Abstract
Background/Objectives: This review summarizes the current knowledge about factors that affect the physical characteristics of urine. It highlights proper urine sample collection and displays factors like diet, hydration status, and medications that can alter urine color, odor, clarity, specific gravity and pH. Results: Urinalysis is a minimally invasive examination of a patient’s health, especially concerning nephrological and endocrinological abnormalities, as well as dietary habits and stimulants used. Certain deviations in appearance, composition or frequency/pain during urination may indicate an ongoing disease process in the body. Based on laboratory results, further medical treatment is determined. The reason for a change in the color of the urine, for its clouding or intense odor may be a disease, as well as the consumption of food, medication, intensive physical exercise or inadequate hydration of the body. Well-standardized procedures for collecting, transporting, preparing and analyzing samples should become the basis for an effective diagnostic strategy in urinalysis. It is worth noting that pharmacists in pharmaceutical care are often the first people to whom a patient turns for health advice and for the interpretation of simple laboratory tests. Acquiring the ability to interpret the results of laboratory tests and the principles of proper sampling for laboratory tests is indispensable in the process of possible counseling and providing reliable answers to patients’ questions. Conclusions: Although urinalysis is not recommended as a routine screening tool for the general population, it can prove to be a valuable source of patient health data in some cases as the data will be useful to physicians and pharmacists to more effectively diagnose and better care for patients.
1. Introduction
There are many physiological processes in the body that result in the production of unnecessary, and sometimes toxic, metabolic products, which are mainly removed by the kidneys. The end product of the multistage purification of the blood is urine, which is 95% water; the remainder is numerous compounds of both organic and inorganic structures. Urinalysis is the most basic examination and provides a great deal of information about a patient’s state of health [1,2]. Based on the laboratory results, further medical treatment is determined. Urinalysis is a minimally invasive examination of a patient’s health especially concerning nephrological and endocrinological abnormalities, as well as dietary habits and stimulants used. The reason for a change in the color of the urine, for its clouding or intense odor may be a disease, as well as the consumption of food, medication, intensive physical exercise or inadequate hydration of the body [2,3,4,5,6,7,8,9,10,11,12], for example, taking vitamins B2 and B12 may result in a bright yellow color of urine [13,14], while supplements containing tryptophan cause a purple color of urine [15]. Excessive supplementation with preparations of iron or black licorice may increase the risk of black-colored urine [16]. A red color is most often indicative of hematuria but can also result from the consumption of foods containing red pigments (e.g., beetroot, berries, blackberries, products containing carotene) [17] or from the use of certain drugs (e.g., nitrofurantoin, metronidazole, rifampicin) [18,19,20]. Red-colored urine, darkening after exposure to sunlight, is also characteristic of an acute porphyria attack [21]. Dehydration will constrict the components of the urine and alter its transparency, specific gravity and pH [6,9,22]. On the other hand, overhydration, taking stimulant drugs or the presence of certain cancers, as well as liver, kidney and adrenal diseases, hypothyroidism, hemolytic jaundice, diabetes mellitus and hypoproteinemia can result in colorless urine [11,23,24,25,26,27,28,29]. There are a number of foods (including asparagus, garlic brassica vegetables, fish and meat proteins, eggs, alcohol, coffee and beans) the consumption of which in some individuals may result in a specific odor, generally associated with the presence of characteristic metabolites [12,30,31,32,33,34,35]. Urine odor is also strongly influenced by diseases such as diabetes mellitus [36]; bacterial and fungal infections [37]; liver, intestinal and pancreatic diseases [34,38]; and genetic diseases such as trimethylaminaemia, phenylketonuria, maple syrup disease or isovaleric acidosis [39,40,41,42,43,44]. A prerequisite for the reliability of the results obtained is the observance of basic sampling principles and awareness of the influence of a number of external factors on the final results.
Well-standardized procedures for collecting, transporting, preparing and analyzing samples should become the basis for an effective diagnostic strategy in urinalysis. The proper collection of the sample guarantees the correct interpretation of the results by the doctor [45,46,47,48,49,50].
This review summarizes the current knowledge about factors that affect the physical characteristics of urine. It highlights proper urine sample collection and displays factors like diet, hydration status and medications that can alter urine color, odor, clarity, specific gravity and pH.
2. Proper Collection of Urine Sample for Analysis
Following basic rules during urine collection is very important to obtain diagnostically reliable results [45,46]. During urinalysis, the first morning sample is generally used, because it has the highest diagnostic value; it is also the best for microbiological examination, i.e., urine culture [47,48,49]. The composition of urine is variable and depends on a great many factors, such as the amounts and types of fluids ingested, food consumed and stimulants or drugs taken. In order to minimize the variability in the composition of urine samples, it is most common to test urine from the first morning micturition, after a prior toilet, from the middle stream (50–150 mL). However, there are cases in which a physician or laboratory diagnostician consulting to prepare for the test may recommend collecting the first stream of urine (e.g., diagnosis of Chlamydia trachomatis) [50]. It is worth noting that with the use of newer nucleic acid amplification techniques (NAATs) and greater sensitivity, the timing of sample collection in a C. trachomatis test may not be as important as thought [51]. The conditions of collection are very important, as the urethra is colonized by bacteria and may contain numerous leukocytes. Therefore, it is necessary to perform a morning toilet to remove various secretions from its outlet, for example, from the prostate in men and the reproductive tract in women [47,48]. Maintaining this basic hygiene will lend credibility to the test results. A typical result indicative of inadequate collection is high titers of bacteria and flat epithelial cells in the absence of leukocytes [47,48,52].
Another option that is usually used for emergency testing or when the patient is unable to collect the first sample is a second urine sample, given 2 to 4 h after the first morning sample [53]. Finally, the so-called random urine sample collected at any time of the day applies primarily to young children and newborns due to the lack of large gaps between micturitions and the poor ability to concentrate the urine [54]. Pouches with hypoallergenic adhesive tape are most commonly used. The genital and anal areas are washed and then the pouch is placed by pressing the adhesive tape against the perineum; the contents are checked every 15 min [54,55]. In any case, the description of the sample delivered to the laboratory should include information about the type of sample collection.
A slightly different type of collection is the daily urine collection (AWD) [56,57]. It is best to use a special graduated container into which each portion of urine should be collected throughout a 24 h period. If possible, it should be stored in a dark and cool place. The rules for collecting such a sample are a little different, namely, the first morning portion of urine should be completely transferred to the toilet and the collection should begin with the second morning urine sample, with the starting time being recorded. Each subsequent portion of urine should be passed in its entirety (without dividing it into initial, middle and final streams) for 24 h from the starting hour. Thus, the last portion of urine collected into the container is the first morning urine sample (omitted when starting the collection) [58,59,60]. At the end of the collection, read the volume of urine collected, mix the whole well (without foaming), and then transfer 50–100 mL into a standard urine container, signed and named, with information about the volume of total urine and the time of the start and end of collection.
In addition to the proper collection of a urine sample, it is extremely important to follow some basic rules before analyzing the urine:
- –
- For a minimum of 24 h before urine collection for laboratory testing, avoid hard work or extreme exercise [52,61] and a high-protein diet [62,63].
- –
- At least one day before the scheduled urine collection for laboratory testing, it is recommended to maintain sexual abstinence [64]. Examination of urine after sexual intercourse can be difficult due to the possible presence of a large number of sperm in the urine, which prevents accurate microscopic evaluation of the urine sediment. There may also be minor damage to the urethra, which will result in the presence of increased epithelium, red blood cells or the presence of bacteria in the urine.
- –
- Collecting urine for laboratory tests during monthly bleeding and 2–3 days before and after menstruation is not recommended (unless otherwise instructed by the referring physician) [65]. When samples are collected during menstruation, the urine is often contaminated with a large number of red blood cells and epithelium, making it difficult to obtain reliable results. This also imposes interpretations in the direction of hematuria and suspicion of, for example, nephrolithiasis or nephrotic syndrome.
- –
- The urine container should be a disposable plastic one, specially purchased for this purpose; if the sample is for microbiological testing, the container should be sterile [52].
- –
- The container with the collected urine should go to the collection point in the shortest time possible (up to a maximum of 2 h at room temperature, or, as a last resort, 4 h at 4 °C because delays can affect the test results, e.g., change in pH, bacterial growth, decomposition of morphotic elements) [52,66]. However, it should be noted that there are opinions that keeping a container of urine in the refrigerator is allowed only if this material is intended for microbiological examination (culture), and it should not last longer than two hours.
- –
- If possible, diuretics should be discontinued, as they affect the amount of urine excreted, as well as changes in electrolyte levels—sodium (Na), potassium (K), calcium (Ca) and others [67]. It is necessary to remember to balance the effect of diuretics, that is, to provide adequate amounts of fluids on a regular basis because otherwise, dehydration can occur. The normal volume of excreted urine is 1000–2500 mL/24 h. The minimum amount of urine needed to excrete the products of protein metabolism with an average supply of protein and dietary salt and the full capacity of the kidneys to thicken the urine is 400–500 mL/day [52,68].
3. The Effects of Diet, Dietary Supplements, Drugs and Exercise on the Physical and Diagnostic Values of Urine Characteristics
The parameters to be evaluated during urinalysis can be divided into the following:
- –
- Physical characteristics: color, odor, transparency, specific gravity and pH [48,52,69].
- –
- Chemical features: protein, glucose, urobilinogen, ketone bodies, bilirubin, uric acid, nitrogenous compounds and others [48,52,69].
- –
- Microscopic analysis of urine [7,70,71] necessary for proper diagnosis in many asymptomatic cases, including urinary tract infections, urinary tract tumors, latent glomerulonephritis or interstitial nephritis. The urine sediment is mainly white and red blood cells, epithelia, rollers, lipids, bacteria, mucus, fungi, parasites and minerals. The result is generally given as a total from 10 fields of view. Microscopic examination of the urine sediment is an integral part of the total urine examination [70,71]. During the final interpretation of the obtained result of the urine sediment analysis, it is necessary to take into account all other parameters, as well as the patient’s clinical symptoms. Mineral compounds may be present in the urine sediment in the form of crystals or amorphous precipitates. These include uric acid crystals, amorphous urates and calcium oxalates. These compounds are often formed in urine that has been stored in a refrigerator prior to testing. It should be noted that manual microscopic examination of urine is time-consuming and requires considerable experience for results interpretation; therefore, in modern laboratories, this process has been automated using flow cytometry and digital microscopy [70,71].
The remainder of this article will discuss the various physical parameters of urine that may be affected by diet/fluids/physical activity/health conditions/medications.
3.1. Changes in the Color of Urine
Normal urine has a straw-yellow, clear color, which indicates adequate hydration of the body. The darker the shade, the greater the likelihood that there is too little water in the body. A change in urine color is an easily noticeable parameter and often worries patients. However, many of the causes of abnormal urine color are benign effects of medications and foods (recently eaten foods and the natural or synthetic dyes they contain) and taking certain medications. Sometimes a change in urine color can also be a sign of illness. If the change in urine color persists for several days, you should see a specialist, have a general urine test, a blood test and, if necessary, a urinary ultrasound.
In general, the color of urine depends on the following:
- –
- The presence of dyes produced by endogenous metabolism, such as urochrome, uroerythrin, urobilinogen [72,73].
- –
- The degree of urine thickening resulting from various causes, discussed in detail later in this article [72].
- –
- Diet (e.g., beets, berries, rhubarb, broad beans, fava beans, aloe vera, carrots, green spinach, purine-rich foods, excess protein, laxatives) [13,14,15,74,75,76,77].
- –
- Drug therapy or reagents used, e.g., chloroquine [13,78], nitrofurantoin [18], phenazopyridine [79], vitamins B [13,14], phenacetin [80], phenytoin [81], methylene blue [82], iron [13,16], methyldopa, rifampicin [83], isoniazid [13], warfarin [13], triamterene [84,85], phenolphthalein [86], phenothiazine [87], sulfasalazine [88], amitriptyline [84,85,89,90], propofol, indigo blue, indigocarmine, cimetidine, indomethacin, doxorubicin, promethazine, rinsapine, sildenafil [84,85,91,92], metronidazole [19,93], sorbitol [13,94], cresol, nitrates [95], tryptophan [96], hydroxycobalamin [97], acetaminophen [98], metoclopramide [90], herbicides [99] and cefozoppran [100].
- –
- Metabolic disorders, e.g., hemolytic anemia, porphyrias [21], liver diseases, cancers [101], alkaptonuria [102], blue diaper syndrome, hypercalcemia, glucose-6-phosphate dehydrogenase deficiency, sickle cell anemia [13] and Lesch-Nyhan disease [103].
- –
- Bacteremia, inflammation in the urinary tract [104,105,106,107].
3.1.1. Dark Yellow/Amber-Colored Urine
May indicate dehydration due to inadequate fluid intake, working or being in a hot room or place [22], heavy physical exercise [108,109,110,111], severe diarrhea, use of laxatives, persistent vomiting or impaired kidney function [55,73,112,113,114,115,116]. This phenomenon is particularly dangerous in children and the elderly. Symptoms of dehydration such as vomiting, diarrhea, sunken fontanel and eyes, irritability and not having a wet diaper for more than 3 h occur in newborns and infants [117]. This poses an immediate life-threatening risk, so medical attention should be sought immediately [118,119,120]. On the other hand, elderly patients become dehydrated as a result of diarrhea, renal disease (including simple uremia and chronic renal failure), frequent constipation, use of laxatives and edema-reducing and hypotensive drugs [121,122,123,124]. Regardless of age, one must replenish fluids to prevent dehydration and reduce the risk of kidney stone formation. The optimal amount is 2–3 L of water per day, depending on gender, physical activity, health status and weather conditions [125].
3.1.2. Colorless Urine
It may be a cause of excessive fluid retention or may occur as a result of the use of certain medications, particularly those that stimulate the kidneys to excrete more urine (diuretics, amitriptyline, tiotropium, cyclophosphamide, vincristine and sulfonylureas). Other causes include glycosides, glipizide, certain cancers (duodenal cancer, pancreatic cancer, thymoma), renal and adrenal disease, hypothyroidism, hemolytic jaundice, diabetes, hypoproteinemia and excessive vasopressin [23,24,25,26,27,28]. However, in most cases, the lack of urine staining usually indicates that one is simply drinking too much water at once (1.5–2 L) [29]. This becomes dangerous when the kidney’s filtration threshold is exceeded. The result is the accumulation of too much water in the bloodstream, which can upset the fluid balance, excessively dilute concentrations of endogenous compounds and flush out minerals and vitamins taken from the diet. Excess water in the intravascular and interstitial spaces can result in edema of peripheral tissues and lungs, hypertension and increased excretion of sodium and water ions by the kidneys.
Excessive accumulation of water in the extracellular space can also result from a variety of diseases and be of a different nature [22,27,126,127,128,129,130], including the following:
- –
- Isotonic—due to excessive supply of isotonic solutions such as sodium chloride in people with impaired renal function, the presence of cardiovascular insufficiency, liver disease, endocrine disorders (Cushing’s syndrome) and glucocorticosteroid treatment, there are increased amounts of water and sodium in the body [130].
- –
- Hypertonic—there is also an excessive amount of sodium in the body, but water retention occurs secondary to the existence of an excessive amount of sodium in the blood [131,132,133]. Causes include excessive supply of hypertonic solutions by parenteral route, renal impairment due to chronic kidney disease or acute renal failure, excessive secretion of antidiuretic hormones (e.g., condition after extensive surgery) and increased secretion of glucocorticoids and mineralocorticoids (shock, adrenal hyperfunction). Hypertonic conductance can also occur in survivors who drink seawater containing a large amount of sodium ions.
- –
- Hypotonic is also referred to as water intoxication, yet another water disorder proceeding with hyponatremia, i.e., a decrease in blood sodium concentration. This can occur as a result of excessive supply of electrolyte-deprived or electrolyte-deficient fluids (e.g., glucose solution) [126,127]. Hypotonic conductance can occur in patients with impaired renal water excretion or in patients with excessive antidiuretic hormone (ADH) secretion, in the course of porphyria, cranial trauma, inflammatory conditions of the brain and lungs (tuberculosis and pulmonary aspergillosis), in certain malignancies (oat cell carcinoma, duodenal and pancreatic cancer, thymoma) and in patients taking such drugs as sulfonylurea derivatives, carbamazepine, amitriptyline, thioridazine, diuretics, cyclophosphamide and vincristine [27,128,129,130,134].
