Enhanced Performance of La0.8Sr0.2FeO3-δ-Gd0.2Ce0.8O2-δ Cathode for Solid Oxide Fuel Cells by Surface Modification with BaCO3 Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials Preparation
2.2. Cell Fabrication and Testing
2.3. Characterizations
3. Results and Discussion
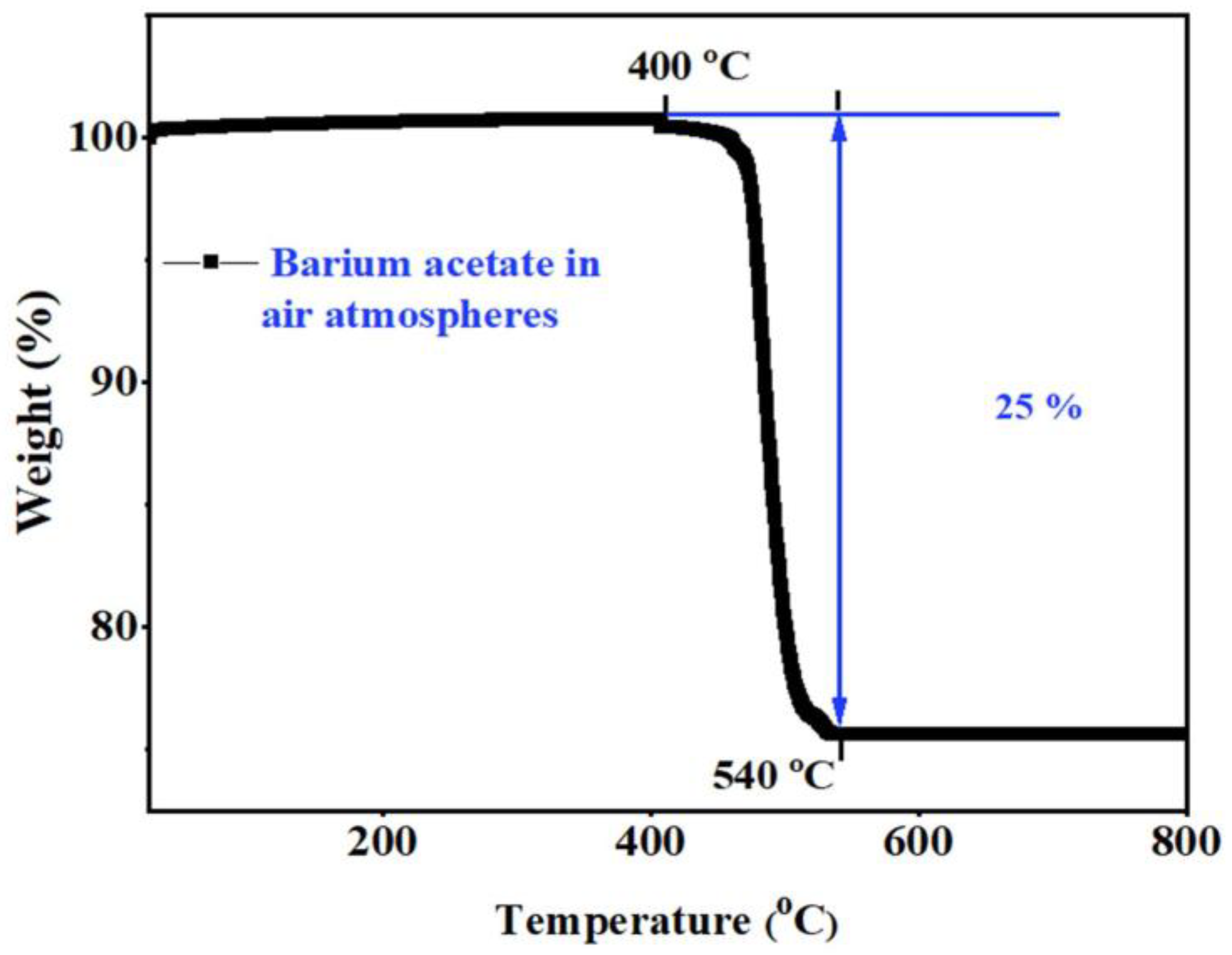
3.1. Thermal Analysis and Chemical Compatibility
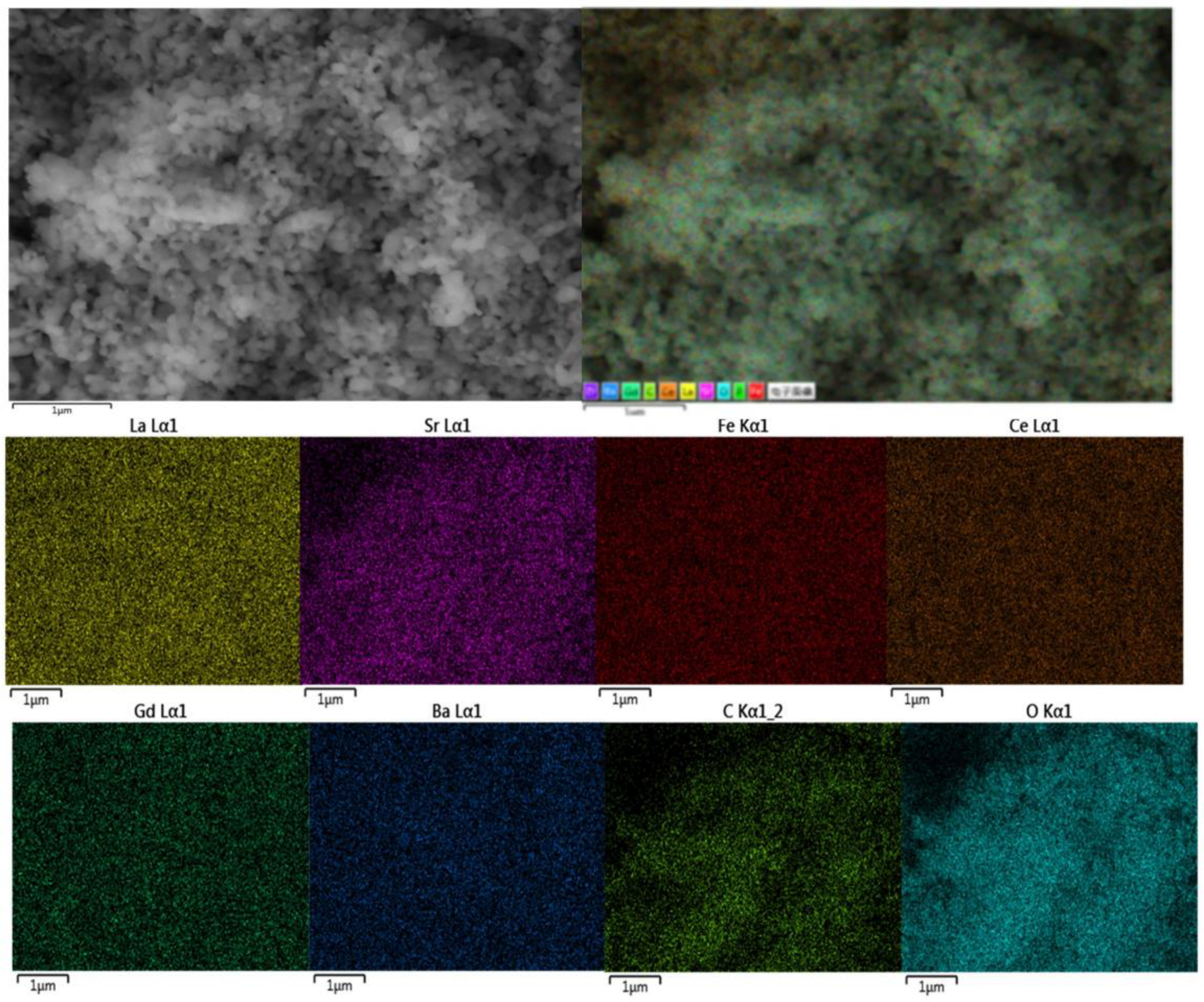
3.2. Microstructure Analysis
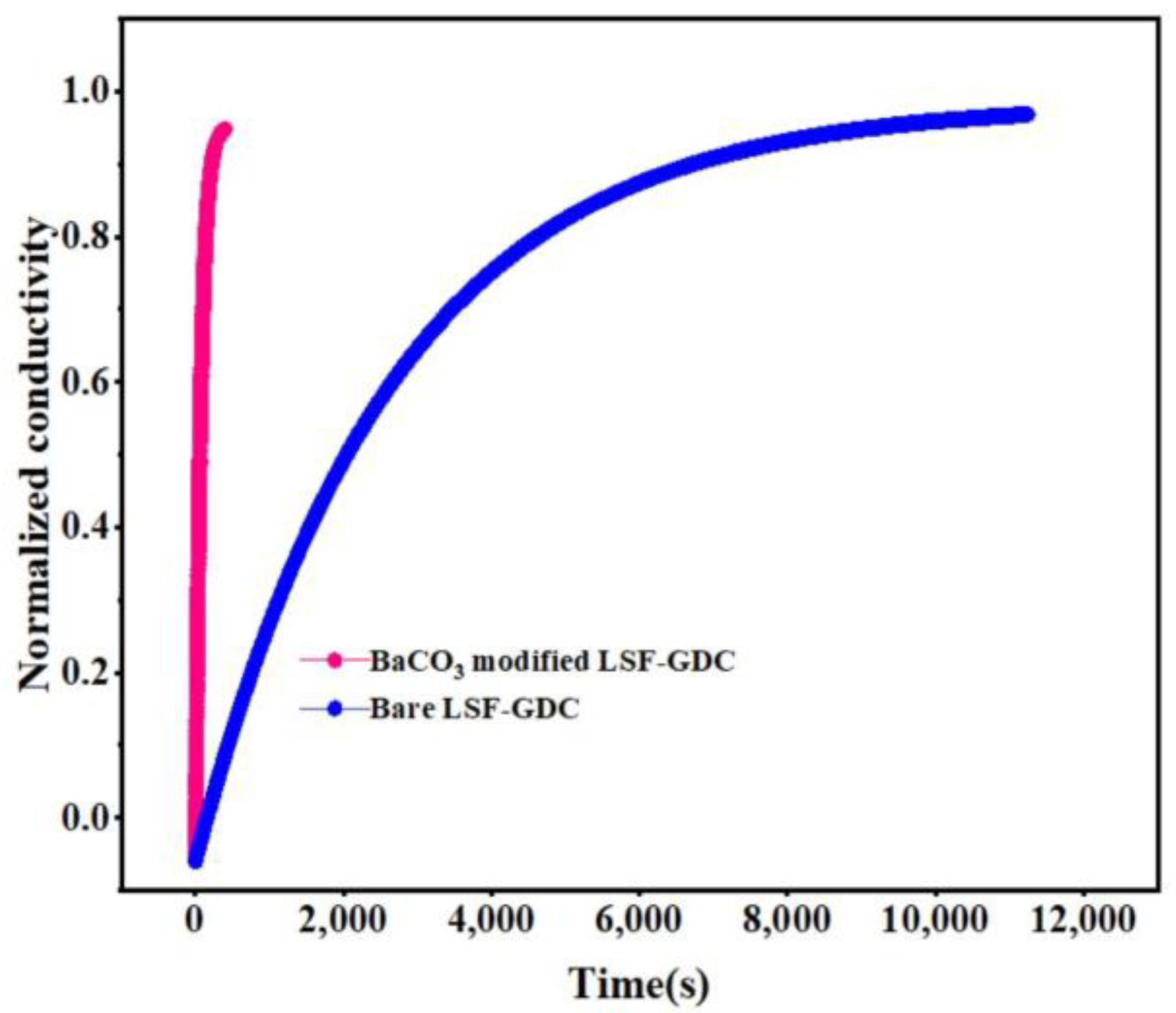
3.3. Electrochemical Activity
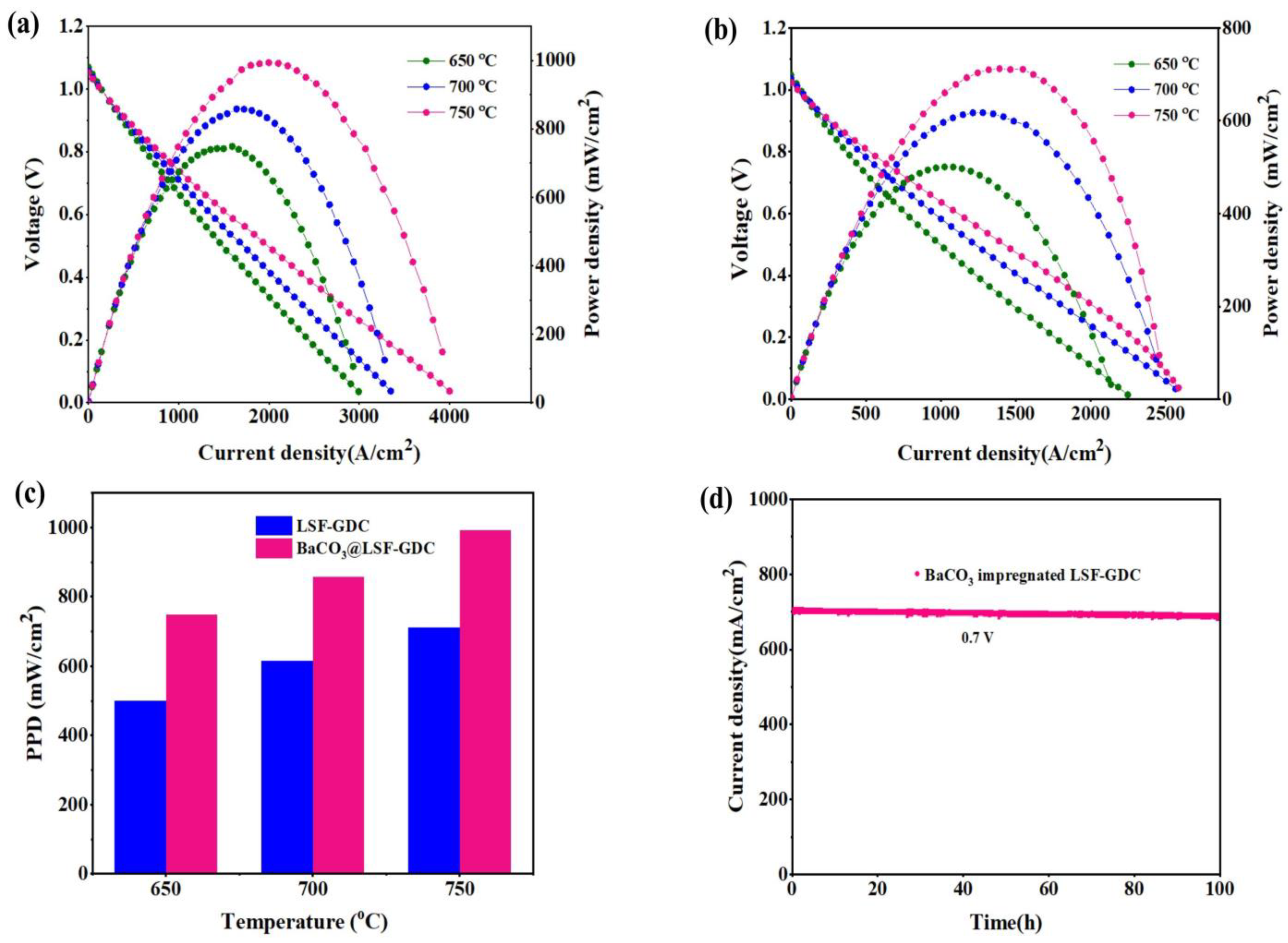
3.4. Cell Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Sun, Z.; Yang, W.; Hong, T.; Zhu, Z.; Zhang, Y.; Wu, X.; Xia, C. Mechanism for the enhanced oxygen reduction reaction of La0.6 Sr0.4Co0.2Fe0.8O3-δ by strontium carbonate. Phys. Chem. Chem. 2017, 19, 503–509. [Google Scholar]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Tung, T.T.; Mouss, M.; Tripathi, K.M.; Kim, T.; Nine, M.J.; Nanjundan, A.K.; Dubal, D.; Losic, D. Coupling graphene microribbons with carbon nanofibers: New carbon hybrids for high-performing lithium and potassium-ion batteries. Sustain. Mater. Technol. 2022, 32, e00393. [Google Scholar] [CrossRef]
- Jung, S.H.; Huong, P.T.; Sahani, S.; Tripathi, K.M.; Park, B.J.; Han, Y.H.; Kim, T.Y. Biomass-Derived Graphene-Based Materials Embedded with Onion-Like Carbons for High Power Supercapacitors. Electrochem. Soc. 2022, 169, 010509. [Google Scholar] [CrossRef]
