Abstract
Aflatoxin B1 (AFB1) contamination in Scutellaria baicalensis poses a serious threat to the safety of traditional Chinese medicinal products. In this study, a sensitive and reliable ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS) method was developed for the quantitative determination of AFB1 in Scutellaria baicalensis. Method optimization included selection of chromatographic columns, mobile phase composition, and mass spectrometric parameters. Sample pretreatment was also optimized to reduce matrix interference and enhance extraction efficiency. The method showed excellent linearity (R2 > 0.999) in the range of 0.1–10.0 µg/L, with a limit of detection (LOD) of 0.03 µg/kg and a limit of quantification (LOQ) of 0.10 µg/kg. Precision and recovery studies demonstrated good repeatability and accuracy, with intra- and inter-day relative standard deviations (RSDs) below 5.2% and recoveries ranging from 88.7% to 103.4%. Application of the method to six commercial Scutellaria baicalensis samples revealed detectable AFB1 in two samples, though all levels were below national safety limits. This method provides a robust tool for routine monitoring of AFB1 in herbal medicines and supports the establishment of quality control systems for Scutellaria baicalensis.
Keywords:
aflatoxin B1; Scutellaria baicalensis; UHPLC-MS/MS; quantitative analysis; method validation Key Contribution:
This study proposes a novel UHPLC-MS/MS method for the sensitive detection of AFB1 in Scutellaria baicalensis, offering improved recovery and lower detection limits compared to previous methods applied to herbal matrices.
1. Introduction
Scutellaria baicalensis (commonly known as Chinese skullcap) is a traditional medicinal herb widely used in East Asia, especially in Chinese medicine, for its anti-inflammatory, antioxidant, and hepatoprotective properties. Its dried root is the main medicinal part and contains abundant flavonoids such as baicalin and wogonoside. Aflatoxin B1 (AFB1), produced primarily by Aspergillus flavus and Aspergillus parasiticus, is a highly toxic Group I carcinogen (Figure 1). It frequently contaminates agricultural products and traditional Chinese medicinal herbs, posing serious health risks such as hepatotoxicity, immunosuppression, and genotoxicity [1]. Under hot and humid storage conditions, traditional Chinese medicinal herbs—especially starchy, oily seeds, rhizomes, and wild-collected species—are prone to fungal growth and subsequent mycotoxin production. The contamination level of AFB1 is significantly influenced by region and storage environment; for instance, the detection rate in southern provinces for Scutellaria baicalensis can reach 10–30% [2,3]. Traditional drying techniques often fail to completely deactivate fungi and effectively suppress toxin production [4,5].
Figure 1.
(a) Structural formula of AFB1. (b) Schematic diagram of the 3D structure of AFB1.
To address the demand for trace-level AFB1 detection in complex matrices such as Scutellaria baicalensis, this study aims to establish a highly sensitive and specific quantitative method based on ultra-high performance liquid chromatography-tandem mass spectrometry (UHPLC-MS/MS). This method seeks to overcome the limitations of traditional HPLC approaches, such as insufficient sensitivity and weak anti-interference capability, while optimizing the sample pretreatment procedures to reduce matrix effects and enhance recovery. This provides reliable technical support for fungal toxin monitoring in herbal medicine.
Due to susceptibility to moisture, Scutellaria baicalensis is highly prone to mildew and subsequent accumulation of AFB1 during cultivation, harvesting, and storage. Although AFB1 detection technology has evolved, achieving the right balance between sensitivity, matrix interference elimination, and cost-efficiency remains a critical challenge. Stringent international regulatory limits (e.g., 2 µg/kg for herbal materials in the European Union) [6] also demand improved analytical solutions. Table 1 summarizes the current mainstream detection methods for Aflatoxin B1, each with its own limitations. Enzyme-Linked Immunosorbent Assay (ELISA), while simple, suffers from high matrix interference and false positives [7]; HPLC with fluorescence detection (HPLC-FLD) lacks sufficient sensitivity (LOD often 1–5 µg/kg) and requires complex derivatization [8,9]; immunoaffinity column-fluorescence methods offer good sensitivity (LOD 0.1–0.5 µg/kg) but at high cost; novel techniques like surface-enhanced Raman scattering (SERS) and electrochemical sensors show promise but face challenges of reproducibility and stability; and GC-MS is highly sensitive but entails complex derivatization and high cost.

Table 1.
Comparison of AFB1 Detection Methods in Traditional Chinese Medicines [8,9,10].
