Abstract
Hemoglobin oxygen (HbO2) saturation is a critical biomarker in patient care, yet conventional measurement approaches are often costly and require extensive calibration. To address these limitations, the present study proposes a novel biosensor derived from paper-based carbon quantum dots (CQDs) fabricated through a one-step thermal treatment. CQDs are carbon-based nanoparticles renowned for their excellent biocompatibility, low toxicity, thermal stability, and remarkable optical properties. To quantify HbO2 saturation, we exploit their photoluminescence, which enables photoinduced electron transfer and fluorescence quenching with hemoglobin. Our results demonstrated that the peak fluorescence intensity of CQDs shows a strong linear correlation with HbO2 saturation. Variations in HbO2 saturation levels were achieved with sodium dithionite and determined using Winterbourn’s equations. Our CQD-based HbO2 saturation measurements closely agreed with those obtained from conventional spectrophotometric analysis. Thus, this investigation highlights the potential of CQDs as a biosensor for effective HbO2 saturation measuring without extensive calibration.
1. Introduction
Hemoglobin oxygen (HbO2) saturation is an essential element in patient care, as red blood cells tightly regulate and determine blood flow in the circulatory system. The lack of measurement of HbO2 saturation levels may result in undetected hypoxia, potentially leading to acute adverse events []. The normal HbO2 saturation level ranges from 95% to 100% and values under 90% are considered a serious deterioration in status with a high risk of hypoxia. Those under 70% are in a life-threatening state []. Hypoxia occurs when oxygen is insufficient at the tissue level and hypoxemia occurs when arterial oxygen tension is below normal []. It is generally known that hypoxemia can lead to hypoxia. Clinically, there is no set standard HbO2 saturation level where hypoxemia occurs []. Thus, it is significant to be able to measure HbO2 saturation over a wider range. Current methods for HbO2 saturation detection include pulse oximetry, near-infrared spectroscopy, hyperspectral imaging, photoacoustic imaging, and arterial blood gas analysis. However, these existing techniques exhibit a trade-off between cost and accuracy, achieving one at the expense of the other. Moreover, it has been recognized that the dynamic measurement of HbO2 saturation changes is challenging with current state-of-the-art technology due to the complexity of calibration and detection protocols. In this study, we designed an innovative nano-sensor using carbon quantum dots (CQDs) to detect the temporal variations of HbO2 saturation level in a blood sample with the goal of achieving greater reliability and simplicity.
There have been some nanostructures used to indirectly measure HbO2 saturation state or levels. According to a previous study [], gold nanoparticle-covered SiO2 substrates can detect hemoglobin’s oxygenation-dependent vibrational markers, such as Fe-O2 stretching and heme modes, to determine oxygenated and de-oxygenated states of hemoglobin using Raman spectroscopy. Furthermore, there are nanostructures using metal-ligands such as Ruthenium or Porphyrin platinum complexes to measure the partial pressure of oxygen in blood. Then, these partial pressure values could be correlated to HbO2 saturation levels following the oxygen-hemoglobin dissociation curve []. An electrical sensor using TiS2 nanosheets and graphene-based optical sensors was also reported to measure partial pressure of oxygen to determine HbO2 saturation []. Such indirect methods of measuring HbO2 saturation using nanostructures highlight the necessity for a direct measurement of HbO2 saturation. Carbon is universally present in all organic matter, and carbon nanoparticles are a nanomaterial formed from pure carbon. In the class of carbon nanoparticles, CQDs are those with a size of less than 10 nm. The properties of CQDs include low toxicity, good biocompatibility, biodegradability, and tunability, attracting attention in the biomedical application field. In addition, CQDs demonstrate intrinsic state luminescence and the quantum confinement effect, a phenomenon where nanoscale energy carriers follow scaling laws based on their size variation [,]. Recently, we successfully demonstrated a fabrication method for CQDs from cellulosic paper for biosensor applications []. The interwoven fibers of paper provide a large surface area for the efficient fabrication of CQDs. With such a synthetic advantage, we found that the fluorescence intensity (FI) of CQDs quenches in the presence of Fe3+ ions and cell-free hemoglobin, consistent with photo-induced electron transfer from oxygen-rich surface groups on CQDs and metal ions. Thus, fluorescence quenching is selective depending on the strength of binding affinities between metal ions and the surface groups on the fluorescent particles.
