Perillaldehyde-Elicited Inhibition of Ochratoxin A Production by Aspergillus carbonarius
Abstract
1. Introduction
2. Results and Discussion
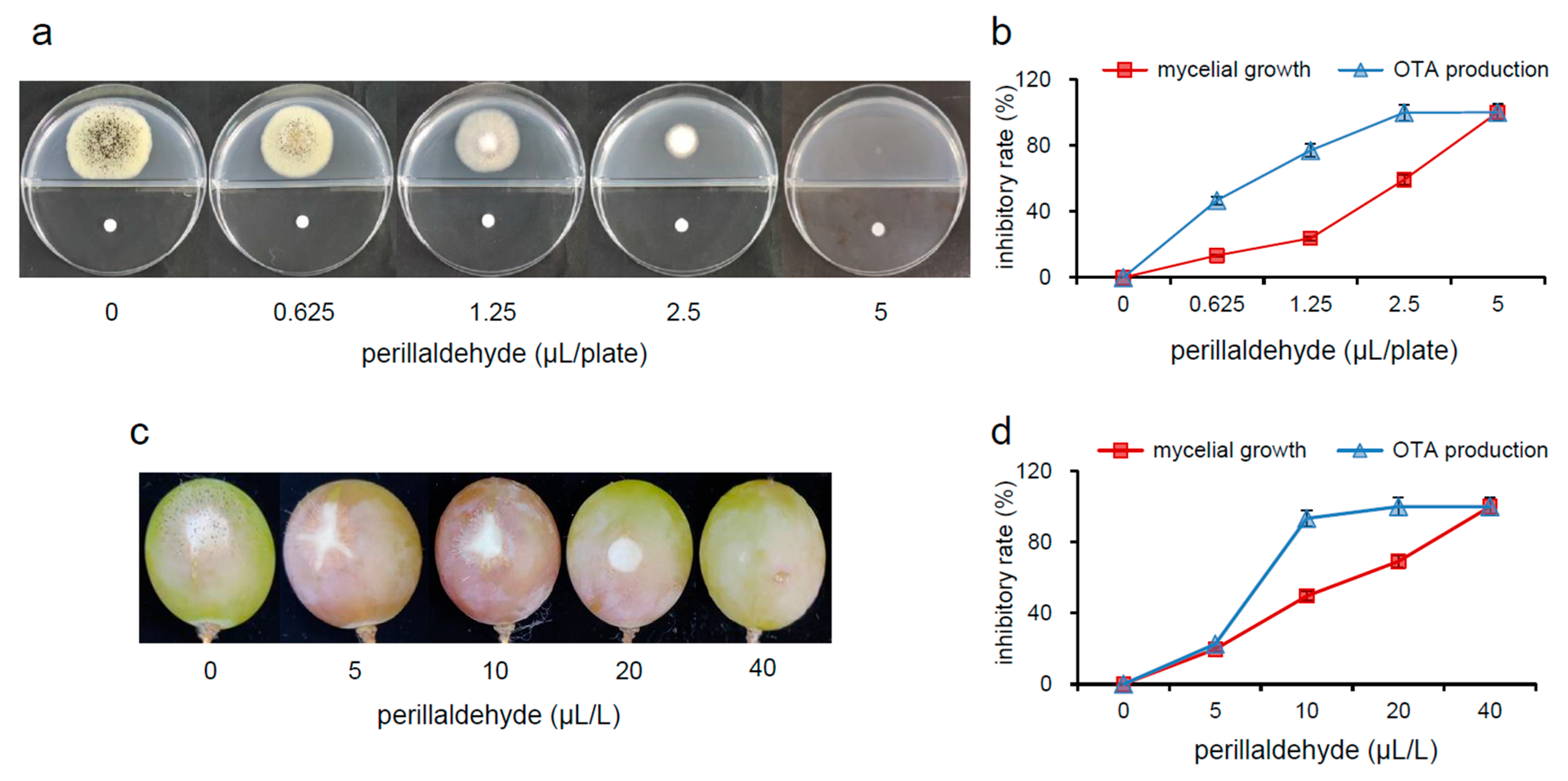
2.1. Inhibitory Effects of Perillaldehyde on Mycelial Growth and OTA Production
2.2. Effects of Perillaldehyde on Mycelial Morphology of A. carbonarius
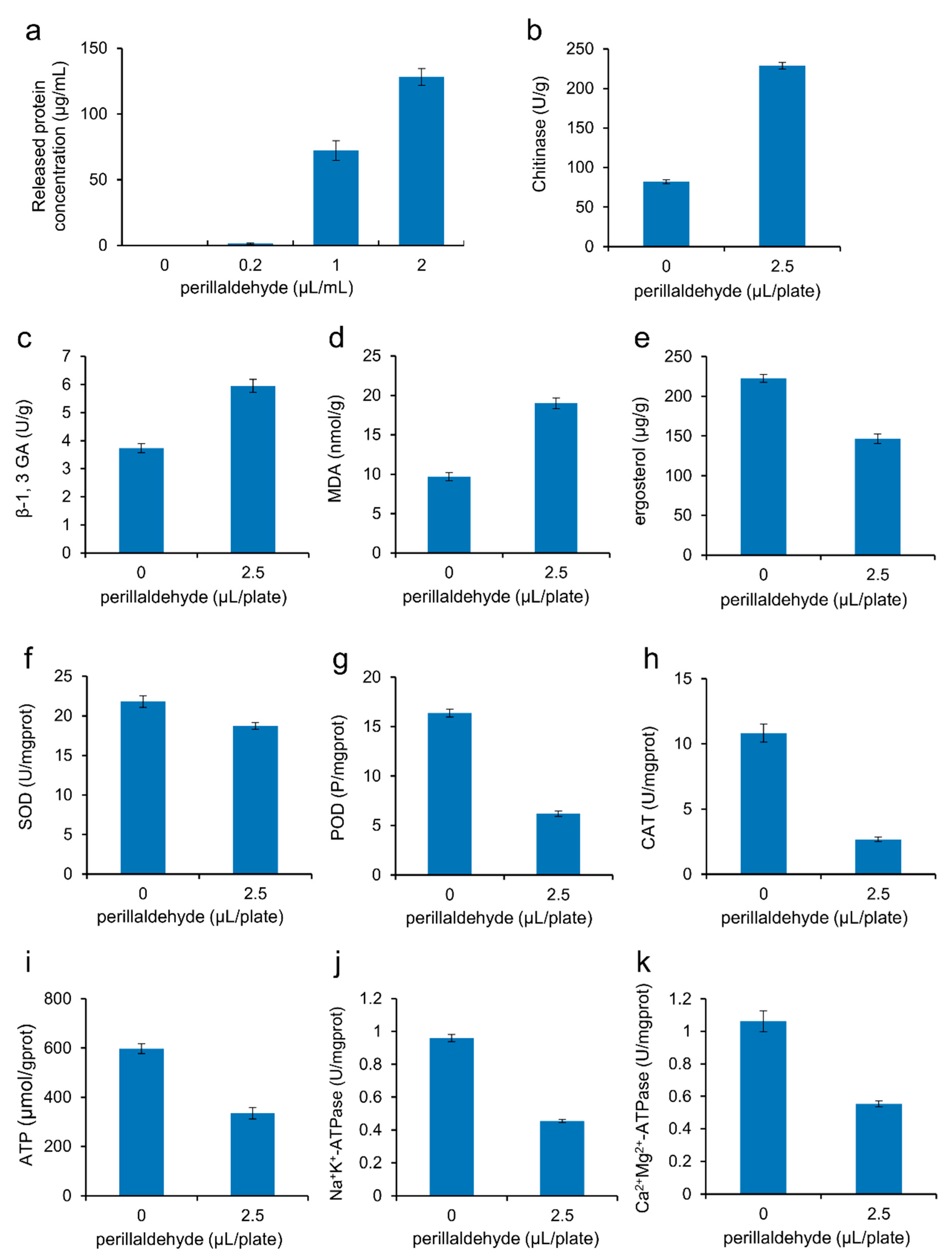