- Dhiman, N.; Pradhan, D.; Mohanty, P. Heteroatom (N and P) enriched nanoporous carbon as an efficient electrocatalyst for the hydrazine oxidation reaction. Fuel 2022, 314, 122722. [Google Scholar] [CrossRef]
- Tsipis, E.V.; Kharton, V.V. Electrode materials and reaction mechanisms in solid oxide fuel cells: A brief review. J. Solid State Electrochem. 2008, 12, 1367–1391. [Google Scholar] [CrossRef]
- Xia, C.; Liu, M. Novel cathodes for low-temperature solid oxide fuel cells. Adv. Mater. 2002, 14, 521–523. [Google Scholar] [CrossRef]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Dyck, C.; Yu, Z.; Krstic, V. Thermal expansion matching of Gd1−xSrxCoO3−δ composite cathodes to Ce0.8Gd0.2O1.95 IT-SOFC electrolytes. Solid State Ion. 2004, 171, 17–23. [Google Scholar] [CrossRef]
- Mastin, J.; Einarsrud, M.-A.; Grande, T. Structural and Thermal Properties of La1-xSrxCoO3-δ. Chem. Mate. 2006, 18, 6047–6053. [Google Scholar] [CrossRef]
- Lee, K.; Manthiram, A. LaSr3Fe3-yCoyO10-δ (0 ≤ y ≤ 1.5) Intergrowth Oxide Cathodes for Intermediate Temperature Solid Oxide Fuel Cells. Chem. Mater. 2006, 18, 1621–1626. [Google Scholar] [CrossRef]
- Ji, H.-I.; Hwang, J.; Yoon, K.J.; Son, J.-W.; Kim, B.-K.; Lee, H.-W.; Lee, J.-H. Enhanced oxygen diffusion in epitaxial lanthanum–strontium–cobaltite thin film cathodes for micro solid oxide fuel cells. Energy Environ. Sci. 2013, 6, 116–120. