Abstract
This study aimed to identify sociodemographic and health indicators of diet quality in pre-frail community-dwelling older adults. Pre-frail older adults are those at risk of progression to clinical manifestations of frailty and are targets for preventative intervention. We previously reported that pre-frail older adults have reasonably good overall diet quality. However, further analyses found a low intake of energy, protein and several micronutrients. Methods: We collected detailed dietary intake from pre-frail (FRAIL scale 1–2) older adults using NZ Intake24, an online version of 24 h multiple pass dietary recall. Diet quality was ascertained with the Diet Quality Index-International (DQI-I). We used regression generalized linear models to determine predictors of diet quality as well as classification and regression tree (CART) analysis to examine the complex relationships between predictors and identified profiles of sub-groups of older adults that predict diet quality. Results: The median age in this sample (n = 468) was 80.0 years (77.0–84.0). Living with others, a high deprivation index and a higher BMI were independent predictors of poorer diet quality. With CART analysis, we found that those with a BMI > 29 kg/m2, living with others and younger than 80 years were likely to have a lower diet quality. Conclusions: We found that BMI, living arrangement and socioeconomic status were independent predictors of diet quality in pre-frail older adults, with BMI being the most important variable in this sample when the interaction of these variables was considered. Future research is needed to determine the similarities and/or differences in the profile of subgroups of older adults with poorer diet quality.
1. Introduction
Frailty is a condition of accumulated decline in physiological reserves resulting in weakened homeostatic responses to stressors [1,2]. The prevalence of physical frailty increases with age, with almost half of individuals aged over 50 years old classified as pre-frail and 12–24% as frail [3]. In a New Zealand survey, Māori living in the community had a 10-to-15 years earlier onset of frailty compared to non-Māori [4]. Older adults displaying features of frailty are vulnerable to poor health outcomes like falls and prolonged hospital stays [1]. Pressure is put on the health sector to work against this inequity and reduce the burden of frailty in the community, especially population groups most at risk.
Poor nutrition can influence frailty indicators such as weight loss and exhaustion [1]. A systematic review and meta-analysis of 10 studies (n = 5447 community-dwelling older adults, mean age 77 years) found that one in four malnourished older adults were pre-frail, and two-thirds were frail [5]. Increasingly, studies have also found higher risks of frailty in older adults with obesity and sarcopenic obesity (low muscle mass and function coupled with high fat mass) [6,7,8,9]. In the NHANES study, pre-frail older adults had greater central adiposity than their robust counterparts [7]. These findings suggest that, even at early stages of frailty progression, or else termed “pre-frailty” [10], both undernutrition and overnutrition may be key risk factors (amongst many others).
Diet quality is a measure of the quality, quantity and variety of the entire diet, allowing for the examination of the association between whole food and health status [11]. Many factors are associated with diet quality in older adults. The complexity of each population group and diet quality determinants make it challenging to single out individual factors as, collectively, there are many factors to consider regarding varying interactions dependent on specific situations [12]. Sociodemographic and health indicators are associated with diet quality in older adults [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. In frail older adults, the higher presence of mobility and functional limitations and poorer health status may impact access to healthy food, predisposing them to the risk of malnutrition [1,5,28].
We have previously described the diet quality of pre-frail older adults in New Zealand [29]. We found that pre-frail older adults had nutritional gaps in energy and nutrient deficiencies and a disproportionate consumption of empty calories. Dietary moderation and balance scores averaged below 50% of the maximum score in the diet quality index–international (DQI-I) tool [29]. The nutritional gaps call into question how clinicians and policymakers can influence diet quality in at-risk groups for frailty prevention. This current paper aimed to identify the predictors of diet quality in pre-frail community-dwelling older adults and identify mutually exclusive subgroups in the sample who shared common characteristics that influence diet quality.
2. Materials and Methods
2.1. Study Sample
Community-dwelling older adults aged 75+ (or 60 for Māori and Pacific people) were recruited across four sites in New Zealand (Auckland, Whangarei, Tauranga and Invercargill) through general practices via postal invitations to participate in the Staying UPright and Eating well Research (SUPER) study. Those who were terminally ill, had advanced dementia or for whom it was medically unsafe to participate in low-intensity exercise as judged by the GP or Māori health provider were not invited to the study.
Those who responded with interest (4791 of 6690 invited) were screened for pre-frailty using the self-administered FRAIL questionnaire. Those scoring 1 or 2 out of 5 in the tool (i.e., pre-frailty) and self-reported being able to stand and able to use the kitchen utensils safely were recruited [30]. Of those screened, 1015 were eligible and 468 pre-frail older adults were recruited. The lower threshold for Māori and Pacific people was implemented to reflect the inequitable earlier occurrence of disability, poor health and mortality in these population groups compared to the general population in New Zealand [31]. All study participants provided written informed consent.
The Southern Health and Disability Ethics Committee, Ministry of Health, New Zealand (Ref 14/STH/101/, 13 August 2014) approved this study.
We utilized the baseline data of the SUPER Study collected between 2016 and 2018 for this analysis.
2.2. Data Collection
Dietary intake was recorded on two separate days using a 24 h multiple pass dietary recall method (24 h-MPR) through the online Intake24 platform developed by Newcastle University [32,33]. The 24 h-MPR has proved to be a feasible dietary assessment method to capture detailed dietary intake in older adults. [34] We adapted the Intake24 online platform to be relevant to New Zealand older adults’ dietary intake based on the 24 h-MPR paper-based version from more than 500 octogenarians. The backend of the Intake24 online platform was updated with the New Zealand FOODfiles database from 2014 to 2016 [35], leading to 158 food codes being withdrawn and 215 updated. Meticulous data verification and coding were completed by qualified nutritionists, ensuring the database fit the purpose of the study. A workflow summary is presented in the supplementary material (Figure S1).
Diet quality was ascertained using the Diet Quality Index-International (DQI-I) tool developed by Kim et al., which measures intake according to four categories: variety (20 points), adequacy (40 points), moderation (30 points) and balance (10 points)—that adds up to a maximum score of 100; a higher score indicates a better diet quality [36]. The DQI-I is validated for international comparisons and has previously been utilized effectively in older population groups [29,37]. Adaptations to the DQI-I for our study were reported previously [29].
Sociodemographic and health information collected using a standardized questionnaire and at-home interviews included: age, sex, ethnic group, education level, New Zealand Deprivation Index, living arrangement, medical conditions, medications, supplements, alcohol, smoking, vision, hearing, Nottingham Extended Activities of Daily Living (NEADL), Montreal Cognitive Assessment (MoCA), Geriatric Depression Scale (GDS) and short physical performance battery (SPPB) [30].
Anthropometric information was measured using a Tanita BC-545N scale and Stadiometer. BMI was calculated as weight (kg)/height (m)2. Waist circumference was measured with a non-stretchable tape taken at the natural narrowing midway between the last rib and the crest of the ilium.
Further data collection details are recorded in the study protocol [30].
2.3. Statistical Tests
2.3.1. Descriptive Data
Quantitative data are presented as median (interquartile range (IQR)) or mean (standard deviation (SD)) depending on data distribution, and categorical data are presented as count (percentage).
2.3.2. Regression and Tree Analysis
Generalized linear models (GLMs), with normal probability distribution and identity link function, were constructed to identify predictors of DQI-I scores. All independent variables (listed in Table 1) except BMI, waist circumference, NEADL and SPPB were categorical. Variables with a p-value less than 0.2 from univariate models were systematically included in the multivariate GLM models to construct parsimonious models. To select a subset of predictors for the models, we added potential predictors of DQI-I scores by groups, i.e., first, we included sociodemographic variables, followed by lifestyle variables (smoking, alcohol), sensory (hearing, vision), body composition (BMI, waist circumference), physical function (NEADL, SPPB) and health status (co-morbidity, prescribed medication, supplement and MoCA). After selecting a subset of predictor variables, we constructed parsimonious models using Akaike Information Criterion and Bayesian Information Criterion. We presented the parsimonious models (Table 2). Further analyses were conducted on DQI-I subcomponents, i.e., diet variety, adequacy, moderation and overall balance.