UHPLC-MS/MS, with advantages of high resolution, sensitivity, and multi-reaction monitoring (MRM) capability, has emerged as a mainstream tool for mycotoxin analysis [10]. Nevertheless, current methods applied to herbal matrices such as Scutellaria baicalensis still suffer from cumbersome pretreatment, significant matrix effects, and signal suppression of trace-level analytes. Therefore, method optimization is imperative to improve analytical performance and practical applicability. Although several validated methods have been established for AFB1 detection in cereals and food matrices, they are not directly applicable to Scutellaria baicalensis due to its unique and complex matrix composition. The presence of abundant flavonoids and polysaccharides in this herb poses significant matrix effects, such as ion suppression and co-elution, which can compromise the accuracy and sensitivity of standard protocols. Therefore, a dedicated method tailored for Scutellaria baicalensis is essential.
This study develops a UHPLC-MS/MS method specifically tailored for quantitative detection of trace AFB1 in Scutellaria baicalensis. Optimization includes chromatographic separation, mass spectrometric parameters (mobile phase, elution program, MRM ion pairs), and sample pretreatment steps (extraction solvent, extraction time, solid–liquid ratio). The method is validated for linearity, LOD, LOQ, precision, and recovery, and applied to the assessment of AFB1 contamination in Scutellaria baicalensis samples from different regions. This work provides methodological reference for mycotoxin analysis in herbal medicine and supports the establishment of quality control systems for Scutellaria baicalensis.
2. Results and Discussion
2.1. Optimization of UHPLC-MS/MS Parameters
2.1.1. Ionization Mode Selection and Collision Energy Optimization
To improve AFB1 detection sensitivity and accuracy, various chromatographic columns, mobile phase compositions, and ion transitions were evaluated. The Agilent ZORBAX Eclipse Plus C18 column demonstrated optimal peak shape and resolution [11]. The addition of 0.1% formic acid to both aqueous and organic phases significantly enhanced ionization efficiency. Methanol was chosen over acetonitrile due to improved peak intensity and stability. The optimal ion transition for quantification was m/z 313.2 →241.2, with m/z 313.2→285.1 used for confirmation. These transitions provided strong signal intensity and selectivity under positive ESI mode. As shown in Figure 2, the ion abundance varies with different collision energies. The highest ion abundance was observed at 120 V, which was consequently selected as the optimal collision energy.
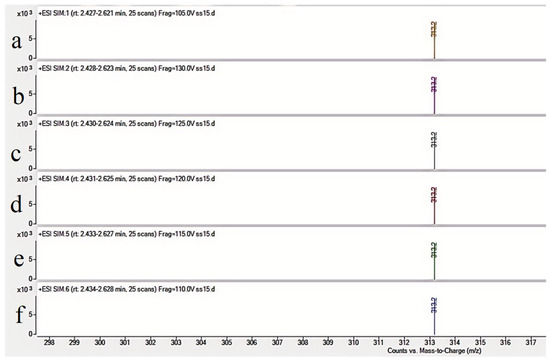
Figure 2.
Precursor ion (m/z 313.2) abundance vs. collision energy ((a): 105 V (b): 130 V (c): 125 V (d): 120 V (e): 115 V (f): 110 V).
Through systematic optimization of collision energy (105–135 V), the highest abundance of the target ion was observed at 120 V (Table 2), which was consequently selected as the optimal collision energy.

Table 2.
Abundance values corresponding to different collision energies.
2.1.2. Optimization of Collision Energy
Collision energy (CE) is a critical parameter affecting ion fragmentation efficiency. Proper optimization of CE enables more effective dissociation of precursor ions into target product ions, significantly enhancing their response intensity. For AFB1 analysis, the CE optimization range was set at 24–32 eV with 2 eV increments to systematically evaluate the abundance variations in product ions under different energy conditions. Experimental results demonstrated that: The quantitative ion transition (m/z 313.2→285.1) reached maximum abundance at 24 eV; the qualitative ion transition (m/z 313.2→241.1) achieved peak intensity at 32 eV. As illustrated in Figure 3 and Figure 4, the abundance profiles of product ions m/z 285.1 and m/z 241.1 are displayed corresponding to different CE values.
