Abstract
Even though there are guidelines for the management of snakebite envenoming (SBE), the use of antibiotics in this pathology remains controversial. The aim of this study is to provide a narrative review of the literature and recommendations based on the best available evidence regarding antibiotic use in SBE. We performed a narrative review of relevant literature regarding SBE and antibiotic use as prophylaxis or treatment. A total of 26 articles were included. There is wide use of antibiotics in SBE; nevertheless, infection was not necessarily documented. The antibiotics used varied according to the study, from beta lactams to lincosamide and nitroimidazoles, and from monotherapy to combined antimicrobials. The most common recommendations were to manage skin and soft tissue infections and avoid infectious complications, but these suggestions are not necessarily based on bacteriological findings. Prophylactic use of antibiotics in SBE is discouraged in most studies. Antibiotic prescription in SBE should be based on the susceptibility of microorganisms isolated from the affected tissue or identified in snakes’ oral cavities. Antibiotics should be reserved only for patients with a demonstrated infection, or those at a high risk of developing an infection, i.e., presenting severe local envenoming, local signs of infection, or those with incorrect manipulation of wounds. Prospective studies are needed to correlate microbiological findings at the wound site and the response to antibiotic use.
Key Contribution:
Antibiotic prescription after snakebite envenoming should be based on local susceptibility to antimicrobial agents and flora identified in snakes’ oral cavities. It should be reserved only for patients with a demonstrated infection or those at a high risk of developing an infection, and not as a prophylactic measure.
1. Introduction
Snakebite envenoming (SBE) is a neglected tropical disease (NTD) responsible for high morbidity and mortality. There are more than 250 species of venomous snakes worldwide that are considered medically important by the World Health Organization (WHO) [1]. More than 5.8 billion people are at risk of encountering a venomous snake, and each year about 2.7 million cases are reported, resulting in 81,000–138,000 deaths [1].
SBE disproportionately affects children in low-income settings [2,3], often leading to permanent physical and psychological sequelae [4]. Due to their smaller size and lower volumes of distribution related to the injected venom, children often present with more severe envenoming, associated with more rapid development of neurotoxicity, coagulopathy, and severe local tissue damage [2,5].
Bacterial infections are a secondary complication of wounds caused by animal bites, including those inflicted by snakes [6,7]. The pathogenic microorganisms causing an infection are not only the ones from the patient’s skin flora but also those present in the snake’s oral cavity. Several studies have isolated bacteria from the oral cavity and venom of several species of snakes, which are likely to be involved in infections in cases of SBE [8,9]. Several studies have shown that bacterial infections are commonly observed in SBE inflicted by a variety of viperid and elapid species in different geographical settings [6,9,10].
Despite the relevance of infectious complications, the burden of infection in snakebites remains largely unknown, and reports tend to show variable findings. Infection rates range from 9 to 77% of patients [11,12], with data in children often being limited and extrapolated from adults. Inappropriate first-aid interventions, such as the use of tourniquets, local application of chemicals or natural products, electric shocks, and incisions at the bite site, among others, are likely to increase the risk of infection [13,14,15].
However, even though snakebites have been shown to have the potential to cause primary infections via the inoculation of infectious agents present in the venom and oral cavity of snakes, and secondary infections as a result of extensive tissue damage and bacterial superinfection, there is no consensus or specific guidelines regarding the use of antibiotics to treat these infections. In many instances, they are used prophylactically or without documenting the occurrence of infection. These antibiotics are often used as initial empirical therapy for many infectious diseases, so their use must be carefully considered.
The objective of this study is to carry out a narrative review of the literature on this topic and provide recommendations based on the best available evidence, which can be applied in centers that manage patients suffering with SBE.
2. Results
From 1980 to 2023, we identified twenty-six publications focused on snakebite and antibiotic use from a range of countries with high incidence of SBE (Figure 1 and Table 1). The description of wound infection associated with SBE is mentioned in many articles, and a considerable percentage of patients developed this complication. Soft tissue infection such as cellulitis or abscess formation was described in 10% to 25% of patients in most studies [6,9,16,17,18,19,20,21,22], but its occurrence could be higher depending on the severity of envenoming [23,24]. In contrast, in some settings the prevalence of infections in SBE patients is lower [25,26,27], probably related to the type of envenoming and management received by patients in these settings.