2.3. Effects of Perillaldehyde on Cell Integrity of A. carbonarius
2.4. Effects of Perillaldehyde on Mycelial Antioxidant Enzymatic Activities of A. carbonarius
2.5. Effects of Perillaldehyde on the Energy Metabolism of A. carbonarius
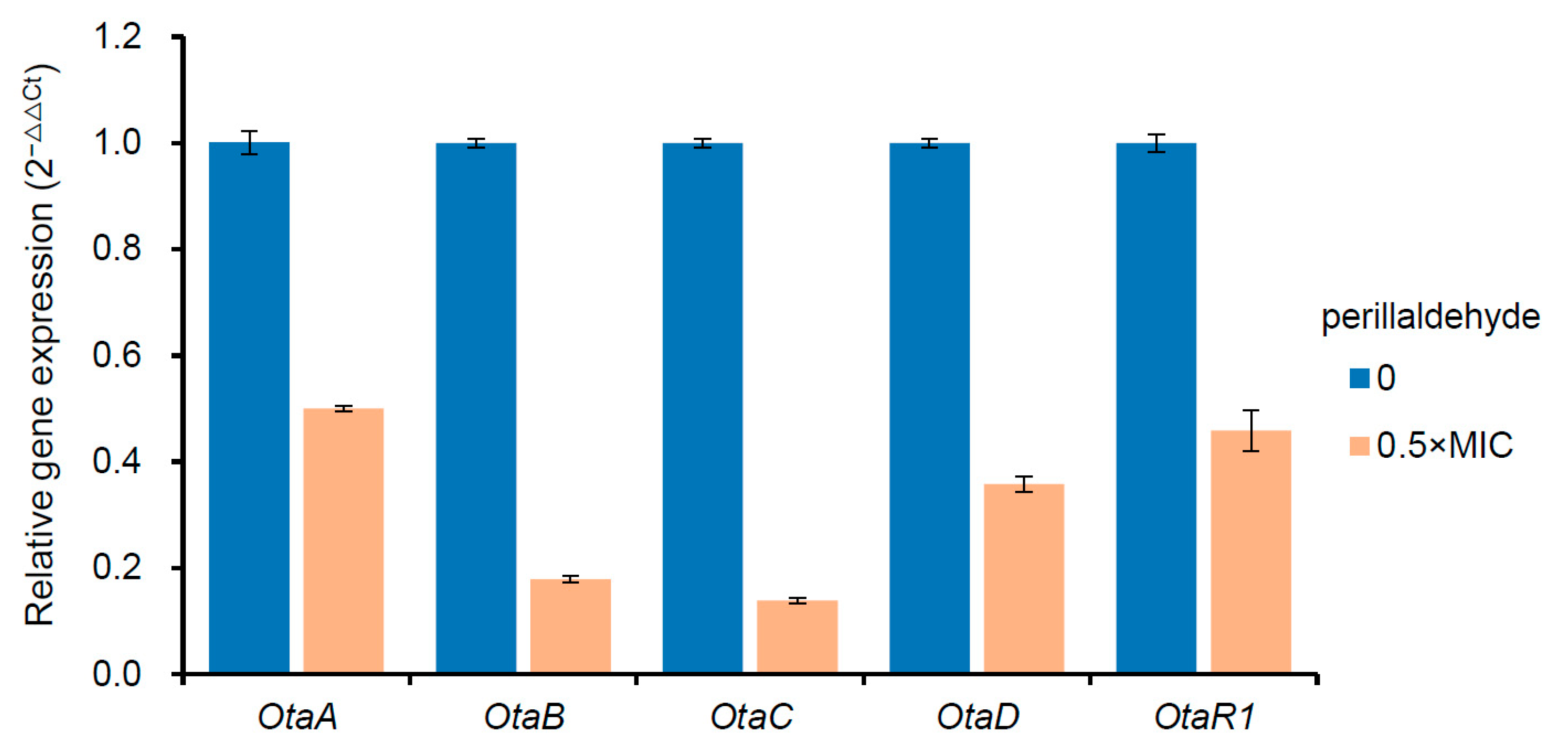
2.6. Comprehensive Transcriptomic Profile Analysis
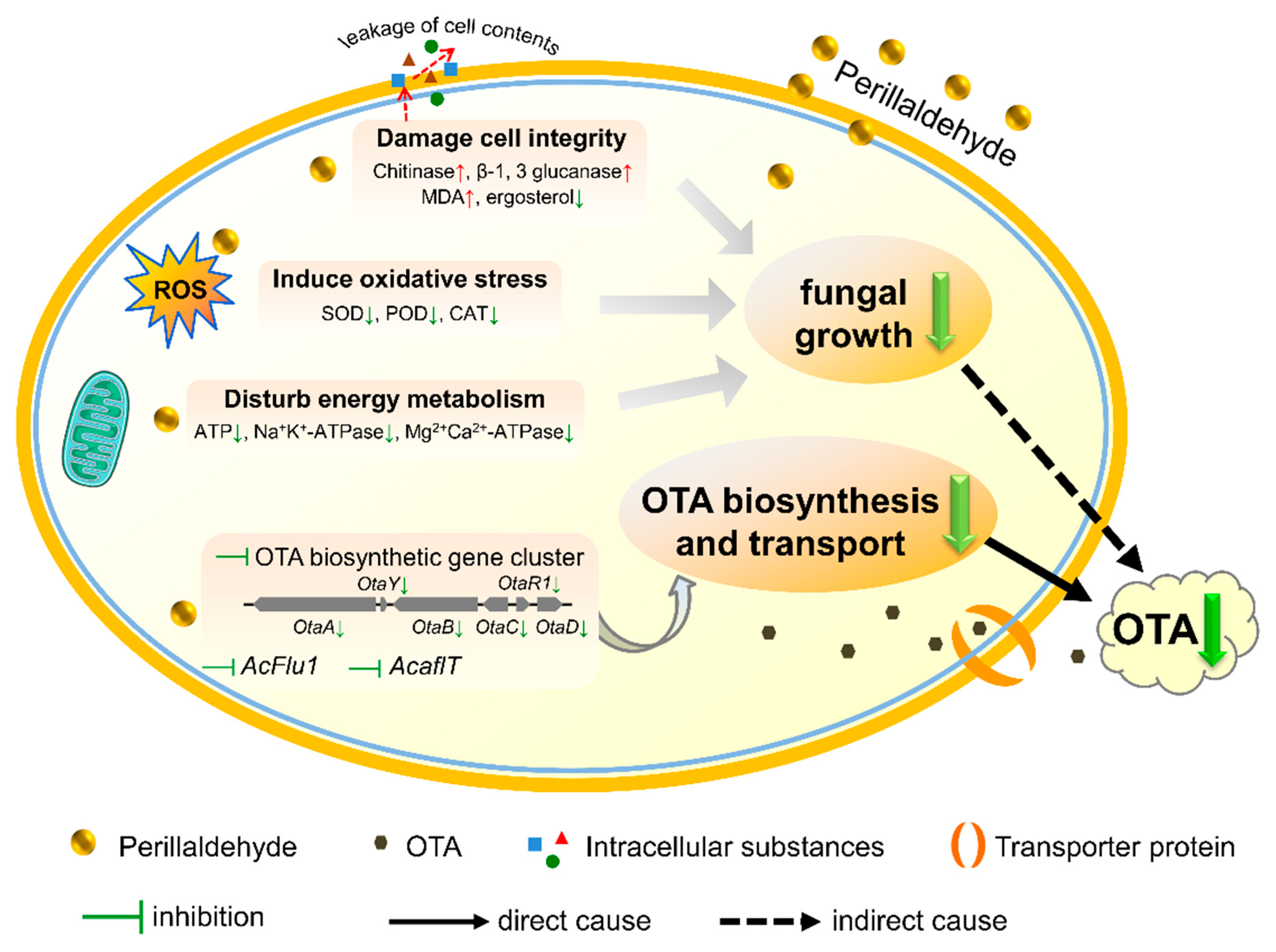
3. Conclusions
4. Materials and Methods
4.1. Strain and Culture Condition
4.2. Analysis of the Antifungal and Antimycotoxigenic Effects of Perillaldehyde on A. carbonarius, In Vitro and In Vivo
4.3. Scanning Electron Microscopy (SEM) Analysis
4.4. Intracellular Protein Release
4.5. Analysis of Metabolism-Related Enzymatic Activities and Substances
4.6. Transcriptome Analysis
4.7. Gene Expression Analysis by Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
4.8. Data Availability
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Agency for Research on Cancer (IARC); World Health Organization (WHO). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Agents Classified by the IARC Monographs. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 6 June 2025).