Building on our earlier findings, we sought to extend their application to the detection of HbO2 saturation. Our previous results demonstrated that CQDs can be used to detect cell-free hemoglobin in blood bags and monitor blood storage conditions, thereby improving blood bank inventory management []. We also observed a degree of variability in the level of fluorescence quenching of CQDs across different blood samples, which we attributed to differences in HbO2 saturation levels. Accordingly, we hypothesized that CQDs are capable of detecting dynamic changes in HbO2 saturation levels. To test this, HbO2 saturation levels were controlled using sodium dithionite as an oxygen scavenger, with reoxygenation occurring over time as oxygen re-entered the solution. Such varying HbO2 saturation values were quantified by optical spectroscopy at selected wavelengths, which were then used to evaluate the optical responses of our CQDs. Finally, the performance of the CQD-based sensor in sensing HbO2 saturation levels was validated by comparison with an existing method.
2. Materials and Methods
2.1. Carbon Quantum Dot (CQD)
We fabricated CQDs from paper through a one-step thermal treatment using a laboratory-grade drying oven (SLN 15, Pol-Eko, Wodzisław Śląski, Poland) as reported recently []. Cellulosic paper with a thickness of 180
and a pore size of 11
(Whatman® Grade 1, GE Healthcare, Buckinghamshire, UK) was laid flat on a stainless-steel tray and baked at 260 °C for 10 min. The temperature of 260 °C was chosen due to its low fluorescence emission variability. To isolate CQDs from the baked cellulose paper, the paper was cut into strips of 800 mg and resuspended in 4 mL of deionized (DI) water. The mixture was then agitated using a vortex mixer (Orbital Vortex Mixer, AIT biotech, Singapore) for 2 min. The excess supernatant containing CQDs was extracted, and the vortex-extraction step was repeated three more times. To remove large cellulose fibers, the supernatant was centrifuged at 15,000× g for 10 min (Sartorius 1-14, Göttingen, Germany), after which the supernatant was filtered through a 0.22-μm microporous membrane (Minisart® High Flow, 6 Sartorius, Goettingen, Germany). Figure 1a provides a brief description of the fabrication procedure. The varying concentrations of CQDs were prepared by oven-drying at 70 °C to accelerate natural evaporation. The quenching property of CQDs to detect the HbO2 saturation levels is described in Figure 1b. The oxygenation level of hemoglobin appears to influence the extent of fluorescence quenching of CQDs in presence of hemoglobin, leading to variability in FI of CQDs. Lower oxygen levels correspond to greater quenching and thus lower absolute FI of CQDs. Yet, the exact mechanism by which oxygen modulates CQD-hemoglobin remains unclear. The absorbance spectrum of CQDs is shown with a peak absorbance at 275 nm (Figure 1c), supporting
–
* transitions of aromatic sp2 domains in the C=C bonds in CQDs []. After the peak absorbance, there is a sharp decrease up to 315 nm, followed by a plateau to zero. These near-zero absorbance values from 550 to 650 nm ensure that CQDs do not interfere with the quantification of HbO2 saturation. CQDs fabricated from cellulosic paper exhibit a peak FI level at 360 nm (Figure 1d). Figure 1e verifies the ability of CQDs to quench in the presence of hemoglobin (p < 0.001). The solution containing CQDs in DI water exhibited a peak FI of 47.88 ± 3.13, whereas the addition of hemoglobin reduced it to 17.76 ± 3.33.
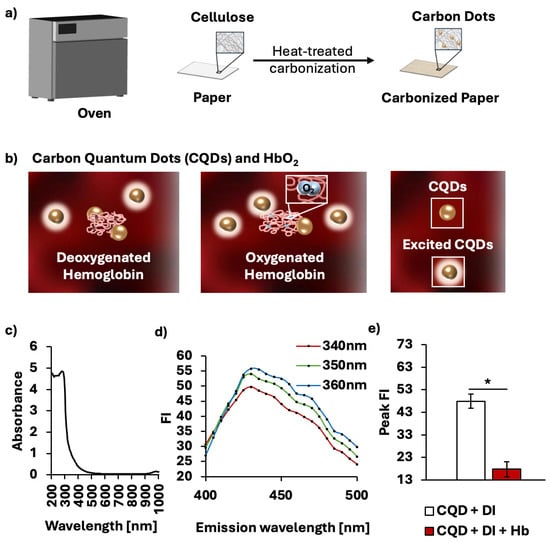
Figure 1.
Synthesis of CQDs and characterization of CQDs in interaction with hemoglobin. (a) Schematic illustration showing one-step thermal fabrication to produce in situ CQDs on paper. (b) Interactions of CQDs and hemoglobin with different oxygenated states. (c) Absorbance spectrum of CQDs from 200 to 1000 nm. (d) Emission spectrum of CQDs with excitation wavelength at 340, 350, 360 nm. (e) Average peak FI with and without cell-free hemoglobin, showing that the fluorescence quenching of CQDs due to hemoglobin is statistically significant. * p < 0.001.