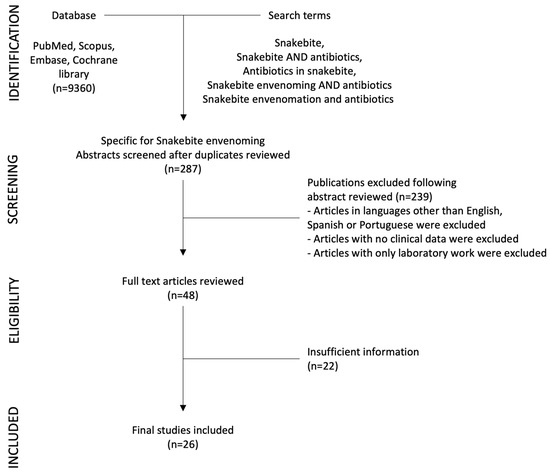
Figure 1.
Diagram of selection of studies.

Table 1.
Included studies with main characteristics, objectives, and principal outcomes and observations.
Risk factors for wound or soft tissue infection were studied in envenomings caused by diverse species of the families Viperidae and Elapidae [7,17]. Although many factors are involved in the development of complications secondary to SBE, a consistently higher incidence of infection was described in patients with clinically moderate to severe envenoming [22], including cases with necrosis [38]. Necrosis, which is associated with tissue damage in envenomings by species of the family Viperidae and some species of the family Elapidae, favors the presence of bacterial infection. Houcke et. al., in their studies in French Guiana, where Bothrops atrox is responsible for most bites, identified necrosis as an independent factor associated with infection in these envenomings (OR 13.15, CI: 4.04–42.84, p < 0.001), along with thrombocytopenia, and rhabdomyolysis [38]. There are other described risk factors for infection, such as self-manipulation of the wound prior to receiving medical attention [34,41,42], envenoming caused by species of Bothrops sp. and by elapid species of the genus Naja that can induce significant tissue damage [7,19,38], or a delay in medical care after the bite [6]. A study in Brazil identified several laboratory parameters that correlated with a higher risk of infection, such as elevated concentration of fibrinogen, alanine aminotransferase (ALT), and C-reactive protein (CRP) [33]. Some of the most common organisms described in the literature as causes of infection in snakebite envenoming are M. morganii, Proteus sp., S. aureus, Enterococcus sp., A. hydrophila, and E. coli (Table 1).
We focused our search on two main aspects: first, the antibiotics that were used to treat SBE cases, and second, the indication of antibiotic use and recommendations. Use of antibiotics in SBE was reported from 12% to as high as 100% of affected patients (Table 1) [36,43,44,45]. They were used either prophylactically or in patients with suspected or confirmed infection. Many antibiotics have been used, including beta-lactams (such as penicillin, amoxicillin–clavulanate, piperacillin–tazobactam, and cephalosporins), aminoglycosides (such as gentamicin and amikacin), nitroimidazoles (metronidazole), lincomycin (clindamycin), or quinolones (Table 1). Older studies included chloramphenicol in some settings [16,21,28,29]. Interestingly, we identified that a sensitivity analysis for identified bacteria was performed in some studies (17 contained reports of antibiotic sensitivity patterns vs. 9 studies that did not report it). Some groups used antibiotics based on the microorganisms most frequently described by other works, or sometimes, they used the reference of sensitivity patterns from other studies.
We also focused our review on the criteria for recommending antibiotic use. Several studies proposed the prophylactic use of antibiotics in SBE owing to the likelihood of bacterial infections in this pathology [16,32,35]. In contrast, most studies emphasize that the prophylactic use of antibiotics should be avoided, and instead suggest that they should be used only when there is evidence of infection in these patients. Three controlled studies in SBE in South America evaluated the use of prophylactic antibiotics. It was found that the incidence of infection was not reduced in patients receiving antibiotics compared to those who did not receive them [21,29,33]. Other studies also argue against the use of prophylactic antibiotics in SBE (Table 1) [23,25,26,37]. Thus, there is a predominant view in the reviewed literature that the prophylactic administration of antibiotics in SBE is not warranted and that they should be used only when there is clinical or bacteriological evidence of infection.