- Deng, H.; Chen, W.; Zhang, B.; Zhang, Y.; Han, L.; Zhang, Q.; Yao, S.; Wang, H.W.; Shen, X.L. Excessive ER-phagy contributes to ochratoxin A-induced apoptosis. Food Chem. Toxicol. 2023, 176, 113793. [Google Scholar] [CrossRef]
- Chen, W.Y.; Han, L.Y.; Yang, R.R.; Wang, H.W.; Yao, S.; Deng, H.Q.; Liu, S.C.; Zhou, Y.; Shen, X.L. Central role of Sigma-1 receptor in ochratoxin A-induced ferroptosis. Arch. Toxicol. 2024, 98, 3323–3336. [Google Scholar] [CrossRef]
- Yao, S.; Chen, W.; Wang, H.; Yang, R.; Zhou, Y.; Liu, S.; Shen, X. Ochratoxin A induces mitochondrial apoptosis and ferroptosis by inhibiting Sigma-1 receptor to disrupt redox and cholesterol homeostasis. Food Sci. Hum. Well 2025, 14, 9250372. [Google Scholar] [CrossRef]
- Pfohl-leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Kizis, D.; Vichou, A.E.; Natskoulis, P.I. Recent advances in mycotoxin analysis and detection of mycotoxigenic fungi in grapes and derived products. Sustainability 2021, 13, 2537. [Google Scholar] [CrossRef]
- Juliany, R.C.; Philip, G.C.; Corliss, A.O.; Steven, C.R. Essential oils as antimicrobials in food systems–a review. Food Control 2015, 54, 111–119. [Google Scholar]
- Wang, D.; Sun, J.; Li, J.; Sun, Z.; Liu, F.; Du, L.; Wang, D. Preparation and characterization of gelatin/zein nanofiber films loaded with perillaldehyde, thymol, or ɛ-polylysine and evaluation of their effects on the preservation of chilled chicken breast. Food Chem. 2022, 373, 131439. [Google Scholar] [CrossRef] [PubMed]
- Honma, M.; Yamada, M.; Yasui, M.; Horibata, K.I.; Sugiyama, K.I.; Masumura, K. In Vivo and in vitro mutagenicity of perillaldehyde and cinnamaldehyde. Genes Environ. 2021, 43, 30. [Google Scholar] [CrossRef]
- Tian, J.; Zeng, X.; Lu, A.; Zhu, A.; Peng, X.; Wang, Y. Perillaldehyde, a potential preservative agent in foods: Assessment of antifungal activity against microbial spoilage of cherry tomatoes. LWT-Food Sci. Technol. 2015, 60, 63–70. [Google Scholar] [CrossRef]
- Tian, H.; Qu, S.; Wang, Y.; Lu, Z.; Zhang, M.; Gan, Y.; Zhang, P.; Tian, J. Calcium and oxidative stress mediate perillaldehyde-induced apoptosis in Candida albicans. Appl. Microbiol. Biot. 2017, 101, 3335–3345. [Google Scholar] [CrossRef]
- Yang, K.; Geng, Q.; Luo, Y.; Xie, R.; Sun, T.; Wang, Z.; Qin, L.; Zhao, W.; Liu, M.; Li, Y. Dysfunction of FadA-cAMP signaling decreases Aspergillus flavus resistance to antimicrobial natural preservative perillaldehyde and AFB1 biosynthesis. Environ. Microbiol. 2022, 24, 1590–1607. [Google Scholar] [PubMed]
- Tian, J.; Wang, Y.; Lu, Z.; Sun, C.; Zhang, M.; Zhu, A.; Peng, X. Perillaldehyde, a promising antifungal agent used in food preservation, triggers apoptosis through a metacaspase-dependent pathway in Aspergillus flavus. J. Agric. Food Chem. 2016, 64, 7404–7413. [Google Scholar] [CrossRef]
- Tian, J.; Wang, Y.; Zeng, H.; Li, Z.; Zhang, P.; Tessema, A.; Peng, X. Efficacy and possible mechanisms of perillaldehyde in control of Aspergillus niger causing grape decay. Int. J. Food Microbiol. 2015, 202, 27–34. [Google Scholar] [CrossRef]