Table 1.
Sample characteristics.

Table 2.
Multivariate regression against DQI-I score and subcomponents.
Classification and regression tree (CART) analysis is a machine learning technique that helps make predictions by untangling interactions between variables and splitting data into subgroups based on a criterion that aims to maximize the distinction of the outcome of interest through a recursive process to produce mutually exclusive and exhaustive subgroups of population that share common characteristics that predict the outcome of interest [38]. We used CART to identify mutually exclusive sub-groups within our sample by partitioning groups based on the interaction between demographic and health characteristics that best describe diet quality. The minimum number of cases for child nodes was set to five (1% of the total sample) [38], and the maximum number of tree depths was 5. A k-fold cross-validation was used to improve confidence in the final trees. We choose k = 10, a commonly used value which divides the dataset into ten equal-sized subsamples, and performed ten iterations of model training and testing. In each iteration, one subsample of data was reserved as the test set, while the remaining nine subsamples were used for training. After all ten iterations were completed, a single final tree model was produced. Separate CART analyses were completed for low-energy reporters (LER = total energy intake/basal metabolic rate ≤ 0.92) and plausible reporters due to the strong relationship between low-energy reporting and DQI-I [29]. We then compared the results obtained from CART with the GLM regression models.
Statistical significance was set at p < 0.05. All statistical tests in this paper were conducted using IBM SPSS Statistics for Macintosh, version 27 (IBM Corp., Armonk, NY, USA).
3. Results
3.1. Sample Descriptives
Of the sample of 468 participants recruited from 27 general practices, three had missing dietary assessments due to technical difficulties. The median age was 80.0 years (77.0–84.0), 59% were female and a majority were of European descent (Table 1).
3.2. Associations: Generalized Linear Models
3.2.1. Univariate Analysis
In univariate analysis, living alone, low deprivation and lower BMI were significantly associated with higher (better) overall DQI-I score (p < 0.05) (Table A1). More specifically, those living alone had a higher score for moderation of food/nutrient intake that may require restriction, e.g., saturated fat and sodium; lower BMI was associated with higher scores in variety and adequacy, and better socioeconomic status was associated with higher adequacy scores.
3.2.2. Multivariate Analysis
Multivariate analyses show that those living with others (p = 0.042), with lower socioeconomic status (p = 0.024) and a higher BMI (p = 0.026) were associated with poorer diet quality (lower DQI-I scores) (Table 2). Living with others and having a higher BMI were associated with lower diet variety scores. Lower socioeconomic status and higher BMI were associated with lower adequacy scores. We observed that impaired cognitive function and regular alcohol consumption were associated with overall balance in dietary score (Table 2).
In subgroup analyses completed among LER, we found no associations between demographic and health variables with DQI-I. In further analyses, we observed various variables associated with adequacy, moderation and balance. Participants living in medium-deprived areas were associated with higher adequacy than those living in highly deprived areas (p = 0.007); the difference was not seen between low and high areas (p = 0.111) (Table S1). For plausible reporters, associations were found for DQI-I and all subcomponents apart from variety. Participants of non-European ethnicity were marginally associated with a lower DQI-I, which is linked to a lower adequacy score (p = 0.003). Smokers were associated with lower adequacy (p = 0.027) than non-smokers (Table S2).
3.3. Relationships between Variables: Classification and Regression Tree (CART) Analysis
We completed a regression tree analysis to identify the profile of subgroups of older adults with poorer diet quality. Variables with a relative importance value above 90% in predicting diet quality were age (100%), BMI (99.2%) and living arrangement (92.7%), followed by vision (85.4%), and each of the remaining variables had a relative importance value less than 50%. In the decision tree model below, splitting the sample into mutually exclusive subgroups (Figure 1), only BMI, living arrangement and age were included; vision was excluded from the final tree model to prioritize more stable predictors.
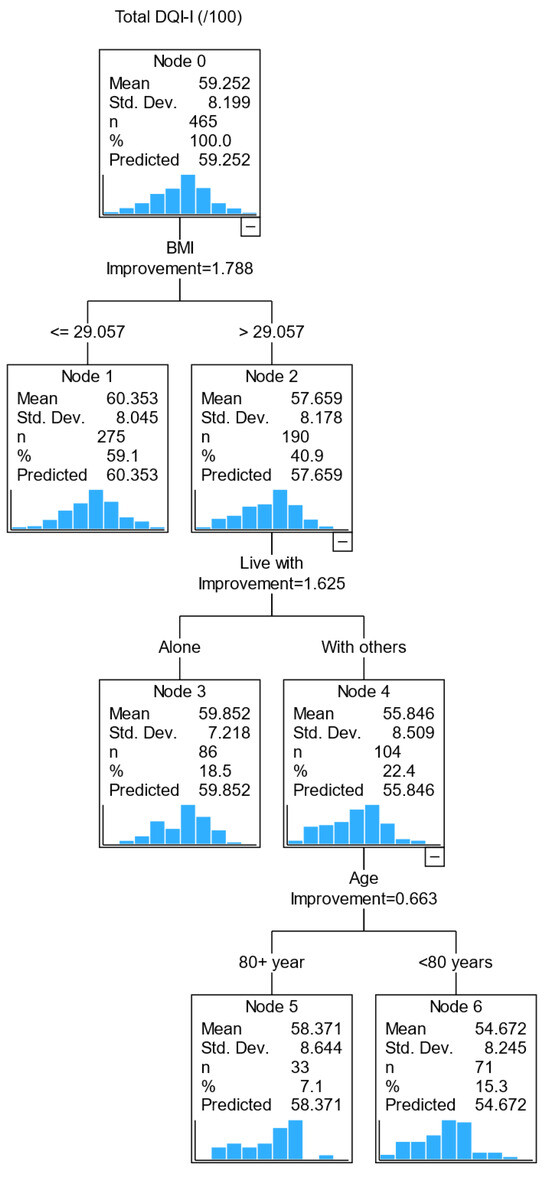
Figure 1.
CART diagram of factors associated with diet quality (DQI-I) in pre-frail older adults.
The first decision point is whether an older adult has a BMI above or below 29 kg/m2 (i.e., the best-split value from the regression tree analysis). A person with a BMI of >29 kg/m2 was predicted to have a lower diet quality than those with a BMI of ≤29 kg/m2. The second decision point was the living arrangement, i.e., living with others or alone. Those with a BMI > 29 kg/m2 and living with others had a lower DQI score than those who lived alone with a similar BMI range. The third decision point was age (<80 and ≥80). Those with a BMI > 29 kg/m2, living with others and less than 80 years old had a lower diet quality than those aged ≥ 80. This model identified that those with a BMI > 29 kg/m2 living with others younger than 80 years were likely to have a lower diet quality.
Separate tree diagrams were constructed for DQI-I subcomponents (Figure S1). In each tree diagram, BMI remains a key (first) independent variable for all subcomponents, except for moderation, where sex was a predictor of moderation score (male sex has a lower moderation score). The second independent variable varies across the DQI-I subcomponents: living arrangement for diet variety, medication for adequacy and NEADL for balance.
When we compared the tree diagrams (CART analysis) to the GLM regression models, we found BMI to be a consistent predictor for the overall DQI-I score and subcomponents’ adequacy and balance scores; sex was also consistently identified for the moderation score in both the GLM model and the CART analysis.