Acute water intoxication can occur independently of the disease, for example, as a result of athletes drinking large amounts of electrolyte-free fluids, e.g., marathon runners, cyclists (up to 5 L) or infants fed very dilute milk mixtures (sodium deficiency) [26,135,136]. Nausea, vomiting, anorexia, pupil irregularity, muscle spasms, seizures and cerebral edema can then occur. If immediate specialized treatment is not given, then damage to the central nervous system, acidosis and even death can occur [137].
3.1.3. Bright Yellow (Fluorescent) Coloration of Urine
Taking B vitamins, especially B2 (riboflavin) and B12 (cyanocobalamin), and numerous multivitamin supplements [13,14]. Very yellow-colored urine after vitamin supplementation is a normal condition and should not cause concern. The color is more intense than usual and often has a bright “canary” hue. Under UV light, a fluorescent effect can be seen [138]. However, if the intense coloration persists after vitamin withdrawal, a doctor should be consulted.
3.1.4. Reddish-Pink/Red Coloration of Urine
Regardless of the intensity, such a color raises concern and its cause should be quickly detected, i.e., the presence of red blood cells or hemoglobin should be confirmed. If blood is indeed present, it can indicate various disorders ranging from kidney function, hematopoietic system or menstrual blood contamination. Red color can include terms such as pink colors, shades of red, orange, brown or black depending on the analyst examining the sample.
The causes of staining with different shades of red can include the following:
- –
- Presence of urate (gout) [139], inflammation of the urinary tract of various etiologies with excessive secretion of uric acid [140]. Hyperuricemia is the excessive accumulation of uric acid due to impaired metabolism of purines. Purines are a group of aromatic organic compounds that occur naturally in foods, especially those rich in protein, or from de novo synthesis and the breakdown of endogenous nucleic acids. Purines are converted into uric acid via metabolic pathways. Excess uric acid is deposited, among other things, in the joints, forming microcrystals and causing gout in addition to facilitating the crystallization of calcium oxalates (urolithiasis). Therefore, in the case of red-orange coloration of urine, and especially in the presence of crystals, hyperuricemia should be considered [139,140]. Some contradictions exist regarding the use of vitamin C as a prophylaxis for gout [141,142,143]. The beneficial effect of ascorbic acid on lowering serum uric acid levels is probably due to its uricosuric effect and inhibition of uric acid synthesis. Taking vitamin C in doses of about 500 mg per day may have a positive effect on lowering uric acid concentrations in healthy individuals [141,142,143]. However, for patients diagnosed with gout, vitamin C supplementation (without medication) has no significant effect [144]. On the other hand, vitamin C is a precursor of oxalic acid, and consumption of high doses (more than 1.500 mg/day), for a prolonged period of time will increase the risk of side effects, especially the risk of kidney stones, so the decision to implement supplementation should be carefully considered [145]. It is believed, however, that this does not apply to natural sources of vitamin C but precisely to dietary supplements.
- –
- Consumption of foods rich in natural and added food dyes (beets, beet leaves, blackberries, blueberries, rhubarb, carrots) [17,74,75,76,94,146]. In this case, the most commonly described symptom is beeturia [17], which is the discoloration of urine after consuming raw, cooked or pickled beets, beet juice or foods enriched with beet extract. The typical color can range from pink to deep red, and the phenomenon occurs in 10–14% of the population, with increased frequency among those with iron deficiency or malabsorption syndrome. The pigments found in beets belong to betacyanins, hence the term betacyaniuria is sometimes used. It is worth noting that the compounds contained in beet (betanin, betalains, betacyanins), after extraction, are used as natural dyes in many foodstuffs such as carrot, orange and tomato fruit juices, tomato concentrates, ketchup, tomato soup, red gelatin, red and purple candies, yogurts, ice cream, icing, sweet fillings for baked goods and sausage products (salami, sausages), as well as in cheeses with additives. The occurrence of red, reddish-purple or even brownish urine as a result of consuming these products is harmless and transient. Similar urine color can be caused by the consumption of rhubarb. The change in urine color in this case, too, is the result of natural dyes found in the edible stalks, which survive the digestion stage in the stomach. Rhubarb stalks also contain small amounts of oxalic acid, which probably protects the pigment from digestive juices and thus contributes to the change in urine color [75,76].
- –
- Accidental contamination with menstrual blood [147].
- –
- Diseases resulting in the presence of free hemoglobin, erythrocytes, myoglobin and porphyrins in urine [13,21,148]. Hemoglobinuria is the excretion in the urine, hemoglobin being the oxygen carrier in the erythrocyte. After the breakdown of red blood cells, a trace amount of free hemoglobin appears in the blood [149]. However, in hemolytic anemia, if massive erythrocyte disintegration occurs, the amount of free hemoglobin increases significantly and, having exceeded the transport capacity of haptoglobin, it begins to appear in the urine and can darken it to a deep reddish color [150,151]. On the other hand, paroxysmal nocturnal hemoglobinuria (PNH, paroxysmal nocturnal hemoglobinuria) is an acquired clonal disease of the hematopoietic stem cell [148,152]. The disease was first described in 1882 in a 29-year-old laborer complaining of fatigue, abdominal pain and severe nocturnal attacks of hemoglobinuria, which worsened after excessive alcohol consumption, after exercise and after administration of iron salts.
Even without the presence of blood, dark red urine can be a sign of a serious hereditary condition, e.g., porphyria or abnormality of heme metabolism [21]. It manifests as dark urine, abdominal pain, photosensitive rashes or neuropsychiatric complaints, among other symptoms. The disease is difficult to detect because it is rare, and sometimes there is no equipment for urine porphyrin analysis, which further delays treatment [21]. Myoglobinuria is the excretion of myoglobin in the urine, which occurs in the course of rhabdomyolysis (damage to striated muscle) [153,154]. Myoglobin is a protein found in striated muscles—skeletal and in the heart—resembling in structure the hemoglobin present in red blood cells. Myoglobin’s primary function is to store oxygen. In the case of significant exertion, when the molecular pressure of oxygen reaches low values in the muscles, myoglobin releases the oxygen molecules attached to it, allowing the muscles to continue working efficiently using high-energy ATP. Only myoglobin released from damaged skeletal muscle and heart cells is found in the bloodstream.
Myoglobin can also enter the urine, impairing kidney function and causing acute kidney failure.
- –
- The use of drugs such as doxorubicin [155], daunorubicin [156], aminophenazone, phenazopyridine [79], quinine [157], chloroquinone [78], L-dopa, hydroquinone [158], naphthol [159], phenytoin, rifampicin [20], metronidazole [19], nitrofurantoin [18], phenacetin [87], phenothiazine [88], salazopyrin [18], barbiturates, lidocaine, diclofenac, clindamycin, erythromycin, clemastine, lutein, progesterone, gestagens, heparin [160] and hydroxycobalamin [97]. In toxicology practice, hydroxycobalamin is sometimes used to treat cyanide poisoning [161]. Traditional treatments rely on drugs such as amyl nitrite, sodium nitrite and sodium thiosulfate; these can cause methemoglobinemia, further reducing the ability of red blood cells to carry oxygen. Hydroxocobalamin works by combining with cyanide to form cyanocobalamin. An unintended but mild effect of its administration is a red tint to the skin and urine. This effect usually passes after a few days [97].
- –
- The cause of red-colored urine may be lead or mercury poisoning, possibly related to kidney damage [162,163].
- –
- Finally, the deliberate addition of blood or dye to a urine sample. Patients with psychiatric disorders add blood or another red substance directly to the sample being analyzed. The non-specific complaints they describe lead to numerous, usually fruitless tests. Simulation is difficult to diagnose and may require repeat urine samples obtained under observation to ultimately discover the primary disorder [164].
3.1.5. Brown Coloration of Urine
Brown or very dark red urine, possibly stored longer, is interpreted as brown and may result from the following:
- –
- Eating larger amounts of rhubarb, broad beans and fava beans [77]. Consumption of foods prepared especially with fava beans changes the color of urine in some people. This is due to fava bean deficiency, or glucose-6-phosphate dehydrogenase deficiency, a congenital disease that leads to the breakdown of red blood cells [165]. Once broken down, the red blood cells release their contents into the blood and eventually into the urine, resulting in dark-colored urine. In people with favaism, eating fava beans triggers a chain of events that leads to blood in the urine and other complications (abdominal pain, vomiting [75].
- –
- Diseases associated with excessive excretion of hemoglobin, myoglobin and porphyrin (described earlier) [13,21,148,153].
- –
- Copper poisoning due to, among other things, hemoglobinuria [166].
- –
- Dehydration, [22] including as a result of strenuous exercise [108,109,110,111].
- –
- Liver diseases (cirrhosis, inflammation). Transparent dark brown urine can be a symptom of jaundice. This is even more certain if at the same time, the stool is discolored and the skin is yellowed [167].
- –
- Cancer metastasis [101]. Brown urine can be a sign of melanocytes in the urinary tract. Metastatic melanoma can lead to a rare condition called diffuse melanosis, causing dark skin, distant internal organ changes and brown or black urine [168].
- –
- Extremely tough exercises [108,109,111,153] during which muscle and/or kidney damage can occur.
- –
- Acetaminophen overdose (increase in p-aminophenol). In the 1980s, it was observed that an overdose of acetaminophen (obtained using an older method of production) in addition to liver failure can cause the appearance of brown urine due to the accumulation of the metabolite p-aminophenol [169].
3.1.6. Black Coloration of Urine
- –
- Excessive supplementation with iron preparations, including in the form of injections, can exacerbate the risk of black urine [16,170]. Patients in this case simply need reassurance that this is a mild side effect of the drug.
- –
- Use of laxatives like cascara (the peel that surrounds coffee beans) or Folium Sennae [171].
- –
- The presence of melanin, which may appear if a cancer producing it (melanoma, melanoblastoma) grows in the body. The presence of melanin in the urine can cause brown discoloration, possibly with a black tinge [168].
- –
- Presence of methemoglobin, for example, as a result of poisoning with nitrates (III/V) or aniline dyes [170,172].
- –
- Presence of homogentisic acid. Alkaptonuria is a rare inherited disorder in which the body has an impaired ability to catabolize tyrosine and phenylalanine leading to the accumulation of homogentisine acid in the body [173]. Clinically, it manifests as arthritis and darkening of the skin and urine. Diagnosis is based on measuring the concentration of homogentisinic acid in the urine. There is no effective pharmacotherapy: high doses of vitamin C and a low-protein diet are recommended [173].
- –
- The use of drugs such as aminophenazone, aminopyrine, antipyrine and quinine [157], chloroquine [13,78], hydroquinone, phenytoin, metronidazole [19,93], nitrates [172], nitrofurantoin [18], phenacetin [80], phenolphthalein [86], phenothiazine [87], salazopyrin, sorbitol and L-dopa α-Methyldopa (the latter two drugs can stimulate melanin synthesis and urinary excretion, a known side effect) [158,174]. Also worth mentioning is cresol, a disinfectant that can sometimes be consumed by alcohol addicts [175].
- –
- Consumption of black licorice in large quantities can temporarily change the color of both stool and urine (from a dark green or almost black color, lasting several days) [75].
3.1.7. Green–Blue Coloration of Urine
Such unusual toying of the urine is most often the result of a certain diet or consumption of products containing synthetic dyes. However, this staining or intermediate staining can also appear after taking certain medications or indicate an infection caused by hepatitis or cancer. Below are a few examples:
- –
- Foods rich in artificial dyes and foods like spinach and green asparagus. However, in some people, eating (especially) asparagus changes the color of urine to slightly green; unfortunately, the smell of urine also changes (ammoniacal) [75]. Carotene pigments are responsible for the color of the urine, while sulfur compounds are responsible for the odor.
- –
- Taking certain medications can cause blue or green urine. For example amitriptyline, doxorubicin, indomethacin, cimetidine, metoclopramide, guaiacol, promethazine, triamterene, rinsapine, propofol, sildenafil and B vitamins [84,85]. The main reason for this phenomenon is the contact of metabolites present in the urine with air and their oxidation to blue–green compounds. This symptom regarding the above-mentioned drugs is mild and not subject to further evaluation when the other results of urinalysis are normal. Other compounds that can cause abnormal urine color are the supplement arbutin (from Arctostaphylos uva-ursi) and methylene blue, used for the treatment of methemoglobinemia, among others. Methemoglobinemia is a hematological disease in which a significant portion of hemoglobin from Fe (II) is replaced by methemoglobin from Fe (III) so that the ability to release oxygen to the tissues is reduced. Cyanosis, shortness of breath, fatigue, headaches and dizziness follow; in extreme cases, it can lead to coma or death. The treatment of methemoglobinemia was one of the first and most important uses of methylene blue (BM) [176,177]. In this case, however, the dose is key—while low doses of BM treat methemoglobinemia, high doses can provoke it (as intravenous injections). It is worth remembering that the use of high doses can also affect the green–blue staining of mucous membranes and urine. The problem is much smaller and virtually unnoticeable when using microdoses, i.e., <100 µg per day [92]. Methylene blue has weak antiseptic properties and is an ingredient in several drugs (such as Uroblue tablets) used to reduce symptoms of bladder inflammation or irritation [85] and as an antifungal, antibacterial and antimalarial drug [85,178,179]. Phosphasal, used for dysuria, also contains methylene blue, which causes blue–green coloration of the urine [85]. Methylene blue is increasingly used for oral mucositis in patients receiving anticancer therapy and sonodynamic effects [180,181]. Blue–green urine is a normal side effect of these drugs and is harmless.
- –
- Dyes (e.g., methylene blue, indigocarmine) used intravenously in the diagnosis of certain kidney and bladder diseases can change the color of patients’ urine temporarily to blue [182,183].
However, the occurrence of green urine may be due to specific medical conditions:
- –
- Blue diaper syndrome (Drummond syndrome) is a rare metabolic disorder that leads to impaired tryptophan absorption. This amino acid is degraded under the influence of bacteria in the intestinal lumen to indole dyes, which are then excreted by the kidneys and, upon contact with air, are oxidized, turning the urine blue. The characteristic symptom is just the presence of blue urine spots on the diaper of a sick infant. Other symptoms are hypercalcemia and nephrocalcinosis. Treatment is dietary restriction (limitation of protein, calcium and vitamin D supply) and antibiotic therapy [184].
- –
- A rare genetic disorder is familial hypercalcemia. Children with this condition urinate blue urine from birth [185].
- –
- Prostatitis, bladder bacteremia caused by urinary tract infections, mainly infection with Pseudomonas aeruginosa. In this case, the history and physical examination indicate an infectious disease, and urine and blood cultures will confirm the diagnosis. Treatment focuses on clearing the infection, not the color of the urine [89,186].
3.1.8. Orange/Red–Orange Coloration of Urine
- –
- Eating lots of raw carrots, drinking carrot juice, taking vitamin supplements (beta-carotene) [94].
- –
- Use of laxatives (fluid loss occurs), such as those containing Folium Sennae [146].
- –
- Excessive bilirubin secretion (in liver disorders) [13].
- –
- The use of drugs such as phenazopyridine [187], rifampicin and high-dose riboflavin [146].
It is also worth mentioning two rare cases of the unexpected appearance of orange-colored urine:
- –
- Accumulation of Citrobacter sedlakii bacteria [188] and colonization of the urinary tract and infection in neonates and immunocompromised patients. Citobacter sedlakii are Gram-negative bacilli of the Enterobacteriaceae family that produce orange indole by degrading tryptophan.
- –
- The second case was associated with uric acid crystalluria [139]. It involved a patient diagnosed with epilepsy. Epileptic seizures can lead to hyperuricemia and renal failure, but orange-colored urine immediately after a seizure has not been reported. Sustained muscle contractions during epileptic seizures cause excessive ATP breakdown and as a result of the breakdown of purine nucleotides, uric acid concentrations increase. The likely causes of uric acid crystallization are acidic urine pH and high uric acid concentration. Thus, uric acid crystalluria should be considered in the differential diagnosis of any patient with reddish-orange colored urine, especially in the presence of macroscopic red–orange crystals [139].
3.1.9. Milky/White Coloration of Urine
It can result from the following:
- –
- Electrolyte disorders resulting in excess calcium and phosphate [189]. Amorphous phosphates in urine are a physiological component of urine that is alkaline or slightly acidic (in shape they resemble fine white-gray sand or in compact form they form putative rollers). Very abundant phosphates detected in urinalysis can be due to a number of causes, from an excessive supply of phosphate in the diet to phosphate lithiasis (calcium phosphates or ammonium–magnesium phosphates (struvite) appear, accompanied by an increase in the specific gravity of the urine) or urinary tract infection (in which case bacteria, erythrocytes and leukocytes may also appear). Phosphate lithiasis is a rare variety of renal deposits accompanying urinary tract infections. The bacteria responsible for the infection alkalize the urine, which promotes the precipitation of phosphate stones. Most often, however, kidney stones are caused by several factors simultaneously.