2.2. Characterization of CQDs
Detailed information on the characterization of paper-derived CQDs can be found in our recent study []. Here, we summarize the materials science data for our CQDs. The morphological characterization of paper-derived CQDs through dynamic light scattering (DLS) confirmed a size distribution within the range of 2.3 to 3.6 nm, with an average diameter of 2.9
0.5 nm, consistent with typical CQD dimensions. Based on FTIR analysis, broad O-H stretching at 3315 cm−1 demonstrates the existence of hydroxyl (-OH) and carboxyl (-COOH) groups. The peak at 2889 cm−1 shows a C-H stretching vibration. The peak at 1631 cm−1 indicates C=C bonds, while the peak at 1024 cm−1 indicates C-O stretching. Additionally, cellulose-related bands are observed at 892, 1109, and 1155 cm−1. Moreover, XPS analysis supported the FTIR findings through C1s peaks at 283.0 eV (C=C), 284.7 eV (C-C), and 286.1 eV (C-O), as well as the O1s peak at 532.5 eV (C-O). Raman spectroscopy showed peaks between 1095 and 1122 cm−1, the axial deformation of C-O, matching cellulose bands. Our CQDs showed significant fluorescence quenching by Fe3+ and Cu2+, which can be attributed to strong binding between ions and oxygenated groups (-OH, -COOH). In addition, the strongest quenching was observed by hemoglobin, as also confirmed in this study.
2.3. Blood Sample Preparation
Fresh porcine whole blood was collected from the abattoir (Primary Industries, Singapore) in 3.2% sodium citrate. Porcine blood was centrifuged at 5000× g for 10 min (Megafuge 8, Thermo Scientific, Waltham, MA, USA). The plasma and buffy coat were removed from the sample. Packed red blood cells (PRBCs) were prepared by washing three times with 1X phosphate-buffered saline solution. Then, the PRBC was suspended in DI water at 40% hematocrit to induce lysis. To confirm that the RBCs are fully lysed, the RBC solution was ultrasonicated. The total hemoglobin concentration was then measured using a hemoglobin analyzer (Hb 301, Hemocue, Ängelholm, Sweden).
2.4. HbO2 Saturation Level Control
To achieve different HbO2 saturation levels, we used sodium dithionite (Na2S2O4) (157953, Sigma Aldrich, Darmstadt, Germany) to remove oxygen from the sample. To ensure the stability of the sodium dithionite solution, a high volume of solution was freshly prepared daily: a varying amount of solid sodium dithionite was added to a 15-mL centrifuge tube containing 10 mL of DI water. Then, all samples were sealed properly to avoid the uncontrolled introduction of oxygen from the air. Dithionite induces hemoglobin to release oxygen by lowering the oxygen concentration in the solution. When used at a proper concentration, sodium dithionite can reoxygenate the blood sample over time and reverse its effects []. For validation of its effects, an electrochemical oxygen gas sensor probe (OX-10-17161, Unisense, Aarhus, Denmark) was utilized.
2.5. HbO2 Saturation Level Measurement
In all experiments, a hemoglobin concentration of 0.25 g dL−1 was chosen to ensure a good balance between minimizing noise and reliably detecting the oxygenation absorbance peaks. To quantify HbO2 saturation, the concentration of oxyhemoglobin (OxyHb) and deoxyhemoglobin (DeoxyHb) was determined using equations derived from Winterbourn extinction coefficients as follows [,,]:
Then, HbO2 is determined by the following equations:
Oxyhemoglobin is the form of hemoglobin with bound oxygen molecules, while deoxyhemoglobin is the form of hemoglobin with no oxygen molecules. Each form of hemoglobin was calculated using the Winterbourn equation, based on absorbance values at reported wavelengths (560, 576, and 630 nm). Absorbance values of samples were obtained through a multimode microplate reader (Varioskan LUX, Thermo Fisher Scientific, Waltham, MA, USA). At these wavelengths, one component is at maximal absorbance with minimal interference from others, ensuring accuracy of measurements [,]. Then, the HbO2 saturation level was subsequently determined by the ratio of oxyhemoglobin to the sum of oxyhemoglobin and deoxyhemoglobin, yielding a value ranging from 0 to 1.
