NA | |
| Neoadjuvant Chemotherapy vs. Surgery | ||||||||
| Imano 2010 [18] | 16 | Neo + Cis + 5-FU | NC | II–III | 16 (100) | A | 58 (±12) | 13 (81) |
| 16 | Surg | S | II–III | 16 (100) | A | 60 (±8) | 9 (56) | |
| Schuhmacher 2010 [19] | 72 | Neo + Cis + 5-FU/Lv | NC | III–IV | 67 (96) | W | 56 (38–70) | 50 (69) |
| 72 | Surg | S | III–IV | 63 (93) | W | 58 (26–69) | 50 (69) | |
| Zhao 2006 [20] | 20 | Neo + 5-FU/Lv | NC | I–IV | NR | A | 58 (32–70) * | NR |
| 20 | Surg | S | I–IV | NR | A | NR | ||
| Hartgrink 2004 [21] | 29 | Neo + Doxo + 5-FU/Lv + MTX | NC | I–IV | D1 | W | 60 (34–75) * | 32 (54) |
| 30 | Surg | S | I–IV | W | ||||
| Neoadjuvant Chemotherapy vs. Adjuvant Chemotherapy | ||||||||
| Fazio 2016 [22] | 34 | Neo + Dtx + Cis + 5-FU | NC | I–IV | 62 (90) | W | 57 (25–75) | 23 (68) |
| 35 | Dtx + Cis + 5-FU | AC | I–IV | W | 59 (39–76) | 24 (69) | ||
| Studies | No. | Regimen | Node | Stage 1 | D2 or > LND No. (%) | Descent | Age, Median, (Range), y | Men No. (%) |
|---|---|---|---|---|---|---|---|---|
| Anthracycline + Fluoropyrimidine vs. Observation | ||||||||
| Neri 2001 [24] | 69 | Epi + 5-FU/Lv | AF | II–III | 9 (13) | W | 62 (37–73) | 50 (72.5) |
| 68 | Observation | Obs | II–III | 10 (15) | W | 64 (35–74) | 48 (70.6) | |
| Krook 1991 [25] | 61 | Doxo + 5-FU | AF | I–III | NR | W | 63 (33–77) | 47 (77) |
| 64 | Observation | Obs | I–III | NR | W | 62 (38–78) | 51 (80) | |
| Anthracycline + Doublet vs. Observation | ||||||||
| Kulig 2010 [26] | 141 | Doxo + Eto + Cis | ATr | I–III | 112 (79) | W | 61 (58–67) | 100 (71) |
| 154 | Observation | Obs | I–III | 123 (80) | W | 64 (61–66) | 111 (72) | |
| Di Costanzo 2008 [27] | 130 | Epi + Cis + 5-FU/Lv | ATr | I–III | 71 (55) | W | 59 | 79 (61) |
| 128 | Observation | Obs | I–III | 72 (56) | W | 59 | 78 (61) | |
| De Vita 2007 [28] | 112 | Epi + Eto + 5-FU/Lv | ATr | I–III | 0 | W | 63 (39–70) | 66 (59) |
| 113 | Observation | Obs | I–III | 0 | W | 62 (41–70) | 65 (58) | |
| Tentes 2006 [29] | 20 | Doxo + MMC + 5-FU | ATr | II–III | 20 (100) | W | 65 (±10) * | 14 (70) |
| 20 | Observation | Obs | II–III | 20 (100) | W | 65 (±11) * | 11 (55) | |
| Tsavaris 1996 [30] | 42 | Epi + MMC + 5-FU | ATr | III | NR | W | 53 (41–65) * | 32 (76) |
| 42 | Observation | Obs | III | NR | W | 57 (35–66) * | 25 (60) | |
| Lise 1995 [31] | 155 | Doxo + MMC + 5-FU | ATr | II–III | 84 (27) | W | <71 years | 94 (61) |
| 159 | Observation | Obs | II–III | W | <71 years | 108 (68) | ||
