Abstract
In this work, the effect of aqueous dispersions of graphene oxide (GO) and nanosized graphene oxide (nGO) on the enzymatic polymerization of polyaniline (PANI) was studied. The enzymatic polymerization of PANI was carried out in aqueous medium using toluenesulfonic acid (TSA) as the dopant, horseradish peroxidase (HRP) as the catalyst, and hydrogen peroxide (H2O2) as the oxidant, using 1.0, 2.5, and 5.0 wt% of GO and nGO. The morphology of PANI-GO/nGO composites was studied by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Further characterization was performed by thermogravimetric analysis (TGA) and spectroscopic techniques such as ultraviolet–visible (UV–Vis), Fourier-transform infrared (FTIR), Raman and X-ray photoelectronics (XPS). SEM images showed that during enzymatic polymerization, PANI completely covers the GO/nGO sheets. Furthermore, physicochemical results confirmed the production of a hybrid PANI-GO/nGO material with Van der Waals-type interactions between the oxygen-based functional groups of GO and the secondary amino bond (-NH-) of PANI. Also, cyclic voltammetry experiments were carried out in situ during the polymerization of PANI-GO/nGO films. The electrochemical response of PANI-GO/nGO composites reflects two broad oxidation peaks around 300 mV and 500 mV during anodic scanning, with reversible oxidation during cathodic scanning. Classical molecular dynamics simulations were used to understand the mechanism of the composite film’s growth.
1. Introduction
The synthesis of conductive polymers with graphene derivatives to create new composites is widely studied due to its numerous applications such as supercapacitors [1,2], biosensing [3,4], optoelectronics [5,6], and water treatment [7,8], among others. Polyaniline (PANI) is one of the most interesting conductive polymers due to its easy synthesis, high environmental stability, and good electrical conductivity [9,10]. PANI is usually synthesized by oxidation in strong acidic media; it might have different oxidation degrees and its transition from one form to another requires an oxidation or reduction reaction [11].
On the other hand, graphene is an electrically conductive material with a 2D shape that can be obtained through chemical synthesis [12], mechanical exfoliation [13], and chemical vapor deposition [14]. Nevertheless, all these methods result in a limited quantity of bulk graphene, and there are still no methods to produce high-quality graphene in large quantities. Graphene oxide (GO) is prepared by the oxidation and exfoliation of graphite using strong chemical oxidizers [15] and is typically used as a precursor of a graphene-like material called reduced graphene oxide. GO has a thin-sheet shape with many functional groups such as carboxyl, hydroxyl, and epoxy, which makes it water dispersible; however, these functional groups can be reduced to achieve a graphene-like material that is electrically conductive [16]. In this context, the lateral size of GO sheets is a critical parameter that modulates the reactivity and properties of GO-based composites, so its control and characterization are essential for the rational design of materials and applications [17,18]. Usually, smaller GO sheets have a higher proportion of edges and defects, which increases the density of reactive oxygenated sites and favors chemical and physical interactions with polymeric matrices, biomolecules, and functionalizing agents, therefore improving its dispersion and interfacial adhesion in nanocomposites [19]. On the other hand, GO sheets with very large size might be difficult to disperse and usually require additional treatments such as fragmentation and stabilization [20].
The synthesis of PANI and GO nanocomposites has been developed using various methods, mainly chemical aniline oxidation [21,22]. Enzymatic oxidative polymerization of aniline is an environmentally friendly alternative method of aniline polymerization [23], with the main advantages being that it is a biosynthetic method that utilizes plant-derived enzymes and is classified as ecological, recyclable, and a non-toxic catalyst. This method is well suited to be combined with GO, since due to its functional groups, it has been reported that GO acts as an enzyme immobilizer [24,25], which is an advantage because it improves the stability, catalytic activity, and reusability of enzymes.
On the other hand, it has been recently reported that PANI, mainly modified with graphene materials, shows great potential for use in water treatment due to its affinity for contaminants and low cost [7]. Different preparation and doping methods have been evaluated, along with the properties of the resulting material. In this article, we report the enzymatic polymerization of PANI in aqueous medium using toluenesulfonic acid (TSA) as the dopant, horseradish peroxidase (HRP) as the catalyst, and hydrogen peroxide (H2O2) as the oxidant, adding 1.0, 2.5, and 5.0 wt% of GO and nGO as enzyme immobilizers. Morphological and physicochemical characteristics were evaluated and compared between the two GO sizes. The growth mechanism of the composite film was studied with the aid of classical molecular dynamics simulations.
2. Materials and Methods
Potassium permanganate 99%, p-toluenesulfonic acid monohydrate (TSA) 98.5%, aniline 99%, and graphite flakes were purchased from Sigma-Aldrich, Toluca, Mexico. Horseradish peroxidase (HRP) 150–250 U/mg was acquired from Sigma-Life Science, St. Louis, MO, USA. Ethylenediaminetetraacetic acid (EDTA) ≥ 99.5% and Hydrogen peroxide water solution 30 wt% were procured from Fluka Analytical, Toluca, Mexico. Hydrochloric acid 37 wt% was obtained by Fermont, Monterrey, Mexico. Sulfuric acid 96.6% was supplied from CTR Scientific, Monterrey, Mexico. All reagents were of analytical grade and were used without further purification.