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Zheng, Y.; Chen, J.; Yu, B.; Chen, Y.; Liu, M. Controlling cation segregation in perovskite-based electrodes for high electro-catalytic activity and durability. Chem. Soc. Rev. 2017, 46, 6345–6378. [Google Scholar] [CrossRef] [PubMed]
- Yokokawa, H.; Tu, H.; Iwanschitz, B.; Mai, A. Fundamental mechanisms limiting solid oxide fuel cell durability. J. Power Sources 2008, 182, 400–412. [Google Scholar] [CrossRef]
- Baharuddin, N.A.; Muchtar, A.; Somalu, M.R. Short review on cobalt-free cathodes for solid oxide fuel cells. J. Hydrog. Energy 2017, 42, 9149–9155. [Google Scholar] [CrossRef]
- Ren, Y.; Küngas, R.; Gorte, R.J.; Deng, C. The effect of A-site cation (Ln = La, Pr, Sm) on the crystal structure, conductivity, and oxygen reduction properties of Sr-doped ferrite perovskites. Solid State Ion. 2012, 212, 47–54. [Google Scholar] [CrossRef]
- Fu, X.; Liu, M.; Meng, X.; Lü, S.; Wang, D.; Zhang, Y.; Liu, H.; Song, M.; Li, Z.; Wang, L. Cobalt-free perovskite Ln0.5Sr0.5Fe0.8Cu0.2O3-δ (Ln = Pr, Nd, Sm, and Gd) as cathode for the intermediate-temperature solid oxide fuel cell. Ionics 2020, 26, 1285–1295. [Google Scholar] [CrossRef]
- Vázquez, S.; Basbus, J.; Soldati, A.L.; Napolitano, F.; Serquis, A.; Suescun, L. Effect of the symmetric cell preparation temperature on the activity of Ba0.5Sr0.5Fe0.8Cu0.2O3-δ as cathode for intermediate temperature Solid Oxide Fuel Cells. J. Power Sources. 2015, 274, 318–323. [Google Scholar] [CrossRef]