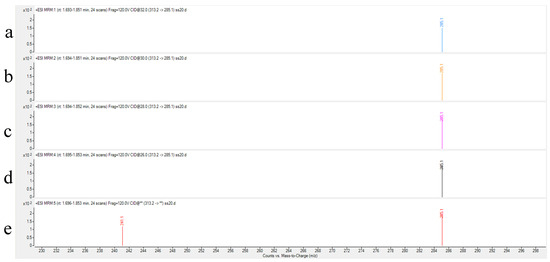
Figure 3.
Abundance of product ion (m/z 285.1) at different collision energies. ((a): 32 eV (b): 30 eV (c): 28 eV (d): 26 eV (e): 24 eV).
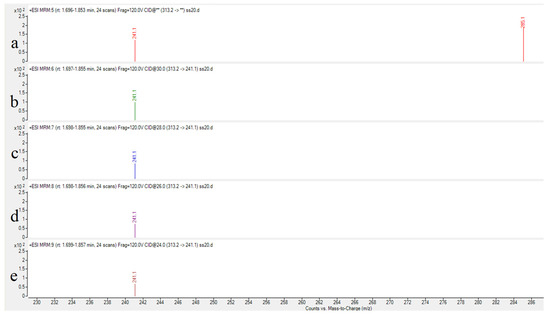
Figure 4.
Abundance of product ion (m/z 241.1) at varied collision energies. ((a): 32 eV (b): 30 eV (c): 28 eV (d): 26 eV (e): 24 eV).
2.1.3. Selection of Precursor and Product Ions
Following collision energy optimization, the protonated molecule [M+H]+ of AFB1 (m/z 313.2) was selected as the precursor ion. Through collision-induced dissociation (CID) and subsequent product ion scanning, fragment ions with high response intensity and excellent stability were identified. The final transitions were established as: Quantitative transition: m/z 313.2→285.1. Qualitative transition: m/z 313.2→241.1(Figure 5). This selective ion pairing strategy significantly enhances both detection specificity (reduced matrix interference) and analytical sensitivity (improved S/N ratio).
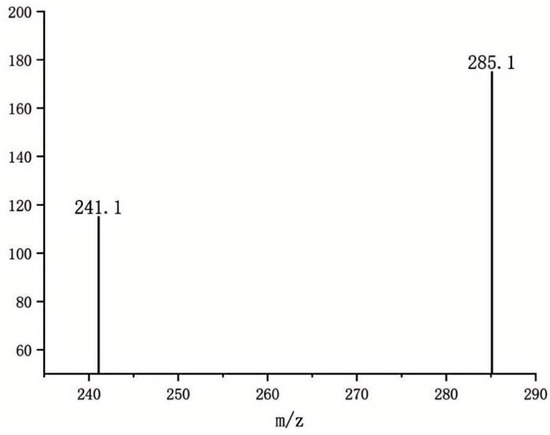
Figure 5.
Abundance of Two Product Ions.
2.1.4. MRM Parameter Configuration
Through systematic optimization, the protonated molecular ion [M+H]+ (m/z 313.2) was selected as the precursor ion, demonstrating the strongest response in positive ion mode. Maximum ion abundance was achieved at a fragmentor voltage of 120 V. The quantitative transition (313.2→285.1) reached peak abundance at 24 eV collision energy, while the qualitative transition (313.2→241.1) peaked at 32 eV (Figure 3 and Figure 4), with complete parameters detailed in Table 3. Additional product ion channels (including m/z 285.1 and 241.1) were evaluated for response intensity, selectivity, and stability to identify optimal MRM combinations (Figure 6).

Table 3.
Parameters for Multiple Reaction Monitoring (MRM) Mode.
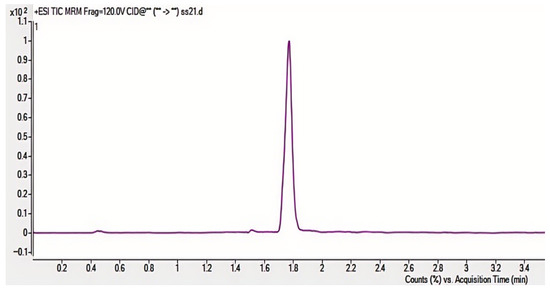
Figure 6.
MRM ion chromatograms.
