Selenium Content in Seafood in Japan
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Selenium Concentration Measurement
2.3. Mercury Concentration Determination
3. Results
| name | species | Japanese name | portion | selenium (mg/kg) |
|---|---|---|---|---|
| alfonsino | Beryx splendens | kinmedai | fillet | 1.27 |
| Japanese bluefish | Scombrops boops | mutsu | fillet with skin | 0.77 |
| Pacific bluefin tuna | Thunnus orientalis | kuromaguro | fillet (low-fat) | 0.75 |
| fillet (high-fat) | 0.72 | |||
| albacore | Thunnus alalunga | bin-naga | fillet | 0.75 |
| skipjack | Euthynnus pelamis | katsuo | fillet with skin | 0.62 |
| Japanese flounder | Paralichthys olivaceus | hirame | fillet with skin c | 0.56 |
| fillet with skin w | 0.42 | |||
| hoki | Macruronus novaezelandiae | hoki | fillet with skin | 0.56 |
| loach | Misgurnus anguillicaudatus | dojou | whole | 0.50 |
| southern black cod | Dissostichus eleginoides | mero | fillet | 0.49 |
| Pacific ocean perch | Sebastes alutus | alaska-menuke | fillet | 0.49 |
| golden-thread | Nemipterus virgatus | itoyoridai | surimi | 0.48 |
| conger pike | Muraenesox cinereus | hamo | fillet with skin | 0.47 |
| yellowtail | Seriola quinqueradiata | buri | fillet with skin w | 0.46 |
| Pacific mackerel | Scomber japonicus | masaba | fillet with skin | 0.40 |
| Pacific herring | Clupea pallasii | nishin | fillet with skin | 0.40 |
| sailfin sandfish | Arctoscopus japonicus | hatahata | fillet with skin | 0.40 |
| gurnard | Chelidonichthys spinosus | houbou | fillet with skin | 0.39 |
| Asian yellowtail | Seriola lalandi | hiramasa | fillet with skin | 0.38 |
| Japanese scallops | Pecten albicans | itayagai | without shell | 0.37 |
| masu salmon | Oncorhynchus masou | sakuramasu | fillet with skin | 0.35 |
| three-line grunt | Parapristipoma trilineatum | isaki | fillet with skin | 0.35 |
| striped jack | Caranx delicatissimus | shima-aji | fillet with skin c | 0.32 |
| Japanese seabass | Lateolabrax japonicus | suzuki | fillet with skin | 0.32 |
| lamprey | Lethenteron japonicum | yatsumeunagi | fillet with skin | 0.32 |
| Pacific halibut | Hippoglossus stenolepis | ohyou | fillet with skin | 0.31 |
| Japanese surf smelt | Hypomesus pretiosus | chika | fillet with skin | 0.31 |
| silver pomfret | Pampus punctatissimus | managatsuo | fillet with skin | 0.31 |
| Japanese common squid | Todarodes pacificus | surumeika | without viscera | 0.30 |
| rainbow trout | Oncorhynchus mykiss | nijimasu | fillet with skin cs | 0.29 |
| fillet with skin cf | 0.26 | |||
| Japanese parrot fish | Oplegnathus fasciatus | ishidai | fillet with skin | 0.29 |
| coho salmon | Oncorhynchus kitsch | ginzake | fillet with skin c | 0.28 |
| Chinook salmon | Oncorhynchus tshawytscha | masunosuke | fillet with skin | 0.28 |
| Japanese whiting | Sillago japonica | shirogisu | fillet with skin | 0.28 |
| flying fish | Cypselurus agoo agoo | tobiuo | fillet with skin | 0.28 |
| sockeye salmon | Oncorhynchus nerka | benizake | fillet with skin | 0.23 |
| Pacific cod | Gadus macrocephalus | madara | fillet with skin | 0.23 |
