Abstract
Background/Objectives: Vitamin D testing has increased significantly in developed countries in recent decades. We aimed to describe trends in vitamin D testing rates and factors associated with testing and vitamin D deficiency in Queensland, Australia (2011–2019). Methods: We used data from the QSkin Sun and Health Study (n = 40,417), a prospective population-based cohort study with linkage to the Medicare Benefits Schedule, Pharmaceutical Benefits Scheme, and pathology laboratories. Main outcomes included age-standardized incidence rate of vitamin D testing; having ≥1 vitamin D test during follow-up; vitamin D deficiency (25-hydroxyvitamin D concentration <50 nmol/L) in the first vitamin D test; and repeat vitamin D tests. Results: The age-standardized incidence rate of testing increased by 2% per quarter during follow-up. Of the 35,250 participants analyzed for associations with testing (median age of 57 years, 52% female), 45% had ≥1 vitamin D test. Among those tested, 56% had no apparent clinical indication for their initial vitamin D test, 21% were vitamin D deficient in their initial test, and 58% had a repeat test. Repeat testing occurred in 56% who were not deficient in their prior test, while only two-thirds of those deficient received a follow-up assessment. Participants who visited a general practitioner ≥2 times in the year prior to follow-up were 60% more likely to have ≥1 vitamin D test compared with those with no visit, but general practitioner (GP) visits were not associated with risk of vitamin D deficiency. Conclusions: These results suggest that initiatives are needed to help clinicians target vitamin D testing in alignment with clinical guidelines.
1. Introduction
There has been a dramatic increase in the rate of vitamin D testing over the past two decades in Australia. Between 2000 and 2011, the rate increased nearly 100-fold [1], leading to concerns about over-testing. Similar trends have emerged across other developed countries, such as the United States [2], Canada [3], France [4], and the United Kingdom [5].
After a review of the evidence [6], in November 2014 the Australian Department of Health introduced new eligibility criteria for government-reimbursed vitamin D tests [7]. These criteria required that vitamin D testing be restricted to people at high risk of deficiency or with clinical indications, after which there was an immediate decrease in testing rates [8]. However, this trend was not sustained [9,10], and by late 2016 the rate had reverted to levels observed before the criteria were introduced [10].
Adherence to the criteria for testing may be suboptimal. A review of a large nationwide dataset (~1.5 million Australian adults) estimated that approximately three-quarters of vitamin D tests ordered by a general practitioner (GP) did not have an obvious clinical indication [8]. Moreover, the prevalence of test results indicating deficiency actually decreased after the introduction of the criteria [10]. Similar patterns of vitamin D testing have been observed in other developed countries [4,11], making Australia’s policy intervention experience relevant internationally.
Identifying factors associated with vitamin D testing may inform further initiatives to optimize the use of testing. An increased likelihood of vitamin D testing has been found among older people, women, migrants, those living in higher socioeconomic areas, and those who visit doctors more frequently [8,12,13,14]. However, no studies to date have investigated testing among individuals who might be at risk of vitamin D deficiency due to sun avoidance (e.g., people with a history of skin cancer), and few studies have explored the issue of repeat testing.
In this paper, we analyze trends in vitamin D testing and the percentage of tests showing vitamin D deficiency between 2011 and 2019 among a population-based cohort in Queensland, Australia, and identify factors associated with vitamin D testing and vitamin D deficiency.
2. Materials and Methods
2.1. Study Population and Data Collection
We used data from the QSkin Sun and Health Study (QSkin), a prospective cohort study established for the study of skin cancer [15]. Between 2010 and 2011, 43,794 residents of Queensland aged 40 to 69 years were recruited into the QSkin Study, using the Australian Electoral Roll as the sampling frame. The QSkin Study was approved by the QIMR Berghofer Medical Research Institute Human Research Ethics Committee (approval number P1309, approval date 21 May 2010).
At baseline all participants provided written consent, including consent to collect information from the Queensland Cancer Register (QCR), pathology laboratories, and hospitals. Participants were also asked to provide optional consent to linkage of their records with the Medicare Benefits Schedule (MBS) and Pharmaceutical Benefits Scheme (PBS). The MBS database holds information on all medical services provided in Australia outside the public hospital system, including in primary care. The PBS records information for prescription medications dispensed that receive a government subsidy (i.e., most commonly prescribed medications). Participants completed a baseline survey that asked about their sociodemographic, phenotypic, lifestyle factors and medical history, including the treatment of skin cancers.