4. Discussion
This paper aims to identify predictors of diet quality in community-dwelling pre-frail older adults and to identify mutually exclusive subgroups in the sample who share common characteristics that influence diet quality. We found that BMI, living arrangement and socioeconomic status were associated with diet quality. In GLM models, those with low socioeconomic status, living with others and higher BMI were independently associated with poorer diet quality.
We observed that a higher BMI was independently associated with lower diet quality, and further analysis showed a similar association for subcomponent diet adequacy. Diet adequacy was ascertained from vegetable, fruit and grain food groups, as well as key nutrients protein, iron, calcium and vitamin C [36]. Socio-economic status is linked to accessibility to food. A systematic review reports that socio-economic status is closely related to food insecurity in older adults, which impacts nutritional status [39]. A severe level of food insecurity characterized by food scarcity and hunger leads to weight loss, while a moderate level of food security characterized by scarcity of nutritional quality food leads to overweight [39]. In our study sample, those living in an area with a high deprivation index had a poorer diet quality.
It is somewhat surprising that living alone was independently associated with better diet quality, contrary to a previous study on octogenarians in which living alone was associated with high nutrition risk [40]; in a younger group of adults (mean age 74 ± 12), there was no conclusive evidence on the link between living arrangement and nutrition risk [41]. This is likely to be attributed to the interplay between living arrangement and other factors; for example, social networks in the neighborhood were shown to attenuate the negative association between living alone and daily fruit and vegetable consumption [42]. Two studies reported that some older adults living on their own relished the opportunity to experiment with different foods while others felt a sense of release from the constraint of eating food that pleased the preferences of others [16,43]. These findings may partly explain the higher diet quality score of those living alone in our study. We recommend further research to untangle the interaction of social networks and diet quality in older adults.
Further analyses into the subcomponents of diet quality found several interesting observations. Firstly, we observed that alcohol intake was associated with diet variety and balance scores. In the NHNES survey on a younger population, total diet quality (measured using the Healthy Eating Index) increased with the frequency of alcohol intake [44]. In our sample, there was no association between diet quality and alcohol consumption. However, those who never had alcohol had a lower diet variety and a higher dietary balance score than those who reported having alcohol more regularly. A lifestyle behavior (no alcohol or supplement) may be a key factor to a better dietary balance score. Another mechanism in play could be a compensating mechanism between diet variety and balance among non-alcohol users; the impact of this compensation over time on physical frailty needs further investigation.
Secondly, we did not find an independent association between smoking status and diet quality, but we observed that non-smokers had better diet adequacy and variety scores than smokers. These are in line with previous findings [45,46]. In the NHNES study, dietary energy density, a marker of diet quality, was associated with smoking status [45]. The authors found that smokers had a significantly higher dietary energy density (associated with poorer diet) than never-smokers [45]. The SRISCAV-LUX study (a younger population age 18–69, mean age approximately 42) reported that cigarette smoking was inversely associated with overall diet quality [46]. Although we did not observe a significant association between smoking status and diet quality, our findings showed a similar trend, as reported previously; the small sample size may have contributed to type II error.
Thirdly, we observed that those who used two or more medications had a lower diet adequacy score than those who used one or none. A similar trend was observed among those with and without cognitive impairment, i.e., the former group had a better dietary balance score. A Canadian study also observed this paradoxical relationship between the number of medications and cognitive function. [47] Reverse causality may contribute to this observation. In the NuAge study, total diet quality was not independently associated with cognition in healthy older adults (mean age 74). [47] The authors postulate that other risk factors may moderate the relationship between diet quality and cognition. Evidence on the effect of single nutrient supplementation on cognitive impairment is inconclusive, and a Mediterranean diet may help reduce the risk of cognitive decline [48].
A final observation on the subcomponents of diet quality was that the male sex was associated with a lower diet moderation score than its female counterpart. We reported previously that sodium intake was higher in males (median (interquartile range): 2140 (1701–2800) mg/day) than in females (1595 (1251–2160) mg/d); this is likely to contribute to a lower moderation score observed in men [29]. When all subcomponents were considered, sex was not an independent predictor of total diet quality in our sample of pre-frail older adults, a finding which is different from previous studies in younger or healthy populations [20,47]. Our findings of a more in-depth insight into predictors of specific aspects of diet quality could be useful for more targeted dietary interventions to impact physical frailty.
Findings from the CART analysis help to untangle the interaction of all variables of interest. We observed that a BMI > 29 kg/m2 was the best-split value in predicting diet quality, i.e., those with BMI > 29 kg/m2 had a lower diet quality (57.6) than those with BMI ≤ 29 kg/m2 (60.4). Further subgroups were identified based on living arrangement, i.e., those with BMI > 29 kg/m2 and living with others have a lower diet quality score than those who live alone. Further analysis showed that BMI remains the most important factor in predicting all subcomponents of diet quality except for moderation (i.e., energy intake from dietary fat, cholesterol, sodium and empty calorie foods), in which the male sex had a lower moderation score than its female counterpart. After BMI, living arrangement was predictive of diet variety; medication was predictive of diet adequacy and NEADL score for the overall balance of macronutrient and fatty acid ratios.
Previous studies found that diet quality is linked to health outcomes. In a longitudinal study of more than 7000 adults with a mean age of 65 years, diet quality was associated inversely with ADL-based disability and depression [49], and the Physicians Health Study found a dose–response relationship between diet quality with pre-frailty and frailty [50]. Enhancing diet quality can be advantageous for older adults. One approach to enhance diet quality in older adults is increasing nutritional literacy (e.g., interpreting food labels), awareness of food choices and knowledge of healthy food swaps.
These three diagrams make it possible for primary care providers to visualize factors important for dietary quality in pre-frail older adults. Nutrition plays a vital role in physical and mental health. Inadequate nutrition can exacerbate pre-frailty progression to the clinical manifestation of frailty [51]. The early identification of factors important for diet quality may facilitate a timely and specific intervention (e.g., education on increasing diet variety and/or specific nutrients, or moderation in empty calories) to optimize physical functionality.
Strengths and Limitations
We collected detailed dietary intake from a sample of community-dwelling older adults using the 24 h multiple pass dietary recall method. To the best of our knowledge, this is the first paper to explore the sociodemographic, socioeconomic and health indicators associated with the diet quality of pre-frail older adults.
This study has several limitations, including the nature of the data analysis. We cannot draw a cause–effect relationship from the results observed in this study. Influence can go both ways. Longitudinal studies are warranted to identify the directions and effects of determinants [12]. We were restricted to completing a training validation for the CART analysis to increase confidence and reduce the risk of bias in the study findings to allow for some external validation [52]. However, with the current sample size (n = 468), k-fold cross-validation is acceptable [38]. A small sample size is prone to prediction errors, including erroneous splits within the tree structures. In our GLM regression models, BMI and living alone were independent predictors of diet quality, agreeing with CART analysis regarding the strength and direction of the association.
This sample includes only pre-frail older adults, as determined using the FRAIL scale. This limits the generalizability of our findings to those who are “robust” or “frail”. Also, the FRAIL scale is composed of five items: fatigue, resistance, ambulation, number of illnesses and unintentional weight loss greater than 5% (12), and it was self-administered, which may be subject to recall bias (medical conditions) or social desirability bias (ambulation, climbing stairs without aid or physical activity levels). While a more objective measure of frailty phenotype (e.g., Fried phenotype of frailty) is desired, a simple test is desired in a primary care setting where resources are constrained. Similarly, finding the right balance between the availability of resources (e.g., trained clinicians) and the robustness of a tool to detect an outcome of interest need is essential. The FRAIL scale has a moderate-to-good agreement with many published frailty measures, including the Fried phenotype of frailty and Comprehensive Geriatric Assessment (CGA) [53].
