The Association Between Egg and Egg-Derived Cholesterol Consumption, and Their Change Trajectories, with Obesity Among Chinese Adults: Results from the China Health and Nutrition Survey
Abstract
1. Introduction
2. Materials and Methods
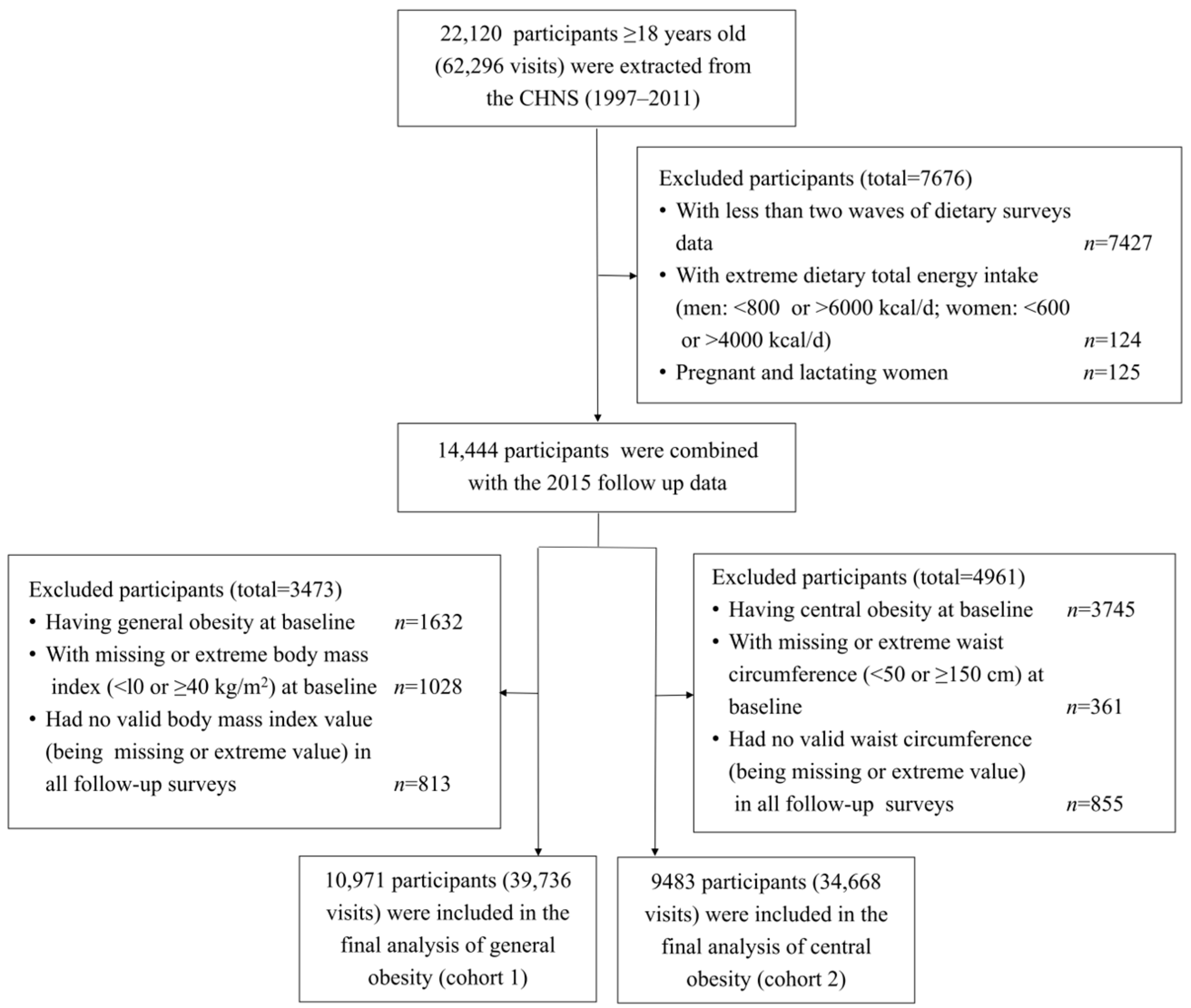
2.1. Study Design and Participants
2.2. Definition of Follow-Up in the Study
2.3. Exposure Assessment
2.4. Outcome Ascertainments
2.5. Assessments of Covariates
2.6. Statistical Analyses
3. Results
3.1. General Characteristics of Participants in the Study
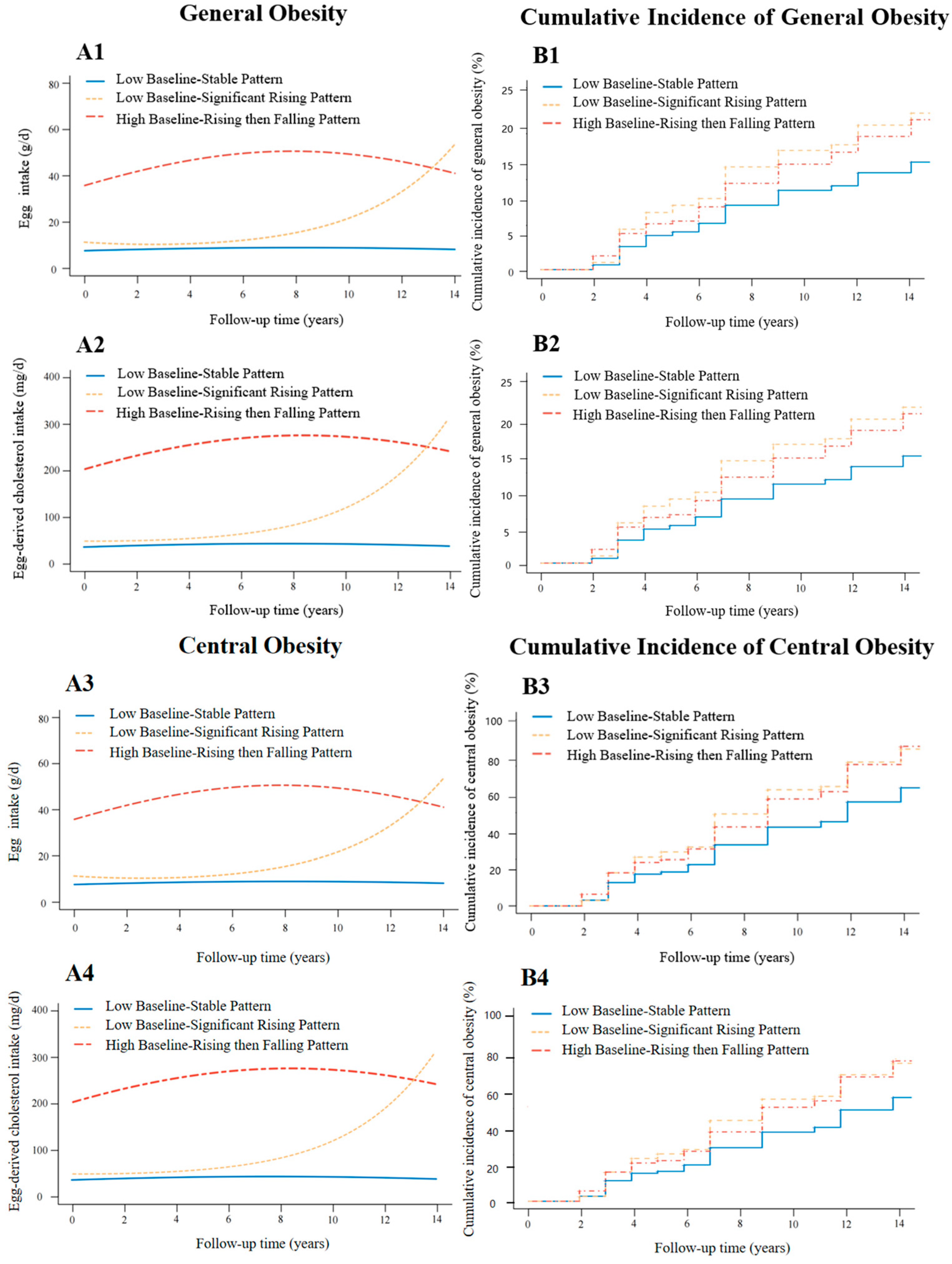
3.2. Trajectory Patterns of Egg and Egg-Derived Cholesterol Consumption of Participants
3.3. Association Between Egg Consumption and Its Change Trajectories and the Risk of Obesity
3.4. Association Between Egg-Derived Cholesterol Consumption and Its Change Trajectories and the Risk of Obesity
3.5. Subgroup Analyses and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohamed, G.A.; Ibrahim, S.R.M.; Elkhayat, E.S.; El Dine, R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014, 52, 269–284. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 August 2023).