Graphene oxide was prepared as reported by Marcano et al. [26] with modifications, followed by prolonged sonication in the case of nGO. The polymerization reaction was carried out in a three-necked flask under a nitrogen atmosphere and with the temperature controlled at 5 °C. A total of 456 mg of TSA, 20 mL of deionized water, and 215 µL of aniline were mixed for 20 min. Subsequently, 2 mL of 1.2 wt% HRP solution at pH = 7 was added, followed by 2 mL of 3.75 wt% H2O2 with the aid of an injection pump over a period of 1 h, and then the solution was allowed to react for 2 h. In enzymatic polymerizations, TSA is used to avoid the rapid inactivation of the enzyme by hydrochloric acid. After the reaction time, the solution was dialyzed for purification. For PANI-GO/nGO composites, aliquots of 1.0, 2.5, and 5.0 wt% of GO/nGO were added before HRP and stirred for 10 min. Additionally, a PANI film and PANI-GO/nGO composite films were produced by introducing a gold-coated quartz crystal into the reactor, which was fitted to a QCM200 Quartz Crystal Microbalance from Stanford Research Systems. The film deposition was monitored using an open-circuit potential technique with an EPSILON potentiostat and a AgCl/Cl reference electrode. Table 1 shows the labels assigned to the synthesized composites.

Table 1.
Labels of PANI and GO/nGO composites.
The morphology of PANI, GO/nGO, and the synthesized composites was evaluated by SEM and TEM using FEI model Nova Nano SEM 200 and FEI model Titan G280300 equipment, Eindhoven, The Netherlands, respectively. The physicochemical properties of the obtained materials were analyzed using UV–Vis spectroscopy on a Lambda 35 PerkinElmer instrument, Shelton, CT, USA, in absorption mode. FTIR spectroscopy was conducted on a Nicolet 6700, Madison, WI, USA, in transmission mode with a KBr pellet. XPS analysis was performed using the Al K (alpha) line on an Axis-Ultra, Kratos, Manchester, UK. Raman spectroscopy was conducted with Renishaw inVia Raman equipment, Gloucestershire, UK, with two different lasers. On the other hand, thermogravimetric analyses were performed on Rigaku TGA/DTA 8120 equipment, Tokyo, Japan, at a heating rate of 10 °C/min with an air flow of 300 mL/min. Likewise, a cyclic voltammetry characterization was performed in a 0.2 N HCl monomer-free electrolyte solution using a platinum wire as the counter electrode and a 20 mV/s scan rate. Classical molecular dynamics simulations of the film’s growth were carried out using OpenMM Ver. 8.0 software and the details are provided in the Supporting Information file.
3. Results
3.1. PANI-GO/nGO Composites Preparation
The following results analyze the composites containing 2.5 wt% of GO and nGO, which are representative samples of the synthesized composites. Figure 1 presents the morphology of the PANI-GO2.5 (Figure 1a,b) and PANI-nGO2.5 (Figure 1c,d) composites. In all the micrographs, we can observe apparently thicker GO/nGO sheets, compared to Figure S1a,b. An increase in the thickness of the sheets is evidenced by the formation of folds (indicated by arrows), this suggests that PANI forms a thin film on the GO/nGO sheets due to the amphiphilic nature of the monomer [27]. Furthermore, mainly in the case of the PANI-GO2.5 composite, it can be observed that part of the PANI formed particle agglomerates and adhered to the surface of the GO sheets, an effect that does not occur with nGO, most likely due to the increase in surface area that the latter presents. However, one noticeable effect that occurs with nGO is that the PANI does not coat each of the nanosheets separately but instead creates a shell that encapsulates an uncontrolled amount of them.
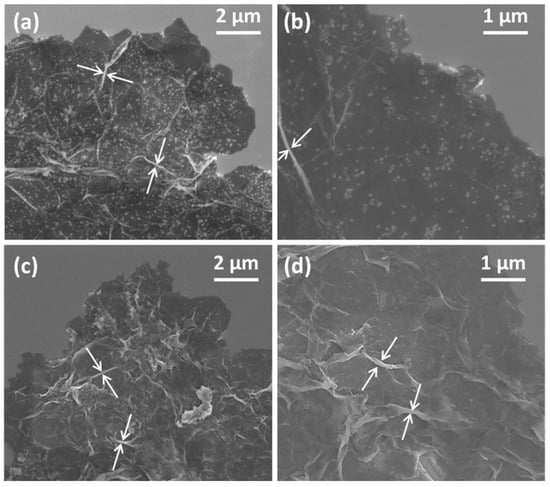
Figure 1.
SEM micrographs of (a,b) PANI-GO2.5, and (c,d) PANI-nGO2.5. The white arrows indicate the wrinkles on the nanocomposite sheets that formed during drying.
TEM micrographs of PANI-GO2.5 (Figure 2a,c) and PANI-nGO2.5 (Figure 2b,d) are shown below. From these images, we can also assume that polymerization occurs on the GO and nGO sheets (Figure 2a,b). Furthermore, in the high-resolution TEM micrographs, the sheets show greater stability to the electron beam of the equipment, confirming that thicker sheets are obtained compared to unrecovered GO. The difference in particle size of the agglomerates seen in the SEM micrographs (Figure 1) can also be observed where the PANI-GO2.5 composite presents larger particles. Similar morphology of fully coated GO sheets with polyaniline has been obtained by chemical polymerization of aniline in the presence of GO sheets [21].