2.2. Outcomes
The main outcomes were: (1) incidence of at least one vitamin D test during follow-up; (2) prevalence of vitamin D deficiency (serum 25-hydroxyvitamin D (25(OH)D) concentration <50 nmol/L) in the first vitamin D test; and (3) incidence of repeat vitamin D tests among participants with at least one vitamin D test. We also described temporal trends (2011 to 2019) in the rates of vitamin D testing and the percentage of tests indicating vitamin D deficiency.
To ascertain outcomes, we first identified all QSkin participants who received at least one government-funded vitamin D test, as recorded in the MBS dataset. For those participants, we then conducted data linkage with the two major private pathology laboratories in Queensland and obtained the results of all vitamin D tests. We matched MBS and pathology records by date and considered records within 30 days as part of a single testing episode. Testing episodes that could not be matched (i.e., those that had only MBS or pathology records), were included in the analyses.
2.3. Explanatory Variables
Details about the derivation of explanatory variables are provided in Supplementary Methods S1. These variables included sociodemographic, phenotypic and lifestyle factors, self-reported history of skin cancers, melanoma diagnoses, prescription of medications that either interfere with vitamin D metabolism or are used to treat conditions associated with vitamin D deficiency, frequency of visits to GPs, unweighted Rx-Risk comorbidity index (a medication-based comorbidity index that quantifies chronic disease burden using prescription drug dispensing data) [16], and the result of any previous vitamin D test. We used Socio-Economic Indexes for Areas (SEIFA) to estimate socioeconomic status [17]. A skin phenotype score, as an indicator of skin cancer risk, was calculated based on phenotypic characteristics and categorized into tertiles. We coded medication prescriptions: as (1) ever prescribed; and (2) recently prescribed (within the last 90 days). Explanatory variables were ascertained from the baseline survey, MBS/PBS/pathology data, and the QCR.
2.4. Assigning an Indication to the First Vitamin D Test
We used the MBS testing criteria for which data were available (i.e., osteoporosis and antiepileptic medication, long-term glucocorticoid use, low sun exposure), and factors known to be associated with vitamin D deficiency (i.e., obesity) to assign the most likely indication for a participant’s first vitamin D test (indications that we were unable to ascertain are shown in Supplementary Methods S3). The hierarchy in which we assigned the indication was as follows: recent prescription of osteoporosis/antiepileptic medication (i.e., first prescribed within 90 days before the test); long-term glucocorticoid use (dispensed at least 4 times in the 12 months before the test); low sun exposure at baseline; and being obese at baseline. We classified participants who did not satisfy any of the criteria as having no apparent indication for vitamin D testing. In a sensitivity analysis, we included Rx-Risk score ≥2 (at the time of the test) as another indication, inserting it between long-term glucocorticoid use and low sun exposure in the original hierarchy.
2.5. Follow-Up
Follow-up began one year after the date of consent. We used the 12-month period after consent to identify people who had ‘prevalent’ vitamin D tests and to derive explanatory variables based on MBS and PBS data. In analyses of associations with having ≥1 vitamin D test, follow-up ended at the earliest of: (1) first vitamin D test; (2) date of death; or (3) 31 December 2019. For analyses of repeat testing, follow-up ended at the earliest of date of death or 31 December 2019, except for analyses of the association with the result of the preceding test, where the date of the first vitamin D test for which the result was unknown was also used to censor participants.
2.6. Eligibility
We excluded participants who did not consent to linkage with the MBS and PBS, had an unknown date of death, or had dates of death preceding dates of vitamin D tests, suggesting incorrect data linkage. For analyses of factors associated with vitamin D testing and deficiency, we also excluded participants who had undergone a vitamin D test or died in the 12 months between consent and the start of follow-up. We decided a priori that sex, age, skin phenotype, and SEIFA were important potential confounders, so participants with missing data for any of these variables were excluded (n = 846, 2.1%). Participants for whom the 25(OH)D concentration was missing for the first test in the MBS data were excluded from the analysis of factors associated with vitamin D deficiency.
2.7. Statistical Analyses
We used the Joinpoint Regression Program (v5.0) [18] to analyze trends in vitamin D testing. Additionally, we described person-based rates of testing and the proportion of tests consistent with vitamin D deficiency by season during the follow-up period. We compared test-based incidence rates within those aged 45–74 years in the QSkin Study cohort with those in the Queensland population (using aggregated MBS data available online from Services Australia) [19].
All other analyses were performed in R v4.3.1. We used Cox regression, log-binomial regression, and Andersen–Gill models with robust standard errors to estimate associations with having a vitamin D test, vitamin D deficiency, and repeat testing, respectively. Analyses of factors associated with having a test were repeated according to time period (2011–2014 versus 2015–2019) (Supplementary Methods S2). For analyses of repeat testing, all exposure variables, except sex, were time-varying, and all models included the number of preceding vitamin D tests. Directed acyclic graphs were used to guide our choice of adjustment variables.
