Abstract
The increasing size of the human population and the shortage of highly valuable proteinaceous ingredients has prompted the international community to scout for new, sustainable, and natural protein resources from invertebrates (e.g., insects) and underutilized legume crops, unexploited terrestrial and aquatic weeds, and fungi. Insect proteins are known for their nutritional value, being rich in proteins with a good balance of essential amino acids and being a valuable source of essential fatty acids and trace elements. Unconventional legume crops were found rich in nutritional, phytochemical, and therapeutic properties, showing excellent abilities to survive extreme environmental conditions. This review evaluates the recent state of underutilized legume crops, aquatic weeds, fungi, and insects intended as alternative protein sources, from ingredient production to their incorporation in food products, including their food formulations and the functional characteristics of alternative plant-based proteins and edible insect proteins as novel foods. Emphasis is also placed on safety issues due to the presence of anti-nutritional factors and allergenic proteins in insects and/or underutilized legumes. The functional and biological activities of protein hydrolysates from different protein sources are reviewed, along with bioactive peptides displaying antihypertensive, antioxidant, antidiabetic, and/or antimicrobial activity. Due to the healthy properties of these foods for the high abundance of bioactive peptides and phytochemicals, more consumers are expected to turn to vegetarianism or veganism in the future, and the increasing demand for such products will be a challenge for the future.
1. Introduction
In the last years, the increase in the human population and depletion of natural resources have become among the most critical issues to be faced worldwide. The current world population comprises 7.5 billion people and is foreseen to increase up to 9–10 billion by 2050 [1]. As a result, the demand for sustainable, healthy, and nourishing foods is constantly raising. Nowadays, food systems represent the major contributors to environmental issues such as global greenhouse gas (GHG) emissions, deforestation, and water consumption [2,3]. Meat production has been found to have a dramatic impact on GHG emissions and consumption of water [4]. Thus, alternative sources of valuable proteins are urgently needed.
For a long time, plant proteins were deemed to possess a minor nutritional value compared to meat proteins, even though this trend is recently reversing [5]. As plant proteins come along with other plant ingredients and accompanying nutrients, a direct comparison with meat proteins in terms of overall benefit has been hardly achieved. A product with over 30% protein content (dry matter) and low-fat content is considered an excellent meat substitute [6]. It is also worth noting that substitutes or alternatives to meat products should be characterized by their similarity to meat protein digestibility-corrected amino acid score (PDCAAS) [7,8].
The technological functions of proteins are linked to their origin and concentration in the food ingredient. Based on their functioning properties, such as water-binding capacity, many proteins can be used in preparing of meat alternatives. Typical proteins exploited for this purpose are pea protein isolate, soy protein isolate, wheat gluten blends, etc. [9]. Gluten proteins, and containing glutenins and gliadins, are used to prepare meat alternatives, imparting the chewiness and structure to the products [10]. Seitan is a pure gluten of meat alternative that is largely consumed in vegetarian and vegan diets and can be found in the form of burgers, sausages, and nuggets seasoned and colored, for example, using microbial natural pigments. Similar to seitan, tofu is a product of thermal coagulation of soybean proteins promoted by calcium ions, which is a versatile alternative to dairy products. On the other side, drawbacks in using gluten for meat alternative analogues rely on the allergenic potential towards allergic individuals and people suffering from celiac disease, limiting its use. In addition to allergic effects, the addition of alternative proteins such as soy proteins to food formulations confer bitter and astringent flavor, limiting the consumer’s acceptability. Thus, novel sources of proteins are to be investigated. Fungi-based meat alternatives look and taste just like meat, and their environmental impact is next to nothing [11]; proteins from edible fungi are also attracting interest for their healthy nutritional profile [12,13]. On the other side, insect proteins are also exploited in food production since they show good oil and water retention capacity, emulsion, and foaming activities [14]. Several proteins derived from legumes represent a good choice for their gel-forming abilities, crucial for immobilizing fat and entrapping water within the matrix of emulsion-type alternative protein products [15].
Plant-based products, such as vegetables and pulses, have gained increasing attention over the time, representing an excellent source of proteins and phytochemicals. In this regard, it has been documented that diets rich in these products may prevent the induction of a wide range of diseases [16]. Over the last decade, the re-introduction of underutilized (neglected, minor, or orphan) crops were found to be an excellent strategy to improve global food security [17]. Underutilized crops are referred to species cultivated in their centers of origins but less used at the global level because they are non-competitive in terms of agronomic, economic, or cultural properties compared to those most cultivated. They are rich in nutritional, phytochemical, and therapeutic qualities [17]. In addition, their ability to survive extreme environmental conditions makes these species a good choice to face climate change and the vulnerability of agriculture and horticulture [18]. Alongside these categories of protein sources, leaves also represent a valuable source of plant proteins to be implemented in the formulation of meat alternatives. Dried powder obtained from fresh leaves can contain up to 30% of the protein content. Beyond terrestrial panacea plants, some aquatic weed species such as duckweed are superfood highly consumed in Asia with a high protein content attracting enormous interest as alternative protein sources to be used in human nutrition.
Insects are a common food for 2 billion people across the globe [19], and over 2000 edible species are known. Because of their high protein content and nutritional value, edible insects, especially crickets, are currently considered a solution to the growing protein demand worldwide [20,21]. Despite its widespread consumption in eastern countries, the European market appears to convert to such new eating habit based on insect foods. However, the growing need for alternative sources of proteins other than meat and their sustainable growing conditions is promoting their acceptance in western countries for the several advantages offer. Considering the urgent need for alternative sources of proteins, on 3 January 2023, the European Union allowed the placing on the market of Acheta domesticus, i.e., the domestic cricket, in partially defatted powder as a novel food [22]; yellow mealworm and migratory locus were previously approved [23]. Most insects are high in micronutrients such as potassium, calcium, iron, magnesium, and selenium and are also a good source of proteins [24]. An overview of the evolution of the European legislation issued on novel food is reported in Table 1.
In most cases, they have higher quality proteins than plant and meat proteins, in terms of their nutritional value, total protein content, essential amino acid composition, and protein efficiency [25]. According to studies conducted by Mason et al. [19], the production of one gram of beef requires 21 times more water than the production of the same amount of protein from cricket, making this protein source a valuable and sustainable alternative to the conventional meat-based proteins [26,27]. Insects have great biodiversity and biomass, potentially being a natural resource to produce new bioactive peptides [28]. Several studies show that insect peptides/polypeptides have antihypertensive, antioxidant, antidiabetic, and antimicrobial activities [21,29,30,31]. Biotechnology appears to be a promising approach to mass production of insect bioactive peptides. A risk of triggering allergic reactions based on cross-allergenic reactions to crustacean and house dust mite proteins is likely to occur in sensitive individuals [32,33].

Table 1.
Chronologically ordered set of the released legal documents regarding insect production for food and feed.
Table 1.
Chronologically ordered set of the released legal documents regarding insect production for food and feed.
| Name of the Document | Year | Reference |
|---|---|---|
| Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No. 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No. 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No. 1852/2001. | 2015 | [34] |
| EFSA Scientific Opinion “Risk profile related to the production and consumption of insects as food and feed” issued 8 October 2015. | 2015 | [35] |
| IPIFF information document “Regulation (EU) 2015/2283 on novel foods- Briefing paper on the provisions relevant to the commercialisation of insect-based products intended for human consumption in the EU." | 2015 | [36] |
| Commission Regulation (EU) 2017/893 of 24 May 2017 amending Annexes I and IV to Regulation (EC) No. 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No. 142/2011 as regards the provisions on processed animal protein. | 2017 | [37] |
| Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 laying down administrative and scientific requirements for applications referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. | 2017 | [38] |
| ‘Novel Food’ Report: Opinion on the Risk Profile for House Cricket (Acheta domesticus) by the Swedish University of Agricultural Sciences (EFSA funded report, adopted on 6 July 2018). | 2018 | [39] |
This review aims to explore novel alternative proteins to meat, including underutilized legumes, aquatic weeds, edible fungi, and insects. The food applications and functional activities related to their derived peptides will also be highlighted in order to underline recent advances and shed light on unexplored fields. In the last section, safety issues related to the introduction of some novel foods in the market will also be discussed.
2. Underutilized Legume Crops
In plant realm, Fabaceae (Leguminosae) family is a source of seeds known as pulses, showing the best compromise between sustainability and nutritional value, in terms of dietary proteins and other essential nutrients, especially in countries where animal protein sources are scarce and considered expensive or avoided for religious reasons (i.e., India) [40,41]. Despite the high number of legume species currently known, only some show well-established domestication and cultivation, such as soybean (Glycine max L.), cowpea (Vigna unguiculata L.), peanut (Arachis hypogaea L.), common beans (Phaseolus vulgaris L.), pea (Pisum sativum L.), lentil (Lens culinaris), and chicken pea (Cicer arietinum L.). Other legumes less known and less exploited are counted among the underutilized and orphan species. This category includes grass pea (Lathyrus sativus L.), lupine (Lupinus Albus), winged bean (Psophocarpus tetragonolobus), and bambara groundnut (Vigna subterranea), shown in Figure 1, which are considered underutilized legumes with promising potentials because of their hardiness, remarkable nutritional profiles, and desirable protein content, especially in their seeds. Orphan legumes that are comparable with soybean in terms of nutritional value are described.

Figure 1.
Underutilized legumes: (a) Winged beans (b) Grasspea (c) Lupins (d) Bambara groundnut.
Grass pea (Lathyrus sativus L.) is a member of the Fabaceae (Leguminosae) family, subfamily Papilionoideae, tribe Vicieae, representing an important annual legume crop cultivated in several areas of the world in South, Southeast Asia, Middle East, Eastern Europe, North America, South America, and East Africa [42,43], with production estimated at 1.2 million tons [44]. Grass pea seeds are used as a pulse, dahl, and flour to prepare different savories, sweets, and snacks. Seeds contain about 8.6–34.6% proteins, higher than chickpea (18%), field pea (21%), French bean (20%), and similar to other legumes, they are rich in lysine (18.4–20.4 mg/kg) but low in sulfur-containing amino acids, ranging from 3.8–4.3 mg/kg in cysteine and 2.5–2.8 mg/kg in methionine [43]. Interestingly, grass pea was found to be the only known dietary source of l-homoarginine, which has been shown to provide benefits in cardiovascular disease treatments. Therefore, as a nutraceutical, grass pea is an excellent example of a potential “functional food” [45,46]. This legume was found to be an excellent source of phenolics [47]. Despite its high nutritional value, grass pea consumption is still limited, likely because of its high content of the neuroexcitatory β-N-oxalyl-l-α, β- diaminopropionic acid (β-ODAP), which causes the neurodegenerative disease called neurolathyrism if overconsumed [48]. However, food processing, such as boiling, was shown to reduce β-ODAP concentration (down to 30% after 90 min boiling) [49]. Over the last few decades, several breeding programs tailored to produce grass pea varieties at reduced β-ODAP content were put in place, and different cultivar with a β-ODAP content <0.1%, have been released [44]. However, the stability of such varieties needs to be assessed over the long term because of the prime influence of climatic and edaphic conditions, along with the strong genotype × environment effect on the final β-ODAP content [50,51,52]. From an agricultural point of view, grass pea shows resistance to harsh environmental conditions [53], such as drought, heat, soil infertility, floods, and many ranges of biotic stresses, and minimal inputs and cost are required for its cultivation. Because of its valuable agronomic and nutritional properties, grass pea figures as a sustainable and nutritional legume. Therefore, its reintroduction into the human diet as a potential functional food would be desirable. As for industrial application, grass pea protein-based films and coatings for the food industry are reported [54], whilst the use of grass pea proteins in food formulation is still limited, probably due to their anti-nutritional factors [55].
Lupinus is a large genus comprising around 200 species, among which four are cultivated in different world regions, namely L. angustifolius (narrow-leafed), L. albus (white), L. luteus (yellow), and L. mutabilis (Andean). Australia is the leading producer of lupin, followed by Poland, Russia, Morocco, and Germany. From 1999 to 2018, the total area of lupin cultivation decreased by about one-third from 1.48 to 0.98 Mha, and total production declined by nearly half from 2.1 to 1.2 Mt [56]. Lupinus albus was the main Lupinus species cultivated in the Mediterranean region, and it is considered a valuable nutritive source containing lower amounts of fat (~6%), a high number of essential amino acids, important dietary minerals, and higher protein (~40%) and dietary fiber (~28%) contents [57]. Due to comparable quantities of proteins with similar amino acid profile, lupin is considered a cheap alternative to other legumes, such as soybean. Interestingly, lupin was found additionally rich in phytochemical, such as importantly bioactive peptides, alkaloids, polyphenols, phytosterols, tocopherols, that make this legume flour a potential food ingredient, especially for bakery products [58]. As an implication of human health, the high fiber and the antioxidant, antihyperlipidemic, and anti-inflammatory activities of the phytochemical of lupin act against various chronic diseases [58]. In contrast to grass pea, the usage of lupin proteins to develop novel foods (e.g., cheese analogue; gluten-free pasta, cookies, and cakes) [59,60,61,62] or to improve food nutritional value was widely explored [61,63]. Lupin protein supplementation was also found to affect positively organoleptic and textural properties of foods [64].
Winged bean (P. tetragonolobus) is a vining plant commonly found in humid countries such as Sri Lanka and Malaysia [65]. Similar to other legumes, the winged bean can fix atmospheric nitrogen, thus promoting the soil fertility and production of other crops such as rice [66]. Although its cultivation to a large scale is limited because of the high variability and low yield [67], the winged bean could be considered a multipurpose legume in terms of agricultural and nutritional properties. From an agricultural perspective, its cultivation requires low external inputs, making it suitable to be cultivated in areas where water scarcity and hot temperatures are increasing because of climate change [17]. In addition, it could be considered a good source of nutrients. Indeed, apart from having a high protein content equivalent to the soybean, its seeds contain a good balance of amino acids, including lysine, which is poor in cereal-based diets [68]. Some minerals found in this plant have been reported to be higher than soybean, including thiamin, riboflavin, and niacin [69]. Winged bean seeds are also rich in oil, particularly unsaturated oil, which is rich in Vitamin E. In addition, all parts of the plant (except the stems) are edible, palatable, and nutritious [70]. The high nutritional value of this legume, combined with its ability to grow in harsh environmental/climatic conditions, make the winged bean a key functional food, also alternative to meat consumption, which cultivation could be implemented, especially in semi-arid and hot regions where the under nourishing diet affect people’s health. Despite its high nutritional value, studies reporting the supplementation of winged bean in food formulation are scarce [71,72,73]. Recently, Saloko et el. reported that the addition of the mixture 9%-winged bean flour: 6% konjac flour in corn noodles significantly affects the final content of water, protein, dietary fiber, fat, and calcium, as well as improving some technological and organoleptic properties of the food [71].
The Bambara groundnut (V. subterrenea) is another underutilized legume with comparable potential content to soybean. This legume is indigenous to Africa, where it is considered the third most important leguminous crop after cowpea (V. unguiculata) and peanut (Arachis hypogaea) [74]. From a nutritional point of view, Bambara groundnut seeds contain 63% carbohydrate, 22% protein, and 6.6% fat [75], showing a total energy value greater than that of other common pulses, such as cowpea, lentil (Lens esculenta), and pigeonpea (Cajanus cajan). Bambara groundnut was found to be a good source of fiber, calcium, iron, and potassium, and among the different varieties consumed, the red seeds contain almost twice as much iron as the cream seeds, thus representing a good choice in areas where iron deficiency is a problem [75]. Because of its valuable nutritional value, Bambara groundnut was proposed as an ingredient to implement foods for infant weaning to improve dietary intakes of children in countries characterized by protein-to-energy malnutrition [76,77]. In addition to infant formulations, flour blends of Bambara groundnut were used for preparing high protein snack products. Snacks produced from blends of Bambara groundnut, cassava, and soybean at different levels reported protein content varying between 14.25% and 16.25%, of which the high protein content and acceptability was attributed to that containing 80% Bambara groundnut [78]. Using Bambara groundnut in enriching breakfast cereal and pasta, traditional foods and milk-based products are also reported [79]. Food applications of these alternative protein sources are given in Figure 2. The levels of starch and amylose (up to 53% of seed of starch and 15.7–35.3% of amylose in starch) and dietary fiber content (up to 10.3% of seed) make this crop suitable for the control of diabetes and high cholesterol correlated disease [80]. Similar to other legumes, Bambara groundnut shows high valuable agronomic properties, such as drought tolerant [81] and ability to grow in poor soils where most common crops do not thrive [82]. In the light of its nutritional and agronomic properties, the cultivation and consumption of Bambara groundnut has the potential to positively contribute to food and nutritional security at both the regional and global levels.
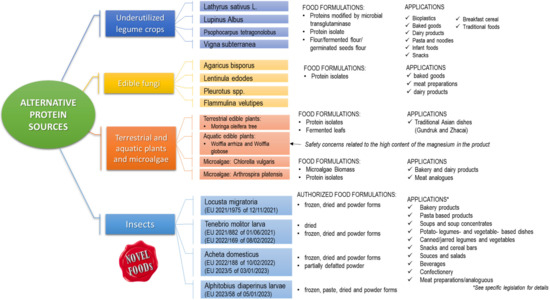
Figure 2.