- –
- Urinary tract infections (purulent infections with E. Coli species, also of the genus Proteus) [190,191]. Severe urinary tract infections can contribute to the appearance of white-colored urine, as purulent fluid can enter the bladder. In addition, the condition of so-called sterile leukocyturia (the presence of purulent fluid in the patient, but without the presence of bacteria), which is associated with urinary tract tuberculosis, should also be considered [192].
- –
- Chyluria (the presence of chyle in the urine) [8]. It results from a malformation of the lymphatic system or is caused by obstruction of the lymphatic system caused by tumor growths or closure of the system by parasites (mainly pinworms). Also, poisoning by mosquito-borne nematodes (filariasis) causes abnormal communication of the lymphatic system with the urinary tract [193]. Another cause can also be a lymphatic fistula [194].
- –
- Sometimes it can be caused by the presence of uric acid crystals from purine-rich foods (such as anchovies, herring and red meat) [195].
3.1.10. Purple Coloration of Urine
- –
- The so-called purple urinary pouch syndrome (PUBS) occurs in patients in post-obstructive states or in severe intestinal failure in patients with catheter insertion [196,197]. It indicates bacterial infection (most often by Providencia stuartti and rettgeri, Proteus mirabilis, Pseudomonas auruginosa, Klebsiella pneumoniae, Escherichia coli, Morganella and Citrobacter species, Enterococci and group B Streptococci) and the presence of tryptophan. Tryptophan in the digestive tract is converted to indoxyl, which is metabolized in the liver and excreted by the kidneys. Bacteria in the urine form indigo and indirubin, which together cause purple urine, staining the Foley catheter (made of polyvinyl chloride) [198]. Generally, purple-stained urine is associated with Gram-negative bacteriuria and usually resolves with antibiotic treatment and catheter change.
- –
- Increased dietary tryptophan content is increased substrate availability for conversion [15]. Protein-rich foods include fish (tuna and cod), cheeses (mozzarella), milk, lean meats and soy products. Pumpkin seeds contain the most tryptophan—576 mg/100 g. Although the tryptophan content of these products is high, they can be consumed without concern—it is not high enough to fear an excess of tryptophan in the body. However, tryptophan used as an ingredient in many supplements to combat sleep problems, prolonged stress and migraines, and to raise overall energy levels in the body may pose some risks. Increased urinary alkalinity facilitates indoxyl oxidation [199,200].
- –
- Severe constipation also promotes bacterial overgrowth, thereby increasing the degradation of dietary tryptophan [30].
The factors that can lead to a change in the color of urine are presented in Table 1.

Table 1.
Factors that can change the color of urine.
3.2. Changes in the Clarity and Transparency of Urine
Properly fresh urine is usually clear and transparent, without visible particles of suspended sediment. Opaque, cloudy urine that persists for several days has an unpleasant odor, indicative of a urinary tract infection or the presence of kidney stones [201] (Figure 1).
Possible causes of urine turbidity (Figure 1):
- –
- Dehydration resulting in, among other things, the presence of an excessive concentration of urine’s aggregated and unaggregated elements. In addition, insufficient drinking of fluids can lead to the formation of stones in the bladder [22,72,121,125].
- –
- Excretion of large leukocytes, erythrocytes, epithelia, urate and phosphate as a result of various pathological conditions [52,202,203].
- –
- Bacteriuria (urinary tract/kidney infection) [104,204,205].
- –
- Lipiduria—the presence of even trace amounts of lipids in the urine, caused mainly by chyluria [206] and trauma [207]. Lipiduria can also be caused by the presence of free cholesterol, cholesterol esters, triglycerides, free fatty acids and phospholipids [208,209,210,211]. In patients with low-grade proteinuria [211], lipid droplets are thought to originate from renal cysts containing degraded blood.
- –
- Lactic staining of urine described earlier can also cause the interpretation of a cloudy sample [8,189,190,194].
- –
- High concentrations of urate and oxalate, loss of amorphous phosphate and carbonate deposits (urine pH changes) [212,213,214].
- –
- Long storage of the sample under room conditions [215,216,217].
- –
- Yeast infections—Candida albicans in the vagina—resulting in the formation of a thick, cheesy, white discharge that mixes with urine [218,219].
- –
- Kidney stones—cloudy urine is one of many symptoms—plus body pain (located in the side, back, below the ribs, groin or abdomen), frequent urination, fever and chills [52].
- –
- Increased cervical mucus production during ovulation in women [220] may produce more malleable, milky or creamy mucus that mixes with urine to give it a cloudy appearance. This is considered a normal occurrence, but if the discharge has an unpleasant odor or a different color, a doctor should be consulted.
- –
- Bacterial vaginitis—appearance of a grayish-white discharge that can mix with urine and make it cloudy [221,222].
- –
- Sexually transmitted diseases such as gonorrhea and chlamydiosis can cause cloudy urine because they increase the production of a gray or white discharge in the vagina that can mix with urine [223,224,225].
- –
- Prostatitis. One of the symptoms of this disease caused by a bacterial infection is discharge from the urethra, which can mix with urine and cause it to become cloudy. Benign prostatic growth, on the other hand, can make it difficult to empty the bladder and result in excessive bladder retention and a tendency to form stones [52,226].
- –
- Late stages of prostate cancer—blood or sediment may accumulate in the obstructed bladder. If these are excreted with the urine, they can cause it to become cloudy. Also, prostatectomy surgery can sometimes lead to cloudy urine [226,227,228]. This may be due to the use of a catheter during the healing process.
- –
- Diabetes—cloudy urine can indicate type 1 or type 2 diabetes (plus other symptoms). Hyperglycemia can also lead to kidney disorders or increase the risk of urinary tract infections—both conditions can cause urine to become cloudy—as well as damage blood vessels in the kidneys, leading to their malfunction. In addition, high blood sugar levels can cause proteins to be present in the urine, which reduces surface tension and can make the urine foamy [229,230,231].
- –
- Pyuria, or an elevated white blood cell count that can pass into the urine due to diseases such as urinary tract infections (UTIs), gonorrhea, interstitial cystitis, tuberculosis, pneumonia and kidney disease [204,232,233] and from taking medications such as diuretics, penicillin and other antibiotics, proton pump inhibitors (e.g., omeprazole) and non-steroidal anti-inflammatory drugs such as aspirin and ibuprofen [234,235,236].
- –
- Intestinal fistula—refers to the presence of an opening between the bladder and the intestine. Intestinal fistulas usually occur due to diseases such as diverticulitis, Crohn’s disease or colon cancer and can cause symptoms such as cloudy urine and abdominal bloating [237].
- –
- Hyperuricosuria, that is, a high concentration of uric acid in the urine, can develop in people who frequently consume high-purine foods such as anchovies, shellfish, sardines, offal, peas, spinach, asparagus, mushrooms, yeast and alcoholic beverages [238,239,240].
- –
- Ketosis—a condition in which high concentrations of ketone bodies appear in the urine. Ketosis occurs when the body begins to use fatty acids instead of glucose for energy. Many factors can cause ketosis, including low-carbohydrate diets, eating disorders, digestive disorders, prolonged diarrhea or vomiting, high-intensity exercise and pregnancy. A high number of ketone bodies in the urine can make it lipemic in color, which can sometimes be interpreted as a cloudy coloration [241].
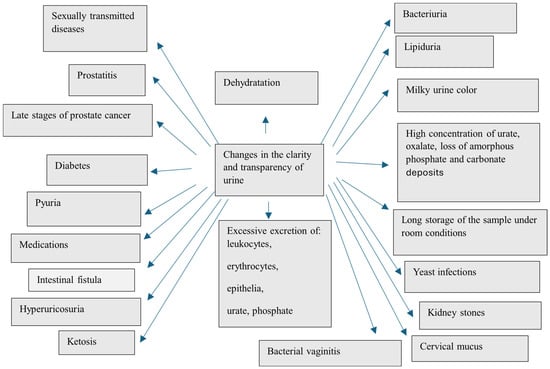
Figure 1.
Factors that can change the clarity and transparency of urine [8,27,52,72,104,121,125,189,190,194,202,203,220,221,222,223,224,225,226,227,228,229,230,231,232,233,234,235,236,237,238,239,240,241].
3.3. Changes in the Odor of Urine
The presence of an inappropriate urine odor is not always associated with the onset of a serious disease. In most cases, change in the odor of urine is the result of a specific diet or a lower tolerance to food products that contain, for example, a high concentration of sulfur. The presence of volatile organic compounds (VOCs) in urine is the result of intermediate or final metabolic transformations of various compounds [242]. In urine, they are often found in relatively higher concentrations, making them easier to detect [243]. Urine odor is highly variable, depending on diet, comorbidities, medications taken, and body condition, including, for example, dehydration (Figure 2).
- –
- The urine of cancer patients contains volatile organic compounds (VOCs) produced specifically in response to a particular cancer, such as the prostate gland, [244,245] ovary, lung and bladder [246,247,248]. Serum prostate-specific antigen is the most commonly used biomarker for prostate cancer and urinary volatile organic compounds have been proposed as alternative biomarkers. Researchers have tried to determine whether certain VOCs in urine can cause a specific urine odor associated with cancer. These studies have confirmed the potential of using urine for diagnostic purposes. Recent work has also shown that characteristic VOC profiles in urine can be associated with infectious diseases [249,250].
- –
- Foodstuffs containing substances whose metabolites can cause the appearance of a specific odor, e.g., asparagus (methyl mercaptan) [12,251,252], garlic (allyl mercaptan, allyl methyl sulfide (AMS), diallyl disulfide (DADS) and diallyl sulfoxide (DASO 2) [31,32], fish protein, meat [30,33], brassica vegetables—cabbage, broccoli, kale, brussels sprouts, cauliflower—oriental spices, eggs, alcohol, cheese, coffee or cooked beans or taking B vitamins [34,35].
- –
- A state of dehydration and thus urine congestion or simple incontinence and involuntary urine leakage can cause a specific pungent odor [253].
- –
- Fungal infection of the urinary tract—yeast-like odor [37].
- –
- Diabetes mellitus causing urine to smell fruity indicates blood glucose levels are too high [36]. In contrast, ketoacidosis causes acetone odor (the smell of sour apples) and indicates diabetic ketoacidosis. Ketone bodies (of which acetone is the main representative) appear in the urine and cause metabolic acidosis, a state of disruption of the body’s acid–base balance [254].
- –
- Liver, intestinal and pancreatic diseases cause a musty odor. Cases have been described with transient trimethylaminuria (TMA) associated with acute intestinal inflammation induced by dietary protein with urinary excretion of TMA and concurrent disease [34,38]. Another source of TMA in urine may be intestinal flora, mainly Anaerococcus, Providencia, Edwardsiella, Clostridium, Collinsella, Desulfovibrio, Lactobacillus and Proteus [251].
- –
- Bacterial infections (Escherichia coli, Salmonella enterica), kidney stones, kidney disease—smell of ammonia, hydrogen sulfide, spoiled meat [34,255,256]. The main contributors to the production of the gaseous methanethiol that determines rotten odor are intestinal bacteria such as E. coli, Citrobacter and Proteus. Methanethiol is absorbed in the intestines then enters the blood and is excreted in the urine [257].
- –
- Genetic diseases:
- –
- Trimethylaminuria (trimethylaminemia) (also storing urine at high temperatures for too long) causes a fishy urine odor. The primary problem associated with trimethylaminuria is a deficiency in the enzyme FMO3 (flavin-containing monooxygenase). This enzyme is involved in the conversion of trimethylamine to trimethylamine oxide; when such conversions do not take place, trimethylamine is excreted from the body in, among other things, urine and sweat. The disease is inherited in an autosomal recessive manner (mutations in the genes encoding FMO3), which means that abnormal genes must be inherited from both parents in order to contract the disease [39].
- –
- Phenylketonuria—mousey urine odor caused by this genetic metabolic disease, which involves the accumulation of one of the essential amino acids, phenylalanine, in excess in the body. Excessively high levels of phenylalanine in the blood lead to gradual depletion of the nervous system [40].
- –
- Maple syrup disease—the smell of burnt sugar in medical terminology, also known as branched-chain amino acid ketoaciduria. This is a genetically determined metabolic disorder in which the body fails to break down the branched-chain amino acids leucine, isoleucine and valine (these are commonly found in foods, especially those rich in proteins) [4]. As a consequence, these substances and their toxic breakdown products (α-keto acids) accumulate in the body, leading to gradual poisoning and, if untreated, death. The cause of maple syrup disease is a deficiency in the activity of the enzyme complex of α-ketoacid dehydrogenases. It is caused by mutations in the BCKDHA, BCKDHB and DBT genes, which contain instructions on how to produce the aforementioned enzyme complex. The disease is inherited autosomal recessively. Alpha-keto acids and amino acids such as leucine, isoleucine and valine accumulate in the blood and urine (among others). The disease is diagnosed in the first days after a child’s birth [4,41].
- –
- Type I tyrosinemia—the urine of patients gives off an odor of rancid butter or rotten mushrooms. In tyrosinemia, tyrosine is not properly metabolized as a result of the lack of fumarylacetoacetate hydrolase and tyrosine aminotransferase. The accumulation of excess tyrosine and toxic metabolites causes liver and kidney damage. Tyrosinemia is a disease inherited in an autosomal recessive manner [42].
- –
- Hypermethioninemia is characterized by an excess of methionine in the blood of patients due to abnormal metabolism of this substance (mutations in the MAT1A, GNMT, or AHCY genes), as well as the smell of a cooked cabbage odor in their breath, sweat or urine. In many patients, hypermethioninemia may not produce visible symptoms; however, it can lead to tissue damage (e.g., liver, cerebellum) [34,43].
- –
- Isovaleric acidosis is a rare genetic metabolic disorder caused by a mutation in the IVD gene. The disease is characterized by, among other things, a “sweaty feet” odor secreted in the urine [44]. The disorder is based on the body’s inability to break down leucine, due to reduced or absent activity of one of the enzymes, isovaleryl-CoA dehydrogenase. As a result, harmful metabolic products (organic acids, mainly isovaleric acid) accumulate in the body of the affected child, damaging the nervous system and internal organs and causing serious health problems. The symptoms of isovaleric acidosis can range in severity from mild to life-threatening.
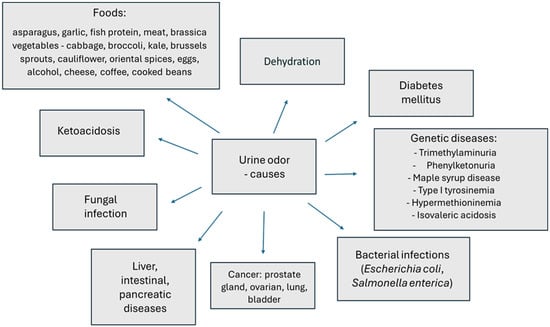
Figure 2.
Factors that can change odor in urine [12,30,31,32,33,34,36,37,38,39,40,41,42,43,44,244,245,246,247,248,251,252,253,254,255,257].
3.4. Changes in the Specific Gravity of Urine
The specific gravity of urine (relative density of urine) depends on the amount of solutes and the amount of diuresis and renal tubular function (as well as kidney function, amount of fluids drunk, blood Ca and K concentrations, protein supply and sodium) [258,259,260]. In adults, normal urine weight ranges from 1.015–1.025 g/mL and is inversely proportional to fluid supply [258]. There are also data reporting that the specific gravity under physiological conditions is 1.001–1.035 g/mL. A large increase in the specific gravity of urine (>1.035 g/mL) may be due to excess of, among other things, glucose/protein/ethanol/radiological contrast (immediately after the examination)/excessive sweating/persistent vomiting/diarrhea and associated dehydration [47,52,73,112,113,114,115,116,259,261,262,263,264]. Each mg/dL of glucose in urine results in a 0.004 increase in urine specific gravity [262]. Each mg/dL of protein in urine results in a 0.003 increase in specific gravity and each 3 °C above the instrument calibration temperature results in a 0.001 increase in specific gravity [47,52,259,263].
The specific gravity of urine can increase due to the following:
- –
- A diet containing vegetables and fruits rich in potassium (bananas, potatoes, dried figs, apples, apricots, leafy vegetables, but to a much greater extent supplementation with potassium preparations) [265]. High urinary potassium concentrations can also indicate two conditions: too much potassium in the serum (hyperkalemia) or too much loss of this element by the kidneys as a result of kidney damage or diseases that disrupt their function.