2.2. Optimization and Evaluation of Extraction Conditions
2.2.1. Effect of Stirring Time on Extraction Efficiency
As shown in Figure 7, the extraction yield of AFB1 exhibited an initial increase followed by stabilization and subsequent decline with prolonged stirring time: At 20 min, the concentration was 26.45 μg·kg−1, which significantly increased to 28.89 μg·kg−1 by 30 min (p < 0.05). No statistically significant difference was observed between 30 min (28.89 μg·kg−1) and 40 min (28.85 μg·kg−1) (p > 0.05), indicating a stabilization phase. However, further extension to 50 min reduced the yield to 25.60 μg·kg−1, suggesting potential co-extraction of interfering compounds or analyte degradation.
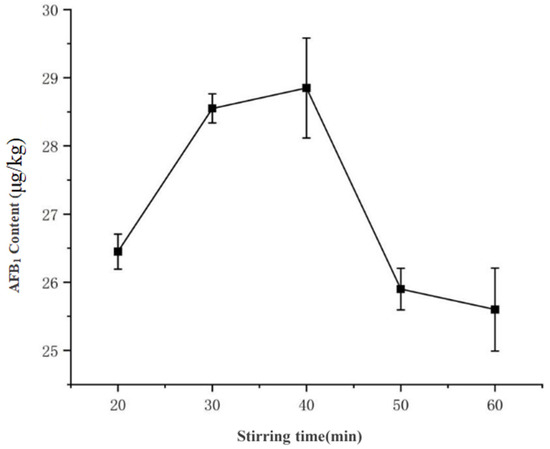
Figure 7.
Effect of magnetic stirring duration on AFB1 extraction efficiency.
Therefore, 30 min was selected as the optimal stirring duration, a finding consistent with the “increase-peak-decline” pattern reported by Zhao et al. [12] in their response surface methodology optimization of aflatoxin extraction from peanuts for rapid detection applications.
2.2.2. Optimization of Extraction Solvent Composition
Under fixed stirring duration (30 min), the solvent composition was systematically evaluated, with pure acetonitrile demonstrating optimal extraction efficiency (28.55 μg/kg AFB1). A significant negative correlation was observed as increasing water content reduced yields to 10.50 μg/kg (4% water) and 4.20 μg/kg (16% water), leading to selection of pure acetonitrile, consistent with Li et al. [13] in ELISA-based AFB1 detection from soy sauce. The results are presented in Figure 8, showing the clear inverse relationship between water proportion and extraction efficiency.
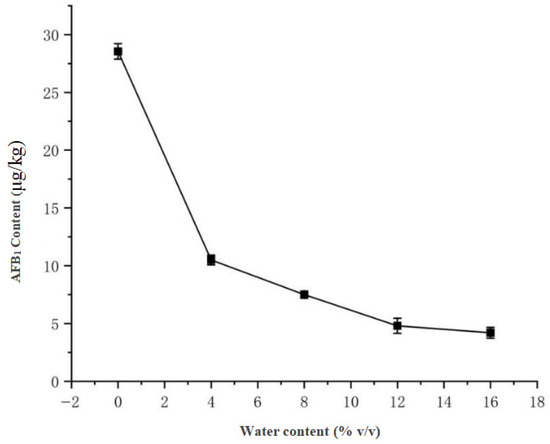
Figure 8.
Effect of water content on AFB1 extraction efficiency.
2.2.3. Optimization of Solid-to-Liquid Ratio
With fixed magnetic stirring for 30 min and pure acetonitrile as the extraction solvent, the results (Figure 9) demonstrated: AFB1 concentrations remained stable at 20–25 μg/kg with ratios of 1:5 to 1:7 (g/mL), but increased significantly to 56.72 μg/kg at 1:8 and peaked at 67.86 μg/kg with a 1:9 ratio. This indicates that smaller solid-to-liquid ratios (1:9) provide more sufficient solvent volume to enhance AFB1 release, a finding consistent with Li et al. [13] in their ELISA-based detection of aflatoxin B1 in soy sauce. Therefore, the 1:9 ratio was selected as optimal.
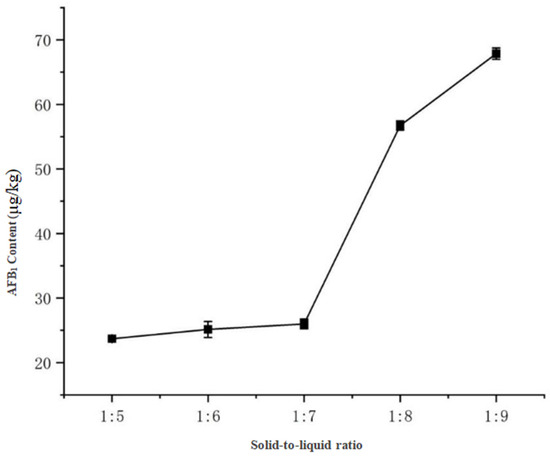
Figure 9.