| Coombes 1990 [32] | 133 | Doxo + MMC + 5-FU | ATr | II–III | NR | W | 57 * | 93 (70) |
| 148 | Observation | Obs | II–III | NR | W | 57 * | 98 (68) | |
| Anthracycline + Etoposide + Cisplatin + Fluoropyrimidine vs. Observation | ||||||||
| Bajetta 2002 [33] | 135 | Doxo + Eto + Cis + 5-FU/Lv | AECF | II–III | Maj. | W | 57 (23–70) | 81 (59) |
| 136 | Observation | Obs | II–III | Maj. | W | 57 (31–70) | 93 (68) | |
| Anthracycline + Doublet vs. Fluoropyrimidine | ||||||||
| Cascinu 2007 [34] | 201 | Epi + Cis + 5-FU/Lv | ATr | II–III | 312 (79) | W | 58 | 135 (67) |
| 196 | 5FU/Lv | F | II–III | W | 59 | 120 (61) | ||
| Lee 2004 [35] | 32 | Epi + Cis + 5-FU/Lv | ATr | III | 32 (100) | A | 53 (31–61) | 13 (41) |
| 29 | 5-FU | F | III | 29 (100) | A | 52 (26–66) | 13 (45) | |
| Anthracycline + Fluoropyrimidine vs. Mitomycin C + Fluoropyrimidine vs. Fluoropyrimidine | ||||||||
| Tsujinaka 2000 [36] | 61 | Epi + 5-FU | AF | I–II | 60 (98) | A | ≤75 years | 38 (62) |
| 62 | MMC + 5-FU | MF | I–II | 61 (98) | A | ≤75 years | 44 (71) | |
| 62 | 5-FU | F | I–II | 61 (98) | A | ≤75 years | 44 (71) | |
| Anthracycline + Doublet vs. Mitomycin C + Fluoropyrimidine vs. Fluoropyrimidine | ||||||||
| Chang 2002 [37] | 131 | Doxo + MMC + 5-FU | ATr | I–III | 131 (100) | A | 51 (26–70) | 100 (76) |
| 131 | MMC + 5-FU | MF | I–III | 131 (100) | A | 54 (23–74) | 96 (73) | |
| 133 | 5-FU | F | I–III | 133 (100) | A | 53 (21–75) | 99 (74) | |
| Cisplatin + Fluoropyrimidine vs. Observation | ||||||||
| Bouche 2005 [38] | 127 | Cis + 5-FU | CF | II–III | 70 (27) | W | 60 (32–82) | 93 (73) |
| 133 | Observation | Obs | II–III | W | 62 (31–83) | 93 (70) | ||
| Chipponi 2004 [39] | 93 | Cis + 5-FU/Lv | CF | II–III | D1+D2 | W | 59 * | 58 (62) |
| 103 | Observation | Obs | II–III | W | 63 * | 71 (69) | ||
| Fluoropyrimidine vs. Observation | ||||||||
| Sasako 2011 [40] | 529 | S-1 | F | II–III | 529 (100) | A | 63 (27–80) | 367 (69) |
| 530 | Observation | Obs | II–III | 530 (100) | A | 63 (33–80) | 369 (70) | |
| Nakajima 2007 [41] | 93 | UFT | F | II–III | 93 (100) | A | 63 | 75 (70) |
| 95 | Observation | Obs | II–III | 95 (100) | A | 64 | 77 (73) | |
| Mitomycin C vs. Observation | ||||||||
| Grau 1993 [42] | 68 | MMC | M | I–III | NR | W | 56 * | 44 (65) |
| 66 | Observation | Obs | I–III | NR | W | 57 * | 44 (67) | |
| Mitomycin C + Fluoropyrimidine vs. Observation | ||||||||
| Cirera 1999 [43] | 76 | MMC + Tgf | MF | I–III | 76 (100) | W | 61 * | 52 (68) |
| 72 | Observation | Obs | I–III | 72 (100) | W | 61 * | 42 (58) | |