The 24 h MPR dietary assessment method is subjected to biases, including social desirability, memory and seasonal variability. However, it minimizes participants’ burden and employing multiple passes allows for detailed information about portion sizes, recipes and preparation methods. This method was used by the New Zealand Adult National Nutrition Survey [54] and validated in advanced age [34,55]. The online platform relies on good network coverage and comprehensiveness of the food composition databases.
Both Māori and Pasifika people are disproportionately represented in poorer health outcomes. Under the Treaty of Waitangi and the goal of reducing inequalities in health, this study was designed to include Māori and Pasifika elders. The study sample included only a small proportion of Māori and Pasifika participants (9%). The proportion of Māori and Pasifika adults aged 60+ in the New Zealand population in 2013 was 6.6% [56] {Statistics New Zealand, #2753}. With the doubling of Māori and Pasifika elders in the next two decades, more robust research with Māori and Pasifika elders is warranted to enhance the understanding of the links between diet quality and frailty.
Future research is needed to identify predictors of diet quality in other settings and beyond pre-frail older adults to determine similarities and/or differences of subgroups of older adults who may benefit from timely and targeted nutrition intervention to improve diet quality.
5. Conclusions
Sociodemographic and health factors are associated with dietary intake in older adults. We found that BMI, living arrangement and socioeconomic status were associated with diet quality in pre-frail older adults, and the profile of subgroups with poorer diet quality were identified. The findings from this paper add a different dimension of appropriate screening strategies to improve the nutritional status of older adults at risk of frailty.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu15204416/s1, Figure S1: Summary of systems and changes implemented for cleaning Intake24 data, Figure S2: CART diagram of factors associated with DQI-I subcomponents in pre-frail older adults, Figure S3: CART diagram of factors associated with DQI-I and subcomponents in pre-frail older adults for LER only. Figure S4: CART diagram of factors associated with DQI-I and subcomponents in pre-frail older adults for low energy reporter (LER) only. Table S1: Multivariate regression against DQI-I score and subcomponents for Low Energy Reporter (LER) only, Table S2: Multivariate regression against DQI-I score and subcomponents for plausible reporters only.
Author Contributions
Conceptualization, R.T. and E.T. (Esther Tay); methodology, E.T. (Esther Tay) and R.T.; software, M.R.; validation, E.T. (Esther Tay), M.R. and R.T.; formal analysis, E.T. (Esther Tay) and D.B.; investigation, E.T. (Esther Tay) and R.T.; resources, R.T.; data curation, E.T. (Esther Tay) and R.T.; writing—original draft preparation, E.T. (Esther Tay) and R.T.; writing—review and editing, R.T., M.R., N.K., D.L.W., R.E., M.C. and A.P.; supervision, R.T.; project administration, E.T. (Esther Tay) and E.T. (Evelingi Tupou); funding acquisition, R.T., N.K. and D.L.W. All authors have read and agreed to the published version of the manuscript.
Funding
The SUPER study was funded by the National Science Challenges: Ageing Well, Ministry of Business Innovation and Employment (UoA Ref: 3710944; UoO Ref 13538). The Health Research Council New Zealand funded the feasibility phase of the SUPER Study. (UoA Ref 3715401; HRC 14/604).
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Southern Health and Disability Ethics Committee (Ref 14/STH/101) Ministry of Health New Zealand.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
We acknowledge the contribution from the SUPER study participants, the study site coordinators, and interviewers/assessors. We acknowledge the local Primary Healthcare Organizations (East Health Trust, Manaia Health, Western Bay of Plenty and Well South) for enabling recruitment through general practices, and Anna Rolleston for facilitating recruitment from the Centre of Health. We also acknowledge Eruera Maxted (Lakes District Health Board) for supporting the dietary assessment during the pilot study phase. Margaret Dando from Age Concern Otago for providing training to the SAYGO facilitators, Older Persons’ Health Canterbury District Health Board for providing training to the Senior Chef facilitators and the local stakeholders (Anglican Care Whangarei, Howick Communicare, Regent Community Trust 4Cs, Senior Citizens Whangarei, local Age Concern) for being instrumental in the delivery of the program.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A

Table A1.
Univariate characteristics associated with DQI-I and subcomponents.
Table A1.
Univariate characteristics associated with DQI-I and subcomponents.
| Demographic and Health Variables | DQI-I | Variety | Adequacy | Moderation | Balance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Std. Error | p-Value | B | Std. Error | p-Value | B | Std. Error | p-Value | B | Std. Error | p-Value | B | Std. Error | p-Value | |
| Age, (<80 years; reference: 80+ years) | −1.474 | 0.7572 | 0.052 | −0.247 | 0.3085 | 0.423 | −0.426 | 0.4101 | 0.299 | −0.622 | 0.3931 | 0.114 | −0.178 | 0.1395 | 0.202 |
| Sex (male; reference: female) | −0.776 | 0.7718 | 0.314 | 0.375 | 0.3132 | 0.231 | 0.083 | 0.4173 | 0.843 | −1.394 | 0.3953 | <0.001 | 0.159 | 0.1418 | 0.261 |
| Ethnic group (European; reference: non-European) | 2.260 | 1.2934 | 0.081 | 0.458 | 0.5264 | 0.384 | 1.381 | 0.6979 | 0.048 | 0.117 | 0.6727 | 0.862 | 0.305 | 0.2381 | 0.200 |
| Education level (primary; reference: tertiary) | 0.041 | 2.2660 | 0.985 | −0.108 | 0.9196 | 0.907 | −1.280 | 1.2224 | 0.295 | 1.201 | 1.1728 | 0.306 | 0.227 | 0.4164 | 0.585 |
| (Secondary; reference: tertiary) | −0.079 | 0.7784 | 0.919 | −0.225 | 0.3159 | 0.477 | −0.205 | 0.4199 | 0.625 | 0.365 | 0.4029 | 0.365 | −0.014 | 0.1430 | 0.924 |
| Live with (alone; reference: others) | 1.578 | 0.7619 | 0.038 | 0.356 | 0.3103 | 0.251 | 0.341 | 0.4131 | 0.409 | 0.999 | 0.3941 | 0.011 | −0.118 | 0.1406 | 0.399 |
| Deprivation (reference: high) Low | 1.959 | 0.9405 | 0.037 | 0.604 | 0.3827 | 0.114 | 1.137 | 0.5072 | 0.025 | −0.082 | 0.4900 | 0.867 | 0.300 | 0.1731 | 0.083 |
| Medium | 1.621 | 0.9595 | 0.091 | 0.474 | 0.3904 | 0.225 | 1.112 | 0.5174 | 0.032 | −0.244 | 0.4998 | 0.626 | 0.279 | 0.1766 | 0.115 |
