Resveratrol: A Multifaceted Guardian against Anxiety and Stress Disorders—An Overview of Experimental Evidence
Abstract
1. Introduction
2. Exploring the Neuroprotective Potential of Resveratrol
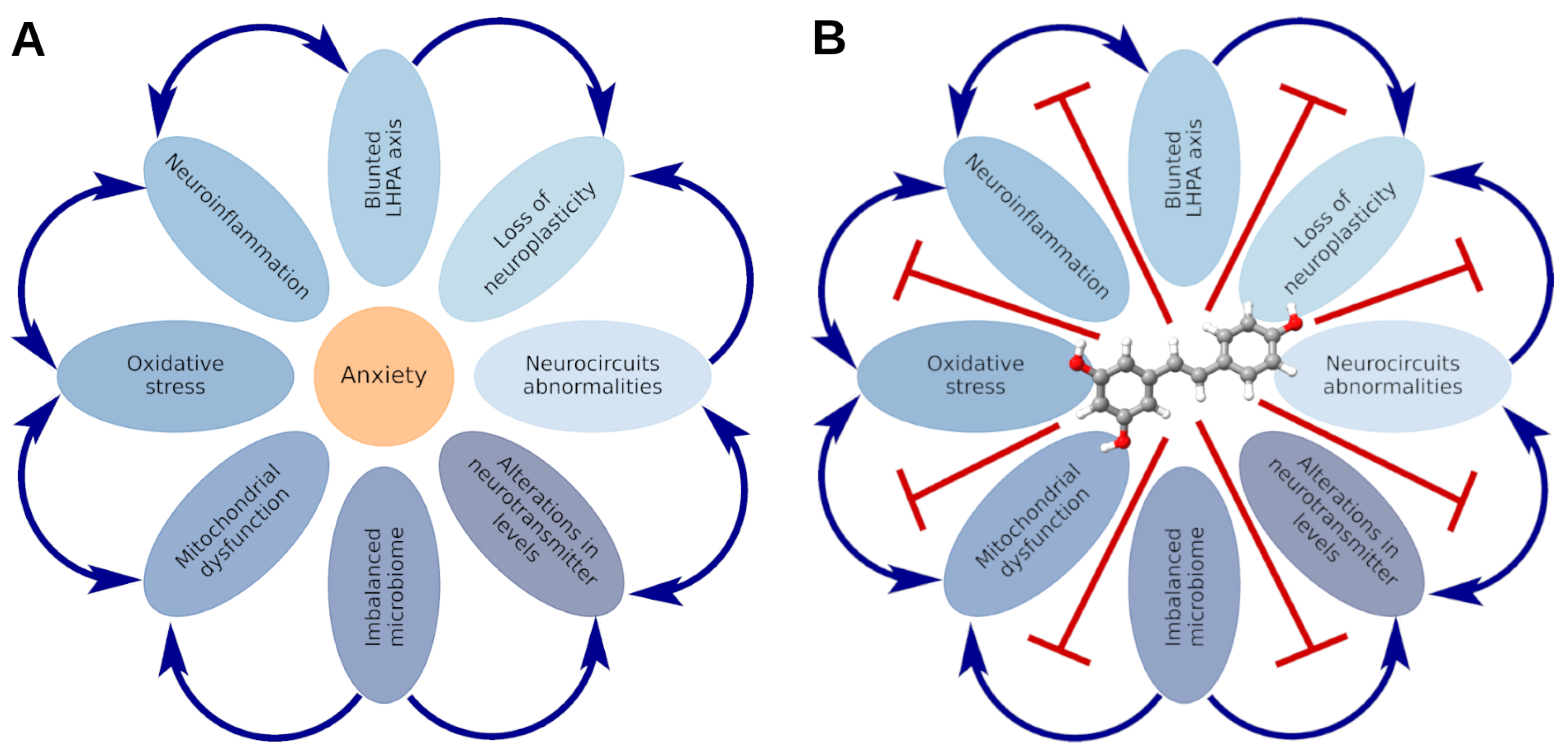
3. Stress-Related Anxiety Disorders: State of the Art
4. Abnormalities in Neuronal Circuits and Neurotransmitter Levels in the Pathogenesis of Stress-Related Anxiety Disorders and the Efficacy of RES in Their Correction
5. Aberrations in Cerebral Blood Flow in the Pathogenesis of Stress-Related Anxiety Disorders and the Efficacy of RES in Their Amelioration
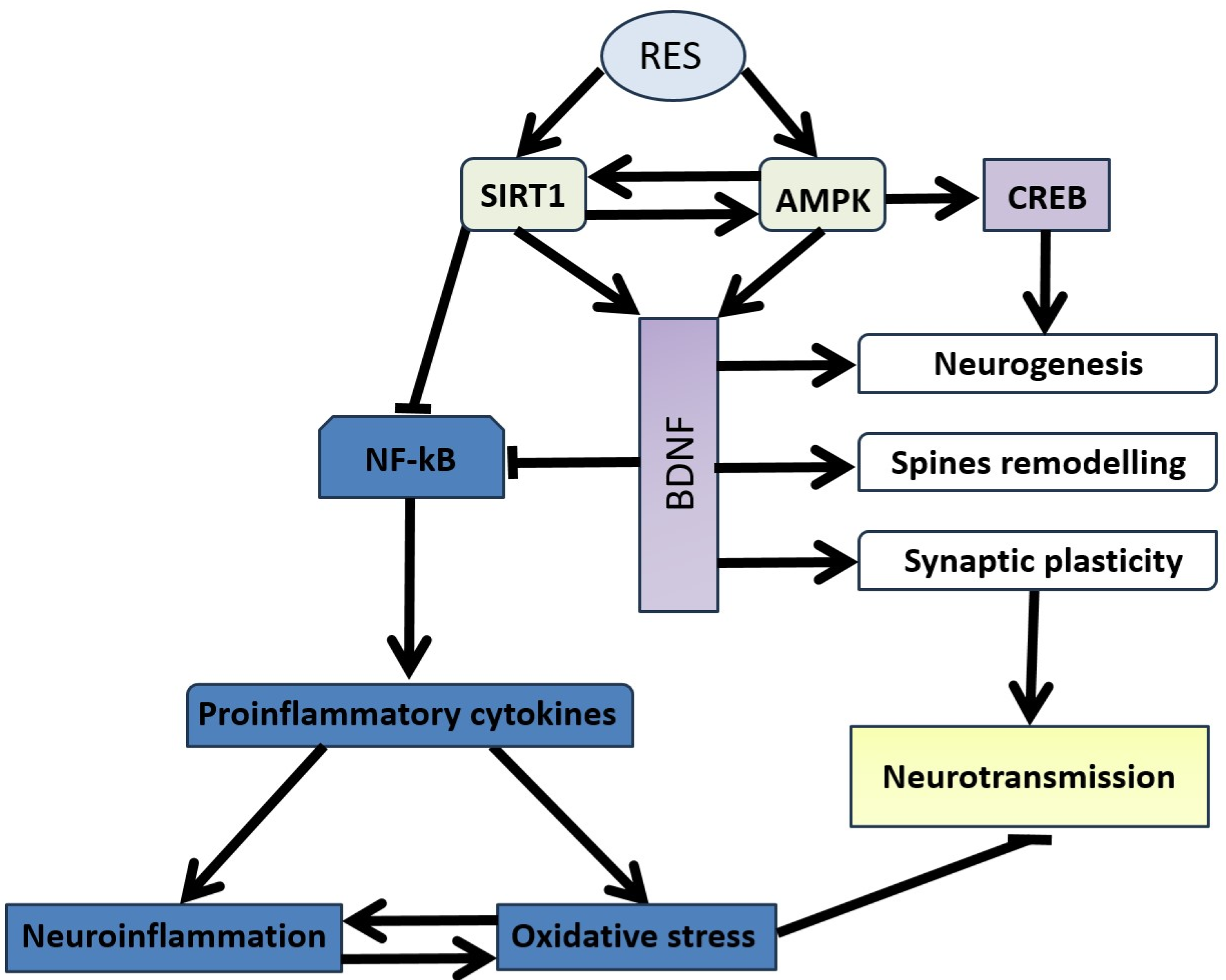
6. Neuronal Plasticity Abnormalities in the Pathogenesis of Stress-Related Anxiety Disorders and the Efficacy of Resveratrol in Correction
7. Peripheral Inflammation and Neuroinflammation in the Pathogenesis of Stress-Related Anxiety Disorders and the Efficacy of Resveratrol in Their Treatment
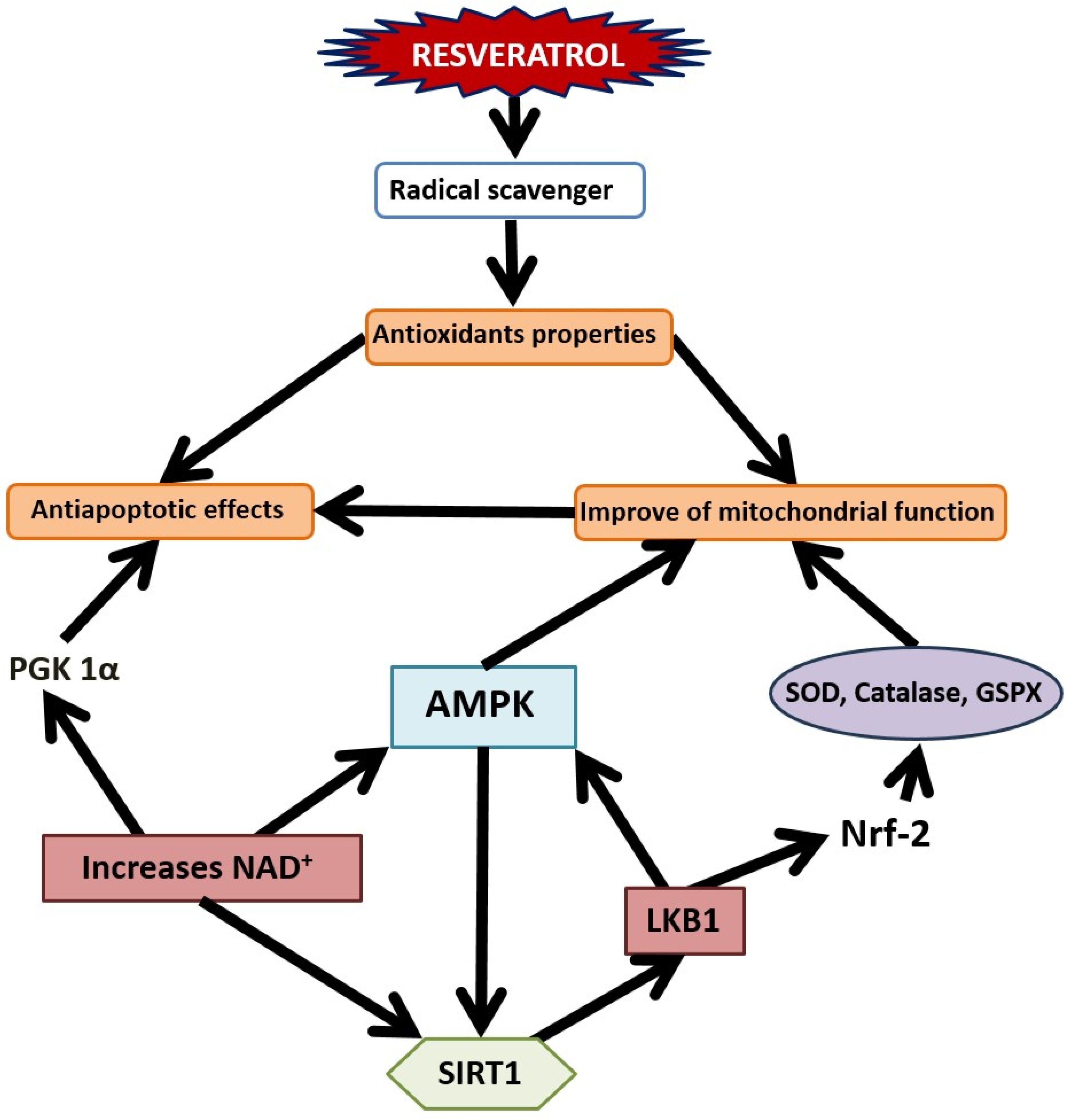
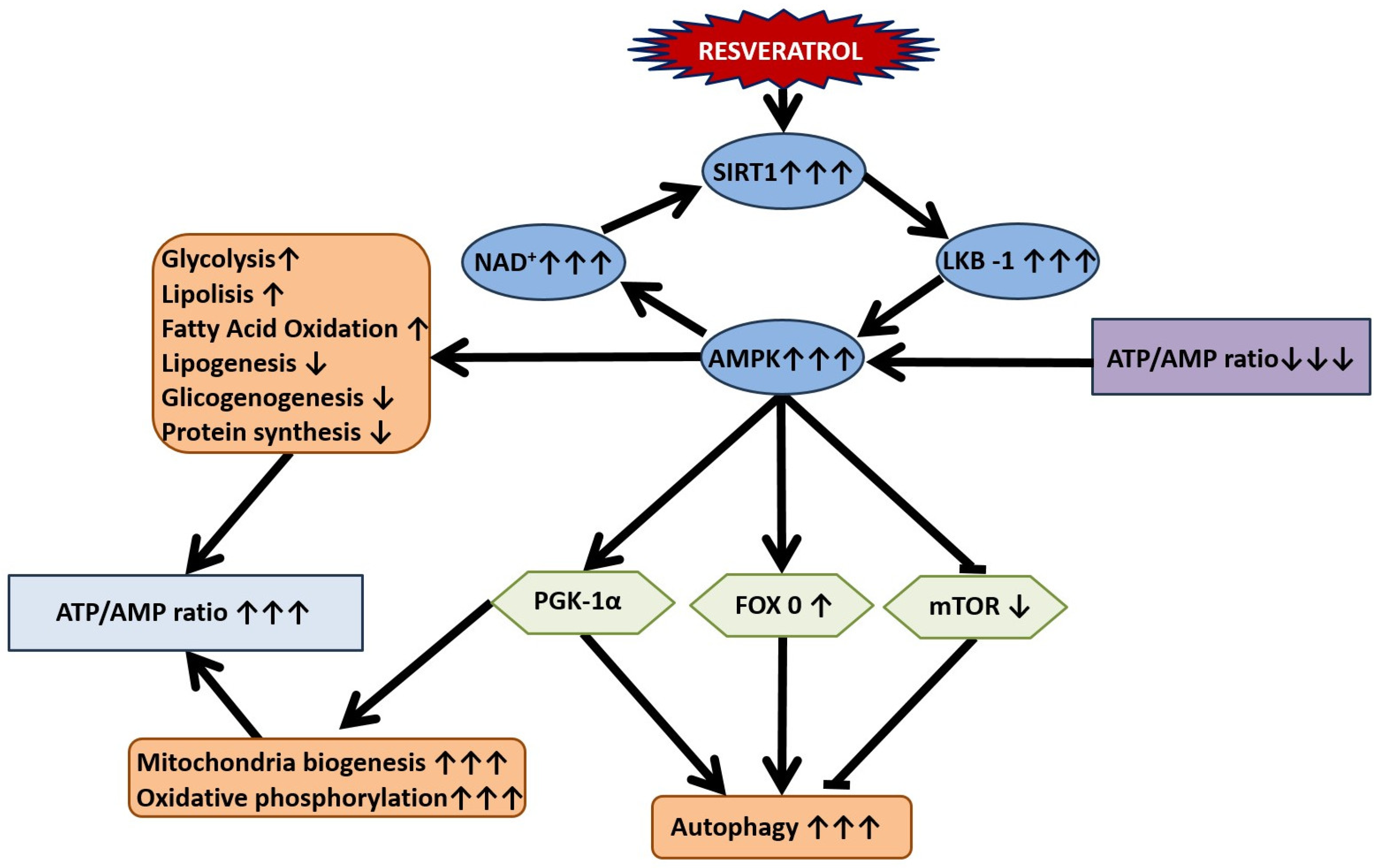
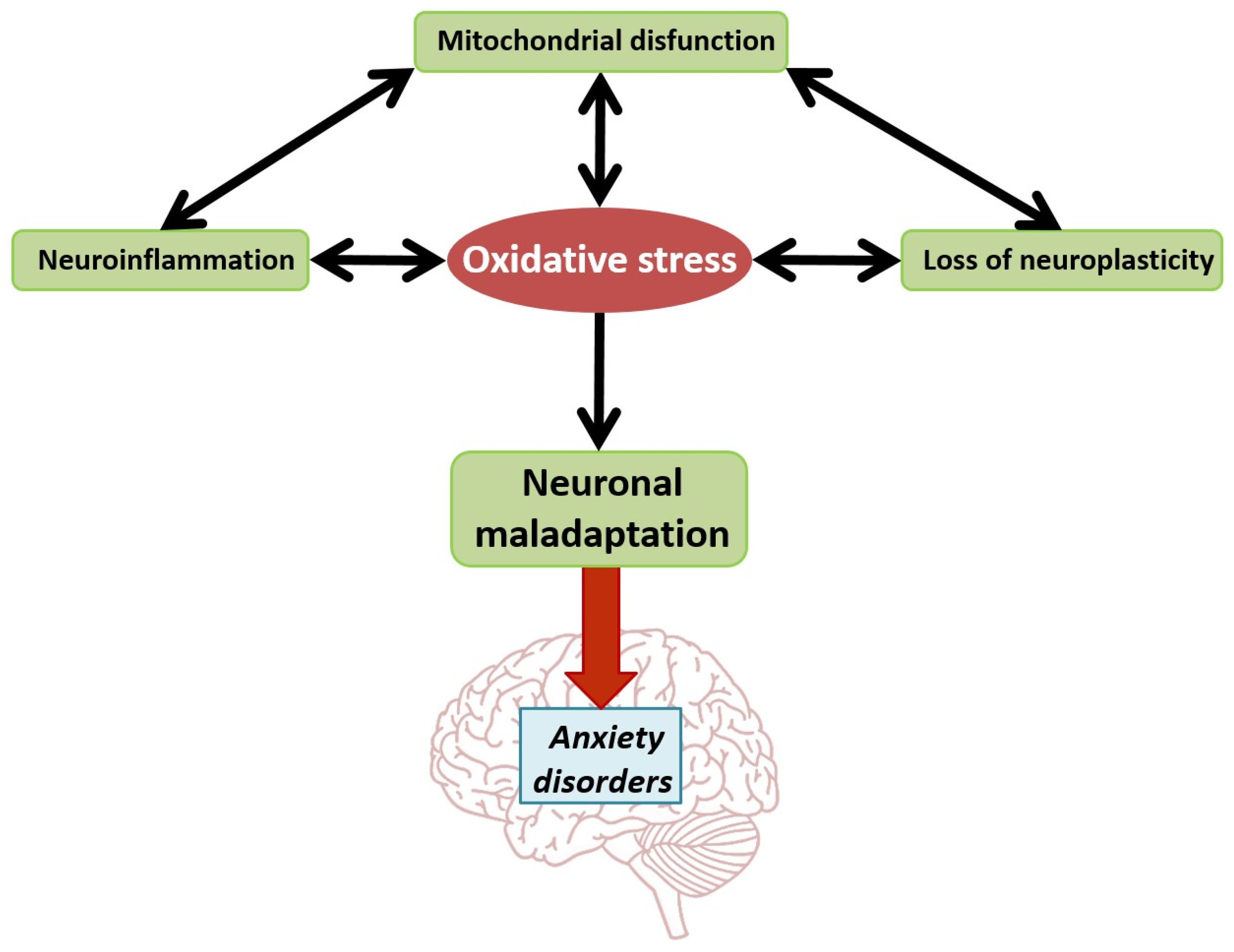
8. Mitochondrial Dysfunction in the Pathogenesis of Stress-Related Anxiety Disorders and the Efficacy of RES in Their Correction
9. Oxidative Stress: Implications in the Pathogenesis of Stress-Related Anxiety Disorders and the Therapeutic Potential of RES
10. Blunted LHPA Axis Function in the Pathogenesis of Stress-Related Anxiety Disorders and the Efficacy of RES in Their Correction
11. Abnormalities in the Gut–Brain and Liver–Brain Axes in the Pathogenesis of Stress-Related Anxiety Disorders and the Efficacy of Resveratrol in Their Correction
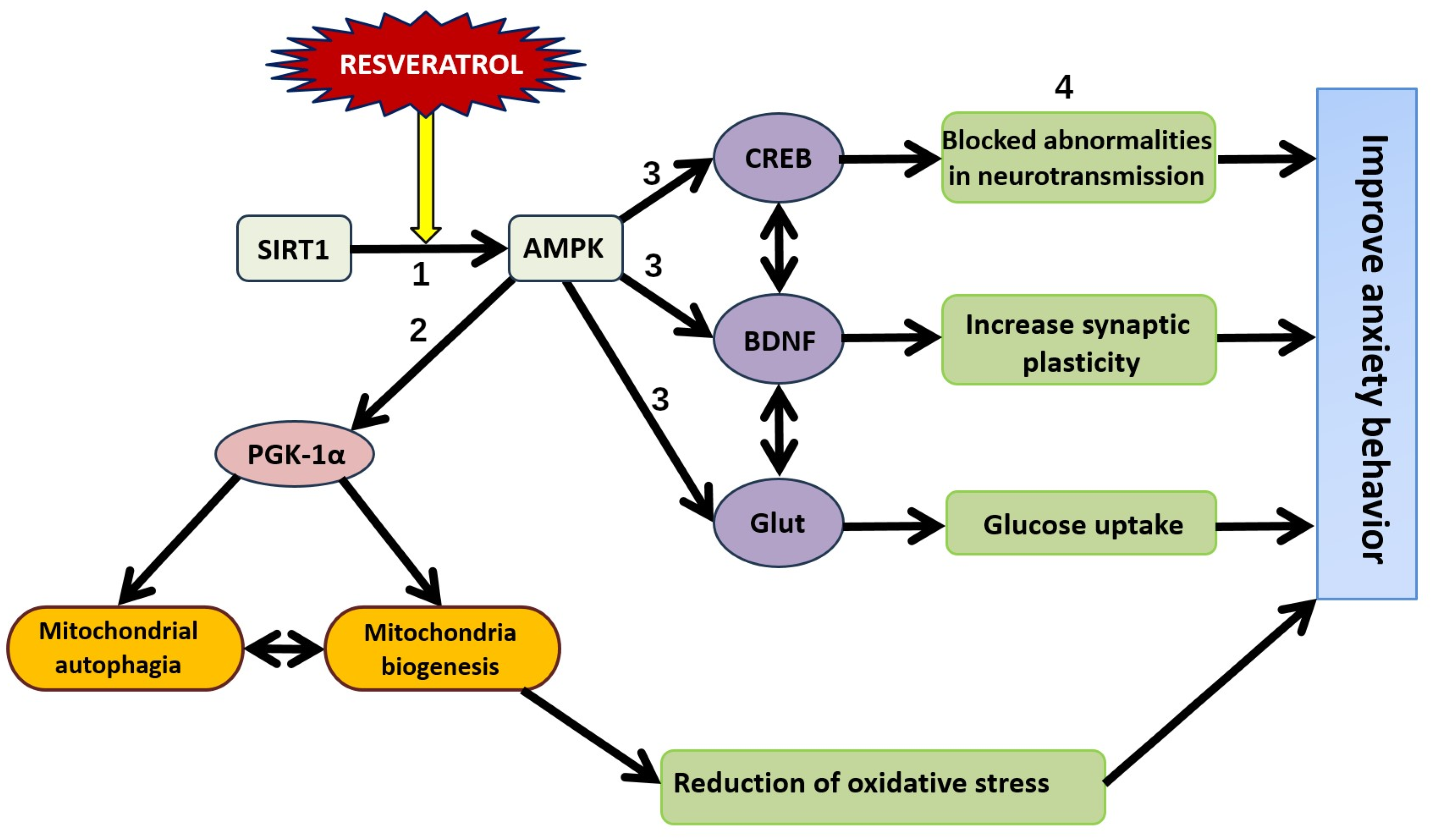
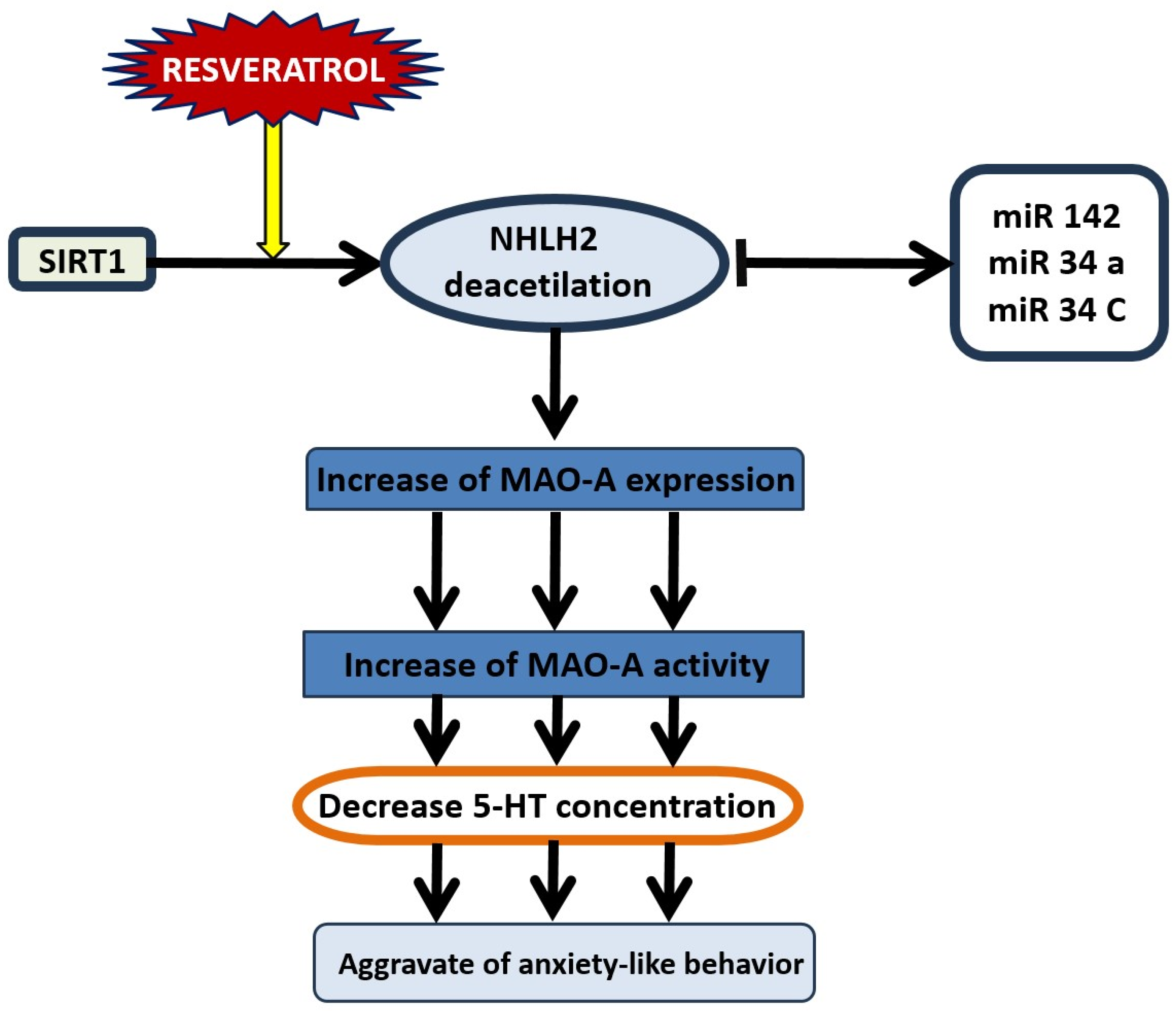
12. SIRT1-Dependent Pathways and the Dual Effect of Resveratrol on Anxiety Behavior
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Prakash, V.; Bose, C.; Sunilkumar, D.; Cherian, R.M.; Thomas, S.S.; Nair, B.G. Resveratrol as a Promising Nutraceutical: Implications in Gut Microbiota Modulation, Inflammatory Disorders, and Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 3370. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Samtiya, M.; Dhewa, T.; Mishra, V.; Aluko, R.E. Health benefits of polyphenols: A concise review. J. Food Biochem. 2022, 46, e14264. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef] [PubMed]
- Gugleva, V.; Zasheva, S.; Hristova, M.; Andonova, V. Topical use of resveratrol: Technological aspects. Pharmacia 2020, 67, 8994. [Google Scholar] [CrossRef]
- Chow, H.H.S.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol Modulates Drug- and Carcinogen-Metabolizing Enzymes in a Healthy Volunteer Study. Cancer Prev. Res. 2010, 3, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- VanElzakker, M.B.; Kathryn Dahlgren, M.; Caroline Davis, F.; Dubois, S.; Shin, L.M. From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol. Learn. Mem. 2014, 113, 3–18. [Google Scholar] [CrossRef]
- Johnson, L.R.; McGuire, J.; Lazarus, R.; Palmer, A.A. Pavlovian fear memory circuits and phenotype models of PTSD. Neuropharmacology 2012, 62, 638–646. [Google Scholar] [CrossRef]
- Tye, K.M. Neural Circuit Motifs in Valence Processing. Neuron 2018, 100, 436–452. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Chakraborty, M.; Chanda, A.; Alqahtani, T.; Kumer, A.; Dhara, B.; Chattopadhyay, M. Neuroendocrine and cellular mechanisms in stress resilience: From hormonal influence in the CNS to mitochondrial dysfunction and oxidative stress. J. Cell. Mol. Med. 2024, 28, 18220. [Google Scholar] [CrossRef]
- Morella, I.M.; Brambilla, R.; Morè, L. Emerging roles of brain metabolism in cognitive impairment and neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2022, 142, 104892. [Google Scholar] [CrossRef]
- Kaplan, G.B.; Dadhi, N.A.; Whitaker, C.S. Mitochondrial dysfunction in animal models of PTSD: Relationships between behavioral models, neural regions, and cellular maladaptation. Front. Physiol. 2023, 14, 1105839. [Google Scholar] [CrossRef]
- García-Bueno, B.; Caso, J.R.; Leza, J.C. Stress as a neuroinflammatory condition in brain: Damaging and protective mechanisms. Neurosci. Biobehav. Rev. 2008, 32, 1136–1151. [Google Scholar] [CrossRef]
- Zhao, W.; Spiers, J.G.; Vassileff, N.; Khadka, A.; Jaehne, E.J.; van den Buuse, M.; Hill, A.F. microRNA-146a modulates behavioural activity, neuroinflammation, and oxidative stress in adult mice. Mol. Cell. Neurosci. 2023, 124, 103820. [Google Scholar] [CrossRef]
- Schumpert, C.A.; Anderson, C.; Dudycha, J.L.; Patel, R.C. Involvement of Daphnia pulicaria Sir2 in regulating stress response and lifespan. Aging 2016, 8, 402–417. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, S.; Sun, J.; Li, Y. The neuroprotective effects of resveratrol preconditioning in transient global cerebral ischemia-reperfusion in mice. Turk. Neurosurg. 2015, 26, 550–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Porro, C.; Cianciulli, A.; Calvello, R.; Panaro, M. Reviewing the Role of Resveratrol as a Natural Modulator of Microglial Activities. Curr. Pharm. Des. 2015, 21, 5277–5291. [Google Scholar] [CrossRef] [PubMed]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef] [PubMed]
- Magnifico, S.; Saias, L.; Deleglise, B.; Duplus, E.; Kilinc, D.; Miquel, M.; Viovy, J.; Brugg, B.; Peyrin, J. NAD+ acts on mitochondrial SirT3 to prevent axonal caspase activation and axonal degeneration. FASEB J. 2013, 27, 4712–4722. [Google Scholar] [CrossRef]
- Renaud, J.; Bournival, J.; Zottig, X.; Martinoli, M.G. Resveratrol Protects DAergic PC12 Cells from High Glucose-Induced Oxidative Stress and Apoptosis: Effect on p53 and GRP75 Localization. Neurotox. Res. 2013, 25, 110–123. [Google Scholar] [CrossRef]
- Bureau, G.; Longpré, F.; Martinoli, M. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2007, 86, 403–410. [Google Scholar] [CrossRef]
- Sun, Q.; Kang, R.; Chen, K.; Liu, K.; Ma, Z.; Liu, C.; Deng, Y.; Liu, W.; Xu, B. Sirtuin 3 is required for the protective effect of Resveratrol on Manganese-induced disruption of mitochondrial biogenesis in primary cultured neurons. J. Neurochem. 2020, 156, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.; Geetha, T.; Broderick, T.; Babu, J. Resveratrol Protects β Amyloid-Induced Oxidative Damage and Memory Associated Proteins in H19-7 Hippocampal Neuronal Cells. Curr. Alzheimer Res. 2015, 12, 147–156. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Tang, F.; Guo, S.; Yang, Q.; Zhang, J. Resveratrol Treatment in Different Time-Attenuated Neuronal Apoptosis after Oxygen and Glucose Deprivation/Reoxygenation via Enhancing the Activation of Nrf-2 Signaling Pathway In Vitro. Cell Transplant. 2018, 27, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Yue, Q.; Guo, W.; Li, T.; Zhang, J.; Li, G.; Liu, Z.; Sun, J. Neuroprotection of resveratrol against neurotoxicity induced by methamphetamine in mouse mesencephalic dopaminergic neurons. BioFactors 2015, 41, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Moldzio, R.; Radad, K.; Krewenka, C.; Kranner, B.; Duvigneau, J.C.; Rausch, W.D. Protective effects of resveratrol on glutamate-induced damages in murine brain cultures. J. Neural Transm. 2013, 120, 1271–1280. [Google Scholar] [CrossRef]
- Alvira, D.; Yeste-Velasco, M.; Folch, J.; Verdaguer, E.; Canudas, A.; Pallàs, M.; Camins, A. Comparative analysis of the effects of resveratrol in two apoptotic models: Inhibition of complex I and potassium deprivation in cerebellar neurons. Neuroscience 2007, 147, 746–756. [Google Scholar] [CrossRef]
- Lin, C.H.; Nicol, C.J.; Cheng, Y.C.; Yen, C.; Wang, Y.S.; Chiang, M.C. Neuroprotective effects of resveratrol against oxygen glucose deprivation induced mitochondrial dysfunction by activation of AMPK in SH-SY5Y cells with 3D gelatin scaffold. Brain Res. 2020, 1726, 146492. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, R. Mammalian Sirt1: Insights on its biological functions. Cell Commun. Signal. 2011, 9, 11. [Google Scholar] [CrossRef]