Food formulations and relative applications of different sources of proteins alternative to meat including underutilized legume crops, edible fungi, terrestrial, aquatic plants and microalgae, and insects.
3. Edible Fungi
Proteins from edible fungi are gaining enormous interest in their healthy nutritional profile. The most cultivated fungal species are Agaricus bisporus (button mushroom, white or brown, or portobello, representing about 40% of worldwide production), Lentinula edodes (about 25% of worldwide production), Pleurotus spp. (mostly P. ostreatus), and Flammulina velutipes [83]. Examples of Pleurotus eryngii and Flammulina velutipes grown on ad the appropriate substrate are reported in Figure 3.

Figure 3.
Edible fungi: panel (a) Flammulina velutipes grown on Potato Destrose Agar (PDA) for 12 days at 25 °C; panel (b) Pleurotus eryngii grown on PDA for 12 days at 25 °C.
Edible fungi have been widely studied as alternative sources of proteins; therefore, this section will provide an overall discussion. The amount of protein in edible fungi varies from 19 to 45% of dry matter (DM), depending on species, stage of maturation, parts of fungus, substrate, and technological process used for their cultivation [13]. The application of biotechnology (e.g., bio-refinery and solid state fermentation) in a bio-based circular economy to produce fungal proteins is encouraged and is being currently investigated [84,85]. The production of multiple secreted proteins or specific target proteins can be carried out using native strains or engineered by highly specialized fungal hosts that can perform various post-translational processing, making them more suitable than bacterial or yeast hosts [86]. Protein yields are also affected by the growth substrate; fungal cultivation on agro-industrial wastes (e.g., corn stover, paddy straw, peat extracts; ram horn hydrolysate) significantly increases the protein content, reaching a percentage higher than 45% DM [87]. Today, a derivative of Fusarium venenatu, an authorized protein source for sale by the UK Government since the 1990s, is produced to a large scale in a continuous fermentation process and exported globally under the brand name QuornTM [88]. Proteins from fungal dried powder (also named “mycoprotein”) are typically used to fortify various foods due to their high nutritional value. Fungal proteins are highly considered substituting partially or entirely animal-based protein foods such as meats [89]. Toxicology studies have ascertained that mycoproteins include no negative effects on the normal growth of humans and animals [90]. Amino acid analysis of Pleurotus spp. has demonstrated the presence of all essential amino acids [91], with leucine, aspartic acid, phenylalanine, and lysine being the most abundant. Umami amino acids were also found together with non-essential amino acids such as gamma- aminobutyric acid (GABA) and ornithine [92]. A recent study carried out on human volunteers has demonstrated that the biological value of proteins in mycoproteins is similar to milk proteins. The protein-to-energy ratio (PER) for nine species of edible mushrooms with a protein content from 16 to 37% was studied by Bach et al. in 2017 [93] and ranged from 0.051 to 0.098. These values were similar to those registered for foods such as beef jerky, whole milk, and lentils [13]. By considering the range of protein amount reported above, 100 g of dried mushrooms can cover from 29.41% to 66.00% of the Recommended Dietary Allowance (RDA) for men and from 35.80% to 80.35% for women [13]. Gonzalez et al. in 2020 [13] reported the True Protein Digestibility (TPD) for some edible mushrooms determined by using Sprague-Dawley rats as animal models. Values highlight a large variability (ranging from 42 to 80%) because of the different chemical composition, also within the same species, and probably because of conditions of cultivation or stage of the mushroom maturation. Less variable data are reported for the Biological Value (BV). In particular, 63.72, 71.94, 74.82 and 77.18 were reported for Lentinus lepidus, P. sajor-caju, P. ostreatus, and L. edodes proteins, respectively. These values showed that a high amount of amino acids is absorbed by the gut that is retained by the body [13], confirming the high nutritional value of mycoproteins. In addition to their nutritional properties, proteins from edible mushrooms have shown interesting biological activities that may improve health, treat and/or preventing disease [93]. Indeed, many proteins with interesting biological activities have been discovered and isolated from edible fungi, such as lectins, fungal immunomodulatory proteins (FIP), ribosome-inactivating proteins (RIP), antimicrobial or antifungal proteins, ribonucleases, and laccases [94]. Beneficial effects have also been attributed to mushroom-derived bioactive peptides [95]. Thus, several studies report the beneficial effects associated with mycoprotein ingestion [96,97,98]. New epidemiological work [98] studying mycoprotein-based food intake using data from the UK National Diet and Nutrition Survey shows that higher mycoprotein intakes are associated with significantly lower markers of glycaemia, improved fiber intake profiles, and diet quality scores. Thus, considering the beneficial effects, the use of mycoproteins has been reported in a wide variety of food products, including baked goods (such as pasta, breads, and cookies, refs. [99,100,101,102,103]), meat products [89], and dairy products [104], as shown in Figure 2. Beyond the nutritional value, improvements in physicochemical and/or organoleptic characteristics have also been reported. Recently, a recombinant Ery4 laccase, purified from P. eryngii and expressed in S. cerevisiae, was added to the manufacturing protocol of a dairy product, resulting in an increase in antioxidant properties and texture and protein content, with a product yield 10% higher than control sample [105,106]. This protein also proved to have the potential to degrade mycotoxin, contributing to increasing the safety standards of food products [107,108].
4. Terrestrial and Aquatic Plants and Microalgae
4.1. Terrestrial and Aquatic Plants
Leaves can also represent a valuable source of plant proteins to be implemented in the formulation of meat alternative products. More than 70% of the proteins are in the chloroplast, where ribulose bisphosphate carboxylase (RuBisCO), an enzyme involved in photosynthesis, is the dominating protein [109]. The protein content of a leaf evolves during its development, resulting in a progressive degradation to free amino acids starting from the early senescence [109]. Moringa oleifera tree (also known as drumstick tree), and Wolffia arrhiza and Wolffia globosa (also known as duckweed), are only two examples of fully edible plants with a high protein content. However, the bio-accessibility of nutrients, particularly proteins, in vegetable foods is impaired by non-starch polysaccharides (cellulose, hemicellulose), polyphenols, and anti-nutritional factors (inhibitors of proteases and α-amylases) that interfere with food proteins and digestive enzymes [110]. The isolation of proteins from the vegetable matrices helps to improve the digestibility by removing fibers [111]. However, food-grade extraction procedures, including, for example, the two-step alkaline extraction/isoelectric precipitation, used for preparing protein isolates from seeds [112,113], fails when applied to leaf powder because of the low solubility of membrane proteins compared to seed storage proteins. Moringa oleifera Lam (Moringaceae) is a valuable perennial plant cultivated in tropical and subtropical regions. In Figure 4, an example of fresh Moringa oleifera plant, its dried leaf, and leaf powder to be employed in different preparations is depicted. Moringa is considered a superfood in India with several edible parts, including leaves, roots, seeds, bark, fruit, flowers, and immature pods. Being rich in proteins, vitamins and minerals, the leaves are part of the diet of pregnant women and weaning children with in vivo proved beneficial effects [114,115]. D’Auria et al. reviewed the nutritional profile and the food applications of the Moringa oleifera seeds and leaves [116]. The enzyme-assisted extraction of proteins from moringa leaf powder, using a cellulolytic enzyme mixture, by breaking down the matrix structure, made it possible to prepare a high protein concentrate (55.7%, w/w) that would not be achievable with alkaline extraction alone [117]. From a nutritional standpoint, the concentration process improved dramatically the in vitro digestibility of the Moringa leaf protein isolate by over 35% compared to the whole leaf powder, with a value (digestibility score of 99.9% and PDCASS 91.42%) that appears to be comparable to whey proteins and perfectly in line with the FAO requirements [117,118]. An alternative to protein isolation for improving the digestibility of leaves is the fermentation. Shi et al. [119] demonstrated that solid-state fermentation of drumstick leaf flour induced by Aspergillus niger, Candida utilis and Bacillus subtilis allowed to obtaining of high-quality proteins, increased concentrations of small peptides and amino acids, as well as reduced concentrations of anti-nutritional factors such as tannins, phytic acid, glucosinolates, and sugars. Overall, great interest is emerging towards the exploitation of Moringa as a value-added ingredient for both bakery products (e.g., bread, biscuit, cake, fresh and dried pasta, and snacks) and meat products [120] (See Figure 2). However, the supplementation of Moringa in the formulation of meat products still represents a partially explored research field, mainly when considering the bioaccessibility and bioavailability of M. oleifera bioactives, thus requiring future research works. It is important to highlight that plant extracts (such as those from Moringa) are recently gaining wide popularity in the meat industry since they are perceived by consumers as safe and included in the Generally Recognized as Safe (GRAS) category [121].

Figure 4.
Moringa oleifera (a) fresh leaf (b) dried leaf (c) leaf powder.
While M. oleifera is a terrestrial panacea plant, duckweed is an aquatic superfood consumed in Asia [122]. It is a monocotyledonous plant of the family Lemnacae, including five genera (Spirodela, Landoltia, Lemna, Wolffiella, and Wolffia) shown in Figure 5, and belonging to the same genera as the Wolffia, which are aquatic [123]. On a dry base, the protein content of duckweed can be as high as 43%, including all essential amino acids [122]. The plant has been recently assessed for its safety as a food ingredient by EFSA in both forms, as powder [124,125] and as fresh vegetable, such as spinach [126]. The NDA panel of EFSA concluded that the high content of magnesium in the product would be of safety concern, and the safety of use of the novel products could not be established. However, the high protein content of the powder and its amino acid profile may push the use of protein isolates as an ingredient in the near future. The preparation of an alkaline extract from a Lemna gibba powder allowed the preparation of a 67.2% protein concentrate (w/w) with good solubility at pH higher than 6 and a similar gel strength at pH 4 to 7, comparable to soy proteins but lower than egg white proteins [127]. It is worth mentioning that the authors used a nitrogen-to-protein conversion factor of 5.8, as suggested by Martin et al. for the spinach RuBisCO, which is the most abundant protein in the leaf [128]. Protein isolates from W. globose, obtained using ultrasound-assisted extraction, also showed antimicrobial properties and better emulsifying stability compared to whey proteins for at least 24 h. Duckweed nutrient content and metabolite composition have gained extensive attention, particularly in the animal feed industry [129]. The application of duckweed as plant-based ingredient for future food products is very limited [129]. Figure 2 provides an overview of food applications described in the literature of alternative proteins extracted from these sources.

Figure 5.
Duckweed genera: (a) Lemna minor, (b) Wolffia, (c) Spirodela polyrhiza, (d) Landoltia punctata, (e) Wolfiella gladiate.
4.2. Microalgae
Microalgae are underwater aquatic plants that have been suggested as food ingredients for their high protein content ranging between 40–70%, depending on the species, poly-unsaturated fatty acids, and micronutrients (minerals and vitamins) [130,131,132,133]. Similar to other plants, microalgae synthetize RuBisCO, even though not to high levels as terrestrial and superficial aquatic plants such as the duckweed [134]. The essential amino acid profile of microalgae appears to be in line with those of soybean and egg proteins [133]. The mainstream microalgae explored as novel ingredients are Chlorella vulgaris and Arthrospira platensis also known as Spirulina. While Chlorella sp. is not classified as a novel food within the European Union, meaning they do not fall under Regulation (EU) 2015/2283 [34], Spirulina sp. is to be classified as a novel ingredient [135] and therefore requires a dedicated safety and nutritional assessment. Efforts have been made to look for technological approaches for improving the yield of productions through breeding and strain selection and genetic engineering [136]. Among the limitations associated with the use of whole microalgae biomass as the ingredient is the “grassy-fishy” flavor/aroma and the color [137,138]. Research efforts made possible the preparation of honey/yellow and the while Chlorella powders that are obtained from chlorophyll-free strains through the selection of low chlorophyll strains and the chemically induced random mutagenesis [139,140]. Both mutant strains of Chlorella have high protein content (40–50%) and essential fatty acids and are already a reality in the market commercialized by Allmicroalgae. The presence of the cellular walls, representing the 10% of the dry weight, is among the major obstacle to protein digestibility of the microalgae biomass. However, data on digestibility appears to differ depending on the species, from as high as 78% from the A. platensis, having a wall mainly composed of the peptidoglycan layer, to 45% Nannochloropsis oceanica and Chlorella [141,142,143]. Microalgae biomass has been used in the formulation of several food products from bakery to dairy products [138,144,145,146,147]. Despite the direct use of biomass would be economically preferable, cell walls and oil also have a negative impact on the texturing properties of the meat analogues prepared using microalgae [146,148]. The preparation of microalgae protein isolates follows a three-step approach with the mechanical or enzyme-assisted cell disruption, solubilization, and the concentration. Microalgae protein extracts are used for complementing formulations extruded for preparing meat analogues. Microbial proteins show excellent performances as functional ingredients, with excellent emulsifying and foaming properties with reduced dependency on pH and superior interfacial stabilization than animal proteins [149].
5. Insects
Insects are deemed a good source of micronutrients and proteins, with an average protein content of 40%, ranging from 20% up to over 70%, depending on the species [150]. Insects are a common food for 2 billion people in 119 countries across the globe. Over 2000 species are edible, and the most exploited as protein sources are Coleoptera Beetles, followed by Lepidoptera Caterpillars, Hemynoptera, wasps, bees, and ants. Although more familiar for Asiatic population, with a widespread consumption in India, China, Thailand, South Korea, Japan, but also Mexico, Brazil, and several African regions, insects are still novel foods for Western countries [20,21]. Due to the growing need for alternative sources of proteins and the attractive advantages shown in cultivating insects in terms of higher sustainability, such tendency of consuming insects-based foods is progressively revolutionizing Western eating habits and the European market. Three insect species that are widely bred in Europe (Tenebrio molitor, Gryllodes sigillatus, Schisocerca gregaria) are considered to have the biggest potential as food components since they contain the highest amount of protein estimated to be 52, 70, and 76%, respectively [151]. On the other hand, it has to be emphasized that often, the protein content can be overestimated due to the presence of a non-protein nitrogen [152]. Although insect proteins are characterized by high threonine and lysine content and low levels of methionine or tryptophan, the essential amino acids’ score for insects ranges from 46% to 96%, exceeding the lowest recommended level for human diets (>40%). The quantity of the same amino acids is even higher in insects than in plants and animals [153]. In addition, insect proteins are more digestible than plant proteins and exhibit lower digestibility than animal proteins [154]. Insect-based proteins are characterized by a low level of solubility ranging from 3% to 45%, which may be improved by enzymatic hydrolysis [155]. Therefore, the application of insect proteins is recommended for foods that do not require high solubility, such as meat analogs. Insect proteins generally have good oil and water retention capacity, emulsion activity, and foaming activity. Among them, edible grasshopper (S. gregaria) and honeybee (Apis mellifera) display highly emulsifying properties comparable to whey protein, showing their potential as alternative emulsifiers [156]. However, the functional characteristics of insects are different and strictly related to the species. For example, protein extracts obtained by conventional means such as Hermetia illucens and other insect species have poor solubility in aqueous media, and this may impair their nutritional value, biological activity, functionalities [157]. The proteins of Protaetia brevitarsis and Allo-Myrina dichotoma species exhibit higher protein thermal stability and emulsification activity than T. molitor, and, as result, they show better manufacturing characteristics [158]. On the contrary, the higher concentration of polyphenols in the protein concentrate of Hermetia illucens can reduce its emulsification activity [159]. Therefore, to increase the use of insect proteins in food production, the functional characteristics of insect proteins should be improved. Innovative processes have been optimized to increase specific abilities of insect proteins such as ultrasound [160], ultra-high pressure, pH-shifting technology [161], and cold atmospheric pressure plasma processing [162]. As already stated, the insects are considered novel foods within European countries and comply with specific regulations for the production, transportation, storage, and commercialization, both as animal feed and food. A blooming of European directives and regulations have been issued in the last 10 years in order to regulate the production and commercialization of novel foods as summarized in Table 1. In the last five years, the European Commission (EC) regulated the production, transport, and storage conditions of insect-based meal allowed in aquafeed from black soldier fly (BSF) (Hermetia illucens), common housefly (Musca domestica), yellow mealworm (T. molitor), lesser mealworm (Alphitobius diaperinus), house cricket (Acheta domesticus), banded cricket (Gryllodes sigillatus), field cricket (Gryllus assimilis), and Silkworm (Bombyx mori) [37,163]. In August 2021, the EC adopted the decision to allow the use of insect-processed animal proteins in formulated pig and poultry feeds [163]. The International Platform of Insects for Food and Feed (IPIFF)—namely the European umbrella organization representing stakeholders active in the production of insects for food and feed—is encouraging European member states to implement regulations from the European Commission [164], supported by favorable opinion of the EFSA Panel on Nutrition, Novel Foods, and Food Allergens (NDA) [165]. The commercialization of Locusta migratoria (EU 2021/1975 of 12/11/2021 [166]), T. molitor larva (EU 2021/882 of 01/06/2021 [167], EU 2022/169 of 08/02/2022 [168]), A. domesticus [EU 2022/188 of 10/02/2022 [169], EU 2023/5 of 03/01/2023 [22]), and A. diaperinus larvae (EU 2023/58 of 05/01/2023 [170]) as novel foods (under the frame of Regulation (EU) 2015/2283 [34]) has been authorized in specific formulations and applied to specific food categories listed in Table 2.