| Medical conditions (1; reference: 2+) | 0.120 | 0.8882 | 0.892 | −0.004 | 0.3606 | 0.992 | −0.244 | 0.4796 | 0.611 | 0.129 | 0.4604 | 0.779 | 0.238 | 0.1629 | 0.143 |
| Vision (impaired; reference: non impaired) | −0.569 | 1.5001 | 0.705 | −0.546 | 0.6086 | 0.370 | 0.513 | 0.8100 | 0.527 | −0.108 | 0.7778 | 0.890 | −0.428 | 0.2751 | 0.120 |
| Hearing (impaired; reference: non impaired) | 1.966 | 1.4367 | 0.171 | −0.008 | 0.5845 | 0.989 | 1.046 | 0.7760 | 0.178 | 0.950 | 0.7450 | 0.202 | −0.022 | 0.2646 | 0.934 |
| BMI, kg/m2 | −0.194 | 0.0769 | 0.012 | −0.062 | 0.0312 | 0.047 | −0.093 | 0.0416 | 0.025 | −0.023 | 0.0401 | 0.574 | −0.016 | 0.0141 | 0.258 |
| Waist circumference, cm | −0.055 | 0.0298 | 0.064 | −0.009 | 0.0121 | 0.461 | −0.022 | 0.0160 | 0.165 | −0.022 | 0.0155 | 0.150 | −0.002 | 0.0055 | 0.753 |
| Medications (1; reference: 2+) | −1.160 | 1.2710 | 0.361 | 0.403 | 0.5162 | 0.435 | −1.236 | 0.6847 | 0.071 | −0.248 | 0.6594 | 0.707 | −0.079 | 0.2338 | 0.735 |
| Supplements (0; reference: 1+) | −0.133 | 0.7653 | 0.862 | −0.274 | 0.3105 | 0.377 | −0.278 | 0.4132 | 0.501 | 0.106 | 0.3968 | 0.790 | 0.314 | 0.1399 | 0.025 |
| Alcohol consumption (never; reference: regular) | −0.369 | 1.0012 | 0.713 | −0.806 | 0.4055 | 0.047 | −0.311 | 0.5409 | 0.565 | 0.428 | 0.5192 | 0.410 | 0.321 | 0.1835 | 0.081 |
| (Occasional; reference: regular) | 0.806 | 0.8704 | 0.355 | −0.289 | 0.3525 | 0.412 | 0.316 | 0.4702 | 0.501 | 0.501 | 0.4514 | 0.267 | 0.278 | 0.1596 | 0.082 |
| Smoking (non-smoker; reference: smoker) | 4.936 | 3.6754 | 0.179 | 2.202 | 1.4917 | 0.140 | 3.936 | 1.9806 | 0.047 | −2.404 | 1.9059 | 0.207 | 1.202 | 0.6746 | 0.075 |
| NEADL score | 0.219 | 0.1662 | 0.188 | 0.052 | 0.0676 | 0.444 | 0.143 | 0.0897 | 0.112 | 0.042 | 0.0863 | 0.623 | −0.018 | 0.0306 | 0.562 |
| SPPB score | 0.187 | 0.1785 | 0.295 | 0.172 | 0.0721 | 0.017 | 0.123 | 0.0963 | 0.202 | −0.099 | 0.0925 | 0.286 | −0.009 | 0.0328 | 0.781 |
| MoCA score (not impaired; reference: impaired) | −0.507 | 0.9252 | 0.583 | −0.307 | 0.3752 | 0.414 | −0.413 | 0.4986 | 0.408 | 0.700 | 0.4779 | 0.143 | −0.488 | 0.1686 | 0.004 |
| GDS score (no depression; reference: depression) | −0.228 | 1.0013 | 0.820 | −0.124 | 0.4065 | 0.760 | 0.028 | 0.5409 | 0.959 | −0.313 | 0.5190 | 0.546 | 0.181 | 0.1839 | 0.324 |
Italicized p-values are significant at the 0.05 level.

Table A2.
Univariate characteristics associated with DQI-I and subcomponents for LER only.
Table A2.
Univariate characteristics associated with DQI-I and subcomponents for LER only.
| Demographic and Health Variables | DQI-I | Variety | Adequacy | Moderation | Balance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Std. Error | p-Value | B | Std. Error | p-Value | B | Std. Error | p-Value | B | Std. Error | p-Value | B | Std. Error | p-Value | |
| Age, (<80 years; reference: 80+ years) | −1.838 | 1.3889 | 0.186 | −0.344 | 0.5442 | 0.527 | −0.754 | 0.7696 | 0.327 | −0.600 | 0.6749 | 0.374 | −1.404 | 2.4930 | 0.573 |
| Sex (male; reference: female) | −0.732 | 1.4186 | 0.606 | −0.112 | 0.5537 | 0.840 | 0.697 | 0.7826 | 0.373 | −1.763 | 0.6726 | 0.009 | 4.461 | 2.5103 | 0.076 |
| Ethnic group (European; reference: non-European) | −0.733 | 2.4075 | 0.761 | 0.019 | 0.9393 | 0.983 | 0.098 | 1.3309 | 0.941 | −1.502 | 1.1601 | 0.195 | 6.520 | 4.2694 | 0.127 |
| Education level (primary; reference: tertiary) | 0.734 | 4.4166 | 0.868 | 1.427 | 1.7175 | 0.406 | 0.094 | 2.4257 | 0.969 | 0.542 | 2.1298 | 0.799 | −13.295 | 7.8215 | 0.089 |
| (Secondary; reference: tertiary) | −0.736 | 1.4238 | 0.605 | −0.325 | 0.5537 | 0.557 | −1.146 | 0.7820 | 0.143 | 0.896 | 0.6866 | 0.192 | −1.615 | 2.5214 | 0.522 |
| Live with (alone; reference: others) | 2.731 | 1.4060 | 0.052 | 1.038 | 0.5488 | 0.058 | 1.034 | 0.7822 | 0.186 | 0.956 | 0.6851 | 0.163 | −2.985 | 2.5313 | 0.238 |
| Deprivation (low; reference: high) | 2.422 | 1.7735 | 0.172 | 0.049 | 0.6975 | 0.943 | 1.910 | 0.9638 | 0.047 | 0.030 | 0.8638 | 0.972 | 4.318 | 3.1674 | 0.173 |
| (Medium; reference: high) | 2.507 | 1.7245 | 0.146 | 0.106 | 0.6782 | 0.876 | 2.570 | 0.9372 | 0.006 | −0.630 | 0.8400 | 0.453 | 4.612 | 3.0799 | 0.134 |
| Medical conditions (1; reference: 2+) | −1.263 | 1.5795 | 0.424 | −0.183 | 0.6172 | 0.767 | −0.657 | 0.8731 | 0.452 | −0.709 | 0.7646 | 0.354 | 2.858 | 2.8181 | 0.311 |
| Vision (impaired; reference: non impaired) | −4.390 | 2.7860 | 0.115 | −1.730 | 1.0865 | 0.111 | −0.623 | 1.5515 | 0.688 | −1.651 | 1.3539 | 0.223 | −3.856 | 5.0079 | 0.441 |
| Hearing (impaired; reference: non impaired) | 1.092 | 2.4885 | 0.661 | −0.430 | 0.9706 | 0.658 | 0.514 | 1.3755 | 0.709 | 1.280 | 1.2017 | 0.287 | −2.720 | 4.4429 | 0.540 |
| BMI, kg/m2 | −0.001 | 0.1381 | 0.994 | 0.066 | 0.0535 | 0.220 | 0.125 | 0.0757 | 0.098 | −0.153 | 0.0657 | 0.020 | −0.388 | 0.2447 | 0.113 |
| Waist circumference, cm | 0.046 | 0.0558 | 0.406 | 0.028 | 0.0217 | 0.199 | 0.082 | 0.0298 | 0.006 | −0.059 | 0.0268 | 0.029 | −0.050 | 0.1007 | 0.618 |
| Medications (1; reference: 2+) | −2.636 | 2.0071 | 0.189 | 0.090 | 0.7873 | 0.909 | −1.769 | 1.1062 | 0.110 | −0.823 | 0.9755 | 0.399 | −1.339 | 3.6044 | 0.710 |
| Supplements (0; reference: 1+) | −0.095 | 1.4198 | 0.947 | −0.367 | 0.5530 | 0.507 | −0.393 | 0.7840 | 0.616 | 0.121 | 0.6877 | 0.860 | 5.436 | 2.4976 | 0.030 |
| Alcohol consumption (never; reference: regular) | −2.726 | 1.8244 | 0.135 | −0.643 | 0.7194 | 0.371 | −1.759 | 1.0080 | 0.081 | 0.045 | 0.8956 | 0.960 | −3.688 | 3.2937 | 0.263 |
| (Occasional; reference: regular) | 1.112 | 1.5913 | 0.485 | 0.185 | 0.6275 | 0.768 | 0.318 | 0.8793 | 0.718 | 0.614 | 0.7812 | 0.432 | −0.049 | 2.8729 | 0.986 |
| Smoking (non-smoker; reference: smoker) | 5.788 | 8.5987 | 0.501 | 1.192 | 3.3576 | 0.723 | 5.772 | 4.7360 | 0.223 | −0.797 | 4.1710 | 0.849 | −3.793 | 15.3810 | 0.805 |
| NEADL score | 0.057 | 0.2766 | 0.836 | 0.009 | 0.1079 | 0.935 | 0.006 | 0.1529 | 0.969 | 0.063 | 0.1339 | 0.638 | −0.207 | 0.4940 | 0.676 |
| SPPB score | −0.080 | 0.3031 | 0.791 | 0.049 | 0.1182 | 0.681 | −0.035 | 0.1675 | 0.836 | −0.112 | 0.1466 | 0.444 | 0.179 | 0.5414 | 0.740 |
| MoCA score (not impaired; reference: impaired) | 1.281 | 1.7996 | 0.477 | 0.604 | 0.6973 | 0.386 | 0.868 | 0.9930 | 0.382 | 0.050 | 0.8717 | 0.954 | −2.413 | 3.2173 | 0.453 |
| GDS score (no depression; reference: depression) | 0.079 | 1.8228 | 0.965 | 0.909 | 0.7071 | 0.199 | −0.094 | 1.0073 | 0.926 | −0.783 | 0.8807 | 0.374 | 0.474 | 3.2561 | 0.884 |
Italicized p-values are significant at the 0.05 level.