2.6. Validation of CQDs for Oxygen Sensing
We have demonstrated that the peak FI of CQDs changes with the saturation levels of HbO2. As the saturation of HbO2 increased, there was a corresponding increase in the peak FI observed in a solution containing 37.5 μL of 0.25 g dL−1 hemoglobin, 75.0 μL of CQDs and 37.5 μL of sodium dithionite solution. To measure the peak FI of hemoglobin solutions with CQDs added at varying HbO2 saturation levels, individual wells of a transparent 96-well plate (650901, Greiner Bio-One, Kremsmünster, Austria) were sequentially analyzed. The microplate reader (Varioskan LUX, Thermo Fisher Scientific, Waltham, MA, USA) measures the absorbance for HbO2 saturation and fluorescence at an excitation wavelength of 360 nm to find the peak FI. To validate our measurements of HbO2 saturation, we employed a spectrophotometric method adapted from a pulse oximetry-based method, incorporating the Beer-Lambert Law and the molar extinction coefficient [,].
2.7. Statistical Analysis
Statistical analyses were performed in RStudio (version 2024.12.1+563; Posit, Boston, MA, USA). All measurements were performed in triplicate, and results are presented as mean ± standard deviation (SD). Sensitivity testing was performed through analysis of covariance (ANCOVA), and other parametric samples were assessed through a two-tailed t-test with unequal variance. Statistical significance was indicated by a p-value less than 0.05.
3. Results and Discussion
The oxygen scavenging ability of sodium dithionite was validated using an electrochemical oxygen gas sensor probe that measures voltage running through the solution in real-time after calibration. At the initial state (t = 0), after the addition of the sodium dithionite solution to the hemoglobin solution, hemoglobin was fully deoxygenated and reoxygenated over time (Figure 2a). HbO2 saturation can be spectroscopically distinguished in the Q-band region. At t = 0, the absorbance spectrum in Figure 2b exhibited a single Q-band peak at 555 nm, characteristic of deoxygenated hemoglobin [,], correlating to a low HbO2 saturation. During the measurement, the absorbance spectrum changed, showing two peaks at 540 and 575 nm, indicating that the hemoglobin solution had been successfully reoxygenated, characteristic of oxygenated hemoglobin. The effect of hemoglobin concentration on the peak FI emitted by two different concentrations (1X and 2X) of CQDs was investigated. The peak FI emission shows a similar increase with decreasing hemoglobin concentration (Figure 2c) at both CQD concentrations. While 2X concentration began with a higher peak FI compared to 1X concentration, both progressively quenched with increasing hemoglobin concentrations. As CQDs are constantly excited whilst measuring different HbO2 saturation levels, the photostability of CQDs under prolonged exposure to UV light was investigated (Figure 2d). When CQDs are excited, the fluorophore is structurally unstable and is susceptible to slight degradation. We found the photobleaching effect on CQDs to be significant (p < 0.005) with a negative linear relationship (R2 = 0.9004). In this study, HbO2 saturation levels were controlled in a time-dependent manner using sodium dithionite, necessitating the excitation of CQDs multiple times within a short timeframe. Thus, to minimize the influence of photobleaching on the interpretation of CQDs as a biosensor, the loss of FI due to photobleaching was compensated during the data analysis.
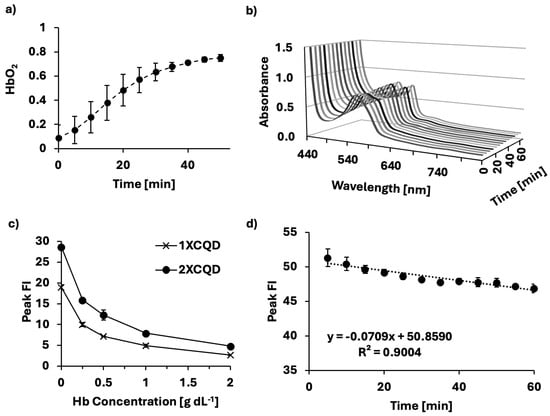
Figure 2.
Methodology for producing a biosensor of HbO2 saturation levels. (a) Sodium dithionite was used to control different hemoglobin oxygen saturation levels over 1 h at 37 °C. (b) Absorbance spectrum of porcine blood samples during the process of oxygenating, measured at 5-min intervals for 1 h from deoxidized states to oxidized states (deoxidized states achieved using sodium dithionite). (c) Relationship between different concentrations of CQDs and different hemoglobin concentrations demonstrating dose-dependent quenching of CQDs due to hemoglobin. (d) Photobleaching effect throughout the experiment (Slope =
, SD = 0.0075, p < 0.005).