| Kim 1992 [44] | 77 | MMC + 5-FU | MF | III | 77 (100) | A | (30–70) | NR |
| 94 | Observation | Obs | III | 94 (100) | A | (30–70) | NR | |
| Mitomycin C + Fluoropyrimidine vs. Mitomycin C | ||||||||
| Grau 1998 [45] | 40 | MMC + Tgf | MF | I–III | D1+D2 | W | 62 (36–75) | 27 (68) |
| 45 | MMC | M | I–III | W | 63 (22–75) | 27 (60) | ||
| Mitomycin C + Cisplatin + Fluoropyrimidine vs. Mitomycin C + Fluoropyrimidine | ||||||||
| Kang 2013 [46] | 431 | MMC + Cis + 5DFUR | MCF | II–III | 431 (100) | A | 55 (20–70) | 294 (68) |
| 424 | MMC + 5DFUR | MF | II–III | 424 (100) | A | 56 (29–70) | 294 (69) | |
| Mitomycin C + Cisplatin + Fluoropyrimidine vs. Cisplatin + Fluoropyrimidine | ||||||||
| Shimoyama 1999 [47] | 12 | MMC + Cis + UFT (600 mg) | MCF | I–III | D1+D2 | A | 65 (±8) | 13 (77) |
| 17 | Cis + UFT | CF | I–III | A | 64 (±8) | 8 (67) | ||
| Oxaliplatin + Fluoropyrimidine vs. Observation | ||||||||
| Noh 2014 [48] | 520 | Ox + Cap | OxF | II–III | 520 (100) | A | 56 (±11) * | 373 (72) |
| 515 | Observation | Obs | II–III | 515 (100) | A | 56 (±11) * | 358 (70) | |
| Oxaliplatin + Fluoropyrimidine vs. Fluoropyrimidine | ||||||||
| Zhang 2011 [49] | 42 | Ox + 5-FU/Lv | OxF | II–III | 42 (100) | A | 48 | 25 (60) |
| 38 | 5-FU/Lv | F | II–III | 38 (100) | A | 54 | 24 (63) | |
| Oxaliplatin + Fluoropyrimidine Prolonged vs. Oxaliplatin + Fluoropyrimidine | ||||||||
| Feng 2015 [50] | 152 | Ox + Cap (Prolonged) | OxFPr | II–III | 152 (100) | A | 61 (±11) | 104 (67) |
| 155 | Ox + Cap | OxF | II–III | 155 (100) | A | 60 (±10) | 99 (65) | |
| Radiotherapy + Chemotherapy vs. Observation | ||||||||
| Smalley 2012 [6] | 281 | RT + 5-FU/Lv | RCh | I–III | 54 (10) | W | 60 (25–87) | 202 (72) |
| 275 | Observation | Obs | I–III | W | 59 (23–80) | 195 (71) | ||
| Radiotherapy + Chemotherapy vs. Fluoropyrimidine | ||||||||
| Kim 2012 [51] | 46 | RT + 5-FU/Lv | RCh | III | 46 (100) | A | 9> 60 | 34 (74) |
| 44 | 5-FU/Lv | F | III | 44 (100) | A | 14>60 | 25 (57) | |
| Yu 2012 [52] | 34 | RT + 5-FU/Lv | RCh | II–III | D1+D2 | A | NR | NR |
| 34 | 5-FU/Lv | F | II–III | A | NR | NR | ||
| Zhu 2012 [53] | 186 | RT + 5-FU/Lv | RCh | I–III | 205 (100) | A | 56 (38–73) | 135 (73) |
| 165 | 5-FU/Lv | F | I–III | 175 (100) | A | 59 (42–75) | 126 (76) | |
| Radiotherapy + Chemotherapy vs. Cisplatin + Fluoropyrimidine | ||||||||
| Park 2015 [54] | 230 | RT + Cis + Cap | RCh | I–III | 230 (100) | A | 56 (28–76) | 143 (62) |