- –
- Frequent consumption of meat, fish and dairy products [266,267].
- –
- Dehydration, fever (excessive sweating), diarrhea, persistent vomiting, proteinuria, glucosuria, certain drugs (e.g., mannitol, dextran) and urinary excretion of certain exogenous substances (ethanol) [262,268].
Regardless of the degree of hydration of the body, consistent persistence of the relative density of urine at around 1.010–1.012 is a sign of isosthenuria and indicates a complete loss of the ability of the kidneys to thicken and dilute urine [269].
The specific gravity of urine is lowered by the following:
- –
- Long-term low-protein diet [263].
- –
- Hypotonic conductance, hypothermia, alkalosis (increase in plasma pH), renal tubular insufficiency, endocrine disorders (e.g., uremia) [27,126,270].
- –
- Electrolyte disturbances—use of laxatives, frequent vomiting, use of diuretics (decrease in K, increase in serum Ca) [271,272].
- –
- Kidney diseases [273].
- –
- Adulteration of urine with various additives. This phenomenon can be considered both in vitro and in vivo [217,274,275]. In vivo adulteration occurs when a patient attempts to dilute the urine enough to bring the drug or drug concentrations below the detection limit, such as by drinking large amounts of fluids, herbal products or diuretics. For example, if the test shows creatinine values below the limit of quantification and a specific gravity of 1.004 g/mL, this indicates dilution of the urine [275]. In vitro adulteration, on the other hand, involves adding compounds to the urine sample that interfere with immunoassays or modify the structure of the molecule being tested so that it can no longer be detected [276,277,278,279,280]. Often used for this are vinegar, salt, bleach, fruit juices (grapefruit, lemon) or eye drops, and substances such as nitrites, hydrogen peroxide, pyridine or antiglutaric: glutaraldehyde [280,281]. The mechanisms of action of the aforementioned substances vary, depending on their physicochemical properties. For example, bleach has a direct effect on reagents and causes false-positive or false-negative results, depending on the immunoassay. Most adulterants are oxidants. They can react with, for example, a metabolite of tetrahydrocannabinol or with an antibody in an immunoassay, producing false-negative results in both the assay and gas chromatography–mass spectrometry (GC-MS) [280]. Glutaraldehyde is also used as a sterilizing or cleaning agent in hospitals, for example. It can cause false-negative results by reducing the optical density for detecting cannabis, amphetamines, methadone, opiates, cocaine and their metabolites by immunoassays. Finally, some adulterants can interfere with the extraction procedure itself [276].
3.5. Changes in the pH of the Urine
The urine reaction is a result of the diet used and the metabolic processes that take place in the kidneys, liver and lungs [217,282,283]. Urine pH ranges from 4.5 to 8; it is assumed that normal urine has a slightly acidic reaction, with pH = 5.5–6.0. Urine collected from the first morning micturition is usually slightly acidic (pH of about 6.5) [284]. The median pH of 24 h urine is about 6 [282]. This pH is the optimal for solubility of both uric acid and phosphate salts, which protect against the formation of stones in the urinary tract. At lower pH, the solubility of uric acid decreases, increasing the risk of crystals, while with an increase in pH above 6, the solubility of calcium phosphate decreases, increasing the risk of renal stones (as brushite and apatite). Thus, urine pH is an important trigger for the formation of various types of stones in the urinary tract. The modern diet is characterized by the ubiquity of sodium chloride. Our ancestors fed mainly on plant foods rich in potassium-alkaline salts. This generates a lot of health problems resulting from the dissonance of genetic conditions and the mismatch between modern menus.
A strongly acidic urine reaction can be caused by the following (among other things):
- –
- Protein-rich diet (especially meat-rich) [283,285,286,287].
- –
- Dehydration [22,110,114,115,116,122,261].
- –
- Starvation [283,288].
- –
- Systemic acidosis (ketoacidosis after ingestion of methanol or ethyl glycol), use of acidifying drugs, fever, some bacterial infections [283,289,290,291].
A strongly alkaline urine reaction can be caused by the following (among other things):
- –
- A vegetarian diet with lots of fruits and vegetables (low in protein) and citrus juices. [283,285,286,292]
- –
- Urinary tract infection with urease-containing bacteria (breakdown of urea to ammonia) [293,294].
- –
- Long (>2.5 h) storage of urine samples → accelerated bacterial growth, increased breakdown of amino acids and urea [201,258,284].
- –
- Use of certain medications (sodium bicarbonate, potassium citrate or acetazolamide) [295,296] kidney stones [292,297]. Bicarbonate intake, therefore, increases the buffering capacity of the body and has a strong alkalizing effect. Bicarbonate is a natural component of mineral water. A study of healthy subjects under standardized conditions showed a significant and sustained increase in urinary pH per day from 6.10 to 6.59 and citrate excretion from 3.045 to 4.554 mmol/24 h after consuming mineral water containing 3388 mg/L bicarbonate [292].
4. Conclusions
Testing a urine sample is usually the first stage in carrying out a general assessment before blood tests are needed. The composition of urine is variable and depends on a great many factors, such as the amounts and types of fluids ingested, food consumed and stimulants or drugs taken. It is essential to have knowledge of the factors that affect urine, including its color, clarity, odor and specific gravity. Well-standardized procedures for collecting, transporting, preparing and analyzing samples should become the basis for an effective diagnostic strategy in urinalysis. Although urinalysis is not recommended as a routine screening tool for the general population, in some cases it can alert the patients to abnormalities in the body and sensitize the medical staff for prompt/further medical action.
Author Contributions
D.S., search strategy, study quality assessment and data extraction, interpretation of data, drafting of the manuscript, manuscript writing; B.B.-K., search strategy supervision, manuscript writing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We thank Grażyna Sygitowicz for providing advice about this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Núñez, J.-F.M. The Normal Ageing Kidney—Morphology and Physiology. Rev. Clin. Gerontol. 2008, 18, 175–197. [Google Scholar] [CrossRef]
- Long, B.; Koyfman, A. The Emergency Department Diagnosis and Management of Urinary Tract Infection. Emerg. Med. Clin. North. Am. 2018, 36, 685–710. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, M.; Feng, W.; Xu, Z.; Wang, Y.; Shi, L.; Zhang, J. Alteration of Angiopoietin-Like Protein 4 Levels in Serum or Urine Correlate with Different Biochemical Markers in Hyperlipidemia-Related Proteinuria. Biomed Res. Int. 2020, 2020, 5281251. [Google Scholar] [CrossRef]
- Robarge, M.E.; Beever, J.E.; Lenz, S.D.; Lynch, C.J.; Wigle, W.L. Maple Syrup Urine Disease in a Central Indiana Hereford Herd. Case Rep. Vet. Med. 2015, 2015, e204037. [Google Scholar] [CrossRef]
- Basheer, A.; Mookkappan, S.; Shanmugham, V.; Natarajan, N.; Kulirankal, K. Black Coloured Urine Following Organophosphorus Poisoning: Report of Two Cases. Case Rep. Crit. Care 2014, 2014, e706021. [Google Scholar] [CrossRef]
- Perrier, E.T.; Buendia-Jimenez, I.; Vecchio, M.; Armstrong, L.E.; Tack, I.; Klein, A. Twenty-Four-Hour Urine Osmolality as a Physiological Index of Adequate Water Intake. Dis. Markers 2015, 2015, 231063. [Google Scholar] [CrossRef]
- Oyaert, M.; Delanghe, J. Progress in Automated Urinalysis. Ann. Lab. Med. 2019, 39, 15–22. [Google Scholar] [CrossRef]
- Stainer, V.; Jones, P.; Juliebø, S.Ø.; Beck, R.; Hawary, A. Chyluria: What Does the Clinician Need to Know? Ther. Adv. Urol. 2020, 12, 1756287220940899. [Google Scholar] [CrossRef]
- Perrier, E.T.; Bottin, J.H.; Vecchio, M.; Lemetais, G. Criterion Values for Urine-Specific Gravity and Urine Color Representing Adequate Water Intake in Healthy Adults. Eur. J. Clin. Nutr. 2017, 71, 561–563. [Google Scholar] [CrossRef]
- Schapira, M.; Manor, O.; Golan, N.; Kalo, D.; Mordehay, V.; Kirshenbaum, N.; Goldsmith, R.; Chefetz, B.; Paltiel, O. Involuntary Human Exposure to Carbamazepine: A Cross-Sectional Study of Correlates across the Lifespan and Dietary Spectrum. Environ. Int. 2020, 143, 105951. [Google Scholar] [CrossRef]
- Perrier, E.T.; Johnson, E.C.; McKenzie, A.L.; Ellis, L.A.; Armstrong, L.E. Urine Colour Change as an Indicator of Change in Daily Water Intake: A Quantitative Analysis. Eur. J. Nutr. 2016, 55, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Ramamoorthy, A.; Sadler, B.M.; van Hasselt, J.G.C.; Elassaiss-Schaap, J.; Kasichayanula, S.; Edwards, A.Y.; van der Graaf, P.H.; Zhang, L.; Wagner, J.A. Crowdsourced Asparagus Urinary Odor Population Kinetics. CPT Pharmacomet. Syst. Pharmacol. 2018, 7, 34–41. [Google Scholar] [CrossRef]
- Aycock, R.D.; Kass, D.A. Abnormal Urine Color. South. Med. J. 2012, 105, 43–47. [Google Scholar] [CrossRef]
- Kenefick, R.W.; Heavens, K.R.; Dennis, W.E.; Caruso, E.M.; Guerriere, K.I.; Charkoudian, N.; Cheuvront, S.N. Quantification of Chromatographic Effects of Vitamin B Supplementation in Urine and Implications for Hydration Assessment. J. Appl. Physiol. 2015, 119, 110–115. [Google Scholar] [CrossRef]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11, 1178646918802282. [Google Scholar] [CrossRef]
- Fernández, S.; Castro, P.; Nogué, S.; Nicolás, J.M. Acute Iron Intoxication: Change in Urine Color during Chelation Therapy with Deferoxamine. Intensive Care Med. 2014, 40, 104. [Google Scholar] [CrossRef]
- Clement, Z.; Ashford, M. Beeturia Mimicking Painless Hematuria. J. Curr. Surg. 2011, 1, 33–34. [Google Scholar]
- Wijma, R.A.; Huttner, A.; Koch, B.C.P.; Mouton, J.W.; Muller, A.E. Review of the Pharmacokinetic Properties of Nitrofurantoin and Nitroxoline. J. Antimicrob. Chemother. 2018, 73, 2916–2926. [Google Scholar] [CrossRef]
- Revollo, J.Y.; Lowder, J.C.; Pierce, A.S.; Twilla, J.D. Urine Discoloration Associated With Metronidazole: A Rare Occurrence. J. Pharm. Technol. 2014, 30, 54–56. [Google Scholar] [CrossRef]
- Sacks, A.; Davis, M.K. A Curious Case of Red-Brown Urine in a Child Taking Mesalamine. J. Pediatr. Gastroenterol. Nutr. 2013, 56, e38. [Google Scholar] [CrossRef]
- Thadani, H.; Deacon, A.; Peters, T. Diagnosis and Management of Porphyria. BMJ 2000, 320, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.; Corbett, J.; Forni, L.; Hooper, L.; Hughes, F.; Minto, G.; Moss, C.; Price, S.; Whyte, G.; Woodcock, T.; et al. A Multidisciplinary Consensus on Dehydration: Definitions, Diagnostic Methods and Clinical Implications. Ann. Med. 2019, 51, 232–251. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B. Fluid Overload. Front. Vet. Sci. 2021, 8, 668688. [Google Scholar] [CrossRef]
- Goh, K.P. Management of Hyponatremia. AFP 2004, 69, 2387–2394. [Google Scholar]
- Fortune, B.E.; Garcia-Tsao, G. Hypervolemic Hyponatremia: Clinical Significance and Management. Clin. Liver Dis. 2013, 2, 109–112. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Johnson, E.C. Water Intake, Water Balance, and the Elusive Daily Water Requirement. Nutrients 2018, 10, 1928. [Google Scholar] [CrossRef]
- Thornton, S.N. Thirst and Hydration: Physiology and Consequences of Dysfunction. Physiol. Behav. 2010, 100, 15–21. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Kopetschny, B.H.; Badenhorst, C.E. The Hydrating Effects of Hypertonic, Isotonic and Hypotonic Sports Drinks and Waters on Central Hydration During Continuous Exercise: A Systematic Meta-Analysis and Perspective. Sports Med. 2022, 52, 349–375. [Google Scholar] [CrossRef]
- Gandy, J. Water Intake: Validity of Population Assessment and Recommendations. Eur. J. Nutr. 2015, 54 (Suppl. S2), 11–16. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Y.-B. Intestinal Microbiota and Chronic Constipation. Springerplus 2016, 5, 1130. [Google Scholar] [CrossRef]
- Scheffler, L.; Sauermann, Y.; Heinlein, A.; Sharapa, C.; Buettner, A. Detection of Volatile Metabolites Derived from Garlic (Allium Sativum) in Human Urine. Metabolites 2016, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, L.; Sharapa, C.; Buettner, A. Quantification of Volatile Metabolites Derived From Garlic (Allium Sativum) in Human Urine. Front. Nutr. 2019, 6, 43. [Google Scholar] [CrossRef]
- Miller, N.B.; Beigelman, A.; Utterson, E.; Shinawi, M. Transient Massive Trimethylaminuria Associated with Food Protein-Induced Enterocolitis Syndrome. JIMD Rep. 2014, 12, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Mogilnicka, I.; Bogucki, P.; Ufnal, M. Microbiota and Malodor—Etiology and Management. Int. J. Mol. Sci. 2020, 21, 2886. [Google Scholar] [CrossRef]
- Simmons, T.L. What’s That Smell? A 10-Year-Old Female With a Strong Odor. Pediatr. Nurs. 2013, 39, 37–38. [Google Scholar]
- Unger, J. Measuring the Sweet Smell of Success in Diabetes Management. Ann. Transl. Med. 2014, 2, 119. [Google Scholar] [CrossRef]
- Kauffman, C.A. Candiduria. Clin. Infect. Dis. 2005, 41, S371–S376. [Google Scholar] [CrossRef]