- Gertz, M.; Nguyen, G.T.T.; Fischer, F.; Suenkel, B.; Schlicker, C.; Fränzel, B.; Tomaschewski, J.; Aladini, F.; Becker, C.; Wolters, D.; et al. A Molecular Mechanism for Direct Sirtuin Activation by Resveratrol. PLoS ONE 2012, 7, e49761. [Google Scholar] [CrossRef]
- Cao, D.; Wang, M.; Qiu, X.; Liu, D.; Jiang, H.; Yang, N.; Xu, R.M. Structural basis for allosteric, substrate-dependent stimulation of SIRT1 activity by resveratrol. Genes Dev. 2015, 29, 1316–1325. [Google Scholar] [CrossRef]
- Chen, M.; Tan, J.; Jin, Z.; Jiang, T.; Wu, J.; Yu, X. Research progress on Sirtuins (SIRTs) family modulators. Biomed. Pharmacother. 2024, 174, 116481. [Google Scholar] [CrossRef] [PubMed]
- Jęśko, H.; Wencel, P.; Strosznajder, R.P.; Strosznajder, J.B. Sirtuins and Their Roles in Brain Aging and Neurodegenerative Disorders. Neurochem. Res. 2016, 42, 876–890. [Google Scholar] [CrossRef] [PubMed]
- Kratz, E.M.; Sołkiewicz, K.; Kubis-Kubiak, A.; Piwowar, A. Sirtuins as Important Factors in Pathological States and the Role of Their Molecular Activity Modulators. Int. J. Mol. Sci. 2021, 22, 630. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Rooklin, D.; Fang, H.; Zhang, Y. Resveratrol serves as a protein-substrate interaction stabilizer in human SIRT1 activation. Sci. Rep. 2016, 6, 38186. [Google Scholar] [CrossRef]
- Ghosh, S.; Liu, B.; Zhou, Z. Resveratrol activates SIRT1 in a Lamin A-dependent manner. Cell Cycle 2013, 12, 872–876. [Google Scholar] [CrossRef]
- Cantó, C.; Auwerx, J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009, 20, 98–105. [Google Scholar] [CrossRef]
- Zeqiraj, E.; Filippi, B.M.; Deak, M.; Alessi, D.R.; van Aalten, D.M.F. Structure of the LKB1-STRAD-MO25 Complex Reveals an Allosteric Mechanism of Kinase Activation. Science 2009, 326, 1707–1711. [Google Scholar] [CrossRef]
- Amat, R.; Planavila, A.; Chen, S.L.; Iglesias, R.; Giralt, M.; Villarroya, F. SIRT1 Controls the Transcription of the Peroxisome Proliferator-activated Receptor-γ Co-activator-1α (PGC-1α) Gene in Skeletal Muscle through the PGC-1α Autoregulatory Loop and Interaction with MyoD. J. Biol. Chem. 2009, 284, 21872–21880. [Google Scholar] [CrossRef]
- Rius-Perez, S.; Torres-Cuevas, I.; Millan, I.; Ortega, A.L.; Perez, S. PGC-1α, Inflammation, and Oxidative Stress: An Integrative View in Metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 11452696. [Google Scholar] [CrossRef]
- Stein, S.C.; Woods, A.; Jones, N.A.; Davison, M.D.; Carling, D. The regulation of AMP-activated protein kinase by phosphorylation. Biochem. J. 2000, 345 Pt 3, 437–443. [Google Scholar] [CrossRef]
- Koronowski, K.B.; Khoury, N.; Saul, I.; Loris, Z.B.; Cohan, C.H.; Stradecki-Cohan, H.M.; Dave, K.R.; Young, J.I.; Perez-Pinzon, M.A. Neuronal SIRT1 (Silent Information Regulator 2 Homologue 1) Regulates Glycolysis and Mediates Resveratrol-Induced Ischemic Tolerance. Stroke 2017, 48, 3117–3125. [Google Scholar] [CrossRef] [PubMed]
- Price, N.; Gomes, A.; Ling, A.; Duarte, F.; Martin-Montalvo, A.; North, B.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.; et al. SIRT1 Is Required for AMPK Activation and the Beneficial Effects of Resveratrol on Mitochondrial Function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Ungurianu, A.; Zanfirescu, A.; Margină, D. Sirtuins, resveratrol and the intertwining cellular pathways connecting them. Ageing Res. Rev. 2023, 88, 101936. [Google Scholar] [CrossRef] [PubMed]
- Rasouri, S.; Lagouge, M.; Auwerx, J. SIRT1/PGC-1: Un axe neuroprotecteur? Méd. Sci. 2007, 23, 840–844. [Google Scholar] [CrossRef]
- Zhang, Z.; Fang, J.; Zhou, J.; Ding, F.; Zhou, G.; Zhao, X.; Zhuang, Z.; Lu, Y. Pterostilbene Attenuates Subarachnoid Hemorrhage-Induced Brain Injury through the SIRT1-Dependent Nrf2 Signaling Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 3550204. [Google Scholar] [CrossRef]
- Shayganfard, M. Molecular and biological functions of resveratrol in psychiatric disorders: A review of recent evidence. Cell Biosci. 2020, 10, 128. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, C.K.; LaVoie, H.A.; DiPette, D.J.; Singh, U.S. Resveratrol Restores Nrf2 Level and Prevents Ethanol-Induced Toxic Effects in the Cerebellum of a Rodent Model of Fetal Alcohol Spectrum Disorders. Mol. Pharmacol. 2011, 80, 446–457. [Google Scholar] [CrossRef]
- Lu, X.; Xu, H.; Sun, B.; Zhu, Z.; Zheng, D.; Li, X. Enhanced Neuroprotective Effects of Resveratrol Delivered by Nanoparticles on Hydrogen Peroxide-Induced Oxidative Stress in Rat Cortical Cell Culture. Mol. Pharm. 2013, 10, 2045–2053. [Google Scholar] [CrossRef]
- Pereira, T.C.B.; Rico, E.P.; Rosemberg, D.B.; Schirmer, H.; Dias, R.D.; Souto, A.A.; Bonan, C.D.; Bogo, M.R. Zebrafish as a Model Organism to Evaluate Drugs Potentially Able to Modulate Sirtuin Expression. Zebrafish 2011, 8, 9–16. [Google Scholar] [CrossRef]
- Peñalver, P.; Belmonte-Reche, E.; Adán, N.; Caro, M.; Mateos-Martín, M.L.; Delgado, M.; González-Rey, E.; Morales, J.C. Alkylated resveratrol prodrugs and metabolites as potential therapeutics for neurodegenerative diseases. Eur. J. Med. Chem. 2018, 146, 123–138. [Google Scholar] [CrossRef]
- Kumar, P.; Padi, S.S.; Naidu, P.S.; Kumar, A. Effect of resveratrol on 3-nitropropionic acid-induced biochemical and behavioural changes: Possible neuroprotective mechanisms. Behav. Pharmacol. 2006, 17, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Pu, C.; Yang, E.; Zhang, H.; Feng, Y.; Luo, P.; Yang, Y.; Zhang, L.; Li, X.; Jiang, X.; et al. Macrophage/Microglia Sirt3 Contributes to the Anti-inflammatory Effects of Resveratrol Against Experimental Intracerebral Hemorrhage in Mice. Cell. Mol. Neurobiol. 2023, 43, 2871–2882. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.G.; Yon, J.M.; Lin, C.; Jung, A.Y.; Jung, K.Y.; Nam, S.Y. Combined treatment with capsaicin and resveratrol enhances neuroprotection against glutamate-induced toxicity in mouse cerebral cortical neurons. Food Chem. Toxicol. 2012, 50, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Fonseca-Kelly, Z.; Callinan, C.; Zuo, L.; Sachdeva, M.M.; Shindler, K.S. SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front. Cell. Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef]
- William Raja, T.R.; Duraipandiyan, V.; Ignacimuthu, S.; Janakiraman, U.; Packiam, S.M. Role of Polyphenols in Alleviating Alzheimer’s Disease: A Review. Curr. Med. Chem. 2023, 30, 4032–4047. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, T.; Li, W.; Gao, N.; Zhang, T. Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol. Lett. 2018, 282, 100–108. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J.-Physiol.-Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [CrossRef]
- Pshenichnyuk, S.A.; Komolov, A.S. Dissociative Electron Attachment to Resveratrol as a Likely Pathway for Generation of the H2 Antioxidant Species Inside Mitochondria. J. Phys. Chem. Lett. 2015, 6, 1104–1110. [Google Scholar] [CrossRef]
- Pollicino, F.; Veronese, N.; Dominguez, L.J.; Barbagallo, M. Mediterranean diet and mitochondria: New findings. Exp. Gerontol. 2023, 176, 112165. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, J.; Wang, Y.; Hao, Q.; Chen, H.; Cheng, X. Sirt1 Regulates Oxidative Stress in Oxygen-Glucose Deprived Hippocampal Neurons. Front. Pediatr. 2020, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Xiong, H.; Chen, S.; Lai, L.; Yang, H.; Xu, Y.; Pang, J.; Su, Z.; Lin, H.; Zheng, Y. Modulation of miR-34a/SIRT1 signaling protects cochlear hair cells against oxidative stress and delays age-related hearing loss through coordinated regulation of mitophagy and mitochondrial biogenesis. Neurobiol. Aging 2019, 79, 30–42. [Google Scholar] [CrossRef]