Table A3.
Univariate characteristics associated with DQI-I and subcomponents for Plausible reporters only.
Table A3.
Univariate characteristics associated with DQI-I and subcomponents for Plausible reporters only.
| Demographic and Health Variables | DQI-I | Variety | Adequacy | Moderation | Balance | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | Std. Error | p-Value | B | Std. Error | p-Value | B | Std. Error | p-Value | B | Std. Error | p-Value | B | Std. Error | p-Value | |
| Age, (<80 years; reference: 80+ years) | −1.252 | 0.8823 | 0.156 | −0.108 | 0.3431 | 0.753 | −0.157 | 0.4212 | 0.709 | −0.761 | 0.4480 | 0.090 | −2.258 | 1.6636 | 0.175 |
| Sex (male; reference: female) | −0.730 | 0.8991 | 0.417 | 0.603 | 0.3472 | 0.083 | −0.188 | 0.4282 | 0.661 | −1.190 | 0.4527 | 0.009 | 0.450 | 1.6962 | 0.791 |
| Ethnic group (European; reference: non-European) | 3.720 | 1.4864 | 0.012 | 0.680 | 0.5807 | 0.242 | 2.026 | 0.7053 | 0.004 | 0.864 | 0.7618 | 0.257 | 1.508 | 2.8284 | 0.594 |
| Education level (primary; reference: tertiary) | −0.399 | 2.5654 | 0.876 | −0.879 | 0.9930 | 0.376 | −2.029 | 1.2146 | 0.095 | 1.627 | 1.3014 | 0.211 | 8.825 | 4.8104 | 0.067 |
| (Secondary; reference: tertiary) | 0.228 | 0.9074 | 0.802 | −0.238 | 0.3512 | 0.499 | 0.190 | 0.4296 | 0.659 | 0.192 | 0.4603 | 0.676 | 0.836 | 1.7014 | 0.623 |
| Live with (alone; reference: others) | 0.805 | 0.8860 | 0.363 | −0.106 | 0.3439 | 0.757 | −0.237 | 0.4221 | 0.574 | 1.182 | 0.4461 | 0.008 | −0.335 | 1.6721 | 0.841 |
| Deprivation (low; reference: high) | 1.705 | 1.0818 | 0.115 | 0.750 | 0.4183 | 0.073 | 0.658 | 0.5155 | 0.202 | 0.020 | 0.5522 | 0.970 | 2.769 | 2.0418 | 0.175 |
| (Medium; reference: high) | 1.378 | 1.1286 | 0.222 | 0.821 | 0.4363 | 0.060 | 0.630 | 0.5378 | 0.241 | −0.238 | 0.5760 | 0.680 | 1.646 | 2.1300 | 0.440 |
| Medical conditions (1; reference: 2+) | 1.014 | 1.0485 | 0.333 | 0.256 | 0.4068 | 0.529 | 0.208 | 0.4997 | 0.677 | 0.369 | 0.5336 | 0.489 | 1.815 | 1.9767 | 0.359 |
| Vision (impaired; reference: non impaired) | 1.257 | 1.7273 | 0.467 | 0.031 | 0.6702 | 0.963 | 1.107 | 0.8205 | 0.177 | 0.562 | 0.8785 | 0.523 | −4.422 | 3.2490 | 0.173 |
| Hearing (impaired; reference: non impaired) | 2.267 | 1.7240 | 0.188 | 0.040 | 0.6702 | 0.953 | 1.069 | 0.8206 | 0.193 | 1.002 | 0.8772 | 0.254 | 1.581 | 3.2574 | 0.627 |
| BMI, kg/m2 | −0.171 | 0.1028 | 0.096 | 0.000 | 0.0399 | 0.991 | −0.011 | 0.0491 | 0.817 | −0.134 | 0.0520 | 0.010 | −0.249 | 0.1936 | 0.198 |
| Waist circumference, cm | −0.064 | 0.0367 | 0.083 | 0.013 | 0.0143 | 0.345 | −0.015 | 0.0175 | 0.383 | −0.056 | 0.0185 | 0.002 | −0.055 | 0.0695 | 0.428 |
| Medications (1; reference: 2+) | 0.726 | 1.6297 | 0.656 | 1.286 | 0.6278 | 0.041 | 0.122 | 0.7759 | 0.875 | −0.556 | 0.8283 | 0.502 | −1.265 | 3.0719 | 0.681 |
| Supplements (0; reference: 1+) | 0.053 | 0.8877 | 0.953 | −0.118 | 0.3441 | 0.733 | −0.017 | 0.4225 | 0.969 | −0.023 | 0.4514 | 0.960 | 2.096 | 1.6690 | 0.209 |
| Alcohol consumption (never; reference: regular) | 1.012 | 1.1664 | 0.386 | −0.817 | 0.4504 | 0.070 | 0.564 | 0.5548 | 0.309 | 0.581 | 0.5930 | 0.327 | 6.840 | 2.1656 | 0.002 |
| (Occasional; reference: regular) | 0.755 | 1.0085 | 0.454 | −0.405 | 0.3894 | 0.298 | 0.461 | 0.4797 | 0.337 | 0.311 | 0.5127 | 0.544 | 3.883 | 1.8724 | 0.038 |
| Smoking (non-smoker; reference: smoker) | 5.214 | 3.9235 | 0.184 | 2.823 | 1.5169 | 0.063 | 4.061 | 1.8586 | 0.029 | −3.235 | 1.9923 | 0.104 | 15.647 | 7.3633 | 0.034 |
| NEADL score | 0.266 | 0.2050 | 0.195 | 0.038 | 0.0796 | 0.632 | 0.164 | 0.0974 | 0.092 | 0.077 | 0.1044 | 0.462 | −0.132 | 0.3873 | 0.733 |
| SPPB score | 0.195 | 0.2209 | 0.377 | 0.135 | 0.0854 | 0.114 | 0.029 | 0.1052 | 0.784 | 0.047 | 0.1124 | 0.674 | −0.162 | 0.4167 | 0.697 |
| MoCA score (not impaired; reference: impaired) | −0.982 | 1.0486 | 0.349 | −0.505 | 0.4061 | 0.213 | −0.650 | 0.4985 | 0.192 | 0.777 | 0.5322 | 0.144 | −6.024 | 1.9498 | 0.002 |
| GDS score (no depression; reference: depression) | −0.397 | 1.1694 | 0.734 | −0.683 | 0.4518 | 0.131 | 0.037 | 0.5567 | 0.947 | −0.016 | 0.5948 | 0.979 | 2.647 | 2.1996 | 0.229 |
Italicized p-values are significant at the 0.05 level.