3. Discussion
We identified twenty-six articles that fulfilled the search criteria on the topic of antibiotic use in SBE. The incidence of infection in SBE is highly variable and depends on several factors. Infections might result from the inoculation of bacteria present in the oral cavity and the venom of snakes [8,46], as well as from bacterial superinfection secondary to local tissue damage and disruption of the skin integrity. Identification of risk factors of infection is essential to determine the cases in which the rational use of antibiotics is indicated. The reviewed literature mentions that the risk of wound infection is higher in patients with moderate or severe envenoming, self-manipulation of the wound [25,26,38], bites inflicted by species that cause pronounced local tissue necrosis such as those of the genera Bothrops in Latin America [10,47] and cytotoxic Naja species [19], or a delay in the access of medical care after the bite [6].
The role of venom-induced local tissue damage, i.e., necrosis and ischemia, as a factor that favors infection, has been demonstrated experimentally [48]. The snake species causing envenoming is also important to consider when suspecting infection. Different snake species predominate in different regions, and variations in the associated pathologies are likely to play a role in the incidence of infections [34,49,50].
In the studies reviewed here, broad-spectrum antibiotics were generally used, either alone or, more often, as combinations, with a predominance of third-generation cephalosporins, ampicillin, metronidazole, clindamycin, and occasionally oral ciprofloxacin (Table 1). There is no consensus regarding which antibiotics to use in SBE, and several studies recommend selecting the antibiotics based on the predominant bacteria of the mouth of snakes [16,30,40,51]. In addition, care should be taken to consider the possible adverse effects of some antibiotics in the context of the pathophysiology of envenoming. For example, in the case of aminoglycosides, their use might be detrimental in the case of neurotoxic envenoming owing to the possible exacerbation of clinical symptoms secondary to the blocking effect at the neuromuscular junctions, and its nephrotoxic side effect. On the other hand, although amoxicillin–clavulanate is recommended for the treatment of soft tissue infections for other animal bites, its use in SBE is controversial, and several studies do not support its use [11,22,33]. Also, the effect of antibiotics in other organs, such as the impact some of them have on renal function, may also be detrimental in a disease in which renal compromise is part of the findings in severe envenoming [52,53].
The routine use of antibiotics as prophylaxis after snakebite has been proposed by some authors and is routinely applied in several hospital settings. However, this practice is controversial, and in most studies analyzed there is a consensus against it, since it is not supported by clinical evidence in controlled trials. Therefore, a rational use of antibiotics is mandatory in every disease associated with infection, given the emergence of multi-resistant bacteria, and SBE is not an exception [54,55]. In ideal conditions, before starting antibiotics, aerobic and anaerobic cultures should be carried out to identify the infecting microorganisms and to select the most effective antibiotics. However, in many rural settings of sub-Saharan Africa, Asia, and Latin America, this may not be possible due to limited resources. Therefore, in many health facilities in regions of high incidence of SBE, the identification of patients that require antibiotic therapy is usually based on clinical evidence of infection, which is often associated with prominent tissue damage as a consequence of envenoming.
Our review has limitations. The literature regarding antibiotics in SBE is heterogeneous, and randomized studies comparing antibiotic use are limited. The use of antibiotics described in the publications was based on standard of care in individual settings, making comparisons difficult. Nevertheless, the description of antibiotics used in different studies show a general picture of the management of infections in snakebite envenoming.
4. Conclusions
The use of antibiotics in SBE is a common practice, and in some cases, it is used prophylactically. Although the literature on the subject is heterogenous, there is a growing consensus that antibiotics should not be used in all cases of SBE, and instead they should be reserved only for patients with a demonstrated infection, or those at a high risk of developing an infection, i.e., presenting severe local envenoming, local signs of infection, or those with incorrect manipulation of wounds. Prospective studies need to be conducted to establish the actual incidence of infection in SBE in different settings, to correlate microbiological findings and pathology at the wound site, as well as to select the most effective antibiotic therapy. There is also a need to generate guidelines and conduct prospective studies on this relevant aspect of SBE.
5. Materials and Methods
To identify published studies in the field, we reviewed the most relevant literature on this subject. The goal was to gather information regarding SBE and antibiotic use, as well as recommendations for prophylaxis with antibiotics or treatment for established infections. Previous publications on these topics were analyzed in detail, and general trends were identified.