- Wang, N.; Luo, Z.; Jin, M.; Sheng, W.; Wang, H.T.; Long, X.; Wu, Y.; Hu, P.; Xu, H.; Zhang, X. Exploration of age-related mitochondrial dysfunction and the anti-aging effects of resveratrol in zebrafish retina. Aging 2019, 11, 3117–3137. [Google Scholar] [CrossRef] [PubMed]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. SIRT1 Modulation of the Acetylation Status, Cytosolic Localization, and Activity of LKB1. J. Biol. Chem. 2008, 283, 27628–27635. [Google Scholar] [CrossRef]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; Auwerx, J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009, 458, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2017, 19, 121–135. [Google Scholar] [CrossRef]
- Chen, M.; Yan, R.; Luo, J.; Ning, J.; Zhou, R.; Ding, L. The Role of PGC-1α-Mediated Mitochondrial Biogenesis in Neurons. Neurochem. Res. 2023, 48, 2595–2606. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhu, J.X.; Xie, W.; Le, W.; Fan, Z.; Jankovic, J.; Pan, T. Resveratrol-Activated AMPK/SIRT1/Autophagy in Cellular Models of Parkinson’s Disease. Neurosignals 2011, 19, 163–174. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, T.; Dong, S.; Guo, Y.; Jankovic, J.; Xu, H.; Wu, Y. Rotenone affects p53 transcriptional activity and apoptosis via targeting SIRT1 and H3K9 acetylation in SH-SY5Y cells. J. Neurochem. 2015, 134, 668–676. [Google Scholar] [CrossRef]
- Pineda-Ramírez, N.; Alquisiras-Burgos, I.; Ortiz-Plata, A.; Ruiz-Tachiquín, M.E.; Espinoza-Rojo, M.; Aguilera, P. Resveratrol Activates Neuronal Autophagy Through AMPK in the Ischemic Brain. Mol. Neurobiol. 2019, 57, 1055–1069. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cantó, C. The molecular targets of resveratrol. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2015, 1852, 1114–1123. [Google Scholar] [CrossRef]
- Kanthasamy, K.; Gordon, R.; Jin, H.; Anantharam, V.; Ali, S.; Kanthasamy, G.A.; Kanthasamy, A. Neuroprotective Effect of Resveratrol Against Methamphetamine-Induced Dopaminergic Apoptotic Cell Death in a Cell Culture Model of Neurotoxicity. Curr. Neuropharmacol. 2011, 9, 49–53. [Google Scholar] [CrossRef]
- Renaud, J.; Martinoli, M.G. Resveratrol as a Protective Molecule for Neuroinflammation: A Review of Mechanisms. Curr. Pharm. Biotechnol. 2014, 15, 318–329. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Y.; Mueller-Steiner, S.; Chen, L.F.; Kwon, H.; Yi, S.; Mucke, L.; Gan, L. SIRT1 Protects against Microglia-dependent Amyloid-β Toxicity through Inhibiting NF-κB Signaling. J. Biol. Chem. 2005, 280, 40364–40374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yuan, L.; Zhang, Q.; Gao, Y.; Liu, G.; Xiu, M.; Wei, X.; Wang, Z.; Liu, D. Resveratrol attenuates hypoxia-induced neurotoxicity through inhibiting microglial activation. Int. Immunopharmacol. 2015, 28, 578–587. [Google Scholar] [CrossRef]
- Liu, J.; Liao, H.; Chen, Y.; Zhu, H.; Li, X.; Liu, J.; Xiang, Q.; Zeng, F.; Yang, Q. Resveratrol Inhibits Oxidative Stress and Regulates M1/M2-Type Polarization of Microglia via Mediation of the Nrf2/Shh Signaling Cascade after OGD/R Injury In Vitro. J. Pers. Med. 2022, 12, 2087. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Liu, Z.; Wei, J.; Lu, L.; Huang, Y.; Luo, L.; Xie, H. Protective effect of SIRT1 on toxicity of microglial-derived factors induced by LPS to PC12 cells via the p53-caspase-3-dependent apoptotic pathway. Neurosci. Lett. 2013, 553, 72–77. [Google Scholar] [CrossRef]
- Tufekci, K.U.; Eltutan, B.I.; Isci, K.B.; Genc, S. Resveratrol Inhibits NLRP3 Inflammasome-Induced Pyroptosis and miR-155 Expression in Microglia Through Sirt1/AMPK Pathway. Neurotox. Res. 2021, 39, 1812–1829. [Google Scholar] [CrossRef]
- Tang, X.L.; Wang, X.; Fang, G.; Zhao, Y.L.; Yan, J.; Zhou, Z.; Sun, R.; Luo, A.L.; Li, S.Y. Resveratrol ameliorates sevoflurane-induced cognitive impairment by activating the SIRT1/NF-κB pathway in neonatal mice. J. Nutr. Biochem. 2021, 90, 108579. [Google Scholar] [CrossRef]
- Wan, L.; Jia, R.M.; Ji, L.L.; Qin, X.M.; Hu, L.; Hu, F.; Han, Y.; Pan, Y.B.; Jiang, C.Y.; Liu, W.T. AMPK-autophagy-mediated inhibition of microRNA-30a-5p alleviates morphine tolerance via SOCS3-dependent neuroinflammation suppression. J. Neuroinflamm. 2022, 19, 25. [Google Scholar] [CrossRef] [PubMed]
- Ueki, K.; Kondo, T.; Kahn, C.R. Suppressor of Cytokine Signaling 1 (SOCS-1) and SOCS-3 Cause Insulin Resistance through Inhibition of Tyrosine Phosphorylation of Insulin Receptor Substrate Proteins by Discrete Mechanisms. Mol. Cell. Biol. 2004, 24, 5434–5446. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, X.; Han, J.; Zhong, Y.; Li, Q.; An, Y. MiR-324/SOCS3 Axis Protects Against Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury and Regulates Myocardial Ischemia via TNF/NF-κB Signaling Pathway. Int. Heart J. 2020, 61, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
- Ravanan, P.; Srikumar, I.F.; Talwar, P. Autophagy: The spotlight for cellular stress responses. Life Sci. 2017, 188, 53–67. [Google Scholar] [CrossRef]
- Ng, F. SIRT1 in the brain—Connections with aging-associated disorders and lifespan. Front. Cell. Neurosci. 2015, 9, 64. [Google Scholar] [CrossRef]
- Cavaliere, G.; Trinchese, G.; Penna, E.; Cimmino, F.; Pirozzi, C.; Lama, A.; Annunziata, C.; Catapano, A.; Mattace Raso, G.; Meli, R.; et al. High-Fat Diet Induces Neuroinflammation and Mitochondrial Impairment in Mice Cerebral Cortex and Synaptic Fraction. Front. Cell. Neurosci. 2019, 13, 509. [Google Scholar] [CrossRef]
- Kauer-Sant’Anna, M.; Kapczinski, F.; Andreazza, A.C.; Bond, D.J.; Lam, R.W.; Young, L.T.; Yatham, L.N. Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder. Int. J. Neuropsychopharmacol. 2008, 12, 447. [Google Scholar] [CrossRef]
- Ding, H.; Chen, J.; Su, M.; Lin, Z.; Zhan, H.; Yang, F.; Li, W.; Xie, J.; Huang, Y.; Liu, X.; et al. BDNF promotes activation of astrocytes and microglia contributing to neuroinflammation and mechanical allodynia in cyclophosphamide-induced cystitis. J. Neuroinflamm. 2020, 17, 19. [Google Scholar] [CrossRef]
- Zhang, F.; Lu, Y.F.; Wu, Q.; Liu, J.; Shi, J.S. Resveratrol promotes neurotrophic factor release from astroglia. Exp. Biol. Med. 2012, 237, 943–948. [Google Scholar] [CrossRef]
- Shen, J.; Xu, L.; Qu, C.; Sun, H.; Zhang, J. Resveratrol prevents cognitive deficits induced by chronic unpredictable mild stress: Sirt1/miR-134 signalling pathway regulates CREB/BDNF expression in hippocampus in vivo and in vitro. Behav. Brain Res. 2018, 349, 1–7. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Y.Y.; Liu, H.; Lu, Y.F.; Wu, Q.; Liu, J.; Shi, J.S. Resveratrol Produces Neurotrophic Effects on Cultured Dopaminergic Neurons through Prompting Astroglial BDNF and GDNF Release. Evid.-Based Complement. Altern. Med. 2012, 2012, 937605. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.P.; Chang, W.T.; Chen, L.; Chen, H.H.; Chan, M.H. Differential inhibitory effects of resveratrol on excitotoxicity and synaptic plasticity: Involvement of NMDA receptor subtypes. Nutr. Neurosci. 2019, 24, 443–458. [Google Scholar] [CrossRef]
- Cui, S.Y.; Yang, M.X.; Zhang, Y.H.; Zheng, V.; Zhang, H.T.; Gurney, M.E.; Xu, Y.; O’Donnell, J.M. Protection from Amyloid β Peptide–Induced Memory, Biochemical, and Morphological Deficits by a Phosphodiesterase-4D Allosteric Inhibitor. J. Pharmacol. Exp. Ther. 2019, 371, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Lissin, D.V.; Carroll, R.C.; Nicoll, R.A.; Malenka, R.C.; Zastrow, M.V. Rapid, Activation-Induced Redistribution of Ionotropic Glutamate Receptors in Cultured Hippocampal Neurons. J. Neurosci. 1999, 19, 1263–1272. [Google Scholar] [CrossRef]
- Kim, J.H.; Chung, K.H.; Hwang, Y.R.; Park, H.R.; Kim, H.J.; Kim, H.G.; Kim, H.R. Exposure to RF-EMF Alters Postsynaptic Structure and Hinders Neurite Outgrowth in Developing Hippocampal Neurons of Early Postnatal Mice. Int. J. Mol. Sci. 2021, 22, 5340. [Google Scholar] [CrossRef]
