Dynamic Duo—The Salmonella Cytolethal Distending Toxin Combines ADP-Ribosyltransferase and Nuclease Activities in a Novel Form of the Cytolethal Distending Toxin
Abstract
:1. Introduction
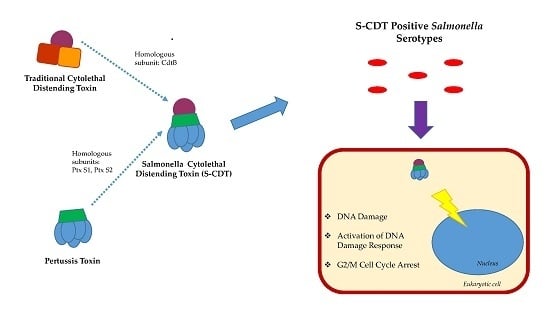
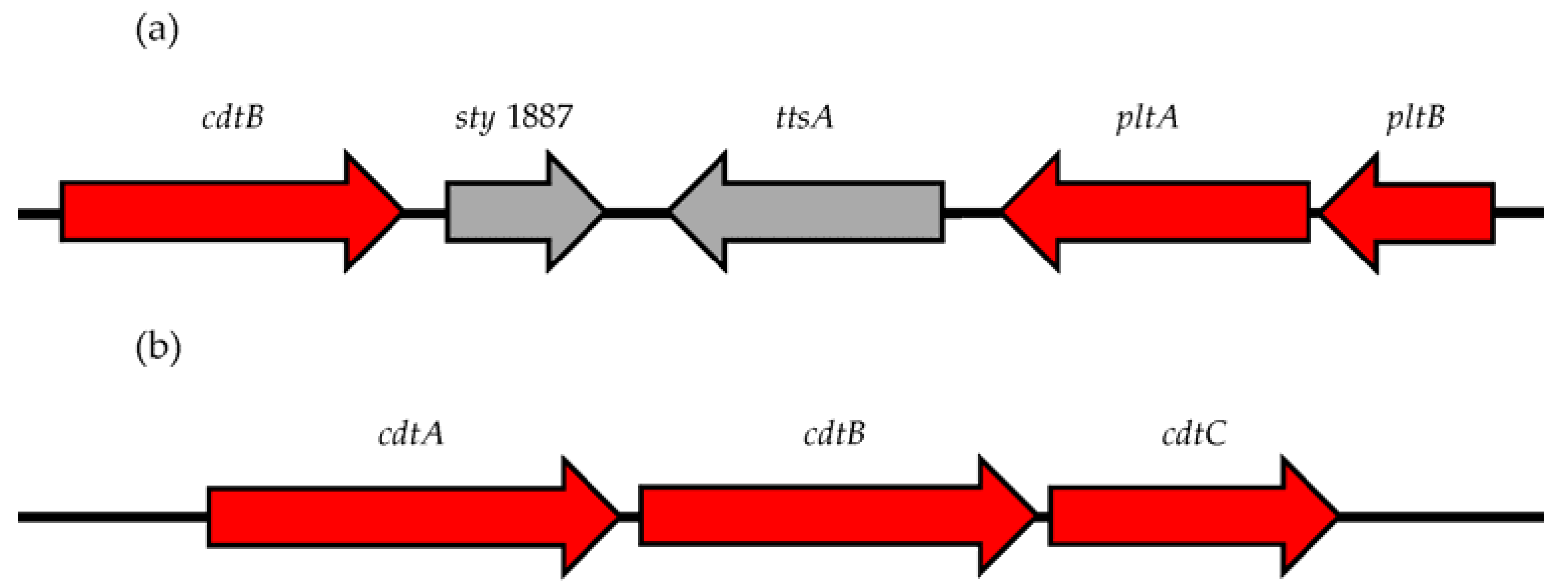
2. Salmonella Encodes a Novel Form of CDT
3. Regulation of S-CDT Expression
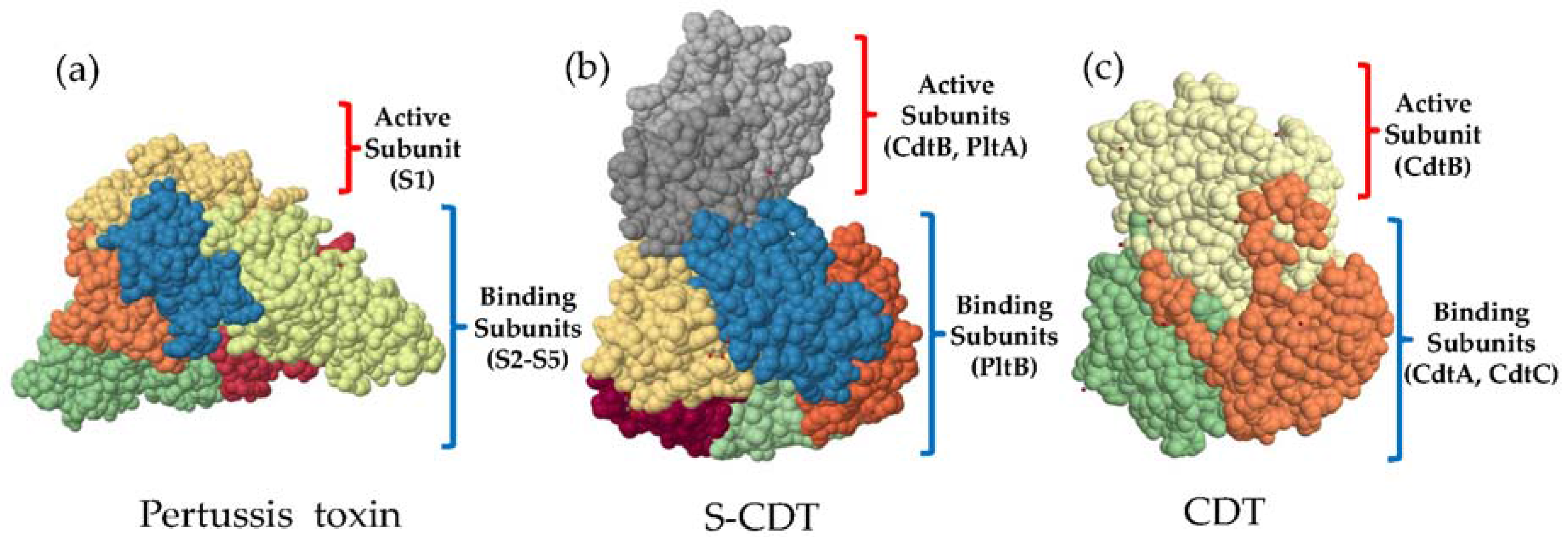
4. ArtA and ArtB and Their Relationship to S-CDT
5. Structure and Function of S-CDT
6. Mechanism of Action
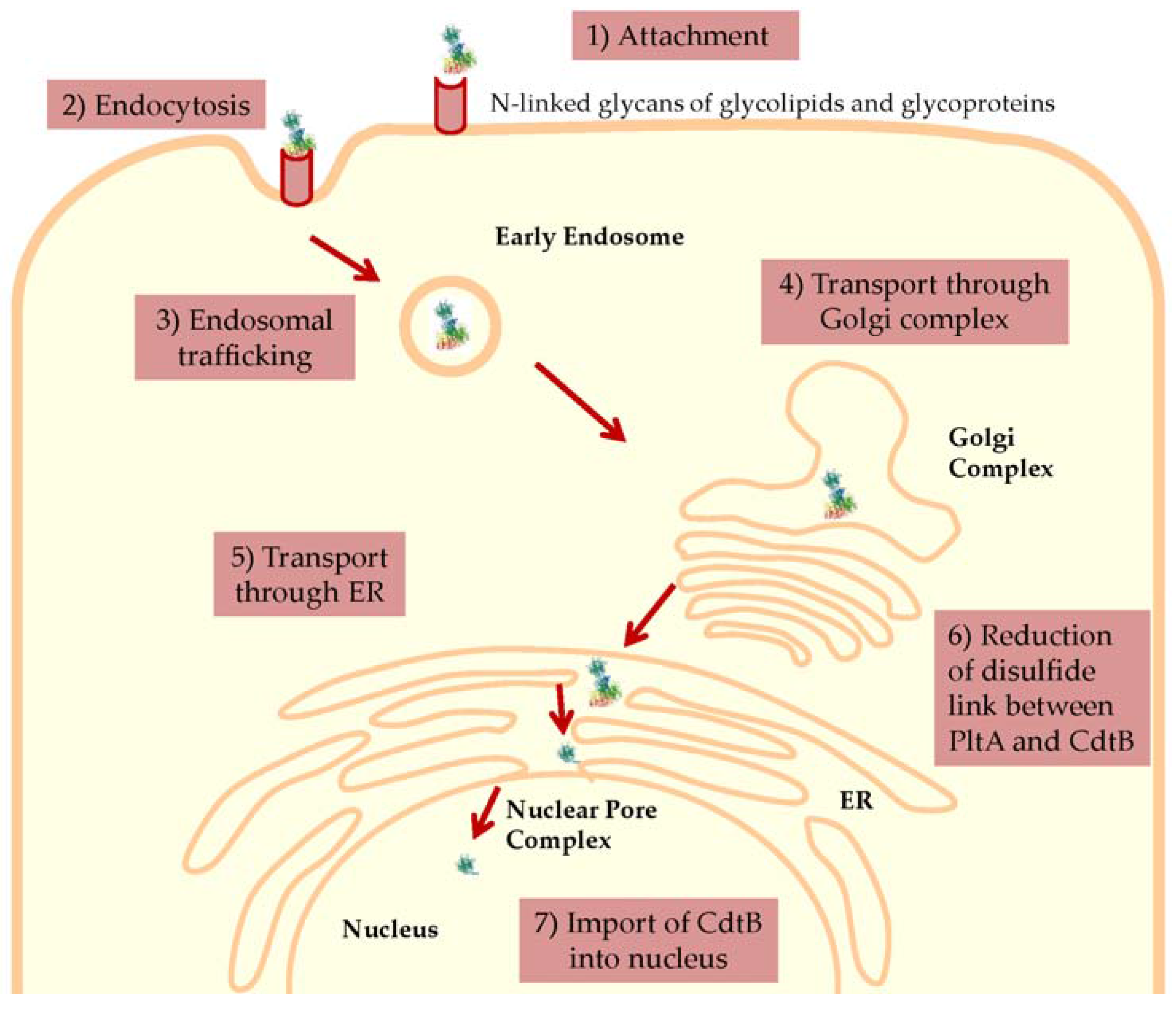
6.1. S-CDT Uses Multiple Host Cell Receptors Enabling it to Intoxicate a Wide Variety of Cell Types
6.2. Entry and Trafficking of S-CDT
7. S-CDT’s Role in Virulence
7.1. DNA Damage and Induction of the DNA Damage Response
7.2. Apoptosis of Immune Cells and Host Immune Suppression
7.3. Tumorigenesis and Carcinogenic Potential
7.4. Administration of S-CDT May Recapitulate Symptoms of Typhoid Fever
7.5. Persistence and Chronic Infection
8. Discussion and Future Directions
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| S-CDT | Salmonella Cytolethal Distending Toxin |
| CDT | Cytolethal Distending Toxin |
| NTS | nontyphoidal Salmonella |
| DDR | DNA Damage Response |
| SCV | Salmonella Containing Vacuole |
References
- Brenner, F.; Villar, R.; Angulo, F.; Tauxe, R.; Swaminathan, B. Salmonella nomenclature. J. Clin. Microbiol. 2000, 38, 2465–2467. [Google Scholar] [PubMed]
- Issenhuth-Jeanjean, S.; Roggentin, P.; Mikoleit, M.; Guibourdenche, M.; de Pinna, E.; Nair, S.; Fields, P.I.; Weill, F.-X. Supplement 2008–2010 (no. 48) to the White–Kauffmann–Le Minor scheme. Res. Microbiol. 2014, 165, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Crump, J.A.; Mintz, E.D. Global trends in typhoid and paratyphoid fever. Clin. Infect. Dis. 2010, 50, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.-A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the united states—Major pathogens. Emerg. Infect. Dis. 2011, 17, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Majowicz, S.E.; Musto, J.; Scallan, E.; Angulo, F.J.; Kirk, M.; O’Brien, S.J.; Jones, T.F.; Fazil, A.; Hoekstra, R.M. The global burden of nontyphoidal Salmonella gastroenteritis. Clin. Infect. Dis. 2010, 50, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.F.; Ingram, L.A.; Cieslak, P.R.; Vugia, D.J.; Tobin-D’Angelo, M.; Hurd, S.; Medus, C.; Cronquist, A.; Angulo, F.J. Salmonellosis outcomes differ substantially by serotype. J. Infect. Dis. 2008, 198, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Uzzau, S.; Brown, D.J.; Wallis, T.; Rubino, S.; Leori, G.; Bernard, S.; Casadesús, J.; Platt, D.J.; Olsen, J.E. Host adapted serotypes of Salmonella enterica. Epidemiol. Infect. 2000, 125, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Gao, X.; Galán, J.E. Structure and function of the Salmonella Typhi chimaeric A2B5 typhoid toxin. Nature 2013, 499, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Porwollik, S.; Dagan, A.; Marzel, A.; Schorr, Y.I.; Desai, P.T.; Agmon, V.; McClelland, M.; Rahav, G.; Gal-Mor, O. Virulence gene profiling and pathogenicity characterization of non-typhoidal Salmonella accounted for invasive disease in humans. PLoS ONE 2013, 8, e58449. [Google Scholar] [CrossRef] [PubMed]