As shown in Figure 3a, there was a strong linear relation between the HbO2 saturation levels and the peak FI of CQDs in the presence of hemoglobin. This linear increase in peak FI corresponded to increasing HbO2 saturation, highlighting the potential of peak FI as a biosensor for oxygenation levels of hemoglobin in the samples. To further test the influence of the concentration of CQDs in this measurement, 1X and 2X CQD concentrations were examined. Both concentrations resulted in a high correlation (R21XCQD = 0.9149, R22XCQD = 0.9125). Compared to the case of 2XCQD, the 1XCQD results showed higher peak FI variability (Figure 3a). Furthermore, there was a significant increase in the sensitivity of HbO2 saturation detection with an increase in the concentration of CQDs (Figure 3b). Using 2XCQD for the HbO2 sensor, we found the higher sensitivity of 2.96
0.18, whereas with 1XCQD, 1.49 ± 0.097. The linear detection range of HbO2 saturation was from 0.2110 to 0.7851. The limits of detection and quantification (LOD and LOQ) were determined to be 0.18 and 0.58, respectively (n = 4). The following formulas were used: LOD = (3 × SDHbO2 = 0.21101)/slope and LOQ = (10 × SDHbO2 = 0.21101)/slope, where SDHbO2 = 0.21101 is the standard deviation at HbO2 of 0.21101. HbO2 of 0.21101 is chosen as it is the lowest hemoglobin oxygen saturation level measured. Lower HbO2 levels could not be achieved since oxygen from ambient air was impulsively introduced during spectroscopic measurement for peak FI after deoxygenation with sodium dithionite.
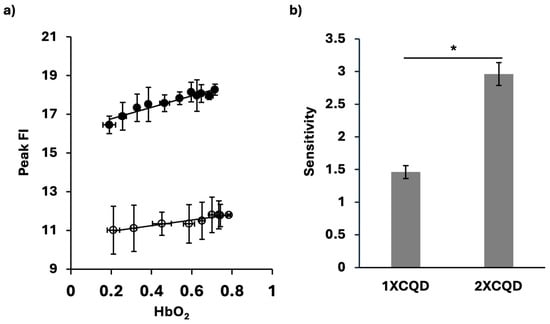
Figure 3.
A biosensor of HbO2 using CQDs for peak FI. (a) Peak FI of CQDs at different HbO2 saturation levels using 1XCQD concentration (○) and 2XCQD concentration (●). For 1XCQD, y = 1.4573x + 10.663, R21XCQD = 0.9149, p < 0.0001. For 2XCQD, y = 2.9587x + 16.174, R22XCQD = 0.9125, p < 0.0001. Each data point reflects measurements from samples (n = 40 for 1XCQD and n = 44 for 2XCQD). (b) HbO2 saturation level sensitivity based on the slope. Using 2XCQD concentration showed a higher sensitivity of 2.96 compared to using 1XCQD of 1.46. * p < 0.001. All the tests were conducted at 37 °C.
The result confirmed that our CQD method can quantify dynamic changes in hemoglobin saturation. To test the accuracy of our measurements, we compared ours with those obtained using an adapted pulse oximetry method. For this comparison test, the 1XCQD concentration was selected to assess its minimal accuracy in our measurement of HbO2 saturation levels. As previously stated, the different levels of HbO2 saturation were time-dependent as the blood sample re-oxygenated over time after the addition of sodium dithionite solution. Samples for both our CQDs and the conventional pulse-oximetry methods were exposed to air with identical surface areas, thereby controlling the rate of hemoglobin oxygenation over time. In Figure 4, the HbO2 saturation level values (n = 16) obtained from the adapted pulse oximetry method were compared with those from our CQD method (n = 16). Our peak FIs of CQDs in hemoglobin solution results were in good agreement with those obtained from the conventional system, exhibiting a strong linear correlation (R2 = 0.9279) (Figure 4a). Bland–Altman analysis (Figure 4b) showed a mean bias of 0.0406 and an SD of 0.0647 relative to the conventional method. The upper and lower limits of agreement were 0.167 and −0.0863, respectively. Hence, it demonstrated that our CQDs for HbO2 saturation level sensing would be as accurate as the conventional method.
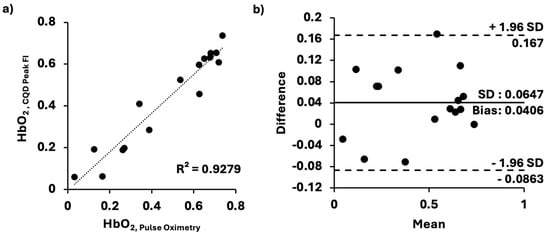
Figure 4.
Comparison between our and conventional pulse oximetry methods. (a) HbO2 saturation levels determined by our method and pulse oximetry; linear relationship (y = 0.9255x − 0.0054, SD = 0.0658, p < 0.0001), closely approximating an identity function. (b) Bland–Altman plot comparing the two methods. All the data were evaluated at 37 °C on a clear 96-well plate under the same conditions.