| walleye pollock | Theragra chalcogramma | suketoudara | fillet with skin | 0.22 |
| Atlantic mackerel | Scomber scombrus | taiseiyousaba | fillet with skin | 0.23 |
| mullet | Mugil cephalus | mabora | fillet with skin | 0.21 |
| char | Salvelinus pluvius | iwana | fillet with skin c | 0.21 |
| short-neck clam | Ruditapes philippinarum | asari | without shell | 0.21 |
| amago salmon | Oncorhynchus masou ishikawae | amago | fillet with skin c | 0.20 |
| hairtail | Trichiurus lepturus | tachiuo | fillet with skin | 0.19 |
| ocellate puffer | Takifugu rubripes | torafugu | fillet c | 0.17 |
| walleye pollock | Theragra chalcogramma | suketoudara | surimi | 0.16 |
| crucian carp | Carassius auratus | funa | fillet with skin | 0.16 |
| Coho salmon | Oncorhynchus kitsch | ginzake | fillet with skin w | 0.15 |
| Atlantic salmon | Salmo salar | taiseiyousake | fillet with skin | 0.15 |
| purple puffer | Takifugu porphyreus | mafugu | fillet | 0.14 |
| Japanese eel | Anguilla japonica | unagi | fillet with skin c | 0.12 |
| name | species | Japanese name | portion | selenium (mg/kg) |
|---|---|---|---|---|
| Pacific cod | Gadus macrocephalus | madara | testis | 0.14 |
| Pacific herring | Clupea pallasii | nishin | ovary | 1.07 |
| mullet | Mugil cephalus | mabora | salted ovary | 1.20 |
| tissue | n | Se (mg/kg) | Hg (mg/kg) |
|---|---|---|---|
| spleen | 6 | 24.8 ± 7.18 | 2.35 ± 0.06 |
| blood | 9 | 17.8 ± 9.32 | 0.61 ± 0.26 |
| hepatopancreas | 6 | 8.09 ± 2.76 | 3.92 ± 1.51 |
| heart | 6 | 4.38 ± 1.04 | 1.24 ± 0.34 |
| red muscle | 6 | 2.71 ± 1.04 | 1.08 ± 0.20 |
| white muscle | 24 | 1.27 ± 0.77 | 1.19 ± 0.43 |
| brain | 6 | 1.73 ± 0.22 | 1.62 ± 0.35 |
| ovary | 6 | 2.43 ± 0.58 | 0.40 ± 0.12 |
| testis | 6 | 1.10 ± 0.18 | 0.23 ± 0.12 |
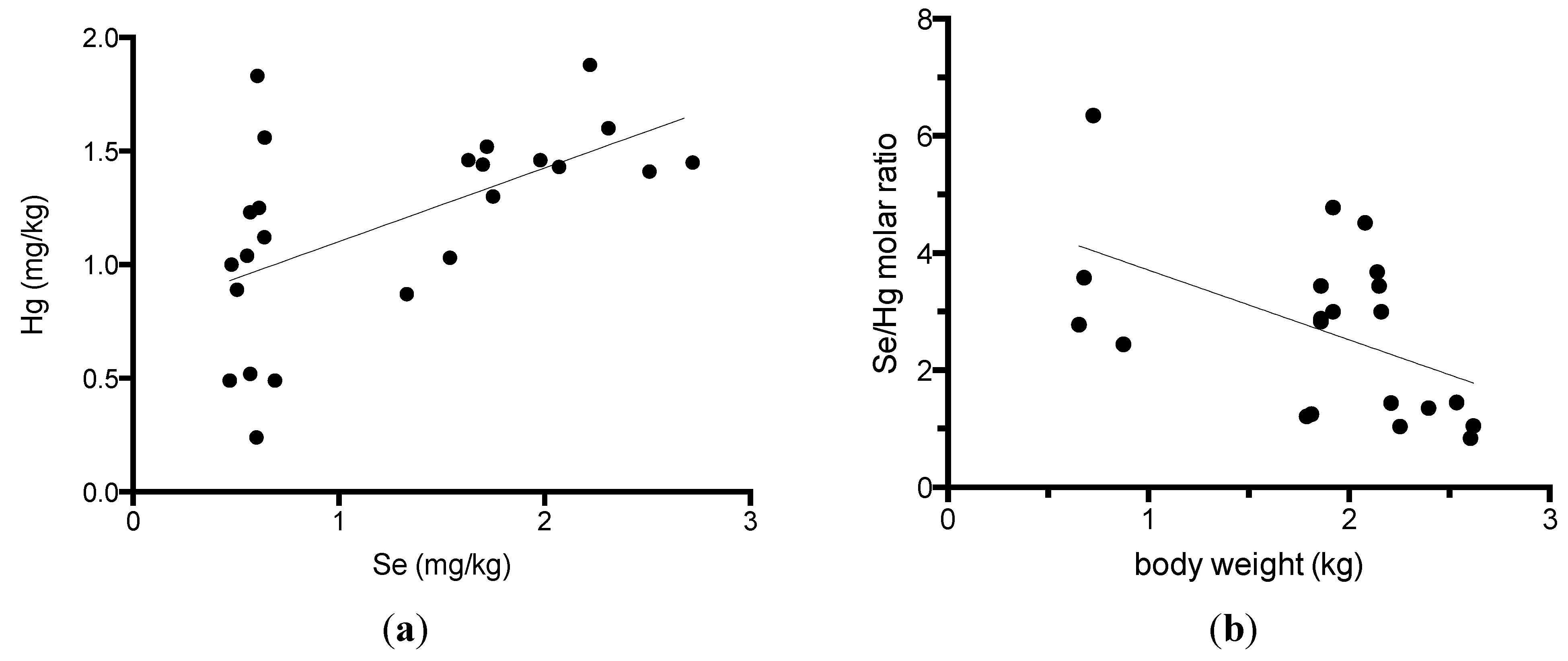
4. Discussion
Acknowledgments
Conflict of Interest
References
- Combs, G.F.; Combs, S.B. The Role of Selenium in Nutrition; Academic Press: Orlando, FL, USA, 1986; pp. 1–532. [Google Scholar]
- Himeno, S.; Imura, N. New aspects of physiological and pharmacological roles of selenium. J. Health Sci. 2000, 46, 1–6. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, S1484–S1491. [Google Scholar]
- Yamashita, Y.; Yamashita, M. Identification of a novel selenium-containing compound, selenoneine, as the predominant chemical form of organic selenium in the blood of bluefin tuna. J. Biol. Chem. 2010, 285, 18134–18138. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yabu, T.; Yamashita, M. Discovery of the strong antioxidant selenoneine in tuna and selenium redox metabolism. World J. Biol. Chem. 2010, 1, 144–150. [Google Scholar] [CrossRef]
- Yamashita, Y.; Amlund, H.; Suzuki, T.; Hara, T.; Hossain, A.M.; Yabu, T.; Touhata, K.; Yamashita, M. Selenoneine, total selenium, and total mercury content in the muscle of fishes. Fish. Sci. 2010, 77, 679–686. [Google Scholar]
- Ganther, H.; Goudie, C.; Sunde, M.; Kopeckey, M.; Wagner, S.; Hoekstra, W. Selenium: Relation to decreased toxicity of methylmercury added to diets containing tuna. Science 1972, 175, 1122–1124. [Google Scholar]