3. Results
We included 40,417 QSkin participants in the analysis of trends in vitamin D testing, and 35,250 in analyses of factors associated with testing (Figure 1). The median age at cohort entry was 57 years, 52% were female, 94% were of White European descent, 15% reported low sun exposure (≤3.5 h/week), and 40% reported prior skin cancer excisions (Table 1). Test results were available from the pathology laboratories for 83% of testing episodes recorded in the MBS. We could not capture the results of vitamin D tests from all laboratories, resulting in 17% of MBS testing episodes lacking a test result.
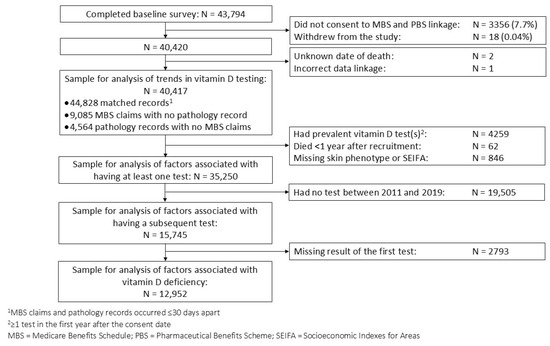
Figure 1.
Flowchart of participant selection for main analyses.

Table 1.
Associations between participant characteristics and having at least one vitamin D test between 2011 and 2019.
3.1. Trends in Vitamin D Testing and Deficiency
Between 2011 and 2019, the person-based incidence of vitamin D testing was 602 per 10,000 person-years. On average, the age-standardized incidence rate of testing increased by 2.0% (95% confidence interval [CI] 1.6 to 2.6) per quarter between 2011 and 2019. Joinpoint regression identified three inflection points (Q1 2013; Q4 2014; Q3 2015) delineating four separate trend intervals, with average quarterly percent changes of 8.4% (95% CI 6.0 to 14.3), −0.5% (95% CI −3.9 to 3.5), −13.7% (95% CI −16.5 to 1.9), and 3.0% (95% CI 2.3 to 3.8), respectively (Figure 2, Table S1). The test-based incidence rate in the QSkin cohort was similar to that in the Queensland population, and the high rate of testing has persisted in the Queensland population up to the end of 2023 (Figure S1). Sex-specific (Figure 2, Table S1) and age-specific (Figure S2) trends were similar to the overall trend. Overall, 32% of participants had two or more vitamin D tests (Figure S3).
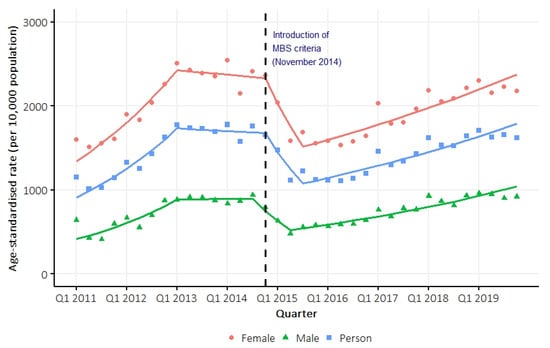
Figure 2.
Overall and sex-specific age-standardized (Australian population 2001) person-based incidence rate of vitamin D testing between 2011 and 2019. Trend lines were estimated using Joinpoint regression models.
The median 25(OH)D concentration remained stable over time at approximately 70 nmol/L (Figure 3a). In 2011, 17% of the tests indicated deficiency, compared with 13% in 2019 (Figure 3b).
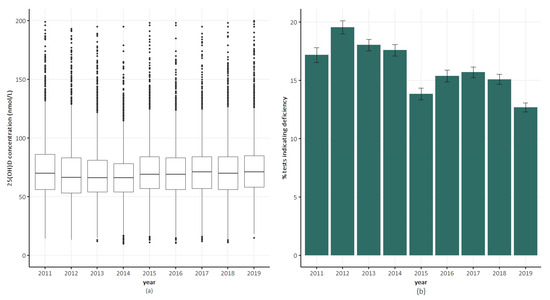
Figure 3.
Trends in serum 25(OH)D concentrations and vitamin D deficiency in QSkin participants over time. (a) Box plots of serum 25(OH)D concentration. (b) Percentage of tests indicated vitamin D deficiency (serum 25(OH)D concentration <50 nmol/L) in a calendar year. Error bars indicate ±1 standard error.