| 228 | Cis + Cap | CF | I–III | 228 (100) | A | 56 (22–77) | 153 (67) | |
| Kwon 2010 [55] | 31 | RT + Cis + Cap + 5-FU | RCh | III | 31 (100) | A | 8 ≥ 60 | 21 (68) |
| 30 | Cis + 5-FU | CF | III | 30 (100) | A | 14 ≥ 60 | 23 (77) | |
| Radiotherapy + Chemotherapy vs. Taxane + Cisplatin | ||||||||
| Bamias 2010 [56] | 72 | RT + Dtx + Cis/Car | RCh | II–III | D0+D1+D2 | W | 63 (32–75) | 48 (67) |
| 71 | Dtx + Cis/Car | TC | II–III | W | 62 (41–79) | 52 (73) | ||
| Taxane + Fluoropyrimidine vs. Cisplatin + Fluoropyrimidine | ||||||||
| Lee 2016 [57] | 75 | Dtx + S-1 | TF | III | 75 (100) | A | NR | NR |
| 78 | Cis + S-1 | CF | III | 78 (100) | A | NR | NR | |
| Taxane + Irinotecan + Cisplatin + Fluoropyrimidine vs. Fluoropyrimidine or Mitomycin C | ||||||||
| Bajetta 2014 [58] | 562 | Dtx + IRI + Cis + 5-FU/Lv | TICF | II–III | 796 (72) | W | ≤75 years | NR |
| 538 | 5-FU/Lv | F | II–III | W | ≤75 years | NR | ||
| Di Bartolomeo 2006 [59] | 85 | Dtx + IRI + Cis + 5-FU/Lv | TICF | II–III | 66 (77) | W | 10 ≥ 70 | 60 (71) |
| 81 | MMC | M | II–III | 62 (76) | W | 8 ≥ 70 | 55 (68) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van den Ende, T.; ter Veer, E.; Machiels, M.; Mali, R.M.A.; Abe Nijenhuis, F.A.; de Waal, L.; Laarman, M.; Gisbertz, S.S.; Hulshof, M.C.C.M.; van Oijen, M.G.H.; et al. The Efficacy and Safety of (Neo)Adjuvant Therapy for Gastric Cancer: A Network Meta-analysis. Cancers 2019, 11, 80. https://doi.org/10.3390/cancers11010080
van den Ende T, ter Veer E, Machiels M, Mali RMA, Abe Nijenhuis FA, de Waal L, Laarman M, Gisbertz SS, Hulshof MCCM, van Oijen MGH, et al. The Efficacy and Safety of (Neo)Adjuvant Therapy for Gastric Cancer: A Network Meta-analysis. Cancers. 2019; 11(1):80. https://doi.org/10.3390/cancers11010080
Chicago/Turabian Stylevan den Ende, Tom, Emil ter Veer, Mélanie Machiels, Rosa M. A. Mali, Frank A. Abe Nijenhuis, Laura de Waal, Marety Laarman, Suzanne S. Gisbertz, Maarten C. C. M. Hulshof, Martijn G. H. van Oijen, and et al. 2019. "The Efficacy and Safety of (Neo)Adjuvant Therapy for Gastric Cancer: A Network Meta-analysis" Cancers 11, no. 1: 80. https://doi.org/10.3390/cancers11010080
APA Stylevan den Ende, T., ter Veer, E., Machiels, M., Mali, R. M. A., Abe Nijenhuis, F. A., de Waal, L., Laarman, M., Gisbertz, S. S., Hulshof, M. C. C. M., van Oijen, M. G. H., & van Laarhoven, H. W. M. (2019). The Efficacy and Safety of (Neo)Adjuvant Therapy for Gastric Cancer: A Network Meta-analysis. Cancers, 11(1), 80. https://doi.org/10.3390/cancers11010080