- Liu, X.; Tang, M.; He, T.Y.; Zhao, S.; Li, H.Z.; Li, Z.; Guo, Y.X.; Wang, X.L. Resveratrol Improves Paclitaxel-Induced Cognitive Impairment in Mice by Activating SIRT1/PGC-1α Pathway to Regulate Neuronal State and Microglia Cell Polarization. Drug Des. Dev. Ther. 2023, 17, 1125–1138. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.D.; Chen, X.X.; Yang, L.J.; Gao, X.R.; Xia, Q.R.; Qi, C.C.; Ge, J.F. Resveratrol ameliorates learning and memory impairments induced by bilateral hippocampal injection of streptozotocin in mice. Neurochem. Int. 2022, 159, 105385. [Google Scholar] [CrossRef]
- Saviola, F.; Pappaianni, E.; Monti, A.; Grecucci, A.; Jovicich, J.; De Pisapia, N. Trait and state anxiety are mapped differently in the human brain. Sci. Rep. 2020, 10, 11112. [Google Scholar] [CrossRef]
- Govic, A.; Nasser, H.; Levay, E.A.; Zelko, M.; Ebrahimie, E.; Mohammadi Dehcheshmeh, M.; Kent, S.; Penman, J.; Hazi, A. Long-Term Calorie Restriction Alters Anxiety-like Behaviour and the Brain and Adrenal Gland Transcriptomes of the Ageing Male Rat. Nutrients 2022, 14, 4670. [Google Scholar] [CrossRef]
- Lu, K.; Jia, X.; Wu, J.; Wang, Q.; Liang, X.F. Neuropeptide Y receptor Y2 (npy2r) deficiency reduces anxiety and increases food intake in Japanese medaka (Oryzias latipes). Front. Cell Dev. Biol. 2023, 11, 1273006. [Google Scholar] [CrossRef]
- Robinette, T.M.; Nicholatos, J.W.; Francisco, A.B.; Brooks, K.E.; Diao, R.Y.; Sorbi, S.; Ricca, V.; Nacmias, B.; Brieño-Enríquez, M.A.; Libert, S. SIRT1 accelerates the progression of activity-based anorexia. Nat. Commun. 2020, 11, 2814. [Google Scholar] [CrossRef] [PubMed]
- Staples, L.G. Predator odor avoidance as a rodent model of anxiety: Learning-mediated consequences beyond the initial exposure. Neurobiol. Learn. Mem. 2010, 94, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, A.; Truitt, W.; Rainnie, D.; Sajdyk, T. Role of stress, corticotrophin releasing factor (CRF) and amygdala plasticity in chronic anxiety. Stress 2005, 8, 209–219. [Google Scholar] [CrossRef]
- Musazzi, L.; Tornese, P.; Sala, N.; Popoli, M. What Acute Stress Protocols Can Tell Us About PTSD and Stress-Related Neuropsychiatric Disorders. Front. Pharmacol. 2018, 9, 758. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, J.; Guo, W. Emotional Roles of Mono-Aminergic Neurotransmitters in Major Depressive Disorder and Anxiety Disorders. Front. Psychol. 2018, 9, 2201. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Elevated Plus Maze Test for Measuring Anxiety-Like Behavior in Rodents. In Pre-Clinical Models; Springer: New York, NY, USA, 2018; pp. 69–74. [Google Scholar] [CrossRef]
- de Boer, S.F.; Buwalda, B.; Koolhaas, J.M. Untangling the neurobiology of coping styles in rodents: Towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci. Biobehav. Rev. 2017, 74, 401–422. [Google Scholar] [CrossRef]
- Ullmann, E.; Perry, S.W.; Licinio, J.; Wong, M.L.; Dremencov, E.; Zavjalov, E.L.; Shevelev, O.B.; Khotskin, N.V.; Koncevaya, G.V.; Khotshkina, A.S.; et al. From Allostatic Load to Allostatic State—An Endogenous Sympathetic Strategy to Deal with Chronic Anxiety and Stress? Front. Behav. Neurosci. 2019, 13, 47. [Google Scholar] [CrossRef]
- Tseilikman, O.B.; Kozochkin, D.A.; Manukhina, E.B.; Downey, H.F.; Misharina, M.E.; Komelkova, M.V.; Nikitina, A.A.; Golodnii, S.V.; Dodohova, M.A.; Tseilikman, V.E. Predicting anxiety responses to halogenated glucocorticoid drugs using the hexobarbital sleep time test. Stress 2016, 19, 390–394. [Google Scholar] [CrossRef]
- Meier, S.M.; Deckert, J. Genetics of Anxiety Disorders. Curr. Psychiatry Rep. 2019, 21, 16. [Google Scholar] [CrossRef]
- Tseilikman, V.E.; Tseilikman, O.B.; Pashkov, A.A.; Ivleva, I.S.; Karpenko, M.N.; Shatilov, V.A.; Zhukov, M.S.; Fedotova, J.O.; Kondashevskaya, M.V.; Downey, H.F.; et al. Mechanisms of Susceptibility and Resilience to PTSD: Role of Dopamine Metabolism and BDNF Expression in the Hippocampus. Int. J. Mol. Sci. 2022, 23, 14575. [Google Scholar] [CrossRef]
- Rao, U. Comorbidity between depressive and addictive disorders in adolescents: Role of stress and hpa activity. US Psyc. 2010, 3, 39–43. [Google Scholar] [PubMed]
- Calhoon, G.G.; Tye, K.M. Resolving the neural circuits of anxiety. Nat. Neurosci. 2015, 18, 1394–1404. [Google Scholar] [CrossRef]
- Jarrin, S.; Finn, D.P. Optogenetics and its application in pain and anxiety research. Neurosci. Biobehav. Rev. 2019, 105, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Britt, J.; McDevitt, R.; Reed, S. Optogenetics in preclinical neuroscience and psychiatry research: Recent insights and potential applications. Neuropsychiatr. Dis. Treat. 2014, 10, 1369–1379. [Google Scholar] [CrossRef][Green Version]
- Alexandra Kredlow, M.; Fenster, R.J.; Laurent, E.S.; Ressler, K.J.; Phelps, E.A. Prefrontal cortex, amygdala, and threat processing: Implications for PTSD. Neuropsychopharmacology 2021, 47, 247–259. [Google Scholar] [CrossRef]
- Akirav, I.; Maroun, M. The Role of the Medial Prefrontal Cortex-Amygdala Circuit in Stress Effects on the Extinction of Fear. Neural Plast. 2007, 2007, 030873. [Google Scholar] [CrossRef]
- Perumal, M.B.; Sah, P. Inhibitory Circuits in the Basolateral Amygdala in Aversive Learning and Memory. Front. Neural Circuits 2021, 15, 633235. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Simpson, K.L.; Weaver, K.J.; Lin, R.C. Differential Distribution Patterns From Medial Prefrontal Cortex and Dorsal Raphe to the Locus Coeruleus in Rats. Anat. Rec. 2012, 295, 1192–1201. [Google Scholar] [CrossRef]
- Marek, R.; Strobel, C.; Bredy, T.W.; Sah, P. The amygdala and medial prefrontal cortex: Partners in the fear circuit. J. Physiol. 2013, 591, 2381–2391. [Google Scholar] [CrossRef]
- McEwen, B.S.; Nasca, C.; Gray, J.D. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2015, 41, 3–23. [Google Scholar] [CrossRef]
- Olivier, J.D.A.; Olivier, B. Translational Studies in the Complex Role of Neurotransmitter Systems in Anxiety and Anxiety Disorders. In Anxiety Disorders; Springer: Singapore, 2020; pp. 121–140. [Google Scholar] [CrossRef]
- Ardianto, C.; Budiatin, A.S.; Sumartha, I.N.B.; Nurrahmi, N.; Rahmadi, M.; Khotib, J. Resveratrol ameliorates physical and psychological stress-induced depressive-like behavior. J. Basic Clin. Physiol. Pharmacol. 2021, 32, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.B.; Chen, X.Q.; Hu, G.Y. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Res. 2006, 1111, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ro, J.H.; Liu, C.C.; Lin, M.C. Resveratrol Mitigates Cerebral Ischemic Injury by Altering Levels of Trace Elements, Toxic Metal, Lipid Peroxidation, and Antioxidant Activity. Biol. Trace Elem. Res. 2020, 199, 3718–3727. [Google Scholar] [CrossRef]
- Parveen, A.; Alqahtani, F.; Javaid, S.; Ashraf, W.; Siddique, F.; Rawat, R.; Rasool, M.F.; Ahmad, T.; Alasmari, F.; Imran, I. Anxiolytic potential of resveratrol and rufinamide combination by modulating GABA-ergic transmission: Insights from experiments, molecular docking and dynamics simulations. J. Physiol. Pharmacol. 2023, 74, 489–506. [Google Scholar]
- Sarubbo, F.; Ramis, M.R.; Aparicio, S.; Ruiz, L.; Esteban, S.; Miralles, A.; Moranta, D. Improving effect of chronic resveratrol treatment on central monoamine synthesis and cognition in aged rats. AGE 2015, 37, 37. [Google Scholar] [CrossRef]
- Shuto, T.; Kuroiwa, M.; Koga, Y.; Kawahara, Y.; Sotogaku, N.; Toyomasu, K.; Nishi, A. Acute effects of resveratrol to enhance cocaine-induced dopamine neurotransmission in the striatum. Neurosci. Lett. 2013, 542, 107–112. [Google Scholar] [CrossRef]