- Yamazaki, H.; Fujieda, M.; Cashman, J.R.; Kamataki, T. Mild Trimethylaminuria Observed in a Japanese Cohort with Liver Damage. Am. J. Med. 2005, 118, 803–805. [Google Scholar] [CrossRef]
- Schmidt, A.C.; Leroux, J.-C. Treatments of Trimethylaminuria: Where We Are and Where We Might Be Heading. Drug Discov. Today 2020, 25, 1710–1717. [Google Scholar] [CrossRef]
- Van Spronsen, F.J.; Blau, N.; Harding, C.; Burlina, A.; Longo, N.; Bosch, A.M. Phenylketonuria. Nat. Rev. Dis. Primers 2021, 7, 36. [Google Scholar] [CrossRef]
- Blackburn, P.R.; Gass, J.M.; Vairo, F.P.E.; Farnham, K.M.; Atwal, H.K.; Macklin, S.; Klee, E.W.; Atwal, P.S. Maple Syrup Urine Disease: Mechanisms and Management. Appl. Clin. Genet. 2017, 10, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.R. The Genetic Tyrosinemias. Am. J. Med. Genet. C Semin. Med. Genet. 2006, 142C, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Barić, I.; Staufner, C.; Augoustides-Savvopoulou, P.; Chien, Y.-H.; Dobbelaere, D.; Grünert, S.C.; Opladen, T.; Petković Ramadža, D.; Rakić, B.; Wedell, A.; et al. Consensus Recommendations for the Diagnosis, Treatment and Follow-up of Inherited Methylation Disorders. J. Inherit. Metab. Dis. 2017, 40, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.M.; Lee, B.H.; Kim, G.-H.; Kim, Y.-M.; Choi, J.-H.; Yoo, H.-W. Chronic Intermittent Form of Isovaleric Aciduria in a 2-Year-Old Boy. Korean J. Pediatr. 2013, 56, 351–354. [Google Scholar] [CrossRef]
- Queremel Milani, D.A.; Jialal, I. Urinalysis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Miler, M.; Šimundić, A.-M. Low Level of Adherence to Instructions for 24-Hour Urine Collection among Hospital Outpatients. Biochem. Medica 2013, 23, 316–320. [Google Scholar] [CrossRef]
- Echeverry, G.; Hortin, G.L.; Rai, A.J. Introduction to Urinalysis: Historical Perspectives and Clinical Application. In The Urinary Proteome: Methods and Protocols; Rai, A.J., Ed.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2010; pp. 1–12. ISBN 978-1-60761-711-2. [Google Scholar]
- Magiorkinis, E.; Diamantis, A. The fascinating story of urine examination: From uroscopy to the era of microscopy and beyond. Diagn. Cytopathol. 2015, 43, 1020–1036. [Google Scholar]
- Chinegwundoh, F. Urine Sample Collection: Issues and a Solution. Trends Urol. Men’s Health 2018, 9, 16–18. [Google Scholar] [CrossRef]
- Meyer, T. Diagnostic Procedures to Detect Chlamydia trachomatis Infections. Microorganisms 2016, 4, 25. [Google Scholar] [CrossRef]
- Mangin, D.; Murdoch, D.; Wells, J.E.; Coughlan, E.; Bagshaw, S.; Corwin, P.; Chambers, S.; Toop, L. Chlamydia Trachomatis Testing Sensitivity in Midstream Compared with First-Void Urine Specimens. Ann. Fam. Med. 2012, 10, 50–53. [Google Scholar] [CrossRef]
- Hitzeman, N.; Greer, D.; Carpio, E. Office-Based Urinalysis: A Comprehensive Review. Am. Fam. Physician 2022, 106, 27–35. [Google Scholar]
- Kawamura, M.; Ohmoto, A.; Hashimoto, T.; Yagami, F.; Owada, M.; Sugawara, T. Second Morning Urine Method Is Superior to the Casual Urine Method for Estimating Daily Salt Intake in Patients with Hypertension. Hypertens. Res. 2012, 35, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.; Temple-Smith, M.; Sanci, L. Urine Sample Collection from Young Pre-Continent Children: Common Methods and the New Quick-Wee Technique. Br. J. Gen. Pract. 2019, 70, 42–43. [Google Scholar] [CrossRef]
- O’Brien, K.; Edwards, A.; Hood, K.; Butler, C.C. Prevalence of Urinary Tract Infection in Acutely Unwell Children in General Practice: A Prospective Study with Systematic Urine Sampling. Br. J. Gen. Pract. 2013, 63, e156–e164. [Google Scholar] [CrossRef]
- Boyd, C.; Wood, K.; Whitaker, D.; Ashorobi, O.; Harvey, L.; Oster, R.; Holmes, R.P.; Assimos, D.G. Accuracy in 24-Hour Urine Collection at a Tertiary Center. Rev. Urol. 2018, 20, 119–124. [Google Scholar]
- Résimont, G.; Gadisseur, R.; Lutteri, L.; Krzesinski, J.M.; Cavalier, E.; Delanaye, P. How I explore… a proteinuria. Rev. Med. Liege 2018, 73, 519–525. [Google Scholar]
- Iglesia, I.; Santaliestra-Pasías, A.M.; Bel-Serrat, S.; Sadalla-Collese, T.; Miguel-Berges, M.L.; Moreno, L.A. Fluid Consumption, Total Water Intake and First Morning Urine Osmolality in Spanish Adolescents from Zaragoza: Data from the HELENA Study. Eur. J. Clin. Nutr. 2016, 70, 541–547. [Google Scholar] [CrossRef]
- Suh, H.; Summers, L.G.; Seal, A.D.; Colburn, A.T.; Mauromoustakos, A.; Perrier, E.T.; Bottin, J.H.; Kavouras, S.A. Afternoon Urine Osmolality Is Equivalent to 24 h for Hydration Assessment in Healthy Children. Eur. J. Clin. Nutr. 2020, 74, 884–890. [Google Scholar] [CrossRef]
- Nabavizadeh, P.; Ghadermarzi, S.; Fakhri, M. A New Method to Make 24-Hour Urine Collection More Convenient: A Validity Study. Int. J. Nephrol. 2014, 2014, e718147. [Google Scholar] [CrossRef]
- Varma, P.P.; Sengupta, P.; Nair, R.K. Post Exertional Hematuria. Ren. Fail. 2014, 36, 701–703. [Google Scholar] [CrossRef]
- Alwis, U.S.; Delanghe, J.; Dossche, L.; Walle, J.V.; Van Camp, J.; Monaghan, T.F.; Roggeman, S.; Everaert, K. Could evening dietary protein intake play a role in nocturnal polyuria? J. Clin. Med. 2020, 9, 2532. [Google Scholar] [CrossRef]
- Weiner, I.D.; Mitch, W.E.; Sands, J.M. Urea and Ammonia Metabolism and the Control of Renal Nitrogen Excretion. Clin. J. Am. Soc. Nephrol. 2015, 10, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Domachevsky, L.; Grupper, M.; Shochat, T.; Adir, Y. Proteinuria on Dipstick Urine Analysis after Sexual Intercourse. BJU Int. 2006, 97, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Yanai, H.; Shukuya, K.; Chiba, H.; Kobayashi, K.; Matsuno, K. Effects of Midstream Collection and the Menstrual Cycle on Urine Particles and Dipstick Urinalysis among Healthy Females. Clin. Chem. 2003, 49, 188–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pernille, H.; Lars, B.; Marjukka, M.; Volkert, S.; Anne, H. Sampling of urine for diagnosing urinary tract infection in general practice—First-void or mid-stream urine? Scand. J. Prim. Health Care 2019, 37, 113–119. [Google Scholar] [CrossRef]
- Wile, D. Diuretyki: Przegląd. Ann. Clin. Biochem. 2012, 49, 419–431. [Google Scholar] [CrossRef]
- Moore, K.; Thompson, C.; Trainer, P. Disorders of Water Balance. Clin. Med. 2003, 3, 28–33. [Google Scholar] [CrossRef]
- Holm, A.; Aabenhus, R. Urine Sampling Techniques in Symptomatic Primary-Care Patients: A Diagnostic Accuracy Review. BMC Fam. Pract. 2016, 17, 72. [Google Scholar] [CrossRef]
- Cho, S.Y.; Hur, M. Advances in Automated Urinalysis Systems, Flow Cytometry and Digitized Microscopy. Ann. Lab. Med. 2019, 39, 1–2. [Google Scholar] [CrossRef]
- Tworek, J.A.; Wilkinson, D.S.; Walsh, M.K. The Rate of Manual Microscopic Examination of Urine Sediment. Arch. Pathol. Lab. Med. 2008, 132, 1868–1873. [Google Scholar]
- Urine Analysis: Physical Examination, and Interpretation (Part 3)—Labpedia.Net 2020. Available online: https://labpedia.net/urine-analysis-physical-examination-and-interpretations-part-3/ (accessed on 15 September 2024).
- Kavouras, S.A.; Johnson, E.C.; Bougatsas, D.; Arnaoutis, G.; Panagiotakos, D.B.; Perrier, E.; Klein, A. Validation of a Urine Color Scale for Assessment of Urine Osmolality in Healthy Children. Eur. J. Nutr. 2016, 55, 907–915. [Google Scholar] [CrossRef]
- Foot, C.L.; Fraser, J.F. Uroscopic Rainbow: Modern Matula Medicine. Postgrad. Med. J. 2006, 82, 126–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lixandru, M.; Foods That Change Urine Color. Nature Word. Available online: https://www.natureword.com/foods-that-change-urine-color/ (accessed on 15 September 2024).
- Koratala, A.; Chamarthi, G.; Segal, M.S. Not All That Is Red Is Blood: A Curious Case of Chromaturia. Clin. Case Rep. 2018, 6, 1179–1180. [Google Scholar] [CrossRef] [PubMed]
- Montasser, D.; Benyahia, M.; Zajjari, Y.; Kabbaj, D.; Alayoud, A.; Allam, M.; Oualim, Z. Acute Renal Failure in Favism Revealing Familial Glucose-6-Phosphate Dehydrogenase Deficiency. Indian. J. Nephrol. 2012, 22, 67–68. [Google Scholar]
- von Bergen, T.N.; Blount, M.A. Chronic Use of Chloroquine Disrupts the Urine Concentration Mechanism by Lowering cAMP Levels in the Inner Medulla. Am. J. Physiol. -Ren. Physiol. 2012, 303, F900–F905. [Google Scholar] [CrossRef][Green Version]
- Rehfuss, A.; Mahon, J.; Sorokin, I.; Smith, C.; Stein, B.S. Phenazopyridine: A Preoperative Way to Identify Ureteral Orifices. Urology 2018, 115, 36–38. [Google Scholar] [CrossRef]
- Kadian, S.; Chakraborty, S.; Vuppalapati, S.; Agrawal, S. Phenytoin-Induced Red Discoloration of Urine in a Pediatric Neurosurgical Patient: An Unusual Finding. J. Neuroanaesth. Crit. Care 2023, 10, 72–74. [Google Scholar]
- Rosenberg, J.W. Effect of Dilantin on Color of Urine. Urology 1982, 20, 226. [Google Scholar] [CrossRef]
- Lam, C.-W.; Wong, S.Y.J. A Case of Green Urine Due to a Traditional Chinese Medicine Containing Methylene Blue. N. Z. Med. J. 2010, 123, 71–76. [Google Scholar]
- Palanduz, A.; Gultekin, D.; Kayaalp, N. Follow-up of Compliance with Tuberculosis Treatment in Children: Monitoring by Urine Tests. Pediatr. Pulmonol. 2003, 36, 55–57. [Google Scholar] [CrossRef]
- Meng, Q.H.; Handy, B.; Wagar, E.A. It’s Not Easy Being Blue-Green. Ann. Lab. Med. 2013, 33, 457–458. [Google Scholar] [CrossRef]
- Cotten, S.W.; McCudden, C.R. What Is Your Guess? The Case of the Blue-Green Urine. Clin. Chem. 2011, 57, 646–647. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Guo, J.; Wnag, R.; Gou, Y. The effects of medical phenolphthalein on the urine analysis and distinctio. Henan Med. Res. 2003, 12, 145–147. [Google Scholar]
- O’Leary, J.C.; Li, Q.; Marinec, P.; Blair, L.J.; Congdon, E.E.; Johnson, A.G.; Jinwal, U.K.; Koren, J.; Jones, J.R.; Kraft, C.; et al. Phenothiazine-Mediated Rescue of Cognition in Tau Transgenic Mice Requires Neuroprotection and Reduced Soluble Tau Burden. Mol. Neurodegener. 2010, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Smeets, T.; van Hunsel, F. Red-Brown Urine Discolouration in Two Patients Taking Mesalamine. Drug Saf. Case Rep. 2016, 3, 6. [Google Scholar] [CrossRef][Green Version]
- Pinto, M.P.B. Green Urine—Understanding Its Importance. J. Lab. Med. 2018, 42, 213–214. [Google Scholar] [CrossRef]
- Pak, F. Green Urine: An Association with Metoclopramide. Nephrol. Dial. Transplant. 2004, 19, 2677. [Google Scholar] [CrossRef][Green Version]
- Gillett, M.J.; Burnett, J.R. Medications and Green Urine. Intern. Med. J. 2006, 36, 64–66. [Google Scholar] [CrossRef]
- Peter, C.; Hongwan, D.; Küpfer, A.; Lauterburg, B.H. Pharmacokinetics and Organ Distribution of Intravenous and Oral Methylene Blue. Eur. J. Clin. Pharmacol. 2000, 56, 247–250. [Google Scholar] [CrossRef]
- Bruce, T.A. Dark Urine Related to Metronidazole Therapy. JAMA 1971, 218, 1832. [Google Scholar]
- Viswanathan, S. Urine Bag as a Modern Day Matula. ISRN Nephrol. 2013, 2013, 215690. [Google Scholar] [CrossRef]
- Wilson, M.L.; Gaido, L. Laboratory Diagnosis of Urinary Tract Infections in Adult Patients. Clin. Infect. Dis. 2004, 38, 1150–1158. [Google Scholar] [CrossRef] [PubMed]
- Poeggeler, B.; Singh, S.K.; Pappolla, M.A. Tryptophan in Nutrition and Health. Int. J. Mol. Sci. 2022, 23, 5455. [Google Scholar] [CrossRef] [PubMed]
- Cescon, D.W.; Juurlink, D.N. Discoloration of Skin and Urine after Treatment with Hydroxocobalamin for Cyanide Poisoning. CMAJ 2009, 180, 251. [Google Scholar] [CrossRef][Green Version]
- Bonkovsky, H.L.; Kane, R.E.; Jones, D.P.; Galinsky, R.E.; Banner, B. Acute Hepatic and Renal Toxicity from Low Doses of Acetaminophen in the Absence of Alcohol Abuse or Malnutrition: Evidence for Increased Susceptibility to Drug Toxicity Due to Cardiopulmonary and Renal Insufficiency. Hepatology 1994, 19, 1141–1148. [Google Scholar]
- Shim, Y.-S.; Gil, H.-W.; Yang, J.-O.; Lee, E.-Y.; Kim, S.-H.; Hong, S.-Y. A Case of Green Urine after Ingestion of Herbicides. Korean J. Intern. Med. 2008, 23, 42–44. [Google Scholar] [CrossRef]
- Kento, F.; Naoya, I. Dark Urine in a Dialysis Patient Treated for a Bloodstream Infection. Kidney360 2022, 3, 582–583. [Google Scholar] [CrossRef]
- Gambichler, T.; Stücker, M.; Kerner, K.; Weiner, S.; Waldherr, R.; Altmeyer, P.; Kreuter, A. Acute Kidney Injury in a Patient with Melanuria, Diffuse Melanosis, and Metastatic Malignant Melanoma. Am. J. Clin. Dermatol. 2008, 9, 267–270. [Google Scholar] [CrossRef]
- Turiansky, G.W.; Levin, S.W. Bluish Patches on the Ears and Axillae with Dark Urine: Ochronosis and Alkaptonuria. Int. J. Dermatol. 2001, 40, 333–335. [Google Scholar] [CrossRef]
- Gasperini, S.; Stagi, S.; Gasperini, U.; Guerrini, R.; la Marca, G.; Donati, M.A. Orange-Colored Diapers as First Sign of Lesch-Nyhan Disease in an Asymptomatic Infant. Pediatr. Nephrol. 2010, 25, 2373–2374. [Google Scholar] [CrossRef][Green Version]
- Behzadi, P.; Behzadi, E.; Yazdanbod, H.; Aghapour, R.; Akbari Cheshmeh, M.; Salehian Omran, D. A Survey on Urinary Tract Infections Associated with the Three Most Common Uropathogenic Bacteria. Maedica 2010, 5, 111–115. [Google Scholar]