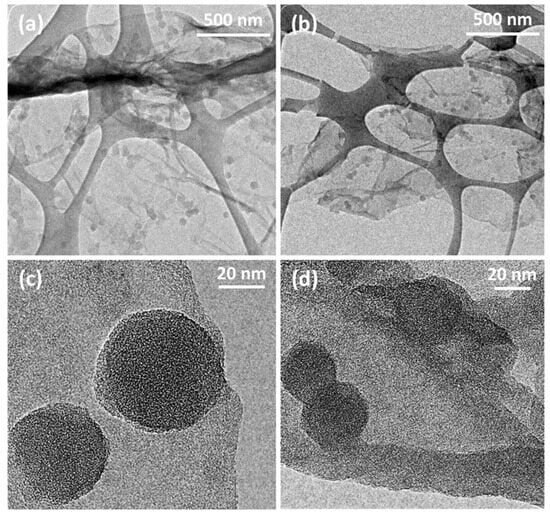
Figure 2.
TEM micrographs of (a,c) PANI-GO2.5 and (b,d) PANI-nGO2.5, mounted on a copper grid and coated with a discontinuous silicon film.
Figure 3 shows the SEM images of the films formed in situ on gold-coated quartz crystals fitted to a QCM200 Quartz Crystal balance. Figure 3a,d show the PANI film where a granular morphology with colloidal particles adhered to the surface is observed. On the other hand, Figure 3b,c show the PANI-GO2.5 and PANI-nGO2.5 films, respectively, where they are distinguished GO and nGO sheets, as well as PANI colloidal particles adhered to the graphene material. Furthermore, Figure 3d–f show a difference in size of the PANI synthesized alone and when it appears adhered to the GO and nGO sheets, where smaller adhered particles can be distinguished when nGO is used in the synthesis, as discussed in the previous morphology results.
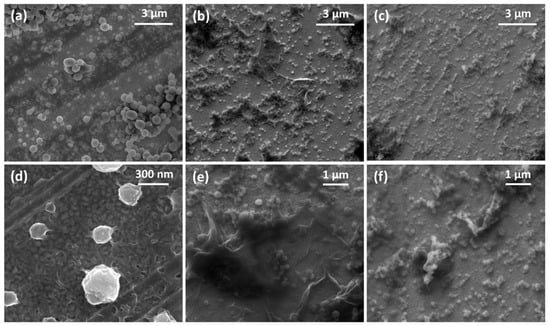
Figure 3.
SEM micrographs of the in situ enzymatically obtained films of (a,d) PANI, (b,e) PANI-GO2.5, and (c,f) PANI-nGO2.5.
The enzymatic polymerization reactions were simultaneously monitored by the open circuit potential (OCP) technique in the quartz crystal balance method (QCM), in order to analyze the oxidation stage and the deposition of the films by microgravimetric measurements. The evolution of the OCP during enzymatic polymerization, together with the gravimetric response and the change in the quartz crystal resistance as a function of time are graphically represented in Figure S3.
3.2. Composites Characterization
3.2.1. Ultraviolet–Visible Spectroscopy
The UV–Vis spectra of PANI and PANI-GO/nGO composites are shown in Figure 4. The absorption spectrum of PANI (black) displays the characteristic bands of polyaniline emeraldine base formed at 320 nm and 630 nm, due to the π-π* transition of benzenoid rings and the absorption of quinoid rings, respectively [28]. On the other hand, the UV–Vis spectra of PANI-GO/nGO composites exhibit, in addition to the PANI bands just mentioned, the characteristic absorption band of GO at 230 nm, attributed to the π-π* transition [29]. Also, composite spectra experience a band shift towards the blue color. This change could be attributed to the non-covalent interactions between the functional groups of GO and those of PANI’s structure, generating hydrogen-bonding interaction >C=O∙∙∙∙H-N< [30]. This is because hydrogen bonding between PANI and GO disrupts the electronic conjugation of PANI, resulting in a reduction in the effective conjugation length.
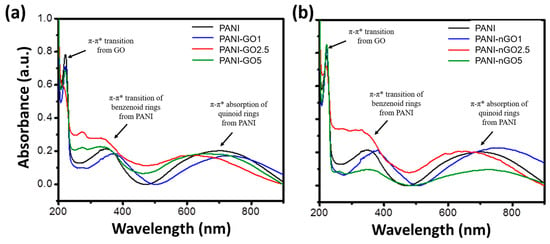
Figure 4.
UV–Vis spectra of PANI and PANI-GO (a) and PANI-nGO (b) composites.
3.2.2. Fourier-Transform Infrared Spectroscopy
The samples were analyzed by FTIR to identify the functional groups created between PANI and GO. Figure 5a shows the spectra of PANI-GO composites, while Figure 5b presents the PANI-nGO composites, in both cases compared to the spectrum of PANI. In all spectra, the predominant bands are those corresponding to the characteristic functional groups of PANI, since the highest concentration in the material corresponds to this polymer. Principal bands are located around 1599 cm−1, 1500 cm−1, 1375 cm−1, and 1305 cm−1, corresponding to the stretching vibrations of the quinoid C-C, benzenoid C-C, C-N, and C=N bonds, respectively [31]. Furthermore, in-plane bending of the C-H bond produces an absorption band at 1170 cm−1, whereas out-of-plane bending generates an absorption band at 830 cm−1 [32]. In the case of the composites, a band at 3383 cm−1 associated with the presence of free N-H bonds is observed, as well as a new band around 3200 cm−1, assigned to the formation of hydrogen bonds between PANI and the carbonyl groups of GO [33,34]. In addition, TSA residues were identified by its characteristic bands around 1100 cm−1 and 1030 cm−1, corresponding to the asymmetric and symmetric stretching vibrations of the S=O bond, and at 696 cm−1 for S-O stretching [35].