- Gu, Z.; Chu, L.; Han, Y. Therapeutic effect of resveratrol on mice with depression. Exp. Ther. Med. 2019, 17, 3061–3064. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, S.; Kharlamov, E.A.; Miller, E.R.; Kelly, K.M. Hippocampal neuropeptide Y protein expression following controlled cortical impact and posttraumatic epilepsy. Epilepsy Behav. 2018, 87, 188–194. [Google Scholar] [CrossRef]
- Nwokafor, C.; Serova, L.I.; Nahvi, R.J.; McCloskey, J.; Sabban, E.L. Activation of NPY receptor subtype 1 by [D-His26]NPY is sufficient to prevent development of anxiety and depressive like effects in the single prolonged stress rodent model of PTSD. Neuropeptides 2020, 80, 102001. [Google Scholar] [CrossRef]
- Harada, N.; Zhao, J.; Kurihara, H.; Nakagata, N.; Okajima, K. Resveratrol improves cognitive function in mice by increasing production of insulin-like growth factor-I in the hippocampus. J. Nutr. Biochem. 2011, 22, 1150–1159. [Google Scholar] [CrossRef]
- Tong, J.; Gao, J.; Liu, Q.; He, C.; Zhao, X.; Qi, Y.; Yuan, T.; Li, P.; Niu, M.; Wang, D.; et al. Resveratrol derivative excited postsynaptic potentiation specifically via PKCβ-NMDA receptor mediation. Pharmacol. Res. 2020, 152, 104618. [Google Scholar] [CrossRef]
- Kondashevskaya, M.V.; Downey, H.F.; Tseilikman, V.E.; Alexandrin, V.V.; Artem’yeva, K.A.; Aleksankina, V.V.; Tseilikman, O.B.; Pashkov, A.A.; Goryacheva, A.V.; Ivleva, I.S.; et al. Cerebral Blood Flow in Predator Stress-Resilient and -Susceptible Rats and Mechanisms of Resilience. Int. J. Mol. Sci. 2022, 23, 14729. [Google Scholar] [CrossRef]
- Tseilikman, V.; Akulov, A.; Shevelev, O.; Khotskina, A.; Kontsevaya, G.; Moshkin, M.; Fedotova, J.; Pashkov, A.; Tseilikman, O.; Agletdinov, E.; et al. Paradoxical Anxiety Level Reduction in Animal Chronic Stress: A Unique Role of Hippocampus Neurobiology. Int. J. Mol. Sci. 2022, 23, 9151. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Nishimura, J.I.; Kobayashi, S.; Kimura, F. Long-term glucocorticoid treatments decrease local cerebral blood flow in the rat hippocampus, in association with histological damage. Neuroscience 1997, 79, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kakino, Y.; Hattori, Y.; Iwashita, M.; Uchiyama, H.; Noda, K.; Yoshimoto, T.; Iida, H.; Ihara, M. Long-Term Resveratrol Intake for Cognitive and Cerebral Blood Flow Impairment in Carotid Artery Stenosis/Occlusion. J. Stroke 2024, 26, 64–74. [Google Scholar] [CrossRef]
- Garrigue, P.; Mounien, L.; Champion, S.; Mouhajir, Y.; Pechere, L.; Guillet, B.; Landrier, J.F.; Seree, E. Long-term administration of resveratrol at low doses improves neurocognitive performance as well as cerebral blood flow and modulates the inflammatory pathways in the brain. J. Nutr. Biochem. 2021, 97, 108786. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.; Lei, M.; Chen, Y.; Tian, S.; Li, C.; Zhang, B. How Oxidative Stress Induces Depression? ASN Neuro 2023, 15, 17590914231181037. [Google Scholar] [CrossRef]
- Leal, G.; Comprido, D.; Duarte, C.B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 2014, 76, 639–656. [Google Scholar] [CrossRef]
- Lee, C.W.; Fang, Y.P.; Chu, M.C.; Chung, Y.J.; Chi, H.; Tang, C.W.; So, E.C.; Lin, H.C.; Lin, H.C. Differential mechanisms of synaptic plasticity for susceptibility and resilience to chronic social defeat stress in male mice. Biochem. Biophys. Res. Commun. 2021, 562, 112–118. [Google Scholar] [CrossRef]
- Dong, E.; Guidotti, A.; Zhang, H.; Pandey, S.C. Prenatal stress leads to chromatin and synaptic remodeling and excessive alcohol intake comorbid with anxiety-like behaviors in adult offspring. Neuropharmacology 2018, 140, 76–85. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, D.; Cheng, S.; Zhang, L.; Shi, Z.; Qin, J.; Zhang, Z.; Wang, H. Prenatal ethanol exposure enhances the susceptibility to depressive behavior of adult offspring rats fed a high-fat diet by affecting BDNF-associated pathway. Int. J. Mol. Med. 2019, 45, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Hao, B.; Dai, Y.; Xue, L.; Shi, Y.; Liu, L.; Xuan, S.; Yang, N.; Wang, X.; Zhao, H. Deficiency of Tet3 in nucleus accumbens enhances fear generalization and anxiety-like behaviors in mice. Brain Pathol. 2022, 32, 13080. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.H.; Song, S.Q.; Xu, Y. Resveratrol ameliorates chronic unpredictable mild stress-induced depression-like behavior: Involvement of the HPA axis, inflammatory markers, BDNF, and Wnt/β-catenin pathway in rats. Neuropsychiatr. Dis. Treat. 2017, 13, 2727–2736. [Google Scholar] [CrossRef]
- Liaqat, H.; Parveen, A.; Kim, S.Y. Antidepressive Effect of Natural Products and Their Derivatives Targeting BDNF-TrkB in Gut–Brain Axis. Int. J. Mol. Sci. 2022, 23, 14968. [Google Scholar] [CrossRef]
- Mehta, K.; Pandey, K.K.; Kaur, B.; Dhar, P.; Kaler, S. Resveratrol attenuates arsenic-induced cognitive deficits via modulation of Estrogen-NMDAR-BDNF signalling pathway in female mouse hippocampus. Psychopharmacology 2021, 238, 2485–2502. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Chen, S.; Gao, F.; Hu, B.; Wang, Y.; Ni, S.; Kou, H.; Song, Z.; Qing, X.; Wang, S.; et al. Resveratrol-enhanced SIRT1-mediated osteogenesis in porous endplates attenuates low back pain and anxiety behaviors. FASEB J. 2021, 35, e21414. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, S.Y.; Ma, Q.; Shi, J.; Yu, Y.; Li, J.X.; Zheng, L.; Zhang, Y.; Si, J.M.; Yu, Y.C. trans-Resveratrol Ameliorates Stress-Induced Irritable Bowel Syndrome-Like Behaviors by Regulation of Brain-Gut Axis. Front. Pharmacol. 2018, 9, 631. [Google Scholar] [CrossRef]
- Abe-Higuchi, N.; Uchida, S.; Yamagata, H.; Higuchi, F.; Hobara, T.; Hara, K.; Kobayashi, A.; Watanabe, Y. Hippocampal Sirtuin 1 Signaling Mediates Depression-like Behavior. Biol. Psychiatry 2016, 80, 815–826. [Google Scholar] [CrossRef]
- Zhu, X.; Li, W.; Li, Y.; Xu, W.; Yuan, Y.; Zheng, V.; Zhang, H.; O’Donnell, J.M.; Xu, Y.; Yin, X. The antidepressant- and anxiolytic-like effects of resveratrol: Involvement of phosphodiesterase-4D inhibition. Neuropharmacology 2019, 153, 20–31. [Google Scholar] [CrossRef]
- Barnes, P.J. Mechanisms and resistance in glucocorticoid control of inflammation. J. Steroid Biochem. Mol. Biol. 2010, 120, 76–85. [Google Scholar] [CrossRef]
- Mourtzi, N.; Sertedaki, A.; Charmandari, E. Glucocorticoid Signaling and Epigenetic Alterations in Stress-Related Disorders. Int. J. Mol. Sci. 2021, 22, 5964. [Google Scholar] [CrossRef] [PubMed]
- Sarapultsev, A.; Sarapultsev, P.; Dremencov, E.; Komelkova, M.; Tseilikman, O.; Tseilikman, V. Low glucocorticoids in stress-related disorders: The role of inflammation. Stress 2020, 23, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Maloley, P.; England, B.; Sayles, H.; Thiele, G.; Michaud, K.; Sokolove, J.; Cannon, G.; Reimold, A.; Kerr, G.; Baker, J.; et al. Post-traumatic stress disorder and serum cytokine and chemokine concentrations in patients with rheumatoid arthritis. Semin. Arthritis Rheum. 2019, 49, 229–235. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; Mellon, S.H.; Yehuda, R.; Grenon, S.M.; Flory, J.D.; Bierer, L.M.; Abu-Amara, D.; Coy, M.; Makotkine, I.; et al. Increased pro-inflammatory milieu in combat related PTSD—A new cohort replication study. Brain Behav. Immun. 2017, 59, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Fairless, R.; Bading, H.; Diem, R. Pathophysiological Ionotropic Glutamate Signalling in Neuroinflammatory Disease as a Therapeutic Target. Front. Neurosci. 2021, 15, 741280. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.B.; Blakely, R.D.; Hewlett, W.A. The Proinflammatory Cytokines Interleukin-1beta and Tumor Necrosis Factor-Alpha Activate Serotonin Transporters. Neuropsychopharmacology 2006, 31, 2121–2131. [Google Scholar] [CrossRef]
- Shen, J.D.; Zhang, Y.W.; Wang, B.Y.; Bai, L.; Lu, S.F.; Zhu, L.L.; Bai, M.; Li, Y.C.; Xu, E.P. Effects of resveratrol on the levels of ATP, 5-HT and GAP-43 in the hippocampus of mice exposed to chronic unpredictable mild stress. Neurosci. Lett. 2020, 735, 135232. [Google Scholar] [CrossRef]