The capacity of CQDs as a novel biosensor is closely related to the synthesis method used. Broadly speaking, CQD synthesis routes can be classified into two main approaches: “top-down” and “bottom-up.” Our CQDs were obtained via the “top-down” approach, in which exfoliation and cutting of large carbon materials are performed after carbonization. Our CQDs were fabricated from cellulosic paper through thermal treatment at 260 °C for 10 min, a process that is quick, simple, and cost-efficient. Moreover, this process avoids hazardous chemicals and the use of high energy, unlike the traditional method. Thus, such simplicity marks a significant advancement toward the biosensing potential of CQDs to detect HbO2 saturation levels. While several existing methods are available to quantify HbO2 saturation (Supplementary Table S1), there is a clear need for a rapid yet reliable method to detect the dynamic changes in HbO2 saturation. Pulse oximetry is often regarded as the fifth vital sign, making it a widely accepted and valid diagnostic tool [,]. However, its accuracy to assess oxygenation drops to 83.2% when peripheral oxygen saturation falls below 90% []. Detecting low HbO2 saturation levels is crucial for determining whether to initiate or increase oxygen therapy. Hyperspectral imaging provides high spatial resolution of skin and tissue oxygen saturation [,]. Yet, it comes at a significantly high cost and requires a substantial amount of time to perform the analysis [,,]. Moreover, all the existing methods require a complex measurement procedure and periodic calibration to ensure accuracy and reliability. On the other hand, our CQDs enable the development of a nanosensor to rapidly detect the HbO2 saturation level using a microplate reader. Since sodium dithionite induces time-dependent deoxygenation and reoxygenation, continuous excitation of CQDs over a short period was required in our experimental setup. However, in a clinical setting, such continuous excitation of CQDs over a short period would not be necessary. In addition, our CQDs show no significant change in FI after being stored for 42 days, demonstrating a good photostability []. Unlike other methods that rely on multiple absorbance wavelengths, our CQD-based approach benefits from a straightforward linear relationship between peak FI and HbO2 saturation levels.
To generate controlled oxygenation states for testing, we used sodium dithionite, which reliably induces deoxygenation in hemoglobin, followed by gradual reoxygenation upon exposure to ambient air [,,]. This reproducible cycle makes dithionite one of the most practical reagents for generating varying HbO2 states in vitro. However, it should be noted that sodium dithionite may act as a potent reducing agent, lowering the partial oxygen pressure, when it is added to the hemoglobin solution. This reduction generates hydrogen peroxide and other unstable oxidation products, such as the superoxide anion radical and the reactive sulfur dioxide radical []. These byproducts may introduce uncontrolled and unknown reactions that interfere with the heme-binding response []. Although such species can, in principle, interfere with hemoglobin and complicate spectral interpretation, their effects are generally short-lived and transient. Notably, a classic study by K. Hamada et al. [] reported that, under acidic conditions or in the presence of cyanide, transient intermediates with absorbance peaks at 405 and 417 nm were observed. However, our conditions—cell-free porcine hemoglobin in DI water without cyanide—differed from those reports. Moreover, these wavelengths do not overlap with the analytical wavelengths (560, 576, 630 nm) used for HbO2 determination in this study. Hence, our measurements of HbO2 saturation levels are unlikely to be affected by the byproducts.
CQD-based biosensor, although it remains in vitro, can extend toward clinical use. The normal hemoglobin level for males is 14 to 18 g dL−1 and for females is 12 to 16 g dL−1 []. Each microplate well for measuring HbO2 will require a sample amount of 37.5 μL with a concentration of 0.25 g dL−1. Owing to the low volume required for measuring HbO2 level, a single drop of blood (35 μL) from the patient would allow testing in ~60 wells.
4. Conclusions
Our study investigated the potential of a one-step, heat-based synthesis of CQDs in the development of a carbon-based oxygen sensor. There was a strong linear correlation between the HbO2 saturation levels and the peak FI of CQDs in the presence of hemoglobin. Compared to conventional methods for sensing HbO2 saturation, our CQD sensor provided rapid detection capability through a microplate reader and stable measurements of dynamic changes in HbO2 saturation ranging from 0.21 to 0.79. The limits of detection and quantification of our CQD sensor were 0.18 and 0.58, respectively. Demonstrating reliability of CQD measurements, comparison between our CQD method and the conventional pulse oximetry method resulted in a strong linear relationship (n = 16, R2 = 0.9279,) and Bland–Altman analysis gave a mean bias of 0.0406 with SD = 0.0647, yielding 95% limits of agreement. With further optimization, this approach could be extended to clinical use under various physiological and pathological conditions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/mi16111261/s1, Supplementary Table S1: Methods of measuring HbO2. References [,,,,,,,] are cited in the supplementary materials.