Table 2.
Summary of the insect-based products whose placing on the market has been authorized within the European Union as novel food in specific formulations and food categories.
About house cricket, EFSA has highlighted that the consumption of the evaluated insect proteins may potentially lead to allergic symptoms. It was also noted that allergens present in the substrate fed to insects, when the whole insect is meant as the ingredient, may reach the ultimate consumers through the consumed insect [171]. The current market trends in modern sedentary lifestyles have driven the development of new functional products that can fulfill consumers’ demand for a healthy diet. Bread, in particular, is a vital staple food and is consumed throughout the world because it is rich in proteins but lacks some essential amino acids (lysine and trilysine) [172,173,174]. Innovative additives, such as insect flour, can be utilized in several bread formulations due to their high protein content, contributing to the enhanced quality and increased nutritional value of the final product [173,175]. For example, sourdough obtained from cricket powder hydrolysates has been reported to be rich in health-promoting molecules such as arachidonic acid and linolenic acid, which could be used for innovative bread production with high nutritional and functional value [176]. Previous studies have shown that wheat bread fortification with various edible insect flours, such as mealworm, buffalo worm, cricket [177,178], and the larvae of the black soldier fly [173,179] has improved the biological value of bread due to the high protein content characteristics. Pauter et al. [180] used 10% cricket powder to increase the protein content of muffins by 1.4-fold. In addition, da Rosa Machado and Thys [181] reported that the enrichment of cricket powder in gluten-free bread can lead to a final high protein content (8.53–12.52, wt%). The same trend was reported by Nissen et al. 2020 [182], where cricket powder was reported to provide gluten-free sourdough bread with high nutritional value proteins. In addition to the nutritional value, cricket enrichment helps products have a unique aroma [182] and improved texture, namely increased hardness and improved consistency [183]. Currently, more than 160 patents and around 500 articles have appeared in the literature about the applications of edible insects [184]. Figure 2 reports some main food applications referred to products fortified with insects in different authorized formulations.
6. Bioactive Properties of Non-Meat Proteins
Biologically active peptides (BPAs) are derived from proteins and exert a positive effect on humans due its health-promoting properties. They have a molecular weight lower than 6 kDa and contain between 3 and 20 amino acids residues that remain inactive in the parent protein and exhibit their activity after release and transport to the active site [185]. Structure–activity relationships are of importance, and the amino acid sequence of the peptide influences its biological activity. The presence of amino acids such as alanine, cysteine, histidine, lysine, leucine, methionine, proline, valine, tryptophan, and tyrosine may contribute to the antioxidant activity of peptides [186], whilst Pro, Lys, or aromatic amino acid residues contribute to Angiotensin Converting Enzyme (ACE) inhibitory activity [187]. BAPs can be released from food proteins during gastrointestinal digestion and through fermentation, germination, enzymatic hydrolysis, and during food processing. Novel processing technologies, such as high pressure, microwave, and pulsed electric field, have recently emerged to overcome the problems associated with the conventional hydrolysis methods [188]. Apart from the already mentioned above, BAPs exhibit a broad range of activities and can be involved in the gastrointestinal system (anti-obesity and satiety peptides), the cardiovascular system, the immune system (antimicrobial, antibiofilm, antiviral, cytomodulatory peptides), and the nervous system (opioid peptides) [189,190,191,192]. Most studied BPAs are derived from protein of animal origin [192]. However, alternative sources have been explored to discover novel sequences addressed to drug targets. Despite the bioactivity of several novel food ingredients described in the literature, the bioactive sequence is not known due to a lack of a tailored pipeline for the isolation and mass spectrometry mapping. On the other hand, protein hydrolysates are often mapped by mass spectrometry, and prediction of bioactivity is made based on bioinformatics, without an in vitro validation. Table 3 reports a list of active peptides from alternative sources. To the best of our knowledge, the reported sequences are the only identified ones since most studies involved protein hydrolysates and their partially purified fractions.

Table 3.
Bioactive peptides from proteins alternative to meat.
Enzymatically-derived BPAs from proteins of major legume species (soybean, cowpea, groundnut, common beans, pea, and chicken pea) display a wide spectrum of biological activities ranging from nutraceutical to therapeutic potential. The hydrolysis process occurs in vitro by using food grade enzymes (alcalase) or during food processing (fruit ripening process), the fermentation process (to obtain soy sauce, tempe, natto, and other fermented products), or the germination process (producing soybean sprouts, green bean sprouts, and sprouts products others) [248]. Except from lupin, peptides released from minor legume proteins still represent an unexplored field worthy to be deeply investigated. Lupin-derived peptides have shown opioid, immune-modulating effects, as well as the ability to reduce glucose, cholesterol, and blood pressure (antihypertensive), depending on the type of investigation (in vivo and in vitro) as depicted in Table 3. This last evidence suggests that these bioactive peptides could probably be useful nutraceutical components in the development of anti-diabetic foods [249]. Papain was found to be the most efficient protease for the production of winged bean seed hydrolysate with potent ACE inhibitory and antioxidant activities [250]. ACE inhibitory properties were also demonstrated in vivo by lowering blood pressure in rat models, fostering the potential of winged bean-derived peptides as a functional food ingredient [251]. Similar biological effects were showed by Bambara groundnut protein hydrolysate and their peptide fractions [252]. It was also demonstrated that BAPs produced from Bambara bean protein hydrolysates demonstrated antihyperglycaemic activity (dipeptidyl peptidase, DPP-IV, inhibitory activity), cardio-protecting effects by ACE inhibition, and resistance to simulated gastro-intestinal digestion [253]. Putatively, due to the high content of anti-nutritional factors and to the best of our knowledge, grass pea-derived peptides have not yet been reported. Proteins and peptides endowed with biological activity from edible fungi are widely reported (Table 3); several biological effects have been reported [254], which make this alternative source of meat promising for exploitation in the development of functional foods. To date, fungal cell factories for efficient and sustainable production of proteins and peptides endowed with technological and healthy properties represent a current challenge [86]. Likewise, edible fungi, M. oleifera seed or leaves protein hydrolysates and related purified peptides showed several beneficial effects, including antioxidant and anti-inflammatory [255], hypoglycemic [231], antimicrobial [232], antihypertensive [229] properties (see Table 3). Partially purified peptides fractions suitable for the regulation of hyperuricemia were also obtained from tryptic hydrolysate of M. oleifera leaves. These fractions contained the most abundant peptides TSIVGNV, ASGGIHV, GNAPGAV, AGEENAG, and VTPQPGVPPEEA, which after gastrointestinal digestion released di-/tripeptides responsible for the inhibition of xanthine oxidase, used as a target in the treatment of hyperuricemia [256]. Most studies also focused on the genus Lemna, although scant information is available on the biological and functional properties of the released peptides. Antimicrobial activity, ACE inhibitory, and DPPH scavenging activities were found for enzymatic hydrolysates obtained by W. globose, Lemna minor, and Chlorella sorokiniana proteins [257,258]. Microalgae peptides that mimic the functions of mediators involved in pathologic processes responsible for vascular damage playing a putative role in the prevention of cardiovascular disease were discussed by Li et al. [212]. Cancer-inhibiting or immunomodulating effects of microalgae have been reported, although the number of reports on bioactive peptide sequences is very limited [259]. These studies, antioxidant and ACE potential have been confirmed in vitro for other microalgae hydrolysates obtained from C. vulgaris, Porphyra dioica, Fucus spiralis [234,260,261]. Bioactive peptides with anti-cancer and immunomodulating activities have also been described in the literature for C. vulgaris, C. pyrenoidosa and Dunaliella salina [262,263,264,265]. The most commonly followed method of bioactive peptides generation from microalgae involves enzymatic digestion of microalgae protein concentrates followed by fractionation using milder techniques such as UF at different membrane pore sizes (3–30 kDa) followed by chromatographic purification. The consumption of insect proteins, silkworm pupa, has been associated with a reduced risk of many diseases, such as cardiovascular diseases (hypertension and hyperlipidemia), and cancer (hepatoma and gastric cancer) [266]. The release of bioactive peptides is suggested based on biological effects (Table 3). Indeed, silkworm pupa hydrolysate was reported to possess several bioactive properties, such as inhibiting ACE activity, antioxidant activity, immunomodulatory activity, improving hypercholesterolemia (Table 3), beyond a decrease in the allergenicity of silkworm pupa protein [267]. The antitumor activity of silkworm pupae protein hydrolysates was also investigated [268,269], although more evidence will be needed to validate these results. Techniques, such as ultrasound, have been applied to assist enzymatic hydrolysis and increase production of bioactive compounds [270]. One of the first and most frequently studied insect peptide hydrolysates was derived from the proteins of the species Bombyx mori. The ACE inhibitory properties were the predominant bioactivity determined. The ACE-inhibitory peptide sequences identified from protein of B. mori pupae were, for example, KHV, ASL, and GNPWM (Table 3). Peptides showing this activity were also identified in cricket (Gryllodes sigillatus), mealworm (T. molitor), and locust protein (Schistocerca gregaria) [271].
7. Safety Issues Linked to Emerging Proteins and Derived Products
Despite technological and industrial application, safety aspects of alternative proteins remain poorly described and tackled. Thus, in this section, we attempt to summarize their current food safety status, including the environmental impact and regulatory framework.
Despite their proven high nutritional value, minor/orphan legume crops consumption could represent a relevant risk for human health because of the intrinsic toxicity of some of these plants. As previously mentioned, the major nutritional problem of grass pea is a neurotoxic non-protein amino acid, b-N-oxalyl-L-a, b-diaminopropionic acid (β-ODAP) in its seeds. Over-consumption of these seeds as a staple food in an unbalanced diet for 3–4 months can cause a disease known as neurolathyrism, which manifests as paralysis of the leg muscles, muscular rigidity, and weakness in humans and domestic animals. The concentration of the β-form is about 95% of the total ODAP and its content in grass pea seeds strongly depends on environmental factors [272] and, by the variety cultivated, indeed a wide range of variation for ODAP content (0.02–2.59%) was observed in grass pea germplasm. Several efforts have been made in the past to reduce this neurotoxin to a safe level for human consumption spanning from the development of grass pea varieties with low ODAP to a low-cost agronomic practices and post-harvest processing [44]. Breeding programs aimed at combining low ODAP content with high valuable agronomic properties led to different grass pea lines with < 0.1% toxin content. Anyway, because of the prime influence of environmental conditions on ODAP concentration, more research tailored to investigate the performance of these genetic lines in field needs to be undertaken [273]. As for detoxifying processing, the combination of soaking and boiling were found to be the most promising strategy to reduce ODAP content up to 67% [44]. Very little information is available on the allergens of grass pea. In 2018, Xu et al., identified an abundant 47 kDa protein in grass pea, which further studies demonstrated to have a homology to the 47 kDa vicilin from pea and Len c 1 from lentil. Interestingly, this grass pea 47 KDa protein was recognized by immunoglobulin E (IgE) antibodies from sera of several patients allergic to peanut, leading to supposed cross-reactivity between grass pea and peanut [274]. Lupine is another orphan legume able to cause severe intoxications (e.g., trembling, shaking, excitation, and convulsion) on humans if over-consumed. This critical safety issue is mainly ascribed to the high content of quinolizidine alkaloids especially in lupine bitter species. These last show a general content of alkaloids ranging from 1 to 3% in the dry weight of their seeds, among which the lupanine, a molecule belonging to quinolizidine alkaloids, that represents the major component. Other minor quinolizidine alkaloids found were albine, hydroxylupanine, sparteine, anagyrine, lupinine, and angustifolin. The high content of quinolizidine alkaloids group was found associated with severe intoxication [61]. Despite its potential toxicity, bitter lupines are traditionally consumed after soaking and cooking that can reduce the content of alkaloids [275]. To protect consumer’s health, the health authorities of several countries fixed the maximum limit of alkaloids in lupine below 200 mg/kg [276]. In line with this recommendation, modern breeding programs focused on developing lupine varieties with low-alkaloid content, the so-called “cultivated sweet” lupine varieties [277]. Sweet lupine seeds show a mean total alkaloid content ranging from 0.3 to 0.5% in the dry weight of their seeds, depending on the species, geography, and climate. Sweet lupines preserve the good agronomic properties of wild lupin, such as high adaptability to temperate and cold climates, low-fertile soils, harsh conditions, and high nitrogen fixation ability [277,278,279], demonstrating high suitability for low-input agriculture. Lupine allergenicity represents another critical issue to be taken into consideration while suggesting this legume consumption. The recent FAO/WHO expert consultation of food allergy, because of the lack of data on potency and prevalence, excluded lupin from the list of priority allergens, making it a problem for a few regions [280]. It has been reported in fact that lupine sensitivity is more prevalent in specific areas, including Mediterranean countries and Australia, where it is more consumed, and where severe allergy symptoms have been recorded, especially for cross-reactivity with peanut and other legumes (lentils, peas, and soybean) [281]. According to the European legislation, the European Union Regulation No. 1169/2011 included lupine among the 14 ingredients that must be declared on food labels because it can trigger allergies or intolerances [282]. According to the Allergen Nomenclature Subcommittee of the World Health Organization and International Union of Immunological Societies [283], the following proteins globulins (α-, β-, and γ-conglutin), 2S albumins (δ-conglutin), and some minor fractions, such as pathogenesis-related (PR)-10 proteins (Lup a 4 and Lup l 4), nonspecific lipid transfer proteins (nsLTP) (Lup an 3), and profilins (Lup a 5) represent the main lupine allergens currently identified [281]. Different strategies have been put in place for reducing lupine allergy, including enzymatic hydrolysis and thermal treatments, such as boiling, autoclaving, microwave heating and extrusion cooking with promising results obtained for enzymatic hydrolysis [284] and prolonged autoclaving [285]. Similar to other legume seeds, winged bean and Bambara groundnut contain several anti-nutritive factors (ANFs), which could limit the consumption of these foods. Tannins, lectins, flatulence factors, phytoglutenins, saponins, and cyanogenic glycosides reduce the nutritional value of winged bean [286], while phytates and tannins are the main ANFs of Bambara groundnut. ANFs are renowned for affecting the bioavailability of nutrients and minerals because of chelation reactions that could occur during digestion, reducing the level of nutrients potentially absorbable [287]. Tannins and phenolic compounds, not specifically, could inhibit enzyme activity and interact with food proteins to form complexes by reducing their quality [288]. However, different processing methods have been put in place to eliminate safely these substances without reducing their nutritional composition. For example, the use of moist heat (such as autoclaving and boiling) or soaking has been shown to significantly reduce the ANFs in winged bean, while preserving high value nutrients [289]. Fermentation of the seeds and other processing methods could be successfully used for reducing ANFs content in Bambara groundnut as reported in some studies [290].
The main food safety hazard associated with mycoproteins is allergens. The ingestion of QuornTM -based foods caused allergic reactions (hives and anaphylaxis) in individuals with a history of mold allergies; the IgE-mediated allergy to mycoprotein may be caused by the acidic ribosomal protein P2 [291]. Despite their nutritional values, microalgae may also carry harmful substances, such as heavy metals and toxins, particularly because of contaminations. Although lower than allowed levels, certain food supplements derived from Spirulina spp. and Chlorella spp. contained cadmium, mercury and this was likely due to the lack of quality control measures [292].
Toxins in microalgal dietary supplements were found when the environment is contaminated with toxin-producing cyanobacteria; in particular, the production of toxins (such as anatoxin-a, cylindrospermopsin, microcystins and saxitoxins), may result from direct toxin production by microalgae (Aphanizomenon flos-aquae) or cross-contamination with other toxin-producing cyanobacteria. Contamination by pathogenic microorganisms and IgE cross-reactivity poses additional safety issues associated with microalgal food products [291].