- Zarebavani, M.; Baghaei Naeini, F.; Farahvash, A.; Moradi, F.; Dashti, N. Resveratrol attenuates chronic social isolation stress-induced affective disorders: Involvement of NF-κB/NLRP3 axis. J. Biochem. Mol. Toxicol. 2023, 37, 23311. [Google Scholar] [CrossRef]
- Wei, R.M.; Zhang, Y.M.; Feng, Y.Z.; Zhang, K.X.; Zhang, J.Y.; Chen, J.; Luo, B.L.; Li, X.Y.; Chen, G.H. Resveratrol ameliorates maternal separation-induced anxiety- and depression-like behaviors and reduces Sirt1-NF-kB signaling-mediated neuroinflammation. Front. Behav. Neurosci. 2023, 17, 1172091. [Google Scholar] [CrossRef]
- Molla, B.; Heredia, M.; Campos, A.; Sanz, P. Pharmacological Modulation of Glutamatergic and Neuroinflammatory Pathways in a Lafora Disease Mouse Model. Mol. Neurobiol. 2022, 59, 6018–6032. [Google Scholar] [CrossRef]
- Tian, Q.; Fan, X.; Ma, J.; Han, Y.; Li, D.; Jiang, S.; Zhang, F.; Guang, H.; Shan, X.; Chen, R.; et al. Resveratrol ameliorates lipopolysaccharide-induced anxiety-like behavior by attenuating YAP-mediated neuro-inflammation and promoting hippocampal autophagy in mice. Toxicol. Appl. Pharmacol. 2020, 408, 115261. [Google Scholar] [CrossRef] [PubMed]
- Ploski, J.E.; Vaidya, V.A. The Neurocircuitry of Posttraumatic Stress Disorder and Major Depression: Insights Into Overlapping and Distinct Circuit Dysfunction—A Tribute to Ron Duman. Biol. Psychiatry 2021, 90, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Liaw, K.; Sharma, R.; Zhang, Z.; Kannan, S.; Kannan, R.M. Targeting Mitochondrial Dysfunction and Oxidative Stress in Activated Microglia using Dendrimer-Based Therapeutics. Theranostics 2018, 8, 5529–5547. [Google Scholar] [CrossRef] [PubMed]
- Javani, G.; Babri, S.; Farajdokht, F.; Ghaffari-Nasab, A.; Mohaddes, G. Mitochondrial transplantation improves anxiety- and depression-like behaviors in aged stress-exposed rats. Mech. Ageing Dev. 2022, 202, 111632. [Google Scholar] [CrossRef]
- Larrieu, T.; Cherix, A.; Duque, A.; Rodrigues, J.; Lei, H.; Gruetter, R.; Sandi, C. Hierarchical Status Predicts Behavioral Vulnerability and Nucleus Accumbens Metabolic Profile Following Chronic Social Defeat Stress. Curr. Biol. 2017, 27, 2202–2210.e4. [Google Scholar] [CrossRef]
- Hollis, F.; van der Kooij, M.A.; Zanoletti, O.; Lozano, L.; Cantó, C.; Sandi, C. Mitochondrial function in the brain links anxiety with social subordination. Proc. Natl. Acad. Sci. USA 2015, 112, 15486–15491. [Google Scholar] [CrossRef]
- Papilloud, A.; Weger, M.; Bacq, A.; Zalachoras, I.; Hollis, F.; Larrieu, T.; Battivelli, D.; Grosse, J.; Zanoletti, O.; Parnaudeau, S.; et al. The glucocorticoid receptor in the nucleus accumbens plays a crucial role in social rank attainment in rodents. Psychoneuroendocrinology 2020, 112, 104538. [Google Scholar] [CrossRef]
- Filiou, M.D.; Sandi, C. Anxiety and Brain Mitochondria: A Bidirectional Crosstalk. Trends Neurosci. 2019, 42, 573–588. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S.; Epel, E.S.; Sandi, C. An energetic view of stress: Focus on mitochondria. Front. Neuroendocrinol. 2018, 49, 72–85. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S. Mitochondria impact brain function and cognition. Proc. Natl. Acad. Sci. USA 2013, 111, 7–8. [Google Scholar] [CrossRef]
- Zhao, J.; Ye, L.; Liu, Z.; Cui, Y.; Deng, D.; Bai, S.; Yang, L.; Shi, Y.; Liu, Z.; Zhang, R. Protective Effects of Resveratrol on Adolescent Social Isolation-Induced Anxiety-Like Behaviors via Modulating Nucleus Accumbens Spine Plasticity and Mitochondrial Function in Female Rats. Nutrients 2022, 14, 4542. [Google Scholar] [CrossRef]
- Kim, H.D.; Hesterman, J.; Call, T.; Magazu, S.; Keeley, E.; Armenta, K.; Kronman, H.; Neve, R.L.; Nestler, E.J.; Ferguson, D. SIRT1 Mediates Depression-Like Behaviors in the Nucleus Accumbens. J. Neurosci. 2016, 36, 8441–8452. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, S.; Misrani, A.; Huang, H.X.; Zhang, Z.Y.; Li, Q.W.; Long, C. Resveratrol Attenuates Chronic Unpredictable Mild Stress-Induced Alterations in the SIRT1/PGC1α/SIRT3 Pathway and Associated Mitochondrial Dysfunction in Mice. Mol. Neurobiol. 2023, 60, 5102–5116. [Google Scholar] [CrossRef]
- Hovatta, I.; Juhila, J.; Donner, J. Oxidative stress in anxiety and comorbid disorders. Neurosci. Res. 2010, 68, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.S.; Lee, S.H.; Seo, H.; Park, G. Changes in RBM47 expression based on the timing of melatonin administration and its effects on Nrf2 activity in the hippocampus. Free. Radic. Biol. Med. 2023, 208, 794–806. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, S.; Oryan, S.; Digaleh, H.; Shaerzadeh, F.; Khodagholi, F.; Maghsoudi, N.; Zarrindast, M.R. Involvement of Nrf2 in Development of Anxiety-Like Behavior by Linking Bcl2 to Oxidative Phosphorylation: Estimation in Rat Hippocampus, Amygdala, and Prefrontal Cortex. J. Mol. Neurosci. 2014, 55, 492–499. [Google Scholar] [CrossRef]
- Schriever, S.C.; Zimprich, A.; Pfuhlmann, K.; Baumann, P.; Giesert, F.; Klaus, V.; Kabra, D.G.; Hafen, U.; Romanov, A.; Tschöp, M.H.; et al. Alterations in neuronal control of body weight and anxiety behavior by glutathione peroxidase 4 deficiency. Neuroscience 2017, 357, 241–254. [Google Scholar] [CrossRef]
- Olsen, R.H.J.; Johnson, L.A.; Zuloaga, D.G.; Limoli, C.L.; Raber, J. Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J. Neurochem. 2013, 125, 303–313. [Google Scholar] [CrossRef]
- Khalifeh, S.; Oryan, S.; Khodagholi, F.; Digaleh, H.; Shaerzadeh, F.; Maghsoudi, N.; Zarrindast, M.R. Complexity of Compensatory Effects in Nrf1 Knockdown: Linking Undeveloped Anxiety-Like Behavior to Prevented Mitochondrial Dysfunction and Oxidative Stress. Cell. Mol. Neurobiol. 2015, 36, 553–563. [Google Scholar] [CrossRef]
- Sergeeva, S.; Bagryanskaya, E.; Korbolina, E.; Kolosova, N. Development of behavioural dysfunctions in accelerated-senescence OXYS rats is associated with early postnatal alterations in brain phosphate metabolism. Exp. Gerontol. 2006, 41, 141–150. [Google Scholar] [CrossRef]
- Sharma, R.; Kumarasamy, M.; Parihar, V.K.; Ravichandiran, V.; Kumar, N. Monoamine Oxidase: A Potential Link in Papez Circuit to Generalized Anxiety Disorders. Cns Neurol. Disord.-Drug Targets 2024, 23, 638–655. [Google Scholar] [CrossRef]
- Geng, X.; Wu, H.; Li, Z.; Li, C.; Chen, D.; Zong, J.; Liu, Z.; Wei, S.; Peng, W. Jie-Yu-He-Huan Capsule Ameliorates Anxiety-Like Behaviours in Rats Exposed to Chronic Restraint Stress via the cAMP/PKA/CREB/BDNF Signalling Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 1703981. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ling, Y.; Zhang, Y.; Liu, L.; Qiu, Y.; Liu, Y.; Yin, Y. The role of EndophilinA1 in chronic unpredicted mild stress-induced depression model mice. Int. Immunopharmacol. 2023, 124, 111023. [Google Scholar] [CrossRef] [PubMed]
- Oettinghaus, B.; Schulz, J.M.; Restelli, L.M.; Licci, M.; Savoia, C.; Schmidt, A.; Schmitt, K.; Grimm, A.; Morè, L.; Hench, J.; et al. Synaptic dysfunction, memory deficits and hippocampal atrophy due to ablation of mitochondrial fission in adult forebrain neurons. Cell Death Differ. 2015, 23, 18–28. [Google Scholar] [CrossRef]
- Chiu, W.; Chen, C.; Lee, T.; Chen, Z.; Ke, P.; Chiang, A. Oxidative stress enhances AP-1 and NF-κB-mediated regulation of β2-Glycoprotein I gene expression in hepatoma cells. J. Cell. Biochem. 2010, 111, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wahab, B.A.; Abdel-Wahab, M.M. Protective effect of resveratrol against chronic intermittent hypoxia-induced spatial memory deficits, hippocampal oxidative DNA damage and increased p47Phox NADPH oxidase expression in young rats. Behav. Brain Res. 2016, 305, 65–75. [Google Scholar] [CrossRef]