3.2. Factors Associated with Having a Vitamin D Test
Of the participants included in analyses of factors associated with testing, 45% (n = 15,745) had at least one vitamin D test during follow-up. We found no apparent indication for the first vitamin D test in 56% of those tested; obesity and low sun exposure were the most common indications (22% and 19% of those tested, respectively) (Table 2). When we considered Rx-Risk score ≥2 to be an indication in the sensitivity analysis, it became the most common indication (49% of those tested), and the percentage of tests with no apparent indication substantially decreased to 32% (Table S2).

Table 2.
Distribution of most likely indication for the first vitamin D test and result of the test.
Rates of testing were generally higher in spring (Figure S4). The factors most strongly associated with testing were recent prescription of medication to treat osteoporosis (hazard ratio (HR), 5.3; 95% CI 4.4 to 6.5), and being female (HR, 2.5; 95% CI 2.4 to 2.6). Other factors associated with increased likelihood of testing were: increasing age; non-European ancestry; greater socioeconomic advantage; prescription of antiepileptic medication and menopausal hormone therapy (MHT); higher Rx-Risk index; and more GP visits. People with a more sun-sensitive phenotype were more likely to be tested and, while somewhat inconclusive, there was evidence of more testing in people diagnosed with skin cancer. People who spent more time outdoors were less likely to be tested, but there was no association with sunscreen use (Table 1). The strength of the associations with sex, age, ancestry, and socioeconomic status was somewhat attenuated after the introduction of MBS criteria in November 2014, whereas the associations with recent prescription of osteoporosis and antiepileptic medication were stronger (Table S3).
3.3. Factors Associated with Being Vitamin D Deficient at the First Vitamin D Test
Among those for whom we obtained the result of the first vitamin D test (n = 12,952), the median 25(OH)D concentration was 65 nmol/L; 21% of participants were vitamin D deficient. The percentage deficient was higher among those with versus without an apparent indication for a test (28% vs. 16%) (Table 2). By season, the lowest 25(OH)D concentration and highest percentage of tests indicating deficiency were observed in winter, followed by spring (Figure S5). The factor most strongly associated with vitamin D deficiency was obesity (risk ratio (RR) 2.0; 95% CI 1.8 to 2.1). Other factors associated with increased risk of deficiency were being female, non-European ancestry, greater socioeconomic advantage, smoking, and prescription of antiepileptic medication (Table 3). There was a small increase in the risk of deficiency among those with a skin phenotype that increased their predisposition to skin cancers, but a decreased risk in those with a skin cancer diagnosis. Factors associated with a lower risk of deficiency were increasing sun exposure, sunscreen use, alcohol consumption, and use of osteoporosis medication (Table 3).

Table 3.
Associations between participant characteristics and vitamin D deficiency 1, based on the first test for each participant (n = 12,952 2).
3.4. Factors Associated with Having a Repeat Vitamin D Test
Among participants who were tested, 58% (n = 9099) had at least one repeat test. The median time between consecutive tests was around 12 months overall (Figure S6) for most participant subgroups, but it was approximately 6 months following tests indicating moderate to severe vitamin D deficiency (less than 30 nmol/L) (Table S4). Just over half (56%) of those not vitamin D deficient on the first test had a repeat test (median gap 12 months). Among those who were deficient, 69% had a repeat test, but it was after 12 months in 38% of these individuals.
The likelihood of repeat testing increased with vitamin D deficiency, female sex, advancing age, more GP visits, and a higher Rx-Risk index (Table 4).

Table 4.
Associations between participant characteristics and repeat testing among people who had at least one test between 2011 and 2019 (n = 15,745).
4. Discussion
Vitamin D is crucial for musculoskeletal health [20], and has potential effects on cancer mortality [21,22], infection [23,24,25], and autoimmune disease [26,27]. It is thus important to avoid vitamin D deficiency. Vitamin D testing is advised for populations at high risk of deficiency as a case-finding strategy [28,29], but routine population screening is not recommended [30]. In this cohort of over 40,000 Australians, we found a relatively high incidence of testing, with nearly half (45%) of all participants having had at least one test between 2011 and 2019.
We found that rates of vitamin D testing followed patterns previously reported [9,14]. The late 2012 change in direction appears to reflect growing clinical and policy awareness about high rates of testing that preceded the formal policy intervention. Published evidence of overtesting emerged in 2012–2013 [1], and data show that testing rates were already declining before the formal November 2014 MBS criteria were introduced [9,14]. The introduction of MBS eligibility criteria in November 2014 initially reduced testing rates, but the subsequent bounce-back by mid-2015 demonstrates that these criteria alone were insufficient to sustain the reduction in vitamin D testing.