Effect of solid-to-liquid ratio on AFB1 extraction efficiency.
2.3. Method Validation Results
The calibration curve showed excellent linearity in the 0.05-50.00 µg/kg range, with R2 > 0.999 (Figure 10). The LOD and LOQ of AFB1 in Scutellaria baicalensis were 0.03 µg/kg and 0.10 µg/kg, respectively, which meet regulatory requirements for trace detection (Table 4). Precision analysis revealed intra-day RSDs of 2.1–4.5% and inter-day RSDs of 3.0–5.2%, indicating good repeatability. Average recoveries ranged from 88.7% to 103.4% at three spiking levels (0.5, 2.0, and 5.0 µg/kg), with RSDs below 6.0%, demonstrating acceptable accuracy and reliability of the method.
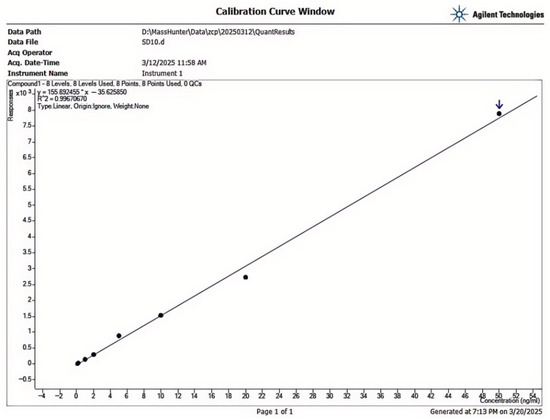
Figure 10.
AFB1 Standard Curve.

Table 4.
Validation Parameters for the Quantitative Determination of AFB1 in Scutellaria baicalensis by UHPLC-MS/MS.
Spiked recovery tests were conducted by adding AFB1 standard solutions at three concentration levels (0.10 μg/kg, 2.00 μg/kg, and 10.00 μg/kg) to pre-processed Scutellaria baicalensis samples. Under the optimized conditions, the method demonstrated excellent accuracy with a mean recovery rate of 96.10% and high precision (RSD = 1.9%, n = 6). These results confirm the reliability and accuracy of the established method for quantitative determination of AFB1 in Scutellaria baicalensis (Table 5).

Table 5.
Spiked recovery test results of AFB1 in Scutellaria baicalensis.
2.4. AFB1 Contamination in Commercial Samples
A total of 12 batches of Scutellaria baicalensis from different provinces were analyzed, including samples from Lanzhou (Gansu), Bozhou (Anhui), Yancheng (Jiangsu), Anguo (Hebei), and Chengdu (Sichuan). Among them, 3 batches of samples were detected with AFB1, accounting for 25% of the total. The concentration of AFB1 in Lanzhou (Gansu) was the highest at 6.28 μg/kg, which was 1.25 times higher than the limit specified in the Chinese Pharmacopoeia. The AFB1 content in samples from other regions did not exceed the limit. The observed regional differences in AFB1 contamination may be associated with several factors. Firstly, cultivation conditions such as soil type and irrigation practices can influence fungal growth. Secondly, post-harvest storage conditions, including temperature and humidity control, play a critical role in preventing mycotoxin accumulation. Finally, climatic variations among regions (e.g., relative humidity and average temperature during harvest and storage) may also contribute to the observed discrepancies. Further studies with larger sample sizes and detailed metadata collection will help to clarify these associations. As shown in Table 6.

Table 6.
AFB1 levels in Scutellaria from different regions.
3. Conclusions
A sensitive and reliable UHPLC-MS/MS method was successfully developed for the quantitative analysis of AFB1 in Scutellaria baicalensis. The method features low detection limits, high precision, and good recovery. It is suitable for routine monitoring of AFB1 contamination in herbal medicine. This method can aid in establishing robust quality control systems for Scutellaria baicalensis and contributes to ensuring the safety of traditional Chinese medicinal products. Despite the method’s strong performance, several limitations remain. The high cost of UHPLC-MS/MS instrumentation may limit its widespread adoption in resource-limited settings. Furthermore, the method’s robustness across different laboratories and operators has not yet been fully validated. Future research should focus on inter-laboratory validation, long-term stability testing, and the development of simplified sample preparation protocols. Large-scale surveillance of Scutellaria baicalensis from broader geographic sources will also be essential to ensure quality and safety consistency.