- Baghaei Naeini, F.; Hassanpour, S.; Asghari, A. Resveratrol exerts anxiolytic-like effects through anti-inflammatory and antioxidant activities in rats exposed to chronic social isolation. Behav. Brain Res. 2023, 438, 114201. [Google Scholar] [CrossRef]
- Shukla, P.; Akotkar, L.; Aswar, U. Resveratrol attenuates early life stress induced depression in rats: Behavioural and neurochemical evidence. Neurosci. Lett. 2024, 820, 137606. [Google Scholar] [CrossRef]
- López, J.F.; Akil, H.; Watson, S.J. Neural circuits mediating stress. Biol. Psychiatry 1999, 46, 1461–1471. [Google Scholar] [CrossRef]
- de Kloet, E.R.; de Kloet, S.F.; de Kloet, C.S.; de Kloet, A.D. Top-down and bottom-up control of stress-coping. J. Neuroendocrinol. 2019, 31, 12675. [Google Scholar] [CrossRef]
- de Kloet, E.R.; Derijk, R. Signaling Pathways in Brain Involved in Predisposition and Pathogenesis of Stress-Related Disease: Genetic and Kinetic Factors Affecting the MR/GR Balance. Ann. N. Y. Acad. Sci. 2004, 1032, 14–34. [Google Scholar] [CrossRef]
- Adcock, I.M.; Cosio, B.; Tsaprouni, L.; Barnes, P.J.; Ito, K. Redox Regulation of Histone Deacetylases and Glucocorticoid-Mediated Inhibition of the Inflammatory Response. Antioxid. Redox Signal. 2005, 7, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Lafuente, C.L.; Kalynchuk, L.E.; Caruncho, H.J.; Ausió, J. The Role of MeCP2 in Regulating Synaptic Plasticity in the Context of Stress and Depression. Cells 2022, 11, 748. [Google Scholar] [CrossRef] [PubMed]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2011, 13, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Numakawa, T.; Kajihara, R. An Interaction between Brain-Derived Neurotrophic Factor and Stress-Related Glucocorticoids in the Pathophysiology of Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 1596. [Google Scholar] [CrossRef]
- Lambert, W.M.; Xu, C.F.; Neubert, T.A.; Chao, M.V.; Garabedian, M.J.; Jeanneteau, F.D. Brain-Derived Neurotrophic Factor Signaling Rewrites the Glucocorticoid Transcriptome via Glucocorticoid Receptor Phosphorylation. Mol. Cell. Biol. 2013, 33, 3700–3714. [Google Scholar] [CrossRef]
- Kelly, J.R.; Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G.; Hyland, N.P. Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015, 9, 392. [Google Scholar] [CrossRef]
- Yehuda, R.; Seckl, J. Minireview: Stress-Related Psychiatric Disorders with Low Cortisol Levels: A Metabolic Hypothesis. Endocrinology 2011, 152, 4496–4503. [Google Scholar] [CrossRef]
- Tseilikman, V.; Dremencov, E.; Maslennikova, E.; Ishmatova, A.; Manukhina, E.; Downey, H.F.; Klebanov, I.; Tseilikman, O.; Komelkova, M.; Lapshin, M.S.; et al. Post-Traumatic Stress Disorder Chronification via Monoaminooxidase and Cortisol Metabolism. Horm. Metab. Res. 2019, 51, 618–622. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Qiu, Z.K.; He, J.L.; Liu, X.; Chen, J.S.; Wang, Y.L. Resveratrol ameliorated the behavioral deficits in a mouse model of post-traumatic stress disorder. Pharmacol. Biochem. Behav. 2017, 161, 68–76. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed]
- Kuwahara, A.; Matsuda, K.; Kuwahara, Y.; Asano, S.; Inui, T.; Marunaka, Y. Microbiota-gut-brain axis: Enteroendocrine cells and the enteric nervous system form an interface between the microbiota and the central nervous system. Biomed. Res. 2020, 41, 199–216. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Wang, G.; Song, X.; Qian, Y.; Liao, Z.; Sui, L.; Ai, L.; Xia, Y. Understanding the Connection between Gut Homeostasis and Psychological Stress. J. Nutr. 2023, 153, 924–939. [Google Scholar] [CrossRef] [PubMed]
- Petakh, P.; Oksenych, V.; Kamyshna, I.; Boisak, I.; Lyubomirskaya, K.; Kamyshnyi, O. Exploring the interplay between posttraumatic stress disorder, gut microbiota, and inflammatory biomarkers: A comprehensive meta-analysis. Front. Immunol. 2024, 15, 1349883. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Ahmad, R.; Moshfegh, C.M.; Sankarasubramanian, J.; Joshi, V.; Elkhatib, S.K.; Chhonker, Y.S.; Murry, D.J.; Talmon, G.A.; Guda, C.; et al. Repeated Social Defeat Stress Induces an Inflammatory Gut Milieu by Altering the Mucosal Barrier Integrity and Gut Microbiota Homeostasis. Biol. Psychiatry Glob. Open Sci. 2023, 3, 824–836. [Google Scholar] [CrossRef]
- Jing, W.; Bi, C.; Fang, Z.; Qian, C.; Chen, J.; Yu, J.; Tian, G.; Ye, M.; Liu, Z. Neuropsychiatric sequelae after liver transplantation and their possible mechanism via the microbiota–gut–liver–brain axis. Biomed. Pharmacother. 2023, 163, 114855. [Google Scholar] [CrossRef]
- Russo, S.; Chan, K.; Li, L.; Parise, L.; Cathomas, F.; LeClair, K.; Shimo, Y.; Lin, H.Y.; Durand-de Cuttoli, R.; Aubry, A.; et al. Stress-activated brain-gut circuits disrupt intestinal barrier integrity and social behaviour. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Li, L.F.; Zou, H.W.; Song, B.L.; Wang, Y.; Jiang, Y.; Li, Z.L.; Niu, Q.H.; Liu, Y.J. Increased Lactobacillus Abundance Contributes to Stress Resilience in Mice Exposed to Chronic Social Defeat Stress. Neuroendocrinology 2022, 113, 563–576. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Teng, T.; Jiang, Y.; Xiang, Y.; Fan, L.; Yu, Y.; Zhou, X.; Xie, P. Comparative analysis of gut microbiota and fecal metabolome features among multiple depressive animal models. J. Affect. Disord. 2022, 314, 103–111. [Google Scholar] [CrossRef]
- Yu, Y.C.; Li, J.; Zhang, M.; Pan, J.C.; Yu, Y.; Zhang, J.B.; Zheng, L.; Si, J.M.; Xu, Y. Resveratrol Improves Brain-Gut Axis by Regulation of 5-HT-Dependent Signaling in the Rat Model of Irritable Bowel Syndrome. Front. Cell. Neurosci. 2019, 13, 30. [Google Scholar] [CrossRef]
- Favari, C.; Rinaldi de Alvarenga, J.F.; Sánchez-Martínez, L.; Tosi, N.; Mignogna, C.; Cremonini, E.; Manach, C.; Bresciani, L.; Del Rio, D.; Mena, P. Factors driving the inter-individual variability in the metabolism and bioavailability of (poly)phenolic metabolites: A systematic review of human studies. Redox Biol. 2024, 71, 103095. [Google Scholar] [CrossRef]
- Tseilikman, V.E.; Fedotova, J.O.; Tseilikman, O.B.; Novak, J.; Karpenko, M.N.; Maistrenko, V.A.; Lazuko, S.S.; Belyeva, L.E.; Kamel, M.; Buhler, A.V.; et al. Resistance to Resveratrol Treatment in Experimental PTSD Is Associated with Abnormalities in Hepatic Metabolism of Glucocorticoids. Int. J. Mol. Sci. 2023, 24, 9333. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, J.; Zhang, M.; Yang, W.; Qin, W.; Zheng, Q.; Chu, Y.; Wu, Y.; Wu, D.; Yuan, X. 11β-HSD1 Inhibitor Alleviates Non-Alcoholic Fatty Liver Disease by Activating the AMPK/SIRT1 Signaling Pathway. Nutrients 2022, 14, 2358. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.; Tseilikman, V.E.; Tseilikman, O.B.; Lazuko, S.S.; Belyeva, L.E.; Rahmani, A.; Fedotova, J. Can Resveratrol Influence the Activity of 11β-Hydroxysteroid Dehydrogenase Type 1? A Combined In Silico and In Vivo Study. Pharmaceuticals 2023, 16, 251. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Bobryshev, Y.V.; Chekhonin, V.P. Epigenetic Alterations in DNA and Histone Modifications Caused by Depression and Antidepressant Drugs: Lessons from the Rodent Models. Curr. Pharm. Des. 2018, 23, 6828–6840. [Google Scholar] [CrossRef]
- Kovanen, L.; Donner, K.; Partonen, T. SIRT1 Polymorphisms Associate with Seasonal Weight Variation, Depressive Disorders, and Diastolic Blood Pressure in the General Population. PLoS ONE 2015, 10, e0141001. [Google Scholar] [CrossRef]
- Xu, L.; Xu, S.; Lin, L.; Gu, X.; Fu, C.; Fang, Y.; Li, X.; Wang, X. High-fat Diet Mediates Anxiolytic-like Behaviors in a Time-dependent Manner Through the Regulation of SIRT1 in the Brain. Neuroscience 2018, 372, 237–245. [Google Scholar] [CrossRef]
- Dang, R.; Wang, M.; Li, X.; Wang, H.; Liu, L.; Wu, Q.; Zhao, J.; Ji, P.; Zhong, L.; Licinio, J.; et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. J. Neuroinflamm. 2022, 19, 41. [Google Scholar] [CrossRef]