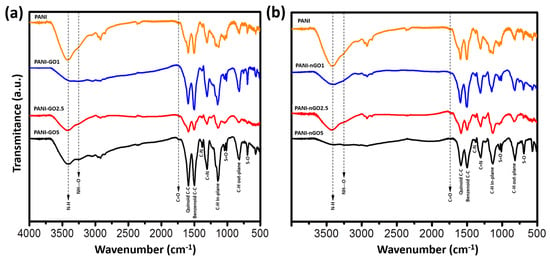
Figure 5.
FTIR spectra of (a) PANI-GO and (b) PANI-nGO composites, compared to PANI.
3.2.3. X-Ray Photoelectron Spectroscopy
Figure 6a shows the low-resolution XPS spectra of the GO, PANI, and PANI-GO2.5 samples. It is observed that GO (black) has a carbon- and oxygen-based composition due to the photoemission peaks corresponding to O 1s around 550 eV of binding energy and C 1s between approximately 280 and 290 eV. On the other hand, PANI (blue) and PANI-GO2.5 (red) show, in addition to the C 1s and O 1s peaks, the presence of nitrogen (N 1s) and sulfur (S 2p) around 400 eV and 168 eV, respectively [34]. A higher amount of oxygen is evident for GO, having an O/C ratio of 0.48, as opposed to an O/C ratio of approximately 0.22 for PANI and the representative composite. The above reaffirms that PANI completely covers GO, otherwise the O/C ratio for the composites would be greater than 0.22. For a detailed analysis of the interaction between PANI and GO, the C 1s peak was deconvoluted. GO (Figure 6b) presents three signals, the first corresponds to the C 1s photoemission of C-C and C-H carbons in the sp2 state (284.6 eV), a second signal with greater intensity is observed at 286.5 eV corresponding to carbon with hydroxyl and oxygen groups (C-O-H and C-O-C), and finally, with a binding energy of 288 eV, there is a third signal corresponding to the carbonyl group C=O [36]. In the PANI spectrum (Figure 6c), the signals at 284.5 eV, 286.6 eV, and 288.1 eV are also identified, reported for the C-C/C-H, O-C-O/C-N, and C=O electronic environments of PANI [34]. The PANI-GO2.5 composite (Figure 6d) presents the same components as pure PANI, indicating that its structure is not modified and that the exposed surface of the composites is basically PANI.
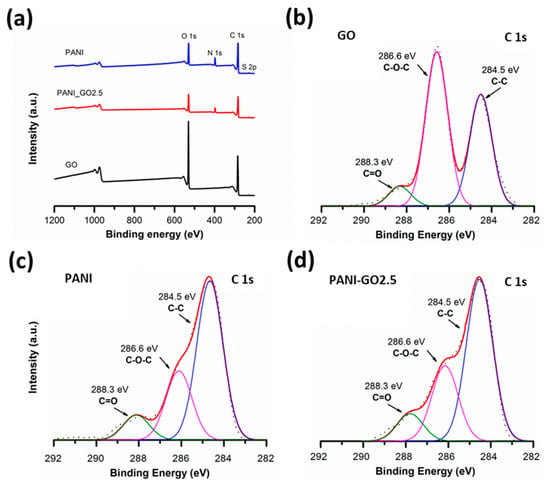
Figure 6.
(a) Survey XPS spectra and high-resolution XPS spectra of C 1s peak for (b) GO, (c) PANI, and (d) PANI-GO2.5.
3.2.4. Raman Spectroscopy
The Raman spectra of the PANI-GO/nGO composites are shown in Figure 7, with the spectrum of PANI as a reference. All spectra show bands from 400 to 1000 cm−1 related to deformation vibrations of the benzene rings, in addition to more intense bands (indicated by arrows) around 1180, 1300–1370, 1500, and 1595 cm−1, related to the C–H vibrations of aromatic rings, C-N stretching band of an aromatic amine, N–H deformation vibrations, and C-C stretching vibrations of the benzene ring, respectively [37,38]. On the other hand, GO is characterized by two intense peaks at 3446 and 1552 cm−1, explained by the presence of residual -OH groups and aromatic C=C stretching vibrations, respectively. However, the spectra of PANI-GO/nGO composites show a weak signal of these bands, which represents a reduction in GO during PANI polymerization [39].
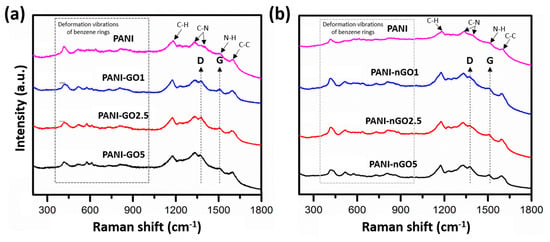
Figure 7.
Raman spectra of (a) PANI-GO and (b) PANI-nGO composites, using moving average for elimination of spectral noise.
3.2.5. Thermogravimetric Analysis
Thermogravimetric analysis of GO with large and small sheet size and their corresponding PANI hybrids are shown in Figure 8a,b, respectively. Regardless of the sheet size, both graphene oxide samples showed similar thermal behavior. The first loss around 100 °C corresponds to bound water and the second weight loss around 200 °C to GO thermal reduction. The third and final weight loss around 450 °C is the thermally reduced graphene oxide oxidation, which leaves a very low amount of ashes. On the other hand, the thermograms of the PANI and GO composites show three weight losses, the first one below 100 °C is associated with the loss of solvents or moisture absorbed in the sample; the second loss is found between 200 and 300 °C, associated with the functional groups present in GO/nGO; the last and third weight loss is found around 400 and 600 °C, because the polymer begins to thermally degrade [40].