A search for biomedical literature in PubMed, Scopus, Embase, and Cochrane library databases was carried out using the following terms: Snakebite, Snakebite AND antibiotics, Antibiotics in snakebite, Snakebite envenoming AND antibiotics, Snakebite envenomation AND antibiotics. We found a total of 9360 articles, of which 287 specifically discussed antibiotic use in SBE. Abstracts and articles were reviewed by two of the authors (HB-C, MLA-A). We excluded those in languages other than English, Spanish or Portuguese, when no clinical data were included and when only laboratory work was reported. Case reports and small case series were also excluded.
Author Contributions
Conceptualization, M.L.A.-A.; methodology, M.L.A.-A., J.M.G. and H.B.-C.; formal analysis, M.L.A.-A., J.M.G. and H.B.-C.; investigation, M.L.A.-A., J.M.G. and H.B.-C.; resources, M.L.A.-A., J.M.G. and H.B.-C.; data curation, M.L.A.-A., J.M.G. and H.B.-C.; writing—original draft preparation, M.L.A.-A., J.M.G. and H.B.-C.; writing—review and editing, M.L.A.-A., J.M.G. and H.B.-C.; visualization, M.L.A.-A., J.M.G. and H.B.-C.; supervision, M.L.A.-A., J.M.G. and H.B.-C.; project administration, M.L.A.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No original data were generated in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Minghui, R.; Malecela, M.N.; Cooke, E.; Abela-Ridder, B. WHO’s Snakebite Envenoming Strategy for prevention and control. Lancet Glob. Health 2019, 7, e837–e838. [Google Scholar] [CrossRef] [PubMed]
- Bhaumik, S.; Menon, G.R.; Habib, A.G. Prioritising snakebite in the child and adolescent health agenda. Lancet Child. Adolesc. Health 2023, 7, 753–755. [Google Scholar] [CrossRef] [PubMed]
- Iliyasu, G.; Dayyab, F.M.; Michael, G.C.; Hamza, M.; Habib, M.A.; Gutierrez, J.M.; Habib, A.G. Case fatality rate and burden of snakebite envenoming in children—A systematic review and meta-analysis. Toxicon 2023, 234, 107299. [Google Scholar] [CrossRef] [PubMed]
- Le Geyt, J.; Pach, S.; Gutierrez, J.M.; Habib, A.G.; Maduwage, K.P.; Hardcastle, T.C.; Hernandez Diaz, R.; Avila-Aguero, M.L.; Ya, K.T.; Williams, D.; et al. Paediatric snakebite envenoming: Recognition and management of cases. Arch. Dis. Child. 2021, 106, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, V.Y.; Ahmed, M.; Colaco, S.M. Clinical profile of snake bite in children in rural India. Iran. J. Pediatr. 2013, 23, 632–636. [Google Scholar] [PubMed]
- Brenes-Chacon, H.; Ulloa-Gutierrez, R.; Soriano-Fallas, A.; Camacho-Badilla, K.; Valverde-Munoz, K.; Avila-Aguero, M.L. Bacterial Infections Associated with Viperidae Snakebites in Children: A 14-Year Experience at the Hospital Nacional de Ninos de Costa Rica(dagger). Am. J. Trop. Med. Hyg. 2019, 100, 1227–1229. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Hsueh, J.H.; Liu, W.C.; Yang, K.C.; Hsu, K.C.; Lin, C.T.; Ho, Y.Y.; Chen, L.W. Contributing Factors for Complications and Outcomes in Patients with Snakebite: Experience in a Medical Center in Southern Taiwan. Ann. Plast. Surg. 2017, 78, S32–S36. [Google Scholar] [CrossRef]
- Jorge, M.T.; de Mendonca, J.S.; Ribeiro, L.A.; da Silva, M.L.; Kusano, E.J.; Cordeiro, C.L. [Bacterial flora of the oral cavity, fangs and venom of Bothrops jararaca: Possible source of infection at the site of bite]. Rev. Inst. Med. Trop. Sao Paulo 1990, 32, 6–10. [Google Scholar] [CrossRef]
- Chen, C.M.; Wu, K.G.; Chen, C.J.; Wang, C.M. Bacterial infection in association with snakebite: A 10-year experience in a northern Taiwan medical center. J. Microbiol. Immunol. Infect. 2011, 44, 456–460. [Google Scholar] [CrossRef]