- GBD 2015 Obesity Collaborators; Afshin, A.; Forouzanfar, M.; Reitsma, M.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, G. Obesity and obesity-related disorders—Editorial. Int. J. Mol. Sci. 2024, 25, 7954. [Google Scholar] [CrossRef] [PubMed]
- Lesnierowski, G.; Stangierski, J. What’s new in chicken egg research and technology for human health promotion?—A review. Trends Food Sci. Technol. 2018, 71, 46–51. [Google Scholar] [CrossRef]
- Melough, M.M.; Chung, S.J.; Fernandez, M.L.; Chun, O.K. Association of eggs with dietary nutrient adequacy and cardiovascular risk factors in US adults. Public Health Nutr. 2019, 22, 2033–2042. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhuang, P.; Zhan, C.; Shen, X.; Jiao, J.; Zhang, Y. Egg and dietary cholesterol consumption and the prevalence of metabolic syndrome: Findings from a population-based nationwide cohort. J. Acad. Nutr. Diet. 2022, 122, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.D.; Hou, T.; Ludwig, D.S.; Rimm, E.B.; Willett, W.; Hu, F.B.; Mozaffarian, D. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: Results from 3 prospective cohorts. Am. J. Clin. Nutr. 2015, 101, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Emrani, A.S.; Beigrezaei, S.; Zademohammadi, F.; Salehi-Abargouei, A. The effect of whole egg consumption on weight and body composition in adults: A systematic review and meta-analysis of clinical trials. Syst. Rev. 2023, 12, 125. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhao, Y.; Li, Q.; Dang, S.; Yan, H. Body fat mass, fat distribution and egg consumption: A population-based study in Chinese adults. J. Am. Coll. Nutr. 2020, 39, 528–536. [Google Scholar] [CrossRef]
- Park, S.J.; Jung, J.H.; Choi, S.W.; Lee, H.J. Association between egg consumption and metabolic disease. Korean J. Food Sci. Anim. Resour. 2018, 38, 209–223. [Google Scholar] [PubMed]
- Kuang, H.; Yang, F.; Zhang, Y.; Wang, T.; Chen, G. The impact of egg nutrient composition and its consumption on cholesterol homeostasis. Cholesterol 2018, 2018, 6303810. [Google Scholar] [CrossRef]
- US Department of Agriculture Agricultural Research Service, Nutrient Data Laboratory. USDA National Nutrient Database for Standard Reference, Release 28. Version Current: September 2015. Available online: https://searchworks.stanford.edu/view/12321932 (accessed on 17 April 2024).