- Gargi, A.; Reno, M.; Blanke, S.R. Bacterial toxin modulation of the eukaryotic cell cycle: Are all cytolethal distending toxins created equally? Front. Cell. Infect. Microbiol. 2012, 2, 124. [Google Scholar] [CrossRef] [PubMed]
- Spanò, S.; Ugalde, J.E.; Galán, J.E. Delivery of a Salmonella Typhi exotoxin from a host intracellular compartment. Cell Host Microbe 2008, 3, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Haghjoo, E.; Galán, J.E. Salmonella Typhi encodes a functional cytolethal distending toxin that is delivered into host cells by a bacterial-internalization pathway. Proc. Natl. Acad. Sci. USA 2004, 101, 4614–4619. [Google Scholar] [CrossRef] [PubMed]
- Den Bakker, H.C.; Switt, A.I.M.; Govoni, G.; Cummings, C.A.; Ranieri, M.L.; Degoricija, L.; Hoelzer, K.; Rodriguez-Rivera, L.D.; Brown, S.; Bolchacova, E. Genome sequencing reveals diversification of virulence factor content and possible host adaptation in distinct subpopulations of Salmonella enterica. BMC Genom. 2011, 12, 425. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Lior, H. Response of chinese hamster ovary cells to a cytolethal distending toxin (CDT) of Escherichia coli and possible misinterpretation as heat-labile (LT) enterotoxin. FEMS Microbiol. Lett. 1987, 43, 19–23. [Google Scholar] [CrossRef]
- Asakura, M.; Samosornsuk, W.; Taguchi, M.; Kobayashi, K.; Misawa, N.; Kusumoto, M.; Nishimura, K.; Matsuhisa, A.; Yamasaki, S. Comparative analysis of cytolethal distending toxin (CDT) genes among Campylobacter jejuni, C. coli and C. fetus strains. Microb. Pathog. 2007, 42, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, C.A.; Balbo, P.B.; Pesci, E.C.; Cottle, D.L.; Mirabito, P.M.; Pickett, C.L. Campylobacter jejuni cytolethal distending toxin causes a G2-phase cell cycle block. Infect. Immun. 1998, 66, 1934–1940. [Google Scholar] [PubMed]
- Mooney, A.; Clyne, M.; Curran, T.; Doherty, D.; Kilmartin, B.; Bourke, B. Campylobacter upsaliensis exerts a cytolethal distending toxin effect on Hela cells and T lymphocytes. Microbiology 2001, 147, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Kamei, K.; Asakura, M.; Somroop, S.; Hatanaka, N.; Hinenoya, A.; Nagita, A.; Misawa, N.; Matsuda, M.; Nakagawa, S.; Yamasaki, S. A PCR-RFLP assay for the detection and differentiation of Campylobacter jejuni, C. coli, C. fetus, C. hyointestinalis, C. lari, C. helveticus and C. upsaliensis. J. Med. Microbiol. 2014, 63, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Cope, L.D.; Lumbley, S.; Latimer, J.L.; Klesney-Tait, J.; Stevens, M.K.; Johnson, L.S.; Purven, M.; Munson, R.S.; Lagergard, T.; Radolf, J.D. A diffusible cytotoxin of Haemophilus ducreyi. Proc. Natl. Acad. Sci. USA 1997, 94, 4056–4061. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhang, Q.; Zhao, J.; Jin, M. Haemophilus parasuis encodes two functional cytolethal distending toxins: CdtC contains an atypical cholesterol recognition/interaction region. PLoS ONE 2012, 7, e32580. [Google Scholar]