- Erhunmwunsee, F.; Pan, C.; Yang, K.; Li, Y.; Liu, M.; Tian, J. Recent development in biological activities and safety concerns of perillaldehyde from perilla plants: A review. Crit. Rev. Food Sci. Nutr. 2021, 62, 6328–6340. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wu, F.; Liu, F.; Wang, Q.; Zhang, X.; Selvaraj, J.N.; Zhao, Y.; Xing, F.; Yin, W.; et al. A consensus ochratoxin A biosynthetic pathway: Insights from the genome sequence of Aspergillus ochraceus and a comparative genomic analysis. Appl. Environ. Microbiol. 2018, 84, e01009-18. [Google Scholar] [CrossRef]
- Ferrara, M.; Gallo, A.; Perrone, G.; Baker, S.E. Comparative genomic analysis of ochratoxin A biosynthetic cluster in producing fungi: New evidence of a cyclase gene involvement. Front. Microbiol. 2020, 11, 3289–3300. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, L.; Jiang, D.; Wang, M.; Liu, H.; Yu, H.; Yao, W. Transcriptomic analysis of inhibition by eugenol of ochratoxin A biosynthesis and growth of Aspergillus carbonarius. Food Control 2022, 135, 108788. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, N.; Wang, D.; Wang, M. Effects of essential oil citral on the growth, mycotoxin biosynthesis and transcriptomic profile of Alternaria alternata. Toxins 2019, 11, 553. [Google Scholar] [CrossRef]
- Brandao, R.M.; Ferreira, V.R.F.; Batista, L.R.; Alves, E.; Lira, N.D.; Bellete, B.S.; Scolforo, J.R.S.; Cardoso, M.D. Antifungal and antimycotoxigenic effect of the essential oil of Eremanthus erythropappus on three different Aspergillus species. Flavour Frag. J. 2020, 35, 524–533. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.P.; Munro, C.A. The fungal cell wall: Structure, biosynthesis, and function. Microbiol. Spectr. 2017, 5, FUNK–0035–2016. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zheng, Y.; Zhou, A.; Neng, J.; Wu, D.; Shen, X.; Lou, X.; Yang, K. Transcriptomic analysis reveals the inhibition mechanism of pulsed light on fungal growth and ochratoxin A biosynthesis in Aspergillus carbonarius. Food Res. Int. 2023, 165, 112501. [Google Scholar] [CrossRef]
- Adams, D.J. Fungal cell wall chitinases and glucanases. Microbiology 2004, 150, 2029–2035. [Google Scholar] [CrossRef]
- Roncero, C.; de Aldana, C.R.V. Glucanases and chitinases. In The Fungal Cell Wall; Latgé, J.P., Ed.; Springer: Cham, Switzerland, 2019; Volume 425, pp. 131–166. [Google Scholar]
- Li, R.; Wu, X.; Yin, X.; Liang, J.; Li, M. The natural product citral can cause significant damage to the hyphal cell walls of Magnaporthe grisea. Molecules 2014, 19, 10279–10290. [Google Scholar] [CrossRef]
- Gao, X.; Liu, J.; Li, B.; Xie, J. Antibacterial activity and antibacterial mechanism of lemon verbena essential oil. Molecules 2023, 28, 102. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Zhang, R.; Yao, W. Major components in Lilac and Litsea cubeba essential oils kill Penicillium roqueforti through mitochondrial apoptosis pathway. Ind. Crop Prod. 2020, 149, 112349. [Google Scholar] [CrossRef]
- Kalagatur, N.K.; Ghosh, O.S.N.; Sundararaj, N.; Mudili, V. Antifungal activity of chitosan nanoparticles encapsulated with Cymbopogon martinii essential oil on plant pathogenic fungi Fusarium graminearum. Front. Pharmacol. 2018, 9, 610. [Google Scholar] [CrossRef]