- Long, W.; Xu, H.; He, T. Preparation and electrochemical performance of cobalt-free cathode material Ba0.5Sr0.5Fe0.9Nb0.1O3-δ for intermediate-temperature solid oxide fuel cells. Chem. Res. Chin. Univ. 2014, 30, 806–810. [Google Scholar] [CrossRef]
- Hansen, K.K. Evaluation of based SOFC cathodes using cone-shaped electrodes and EIS. Solid State Ion. 2020, 344, 115096. [Google Scholar] [CrossRef]
- Paiva, J.A.E.; Daza, P.C.C.; Rodrigues, F.A.; Ortiz-Mosquera, J.F.; da Silva, C.R.M.; Munoz, M.M.; Meneses, R.A.M. Synthesis and electrical properties of strontium-doped lanthanum ferrite with perovskite-type structure. Ceram. Int. 2020, 46, 18419–18427. [Google Scholar] [CrossRef]
- Tatko, M.; Mosiałek, M.; Kędra, A.; Bielańska, E.; Ruggiero-Mikołajczyk, M.; Nowak, P. Thermal shock resistant composite cathode material Sm0.5Sr0.5CoO3–δ–La0.6Sr0.4FeO3–δ for solid oxide fuel cells. J. Solid State Electrochem. 2016, 20, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Li, J.; Wang, L.; Guo, X.; Sun, H.; Zhang, H.; Hu, Q. Improved electrochemical performance of Bi-doped La0.8Sr0.2FeO3-δ nanofiber cathode for IT-SOFCs via electrospinning. Ceram. Int. 2021, 47, 534–540. [Google Scholar] [CrossRef]
- Khoshkalam, M.; Tripković, Ð.; Tong, X.; Faghihi-Sani, M.A.; Chen, M.; Hendriksen, P.V. Improving oxygen incorporation rate on (La0.6Sr0.4)0.98FeO3-δ via Pr2Ni1-xCuxO4+δ surface decoration. J. Power Sources 2020, 457, 228035. [Google Scholar] [CrossRef]