- Belibasakis, G.N.; Mattsson, A.; Wang, Y.; Chen, C.; Johansson, A. Cell cycle arrest of human gingival fibroblasts and periodontal ligament cells by Actinobacillus actinomycetemcomitans: Involvement of the cytolethal distending toxin. Apmis 2004, 112, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Young, V.B.; Knox, K.A.; Schauer, D.B. Cytolethal distending toxin sequence and activity in the enterohepatic pathogen Helicobacter hepaticus. Infect. Immun. 2000, 68, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.S.; Ge, Z.; Shen, Z.; Dewhirst, F.E.; Fox, J.G. Cytolethal distending toxin: A potential virulence factor for Helicobacter cinaedi. J. Infect. Dis. 2003, 188, 1892–1897. [Google Scholar] [CrossRef] [PubMed]
- Young, V.B.; Chien, C.-C.; Knox, K.A.; Taylor, N.S.; Schauer, D.B.; Fox, J.G. Cytolethal distending toxin in avian and human isolates of Helicobacter pullorum. J. Infect. Dis. 2000, 182, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, N.P.; Dassanayake, R.P.; Kuszynski, C.A.; Duhamel, G.E. Contribution of Helicobacter hepaticus cytolethal distending toxin subunits to human epithelial cell cycle arrest and apoptotic death in vitro. Helicobacter 2013, 18, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Bratti, X.; Frisan, T.; Thelestam, M. The cytolethal distending toxins induce DNA damage and cell cycle arrest. Toxicon 2001, 39, 1729–1736. [Google Scholar] [CrossRef]
- Okuda, J.; Fukumoto, M.; Takeda, Y.; Nishibuchi, M. Examination of diarrheagenicity of cytolethal distending toxin: Suckling mouse response to the products of the CdtABC genes of Shigella dysenteriae. Infect. Immun. 1997, 65, 428–433. [Google Scholar] [PubMed]
- Shima, A.; Hinenoya, A.; Asakura, M.; Sugimoto, N.; Tsukamoto, T.; Ito, H.; Nagita, A.; Faruque, S.M.; Yamasaki, S. Molecular characterizations of cytolethal distending toxin produced by Providencia alcalifaciens strains isolated from patients with diarrhea. Infect. Immun. 2012, 80, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Parkhill, J.; Dougan, G.; James, K.; Thomson, N.; Pickard, D.; Wain, J.; Churcher, C.; Mungall, K.; Bentley, S.; Holden, M. Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18. Nature 2001, 413, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Locht, C.; Coutte, L.; Mielcarek, N. The ins and outs of pertussis toxin. FEBS J. 2011, 278, 4668–4682. [Google Scholar] [CrossRef] [PubMed]
- Paton, A.W.; Paton, J.C. Escherichia coli subtilase cytotoxin. Toxins 2010, 2, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Mezal, E.H.; Bae, D.; Khan, A.A. Detection and functionality of the CdtB, PltA, and PltB from Salmonella enterica serovar Javiana. Pathog. Dis. 2014, 72, 95–103. [Google Scholar] [PubMed]