As shown previously [8,10], the prevalence of deficiency in tested samples decreased over time. The reason for this trend is unknown, but is most likely related to increased awareness of vitamin D deficiency and growing use of vitamin D supplementation in recent years. If true, this raises the question of whether initial vitamin D tests are needed in patients who are already taking vitamin D supplements.
In contravention of Australian government guidelines, 32% of participants had no apparent indication for their first test even when we considered factors strongly associated with vitamin deficiency but not included in the MBS criteria as indications for testing (i.e., obesity, co-morbidities). Additionally, repeat testing occurred in over half of those who were not deficient. These findings, along with the positive association between more GP visits and testing (without a corresponding increase in risk of deficiency), suggest that a degree of untargeted screening is occurring.
Australian guidelines recommend that individuals found to be deficient and initiated on supplementation should be retested within 3–4 months [29]. Only two-thirds of those deficient were retested, and approximately 38% occurred more than 12 months after the test identifying vitamin D deficiency, providing further evidence that testing is not always targeted appropriately.
The Royal College of Pathologists of Australasia and Royal Australian College of General Practitioners recommend vitamin D testing in people chronically lacking in sun exposure [28,29]. We found low sun exposure to be the apparent indicator for testing in approximately 19% of those tested. However, only 5–10 min/day outdoors is needed to maintain adequate vitamin D status in Queensland (provided sufficient skin is exposed), so many of these participants may not have been underexposed. A newly released position statement might be able to assist clinicians in identifying patients with low sun exposure [31] who would then be eligible for testing. Alternatively, supplementation could be initiated without testing according to Australian nutrition guidelines which recommend that those patients should consume 400–600 IU of vitamin D per day [32]. These guidelines are consistent with the United States Endocrine Society recommendations, which implicitly assume that people have sufficient sun exposure or supplementary vitamin D to avoid vitamin D deficiency [30]. Ensuring that individuals meet these guidelines may obviate the need for testing in many people. Additional research is needed to understand clinicians’ use of the low sun exposure criterion to triage patients for vitamin D testing and their perspective on supplementation versus testing.
We included Rx-Risk to reflect real-world clinical decision-making, recognizing that GPs may order vitamin D testing to investigate whether vitamin D deficiency could be contributing to patients’ chronic conditions or overall health status, or conversely, whether chronic health conditions might lead to reduced vitamin D. This approach accommodates clinical judgment beyond the specific MBS criteria, acknowledging that clinicians may consider vitamin D testing as part of a broader diagnostic workup for patients with multiple comorbidities. We positioned Rx-Risk (≥2) between long-term glucocorticoid use and lifestyle factors (low sun exposure, obesity) because it represents intermediate clinical risk. Specifically, we considered indications included in the MBS criteria (osteoporosis/antiepileptic drugs, glucocorticoids) as having the strongest clinical rationale, followed by chronic disease burden (Rx-Risk), and finally lifestyle/demographic factors, which were collected at baseline. This hierarchy reflects decreasing clinical specificity for vitamin D testing indications.
A limitation of this study is that data about vitamin D testing were only available in the QSkin cohort up to 2019. However, there has been no decrease in the rate of testing in Queensland in the past 5 years, suggesting that there remains a need to optimize testing to target those most at risk of vitamin D deficiency. In addition, our analysis was limited by the inability to assess vitamin D supplement use, as these supplements are generally not PBS-subsidized in Australia and therefore not available in the linked pharmaceutical data. Since vitamin D supplementation is a key determinant of vitamin D status, understanding supplementation trends could provide valuable insights into the appropriateness and drivers of vitamin D testing. Furthermore, information for some testing indications specified by the MBS was not available in our dataset (see Supplementary Methods S3); therefore, we may have misclassified some tests as being without indication. However, these missing indications mostly occur rarely (e.g., gastrointestinal malabsorption) so the misclassification is likely to have been small.