Using insects as food ingredients involves different levels of risk: biological, chemical, and allergenic risk [293]. Legal documents ensuring safety of insects-based foods and feed are chronologically reported in Table 1. About the biological risk, processing of the insects (e.g., hot slaughtering and cooking) can help moderate and eliminate the microbiological risk [294]. Contamination by chemicals is of enormous concern as reported by EFSA [35], with specific regard to heavy metals (cadmium, mercury, lead, and arsenic) and the accumulated pollutants from the environment (e.g., hormones and pesticides) [295]. A crucial key step in controlling chemical risks relates to the breeding stage and transformation process [296]. In addition to biological and chemical risks, the allergen risk of triggering a severe reaction in sensitive consumers is basically due to the presence of tropomyosin and arginine kinase—two major proteins common in different insects and responsible for allergic reactions [32,297,298]. These are renowned pan-allergens known for their cross-reactions with homologous proteins of crustaceans and dust mites [299]. The results reported by De Marchi et al. [26] revealed that tropomyosin identified in cricket was the most significant IgE binding protein, and cross-reactivity of it with the shrimp tropomyosin was verified by ELISA. Silkworm bodies contain allergens in their different growth stages (larva, pupa, moth) and their metabolites (silk, molting, feces) and 45 potential allergens were found in silkworms and some of them detected also in larvae, pupae, moths, silk, slough, and feces [300]. Thus, the risk of developing allergic reactions by eating insects poses an actual concern in allergic consumers. The results of the homology comparison showed that the allergens of silkworms were likely to cross-react with the allergens of Dermatophagoides farinae, Aedes aegypti, Tyrophagus putrescentiae, Triticum aestivum, etc. [300]. Based on closely related phylogenesis, the frequently reported IgE-binding cross-reactions between these allergens from different sources may cause unexpected cross-allergic reactions in susceptible individuals [51]. Due to the close taxonomic relationship between arthropods and crustaceans, patients allergic to house dust mites, shrimp, and mealworms should be cautious in eating insects [296,301]. The proper labeling of food containing insects is a correct measure to be implemented by food manufacturers to protect allergic consumers’ health [302].
Overall, alongside proper labeling, also the current digital tools and e-commerce platforms available facilitating access to food and selection from the virtual shelves should be implemented with information on the safety and functional properties of foods, in particular novel foods, in order to protect the consumers’ health including vulnerable ones [303].
8. Conclusions and Future Perspectives
The efforts of recent years in scouting for novel proteins sources alternative to meat and the increasing use of these non-meat proteins in the development of innovative foods proved to attract consumers for being healthy and eco-sustainable. The most exploited non-meat protein sources including algae, insects, fungi, and underutilized crops capable of growing also in harsh conditions were deemed promising because they are environmentally friendly and able to tackle the impact of the climate change worldwide. Fabaceae (Leguminosae) family is a source of seeds known as pulses, showing the best compromise between sustainability and nutritional value also including grass pea, lupine, winged bean, Bambara groundnut and are considered underutilized legumes with promising potentials, because of their hardiness, remarkable nutritional profiles, and desirable protein content, especially in their seeds with a good resistance to draught conditions. Edible mushrooms are rich in water and provide proteins, dietary fiber, chitin, vitamins (folate and B12), and some minerals. They are poor in fat and sodium and can be proposed as part of a well-balanced diet. Aquatic plants and microalgae are underwater aquatic plants, which have been suggested as food ingredients for their high, poly-unsaturated fatty acids and micronutrients (minerals and vitamins). Insects are deemed a good source of micronutrients and proteins with an average protein content of 40%. Over 2000 species are edible and the most exploited as protein sources are Coleoptera Beetles, followed by Lepidoptera Caterpillars, Hemynoptera, wasps, bees, and ants. Due to the growing need for alternative sources of proteins and the attractive advantages shown in cultivating insects in terms of the higher sustainability, such tendency of consuming insects-based foods is progressively revolutionizing western eating habits, having recently entered also the European market. To summarize, an increase in demand for plant-based protein is expected to be a consolidated trend in the coming years as the international community looks for new sources of proteins to meet the needs of a growing population. Due to the healthy properties described for these foods and the high abundance of bioactive peptides and phytochemicals promoting antioxidant defense and reducing inflammation, more consumers in future will turn to vegetarianism and veganism, which will also contribute significantly to the demand for such products. On another perspective, despite the recommendations to increase plant-food consumption in human diet, there have been some concerns raised about the potential presence of allergens or chemical contamination in the case of insects posing some safety hazard to consumers. On one hand, the high demand for novel and existing sustainable protein sources (e.g., legumes, insects, algae, and cultured meat) to replace animal-based sources is dramatically increasing. This change in protein consumption calls for future re-evaluation of the current methods to assess their quality and bioavailability, due to the fact that the food diet is massively changing. This fosters a deeper investigation in the future, including re-evaluating the two conventional scores currently in use: PDCAAS (protein digestibility-corrected amino acid score) and DIAAS (proteins indispensable amino acid score) because of their limitations. This is necessary in order to address plant and novel proteins’ quality.
Author Contributions
Conceptualization, L.M. and L.Q.; writing—original draft preparation, L.Q., C.N., E.D.A., A.L. and L.M.; writing—review and editing, R.P., F.R. and L.M.; funding acquisition, L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Alifun Project “Sviluppo di ALImenti FUNzionali per l’innovazione dei prodotti alimentari di tradizione italiana”, grant PON ARS01_00783. TheNutribox Project grant 20431-21 and Nutribox-App ref. 21361-19 funded by EITFood were also acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations. Report of the Secretary-General on the Work of the Organization (A/74/1, 74th Session); United Nations: New York, NY, USA, 2019; Available online: https://www.un.org/annualreport/2019/files/2019/09/Annual-report-SG-2019-EN-Complete-Web.pdf (accessed on 24 February 2023).
- Vermeulen, S.J.; Campbell, B.M.; Ingram, J.S. Climate change and food systems. Annu. Rev. Environ. Resour. 2012, 37, 195–222. [Google Scholar] [CrossRef]
- HLPE. Water for Food Security and Nutrition. A Report by the High Level Panel of Experts on Food Security and Nutrition of the Committee on World Food Security, Rome 2015. 2015. Available online: www.fao.org/cfs/cfs-hlpe (accessed on 24 February 2023).
- Charles, H.; Godfray, J.; Aveyard, P.; Garnett, T.; Hall, J.W.; Key, T.J.; Jebb, S.A. Meat consumption, health, and the environment. Science 2018, 361, 6399. [Google Scholar]
- Richter, C.K.; Skulas-Ray, A.C.; Champagne, C.M.; Kris-Etherton, P.M. Plant protein and animal proteins: Do they differentially affect cardiovascular disease risk? Adv. Nutr. 2015, 6, 712–728. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulou, K.; Dekkers, B.; van Der Goot, A.J. Plant-based meat analogues. In Sustainable Meat Production and Processing; Galanakis, C.M., Ed.; Academic Press: London, UK, 2019; pp. 103–126. [Google Scholar]
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel protein sources for applications in meat-alternative products—Insight and challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Zahari, I.; Östbring, K.; Purhagen, J.K.; Rayner, M. Plant-Based Meat Analogues from Alternative Protein: A Systematic Literature Review. Foods 2022, 11, 2870. [Google Scholar] [CrossRef] [PubMed]
- Schreuders, F.K.; Sagis, L.M.; Bodnár, I.; Erni, P.; Boom, R.M.; van der Goot, A.J. Mapping the texture of plant protein blends for meat analogues. Food Hydrocoll. 2021, 118, 106753. [Google Scholar] [CrossRef]
- Chiang, J.H.; Loveday, S.M.; Hardacre, A.K.; Parker, M.E. Effects of soy protein to wheat gluten ratio on the physicochemical properties of extruded meat analogues. Food Struct. 2019, 19, 100102. [Google Scholar] [CrossRef]
- Bhuvaneswari, M.; Sivakumar, N. Fungi: A Potential Future Meat Substitute. In Fungi in Sustainable Food Production; Dai, X., Sharma, M., Chen, J., Eds.; Springer: Cham, Switzerland, 2021; pp. 181–195. [Google Scholar]
- González, A.; Cruz, M.; Losoya, C.; Nobre, C.; Loredo, A.; Rodríguez, R.; Contreras, J.; Belmares, R. Edible mushrooms as a novel protein source for functional foods. Food Funct. 2020, 11, 7400–7414. [Google Scholar] [CrossRef]
- Molfetta, M.; Morais, E.G.; Barreira, L.; Bruno, G.L.; Porcelli, F.; Ducat-Bony, E.; Bonnarme, P.; Minervini, F. Protein Sources Alternative to Meat: State of the Art and Involvement of Fermentation. Foods 2022, 11, 2065. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of functional properties of edible insects and protein preparations thereof. LWT 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Sha, L.; Xiong, Y.L. Plant protein-based alternatives of reconstructed meat: Science, technology, and challenges. Trends Food Sci. Technol. 2020, 102, 51–61. [Google Scholar] [CrossRef]
- Hertzler, S.R.; Lieblein-Boff, J.C.; Weiler, M.; Allgeier, C. Plant proteins: Assessing their nutritional quality and effects on health and physical function. Nutrients 2020, 12, 3704. [Google Scholar] [CrossRef] [PubMed]
- Massawe, F.; Mayes, S.; Cheng, A. Crop diversity: An unexploited treasure trove for food security. Trends Plant Sci. 2016, 21, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Gregory, P.J.; Mayes, S.; Hui, C.H.; Jahanshiri, E.; Julkifle, A.; Kuppusamy, G.; Kuan, H.W.; Lin, T.X.; Massawe, F.; Suhairi, T.A.S.T.M.; et al. Crops For the Future (CFF): An overview of research efforts in the adoption of underutilised species. Planta 2019, 250, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B.; Black, R.; Booth, S.L.; Brentano, A.; Broadbent, B.; Connolly, P.; Finley, J.; Goldin, J.; Griffin, Y.; Hagen, K.; et al. Fostering strategies to expand the consumption of edible insects: The value of a tripartite coalition between academia, industry, and government. Curr. Dev. Nutr. 2018, 2, nzy056. [Google Scholar] [CrossRef] [PubMed]
- Gravel, A.; Doyen, A. The use of edible insect proteins in food: Challenges and issues related to their functional properties. Innov. Food Sci. Emerg. Technol. 2020, 59, 102272. [Google Scholar] [CrossRef]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef]
- Commission Implementing Regulation (EU) 2023/5 of 3 January 2023 Authorising the Placing on the Market of Acheta Domesticus (House Cricket) Partially Defatted Powder as a Novel Food and Amending Implementing Regulation (EU) 2017/2470; European Union: Luxemburg, 2023.
- Precup, G.; Ververis, E.; Azzollini, D.; Rivero-Pino, F.; Zakidou, P.; Germini, A. The Safety Assessment of Insects and Products Thereof as Novel Foods in the European Union. In Novel Foods and Edible Insects in the European Union; Scaffardi, L., Formici, G., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Veldkamp, T.; Dong, L.; Paul, A.; Govers, C.C.F.M. Bioactive properties of insect products for monogastric animals—A review. J. Insects Food Feed. 2022, 8, 1027–1040. [Google Scholar] [CrossRef]
- Rumpold, B.A.; Schlüter, O.K. Nutritional composition and safety aspects of edible insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- De Marchi, L.; Mainente, F.; Leonardi, M.; Scheurer, S.; Wangorsch, A.; Mahler, V.; Pilolli, R.; Sorio, D.; Zoccatelli, G. Allergenicity assessment of the edible cricket Acheta domesticus in terms of thermal and gastrointestinal processing and IgE cross-reactivity with shrimp. Food Chem. 2021, 359, 129878. [Google Scholar] [CrossRef]
- Kamemura, N.; Sugimoto, M.; Tamehiro, N.; Adachi, R.; Tomonari, S.; Watanabe, T.; Mito, T. Cross-allergenicity of crustacean and the edible insect Gryllus bimaculatus in patients with shrimp allergy. Mol. Immunol. 2019, 106, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current trends of bioactive peptides—New sources and therapeutic effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef] [PubMed]
- da Silva Lucas, A.J.; de Oliveira, L.M.; Da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Sosa, D.A.T.; Fogliano, V. Potential of insect-derived ingredients for food applications. In Insect Physiology and Ecology; Shields, V.D.C., Ed.; InTech: London, UK, 2017; pp. 215–231. [Google Scholar]
- Hall, F.; Liceaga, A. Effect of microwave-assisted enzymatic hydrolysis of cricket (Gryllodes sigillatus) protein on ACE and DPP-IV inhibition and tropomyosin-IgG binding. J. Funct. Foods 2020, 64, 103634. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Cunha, L.M.; Sousa-Pinto, B.; Fonseca, J. Allergic risks of consuming edible insects: A systematic review. Mol. Nutr. Food Res. 2018, 62, 1700030. [Google Scholar] [CrossRef]
- Pali-Scholl, I.; Meinlschmidt, P.; Larenas-Linnemann, D.; Purschke, B.; Hofstetter, G.; Rodriguez-Monroy, F.A.; Einhorn, L.; Mothes-Luksch, N.; Jensen-Jarolim, E.; Jager, H. Edible insects: Cross-recognition of IgE from crustacean-and house dust mite allergic patients, and reduction of allergenicity by food processing. World Allergy Organ. J. 2019, 12, 100006. [Google Scholar] [CrossRef]
- Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on Novel Foods, Amending Regulation (EU) No. 1169/2011 of the European Parliament and of the Council and Repealing Regulation (EC) No. 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No. 1852/2001; European Union: Luxemburg, 2001.
- EFSA Scientific Committee. Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [Google Scholar] [CrossRef]
- IPIFF Information Document “Regulation (EU) 2015/2283 on Novel Foods-Briefing Paper on the Provisions Relevant to the Commercialisation of Insect-Based Products Intended for Human Consumption in the EU; European Union: Luxemburg, 2015.
- Commission Regulation (EU) 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No. 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No. 142/2011 as Regards the Provisions on Processed Animal Protein; European Union: Luxemburg, 2017.
- Commission Implementing Regulation (EU) 2017/2469 of 20 December 2017 Laying Down Administrative and Scientific Requirements for Applications Referred to in Article 10 of Regulation (EU) 2015/2283 of the European Parliament and of the Council on Novel Foods; European Union: Luxemburg, 2017.
- Novel Food’ Report: Opinion on the Risk Profile for House Cricket (Acheta domesticus) by the Swedish University of Agricultural Sciences (EFSA Funded Report, Adopted on 6 July 2018); EFSA: Parma, Italy, 2018.