- Sakamoto, S.; Miyazawa, K.; Yasui, T.; Iguchi, T.; Fujita, M.; Nishimatsu, H.; Masaki, T.; Hasegawa, T.; Hibi, H.; Arakawa, T.; et al. Chronological Changes in Epidemiological Characteristics of Lower Urinary Tract Urolithiasis in Japan. Int. J. Urol. 2019, 26, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Alperin, M.; Burnett, L.; Lukacz, E.; Brubaker, L. The Mysteries of Menopause and Urogynecologic Health: Clinical and Scientific Gaps. Menopause 2019, 26, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Willis, G.C.; Tewelde, S.Z. The Approach to the Patient with Hematuria. Emerg. Med. Clin. N. Am. 2019, 37, 755–769. [Google Scholar] [CrossRef]
- Lopez, R.M.; Lund, D.C.; Tritsch, A.J.; Liebl, V. Relationship Between Pre- and Post-Exercise Body Mass Changes and Pre-Exercise Urine Color in Female Athletes. Front. Sports Act. Living 2022, 4, 791699. [Google Scholar] [CrossRef]
- Rehrer, N.J. Fluid and Electrolyte Balance in Ultra-Endurance Sport. Sports Med. 2001, 31, 701–715. [Google Scholar] [CrossRef]
- Maughan, R.J.; Leiper, J.B.; Shirreffs, S.M. Factors Influencing the Restoration of Fluid and Electrolyte Balance after Exercise in the Heat. Br. J. Sports Med. 1997, 31, 175–182. [Google Scholar] [CrossRef]
- Abbasi, I.S.; Lopez, R.M.; Kuo, Y.-T.; Shapiro, B.S. Efficacy of an Educational Intervention for Improving the Hydration Status of Female Collegiate Indoor-Sport Athletes. J. Athl. Train. 2021, 56, 829–835. [Google Scholar] [CrossRef]
- McKenzie, A.L.; Muñoz, C.X.; Armstrong, L.E. Accuracy of Urine Color to Detect Equal to or Greater Than 2% Body Mass Loss in Men. J. Athl. Train. 2015, 50, 1306–1309. [Google Scholar] [CrossRef]
- Hahn, R.G.; Waldréus, N. An Aggregate Urine Analysis Tool to Detect Acute Dehydration. Int. J. Sport. Nutr. Exerc. Metab. 2013, 23, 303–311. [Google Scholar]
- Armstrong, L.E.; Soto, J.A.; Hacker, F.T.; Casa, D.J.; Kavouras, S.A.; Maresh, C.M. Urinary Indices during Dehydration, Exercise, and Rehydration. Int. J. Sport Nutr. 1998, 8, 345–355. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Ganio, M.S.; Casa, D.J.; Lee, E.C.; McDermott, B.P.; Klau, J.F.; Jimenez, L.; Le Bellego, L.; Chevillotte, E.; Lieberman, H.R. Mild Dehydration Affects Mood in Healthy Young Women. J. Nutr. 2012, 142, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Bunn, D.K.; Abdelhamid, A.; Gillings, R.; Jennings, A.; Maas, K.; Millar, S.; Twomlow, E.; Hunter, P.R.; Shepstone, L.; et al. Water-Loss (Intracellular) Dehydration Assessed Using Urinary Tests: How Well Do They Work? Diagnostic Accuracy in Older People. Am. J. Clin. Nutr. 2016, 104, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Pringle, K.; Shah, S.P.; Umulisa, I.; Munyaneza, R.B.M.; Dushimiyimana, J.M.; Stegmann, K.; Musavuli, J.; Ngabitsinze, P.; Stulac, S.; Levine, A.C. Comparing the accuracy of the three popular clinical dehydration scales in children with diarrhea. Int. J. Emerg. Med. 2011, 4, 58. [Google Scholar] [PubMed]
- Geurts, D.; Steyerberg, E.W.; Moll, H.; Oostenbrink, R. How to Predict Oral Rehydration Failure in Children With Gastroenteritis. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 503–508. [Google Scholar] [CrossRef]
- Prisco, A.; Capalbo, D.; Guarino, S.; Del Giudice, E.M.; Marzuillo, P. How to Interpret Symptoms, Signs and Investigations of Dehydration in Children with Gastroenteritis. Arch. Dis. Child. Educ. Pract. 2021, 106, 114–119. [Google Scholar] [CrossRef]
- GBD Diarrhoeal Diseases Collaborators. Estimates of Global, Regional, and National Morbidity, Mortality, and Aetiologies of Diarrhoeal Diseases: A Systematic Analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef]
- El-Sharkawy, A.M.; Sahota, O.; Maughan, R.J.; Lobo, D.N. The pathophysiology of fluid and electrolyte balance in the older adult surgical patient. Clin. Nutr. 2014, 33, 6–13. [Google Scholar]
- El-Sharkawy, A.M.; Watson, P.; Neal, K.R.; Ljungqvist, O.; Maughan, R.J.; Sahota, O.; Lobo, D.N. Hydration and Outcome in Older Patients Admitted to Hospital (The HOOP Prospective Cohort Study). Age Ageing 2015, 44, 943–947. [Google Scholar] [CrossRef]
- Fortes, M.B.; Owen, J.A.; Raymond-Barker, P.; Bishop, C.; Elghenzai, S.; Oliver, S.J.; Walsh, N.P. Is This Elderly Patient Dehydrated? Diagnostic Accuracy of Hydration Assessment Using Physical Signs, Urine, and Saliva Markers. J. Am. Med. Dir. Assoc. 2015, 16, 221–228. [Google Scholar] [CrossRef]
- Schlanger, L.E.; Bailey, J.L.; Sands, J.M. Electrolytes in the aging. Adv. Chronic Kidney Dis. 2010, 17, 308–319. [Google Scholar]
- Shaheen, N.A.; Alqahtani, A.A.; Assiri, H.; Alkhodair, R.; Hussein, M.A. Public knowledge of dehydration and fluid intake practices: Variation by participants’ characteristics. BMC Public Health 2018, 18, 1346. [Google Scholar]
- Hew-Butler, T.; Collins, M.; Bosch, A.; Sharwood, K.; Wilson, G.; Armstrong, M.; Jennings, C.; Swart, J.; Noakes, T. Maintenance of Plasma Volume and Serum Sodium Concentration despite Body Weight Loss in Ironman Triathletes. Clin. J. Sport. Med. 2007, 17, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Knechtle, B.; Senn, O.; Imoberdorf, R.; Joleska, I.; Wirth, A.; Knechtle, P.; Rosemann, T. Maintained total body water content and serum sodium concentrations despite body mass loss in female ultra-runners drinking ad libitum during a 100 km race. Asia Pac. J. Clin. Nutr. 2010, 19, 83–90. [Google Scholar] [PubMed]
- Chamney, P.W.; Wabel, P.; Moissl, U.M.; Müller, M.J.; Bosy-Westphal, A.; Korth, O.; Fuller, N.J. A Whole-Body Model to Distinguish Excess Fluid from the Hydration of Major Body Tissues. Am. J. Clin. Nutr. 2007, 85, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Charra, B. Fluid Balance, Dry Weight, and Blood Pressure in Dialysis. Hemodial. Int. 2007, 11, 21–31. [Google Scholar] [CrossRef]
- Załuska, W.; Dzimira, S. Hypervolemia—Pathophysiology and clinical manifestation. Forum Nefrologiczne 2012, 5, 68–72. Available online: https://docplayer.pl/65999381-Przewodnienie-patofizjologia-i-klinika.html (accessed on 12 January 2023).
- Broll, M.; John, S. Hypernatremia. Med. Klin. Intensivmed. Notfmed. 2020, 115, 263–274. [Google Scholar] [CrossRef]
- Dornelles, M.; Dornelles, E.P.; Dornelles, L.P. Hypertonic Saline in ICU Setting: What Is Its Position? A Systematic Review and Empirical Analysis. Ain-Shams J. Anesthesiol. 2022, 14, 55. [Google Scholar] [CrossRef]
- Severs, D.; Rookmaaker, M.B.; Hoorn, E.J. Intravenous Solutions in the Care of Patients with Volume Depletion and Electrolyte Abnormalities. Am. J. Kidney Dis. 2015, 66, 147–153. [Google Scholar] [CrossRef]
- Ząbek, M.; Szpytma, T.; Gliński, B. Extreme Hyponatremia Due to the Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH) in a Patient with Small Cell Lung Cancer—A Case Report and Literature Review. Contemp. Oncol. 2005, 9, 312–315. [Google Scholar]
- Armstrong, L.E. Rehydration during Endurance Exercise: Challenges, Research, Options, Methods. Nutrients 2021, 13, 887. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Lee, J.A.; Oh, S.; Choi, C.W.; Kim, E.-K.; Kim, H.-S.; Kim, B.I.; Choi, J.-H. Risk Factors for Late-Onset Hyponatremia and Its Influence on Neonatal Outcomes in Preterm Infants. J. Korean Med. Sci. 2015, 30, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Farrell, D.J.; Bower, L. Fatal Water Intoxication. J. Clin. Pathol. 2003, 56, 803–804. [Google Scholar]
- Ramanujam, V.M.S.; Anderson, K.E.; Grady, J.J.; Nayeem, F.; Lu, L.-J.W. Riboflavin as an Oral Tracer for Monitoring Compliance in Clinical Research. Open Biomark. J. 2011, 2011, 1–7. [Google Scholar]
- George, N.; Vivekanandan, M.; Karnam, A.H.F. Reddish-Orange Discoloration of Urine Due to Uric Acid Crystalluria after Recurrent Seizures. Kidney Int. 2012, 81, 1281. [Google Scholar] [CrossRef][Green Version]
- Giordano, C.; Karasik, O.; King-Morris, K.; Asmar, A. Uric Acid as a Marker of Kidney Disease: Review of the Current Literature. Dis. Markers 2015, 2015, 382918. [Google Scholar] [CrossRef]
- Choi, H.K.; Gao, X.; Curhan, G. Vitamin C Intake and the Risk of Gout in Men: A Prospective Study. Arch. Intern. Med. 2009, 169, 502–507. [Google Scholar] [CrossRef]
- Juraschek, S.P.; Miller, E.R., III; Gelber, A.C. Effect of Oral Vitamin C Supplementation on Serum Uric Acid: A Meta-Analysis of Randomized Controlled Trials. Arthritis Care Res. 2011, 63, 1295–1306. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Appel, L.J.; Choi, M.J.; Gelber, A.C.; Charleston, J.; Norkus, E.P.; Miller, E.R., III. The Effects of Vitamin C Supplementation on Serum Concentrations of Uric Acid: Results of a Randomized Controlled Trial. Arthritis Rheum. 2005, 52, 1843–1847. [Google Scholar] [CrossRef]
- Stamp, L.K.; O’Donnell, J.L.; Frampton, C.; Drake, J.M.; Zhang, M.; Chapman, P.T. Clinically Insignificant Effect of Supplemental Vitamin C on Serum Urate in Patients With Gout: A Pilot Randomized Controlled Trial. Arthritis Rheum. 2013, 65, 1636–1642. [Google Scholar] [CrossRef]
- Massey, L.K.; Liebman, M.; Kynast-Gales, S.A. Ascorbate Increases Human Oxaluria and Kidney Stone Risk. J. Nutr. 2005, 135, 1673–1677. [Google Scholar] [CrossRef] [PubMed]
- Mégarbane, B.; Delahaye, A.; Goldgran-Tolédano, D.; Baud, F.J. Antidotal treatment of cyanide poisoning. J. Chin. Med. Assoc. 2003, 66, 193–203. [Google Scholar] [PubMed]
- Wainman, B.C.; Kesner, J.S.; Martin, I.D.; Meadows, J.W.; Krieg, E.F.; Nieboer, E.; Tsuji, L.J. Menstrual Cycle Perturbation by Organohalogens and Elements in the Cree of James Bay, Canada. Chemosphere 2016, 149, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Bessler, M.; Hiken, J. The Pathophysiology of Disease in Patients with Paroxysmal Nocturnal Hemoglobinuria. Hematology 2008, 2008, 104–110. [Google Scholar] [CrossRef]
- Deters, A.; Kulozik, A.E. Hemoglobinuria. In Practical Algorithms in Pediatric Hematology and Oncology; Karger AG: Basel, Switzerland, 2003. [Google Scholar]
- Dhaliwal, G.; Cornett, P.A.; Lawrence, M.; Tierney, J. Hemolytic Anemia. AFP 2004, 69, 2599–2607. [Google Scholar]
- Shih, A.W.Y.; McFarlane, A.; Verhovsek, M. Haptoglobin Testing in Hemolysis: Measurement and Interpretation. Am. J. Hematol. 2014, 89, 443–447. [Google Scholar] [CrossRef]
- Luzzatto, L. PNH from Mutations of Another PIG Gene. Blood 2013, 122, 1099–1100. [Google Scholar] [CrossRef][Green Version]
- Bosch, X.; Poch, E.; Grau, J.M. Rhabdomyolysis and Acute Kidney Injury. N. Engl. J. Med. 2009, 361, 62–72. [Google Scholar] [CrossRef]
- Banasik, M.; Kuźniar, J.; Kusztal, M.; Porazko, T.; Weyde, W.; Klinger, M. Myoglobinuria Caused by Exertional Rhabdomyolysis Misdiagnosed as Psychiatric Illness. Med. Sci. Monit. 2008, 14, CS1–CS4. [Google Scholar]
- Wei, C.-L.H.; Lee, D.-J.; Wu, C.-C. Doxorubicin-Associated Red-Colored Dialysate. Kidney Int. 2022, 101, 1091. [Google Scholar] [CrossRef]
- Shakya, P.; Sharma Nepal, A. Daunorubicin Induced Stevens-Johnson Syndrome: A Case Report. Clin. Case Rep. 2021, 9, e04475. [Google Scholar] [CrossRef] [PubMed]
- Mahamadou, D.; Hassane, D.M.; Zeinabou, M.T.M.; Aboubacar, I.; Osseini, A.; Harissou, A.; Abdoul-Aziz, G.; Ibrahim, A.; Maman Laminou, I.; Eric, A. A Report of Four Cases of Blackwater Fever after Quinine Treatment at Zinder National Hospital, Niger Republic. Case Rep. Infect. Dis. 2019, 2019, 2346087. [Google Scholar] [CrossRef] [PubMed]
- Cardwell, J.B. Red Urine Associated with Methyldopa TREATMENT. Lancet 1969, 2, 326. [Google Scholar] [CrossRef] [PubMed]
- Volney, G.; Tatusov, M.; Yen, A.C.; Karamyan, N. Naphthalene Toxicity: Methemoglobinemia and Acute Intravascular Hemolysis. Cureus 2018, 10, e3147. [Google Scholar] [CrossRef] [PubMed]
- Krishnamurthy, M.; Freedman, M.L. Complications of Anticoagulation with Heparin. AMA J. Ethics 2005, 7, 297–300. [Google Scholar] [CrossRef]
- Purvis, M.V.; Rooks, H.; Young Lee, J.; Longerich, S.; Kahn, S.A. Prehospital Hydroxocobalamin for Inhalation Injury and Cyanide Toxicity in the United States—Analysis of a Database and Survey of Ems Providers. Ann. Burn. Fire Disasters 2017, 30, 126–128. [Google Scholar]
- Ekawanti, A.; Krisnayanti, B.D. Effect of Mercury Exposure on Renal Function and Hematological Parameters among Artisanal and Small-Scale Gold Miners at Sekotong, West Lombok, Indonesia. J. Health Pollut. 2015, 5, 25–32. [Google Scholar] [CrossRef]
- Tsai, M.-T.; Huang, S.-Y.; Cheng, S.-Y. Lead Poisoning Can Be Easily Misdiagnosed as Acute Porphyria and Nonspecific Abdominal Pain. Case Rep. Emerg. Med. 2017, 2017, 9050713. [Google Scholar] [CrossRef]
- Jaghab, K.; Skodnek, K.B.; Padder, T.A. Munchausen’s Syndrome and Other Factitious Disorders in Children. Psychiatry 2006, 3, 46–55. [Google Scholar]
- Lee, S.W.H.; Chaiyakunapruk, N.; Lai, N.M. What G6PD-Deficient Individuals Should Really Avoid. Br. J. Clin. Pharmacol. 2017, 83, 211–212. [Google Scholar] [CrossRef]
- Borobia, M.; Villanueva-Saz, S.; Ruiz de Arcaute, M.; Fernández, A.; Verde, M.T.; González, J.M.; Navarro, T.; Benito, A.A.; Arnal, J.L.; De Las Heras, M.; et al. Copper Poisoning, a Deadly Hazard for Sheep. Animals 2022, 12, 2388. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.F.; Wasserman, J. Are Unexpected Positive Dipstick Urine Bilirubin Results Clinically Significant? A Retrospective Review. Lab. Med. 2014, 45, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Maj, J.; Jankowska-Konsur, A.; Gruber, J.; Wozniak, Z.; Nockowski, P.; Hryncewicz-Gwózdz, A. Diffuse Melanosis Cutis Related to Dermal Micrometastases as the First Clinical Symptom of Distant Metastatic Malignant Melanoma: Case Report. Medicine 2017, 96, e6470. [Google Scholar] [CrossRef] [PubMed]