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A 2001, 56, M146–M157. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2020, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.; Twitchin, S.; Kletchko, S.; Ryan, F. The living environments of community-dwelling older people who become frail: Another look at the living standards of older New Zealanders survey. Soc. Policy J. N. Z. 2006, 28, 133–157. [Google Scholar]
- Verlaan, S.; Ligthart-Melis, G.C.; Wijers, S.L.J.; Cederholm, T.; Maier, A.B.; de van der Schueren, M.A.E. High Prevalence of Physical Frailty Among Community-Dwelling Malnourished Older Adults–A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2017, 18, 374–382. [Google Scholar] [CrossRef]
- Hirani, V.; Naganathan, V.; Blyth, F.; Le Couteur, D.G.; Seibel, M.J.; Waite, L.M.; Handelsman, D.J.; Cumming, R.G. Longitudinal associations between body composition, sarcopenic obesity and outcomes of frailty, disability, institutionalisation and mortality in community-dwelling older men: The Concord Health and Ageing in Men Project. Age Ageing 2016, 46, 413–420. [Google Scholar] [CrossRef]
- Crow, R.S.; Lohman, M.C.; Titus, A.J.; Cook, S.B.; Bruce, M.L.; Mackenzie, T.A.; Bartels, S.J.; Batsis, J.A. Association of Obesity and Frailty in Older Adults: NHANES 1999–2004. J. Nutr. Health Aging 2019, 23, 138–144. [Google Scholar] [CrossRef]
- Villareal, D.T.; Banks, M.; Siener, C.; Sinacore, D.R.; Klein, S. Physical Frailty and Body Composition in Obese Elderly Men and Women. Obes. Res. 2004, 12, 913–920. [Google Scholar] [CrossRef]
- Woo, J.; Leung, J.; Kwok, T. BMI, Body Composition, and Physical Functioning in Older Adults. Obesity 2007, 15, 1886–1894. [Google Scholar] [CrossRef]
- Lang, P.O.; Michel, J.P.; Zekry, D. Frailty Syndrome: A Transitional State in a Dynamic Process. Gerontology 2009, 55, 539–549. [Google Scholar] [CrossRef]
- Wirt, A.; Collins, C.E. Diet quality—What is it and does it matter? Public Health Nutr. 2009, 12, 2473–2492. [Google Scholar] [CrossRef] [PubMed]
- Payette, H.; Shatenstein, B. Determinants of Healthy Eating in Community-dwelling Elderly People. Can. J. Public Health 2005, 96, S30–S35. [Google Scholar] [CrossRef]
- Thiele, S.; Mensink, G.B.M.; Beitz, R. Determinants of diet quality. Public Health Nutr. 2004, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, M.G.; Milte, C.M.; Crawford, D.; McNaughton, S.A. A Revised Australian Dietary Guideline Index and Its Association with Key Sociodemographic Factors, Health Behaviors and Body Mass Index in Peri-Retirement Aged Adults. Nutrients 2016, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Okada, K.; Matsushita, E.; Uno, C.; Satake, S.; Arakawa Martins, B.; Kuzuya, M. Sex-Specific Association between Social Frailty and Diet Quality, Diet Quantity, and Nutrition in Community-Dwelling Elderly. Nutrients 2020, 12, 2845. [Google Scholar] [CrossRef]
- Host, A.; McMahon, A.-T.; Walton, K.; Charlton, K. Factors Influencing Food Choice for Independently Living Older People—A Systematic Literature Review. J. Nutr. Gerontol. Geriatr. 2016, 35, 67–94. [Google Scholar] [CrossRef]
- Bloom, I.; Edwards, M.; Jameson, K.A.; Syddall, H.E.; Dennison, E.; Gale, C.R.; Baird, J.; Cooper, C.; Sayer, A.A.; Robinson, S. Influences on diet quality in older age: The importance of social factors. Age Ageing 2017, 46, 277–283. [Google Scholar] [CrossRef]
- Atkins, J.L.; Ramsay, S.E.; Whincup, P.H.; Morris, R.W.; Lennon, L.T.; Wannamethee, S.G. Diet quality in older age: The influence of childhood and adult socio-economic circumstances. Br. J. Nutr. 2015, 113, 1441–1452. [Google Scholar] [CrossRef]
- Bailey, R.L.; Harris Ledikwe, J.; Smiciklas-Wright, H.; Mitchell, D.C.; Jensen, G.L. Persistent oral health problems associated with comorbidity and impaired diet quality in older adults. J. Am. Diet. Assoc. 2004, 104, 1273–1276. [Google Scholar] [CrossRef]
- Deierlein, A.L.; Morland, K.B.; Scanlin, K.; Wong, S.; Spark, A. Diet Quality of Urban Older Adults Age 60 to 99 Years: The Cardiovascular Health of Seniors and Built Environment Study. J. Acad. Nutr. Diet. 2014, 114, 279–287. [Google Scholar] [CrossRef][Green Version]
- Schoufour, J.D.; de Jonge, E.A.L.; Kiefte-de Jong, J.C.; van Lenthe, F.J.; Hofman, A.; Nunn, S.P.T.; Franco, O.H. Socio-economic indicators and diet quality in an older population. Maturitas 2018, 107, 71–77. [Google Scholar] [CrossRef]
- de Souza Fernandes, D.P.; Duarte, M.S.L.; Pessoa, M.C.; do Carmo Castro Franceschini, S.; Ribeiro, A.Q. Evaluation of diet quality of the elderly and associated factors. Arch. Gerontol. Geriatr. 2017, 72, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Freitas, T.; Previdelli, A.; Ferreira, M.; Marques, K.; Goulart, R.; Aquino, R. Factors associated with diet quality of older adults. Rev. Nutr. 2017, 30, 297–306. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.J.; Kim, K. Do Where the Elderly Live Matter? Factors Associated with Diet Quality among Korean Elderly Population Living in Urban Versus Rural Areas. Nutrients 2020, 12, 1314. [Google Scholar] [CrossRef]
- Bloom, I.; Lawrence, W.; Barker, M.; Baird, J.; Dennison, E.; Sayer, A.A.; Cooper, C.; Robinson, S. What influences diet quality in older people? A qualitative study among community-dwelling older adults from the Hertfordshire Cohort Study, U.K. Public Health Nutr. 2017, 20, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Nohan, A.F.; Adznam, S.N.A.; Jamaluddin, R.; Norazman, C.W. Diet quality and its associated factors among community dwelling older adults in urban district in Kuala Lumpur, Malaysia. Malays. J. Med. Health Sci. 2020, 16, 153–162. [Google Scholar]
- Wong, J.E.; Haszard, J.J.; Howe, A.S.; Parnell, W.R.; Skidmore, P.M.L. Development of a Healthy Dietary Habits Index for New Zealand Adults. Nutrients 2017, 9, 454. [Google Scholar] [CrossRef]
- Kim, C.-O. Food choice patterns among frail older adults: The associations between social network, food choice values, and diet quality. Appetite 2016, 96, 116–121. [Google Scholar] [CrossRef]
- Tay, E.; Barnett, D.; Leilua, E.; Kerse, N.; Rowland, M.; Rolleston, A.; Waters, D.L.; Edlin, R.; Connolly, M.; Hale, L.; et al. The Diet Quality and Nutrition Inadequacy of Pre-Frail Older Adults in New Zealand. Nutrients 2021, 13, 2384. [Google Scholar] [CrossRef]
- Teh, R.; Kerse, N.; Waters, D.L.; Hale, L.; Pillai, A.; Leilua, E.; Tay, E.; Rolleston, A.; Edlin, R.; Maxted, E.; et al. Study protocol of a randomised controlled trial to examine the impact of a complex intervention in pre-frail older adults. Aging Clin. Exp. Res. 2019, 31, 1407–1417. [Google Scholar] [CrossRef]
- Statistics New Zealand. National and Subnational Period Life Tables: 2017–2019; New Zealand Government: Wellington, New Zealand, 2021. [Google Scholar]
- Simpson, E.; Bradley, J.; Poliakov, I.; Jackson, D.; Olivier, P.; Adamson, A.J.; Foster, E. Iterative Development of an Online Dietary Recall Tool: INTAKE24. Nutrients 2017, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Dietary Intake Data were Collected Using Intake24.org (NZ 2018): An Open Source Dietary Assessment Research Tool, Freely Available to Researchers, Maintained and Developed by the Nutrition Measurement Platform, MRC Epidemiology Unit, University of Cambridge, in Collaboration with Open Lab, Newcastle University. Available online: https://www.mrc-epid.cam.ac.uk/research/measurement-platform/dietary-assessment/intake24/ (accessed on 20 August 2023).