- Chapman, M.A. Transcriptome sequencing and marker development for four underutilized legumes. Appl. Plant Sci. 2015, 3, 1400111. [Google Scholar] [CrossRef]
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future protein supply and demand: Strategies and factors influencing a sustainable equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Dixit, G.P.; Parihar, A.K.; Bohra, A.; Singh, N.P. Achievements and prospects of grass pea (Lathyrus sativus L.) improvement for sustainable food production. Crop J. 2016, 4, 407–416. [Google Scholar] [CrossRef]
- Ramya, K.R.; Tripathi, K.; Pandey, A.; Barpete, S.; Gore, P.G.; Raina, A.P.; Khawar, K.M.; Swain, N.; Sarker, A. Rediscovering the Potential of Multifaceted Orphan Legume Grasspea-a Sustainable Resource with High Nutritional Values. Front. Nutr. 2022, 8, 1327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bejiga, G.; Ahmed, S.; Nakkoul, H.; Sarker, A. Genetic improvement of grass pea for low neurotoxin (β-ODAP) content. Food Chem. Toxicol. 2011, 49, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Rao, S.L.N. Lessons from neurolathyrism: A disease of the past & the future of Lathyrus sativus (Khesari dal). Indian J. Med. Res. 2013, 138, 32. [Google Scholar] [PubMed]
- Llorent-Martinez, E.J.; Zengin, G.; Fernández-de Córdova, M.L.; Bender, O.; Atalay, A.; Ceylan, R.; Mollica, A.; Mocan, A.; Uysal, S.; Guler, G.O.; et al. Traditionally used Lathyrus species: Phytochemical composition, antioxidant activity, enzyme inhibitory properties, cytotoxic effects, and in silico studies of L. czeczottianus and L. nissolia. Front. Pharmacol. 2017, 8, 83. [Google Scholar] [CrossRef]
- Fratianni, F.; Cardinale, F.; Cozzolino, A.; Granese, T.; Albanese, D.; Di Matteo, M.; Zaccardelli, M.; Coppola, R.; Nazzaro, F. Polyphenol composition and antioxidant activity of different grass pea (Lathyrus sativus), lentils (Lens culinaris), and chickpea (Cicer arietinum) ecotypes of the Campania region (Southern Italy). J. Funct. Food. 2014, 7, 551–557. [Google Scholar] [CrossRef]
- Vaz Patto, M.C.; Rubiales, D. Lathyrus diversity: Available resources with relevance to crop improvement—L. sativus and L. cicera as case studies. Ann. Bot. 2014, 113, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Barpete, S.; Gupta, P.; Khawar, K.M.; Kumar, S. Effect of cooking methods on protein content and neurotoxin (β-ODAP) concentration in grass pea (Lathyrus sativus L.) grains. CyTA J. Food 2021, 19, 448–456. [Google Scholar] [CrossRef]
- Fikre, A.; Negwo, T.; Kuo, Y.H.; Lambein, F.; Ahmed, S. Climatic, edaphic and altitudinal factors affecting yield and toxicity of Lathyrus sativus grown at five locations in Ethiopia. Food Chem. Toxicol. 2011, 49, 623–630. [Google Scholar] [CrossRef]
- Jiao, C.J.; Jiang, J.L.; Ke, L.M.; Cheng, W.; Li, F.M.; Li, Z.X.; Wang, C.Y. Factors affecting β-ODAP content in Lathyrus sativus and their possible physiological mechanisms. Food Chem. Toxicol. 2011, 49, 543–549. [Google Scholar] [CrossRef]
- Girma, D.; Korbu, L. Genetic improvement of grass pea (Lathyrus sativus) in Ethiopia: An unfulfilled promise. Plant Breed. 2012, 131, 231–236. [Google Scholar] [CrossRef]
- Tripathi, K.; Pamarthi, R.K.; Gowthami, R.; Gore, P.G.; Gayacharan, C.; Barpete, S.; Singh, N.; Sarker, A.; Kumar, A. Deciphering morpho-taxonomic variability in Lathyrus species. Indian J. Plant Genet. Resour. 2021, 34, 279–289. [Google Scholar] [CrossRef]
- Mihalca, V.; Kerezsi, A.D.; Weber, A.; Gruber-Traub, C.; Schmucker, J.; Vodnar, D.C.; Dulf, F.V.; Socaci, S.A.; Farcas, A.; Muresan, C.I.; et al. Protein-based films and coatings for food industry applications. Polymers 2021, 13, 769. [Google Scholar] [CrossRef] [PubMed]
- Teterycz, D.; Sobota, A.; Zarzycki, P.; Latoch, A. Legume flour as a natural colouring component in pasta production. J. Food Sci. Technol. 2020, 57, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Semba, R.D.; Ramsing, R.; Rahman, N.; Kraemer, K.; Bloem, M.W. Legumes as a sustainable source of protein in human diets. Global Food Secur. 2021, 28, 100520. [Google Scholar] [CrossRef]
- Guemes-Vera, N.; Martinez-Herrera, J.; Hernandez-Chavez, J.F.; Yanez-Fernandez, J.; Totosaus, A. Comparison of chemical composition and protein digestibility, carotenoids, tanins and alkaloids content of wild lupinus varieties flour. Pak. J. Nutr. 2012, 11, 676. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Huma, Z.E.; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Awad, R.A.; Salama, W.M.; Farahat, A.M. Effect of lupine as cheese base substitution on technological and nutritional properties of processed cheese analogue. Acta Sci. Pol. Technol. Aliment. 2014, 13, 55–64. [Google Scholar] [CrossRef]
- Albuja-Vaca, D.; Yepez, C.; Vernaza, M.G.; Navarrete, D. Gluten-free pasta: Development of a new formulation based on rice and lupine bean flour (Lupinus mutabilis) using a mixture-process design. Food Sci. Technol. 2019, 40, 408–414. [Google Scholar] [CrossRef]
- Boukid, F.; Pasqualone, A. Lupine (Lupinus spp.) proteins: Characteristics, safety and food applications. Eur. Food Res. Technol. 2022, 248, 345–356. [Google Scholar] [CrossRef]
- Linares-García, L.; Repo-Carrasco-Valencia, R.; Glorio Paulet, P.; Schoenlechner, R. Development of gluten-free and egg-free pasta based on quinoa (Chenopdium quinoa Willd) with addition of lupine flour, vegetable proteins and the oxidizing enzyme POx. Eur. Food Res. Technol. 2019, 245, 2147–2156. [Google Scholar] [CrossRef]
- Çoban, D.İ.; Babiker, E.E.; Al Juhaimi, F.; Uslu, N.; Özcan, M.M.; Ghafoor, K.; Ahmed, I.A.M.; Almusallam, I.A. Fatty acid composition, mineral contents, and glycemic index values of chips produced with different cooking methods and lupine (Lupinus albus L.) flour formulations. J. Food Process. Preserv. 2021, 45, e15161. [Google Scholar] [CrossRef]
- López, E.P.; Goldner, M.C. Influence of storage time for the acceptability of bread formulated with lupine protein isolate and added brea gum. LWT Food Sci. Technol. 2015, 64, 1171–1178. [Google Scholar] [CrossRef]
- Lepcha, P.; Egan, A.N.; Doyle, J.J.; Sathyanarayana, N. A review on current status and future prospects of winged bean (Psophocarpus tetragonolobus) in tropical agriculture. Plant Foods Hum. Nutr. 2017, 72, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Islam, A.M.; Azirun, S.M.; Boyce, A.N. Tropical legume crop rotation and nitrogen fertilizer effects on agronomic and nitrogen efficiency of rice. Sci. World J. 2014, 2014, 490841. [Google Scholar] [CrossRef] [PubMed]
- Wong, Q.N.; Massawe, F.; Mayes, S. Improving winged bean (Psophocarpus tetragonolobus) productivity: An analysis of the determinants of productivity. In Proceedings of the XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014), Brisbane, Australia, 17–24 August 2014. [Google Scholar]
- Maphosa, Y.; Jideani, V.A. The role of legumes in human nutrition. In Functional Food—Improve Health through Adequate Food; Hueda, M.C., Ed.; Intech: London, UK, 2017; pp. 1–13. [Google Scholar]
- Jaffe, W.G.; Korte, R. Nutritional characteristics of the winged bean in rats. Nutr. Rep. Int. 1976, 14, 449–455. [Google Scholar]
- Mohanty, C.S.; Pradhan, R.C.; Singh, V.; Singh, N.; Pattanayak, R.; Prakash, O.; Chanotiya, C.S.; Rout, P.K. Physicochemical analysis of Psophocarpus tetragonolobus (L.) DC seeds with fatty acids and total lipids compositions. J. Food Sci. Technol. 2015, 52, 3660–3670. [Google Scholar] [CrossRef]
- Saloko, S.; Cicillia, S.; Rakmah, S. The Effect Addition of Winged Bean and Konjac Flour on the Quality of Instant Cassava-Corn Noodles. Nusant. Sci. Technol. Proc. 2020, 2020, 8–20. [Google Scholar]
- Nmorka, G.O.; Okezie, B.O. Nutritional quality of winged bean composite breads. Cereal Chem. 1983, 60, 198–202. [Google Scholar]
- Kailasapathy, K.; Perera, P.A.J.; Macneil, J.H. Improved Nutritional Value in Wheat Bread by Fortification with Full-Fat Winged Bean Flour (Psophocarpus tetragonolobus L. DC). J. Food Sci. 1985, 50, 1693–1696. [Google Scholar] [CrossRef]
- Aremu, M.O.; Olaofe, O.; Akintayo, E.T. Chemical composition and physicochemical characteristics of two varieties of bambara groundnut (Vigna subterrenea) flours. J. Appl. Sci. 2006, 6, 1900–1903. [Google Scholar] [CrossRef]
- Basu, S.; Roberts, J.A.; Azam-Ali, S.N.; Mayes, S. Bambara Groundnut. In Pulses, Sugar and Tuber Crops; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 3, pp. 159–173. [Google Scholar]
- Ijarotimi, O.S. Protein and hematological evaluations of infant formulated from cooking banana fruits (Musa spp, ABB genome) and fermented bambara groundnut (Vigna subterranean L. Verdc) seeds. Nutr. Res. Pract. 2008, 2, 165–170. [Google Scholar] [CrossRef]
- Ijarotimi, O.S.; Keshinro, O.O. Formulation and nutritional quality of infant formula produced from germinated popcorn, Bambara groundnut and African locust bean flour. J. Microbiol. Biotechnol. Food Sci. 2012, 1, 1358–1388. [Google Scholar]
- Adebayo-Oyetoro Abiodun, O.; Shotunde, A.B.; Adeyeye Samuel, A.O.; Ogundipe, O.O. Quality evaluation of millet-based fura powder supplemented with Bambara groundnut. Int. J. Food Sci. Nutr. Diet 2017, 6, 358–362. [Google Scholar] [CrossRef]
- Nwadi, O.M.; Uchegbu, N.; Oyeyinka, S.A. Enrichment of food blends with bambara groundnut flour: Past, present, and future trends. Legume Sci. 2020, 2, e25. [Google Scholar] [CrossRef]
- Halimi, R.A.; Barkla, B.J.; Mayes, S.; King, G.J. The potential of the underutilized pulse bambara groundnut (Vigna subterranea (L.) Verdc.) for nutritional food security. J. Food Compos. Anal. 2019, 77, 47–59. [Google Scholar] [CrossRef]
- Chai, H.H.; Massawe, F.; Mayes, S. Effects of mild drought stress on the morpho-physiological characteristics of a bambara groundnut segregating population. Euphytica 2016, 208, 225–236. [Google Scholar] [CrossRef]
- Azam-Ali, S.N.; Sesay, A.; Karikari, S.K.; Massawe, F.J.; Aguilar-Manjarrez, J.; Bannayan, M.; Hampson, K.J. Assessing the potential of an underutilized crop—A case study using bambara groundnut. Exp. Agric. 2001, 37, 433–472. [Google Scholar] [CrossRef]
- Marçal, S.; Sousa, A.S.; Taofiq, O.; Antunes, F.; Morais, A.M.; Freitas, A.C.; Barros, L.; Ferreira, I.C.F.R.; Pintado, M. Impact of postharvest preservation methods on nutritional value and bioactive properties of mushrooms. Trends Food Sci. Technol. 2021, 110, 418–431. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; de Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef]
- Schweiggert-Weisz, U.; Eisner, P.; Bader-Mittermaier, S.; Osen, R. Food proteins from plants and fungi. Curr. Opin. Food Sci. 2020, 32, 156–162. [Google Scholar] [CrossRef]
- Lübeck, M.; Lübeck, P.S. Fungal cell factories for efficient and sustainable production of proteins and peptides. Microorganisms 2022, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Bakratsas, G.; Polydera, A.; Katapodis, P.; Stamatis, H. Recent trends in submerged cultivation of mushrooms and their application as a source of nutraceuticals and food additives. Future Foods 2021, 4, 100086. [Google Scholar] [CrossRef]
- Wiebe, M.G. Myco-protein from Fusarium venenatum: A well-established product for human consumption. Appl. Microbiol. Biotechnol. 2002, 58, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montes, A.; Rangel-Vargas, E.; Lorenzo, J.M.; Romero, L.; Santos, E.M. Edible mushrooms as a novel trend in the development of healthier meat products. Curr. Opin. Food Sci. 2021, 37, 118–124. [Google Scholar] [CrossRef]
- Finnigan, T.; Needham, L.; Abbott, C. Mycoprotein: A healthy new protein with a low environmental impact. In Sustainable Protein Sources; Nadathur, R.S., Scalin, L., Wanasundara, J.P.D., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 305–325. [Google Scholar]
- Manu-Tawiah, W.; Martin, A.M. Chemical composition of Pleurotus ostreatus mycelial biomass. Food Microbiol. 1987, 4, 303–310. [Google Scholar] [CrossRef]
- Mumpuni, A.; Ekowati, N.; Purnomowati, P.; Purwati, E.S. Growth and protein content establishment of Pleurotus ostreatus on liquid and solid medium. Biosaintifika J. Biol. Biol. Educ. 2017, 9, 572–578. [Google Scholar] [CrossRef]
- Bach, F.; Helm, C.V.; Bellettini, M.B.; Maciel, G.M.; Haminiuk, C.W.I. Edible mushrooms: A potential source of essential amino acids, glucans and minerals. Int. J. Food Sci. Technol. 2017, 52, 2382–2392. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Chen, Y.; Liu, T.; Zhang, S.; Fan, H.; Liu, H.; Li, Y. Healthy function and high valued utilization of edible fungi. Food Sci. Hum. Wellness 2021, 10, 408–420. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, M.; Wu, S.; Liao, X.; Wang, J.; Wu, Q.; Zhuang, M.; Ding, Y. A review on mushroom-derived bioactive peptides: Preparation and biological activities. Food Res. Int. 2020, 134, 109230. [Google Scholar] [CrossRef]
- Derbyshire, E.J.; Delange, J. Fungal protein—What is it and what is the health evidence? A systematic review focusing on mycoprotein. Front. Sustain. Food Syst. 2021, 5, 581682. [Google Scholar] [CrossRef]
- Cherta-Murillo, A.; Lett, A.M.; Frampton, J.; Chambers, E.S.; Finnigan, T.J.; Frost, G.S. Effects of mycoprotein on glycaemic control and energy intake in humans: A systematic review. Br. J. Nutr. 2020, 123, 1321–1332. [Google Scholar] [CrossRef] [PubMed]
- Cherta-Murillo, A.; Frost, G.S. The association of mycoprotein-based food consumption with diet quality, energy intake and non-communicable diseases’ risk in the UK adult population using the National Diet and Nutrition Survey (NDNS) years 2008/2009–2016/2017: A cross-sectional study. Br. J. Nutr. 2022, 127, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Ishara, J.R.; Sila, D.N.; Kenji, G.M.; Buzera, A.K.; Mushagalusa, G.N. Nutritional and Physical Attributes of Maize-mushroom Complementary Porridges as Influenced by Mushroom Species and Ratio. Am. J. Food Nutr. 2018, 6, 17–27. [Google Scholar]
- Azeez, L.A.; Adedokun, S.O.; Adeoti, A.O.; Babalola, J.O. Quality characteristics of fortified bread produced from cassava and mushroom flours. J. Food Process. Technol. 2018, 9, 724. [Google Scholar]
- Oyetayo, V.O.; Oyedeji, R.R. Proximate and mineral composition of bread fortified with mushroom (Plerotus ostreatus and Calocybe indica). Microbiol. Res. J. Int. 2017, 19, 1–9. [Google Scholar] [CrossRef]
- Cornelia, M.; Chandra, J. Utilization of white oyster mushroom powder (Pleurotus ostreatus (Jacq.) P. Kumm.) in the making of biscuit as emergency food product. EurAsian J. Biosci. 2019, 13, 1859–1866. [Google Scholar]
- Rathore, H.; Sehwag, S.; Prasad, S.; Sharma, S. Technological, nutritional, functional and sensorial attributes of the cookies fortified with Calocybe indica mushroom. J. Food Meas. Charact. 2019, 13, 976–987. [Google Scholar] [CrossRef]
- Khider, M.; Seoudi, O.; Abdelaliem, Y.F. Functional processed cheese spreads with high nutritional value as supplemented with fresh and dried mushrooms. Int. J. Nutr. Food Sci. 2017, 6, 45–52. [Google Scholar] [CrossRef]
- Loi, M.; Quintieri, L.; Fanelli, F.; Caputo, L.; Mulè, G. Application of a recombinant laccase-chlorogenic acid system in protein crosslink and antioxidant properties of the curd. Food Res. Int. 2018, 106, 763–770. [Google Scholar] [CrossRef]
- Loi, M.; Quintieri, L.; De Angelis, E.; Monaci, L.; Logrieco, A.F.; Caputo, L.; Mulè, G. Yield improvement of the Italian fresh Giuncata cheese by laccase–induced protein crosslink. Int. Dairy J. 2020, 100, 104555. [Google Scholar] [CrossRef]
- Loi, M.; Fanelli, F.; Zucca, P.; Liuzzi, V.C.; Quintieri, L.; Cimmarusti, M.T.; Monaci, L.; Haidukowski, M.; Logrieco, A.F.; Sanjust, E.; et al. Aflatoxin B1 and M1 degradation by Lac2 from Pleurotus pulmonarius and redox mediators. Toxins 2016, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Glazunova, O.; Fedorova, T.; Logrieco, A.F.; Mulè, G. Fungal Laccases: The Forefront of Enzymes for Sustainability. J. Fungi 2021, 7, 1048. [Google Scholar] [CrossRef] [PubMed]
- Buchanan-Wollaston, V. Postharvest physiology|Senescence, Leaves. In Encyclopedia of Applied Plant Sciences; Elsevier: Amsterdam, The Netherlands, 2003; pp. 808–816. [Google Scholar]
- Moughan, P.J. Population protein intakes and food sustainability indices: The metrics matter. Glob. Food Secur. 2021, 29, 100548. [Google Scholar] [CrossRef]
- Guillin, F.; Gaudichon, C.; Guérin-Deremaux, L.; Lefranc-Millot, C.; Tomé, D.; Khodorova, N.; Airinei, G.; Calvez, J. Real ileal digestibility of pea protein compared to casein in healthy humans. Curr. Dev. Nutr. 2020, 4, 634. [Google Scholar] [CrossRef]
- Mamone, G.; Picariello, G.; Ramondo, A.; Nicolai, M.A.; Ferranti, P. Production, digestibility and allergenicity of hemp (Cannabis sativa L.) protein isolates. Food Res. Int. 2019, 115, 562–571. [Google Scholar] [CrossRef]
- Preece, K.E.; Hooshyar, N.; Zuidam, N.J. Whole soybean protein extraction processes: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 163–172. [Google Scholar] [CrossRef]
- Basri, H.; Hadju, V.; Zulkifli, A.; Syam, A.; Indriasari, R. Effect of Moringa oleifera supplementation during pregnancy on the prevention of stunted growth in children between the ages of 36 to 42 months. J. Public Health Res. 2021, 10, 2207. [Google Scholar] [CrossRef]
- Boateng, L.; Ashley, I.; Ohemeng, A.; Asante, M.; Steiner-Asiedu, M. Improving blood retinol concentrations with complementary foods fortified with Moringa oleifera leaf powder—A pilot study. Yale J. Biol. Med. 2018, 91, 83–94. [Google Scholar]
- D’Auria, G.; Nitride, C.; Ferranti, P. Moringa oleifera Lam. proteins: Properties and food applications. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Benhammouche, T.; Melo, A.; Martins, Z.; Faria, M.A.; Pinho, S.C.M.; Ferreira, I.M.L.P.V.O.; Zaidi, F. Nutritional quality of protein concentrates from moringa oleifera leaves and in vitro digestibility. Food Chem. 2021, 348, 128858. [Google Scholar] [CrossRef]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition: Report of an FAQ Expert Consultation; FAO: Rome, Italy, 2013; Volume 92. [Google Scholar]
- Shi, H.; Yang, E.; Li, Y.; Chen, X.; Zhang, J. Effect of solid-state fermentation on nutritional quality of leaf flour of the drumstick tree (Moringa oleifera Lam.). Front. Bioeng. Biotechnol. 2021, 9, 626628. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Montesano, D.; Lucini, L. The potential of Moringa oleifera in food formulation: A promising source of functional compounds with health-promoting properties. Curr. Opin. Food Sci. 2021, 42, 257–269. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Rocchetti, G.; Pateiro, M.; Lucini, L.; Domínguez, R.; Lorenzo, J.M. Addition of plant extracts to meat and meat products to extend shelf-life and health-promoting attributes: An overview. Curr. Opin. Food Sci. 2020, 31, 81–87. [Google Scholar] [CrossRef]
- de Beukelaar, M.F.A.; Zeinstra, G.G.; Mes, J.J.; Fischer, A.R.H. Duckweed as human food. The influence of meal context and information on duckweed acceptability of dutch consumers. Food Qual. Prefer. 2019, 71, 76–86. [Google Scholar] [CrossRef]
- Bog, M.; Appenroth, K.-J.; Sree, K.S. Duckweed (Lemnaceae): Its molecular taxonomy. Front. Sustain. Food Syst. 2019, 3, 117. [Google Scholar] [CrossRef]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of water lentil powder from lemnaceae as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06845. [Google Scholar]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Wolffia globosa powder as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06938. [Google Scholar] [PubMed]
- Turck, D.; Bohn, T.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Lemna Minor and Lemna Gibba whole plant material as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, e07598. [Google Scholar] [PubMed]
- Nieuwland, M.; Geerdink, P.; Engelen-Smit, N.P.E.; van der Meer, I.M.; America, A.H.P.; Mes, J.J.; Kootstra, A.M.J.; Henket, J.T.M.M.; Mulder, W.J. Isolation and gelling properties of duckweed protein concentrate. ACS Food Sci. Technol. 2021, 1, 908–916. [Google Scholar] [CrossRef]
- Martin, A.H.; Castellani, O.; de Jong, G.A.; Bovetto, L.; Schmitt, C. Comparison of the functional properties of RuBisCO protein isolate extracted from sugar beet leaves with commercial whey protein and soy protein isolates. J. Sci. Food Agric. 2019, 99, 1568–1576. [Google Scholar] [CrossRef]
- Yahaya, N.; Hamdan, N.H.; Zabidi, A.R.; Mohamad, A.M.; Suhaimi, M.L.H.; Johari, M.A.A.M.; Yahya, H.N.; Yahya, H. Duckweed as a future food: Evidence from metabolite profile, nutritional and microbial analyses. Future Foods 2022, 5, 100128. [Google Scholar] [CrossRef]
- Freitas, H.R. Chlorella Vulgaris as a Source of Essential Fatty Acids and Micronutrients: A Brief Commentary. Open Plant Sci. J. 2017, 10, 92–99. [Google Scholar] [CrossRef]
- Barta, D.G.; Coman, V.; Vodnar, D.C. Microalgae as sources of Omega-3 polyunsaturated fatty acids: Biotechnological aspects. Algal Res. 2021, 58, 102410. [Google Scholar] [CrossRef]
- Ursu, A.-V.; Marcati, A.; Sayd, T.; Sante-Lhoutellier, V.; Djelveh, G.; Michaud, P. Extraction, fractionation and functional properties of proteins from the microalgae Chlorella vulgaris. Bioresour. Technol. 2014, 157, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.W. Micro-Algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; dos Reis Coimbra, J.S.; de Oliveira Leite, M.; Albino, L.F.T.; Martins, M.A. Microalgae Proteins: Production, Separation, Isolation, Quantification, and Application in Food and Feed. Crit. Rev. Food Sci. Nutr. 2021, 61, 1976–2002. [Google Scholar] [CrossRef]
- Consultation Request for the Determination of the Novel Food Status ARTICLE 4 of Regulation (EU) 2015/2283; European Union: Luxemburg, 2022; Available online: https://food.ec.europa.eu/system/files/2022-03/novel-food_consult-status_chlorella-sp.pdf (accessed on 24 February 2023).