- Yang, Y.; Wang, G.; Pan, X.; Chinese Center for Disease Control and Prevention; Institute of Nutrition and Food Safety. China Food Composition Table 2002; Peking University Medical Press: Beijing, China, 2002; p. 393. [Google Scholar]
- Berger, S.; Raman, G.; Vishwanathan, R.; Jacques, P.F.; Johnson, E.J. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Rosqvist, F.; Kullberg, J.; Ståhlman, M.; Cedernaes, J.; Heurling, K.; Johansson, H.E.; Iggman, D.; Wilking, H.; Larsson, A.; Eriksson, O.; et al. Overeating saturated fat promotes fatty liver and ceramides compared with polyunsaturated fat: A randomized trial. J. Clin. Endocrinol. Metab. 2019, 104, 6207–6219. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.; Ruxton, C.H.S. Eggs: Healthy or risky? A review of evidence from high quality studies on hen’s eggs. Nutrients 2023, 15, 2657. [Google Scholar] [CrossRef]
- Popkin, B.M.; Du, S.; Zhai, F.; Zhang, B. Cohort Profile: The China Health and Nutrition Survey—Monitoring and understanding socio-economic and health change in China, 1989–2011. Int. J. Epidemiol. 2010, 39, 1435–1440. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhai, F.Y.; Du, S.F.; Popkin, B.M. The China Health and Nutrition Survey, 1989–2011. Obes. Rev. 2014, 15 (Suppl. 1), 2–7. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, B.; Zhai, F.; Wang, H.; Zhang, J.; Du, W.; Su, C.; Zhang, J.; Jiang, H.; Popkin, B.M. Fatty and lean red meat consumption in China: Differential association with Chinese abdominal obesity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 869–876. [Google Scholar] [CrossRef] [PubMed]
- The Working Group on Obesity in China (WGOC). Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population. Chin. J. Epidemiol. 2014, 23, 5–10. [Google Scholar]
- Pan, K.; Smith, L.P.; Batis, C.; Popkin, B.M.; Kenan, W.R. Increased energy intake and a shift towards high-fat, non-staple high-carbohydrate foods amongst China’s older adults, 1991–2009. J. Aging Res. Clin. Pract. 2014, 3, 107–115. [Google Scholar] [PubMed]
- Li, Y.; Wang, D.D.; Ley, S.H.; Vasanti, M.; Howard, A.G.; He, Y.; Hu, F.B. Time trends of dietary and lifestyle factors and their potential impact on diabetes burden in China. Diabetes Care 2017, 40, 1685–1694. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Jiang, H.; Liang, X.; Wang, Y.; Lu, W. Associations of BMI and waist circumference with all-cause mortality: A 22-year cohort study. Obesity 2019, 27, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Proust-Lima, C.; Philipps, V.; Liquet, B. Estimation of Extended Mixed Models Using Latent Classes and Latent Processes: The R Package lcmm. J. Stat. Softw. 2017, 78, 1–56. [Google Scholar] [CrossRef]
- Li, S.; Li, X. Demand and trend of Chinese residents′ egg consumption. China Poult. 2018, 40, 1–7. [Google Scholar]