4. Materials and Methods
4.1. Materials and Reagents
Acetonitrile and methanol (MS grade) were obtained from Thermo Fisher Scientific (Waltham, MA, USA); analytical-grade acetonitrile was purchased from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, China). Purified water was sourced from Watsons Beverage Technology Co., Ltd. (Guangzhou, China). The Aflatoxin B1 standard solution (purity 98%) was obtained from Hebei Bailin Superfine Materials Co., Ltd. (Langfang, China) Six Scutellaria baicalensis decoction pieces were sourced from different provinces as detailed in Table 7.

Table 7.
Appearance and origin of Scutellaria baicalensis samples from different regions.
4.2. Instruments and Equipment
The instrumentation included a QW-DCY-KS12A nitrogen evaporator (Hangzhou Qiwei Instruments Co., Ltd., Hangzhou, China), EL204-IC electronic balance (Shanghai Yueping Scientific Instrument Co., Ltd., Shanghai, China), G6470B UHPLC-MS/MS system (Agilent Technologies, Santa Clara, CA, USA), 150 high-speed pulverizer (Yongkang Minye Industry & Trade Co., Ltd., Yongkang, China ), H1850 centrifuge (Xiangyi Laboratory Instrument Development Co., Ltd., Changsha, China)), 906-201 vortex mixer (Hefei Aibensen Scientific Instruments Co., Ltd., Hefei, China), and 101-00BS electric thermostatic drying oven (Shanghai Lichen Bangxi Instrument Technology Co., Ltd., Shanghai, China).
4.3. Preparation of Standard Solutions and Sample Pretreatment
The AFB1 standard stock solution was prepared by diluting the reference standard to a concentration of 1.0 mg/L with methanol and stored in amber glass bottles at 4 °C. A series of working standard solutions at concentrations of 0.1, 0.5, 1.0, 2.0, 5.0, and 10.0 µg/kg [14,15,16,17] were obtained by serial dilution.
Each Scutellaria baicalensis sample was pulverized, passed through a 40-mesh sieve, and stored in a sealed, light-protected container. Accurately weighed 2.000 g of sample was transferred into a 50 mL centrifuge tube. Then, 20 mL of 80% acetonitrile aqueous solution was added, and the tube was vortexed for 3 min. Ultrasonic extraction was performed at room temperature for 40 min, followed by centrifugation at 4500 rpm for 5 min [18]. An aliquot of 10 mL supernatant was transferred to a new centrifuge tube and evaporated to dryness under a gentle nitrogen stream at 40 °C. The residue was reconstituted with 1.0 mL methanol-water (50:50, v/v), vortexed for 1 min, and filtered through a 0.22 µm membrane filter before UHPLC-MS/MS analysis [19,20] (Figure 11).

Figure 11.
AFB1 Extraction Flowchart.
4.4. Chromatographic and Mass Spectrometric Conditions
Chromatographic separation was carried out on an Agilent ZORBAX Eclipse Plus C18 column (2.1 mm × 100 mm, 1.8 µm) using gradient elution. The mobile phase consisted of water with 0.1% formic acid (A) and methanol with 0.1% formic acid (B), delivered at a flow rate of 0.3 mL/min. The elution program was as follows [21]: 0–1.5 min, linear gradient to 95% B; 1.5–4.2 min, held at 95% B; 4.2–4.3 min, returned to 25% B and re-equilibrated. As shown in Table 8.

Table 8.
Mobile Phase Elution Gradient.
The mass spectrometer operated in positive electrospray ionization (ESI+) mode with multiple reaction monitoring (MRM) [22]. The ion source conditions were: gas temperature 350 °C, gas flow 10 L/min, nebulizer pressure 40 psi, capillary voltage 4000 V. The precursor ion of AFB1 was m/z 313.2, and product ions for quantification and confirmation were m/z 241.2 and m/z 285.1, respectively. As shown in Table 9.

Table 9.
Mass Spectrometer Parameters.
4.5. Single-Factor Experiments
- 1.
- Magnetic Stirring Time
The ratio of acetonitrile-water solution was fixed at 100:0 (v/v), and the solid-to-liquid ratio was set at 1:5 (g/mL). The stirring time gradient was set at 20, 30, 40, 50, and 60 min. Each group was tested in triplicate, and the AFB1 concentration was calculated based on peak area measurements using UHPLC-MS/MS.