- Williams, K.; Gokulan, K.; Shelman, D.; Akiyama, T.; Khan, A.; Khare, S. Cytotoxic mechanism of cytolethal distending toxin in nontyphoidal Salmonella serovar (Salmonella Javiana) during macrophage infection. DNA Cell Biol. 2015, 34, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rivera, L.D.; Bowen, B.M.; den Bakker, H.C.; Duhamel, G.E.; Wiedmann, M. Characterization of the cytolethal distending toxin (typhoid toxin) in non-typhoidal salmonella serovars. Gut Pathog. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.T.; Porwollik, S.; Long, F.; Cheng, P.; Wollam, A.; Clifton, S.W.; Weinstock, G.M.; McClelland, M. Evolutionary genomics of Salmonella enterica subspecies. mBio 2013, 4, e00579-12. [Google Scholar] [CrossRef] [PubMed]
- Allard, M.W.; Luo, Y.; Strain, E.; Li, C.; Keys, C.E.; Son, I.; Stones, R.; Musser, S.M.; Brown, E.W. High resolution clustering of Salmonella enterica serovar Montevideo strains using a next-generation sequencing approach. BMC Genom. 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.S.; Ong, G.; Song, K.P. Introns in the cytolethal distending toxin gene of Actinobacillus actinomycetemcomitans. J. Bacteriol. 2005, 187, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Tazumi, A.; Hirayama, J.; Hayashi, K.; Tasaki, E.; Asakura, M.; Yamasaki, S.; Moore, J.; Millar, B.; Matsubara, K. Expression and analysis of a cytolethal distending toxin (CDT) gene operon in Campylobacter lari. Br. J. Biomed. Sci. 2012, 69, 26. [Google Scholar] [PubMed]
- Haghjoo, E.; Galán, J.E. Identification of a transcriptional regulator that controls intracellular gene expression in Salmonella Typhi. Mol. Microbiol. 2007, 64, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Guidi, R.; Levi, L.; Rouf, S.F.; Puiac, S.; Rhen, M.; Frisan, T. Salmonella enterica delivers its genotoxin through outer membrane vesicles secreted from infected cells. Cell. Microbiol. 2013, 15, 2034–2050. [Google Scholar] [CrossRef] [PubMed]
- Charles, R.C.; Harris, J.B.; Chase, M.R.; Lebrun, L.M.; Sheikh, A.; LaRocque, R.C.; Logvinenko, T.; Rollins, S.M.; Tarique, A.; Hohmann, E.L. Comparative proteomic analysis of the PhoP regulon in Salmonella enterica serovar Typhi versus Typhimurium. PLoS ONE 2009, 4, e6994. [Google Scholar] [CrossRef] [PubMed]
- Hodak, H.; Galan, J.E. A Salmonella Typhi homologue of bacteriophage muramidases controls typhoid toxin secretion. EMBO Rep. 2013, 14, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Tanaka, K.; Nishimori, K.; Makino, S.-i.; Kanno, T.; Ishihara, R.; Hatama, S.; Kitano, R.; Kishima, M.; Sameshima, T. The artAB genes encode a putative ADP-ribosyltransferase toxin homologue associated with Salmonella enterica serovar Typhimurium DT104. Microbiology 2005, 151, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- UniProtKB–Q8Z6A3 (Q8Z6A3_SALTI). Available online: http://www.uniprot.org/uniprot/Q8Z6A3 (accessed on 3 March 2016).