- Torres-Tiji, Y.; Fields, F.J.; Mayfield, S.P. Microalgae as a Future Food Source. Biotechnol. Adv. 2020, 41, 107536. [Google Scholar] [CrossRef]
- Lafarga, T. Effect of Microalgal Biomass Incorporation into Foods: Nutritional and Sensorial Attributes of the End Products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Matos, Â.P.; Novelli, E.; Tribuzi, G. Use of Algae as Food Ingredient: Sensory Acceptance and Commercial Products. Front. Food Sci. Technol. 2022, 2, 989801. [Google Scholar] [CrossRef]
- Schüler, L.; Greque de Morais, E.; Trovão, M.; Machado, A.; Carvalho, B.; Carneiro, M.; Maia, I.; Soares, M.; Duarte, P.; Barros, A.; et al. Isolation and Characterization of Novel Chlorella vulgaris Mutants with Low Chlorophyll and Improved Protein Contents for Food Applications. Front. Bioeng. Biotechnol. 2020, 8, 469. [Google Scholar] [CrossRef]
- Urano, N.; Fujii, K. Effect of Osmotic Stabilizers on Protoplast Generation of Chlorella ellipsoidea Yellow/White Color Mutants. J. Biosci. Bioeng. 2000, 90, 567–569. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of Interest as Food Source: Biochemical Composition and Digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Canelli, G.; Tarnutzer, C.; Carpine, R.; Neutsch, L.; Bolten, C.J.; Dionisi, F.; Mathys, A. Biochemical and Nutritional Evaluation of Chlorella and Auxenochlorella Biomasses Relevant for Food Application. Front. Nutr. 2020, 7, 565996. [Google Scholar] [CrossRef] [PubMed]
- Kose, A.; Ozen, M.O.; Elibol, M.; Oncel, S.S. Investigation of in Vitro Digestibility of Dietary Microalga Chlorella vulgaris and Cyanobacterium Spirulina platensis as a Nutritional Supplement. 3 Biotech 2017, 7, 170. [Google Scholar] [CrossRef]
- Şahin, O.I. Effect of Spirulina Biomass Fortification for Biscuits and Chocolates. Turk. J. Agric. Food Sci. Technol. 2019, 7, 583. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae Biomass as an Alternative Ingredient in Cookies: Sensory, Physical and Chemical Properties, Antioxidant Activity and in Vitro Digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, T.; Chen, S.H.Y.; Liu, B.; Sun, P.; Sun, H.; Chen, F. The Potentials and Challenges of Using Microalgae as an Ingredient to Produce Meat Analogues. Trends Food Sci. Technol. 2021, 112, 188–200. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in Microalgae Incorporation into Innovative Food Products with Potential Health Benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Bertsch, P.; Böcker, L.; Mathys, A.; Fischer, P. Proteins from Microalgae for the Stabilization of Fluid Interfaces, Emulsions, and Foams. Trends Food Sci. Technol. 2021, 108, 326–342. [Google Scholar] [CrossRef]
- Elhassan, M.; Wendin, K.; Olsson, V.; Langton, M. Quality Aspects of Insects as Food-Nutritional, Sensory, and Related Concepts. Foods 2019, 8, 95. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected Species of Edible Insects as a Source of Nutrient Composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.C.; Miller, A.C.; Miller, M.E.; Xiao, H.; Wu, X. Potential Health Benefits of Edible Insects. Crit. Rev. Food Sci. Nutr. 2022, 62, 3499–3508. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and Sensory Quality of Edible Insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Purschke, B.; Meinlschmidt, P.; Horn, C.; Rieder, O.; Jäger, H. Improvement of Techno-Functional Properties of Edible Insect Protein from Migratory Locust by Enzymatic Hydrolysis. Eur. Food Res. Technol. 2018, 244, 999–1013. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, L.; Hinrichs, J.; Weiss, J. Cultivation and Downstream Processing of Microalgae and Cyanobacteria to Generate Protein-Based Technofunctional Food Ingredients. Crit. Rev. Food Sci. Nutr. 2020, 60, 2961–2989. [Google Scholar] [CrossRef]
- Kim, T.K.; Lee, M.H.; Yu, M.H.; Yong, H.I.; Jang, H.W.; Jung, S.; Choi, Y.S. Thermal stability and rheological properties of heat-induced gels prepared using edible insect proteins in a model system. LWT 2020, 134, 110270. [Google Scholar] [CrossRef]
- Wang, J.; Jousse, M.; Jayakumar, J.; Fernández-Arteaga, A.; de Lamo-Castellví, S.; Ferrando, M.; Güell, C. Black Soldier Fly (Hermetia illucens) Protein Concentrates as a Sustainable Source to Stabilize O/W Emulsions Produced by a Low-Energy High-Throughput Emulsification Technology. Foods 2021, 10, 1048. [Google Scholar] [CrossRef]
- Pan, J.; Xu, H.; Cheng, Y.; Mintah, B.K.; Dabbour, M.; Yang, F.; Chen, W.; Zhang, Z.; Dai, C.; He, R.; et al. Recent Insight on Edible Insect Protein: Extraction, Functional Properties, Allergenicity, Bioactivity, and Applications. Foods 2022, 11, 2931. [Google Scholar] [CrossRef]
- Palanisamy, M.; Töpfl, S.; Berger, R.G.; Hertel, C. Physico-Chemical and Nutritional Properties of Meat Analogues Based on Spirulina/Lupin Protein Mixtures. Eur. Food Res. Technol. 2019, 245, 1889–1898. [Google Scholar] [CrossRef]
- Weinrich, R.; Elshiewy, O. Preference and Willingness to Pay for Meat Substitutes Based on Micro-Algae. Appetite 2019, 142, 104353. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2021/1372 of 17 August 2021 Amending Annex IV to Regulation (EC) No. 999/2001 of the European Parliament and of the Council as Regards the Prohibition to Feed Non-Ruminant Farmed Animals, Other Than Fur Animals, with Protein Derived from Animals; European Union: Luxemburg, 2021.
- IPIFF. IPIFF’s Policy Priorities towards 2025. The Insect Sector Milestones towards Sustainable Food Supply Chains. Available online: https://ipiff.org/wp-content/uploads/2020/05/IPIFF-RegulatoryBrochure-update07-2020-1.pdf (accessed on 24 February 2022).
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA). Safety of dried yellow mealworm (Tenebrio molitor larva) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, e06343. [Google Scholar]
- Commission Implementing Regulation (EU) 2021/1975 of 12 November 2021 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Locusta migratoria as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council and Amending Commission Implementing Regulation (EU) 2017/2470; European Union: Luxemburg, 2021.
- Commission Implementing Regulation (EU) 2021/882 of 1 June 2021 Authorising the Placing on the Market of Dried Tenebrio molitor larva as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470; European Union: Luxemburg, 2021.
- Commission Implementing Regulation (EU) 2022/169 of 8 February 2022 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Yellow Mealworm (Tenebrio molitor larva) as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470; European Union: Luxemburg, 2022.
- Commission Implementing Regulation (EU) 2022/188 of 10 February 2022 Authorising the Placing on the Market of Frozen, Dried and Powder Forms of Acheta domesticus as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/247; European Union: Luxemburg, 2022.
- Commission Implementing Regulation (EU) 2023/58 of 5 January 2023 Authorising the Placing on the Market of the Frozen, Paste, Dried and Powder Forms of Alphitobius diaperinus Larvae (Lesser Mealworm) as a Novel Food and Amending Implementing Regulation (EU) 2017/2470; European Union: Luxemburg, 2023.