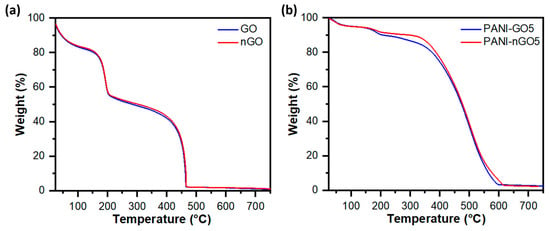
Figure 8.
Thermogravimetric analysis of (a) graphene oxide samples with large (GO) and small (nGO) lateral size, and (b) nanocomposites of polyaniline and graphene oxide with large (PANI-GO5) and small (PANI-nGO5) lateral size.
3.2.6. Simultaneous Cyclic Voltammetry and Quartz-Crystal Microbalance Study
During the polymerizations, we noticed that a thin conformal film of PANI and GO was also obtained (Figure 3). We deposited these films in gold-coated quartz-crystal and studied these films by combining cyclic voltammetry and microbalance measurements; the results are presented in Figure 9. The behavior of PANI, PANI-GO2.5, and PANI-nGO2.5 composites presents similarity, which is discussed below. The changes in resistance (Figure 9(a1–a3)) are due to changes in the energy dissipation of the quartz crystal [41,42], mainly due to changes in the damping capacity of the deposited film, which in turn result from changes in its mechanical properties. This change in the resistance of the quartz crystal is consistent with the mass changes (Figure 9(b1–b3)); both curves present the same shapes, indicating that there is no significant change in the elastic properties of the films during the test. Furthermore, the films show two broad oxidation peaks around 300 mV and 500 mV during anodic scanning (Figure 9(c1–c3)), indicating transitions between different oxidation states of PANI, specifically, from leucoemeraldine to emeraldine and from emeraldine to pernigraniline [42]. The respective reductions are shown during cathodic scanning, indicating that the oxidation is reversible. During potential scanning, the polymer becomes dedoped during the leucoemeraldine stage, reflecting a mass loss with a frequency shift (Figure 9(b1)) corresponding to a mass amount of around 4 µg/cm2 for PANI and higher for the composites (Figure 9(b2,b3)), due to the loss and absorption of chlorine anions during the doping–redoping process [43].
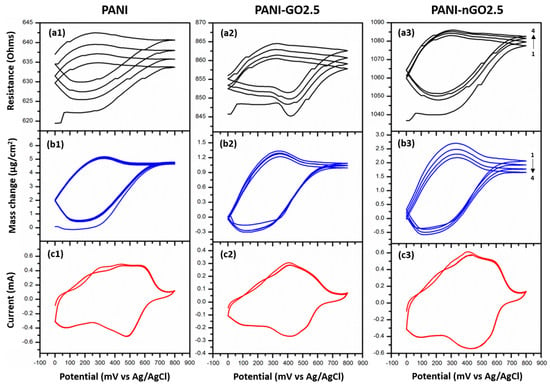
Figure 9.
Simultaneous cyclic voltammetry/quartz-crystal microbalance characterization of PANI (left), PANI-GO2.5 (middle), and PANI-nGO2.5 (right) enzymatically synthesized films. (a1–a3) Cyclic voltammetry, (b1–b3) mass change, and (c1–c3) open circuit potential of the crystal during the enzymatic reaction.
3.2.7. Molecular Dynamics Simulation
The mechanism of film growth was studied with the help of molecular dynamics simulations and selected frames of a simulation are shown sequentially in Figure 10. At the beginning of the simulation, the GO sheets were solvated with water molecules, while the PANI octamers rapidly adsorbed onto the gold electrode surface. These short PANI chains represent oligomers that act as nuclei for the subsequent formation of PANI precipitates. After approximately 15 ns, the PANI molecules flattened against the electrode, indicating strong interactions with the surface. The GO sheets occasionally interacted with each other through hydrogen bonding [20]; however, these contacts were weak and did not lead to irreversible aggregation. In contrast, when the PANI films interacted with the GO sheets, they established both hydrogen bonds and π–π stacking interactions between the aromatic rings of PANI and the sp2-rich regions of GO (Figure 10b). These interactions resulted in the irreversible formation of PANI–GO aggregates with reduced solubility. Once the PANI-GO complexes formed, the PANI molecules progressively dragged the GO sheets toward the electrode, acting as binding elements that promoted the deposition of the PANI–GO complexes onto the surface (Figure 10c,d) [37]. These simulations therefore support a mechanism of composite film formation driven by the binding ability of PANI, since GO is highly hydrophilic and alone exhibits low affinity for the gold electrode.
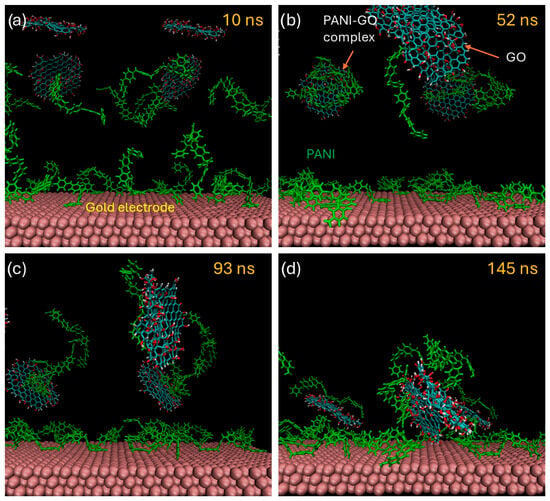
Figure 10.
Different stages of the film’s growth as simulated by classical molecular dynamics. (a) Initial stage of the simulation cell with solvated GO sheets and adsorbed PANI chains on the electrode. (b) Formation of PANI-GO complexes, (c) PANI-GO complexes being dragged towards the electrode. (d) Irreversible adsorption of PAN-GO complex on the surface of the gold electrode coated with PANI molecules.