- B Keogh, J.; M Clifton, P. Energy intake and satiety responses of eggs for breakfast in overweight and obese adults—A crossover study. Int. J. Environ. Res. Public Health 2020, 17, 5583. [Google Scholar] [CrossRef]
- Leidy, H.J.; Clifton, P.M.; Astrup, A.; Wycherley, T.P.; Westerterp-Plantenga, M.S.; Luscombe-Marsh, N.D.; Woods, S.C.; Mattes, R.D. The role of protein in weight loss and maintenance. Am. J. Clin. Nutr. 2015, 101, 1320S–1329S. [Google Scholar] [CrossRef] [PubMed]
- Cawthon, C.R.; de La Serre, C.B. The critical role of CCK in the regulation of food intake and diet-induced obesity. Peptides 2021, 138, 170492. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Dietary fats and the gut microbiota: Their impacts on lipid-induced metabolic syndrome. J. Funct. Foods 2022, 91, 105026. [Google Scholar] [CrossRef]
- Garcia, K.; Ferreira, G.; Reis, F.; Viana, S. Impact of dietary sugars on gut microbiota and metabolic health. Diabetology 2022, 3, 549–560. [Google Scholar] [CrossRef]
- Raja, V.; Aguiar, C.; Alsayed, N.; Chibber, Y.S.; ElBadawi, H.; Ezhov, M.; Hermans, M.P.; Pandey, R.C.; Ray, K.K.; Tokgözoglu, L.; et al. Non-HDL-cholesterol in dyslipidemia: Review of the state-of-the-art literature and outlook. Atherosclerosis 2023, 383, 117312. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Sun, L.; Zong, G.; Song, B.; Zheng, H.; Jin, Q.; Li, H.; Lin, X. Genetic susceptibility, dietary cholesterol intake, and plasma cholesterol levels in a Chinese population. J. Lipid Res. 2020, 61, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Mardi, P.; Abdi, F.; Ehsani, A.; Seif, E.; Djalalinia, S.; Heshmati, J.; Shahrestanaki, E.; Gorabi, A.M.; Qorbani, M. Is non-high-density lipoprotein associated with metabolic syndrome? A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 957136. [Google Scholar]
- Spence, J.D.; Jenkins, D.J.; Davignon, J. Dietary cholesterol and egg yolks: Not for patients at risk of vascular disease. Can. J. Cardiol. 2010, 26, e336–e339. [Google Scholar] [CrossRef] [PubMed]
- Hajri, T. Effects of oxidized lipids and lipoproteins on cardiac function. Front. Biosci.-Landmark 2018, 23, 1822–1847. [Google Scholar] [CrossRef]
- Zhou, H.; Urso, C.J.; Jadeja, V. Saturated fatty acids in obesity-associated inflammation. J. Inflamm. Res. 2020, 13, 1–14. [Google Scholar] [CrossRef]
- Silva Figueiredo, P.; Carla Inada, A.; Marcelino, G.; Maiara Lopes Cardozo, C.; de Cássia Freitas, K.; de Cássia Avellaneda Guimarães, R.; Pereira de Castro, A.; Aragão do Nascimento, V.; Aiko Hiane, P. Fatty acids consumption: The role metabolic aspects involved in obesity and its associated disorders. Nutrients 2017, 9, 1158. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, L.C.; Brière, F.; Tremblay, A.J.; Rancourt-Bouchard, M.; Drouin-Chartier, J.P.; Corbeil, J.; Lemelin, V.; Charest, A.; Schaefer, E.J.; Lamarche, B.; et al. Substitution of dietary monounsaturated fatty acids from olive oil for saturated fatty acids from lard increases low-density lipoprotein apolipoprotein B-100 fractional catabolic rate in subjects with dyslipidemia associated with insulin resistance: A randomized controlled trial. Am. J. Clin. Nutr. 2024, 119, 1270–1279. [Google Scholar] [PubMed]