5. Conclusions
In conclusion, this study provides compelling evidence of widespread and sustained growth in vitamin D testing in Australia. Coordinated initiatives are warranted to ensure vitamin D testing is more appropriately targeted to high-risk populations, improving resource allocation while maintaining optimal patient care. Since vitamin D supplementation is safe, effective, and relatively cheap, it may be a better use of healthcare resources to initiate supplementation, in the absence of testing, in people without clinical indications but who receive minimal sun exposure and/or have a high body mass index.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu17152549/s1: Figure S1: Test-based age-standardized rates of vitamin D testing among the Queensland population and the QSkin cohort (aged 45–74); Figure S2: Age-specific person-based incidence rate of vitamin D testing between 2011 and 2019; Figure S3: Number of tests per person between 2011 and 2019; Figure S4: Person-based age-standardized incidence rate of vitamin D testing by season between 2011 and 2019; Figure S5. (a) 25(OH)D concentration; and (b) percentage of vitamin D tests indicating deficiency, according to season in tests conducted in QSkin participants between 2011 and 2019; Figure S6. Time between consecutive vitamin D tests among participants who had at least 2 vitamin D tests between 2011 and 2019; Table SA: List of explanatory variables, corresponding outcome(s), and how they were treated in regression models; Table SB: Rx-Risk comorbidity category and corresponding anatomical therapeutic chemical code; Table SC: Logistic regression model of the association between skin phenotypes and skin cancer outcome, adjusting for sex and age at baseline; Table S1: Average percent changes in age-standardized person-based rates of vitamin D testing in QSkin participants between 2011 and 2019; results presented for all participants and according to sex; Table S2: Sensitivity analysis: Distribution of most likely indication for the first vitamin D test, and result of the test according to whether test was indicated; including Rx-Risk comorbidity index ≥2 as an indication; Table S3: Associations between participant characteristics and having at least one vitamin D test, stratified by time period (prior to 2015 versus 2015 onwards); Table S4: Distribution of number of vitamin D tests and time between consecutive tests between 2011 and 2019, according to selected participant characteristics. Reference [33] is cited in the supplementary materials.
Author Contributions
Conceptualization, D.C.W. and R.E.N.; methodology, V.T., D.S.A.M., M.W., D.C.W. and R.E.N.; software, V.T.; formal analysis, V.T.; resources, C.M.O., D.C.W. and R.E.N.; data curation, N.P.; writing—V.T.; writing—review and editing, D.S.A.M., C.M.O., N.P., M.W., D.C.W. and R.E.N.; visualization, V.T.; supervision, D.S.A.M., M.W., D.C.W. and R.E.N.; project administration, C.M.O.; funding acquisition, C.M.O. and D.C.W. All authors have read and agreed to the published version of the manuscript.
Funding
The QSkin Sun and Health Study was supported by the National Health and Medical Research Council (NHMRC) of Australia (grant number APP1185416).
Institutional Review Board Statement
The QSkin Sun and Health Study was approved by the QIMR Berghofer Medical Research Institute Human Research Ethics Committee (approval number P1309, approval date 21 May 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data underlying this article may be shared on request to the corresponding author, subject to ethical and regulatory clearances from the host institution.
Acknowledgments
We extend our thanks to all QSkin participants who committed to this research. We acknowledge Services Australia, QML Pathology, and Sullivan Nicolaides Pathology for supplying the MBS and PBS, and 25 hydroxy vitamin D results used in this analysis.
Conflicts of Interest
Vu Tran is supported by The University of Queensland and QIMR Berghofer PhD Scholarship. David Whiteman is supported by an investigator grant from the National Health Medical and Research Council Research (2026567), a consultancy fee from the New Zealand Cancer Control Agency, and support for attending the 2025 World Congress of Melanoma, 2024 New Zealand Melanoma Summit, and 2023 EADO Congress. Catherine Olsen is supported by the New Zealand Public Health Agency and Maurice Blackburn Lawyers for her advisory role. David Whiteman and Catherine Olsen are supported by NHMRC CTCS grant number APP1185416. The funders had no role in the planning, writing or publication of the work, or any role in study design, data collection, analysis or interpretation, reporting or publication.