- Dawson-Andoh, B.E. Ergosterol content as a measure of biomass of potential biological control fungi in liquid cultures. Holz Roh-Werkst. 2002, 60, 115–117. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef] [PubMed]
- Yaakoub, H.; Mina, S.; Calenda, A.; Bouchara, J.P.; Papon, N. Oxidative stress response pathways in fungi. Cell Mol. Life Sci. 2022, 79, 333. [Google Scholar]
- Vatansever, F.; de Melo, W.C.M.A.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R.; et al. Antimicrobial strategies centered around reactive oxygen species-bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef]
- Czégény, G.; Rácz, A. Phenolic peroxidases: Dull generalists or purposeful specialists in stress responses? J. Plant Physiol. 2023, 280, 153884. [Google Scholar] [CrossRef]
- Hu, Z.; Lu, C.; Zhang, Y.; Tong, W.; Du, L.; Liu, F. Proteomic analysis of Aspergillus flavus reveals the antifungal action of Perilla frutescens essential oil by interfering with energy metabolism and defense function. LWT-Food Sci. Technol. 2021, 154, 112660. [Google Scholar] [CrossRef]
- Pan, C.; Li, Y.; Yang, K.; Famous, E.; Ma, Y.; He, X.; Geng, Q.; Liu, M.; Tian, J. The molecular mechanism of perillaldehyde inducing cell death in Aspergillus flavus by inhibiting energy metabolism revealed by transcriptome sequencing. Int. J. Mol. Sci. 2020, 21, 1518. [Google Scholar] [CrossRef]
- Pedersen, P.L.; Carafoli, E. Ion motive ATPases. I. Ubiquity, properties, and significance to cell function. Trends Biochem. Sci. 1987, 12, 146–150. [Google Scholar] [CrossRef]
- Gozalbo, D.; Roig, P.; Villamón, E.; Gil, M.L. Candida and candidiasis: The cell wall as a potential molecular target for antifungal therapy. Curr. Drug Targets 2004, 4, 117–135. [Google Scholar] [CrossRef] [PubMed]
- Lepesheva, G.I.; Waterman, M.R. Sterol 14 alpha-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim. Biophys. Acta 2007, 1770, 467–477. [Google Scholar] [CrossRef]
- Aaron, K.E.; Pierson, C.A.; Lees, N.D.; Bard, M. The Candida albicans ERG26 gene encoding the C-3 sterol dehydrogenase (C-4 decarboxylase) is essential for growth. Fems. Yeast Res. 2002, 2, 93–101. [Google Scholar]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef]
- Martín, J.F.; Casqueiro, J.; Liras, P. Secretion systems for secondary metabolites: How producer cells send out messages of intercellular communication. Curr. Opin. Microbiol. 2005, 8, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Wang, Z.; Cheng, D.; Chen, X.; Chen, Y.; Ma, Z. The ATP-binding protein FgArb1 is essential for penetration, infectious and normal growth of Fusarium graminearum. New Phytol. 2018, 219, 1447–1466. [Google Scholar] [CrossRef]
- Xue, W.; Yin, Y.; Ismail, F.; Hu, C.; Zhou, M.; Cao, X.; Li, S.; Sun, X. Transcription factor ccg-8 plays a pivotal role in azole adaptive responses of Neurospora crassa by regulating intracellular azole accumulation. Curr. Genet. 2019, 65, 735–745. [Google Scholar] [CrossRef]