- 2.
- Water Ratio in Acetonitrile-Water Solution
The solid-to-liquid ratio was fixed at 1:5 (g/mL), and the stirring time was set at 30 min. The acetonitrile-water solution gradient was set at 100:0, 96:4, 92:8, 88:12, and 84:16 (v/v). Each group was tested in triplicate, and the AFB1 concentration was determined via UHPLC-MS/MS peak area analysis.
- 3.
- Solid-to-Liquid Ratio
The acetonitrile-water solution ratio was fixed at 100:0 (v/v), and the stirring time was set at 30 min. The solid-to-liquid ratio gradient was set at 1:5, 1:6, 1:7, 1:8, and 1:9 (g/mL). Each group was tested in triplicate, and the AFB1 concentration was calculated based on UHPLC-MS/MS peak area measurements.
4.6. Method Validation
The method was validated for linearity [23], limit of detection (LOD), limit of quantification (LOQ), precision, and recovery. Linearity was assessed using matrix-matched calibration curves constructed by spiking blank Scutellaria baicalensis extracts with AFB1 at six concentration levels (0.1–10.0 µg/L), with the coefficient of determination (R2) required to be ≥0.999. The LOD and LOQ were determined based on signal-to-noise ratios (S/N) of 3 and 10, respectively.
Intra-day and inter-day precision were evaluated by analyzing spiked samples at three concentration levels (0.5, 2.0, 5.0 µg/kg) in six replicates, expressed as relative standard deviation (RSD%) [24]. Recovery experiments were conducted by spiking blank samples at the same levels and calculating the mean percentage of AFB1 recovered.
Author Contributions
Conceptualization, Y.L.; methodology, Y.L. and C.Z.; software, Y.L. and Y.-Y.L.; validation, J.X. and Y.L.; formal analysis, Y.L.; investigation, C.Z. and Y.L.; resources, Y.L. and Y.-Y.L.; data curation, Y.L. and C.Z.; writing—original draft preparation, Y.L.; writing—review and editing, Y.L. and J.G.; visualization, C.Z.; supervision, Y.-Y.L.; project administration, Y.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Henan Provincial Demonstration Course Program for General Education (Grant number PX-99232277), the 2025 Henan Provincial College Students’ Innovation Training Program (Grant number S202512949015), the Scientific Research Foundation for Doctors of Zhengzhou Normal University (grant number 702457) and the Zhengzhou Normal University Special Demonstration Course Program for the Integration of Professional Education and Innovation & Entrepreneurship (Grant number ZCRHTSKC-0001242816). The APC was self-funded by the corresponding author.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. (Authors are going to explore further function and the data will be available on request).
Conflicts of Interest
The authors declare that they have no conflicts of interest. The funders had no role in the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
References
- National Institutes for Food and Drug Control. Pharmacopoeia of the People’s Republic of China; National Institutes for Food and Drug Control: Beijing, China, 2020; Volume 1. [Google Scholar]
- Wang, H.-S.; Liu, L.; Chen, W.-B. Investigation of mold and mycotoxin contamination in Chinese medicinal herbs and decoction pieces. China J. Chin. Mater. Medica 2005, 30, 678–681. [Google Scholar]
- Yang, K.-B.; Huang, S.-F. Research progress on mildew and prevention of Chinese medicinal materials. China J. Chin. Mater. Medica 2011, 36, 645–648. [Google Scholar]
- European Commission. Commission Regulation (EU) No 2021/1317 of 10 August 2021 on maximum levels for mycotoxins in foodstuffs. Off. J. Eur. Union 2021, 286, 1–4. [Google Scholar]
- Li, Y.; Wu, Y.-P. Research progress in immunoassays for mycotoxin detection. Food Sci. 2012, 33, 319–323. [Google Scholar]