- Piché, M.E.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: An update. Prog. Cardiovasc. Dis. 2018, 61, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Koliaki, C.; Liatis, S.; Kokkinos, A. Obesity and cardiovascular disease: Revisiting an old relationship. Metabolism 2019, 92, 98–107. [Google Scholar] [CrossRef]
- Baranowski, T. 24-Hour Recall and Diet Record Methods. In Nutritional Epidemiology, 2nd ed.; Willett, W., Ed.; Oxford University Press: New York, NY, USA, 1998; pp. 50–73. [Google Scholar]
| Characteristics | General Obesity | Central Obesity | ||||
|---|---|---|---|---|---|---|
| No | Yes | Total | No | Yes | Total | |
| (n = 9821, 89.5%) | (n = 1150, 10.5%) | (n = 10,971) | (n = 6040, 69.0%) | (n = 3443, 31.0%) | (n = 9483) | |
| Duration of follow-up, years, Mean ± SD | 9.14 ± 5.23 | 6.61 ± 4.25 | 8.87 ± 5.19 | 8.26 ± 4.98 | 6.35 ± 3.96 | 7.56 ± 4.73 |
| Egg intake, g/d, Mean ± SD | 21.86 ± 29.99 | 24.76 ± 31.41 | 22.16 ± 30.15 | 19.72 ± 28.40 | 22.16 ± 30.01 | 20.61 ± 29.02 |
| Egg-derived cholesterol intake, mg/d, Mean ± SD | 124.66 ± 171.02 | 141.19 ± 179.11 | 126.39 ± 171.95 | 112.48 ± 161.94 | 126.38 ± 171.14 | 117.52 ± 165.46 |
| Men, n (%) | 4702 (47.9) | 531 (46.2) | 5233 (47.7) | 3044 (50.4) | 1505 (43.7) | 4549 (48.0) |
| Age (years), Mean ± SD | 42.32 ± 15.60 | 42.35 ± 12.85 | 42.33 ± 15.33 | 40.09 ± 15.96 | 43.39 ± 13.26 | 41.29 ± 15.12 |
| Han nationality, n (%) | 8496 (86.5) | 1036 (90.1) | 9532 (86.9) | 5135 (85.0) | 3040 (88.3) | 8175 (86.2) |
| Marital status, n (%) | ||||||
| Unmarried | 1454 (14.8) | 97 (8.5) | 1551 (14.1) | 1216 (20.2) | 260 (7.6) | 1476 (15.6) |
| Married | 7693 (78.3) | 991 (86.2) | 8684 (79.2) | 4426 (73.3) | 2978 (86.5) | 7404 (78.1) |
| Divorced/separated/widowed | 599 (6.1) | 51 (4.4) | 650 (5.9) | 352 (5.8) | 171 (5.0) | 523 (5.5) |
| Education level, n (%) | ||||||
| Illiterate | 2150 (21.9) | 251 (21.9) | 2401 (21.9) | 1157 (19.2) | 877 (25.5) | 2034 (21.4) |
| Primary school | 2022 (20.6) | 270 (23.5) | 2292 (20.9) | 1203 (19.9) | 806 (23.4) | 2009 (21.2) |
| Middle school | 3073 (31.2) | 348 (30.3) | 3421 (31.2) | 2021 (33.5) | 1007 (29.2) | 3028 (31.9) |
| High school and above | 2377 (24.2) | 244 (21.2) | 2621 (23.9) | 1523 (25.2) | 678 (19.7) | 2201 (23.2) |
| Income group, n (%) | ||||||
| Low | 4288 (43.6) | 511 (44.5) | 4799 (43.7) | 2710 (44.9) | 1582 (45.9) | 4292 (45.3) |
| Medium | 3743 (38.1) | 463 (40.3) | 4206 (38.3) | 2272 (37.6) | 1368 (39.8) | 3640 (38.4) |
| High | 1723 (17.5) | 162 (14.1) | 1885 (17.2) | 1022 (16.9) | 459 (13.3) | 1481 (15.6) |
| Community type, n (%) | ||||||