- Wang, H.; Paton, J.C.; Herdman, B.P.; Rogers, T.J.; Beddoe, T.; Paton, A.W. The B subunit of an AB5 toxin produced by Salmonella enterica serovar Typhi up-regulates chemokines, cytokines, and adhesion molecules in human macrophage, colonic epithelial, and brain microvascular endothelial cell lines. Infect. Immun. 2013, 81, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Hazes, B.; Boodhoo, A.; Cockle, S.A.; Read, R.J. Crystal structure of the pertussis toxin–ATP complex: A molecular sensor. J. Mol. Biol. 1996, 258, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Nešić, D.; Hsu, Y.; Stebbins, C.E. Assembly and function of a bacterial genotoxin. Nature 2004, 429, 429–433. [Google Scholar] [CrossRef] [PubMed]
- UniProtKB–Q8Z6A4 (Q8Z6A4_SALTI). Available online: http://www.uniprot.org/uniprot/Q8Z6A4 (accessed on 26 January 2016).
- Carbonetti, N.H. Pertussis toxin and adenylate cyclase toxin: Key virulence factors of Bordetella pertussis and cell biology tools. Future Microbiol. 2010, 5, 455–469. [Google Scholar] [CrossRef] [PubMed]
- Aktories, K. Bacterial protein toxins that modify host regulatory GTPases. Nat. Rev. Microbiol. 2011, 9, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Jinadasa, R.N.; Bloom, S.E.; Weiss, R.S.; Duhamel, G.E. Cytolethal distending toxin: A conserved bacterial genotoxin that blocks cell cycle progression, leading to apoptosis of a broad range of mammalian cell lineages. Microbiology 2011, 157, 1851–1875. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; Dlakić, M.; Walker, L.P.; Besack, D.; Jaffe, E.; LaBelle, E.; Boesze-Battaglia, K. A novel mode of action for a microbial-derived immunotoxin: The cytolethal distending toxin subunit B exhibits phosphatidylinositol 3, 4, 5-triphosphate phosphatase activity. J. Immunol. 2007, 178, 5099–5108. [Google Scholar] [CrossRef] [PubMed]
- DiRienzo, J.M. Uptake and processing of the cytolethal distending toxin by mammalian cells. Toxins 2014, 6, 3098–3116. [Google Scholar] [CrossRef] [PubMed]
- Akifusa, S.; Heywood, W.; Nair, S.P.; Stenbeck, G.; Henderson, B. Mechanism of internalization of the cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Microbiology 2005, 151, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, A.; Maldonado-Arocho, F.J.; Gargi, A.; Cardwell, M.M.; Prouty, M.G.; Blanke, S.R.; Bradley, K.A. Cytolethal distending toxin family members are differentially affected by alterations in host glycans and membrane cholesterol. J. Biol. Chem. 2010, 285, 18199–18207. [Google Scholar] [CrossRef] [PubMed]
- Gargi, A.; Tamilselvam, B.; Powers, B.; Prouty, M.G.; Lincecum, T.; Eshraghi, A.; Maldonado-Arocho, F.J.; Wilson, B.A.; Bradley, K.A.; Blanke, S.R. Cellular interactions of the cytolethal distending toxins from Escherichia coli and Haemophilus ducreyi. J. Biol. Chem. 2013, 288, 7492–7505. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, L.A.; Dreyfus, L.A. Carbohydrate-binding specificity of the Escherichia coli cytolethal distending toxin CdtA and CdtC subunits. Infect. Immun. 2005, 73, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Mise, K.; Akifusa, S.; Watarai, S.; Ansai, T.; Nishihara, T.; Takehara, T. Involvement of ganglioside GM3 in G2/M cell cycle arrest of human monocytic cells induced by Actinobacillus actinomycetemcomitans cytolethal distending toxin. Infect. Immun. 2005, 73, 4846–4852. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Cortes-Bratti, X.; Guidi, R.; Frisan, T. The biology of the cytolethal distending toxins. Toxins 2011, 3, 172–190. [Google Scholar] [CrossRef] [PubMed]
- Chong, D.C.; Paton, J.C.; Thorpe, C.M.; Paton, A.W. Clathrin-dependent trafficking of subtilase cytotoxin, a novel AB5 toxin that targets the endoplasmic reticulum chaperone BiP. Cell. Microbiol. 2008, 10, 795–806. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Teter, K.; Lilley, B.N.; Stenerlöw, B.; Holmes, R.K.; Ploegh, H.L.; Sandvig, K.; Thelestam, M.; Frisan, T. Cellular internalization of cytolethal distending toxin: A new end to a known pathway. Cell. Microbiol. 2005, 7, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, A.; Dixon, S.D.; Tamilselvam, B.; Kim, E.J.-K.; Gargi, A.; Kulik, J.C.; Damoiseaux, R.; Blanke, S.R.; Bradley, K.A. Cytolethal distending toxins require components of the ER-associated degradation pathway for host cell entry. PLOS Pathog. 2014. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M. Orthobunyaviruses: Recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Fedor, Y.; Vignard, J.; Nicolau-Travers, M.L.; Boutet-Robinet, E.; Watrin, C.; Salles, B.; Mirey, G. From single-strand breaks to double-strand breaks during s-phase: A new mode of action of the Escherichia coli cytolethal distending toxin. Cell. Microbiol. 2013, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bezine, E.; Vignard, J.; Mirey, G. The cytolethal distending toxin effects on mammalian cells: A DNA damage perspective. Cells 2014, 3, 592–615. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Kim, S.-T.; Lim, D.-S.; Kastan, M.B. Two molecularly distinct G2/M checkpoints are induced by ionizing irradiation. Mol. Cell. Biol. 2002, 22, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; Demuth, D.R.; Zekavat, A. Exposure of lymphocytes to high doses of Actinobacillus actinomycetemcomitans cytolethal distending toxin induces rapid onset of apoptosis-mediated DNA fragmentation. Infect. and Immun. 2006, 74, 2080–2092. [Google Scholar] [CrossRef] [PubMed]