- Yoon, E.; Babar, A.; Choudhary, M.; Kutner, M.; Pyrsopoulos, N. Acetaminophen-Induced Hepatotoxicity: A Comprehensive Update. J. Clin. Transl. Hepatol. 2016, 4, 131–142. [Google Scholar] [CrossRef]
- Koubaissi, S.A.; Al Assaad, R.G.; Itani, Z.; Bouakl, I. Black Urine and Methemoglobinemia in the Setting of Sepsis Due to Clostridium Perfringens. Clin. Med. Insights Case Rep. 2020, 13, 1179547620981894. [Google Scholar] [CrossRef]
- Liu, S.-W.; Lin, C.-C.; How, C.-K. A Man With Black Urine. Ann. Emerg. Med. 2009, 53, 843. [Google Scholar] [CrossRef]
- Manassaram, D.M.; Backer, L.C.; Messing, R.; Fleming, L.E.; Luke, B.; Monteilh, C.P. Nitrates in Drinking Water and Methemoglobin Levels in Pregnancy: A Longitudinal Study. Environ. Health 2010, 9, 60. [Google Scholar] [CrossRef]
- Annamalai, A.K.; Gurnell, M. Black Urine—Alkaptonuria. QJM An. Int. J. Med. 2022, 115, 397–398. [Google Scholar] [CrossRef]
- Altmann, P.; Mansell, M.A. Black Urine. Postgrad. Med. J. 1980, 56, 877–878. [Google Scholar] [CrossRef]
- Seak, C.-K.; Lin, C.-C.; Seak, C.-J.; Hsu, T.-Y.; Chang, C.-C. A Case of Black Urine and Dark Skin—Cresol Poisoning. Clin. Toxicol. 2010, 48, 959–960. [Google Scholar] [CrossRef]
- Baraka, A.S.; Ayoub, C.M.; Yazbeck-Karam, V.; Kaddoum, R.N.; Gerges, F.J.; Hadi, U.M.; Dagher, C.M. Prophylactic Methylene Blue in a Patient with Congenital Methemoglobinemia. Can. J. Anaesth. 2005, 52, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Bradberry, S.M. Occupational Methaemoglobinaemia. Mechanisms of Production, Features, Diagnosis and Management Including the Use of Methylene Blue. Toxicol. Rev. 2003, 22, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Crossley, K.B. Methylene Blue—A Therapeutic Dye for All Seasons? J. Chemother. 2002, 14, 431–443. [Google Scholar] [CrossRef]
- Schirmer, R.H.; Coulibaly, B.; Stich, A.; Scheiwein, M.; Merkle, H.; Eubel, J.; Becker, K.; Becher, H.; Müller, O.; Zich, T.; et al. Methylene Blue as an Antimalarial Agent. Redox Rep. 2003, 8, 272–275. [Google Scholar] [CrossRef]
- Roldan, C.J.; Huh, B.; Song, J.; Nieto, Y.; Osei, J.; Chai, T.; Nouri, K.; Koyyalagunta, L.; Bruera, E. Methylene Blue for Intractable Pain from Oral Mucositis Related to Cancer Treatment: A Randomized Phase 2 Clinical Trial. BMC Med. 2022, 20, 377. [Google Scholar] [CrossRef]
- Komori, C.; Okada, K.; Kawamura, K.; Chida, S.; Suzuki, T. The Sonodynamic Antitumor Effect of Methylene Blue on Sarcoma180 Cells In Vitro. Anticancer. Res. 2009, 29, 2411–2415. [Google Scholar]
- Joel, A.B.; Mueller, M.D.; Pahira, J.J.; Mordkin, R.M. Nonvisualization of Intravenous Methylene Blue in Patients with Clinically Normal Renal Function. Urology 2001, 58, 607. [Google Scholar] [CrossRef]
- Yusim, Y.; Livingstone, D.; Sidi, A. Blue Dyes, Blue People: The Systemic Effects of Blue Dyes When Administered via Different Routes. J. Clin. Anesth. 2007, 19, 315–321. [Google Scholar] [CrossRef]
- Distelmaier, F.; Herebian, D.; Atasever, C.; Beck-Woedl, S.; Mayatepek, E.; Strom, T.M.; Haack, T.B. Blue Diaper Syndrome and PCSK1 Mutations. Pediatrics 2018, 141, S501–S505. [Google Scholar] [CrossRef]
- Drummond, K.N.; Michael, A.F.; Ulstrom, R.A.; Good, R.A. The blue diaper syndrome: Familial hypercalcemia with nephrocalcinosis and indicanuria; a new familial disease, with definition of the metabolic abnormality. Am. J. Med. 1964, 37, 928–948. [Google Scholar] [CrossRef]
- Olejnickova, K.; Hola, V.; Ruzicka, F. Catheter-Related Infections Caused by Pseudomonas Aeruginosa: Virulence Factors Involved and Their Relationships. Pathog. Dis. 2014, 72, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.K.; Mirza, N. Elderly Woman with Orange Urine and Purple Hands; Elsevier Limited: Rochester, UK, 2008; Volume 83, p. 744. [Google Scholar]
- Demirdas, S.; Schröder, C.H. An Infant with Orange-Colored Urine. Pediatr. Nephrol. 2010, 25, 381. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moe, S.M. Disorders Involving Calcium, Phosphorus, and Magnesium. Prim. Care 2008, 35, 215–237. [Google Scholar] [CrossRef]
- Johnson, J.R.; Gajewski, A.; Lesse, A.J.; Russo, T.A. Extraintestinal Pathogenic Escherichia Coli as a Cause of Invasive Nonurinary Infections. J. Clin. Microbiol. 2003, 41, 5798–5802. [Google Scholar] [CrossRef]
- Mefod’ev, V.V.; Khokhliavina, R.M.; Kozlov, L.B. Etiology of purulent septic diseases and the antibiotic resistance of the isolated causative agents. Zh Mikrobiol. Epidemiol. Immunobiol. 2002, 119–120. [Google Scholar]
- Kumar, S.; Shankaregowda, S.A.; Choudhary, G.R.; Singla, K. Rare Presentation of Genitourinary Tuberculosis Masquerading as Renal Cell Carcinoma: A Histopathological Surprise. J. Clin. Imaging Sci. 2014, 4, 26. [Google Scholar] [CrossRef]
- Srivastava, S.; Tiwari, V.; Sen, M. Rare Cases of Filarial Chyluria in Children. IMCRJ 2022, 15, 135–139. [Google Scholar] [CrossRef]
- Knier, B.; Büschges-Seraphin, B.; Hilgers, K.F.; Amann, K.U.; Uder, M.; Eckardt, K.-U.; Jacobi, J. From Red to White Urine: A Patient’s Nightmare with a Rather Benign Outcome. BMC Nephrol. 2012, 13, 7. [Google Scholar] [CrossRef]
- Horner, K.B.; Sas, D.J. White Urine in an Asymptomatic Child. J. Pediatr. 2011, 159, 351. [Google Scholar] [CrossRef]
- Saraireh, M.; Gharaibeh, S.; Araydah, M.; Al Sharie, S.; Haddad, F.; Alrababah, A. Violet Discoloration of Urine: A Case Report and a Literature Review. Ann. Med. Surg. 2021, 68, 102570. [Google Scholar] [CrossRef]
- Kumar, R.; Devi, K.; Kataria, D.; Kumar, J.; Ahmad, I. Purple Urine Bag Syndrome: An Unusual Presentation of Urinary Tract Infection. Cureus 2021, 13, e16319. [Google Scholar] [CrossRef] [PubMed]
- Vetter, M.; Kaufmann, M.D.; Neurath, M.F.; Kremer, A.E. Purple Urine in a Patient after Recovery from a SARS-CoV-2 Infection. Int. J. Infect. Dis. 2021, 105, 472–473. [Google Scholar] [CrossRef] [PubMed]
- DeRon, N.; Legan, C. Purple Urine Bag Syndrome: A Rare and Surprising Clinical Presentation. Cureus 2023, 15, e33354. [Google Scholar]
- Kalsi, D.S.; Ward, J.; Lee, R.; Handa, A. Purple Urine Bag Syndrome: A Rare Spot Diagnosis. Dis. Markers 2017, 2017, 9131872. [Google Scholar] [CrossRef]
- DIAGNOSTYKA LABORATORYJNA. Available online: https://docplayer.pl/898742-Diagnostyka-laboratoryjna.html (accessed on 26 January 2023).
- Schumann, G.B.; Greenberg, N.F. Usefulness of Macroscopic Urinalysis as a Screening Procedure. A Preliminary Report. Am. J. Clin. Pathol. 1979, 71, 452–456. [Google Scholar] [CrossRef]
- Roth, S.; Renner, E.; Rathert, P. Microscopic Hematuria: Advances in Identification of Glomerular Dysmorphic Erythrocytes. J. Urol. 1991, 146, 680–684. [Google Scholar] [CrossRef]
- Nicolle, L.E. Asymptomatic Bacteriuria: Review and Discussion of the IDSA Guidelines. Int. J. Antimicrob. Agents 2006, 28 (Suppl. S1), S42–S48. [Google Scholar] [CrossRef]
- Ashraf, M.S.; Gaur, S.; Bushen, O.Y.; Chopra, T.; Chung, P.; Clifford, K.; Hames, E.; Hertogh, C.M.P.M.; Krishna, A.; Mahajan, D.; et al. Diagnosis, Treatment, and Prevention of Urinary Tract Infections in Post-Acute and Long-Term Care Settings: A Consensus Statement From AMDA’s Infection Advisory Subcommittee. J. Am. Med. Dir. Assoc. 2020, 21, 12–24.e2. [Google Scholar] [CrossRef]
- Burnett, J.R.; Sturdy, G.G.; Smith, S.J.; Ten, Y.; McComish, M.J. “Milky” Urine: A Case of Chyluria. Med. J. Aust. 2004, 180, 1–8. [Google Scholar]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Barberi, I.; Gitto, E. Milky Urine in a Premature Infant: Questions. Pediatr. Nephrol. 2014, 29, 2319. [Google Scholar] [CrossRef][Green Version]
- Hotta, O.; Yusa, N.; Kitamura, H.; Taguma, Y. Urinary Macrophages as Activity Markers of Renal Injury. Clin. Chim. Acta 2000, 297, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Becker, G.J.; Nicholls, K. Lipiduria—With special relevance to Fabry disease. Clin. Chem. Lab. Med. 2015, 53, 1465–1470. [Google Scholar]
- Braden, G.L.; Sanchez, P.G.; Fitzgibbons, J.P.; Stupak, W.J.; Germain, M.J. Urinary Doubly Refractile Lipid Bodies in Nonglomerular Renal Diseases. Am. J. Kidney Dis. 1988, 11, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.A.; Cuppage, F.E.; Grantham, J.J. Urinary Lipid Bodies in Polycystic Kidney Disease. Am. J. Kidney Dis. 1985, 5, 49–53. [Google Scholar] [CrossRef]
- Punj, J.; Anand, R.; Darlong, V.; Pandey, R. Milky Urine! A Cause for Concern? Indian. J. Anaesth. 2013, 57, 87–88. [Google Scholar] [CrossRef]
- Vera, M.; Molano, A.; Rodríguez, P. Turbid White Urine. NDT Plus 2010, 3, 45–47. [Google Scholar] [CrossRef]
- Gopala, S.K.; Joe, J. Effect of Calcium Content of Diet on Crystal Formation in Urine of Patients with Calcium Oxalate Stones: A Randomized Crossover Clinical Trial. Afr. J. Urol. 2021, 27, 124. [Google Scholar] [CrossRef]
- Fogazzi, G.B.; Verdesca, S.; Garigali, G. Urinalysis: Core Curriculum 2008. Am. J. Kidney Dis. 2008, 51, 1052–1067. [Google Scholar] [CrossRef]
- Delanghe, J.; Speeckaert, M. Preanalytical Requirements of Urinalysis. Biochem. Med. 2014, 24, 89–104. [Google Scholar] [CrossRef]
- Cook, J.D.; Strauss, K.A.; Caplan, Y.H.; Lodico, C.P.; Bush, D.M. Urine pH: The Effects of Time and Temperature after Collection. J. Anal. Toxicol. 2007, 31, 486–496. [Google Scholar] [CrossRef]
- Wang, L.; Ji, X.; Sun, G.; Qin, Y.; Gong, M.; Zhang, J.; Li, N.; Na, Y. Fungus Ball and Emphysematous Cystitis Secondary to Candida Tropicalis: A Case Report. Can. Urol. Assoc. J. 2015, 9, e683–e686. [Google Scholar] [CrossRef] [PubMed]
- Jonczyk, P.; Szczerba, K.; Kandefer, B.; Potempa, M.; Tynior, W.; Kajdaniuk, D. Rzadkie przypadki bezoarów w drogach moczowych u pacjentów diabetologicznych—przegląd doniesień kazuistycznych. Diabetol. Prakt. 2016, 2, 150–157. [Google Scholar]
- Najmabadi, S.; Schliep, K.C.; Simonsen, S.E.; Porucznik, C.A.; Egger, M.J.; Stanford, J.B. Cervical Mucus Patterns and the Fertile Window in Women without Known Subfertility: A Pooled Analysis of Three Cohorts. Hum. Reprod. 2021, 36, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Datcu, R.; Gesink, D.; Mulvad, G.; Montgomery-Andersen, R.; Rink, E.; Koch, A.; Ahrens, P.; Jensen, J.S. Bacterial Vaginosis Diagnosed by Analysis of First-Void-Urine Specimens. J. Clin. Microbiol. 2014, 52, 218–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naicker, D.; Ramsuran, V.; Naicker, M.; Dessai, F.; Giandhari, J.; Tinarwo, P.; Abbai, N. Strong Correlation between Urine and Vaginal Swab Samples for Bacterial Vaginosis. S. Afr. J. Infect. Dis. 2021, 36, 199. [Google Scholar] [CrossRef]
- Maeda, K.; Shigemura, K.; Yang, Y.; Nakano, Y.; Arakawa, S.; Fujisawa, M. A Case of Refractory Urethritis with Repeated Doctor Shopping. IJU Case Rep. 2022, 5, 129–131. [Google Scholar] [CrossRef]
- Meites, E.; Gaydos, C.A.; Hobbs, M.M.; Kissinger, P.; Nyirjesy, P.; Schwebke, J.R.; Secor, W.E.; Sobel, J.D.; Workowski, K.A. A Review of Evidence-Based Care of Symptomatic Trichomoniasis and Asymptomatic Trichomonas Vaginalis Infections. Clin. Infect. Dis. 2015, 61 (Suppl. S8), S837–S848. [Google Scholar] [CrossRef]
- Sherrard, J.; Wilson, J.; Donders, G.; Mendling, W.; Jensen, J.S. 2018 European (IUSTI/WHO) International Union against Sexually Transmitted Infections (IUSTI) World Health Organisation (WHO) Guideline on the Management of Vaginal Discharge. Int. J. STD AIDS 2018, 29, 1258–1272. [Google Scholar] [CrossRef]
- Khan, F.U.; Ihsan, A.U.; Khan, H.U.; Jana, R.; Wazir, J.; Khongorzul, P.; Waqar, M.; Zhou, X. Comprehensive Overview of Prostatitis. Biomed. Pharmacother. 2017, 94, 1064–1076. [Google Scholar] [CrossRef]
- Rabani, S.M.R. Gossypiboma (Retained Bladder Surgical Sponge): A Case Report. Armaghane Danesh Bimon. J. 2006, 11, 123–128. [Google Scholar]
- Wolfe, T.; Smith, C.; Jacob, R. Acute Bacterial Prostatitis in an Adolescent Patient Following Blunt Trauma. Taylor Fr. 2018, 31, 107–108. [Google Scholar] [CrossRef]
- Fünfstück, R.; Nicolle, L.E.; Hanefeld, M.; Naber, K.G. Urinary Tract Infection in Patients with Diabetes Mellitus. Clin. Nephrol. 2012, 77, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.E. Urinary Tract Infections in Patients with Diabetes Mellitus: Epidemiology, Pathogenesis and Treatment. Int. J. Antimicrob. Agents 2008, 31, 54–57. [Google Scholar] [CrossRef]
- Rossing, P.; Caramori, M.L.; Chan, J.C.N.; Heerspink, H.J.L.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. KDIGO 2022 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2022, 102, S1–S127. [Google Scholar] [CrossRef]
- Giesen, C.D.; Greeno, A.M.; Thompson, K.A.; Patel, R.; Jenkins, S.M.; Lieske, J.C. Performance of Flow Cytometry to Screen Urine for Bacteria and White Blood Cells Prior to Urine Culture. Clin. Biochem. 2013, 46, 810–813. [Google Scholar] [CrossRef]
- Nelson, D.B.; Cunningham, F.G.; Lindheimer, M.D. Sterile Pyuria. N. Engl. J. Med. 2015, 372, 2372–2373. [Google Scholar] [CrossRef]
- Jurado-Escobar, R.; Doña, I.; Bogas-Herrera, G.; Pérez-Sánchez, N.; Salas, M.; Laguna, J.J.; Muñoz-Cano, R.; Mayorga, C.; Torres, M.J.; Cornejo-García, J.A. Platelet-Adherent Leukocytes Associated With Cutaneous Cross-Reactive Hypersensitivity to Nonsteroidal Anti-Inflammatory Drugs. Front. Pharmacol. 2020, 11, 594427. [Google Scholar]
- Bates, B. Interpretation of Urinalysis and Urine Culture for UTI Treatment. Pharm. Fac. Scholarsh. 2013, 38, 65–68. [Google Scholar]