4. Conclusions
Graphene oxide (GO) is obtained by the chemical oxidation of graphite flakes, resulting in graphite oxide, which can then be exfoliated using ultrasound to produce GO nanosheets. Prolonged ultrasonic treatment of GO dispersions further fragments the sheets to nanoscale dimensions. In situ enzymatic polymerization, catalyzed by horseradish peroxidase, allows the synthesis of polyaniline–graphene oxide (PANI-GO/nGO) nanocomposites. The amount of GO used does not inhibit the catalytic activity of the enzyme during polymerization. PANI forms nanocomposites by growing directly onto the GO nanosheets, regardless of their size. Furthermore, both PANI and PANI-GO/nGO nanocomposite films are successfully deposited in situ onto gold-coated quartz substrates during the polymerization process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/mi16111287/s1, Figure S1: SEM micrographs of (a) GO, (b) nGO, and (c) PANI; Figure S2: TEM micrographs of aqueous solutions of (a and d) GO, (b and e) nGO, and (c and f) PANI; mounted on a copper grid and coated with a discontinuous silicon film; Figure S3: Time-dependent monitoring of film formation by in situ enzymatic polymerization of polyaniline and polyaniline composites with graphene oxide. (a) Change in quartz crystal resistance, (b) gravimetric response of the quartz crystal, and (c) evolution of the open circuit potential. Figure S4: Molecular models of the polyaniline 8-mer and the nano-sized graphene oxide used in the simulations. Figure S5: Methodology used for measuring the thickness of the GO-PANI hybrids. (a) TEM image of the PANI-GO hybrid. (b) Scheme showing the morphology and orientation of the wrinkle, (c) Intensity profile used for measuring the thickness of the sheet. References [44,45,46,47,48] are cited in supplementary file.
Author Contributions
Methodology, C.G.-B.; resources, S.S.-G. and R.C.-S.; writing—original draft preparation, C.G.-B.; writing—review and editing, R.C.-S. and S.S.-G.; supervision, R.C.-S. and S.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by the secretary of public education (SEP), grant number PROMEP/103.5/09/4985, by the fund for science and technology (FONCYT), project COAH-2025-C25-B004, and by the center for research in applied chemistry (CIQA) through project 6787-F.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
C. Guerrero-Bermea acknowledges CONACyT for a scholarship for graduate studies. The authors thank Lizeth Garcia for her technical support in polyaniline synthesis and Guadalupe Méndez for her technical support in thermogravimetric analysis.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sahoo, P.K.; Kumar, N.; Jena, A.; Mishra, S.; Lee, C.-P.; Lee, S.-Y.; Park, S.-J. Recent progress in graphene and its derived hybrid materials for high-performance supercapacitor electrode applications. RSC Adv. 2024, 14, 1284–1303. [Google Scholar] [CrossRef]
- Khaldi, K.; Ezzat, A.O.; Alkoudsi, B.D.; Abd-Elkader, O.H.; Sabantina, L.; Benyoucef, A. Chemical synthesis of quaternary hybrid conducting polymers@Cr2O3-graphene oxide electrodes with excellent performance in supercapacitors. J. Inorg. Organomet. Polym. Mater. 2025, 35, 58–71. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, G.H.; Singh, G.; More, N.; Keerthana, M.; Sharma, A.; Jawade, D.; Balu, A.; Kapusetti, G. Ultrahigh sensitive graphene oxide/conducting polymer composite-based biosensor for cholesterol and bilirubin detection. Biosens. Bioelectron. X 2023, 13, 100290. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, D.P.; Muñoz, R.; Amami, M.; Singh, R.K.; Singh, S.; Kumar, V. Graphene-based materials for biotechnological and biomedical applications: Drug delivery, bioimaging and biosensing. Mater. Today Chem. 2023, 33, 101750. [Google Scholar] [CrossRef]
- Wu, J.; Lin, H.; Moss, D.J.; Loh, K.P.; Jia, B. Graphene oxide for photonics, electronics and optoelectronics. Nat. Rev. Chem. 2023, 7, 162–183. [Google Scholar] [CrossRef]
- Sabet, M. Advanced graphene–polymer composites: Synthesis, properties, and applications in electronics and optoelectronics. J. Mater. Sci. 2025, 60, 6807–6849. [Google Scholar] [CrossRef]
- Hao, J.; Wang, L.; Wang, X.; Wang, J.; He, M.; Zhang, X.; Wang, J.; Nie, L.; Li, J. Preparation, modification and antifouling properties of polyaniline conductive membranes for water treatment: A comprehensive review. Environ. Sci. Water Res. Technol. 2024, 10, 105–127. [Google Scholar] [CrossRef]
- Bi, A.; Mahmood, S.; Garg, N.; Thakur, S.; Chaudhry, S.A.; Yadav, S.; Gupta, M.; Bechelany, M.; Prakash, J.; Haque, Z. Graphene oxide/polyindole nanocomposite: A highly efficient multi-cyclic, stable and sustainable photocatalyst platform for wastewater remediation under visible light. Mater. Adv. 2025, 6, 6320–6336. [Google Scholar] [CrossRef]