- Dai, G.L.; Yang, X.Y.; Chen, S.S.; Wang, Y.Q.; Liu, M.C.; Cao, Y.; Li, F.R.; Ma, C.Y.; Ju, W.Z. Effects of Jiaotai Pills on CUMS-induced depression model in mice based on changes of SIRT1 expression in hippocampus. Zhongguo Zhong Yao Zhi 2021, 46, 6511–6519. [Google Scholar]
- Fan, J.; Guang, H.; Zhang, H.; Chen, D.; Ding, L.; Fan, X.; Xue, F.; Gan, Z.; Wang, Y.; Mao, S.; et al. SIRT1 Mediates Apelin-13 in Ameliorating Chronic Normobaric Hypoxia-induced Anxiety-like Behavior by Suppressing NF-κB Pathway in Mice Hippocampus. Neuroscience 2018, 381, 22–34. [Google Scholar] [CrossRef]
- Brynildsen, J.K.; Lee, B.G.; Perron, I.J.; Jin, S.; Kim, S.F.; Blendy, J.A. Activation of AMPK by metformin improves withdrawal signs precipitated by nicotine withdrawal. Proc. Natl. Acad. Sci. USA 2018, 115, 4282–4287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, P.; Cheng, P.; Zhang, Z.; Yang, S.; Wang, J.; Wang, X.; Zhu, G. Exploring the effect of Anshen Dingzhi prescription on hippocampal mitochondrial signals in single prolonged stress mouse model. J. Ethnopharmacol. 2024, 323, 117713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, S.F.; Yun, Q.; Liu, W.J.; Guo, M.N.; Zhu, Y.Q.; Liu, Z.Z.; Qian, J.J.; Zhang, W.N. Ameliorative effect of SIRT1 in postpartum depression mediated by upregulation of the glucocorticoid receptor. Neurosci. Lett. 2021, 761, 136112. [Google Scholar] [CrossRef] [PubMed]
- Libert, S.; Pointer, K.; Bell, E.; Das, A.; Cohen, D.; Asara, J.; Kapur, K.; Bergmann, S.; Preisig, M.; Otowa, T.; et al. SIRT1 Activates MAO-A in the Brain to Mediate Anxiety and Exploratory Drive. Cell 2011, 147, 1459–1472. [Google Scholar] [CrossRef]
- Li, W.; Guo, B.; Tao, K.; Li, F.; Liu, Z.; Yao, H.; Feng, D.; Liu, X. Inhibition of SIRT1 in hippocampal CA1 ameliorates PTSD-like behaviors in mice by protections of neuronal plasticity and serotonin homeostasis via NHLH2/MAO-A pathway. Biochem. Biophys. Res. Commun. 2019, 518, 344–350. [Google Scholar] [CrossRef]
- Wang, Z.; Gu, J.; Wang, X.; Xie, K.; Luan, Q.; Wan, N.; Zhang, Q.; Jiang, H.; Liu, D. Antidepressant-like activity of resveratrol treatment in the forced swim test and tail suspension test in mice: The HPA axis, BDNF expression and phosphorylation of ERK. Pharmacol. Biochem. Behav. 2013, 112, 104–110. [Google Scholar] [CrossRef]
- Ji, L.L.; Ye, Y.; Nie, P.Y.; Peng, J.B.; Fu, C.H.; Wang, Z.Y.; Tong, L. Dysregulation of miR-142 results in anxiety-like behaviors following single prolonged stress. Behav. Brain Res. 2019, 365, 157–163. [Google Scholar] [CrossRef]
- Li, G.; Wang, G.; Shi, J.; Xie, X.; Fei, N.; Chen, L.; Liu, N.; Yang, M.; Pan, J.; Huang, W.; et al. trans-Resveratrol ameliorates anxiety-like behaviors and fear memory deficits in a rat model of post-traumatic stress disorder. Neuropharmacology 2018, 133, 181–188. [Google Scholar] [CrossRef]
- Tseilikman, V.E.; Shatilov, V.A.; Zhukov, M.S.; Buksha, I.A.; Epitashvily, A.E.; Lipatov, I.A.; Aristov, M.R.; Koshelev, A.G.; Karpenko, M.N.; Traktirov, D.S.; et al. Limited Cheese Intake Paradigm Replaces Patterns of Behavioral Disorders in Experimental PTSD: Focus on Resveratrol Supplementation. Int. J. Mol. Sci. 2023, 24, 14343. [Google Scholar] [CrossRef]
- Juliani, P.Z.; Rodrigues, T.; Bressan, G.N.; Camponogara, C.; Oliveira, S.M.; Brucker, N.; Fachinetto, R. Effects of association between resveratrol and ketamine on behavioral and biochemical analysis in mice. J. Neural Transm. 2024, 131, 971–986. [Google Scholar] [CrossRef]
- Bautista-Aguilera, O.M.; Alonso, J.M.; Catto, M.; Iriepa, I.; Knez, D.; Gobec, S.; Marco-Contelles, J. N-Hydroxy-N-Propargylamide Derivatives of Ferulic Acid: Inhibitors of Cholinesterases and Monoamine Oxidases. Molecules 2022, 27, 7437. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A. Tribulin and Endogenous MAO-Inhibitory Regulation In Vivo. Neurotoxicology 2004, 25, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Rideout, D.A.; Rakita, S.S.; Gower, W.R.; You, M.; Murr, M.M. Does LKB1 Mediate Activation of Hepatic AMP-Protein Kinase (AMPK) and Sirtuin1 (SIRT1) After Roux-en-Y Gastric Bypass in Obese Rats? J. Gastrointest. Surg. 2010, 14, 221–228. [Google Scholar] [CrossRef] [PubMed]
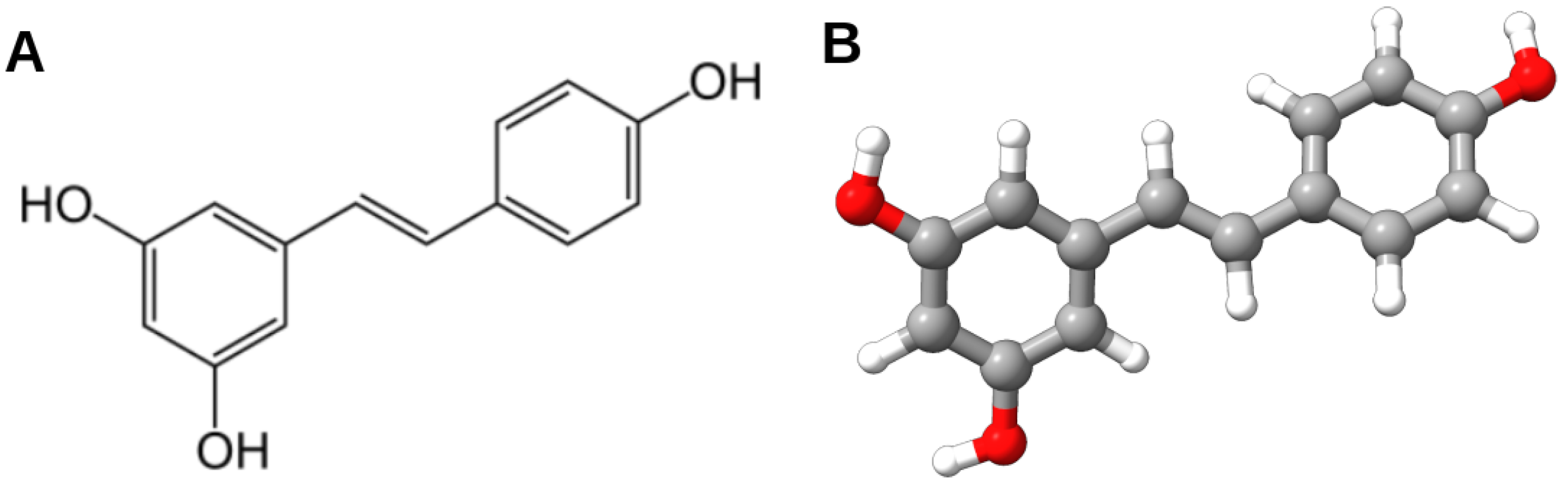
| Organism Neuronal Culture | Molecular Target | Effects of RES | References |
|---|---|---|---|
| PC 12 cells | p53 | RES prevents the proapoptotic increase in nuclear p53 induced by high glucose levels. | [19] |
| N9 microglial cells | NF-B | RES reduces apoptotic neuronal cell death induced by neuroinflammation. | [20] |
| primary cultured neurons | SIRT1, SIRT3, PGC1 | RES may ameliorate manganese (Mn)-induced neuronal injury and mitochondrial dysfunction in primary cultured neurons by activating the SIRT1/PGC-1 signaling pathway, with SIRT3 being essential for promoting mitochondrial biogenesis and attenuating Mn-induced mitochondrial dysfunction. | [21] |
| H19-7 hippocampal neuronal cells | superoxide dismutase, catalase, glutathione reductase | RES treatment attenuated the accumulation of lipid peroxide levels, upregulated antioxidant activities, and improved the expression of memory-associated proteins in A-treated H19-7 cells. | [22] |
| primary cultures of rat cortical neurons | Nrf-2 | RES treatment at various time points increased neuronal viability and inhibited neuronal apoptosis in vitro, at least in part, by enhancing the activation of the Nrf-2 signaling pathway. | [23] |
| culture of dopaminergic neurons | intracellular free calcium, reactive oxygen species | RES enhances cell viability and reduces apoptosis by attenuating MA-induced reactive oxygen species (ROS) production and calcium overload. It protects dopaminergic neurons from cytotoxicity by inhibiting and oxidative stress. | [24] |
| mice mesencephalic and cortical primary cultures | complex III of mitochondrial respiratory chain | A significant reduction in glutamate-induced radical formation was observed in cultures treated with resveratrol, demonstrating the antioxidant potential of RES. | [25] |
| rat cerebellar granule neurons (CGNs) | mitochondrial complex I, SIRT1 | RES exhibits beneficial effects against mitochondrial dysfunction and prevents cell death in neuronal cells. | [26] |
| SH-SY5Y cells | AMPK | RES rescues SH-SY5Y cells from oxygen-glucose deprivation (OGD)-mediated mitochondrial deficiency and restores the transcript expression levels of PGC-1 and mitochondrial genes. | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseilikman, V.E.; Tseilikman, O.B.; Yegorov, O.N.; Brichagina, A.A.; Karpenko, M.N.; Tseilikman, D.V.; Shatilov, V.A.; Zhukov, M.S.; Novak, J. Resveratrol: A Multifaceted Guardian against Anxiety and Stress Disorders—An Overview of Experimental Evidence. Nutrients 2024, 16, 2856. https://doi.org/10.3390/nu16172856
Tseilikman VE, Tseilikman OB, Yegorov ON, Brichagina AA, Karpenko MN, Tseilikman DV, Shatilov VA, Zhukov MS, Novak J. Resveratrol: A Multifaceted Guardian against Anxiety and Stress Disorders—An Overview of Experimental Evidence. Nutrients. 2024; 16(17):2856. https://doi.org/10.3390/nu16172856
Chicago/Turabian StyleTseilikman, Vadim E., Olga B. Tseilikman, Oleg N. Yegorov, Alina A. Brichagina, Marina N. Karpenko, David V. Tseilikman, Vladislav A. Shatilov, Maxim S. Zhukov, and Jurica Novak. 2024. "Resveratrol: A Multifaceted Guardian against Anxiety and Stress Disorders—An Overview of Experimental Evidence" Nutrients 16, no. 17: 2856. https://doi.org/10.3390/nu16172856
APA StyleTseilikman, V. E., Tseilikman, O. B., Yegorov, O. N., Brichagina, A. A., Karpenko, M. N., Tseilikman, D. V., Shatilov, V. A., Zhukov, M. S., & Novak, J. (2024). Resveratrol: A Multifaceted Guardian against Anxiety and Stress Disorders—An Overview of Experimental Evidence. Nutrients, 16(17), 2856. https://doi.org/10.3390/nu16172856