- Lin, Q.; Lin, J.; Liu, T.; Xia, C.; Chen, C. Solid oxide fuel cells supported on cathodes with large straight open pores and catalyst-decorated surfaces. Solid State Ion. 2018, 323, 130–135. [Google Scholar] [CrossRef]
- Hong, T.; Chen, F.; Xia, C. Barium carbonate nanoparticle to enhance oxygen reduction activity of strontium doped lanthanum ferrite for solid oxide fuel cell. J. Power Sources 2015, 278, 741–750. [Google Scholar] [CrossRef]
- Hong, T.; Brinkman, K.S.; Xia, C. Barium Carbonate Nanoparticles as Synergistic Catalysts for the Oxygen Reduction Reaction on La0.6Sr0.4Co0.2Fe0.8O3-δ Solid-Oxide Fuel Cell Cathodes. Chem. Electro. Chem. 2016, 3, 805–813. [Google Scholar]
- Pechini, M.P. Method of preparing lead and alkaline earth titanates and niobates and coating method using the same to form a capacitor. U.S. Patent 3,330,697, 26 August 1967. [Google Scholar]
- Hong, T.; Lee, S.; Ohodnicki, P.; Brinkman, K. A highly scalable spray coating technique for electrode infiltration: Barium carbonate infiltrated La0.6Sr0.4Co0.2Fe0.8O3-δ perovskite structured electrocatalyst with demonstrated long-term durability. Int. J. Hydrog. Energy 2017, 42, 24978–24988. [Google Scholar] [CrossRef]