- Lee, J.; Mark, R.G.; Celi, L.A.; Danziger, J. Proton Pump Inhibitors Are Not Associated with Acute Kidney Injury in Critical Illness. J. Clin. Pharmacol. 2016, 56, 1500–1506. [Google Scholar] [CrossRef]
- Han, S.-R.; Kim, H.-J.; Yoo, R.N.; Shin, S.H.; Kim, G.; Cho, H.M.; Lee, S.-J.; Lee, H.-I. Enterovesical Fistula From Meckel Diverticulum. Ann. Coloproctol. 2021, 37, S1–S3. [Google Scholar] [CrossRef]
- Villegas, R.; Xiang, Y.-B.; Elasy, T.; Xu, W.-H.; Cai, H.; Cai, Q.; Linton, M.; Fazio, S.; Zheng, W.; Shu, X.-O. Purine-Rich Foods, Protein Intake, and the Prevalence of Hyperuricemia: The Shanghai Men’s Health Study. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, N. Dietary Factors and Hyperuricaemia. Curr. Pharm. Des. 2005, 11, 4133–4138. [Google Scholar] [CrossRef] [PubMed]
- Zgaga, L.; Theodoratou, E.; Kyle, J.; Farrington, S.M.; Agakov, F.; Tenesa, A.; Walker, M.; McNeill, G.; Wright, A.F.; Rudan, I.; et al. The Association of Dietary Intake of Purine-Rich Vegetables, Sugar-Sweetened Beverages and Dairy with Plasma Urate, in a Cross-Sectional Study. PLoS ONE 2012, 7, e38123. [Google Scholar] [CrossRef]
- Brewster, S.; Curtis, L.; Poole, R. Urine versus Blood Ketones. Pract. Diabetes 2017, 34, 13–15. [Google Scholar] [CrossRef]
- Gao, Q.; Lee, W.-Y. Urinary Metabolites for Urological Cancer Detection: A Review on the Application of Volatile Organic Compounds for Cancers. Am. J. Clin. Exp. Urol. 2019, 7, 232–248. [Google Scholar]
- Silva, C.L.; Passos, M.; Câmara, J.S. Solid Phase Microextraction, Mass Spectrometry and Metabolomic Approaches for Detection of Potential Urinary Cancer Biomarkers—A Powerful Strategy for Breast Cancer Diagnosis. Talanta 2012, 89, 360–368. [Google Scholar] [CrossRef]
- Khalid, T.; Aggio, R.; White, P.; De Lacy Costello, B.; Persad, R.; Al-Kateb, H.; Jones, P.; Probert, C.S.; Ratcliffe, N. Urinary Volatile Organic Compounds for the Detection of Prostate Cancer. PLoS ONE 2015, 10, e0143283. [Google Scholar] [CrossRef]
- Kumar, M.; Mandal, S.; Goel, A. Olfactory Detection of Prostate Cancer by Dogs Sniffing Urine: A Step Forward in Early Diagnosis. Indian. J. Urol. 2011, 27, 430. [Google Scholar]
- Willis, C.M.; Church, S.M.; Guest, C.M.; Cook, W.A.; McCarthy, N.; Bransbury, A.J.; Church, M.R.T.; Church, J.C.T. Olfactory Detection of Human Bladder Cancer by Dogs: Proof of Principle Study. BMJ 2004, 329, 712. [Google Scholar] [CrossRef]
- Grayson, K.; Gregory, E.; Khan, G.; Guinn, B.-A. Urine Biomarkers for the Early Detection of Ovarian Cancer—Are We There Yet? Biomark. Cancer 2019, 11, 1179299X19830977. [Google Scholar] [CrossRef]
- Shirasu, M.; Touhara, K. The scent of disease: Volatile organic compounds of the human body related to disease and disorder. J. Biochem. 2011, 150, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.; Nanda, R.; Chakraborty, T. Clinical Application of Volatile Organic Compound Analysis for Detecting Infectious Diseases. Clin. Microbiol. Rev. 2013, 26, 462–475. [Google Scholar] [CrossRef]
- Banday, K.M.; Pasikanti, K.K.; Chan, E.C.Y.; Singla, R.; Rao, K.V.S.; Chauhan, V.S.; Nanda, R.K. Use of Urine Volatile Organic Compounds to Discriminate Tuberculosis Patients from Healthy Subjects. Anal. Chem. 2011, 83, 5526–5534. [Google Scholar] [CrossRef]
- Mitchell, S.C. Asparagus, Urinary Odor, and 1,2-Dithiolane-4-Carboxylic Acid. Perspect. Biol. Med. 2013, 56, 341–351. [Google Scholar] [CrossRef]
- Pelchat, M.L.; Bykowski, C.; Duke, F.F.; Reed, D.R. Excretion and Perception of a Characteristic Odor in Urine after Asparagus Ingestion: A Psychophysical and Genetic Study. Chem. Senses 2011, 36, 9–17. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.-H.; Choi, S.O.; Sa, I.Y.; Oh, S.Y. Major Odorants Released as Urinary Volatiles by Urinary Incontinent Patients. Sensors 2013, 13, 8523–8533. [Google Scholar] [CrossRef]
- Nyenwe, E.A.; Kitabchi, A.E. The Evolution of Diabetic Ketoacidosis: An Update of Its Etiology, Pathogenesis and Management. Metabolism 2016, 65, 507–521. [Google Scholar] [CrossRef]
- He, X.; Slupsky, C.M. Metabolic Fingerprint of Dimethyl Sulfone (DMSO2) in Microbial-Mammalian Co-Metabolism. J. Proteome Res. 2014, 13, 5281–5292. [Google Scholar] [CrossRef]
- Pal, V.K.; Bandyopadhyay, P.; Singh, A. Hydrogen Sulfide in Physiology and Pathogenesis of Bacteria and Viruses. IUBMB Life 2018, 70, 393–410. [Google Scholar] [CrossRef]
- Visser, E.H.; Berkhout, D.J.C.; Singh, J.; Vermeulen, A.; Ashtiani, N.; De Boer, N.K.; van Wijk, J.A.E.; de Meij, T.G.; Bökenkamp, A. Smell—Adding a New Dimension to Urinalysis. Biosensors 2020, 10, 48. [Google Scholar] [CrossRef]
- Flasar, C. What Is Urine Specific Gravity? Nursing 2022 2008, 38, 14. [Google Scholar] [CrossRef]
- Newman, D.J.; Pugia, M.J.; Lott, J.A.; Wallace, J.F.; Hiar, A.M. Urinary Protein and Albumin Excretion Corrected by Creatinine and Specific Gravity. Clin. Chim. Acta 2000, 294, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Buaprasert, P.; Piyapaisarn, S.; Vanichkulbodee, A.; Kamsom, A.; Sri-on, J. Prevalence and Risk Factors of Hypertonic Dehydration among Older Patients Admitted to the Emergency Department: A Prospective Cross-Sectional Study. Geriatr. Gerontol. Int. 2021, 21, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, E.; Buyukhatipoglu, H.; Aktaran, S.; Geyik, R. The Value of Urine Specific Gravity in Detecting Diabetes Insipidus in a Patient with Uncontrolled Diabetes Mellitus: Urine Specific Gravity in Differential Diagnosis. J. Gen. Intern. Med. 2006, 21, C1–C2. [Google Scholar] [CrossRef] [PubMed]
- Tałałaj, M.; Paczyńska, M. Urine analysis with assessment of renal efficiency—An underestimated component of diagnostics in general practice. Postępy Nauk. Med. 2007, 4, 119–124. [Google Scholar]
- Cavanaugh, C.; Perazella, M.A. Urine Sediment Examination in the Diagnosis and Management of Kidney Disease: Core Curriculum 2019. Am J Kidney Dis 2019, 73, 258–272. [Google Scholar] [CrossRef] [PubMed]
- Jędrusik, P.; Symonides, B.; Wojciechowska, E.; Gryglas, A.; Gaciong, Z. Diagnostic Value of Potassium Level in a Spot Urine Sample as an Index of 24-Hour Urinary Potassium Excretion in Unselected Patients Hospitalized in a Hypertension Unit. PLoS ONE 2017, 12, e0180117. [Google Scholar] [CrossRef]
- Cuenca-Sánchez, M.; Navas-Carrillo, D.; Orenes-Piñero, E. Controversies Surrounding High-Protein Diet Intake: Satiating Effect and Kidney and Bone Health. Adv. Nutr. 2015, 6, 260–266. [Google Scholar] [CrossRef]
- Martin, W.F.; Cerundolo, L.H.; Pikosky, M.A.; Gaine, P.C.; Maresh, C.M.; Armstrong, L.E.; Bolster, D.R.; Rodriguez, N.R. Effects of Dietary Protein Intake on Indexes of Hydration. J. Am. Diet. Assoc. 2006, 106, 587–589. [Google Scholar] [CrossRef]
- Logan-Sprenger, H.M.; Spriet, L.L. The Acute Effects of Fluid Intake on Urine Specific Gravity and Fluid Retention in a Mildly Dehydrated State. J. Strength. Cond. Res. 2013, 27, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Castaño Bilbao, I.; Slon Roblero, M.F.; García-Fernández, N. Estudios de función renal: Función glomerular y tubular. Análisis De La Orina. Nefrol. 2009, 2, 17–30. [Google Scholar]
- Ferlin, M.L.S.; Sales, D.S.; Celini, F.P.M.; Martinelli Junior, C.E. Central Diabetes Insipidus: Alert for Dehydration in Very Low Birth Weight Infants during the Neonatal Period. A Case Report. Sao Paulo Med. J. 2014, 133, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Savoie, F.A.; Dion, T.; Asselin, A.; Goulet, E.D.B. Sodium-Induced Hyperhydration Decreases Urine Output and Improves Fluid Balance Compared with Glycerol- and Water-Induced Hyperhydration. Appl. Physiol. Nutr. Metab. 2015, 40, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mehler, P.S.; Walsh, K. Electrolyte and Acid-Base Abnormalities Associated with Purging Behaviors. Int. J. Eat. Disord. 2016, 49, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Nainggolan, G.; Soemarko, D.; Siregar, P.; Lydia, A.; Bardosono, S.; Prijanti, A.R.; Aulia, D. Diagnostic Role of Urine Specific Gravity to Detect Kidney Impairment on Heat-Exposed Workers in a Shoe Factory in Indonesia: A Cross-Sectional Study. BMJ Open 2021, 11, e047328. [Google Scholar] [CrossRef]
- Goggin, M.M.; Tann, C.-M.; Miller, A.; Nguyen, A.; Janis, G.C. Catching Fakes: New Markers of Urine Sample Validity and Invalidity. J. Anal. Toxicol. 2017, 41, 121–126. [Google Scholar] [CrossRef]
- Feldhammer, M.; Saitman, A.; Nguyen, L.; Milstid, B. Dilution of Urine Followed by Adulteration in an Attempt to Deceive the Laboratory. J. Anal. Toxicol. 2019, 43, e7–e9. [Google Scholar] [CrossRef]
- Feliu, C.; Cazaubon, Y.; Fouley, A.; Guillemin, H.; Gozalo, C.; Djerada, Z. A Slight Smell of Lemon. Ther. Drug Monit. 2017, 39, 205. [Google Scholar] [CrossRef]
- Peace, M.R.; Tarnai, L.D. Performance Evaluation of Three On-Site Adulterant Detection Devices for Urine Specimens. J. Anal. Toxicol. 2002, 26, 464–470. [Google Scholar] [CrossRef]
- Phan, H.M.; Yoshizuka, K.; Murry, D.J.; Perry, P.J. Drug Testing in the Workplace. Pharmacotherapy 2012, 32, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Burrows, D.L.; Nicolaides, A.; Rice, P.J.; Dufforc, M.; Johnson, D.A.; Ferslew, K.E. Papain: A Novel Urine Adulterant. J. Anal. Toxicol. 2005, 29, 275–295. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, A.H.; Bristol, B.; Sexton, K.; Cassella-McLane, G.; Holtman, V.; Hill, D.W. Adulteration of Urine by “Urine Luck. ” Clin. Chem. 1999, 45, 1051–1057. [Google Scholar] [PubMed]
- Tsai, S.-C.J.; ElSohly, M.A.; Dubrovsky, T.; Twarowska, B.; Towt, J.; Salamone, S.J. Determination of Five Abused Drugs in Nitrite-Adulterated Urine by Immunoassays and Gas Chromatography-Mass Spectrometry. J. Anal. Toxicol. 1998, 22, 474–480. [Google Scholar] [CrossRef][Green Version]
- Worcester, E.M.; Bergsland, K.J.; Gillen, D.L.; Coe, F.L. Mechanism for Higher Urine pH in Normal Women Compared with Men. Am. J. Physiol. -Ren. Physiol. 2018, 314, F623–F629. [Google Scholar] [CrossRef]
- Frassetto, L.; Morris, R.C.; Sellmeyer, D.E.; Todd, K.; Sebastian, A. Diet, Evolution and Aging—The Pathophysiologic Effects of the Post-Agricultural Inversion of the Potassium-to-Sodium and Base-to-Chloride Ratios in the Human Diet. Eur. J. Nutr. 2001, 40, 200–213. [Google Scholar] [CrossRef]
- Miszewska-Szyszkowska, D.; Durlik, M. Najczęstsze Błędy w Interpretacji Wyników Badania Ogólnego moczu. Med. Po Dyplomie 2012, 11, 1–6. Available online: https://podyplomie.pl/medycyna/10583,najczestsze-bledy-w-interpretacji-wynikow-badania-ogolnego-moczu (accessed on 10 January 2023).
- Welch, A.A.; Mulligan, A.; Bingham, S.A.; Khaw, K.-T. Urine pH Is an Indicator of Dietary Acid-Base Load, Fruit and Vegetables and Meat Intakes: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Population Study. Br. J. Nutr. 2008, 99, 1335–1343. [Google Scholar] [CrossRef]
- Pizzorno, J.; Frassetto, L.A.; Katzinger, J. Diet-induced acidosis: Is it real and clinically relevant? Br. J. Nutr. 2010, 103, 1185–1194. [Google Scholar] [CrossRef]
- Murakami, K.; Sasaki, S.; Takahashi, Y.; Uenishi, K. Japan Dietetic Students’ Study for Nutrition and Biomarkers Group Association between Dietary Acid-Base Load and Cardiometabolic Risk Factors in Young Japanese Women. Br. J. Nutr. 2008, 100, 642–651. [Google Scholar] [CrossRef]
- Yancy, W.S.; Olsen, M.K.; Dudley, T.; Westman, E.C. Acid-Base Analysis of Individuals Following Two Weight Loss Diets. Eur. J. Clin. Nutr. 2007, 61, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Kiwull-Schöne, H.; Kiwull, P.; Manz, F.; Kalhoff, H. Food Composition and Acid-Base Balance: Alimentary Alkali Depletion and Acid Load in Herbivores. J. Nutr. 2008, 138, 431S–434S. [Google Scholar] [CrossRef]
- Adeva, M.M.; Souto, G. Diet-Induced Metabolic Acidosis. Clin. Nutr. 2011, 30, 416–421. [Google Scholar] [CrossRef]
- Kozłowski, B.; Szczęśniak, A.; Włodarski, R.; Piekarz, T. Kwasica mleczanowa z pH < 6, 8—Zatrucie alkoholem niespożywczym czy kwasica towarzysząca leczeniu metforminą? Opis przypadku. Anestezjol. I Ratow. 2015, 9, 180–185. [Google Scholar]
- Siener, R. Can the manipulation of urinary ph by beverages assist with the prevention of stone recurrence? Urolithiasis 2016, 44, 51–56. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Chen, Y.-H.; Lu, P.-L.; Lin, W.-R.; Chen, T.-C.; Lin, C.-Y. Proteus Mirabilis Urinary Tract Infection and Bacteremia: Risk Factors, Clinical Presentation, and Outcomes. J. Microbiol. Immunol. Infect. 2012, 45, 228–236. [Google Scholar] [CrossRef]
- Ronald, A. The Etiology of Urinary Tract Infection: Traditional and Emerging Pathogens. Am. J. Med. 2002, 113 (Suppl. 1A), S14–S19. [Google Scholar] [CrossRef]
- Sterrett, S.P.; Penniston, K.L.; Wolf, J.S.; Nakada, S.Y. Acetazolamide Is an Effective Adjunct for Urinary Alkalization in Patients with Uric Acid and Cystine Stone Formation Recalcitrant to Potassium Citrate. Urology 2008, 72, 278–281. [Google Scholar] [CrossRef]
- Koff, S.G.; Paquette, E.L.; Cullen, J.; Gancarczyk, K.K.; Tucciarone, P.R.; Schenkman, N.S. Comparison between Lemonade and Potassium Citrate and Impact on Urine pH and 24-Hour Urine Parameters in Patients with Kidney Stone Formation. Urology 2007, 69, 1013–1016. [Google Scholar] [CrossRef]
- Wagner, C.A.; Mohebbi, N. Urinary pH and Stone Formation. J. Nephrol. 2010, 23 (Suppl. S16), S165–S169. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).