- Mangerich, A.; Knutson, C.G.; Parry, N.M.; Muthupalani, S.; Ye, W.; Prestwich, E.; Cui, L.; McFaline, J.L.; Mobley, M.; Ge, Z. Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer. Proc. Natl. Acad. Sci. 2012, 109, E1820–E1829. [Google Scholar] [CrossRef] [PubMed]
- Hassane, D.C.; Lee, R.B.; Pickett, C.L. Campylobacter jejuni cytolethal distending toxin promotes DNA repair responses in normal human cells. Infect. Immun. 2003, 71, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Alaoui-El-Azher, M.; Mans, J.J.; Baker, H.V.; Chen, C.; Progulske-Fox, A.; Lamont, R.J.; Handfield, M. Role of the ATM-checkpoint kinase 2 pathway in CDT-mediated apoptosis of gingival epithelial cells. PLoS ONE 2010, 5, e11714. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sharipo, A.; Chaves-Olarte, E.; Masucci, M.G.; Levitsky, V.; Thelestam, M.; Frisan, T. The Haemophilus ducreyi cytolethal distending toxin activates sensors of DNA damage and repair complexes in proliferating and non-proliferating cells. Cell. Microbiol. 2002, 4, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Nakashima, K.; Nagasawa, T.; Abiko, Y.; Furuichi, Y. Involvement of toll-like receptor 2 in apoptosis of Aggregatibacter actinomycetemcomitans-infected Thp-1 cells. J. Microbiol. Immunol. Infect. 2013, 46, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Rabin, S.D.; Flitton, J.G.; Demuth, D.R. Aggregatibacter actinomycetemcomitans cytolethal distending toxin induces apoptosis in nonproliferating macrophages by a phosphatase-independent mechanism. Infect. Immun. 2009, 77, 3161–3169. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Bratti, X.; Karlsson, C.; Lagergård, T.; Thelestam, M.; Frisan, T. The Haemophilus ducreyi cytolethal distending toxin induces cell cycle arrest and apoptosis via the DNA damage checkpoint pathways. J. Biol. Chem. 2001, 276, 5296–5302. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Majam, G.; Guerry, P. Intracellular survival of Campylobacter jejuni in human monocytic cells and induction of apoptotic death by cytholethal distending toxin. Infect. Immun. 2005, 73, 5194. [Google Scholar] [CrossRef] [PubMed]
- Bielaszewska, M.; Sinha, B.; Kuczius, T.; Karch, H. Cytolethal distending toxin from shiga toxin-producing Escherichia coli O157 causes irreversible G2/M arrest, inhibition of proliferation, and death of human endothelial cells. Infect Immun. 2005, 73, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Liyanage, N.P.; Manthey, K.C.; Dassanayake, R.P.; Kuszynski, C.A.; Oakley, G.G.; Duhamel, G.E. Helicobacter hepaticus cytolethal distending toxin causes cell death in intestinal epithelial cells via mitochondrial apoptotic pathway. Helicobacter 2010, 15, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Asakura, H.; Momose, Y.; Ryu, C.-H.; Kasuga, F.; Yamamoto, S.; Kumagai, S.; Igimi, S. Providencia alcalifaciens causes barrier dysfunction and apoptosis in tissue cell culture: Potent role of lipopolysaccharides on diarrheagenicity. Food Add. Contam. Part A 2013, 30, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Guidi, R.; Guerra, L.; Levi, L.; Stenerlöw, B.; Fox, J.G.; Josenhans, C.; Masucci, M.G.; Frisan, T. Chronic exposure to the cytolethal distending toxins of Gram-negative bacteria promotes genomic instability and altered DNA damage response. Cell.Microbiol. 2013, 15, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Lawrence, T.; Nizet, V. Innate immunity gone awry: Linking microbial infections to chronic inflammation and cancer. Cell 2006, 124, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Feng, Y.; Rogers, A.; Rickman, B.; Whary, M.; Xu, S.; Clapp, K.; Boutin, S.; Fox, J. Cytolethal distending toxin promotes Helicobacter cinaedi-associated typhlocolitis in interleukin-10-deficient mice. Infect. Immun. 2009, 77, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Rogers, A.B.; Feng, Y.; Lee, A.; Xu, S.; Taylor, N.S.; Fox, J.G. Bacterial cytolethal distending toxin promotes the development of dysplasia in a model of microbially induced hepatocarcinogenesis. Cell. microbiol. 2007, 9, 2070–2080. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Feng, Y.; Whary, M.T.; Nambiar, P.R.; Xu, S.; Ng, V.; Taylor, N.S.; Fox, J.G. Cytolethal distending toxin is essential for Helicobacter hepaticus colonization in outbred swiss webster mice. Infect. Immun. 2005, 73, 3559–3567. [Google Scholar] [CrossRef] [PubMed]