Abbreviations
The following abbreviations are used in this manuscript:
| MBS | Medicare Benefits Schedule |
| PBS | Pharmaceutical Benefits Scheme |
| GP | General practitioner |
| QSkin | QSkin Sun and Health Study |
| 25(OH)D | 25-hydroxyvitamin D |
| QCR | Queensland Cancer Register |
| SEIFA | Socio-Economic Indexes for Areas |
| HR | Hazard ratio |
| RR | Risk ratio |
| IR | Incidence rate |
| CI | Confidence interval |
| BMI | Body mass index |
| Ref | Reference category |
| MHT | Menopausal hormone therapy |
| NHMRC | National Health and Medical Research Council |
References
- Bilinski, K.; Boyages, S. Evidence of overtesting for vitamin D in Australia: An analysis of 4.5 years of Medicare Benefits Schedule (MBS) data. BMJ Open 2013, 3, e002955. [Google Scholar] [CrossRef]
- Shahangian, S.; Alspach, T.D.; Astles, J.R.; Yesupriya, A.; Dettwyler, W.K. Trends in laboratory test volumes for Medicare part B reimbursements, 2000–2010. Arch. Pathol. Lab. Med. 2013, 138, 189–203. [Google Scholar] [CrossRef]
- Felicia, T.; Ian, C.-Y.; Alan, G.; Vipin, B.; Angela, R. Reducing overutilisation of serum vitamin D testing at a tertiary care centre. BMJ Open Qual. 2020, 9, e000929. [Google Scholar] [CrossRef] [PubMed]
- Caillet, P.; Goyer-Joos, A.; Viprey, M.; Schott, A.-M. Increase of vitamin D assays prescriptions and associated factors: A population-based cohort study. Sci. Rep. 2017, 7, 10361. [Google Scholar] [CrossRef]
- Crowe, F.L.; Jolly, K.; MacArthur, C.; Manaseki-Holland, S.; Gittoes, N.; Hewison, M.; Scragg, R.; Nirantharakumar, K. Trends in the incidence of testing for vitamin D deficiency in primary care in the UK: A retrospective analysis of The Health Improvement Network (THIN), 2005–2015. BMJ Open 2019, 9, e028355. [Google Scholar] [CrossRef]
- Australian Government Department of Health. MBS Reviews-Vitamin D Testing Report; Australian Government Department of Health: Canberra, Australia, 2014.
- Australian Government Department of Health. Medicare Benefits Schedule Book; Australian Government Department of Health: Canberra, Australia, 2014.
- Gonzalez-Chica, D.; Stocks, N. Changes to the frequency and appropriateness of vitamin D testing after the introduction of new Medicare criteria for rebates in Australian general practice: Evidence from 1.5 million patients in the NPS MedicineInsight database. BMJ Open 2019, 9, e024797. [Google Scholar] [CrossRef]
- Gordon, L.; Waterhouse, M.; Reid, I.R.; Neale, R.E. The vitamin D testing rate is again rising, despite new MBS testing criteria. Med. J. Aust. 2020, 213, 155–155.e1. [Google Scholar] [CrossRef]
- John, A.S.; Morris, H.; Richardson, A.; Lidbury, B.; Ward, G.; Badrick, T. Vitamin D testing: Impact of changes to testing guidelines on detection of patients at risk of vitamin D deficiency. Ann. Clin. Biochem. 2020, 58, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Felcher, A.H.; Gold, R.; Mosen, D.M.; Stoneburner, A.B. Decrease in unnecessary vitamin D testing using clinical decision support tools: Making it harder to do the wrong thing. J. Am. Med. Inform. Assoc. 2017, 24, 776–780. [Google Scholar] [CrossRef]
- Gowda, U.; Smith, B.J.; Wluka, A.E.; Fong, D.P.S.; Kaur, A.; Renzaho, A.M.N. Vitamin D testing patterns among general practitioners in a major Victorian primary health care service. Aust. N. Z. J. Public Health 2016, 40, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Tapley, A.; Magin, P.; Morgan, S.; Henderson, K.; Scott, J.; Thomson, A.; Spike, N.; McArthur, L.; Driel, M.; McElduff, P.; et al. Test ordering in an evidence free zone: Rates and associations of Australian general practice trainees’ vitamin D test ordering. J. Eval. Clin. Pract. 2015, 21, 1151–1156. [Google Scholar] [CrossRef]
- Wilson, L.F.; Xu, Z.; Mishra, G.D.; Dobson, A.J.; Doust, J. Did changes to recommended testing criteria affect the rate of vitamin D testing among Australian women. Arch. Osteoporos. 2020, 15, 162. [Google Scholar] [CrossRef]
- Olsen, C.M.; Green, A.C.; Neale, R.E.; Webb, P.M.; Cicero, R.A.; Jackman, L.M.; O’Brien, S.M.; Perry, S.L.; Ranieri, B.A.; Whiteman, D.C.; et al. Cohort profile: The QSkin Sun and Health Study. Int. J. Epidemiol. 2012, 41, 929–929i. [Google Scholar] [CrossRef]
- Lu, C.Y.; Barratt, J.; Vitry, A.; Roughead, E. Charlson and Rx-Risk comorbidity indices were predictive of mortality in the Australian health care setting. J. Clin. Epidemiol. 2011, 64, 223–228. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Socio-Economic Indexes for Areas (SEIFA): Technical Paper. Available online: https://www.abs.gov.au/statistics/detailed-methodology-information/concepts-sources-methods/socio-economic-indexes-areas-seifa-technical-paper/latest-release (accessed on 31 January 2024).