- Adamson, A.; Davies, K.; Wham, C.; Kepa, M.; Foster, E.; Jones, A.; Mathers, J.; Granic, A.; Teh, R.; Moyes, S.; et al. Assessment of Dietary Intake in Three Cohorts of Advanced Age in Two Countries: Methodology Challenges. J. Nutr. Health Aging 2023, 27, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Sivakumaran, S.; Huffman, L.; Gilmore, Z.; Sivakumaran, S. New Zealand FOODfiles 2016 Manual; The New Zealand Institute for Plant & Food Research Limited and Ministry of Health: Auckland, New Zealand, 2017. [Google Scholar]
- Kim, S.; Haines, P.S.; Siega-Riz, A.M.; Popkin, B.M. The Diet Quality Index-International (DQI-I) Provides an Effective Tool for Cross-National Comparison of Diet Quality as Illustrated by China and the United States. J. Nutr. 2003, 133, 3476–3484. [Google Scholar] [CrossRef]
- Chan, R.; Leung, J.; Woo, J. Dietary Patterns and Risk of Frailty in Chinese Community-Dwelling Older People in Hong Kong: A Prospective Cohort Study. Nutrients 2015, 7, 7070–7084. [Google Scholar] [CrossRef] [PubMed]
- Lemon, S.C.; Roy, J.; Clark, M.A.; Friedmann, P.D.; Rakowski, W. Classification and regression tree analysis in public health: Methodological review and comparison with logistic regression. Ann. Behav. Med. 2003, 26, 172–181. [Google Scholar] [CrossRef]
- Pereira, M.H.Q.; Pereira, M.L.A.S.; Campos, G.C.; Molina, M.C.B. Food insecurity and nutritional status among older adults: A systematic review. Nutr. Rev. 2021, 80, 631–644. [Google Scholar] [CrossRef]
- Wham, C.A.; Teh, R.; Moyes, S.; Dyall, L.; Kepa, M.; Hayman, K.; Kerse, N. Health and social factors associated with nutrition risk: Results from life and living in advanced age: A cohort study in New Zealand (LILACS NZ). J. Nutr. Health Aging 2015, 19, 637–645. [Google Scholar] [CrossRef]
- O’Keeffe, M.; Kelly, M.; O’Herlihy, E.; O’Toole, P.W.; Kearney, P.M.; Timmons, S.; O’Shea, E.; Stanton, C.; Hickson, M.; Rolland, Y.; et al. Potentially modifiable determinants of malnutrition in older adults: A systematic review. Clin. Nutr. Off. J. Eur. Soc. Parenter. Enter. Nutr. 2019, 38, 2477–2498. [Google Scholar] [CrossRef]
- Choi, Y.J.; Ailshire, J.A.; Crimmins, E.M. Living alone, social networks in neighbourhoods, and daily fruit and vegetable consumption among middle-aged and older adults in the USA. Public Health Nutr. 2020, 23, 3315–3323. [Google Scholar] [CrossRef]
- Lane, K.; Poland, F.; Fleming, S.; Lambert, N.; Macdonald, H.; Potter, J.; Raats, M.; Skidmore, P.; Vince, C.; Wellings, A.; et al. Older women’s reduced contact with food in the Changes Around Food Experience (CAFE) study: Choices, adaptations and dynamism. Ageing Soc. 2014, 34, 645–669. [Google Scholar] [CrossRef]
- Breslow, R.A.; Guenther, P.M.; Smothers, B.A. Alcohol Drinking Patterns and Diet Quality: The 1999–2000 National Health and Nutrition Examination Survey. Am. J. Epidemiol. 2006, 163, 359–366. [Google Scholar] [CrossRef]
- MacLean, R.R.; Cowan, A.; Vernarelli, J.A. More to gain: Dietary energy density is related to smoking status in US adults. BMC Public Health 2018, 18, 365. [Google Scholar] [CrossRef] [PubMed]
- Alkerwi Aa Baydarlioglu, B.; Sauvageot, N.; Stranges, S.; Lemmens, P.; Shivappa, N.; Hébert, J.R. Smoking status is inversely associated with overall diet quality: Findings from the ORISCAV-LUX study. Clin. Nutr. 2017, 36, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Shatenstein, B.; Ferland, G.; Belleville, S.; Gray-Donald, K.; Kergoat, M.-J.; Morais, J.; Gaudreau, P.; Payette, H.; Greenwood, C. Diet quality and cognition among older adults from the NuAge study. Exp. Gerontol. 2012, 47, 353–360. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Andreyeva, T. Diet Quality and Health in Older Americans. Nutrients 2022, 14, 1198. [Google Scholar] [CrossRef] [PubMed]
- Ward, R.E.; Orkaby, A.R.; Chen, J.; Hshieh, T.T.; Driver, J.A.; Gaziano, J.M.; Djousse, L. Association between Diet Quality and Frailty Prevalence in the Physicians’ Health Study. J. Am. Geriatr. Soc. 2020, 68, 770–776. [Google Scholar] [CrossRef]
- Feart, C. Nutrition and frailty: Current knowledge. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 95, 109703. [Google Scholar] [CrossRef]
- Wray, C.M.; Byers, A.L. Methodological Progress Note: Classification and Regression Tree Analysis. J. Hosp. Med. 2020, 15, 549–551. [Google Scholar] [CrossRef]
- Aguayo, G.A.; Donneau, A.-F.; Vaillant, M.T.; Schritz, A.; Franco, O.H.; Stranges, S.; Malisoux, L.; Guillaume, M.; Witte, D.R. Agreement Between 35 Published Frailty Scores in the General Population. Am. J. Epidemiol. 2017, 186, 420–434. [Google Scholar] [CrossRef]
- University of Otago and Ministry of Health. A Focus on Nutrition: Key Findings of the 2008/09 New Zealand Adult Nutrition Survey; Ministry of Health: Wellington, New Zealand, 2011. [Google Scholar]
- Adamson, A.J.; Collerton, J.; Davies, K.; Foster, E.; Jagger, C.; Stamp, E.; Mathers, J.C.; Kirkwood, T.; Newcastle 85+ Study Core Team. Nutrition in advanced age: Dietary assessment in the Newcastle 85+ study. Eur. J. Clin. Nutr. 2009, 63, S6–S18. [Google Scholar] [CrossRef] [PubMed]
- Statistics New Zealand. Ethnic Group by Age and Sex, for the Census Usually Resident Population Count, 2013 and 2018 Census. Available online: https://nzdotstat.stats.govt.nz/wbos/Index.aspx?DataSetCode=TABLECODE8320 (accessed on 20 August 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).