- Guerra, L.; Nemec, K.N.; Massey, S.; Tatulian, S.A.; Thelestam, M.; Frisan, T.; Teter, K. A novel mode of translocation for cytolethal distending toxin. Biochim. Biophys Acta (BBA)-Mol. Cell Res. 2009, 1793, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Fahrer, J.; Huelsenbeck, J.; Jaurich, H.; Dörsam, B.; Frisan, T.; Eich, M.; Roos, W.P.; Kaina, B.; Fritz, G. Cytolethal distending toxin (cdt) is a radiomimetic agent and induces persistent levels of DNA double-strand breaks in human fibroblasts. DNA Repair 2014, 18, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Buc, E.; Dubois, D.; Sauvanet, P.; Raisch, J.; Delmas, J.; Darfeuille-Michaud, A.; Pezet, D.; Bonnet, R. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 2013, 8, e56964. [Google Scholar] [CrossRef] [PubMed]
- Nalbant, A.; Zadeh, H. Actinobacillus actinomycetemcomitans induces apoptosis of T lymphocytes by the Fas and Fas ligand pathway. Oral microbiol. Immunol. 2002, 17, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Ohara, M.; Hayashi, T.; Kusunoki, Y.; Miyauchi, M.; Takata, T.; Sugai, M. Caspase-2 and caspase-7 are involved in cytolethal distending toxin-induced apoptosis in Jurkat and MOLT-4 T-cell lines. Infect. Immun. 2004, 72, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Shenker, B.J.; Hoffmaster, R.H.; Zekavat, A.; Yamaguchi, N.; Lally, E.T.; Demuth, D.R. Induction of apoptosis in human t cells by Actinobacillus actinomycetemcomitans cytolethal distending toxin is a consequence of G2 arrest of the cell cycle. J. Immunol. 2001, 167, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Wising, C.; Azem, J.; Zetterberg, M.; Svensson, L.A.; Ahlman, K.; Lagergård, T. Induction of apoptosis/necrosis in various human cell lineages by Haemophilus ducreyi cytolethal distending toxin. Toxicon 2005, 45, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–232. [Google Scholar] [PubMed]
- Arthur, J.C.; Perez-Chanona, E.; Mühlbauer, M.; Tomkovich, S.; Uronis, J.M.; Fan, T.-J.; Campbell, B.J.; Abujamel, T.; Dogan, B.; Rogers, A.B. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 2012, 338, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Scanu, T.; Spaapen, R.M.; Bakker, J.M.; Pratap, C.B.; Wu, L.; Hofland, I.; Broeks, A.; Shukla, V.K.; Kumar, M.; Janssen, H. Salmonella manipulation of host signaling pathways provokes cellular transformation associated with gallbladder carcinoma. Cell Host Microbe 2015, 17, 763–774. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Escobedo, G.; Marshall, J.M.; Gunn, J.S. Chronic and acute infection of the gall bladder by Salmonella Typhi: Understanding the carrier state. Nat. Rev. Microbiol. 2011, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, V.; Eslick, G. Systematic review with meta-analysis: The relationship between chronic Salmonella Typhi carrier status and gall-bladder cancer. Aliment. Pharmacol. Ther. 2014, 39, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Willinger, T.; Rongvaux, A.; Eynon, E.E.; Stevens, S.; Manz, M.G.; Flavell, R.A.; Galán, J.E. A mouse model for the human pathogen Salmonella Typhi. Cell Host Microbe 2010, 8, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, S.C.; Forest, C.G.; Lepage, C.; Leclerc, J.-M.; Daigle, F. So similar, yet so different: Uncovering distinctive features in the genomes of Salmonella enterica serovars Typhimurium and Typhi. FEMS Microbiol. Lett. 2010, 305, 1–13. [Google Scholar] [CrossRef] [PubMed]
- De Jong, H.K.; Parry, C.M.; van der Poll, T.; Wiersinga, W.J. Host–pathogen interaction in invasive salmonellosis. PloS Pathog. 2012. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.D.; Falkow, S. Salmonellosis: Host immune responses and bacterial virulence determinants. Annu. Rev. Immunol. 1996, 14, 533–561. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.M.; Black, R.E.; Lanata, C. Precise estimation of the numbers of chronic carriers of Salmonella Typhi in Santiago, Chile, an endemic area. J. Infect. Dis. 1982, 146, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Merselis, J.G.; Kaye, D.; Connolly, C.S.; Hook, E.W. Quantitative bacteriology of the typhoid carrier state. Am. J. Trop. Med. Hyg. 1964, 13, 425–429. [Google Scholar] [PubMed]
- Gunn, J.S.; Marshall, J.M.; Baker, S.; Dongol, S.; Charles, R.C.; Ryan, E.T. Salmonella chronic carriage: Epidemiology, diagnosis, and gallbladder persistence. Trends Microbiol. 2014, 22, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, S.; Revathi, G.; Kariuki, N.; Kiiru, J.; Mwituria, J.; Muyodi, J.; Githinji, J.W.; Kagendo, D.; Munyalo, A.; Hart, C.A. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: Zoonotic or anthroponotic transmission? J. Med. Microbiol. 2006, 55, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Lax, A.J. Bacterial toxins and cancer—A case to answer? Nat. Rev. Microbiol. 2005, 3, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kåhrström, C.T. Bacterial pathogenesis: E. coli claims the driving seat for cancer. Nat. Rev. Microbiol. 2012, 10, 670–671. [Google Scholar]