- Leng, Y.; Chan, S.H.; Liu, Q. Development of LSCF-GDC composite cathodes for low-temperature solid oxide fuel cells with thin-film GDC electrolyte. Int. J. Hydrog. Energy 2008, 33, 3808–3817. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Ren, Y.; Li, Y.; Xia, C. Magnesium oxide as a synergistic catalyst for oxygen reduction reaction on strontium doped lanthanum cobalt ferrite. Int. J. Hydrog. Energy 2018, 43, 3797–3802. [Google Scholar] [CrossRef]
- Zhang, L.; Hong, T.; Li, Y.; Xia, C. CaO effect on the electrochemical performance of lanthanum strontium cobalt ferrite cathode for the intermediate-temperature solid oxide fuel cell. Int. J. Hydrog. Energy 2017, 42, 17242–17250. [Google Scholar] [CrossRef]
- Hwang, U.-Y.; Park, H.-S.; Koo, K.-K. The behavior of barium acetate and titanium isopropoxide during the formation of crystalline barium titanate. Ind. Eng. Chem. Res. 2004, 43, 728–734. [Google Scholar] [CrossRef]
- Guan, C.; Wang, Y.; Chen, K.; Xiao, G.; Lin, X.; Zhou, J.; Song, S.; Wang, J.-Q.; Zhu, Z.; Zhou, X.-D. Molten salt synthesis of Nb-doped (La, Sr) FeO3 as the oxygen electrode for reversible solid oxide cells. Mater. Lett. 2019, 245, 114–117. [Google Scholar] [CrossRef]
- Gao, Z.; Barnett, S.A. Reduced-Temperature Firing of Anode Supported Solid Oxide Fuel Cells. ECS Trans. 2013, 58, 231. [Google Scholar] [CrossRef]
- Von Dollen, P.; Barnett, S. A Study of Screen Printed Yttria Stabilized Zirconia Layers for Solid Oxide Fuel Cells. J. Am. Ceram Soc. 2005, 88, 3361–3368. [Google Scholar] [CrossRef]