- Ralston, N.V.C.; Ralston, C.R.; Blackwell, J.L.; Raymond, L.J. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology 2008, 29, 802–811. [Google Scholar] [CrossRef]
- Ralston, N.V.C.; Blackwell, J.L.; Raymond, L.J. Importance of molar ratios in selenium—dependent protection against methylmercury toxicity. Biol. Trace Elem. Res. 2007, 119, 255–268. [Google Scholar] [CrossRef]
- Yamashita, Y.; Omura, Y.; Okazaki, E. Total mercury and methylmercury levels in commercially important fishes in Japan. Fish. Sci. 2005, 71, 1029–1035. [Google Scholar]
- Watkinson, J.H. Fluorometric determination of selenium in biological material with 2,3-diaminonapththalene. Anal. Chem. 1966, 38, 92–97. [Google Scholar] [CrossRef]
- Kryukox, G.V.; Gladyshev, V.N. Selenium metabolism in zebrafish: Multiplicity of selenoprotein genes and expression of a protein containing 17 selenocysteine residues. Genes Cells 2000, 5, 1049–1060. [Google Scholar] [CrossRef]
- Nagai, T.; Inada, J.; Hmada, M.; Kai, N.; Tanoue, Y.; Kaminishi, Y.; Nakagawa, H.; Fujiki, K.; Nakao, M. Distribution of glutathione peroxidase activity in fish. Fish. Sci. 1999, 65, 665–666. [Google Scholar] [CrossRef]
- Thompson, J.L.; See, V.H.L.; Thomas, P.M.; Schuller, K.A. Cloning and characterization of two glutathione peroxidase cDNAs from southern bluefin tuna (Thunnus maccoyii). Comp. Biochem. Physiol. 2010, 156, 287–297. [Google Scholar]
- Yamashita, Y.; Yabu, T.; Touhata, K.; Yamashita, M. Purification and characterization of glutathione peroxidase 1 in the red muscle of bluefin tuna. Fish. Sci. 2012, 79, 407–411. [Google Scholar]
- Vendeland, S.C.; Beilstein, M.A.; Chen, C.L.; Jensen, O.N.; Barofsky, E.; Whanger, P.D. Purification and properties of selenoprotein W from rat muscle. J. Biol. Chem. 1993, 268, 17103–17107. [Google Scholar]
- Arnér, E.S. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim. Biophys. Acta 2009, 1790, 495–526. [Google Scholar] [CrossRef]
- Ralston, N.V.; Raymond, L.J. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology 2010, 278, 112–123. [Google Scholar] [CrossRef]
- Yamashita, M.; Yamashita, Y.; Suzuki, T.; Kani, K.; Mizusawa, N.; Imamura, S.; Takemoto, T.; Hara, T.; Hossain, M.A.; Yabu, T.; Touhata, K. National Research Institute of Fisheries Science, Yokohama, Japan. Selenoneine, a novel selenium-containing compound, mediates detoxification mechanisms against methylmercury accumulation and toxicity in zebrafish embryo. Mar. Biotechnol. 2013. submitted. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamashita, Y.; Yamashita, M.; Iida, H. Selenium Content in Seafood in Japan. Nutrients 2013, 5, 388-395. https://doi.org/10.3390/nu5020388
Yamashita Y, Yamashita M, Iida H. Selenium Content in Seafood in Japan. Nutrients. 2013; 5(2):388-395. https://doi.org/10.3390/nu5020388
Chicago/Turabian StyleYamashita, Yumiko, Michiaki Yamashita, and Haruka Iida. 2013. "Selenium Content in Seafood in Japan" Nutrients 5, no. 2: 388-395. https://doi.org/10.3390/nu5020388
APA StyleYamashita, Y., Yamashita, M., & Iida, H. (2013). Selenium Content in Seafood in Japan. Nutrients, 5(2), 388-395. https://doi.org/10.3390/nu5020388