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA). Safety of partially defatted house cricket (Acheta domesticus) powder as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2022, 20, 7258. [Google Scholar]
- Cardoso, R.V.C.; Fernandes, A.; Gonzalez-Paramas, A.M.; Barros, L.; Ferreira, I. Flour fortification for nutritional and health improvement: A review. Food Res. Int. 2019, 125, 108576. [Google Scholar] [CrossRef]
- Montevecchi, G.; Licciardello, F.; Masino, F.; Miron, L.T.; Antonelli, A. Fortification of wheat flour with black soldier fly prepupae. Evaluation of technological and nutritional parameters of the intermediate doughs and final baked products. Innov. Food Sci. Emerg. Technol. 2021, 69, 102666. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Bonaccorsi, G.; Lorini, C.; Cini, E. Assessment of the rheological properties and bread characteristics obtained by innovative protein sources (Cicer arietinum, Acheta domesticus, Tenebrio molitor): Novel food or potential improvers for wheat flour? LWT 2020, 118, 108867. [Google Scholar] [CrossRef]
- Kowalski, S.; Mikulec, A.; Mickowska, B.; Skotnicka, M.; Mazurek, A. Wheat bread supplementation with various edible insect flours. Influence of chemical composition on nutritional and technological aspects. LWT 2022, 159, 113220. [Google Scholar] [CrossRef]
- Patrignani, F.; Parrotta, L.; Del Duca, S.; Vannini, L.; Camprini, L.; Dalla Rosa, M.; Schlüter, O.; Lanciotti, R. Potential of Yarrowia lipolytica and Debaryomyces hansenii strains to produce high quality food ingredients based on cricket powder. LWT 2020, 119, 108866. [Google Scholar] [CrossRef]
- Lau, C.C.; Abdullah, N.; Shuib, A.S.; Aminudin, N. Novel angiotensin I-converting enzyme inhibitory peptides derived from edible mushroom Agaricus bisporus (J.E. Lange) Imbach identified by LC-MS/MS. Food Chem. 2014, 148, 396–401. [Google Scholar] [CrossRef]
- Choi, H.S.; Cho, H.Y.; Yang, H.C.; Ra, K.S.; Suh, H.J. Angiotensin I-converting enzyme inhibitor from Grifola frondosa. Food Res. Int. 2001, 34, 177–182. [Google Scholar] [CrossRef]
- González, C.M.; Garzón, R.; Rosell, C.M. Insects as ingredients for bakery goods. A comparison study of H. illucens, A. domestica and T. molitor flours. Innov. Food Sci. Emerg. Technol. 2019, 51, 205–210. [Google Scholar] [CrossRef]
- Pauter, P.; Różańska, M.; Wiza, P.; Dworczak, S.; Grobelna, N.; Sarbak, P.; Kowalczewski, P. Effects of the replacement of wheat flour with cricket powder on the characteristics of muffins. Acta Sci. Pol. Technol. Aliment. 2018, 17, 227–233. [Google Scholar] [PubMed]
- da Rosa Machado, C.; Thys, R.C.S. Cricket powder (Gryllus assimilis) as a new alternative protein source for gluten-free breads. Innov. Food Sci. Emerg. Technol. 2019, 56, 102180. [Google Scholar] [CrossRef]
- Nissen, L.; Samaei, S.P.; Babini, E.; Gianotti, A. Gluten free sourdough bread enriched with cricket flour for protein fortification: Antioxidant improvement and Volatilome characterization. Food Chem. 2020, 333, 127410. [Google Scholar] [CrossRef] [PubMed]
- Kowalczewski, P.Ł.; Walkowiak, K.; Masewicz, Ł.; Bartczak, O.; Lewandowicz, J.; Kubiak, P.; Baranowska, H.M. Gluten-free bread with cricket powder-mechanical properties and molecular water dynamics in dough and ready product. Foods 2019, 8, 240. [Google Scholar] [CrossRef]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible insects as a protein source: A review of public perception, processing technology, and research trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef]
- Maqsoudlou, A.; Mahoonak, A.S.; Mora, L.; Mohebodini, H.; Toldrá, F.; Ghorbani, M. Peptide identification in alcalase hydrolysated pollen and comparison of its bioactivity with royal jelly. Food Res. Int. 2019, 116, 905–915. [Google Scholar] [CrossRef]
- Sarmadi, B.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Tondo, A.R.; Caputo, L.; Mangiatordi, G.F.; Monaci, L.; Lentini, G.; Logrieco, A.F.; Montaruli, M.; Nicolotti, O.; Quintieri, L. Structure-based identification and design of angiotensin converting enzyme-inhibitory peptides from whey proteins. J. Agric. Food Chem. 2019, 68, 541–548. [Google Scholar] [CrossRef]
- Tadesse, S.A.; Emire, S.A. Production and processing of antioxidant bioactive peptides: A driving force for the functional food market. Heliyon 2020, 6, e04765. [Google Scholar] [CrossRef] [PubMed]
- Quintieri, L.; Caputo, L.; Monaci, L.; Deserio, D.; Morea, M.; Baruzzi, F. Antimicrobial efficacy of pepsin-digested bovine lactoferrin on spoilage bacteria contaminating traditional Mozzarella cheese. Food Microbiol. 2012, 31, 64–71. [Google Scholar] [CrossRef]
- Quintieri, L.; Caputo, L.; Monaci, L.; Cavalluzzi, M.M.; Denora, N. Lactoferrin-derived peptides as a control strategy against skinborne staphylococcal biofilms. Biomedicines 2020, 8, 323. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, N.; Caputo, L.; Quintieri, L.; Monaci, L.; Ciriaco, F.; Nicolotti, O. Rational Discovery of Antiviral Whey Protein-Derived Small Peptides Targeting the SARS-CoV-2 Main Protease. Biomedicines 2022, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, A.; Vázquez, A. Bioactive peptides: A review. Food Qual. Saf. 2017, 1, 29–46. [Google Scholar] [CrossRef]
- Jang, J.H.; Jeong, S.C.; Kim, J.H.; Lee, Y.H.; Ju, Y.C.; Lee, J.S. Characterisation of a new antihypertensive angiotensin I-converting enzyme inhibitory peptide from Pleurotus cornucopiae. Food Chem. 2011, 127, 412–418. [Google Scholar] [CrossRef]
- Koo, K.C.; Lee, D.H.; Kim, J.H.; Yu, H.E.; Park, J.S.; Lee, J.S. Production and characterization of antihypertensive angiotensin I-converting enzyme inhibitor from Pholiota adiposa. J. Microbiol. Biotechnol. 2006, 16, 757–763. [Google Scholar]
- Lee, D.H.; Kim, J.H.; Park, J.S.; Choi, Y.J.; Lee, J.S. Isolation and characterization of a novel angiotensin I-converting enzyme inhibitory peptide derived from the edible mushroom Tricholoma giganteum. Peptides 2004, 25, 621–627. [Google Scholar]
- Kang, M.G.; Kim, Y.H.; Bolormaa, Z.; Kim, M.K.; Seo, G.S.; Lee, J.S. Characterization of an antihypertensive angiotensin I-converting enzyme inhibitory peptide from the edible mushroom Hypsizygus marmoreus. BioMed Res. Int. 2013, 2013, 283964. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Li, K.; Peng, X.; Wang, Z.; Ding, L.; Liu, L.; Xu, P.; Liu, G.Q. Isolation and characterization of three antihypertension peptides from the mycelia of Ganoderma Lucidum (Agaricomycetes). J. Agric. Food Chem. 2019, 67, 8149–8159. [Google Scholar] [CrossRef]
- Lau, C.C.; Abdullah, N.; Aminudin, N.; Shuib, A.S. Effect of freeze-drying process on the property of angiotensin I-converting enzyme inhibitory peptides in grey oyster mushrooms. Dry. Technol. 2013, 31, 1693–1700. [Google Scholar] [CrossRef]
- Geng, X.; Tian, G.; Zhang, W.; Zhao, Y.; Zhao, L.; Wang, H.; Ng, T.B. A Tricholoma matsutake peptide with angiotensin converting enzyme inhibitory and antioxidative activities and antihypertensive effects in spontaneously hypertensive rats. Sci. Rep. 2016, 6, 24130. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.W.; Ng, T.B.; Wang, H.X. Antiproliferative and antimitogenic activities in a peptide from puffball mushroom Calvatia caelata. Biochem. Biophys. Res. Commun. 2001, 289, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Ngai, P.H.; Wang, H.X.; Ng, T.B. Purification and characterization of a ubiquitin-like peptide with macrophage stimulating, antiproliferative and ribonuclease activities from the mushroom Agrocybe cylindracea. Peptides 2003, 24, 639–645. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B.; Wang, H.; Sze, S.C.W.; Zhang, K.Y.; Li, Q.; Lu, X. Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine 2011, 18, 387–392. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Cao, W.; Yao, K.; Liu, Z.; Guo, J. Anti-inflammation and antioxidant effect of Cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Metab. Brain Dis. 2012, 27, 159–165. [Google Scholar] [CrossRef]
- Ngai, P.H.; Zhao, Z.; Ng, T.B. Agrocybin, an antifungal peptide from the edible mushroom Agrocybe cylindracea. Peptides 2005, 26, 191–196. [Google Scholar] [CrossRef]
- Wang, H.; Ng, T.B. Eryngin, a novel antifungal peptide from fruiting bodies of the edible mushroom Pleurotus eryngii. Peptides 2004, 25, 1–5. [Google Scholar] [CrossRef]
- Ye, X.Y.; Ng, T.B. A Novel Peptide with ribonuclease and translation-inhibitory activities from fruiting bodies of the oyster mushroom Pleurotus ostreatus. J. Pept. Sci. 2002, 8, 235–240. [Google Scholar] [CrossRef]
- Chu, K.T.; Xia, L.; Ng, T.B. Pleurostrin, an antifungal peptide from the oyster mushroom. Peptides 2005, 26, 2098–2103. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.X.; Ng, T.B. A peptide with HIV-1 reverse transcriptase inhibitory activity from the medicinal mushroom Russula paludosa. Peptides 2007, 28, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, X.; Li, W. Antioxidant, antitumor and immunostimulatory activities of the polypeptide from Pleurotus eryngii mycelium. Int. J. Biol. Macromol. 2017, 97, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.W.; Jeong, S.C.; Lee, D.H.; Park, J.S.; Lee, J.S. Isolation and characterization of a novel platelet aggregation inhibitory peptide from the medicinal mushroom, Inonotus obliquus. Peptides 2006, 27, 1173–1178. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Huo, C.; Qian, Y.; Ren, D.; Lu, J. Ultra-high-pressure processing improves proteolysis and release of bioactive peptides with activation activities on alcohol metabolic enzymes in vitro from mushroom foot protein. Food Chem. 2017, 231, 25–32. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, S.; Xin, X.; Zhang, B.; Thomas, A.; Charles, A.; Lee, K.S.; Jin, B.R.; Gui, Z. Purification and characterization of a novel immunomodulatory hexapeptide from alcalase hydrolysate of ultramicro-pretreated silkworm (Bombyx mori) pupa protein. J. Asia-Pac. Entomol. 2019, 22, 633–637. [Google Scholar] [CrossRef]
- Sun, S.; Wang, W.; Wang, N.; Zhang, Y.; Zhu, Z.; Li, X.; Wang, J.; Chen, Q.; Ahmed Sadiq, F.; Yang, H.; et al. HPP and SGQR peptides from silkworm pupae protein hydrolysates regulated biosynthesis of cholesterol in HepG2 cell line. J. Funct. Foods 2021, 77, 104328. [Google Scholar] [CrossRef]
- He, W.; Huang, H.; He, J.; Subhan, S.; Peng, Y.; Huang, M.; He, H.; Tang, Y.; Zhao, Z. Amino acids imprinted ZIF-8s for the highly efficient and selective adsorption of antioxidant peptides from silkworm pupa protein. Food Res. Int. 2022, 157, 111406. [Google Scholar] [CrossRef]
- Xia, J.; Ge, C.; Yao, H. Antimicrobial Peptides from Black Soldier Fly (Hermetia illucens) as Potential Antimicrobial Factors Representing an Alternative to Antibiotics in Livestock Farming. Animals 2021, 11, 1937. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.; Reddivari, L.; Liceaga, A.M. Identification and Characterization of Edible Cricket Peptides on Hypertensive and Glycemic In Vitro Inhibition and Their Anti-Inflammatory Activity on RAW 264.7 Macrophage Cells. Nutrients 2020, 12, 3588. [Google Scholar] [CrossRef] [PubMed]
- Kay, I.; Coast, G.M.; Cusinato, O.; Wheeler, C.H.; Totty, N.F.; Goldsworthy, G.J. Isolation and Characterization of a Diuretic Peptide from Acheta domesticus. Evidence for a Family of Insect Diuretic Peptides. Biol. Chem. 1991, 372, 505–512. [Google Scholar]
- Brai, A.; Immacolata Trivisani, C.; Vagaggini, C.; Stella, R.; Angeletti, R.; Iovenitti, G.; Francardi, V.; Dreassi, E. Proteins from Tenebrio molitor: An interesting functional ingredient and a source of ACE inhibitory peptides. Food Chem. 2022, 393, 133409. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.R.; Lee, S.O. Novel hepatoprotective peptides derived from protein hydrolysates of mealworm (Tenebrio molitor). Food Res. Int. 2020, 133, 109194. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Vistoli, G. Peptides derived from soy and lupin protein as dipeptidyl-peptidase IV inhibitors. in vitro biochemical screening and in silico molecular modeling study. J. Agric. Food Chem. 2016, 64, 9601–9606. [Google Scholar] [CrossRef] [PubMed]
- Kamran, F.; Phillips, M.; Reddy, N. Functional properties of Australian blue lupin (Lupinus angustifolius) protein and biological activities of protein hydrolysates. Legume Sci. 2021, 3, e65. [Google Scholar] [CrossRef]
- Aluko, R.E.; Girgih, A.T.; He, R.; Malomo, S.; Li, H.; Offengenden, M.; Wu, J.P. Structural and functional characterization of yellow field pea seed (Pisum sativum L.) protein-derived antihypertensive peptides. Food Res. Int. 2015, 77, 10–16. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Arnoldi, A.; Aiello, G. Erratum to: YDFYPSSTKDQQS (P3), a peptide from lupin protein, absorbed by Caco-2 cells, modulates cholesterol metabolism in HepG2 cells via SREBP-1 activation. J. Food Biochem. 2019, 43, e12524. [Google Scholar] [CrossRef]
- Babini, E.; Tagliazucchi, D.; Martini, S.; Dei Più, L.; Gianotti, A. LC-ESI-QTOF-MS identification of novel antioxidant peptides obtained by enzymatic and microbial hydrolysis of vegetable proteins. Food Chem. 2017, 228, 186–196. [Google Scholar] [CrossRef]
- Millan-Linares, M.C.; Toscano, R.; Lemus-Conejo, A.; Martin, M.E.; Pedroche, J.; Millan, F.; Montserrat-de la Paz, S. GPETAFLR, a biopeptide from Lupinus angustifolius L., protects against oxidative and inflammatory damage in retinal pigment epithelium cells. J. Food Biochem. 2019, 43, e12995. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, X.; Ren, G.; Wu, C.; Qin, P.Y.; Yao, Y. Peptides from extruded lupin (Lupinus albus L.) regulate inflammatory activity via the p38 MAPK signal transduction pathway in RAW 264.7 cells. J. Agric. Food Chem. 2020, 32, 1–8. [Google Scholar] [CrossRef]
- Muñoz, E.B.; Luna-vital, D.A.; Fornasini, M.; Baldeón, M.E.; Gonzalez, E.; Mejia, D. Gamma-conglutin peptides from Andean lupin legume (Lupinus mutabilis Sweet) enhanced glucose uptake and reduced gluconeogenesis in vitro. J. Funct. Foods 2018, 45, 339–347. [Google Scholar] [CrossRef]
- Yea, C.S.; Ebrahimpour, A.; Hamid, A.A.; Bakar, J.; Muhammad, K.; Saari, N. Winged bean [Psophorcarpus tetragonolobus (L.) DC] seeds as an underutilised plant source of bifunctional proteolysate and biopeptides. Food Funct. 2014, 5, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wang, Y.; Wang, M.; Wang, Z.; Wang, X.; Ju, X.; He, R. Antihypertensive activity of the ACE–renin inhibitory peptide derived from Moringa oleifera protein. Food Funct. 2021, 12, 8994–9006. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.L.; Cai, S.Y.; Gao, M.; Chu, X.M.; Pan, X.Y.; Gong, K.K.; Xiao, C.W.; Chen, Y.; Zhao, Y.Q.; Wang, B.; et al. Purification of antioxidant peptides of Moringa oleifera seeds and their protective effects on H2O2 oxidative damaged Chang liver cells. J. Funct. Foods 2020, 64, 103698. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Xu, F.; Xie, J.; Gao, X.; Li, L.; Tian, Y.; Sheng, J. Characterization of the structure, stability, and activity of hypoglycemic peptides from Moringa oleifera seed protein hydrolysates. Food Funct. 2022, 13, 3481–3494. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, L.; Huang, Z.; Zhao, Q.; Fan, J.; Tian, Y.; Huang, A. Isolation, identification and characterization of a novel antimicrobial peptide from Moringa oleifera seeds based on affinity adsorption. Food Chem. 2023, 398, 133923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; He, L.; Wang, X.; Ding, X.; Li, L.; Tian, Y.; Huang, A. Characterization of a Novel Antimicrobial Peptide Isolated from Moringa oleifera Seed Protein Hydrolysates and Its Membrane Damaging Effects on Staphylococcus aureus. J. Agric. Food Chem. 2022, 70, 6123–6133. [Google Scholar] [CrossRef]
- Cermeño, M.; Stack, J.; Tobin, P.R.; O’Keeffe, M.B.; Harnedy, P.A.; Stengel, D.B.; FitzGerald, R.J. Peptide Identification from a Porphyra dioica Protein Hydrolysate with Antioxidant, Angiotensin Converting Enzyme and Dipeptidyl Peptidase IV Inhibitory Activities. Food Funct. 2019, 10, 3421–3429. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kang, N.; Kim, E.-A.; Kang, M.C.; Lee, S.-H.; Kang, S.-M.; Lee, J.-B.; Jeon, B.-T.; Kim, S.-K.; Park, S.-J.; et al. A Novel Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptide from a Marine Chlorella ellipsoidea and Its Antihypertensive Effect in Spontaneously Hypertensive Rats. Process Biochem. 2012, 47, 2005–2011. [Google Scholar] [CrossRef]