- Gunter, A.B.; Hermans, A.; Bosinich, W.; Johnson, D.A.; Harris, L.J.; Gleddie, S. Protein engineering of Saccharomyces cerevisiae transporter Pdr5p identifies key residues that impact Fusarium mycotoxin export and resistance to inhibition. MicrobiologyOpen 2016, 5, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Geisen, R.; Schmidt-Heydt, M.; Karolewiez, A. A gene cluster of the ochratoxin A biosynthetic genes in Penicillium. Mycotoxin Res. 2006, 22, 134–141. [Google Scholar] [CrossRef]
- Crespo-Sempere, A.; Gonzáles-Candelas, L.; Martínez-Culabras, P.V. Genes differentially expressed by Aspergillus carbonarius strains under ochratoxin A producing conditions. Int. J. Food Microbiol. 2010, 142, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Gerin, D.; De Miccolis Angelini, R.M.; Pollastro, S.; Faretra, F. RNA-Seq reveals OTA-related gene transcriptional changes in Aspergillus carbonarius. PLoS ONE 2016, 11, e0147089. [Google Scholar] [CrossRef]
- Yu, J.; Chang, P.K.; Ehrlich, K.C.; Cary, J.W.; Bhatnagar, D.; Cleveland, T.E.; Payne, G.A.; Linz, J.E.; Woloshuk, C.P.; Bennett, J.W. Clustered pathway genes in aflatoxin biosynthesis. Appl. Environ. Microbiol. 2004, 70, 1253–1262. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genom. Proteom. Bioinf. 2021, 19, 578–583. [Google Scholar] [CrossRef]
- CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2022, 50, D27–D38. [Google Scholar] [CrossRef] [PubMed]



| Gene ID | log2(PA/CK) | q-Value | Description |
|---|---|---|---|
| ASPCADRAFT_173482 | −1.832 | 0.000 | OTApks, Ochratoxin A biosynthesis cluster protein A (OtaA) |
| ASPCADRAFT_209537 | −4.863 | 0.000 | OTAcyc, probable cyclase, Ochratoxin A biosynthesis cluster protein Y (OtaY) |
| ASPCADRAFT_132610 | −3.353 | 0.000 | OTAnrps, Ochratoxin A biosynthesis cluster protein B (OtaB) |
| ASPCADRAFT_517149 | −4.052 | 0.000 | P450, Cytochrome P450 monooxygenase, Ochratoxin A biosynthesis cluster protein C (OtaC) |
| ASPCADRAFT_7821 | −2.387 | 0.001 | bZIP, Transcription factor, Ochratoxin A biosynthesis cluster protein R1 (OtaR1) |
| ASPCADRAFT_209543 | −3.007 | 0.000 | OTAhal, Flavin-dependent halogenase, Ochratoxin A biosynthesis cluster protein D (OtaD) |
| ASPCADRAFT_135554 | −5.220 | 0.000 | Homology with the Flu1 gene, which is involved in OTA secretion and transport |
| ASPCADRAFT_173225 | −1.370 | 0.007 | Homology with the aflT gene, which is associated with the exogenesis and transport of aflatoxins |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Wang, L.; Jiang, N.; Yan, J.; Mei, J.; Wang, M. Perillaldehyde-Elicited Inhibition of Ochratoxin A Production by Aspergillus carbonarius. Toxins 2025, 17, 530. https://doi.org/10.3390/toxins17110530
Jiang D, Wang L, Jiang N, Yan J, Mei J, Wang M. Perillaldehyde-Elicited Inhibition of Ochratoxin A Production by Aspergillus carbonarius. Toxins. 2025; 17(11):530. https://doi.org/10.3390/toxins17110530
Chicago/Turabian StyleJiang, Dongmei, Liuqing Wang, Nan Jiang, Jiaqi Yan, Jingzhi Mei, and Meng Wang. 2025. "Perillaldehyde-Elicited Inhibition of Ochratoxin A Production by Aspergillus carbonarius" Toxins 17, no. 11: 530. https://doi.org/10.3390/toxins17110530
APA StyleJiang, D., Wang, L., Jiang, N., Yan, J., Mei, J., & Wang, M. (2025). Perillaldehyde-Elicited Inhibition of Ochratoxin A Production by Aspergillus carbonarius. Toxins, 17(11), 530. https://doi.org/10.3390/toxins17110530