- Kim, H.-J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Australian Government-Services Australia. Medicare Items Reports. Available online: http://medicarestatistics.humanservices.gov.au/statistics/mbs_item.jsp (accessed on 10 March 2023).
- Wolff, A.E.; Jones, A.N.; Hansen, K.E. Vitamin D and musculoskeletal health. Nat. Clin. Pract. Rheumatol. 2008, 4, 580–588. [Google Scholar] [CrossRef]
- Kuznia, S.; Zhu, A.; Akutsu, T.; Buring, J.E.; Camargo, C.A., Jr.; Cook, N.R.; Chen, L.-J.; Cheng, T.-Y.D.; Hantunen, S.; Lee, I.M.; et al. Efficacy of vitamin D3 supplementation on cancer mortality: Systematic review and individual patient data meta-analysis of randomised controlled trials. Ageing Res. Rev. 2023, 87, 101923. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; de Fazio, A.; Protani, M.M.; Ibiebele, T.I.; Nagle, C.M.; Brand, A.H.; Blomfield, P.I.; Grant, P.; Perrin, L.C.; Neale, R.E. Circulating 25-hydroxyvitamin D and survival in women with ovarian cancer. Am. J. Clin. Nutr. 2015, 102, 109–114. [Google Scholar] [CrossRef]
- Bergman, P.; Lindh, A.U.; Björkhem-Bergman, L.; Lindh, J.D. Vitamin D and respiratory tract infections: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.; Rahman, A.; Majidi, A.; Waterhouse, M.; Neale, R.E. Acute respiratory tract infection and 25-hydroxyvitamin D concentration: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2019, 16, 3020. [Google Scholar] [CrossRef]
- Pham, H.; Waterhouse, M.; Baxter, C.; Romero, B.D.; McLeod, D.S.A.; Armstrong, B.K.; Ebeling, P.R.; English, D.R.; Hartel, G.; O’Connell, R.L.; et al. Vitamin D supplementation and hospitalization for infection in older adults: A post-hoc analysis of data from the Australian D-Health Trial. Am. J. Clin. Nutr. 2023, 117, 350–356. [Google Scholar] [CrossRef]
- Hahn, J.; Cook, N.R.; Alexander, E.K.; Friedman, S.; Walter, J.; Bubes, V.; Kotler, G.; Lee, I.M.; Manson, J.E.; Costenbader, K.H. Vitamin D and marine omega 3 fatty acid supplementation and incident autoimmune disease: VITAL randomized controlled trial. BMJ 2022, 376, e066452. [Google Scholar] [CrossRef]
- McLaughlin, L.; Clarke, L.; Khalilidehkordi, E.; Butzkueven, H.; Taylor, B.; Broadley, S.A. Vitamin D for the treatment of multiple sclerosis: A meta-analysis. J. Neurol. 2018, 265, 2893–2905. [Google Scholar] [CrossRef] [PubMed]
- The Royal Australian College of General Practitioners. First Do No Harm: A Guide to Choosing Wisely in General Practice. Available online: https://www.racgp.org.au/clinical-resources/clinical-guidelines/key-racgp-guidelines/view-all-racgp-guidelines/first-do-no-harm/gp-resources/vitamin-d-testing (accessed on 20 April 2024).
- The Royal College of Pathologists of Australasia. Position Statement: Use and Interpretation of Vitamin D Testing. Available online: https://www.rcpa.edu.au/Library/College-Policies/Position-Statements/Use-and-Interpretation-of-Vitamin-D-Testing (accessed on 22 May 2024).
- Demay, M.B.; Pittas, A.G.; Bikle, D.D.; Diab, D.L.; Kiely, M.E.; Lazaretti-Castro, M.; Lips, P.; Mitchell, D.M.; Murad, M.H.; Powers, S.; et al. Vitamin D for the prevention of disease: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2024, 109, 1907–1947. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.; Janda, M. Sun Exposure Summit Position Statement; The University of Queensland: Brisbane, Australia, 2023; Available online: https://doi.org/10.14264/f9e3744 (accessed on 20 May 2024).
- National Health and Medical Research Council (NHMRC). Nutrient Reference Values for Australia and New Zealand Including Recommended Dietary Intakes; National Health and Medical Research Council (NHMRC): Canberra, Australia, 2017.
- Pratt, N.L.; Kerr, M.; Barratt, J.D.; Kemp-Casey, A.; Ellett, L.M.; Ramsay, E.; Roughead, E.E. The validity of the Rx-Risk Comorbidity Index using medicines mapped to the Anatomical Therapeutic Chemical (ATC) Classification System. BMJ Open 2018, 8, e021122. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).