- Li, F.; Zhang, J.-G. Research progress on mycotoxin detection methods. Food Ind. 2015, 36, 280–284. [Google Scholar]
- Liu, W.-B.; Hao, J.; Liu, L. Application of electronic nose in mycotoxin detection. Food Ferment. Ind. 2019, 45, 289–294. [Google Scholar]
- Lyu, Y.-Y.; Wang, H.; Liu, X.-L. Research progress in application of liquid chromatography-tandem mass spectrometry in mycotoxin detection. Chin. J. Food Hyg. 2020, 32, 424–429. [Google Scholar]
- Wang, S.-M.; Xu, Y.; Mao, D.; Zheng, R.; Wang, K.; Ji, S. Determination of aflatoxins G2, G1, B2 and B1 in Semen Persicae by HPLC-MS/MS. Chin. J. Pharm. Anal. 2005, 26, 610–632. [Google Scholar]
- Chen, Y.; Chen, C.-J.; Li, J.; Luan, L.; Liu, X.; Wu, Y. Determination of 10 mycotoxins in Panax notoginseng by ultra performance liquid chromatography-tandem mass spectrometry. Chin. J. Pharm. Anal. 2015, 34, 81–85. [Google Scholar]
- Wu, H.-T.; Li, M.-M.; Guan, E.-Q.; Zhang, L.; Chen, Y.-X.; Liu, Z.-F.; Wang, J.-K.; Zhao, Q.; Yang, P.; Xu, R.; et al. Simultaneous determination of four mycotoxins in corn by Q-Orbitrap liquid chromatography-mass spectrometry. Food Ferment. Ind. 2022, 48, 245–251. [Google Scholar]
- Zhao, P.; Zhang, Y.Y.; Zhou, J.H.; Chen, F. Optimization of Aflatoxin Extraction from Peanuts Using Response Surface Methodology and Its Application in Rapid Detection Evaluation. J. Nanjing Norm. Univ. (Eng. Technol. Ed.) 2022, 22, 72–82. [Google Scholar]
- Li, J.; Li, X.; Qi, Y.; Tian, T.X.; Zhang, Z.J.; Long, L.S. Detection of Aflatoxin B1 in Soy Sauce by Enzyme-Linked Immunosorbent Assay. J. Food Saf. Qual. 2016, 7, 4736–4740. [Google Scholar]
- Wen, H.-J.; Liao, Z.-Q.; Jin, G.-Y. Advances in mycotoxin detection methods. Drug Stand. China 2023, 24, 465–475. [Google Scholar]
- Huang, Q.-W. Study on Analytical Methods and Risk Assessment of Mycotoxins. Ph.D. Thesis, Zhejiang University, Hangzhou, China, 2021. [Google Scholar]
- Luo, X.; Sun, Z.-X. Research progress on detection methods of aflatoxins. Carcinog. Teratog. Mutagen. 2024, 36, 417–420. [Google Scholar]
- Duan, F.-F.; Li, J.-Y.; Li, X.-Q. Detection of six common mycotoxins in grains by immunoaffinity column clean-up and HPLC. Grain Storage 2024, 53, 74–79. [Google Scholar]
- Shan, L.-N.; Wang, Y.-D.; Dou, X.-W.; Duan, Y.; Yang, S.; Wang, J.; Yang, M. Comparative study on aflatoxin contamination in four commonly used bulk traditional Chinese medicinal slices. Mod. Tradit. Chin. Med. Mater. Medica-World Sci. Technol. 2020, 22, 3718–3725. [Google Scholar]
- Yang, H. Study on Detection Methods for Pesticide Residues and Mycotoxins in Citrus Medicinal Materials Based on High-Resolution Mass Spectrometry. Master’s Thesis, Chengdu University of Traditional Chinese Medicine, Chengdu, China, 2023. [Google Scholar]
- Chu, H.-Q.; Zhuge, N.-J.; Chen, S.-S.; Wu, Q.; Yu, X.; Yang, C. Research progress on common detection methods for mycotoxins in grain and oil foods. J. Food Saf. Qual. 2025, 16, 1–9. [Google Scholar]
- Fang, J.-H.; Pan, X.-J.; Yin, F. Simultaneous determination of eight mycotoxins in Coix seed by QuEChERS-UPLC-MS/MS. J. Zhejiang Sci-Tech Univ. (Nat. Sci.) 2025, 53, 254–262. [Google Scholar]
- Yi, X.-J. Study on Detection Methods and Degradation Products of Aflatoxins in Food. Master’s Thesis, Yantai University, Yantai, China, 2020. [Google Scholar]
- Guan, L.-J. Study on LC-MS/MS Method for Detection of 24 Mycotoxins in Honey. Master’s Thesis, Yantai University, Yantai, China, 2019. [Google Scholar]
- Deng, X.-Y.; Zhang, J.-X.; Zhang, J.-H. Determination of 15 mycotoxins in food by UPLC-MS/MS. China Food Saf. Mag. 2021, 1, 69–74. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).