- Otero-Patino, R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon 2009, 54, 998–1011. [Google Scholar] [CrossRef]
- Resiere, D.; Gutierrez, J.M.; Neviere, R.; Cabie, A.; Hossein, M.; Kallel, H. Antibiotic therapy for snakebite envenoming. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190098. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.K.; Vardanega, J.; Smith, S.; White, J.; Little, M.; Hanson, J. The Incidence of Infection Complicating Snakebites in Tropical Australia: Implications for Clinical Management and Antimicrobial Prophylaxis. J. Trop. Med. 2023, 2023, 5812766. [Google Scholar] [CrossRef] [PubMed]
- Parker-Cote, J.; Meggs, W.J. First Aid and Pre-Hospital Management of Venomous Snakebites. Trop. Med. Infect. Dis. 2018, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Gil-Alarcon, G.; Sanchez-Villegas Mdel, C.; Hugo Reynoso, V. Pre-hospital treatment of ophidian accidents: Review, update, and current problems. Gac. Med. Mex. 2011, 147, 195–208. [Google Scholar] [PubMed]
- Hardcastle, T.C.; Kajee, M.; Lachenicht, K.; Van der Walt, N. Approach to the diagnosis and management of snakebite envenomation in South Africa in humans. S. Afr. Med. J. 2023, 113, 10–18. [Google Scholar] [CrossRef]
- Kerrigan, K.R. Bacteriology of snakebite abscess. Trop. Doct. 1992, 22, 158–160. [Google Scholar] [CrossRef]
- Wagener, M.; Naidoo, M.; Aldous, C. Wound infection secondary to snakebite. S. Afr. Med. J. 2017, 107, 315–319. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, Y.C.; Goh, Z.N.L.; Seak, C.K.; Seak, J.C.; Shi-Ying, G.; Seak, C.J.; Spot, I. Wound Infections of Snakebites from the Venomous Protobothrops mucrosquamatus and Viridovipera stejnegeri in Taiwan: Bacteriology, Antibiotic Susceptibility, and Predicting the Need for Antibiotics-A BITE Study. Toxins 2020, 12, 575. [Google Scholar] [CrossRef]
- Yeh, H.; Gao, S.Y.; Lin, C.C. Wound Infections from Taiwan Cobra (Naja atra) Bites: Determining Bacteriology, Antibiotic Susceptibility, and the Use of Antibiotics-A Cobra BITE Study. Toxins 2021, 13, 183. [Google Scholar] [CrossRef]
- Mendes, V.; Pereira, H.D.S.; Elias, I.C.; Soares, G.S.; Santos, M.; Talhari, C.; Cordeiro-Santos, M.; Monteiro, W.M.; Sachett, J.A.G. Secondary infection profile after snakebite treated at a tertiary referral center in the Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2022, 55, e0244. [Google Scholar] [CrossRef]
- Kerrigan, K.R.; Mertz, B.L.; Nelson, S.J.; Dye, J.D. Antibiotic prophylaxis for pit viper envenomation: Prospective, controlled trial. World J. Surg. 1997, 21, 369–372, discussion 372–363. [Google Scholar] [CrossRef]
- Resiere, D.; Mehdaoui, H.; Neviere, R.; Olive, C.; Severyns, M.; Beaudoin, A.; Florentin, J.; Brouste, Y.; Banydeen, R.; Cabie, A.; et al. Infectious Complications Following Snakebite by Bothrops lanceolatus in Martinique: A Case Series. Am. J. Trop. Med. Hyg. 2020, 102, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumaran, S.; Salim, A.; Almeida, J.R.; Williams, J.; Vijayakumar, P.; Thirunavukarasu, A.; Christopoulos, M.A.; Williams, H.F.; Thirumalaikolundusubramanian, P.; Patel, K.; et al. The Effectiveness of Antibiotics in Managing Bacterial Infections on Bite Sites following Snakebite Envenomation. Toxins 2023, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.C.; Liu, P.Y.; Hung, D.Z.; Lai, W.C.; Huang, S.T.; Hung, Y.M.; Yang, C.C. Bacteriology of Naja atra Snakebite Wound and Its Implications for Antibiotic Therapy. Am. J. Trop. Med. Hyg. 2016, 94, 1129–1135. [Google Scholar] [CrossRef]