- Sheih, I.-C.; Fang, T.J.; Wu, T.-K. Isolation and Characterisation of a Novel Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptide from the Algae Protein Waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Cherng, J.Y.; Liu, C.C.; Shen, C.R.; Lin, H.H.; Shih, M.F. Beneficial Effects of Chlorella-11 Peptide on Blocking LPS-Induced Macrophage Activation and Alleviating Thermal Injury-Induced Inflammation in Rats. Int. J. Immunopathol. Pharmacol. 2010, 23, 811–820. [Google Scholar] [CrossRef]
- Shih, M.; Chen, L.; Cherng, J. Chlorella 11-Peptide Inhibits the Production of Macrophage-Induced Adhesion Molecules and Reduces Endothelin-1 Expression and Endothelial Permeability. Mar. Drugs 2013, 11, 3861–3874. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-C.; Heo, S.-Y.; Choi, S.-W.; Qian, Z.-J.; Heo, S.-J.; Kang, D.-H.; Kim, N.; Jung, W.-K. A Heptameric Peptide Isolated from the Marine Microalga Pavlova Lutheri Suppresses PMA-Induced Secretion of Matrix Metalloproteinase-9 through the Inactivation of the JNK, P38, and NF-ΚB Pathways in Human Fibrosarcoma Cells. J. Appl. Phycol. 2018, 30, 2367–2378. [Google Scholar] [CrossRef]
- Oh, G.-W.; Ko, S.-C.; Heo, S.-Y.; Nguyen, V.-T.; Kim, G.; Jang, C.H.; Park, W.S.; Choi, I.-W.; Qian, Z.-J.; Jung, W.-K. A Novel Peptide Purified from the Fermented Microalga Pavlova lutheri Attenuates Oxidative Stress and Melanogenesis in B16F10 Melanoma Cells. Process Biochem. 2015, 50, 1318–1326. [Google Scholar] [CrossRef]
- Suetsuna, K.; Chen, J.-R. Identification of Antihypertensive Peptides from Peptic Digest of Two Microalgae, Chlorella vulgaris and Spirulina platensis. Mar. Biotechnol. 2001, 3, 305–309. [Google Scholar] [CrossRef]
- Sun, Y.; Chang, R.; Li, Q.; Li, B. Isolation and Characterization of an Antibacterial Peptide from Protein Hydrolysates of Spirulina platensis. Eur. Food Res. Technol. 2016, 242, 685–692. [Google Scholar] [CrossRef]
- Yu, J.; Hu, Y.; Xue, M.; Dun, Y.; Li, S.; Peng, N.; Liang, Y.; Zhao, S. Purification and Identification of Antioxidant Peptides from Enzymatic Hydrolysate of Spirulina platensis. J. Microbiol. Biotechnol. 2016, 26, 1216–1223. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X. Inhibitory Effects of Small Molecular Peptides from Spirulina (Arthrospira) platensis on Cancer Cell Growth. Food Funct. 2016, 7, 781–788. [Google Scholar] [CrossRef]
- Lu, J.; Ren, D.-F.; Xue, Y.-L.; Sawano, Y.; Miyakawa, T.; Tanokura, M. Isolation of an Antihypertensive Peptide from Alcalase Digest of Spirulina platensis. J. Agric. Food Chem. 2010, 58, 7166–7171. [Google Scholar] [CrossRef]
- Kim, N.-H.; Jung, S.-H.; Kim, J.; Kim, S.-H.; Ahn, H.-J.; Song, K. Bin. Purification of an Iron-Chelating Peptide from Spirulina Protein Hydrolysates. J. Korean Soc. Appl. Biol. Chem. 2014, 57, 91–95. [Google Scholar] [CrossRef]
- Safitri Nur, M.; Herawati, E.Y.; Hsu, J.-L. Antioxidant Activity of Purified Active Peptide Derived from Spirulina platensis Enzymatic Hydrolysates. Res. J. Life Sci. 2017, 4, 119–128. [Google Scholar] [CrossRef]
- Popoola, J.; Ojuederie, O.; Omonhinmin, C.; Adegbite, A. Neglected and underutilized legume crops: Improvement and future prospects. In Recent Advances in Grain Crops Research; Shah, F., Khan, Z., Iqbal, A., Turan, M., Olgun, M., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Okagu, I.U.; Ndefo, J.C.; Aham, E.C.; Obeme-Nmom, J.I.; Agboinghale, P.E.; Aguchem, R.N.; Nechi, R.N.; Lammi, C. Lupin-derived bioactive peptides: Intestinal transport, bioavailability and health benefits. Nutrients 2021, 13, 3266. [Google Scholar] [CrossRef] [PubMed]
- Chay, S.Y.; Tan, W.K.; Saari, N. Preparation and characterisation of nanoliposomes containing winged bean seeds bioactive peptides. J. Microencapsul. 2015, 32, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Chay, S.Y.; Salleh, A.; Sulaiman, N.F.; Abidin, N.Z.; Hanafi, M.A.; Zarei, M.; Saari, N. Blood-pressure lowering efficacy of winged bean seed hydrolysate in spontaneously hypertensive rats, peptide characterization and a toxicity study in Sprague-Dawley rats. Food Funct. 2018, 9, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Arise, A.K.; Adeola, M.; Alashi, A.M.; Nwachukwu, I.D.; Malomo, S.A.; Aluko, R.E.; Amonsou, E.O. Inhibitory properties of Bambara groundnut protein hydrolysate and peptide fractions against angiotensin-converting enzymes, renin and free radicals. J. Sci. Food Agric. 2017, 97, 2834–2841. [Google Scholar] [CrossRef]
- Mune Mune, M.A.; Minka, S.R.; Henle, T. Investigation on antioxidant, angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activity of Bambara bean protein hydrolysates. Food Chem. 2018, 250, 162–169. [Google Scholar] [CrossRef]
- Landi, N.; Clemente, A.; Pedone, P.V.; Ragucci, S.; Di Maro, A. An Updated Review of Bioactive Peptides from Mushrooms in a Well-Defined Molecular Weight Range. Toxins 2022, 14, 84. [Google Scholar] [CrossRef]
- Avilés-Gaxiola, S.; León-Félix, J.; Jiménez-Nevárez, Y.B.; Angulo-Escalante, M.A.; Ramos-Payán, R.; Colado-Velázquez III, J.; Heredia, J.B. Antioxidant and anti-inflammatory properties of novel peptides from Moringa oleifera Lam. leaves. S. Afr. J. Bot. 2021, 141, 466–473. [Google Scholar] [CrossRef]
- Tian, Y.; Lin, L.; Zhao, M.; Peng, A.; Zhao, K. Xanthine oxidase inhibitory activity and antihyperuricemic effect of Moringa oleifera Lam. leaf hydrolysate rich in phenolics and peptides. J. Ethnopharmacol. 2021, 270, 113808. [Google Scholar] [CrossRef]
- Tran, H.C.; Le, H.A.T.; Le, T.T.; Phan, V.M. Effects of enzyme types and extraction conditions on protein recovery and antioxidant properties of hydrolysed proteins derived from defatted Lemna minor. Appl. Sci. Eng. Prog. 2021, 14, 360–369. [Google Scholar] [CrossRef]
- Duangjarus, N.; Chaiworapuek, W.; Rachtanapun, C.; Ritthiruangdej, P.; Charoensiddhi, S. Antimicrobial and Functional Properties of Duckweed (Wolffia globosa) Protein and Peptide Extracts Prepared by Ultrasound-Assisted Extraction. Foods 2022, 11, 2348. [Google Scholar] [CrossRef] [PubMed]
- Skjånes, K.; Aesoy, R.; Herfindal, L.; Skomedal, H. Bioactive peptides from microalgae: Focus on anti-cancer and immunomodulating activity. Physiol. Plant. 2021, 173, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.A.; Coscueta, E.R.; Nova, P.; Silva, J.L.; Pintado, M.M. Bioactive Hydrolysates from Chlorella vulgaris: Optimal Process and Bioactive Properties. Molecules 2022, 27, 2505. [Google Scholar] [CrossRef] [PubMed]
- Paiva, L.; Lima, E.; Neto, A.; Baptista, J. Angiotensin I-Converting Enzyme (ACE) Inhibitory Activity, Antioxidant Properties, Phenolic Content and Amino Acid Profiles of Fucus spiralis L. Protein Hydrolysate Fractions. Mar. Drugs 2017, 15, 311. [Google Scholar] [CrossRef] [PubMed]
- Sedighi, M.; Jalili, H.; Ranaei-Siadat, S.-O.; Amrane, A. Potential Health Effects of Enzymatic Protein Hydrolysates from Chlorella vulgaris. Appl. Food Biotechnol. 2016, 3, 160. [Google Scholar]
- Morris, H.J.; Carrillo, O.; Almarales, A.; Bermúdez, R.C.; Lebeque, Y.; Fontaine, R.; Llauradó, G.; Beltrán, Y. Immunostimulant Activity of an Enzymatic Protein Hydrolysate from Green Microalga Chlorella vulgaris on Undernourished Mice. Enzym. Microb. Technol. 2007, 40, 456–460. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X. Separation, Antitumor Activities, and Encapsulation of Polypeptide from Chlorella pyrenoidosa. Biotechnol. Prog. 2013, 29, 681–687. [Google Scholar] [CrossRef]
- Darvish, M.; Jalili, H.; Ranaei-Siadat, S.-O.; Sedighi, M. Potential Cytotoxic Effects of Peptide Fractions from Dunaliella salina Protein Hydrolyzed by Gastric Proteases. J. Aquat. Food Prod. Technol. 2018, 27, 165–175. [Google Scholar] [CrossRef]
- Stull, V.J. Impacts of insect consumption on human health. J. Insects Food Feed 2021, 7, 695–713. [Google Scholar] [CrossRef]
- Dai, Y.; Huang, M.; Xu, Y.; Mu, L.; Gao, J.; Chen, H.; Li, X. Enzymatic hydrolysis of silkworm pupa and its allergenicity evaluation by animal model with different immunization routes. Food Sci. Hum. Wellness 2023, 12, 774–782. [Google Scholar] [CrossRef]
- Li, W.; Mu, L.; Zou, Y.; Wang, W.; Zhao, H.; Wu, X.; Liao, S. Effect of Silkworm Pupa Protein Hydrolysates on Proliferation of Gastric Cancer Cells In-Vitro. Foods 2022, 11, 2367. [Google Scholar] [CrossRef]
- Ji, X.; Wang, J.; Ma, A.; Feng, D.; He, Y.; Yan, W. Effects of silkworm pupa protein on apoptosis and energy metabolism in human colon cancer DLD-1 cells. Food Sci. Hum. Wellness 2022, 11, 1171–1176. [Google Scholar]
- Mintah, B.K.; He, R.; Dabbour, M.; Xiang, J.; Agyekum, A.A.; Ma, H. Techno-functional attribute and antioxidative capacity of edible insect protein preparations and hydrolysates thereof: Effect of multiple mode sonochemical action. Ultrason. Sonochemistry 2019, 58, 104676. [Google Scholar]
- Zielińska, E.; Baraniak, B.; Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551. [Google Scholar]
- Tadesse, W.; Bekele, E. Variation and association of morphological and biochemical characters in grass pea (Lathyrus sativus L.). Euphytica 2003, 130, 315–324. [Google Scholar] [CrossRef]
- Lambein, F.; Travella, S.; Kuo, Y.H.; Van Montagu, M.; Heijde, M. Grass pea (Lathyrus sativus L.): Orphan crop, nutraceutical or just plain food? Planta 2019, 250, 821–838. [Google Scholar] [PubMed]
- Xu, Q.; Song, B.; Liu, F.; Song, Y.; Chen, P.; Liu, S.; Krishnan, H.B. Identification and characterization of β-Lathyrin, an abundant glycoprotein of grass pea (Lathyrus sativus L.), as a potential allergen. J. Agric. Food Chem. 2018, 66, 8496–8503. [Google Scholar] [CrossRef]
- Villacrés, E.; Álvarez, J.; Rosell, C. Effects of two debittering processes on the alkaloid content and quality characteristics of lupin (Lupinus mutabilis Sweet). J. Sci. Food Agric. 2020, 100, 2166–2175. [Google Scholar]
- Berghout, J.A.M.; Marmolejo-Garcia, C.; Berton-Carabin, C.C.; Nikiforidis, C.V.; Boom, R.M.; van der Goot, A.J. Aqueous fractionation yields chemically stable lupin protein isolates. Food Res. Int. 2015, 72, 82–90. [Google Scholar]
- Gulisano, A.; Alves, S.; Martins, J.N.; Trindade, L.M. Genetics and breeding of Lupinus mutabilis: An emerging protein crop. Front. Plant Sci. 2019, 10, 1385. [Google Scholar]
- Burgos-Diaz, C.; Piornos, J.A.; Wandersleben, T.; Ogura, T.; Hernández, X.; Rubilar, M. Emulsifying and foaming properties of different protein fractions obtained from a novel lupin variety AluProt-CGNA(R) (Lupinus luteus). J. Food Sci. 2016, 81, C1699–C1706. [Google Scholar]
- Paraskevopoulou, A.; Provatidou, E.; Tsotsiou, D.; Kiosseoglou, V. Dough rheology and baking performance of wheat flour–lupin protein isolate blends. Food Res. Int. 2010, 43, 1009–1016. [Google Scholar]
- FAO; WHO. Risk Assessment of Food Allergens. Part 1—Review and validation of Codex Alimentarius priority allergen list through risk assessment. In Meeting Report 2022; Food Safety and Quality Series No. 14; FAO: Rome, Italy, 2022. [Google Scholar]
- Villa, C.; Costa, J.; Mafra, I. Lupine allergens: Clinical relevance, molecular characterization, cross-reactivity, and detection strategies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3886–3915. [Google Scholar]
- Regulation (EU) No. 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No. 1924/2006 and (EC) No. 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No. 608/2004; European Union: Luxemburg, 2004.
- WHO/IUIS Allergen Nomenclature. Available online: www.allergen.org (accessed on 25 November 2022).
- Schlegel, K.; Sontheimer, K.; Hickisch, A.; Wani, A.A.; Eisner, P.; Schweiggert-Weisz, U. Enzymatic hydrolysis of lupin protein isolates—Changes in the molecular weight distribution, technofunctional characteristics, and sensory attributes. Food Sci. Nutr. 2019, 7, 2747–2759. [Google Scholar] [PubMed]
- Álvarez-Álvarez, J.; Guillamón, E.; Crespo, J.F.; Cuadrado, C.; Burbano, C.; Rodríguez, J.; Fernández, C.; Muzquiz, M. Effects of extrusion, boiling, autoclaving, and microwave heating on lupine allergenicity. J. Agric. Food Chem. 2005, 53, 1294–1298. [Google Scholar] [PubMed]
- Tan, N.H.; Rahim, Z.H.A.; Khor, H.T.; Wong, K.C. Winged bean (Psophocarpus tetragonolobus) tannin level, phytate content and hemagglutinating activity. J. Agric. Food Chem. 1983, 31, 916–917. [Google Scholar]
- Vatanparast, M.; Shetty, P.; Chopra, R.; Doyle, J.J.; Sathyanarayana, N.; Egan, A.N. Transcriptome sequencing and marker development in winged bean (Psophocarpus tetragonolobus; Leguminosae). Sci. Rep. 2016, 6, 29070. [Google Scholar] [PubMed]
- Kantha, S.S.; Hettiarachchy, N.S.; Erdman, J.W., Jr. Phytic acid, and selected minerals in winged bean flour. Cereal Chem. 1986, 63, 9–13. [Google Scholar]
- Adegboyega, T.T.; Abberton, M.T.; AbdelGadir, A.H.; Dianda, M.; Maziya-Dixon, B.; Oyatomi, O.A.; Ofodile, S.; Babalola, O.O. Nutrient and antinutrient composition of winged bean (Psophocarpus tetragonolobus (L.) DC.) seeds and tubers. J. Food Qual. 2019, 3075208. [Google Scholar]
- Belmiro, R.H.; Tribst, A.A.L.; Cristianini, M. Effects of high pressure processing on common beans (Phaseolus vulgaris L.): Cotyledon structure, starch characteristics, and phytates and tannins contents. Starch-Stärke 2020, 72, 1900212. [Google Scholar]
- Hadi, J.; Brightwell, G. Safety of Alternative Proteins: Technological, Environmental and Regulatory Aspects of Cultured Meat, Plant-Based Meat, Insect Protein and Single-Cell Protein. Foods 2021, 10, 1226. [Google Scholar]
- Rzymski, P.; Niedzielski, P.; Kaczmarek, N.; Jurczak, T.; Klimaszyk, P. The Multidisciplinary Approach to Safety and Toxicity Assessment of Microalgae-Based Food Supplements Following Clinical Cases of Poisoning. Harmful Algae 2015, 46, 34–42. [Google Scholar]
- Pali-Schöll, I.; Verhoeckx, K.; Mafra, I.; Bavaro, S.L.; Mills, E.N.C.; Monaci, L. Allergenic and novel food proteins: State of the art and challenges in the allergenicity assessment. Trends Food Sci. Technol. 2019, 84, 45–48. [Google Scholar]
- Kooh, P.; Jury, V.; Laurent, S.; Audiat-Perrin, F.; Sanaa, M.; Tesson, V.; Federighi, M.; Boué, G. Control of Biological Hazards in Insect Processing: Application of HACCP Method for Yellow Mealworm (Tenebrio molitor) Powders. Foods 2020, 9, 1528. [Google Scholar]
- Ribeiro, J.C.; Sousa-Pinto, B.; Fonseca, J.; Fonseca, S.C.; Cunha, L.M. Edible insects and food safety: Allergy. J. Insects Food Feed 2021, 7, 833–847. [Google Scholar]
- Cappelli, A.; Cini, E.; Lorini, C.; Oliva, N.; Bonaccorsi, G. Insects as food: A review on risks assessments of Tenebrionidae and Gryllidae in relation to a first machines and plants development. Food Control 2020, 108, 106877. [Google Scholar]
- Verhoeckx, K.C.; van Broekhoven, S.; den Hartog-Jager, C.F.; Gaspari, M.; de Jong, G.A.; Wichers, H.J.; van Hoffen, E.; Houben, G.F.; Knulst, A.C. House dust mite (Der p 10) and crustacean allergic patients may react to food containing Yellow mealworm proteins. Food Chem. Toxicol. 2014, 65, 364–373. [Google Scholar]
- Francis, F.; Doyen, V.; Debaugnies, F.; Mazzucchelli, G.; Caparros, R.; Alabi, T.; Blecker, C.; Haubruge, E.; Corazza, F. Limited cross reactivity among arginine kinase allergens from mealworm and cricket edible insects. Food Chem. 2019, 276, 714–718. [Google Scholar]
- De Gier, S.; Verhoeckx, K. Insect (food) allergy and allergens. Mol. Immunol. 2018, 100, 82–106. [Google Scholar] [PubMed]
- He, W.; Li, S.; He, K.; Sun, F.; Mu, L.; Li, Q.; Yi, J.; He, Z.; Liu, Z.; Wu, X. Identification of potential allergens in larva, pupa, moth, silk, slough and feces of domestic silkworm (Bombyx mori). Food Chem. 2021, 362, 130231. [Google Scholar] [PubMed]
- Lamberti, C.; Nebbia, S.; Cirrincione, S.; Brussino, L.; Giorgis, V.; Romito, A.; Marchese, C.; Manfredi, M.; Marengo, E.; Giuffrida, M.G.; et al. Thermal processing of insect allergens and IgE cross-recognition in Italian patients allergic to shrimp, house dust mite and mealworm. Food Res. Int. 2021, 148, 110567. [Google Scholar] [PubMed]
- Garino, C.; Mielke, H.; Knuppel, S.; Selhorst, T.; Broll, H.; Braeuning, A. Quantitative allergenicity risk assessment of food products containing yellow mealworm (Tenebrio molitor). Food Chem. Toxicol. 2020, 142, 111460. [Google Scholar] [PubMed]
- Quintieri, L.; Monaci, L. From reality to virtuality: The NUTRIBOX e-commerce platform. Affidia-J. Food Diagn. 2021, 3, 75–77. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).