Author Contributions
J.L.: Writing—review and editing, Writing—original draft, Visualization, Methodology, Data curation, Discussion. X.R.L.: Writing—review and editing, Conceptualization. J.K.S.T.: Writing—review and editing, Data analysis. S.K.: Conceptualization, Writing—review and editing, Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Porcine blood samples were obtained from the abattoir (Primary Industries, Singapore). As no live animals were handled in this study, institutional ethics approval was not required. All procedures complied with institutional guidelines for the ethical use of animal by-products in research.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Abbreviations
| CQDs | Carbon Quantum Dots |
| HbO2 | Hemoglobin oxygen |
| FI | Fluorescence Intensity |
| DI | Deionized (water) |
| PRBC | Packed Red Blood Cell |
| OxyHb | Oxyhemoglobin |
| DeoxyHb | Deoxyhemoglobin |
| SD | Standard Deviation |
| ANCOVA | Analysis of Covariance |
| R2 | Coefficient of determination |
| p | p-value |
| Na2S2O4 | Sodium Dithionite |
| UV | Ultraviolet |
References
- Hafen, B.B.; Sharma, S. Oxygen Saturation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Peterson, B.K. In Physical Rehabilitation; Cameron, M.H., Monroe, L.G., Eds.; Vital Signs. Chapter 22; W.B. Saunders: Saint Louis, MO, USA, 2007; pp. 598–624. [Google Scholar]
- Samuel, J.; Franklin, C. In Common Surgical Diseases: An Algorithmic Approach to Problem Solving; Myers, J.A., Millikan, K.W., Saclarides, T.J., Eds.; Hypoxemia and Hypoxia. Springer New York: New York, NY, USA, 2008; pp. 391–394. [Google Scholar]
- American Thoracic Society. ATS/ACCP Statement on cardiopulmonary exercise testing. Am. J. Respir. Crit. Care Med. 2003, 167, 211–277. (In English) [Google Scholar] [CrossRef]
- Premasiri, W.R.; Lee, J.C.; Ziegler, L.D. Surface-enhanced Raman scattering of whole human blood, blood plasma, and red blood cells: Cellular processes and bioanalytical sensing. J. Phys. Chem. B 2012, 116, 9376–9386. (In English) [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. Luminescent sensing and imaging of oxygen: Fierce competition to the Clark electrode. BioEssays 2015, 37, 921–928. (In English) [Google Scholar] [CrossRef] [PubMed]
- Johny, V.; Chinmaya, K.V.; Nihal, C.V.M.; Kurian, V.; Rao, G.M.; Ghosh, M.; Ghosh, S. Towards Real-Time Oxygen Sensing: From Nanomaterials to Plasma. Front. Sens. 2022, 2, 826403. (In English) [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. (In English) [Google Scholar] [CrossRef]
- Elugoke, S.E.; Uwaya, G.E.; Quadri, T.W.; Ebenso, E.E. Carbon Quantum Dots: Basics, Properties, and Fundamentals. In Carbon Dots: Recent Developments and Future Perspectives; Chapter 1; ACS Symposium Series, No. 1465; American Chemical Society: Washington, DC, USA, 2024; Volume 1465, pp. 3–42. [Google Scholar]
- Park, S.Y.; Tan, J.K.S.; Mo, X.; Song, Y.; Lim, J.; Liew, X.R.; Chung, H.; Kim, S. Carbon Quantum Dots with Tunable Size and Fluorescence Intensity for Development of a Nano-biosensor. Small 2025, 21, e2404524. (In English) [Google Scholar] [CrossRef]
- Briely-Sabo, K.; Bjornerud, A. Accurate de-oxygenation of ex vivo whole blood using sodium Dithionite. Proc. Intl. Soc. Mag. Reson. Med. 2000, 8, 2025. [Google Scholar]
- Meng, F.; Alayash, A.I. Determination of extinction coefficients of human hemoglobin in various redox states. Anal. Biochem. 2017, 521, 11–19. [Google Scholar] [CrossRef]
- Benesch, R.E.; Benesch, R.; Yung, S. Equations for the spectrophotometric analysis of hemoglobin mixtures. Anal. Biochem. 1973, 55, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Oxidative reactions of hemoglobin. Methods Enzymol. 1990, 186, 265–272. [Google Scholar] [CrossRef]
- Morgan, T.J. The oxyhaemoglobin dissociation curve in critical illness. Crit. Care Resusc. 1999, 1, 93–100. (In English) [Google Scholar] [CrossRef]