- Clark, R.F.; Selden, B.S.; Furbee, B. The incidence of wound infection following crotalid envenomation. J. Emerg. Med. 1993, 11, 583–586. [Google Scholar] [CrossRef] [PubMed]
- LoVecchio, F.; Klemens, J.; Welch, S.; Rodriguez, R. Antibiotics after rattlesnake envenomation. J. Emerg. Med. 2002, 23, 327–328. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.T.; Corsi, J.M.; Boneti, C.; Jackson, R.J.; Smith, S.D.; Kokoska, E.R. Pediatric snakebites: Lessons learned from 114 cases. J. Pediatr. Surg. 2008, 43, 1338–1341. [Google Scholar] [CrossRef]
- Jorge, M.T.; Ribeiro, L.A.; da Silva, M.L.; Kusano, E.J.; de Mendonca, J.S. Microbiological studies of abscesses complicating Bothrops snakebite in humans: A prospective study. Toxicon 1994, 32, 743–748. [Google Scholar] [CrossRef]
- Jorge, M.T.; Malaque, C.; Ribeiro, L.A.; Fan, H.W.; Cardoso, J.L.; Nishioka, S.A.; Sano-Martins, I.S.; Franca, F.O.; Kamiguti, A.S.; Theakston, R.D.; et al. Failure of chloramphenicol prophylaxis to reduce the frequency of abscess formation as a complication of envenoming by Bothrops snakes in Brazil: A double-blind randomized controlled trial. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 529–534. [Google Scholar] [CrossRef]
- Garg, A.; Sujatha, S.; Garg, J.; Acharya, N.S.; Chandra Parija, S. Wound infections secondary to snakebite. J. Infect. Dev. Ctries. 2009, 3, 221–223. [Google Scholar] [CrossRef]
- Huang, L.W.; Wang, J.D.; Huang, J.A.; Hu, S.Y.; Wang, L.M.; Tsan, Y.T. Wound infections secondary to snakebite in central Taiwan. J. Venom. Anim. Toxins incl Trop. Dis. 2012, 18, 272–276. [Google Scholar] [CrossRef]
- Palappallil, D.S. Pattern of Use of Antibiotics Following Snake Bite in a Tertiary Care Hospital. J. Clin. Diagn. Res. 2015, 9, OC05–OC09. [Google Scholar] [CrossRef] [PubMed]
- Sachett, J.A.G.; da Silva, I.M.; Alves, E.C.; Oliveira, S.S.; Sampaio, V.S.; do Vale, F.F.; Romero, G.A.S.; Dos Santos, M.C.; Marques, H.O.; Colombini, M.; et al. Poor efficacy of preemptive amoxicillin clavulanate for preventing secondary infection from Bothrops snakebites in the Brazilian Amazon: A randomized controlled clinical trial. PLoS Negl. Trop. Dis. 2017, 11, e0005745. [Google Scholar] [CrossRef] [PubMed]
- August, J.A.; Boesen, K.J.; Hurst, N.B.; Shirazi, F.M.; Klotz, S.A. Prophylactic Antibiotics Are Not Needed Following Rattlesnake Bites. Am. J. Med. 2018, 131, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Ngo, N.D.; Le, Q.X.; Pham, A.Q.; Nguyen, N.T.; Ha, H.T.; Dinh, M.M.Q.; Le, T.Q. Clinical Features, Bacteriology, and Antibiotic Treatment Among Patients with Presumed Naja Bites in Vietnam. Wilderness Environ. Med. 2020, 31, 151–156. [Google Scholar] [CrossRef]
- Kriengkrairut, S.; Othong, R. Bacterial infection secondary to Trimeresurus species bites: A retrospective cohort study in a university hospital in Bangkok. Emerg. Med. Australas. 2021, 33, 1006–1012. [Google Scholar] [CrossRef]
- Chiang, L.C.; Chaou, C.H.; Li, Y.Y.; Seak, C.J.; Yu, S.R.; Lin, C.C. Management and Prognosis of Snake Envenomation Among Pediatric Patients: A National Database Study. J. Acute Med. 2022, 12, 13–22. [Google Scholar] [CrossRef]
- Houcke, S.; Resiere, D.; Lontsingoula, G.R.; Cook, F.; Lafouasse, P.; Pujo, J.M.; Demar, M.; Matheus, S.; Hommel, D.; Kallel, H. Characteristics of Snakebite-Related Infection in French Guiana. Toxins 2022, 14, 89. [Google Scholar] [CrossRef]
- Hu, S.; Lou, Z.; Shen, Y.; Tu, M. Bacteriological Studies of Venomous Snakebite Wounds in Hangzhou, Southeast China. Am. J. Trop. Med. Hyg. 2022, 107, 925–929. [Google Scholar] [CrossRef]