| City | 1537 (15.6) | 163 (14.2) | 1700 (15.5) | 1014 (16.8) | 491 (14.3) | 1505 (15.9) |
| Suburb | 1708 (17.4) | 209 (18.2) | 1917 (17.5) | 1040 (17.2) | 613 (17.8) | 1653 (17.4) |
| Town | 1785 (18.2) | 241 (20.9) | 2026 (18.5) | 1283 (21.3) | 655 (19.0) | 1938 (20.4) |
| Village | 4722 (48.1) | 564 (49.0) | 5286 (48.2) | 2679 (44.4) | 1673 (48.6) | 4352 (45.9) |
| Regions, n (%) | ||||||
| Northeast | 1890 (19.2) | 299 (26.0) | 2189 (20.0) | 1062 (17.6) | 730 (21.2) | 1792 (18.9) |
| East Coast | 2097 (21.4) | 323 (28.1) | 2420 (22.1) | 1099 (18.2) | 903 (26.2) | 2002 (21.1) |
| Central | 3248 (33.1) | 360 (31.3) | 3608 (32.9) | 1975 (32.7) | 1163 (33.8) | 3138 (33.1) |
| Western | 2586 (26.3) | 168 (14.6) | 2754 (25.1) | 1904 (31.5) | 647 (18.8) | 2551 (26.9) |
| Current smoker, n (%) | 3082 (31.4) | 346 (30.1) | 3428 (31.2) | 1966 (32.5) | 1054 (30.6) | 3020 (31.8) |
| Current drinker, n (%) | 3384 (34.5) | 399 (34.7) | 3783 (34.5) | 2074 (34.3) | 1211 (35.2) | 3285 (34.6) |
| Physical activities, n (%) | ||||||
| Light | 5778 (58.8) | 695 (60.9) | 6473 (59.0) | 3563 (58.9) | 2166 (62.9) | 5729 (60.4) |
| Medium | 1355 (13.8) | 187 (16.3) | 1542 (14.1) | 728 (12.1) | 556 (16.1) | 1284 (13.5) |
| Heavy | 2362 (24.0) | 226 (19.7) | 2588 (23.6) | 1553 (25.7) | 609 (17.7) | 2162 (22.8) |
| Dietary total energy intake, kcal/d, Mean ± SD | 2259.55 ± 646.14 | 2325.67 ± 656.75 | 2266.48 ± 647.55 | 2259.71 ± 649.11 | 2301.64 ± 648.52 | 2274.93 ± 649.17 |
| Meat intake, g/d, Mean ± SD | 72.23 ± 88.28 | 43.88 ± 78.73 | 69.26 ± 87.76 | 84.14 ± 89.85 | 74.22 ± 87.66 | 80.54 ± 89.19 |
| Dietary total protein intake, g/d, Mean ± SD | 68.03 ± 25.77 | 71.03 ± 27.80 | 68.34 ± 26.00 | 67.22 ± 25.03 | 69.30 ± 26.35 | 67.98 ± 25.53 |
| History of stroke, n (%) | 48 (0.5) | 5 (0.4) | 53 (0.5) | 16 (0.3) | 17 (0.5) | 33 (0.3) |
| History of myocardial infarction, n (%) | 29 (0.3) | 5 (0.4) | 34 (0.3) | 16 (0.3) | 6 (0.2) | 22 (0.2) |
| History of diabetes, n (%) | 124 (1.3) | 15 (1.3) | 139 (1.3) | 56 (0.9) | 42 (1.2) | 98 (1.0) |
| Egg Consumption | General Obesity | Central Obesity | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Egg intake (g/d) | ||||||||
| 0.0 | 1.63 (1.36, 1.94) | <0.001 | 1.31 (1.08, 1.58) | 0.013 | 1.37 (1.23, 1.54) | <0.001 | 1.17 (1.04, 1.31) | 0.002 |
| 0.1~50.0 | Reference | Reference | Reference | Reference | ||||
| 50.1~100.0 | 1.35 (1.11, 1.65) | 0.003 | 1.30 (1.07, 1.60) | 0.021 | 1.31 (1.14, 1.50) | <0.001 | 1.31 (1.14, 1.50) | <0.001 |
| >100.0 | 2.31 (1.38, 3.88) | 0.002 | 1.98 (1.17, 3.35) | 0.011 | 1.78 (1.24, 2.56) | 0.002 | 1.64 (1.15, 2.36) | 0.006 |
| Change trajectory patterns of egg intake | ||||||||
| Low Baseline-Stable Pattern | Reference | Reference | Reference | Reference | ||||
| Low Baseline-Significant Rising Pattern | 1.52 (1.23, 1.87) | <0.001 | 1.56 (1.25, 1.93) | <0.001 | 1.44 (1.26, 1.64) | <0.001 | 1.47 (1.29, 1.68) | <0.001 |