- Choi, M.-B.; Singh, B.; Wachsman, E.D.; Song, S.-J. Performance of La0.1Sr0.9Co0.8Fe0.2O3-δ and La0.1Sr0.9Co0.8Fe0.2O3-δ-Ce0.9Gd0.1O2 Oxygen Electrodes with Ce0.9Gd0.1O2 Barrier Layer in Reversible Solid Oxide Fuel Cells. J. Power Sources 2013, 239, 361–373. [Google Scholar] [CrossRef]
- Kilner, J.A.; Burriel, M. Materials for intermediate-temperature solid-oxide fuel cells, Annu. Rev. Mater. Res. 2014, 44, 365–393. [Google Scholar] [CrossRef]



| Bare | Kchem | Modified with | Kchem | T(°C) | Ref. |
|---|---|---|---|---|---|
| La0.8Sr0.2FeO3-δ-Gd0.2Ce0.8O2-δ | 1.256 10−5 | 0.65 mg/cm2 BaCO3 | 2.194 10−4 | 750 | This work |
| La0.8Sr0.2FeO3-δ | 1 10−5 | 0.95 mg/cm2 BaCO3 | 9.9 10−5 | 700 | [25] |
| La0.6Sr0.4Co0.2Fe0.8O3-δ | 1.8 10−5 | 0.85 mg/cm2 BaCO3 | 1.5 10−4 | 700 | [26] |
| La0.6Sr0.4Co0.2Fe0.8O3-δ | 4.0 10−5 | 0.055 mg/cm2 MgO | 9.49 10−5 | 750 | [30] |
| La0.6Sr0.4Co0.2Fe0.8O3-δ | 1.8 10−5 | 0.07 mg/cm2 CaO | 2.81 10−4 | 700 | [31] |
| La0.6Sr0.4Co0.2Fe0.8O3-δ | 2.2 10−5 | SrCO3 | 2.4 10−3 | 700 | [32] |
| Anode | Electrolyte | Buffer Layer | Cathode | PPD (650 °C mW/cm2) | PPD (700 °C mW/cm2) | PPD (750 °C mW/cm2) | Ref. |
|---|---|---|---|---|---|---|---|
| NiO-YSZ | YSZ | GDC | BaCO3@LSF-GDC | 748 | 858 | 993 | This work |
| NiO-YSZ | YSZ | GDC | LSCF-GDC | 410 | 680 | 1030 | [34] |
| NiO-YSZ | YSZ | GDC | LSCF-GDC | 380 | 650 | 1020 | [35] |
| NiO-YSZ | YSZ | GDC | LSCF-GDC | - | 540 | 990 | [36] |
| NiO-YSZ | YSZ | GDC | LSCF-GDC | 580 | 840 | 1080 | [37] |