- Gould, L.H.; Bopp, C.; Strockbine, N.; Atkinson, R.; Baselski, V.; Body, B.; Carey, R.; Crandall, C.; Hurd, S.; Kaplan, R. Recommendations for diagnosis of shiga toxin–Producing Escherichia coli infections by clinical laboratories. MMWR Recomm. Rep. 2009, 58, 1–14. [Google Scholar] [PubMed]
- Wong, C.S.; Jelacic, S.; Habeeb, R.L.; Watkins, S.L.; Tarr, P.I. The risk of the hemolytic–uremic syndrome after antibiotic treatment of Escherichia coli O157:H7 infections. N. Engl. J Med. 2000, 342, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
- Lowy, I.; Molrine, D.C.; Leav, B.A.; Blair, B.M.; Baxter, R.; Gerding, D.N.; Nichol, G.; Thomas, W.D., Jr.; Leney, M.; Sloan, S. Treatment with monoclonal antibodies against Clostridium difficile toxins. N. Engl. J Med. 2010, 362, 197. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J. Botulism. Clin. Infect. Dis. 2005, 41, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Goldwater, P.N.; Bettelheim, K.A. Treatment of enterohemorrhagic Escherichia coli (EHEC) infection and hemolytic uremic syndrome (HUS). BMC Med. 2012, 10, 1. [Google Scholar] [CrossRef] [PubMed]
| Serotype | S-CDT Status 1 | References |
|---|---|---|
| 9,12:I,v:- | − | [9] |
| Agbeni | + | [13] |
| Agona | − | [13] |
| Anatum | − | [13] |
| Arechavaleta | + | [13] |
| Bareilly | − | [13] |
| Barranquilla | + | [13] |
| Berta | − | [13] |
| Braenderup | − | [13] |
| Brandenburg | + | [13] |
| Bredeney | + | [9] |
| Choleraesuis | − | [9,13] |
| Corvallis | + | [13] |
| Cotham | + | [13] |
| Cubana | + | [13] |
| Dublin | − | [9,13] |
| Enteritidis | − | [9,13] |
| Freetown | + | [13] |
| Gaminara | + | [13] |
| Georgia | + | [13] |
| Give | + | [13] |
| Glostrup | + | [13] |
| Hadar | − | [9,13] |
| Hartford | − | [13] |
| Heidelberg | − | [9,13] |
| 4,[5],12:i:- | − | [13] |
| Indiana | + | [13] |
| Infantis | − | [13] |
| Inverness | + | [13] |
| Javiana | + | [13,32] |
| Johannesburg | + | [13] |
| Kiambu | + | [13] |
| Kintambo | + | [13] |
| Kisarawe | + | [13] |
| Luciana | + | [13] |
| Miami | + | [13] |
| Minnesota | + | [13] |
| Mississippi | ± | [13] |
| Montevideo | + | [9,13,36] |
| Muenchen | − | [13] |
| Muenster | + | [13] |
| Newport | − | [9,13] |
| Oranienburg | + | [13] |
| Overschie | + | [13] |
| Panama | + | [13] |
| Paratyphi A | + | [13] |
| Pomona | + | [13] |
| Poona | + | [13] |
| Reading | + | [13] |
| Rubislaw | + | [13] |
| Sandiego | + | [13] |
| Schwarzengrund | + | [9,13] |
| Telelkebir | + | [13] |
| Thompson | − | [13] |
| Typhi | + | [12,29] |
| Typhimurium | − | [9,13] |
| Urbana | + | [13] |
| Virchow | − | [9,13] |
| Wandsworth | + | [13] |
| Pathogenic Outcome of CDT-Mediated Intoxication | Bacterial Species 1 | References |
|---|---|---|
| Cellular Outcomes | ||
| G2/M Phase arrest | A. actinomycetemcomitans | [12,14,15,17,19,22,24,27,28,34,67] |
| C. jejuni | ||
| E. coli | ||
| Haemophilus spp. | ||
| Helicobacter spp. | ||
| P. alcalifaciens | ||
| Shigella spp. | ||
| Salmonella (Typhi and NTS) | ||
| Activation of host cell DNA damage response | A. actinomycetemcomitans | [26,28,40,64,68,69,70,71] |
| C. jejuni | ||
| E. coli | ||
| Haemophilus spp. | ||
| H. ducreyi | ||
| H. hepaticus | ||
| P. alcalifaciens | ||
| S. Typhi | ||
| Induction of autophagy | NTS | [33] |
| Induction of apoptosis | A. actinomycetemcomitans | [33,72,73,74,75,76,77,78] |
| C. jejuni | ||
| E. coli | ||
| H. ducreyi | ||
| Helicobacter spp. | ||
| P. alcalifaciens | ||
| NTS | ||
| Host Outcomes | ||
| Tumorigenesis and neoplastic lesions | H. cinaedi | [79,80,81,82] |
| H. hepaticus | ||
| H. ducreyi | ||
| Typhoid-like illness | S. Typhi | [8] |
| Chronic infection | H. hepaticus | [83] |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, R.; Wiedmann, M. Dynamic Duo—The Salmonella Cytolethal Distending Toxin Combines ADP-Ribosyltransferase and Nuclease Activities in a Novel Form of the Cytolethal Distending Toxin. Toxins 2016, 8, 121. https://doi.org/10.3390/toxins8050121
Miller R, Wiedmann M. Dynamic Duo—The Salmonella Cytolethal Distending Toxin Combines ADP-Ribosyltransferase and Nuclease Activities in a Novel Form of the Cytolethal Distending Toxin. Toxins. 2016; 8(5):121. https://doi.org/10.3390/toxins8050121
Chicago/Turabian StyleMiller, Rachel, and Martin Wiedmann. 2016. "Dynamic Duo—The Salmonella Cytolethal Distending Toxin Combines ADP-Ribosyltransferase and Nuclease Activities in a Novel Form of the Cytolethal Distending Toxin" Toxins 8, no. 5: 121. https://doi.org/10.3390/toxins8050121
APA StyleMiller, R., & Wiedmann, M. (2016). Dynamic Duo—The Salmonella Cytolethal Distending Toxin Combines ADP-Ribosyltransferase and Nuclease Activities in a Novel Form of the Cytolethal Distending Toxin. Toxins, 8(5), 121. https://doi.org/10.3390/toxins8050121