| High Baseline-Rising then Falling Pattern | 1.35 (1.15, 1.59) | <0.001 | 1.38 (1.13, 1.69) | <0.001 | 1.39 (1.25, 1.54) | <0.001 | 1.52 (1.34, 1.72) | <0.001 |
| Egg-Derived Cholesterol Consumption | General Obesity | Central Obesity | ||||||
|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |||||
| HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Quartiles of egg-derived cholesterol intake | ||||||||
| Q1 | 1.45 (1.22, 1.74) | <0.001 | 1.28 (1.06, 1.54) | 0.009 | 1.30 (1.17, 1.45) | <0.001 | 1.20 (1.08, 1.33) | <0.001 |
| Q2 | Reference | Reference | Reference | Reference | ||||
| Q3 | 1.24 (1.04, 1.47) | 0.014 | 1.21 (1.02, 1.44) | 0.030 | 1.10 (1.00, 1.22) | 0.057 | 1.11 (1.01, 1.23) | 0.038 |
| Q4 | 1.49 (1.26, 1.78) | <0.001 | 1.43 (1.19, 1.71) | <0.001 | 1.31 (1.19, 1.45) | <0.001 | 1.32 (1.19, 1.46) | <0.001 |
| Change trajectory patterns of egg-derived cholesterol intake | ||||||||
| Low Baseline-Stable Pattern | Reference | Reference | Reference | Reference | ||||
| Low Baseline-Significant Rising Pattern | 1.52 (1.23, 1.87) | <0.001 | 1.54 (1.25, 1.92) | <0.001 | 1.44 (1.26, 1.64) | <0.001 | 1.46 (1.28, 1.68) | <0.001 |
| High Baseline-Rising then Falling Pattern | 1.35 (1.15, 1.59) | <0.001 | 1.37 (1.15, 1.64) | <0.001 | 1.39 (1.25, 1.54) | <0.001 | 1.47 (1.32, 1.64) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, T.; Chen, B.; Hu, J.; Fan, H.; Zhang, Z.; Zhai, T.; Li, C.; Wang, D.; Xue, W.; Pei, L.; et al. The Association Between Egg and Egg-Derived Cholesterol Consumption, and Their Change Trajectories, with Obesity Among Chinese Adults: Results from the China Health and Nutrition Survey. Nutrients 2025, 17, 333. https://doi.org/10.3390/nu17020333
Tang T, Chen B, Hu J, Fan H, Zhang Z, Zhai T, Li C, Wang D, Xue W, Pei L, et al. The Association Between Egg and Egg-Derived Cholesterol Consumption, and Their Change Trajectories, with Obesity Among Chinese Adults: Results from the China Health and Nutrition Survey. Nutrients. 2025; 17(2):333. https://doi.org/10.3390/nu17020333
Chicago/Turabian StyleTang, Tianhui, Binghua Chen, Jiahao Hu, Hangzhao Fan, Zilan Zhang, Tianyang Zhai, Chunxiao Li, Duolao Wang, Wanli Xue, Leilei Pei, and et al. 2025. "The Association Between Egg and Egg-Derived Cholesterol Consumption, and Their Change Trajectories, with Obesity Among Chinese Adults: Results from the China Health and Nutrition Survey" Nutrients 17, no. 2: 333. https://doi.org/10.3390/nu17020333
APA StyleTang, T., Chen, B., Hu, J., Fan, H., Zhang, Z., Zhai, T., Li, C., Wang, D., Xue, W., Pei, L., Chen, F., Mi, B., & Zhao, Y. (2025). The Association Between Egg and Egg-Derived Cholesterol Consumption, and Their Change Trajectories, with Obesity Among Chinese Adults: Results from the China Health and Nutrition Survey. Nutrients, 17(2), 333. https://doi.org/10.3390/nu17020333