- Majeed, A.H.; Mohammed, L.A.; Hammoodi, O.G.; Sehgal, S.; Alheety, M.A.; Saxena, K.K.; Dadoosh, S.A.; Mohammed, I.K.; Jasim, M.M.; Salmaan, N.U. A review on polyaniline: Synthesis, properties, nanocomposites, and electrochemical applications. Int. J. Polym. Sci. 2022, 2022, 9047554. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Baghdadi, A.H.; Kamarudin, S.K.; Rashid, S.A.; Jakmunee, J.; Shaari, N. Recent progress in polyaniline and its composites: Synthesis, properties, and applications. Eur. Polym. J. 2024, 210, 112948. [Google Scholar] [CrossRef]
- Beygisangchin, M.; Abdul Rashid, S.; Shafie, S.; Sadrolhosseini, A.R.; Lim, H.N. Preparations, properties, and applications of polyaniline and polyaniline thin films—A review. Polymers 2021, 13, 2003. [Google Scholar] [CrossRef]
- Ren, H.; Xia, X.; Sun, Y.; Zhai, Y.; Zhang, Z.; Wu, J.; Li, J.; Liu, M. Electrolyte engineering for the mass exfoliation of graphene oxide across wide oxidation degrees. J. Mater. Chem. A 2024, 12, 23416–23424. [Google Scholar] [CrossRef]
- Chow, D.; Burns, N.; Boateng, E.; van der Zalm, J.; Kycia, S.; Chen, A. Mechanical exfoliation of expanded graphite to graphene-based materials and modification with palladium nanoparticles for hydrogen storage. Nanomaterials 2023, 13, 2588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, X.; Zhang, M.; Zhang, R.; Ta, H.Q.; Sun, J.; Wang, W.; Zhu, W.; Fang, T.; Jia, K. Fast synthesis of large-area bilayer graphene film on Cu. Nat. Commun. 2023, 14, 3199. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Sidhu, J.S.; Verma, M. Influence of reduction duration on reduced graphene oxide for supercapacitor energy storage enhancement. Carbon Trends 2025, 19, 100499. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, Y.; Liu, Q.; Yan, C.; Xiao, B.; Yang, J.; Liu, M.; Zhu, L. Lateral size dependent colloidal stability of graphene oxide in water: Impacts of protein properties and water chemistry. Environ. Sci. Nano 2020, 7, 634–644. [Google Scholar] [CrossRef]
- Sim, H.J.; Li, Z.; Xiao, P.; Lu, H. The influence of lateral size and oxidation of graphene oxide on its chemical reduction and electrical conductivity of reduced graphene oxide. Molecules 2022, 27, 7840. [Google Scholar] [CrossRef]
- Qiu, B.; Sun, T.; Yuan, M.; Zhang, H.; Chen, Y.; Zhou, S.; Heng, Z.; Liang, M.; Zou, H. Effect of different lateral dimension graphene oxide sheets on the interface of carbon fiber reinforced polymer composites. Compos. Sci. Technol. 2021, 213, 108939. [Google Scholar] [CrossRef]
- Szabo, T.; Maroni, P.; Szilagyi, I. Size-dependent aggregation of graphene oxide. Carbon 2020, 160, 145–155. [Google Scholar] [CrossRef]
- Xu, J.; Wang, K.; Zu, S.-Z.; Han, B.-H.; Wei, Z. Hierarchical Nanocomposites of Polyaniline Nanowire Arrays on Graphene Oxide Sheets with Synergistic Effect for Energy Storage. ACS Nano 2010, 4, 5019–5026. [Google Scholar] [CrossRef]
- Yin, J.; Wang, X.; Chang, R.; Zhao, X. Polyaniline decorated graphene sheet suspension with enhanced electrorheology. Soft Matter 2012, 8, 294–297. [Google Scholar] [CrossRef]
- Cruz-Silva, R.; Arizmendi, L.; Del-Angel, M.; Romero, J. pH- and Thermosensitive Polyaniline Colloidal Particles Prepared by Enzymatic Polymerization. Langmuir 2007, 23, 8–12. [Google Scholar] [CrossRef]
- Zhou, W.; Rao, Y.; Zhuang, W.; Ge, L.; Lin, R.; Tang, T.; Wu, J.; Li, M.; Yang, P.; Zhu, C. Improved enzymatic activity by oriented immobilization on graphene oxide with tunable surface heterogeneity. Compos. Part B Eng. 2021, 216, 108788. [Google Scholar] [CrossRef]
- Shang, H.; Wang, J.; Guo, B.; Zhu, H.; Li, H. Immobilization of phospholipase D on Fe3O4@SiO2–graphene oxide nanocomposites: A strategy to improve catalytic stability and reusability in the efficient production of phosphatidylserine. Molecules 2025, 30, 912. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Wang, L.; Ye, Y.; Lu, X.; Wen, Z.; Li, Z.; Hou, H.; Song, Y. Hierarchical nanocomposites of polyaniline nanowire arrays on reduced graphene oxide sheets for supercapacitors. Sci. Rep. 2013, 3, 3568. [Google Scholar] [CrossRef]
- Qutub, N.; Singh, P.; Sabir, S.; Umar, K.; Sagadevan, S.; Oh, W.-C. Synthesis of polyaniline-supported CdS/CdS–ZnS/CdS–TiO2 nanocomposite for efficient photocatalytic applications. Nanomaterials 2022, 12, 1355. [Google Scholar] [CrossRef] [PubMed]
- Tene, T.; Guevara, M.; Benalcázar Palacios, F.; Morocho Barrionuevo, T.P.; Vacacela Gomez, C.; Bellucci, S. Optical properties of graphene oxide. Front. Chem. 2023, 11, 1214072. [Google Scholar] [CrossRef]