| NiO-YSZ | YSZ | GDC | LSCF-GDC | 562 | [38] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desta, H.G.; Yang, Y.; Teketel, B.S.; Yang, Q.; Song, K.; Zhu, S.; Tian, D.; Chen, Y.; Luo, T.; Lin, B. Enhanced Performance of La0.8Sr0.2FeO3-δ-Gd0.2Ce0.8O2-δ Cathode for Solid Oxide Fuel Cells by Surface Modification with BaCO3 Nanoparticles. Micromachines 2022, 13, 884. https://doi.org/10.3390/mi13060884
Desta HG, Yang Y, Teketel BS, Yang Q, Song K, Zhu S, Tian D, Chen Y, Luo T, Lin B. Enhanced Performance of La0.8Sr0.2FeO3-δ-Gd0.2Ce0.8O2-δ Cathode for Solid Oxide Fuel Cells by Surface Modification with BaCO3 Nanoparticles. Micromachines. 2022; 13(6):884. https://doi.org/10.3390/mi13060884
Chicago/Turabian StyleDesta, Halefom G., Yang Yang, Birkneh Sirak Teketel, Quan Yang, Kai Song, Shiyue Zhu, Dong Tian, Yonghong Chen, Tianyong Luo, and Bin Lin. 2022. "Enhanced Performance of La0.8Sr0.2FeO3-δ-Gd0.2Ce0.8O2-δ Cathode for Solid Oxide Fuel Cells by Surface Modification with BaCO3 Nanoparticles" Micromachines 13, no. 6: 884. https://doi.org/10.3390/mi13060884
APA StyleDesta, H. G., Yang, Y., Teketel, B. S., Yang, Q., Song, K., Zhu, S., Tian, D., Chen, Y., Luo, T., & Lin, B. (2022). Enhanced Performance of La0.8Sr0.2FeO3-δ-Gd0.2Ce0.8O2-δ Cathode for Solid Oxide Fuel Cells by Surface Modification with BaCO3 Nanoparticles. Micromachines, 13(6), 884. https://doi.org/10.3390/mi13060884