- Prahl, S. Tabulated Molar Extinction Coefficient for Hemoglobin in Water; Oregon Medical Laser Center: Portland, OR, USA, 1998. [Google Scholar]
- Patel, M.P.; Siu, V.; Silva-Garcia, A.; Xu, Q.; Li, Z.; Oksenberg, D. Development and validation of an oxygen dissociation assay, a screening platform for discovering, and characterizing hemoglobin-oxygen affinity modifiers. Drug Des. Devel. Ther. 2018, 12, 1599–1607. [Google Scholar] [CrossRef]
- Castro, D.; Patil, S.M.; Zubair, M.; Keenaghan, M. Arterial Blood Gas. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse oximetry: Understanding its basic principles facilitates appreciation of its limitations. Respir. Med. 2013, 107, 789–799. (In English) [Google Scholar] [CrossRef]
- Abraham, E.A.; Verma, G.; Arafat, Y.; Acharya, S.; Kumar, S.; Pantbalekundri, N. Comparative Analysis of Oxygen Saturation by Pulse Oximetry and Arterial Blood Gas in Hypoxemic Patients in a Tertiary Care Hospital. Cureus 2023, 15, e42447. [Google Scholar] [CrossRef] [PubMed]
- Becker, P.; Blatt, S.; Pabst, A.; Heimes, D.; Al-Nawas, B.; Kammerer, P.W.; Thiem, D.G.E. Comparison of Hyperspectral Imaging and Microvascular Doppler for Perfusion Monitoring of Free Flaps in an In Vivo Rodent Model. J. Clin. Med. 2022, 11, 4134. [Google Scholar] [CrossRef]
- Calin, M.A.; Boiangiu, I.C.; Parasca, S.V.; Miclos, S.; Savastru, D.; Manea, D. Blood oxygenation monitoring using hyperspectral imaging after flap surgery. Spectrosc. Lett. 2017, 50, 150–155. (In English) [Google Scholar] [CrossRef]
- Sicher, C.; Rutkowski, R.; Lutze, S.; von Podewils, S.; Wild, T.; Kretching, M.; Daeschlein, G. Hyperspectral imaging as a possible tool for visualization of changes in hemoglobin oxygenation in patients with deficient hemodynamics—Proof of concept. Biomed. Eng./Biomed. Tech. 2018, 63, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. Hyperspectral imaging for non-invasive blood oxygen saturation assessment. Photodiagnosis Photodyn. Ther. 2024, 45, 104003. [Google Scholar] [CrossRef]
- Serianni, R.; Barash, J.; Bentley, T.; Sharma, P.; Fontana, J.L.; Via, D.; Duhm, J.; Bunger, R.; Mongan, P.D. Porcine-specific hemoglobin saturation measurements. J. Appl. Physiol. 2003, 94, 561–566. (In English) [Google Scholar] [CrossRef]
- Guensch, D.P.; Michel, M.C.; Huettenmoser, S.P.; Jung, B.; Gulac, P.; Segiser, A.; Longnus, S.L.; Fischer, K. The blood oxygen level dependent (BOLD) effect of in-vitro myoglobin and hemoglobin. Sci. Rep. 2021, 11, 11464. [Google Scholar] [CrossRef]
- Majid, M.A.; Ullah, H.; Alshehri, A.M.; Tabassum, R.; Aleem, A.; Khan, A.R.; Batool, Z.; Nazir, A.; Bibi, I. Development of novel polymer haemoglobin based particles as an antioxidant, antibacterial and an oxygen carrier agents. Sci. Rep. 2024, 14, 3031. [Google Scholar] [CrossRef] [PubMed]
- Herzfeld, J.; Seidel, N.E.; Taylor, M.P.; Droupadi, P.R.; Wang, N.E. Gentle Chemical Deoxygenation of Hemoglobin Solutions. Hemoglobin 1990, 14, 399–411. [Google Scholar] [CrossRef]
- Pires, I.S.; Belcher, D.A.; Palmer, A.F. Quantification of Active Apohemoglobin Heme-Binding Sites via Dicyanohemin Incorporation. Biochemistry 2017, 56, 5245–5259. (In English) [Google Scholar] [CrossRef]
- Hamada, K.; Okazaki, T.; Shukuya, R.; Kaziro, K. The deoxygenation of dilute oxyhemoglobin by sodium dithionite. J. Biochem. 1962, 52, 374–376. (In English) [Google Scholar] [CrossRef] [PubMed]
- Walker, H.K.; Hall, W.D.; Hurst, J.W. Clinical Methods: The History, Physical, and Laboratory Examinations, (In English), 3rd ed.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Nitzan, M.; Nitzan, I.; Arieli, Y. The Various Oximetric Techniques Used for the Evaluation of Blood Oxygenation. Sensors 2020, 20, 4844. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Y.; Yao, J. Photoacoustic tomography of blood oxygenation: A mini review. Photoacoustics 2018, 10, 65–73. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).