- Kandasamy, M.; Seetharaman, A.; Chakraborty, B.; Manohara Babu, I.; William, J.J.; Muralidharan, G.; Jothivenkatachalam, K.; Sivasubramanian, D. Experimental and theoretical investigation of the energy-storage behavior of a polyaniline-linked reduced-graphene-oxide–SnO2 ternary nanohybrid electrode. Phys. Rev. Appl. 2020, 14, 024067. [Google Scholar] [CrossRef]
- Qiu, B.; Wang, J.; Li, Z.; Wang, X.; Li, X. Influence of acidity and oxidant concentration on the nanostructures and electrochemical performance of polyaniline during fast microwave-assisted chemical polymerization. Polymers 2020, 12, 310. [Google Scholar] [CrossRef]
- Morávková, Z.; Šeděnková, I.; Bober, P. The first stages of chemical and electrochemical aniline oxidation—Spectroscopic comparative study. Appl. Sci. 2020, 10, 2091. [Google Scholar] [CrossRef]
- Ab Mutalib, T.N.A.B.T.; Tan, S.J.; Foo, K.L.; Liew, Y.M.; Heah, C.Y.; Abdullah, M.M.A.B. Properties of polyaniline/graphene oxide (PANI/GO) composites: Effect of GO loading. Polym. Bull. 2021, 78, 4835–4847. [Google Scholar] [CrossRef]
- Bautkinová, T.; Sifton, A.; Kutorglo, E.M.; Dendisová, M.; Kopecký, D.; Ulbrich, P.; Mazúr, P.; Laachachi, A.; Hassouna, F. New approach for the development of reduced graphene oxide/polyaniline nanocomposites via sacrificial surfactant-stabilized reduced graphene oxide. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124415. [Google Scholar] [CrossRef]
- Dhawan, K.; Bhardwaj, D.; Singh, R. An efficient nano titania-supported sulfonic acid (n-TSA) catalyzed solvent-free synthesis of isoxazolyl-thiazolidinones. Mater. Today Proc. 2021, 43, 3231–3235. [Google Scholar] [CrossRef]
- Moeini, B.; Linford, M.R.; Fairley, N.; Barlow, A.; Cumpson, P.; Morgan, D.; Fernandez, V.; Baltrusaitis, J. Definition of a new (Doniach–Sunjic–Shirley) peak shape for fitting asymmetric signals applied to reduced graphene oxide/graphene oxide XPS spectra. Surf. Interface Anal. 2022, 54, 67–77. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Liu, J. Facial preparation of covalent modified reduced graphene oxide/polyaniline composite and its stable-enhanced electrochemical performance. Heliyon 2023, 9, e13002. [Google Scholar] [CrossRef] [PubMed]
- Myasoedova, T.N.; Nedoedkova, O.V.; Kalusulingam, R.; Popov, Y.V.; Mikheykin, A.S.; Konstantinov, A.S.; Zhengyou, L.; Mikhailova, T.S.; Shmatko, V.A.; Yalovega, G.E. Fabrication of Ni–polyaniline/graphene oxide composite electrode with high capacitance and water splitting activity. ChemPhysChem 2024, 25, e202300795. [Google Scholar] [CrossRef]
- Scardaci, V.; Compagnini, G. Raman spectroscopy investigation of graphene oxide reduction by laser scribing. C 2021, 7, 48. [Google Scholar] [CrossRef]
- Ghafuri, H.; Keshvari, M.; Eshrati, F.; Hanifehnejad, P.; Emami, A.; Zand, H.R.E. Catalytic efficiency of GO–PANI nanocomposite in the synthesis of N-aryl-1,4-dihydropyridine and hydroquinoline derivatives. Sci. Rep. 2025, 15, 2791. [Google Scholar] [CrossRef]
- Abbood, A.S.; Ibraheem, I.J. Polyaniline nano films synthesis in one step via chemical oxidative polymerization. Baghdad Sci. J. 2024, 21, 15. [Google Scholar] [CrossRef]
- Le, H.T.T.; Liem, N.T.; Giang, N.C.; Hoang, P.H.; Phuong, N.T.M. Improving electrochemical performance of hybrid electrode materials by a composite of nanocellulose, reduced oxide graphene and polyaniline. RSC Adv. 2023, 13, 22375–22388. [Google Scholar] [CrossRef]
- Montes-Rojas, A.; Ramírez-Orizaga, M.; Ávila-Rodríguez, J.G.; Torres-Rodríguez, L.M. Study of polyaniline/poly(sodium 4-styrenesulfonate) composite deposits using an electrochemical quartz crystal microbalance for the modification of a commercial anion exchange membrane. Membranes 2020, 10, 387. [Google Scholar] [CrossRef]
- Bahramian, A. Molecular dynamics simulation of surface morphology and thermodynamic properties of polyaniline nanostructured film. Surf. Interface Anal. 2015, 47, 1–14. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Jo, S.; Brooks, C.; Lee, H.; Im, W. CHARMM-GUI ligand reader and modeler for CHARMM force field generation of small molecules. J. Comput. Chem. 2017, 38, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Kern, N.; Lee, J.; Choi, Y.; Im, W. CHARMM-GUI Multicomponent Assembler for modeling and simulation of complex multicomponent systems. Nat. Commun. 2024, 15, 5459. [Google Scholar] [CrossRef] [PubMed]
- Eastman, P.; Swails, J.; Chodera, J.; McGibbon, R.; Zhao, Y.; Beauchamp, K.; Wang, L.; Simmonett, A.; Harrigan, M.; Stern, C.; et al. OpenMM 7: Rapid development of high performance algorithms for molecular dynamics. PLoS Comput. Biol. 2017, 13, e1005659. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).