- Soares Coriolano Coutinho, J.V.; Fraga Guimaraes, T.; Borges Valente, B.; Gomes Martins de Moura Tomich, L. Epidemiology of secondary infection after snakebites in center-west Brazil. PLoS Negl. Trop. Dis. 2023, 17, e0011167. [Google Scholar] [CrossRef]
- Michael, G.C.; Thacher, T.D.; Shehu, M.I. The effect of pre-hospital care for venomous snake bite on outcome in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 2011, 105, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Singaravelu, K.P.; Pandit, V.R.; Chinnakali, P.; Bammigatti, C. Pre-hospital care and its association with clinical outcome of snakebite victims presenting at a tertiary care referral hospital in South India. Trop. Doct. 2021, 51, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.C.; Traynor, M.; Bruce, J.L.; Bekker, W.; Laing, G.L.; Aho, J.M.; Kong, V.Y.; Klinkner, D.B.; Zielinski, M.D.; Clarke, D.L. Surgical Considerations for Pediatric Snake Bites in Low- and Middle-Income Countries. World J. Surg. 2019, 43, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Tekin, R.; Sula, B.; Cakirca, G.; Aktar, F.; Deveci, O.; Yolbas, I.; Celen, M.K.; Bekcibasi, M.; Palanci, Y.; Dogan, E. Comparison of snakebite cases in children and adults. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2711–2716. [Google Scholar] [PubMed]
- Correa, J.A.; Fallon, S.C.; Cruz, A.T.; Grawe, G.H.; Vu, P.V.; Rubalcava, D.M.; Kaziny, B.; Naik-Mathuria, B.J.; Brandt, M.L. Management of pediatric snake bites: Are we doing too much? J. Pediatr. Surg. 2014, 49, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Resiere, D.; Hossein, M.; Megarbane, B. Snake Bites by Bothrops lanceolatus in Martinique. Med. Sante Trop. 2018, 28, 37–43. [Google Scholar] [CrossRef]
- Oliveira, I.S.; Pucca, M.B.; Cerni, F.A.; Vieira, S.; Sachett, J.; Seabra de Farias, A.; Lacerda, M.; Murta, F.; Baia-da-Silva, D.; Rocha, T.A.H.; et al. Snakebite envenoming in Brazilian children: Clinical aspects, management and outcomes. J. Trop. Pediatr. 2023, 69, fmad010. [Google Scholar] [CrossRef]
- Saravia-Otten, P.; Gutierrez, J.M.; Arvidson, S.; Thelestam, M.; Flock, J.I. Increased infectivity of Staphylococcus aureus in an experimental model of snake venom-induced tissue damage. J. Infect. Dis. 2007, 196, 748–754. [Google Scholar] [CrossRef]
- Saravu, K.; Somavarapu, V.; Shastry, A.B.; Kumar, R. Clinical profile, species-specific severity grading, and outcome determinants of snake envenomation: An Indian tertiary care hospital-based prospective study. Indian. J. Crit. Care Med. 2012, 16, 187–192. [Google Scholar] [CrossRef]
- Nelson, B.K. Snake envenomation. Incidence, clinical presentation and management. Med. Toxicol. Adverse Drug Exp. 1989, 4, 17–31. [Google Scholar] [CrossRef]
- Padhi, L.; Panda, S.K.; Mohapatra, P.P.; Sahoo, G. Antibiotic susceptibility of cultivable aerobic microbiota from the oral cavity of Echis carinatus from Odisha (India). Microb. Pathog. 2020, 143, 104121. [Google Scholar] [CrossRef] [PubMed]
- Chugh, K.S.; Pal, Y.; Chakravarty, R.N.; Datta, B.N.; Mehta, R.; Sakhuja, V.; Mandal, A.K.; Sommers, S.C. Acute renal failure following poisonous snakebite. Am. J. Kidney Dis. 1984, 4, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lindbaek, M.; Berild, D.; Straand, J.; Hjortdahl, P. Influence of prescription patterns in general practice on anti-microbial resistance in Norway. Br. J. Gen. Pract. 1999, 49, 436–440. [Google Scholar]
- Tagwireyi, D.D.; Ball, D.E.; Nhachi, C.F. Routine prophylactic antibiotic use in the management of snakebite. BMC Clin. Pharmacol. 2001, 1, 4. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).